Abstract
An experiment was conducted to test the hypothesis that increased dietary Trp is needed in high-Leu diets for growing pigs to prevent a drop in plasma serotonin and hypothalamic serotonin concentrations and to maintain growth performance of animals. A total of 144 growing pigs (initial weight: 28.2 ± 1.9 kg) were assigned to 9 treatments in a randomized complete block design with 2 blocks, 2 pigs per pen, and 8 replicate pens per treatment. The 9 diets were formulated in a 3 × 3 factorial with 3 levels of dietary Leu (101%, 200%, or 299% standardized ileal digestible [SID] Leu:Lys), and 3 levels of dietary Trp (18%, 23%, or 28% SID Trp:Lys). A basal diet that met requirements for SID Leu and SID Trp was formulated and 8 additional diets were formulated by adding crystalline L-Leu and (or) L-Trp to the basal diet. Individual pig weights were recorded at the beginning of the experiment and at the conclusion of the 21-d experiment. On the last day of the experiment, 1 pig per pen was sacrificed, and blood and hypothalamus samples were collected to measure plasma urea N, plasma serotonin, and hypothalamic serotonin concentrations. Results indicated that increasing dietary Trp increased (P < 0.05) hypothalamic serotonin, whereas increases (P < 0.05) in average daily gain (ADG) and average daily feed intake (ADFI) were observed only in pigs fed diets containing excess Leu. Increasing dietary Leu reduced (P < 0.05) ADG, ADFI, and hypothalamic serotonin. However, the increase in ADG and ADFI caused by dietary Trp was greater if 299% SID Leu:Lys was provided than if 101% SID Leu:Lys was provided (interaction, P < 0.05). Plasma Leu concentration was positively affected by dietary Leu and negatively affected by dietary Trp, but the negative effect of Trp was greater if 299% SID Leu:Lys was provided than if 101% SID Leu:Lys was provided (interaction, P < 0.05). Plasma concentration of Trp was positively affected by increased dietary Trp and increased dietary Leu, but the increase in plasma concentration of Trp was greater if Leu level was at 101% SID Leu:Lys ratio than at 299% SID Leu:Lys ratio (interaction, P < 0.05). In conclusion, increased dietary Leu reduced ADG, ADFI, and hypothalamic serotonin concentration, and influenced metabolism of several indispensable amino acids, but Trp supplementation partly overcame the negative effect of excess Leu. This demonstrates the importance of Trp in regulation of hypothalamic serotonin, and therefore, feed intake of pigs.
Keywords: branched-chain amino acids, leucine, pigs, serotonin, tryptophan
Introduction
Tryptophan is one of indispensable amino acids (AA) that is often limiting for growth in pigs fed corn–soybean meal-based diets (Lewis, 2001; Petersen, 2011). Tryptophan may act as a regulator of feed intake by enhancing serotonin signaling in the brain (Henry et al., 1992) because Trp is a precursor for serotonin, which is a cerebral neurotransmitter that plays an important role in appetite regulation (Zhang et al., 2007). High Trp intake increases feed intake (Henry et al 1992; Ettle and Roth, 2004), and this is partly attributed to increased serotonin synthesis (Shen et al., 2012a). Availability of dietary Trp in the brain is considered the rate-limiting step in hypothalamic serotonin synthesis (Meunier-Salaün et al., 1991; Shen et al., 2012b). However, to be transported into the brain, Trp competes with other large neutral AA (LNAA) such as Val, Leu, Ile, Tyr, and Phe for a common transporter (L-type AA transporter 1) to cross the blood-brain barrier (Le Floc’h and Sève, 2007).
Diets based on corn and corn co-products and sorghum and sorghum co-products are rich in Leu and excess dietary Leu reduces pig feed intake and growth performance (Gatnau et al., 1995; Wiltafsky et al., 2010). Excess dietary Leu may also reduce synthesis of serotonin in the brain (Wessels et al., 2016b) because excess Leu may prevent Trp from being transported from the blood to the brain, which may result in reduced availability of Trp for serotonin synthesis in the brain. Indeed, excess dietary Leu reduces hypothalamic serotonin concentration (Kwon et al., 2019). As a consequence, it is possible that if dietary Leu is in excess of the requirement, extra dietary Trp may be needed to overcome the reduction in serotonin concentrations, but to our knowledge, no information about effects of extra Trp on growth performance and serotonin synthesis have been presented for pigs fed diets containing excess Leu. Therefore, the objective of this experiment was to test the hypothesis that increased dietary Trp is needed in high-Leu diets for growing pigs to prevent drops in both plasma serotonin and hypothalamic serotonin concentrations and to maintain growth performance of animals.
Materials and Methods
All animal care procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois.
Animals, diets, and experimental design
A total of 144 growing pigs with an initial body weight of 28.2 ± 1.9 kg were divided into 2 blocks of 72 pigs and randomly assigned to 9 dietary treatments in a randomized complete block design. There were 2 pigs (1 barrow and 1 gilt) per pen and 4 replicate pens per block for a total of 8 replicate pens per treatment. Pigs were the offspring of Line 359 boars and Camborough sows (Pig Improvement Company, Henderson, TN). A basal diet based on corn, soybean meal, wheat, and barley was formulated to contain 1.003% standardized ileal digestible (SID) Leu which was 100% of the requirement for SID Leu (101% SID Leu:Lys; NRC, 2012; Table 1). Two additional diets were formulated by adding either 1.00% or 2.00% crystalline L-Leu to the basal diet to have SID Leu:Lys ratios of either 200% or 299%. These 3 diets were formulated to have a SID Trp:Lys ratio of 18%. Six additional diets were formulated by adding either 0.05% or 0.10% crystalline L-Trp to each of the 3 original diets. Thus, there was a total of 9 diets that were arranged in a 3 × 3 factorial design with 3 levels of Leu (101%, 200%, or 299% SID Leu:Lys) and 3 levels of SID Trp (18%, 23%, or 28% SID Trp:Lys).
Table 1.
Ingredient composition of experimental diets, as-fed basis
| SID1 Leu:Lys, % | 101 | 200 | 299 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SID Trp:Lys, % | 18 | 23 | 28 | 18 | 23 | 28 | 18 | 23 | 28 |
| Ingredient, % | |||||||||
| Ground corn | 27.91 | 27.91 | 27.91 | 27.91 | 27.91 | 27.91 | 27.91 | 27.91 | 27.91 |
| Soybean meal, 46% CP2 | 17.60 | 17.60 | 17.60 | 17.60 | 17.60 | 17.60 | 17.60 | 17.60 | 17.60 |
| Ground wheat | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Ground barley | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 |
| Soybean oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cornstarch | 0.90 | 0.90 | 0.90 | 0.45 | 0.45 | 0.45 | — | — | — |
| L-Lys HCl | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 |
| DL-Met | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| L-Thr | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| L-Trp | 0.02 | 0.07 | 0.12 | 0.02 | 0.07 | 0.12 | 0.02 | 0.07 | 0.12 |
| L-His | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| L-Leu | — | — | — | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 2.00 |
| L-Val | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| L-Gly | 1.20 | 1.15 | 1.10 | 0.65 | 0.60 | 0.55 | 0.10 | 0.05 | 0.00 |
| Limestone | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Monocalcium phosphate | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin–mineral premix3 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
1SID, standardized ileal digestible.
2CP, crude protein.
3The vitamin–micromineral premix provided the following quantities of vitamins and micro minerals per kilogram of complete diet: Vitamin A as retinyl acetate, 11,136 IU; vitamin D3 as cholecalciferol, 2,208 IU; vitamin E as DL-alpha tocopheryl acetate, 66 IU; vitamin K as menadione dimethylprimidinol bisulfite, 1.42 mg; thiamin as thiamine mononitrate, 0.24 mg; riboflavin, 6.59 mg; pyridoxine as pyridoxine hydrochloride, 0.24 mg; vitamin B12, 0.03 mg; D-pantothenic acid as D-calcium pantothenate, 23.5 mg; niacin, 44.1 mg; folic acid, 1.59 mg; biotin, 0.44 mg; Cu, 20 mg as copper sulfate and copper chloride; Fe, 126 mg as ferrous sulfate; I, 1.26 mg as ethylenediamine dihydriodide; Mn, 60.2 mg as manganese sulfate; Se, 0.3 mg as sodium selenite and selenium yeast; and Zn, 125.1 mg as zinc sulfate.
