Abstract
Background
Early reports have shown that critically ill patients infected with SARS-CoV-2 have a high prevalence of nosocomial pneumonia, particularly ventilator-associated pneumonia (VAP).
Method
In the present study, we determined the bacterial agents isolated from endotracheal aspirate (ETA) cultures of Covid-19 general intensive care patients and evaluated the antibiotic resistance profiles of common bacterial agents compared to the pre-pandemic period.
Results
While a total of 119 significant growths with polymicrobial growths were detected in the ETA cultures of 73 (7.5%) of 971 patients hospitalized in the intensive care unit before the pandemic, 87 significant growths were detected in the ETA cultures of 67 (11.1%) of 602 patients hospitalized in the Covid-19 intensive care unit (ICU) after the pandemic. While 61 (83.6%) of patients in the ICU died before the pandemic, 63 (94.0%) of patients in the Covid-19 ICU died after the pandemic. In terms of age, gender, and mortality, there was no significant difference between the two ICUs (p > 0.05). Before the pandemic, the mean length of stay in the ICU was 33.59 ± 32.89 days, and after the pandemic, it was 13.49 ± 8.03 days. This was a statistically significant difference (p < 0.05). Acinetobacter baumannii (28.5%), Klebsiella pneumoniae (22.6%), Pseudomonas aeruginosa (15.9%), Staphylococcus aureus (6.7%), Escherichia coli (7.5%), Candida spp. (5.0%) were the most prevalent causal microorganisms discovered in pre-pandemic ICU ETA samples, whereas A. baumannii (54.0%), K. pneumoniae (10.3%), P. aeruginosa (6.8%), E. faecium (8%), and Candida spp.(13.7%) were the most common causative microorganisms detected in Covid-19 ICU ETA samples. Except for tigecycline, antibiotic resistance rates in A. baumannii strains increased following the pandemic. Only tobramycin showed a significant difference in the increase of resistance among these antibiotics (p = 0.037). The rate of tigecycline resistance, on the other hand, was 17.6% before the pandemic and 2.2% afterward (p < 0.05). After the pandemic, increased resistance of K. pneumoniae strains to colistin, meropenem, ertapenem, amoxicillin-clavulanic acid, piperacillin-tazobactam, ciprofloxacin, tigecycline, and cefepime antibiotics was observed. However, these increases were not statistically significant. Except for imipenem, antibiotic resistance rates in P. aeruginosa strains increased following the pandemic. The increase in resistance of ceftazidime and levofloxacin was statistically significant (p < 0.05).
Conclusion
As a result, the Covid-19 pandemic requires intensive care follow-ups at an earlier age and with a more mortal course. Although the length of stay in the intensive care unit has been shortened, it is observed that this situation is observed due to early mortality. In P. aeruginosa strains, a significant difference was detected in the resistance increase of the ceftazidime and levofloxacin (p < 0.05) and with the exception of tigecycline, antibiotic resistance rates in A. baumannii strains increased following the pandemic. Only tobramycin showed a significant difference in the increase of resistance among these antibiotics (p = 0.037). Secondary infections in patients create more difficult treatment processes due to both Covid-19 and increasing antibiotic resistance today.
Keywords: SARS-CoV-2, Bacterial co-infection, Antimicrobial stewardship, Clinical outcome
1. Introduction
Coronavirus disease 2019 (Covid-19) was first identified in December 2019 in Wuhan, China, and has spread all over the world. It is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Covid-19 is a respiratory illness with flu-like symptoms, manifesting as dry cough, fever, severe headache, and fatigue [1]. Viral agents causing respiratory tract infections predispose to secondary bacterial infections due to their effects on the immune system [2,3]. Through a variety of mechanisms, viral infections have been shown to enhance bacterial colonization of the airway. Changes in mucus secretion, cell death, hyperplasia, decreased mucosal clearance, decreased oxygen exchange, and impaired surfactant secretion are among the negative effects [4,5]. Depending on the virus, the type of bacteria, and the intensity of the host immune response against a bacterium or virus, each of these effects are caused by a variety of molecular mechanisms. Secondary or bacterial coinfections with other viruses can greatly increase the mortality rate in patients with viral infections according to laboratory, clinical, and epidemiological investigations [6,7]. In the 2009 H1N1 influenza pandemic, 4–33% of hospitalized patients were complicated by bacterial pneumonia [8]. Up to 30% of patients were diagnosed with secondary bacterial infections during the first SARS-CoV outbreak in 2003, and coinfection was positively associated with disease severity [9]. Bacterial coinfections are also prevalent in 2%–65% of cases during normal flu seasons and have been linked to morbidity and mortality [10].
Early reports have shown that critically ill patients infected with SARS-CoV-2 have a high prevalence of nosocomial pneumonia, particularly ventilator-associated pneumonia (VAP) [11]. Multiple broad-spectrum antibacterial agents were utilized during the Covid-19 outbreak, and the great majority of patients hospitalized with Covid-19 were given empirical antimicrobial therapy before secondary bacterial infections were confirmed [11,12]. Guidelines, on the other hand, advocate the use of culture-based approaches to limit overdiagnosis and facilitate appropriate antimicrobial therapy in VAP [13]. Therefore, determining the causative microorganisms and their antibiotic susceptibility in these units is important to both guide empirical treatment and to reduce mortality and morbidity [14].
