Abstract
Introduction
Defects of platelet functional responses in COVID-19 were reported, but their origin and pathophysiological significance are unclear. The objective of this study was to characterize the thrombocytopathy in COVID-19.
Materials and methods
Analysis of platelet functional responses to activation by flow cytometry and aggregometry in 46 patients with confirmed COVID-19 of different severity (non-ICU, ICU, and ECMO) over the course of hospitalization alongside with plasma coagulation, inflammatory markers (CRP, fibrinogen, NETosis assays in smears) was performed.
Results and conclusions
All patients had increased baseline percentage of procoagulant platelets (healthy: 0.9 ± 0.5%; COVID-19: 1.7 ± 0.6%). Patients had decreased agonist-induced platelet GPIb shedding (1.8 ± 0.7 vs 1.25 ± 0.4), P-Selectin exposure (1.51 ± 0.21 vs 1.1 ± 0.3) and aggregation. The values of these parameters among the non-ICU and ICU cohorts differed modestly, while the ECMO cohort differed significantly. Only ECMO patients had pronounced thrombocytopenia. While inflammatory markers improved over time, the observed platelet functional responses changed only moderately. SARS-CoV-2 RNA was found in 8% of blood samples and it did not correlate with platelet counts or responses. All patients had increased NETosis that moderately correlated with platelet dysfunction. High cumulative dosages of LMWH (average > 12,000 IU/day over 5 days) resulted in an improvement in platelet parameters. The observed pattern of platelet refractoriness was reproduced by in vitro pre-treatment of washed platelets with subnanomolar thrombin or perfusion of blood through a collagen-covered flow chamber.
We conclude that platelet dysfunction in COVID-19 is consistent with the intravascular-coagulation-induced refractoriness rather than with an inflammation-induced mechanism or a direct activation by the virus.
Abbreviations: COVID-19, Coronavirus disease 2019; IL, Interleukin; TPO, Thrombopoietin; ACE-2, Angiotensin Converting Enzyme 2; MAPK, Mitogen-activated Protein Kinase; NET, Neutrophil Extracellular TRAP; ARDS, Acute Respiratory Distress Syndrome; CLEC-2, C-type lectin-like receptor 2; VWF, Von Willebrand Factor; GPIb, Glycoprotein Ib; ICU, Intensive Care Unit; ECMO, Extracorporeal Membrane Oxygenation; LMWH, Low Molecular Weight Heparin; PRP, Platelet Rich Plasma; PPP, Platelet Poor Plasma; TRAP, Thrombin Receptor Activating Peptide; PCR, Polymerase Chain Reaction; BSA, Bovine Serum Albumin; ADAM-17, A Disintegrin and Metalloprotease 17; PAR, Protease Activated Receptor; MFI, Mean Fluorescence Intensity; FSC, Forward Light Scattering; PMA, Phorbol Myristate Acetate; LPS, Lipopolysaccharides; GPVI, Glycoprotein VI
Keywords: Blood platelets, Flow cytometry, Extracellular traps, COVID-19, Thrombosis
1. Introduction
SARS-CoV-2 coronavirus outbreak in late 2019 resulted in the global-wise COVID-19 pneumonia pandemic [1]. SARS-CoV-2 causes inadequate immune response [2], excessive blood coagulation [1], [3] and can result in acute respiratory distress syndrome [4]. Most patient handling strategies converge on the necessity of anticoagulant therapy for COVID-19 [1] due to the high frequency of lung microvascular thrombosis in COVID-19 [2], [5]. Autopsy studies have also identified significant amounts of intravascular tissue factor in the lungs of COVID-19 patients [6]. It is believed that this coagulopathy originates from COVID-19-induced cytokine storm [7], [8], a significant increase of the amounts of inflammatory cytokines (IL-1b, IL-6, IL-8) in blood [9]. It has been demonstrated multiple times that cytokine storm is mediated by monocytes and neutrophils, which infiltrate patient lungs upon SARS-CoV-2 infection [2], [10], release cytokines and cause endothelial dysfunction and subsequent activation of blood coagulation [11], [12].
Current ISTH guidelines on thrombosis prevention in COVID-19 patients focus on plasma coagulation, while platelets are neglected [13]. Indeed, clinical studies of anti-platelet therapy in COVID-19 demonstrated that aspirin is the only anti-platelet drug that can significantly affect patient's condition [14]. On the other hand, other studies demonstrated that pre-hospitalization antiplatelet therapy can significantly decrease the mortality from COVID-19 [15]. Platelet dysfunction in COVID-19 has been shown several times and most of the authors agree that platelets are pathologically activated [16], [17], [18], [19]. Indeed, circulating platelets had higher P-selectin and CD63 expression, which demonstrates previous granule release [16], [17], [19], [20]. Furthermore, Hottz et al. detected an increase in thromboxane B2 in COVID-19 patients' blood, which testified to platelet activation happening in the blood [19].