All diets were formulated to be isoenergetic (3,300 kcal ME/kg) and to contain 1.00% SID Lys, which was assumed to be slightly above the SID Lys requirement (0.98%) for 25 to 50 kg pigs (NRC, 2012). Requirements for SID Leu and Trp for 25 to 50 kg pigs are estimated to be 0.99% and 0.17%, respectively (NRC, 2012). These values from NRC (2012) correspond to 101% SID Leu:Lys ratio and 17% SID Trp:Lys ratio. Therefore, diets containing 18% SID Trp:Lys were believed to provide dietary Trp slightly above the requirement. Valine and Ile were included close to 100% of the requirement for pigs, which is common for diets used under commercial conditions. Other indispensable AA were included in all diets in excess of the requirement (NRC, 2012). Glycine was included in all diets to maintain a constant concentration of dietary crude protein at 18% among all diets.
Pigs were housed in pens with concrete slats. Each pen (0.9 × 1.8 m) was equipped with a feeder and a nipple drinker, and pigs had free access to feed and water throughout the experiment. The individual body weight of pigs was recorded at the beginning and at the end of the 21-d experiment. Daily feed allotments were recorded, and the weight of feed left in the feeders was recorded on the last day of the experiment to calculate feed consumption. The average daily gain (ADG), average daily feed intake (ADFI), and average gain to feed ratio were calculated for each pen of pigs and for each treatment group at the conclusion of the experiment.
Sample collection
At the beginning and on day 11 of the experiment, 1 blood sample was collected from the jugular vein of 1 barrow in each pen using a heparinized vacutainer (BD, Franklin Lakes, NJ). On the last d of the experiment, 2 blood samples were collected in heparinized vacutainers and vacutainers containing EDTA (BD) from the same pig in each pen that was used for bleeding at the beginning and on day 11 of the experiment. All samples were centrifuged at 1,500 × g at 4 °C for 15 min to collect plasma and samples were then frozen at -80 °C until analyzed. After bleeding, 1 pig per pen was euthanized by electrocution and then exsanguinated. Brain tissue was removed, and the hypothalamus was isolated and frozen in liquid N and then stored at -80 °C until analysis.
Sample analyses
Samples of corn, soybean meal, wheat, and barley, which were the main ingredients in the diets, and all experimental diets were analyzed for AA [method 982.30 E (a, b, c); AOAC Int., 2007] using an Amino Acid Analyzer (model L 8800; Hitachi High Technologies America Inc., Pleasanton, CA). These samples were also analyzed for N (method 984.13; AOAC Int., 2007) using a Kjeltec 8400 apparatus (FOSS Inc., Eden Prairie, MN). Crude protein was calculated as N × 6.25. All diets and ingredients were also analyzed for dry matter (method 930.15; AOAC Int., 2007), and all diets were analyzed for ash (method 942.05; AOAC Int., 2007). The concentration of acid hydrolyzed ether extract (method AM 5-04; AOAC Int., 2007) in all diets was measured using the ANKOM HCl hydrolysis system and an ANKOM XT15 fat extractor (ANKOM Technologies, Macedon, NY). All diets were also analyzed for gross energy using a bomb calorimeter (model 6400; Parr Instruments, Moline, IL). Plasma from blood in the heparinized tubes was analyzed for plasma urea N using a Beckman Coulter Clinical Chemistry AU analyzer (Beckman Coulter Inc., Brea, CA). The heparinized plasma samples were also analyzed for free AA using an Amino Acid Analyzer (model L 8900; Hitachi High Technologies America Inc., Pleasanton, CA) equipped with a high-performance cation exchange column.
Platelet-free plasma was prepared from anticoagulated blood in the tubes containing EDTA by double centrifugation according to the protocol described by Shen et al. (2012a). The supernatant was filtered with a 0.45-µm syringe filter to remove remaining platelets from the plasma. Concentrations of serotonin in the platelet-free plasma and in the hypothalamus were analyzed using ELISA kits developed for porcine according to the manufacturer’s protocol (GenWay Biotech, Inc., San Diego, CA). To obtain homogenates from the hypothalamus, frozen samples were weighed (0.5 g) and homogenized with buffer solution on ice using a hand-held Tissue Tearor (Biospec Products, Inc., Bartlesville, OK). The homogenate was centrifuged at 15,000 × g at 4 °C for 30 min and the supernatant was used to determine the concentration of tissue-free serotonin in the hypothalamus.
Statistical analyses
Normality of data was verified, and outliers were identified using the UNIVARIATE procedure of SAS (SAS Institute Inc., Cary, NC). Data were analyzed using the MIXED procedure of SAS (SAS Institute. Inc.). The experimental unit was the pen and the model included dietary concentration of SID Leu, dietary concentration of SID Trp, and the interaction between SID Leu and SID Trp as fixed effects and block and replicate within block as random effect. Assumptions of the model were tested using PROC GPLOT and influence options of SAS. Effects of dietary concentration of SID Leu, dietary concentration of SID Trp, and the interaction between SID Leu and SID Trp were considered significant at P ≤ 0.05. If the interaction or one of the main effects was significant, the software NLREG version 6.5 (Sherrod, 2008) was used to determine parameter estimates for the second-order response surface model to increasing dietary concentrations of SID Leu and SID Trp as described by Khuri and Cornell (1996). Parameter estimates of the model that were not significant (P > 0.10) and were not included in a significant interaction, were removed from the model and the estimates were recalculated. The surface response full model was:
Y = a + b × SID Trp + c × SID Trp2 + d × SID Leu + e × SID Leu2 + f × SID Trp × SID Leu + g × SID Trp2 × SID Leu + h × SID Trp × SID Leu2 + i × SID Trp2 × SID Leu2,
where Y is the dependent variable, a is the intercept, b, c, d, e, f, g, h, and i are the coefficients, and SID Trp and SID Leu are the percentage concentrations of dietary SID Trp and SID Leu.