In this study; It was aimed to determine the bacterial agents isolated from ETA cultures of Covid-19 general intensive care patients and to evaluate the antibiotic resistance profiles of common bacterial agents compared to the pre-pandemic period.
2. Materials and methods
On April 1, 2020, general intensive care unit (ICU) 1 was reserved for Covid-19 patients in our hospital. In this study, the results of endotracheal aspirate (ETA) cultures sent from general ICU between April 1, 2019–March 31, 2020, and ETA cultures sent from Covid-19 intensive care patients between April 1, 2020–March 31, 2021 (post-pandemic) were investigated retrospectively. Microorganisms that were evaluated to be the cause of lower respiratory tract infection as a result of the culture of ETA samples taken from the patients were included in the study. To detect Covid-19, reverse transcription real-time polymerase chain reaction was performed from respiratory tract samples.
ETA samples taken into a Luken tube were evaluated in the microbiology laboratory. An equal amount of sterile saline was added to the samples and mixed. Then, 0.01 mL of this mixture was inoculated on 5% sheep blood agar, Eosin-Methylene blue (EMB) agar, and chocolate agar media. The preparation was prepared from the samples for microscopic evaluation and stained by the gram method. The quality of the sample, the predominant microorganisms, and leukocytes were investigated.
The samples were incubated in an incubator at 37 °C for 24–48 h. Growing bacteria were evaluated quantitatively and growths of 105 CFU/mL and above were considered significant [15,16]. When the same agent was grown in more than one ETA culture of the same patient, only the first isolated strain was evaluated. Identification of bacteria and their antibiotic susceptibility were determined by the VITEK 2 (bioMerieux, France) automated identification system. The results were evaluated according to the “European Committee for Antimicrobial Susceptibility Tests ”(EUCAST) standard. This study was approved by the Siirt University Non-Interventional Clinical Research Ethics Committee (Meeting date: May 28, 2021, meeting number: 10241, decision no: 2021/01.01) and the Republic of Turkey Ministry of Health as well (Date: July 03, 2021).
2.1. Statical analysis
Statistical analysis of the data was carried out with the Statistical Package for Social Science for Windows (SPSS) 26 program and it was studied with a confidence level of 95%. A value of p < 0.05 was considered statistically significant. In the study; a Chi-square test was applied to compare the antibiotic resistance profiles of common bacterial agents in ETA cultures of Covid-19 intensive care patients compared to the pre-pandemic period. The length of stay did not show a normal distribution, so the Mann-Whitney test was used. Chi-square test for mortality was used in relation to gender, and independent groups t-test was used in relation to age (showing normal distribution).
3. Results
ETA cultures of 73 (7.5%) of 971 patients hospitalized in the ICU before the pandemic had a total of 119 significant growths, together with polymicrobial growths. 49 (67.1%) of the patients considered as causative were male, 24 (32.9%) were female; the mean age of men was found as 69.45 ± 17.78, and the mean age of women was 76.38 ± 16.30. After the pandemic, there were 87 significant growths in the ETA cultures of 67 (11.1%) of 602 patients hospitalized in the Covid-19 ICU. Of the patients considered as causative, 42 (62.7%) were male, 25 (37.3%) were female; the mean age of men was found as 70.31 ± 11.76, and the mean age of women was found as 72.08 ± 11.49. While 61 (83.6%) of the patients in the ICU died before the pandemic, 63 (94.0%) of the patients in the Covid-19 ICU died after the pandemic. There was no significant difference between the two ICUs in terms of age, gender, and mortality (p > 0.05) (Table 1 ). The mean length of stay in the ICU was found as 33.59 ± 32.89 before the pandemic, and 13.49 ± 8.03 in the post-pandemic ICU. This difference was statistically significant (p < 0.05).
Table 1.
Comparison of pre-pandemic ICU and Covid-19 ICU patients in terms of age, gender, length of stay, and mortality.
| Variable | Pre-pandemic ICU | Covid-19 ICU | Total | Statistics | p value | |
|---|---|---|---|---|---|---|
| Aget | Male | 69.45 ± 17.78 | 70.31 ± 11.76 | 69.85 ± 15.22 | −0.276 | 0.783 |
| Female | 76.38 ± 16.30 | 72.08 ± 11.49 | 74.18 ± 14.07 | 1070 | 0.290 | |
| Total | 71.73 ± 17.50 | 70.97 ± 11.60 | 71.36 ± 14.93 | 0.303 | 0.762 | |
| Length of stayU | Total | 33.59 ± 32.89 | 13.49 ± 8.03 | 23.97 ± 26.31 | 1281.0 | 0.000a |
| MortalityX2 | Alive | 12 (16.4) | 4 (6.0) | 16 (11.4) | 2819 | 0.093 |
| Death | 61 (83.6) | 63 (94.0) | 124 (88.6) | |||
| GenderX2 | Male | 49 (67.1) | 42 (62.7) | 91 (65.0) | 0.139 | 0.710 |
| Female | 24 (32.9) | 25 (37.3) | 49 (35.0) |
p < 0.05 significant relationship/difference, U: Mann Whitney, t: independent group. t, X2: Chi-square.
