Abstract
Background
Obstructive sleep apnea (OSA) is related to multiple complications including insulin resistance (IR), endothelial dysfunction, and increased risk of cardiovascular disease (CVD). The apnea–hypopnea index (AHI) was widely used to measure OSA severity but poorly correlated with complications above. This study aimed to evaluate whether a new metric, the sleep breathing impairment index (SBII), was associated with cardiovascular risk in patients with OSA.
Methods
This study enrolled 140 consecutive male OSA patients without overt atherosclerotic CVD events, including coronary heart disease, stroke, peripheral vascular disease, or heart failure. Data on baseline medical history, anthropometric and polysomnographic parameters, fasting biochemical measurements and endothelial function tests, and common questionnaires were collected. The SBII was calculated by the product of the duration of each obstructive event and the associated desaturation area. The primary outcome was the moderate-to-high Framingham 10-year CVD risk.
Results
The median age of enrolled patients was 40 (35–48) years. Eighty subjects had a moderate-to-high Framingham CVD risk. Patients with SBII in the third and fourth quartile had an increased proportion of moderate-to-high Framingham CVD risk with an adjusted OR 6.28 (95% CI 1.10–36.04) and 11.78 (95% CI 1.25–111.38). Significant association was not demonstrated in AHI and the Framingham CVD risk.
Conclusion
Higher SBII was associated with an increased 10-year CVD risk after adjusting for multiple potential confounding factors. Additional valuable information derived from polysomnography besides AHI deserves to be paid more attention.
Keywords: obstructive sleep apnea, sleep breathing impairment index, apnea-hypopnea index, Framingham cardiovascular risk
Introduction
Obstructive sleep apnea (OSA) is a common condition characterized by repetitive pharyngeal collapse causing intermittent hypoxia, disrupted sleep, and sympathetic activation, with a high prevalence in the general population (23% in women and nearly 50% in men).1 Common symptoms include snoring, hyposomnia, and daytime sleepiness. OSA has been demonstrated to be an independent risk factor for cardiovascular morbidity.2 The surges in sympathetic activity, nocturnal hypoxia, and several adverse determinants associated with OSA such as obesity, insulin resistance (IR), and endothelial dysfunction have been postulated to be the potential pathogenesis of cardiovascular sequelae.3,4 Besides, reactive oxide species induce endothelial dysfunction in the early stages of OSA by increasing the expression of leukocyte-specific and endothelial-specific adhesion molecules, and the endothelial dysfunction may also cause microvascular damage.5 Additionally, OSA is linked with increased oxidative stress, which adversely affect the associated cardio-/cerebrovascular disease in OSA.6 The adverse OSA effect on cardiovascular disease (CVD) has been well established. However, this remains controversial because alternative pieces of evidence revealed different OSA associations or even protective effects.7,8 These findings support the idea that the apnea–hypopnea index (AHI), an extensively used metric to assess OSA severity and predict the clinical outcomes, was no longer appropriate for use as the optimal metric to characterize OSA in clinical practice and research.9
AHI, defined as the number of apnea and hypopnea events per hour of sleep, provided limited information while ignoring the pathophysiologic elements contained in the event-associated desaturation. A few arguments were raised to refute AHI validity. The biological effects of apnea and hypopnea are supposed to be different although the evidence supporting this was limited but was fundamentally equal in AHI calculation. The hypoxemia degree associated with obstruction and the duration of events would surely exert a pathophysiological impact on clinical outcomes but were not reflected in AHI computation. In recent years, several new metrics regarding the more comprehensive OSA evaluation (eg, hypoxic burden,10 obstruction severity,11 or hypoxia load12) have shown an advantage in correlating with diverse sequelae compared with AHI. However, each of them was harboring its insufficiency, and more evidence against the ubiquitous use of AHI with an advanced polysomnographic analysis is warranted.
The current study sought to demonstrate the association between CVD risk and different OSA severities in a cohort of patients free of overt pre-existing CVD, based on the new metric named the sleep breathing impairment index (SBII) that was derived from incorporating the overall characteristics of the obstructive event and its related hypoxia. Thus, SBII rather than AHI was hypothesized to be associated with CVD risk.