Results
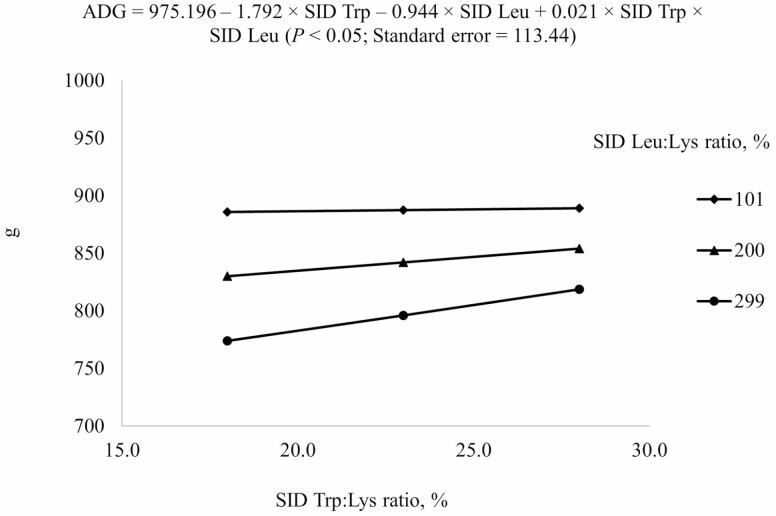
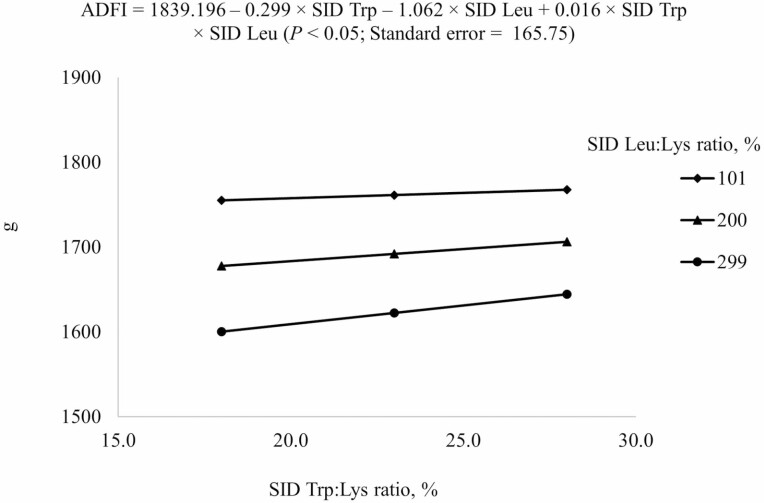
Crude protein and AA concentrations in corn, soybean meal, wheat, and barley were in agreement with reported values (NRC, 2012; Table 2) and analyzed values for crude protein, Leu, Trp, Lys, and most AA in experimental diets were also in agreement with calculated values (Table 3). Inclusion of AA other than Leu and Trp was constant among diets. However, there were some discrepancies between calculated and analyzed concentrations of some AA, which are likely due to errors in sub-sampling or analysis. All animals were healthy and readily consumed their assigned diets throughout the experimental period. The reduced models (P < 0.05) were used to predict ADG and ADFI (Table 4). However, the average gain-to-feed ratio could not be predicted from dietary SID Trp or SID Leu. For ADG and ADFI, the negative linear SID Trp and SID Leu terms, and the interaction between SID Trp and SID Leu were included in the final model (Figures 1 and 2). An interaction (P < 0.05) between SID Leu and SID Trp was observed for ADG and ADFI, because increasing SID Trp increased ADG and ADFI more if Leu level was at 299% SID Leu:Lys ratio than at 101% SID Leu:Lys ratio.
Table 2.
Analyzed nutrient composition of ingredients, as-fed basis
| Item | Corn | SBM1 | Wheat | Barley |
|---|---|---|---|---|
| CP2, % | 7.02 | 46.24 | 11.01 | 11.25 |
| Dry matter, % | 85.56 | 88.80 | 86.58 | 87.64 |
| Indispensable AA, % | ||||
| Arg | 0.32 | 3.28 | 0.52 | 0.55 |
| His | 0.21 | 1.19 | 0.24 | 0.24 |
| Ile | 0.26 | 2.15 | 0.39 | 0.38 |
| Leu | 0.78 | 3.51 | 0.69 | 0.72 |
| Lys | 0.26 | 2.91 | 0.36 | 0.48 |
| Met | 0.14 | 0.61 | 0.19 | 0.19 |
| Phe | 0.32 | 2.36 | 0.45 | 0.49 |
| Thr | 0.26 | 1.79 | 0.32 | 0.38 |
| Trp | 0.06 | 0.63 | 0.13 | 0.12 |
| Val | 0.33 | 2.20 | 0.46 | 0.53 |
| Dispensable AA, % | ||||
| Ala | 0.50 | 1.97 | 0.41 | 0.47 |
| Asp | 0.46 | 5.04 | 0.58 | 0.72 |
| Cys | 0.16 | 0.62 | 0.26 | 0.24 |
| Glu | 1.21 | 8.08 | 2.70 | 2.19 |
| Gly | 0.29 | 1.92 | 0.45 | 0.45 |
| Pro | 0.62 | 2.28 | 0.90 | 0.93 |
| Ser | 0.33 | 1.95 | 0.44 | 0.42 |
| Tyr | 0.19 | 1.71 | 0.27 | 0.27 |
| BCAA3:CP ratio, % | ||||
| Ile:CP | 3.70 | 4.65 | 3.54 | 3.38 |
| Leu:CP | 11.11 | 7.59 | 6.27 | 6.40 |
| Val:CP | 4.70 | 4.76 | 4.18 | 4.71 |
1SBM, soybean meal.
2CP, crude protein.
3BCAA, branched-chain amino acids.
Table 3.
Analyzed nutrient composition of experimental diets, as-fed basis
| SID1 Leu:Lys, % | 101 | 200 | 299 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SID Trp:Lys, % | 18 | 23 | 28 | 18 | 23 | 28 | 18 | 23 | 28 |
| Gross energy, kcal/kg | 4,000 | 4,011 | 4,006 | 4,055 | 4,051 | 4,052 | 4,082 | 4,074 | 4,073 |
| Crude protein, % | 16.46 | 16.63 | 16.40 | 16.63 | 16.61 | 16.70 | 16.62 | 16.58 | 16.58 |
| Dry matter, % | 88.68 | 88.73 | 88.55 | 88.59 | 88.78 | 88.70 | 88.56 | 88.56 | 88.51 |
| Ash, % | 4.65 | 4.56 | 4.49 | 4.37 | 4.46 | 4.32 | 4.37 | 4.65 | 4.43 |
| Acid hydrolyzed ether extract, % | 4.43 | 4.46 | 4.56 | 4.64 | 4.55 | 4.61 | 4.49 | 4.58 | 4.43 |
| Indispensable AA, % | |||||||||
| Arg | 0.95 | 0.91 | 0.89 | 0.95 | 0.92 | 0.93 | 0.84 | 0.88 | 0.87 |
| His | 0.42 | 0.41 | 0.40 | 0.42 | 0.41 | 0.42 | 0.39 | 0.40 | 0.39 |
| Ile | 0.68 | 0.66 | 0.63 | 0.67 | 0.65 | 0.66 | 0.60 | 0.63 | 0.61 |
| Leu | 1.21 | 1.19 | 1.13 | 2.16 | 2.14 | 2.21 | 3.27 | 3.05 | 3.20 |
| Lys | 1.12 | 1.09 | 1.12 | 1.12 | 1.09 | 1.12 | 1.07 | 1.08 | 1.08 |
| Met | 0.31 | 0.29 | 0.29 | 0.31 | 0.28 | 0.29 | 0.26 | 0.31 | 0.27 |
| Phe | 0.77 | 0.75 | 0.72 | 0.77 | 0.74 | 0.76 | 0.69 | 0.73 | 0.72 |
| Thr | 0.65 | 0.69 | 0.69 | 0.70 | 0.69 | 0.69 | 0.65 | 0.69 | 0.66 |
| Trp | 0.18 | 0.24 | 0.28 | 0.19 | 0.23 | 0.28 | 0.20 | 0.23 | 0.27 |
| Val | 0.84 | 0.84 | 0.82 | 0.86 | 0.83 | 0.84 | 0.79 | 0.81 | 0.80 |
| Dispensable AA, % | |||||||||
| Ala | 0.73 | 0.71 | 0.68 | 0.72 | 0.69 | 0.71 | 0.66 | 0.69 | 0.68 |
| Asp | 1.37 | 1.32 | 1.31 | 1.37 | 1.32 | 1.35 | 1.22 | 1.28 | 1.25 |
| Cys | 0.29 | 0.27 | 0.26 | 0.28 | 0.27 | 0.27 | 0.25 | 0.26 | 0.24 |
| Glu | 2.99 | 2.85 | 2.75 | 2.94 | 2.82 | 2.90 | 2.67 | 2.78 | 2.73 |
| Gly | 1.66 | 1.73 | 1.89 | 1.29 | 1.22 | 1.24 | 0.71 | 0.70 | 0.61 |
| Pro | 1.12 | 1.05 | 0.99 | 1.10 | 1.02 | 1.00 | 1.00 | 1.02 | 1.04 |
| Ser | 0.62 | 0.60 | 0.59 | 0.63 | 0.61 | 0.63 | 0.58 | 0.61 | 0.60 |
| Tyr | 0.49 | 0.46 | 0.46 | 0.49 | 0.48 | 0.48 | 0.44 | 0.46 | 0.47 |
1SID = standardized ileal digestible.
Table 4.