Additional evidence of platelet involvement comes from platelet counts. In recently infected COVID-19 patients, platelet count initially rises with a subsequent drop, which is characteristic for an increased platelet consumption [21]. Enhanced consumption also increases TPO synthesis by the liver and leads to platelet overproduction and platelets becoming larger and younger in COVID-19 patients [21], [22], [23]. Our previous in silico studies have additionally demonstrated the important role of these processes in COVID-19 [24]. Above all, mild to intermediate thrombocytopenia was proved to be a negative prognostic marker for COVID-19 patients [20], [21], [22].
There are multiple possible pathways of platelet involvement in the COVID-19 pathology. Zaid et al. have demonstrated the presence of the ACE2 receptor on the platelet surface [17] and Zhang et al. have proposed the platelet MAPK-signaling initiation through ACE2 by SARS-CoV-2 [18]. It has been recently demonstrated that upon SARS-CoV-2 intake, platelets undergo programmed cell death [25]. An increase of platelet vesicles encapsulating viral particles has also been shown [26]. However, ACE-2 absence on the platelets has been reported as well [16], [27]. Therefore, it is disputable, whether SARS-CoV-2 directly impacts platelet responsiveness to activation or not.
Another source of platelet activation in COVID-19 is the immune system. Neutrophil's hyperactivation resulting in NETosis induced blood coagulation and platelet aggregation [28] and an enhanced numbers of platelet-monocyte and platelet-neutrophil hetero-aggregates was shown to be present in blood of COVID-19 patients [20], [29], [30]. Both of these phenomena are characteristic for immunothrombosis [2]. Formation of platelet-monocyte and platelet-neutrophil hetero-aggregates could be caused either by platelet or by immune cell activation [2] and are effective promoters of the blood coagulation [2], [31]. It has also been proposed that in the case of inflammation-induced lung damage and ARDS, platelets become involved in the pathology via platelet CLEC-2 and alveolar macrophage-podoplanin interaction [32]. On the other hand, endothelial dysfunction could cause excessive VWF presence within the blood flow and cause platelet involvement via VWF-platelet GPIb interaction [2]. Finally, alterations in platelet production due to increased TPO levels [16], [22] and megakaryocyte dysfunction [21] can contribute to platelet enhanced reactivity.
Here we aimed to comprehensive testing of the platelet functional responses to conventional activation (PFR) in the COVID-19 patients of different severity (patients from non-ICU, ICU and ECMO cohorts) and to estimate whether platelets play an active role in the pathology. We studied PFR using flow cytometry and aggregometry, tested patient plasma on the SARS-CoV-2 presence, and analyzed NETosis. COVID-19 patients platelet responsiveness was significantly diminished compared to healthy donors, and platelet size and their refractoriness correlated to the disease severity. Platelets of the patients were sensitive to high cumulative dosages of LMWH after 5 days of treatment. All of the COVID-19 patients had increased NETosis, and NETosis level moderately correlated with the increase of platelet size and reduction of their relative P-Selectin expression upon activation. SARS-CoV-2 presence did not correlate to platelet responsiveness and size. In vitro assays of healthy platelets revealed that platelet refractoriness, observed here in COVID-19 patients, could be obtained upon low-dose thrombin pre-stimulation or in case of ongoing collagen-induced thrombus formation. Thus, based on our and previously published findings, we claim that platelets in COVID-19 are less responsive potentially due to hypercoagulation and immune cell activity, and platelets might not be active participants in the COVID-19 pathogenesis.
2. Methods
2.1. Patient inclusion criteria
This study recruited 46 patients with confirmed COVID-19 admitted to 52nd Municipal Hospital, Moscow, Russia, between August 4th and September 18th 2020. Patients required in-hospital admission because of the severity of the disease course based on clinical findings (Table S1). Patients whose condition was burdened with severe pathologies, like cancer, or patients with Charlson Comorbidity Index > 6 (with exception for one patient with CCI 7) were excluded from the study. Patients were divided into three groups depending on the admission unit: the ECMO group, the Intensive Care Unit (ICU) only group, and the non-ICU only group. Some patients received treatment in both ICU and non-ICU groups. All patients received treatment according to the Russian Federation National Guidance for Treating COVID-19. Specifically, the patients were treated with: antimicrobial prophylaxis, antiviral therapy, immune-suppressive therapy (Table S2). The patient received thromboprophylaxis therapy with low-molecular-weight heparin (LMWH). Some patients were also treated with anti-platelet drugs due to the cardiovascular pathologies in anamnesis. Biochemical variables and clinical parameters were recorded at hospital admission. Patients were followed until their discharge from hospital, death or after the 28th day of being studied.
The study was approved by the Independent Ethical Committee of NMRC PHOI decision 3/2020 (19.05.2020), and informed consent was obtained from each patient according to the principles of the declaration of Helsinki.
The healthy donors' blood was obtained at NMRC PHOI blood center between August 6th and September 12th. 43 donors (69,8% male) with median age of 32 (19–60) were included. All the donors had no pathologies and had the median platelet count of 269 (194–362) at the day of blood donation.
2.2. Materials
Annexin V-Alexa647, antibodies to CD62P-Alexa647, CD42b-PE, were from Sony Biotechnology (San Jose, CA, USA). Fucoidan from Fucus vesiculosis, HEPES, bovine serum albumin, TRAP-6 were from Sigma-Aldrich (St Louis, MO, USA). NaCl, Na2HPO4, KCl, NaHCO3, HEPES, glucose, MgCl2 were from Agat-Med (Moscow, RF). VWF was a kind gift from Dr. Pierre Mangin (Strasburg, France).