Methods
Patients
Consecutive patients referred for suspected OSA at the sleep center of Peking Union Medical College Hospital (PUMCH) were screened from November 2020 to May 2021. OSA diagnosis was based on the International Classification of Sleep Disorders, third edition.13 In clinical practice, many more male patients were found seeking consultation than females. Thus, only male OSA patients between 16 and 70 years old were enrolled. The exclusion criteria were (1) having received OSA treatment including continuous positive airway pressure (CPAP) before enrollment; (2) diagnosis of COPD, restless legs syndrome or narcolepsy; (3) pre-existing CVD, including coronary heart disease, stroke, peripheral vascular disease, or heart failure; (4) concurrent use of lipid-lowering medication or insulin; (5) severe chronic debilitating conditions or pregnancy; (6) inability to complete questionnaires; and (7) patients having total sleep time of <4 h. The study was approved by the ethics committees of PUMCH (JS-2632) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant in this study.
Polysomnographic Recording
All participants underwent overnight polysomnography (PSG, Embla N7000, Natus Medical Incorporated, Orlando, FL, USA) from 11 p.m. to 6 a.m., which were recorded and analyzed by a skilled sleep laboratory technician following standard protocols recommended by the American Academy of Sleep Medicine.13 Apnea was defined as cessation of airflow for ≥10 s, whereas hypopnea was identified as >30% decrease in airflow lasting for at least 10 s accompanied by an associated ≥3% oxygen desaturation. Non-OSA, mild OSA, moderate OSA, severe OSA, and very severe OSA were defined as an AHI of <5, 5–15, 15–30, 30–60, and >60, respectively. Moreover, oxygen desaturation index (ODI), percent of time spent with SpO2 <90% (T90), and lowest SpO2 (LSpO2) were obtained.
SBII Measurement
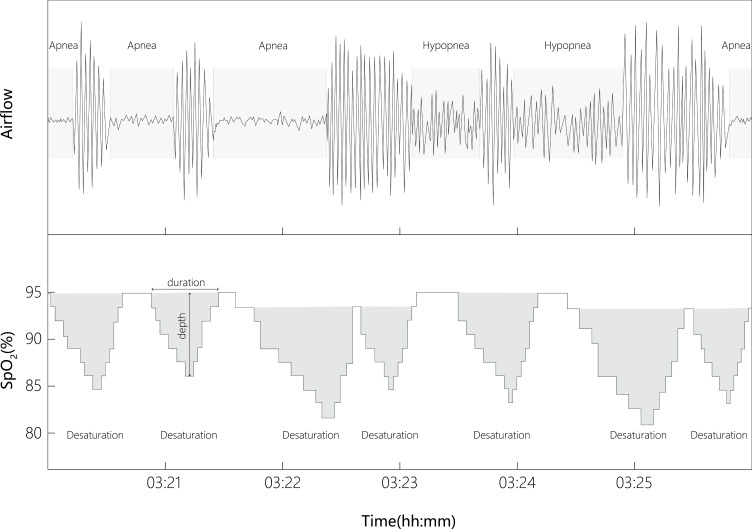
The novel index, SBII, was derived from the exported files of polysomnographic data containing detailed information on the frequency, duration, and degree of analyzed obstructive (apnea and hypopnea) and associated desaturation events with a customized and automated program managed by python. Each event-associated desaturation would be identified when the beginning of individual desaturation was in a 100-s window from the beginning of each respiratory event, and both desaturation and recovery areas (recovery up to the maximum SpO2) were included in the desaturation area. Therefore, each event-associated desaturation area would be considered as a triangle, with its height and base being the desaturation degree and the duration of desaturation, respectively (Figure 1). The SBII was calculated using the sum of the products of the duration of each obstructive event and the associated desaturation area, and then divided by total sleep time. The SBII unit would be (%min2)/h. Rapid eye movement (REM)-SBII and supine-SBII could also then be accordingly obtained.
Figure 1.
The algorithm of sleep breathing impairment index.
Notes: Each event-associated desaturation area would be considered as a triangle, with its height and base being the desaturation degree and the duration of desaturation, respectively. The sleep breathing impairment index was calculated using the sum of the products of the duration of each obstructive event and the associated desaturation area, and then divided by total sleep time. The unit would be (%min2)/h.