The most common causative microorganisms detected in pre-pandemic ICU ETA samples were Acinetobacter baumannii 34 (28.5%), Klebsiella pneumoniae 27 (22.6%), Pseudomonas aeruginosa19 (15.9%), Staphylococcus aureus 8 (6.7%), Escherichia coli 9 (7.5%), Candida spp. 6 (5.0%), while the most common causative microorganisms detected in Covid-19 ICU ETA samples were A. baumannii 47 (54.0%), K. pneumoniae 9 (10.3%), P. aeruginosa 6 (6.8%), E. faecium 7 (8%) and Candida spp.12 (13.7). The distribution of agents in ETA cultures is given in Table 2 . In the pre-pandemic ICU samples, the rates of resistance to amikacin in A. baumannii, K. pneumoniae, and P. aeruginosa were 44.1%, 46.2%, and 21.1%, respectively. However, the rates of resistance to meropenem were detected as 100%, 65.0%, and 31.6%, respectively. Colistin resistance rates in A. baumannii, K. pneumoniae, and P. aeruginosa were 0%, 9.5%, and 5.3%, respectively. Antibiotic resistance rates of S.aureus isolates were found as 37.5% for oxacillin, 75.0% for penicillin, 28.5% for erythromycin,12.5% for clindamycin, 0% for vancomycin, 25.0% for ciprofloxacin, 20.0% for levofloxacin, 20.0% for tetracycline, 12.5% for gentamicin, 0% for daptomycin and 0% for linezolid.
Table 2.
Distribution of bacterial agents isolated from ETA cultures of ICU patients before the pandemic and Covid-19 ICU patients during the pandemic.
| Agent | Pre-pandemic ICU n (%) | Covid-19 ICU n (%) |
|---|---|---|
| Acinetobacter baumannii | 34 (28.5) | 47 (54.0) |
| Klebsiella pneumoniae | 27 (22.6) | 9 (10.3) |
| Pseudomonas aeruginosa | 19 (15.9) | 6 (6.8) |
| Escherichia coli | 9 (7.5) | 1 (1.1) |
| Proteus mirabilis | 8 (6.7) | 0 |
| Enterobacter aerogenes | 2 (1.6) | 0 |
| Stenotrophomonas maltophilia | 0 | 1 (1.1) |
| Sphingomonas paucomobilis | 1 (0.8) | 0 |
| Serratia marcescens | 1 (0.8) | 0 |
| Staphylococcus aureus | 8 (6.7) | 2 (2.2) |
| Enterococcus faecium | 0 | 7 (8.0) |