2.3. Sample preparation for flow cytometry
For flow cytometry assays, the blood of the patients or healthy donors has collected into sodium citrate (3.8% v/v) vacuum tubes (Improvacuter). Sample preparation was performed the same as in [33]. Briefly, blood was left resting for 15 min at room temperature and then diluted 20 times by Tyrode's buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2, pH 7.3) without calcium with hirudin. Samples were then incubated 1:1 with activators (25 μM TRAP-6 and 20 μg/ml collagen) or vehicle in Tyrode's-Hirudin-Calcium (5 mM). After 10 min of incubation, samples were mixed 1:1 with anti-CD62p (P-Selectin), anti-CD42b antibodies (GPIb), and Annexin-V in Tyrode's-Hirudin-Calcium (2.5 mM) buffer and left for 10 min. Then samples were diluted to the platelet concentration of 1000 plt/μl by Tyrode's-Hirudin-Calcium (2.5 mM) buffer and studied using BC Navios Flow Cytometer. The mean fluorescence intensities for the collected data are given in Fig. S1.
2.4. Platelet aggregometry
For platelet aggregometry, PRP was obtained from sodium citrate anti-coagulated blood, centrifugated for 8 min at 150g. To obtain PPP, resting blood was centrifugated for 1250g for 15 min. For aggregometry assays, platelet concentration in PRP was adjusted to 150–250 plt/μl by the addition of PPP, if it was needed. Platelet aggregation upon addition of 5 μM TRAP-6 or 100 μg/ml Fucoidan was measured by SOLAR AP-2110.
2.5. SARS-CoV-2 RNA analysis
For RNA isolation from plasma DSP Virus Kit (Qiagen) was used according to the manufacturer's recommendations. For the virus detection, GeneFinder COVID-19 Plus RealAmp Kit (OSANG Healthcare Co.) and 7500 Fast PCR machine (Applied Biosystems) were used according to the manufacturer's recommendations.
2.6. NETosis assay
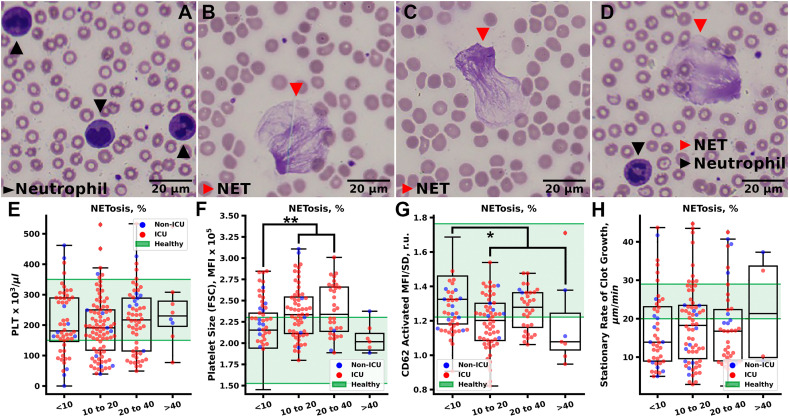
Extracellular traps of blood phagocytes were investigated according to original methodology, based on microscopic analysis of standardized thin blood smear as described earlier [34], [35]. Briefly, 2 μl of fresh whole blood anti-coagulated with sodium citrate were used to prepare blood smears with thin monolayer of cells within 1.5 h after blood collection. Glass slides were obtained from PJSC “Steklopribor” (Ukraine). Smears were fixed for 3.5 min with May-Grünwald solution and stained with Romanovsky stain for 20 min (MiniMed Co. Ltd., Russia) according to May-Grünwald-Giemsa method [36], and investigated using automatic microscopic scanner MECOS C2 (“MEdical COmputer Systems (MECOS)” Company, Russia). At least 200 intact leukocytes and extracellular traps derived from leukocytes were counted (see examples of cells and NETs in Fig. 5A–D). NETosis was assessed as a percent of NETs from all observed leukocytes. Various phagocytes can produce extracellular traps [37], and the described method does not differentiate traps produced by different blood phagocytes. However, since they are mainly represented by neutrophils, %NETs relate primarily to NETosis.
Fig. 5.
NETosis impact on platelets in COVID-19 patients. A–D Typical images of the neutrophils and NETs in blood smears. E – Platelet count did not correlate with NETosis. F, G – Platelet Size (F) was significantly increased in patients with more severe NETosis with a drop for patients with the most severe NETosis. At the same time, relative P-Selectin exposure on activated platelets (G) was significantly diminished in all patients suffering from more severe NETosis. H – Blood plasma coagulation (stationary rate of clot growth in thrombodynamics) was independent of NETosis. Statistical significance was calculated using Mann-Whitney criteria, * corresponds to p < 0.05; ** corresponds to p < 0.01; *** correspond to p < 0.001; no marking corresponds to non-significant differences.