Data Collection, Biochemistry Tests, and Endothelial Function Test
All participants filled out the Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS) questionnaires. Poorer sleep quality was identified as a PSQI ≥8.14 Excessive daytime sleepiness was defined as an ESS score >10.15 Baseline medical history and anthropometric data were obtained. After a 10-min rest, seated blood pressure was consecutively measured thrice on the right arm in the morning with a validated automated electronic device (Omron Healthcare) and then averaged for final analysis. Fasting blood samples were taken in the morning after PSG and then processed within 0.5 h. Serum lipid profiles, glycosylated hemoglobin, insulin, and blood glucose were measured with standard procedure. Moreover, endothelial function was measured by peripheral arterial tonometry (EndoPAT, Itamar Medical Ltd., Israel) in the morning after PSG according to the published guideline.16 The standard protocol consisted of three consecutive 5-min duration of stages: (1) baseline recording; (2) occlusion recording with inflating the blood pressure cuff to 60 mmHg above the baseline systolic blood pressure and above 200 mmHg; and (3) recording after deflation of the cuff. Endothelial dysfunction was defined as a reactive hyperemia index (RHI) value <1.67.
Moreover, the Framingham CVD risk was calculated on the basis of a specific multivariable algorithm and categorized as low (≤6%), moderate-to-high (>6%) risks.17 Homeostasis model assessment of insulin resistance (HOMA-IR) was counted as the product of fasting insulin and fasting glucose (in millimole per liter) divided by 22.5. Moreover, IR was defined as HOMA-IR ≥2.5.18 A body mass index (BMI) >25 was identified as obesity.19
Statistical Analysis
Categorical data were described as numbers (in percentage). Continuous variables were presented as mean ± SD or median (interquartile range, 25–75%) if normally distributed or not. Logistical regression analysis was used to determine the association between SBII and a moderate-to-high Framingham CVD risk. SBII was categorized on the basis of its quartiles for better characterization. Once a significant unadjusted association between SBII quartiles and CVD risk was identified, logistic regression models were constructed to further estimate the adjusted risk: model 1 included BMI and excessive daytime sleepiness; model 2 included AHI, LSpO2, and T90 plus all covariates in model 1; and model 3 included endothelial dysfunction and IR plus all covariates in model 2. In addition, the current study also included ODI or different severity based on AHI (5–15, 15–30, 30–60, and >60) instead of AHI itself in model 2. Data management was performed using SPSS software (version 24.0, NY, USA). A two-sided p < 0.05 was considered statistically significant.
Results
Baseline Characteristics of Enrolled Patients
This study recruited 140 patients with OSA. Table 1 presents the baseline characteristics. The median age of these enrolled patients with OSA was 40 years, and the median AHI was 43.9/h. Among them, 22.1%, 17.1%, 32.1%, and 28.6% had AHI indicating mild (5–15), moderate (15–30), severe (30–60), and very severe (>60) OSA, respectively. Furthermore, 57.1% of subjects had a moderate-to-high Framingham CVD risk (half of them have an RHI <1.67 indicating endothelial dysfunction), and 57.9% were identified to have IR. Table 2 shows that the SBII, REM-SBII, and supine-SBII were higher in the moderate-to-high CVD risk group than in the low CVD risk group. A higher proportion of excessive daytime sleepiness was noted in the moderate-to-high CVD risk group.
Table 1.