| Streptococcus pneumoniae | 4 (3.3) | 2 (2.2) |
|
Candida spp. |
6 (5.0) |
12 (13.7) |
| Total | 119 | 87 |
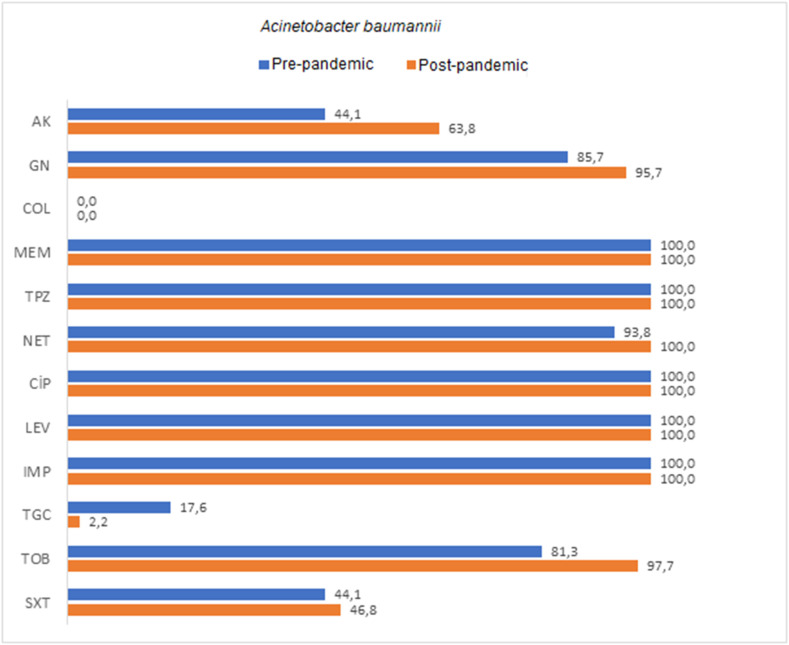
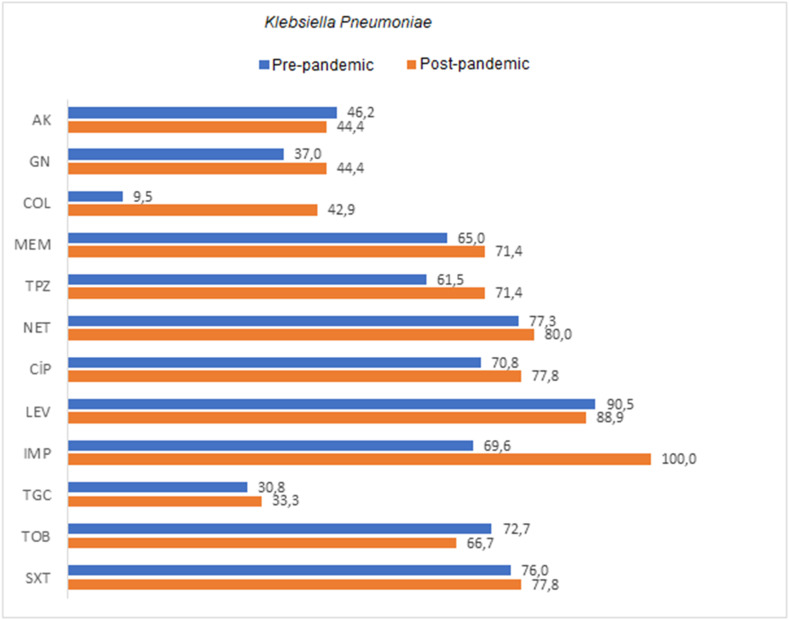
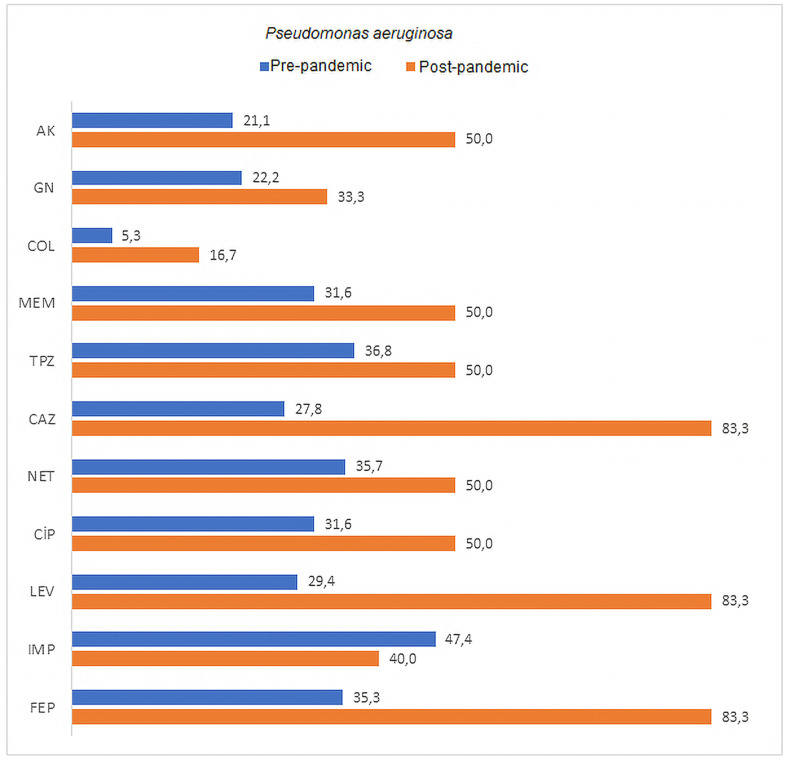
The rates of resistance to amikacin in A. baumannii, K. pneumoniae, and P. aeruginosa in Covid-19 ICU samples were 63.8%, 44.4%, and 50.0%, respectively, while the rates of resistance to meropenem were 100%, 71.4%, and 50.0%, respectively. Colistin resistance rates in A. baumannii, K. pneumoniae, and P. aeruginosa were found as 0%, 42.9%, and 16.7%, respectively. Comparison of the antimicrobial resistance rates of the most commonly isolated agents is shown in Table 3, Table 4, Table 5 , as well as in Fig. 1, Fig. 2, Fig. 3 . An increase in antibiotic resistance rates were observed in A. baumannii strains after the pandemic, except for tigecycline. A significant difference was found in the increase of resistance only for tobramycin among these antibiotics (p = 0.037). Tigecycline resistance rate, on the other hand, was 17.6% before the pandemic and decreased to 2.2% after the pandemic. This difference was statistically significant (p < 0.05). When the antibiotic resistance rates of K. pneumoniae strains were compared, an increase in resistance was observed in gentamicin, colistin, meropenem, ertapenem, amoxicillin-clavulanic acid, piperacillin tazobactam, ciprofloxacin, tigecycline, and cefepime antibiotics after the pandemic. However, these increases were not statistically significant. An increase in antibiotic resistance rates was detected in P. aeruginosa strains after the pandemic, except for imipenem. A significant difference was found in the resistance increase of the studied antibiotics ceftazidime and levofloxacin (p < 0.05).
Table 3.
Comparison of antibiotic resistance rates of Acinetobacter baumannii strains isolated from ETA cultures of pre-pandemic ICU and pandemic-period Covid-19 ICU patients.