2.7. Microslide and flow chamber preparation and whole blood perfusion assays
Microslides for blood perfusion had dimensions of 0.2 × 2 × 100 mm. VWF (2 IU) and BSA (20%) were injected into the microslides and left overnight at 4 °C. Microslides were then perfused by PBS. Whole hirudinated blood was perfused through the VWF or BSA-covered microslides at the 2000 s−1 wall shear rate. Perfused blood was collected and analyzed analogously to patient and healthy donor samples.
The construction and geometry of the parallel-plate flow chambers were described previously [38]. The parameters of the channel were 0.2 × 18 × 0.206 mm. Glass coverslips were coated with either fibrillar collagen type I (0.2 mg/ml) or collagen type I (0.2 mg/ml) and VWF (2 IU) for 1 h 30 min at 37°С, washed with distilled water and then inserted into the flow chambers. Blood was perfused through the chambers with wall shear rates 800 s−1.
2.8. Platelet washing and thrombin pre-treatment
For the in vitro study of thrombin impact on platelet activation, whole citrated blood of healthy donors was centrifuged at 100g for 8 min, and PRP was collected. PRP was diluted 1/3 by sodium citrate (pH 5.5) and centrifuged at 400g for 5 min. Platelet pellet was resuspended in Tyrode's buffer w/o calcium. The resulting platelet suspension was once more diluted 1/3 by sodium citrate (pH 5.5) and centrifuged at 400g for 5 min. Platelet pellet was resuspended by Tyrode's buffer with calcium (2.5 mM) and left resting for 30 min. Then platelets were pre-incubated either with 500 nM of thrombin or with the vehicle for 10 min and then analyzed analogously to whole blood samples of the patients and healthy donors. Washed platelet samples were studied by Agilent Acea Novocyte 3000.
2.9. Statistical analysis
Flow cytometry data were processed using FlowJo (http://www.flowjo.com/) software. Statistical analysis was performed in Python 3.8. Statistical information is given in the figure legends. For comparison between patient groups we have performed the nested [39] data analysis. Briefly, the data corresponding to each patient was averaged and an intercluster correlation [39] (ICC) was calculated. For the missing data linear interpolation was used. For our data ICC was less than 0.5, therefore, we further analyzed only the averaged patients points using standard statistical approaches, as the risk for false-positive result in this case was significantly lesser than upon analysis of the data without taking nested structure into account [39].
3. Results
3.1. Patients' characteristics
The patients enrolled in the current study were either treated only in Therapy Unit (“Non-ICU”, 3 patients), or treated in the Intensive Care Unit (“ICU”, 20 patients), or transferred between Therapy and ICU (15 patients), or treated in ICU with extra-corporal membrane oxygenation (“ECMO”, 8 patients). If patient was transferred between ICU and non-ICU groups, we moved him to the corresponding assay group (Table S1). Overall, the median age was 62.5 years (29 to 82), with male patients predominating (63%). Chronic diseases and pre-hospital treatment were comparable between the treatment groups. The ECMO group had lower platelet count, higher neutrophils count, and C-reactive protein (CRP) level at the beginning of hospitalization than the other groups (Table S1). The applied therapy and thrombotic events during treatment are given in Table S2.
We performed platelet and coagulation testing once in 1–3 days for each patient. The following results are grouped according to the hospital unit in which the patient was treated at the day of the analysis. For convenience, the patients were further divided into “Deceased” (deceased in the Hospital) and “Survived” (discharged with improvement).
3.2. All COVID-19 patients demonstrated platelet dysfunction, which negatively correlated with treatment group
Platelet functional responses to activations were assessed by means of flow cytometry (Fig. 1A–F). Platelets were activated via thrombin receptor PAR1 agonist TRAP-6 and GPVI receptor agonist collagen in order to achieve strong activation of platelets, which presumably occurs during thrombus formation [33]. Baaten et al. have shown that upon such strong stimulation GPIb levels decrease on the platelets [40], probably, due to shedding by ADAM-17 [41]. Platelet GPIb shedding (GPIb MFI on resting/GPIb on activated platelets) and relative P-Selectin exposure (P-Selectin MFI/SD) upon activation were taken as the markers for platelet responsiveness (typical dot-plots of healthy donor and COVID-19 are depicted in Fig. 1A and B, correspondingly).
Fig. 1.
