Abstract
Endocannabinoids are lipid mediators that interact with the same cannabinoid receptors that recognize Δ9-tetrahydrocannabinol (THC), the psychoactive constituent of marijuana, to induce similar effects in the brain and periphery. Alcohol and THC are both addictive substances whose acute use elicits rewarding effects that can lead to chronic and compulsive use via engaging similar signaling pathways in the brain. In the liver, both alcohol and endocannabinoids activate lipogenic gene expression leading to fatty liver disease. This review focuses on evidence accumulated over the last two decades to indicate that both the addictive neural effects of ethanol and its organ toxic effects in the liver and elsewhere are mediated, to a large extent, by endocannabinoids signaling via cannabinoid-1 receptors (CB1R). The therapeutic potential of CB1R blockade, globally or in peripheral tissues only is also discussed.
Keywords: endocannabinoids, cannabinoid receptors, alcohol drinking behavior, alcoholic liver disease
THE ENDOCANNABINOID SYSTEM (ECS)
In recent decades, there has been growing interest in the biology of cannabinoids, fueled in part by the potential health benefits of marijuana and its different constituents but also by the discovery of endogenous cannabinoids (endocannabinoids) and their role in biological functions in health and disease. The story of endocannabinoids started in the late 1980’s with the discovery that the biological effects of Δ9-tetrahydrocannabinol (THC), the psychoactive component of marijuana, are mediated by G-protein-coupled receptors (GPCR) that negatively regulate adenylate cyclase (Devane et al., 1988). This was contrary to the earlier prevailing notion that THC, a highly lipophilic compound, acts by non-specific perturbation of membrane lipids. Within a few years, two such GPCR were identified by molecular cloning: the cannabinoid-1 receptor (CB1R) (Matsuda et al., 1990), which is the most abundant receptor in the mammalian brain but is also expressed at much lower yet functionally relevant levels in most peripheral tissues and the CB2R (Munro et al., 1993), which is expressed predominantly, although not exclusively, in cells of the immune and hematopoietic systems. The presence of receptors in mammalian tissues for a plant-derived substance triggered a search for endogenous ligands. In the 1990’s, a number of endogenous, arachidonic acid-containing lipids were found to bind to and activate CB1R and CB2R with high affinity, the two most widely studied ones being anandamide (arachidonoyl ethanolamide) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG)(Mechoulam et al., 1995; Sugiura et al., 1995), collectively called endocannabinoids. Although other GPCR also recognize some cannabinoids and related endogenous lipids (GPR18, GPR55, GPR119, reviewed in (Irving et al., 2017)), the role of these receptors in the biology of endocannabinoids is less well understood. Endocannabinoids, primarily anandamide, also interact with other targets including Transient Receptor Potential (TRP) channels and Peroxisome Proliferation-Activated Receptors (PPAR), albeit with lower affinity than their affinity for cannabinoid receptors. These interactions and their possible physio-pathological implications have been recently reviewed (Iannotti et al., 2016).
Endocannabinoids are generated on demand in the cell membrane from membrane phospholipid precursors, in response to a rise in intracellular calcium or activation of metabotropic GPCR, including metabotropic glutamate receptors. The immediate membrane precursor of anandamide is N-arachidonoyl phosphatidylethanolamine (NAPE), from which it can be generated by multiple parallel pathways including NAPE-specific PLD, as reviewed recently (Hussain et al., 2017), whereas the metabolism of anandamide is catalyzed primarily by fatty acid amide hydrolase (FAAH)(Cravatt et al., 1996). In the brain, 2-AG is generated from diacylglycerol predominantly via diacylglycerol lipase α (DAGLα), whereas in the liver the same process is catalyzed by DAGLβ (Bisogno et al., 2003). Metabolic degradation of 2-AG is catalyzed primarily by monoacylglycerol lipase (MAGL)(Labar et al., 2010), with minor contributions by α/β hydrolases ABHD6 and ABHD12 (Blankman et al., 2007). The endocannabinoids, their cognate receptors and the enzymatic machinery involved in their biosynthesis and degradation constitute the endocannabinoid system (ECS).
During the twenty-five or so years that have passed since their discovery, endocannabinoids have been implicated in the regulation of a growing number of biological functions both in the brain and in the periphery. Such studies were enabled by the introduction of highly selective and potent inhibitors of cannabinoid receptors (Rinaldi-Carmona et al., 1994; Rinaldi-Carmona et al., 1998) and of the enzymes involved in their biosynthesis (Baggelaar et al., 2015) and degradation (Fegley et al., 2005; Long et al., 2009). Additional critical tools for such studies were mice deficient in CB1R (Ledent et al., 1999; Zimmer et al., 1999), FAAH (Cravatt et al., 2001), MAGL (Taschler et al., 2011) and DAGLα and β (Gao et al., 2010). In the CNS, endocannabinoids had been identified as the long sought retrograde transmitters (Alger, 2002) that are generated in postsynaptic cells and act at CB1R located on presynaptic terminals (Katona et al., 1999; Wilson et al., 2001) to mediate some forms of synaptic plasticity (Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). Particularly relevant to the present review, endocannabinoids acting via CB1R are be obligatory components of the mesolimbic dopaminergic reward pathway (Solinas et al., 2008) that mediates both natural and drug reward (Gessa et al., 1998), including the rewarding effects of alcohol (Hungund et al., 2003). The ECS is also present and functional in peripheral tissues (Maccarrone et al., 2015), including the liver where activation of CB1R promotes de novo lipogenesis (Osei-Hyiaman et al., 2005) and mediates the similar action of ethanol (Jeong et al., 2008). Here we review progress in our understanding of the role of the ECS in both ethanol seeking behavior and in ethanol-induced organ damage, with a focus on recent advances. For many important earlier studies, the reader is referred to excellent recent reviews of this topic (Gianessi et al., 2019; Henderson-Redmond et al., 2016; Maccioni et al., 2010; Pava and Woodward, 2012; Siegmund, 2010; Talani and Lovinger, 2015).
SIMILARITIES BETWEEN ALCOHOL AND CANNABIS USE DISORDERS
Well before the ECS or molecular targets for alcohol were discovered and both ethanol and cannabis were thought to act by non-specific perturbation of membrane lipids, intriguing similarities between alcohol use disorders (AUD) and cannabis use disorders (CUD) had been noted. At the epidemiological level, the proportion of the population that becomes dependent on ethanol or cannabis is the highest in adolescents and gradually declines thereafter for both substances (Grant and Dawson, 1997; Stinson et al., 2006). Furthermore, there is a high degree of comorbidity between AUD and CUD, which is strongest in adolescence (Duncan et al., 2015). Probably the closest indication of a functional link between ethanol and cannabis in early studies was evidence for cross-tolerance between their respective behavioral and neurocognitive effects (reviewed in (Pava and Woodward, 2012).
An age-dependent decline in alcohol preference has also been noted in rodent models of drinking behavior. Ethanol preference and intake by the ethanol preferring C57Bl6J mouse strain was twice as high in young (6–10 wk) than old (26–48 wk) male mice (Wang et al., 2003) and a similar large difference, although at lower levels of ethanol preference, had been noted in the alcohol aversive BALB/c mouse strain (Kakihana and McClearn, 1963). This indicated that the age dependence of alcohol preference manifests not only longitudinally in a given strain but also across strains with genetically determined differences in alcohol preference. This age-dependent decline in alcohol preference was linked to the ECS by the observation that rimonabant treatment of young C57Bl/6J mice reduced their alcohol preference to that seen in older animals, but was ineffective in modifying alcohol preference in the latter or in CB1R knockout mice, which displayed similar low alcohol preference in both age groups (Wang et al., 2003). Young and old mice had similar brain levels of CB1R or endocannabinoids but there was an age-dependent decline in CB1R coupling as measured by GTPγS binding in the limbic forebrain, a group of subcortical structures including the hippocampus, hypothalamus and amygdala involved in the control of emotions and motivational behavior, which may account for the observed age-dependent loss of a CB1R-mediated component in drinking preference (Wang et al., 2003). An analogous finding was the increased CB1R coupling efficiency found in the striatum of alcohol-preferring C57Bl/6J versus alcohol aversive DBA/2J mice (Vinod et al., 2008b). Selectively bred rodent lines have been used to explore the genetic determinants of alcohol-seeking behavior, as reviewed recently (Spence et al., 2009).
Similar to the age-dependence of the effect of rimonabant on drinking, pre-pubertal male C57Bl/6J mice tested at postnatal day (PD) 21 in a binge drinking paradigm had higher alcohol intake than adult (PD 63) mice and the CB1R antagonist AM-251 was more potent in reducing drinking in the younger compared to the older group (Agoglia et al., 2016). Studies with agonists also point to an age-dependent decline in cannabinoid/ethanol interactions. Treatment of pubertal (postnatal day 40) or adult Wistar rats (PD 100) with WIN 55,212–2 caused heightened anxiety and increased alcohol preference in the young but not the adult animals, which was linked to a similar age-dependent, WIN 55,212–2-induced increase in the expression of the NR1 subunit of the NMDA receptor and its regulatory protein CC-Homer (Klugmann et al., 2011), suggesting a role of the glycine binding site of the NMDA receptor in cannabinoid-induced drinking. Indeed, the NMDA/glycine receptor antagonist L-701 was reported to inhibit WIN 55,212–2-induced alcohol drinking (Alen et al., 2009). WIN 55,212–2 similarly increased anxiety and alcohol preference in early adolescent CD1 mice (Frontera et al., 2018).