| Pre-pandemic ICU |
Covid-19 ICU |
Total |
p | ||||
|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | ||
| AK | 19 (55.9) | 15 (44.1) | 17 (36.2) | 30 (63.8) | 36 (44.4) | 45 (55.6) | 0.125 |
| GN | 4 (14.3) | 24 (85.7) | 2 (4.3) | 45 (95.7) | 6 (8) | 69 (92) | 0.188 |
| COL | 33 (100) | 0 (0) | 47 (100) | 0 (0) | 80 (100) | 0 (0) | x |
| MEM | 0 (0) | 34 (100) | 0 (0) | 46 (100) | 0 (0) | 80 (100) | x |
| TPZ | 0 (0) | 17 (100) | 0 (0) | 46 (100) | 0 (0) | 63 (100) | x |
| NET | 2 (6.3) | 30 (93.8) | 0 (0) | 44 (100) | 2 (2.6) | 74 (97.4) | 0.174 |
| CİP | 0 (0) | 33 (100) | 0 (0) | 47 (100) | 0 (0) | 80 (100) | x |
| LEV | 0 (0) | 34 (100) | 0 (0) | 41 (100) | 0 (0) | 75 (100) | x |
| IMP | 0 (0) | 17 (100) | 0 (0) | 41 (100) | 0 (0) | 58 (100) | x |
| TGC | 28 (82.4) | 6 (17.6) | 45 (97.8) | 1 (2.2) | 73 (91.3) | 7 (8.8) | 0.038* |
| TOB | 6 (18.8) | 26 (81.3) | 1 (2.3) | 42 (97.7) | 7 (9.3) | 68 (90.7) | 0.037* |
| SXT | 19 (55.9) | 15 (44.1) | 25 (53.2) | 22 (46.8) | 44 (54.3) | 37 (45.7) | 0.989 |
*p < 0.05 significant relationship, p > 0.05 no significant relationship; Chi-square test – x:Test not possible.
AK: amikacin, GN: gentamicin, COL: colistin, MEM: meropenem, TPZ: piperacillin tazobactam, NET: netilmicin, CIP: ciprofloxacin, LEV: levofloxacin, IMP: imipenem, TGC: tigecycline, TOB: tobramycin, SXT: trimethoprim sulfamethoxazole.
Table 4.
Comparison of antibiotic resistance rates of Klebsiella pneumoniae strains isolated from ETA cultures of pre-pandemic ICU and pandemic-period Covid-19 ICU patients.
| Pre-pandemic ICU |
Covid-19 ICU |
Total |
p | ||||
|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | ||
| AK | 14 (53.8) | 12 (46.2) | 5 (55.6) | 4 (44.4) | 19 (54.3) | 16 (45.7) | 0.999 |
| GN | 17 (63) | 10 (37) | 5 (55.6) | 4 (44.4) | 22 (61.1) | 14 (38.9) | 0.712 |
| COL | 19 (90.5) | 2 (9.5) | 4 (57.1) | 3 (42.9) | 23 (82.1) | 5 (17.9) | 0.082 |
| MEM | 7 (35) | 13 (65) | 2 (28.6) | 5 (71,.4) | 9 (33.3) | 18 (66.7) | 0.999 |
| ETP | 10 (38.5) | 16 (61.5) | 2 (28.6) | 5 (71.4) | 12 (36.4) | 21 (63.6) | 0.999 |
| AMC | 5 (22.7) | 17 (77.3) | 1 (20) | 4 (80) | 6 (22.2) | 21 (77.8) | 0.999 |
| TPZ | 7 (29.2) | 17 (70.8) | 2 (22,2) | 7 (77.8) | 9 (27.3) | 24 (72.7) | 0.999 |
| CAZ | 2 (9.5) | 19 (90.5) | 1 (11.1) | 8 (88.9) | 3 (10) | 27 (90) | 0.999 |
| CİP | 7 (30.4) | 16 (69.6) | 0 (0) | 9 (100) | 7 (21.9) | 25 (78.1) | 0.149 |
| TGC | 18 (69.2) | 8 (30.8) | 6 (66.7) | 3 (33.3) | 24 (68.6) | 11 (31.4) | 0.999 |
| SXT | 6 (27.3) | 16 (72.7) | 3 (33.3) | 6 (66.7) | 9 (29) | 22 (71) | 0.999 |
| FEP | 6 (24) | 19 (76) | 2 (22.2) | 7 (77.8) | 8 (23.5) | 26 (76.5) | 0.999 |
p < 0.05 significant relationship, p > 0.05 no significant relationship; Chi-square test.
AK: amikacin, GN: gentamicin, COL: colistin, MEM: meropenem, ETP: ertapenem, AMC: amoxicillin clavulanic acid, TPZ: piperacillin tazobactam, CAZ: ceftazidime, CIP: ciprofloxacin, TGC: tigecycline, SXT: trimethoprim, sulfamethoxazole, FEP: cefepime.
Table 5.
Comparison of antibiotic resistance rates of Pseudomonas aeruginosa strains isolated from ETA cultures of pre-pandemic ICU and pandemic-period Covid-19 ICU patients.
| Pre-pandemic ICU |
Covid-19 ICU |
Total |
p | ||||
|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | ||
| AK | 15 (78.9) | 4 (21.1) | 3 (50) | 3 (50) | 18 (72) | 7 (28) | 0.298 |
| GN | 14 (77.8) | 4 (22.2) | 4 (66.7) | 2 (33.3) | 18 (75) | 6 (25) | 0.618 |
| COL | 18 (94.7) | 1 (5.3) | 5 (83.3) | 1 (16.7) | 23 (92) | 2 (8) | 0.430 |
| MEM | 13 (68.4) | 6 (31.6) | 3 (50) | 3 (50) | 16 (64) | 9 (36) | 0.630 |
| TPZ | 12 (63.2) | 7 (36.8) | 3 (50) | 3 (50) | 15 (60) | 10 (40) | 0.653 |
| CAZ | 13 (72.2) | 5 (27.8) | 1 (16.7) | 5 (83.3) | 14 (58.3) | 10 (41.7) | 0.049* |
| NET | 9 (64.3) | 5 (35.7) | 3 (50) | 3 (50) | 12 (60) | 8 (40) | 0.642 |
| CİP | 13 (68.4) | 6 (31.6) | 3 (50) | 3 (50) | 16 (64) | 9 (36) | 0.630 |
| LEV | 12 (70.6) | 5 (29.4) | 1 (16.7) | 5 (83.3) | 13 (56.5) | 10 (43.5) | 0.050* |
| IMP | 10 (52.6) | 9 (47.4) | 3 (60) | 2 (40) | 13 (54.2) | 11 (45.8) | 0.999 |
| FEP | 11 (64.7) | 6 (35.3) | 1 (16.7) | 5 (83.3) | 12 (52.2) | 11 (47.8) | 0.069 |
p < 0.05 significant relationship, p > 0.05 no significant relationship; Chi-square test.