Comparison of the functional parameters of the platelets of COVID-19 patients and healthy donors. A, B – Typical dot-plots of CD62p (P-Selectin) vs CD42b (GPIb) of the activated platelets of healthy donor (A) and COVID-19 patient (B). C – Dynamics of the platelet size (FSC MFI), GPIb shedding (CD42 Rst/Act) and P-Selectin exposure (CD62p MFI/SD) of the typical patients from non-ICU (blue), ICU (red) and ECMO (black) units. Green highlights the region of healthy values. D – Size (FSC MFI) of the platelets of the COVID-19 patients was significantly increased compared to healthy donors independently on the patient group. E, F – GPIb shedding and relative P-Selectin exposure were significantly reduced in all patients, with the values of ECMO patients being the lowest. G–H – Typical aggregation curves upon stimulation by 5 μM of TRAP-6 (G) or 100 μg/ml Fucoidan (D). Green curves – healthy donors, blue curves – irreversible aggregation of the COVID-19 patient platelets, red curves – reversible aggregation of the COVID-19 patient platelets. I, J – Platelet aggregation upon stimulation with low doses of TRAP-6 (5 μM) or normal doses of Fucoidan (100 μg/ml) was significantly diminished in all of the patients. Grey dots on all of the box plots represent individual measurements of the patients. Blue dots on all of the plots correspond to averaged measurements of the survivors. Red dots correspond to averaged measurements of the deceased patients. Statistical significance was calculated using Mann-Whitney criteria, * corresponds to p < 0.05; ** corresponds to p < 0.01; *** correspond to p < 0.001; no marking corresponds to non-significant differences. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Since PAR1 governs platelet responses to thrombin more than PAR4 [42], we used the PAR1 activating peptide, TRAP-6, to study aggregation of non-thrombocytopenic patients (Fig. 1G). Aggregometry was also used to study responses to activation through CLEC-2 (by fucoidan [43]; Fig. 1H), which is impossible to study under low platelet concentration in flow cytometry [43].
Neither of the studied parameters of platelet activation exhibited pronounced dynamics (except for the ECMO patients, whose platelet counts, size and activation impaired over time; 3 typical patients from non-ICU, ICU and ECMO groups are given in Fig. 1C). Only 30% of the patients in non-ICU and ICU groups had mild thrombocytopenia (Fig. S1A). On the other hand, platelet size (FSC MFI) was increased for all patients (Fig. 1D, S1B) and weakly negatively correlated with platelet counts (Sp = −0.33, Fig. S2). The fraction of procoagulant platelets was increased in all patients independently on the treatment group, which presumes enhanced platelet death in COVID-19 (Fig. S1C). GPIb amounts on resting platelets were slightly increased in COVID-19 patients (Fig. S1D), GPIb shedding upon activation was significantly reduced (Fig. 1E). Average expression of P-Selectin on the patients' platelets was either decreased or normal (Fig. S1E, F). Patients' relative P-selectin exposure in response to activation was significantly reduced compared to healthy donors (Fig. 1F). Platelet aggregation also significantly decreased (Figs. 1I, J, S3). In 36% patients aggregation in response to PAR1 stimulation was reversible (Fig. 1G), possibly due to a defective granule release [44].
3.3. No significant improvement in the platelet quality was detected in the course of treatment
We further hypothesized that platelet condition of the patient group as a whole could improve in course of treatment as fibrinogen (Figs. S4–S6) and CRP (Fig. S7) levels decreased with the course of therapy. However, no correlation between the length of the patient stay and platelet reactivity was detected for non-ECMO patients (Fig. S8). On ECMO a moderate deterioration of platelet responsiveness to activation with time was observed (Fig. S6).
Then we compared patients with similar period of stay in the corresponding units (Fig. 2 ). Individual dynamics for three patients from each of the therapy groups is given in Figs. S4–S6. For the short-staying patients neither of the studied platelet parameters exhibited significant dynamics (Fig. 2A, D, G, J). However, if the patient spent from one to two weeks in ICU, the platelet counts noticeably decreased (Fig. 2B), while platelet size significantly increased only in ECMO group (Fig. 2E). These data correspond to the platelets of these patients becoming younger [22]. On the other hand, GPIb shedding on the platelets from non-ICU group restored (Fig. 2H), while P-Selectin exposure was stable in non-ICU and ICU groups and decreased in ECMO group (Fig. 2K).
Fig. 2.
Dynamics of the platelet counts, size and activation in COVID-19 patients. A–D – Average platelet count dynamics in patients, who stayed less than in a week in corresponding (non-ICU – blue; ICU – red; ECMO – black) unit (A; non-ICU – n = 10; ICU – n = 17; ECMO – n = 2), from 7 to 14 days (B; non-ICU – n = 8; ICU – n = 11; ECMO – n = 2) and from 14 to 21 days (C; non-ICU – n = 2; ICU – n = 5; ECMO – n = 3). D–F – Average size (FSC MFI) of the platelets of patients, who stayed less than in a week in corresponding unit (D; non-ICU – n = 6; ICU – n = 13; ECMO – n = 2), from 7 to 14 days (E; non-ICU – n = 6; ICU – n = 11; ECMO – n = 2) and from 14 to 21 days (F; non-ICU – n = 2; ICU – n = 3; ECMO – n = 3). G–I – GPIb shedding upon activation of the platelets of patients, who stayed less than in a week in corresponding unit (G; non-ICU – n = 6; ICU – n = 13; ECMO – n = 2), from 7 to 14 days (H; non-ICU – n = 6; ICU – n = 11; ECMO – n = 2) and from 14 to 21 days (I; non-ICU – n = 2; ICU – n = 3; ECMO – n = 3). J–L – Relative P-Selectin expression of the platelets of patients, who stayed less than in a week in corresponding unit (J; non-ICU – n = 6; ICU – n = 13; ECMO – n = 2), from 7 to 14 days (K; non-ICU – n = 6; ICU – n = 11; ECMO – n = 2) and from 14 to 21 days (L; non-ICU – n = 2; ICU – n = 3; ECMO – n = 3). Missing points in individual patient dynamics were filled in using linear interpolation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Finally, for the long-staying patients (2 to 3 weeks) the platelet counts dropped in both ICU and ECMO groups and restored in non-ICU group (Fig. 2C). Furthermore, the observed platelet responses were either stable or exhibited negative dynamics in all therapy groups, except for the non-ICU (Fig. 2F, I, L). Thus, it can be speculated that a noticeable improvement in platelet condition happens only for the least severe COVID-19 patients.