Baseline Characteristics of Participants
| Variables | N = 140 |
|---|---|
| Age, y, | 40 (35–48) |
| BMI, kg/m2 | 27.7 (25.4–29.8) |
| BMI >25, n (%) | 108 (77.1%) |
| RHI <1.67, n (%) | 70 (50%) |
| TG, mmol/L | 1.83 (1.40–2.77) |
| LDL-c, mmol/L | 3.16 (2.53–3.82) |
| HbA1c, % | 5.4 (5.2–5.8) |
| DM, n (%) | 16 (11.4%) |
| HOMA-IR | 2.95 (1.95–4.68) |
| HOMA-IR >2.5, % | 81 (57.9%) |
| Hypertension, n (%) | 46 (32.9%) |
| Current smoking, n (%) | 45 (32.1%) |
| SBII, (%min2)/h | 38.2 (10.2–98.3) |
| REM-SBII, (%min2)/h | 56.6 (10.4–201.8) |
| Supine-SBII, (%min2)/h | 50.2 (14.2–121.9) |
| Waist-to-hip ratio | 0.95 (0.91–0.98) |
| AHI, /h | 43.9 (17.4–63.2) |
| AHI 5–15, n (%) | 31 (22.1%) |
| AHI 15–30, n (%) | 24 (17.1%) |
| AHI 30–60, n (%) | 45 (32.1%) |
| AHI >60, n (%) | 40 (28.6%) |
| ODI, /h | 36.2 (14.9–60.3) |
| LSpO2, % | 82.5 (75.5–88.0) |
| T90, % | 0.75 (0.10–4.80) |
| ESS >10, n (%) | 88 (62.9%) |
| PSQI ≥8, n (%) | 63 (45%) |
| Moderate-to-high CVD risk, n (%) | 80 (57.1%) |
Abbreviations: BMI, body mass index; RHI, reactive hyperemia index; TG, triglyceride; LDL-c, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; HOMA-IR, homeostasis model assessment of insulin resistance; SBII, sleep breathing impairment index; REM, rapid eye movement; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; LSpO2, lowest oxygen saturation by pulse oximetry; T90, percent of time spent with SpO2 below 90%; ESS, Epworth Sleepiness Scale; PSQI, Pittsburgh Sleep Quality Index.
Table 2.
Comparison of Characteristics in the Low Framingham CVD Risk Group and Moderate-to-High CVD Risk Group in OSA Patients
| Variables | Low CVD Risk Group (n=60) | Moderate-to-High CVD Risk Group (n=80) | P value |
|---|---|---|---|
| Age, y, | 35 (31–39) | 46 (40–53) | <0.001 |
| BMI, kg/m2 | 27.7 (25.3–30.1) | 27.8 (25.4–29.8) | 0.919 |
| BMI >25, n (%) | 45 (75%) | 63 (78.8%) | 0.601 |
| RHI <1.67, n (%) | 35 (58.3%) | 35 (43.8%) | 0.088 |
| TG, mmol/L | 1.57 (1.29–2.41) | 1.92 (1.54–3.02) | 0.021 |
| LDL-c, mmol/L | 3.14 (2.53–3.52) | 3.16 (2.54–3.86) | 0.395 |
| HbA1c, % | 5.3 (5.2–5.5) | 5.5 (5.3–6.1) | 0.002 |
| DM, n (%) | 1 (1.7%) | 15 (18.8%) | 0.002 |
| HOMA-IR | 2.81 (1.84–5.18) | 2.96 (2.02–4.60) | 0.928 |
| HOMA-IR >2.5, % | 36 (60%) | 45 (56.3%) | 0.657 |
| Hypertension, n (%) | 10 (16.7%) | 36 (45%) | <0.001 |
| Current smoking, n (%) | 6 (10%) | 39 (48.8%) | <0.001 |
| SBII, (%min2)/h | 15.9 (6.3–77.5) | 53.3 (16.7–110.4) | 0.002 |
| REM-SBII, (%min2)/h | 29.6 (4.5–115.8) | 86.7 (22.1–244.1) | 0.012 |
| Supine-SBII, (%min2)/h | 29.0 (7.9–97.7) | 63.6 (27.0–140.9) | 0.004 |
| Waist-to-hip ratio | 0.94 (0.90–0.99) | 0.95 (0.92–0.98) | 0.391 |
| AHI, /h | 29.4 (10.2–60.0) | 50.1 (23.0–64.6) | 0.016 |
| ODI, /h | 23.9 (10.2–60.0) | 41.6 (20.5–60.6) | 0.037 |
| LSpO2, % | 85 (77–89) | 82 (74–86) | 0.029 |
| T90, % | 0.3 (0–3.15) | 1.35 (0.2–9.35) | 0.009 |
| ESS >10, n (%) | 31 (51.7%) | 57 (71.3%) | 0.018 |
| PSQI ≥8, n (%) | 26 (43.3%) | 37 (46.3%) | 0.731 |
Abbreviations: CVD, cardiovascular disease; OSA, obstructive sleep apnea; BMI, body mass index; RHI, reactive hyperemia index; TG, triglyceride; LDL-c, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; HOMA-IR, homeostasis model assessment of insulin resistance; SBII, sleep breathing impairment index; REM, rapid eye movement; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; LSpO2, lowest oxygen saturation by pulse oximetry; T90, percent of time spent with SpO2 below 90%; ESS, Epworth Sleepiness Scale; PSQI, Pittsburgh Sleep Quality Index.