Least squares means for growth performance of growing pigs fed diets with varying ratios between dietary standardized ileal digestible (SID) Leu and SID Trp, as-fed basis
| SID Leu:Lys, % | 101 | 200 | 299 | SEM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SID Trp:Lys, % | 18 | 23 | 28 | 18 | 23 | 28 | 18 | 23 | 28 | |
| No. of pens | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| BW, kg | ||||||||||
| Day 1 | 28.8 | 28.9 | 29.1 | 28.9 | 28.8 | 29.0 | 28.8 | 28.9 | 29.0 | 0.9 |
| Day 21 | 47.0 | 47.8 | 47.0 | 46.6 | 47.0 | 48.0 | 44.5 | 46.0 | 45.3 | 1.9 |
| ADG, g/d1 | 867 | 898 | 852 | 845 | 869 | 905 | 750 | 815 | 777 | 61 |
| ADFI, g/d2 | 1,675 | 1,724 | 1,630 | 1,657 | 1,656 | 1,720 | 1,519 | 1,584 | 1,506 | 95 |
| Gain-to-feed ratio3 | 0.52 | 0.52 | 0.52 | 0.51 | 0.52 | 0.53 | 0.49 | 0.51 | 0.51 | 0.02 |
1Results indicated that ADG from days 0 to 21 at different combinations of SID Trp and SID Leu could be described by the following model: 975.196 – 1.792 × SID Trp – 0.944 × SID Leu + 0.021 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were < 0.001, 0.343, and 0.665, respectively.
2Results indicated that ADFI from days 0 to 21 at different combinations of SID Trp and SID Leu could be described by the following model: 1839.196 – 0.299 × SID Trp – 1.062 × SID Leu + 0.016 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were <0.001, 0.562, and 0.514, respectively.
3Results indicated that average gain-to-feed ratio could not be predicted from dietary SID Trp or SID Leu.
Figure 1.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and Leu (P < 0.05), for average daily gain (ADG) in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
Figure 2.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for average daily feed intake (ADFI) in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
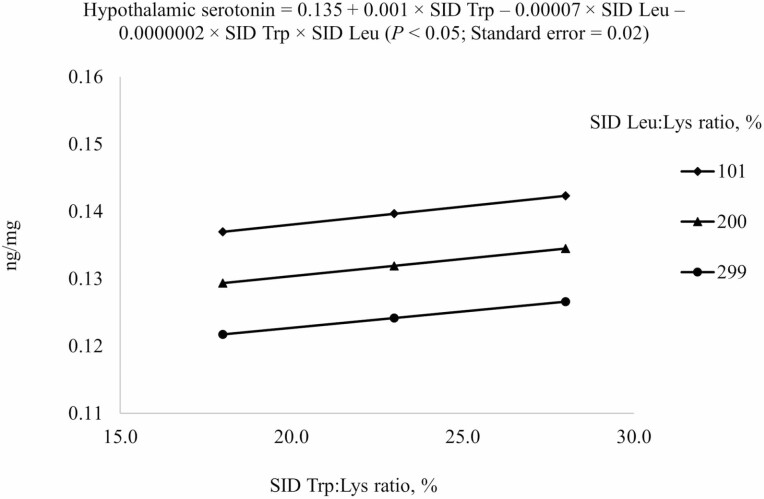
For prediction of serotonin in the hypothalamus, the reduced model (P < 0.05) was used (Table 5). An interaction (P < 0.05) between SID Trp and SID Leu was also observed because increasing SID Trp at high SID Leu concentrations increased hypothalamic serotonin less if Leu level was at 101% SID Leu:Lys ratio than at 299% SID Leu:Lys ratio (Figure 3). However, plasma urea N and plasma serotonin could not be predicted from dietary SID Trp or SID Leu using the chosen model.
Table 5.
Least squares means for concentrations of plasma urea N, plasma serotonin, and hypothalamic serotonin of growing pigs fed diets with varying ratios between dietary standardized ileal digestible (SID) Leu and SID Trp, as-fed basis
| SID Leu:Lys, % | 101 | 200 | 299 | SEM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SID Trp:Lys, % | 18 | 23 | 28 | 18 | 23 | 28 | 18 | 23 | 28 | |
| No. of pens | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| Plasma urea N, μg/mL1 | ||||||||||
| Day 1 | 7.5 | 7.3 | 7.8 | 7.6 | 7.4 | 7.6 | 6.9 | 7.3 | 7.3 | 0.6 |
| Day 11 | 7.5 | 7.5 | 7.4 | 8.4 | 7.5 | 8.0 | 7.8 | 8.8 | 7.6 | 0.7 |
| Day 21 | 8.8 | 7.4 | 7.9 | 8.6 | 7.9 | 8.3 | 7.6 | 8.9 | 8.1 | 1.0 |
| Serotonin | ||||||||||
| Hypothalamus, ng/mg2 | 0.139 | 0.138 | 0.142 | 0.128 | 0.132 | 0.136 | 0.122 | 0.126 | 0.125 | 0.006 |
| Plasma, ng/mL3 | 62.8 | 40.1 | 68.8 | 57.2 | 53.6 | 41.1 | 67.8 | 59.7 | 39.7 | 19.1 |
1Results indicated that plasma urea N could not be predicted from dietary SID Trp or SID Leu.
2Results indicated that serotonin concentration in hypothalamus at different combinations of SID Trp and SID Leu could be described by the following model: 0.135 + 0.001 × SID Trp – 0.00007 × SID Leu – 0.0000002 × SID Trp × SID Leu (P < 0.05); P-value for effects of Leu, Trp, and the interaction between Leu and Trp were 0.006, 0.577, and 0.960, respectively.
3Results indicated that serotonin concentration in plasma could not be predicted from dietary SID Trp or SID Leu.