AK: amikacin, GN: gentamicin, COL: colistin, MEM: meropenem, TPZ: piperacillin tazobactam, CAZ: ceftazidime NET: netilmicin, CIP: ciprofloxacin, LEV: levofloxacin, IMP: imipenem, FEP: cefepime.
Fig. 1.
Comparison of antibiotic resistance rates of Acinetobacter baumannii strains isolated from ETA cultures of pre-pandemic ICU and pandemic-period Covid-19 ICU patients.
AK: amikacin, GN: gentamicin, COL: colistin, MEM: meropenem, TPZ: piperacillin-tazobactam, NET: netilmicin, CIP: ciprofloxacin, LEV: levofloxacin, IMP: imipenem, TGC: tigecycline, TOB: tobramycin, SXT: trimethoprim-sulfamethoxazole.
Fig. 2.
Comparison of antibiotic resistance rates of Klebsiella pneumoniae strains isolated from ETA cultures of pre-pandemic ICU and pandemic-period Covid-19 ICU patients.
AK: amikacin, GN: gentamicin, COL: colistin, MEM: meropenem, ETP: ertapenem, AMC: amoxicillin-clavulanic acid, TPZ: piperacillin-tazobactam, CAZ: ceftazidime, CIP: ciprofloxacin, TGC: tigecycline, SXT: trimethoprim, sulfamethoxazole, FEP: cefepime.
Fig. 3.
Comparison of antibiotic resistance rates of Pseudomonas aeruginosa strains isolated from ETA cultures of pre-pandemic ICU and pandemic-period Covid-19 ICU patients.
AK: amikacin, GN: gentamicin, COL: colistin, MEM: meropenem, TPZ: piperacillin tazobactam, CAZ: ceftazidime NET: netilmicin, CIP: ciprofloxacin, LEV: levofloxacin, IMP: imipenem, FEP: cefepime.
4. Discussion
The pandemic of SARS-CoV-2 infection in early 2020 hit most countries in the world seriously, and one of the biggest challenges posed by this infection was the large number of patients requiring intensive care [17]. In other severe acute respiratory syndrome (SARS) outbreaks have been reported bacterial and fungal superinfections during intensive care unit stays. However, limited data are available on Covid-19 patients. Although many researchers recognize the significance of superinfection, definitive data are still lacking [18,19]. Since some patients used antibiotics before culture, microorganisms cannot grow as a result of culture. In a study conducted in Tehran, Iran, A. baumannii and K. pneumoniae were found to have the highest incidence rates in ICUs [20]. In a study investigating secondary infection of the lower respiratory tract of Covid-19 patients in intensive care, A. baumannii was found to be the most common organism, followed by S. aureus [21]. In a study of respiratory pathogens from lower respiratory tract specimens of adult patients in intensive care units, the most common pathogens detected were K. pneumoniae (21.3%), P. aeruginosa (14.9%), A. baumannii (12.8%), and S. aureus (12.8%) [22]. Genç et al. [23] evaluated the agents of hospital pneumonia in ICU and reported that Gram-negative bacteria A. baumannii, Pseudomonas spp., and Gram-positive bacteria S. aureus were the most common agents. In our study, which focused on bacterial coinfection of the lower respiratory tract of patients in the Covid-19 ICU, A. baumannii was the most common microorganism, followed by K. pneumoniae, as in the pre-pandemic period. In the present study, E. faecium (8%) was found to be the most common Gram-positive bacteria in Covid-19 ICU, and S. aureus (6.7%) in pre-pandemic ICU.
In a study focusing on secondary infection of the lower respiratory tract in Covid-19 ICU patients, the average length of stay in the ICU was found to be approximately 15 days, and 95% of the cases died at the end of the study [21]. In their study, Bardi et al. [24] found the mortality rate of nosocomial infections in Covid-19 ICUs as 36% (51/140) and the average hospitalization period as 14 days. In our study, the hospitalization period was found to be 13.49 ± 8.03 in Covid-19 ICU patients, and 33.59 ± 32.89 in the pre-pandemic period, and this difference was significant (p < 0.05). In the present study, the mortality rate was found to be 94% (64/67) in Covid-19 ICU patients, and 83.6% (62/73) in pre-pandemic ICU patients (p > 0.05).