Association Between SBII and Framingham CVD Risk
The results of multivariate analysis using binary logistic regression analysis are shown in Table 3. Patients with SBII in the third and fourth quartiles had an increased proportion of moderate-to-high Framingham CVD risk with unadjusted odds ratios (ORs) of 3.24 (95% confidence interval [CI], 1.22–8.63) and 5.54 (95% CI, 1.98–15.52), respectively. After adjusting for BMI and excessive daytime sleepiness, AHI, LSpO2, T90, endothelial dysfunction, and IR, the association between SBII in the highest two quartiles and moderate-to-high Framingham CVD risk remained significant in model 3 [OR, 6.28 (95% CI, 1.10–36.04); OR, 11.78 (95% CI, 1.25–111.38)]. Such significant association was not demonstrated in the AHI and the Framingham CVD risk. Moreover, the current study further replaced the AHI in model 2 with ODI or different OSA severity based on AHI (5–15, 15–30, 30–60, and >60), and a similar association between SBII and CVD risk as previously mentioned remained significant (data not shown).
Table 3.
Unadjusted and Adjusted Association of SBII with Moderate-to-High Cardiovascular Risk in Different Models
| SBII Quartiles | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
|---|---|---|---|---|
| Model 0 | Model 1 | Model 2 | Model 3 | |
| First | 1 | 1 | 1 | 1 |
| Second | 2.56 (0.97–6.72) | 2.29 (0.85–6.15) | 3.22 (0.98–10.51) | 3.15 (0.93–10.69) |
| Third | 3.24 (1.22–8.63)a | 3.01 (1.12–8.12)a | 6.34 (1.14–35.17)a | 6.28 (1.10–36.04)a |
| Fourth | 5.54 (1.98–15.52)b | 4.53 (1.55–13.25)b | 12.98 (1.44–116.8)a | 11.78 (1.25–111.38)a |
Notes: Model 1 covariates included BMI and excessive daytime sleepiness; Model 2 included AHI, LSpO2, and T90 plus all covariates in model 1; Model 3 included endothelial dysfunction and insulin resistance plus all covariates in model 2; a<0.05; b<0.01.
Abbreviations: SBII, sleep breathing impairment index; AHI, apnea-hypopnea index; LSpO2, lowest oxygen saturation by pulse oximetry; T90, percent of time spent with SpO2 below 90%.
Discussion
This cross-sectional study demonstrated that a novel parameter, SBII, derived from advanced polysomnographic data analysis, was independently associated with 10-year cardiovascular risk defined by a specific Framingham CVD risk algorithm. This statistically significant association was mainly confirmed between the first and third or fourth SBII quartiles but not between the first and second SBII quartiles and persisted despite adjusting for various important confounders including BMI, excessive daytime sleepiness, AHI (or ODI), LSpO2, T90, endothelial dysfunction, and IR. Meanwhile, no significant association was found between conventional parameters (eg, AHI and higher CVD risk in the logistic regression models), suggesting that SBII as a more comprehensive index may be superior to AHI in measuring the OSA severity and predicting adverse outcomes associated with OSA.
Previous studies have reported the controversial impact of OSA on CVD outcomes. Gottlieb et al reported, in a prospective, community-based cohort study, that OSA was associated with an increased risk of heart failure and was only predictive of coronary heart disease incidence in men ≤70 years old.20 Redline et al performed a study in a community-based sample to address the OSA relationship with ischemic stroke incidence and demonstrated a significant positive effect of AHI on stroke in men with mild to moderate sleep apnea. However, a similar finding was not found in women.21 In contrast, several studies also exist showing an inverse relationship between OSA severity based on ODI (or AHI) and prevalence of CVD morbidity and other clinical outcomes, thus suggesting a putatively protective mechanism called ischemia preconditioning.7,22 Marin et al found that the highest OSA severity was associated with the lower cardiovascular morbidity burden.7 Considering that the effect of hypoxia in OSA on cardiovascular outcomes seems to be a controversial issue, it is not surprising that no consensus was noted regarding the impact of CPAP on improving adverse OSA events.23,24
In addition, measurements of the nocturnal intermittent hypoxia (eg, T90 or ODI were) were also shown to be related to cardiovascular complications. Oldenburg et al insisted that T90 was independently associated with increased all-cause mortality in patients with stable heart failure,25 which is consistent with another study that claimed that T90, as a reflection of nocturnal hypoxemic burden, was an independent predictor of cardiovascular mortality. However, the observation was based on predominantly elderly men.26 Kendzerska et al also found that T90 other than AHI was shown as important predictors of composite cardiovascular outcome.27 However, no specific cutoff of T90 was noted in categorizing OSA severity, making it arbitrary when defining worse hypoxemia with T90 in published literature.