Figure 3.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for hypothalamic serotonin concentrations in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
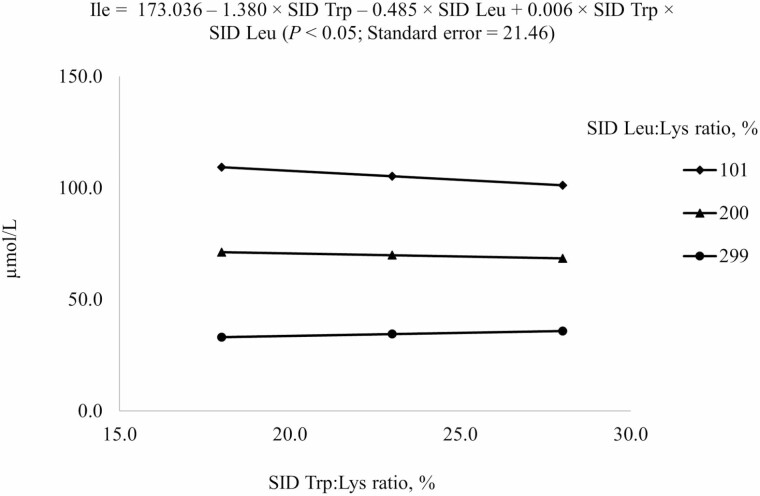
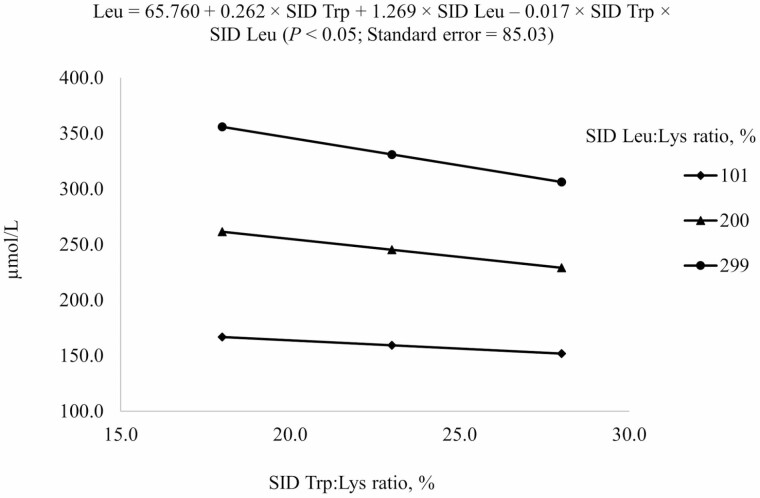
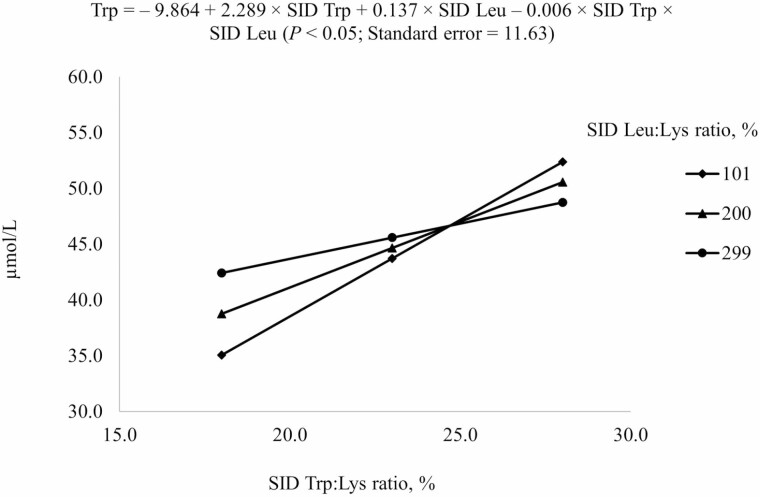
For prediction of free AA concentrations in plasma, reduced models (P < 0.05) were used (Table 6). For Ile, both negative linear SID Trp and SID Leu terms and the interaction between SID Trp and SID Leu were included in the model (Figure 4). Increasing SID Trp at the highest SID Leu level increased Ile concentration in plasma, but increasing SID Trp at the lowest SID Leu levels decreased Ile concentration in plasma (interaction, P < 0.05). For Leu and Trp, positive linear SID Trp and SID Leu terms and the interaction between SID Trp and SID Leu were included in the final model (Figures 5 and 6). Plasma concentration of Leu was reduced by increasing dietary Trp, but the reduction was greater if Leu level was at 299% SID Leu:Lys ratio than at 101% SID Leu:Lys ratio (interaction, P < 0.05; Figure 5). Plasma concentration of Trp increased with increasing dietary Trp but the increase was greater if SID Leu was at the requirement than at 300% of the requirement (interaction, P < 0.05; Figure 6).
Table 6.
Least squares means for concentrations of amino acids (AA) in plasma of growing pigs fed diets with varying ratios between dietary standardized ileal digestible (SID) Leu and SID Trp, as-fed basis
| SID Leu:Lys, % | 101 | 200 | 299 | SEM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SID Trp:Lys, % | 18 | 23 | 28 | 18 | 23 | 28 | 18 | 23 | 28 | |
| Indispensable AA, µmol/L1 | ||||||||||
| Arg | 142.2 | 123.1 | 137.4 | 131.6 | 117.8 | 132.2 | 135.8 | 135.5 | 111.4 | 14.3 |
| His | 54.3 | 43.9 | 45.8 | 50.6 | 49.7 | 61.9 | 51.6 | 53.4 | 46.0 | 4.8 |
| Ile2 | 122.5 | 109.1 | 112.9 | 49.0 | 56.2 | 48.9 | 43.7 | 41.2 | 45.3 | 6.2 |
| Leu3 | 169.2 | 145.6 | 145.5 | 265.0 | 251.1 | 250.4 | 342.4 | 354.2 | 283.9 | 32.0 |
| Lys | 151.3 | 151.3 | 158.7 | 171.7 | 188.2 | 183.8 | 185.4 | 181.4 | 158.3 | 23.1 |
| Met | 46.7 | 40.3 | 44.4 | 42.9 | 39.6 | 44.4 | 46.9 | 43.9 | 40.7 | 5.1 |
| Phe | 74.5 | 61.7 | 66.1 | 65.6 | 69.8 | 70.8 | 74.6 | 72.3 | 74.4 | 4.1 |
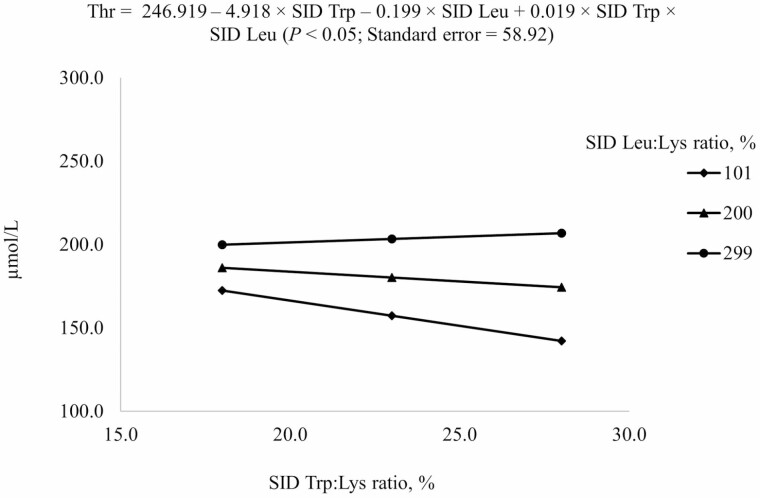
| Thr4 | 186.9 | 161.0 | 152.9 | 169.3 | 147.4 | 164.9 | 206.1 | 224.5 | 209.6 | 20.8 |
| Trp5 | 35.5 | 42.2 | 51.7 | 38.9 | 45.5 | 53.0 | 40.7 | 48.5 | 45.8 | 4.2 |
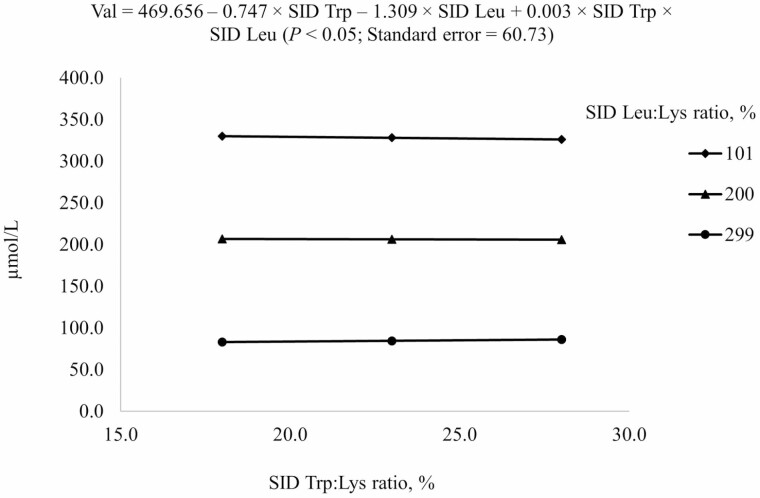
| Val6 | 367.6 | 355.5 | 362.6 | 137.8 | 147.5 | 139.1 | 114.7 | 116.1 | 116.5 | 13.9 |
| Dispensable AA, µmol/L 7 | ||||||||||
| Ala | 463.5 | 483.6 | 459.6 | 431.8 | 514.8 | 511.2 | 509.1 | 526.7 | 612.2 | 47.8 |
| Asp | 26.2 | 27.0 | 25.0 | 26.1 | 28.3 | 27.6 | 27.1 | 30.2 | 28.6 | 2.2 |
| Cys | 2.2 | ND8 | 2.6 | ND | 4.7 | 1.1 | 4.3 | 3.4 | 1.5 | 1.8 |
| Glu | 259.3 | 272.5 | 254.3 | 255.9 | 282.3 | 298.1 | 282.6 | 267.3 | 303.8 | 20.5 |
| Gly | 1,754 | 2,189 | 1,834 | 1,654 | 1,825 | 1,532 | 1,856 | 1,874 | 1,940 | 220 |
| Pro | 252.7 | 272.2 | 274.8 | 252.0 | 264.5 | 268.0 | 273.8 | 279.1 | 332.9 | 20.5 |
| Ser | 157.6 | 192.0 | 167.4 | 153.1 | 183.4 | 152.5 | 175.0 | 170.0 | 198.3 | 17.2 |
| Tyr | 65.7 | 71.6 | 68.3 | 65.9 | 74.1 | 71.1 | 76.3 | 66.4 | 80.8 | 5.7 |
1Results indicated that concentrations of indispensable AA except Ile, Leu, Thr, Trp, and Val in plasma could not be predicted from dietary SID Trp or SID Leu.