There is also a close similarity between the mechanisms and anatomical substrates involved in ethanol and cannabinoid reward, with the mesolimbic dopaminergic reward pathway being involved in both (Cheer et al., 2007). In freely moving rats, i.p. injections of low doses of ethanol caused dose-dependent release of dopamine in the dopaminergic projection area of the nucleus accumbens (NAc) with less release in the subcortical dorsal caudate nucleus, as measured by in vivo microdialysis (Di Chiara and Imperato, 1988). The increase in ethanol-induced dopamine release in the NAc was greater in alcohol preferring than in alcohol-avoiding rats (Bustamante et al., 2008). Interestingly, salsolinol, an endogenous adduct of dopamine and the alcohol metabolite acetaldehyde that is self-administered into the ventral tegmental area (VTA) where it induces a strong place preference, also activate VTA dopaminergic neurons (Xie et al., 2013). Cannabinoids produce similar effects: CB1R activation by systemic administration of THC or the synthetic cannabinoid WIN55,212–2 to rats elicited dopamine release in the NAc shell as detected by microdialysis (Tanda et al., 1997), and increased the spontaneous firing rate of dopamine neurons projecting to the NAc (Gessa et al., 1998). VTA dopaminergic neurons do not express CB1R and the increase in DA release by cannabinoids is believed to be mediated by CB1R on inhibitory GABAergic interneurons, resulting in disinhibition of VTA dopaminergic neurons and enhanced dopamine release in the NAc (Cheer et al., 2004; Lupica et al., 2004). However, the modulation of dopamine release in the NAc by endocannabinoids is more complex, as activation of CB1R on prefronto-cortical glutamatergic terminals in the NAc reduces the release of glutamate and, consequently, dopamine, which may be relevant for limiting the rewarding effects of acute drug exposure, and may play a role in relapse to drug seeking behavior (Mateo et al., 2017). Whether ethanol has a similar action, has not yet been observed.
ETHANOL EFFECTS ON ENDOCANNABINOID PRODUCTION AND CB1R EXPRESSION
There is fairly consistent evidence that either short-term or chronic exposure of various cultured neuronal cell types to ethanol upregulates the production of both anandamide and 2-AG in a calcium-dependent manner (Basavarajappa and Hungund, 1999; Basavarajappa et al., 2008; Basavarajappa et al., 2000) and acute exposure of alcohol also upregulates anandamide in vivo in the NAc of rats (Ceccarini et al., 2013) or in the hippocampus and cortex of immature mice (Subbanna et al., 2013). Whereas chronic exposure to alcohol can also increase endocannabinoid levels, as found for 2-AG in the dorsolateral striatum (DLS)(DePoy et al., 2013), this can result in the downregulation of CB1R expression in the DLS as well as in other brain regions (Ceccarini et al., 2014; Hirvonen et al., 2013; Ortiz et al., 2004), which may be one of the mechanisms that underlies the negative affect associated with chronic alcoholism.
The striatum is involved in the regulation of voluntary behavior and cognition, which are impaired by chronic alcohol consumption. The mechanisms underlying the effects of alcohol may involve changes in synaptic plasticity: low concentrations of alcohol were found to inhibit NMDA receptor-mediated long-term potentiation (LTP), which may be involved in the acute intoxicating effects, whereas higher concentrations of ethanol (50 mM) promoted long-term depression (LTD) in the dorsomedial striatum, a brain region implicated in the learning and selection of goal-directed behaviors (Yin et al., 2007). There is evidence that these effects of ethanol are mediated via interference with endocannabinoid activation of CB1R. In the dorsolateral striatum (DLS), ethanol inhibits CB1R-mediated long-lasting disinhibition of striatal output and also reduces LTD, induced by different stimulation parameters, at a site downstream from CB1R activation (Clarke and Adermark, 2010). In another study, acute exposure of DLS slices to 50 mM ethanol depressed striatal output measured by population spike amplitude, whereas washout of ethanol had the opposite effect, i.e. facilitation, which is mediated via cholinergic interneurons (Adermark et al., 2011a). These findings reveal complex effects of ethanol on synaptic plasticity and the neural circuits involved, although they do not directly address the role of endocannabinoids. The role of endocannabinoids and CB1R in the DLS was explored in a more recent study, in which chronic intermittent exposure of mice to alcohol vapor resulted in the absence of CB1R-mediated LTD in DLS neurons, which was associated with facilitation of various, DLS-dependent learning paradigms (DePoy et al., 2013).
ENDOCANNABINOID EFFECTS ON ALCOHOL DRINKING BEHAVIOR
There are three lines of evidence supporting the role of the ECS in the regulation of alcohol drinking behavior: a) the effects of direct treatment with a cannabinoid agonist; b) the effects of increased endocannabinoid tone achieved by pharmacological blockade or genetic deletion of endocannabinoid degrading enzymes; and c) the effects of selective cannabinoid receptor antagonists or genetic deletion of these receptors.
a). Agonists.
As for agonist effects, an early study in Wistar rats documented dose-dependent increases in the motivation to drink beer by the potent CB1R agonist CP 55,940, using an operant drinking paradigm. A similar effect on the intake of a sucrose solution suggested a general effect on appetite for palatable beverages rather than a specific effect on alcohol (Gallate et al., 1999). Using an operant drinking paradigm, the same group found that THC can reinstate beer drinking by rats but, again, the same was true for sucrose intake (McGregor et al., 2005). More specificity was observed in ethanol-preferring sP rats, in which similar doses of CP 55,940 or somewhat higher doses of WIN 55,212–2 selectively increased ethanol but not sucrose drinking (Colombo et al., 2002). Low doses of WIN 55,212–2 administered either systemically (0.5 mg/kg) or into the posterior VTA (0.25 μg/side) similarly increased ethanol intake in the ‘drinking in the dark’ binge drinking paradigm. This highlighted the role of CB1R in the pVTA in promoting alcohol drinking presumably by disinhibiting VTA dopaminergic neurons (Linsenbardt and Boehm, 2009), in agreement with findings in alcohol-preferring AA rats (Malinen and Hyytia, 2008). In the same study, higher systemic (1–2 mg/kg) or intra-pVTA doses of WIN 55,212–2 (0.5–2.5 μg/side) suppressed alcohol drinking as well as total water intake, most likely as the result of CB1R-induced hypomotility. This illustrates a pitfall of using direct acting CB1R agonists whose behavioral effects may be confounded by their robust display of the ‘tetrad’ responses that are the hallmarks of CB1R activation (hypomotility, catalepsy, hypothermia and hypoalgesia), particularly hypomotility and catalepsy.
Endocannabinoids and CB1R also appear to mediate the inhibitory effect of ethanol on adult hippocampal neurogenesis. Treatment of Wistar rats with a CB1R agonist or an inhibitor of endocannabinoid degradation mimicked the inhibitory effect of alcohol, whereas treatment with CB1R antagonists prevented alcohol-induced inhibition of adult hippocampal neurogenesis (Khatri et al., 2018).
b). Inhibitors of endocannabinoid degrading enzymes
In contrast to the direct effect of synthetic agonists, the biological effects of endocannabinoids including their effects on alcohol drinking behavior may be unmasked by preventing their metabolic degradation. As mentioned before, anandamide is primarily metabolized by FAAH whereas 2-AG is degraded mainly by MAGL. Both these enzymes are tonically active, so their pharmacological inhibition or genetic deletion results in large and selective increases in the tissue levels of their respective substrates. Anandamide involvement in alcohol preference was first suggested by findings in C57Bl/6J mice with genetic deletion of FAAH (Basavarajappa et al., 2006). Female wild-type mice had higher alcohol preference and intake than littermate male mice and germline deletion of FAAH further increased alcohol preference and intake in female but not male mice (Basavarajappa et al., 2006). No such sex differences were found in other studies, in which genetic deletion of FAAH in mice on a C57Bl/6J genetic background resulted in increased preference for alcohol but not for sweetened or bitter solutions, as well as reduced sensitivity to the sedative and motor coordination disruptive effects of ethanol in both male and female mice (Blednov et al., 2007; Vinod et al., 2008a). These effects were replicated by the treatment of wild-type mice with the FAAH inhibitor URB597, whereas URB597 treatment of CB1R−/− mice did not affect the lower baseline alcohol preference of these animals. This supported the role of CB1R in the increased alcohol preference induced by FAAH inhibition or deletion (Vinod et al., 2008a). Consistent with these findings, alcohol preferring AA rats had decreased expression and activity of FAAH in the prefrontal cortex (PFC), and intra-PFC injections of rimonabant reduced alcohol self-administration, whereas intra-PFC injections of URB597 in non-selected Wistar rats increased alcohol self-administration (Hansson et al., 2007). An association between impaired FAAH activity and increased preference for ethanol drinking was also supported by a study using knock-in mice expressing a mutant FAAH. In human subjects, the C385A single nucleotide polymorphism (SNP) in the FAAH gene was reported to be associated with reduced FAAH expression in peripheral tissues and increased incidence of problem drug use (Sipe et al., 2002). A knock-in mouse harboring the same variant allele confirmed its effect in reducing FAAH expression and activity, resulting in a decrease in anxiety-like behaviors (Dincheva et al., 2015). Binge-like alcohol intake was significantly higher in the FAAHA/A mutant than in the FAAHC/C controls with no difference in saccharin or sucrose intake, and alcohol intake was dose-dependently inhibited by a CB1R antagonist (Zhou et al., 2016).
A very different picture emerges from other studies exploring the role of FAAH in the negative affect associated with chronic alcohol use and withdrawal. In a study using Wistar rats and Marchigian Sardinian alcohol preferring (msP) rats, URB597 treatment neither increased voluntary drinking by msP rats nor facilitated operant alcohol self-administration by Wistar rats, even though rimonabant reduced self-administration. URB597 also failed to influence cue- or stress-induced reinstatement, whereas it robustly suppressed withdrawal-induced anxiety (Cippitelli et al., 2008). URB597 treatment was also reported to prevent the alcohol deprivation effect observed when alcohol is reinstated following withdrawal, which was also blocked by rimonabant (Zhou et al., 2017). An analogous finding is the reported effect of the MAGL inhibitor JZL-184 in mitigating the protracted affective disturbance present in alcohol withdrawn mice, which implicates 2-AG in this protective effect (Holleran et al., 2016). Interestingly, the duality in the effects of FAAH inhibition, i.e. promoting the rewarding salience of ethanol versus inhibiting the negative affect of withdrawal, also applies to MAGL inhibition: a 7-day methamphetamine treatment protocol in mice increased alcohol preference, which was inhibited by the CB1R antagonist AM251 but potentiated by the MAGL inhibitor N-arachidonoyl maleimide (Gutierrez-Lopez et al., 2010).