Although more details in these studies could also be responsible for the association of the added burden of hypoxia in OSA with CVD morbidity, an alternative interpretation is that these simple metrics including AHI, ODI, and T90 only considered one single pathophysiological OSA trait and failed to capture other aspects, which exerted an uncertain influence on the effect of OSA treatment on the CVD outcomes. For example, AHI measures nothing but the frequency of obstructive events (apneas and hypopneas), while T90 only calculated the time spent with SpO2 <90% based on standard definitions. This may be part of the reason that large clinical trials failed to show the benefits of CPAP in preventing CVD events.24
A novel metric incorporating comprehensive information about the frequency, duration, and depth of obstructive events and related hypoxia would be essential to reflect OSA complexity. In recent years, such promising parameters derived from reinventing the use of PSG data have shown an advantage for predicting cardiovascular sequelae when compared with AHI. Obstruction severity, a new index incorporating the duration of each apneic or hypopnea event, and the event-associated area above the desaturation curve were proved to be significantly related to mortality in severe OSA.11 However, in the calculation of obstruction severity, the end of event-associated desaturation was determined as the area up to minimum oxygen saturation before recovery, and only hypopneas with a ≥4% desaturation would be included. Furthermore, only the first desaturation in a 60-s window following the beginning of the respiratory events was defined as event-associated desaturation even in cases with more than one desaturation event. Thus, this metric obstruction severity would undoubtedly underestimate the nocturnal hypoxemic OSA burden. Furthermore, Azarbarzin et al presented a measure, hypoxic burden, to predict OSA-related outcomes. The hypoxic burden was demonstrated to be associated with an increased CVD mortality among elderly adults in two large cohorts10 and was shown to be related to higher blood pressure and the risk of heart failure incidence,28 even after adjusting for confounding factors. As a measure of event-related hypoxemia, the algorithm of the hypoxic burden does not reflect the duration of respiratory events and included those smaller degrees of desaturation (<3%), which weakened the ubiquity of this promising parameter. A recent study claimed that, as a marker for low arousal threshold, short respiratory event better predicts mortality in patients with sleep apnea although longer respiratory events seemed to carry heavier pathophysiological burden than shorter events.29 However, the SBII in the current study tackled these aforementioned shortcomings by taking all aspects into consideration, which includes the frequency and duration of obstructive events and the frequency, duration (both the desaturation and recovery areas), and depth of all desaturation events associated with a corresponding obstructive event, making it a more comprehensive index to measure OSA severity comparing with hypoxic burden or obstruction severity.
The current study has some strengths and limitations. The SBII derived from advanced polysomnographic analysis captures more aspects of OSA than many other parameters including hypoxic burden and obstruction severity, resulting in better capturing the pathophysiologic diversity of the disease process. The association between SBII and CVD risk remains significant despite adjusting for confounders including excessive daytime sleepiness, endothelial dysfunction, and IR, which were demonstrated to be CVD risk factors in previous studies. However, limitations are also needed to be noticed. First, this cross-sectional study was conducted in a single center with a small sample size, and only male patients were included. Second, the major outcome in the current study was an indirect metric calculated using the Framingham CVD risk algorithm. However, whether the significant association will remain robust when choosing cardiovascular morbidity in long-term follow-up as the primary outcome is uncertain. Third, the SBII fails to incorporate other OSA elements (eg, loop gain) and reflects no difference in the biological effects of apneas and hypopneas.