Overuse of antimicrobials increases the risk of multi-resistant hospital-acquired secondary infections associated with adverse clinical outcomes [25]. Therefore, the application of empirical antibiotic coverage in Covid-19 patients should be carefully evaluated. Examining the prevalence and etiology of bacterial coinfections in patients with viral respiratory tract infections can be of great help in initiating early and appropriate antimicrobial therapy and improving prognosis. The reported incidence of coinfection in Covid-19 patients ranges from 3.6% to 43% [26]. Acer et al. [27] detected bacterial coinfection in 64 (8.9%) of 720 Covid-19 patients. In a study involving 140 patients with severe Covid-19 admitted to the intensive care unit, 57 patients (40.7%) were reported developing a bacterial or fungal infection during their stay in the intensive care unit [24]. In another study, coinfections with Enterobacterales (34.0%) and Aspergillus fumigatus (18.0%) were detected in critically ill patients (n = 50) admitted to intensive care units [28]. In another study conducted to determine the incidence, 731 intensive care patients who were positive for Covid-19 were examined. The presence of lower respiratory tract infection in 24 of these patients and the presence of bacteremia in 103 of these patients were detected in the culture medium [29]. In another study investigating bacterial and fungal co-infections among Covid-19 patients in the intensive care unit, the pathogen was detected in 58.3% of respiratory tract samples [30]. Rawson et al. [19] reported defined bacterial or fungal co-infection in 62 (8%) of 806 Covid-19 patients; However, 72% of these patients were given systemic antibacterial drugs. In our study, the incidence of bacterial and fungal co-infection in the lower respiratory tract in Covid-19 ICU patients was found to be 11.1%.
Sharifipour et al. [21] showed that A. baumannii isolates grown in ETA cultures of Covid-19 patients in the ICU were highly resistant to all antibiotics tested, except colistin, with a resistance rate of 52%. In the report of the antibiotic resistance research conducted by the Ministry of Health in 2017, while colistin resistance was found to be 3.9% for A. baumannii, imipenem resistance was 97.42%, levofloxacin resistance was 97.19%, meropenem resistance was 97.16%, and tigecycline resistance was 34.193 [31]. In a study evaluating hospital-acquired pneumonia cases in intensive care patients, carbapenem resistance in Acinetobacter strains was determined as 100%, and the rates of resistance to netilmicin and colistin were determined as 48.5% and 0%, respectively [23]. Similarly, in our study, carbapenem resistance in A. baumannii isolates was found to be 100%, colistin resistance was 0%, and netilmicin resistance was found to be 30% before the pandemic and 44% after the pandemic. Colistin has become almost the only option for A. baumannii strains, which are the most common agents in ETA cultures in ICUs in our hospital. In our study, although more intensive care unit admissions were observed in the pre-pandemic period, A. baumannii infection was found much more frequently in patients followed up during the pandemic period. In different studies on resistance, an increase in resistance rates in Gram-negative bacilli has been demonstrated [32,33]. In our study, resistance increase occurred in almost all antibiotics we examined over the years in K. pneumoniae and P. aeruginosa isolates, which are the most common pathogens seen in ETA cultures following A. baumannii in both intensive care units. The increase in resistance in K. pneumoniae strains was not found statistically significant (p > 0.05). The increase in resistance to ceftazidime and levofloxacin was significant in P. aeruginosa strains (p < 0.05). The reason for this increase in resistance may be the increased need for antibiotics in Covid-19 patients or the increased use of unnecessary empirical antibiotics. Given the potential misuse of empirical broad-spectrum antibiotics in severe Covid-19 patients, the focus should be on the careful use of antibiotics based on culture results to reduce the development of resistance [34].
In conclusion, as seen in our study, the Covid-19 pandemic requires intensive care follow-ups at an earlier age and with a more mortal course. Although the length of stay in the intensive care unit has been shortened, it is observed that this situation is observed due to early mortality. In P. aeruginosa strains, a significant difference was detected in the resistance increase of the ceftazidime and levofloxacin (p < 0.05). With the exception of tigecycline, antibiotic resistance rates in A. baumannii strains increased following the pandemic. Only tobramycin showed a significant difference in the increase of resistance among these antibiotics (p = 0.037). The rate of tigecycline resistance, on the other hand, was 17.6% before the pandemic and 2.2% afterward. Secondary infections in patients create more difficult treatment processes due to both Covid-19 and increasing antibiotic resistance today.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Taleghani N., Taghipour F. Diagnosis of COVID-19 for controlling the pandemic: a review of the state-of-the-art. Biosens. Bioelectron. 2020:112830. doi: 10.1016/j.bios.2020.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArdle A.J., Turkova A., Cunnington A.J. When do co-infections matter? Curr. Opin. Infect. Dis. 2018;31:209. doi: 10.1097/QCO.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paget C., Trottein F. Mechanisms of bacterial superinfection post-influenza: a role for unconventional T cells. Front. Immunol. 2019;10:336. doi: 10.3389/fimmu.