These seemingly conflicting findings could be reconciled within the framework of the Koobian concept of opponent processes of addiction, i.e. euphoria caused by acute alcohol followed by the dysphoria that develops with repeated withdrawals (Koob, 2019). Accordingly, the acute increase in anandamide induced by FAAH inhibitors promotes alcohol reward and thus increases its abuse potential, which could be therapeutically countered by CB1R antagonists, whereas the negative affect related to alcohol withdrawal may be mitigated by FAAH or MAGL inhibitors by promoting the CB1R-mediated anti-anxiety actions of anandamide or 2-AG, respectively. This concept gained further support from a CB1R PET study in which acute exposure to alcohol resulted in a global increase in CB1R availability in the brain whereas chronic intake in alcoholic patients caused long lasting reductions in CB1R availability in various brain regions including the ventral striatum, which may underlie the negative affect of prolonged withdrawal and abstinence (Ceccarini et al., 2014). A long-lasting global reduction of CB1R availability in chronic alcoholics was also documented in another CB1R PET study (Hirvonen et al., 2013). As a sign of further complexity, the anxiogenic effect of abstinence that followed chronic exposure to alcohol was found to be reduced by rimonabant (Lallemand et al., 2001; Rubio et al., 2008), which stands in contrast to the similar anxiolytic effects of FAAH or MAGL inhibitors.
A possible explanation of this paradox involves relative contribution of activation of CB1R on GABAergic vs glutamatergic terminals. In the above study by Rubio et al. (2008), ethanol withdrawal was associated with significant reductions in GABA in the PFC, caudate-putamen, globus pallidus and amygdala, and the reductions were reversed in the first 3 regions by rimonabant, which may underlie rimonabant’s anxiolytic effect in this paradigm. Consistently, conditional knockout of CB1R in cortical glutamatergic neurons abolished the anxiolytic effects of low dose CB1R agonists, whereas conditional knockout of CB1R in GABAergic neurons resulted in the loss of the anxiogenic effects of high doses of CB1R agonists (Rey et al., 2012).
c). Effects of blockade or deletion of CB1R
The third, and most decisive, argument to support the role of the ECS in mediating alcohol reward comes from studies documenting the effect of pharmacological antagonism or genetic deletion of CB1R on alcohol preference and intake in different drinking paradigms. Soon after the introduction of rimonabant (SR141716), it was reported that systemic administration of rimonabant or its analog SR147778 reduced voluntary alcohol intake in various rodent models of drinking (Arnone et al., 1997; Colombo et al., 1998; Femenia et al., 2010; Gallate and McGregor, 1999; Lallemand and De Witte, 2006; Lallemand et al., 2001; Wang et al., 2003) and also suppressed the motivational properties of alcohol in both wild-type and alcohol-preferring rodents (Colombo et al., 2004; Dyr et al., 2008; Economidou et al., 2006; Gessa et al., 2005; Malinen and Hyytia, 2008; Serra et al., 2001; Vinod et al., 2008b). Furthermore, rimonabant also attenuated the increased intake in alcohol in response to low doses of morphine, highlighting a functional link between the endogenous opioid system and the ECS in the control of alcohol drinking behavior (Vacca et al., 2002). Similar effects were obtained following localized microinjection of rimonabant into the VTA (Malinen and Hyytia, 2008) and in particular its posterior region (Alvarez-Jaimes et al., 2009), into the nucleus accumbens (NAc) of AA mice (Malinen and Hyytia, 2008) or Wistar rats (Alvarez-Jaimes et al., 2009) and, in some rat lines, into the medial prefrontal cortex (Hansson et al., 2007). The observation of a reduced endocannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) in the VTA of alcohol-preferring sP rats compared to non-preferring sNP rats (Melis et al., 2009) may reflect an underlying increase in the tonic activity of the ECS in the former, which would be reversed by CB1R blockade. Together, these findings suggest that components of the mesolimbic dopaminergic reward pathway are one of the targets through which CB1R blockade inhibits alcohol-seeking behavior.
In an in vivo microdialysis study, alcohol self-administration in male Wistar rats elicited a selective increase in the extracellular levels of 2-AG but not anandamide in microdyalisates from the NAc, which could be blocked by rimonabant pretreatment. This is consistent with the following putative circuitry: 2-AG acting at CB1R on glutamatergic terminals reduces glutamate release onto GABAergic medium spiny neurons projecting to the VTA, resulting in reduced GABAergic tone which disinhibits dopaminergic neurons in the VTA, leading to increased dopamine release in the NAc (Caille et al., 2007). However, additional complexity is indicated by the presence of cholinergic interneurons in the NAc. Stimulation of these neurons directly increases DA release in coordination with PFC glutamatergic afferents without involving the VTA, and this effect is inhibited by CB1R present on PFC terminals (Mateo et al., 2017), as discussed before. Further work is required to understand what controls the balance between these opposing, CB1R-mediated effects on accumbal dopamine release.
Interactions between the ECS and alcohol also occur at additional structures, such as the dorsolateral striatum (DLS) (Adermark et al., 2011b), where such interactions may influence reward-guided striatal learning (Gerdeman et al., 2002) and habit formation (Gremel et al., 2016; Hilario et al., 2007), known reinforcers of addictive behavior. Indeed, addictive behaviors, including AUD, are characterized by a degradation of the prefrontal executive control over behavior, which is shifted to the DLS resulting in the reinforcement of compulsive drug seeking behavior (Kalivas and Volkow, 2005), and a similar shift also applies to cannabis addiction (Gremel et al., 2016; Mason et al., 2012). Recent evidence indicates that chronic alcohol exposure may induce such a shift by selectively reducing the excitability of orbital frontal cortex (OFC) excitatory output to the direct output pathway in the dorsal medial striatum (DMS), which normally supports goal-directed behavior (Renteria et al., 2018).
Endocannabinoids acting via CB1R have been implicated in the striatal drive of compulsive alcohol seeking behavior by several lines of evidence, including morphological, electrophysiological and behavioral changes in response to intermittently exposing mice to ethanol vapor (DePoy et al., 2013). Such exposure to ethanol resulted in the expansion of the dendritic arbor of DLS neurons and downregulation of DLS CB1R signaling including a loss of CB1R-dependent LTD. These changes were associated with a facilitation of DLS-dependent learning, as tested in a variety of behavioral paradigms. These findings indicate that the prolonged down-regulation of DLS CB1R signaling in response to chronic alcohol exposure and the resulting DLS neuronal remodeling mediate the shift from prefrontocortical to dorsal striatal learning and the progression of alcoholism (DePoy et al., 2013). Chronic exposure to THC similarly leads to loss of endocannabinoid-mediated LTD in the DLS associated with increased habit learning, which could be rescued by blockade of small conductance, calcium activated potassium (SK) channels (Nazzaro et al., 2012). It would be interesting to test whether SK channel inhibition can similarly rescue normal, goal-directed behavior in mice chronically exposed to alcohol. A very recent study extends these findings by showing that chronic exposure of adolescent mice to alcohol causes a loss of endocannabinoid-mediated LTD in the dentate gyrus molecular layer (DGML) of the hippocampus that is coupled to impaired recognition memory. These effects last into adulthood and are associated with loss of CB1R in the DGML, reduced CB1R-induced G protein activation and increased expression of MAGL. Furthermore, these long-term deficits can be rescued by MAGL inhibitors (Penasco et al., 2020).
Whereas the effects of rimonabant and related CB1R inverse agonists suggest endocannabinoid involvement, they do not prove it, as inverse agonists may produce their effects in the absence of receptor occupancy by endogenous ligands. On the other hand, a biological response to a CB1R neutral antagonist in the absence of prior treatment with an agonist strongly indicates preexisting tonic activation by an endogenous agonist. Indeed, the CB1R neutral antagonist AM4113 decreased binge-like alcohol drinking in male C57Bl6/J mice using a modified two-bottle drinking in the dark paradigm and attenuated alcohol-induced dopamine release in the NAc, as measured by in vivo microdialysis (Balla et al., 2018). This supports the role of endocannabinoids in promoting both alcohol drinking and the release of dopamine in the NAc, as indicated by the inhibition of both by a CB1R neutral antagonist.
Well before the above study, strong evidence for enhanced endocannabinoid/CB1R tone maintaining high alcohol preference has been provided by multiple reports documenting reduced alcohol drinking and preference in CB1R deficient mice. The first two studies also demonstrated age-dependence of the role of endocannabinoids in driving drinking behavior and its association with reduced G protein coupling of CB1R in the limbic forebrain (Wang et al., 2003) and the loss of alcohol-induced dopamine release in the NAc in mice with global deletion of Cnr1 (Hungund et al., 2003). Subsequent studies confirmed and extended these findings. CB1R deficient mice on a mixed C57Bl/6 × 129/Ola F2 genetic background consumed less alcohol and sucrose and had greatly reduced NPY-induced food intake than their wild-type littermates, which phenocopied the effects of rimonabant (Poncelet et al., 2003). In another study using mice on a C57Bl6/J genetic background, deletion of Cnr1 resulted in a complete loss of withdrawal symptoms and an absence of foot-shock stress-induced reinstatement of alcohol drinking (Racz et al., 2003). The inverse relationship between sensitivity to the acute intoxicating effects of alcohol and predisposition for risky drinking in human alcoholics (Schuckit et al., 2019) has been replicated in CB1R-deficient mice whose reduced ethanol preference was associated with increased sensitivity to the sedative/hypnotic effects of alcohol (Naassila et al., 2004). CB1R-deficient mice also display reduced conditioned place preference for alcohol (Houchi et al., 2005; Thanos et al., 2005) and increased levels of striatal D2 receptors (Houchi et al., 2005).