3.4. Low-molecular-weight heparin (LMWH) therapy has a persistent impact on platelet condition
Next, we addressed the question of what factors have impact on the platelet size and responsiveness in COVID-19. First, we looked into overall correlations between parameters (Figs. S2, S8). Expectedly, the studied platelet parameters correlated well with each other. It is noteworthy that platelet counts had a weak negative correlation with platelet FSC (Sp = −0.33, Figs. S2, S8), again supporting the assumption of an increased platelet consumption in COVID-19. For other clinical parameters of the patients no strong correlations were found between plasma coagulation tests and platelet functional assay (Figs. S2, S8), while the C-reactive protein levels demonstrated a weak yet significant negative correlation with most of the platelet parameters (Figs. S2, S8).
Most of the patients had episodic hypercoagulation which partly was corrected with the LMWH therapy (Fig. S9). To study the impact of the LMWH therapy on platelets, we split the patients into three groups by the cumulative heparin dosage during the observation period (low dosages: <12,000 IU/day; intermediate dosages: from 12,000 to 15,000 IU/day; high dosages: >15,000 IU/day). Only non-ECMO patients were included in this assay. Patients were further divided into three subgroups: all (“All”); patients without secondary infections or CoV-recombinant plasma transfusions (“No Sec.”) and “No Sec.” with CRP < 200 (“CRP < 200”). No significant changes were detected in the platelet condition after three days of LMWH therapy for neither of the groups, except for the “No Sec.” group (Fig. 3A). However, when we increased the studied interval to five (Fig. 3B) or to seven (Fig. 3C) days, platelet condition in the “No Sec.” and “CRP < 200” groups improved significantly for patients who received higher dosages of LMWH in comparison with patients who received lower dosages of LMWH. Platelet count (Fig. 3D) and platelet size (Fig. 3E) were also sensitive to the amount of LMWH therapy in these groups. Pre-hospital anti-platelet therapy or in-hospital immune-suppression by tocilizumab had a limited impact on platelets (Fig. 3F, G).
Fig. 3.
LMWH-therapy has a long-term impact on platelet condition in COVID-19. A–C – Dependence of the changes (Δ) in relative platelet P-Selectin expression upon activation (CD62p MFI/SD) from average LMWH daily therapy during 3 (A), 5 (B) and 7 (D) days. Green – “All” – all non-ECMO patients; Blue – “No Sec.” – non-ECMO patients without secondary infections or CoV-recombinant plasma transfusions; Red – “CRP < 200” - non-ECMO patients without secondary infections or CoV-recombinant plasma transfusions with CRP < 200. D, E - Dependence of the changes (Δ) in platelet count (D) and platelet size (E) from average LMWH daily therapy during 3 (A), 5 (B) and 7 (D) days. Statistical significance was calculated using Mann-Whitney criteria, * corresponds to p < 0.05; ** corresponds to p < 0.01; *** corresponds to p < 0.001. F, G – Anti-platelet drugs (aspirin, cangrelor, F) and immune-suppressive drugs (tocilizumab, G) diminished platelet activation capability. Statistical significance was calculated using Mann-Whitney criteria, * corresponds to p < 0.05; ** corresponds to p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. ECMO therapy resulted in the most pronounced phenotype of platelet dysfunction
The above described severe platelet dysfunction in patients on ECMO requires additional consideration, because ECMO therapy is applied only in the most severe COVID-19 cases and ECMO itself is known to significantly enhance platelet consumption [45], [46]. Here we have studied platelet condition after 18 transfusions of healthy donor platelets to COVID-19 patients on ECMO. Most of the transfusions did not significantly improve the platelet count (Fig. 4A). The platelet FSC significantly reduced only immediately after activation, returning to pre-transfusion values in 1 h (Fig. 4B). The amount of PS-positive platelets increased upon transfusion (Fig. 4C), and the relative P-Selectin exposure non-significantly improved for 6 h (Fig. 4D). After 24 h, the platelet condition returned to its initial state (Fig. 4B–D). We conclude that a severe consumption of platelets is present in COVID-19 patients on ECMO, and observed platelet condition could be considered as a pronounced platelet COVID-19 phenotype.
Fig. 4.