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakaletz L.O. Viral–bacterial co-infections in the respiratory tract. Curr. Opin. Microbiol. 2017;35:30–35. doi: 10.1016/j.mib.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014;12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 6.Jia L., Xie J., Zhao J., Cao D., Liang Y., Hou X., et al. Mechanisms of severe mortality-associated bacterial co-infections following influenza virus infection. Front Cell Infect Microbiol. 2017;7:338. doi: 10.3389/fcimb.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quah J., Jiang B., Tan P.C., Siau C., Tan T.Y. Impact of microbial Aetiology on mortality in severe community-acquired pneumonia. BMC Infect. Dis. 2018;18:1–9. doi: 10.1186/s12879-018-3366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R., III, et al. Critical illness from 2009 pandemic influenza A (H1N1) virus and bacterial co-infection in the United States. Crit. Care Med. 2012;40:1487. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahariadis G., Gooley T.A., Ryall P., Hutchinson C., Latchford M.I., Fearon M.A., et al. Risk of ruling out severe acute respiratory syndrome by ruling in another diagnosis: variable incidence of atypical bacteria coinfection based on diagnostic assays. Cancer Res. J. 2006;13:17–22. doi: 10.1155/2006/862797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertow D.S., Memoli M.J. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres A., Niederman M.S., Chastre J., Ewig S., Fernandez-Vandellos P., Hanberger H., et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur. Respir. J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 14.Çetin E.S., Aynali A., Demirci S., Sanem A., Aridoğan C.B., Aridoğan B.C. Nöroloji yoğun bakım ünitesinde yatan hastalardan izole edilen hastane infeksiyonu etkenleri. Ankara Üniv Tıp Fak Mecm. 2009;62:13–17. [Google Scholar]
- 15.Şafak B., Çİftçİ İ.H., Kiyildi N., Aktepe O.C., Çetİnkaya Z., Altindİş M. Ventilatör ilişkili pnömoni tanısında endotrakeal aspirat kültürleri: 2004-2006 yılları sonuçları. Ankem Derg. 2007;21:81–85. [Google Scholar]
- 16.Çelik D., Yildiz Ş.T., Ilgazli A., Wilke A., Başyiğit İ., Yildiz F., et al. Ventilatör ilişkili pnömoni tanısında bronkoskopik ve bronkoskopik olmayan yöntemlerın tanısal etkinliklerinin karşılaştırılması. Solunum. 2006;8:95–101. [Google Scholar]
- 17.Borobia A.M., Carcas A.J., Arnalich F., et al. medRxiv; 2020. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P., Liu Z., Chen Y., Xiao Y., Huang X., Fan X.-G. Bacterial and fungal infections in COVID-19 patients: a matter of concern. Infect. Control Hosp. Epidemiol. 2020;41:1124–1125. doi: 10.1017/ice.2020.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawson T.M., Moore L.S., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharifi A., Kavoosi F., Hosseini S.M.J., Mosavat A., Ahmadi A. Prevalence of Streptococcus pneumoniae in ventilator-associated pneumonia by real-time PCR. Arch. Clin. Infect. Dis. 2019;14:6. [Google Scholar]
- 21.Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-Ardakani R., Koohpaei A., et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 2020;20:1–7. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.H., Ruan S.-Y., Pan S.-C., Lee T.-F., Chien J.-Y., Hsueh P.-R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J. Microbiol. Immunol. Infect. 2019;52:920–928. doi: 10.1016/j.jmii.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genç Y., Gürkan Y., Mumcuoğlu İ., Kanyilmaz D., Aksoy A., Aksu N. Yoğun bakım hastalarında hastane kaynaklı pnömoni olgularının değerlendirilmesi ve sık görülen bakteriyel etkenlerin antimikrobiyallere dirençlerinin araştırılması. Turk Hij Den Biyol Derg. 2016;73(4):355–364. [Google Scholar]
- 24.Bardi T., Pintado V., Gomez-Rojo M., Escudero-Sanchez R., Lopez A.A., Diez-Remesal Y., et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sticchi C., Alberti M., Artioli S., Assensi M., Baldelli I., Battistini A., et al. Regional point prevalence study of healthcare-associated infections and antimicrobial use in acute care hospitals in Liguria. Italy. J Hosp Infect. 2018;99:8–16. doi: 10.1016/j.jhin.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acer O., Ozudogru O., Zengin F. Investigation of bacterial coinfection among hospitalized patients with COVID-19: a retrospective study in A Turkey training and research hospital. Int. J. Clin. Ski. 2021;15:168. [Google Scholar]
- 28.Rothe K., Feihl S., Schneider J., Wallnöfer F., Wurst M., Lukas M., et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:859–869. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripa M., Galli L., Poli A., Oltolini C., Spagnuolo V., Mastrangelo A., et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin. Microbiol. Infect. 2021;27:451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S., Hua M., Liu X., Du C., Pu L., Xiang P., et al. Full title: bacterial and fungal co-infections among COVID-19 patients in intensive. Microb. Infect. May-Jun 2021;23(4–5) doi: 10.1016/j.micinf.2021.104806. 104806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakanlığı S. 2017. Ulusal Sağlık Hizmeti İlişkili Enfeksiyonlar Sürveyans Ağı Özet Raporu.https://infline saglik gov tr/login aspx Erişim adresi: 2018. [Google Scholar]
- 32.MacVane S.H. Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J. Intensive Care Med. 2017;32:25–37. doi: 10.1177/0885066615619895. [DOI] [PubMed] [Google Scholar]
- 33.Arsi Europe. European Centre for Disease Prevention and Control Stockholm; 2017. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) [Google Scholar]
- 34.Clancy C.J., Nguyen M.H. COVID-19, superinfections and antimicrobial development: what can we expect? Clin. Infect. Dis. 2020;71(10):2736–2743. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]