THERAPEUTIC POTENTIAL OF CB1R BLOCKADE IN ALCOHOLISM
The evidence summarized above strongly implicates endocannabinoids acting via CB1R in mediating the motivational effects of alcohol. It also documents the efficacy of CB1R blockade/inverse agonism in attenuating alcohol preference and intake in rodent models, which had triggered two clinical studies. The first one was a multi-center, proof-of-concept, double-blind, placebo-controlled study evaluating rimonabant’s efficacy in preventing relapse drinking in 260 recently detoxified alcohol-dependent patients (Soyka et al., 2008). Rimonabant at a dose of 2 × 10 mg/day for 3 months failed to produce statistically significant effects, although there was a tendency for reduced relapse rate (47.7% in the placebo group vs. 41.5% in the rimonabant group), and this trend was more marked in patients who relapsed to heavy drinking (from 35.6% to 25.7%). There was also a statistically non-significant trend toward the beneficial effects of rimonabant on secondary outcomes including abstinence duration and percentage of drinking days (Soyka et al., 2008). The second study was a double-blind, placebo-controlled trial involving 49 participants, assessing the efficacy of 20 mg/day rimonabant for two weeks in reducing drinking by non-treatment seeking heavy drinkers. Participants reported their daily alcohol intake during the 2 two weeks of call-in, followed by an ‘inpatient’ self-administration paradigm during which the participants had the option of consuming a total of 8 alcohol drinks. Again, there was no significant effect of rimonabant versus placebo, although there was a tendency for reduced number of drinks consumed in the inpatient component of the study by the rimonabant group (3.3. drinks) compared to the placebo group (4.5 drinks) (George et al., 2010).
A major limitation of both studies was the use of a dose of rimonabant (20 mg/day or ~0.3 mg/kg/day) which only produces ~30% CB1R occupancy (Huestis et al., 2001). This dosing regimen was adopted to minimize neuropsychiatric side effects that occur more frequently at higher doses. It is likely that significant reduction in alcohol drinking only occurs at complete or near-complete blockade of CB1R, by analogy to the effects of naltrexone, a μ opioid receptor antagonist. Both compounds are believed to inhibit alcohol-seeking behavior by blocking the activity of the mesolimbic dopaminergic reward pathway. Used in the same laboratory self-administration paradigm as used by George et al., naltrexone significantly inhibited alcohol drinking at doses of 50–150 mg (O’Malley et al., 2002), which produce 100% occupancy of μ opioid receptors (Weerts et al., 2008). This suggests that the therapeutic potential of CB1R blockade could be resurrected if one could minimize the chance of centrally mediated neuropsychiatric side effects that would allow the use of a dose producing full receptor occupancy.
One such approach may be the use of CB1R neutral antagonists, such as AM4113, which has been found to lack some of the behavioral effects attributed to blockade of CB1R in the CNS, such as nausea (Sink et al., 2008) or an enhanced retention of contextual fear conditioning (Sink et al., 2010a), and was less anxiogenic than the CB1R inverse agonist AM251 (Sink et al., 2010b). However, AM4113 also induced disruptive scratching and grooming behavior in mice similar to rimonabant (Jarbe et al., 2008), a behavior considered to be a sensitive predictor of neuropsychiatric side effects in humans (Tallett et al., 2007). Further work is needed to explore the therapeutic potential and CNS safety of CB1R neutral antagonists.
Another approach to avoid the neuropsychiatric side effects associated with CB1R blockade in the CNS is to use second generation, peripherally restricted antagonists. The behavioral and metabolic profile of two such compounds, the neutral CB1R antagonist AM6545 (Tam et al., 2010) and the CB1R inverse agonist JD5037 (Tam et al., 2012) have been characterized in detail in a mouse model of diet-induced obesity. At doses causing maximal inhibition of weight gain and reversal of the metabolic consequences of obesity, neither compound elicited anxiety-like behavior in the elevated plus maze, nor induced hyperambulatory activity associated with excessive grooming and scratching or blocked cannabinoid-induced catalepsy, effects linked to CB1R in the brain and readily induced by brain-penetrant CB1R antagonists such as rimonabant. Unlike rimonabant, they also failed to displace a CB1R PET ligand from binding sites in the brain.
Using such a therapeutic approach may appear counterintuitive given the undisputed role of CNS structures in the control of alcohol seeking behavior, Surprisingly, JD5037 was equally efficacious with its brain-penetrant parent compound ibipinabant in reducing food intake in mice with diet-induced obesity, another centrally regulated behavior (Tam et al., 2012). The mechanism underlying this unexpected finding turned out to involve rapid reversal of the extreme hyperleptinemia of these animals by peripheral CB1R blockade through the reduction of leptin gene expression and secretion by blocking CB1R in adipocytes and a simultaneous increase in the renal clearance of leptin by blocking CB1R in proximal tubular cells (Tam et al., 2012). In agreement with the role of hyperleptinemia in maintaining leptin resistance in obesity (Knight et al., 2010), its rapid reversal by peripheral CB1R blockade resulted in restoration of leptin sensitivity, so the observed hypophagia and associated weight loss could be attributed to endogenous leptin. Accordingly, CB1R blockade failed to reduce food intake and body weight in leptin deficient ob/ob mice, and in diet-induced obese mice these effects could be inhibited by a pegylated leptin receptor antagonist (Tam et al., 2012).
A recent study explored whether a similar periphery-to-brain axis may be involved in regulating alcohol seeking behavior, using the peripheral CB1R inverse agonist JD5037 in a two bottle/free choice as well as a limited access, drinking in the dark paradigm, and male mice on a C57Bl6/J background (Godlewski et al., 2019). JD5037 treatment significantly reduced ethanol preference and intake in wild-type mice but not in mice deficient in CB1R, ghrelin, or the ghrelin receptor GHSR1A, indicating that the stomach-derived hormone ghrelin may be the link between peripheral CB1R and the CNS structures controlling alcohol drinking behavior. JD5037 treatment of alcohol-drinking mice inhibited the formation of biologically active octanoyl-ghrelin but not its precursor desacyl-ghrelin, suggesting that CB1R modulate ghrelin acylation, catalyzed by the enzyme ghrelin O-acyl transferase or GOAT. This was supported by the reduced alcohol preference which was unaffected by JD5037 treatment in mice deficient in Mboat4, the gene encoding GOAT. Ghrelin-producing MGN3–1 mouse stomach ghrelinoma cells express CB1R and generate high levels of endocannabinoids (Godlewski et al., 2019), similar to primary ghrelin-producing cells of the stomach mucosa (Engelstoft et al., 2013). In MGN3–1 cells incubated with deuterated palmitic acid, JD5037 reduced the level of the substrate octanoyl-carnitine generated from palmitoyl-carnitine by increasing the rate of fatty acid β-oxidation, which reduced the amount of octanoyl-ghrelin generated by these cells. This indicated that blockade of CB1R in ghrelin-producing cells reduces ghrelin-acylation by reducing the availability of its substrate octanoic acid. Finally, blocking vagal afferents, which express GHSR1A, abrogated the ability of either CB1R or GHSR1A blockade to reduce alcohol drinking. This suggests that octanoyl-ghrelin released by ghrelin-producing cells activates GHSR1A on neighboring vagal afferent terminals to generate a neural signal to brain structures involved in the control of alcohol drinking behavior (Godlewski et al., 2019). This mechanism is schematically illustrated in Figure 1.
Figure 1. Endocannabinoids modulate alcohol drinking behavior via a stomach/brain axis.
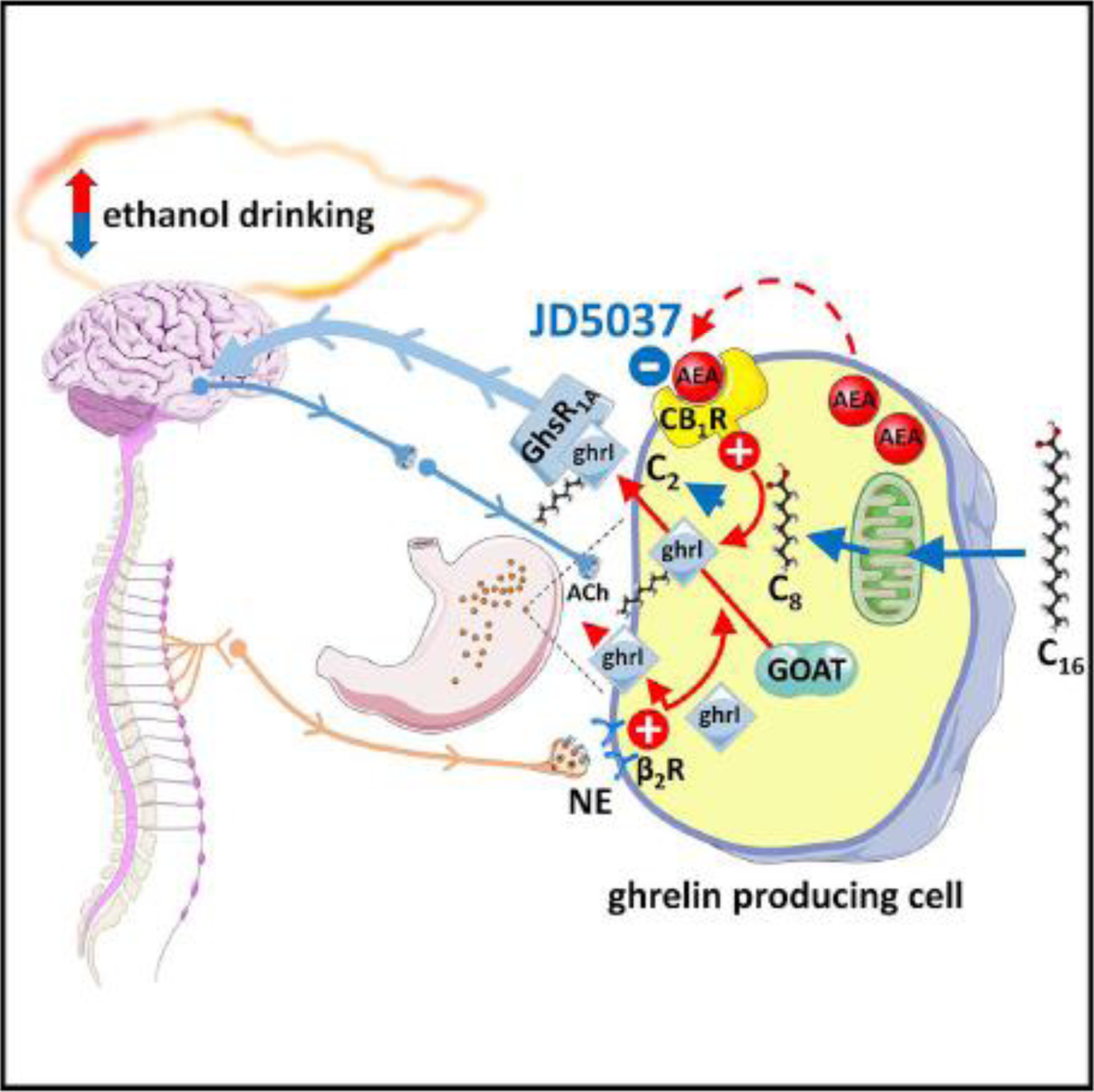
In ghrelin-producing stomach cells, the enzyme ghrelin O-acyl transferase (GOAT) catalyzes the formation of biologically active octanoyl-ghrelin. The source of the octanoic acid (C8) substrate is circulating long-chain fatty acids such as palmitate (C16) which are taken up by the cells and subjected to acyl chain shortening via β-oxidation. Blockade of CB1R on ghrelin-producing stomach cells accelerates the rate of fatty acid β-oxidation, thus reducing the availability of C8 for ghrelin acylation and, consequently, the cellular level of octanoyl-ghrelin without affecting its precursor deasacyl ghrelin. Octanoyl-ghrelin released by the cells activates ghrelin receptors (GhsR1A) located on vagal afferent terminals that signal to the brain to reduce drinking. In contrast to the selective modulation of octanoyl-ghrelin levels by CB1R, norepinephrine (NE) released from sympathetic nerves and activating β2-adrenergic receptors (β2R) stimulates the release of both desacyl- and octanoyl-ghrelin, which also promotes alcohol drinking that can be antagonized by the βR-blocker propranolol.