Impact of ECMO, virus presence, and COVID-19 plasma on platelets. A – Pairwise comparison of the platelet count before and after platelet transfusions to patients on ECMO. B–D – Dynamics of the platelet size (B), number of PS-positive platelets (C), and relative P-Selectin expression upon activation (D): before platelet transfusion (before), 15 min after the platelet transfusion (after), 1 h after the platelet transfusion (1 h), 6 h after the platelet transfusion (6 h) and next day (24 h). E – N- and E- or just N-SARS-CoV-2 genes were present in the plasma of recently hospitalized patients, while no viral presence was detected in the PRP of the patients that spent more time in the hospital. F, G – No viral impact on the relative P-Selectin expression (F) and stationary rate of plasma clot growth (G) was detected. Blue dots correspond to individual measurements of the non-ICU patients, red dots to ICU patients, black dots to ECMO patients. Green regions represent healthy donor ranges. H, I – No impact of COVID-19 plasma on the number of PS-positive platelets (H) and relative P-Selectin expression (I) in the healthy donor PRP was detected (H-H – healthy donor platelets incubated with other healthy donor plasma; H-P – healthy donor platelets incubated with COVID-19 patient plasma). Statistical significance was calculated using Mann-Whitney criteria, * corresponds to p < 0.05; ** corresponds to p < 0.01; *** correspond to p < 0.001; no marking corresponds to non-significant differences. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Viral presence in PRP and COVID-19 plasma has no significant impact on platelet activation
Another potential mechanism of platelet dysfunction in COVID-19 patients is direct platelet activation by SARS-CoV-2. Unexpectedly, in our study the virus (assayed by the presence of viral N- and E-genes or just N-gene) was present in blood plasma of recently enrolled patients, while no viral presence was detected in the samples that were collected on the latter stages of the treatment (Fig. 4E). Viral presence did not correlate with platelet parameters or the rate of plasma clot formation (Fig. 4F, G). We also tested COVID-19 patient plasma impact on healthy donor platelets. After incubating healthy platelet rich plasma with COVID-19 patient's platelet free plasma (1:1 v/v), no COVID-19-like platelet phenotype appeared (Fig. 4H, I).
3.7. NETosis was increased in COVID-19 patients, but its impact on platelet was limited
In order to test the potential impact of the innate immune system activation in COVID-19 patients on the observed platelet functional responses, we studied the correlations of the NETosis with markers of platelet activation. Previously, using myeloperoxidase and neutrophil elastase staining, it has been demonstrated that NETosis can be assessed in blood smears [34], [47]. Furthermore, in the described studies classical inducers of NETosis PMA, LPS and Ionomycin were able to increase amounts of NETs in blood smears [34]. Thus, in agreement with [47], we used blood smears to study NETosis in COVID-19 patients.
All COVID-19 patients had a higher level of NETs than healthy donors (Fig. S10A), which was in perfect agreement with the studies of COVID-19 induced NETosis using more conventional approaches [28]. Patients, that underwent anti-IL6 treatment with Tocilizumab had lower NETosis, than patients that received other kinds of immune-suppressive treatment (Fig. S10B and C, correspondingly). LMWH and viral presence in PRP also had modest impact on NETosis (Fig. S10D and E, correspondingly). Platelet counts did not correlate with NETosis (Fig. 5E), while deviations in platelet size and relative P-Selectin exposure were more pronounced in patients with higher NETosis (Fig. 5F, G). No correlation between NETosis and plasma coagulation parameters was detected (Fig. 5H).
3.8. Platelets pre-treated with low dose thrombin have the same phenotype as COVID-19 patient platelets
Thus far the observed platelet phenotype suggests thrombin-dependent platelet activation in circulation. To prove this point, we strived to reconstruct COVID-19-alike phenotype in vitro (Fig. 6 ). We checked three possible causes. First, GPVI receptor could be shedded on platelets, thus reducing platelet response to collagen. To test this, we have varied collagen concentrations in the experiments with healthy platelets (Fig. 6A, B), as it can be expected that reduced GPVI levels could cause reduced platelet reactivity to GPVI and this can be mimicked by the reduction of collagen concentration in the activation mixture. However, this did not affect platelet capability to become activated, except for the fraction of procoagulant platelets (Fig. S11). As it has been demonstrated that COVID-19 is associated with endothelial dysfunction and excessive VWF levels in the blood flow [2], we aimed to analyze ex vivo effects of platelet interaction with VWF under high shear rate in VWF covered microslide. No significant differences were present between platelets from resting blood, or blood perfused over BSA or VWF covered microslides (Fig. S12A, B). On the other hand, it can be expected that disturbed endothelium could cause collagen exposure [2]. Thus, we assessed ex vivo whether thrombi on collagen could cause platelet dysfunction under normal shear rate (800 s−1). Whole recalcified citrated blood was perfused through the chamber and only last 100 μl of blood perfused over the pre-formed for 5 min thrombi were collected and analyzed. It appeared that collagen-induced thrombus formation could cause significant decrease in the relative P-Selectin expression upon activation (Fig. 6C) and GPIb shedding (Fig. 6D).
Fig. 6.
Assays of the mechanisms of the platelet dysfunction in COVID-19. A, B – Collagen concentration variation with TRAP-6 concentration fixed at 12.5 μM did not have an impact on relative platelet P-selectin exposure (A) and GPIb shedding (B). C, D – Perfusion of the whole blood through BSA, VWF, collagen or collagen and VWF (Coll/VWF) covered flow chamber caused significant decrease in platelet activation capability in case of collagen. E, F –TRAP-6 concentration variation with collagen concentration fixed at 10 μg/ml significantly decreased relative platelet P-selectin exposure (E) and GPIb shedding (F). G, H – Washed platelet pre-treatment with sub-micromolar doses of thrombin for 30 min and subsequent normal stimulation resulted in the diminished relative P-Selectin exposure (G) and GPIb shedding (H) in contrast to vehicle pre-treated samples. Statistical significance was calculated using Mann-Whitney criteria, * corresponds to p < 0.05; ** corresponds to p < 0.01; *** correspond to p < 0.001; no marking corresponds to non-significant differences.