Conclusions
The SBII, as a novel index to measure OSA severity, was associated with an increased 10-year CVD risk after adjusting for multiple potential confounding factors. The SBII is believed to be a more comprehensive measure to better capture the pathophysiologic OSA diversity when evaluating OSA severity and the association with clinical sequelae. Thus, future studies are warranted to test the effect of CPAP on OSA-related outcomes based on this novel metric.
Funding Statement
This study has been funded by the National Key Research and Development Projects of China (No. 2018YFC1315103) and National Natural Science Foundation of China (No. 81570085).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NA, Yaggi HK, Concato J, et al. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–136. doi: 10.1007/s11325-009-0298-7 [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Zou J, Li X, et al. Effect of the interaction between obstructive sleep apnea and lipoprotein(a) on insulin resistance: a large-scale cross-sectional study. J Diabetes Res. 2019;2019:9583286. doi: 10.1155/2019/9583286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanek A, Brozyna-Tkaczyk K, Myslinski W. Oxidative stress markers among obstructive sleep apnea patients. Oxid Med Cell Longev. 2021;2021:9681595. doi: 10.1155/2021/9681595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia – revisited – the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Masa JF, Corral J, Romero A, et al. Protective cardiovascular effect of sleep apnea severity in obesity hypoventilation syndrome. Chest. 2016;150:68–79. doi: 10.1016/j.chest.2016.02.647 [DOI] [PubMed] [Google Scholar]
- 8.Kaculini C, Wallace DJ, Haywood AE, et al. Protective effects of obstructive sleep apnea on outcomes after subarachnoid hemorrhage: a nationwide analysis. Neurosurgery. 2020;87:1008–1015. doi: 10.1093/neuros/nyaa242 [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM. COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. Chest. 2016;149:16–19. doi: 10.1378/chest.14-2261 [DOI] [PubMed] [Google Scholar]
- 10.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muraja-Murro A, Kulkas A, Hiltunen M, et al. The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res. 2013;22:663–669. doi: 10.1111/jsr.12070 [DOI] [PubMed] [Google Scholar]
- 12.Linz D, Colling S, Nussstein W, et al. Nocturnal hypoxemic burden is associated with epicardial fat volume in patients with acute myocardial infarction. Sleep Breath. 2018;22:703–711. doi: 10.1007/s11325-017-1616-0 [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 14.Hoffman NL, O’Connor PJ, Schmidt MD, et al. Differences in sleep between concussed and nonconcussed college students: a matched case-control study. Sleep. 2019:42. doi: 10.1093/sleep/zsy222 [DOI] [PubMed] [Google Scholar]
- 15.Leger D, Stepnowsky C. The economic and societal burden of excessive daytime sleepiness in patients with obstructive sleep apnea. Sleep Med Rev. 2020;51:101275. doi: 10.1016/j.smrv.2020.101275 [DOI] [PubMed] [Google Scholar]
- 16.Bironneau V, Tamisier R, Trzepizur W, et al. Sleep apnoea and endothelial dysfunction: an individual patient data meta-analysis. Sleep Med Rev. 2020;52:101309. doi: 10.1016/j.smrv.2020.101309 [DOI] [PubMed] [Google Scholar]
- 17.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 18.Koren D, Gozal D, Philby MF, et al. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. Eur Respir J. 2016;47:1152–1161. doi: 10.1183/13993003.01430-2015 [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17:819–826. doi: 10.1007/s11325-012-0770-7 [DOI] [PubMed] [Google Scholar]
- 23.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 24.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg O, Wellmann B, Buchholz A, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37:1695–1703. doi: 10.1093/eurheartj/ehv624 [DOI] [PubMed] [Google Scholar]
- 26.Baumert M, Immanuel SA, Stone KL, et al. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J. 2020;41:533–541. doi: 10.1093/eurheartj/ehy838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 2014;11:e1001599. doi: 10.1371/journal.pmed.1001599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azarbarzin A, Sands SA, Taranto-Montemurro L, et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest. 2020;158:739–750. doi: 10.1016/j.chest.2020.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler MP, Emch JT, Rueschman M, et al. Apnea-hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med. 2019;199:903–912. doi: 10.1164/rccm.201804-0758OC [DOI] [PMC free article] [PubMed] [Google Scholar]