Reproduced from Godlewski et al., Cell Metabolism 29:1320–33, 2019.
Interestingly, vagal afferent nerve firing is decreased by signals that increase alcohol intake, such as ghrelin (Date et al., 2002), whereas it is increased by blockade of peripheral ghrelin receptors (Kong et al., 2016) or by cholecystokinin (Date et al., 2002; Schwartz et al., 1997), which also reduces alcohol intake (Geary et al., 2004), The apparent reciprocal relationship between vagal afferent nerve firing and alcohol preference is compatible with the increased incidence of alcoholism following Roux-en-Y gastric bypass surgery, which disrupts gastric innervation (Hajnal et al., 2012), and results in loss of ghrelin regulation of the firing of VTA dopaminergic neurons (Sirohi et al., 2017).
As discussed above, the use of a suboptimal dose of rimonabant in previous clinical trials in order to minimize the risk of CNS-mediated adverse effects was a possible reason for its failure to elicit statistically significant therapeutic effects in AUD. As such a dose limitation does not apply to peripherally restricted CB1R antagonists, future clinical trials to test their therapeutic potential in AUD are warranted.
POSSIBLE INVOLVEMENT OF CB2R IN ALCOHOL DRINKING BEHAVIOR
Although low level expression of CB2R mRNA in the brain has been detected in multiple laboratories (Onaivi et al., 2012; Van Sickle et al., 2005), direct evidence for the presence of CB2R protein in neuronal cells is lacking (Lopez et al., 2018). Nevertheless, some electrophysiological evidence is compatible with the presence of functional CB2R in CNS neurons (Atwood and Mackie, 2010; Stempel et al., 2016). There is limited and conflicting information about the possible involvement of CB2R in the control of alcohol seeking behavior. CB2R knockout mice displayed increased ethanol-induced conditioned place preference (CPP), increased voluntary ethanol intake, increased motivation to drink ethanol and increased acquisition of ethanol self-administration, relative to wild-type controls (Ortega-Alvaro et al., 2015). The phenotype of the knockouts could be replicated in wild-type mice by the similar increase in alcohol-induced CPP and consumption elicited by the CB2R antagonist AM630 and the opposite effects of the agonist JWH633 (Navarrete et al., 2018). The increased ethanol CPP in CB2R KO mice was confirmed by others, although in this study the effects of the genetic knockout could not be replicated by CB2R antagonist treatment (Powers et al., 2015). Further complicating the picture, another group recently reported that both AM630 and JWH633 decrease alcohol reward as measured by alcohol-induced CPP (Martin-Sanchez et al., 2019). Data obtained in genetic knockouts also fall short of delivering conclusive evidence for the role of CB2R in alcohol seeking behavior. In contrast to the increased alcohol preference in mice with global knockout of CB2R, conditional knockout of the Cnr2 gene in midbrain dopaminergic neurons resulted in decreased preference for alcohol and decreased alcohol-induced CPP. In the same study, treatment of wild-type littermate mice with the CB2R agonist JWH-133 similarly reduced alcohol-induced CPP, which is difficult to interpret (Liu et al., 2017). Further work is clearly needed to convincingly implicate CB2R in the control of alcohol reward and alcohol seeking behavior.
ENDOCANNABINOIDS AND ALCOHOLIC LIVER DISEASE
Contrary to earlier notions, hepatocytes were found to express functional CB1R, activation of which promotes hepatic insulin resistance, stimulates de novo lipogenesis by inducing the lipogenic transcription factor SREBP1c and its targets acetyl co-A carboxylase and fatty and synthase, and reduces fatty acid oxidation and (Osei-Hyiaman et al., 2005; Osei-Hyiaman et al., 2008). Indeed, rimonabant was effective not only in reducing body weight in overweight people with the metabolic syndrome but, first among anti-obesity agents, simultaneously improved all of the major metabolic complications, including non-alcoholic fatty liver disease (NAFLD), dyslipidemia and insulin resistance (Despres et al., 2005; Gary-Bobo et al., 2007; Pi-Sunyer et al., 2006; Van Gaal et al., 2005). This supports the notion that increased activity of the endocannabinoid/CB1R system is a pathogenic feature of the metabolic syndrome.
Chronic alcoholism can lead to alcoholic fatty liver disease (AFLD), the underlying molecular mechanisms of which are similar to those involved in NAFLD, including increased de novo lipogenesis (Lieber et al., 1966; You et al., 2002), decreased fatty acid oxidation (You et al., 2004) and insulin resistance (Carr et al., 2013). It is therefore not surprising that endocannabinoids acting at CB1R were found to be major drivers of AFLD (Jeong et al., 2008). This conclusion was based on the findings that mice deficient in CB1R globally or in hepatocytes only are resistant to ethanol-induced steatosis and increased lipogenic gene expression and have increased activity of hepatic carnitine palmitoyltransferase-1, the rate limiting enzyme in fatty acid β-oxidation. Furthermore, ethanol feeding increased hepatic Cnr1 expression and increased the levels of 2-AG (but not anandamide) along with increased gene expression of its biosynthetic enzyme DAGLβ selectively in hepatic stellate cells (HSC). In addition, co-culture of wild-type but not Cnr1−/− hepatocytes with HSC from alcohol drinking mice resulted in upregulation of Cnr1 and lipogenic gene expression in the hepatocytes (Jeong et al., 2008). This supported the hypothesis that paracrine activation CB1R on hepatocytes by HSC-derived 2-AG mediates alcoholic steatosis.
The above findings left open the question of the mechanism by which alcohol drinking increases 2-AG production by HSC. This question has just been answered by recent findings that chronic ethanol intake induces hepatic cysteine deficiency and subsequent glutathione depletion, triggering a compensatory increase in the gene and protein expression of the hepatic cystine/glutamate antiporter xCT which results in increased extracellular levels of glutamate. Alcohol intake also induces the expression of the metabotropic glutamate receptor-5 (mGluR5) selectively in HSC, stimulation of which by the released glutamate induces the production and release of 2-AG by HSC to activate CB1R and triggering de novo lipogenesis in neighboring hepatocytes (Choi et al., 2019). This bidirectional metabolic synapse (schematically illustrated in Figure 2), which is reminiscent of the bidirectional communication in the brain via anterograde and retrograde synaptic transmission, reveals novel targets for the treatment of AFLD. Indeed, genetic deletion or pharmacologic inhibition of either xCT or mGluR5 mitigated alcohol-induced steatosis (Choi et al., 2019).
Figure 2. A ‘bidirectional metabolic synapse’ between hepatocytes and hepatic stellate cells (HSC) mediates endocannabinoid-induced de novo lipogenesis.
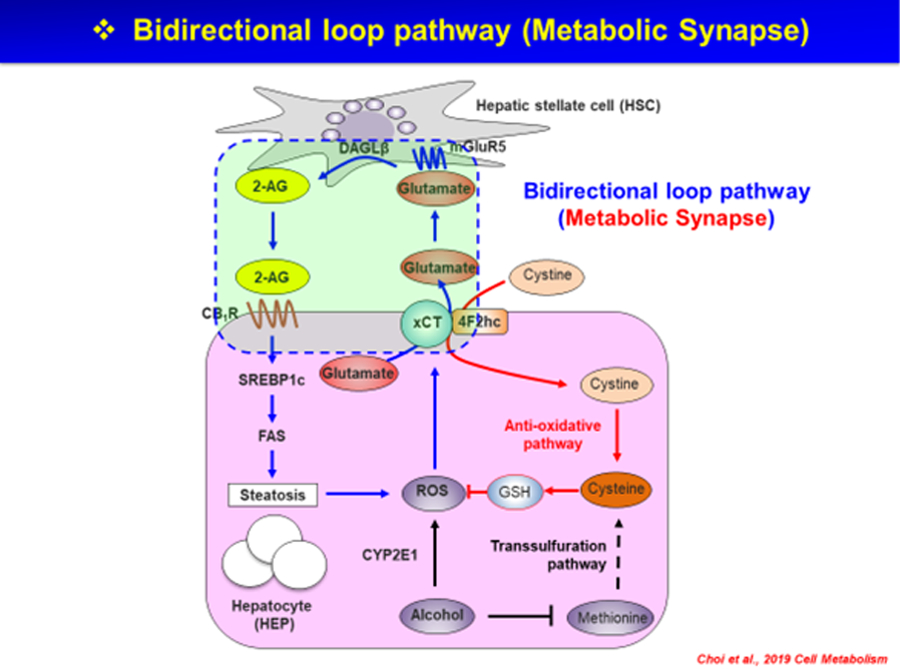
Chronic alcohol consumption induces CYP2E1-mediated production of reactive oxygen species (ROS) by hepatocytes, which is compensated by glutathion (GSH) generation through the uptake of cystine, mediated by the cystine-glutamate antiporter xCT. The parallel release of glutamate stimulates metabolic glutamate receptor-5 (mGluR5) on hepatic stellate cells (HSC) to produce 2-arachidonoyl glycerol (2-AG). 2-AG then activates CB1R on adjacent hepatocytes to induce de novo lipogenesis by inducing the lipogenic transcription factor SREBP-1c and the lipogenic enzyme fatty acid synthase (FAS).