2Results indicated that Ile concentration in plasma at different combinations of SID Trp and SID Leu could be described by the following model: 173.036 – 1.380 × SID Trp – 0.485 × SID Leu + 0.006 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were <0.001, 0.789, and 0.446, respectively.
3Results indicated that Leu concentration in plasma at different combinations of SID Trp and SID Leu could be described by the following model: 65.760 + 0.262 × SID Trp + 1.269 × SID Leu – 0.017 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were < 0.001, 0.399, and 0.761, respectively.
4Results indicated that Thr concentration in plasma at different combinations of SID Trp and SID Leu could be described by the following model: 246.919 – 4.918 × SID Trp – 0.199 × SID Leu + 0.019 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were 0.003, 0.744, and 0.707, respectively.
5Results indicated that Trp concentration in plasma at different combinations of SID Trp and SID Leu could be described by the following model: – 9.864 + 2.289 × SID Trp + 0.137 × SID Leu – 0.006 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were 0.702, 0.003, and 0.517, respectively.
6Results indicated that Val concentration in plasma at different combinations of SID Trp and SID Leu could be described by the following model: 469.656 – 0.747 × SID Trp – 1.309 × SID Leu + 0.003 × SID Trp × SID Leu (P < 0.05); P-values for effects of Leu, Trp, and the interaction between Leu and Trp were <0.001, 0.998, and 0.954, respectively.
7Results indicated that concentrations of dispensable AA in plasma could not be predicted from dietary SID Trp or SID Leu.
8ND, Not detected.
Figure 4.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for plasma Ile concentration in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
Figure 5.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for plasma Leu concentration in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
Figure 6.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for plasma Trp concentration in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
Increasing SID Trp at the highest SID Leu level increased Val concentration in plasma but increasing SID Trp at lower SID Leu levels decreased Val concentration in plasma (interaction, P < 0.05; Figure 7). Increasing SID Trp if 101% SID Leu:Lys was provided increased Thr concentration in plasma, but increasing SID Trp if SID Leu level was above the requirement (200% or 299% SID Leu:Lys) decreased Thr concentration in plasma (interaction, P < 0.05; Figure 8).
Figure 7.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for plasma Val concentration in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
Figure 8.
Predicted values, based on the interaction between standardized ileal digestible (SID) Trp and SID Leu (P < 0.05), for plasma Thr concentration in growing pigs fed diets containing from 18% to 28% SID Trp:Lys and from 101% to 299% SID Leu:Lys.
Discussion
Corn and corn co-products and sorghum and sorghum co-products have high Leu:crude protein ratio compared with other cereal grains and the Leu:Lys ratio in corn and sorghum protein is also greater than in most oilseed meals (NRC, 2012; Sotak et al., 2015). If corn- or sorghum-coproducts supply a large amount of protein in diets, concentrations of dietary Leu will be elevated, which may result in an imbalanced supply of branched-chain AA (BCAA) in diets (Rojo-Gomez, 2011; Yang et al., 2019). For example, if a corn-based diet with 30% conventional corn distillers dried grains with solubles (DDGS) is fed to growing pigs, dietary SID Leu will exceed the requirement by 50% to 100%. If greater inclusion of DDGS or if high protein DDGS is used, the excess of dietary Leu will be even greater (Espinosa and Stein, 2018). It is, therefore, possible that practical diets under certain circumstances contain at least twice as much Leu as required.
Results of this experiment confirm that increased dietary Leu reduced ADG and ADFI, but the interactions between SID Trp and SID Leu demonstrated that the negative effect of excess Leu may partially be ameliorated by increasing dietary Trp, which has also been reported previously (Gatnau et al., 1995; Wiltafsky et al., 2010; Wessels et al., 2016a). However, the observation that both ADG and ADFI were maximized at the lowest Leu concentration indicates that excess Trp cannot completely overcome the negative effects of excess Leu. Pigs fed diets containing excess Leu had lower ADFI than pigs fed diets containing SID Leu at the requirement (NRC, 2012), but pig brain Trp concentrations were not influenced by dietary Leu (Wessels et al., 2016a). Thus, it is possible that Trp concentration in the brain is not the only reason for the reduced ADFI observed in pigs fed diets with excess Leu. An imbalanced supply of AA may induce metabolic losses of specific indispensable AA, resulting in AA deficiency (Jansman et al., 2019). In rats and birds, sensing deficiencies and imbalances of indispensable AA by the anterior piriform cortex of the brain is likely the reason for reduced voluntary feed intake (Gietzen et al., 2004). This may be a protective mechanism to prevent protein breakdown in the brain if AA supply is inadequate (Hao et al., 2005).
The observation that concentrations of plasma Ile and Val were decreased by increasing dietary Leu confirmed that increased dietary Leu reduced availability of free Val and Ile in plasma, which is in agreement with reported data (Duan et al., 2016; Wessels et al., 2016a; Kwon et al., 2019). Plasma levels of Ile and Val in pigs fed the diets containing 101% SID Leu:Lys ratio were also in agreement with recent data (Wessels et al., 2016a; Kwon et al., 2020). Likewise, plasma levels of Ile in pigs fed diets containing excess dietary Leu were in agreement with levels of plasma Ile determined in pigs fed Ile deficient diets (Wiltafsky et al., 2009). It is, therefore, likely that the reduced feed intake that is often observed in pigs fed diets containing excess dietary Leu is partially a result of Ile deficiency, which may have been caused by catabolism of Ile after absorption.
Leucine stimulates catabolism of BCAA in skeletal muscle and liver (Harper et al., 1984). If diets fed to pigs contain excess Leu, catabolism of all 3 BCAA may increase because of the stimulating effect of the Leu metabolite, α-keto isocaproate on BCAA catabolizing enzymes (Wiltafsky et al., 2010). Thus, excess dietary Leu decreases the quantities of Val and Ile that are available for protein synthesis. In addition, excess dietary Leu reduced feed intake and growth performance of pigs (Gatnau et al., 1995; Wiltafsky et al., 2010), which may be a result of the imbalanced supply of BCAA that is a result of increased catabolism of Val and Ile.
The increased concentration of serotonin in the hypothalamus that was observed as dietary Trp increased confirms the importance of Trp as a precursor for serotonin. Availability of Trp in the brain is the rate-limiting step for serotonin biosynthesis (Meunier-Salaün et al., 1991; Shen et al., 2012b). Results also demonstrated the negative effect of Leu on serotonin synthesis, which is likely because excess Leu reduces Trp uptake in the brain due to competition in transportation from blood to brain (Henry et al., 1992; Wessels et al., 2016a). Tryptophan has to compete with the LNAA including BCAA for a common uptake transporter (L-type AA transport system) to cross the blood-brain barrier (Le Floc’h and Sève, 2007), and the Trp to LNAA ratio in plasma is the key determinant for serotonin synthesis in the brain (Henry et al., 1992). Reduced Trp to LNAA ratio in plasma, which may be a result of increased dietary Leu, may, therefore, contribute to a reduced uptake of Trp by the brain. However, in this experiment, the Trp to LNAA ratio in plasma was greater if dietary Leu was at a 299% SID Leu:Lys ratio than at 101% SID Leu:Lys ratio. Increasing levels of one of the LNAA elevates its brain uptake and decreases the uptake of the other LNAA (Fernstrom, 2013), but due to the transporter having different affinity for different LNAA, the Trp to LNAA ratio may not always be a good predictor for uptake of Trp into the brain. Smith (2000) summarized Km (Kaplan–Meier estimate) values for AA uptake into brain through the L-type AA transport system in rats, where Km is the half-saturation concentration in the absence of competitors. The Km for Val (~210 µmol/L) was greater than for Ile (~56 µmol/L) and Leu (~29 µmol/L), indicating that the apparent transport affinity (1/Km) of Leu is almost 10 times greater than that of Val. Therefore, even if the Trp to LNAA ratio is reduced in plasma, increased Leu with decreased Val in plasma may reduce Trp uptake into the brain.
A positive correlation between hypothalamic Trp and hypothalamic serotonin and a negative correlation between hypothalamic Trp and plasma Leu were reported (Wessels et al., 2016b). Reduced availability of Trp in plasma also decreased serotonin synthesis in the hypothalamus, resulting in reduced voluntary feed intake in pigs (Henry et al., 1992). Therefore, the reduced feed intake caused by excess dietary Leu that was observed in the present study may be a result of sensing deficiency for Ile and decreased serotonin concentration in the brain, which were also demonstrated.
Platelets in blood and in the gastrointestinal tracts are the greatest pools of body serotonin in animals (Le Floc’h et al., 2017). Serotonin in blood is mostly synthesized by the enterochromaffin cells of the gastrointestinal tract (Watanabe et al., 2010) and approximately 95% of blood serotonin is stored in the platelets. Blood serotonin concentration did not affect intake of food in humans (Anderson et al., 1985), and dietary Trp did not affect plasma serotonin in pigs although serotonin in the hypothalamus was increased (Shen et al., 2012a; b). Thus, the observation that serotonin concentration in the brain was not related to serotonin concentration in blood, and that plasma serotonin was not affected by dietary Leu or Trp is in agreement with reported data.
Plasma urea N is considered a measure for changes in dietary AA balance and efficiency of AA utilization in pigs (Coma et al., 1995). Excess dietary Leu increased plasma urea N, which likely was a result of increased catabolism of Ile and Val, and therefore, imbalances among other indispensable AA (Kwon et al., 2019). Supply of AA in excess of the requirement may result in increased urea synthesis, because excess AA are catabolized (Eggum, 1970). However, the lack of responses for plasma urea N in this experiment indicates that adding additional dietary Leu or dietary Trp to the diet was not effective in improving the efficiency of protein synthesis.
Because crystalline L-Leu or L-Trp were added to the basal diet that was formulated to provide dietary Leu or Trp at the requirement, increased concentrations of free Leu or Trp in plasma was expected as additional Leu or Trp was added to diets. However, the interaction between SID Trp and SID Leu for both AA in plasma may be a result of improved ADG and ADFI in pigs fed diets with additional Trp, indicating that inclusion of additional Trp is needed in diets with excess dietary Leu.
The reason for the concentration of Thr in plasma was positively affected by addition of Leu to the diet may be that the Thr dehydratase pathway, which may be used in Thr metabolism, may be affected by BCAA catabolizing enzymes in rats (House et al., 2001) and possibly also in pigs. The observation that addition of Trp to the diet containing 299% SID Leu:Lys ratio increased concentrations of plasma Thr may be a result of the greater feed intake in pigs fed diets with greater Trp concentration. However, if pigs were fed diets containing 101% SID Leu:Lys or 199% SID Leu:Lys, addition of Trp had a negative effect on plasma Thr. It is possible that the reason for this observation is that additional dietary Trp results in increased microbial synthesis of indoles in the intestinal tract, which in turn may initiate increased synthesis of mucin, which is rich in Thr. Inclusion of fiber in diets to pigs results in increased requirement for dietary Thr because of increased synthesis of mucin (Mathai et al., 2016), and it is, therefore, possible that the Thr requirement is also increased if the concentration of indoles in the intestinal tract is increased. However, additional research is needed to test this hypothesis.
Conclusion
Increased dietary Leu reduced ADG and ADFI, but Trp supplementation partially overcame the negative effect of excess dietary Leu. Hypothalamic serotonin concentration was decreased as dietary Leu increased as a consequence of reduced uptake of Trp, but hypothalamic serotonin concentration was increased as dietary Trp increased. Changes in BCAA, Trp, and Thr concentrations in plasma were observed by addition of Leu or Trp to the diet, indicating that excess dietary Leu influenced the metabolism of several indispensable AA.
Acknowledgement
The financial support from Ajinomoto Animal Nutrition North America, Inc., Chicago, IL is greatly appreciated.
Glossary
Abbreviations
- AA
amino acids
- ADFI
average daily feed intake
- ADG
average daily gain
- BCAA
branched-chain amino acids
- DDGS
corn distillers dried grains with solubles
- LNAA
large neutral amino acids
- SID
standardized ileal digestibility
Conflict of Interest Statement
J.A.S. is an employee at Ajinomoto Animal Nutrition, a global supplier of crystalline AA to the food and feed industries. W.B.K. and H.H.S. have no real or perceived conflicts of interest.
Literature Cited
- Anderson, G. M., Feibel F. C., Wetlaufer L. A., Schlicht K. R., Ort S. M., and Cohen D. J.. . 1985. Effect of a meal on human whole blood serotonin. Gastroenterology 88(1 Pt 1):86–89. doi: 10.1016/s0016-5085(85)80137-2. [DOI] [PubMed] [Google Scholar]
- AOAC Int. 2007. Official methods of analysis of AOAC Int. 18th ed. Rev. 2. Howitz W., and Latimer G. W. Jr, Gaithersburg, MD: AOAC International. [Google Scholar]
- Coma, J., Carrion D., and Zimmerman D. R.. . 1995. Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs. J. Anim. Sci. 73:472–481. doi: 10.2527/1995.732472x. [DOI] [PubMed] [Google Scholar]
- Duan, Y. H., Zeng L. M., Li F. N., Li Y. H., Tan B. E., Ji Y. J., Kong X. F., Tang Y. L., Zhang Y. Z., and Yin Y. L.. . 2016. Effects of dietary branched-chain amino acid ratio on growth performance and serum amino acid pool of growing pigs. J. Anim. Sci. 94:129–134. doi: 10.2527/jas.2015-9527. [DOI] [Google Scholar]
- Eggum, B. O. 1970. Blood urea measurement as a technique for assessing protein quality. Br. J. Nutr. 24:983–988. doi: 10.1079/bjn19700101. [DOI] [PubMed] [Google Scholar]
- Espinosa, C. D., and Stein H. H.. . 2018. High-protein distillers dried grains with solubles produced using a novel front-end- back-end fractionation technology has greater nutritional value than conventional distillers dried grains with solubles when fed to growing pigs. J. Anim. Sci. 96:1869–1876. doi: 10.1093/jas/sky052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettle, T., and Roth F. X.. . 2004. Specific dietary selection for tryptophan by the piglet. J. Anim. Sci. 82:1115–1121. doi: 10.2527/2004.8241115x. [DOI] [PubMed] [Google Scholar]
- Fernstrom, J. D. 2013. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 45:419–430. doi: 10.1007/s00726-012-1330-y. [DOI] [PubMed] [Google Scholar]
- Gatnau, R., Zimmerman D. R., Nissen S. L., Wannemuehler M., and Ewan R. C.. . 1995. Effects of excess dietary leucine and leucine catabolites on growth and immune responses in weanling pigs. J. Anim. Sci. 73:159–165. doi: 10.2527/1995.731159x. [DOI] [PubMed] [Google Scholar]
- Gietzen, D. W., Ross C. M., Hao S., and Sharp J. W.. . 2004. Phosphorylation of eIF2 is involved in the signaling of indispensable amino acid deficiency in the anterior piriform cortex of the brain in rats. J. Nutr. 134:717–723. doi: 10.1093/jn/134.4.717. [DOI] [PubMed] [Google Scholar]