Reproduced from Choi et al., Cell Metabolism, 30:877–889, 2019.
Interestingly, both these proteins have been implicated in the control of alcohol drinking behavior. Certain β-lactam antibiotics were found to reduce voluntary alcohol intake in P rats and in parallel to increase astrocytic expression of xCT in the PFC and NAc (Alasmari et al., 2015), suggesting that inhibition of xCT activity could increase alcohol preference and drinking, an undesirable effect opposite to the beneficial reduction in alcoholic steatosis. In contrast, mGluR5 inhibition reduces alcohol-seeking behavior both in mice and alcoholic patients (Bird et al., 2008; Holmes et al., 2013), similar to its beneficial inhibitory effect on hepatic steatosis, as discussed above. Thus, engaging the same pharmacological target (mGluR5) can mitigate alcoholism as well as alcoholic liver disease. The same principle may apply to peripherally restricted CB1R antagonists, which may not only be effective in curbing the drive to drink alcohol via a stomach/brain axis (Godlewski et al., 2019), but have also been shown to mitigate alcohol-induced steatosis (Amato et al., 2018). Furthermore, the mechanism identified in mice may also operate in human alcoholism, as suggested by the finding of increased plasma glutamate levels in patients with AFLD and alcoholic steatohepatitis compared to healthy controls or patients with cirrhosis, and changes in gene expression identified in the livers of alcohol-fed compared to control mice were also evident in liver tissue from patients with AFLD vs. controls without steatosis (Choi et al., 2019).
Cytochrome P450 2E1 (CYP2E1) is a key enzyme involved in alcohol-induced generation of reactive oxygen species (ROS) which cause oxidative stress-related tissue damage. The pathway linking alcohol and increased expression of CYP2E1 involves CB1R and the nuclear receptor estrogen-related receptor γ (ERRγ), as indicated by the absence of alcohol-induced CYP2E1 expression and ROS production in Cnr1−/− mice or mice with liver-specific deletion of the gene encoding EERγ (Kim et al., 2013). Alcohol-induced steatosis and ROS-mediated tissue damage can both progress to the development of liver fibrosis, so it is not surprising that the ECS has been implicated in hepatic fibrogenesis (Siegmund, 2010; Teixeira-Clerc et al., 2010; Teixeira-Clerc et al., 2006), including alcoholic liver fibrosis (Patsenker et al., 2011; Trebicka et al., 2011), with CB1R and CB2R being pro- and antifibrogenic, respectively (Mallat et al., 2013).
In contrast to the role of CB1R in promoting ALD, activation of CB2R may protect against alcoholic steatohepatitis. The CB2R agonist JWH-133 mitigated alcohol-induced hepatic inflammation and steatosis by suppressing pro-inflammatory M1 polarization and promoting the M2 phenotype of Kupffer cells (Louvet et al., 2011). The CB2R-mediated anti-inflammatory effects in Kupffer cells were subsequently found to involve activation of the autophagy pathway (Denaes et al., 2016). Β-Caryophyllene (BCP) is a plant-derived substance with CB2R agonist and anti-inflammatory properties (Gertsch et al., 2008). When chronic plus binge alcohol-fed mice were chronically treated with BCP, the hepatic steatosis and inflammatory changes such as neutrophil infiltration and M1 phenotypic switch of Kupffer cells were significantly ameliorated, and these effect were more prominent in wild-type than in CB2R−/− mice, indicating a protective effect mediated by CB2R activation (Varga et al., 2018). Together, these findings point to the therapeutic potential of CB2R agonists in ALD.
ENDOCANNABINOIDS, CB1R AND ALCOHOL BINGE-INDUCED CARDIOVASCULAR DYSFUNCTION
Endocannabinoids have robust effects on the cardiovascular system, predominantly via activation of CB1R. CB1R are present in both the rodent (Batkai et al., 2007) and human heart (Valenta et al., 2018) where its activation reduces cardiac contractility (Batkai et al., 2007), whereas activation of CB1R in the vascular endothelium leads to long-lasting, profound vasodilation and hypotension (Batkai et al., 2001; Lake et al., 1997). In a recent study in mice, a single dose of 5 g/kg ethanol delivered by gavage elicited a profound decrease in cardiac contractility, detected 3 hours later by intraventricular pressure-volume catheterization and echocardiography and paralleled by an increase in myocardial anandamide (but not 2-AG) levels. This was associated with hypotension and a redistribution of blood flow from the kidneys and mesenteric bed to skeletal muscle, the latter probably resulting from a reflex increase in sympathetic tone. All these changes were reversed or attenuated by a bolus dose of rimonabant, indicating CB1R activation as the underlying mechanism (Paloczi et al., 2019).
SUMMARY
Endocannabinods are ubiquitous lipid mediators involved in the control of a broad range of biological functions both in the CNS and in peripheral tissues. In the brain, endocannabinoid signaling via CB1R in the mesolimbic dopaminergic pathway mediate drug including alcohol reward, whereas CB1R-signaling in the dorsolateral striatum has been implicated in compulsive habitual alcohol-seeking behavior. In the liver, CB1R in hepatocytes play a key role in alcoholic fatty liver disease and CB1R in cardiomyocytes have been linked to binge alcohol-induced acute cardiovascular disturbance. Blockade of CB1R mitigates all these effects of alcohol drinking but its therapeutic exploitation has been thwarted by CNS-mediated side effects. Recently developed, peripherally restricted CB1R antagonists have been shown to retain all the beneficial effects of global CB1R blockade, including the reduction of voluntary alcohol intake via a stomach/brain signaling axis, without its neuropsychiatric liability. Such compounds therefore have therapeutic potential in the management of both addictive drinking as well as the organ toxicity associated with chronic alcoholism.
ACKNOWLEDGEMENTS
Work from the author’s laboratory has been supported by intramural research funds of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The author declares that no financial conflict of interest exists.
REFERENCES
- Adermark L, Clarke RB, Soderpalm B and Ericson M (2011a) Ethanol-induced modulation of synaptic output from the dorsolateral striatum in rat is regulated by cholinergic interneurons. Neurochem Int 58:693–699. [DOI] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Ericson M and Soderpalm B (2011b) Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology 61:1160–1165. [DOI] [PubMed] [Google Scholar]
- Agoglia AE, Holstein SE, Eastman VR and Hodge CW (2016) Cannabinoid CB1 receptor inhibition blunts adolescent-typical increased binge alcohol and sucrose consumption in male C57BL/6J mice. Pharmacol Biochem Behav 143:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Abuhamdah S and Sari Y (2015) Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neurosci Lett 600:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alen F, Santos A, Moreno-Sanz G, Gonzalez-Cuevas G, Gine E, Franco-Ruiz L, Navarro M and Lopez-Moreno JA (2009) Cannabinoid-induced increase in relapse-like drinking is prevented by the blockade of the glycine-binding site of N-methyl-D-aspartate receptors. Neuroscience 158:465–473. [DOI] [PubMed] [Google Scholar]
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68:247–286. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I and Parsons LH (2009) Regional Influence of Cannabinoid CB1 Receptors in the Regulation of Ethanol Self-Administration by Wistar Rats. Open Neuropsychopharmacol J 2:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato GS, Manke A, Harris DL, Wiethe RW, Vasukuttan V, Snyder RW, Lefever TW, Cortes R, Zhang Y, Wang S, Runyon SP and Maitra R (2018) Blocking Alcoholic Steatosis in Mice with a Peripherally Restricted Purine Antagonist of the Type 1 Cannabinoid Receptor. J Med Chem 61:4370–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P and Le Fur G (1997) Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132:104–106. [DOI] [PubMed] [Google Scholar]
- Atwood BK and Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggelaar MP, Chameau PJ, Kantae V, Hummel J, Hsu KL, Janssen F, van der Wel T, Soethoudt M, Deng H, den Dulk H, Allara M, Florea BI, Di Marzo V, Wadman WJ, Kruse CG, Overkleeft HS, Hankemeier T, Werkman TR, Cravatt BF and van der Stelt M (2015) Highly Selective, Reversible Inhibitor Identified by Comparative Chemoproteomics Modulates Diacylglycerol Lipase Activity in Neurons. J Am Chem Soc 137:8851–8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla A, Dong B, Shilpa BM, Vemuri K, Makriyannis A, Pandey SC, Sershen H, Suckow RF and Vinod KY (2018) Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology 131:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS and Hungund BL (1999) Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem 72:522–528. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I and Arancio O (2008) Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem 107:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB and Hungund BL (2000) Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta 1535:78–86. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB and Hungund BL (2006) Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology 50:834–844. [DOI] [PubMed] [Google Scholar]
- Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbaschek R, Garcia N Jr., Sanyal AJ and Kunos G (2001) Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 7:827–832. [DOI] [PubMed] [Google Scholar]
- Batkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P and Kunos G (2007) Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol 293:H1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E and Lawrence AJ (2008) Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol 11:765–774. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V and Doherty P (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM and Cravatt BF (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL 2nd, Walker D and Harris RA (2007) Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology 32:1570–1582. [DOI] [PubMed] [Google Scholar]
- Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y and Herrera-Marschitz M (2008) Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. Eur J Pharmacol 591:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG and Parsons LH (2007) Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci 27:3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RM, Dhir R, Yin X, Agarwal B and Ahima RS (2013) Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol Clin Exp Res 37:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Casteels C, Koole M, Bormans G and Van Laere K (2013) Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: a combined PET and microdialysis study. Eur J Nucl Med Mol Imaging 40:1582–1594. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S and Van Laere K (2014) Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci 34:2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE and Wightman RM (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24:4393–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE and Wightman RM (2007) Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WM, Kim HH, Kim MH, Cinar R, Yi HS, Eun HS, Kim SH, Choi YJ, Lee YS, Kim SY, Seo W, Lee JH, Shim YR, Kim YE, Yang K, Ryu T, Hwang JH, Lee CH, Choi HS, Gao B, Kim W, Kim SK, Kunos G and Jeong WI (2019) Glutamate Signaling in Hepatic Stellate Cells Drives Alcoholic Steatosis. Cell Metab 30:877–889 e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D and Ciccocioppo R (2008) Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 198:449–460. [DOI] [PubMed] [Google Scholar]
- Clarke RB and Adermark L (2010) Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology 58:799–805. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R and Gessa GL (1998) Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 63:PL113–117. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM and Gessa L (2002) Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 159:181–187. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Carai MA and Gessa GL (2004) Suppressing effect of the cannabinoid CB1 receptor antagonist, SR 141716, on alcohol’s motivational properties in alcohol-preferring rats. Eur J Pharmacol 498:119–123. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR and Lichtman AH (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98:9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA and Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K and Nakazato M (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123:1120–1128. [DOI] [PubMed] [Google Scholar]
- Denaes T, Lodder J, Chobert MN, Ruiz I, Pawlotsky JM, Lotersztajn S and Teixeira-Clerc F (2016) The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease Via a Macrophage Autophagy-Dependent Pathway. Sci Rep 6:28806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC and Holmes A (2013) Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A 110:14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Golay A and Sjostrom L (2005) Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS and Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A and Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Chiara G and Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ and Lee FS (2015) FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 6:6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SC, Gau JM, Farmer RF, Seeley JR, Kosty DB and Lewinsohn PM (2015) Comorbidity and temporal relations of alcohol and cannabis use disorders from youth through adulthood. Drug Alcohol Depend 149:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyr W, Ligieza J and Kostowski W (2008) The effect of cannabinoid CB(1) receptor antagonist rimonabant (SR-141716) on ethanol drinking in high-preferring rats. Alcohol 42:509–512. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L and Ciccocioppo R (2006) Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 183:394–403. [DOI] [PubMed] [Google Scholar]
- Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM and Schwartz TW (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2:376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G and Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3’-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358. [DOI] [PubMed] [Google Scholar]
- Femenia T, Garcia-Gutierrez MS and Manzanares J (2010) CB1 receptor blockade decreases ethanol intake and associated neurochemical changes in fawn-hooded rats. Alcohol Clin Exp Res 34:131–141. [DOI] [PubMed] [Google Scholar]
- Frontera JL, Gonzalez Pini VM, Messore FL and Brusco A (2018) Exposure to cannabinoid agonist WIN 55,212–2 during early adolescence increases alcohol preference and anxiety in CD1 mice. Neuropharmacology 137:268–274. [DOI] [PubMed] [Google Scholar]
- Gallate JE and McGregor IS (1999) The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl) 142:302–308. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE and McGregor IS (1999) Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol 370:233–240. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA and Doherty P (2010) Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrie P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM and Bensaid M (2007) Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 46:122–129. [DOI] [PubMed] [Google Scholar]
- Geary N, Wolfe A, Polidori C, Policani F and Massi M (2004) Exogeneous and endogenous CCK inhibit ethanol ingestion in Sardinian alcohol-preferring rats. Peptides 25:1185–1194. [DOI] [PubMed] [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Heilig M, Ramchandani VA, Geyer C, Spero DE, Singley ED, O’Malley SS, Bishai R, Rawlings RR and Kunos G (2010) Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology (Berl) 208:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J and Lovinger DM (2002) Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5:446–451. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M and Zimmer A (2008) Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A 105:9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL and Diana M (1998) Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341:39–44. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA and Colombo G (2005) Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol 40:46–53. [DOI] [PubMed] [Google Scholar]
- Gianessi CA, Groman SM, Thompson SL, Jiang M, van der Stelt M and Taylor JR (2019) Endocannabinoid contributions to alcohol habits and motivation: Relevance to treatment. Addict Biol:e12768. [DOI] [PMC free article] [PubMed]
- Godlewski G, Cinar R, Coffey NJ, Liu J, Jourdan T, Mukhopadhyay B, Chedester L, Liu Z, Osei-Hyiaman D, Iyer MR, Park JK, Smith RG, Iwakura H and Kunos G (2019) Targeting Peripheral CB1 Receptors Reduces Ethanol Intake via a Gut-Brain Axis. Cell Metabolism 29:1320–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF and Dawson DA (1997) Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 9:103–110. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM and Costa RM (2016) Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron 90:1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Lopez MD, Llopis N, Feng S, Barrett DA, O’Shea E and Colado MI (2010) Involvement of 2-arachidonoyl glycerol in the increased consumption of and preference for ethanol of mice treated with neurotoxic doses of methamphetamine. Br J Pharmacol 160:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND and Thanos PK (2012) Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One 7:e49121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH and Heilig M (2007) Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology 32:117–126. [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond AN, Guindon J and Morgan DJ (2016) Roles for the endocannabinoid system in ethanol-motivated behavior. Prog Neuropsychopharmacol Biol Psychiatry 65:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MR, Clouse E, Yin HH and Costa RM (2007) Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB and Heilig M (2013) Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry 18:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, Rocco LE, Patel S and Winder DG (2016) Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology 41:2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R and Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 229:539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M and Naassila M (2005) CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology 30:339–349. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET and Frank RA (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322–328. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS and Vadasz C (2003) Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem 84:698–704. [DOI] [PubMed] [Google Scholar]
- Hussain Z, Uyama T, Tsuboi K and Ueda N (2017) Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1546–1561. [DOI] [PubMed] [Google Scholar]
- Iannotti FA, Di Marzo V and Petrosino S (2016) Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog Lipid Res 62:107–128. [DOI] [PubMed] [Google Scholar]
- Irving A, Abdulrazzaq G, Chan SLF, Penman J, Harvey J and Alexander SPH (2017) Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Adv Pharmacol 80:223–247. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, LeMay BJ, Olszewska T, Vemuri VK, Wood JT and Makriyannis A (2008) Intrinsic effects of AM4113, a putative neutral CB1 receptor selective antagonist, on open-field behaviors in rats. Pharmacol Biochem Behav 91:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong WI, Osei-Hyiaman D, Park O, Liu J, Batkai S, Mukhopadhyay P, Horiguchi N, Harvey-White J, Marsicano G, Lutz B, Gao B and Kunos G (2008) Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab 7:227–235. [DOI] [PubMed] [Google Scholar]
- Kakihana R and McClearn GE (1963) Development of Alcohol Preference in Balb/C Mice. Nature 199:511–512. [DOI] [PubMed] [Google Scholar]
- Kalivas PW and Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K and Freund TF (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri D, Laroche G, Grant ML, Jones VM, Vetreno RP, Crews FT and Mukhopadhyay S (2018) Acute Ethanol Inhibition of Adult Hippocampal Neurogenesis Involves CB1 Cannabinoid Receptor Signaling. Alcohol Clin Exp Res 42:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Kim YH, Jang HH, Park J, Kim JR, Koh M, Jeong WI, Koo SH, Park TS, Yun CH, Park SB, Chiang JYL, Lee CH and Choi HS (2013) Estrogen-related receptor gamma controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 62:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Klippenstein V, Leweke FM, Spanagel R and Schneider M (2011) Cannabinoid exposure in pubertal rats increases spontaneous ethanol consumption and NMDA receptor associated protein levels. Int J Neuropsychopharmacol 14:505–517. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Hannan KS, Greenberg ML and Friedman JM (2010) Hyperleptinemia is required for the development of leptin resistance. PLoS One 5:e11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Chuddy J, Stock IA, Loria PM, Straub SV, Vage C, Cameron KO, Bhattacharya SK, Lapham K, McClure KF, Zhang Y and Jackson VM (2016) Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo. Br J Pharmacol 173:1452–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2019) Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry [DOI] [PubMed]
- Kreitzer AC and Regehr WG (2001) Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29:717–727. [DOI] [PubMed] [Google Scholar]
- Labar G, Wouters J and Lambert DM (2010) A review on the monoacylglycerol lipase: at the interface between fat and endocannabinoid signalling. Curr Med Chem 17:2588–2607. [DOI] [PubMed] [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR and Kunos G (1997) Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther 281:1030–1037. [PubMed] [Google Scholar]
- Lallemand F and De Witte P (2006) SR147778, a CB1 cannabinoid receptor antagonist, suppresses ethanol preference in chronically alcoholized Wistar rats. Alcohol 39:125–134. [DOI] [PubMed] [Google Scholar]
- Lallemand F, Soubrie PH and De Witte PH (2001) Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic ethanol administration. Alcohol Clin Exp Res 25:1317–1323. [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W and Parmentier M (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401–404. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Spritz N and DeCarli LM (1966) Role of dietary, adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J Clin Invest 45:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN and Boehm SL 2nd (2009) Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience 164:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Canseco-Alba A, Zhang HY, Tagliaferro P, Chung M, Dennis E, Sanabria B, Schanz N, Escosteguy-Neto JC, Ishiguro H, Lin Z, Sgro S, Leonard CM, Santos-Junior JG, Gardner EL, Egan JM, Lee JW, Xi ZX and Onaivi ES (2017) Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep 7:17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH and Cravatt BF (2009) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vazquez C, Amores M, Ruiz-Perez G, Garcia-Garcia E, Beatka M, Tolon RM, Dittel BN, Hillard CJ and Romero J (2018) Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation 15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, Pecker F, Mallat A and Lotersztajn S (2011) Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 54:1217–1226. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC and Hoffman AF (2004) Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol 143:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bab I, Biro T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA and Zimmer A (2015) Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36:277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Colombo G and Carai MA (2010) Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets 9:55–59. [DOI] [PubMed] [Google Scholar]
- Malinen H and Hyytia P (2008) Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res 32:1976–1983. [DOI] [PubMed] [Google Scholar]