- Hao, S., Sharp J. W., Ross-Inta C. M., McDaniel B. J., Anthony T. G., Wek R. C., Cavener D. R., McGrath B. C., Rudell J. B., Koehnle T. J., . et al. 2005. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- Harper, A. E., Miller R. H., and Block K. P.. . 1984. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Henry, Y., Sève B., Colléaux Y., Ganier P., Saligaut C., and Jégo P.. . 1992. Interactive effects of dietary levels of tryptophan and protein on voluntary feed intake and growth performance in pigs, in relation to plasma free amino acids and hypothalamic serotonin. J. Anim. Sci. 70:1873–1887. doi: 10.2527/1992.7061873x. [DOI] [PubMed] [Google Scholar]
- House, J. D., Hall B. N., and Brosnan J. T.. . 2001. Threonine metabolism in isolated rat hepatocytes. Am. J. Physiol. Endocrinol. Metab. 281:E1300–E1307. doi: 10.1152/ajpendo.2001.281.6.E1300. [DOI] [PubMed] [Google Scholar]
- Jansman, A. J. M., Cirot O., Corrent E., Lambert W., Ensink J., and van Diepen J. T. M.. . 2019. Interaction and imbalance between indispensable amino acids in young piglets. Animal 13:941–949. doi: 10.1017/S175173111800263X. [DOI] [PubMed] [Google Scholar]
- Khuri, A. I., and Cornell J. A.. . 1996. Response surfaces: designs and analyses. 2nd ed. Gainesville, FL: Marcel Dekker, Inc. [Google Scholar]
- Kwon, W. B., Touchette K. J., Simongiovanni A., Syriopoulos K., Wessels A., and Stein H. H.. . 2019. Excess dietary leucine in diets for growing pigs reduces growth performance, biological value of protein, protein retention, and serotonin synthesis. J. Anim. Sci. 97:4282−4292. doi: 10.1093/jas/skz259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, W. B., Soto J. A., and Stein H. H.. . 2020. Effects on nitrogen balance and metabolism of branched-chain amino acids by growing pigs of supplementing isoleucine and valine to diets with adequate or excess concentrations of dietary leucine. J. Anim. Sci. 98:skaa346. doi: 10.1093/jas/skaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc’h, N., Simongiovanni A., Corrent E., and Matte J. J.. . 2017. Comparison of plasma tryptophan-related metabolites in crossbred Piétrain and Duroc pigs. J. Anim. Sci. 95:1606–1613. doi: 10.2527/jas.2016.1179. [DOI] [PubMed] [Google Scholar]
- Le Floc’h, N., and Sève B.. . 2007. Biological roles of tryptophan and its metabolism. Potential implications for pig feeding. Livest. Sci. 112:23–32. doi: 10.1016/j.livsci.2007.07.002. [DOI] [Google Scholar]
- Lewis, A. J. 2001. Amino acids in swine nutrition. InLewis A. J. and Southern L. L., editors. Swine nutrition, 2nd ed. Boca Raton, FL:CRC Press; p. 131–150. [Google Scholar]
- Mathai, J. K., Htoo J. K., Thomson J., Touchette K. J., and Stein H. H.. . 2016. Effects of dietary fiber on the ideal standardized ileal digestible threonine:lysine ratio for 25 to 50 kg growing gilts. J. Anim. Sci. 94:4217–4230. doi: 10.2527/jas.2016.0680. [DOI] [PubMed] [Google Scholar]
- Meunier-Salaün, M. C., Monnier M., Colléaux Y., Sève B., and Henry Y.. . 1991. Impact of dietary tryptophan and behavioral type on behavior, plasma cortisol, and brain metabolites of young pigs. J. Anim. Sci. 69:3689–3698. doi: 10.2527/1991.6993689x. [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient Requirements of Swine. 11th rev. ed.Washington, DC:National Academies Press. [Google Scholar]
- Petersen, G. I. 2011. Estimation of the ideal standardized ileal digestible tryptophan:lysine ratio in 10 to 20 kg pigs. [PhD diss].Urbana-Champaign: Univeristy of Illinois. [Google Scholar]
- Rojo-Gomez, A. 2011. Evaluation of the effects of branched-chain amino acids and corn-distillers dried grains by-products on the growth performance, carcass and meat quality characteristics of pigs [PhD diss]. Urbana, IL: University of Illinois. [Google Scholar]
- Shen, Y. B., Voilqué G., Kim J. D., Odle J., and Kim S. W.. . 2012a. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J. Anim. Sci. 90:2264–2275. doi: 10.2527/jas.2011-4203. [DOI] [PubMed] [Google Scholar]
- Shen, Y. B., Voilqué G., Odle J., and Kim S. W.. . 2012b. Dietary L-tryptophan supplementation with reduced large neutral amino acids enhances feed efficiency and decreases stress hormone secretion in nursery pigs under social-mixing stress. J. Nutr. 142:1540–1546. doi: 10.3945/jn.112.163824. [DOI] [PubMed] [Google Scholar]
- Sherrod, P. H. 2008. Nonlinear regression analysis program (NLREG) version 6.5 (advanced). Nashville, TN: Philip H. Sherrod. [Google Scholar]
- Smith, Q. R. 2000. Transport of glutamate and other amino acids at the blood brain barrier. J. Nutr. 130:1016S–1022S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Sotak, K. M., Houser T. A., Goodband R. D., Tokach M. D., Dritz S. S., DeRouchey J. M., Goehring B. L., Skaar G. R., and Nelssen J. L.. . 2015. The effects of feeding sorghum dried distillers grains with solubles on finishing pig growth performance, carcass characteristics, and fat quality. J. Anim. Sci. 93:2904–2915. doi: 10.2527/jas.2014-8022. [DOI] [PubMed] [Google Scholar]
- Watanabe, H., Akasaka D., Ogasawara H., Sato K., Miyake M., Saito K., Takahashi Y., Kanaya T., Takakura I., Hondo T., . et al. 2010. Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover. Endocrinology 151:4776–4786. doi: 10.1210/en.2009-1349. [DOI] [PubMed] [Google Scholar]
- Wessels, A. G., Kluge H., Hirche F., Kiowski A., Schutkowski A., Corrent E., Bartelt J., König B., and Stangl G. I.. . 2016a. High Leucine diets stimulate cerebral branched-chain amino acid degradation and modify serotonin and ketone body concentrations in a pig model. PLoS One 11:e0150376. doi: 10.1371/journal.pone.0150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels, A. G., Kluge H., Hirche F., Kiowski A., Bartelt J., Corrent E., and Stangl G. I.. . 2016b. High leucine intake reduces the concentration of hypothalamic serotonin in piglets. J. Anim. Sci. 94:26–29. doi: 10.2527/jas2015-9728. [DOI] [Google Scholar]
- Wiltafsky, M. K., Bartelt J., Relandeau C., and Roth F. X.. . 2009. Estimation of the optimum ratio of standardized ileal digestible isoleucine to lysine for eight- to twenty-fivekilogram pigs in diets containing spray-dried blood cells or corn gluten feed as a protein source. J. Anim. Sci. 87:2554–2564. doi: 10.2527/jas.2008-1320. [DOI] [PubMed] [Google Scholar]
- Wiltafsky, M. K., Pfaffl M. W., and Roth F. X.. . 2010. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 103:964–976. doi: 10.1017/S0007114509992212. [DOI] [PubMed] [Google Scholar]
- Yang, Z., Urriola P. E., Hilbrands A. M., Johnston L. J., and Shurson G. C.. . 2019. Growth performance of nursery pigs fed diets containing increasing levels of a novel high-protein corn distillers dried grains with solubles. Transl. Anim. Sci. 3:350–358. doi: 10.1093/tas/txy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Yin J., Li D., Zhou X., and Li X.. . 2007. Tryptophan enhances ghrelin expression and secretion associated with increased food intake and weight gain in weanling pigs. Domest. Anim. Endocrinol. 33:47–61. doi: 10.1016/j.domaniend.2006.04.005. [DOI] [PubMed] [Google Scholar]