- Mallat A, Teixeira-Clerc F and Lotersztajn S (2013) Cannabinoid signaling and liver therapeutics. J Hepatol 59:891–896. [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez A, Warnault V, Montagud-Romero S, Pastor A, Mondragon N, De La Torre R and Valverde O (2019) Alcohol-induced conditioned place preference is modulated by CB2 cannabinoid receptors and modifies levels of endocannabinoids in the mesocorticolimbic system. Pharmacol Biochem Behav 183:22–31. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Buffkins K, Kyle M, Adusumalli M, Begovic A and Rao S (2012) A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology 37:1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, Gildish I, Cachope R, Bellocchio L, Guzman M, Morales M, Cheer JF and Lovinger DM (2017) Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 96:1112–1126 e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC and Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Dam KD, Mallet PE and Gallate JE (2005) Delta9-THC reinstates beer- and sucrose-seeking behaviour in abstinent rats: comparison with midazolam, food deprivation and predator odour. Alcohol Alcohol 40:35–45. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR and et al. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90. [DOI] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Perra S, Colombo G, Muntoni AL and Pistis M (2009) Electrophysiological properties of dopamine neurons in the ventral tegmental area of Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 201:471–481. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL and Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C and Daoust M (2004) Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology 46:243–253. [DOI] [PubMed] [Google Scholar]
- Navarrete F, Garcia-Gutierrez MS and Manzanares J (2018) Pharmacological regulation of cannabinoid CB2 receptor modulates the reinforcing and motivational actions of ethanol. Biochem Pharmacol 157:227–234. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P and Tonini R (2012) SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci 15:284–293. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R and Kreek MJ (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 160:19–29. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T and Kano M (2001) Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29:729–738. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gu S and Liu QR (2012) CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol 26:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Ternianov A, Aracil-Fernandez A, Navarrete F, Garcia-Gutierrez MS and Manzanares J (2015) Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addict Biol 20:43–55. [DOI] [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Perez-Rial S, Palomo T and Manzanares J (2004) Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol 39:88–92. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L and Kunos G (2005) Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C and Kunos G (2008) Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118:3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloczi J, Matyas C, Cinar R, Varga ZV, G.; H, Schindler TH, Kunos G and Pacher P (2019) Alcohol binge-induced cardiovascular dysfunction involves endocannabinoid-CB1-R signaling. JACC: Basic to Translational Science 4:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsenker E, Stoll M, Millonig G, Agaimy A, Wissniowski T, Schneider V, Mueller S, Brenneisen R, Seitz HK, Ocker M and Stickel F (2011) Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med 17:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ and Woodward JJ (2012) A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol 46:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penasco S, Rico-Barrio I, Puente N, Fontaine CJ, Ramos A, Reguero L, Gerrikagoitia I, de Fonseca FR, Suarez J, Barrondo S, Aretxabala X, Garcia Del Cano G, Salles J, Elezgarai I, Nahirney PC, Christie BR and Grandes P (2020) Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor-dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 45:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J and Rosenstock J (2006) Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama 295:761–775. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R and Soubrie P (2003) Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett 343:216–218. [DOI] [PubMed] [Google Scholar]
- Powers MS, Breit KR and Chester JA (2015) Genetic Versus Pharmacological Assessment of the Role of Cannabinoid Type 2 Receptors in Alcohol Reward-Related Behaviors. Alcohol Clin Exp Res 39:2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M and Zimmer A (2003) A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci 23:2453–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Baltz ET and Gremel CM (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun 9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP and Lutz B (2012) Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37:2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D and et al. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC and Le Fur GL (1998) SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 284:644–650. [PubMed] [Google Scholar]
- Rubio M, Fernandez-Ruiz J, de Miguel R, Maestro B, Michael Walker J and Ramos JA (2008) CB1 receptor blockade reduces the anxiogenic-like response and ameliorates the neurochemical imbalances associated with alcohol withdrawal in rats. Neuropharmacology 54:976–988. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Clarke D, Mendoza LA, Kawamura M and Schoen L (2019) Predictors of Increases in Alcohol Problems and Alcohol Use Disorders in Offspring in the San Diego Prospective Study. Alcohol Clin Exp Res 43:2232–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH, White WO and Ladenheim EE (1997) Relationships between gastric motility and gastric vagal afferent responses to CCK and GRP in rats differ. Am J Physiol 272:R1726–1733. [DOI] [PubMed] [Google Scholar]
- Serra S, Carai MA, Brunetti G, Gomez R, Melis S, Vacca G, Colombo G and Gessa GL (2001) The cannabinoid receptor antagonist SR 141716 prevents acquisition of drinking behavior in alcohol-preferring rats. Eur J Pharmacol 430:369–371. [DOI] [PubMed] [Google Scholar]
- Siegmund SV (2010) Role of the endocannabinoid system in alcoholic liver disease. Dig Dis 28:751–755. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA and Salamone JD (2008) The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology 33:946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Collins LE, Markus EJ, Vemuri VK, Makriyannis A and Salamone JD (2010a) The CB1 inverse agonist AM251, but not the CB1 antagonist AM4113, enhances retention of contextual fear conditioning in rats. Pharmacol Biochem Behav 95:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, Markus EJ, Vemuri VK, Makriyannis A and Salamone JD (2010b) Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol 20:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E and Cravatt BF (2002) A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A 99:8394–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Richardson BD, Lugo JM, Rossi DJ and Davis JF (2017) Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity (Silver Spring) 25:1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR and Piomelli D (2008) The endocannabinoid system in brain reward processes. Br J Pharmacol 154:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF and Investigators AS (2008) Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol 28:317–324. [DOI] [PubMed] [Google Scholar]
- Spence JP, Liang T, Liu L, Johnson PL, Foroud T, Carr LG and Shekhar A (2009) From QTL to candidate gene: a genetic approach to alcoholism research. Curr Drug Abuse Rev 2:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A and Schmitz D (2016) Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 90:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R and Grant BF (2006) Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med 36:1447–1460. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S and Basavarajappa BS (2013) Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J Neurosci 33:6350–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A and Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97. [DOI] [PubMed] [Google Scholar]
- Talani G and Lovinger DM (2015) Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol 49:781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE and Rodgers RJ (2007) Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology (Berl) 195:27–39. [DOI] [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, Innis RB, Cheng K, Rice KC, Deschamps JR, Chorvat RJ, McElroy JF and Kunos G (2012) Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A and Kunos G (2010) Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120:2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE and Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050. [DOI] [PubMed] [Google Scholar]
- Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, Schoiswohl G, Preiss-Landl K, Jaeger D, Reiter B, Koefeler HC, Wojciechowski J, Theussl C, Penninger JM, Lass A, Haemmerle G, Zechner R and Zimmermann R (2011) Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem 286:17467–17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Belot MP, Manin S, Deveaux V, Cadoudal T, Chobert MN, Louvet A, Zimmer A, Tordjmann T, Mallat A and Lotersztajn S (2010) Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology 52:1046–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A and Lotersztajn S (2006) CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12:671–676. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A and Volkow ND (2005) Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res 164:206–213. [DOI] [PubMed] [Google Scholar]
- Trebicka J, Racz I, Siegmund SV, Cara E, Granzow M, Schierwagen R, Klein S, Wojtalla A, Hennenberg M, Huss S, Fischer HP, Heller J, Zimmer A and Sauerbruch T (2011) Role of cannabinoid receptors in alcoholic hepatic injury: steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver Int 31:860–870. [DOI] [PubMed] [Google Scholar]
- Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL and Colombo G (2002) Boosting effect of morphine on alcohol drinking is suppressed not only by naloxone but also by the cannabinoid CB1 receptor antagonist, SR 141716. Eur J Pharmacol 445:55–59. [DOI] [PubMed] [Google Scholar]
- Valenta I, Varga ZV, Valentine H, Cinar R, Horti A, Mathews WB, Dannals RF, Steele K, Kunos G, Wahl RL, Pomper MG, Wong DF, Pacher P and Schindler TH (2018) Feasibility Evaluation of Myocardial Cannabinoid Type 1 Receptor Imaging in Obesity: A Translational Approach. JACC Cardiovasc Imaging 11:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O and Rossner S (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD and Sharkey KA (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Matyas C, Erdelyi K, Cinar R, Nieri D, Chicca A, Nemeth BT, Paloczi J, Lajtos T, Corey L, Hasko G, Gao B, Kunos G, Gertsch J and Pacher P (2018) beta-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br J Pharmacol 175:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J and Hungund BL (2008a) Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem 104:233–243. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND and Hungund BL (2008b) Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse 62:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A and Kunos G (2003) Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A 100:1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ and McCaul ME (2008) Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology 33:653–665. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G and Nicoll RA (2001) Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31:453–462. [DOI] [PubMed] [Google Scholar]
- Wilson RI and Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592. [DOI] [PubMed] [Google Scholar]
- Xie G, Krnjevic K and Ye JH (2013) Salsolinol modulation of dopamine neurons. Front Behav Neurosci 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L and Lovinger DM (2007) Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci 25:3226–3232. [DOI] [PubMed] [Google Scholar]
- You M, Fischer M, Deeg MA and Crabb DW (2002) Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 277:29342–29347. [DOI] [PubMed] [Google Scholar]
- You M, Matsumoto M, Pacold CM, Cho WK and Crabb DW (2004) The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127:1798–1808. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang T, Lee F and Kreek MJ (2016) Involvement of Endocannabinoids in Alcohol “Binge” Drinking: Studies of Mice with Human Fatty Acid Amide Hydrolase Genetic Variation and After CB1 Receptor Antagonists. Alcohol Clin Exp Res 40:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Schwartz BI, Giza J, Gross SS, Lee FS and Kreek MJ (2017) Blockade of alcohol escalation and “relapse” drinking by pharmacological FAAH inhibition in male and female C57BL/6J mice. Psychopharmacology (Berl) 234:2955–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M and Bonner TI (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 96:5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]