The third tested possibility is PAR1 receptor desensitizing due to contact with thrombin. To test this, we have varied TRAP-6 concentration, which significantly affected relative P-Selectin expression and GPIb shedding (Fig. 6E, F). To mimic the ongoing hypercoagulation [1], [5], we have pre-treated washed platelets with 0.5 nM of thrombin and let them rest for 30 min and analyzed in the same manner as the whole blood samples. This diminished the relative CD62p expression and GPIb shedding upon activation (Fig. 6G, H). Therefore, it can be stated that platelet phenotype, observed in COVID-19, could be obtained upon platelet contact with pre-formed thrombi or low dose thrombin.
4. Discussion
Platelets' role and potential mechanisms of platelet participation in the COVID-19-induced pathology were the object of speculations since the first reports on active thrombotic processes in COVID-19 patients. Nowadays, there is a set of common hypotheses on these mechanisms: direct viral impact of SARS-CoV-2 particles in blood [17], [18], immunothrombosis [2], [20] and enhanced platelet consumption [20], [30]. Here we demonstrated that NETosis (reflecting immunothrombosis) and viral presence do not correlate with platelet dysfunctions (Fig. 4, Fig. 5), while high doses of LMWH correlated with an improved platelet condition (Fig. 3). The latter is in line with our further findings that COVID-19-like platelet phenotype could be obtained only upon mimicking temporary platelet contact with low doses of thrombin (Fig. 6). Therefore, while all of the aforementioned hypotheses of the platelet dysfunction mechanisms are correct, excessive plasma coagulation appears to have more impact.
Our results are in partial agreement with previously published data that platelets in COVID-19 patients are pre-activated [16], [17], [20]: COVID-19 patients resting platelets were increased in size, and the number of PS+ platelets was higher (Fig. 1) [16], [22]. On the contrary, COVID-19 patients' platelets had reduced GPIb shedding and relative P-selectin exposure (Fig. 1C, E, F). The observed platelet refractoriness was also reflected by the reduced platelet aggregation (Fig. 1I, J). The difference from the previously published works [16], [18] comes from us utilizing live platelets instead of fixed ones.
It is noteworthy, that platelet condition did not change significantly in the course of treatment (Fig. 2), while other clinical parameters of the patients improved (Figs. S4–S7). Weak dynamics in the platelet count and volume was earlier observed in pneumonia caused by influenza, Epstein-Barr virus and SARS-CoV-2 [18], [48], [49]. Hereafter, we analyzed the impact of the cumulative LMWH dose administered to the patients on platelet condition within different time periods. It appeared that LMWH therapy could cause positive changes in the platelet count and condition (Fig. 3A–E) that became significant after 5 days of treatment (Fig. 3B). Thus, platelets in COVID-19 are “slow responders”, which is in line with the findings of Chao et al. [50]. This also implies plasma hypercoagulation significance for platelet dysfunction, described earlier for other diseases [51]. LMWH therapy in our setting had impact only in the absence of excessive inflammation (caused by secondary infections of CoV-recombinant plasma transfusions), while there was no correlation between LMWH therapy and platelet condition in these patients (Fig. 3A–E).
To test how enhanced platelet consumption affects observed COVID-19 patient platelet phenotype, we have studied platelets of the COVID-19 patients on ECMO therapy [52]. In COVID-19 patients receiving ECMO, platelet count and platelet responsiveness dropped in the first 2–3 days after the start of the ECMO therapy (Fig. 2A). Platelet transfusions in ECMO COVID-19 patients did not improve platelet counts: transfused platelets become activated, undergo necrosis and are cleared from the circulation within 24 h (Fig. 4A–D). It is noteworthy that while ECMO-induced decrease in platelet responsiveness has been previously reported [53], [54], in the previous studies platelet transfusion were effective for at least 50% of the patients [53], [55]. On the contrary, only one platelet transfusion in our study caused significant improvement in platelet count, while not affecting platelet responsiveness to activation (Fig. 4). Furthermore, superficial comparison of ECMO patients with influenza-induced and COVID-19-caused ARDS reveals that the survival rate of COVID-19 patients on ECMO is significantly lower than the one of the influenza-patients [56]. Comparison between patient cohorts revealed that ECMO “enhances” the phenotype of the ICU and non-ICU COVID-19 patients (Fig. 1). The observed reduced platelet reactivity thus could be explained by the pre-activation during circulation: activated platelets have already partially shedded their GPIb and secreted their granules, resulting in decreased further activation (Fig. 1, Fig. 2). Such phenomenon has been partially demonstrated before [20].
Although platelet pre-activation by SARS-CoV-2 has been demonstrated [18], we did not find any significant correlation between platelet responsiveness and viremia (Fig. 4E–G). Incubation of COVID-19 plasma with healthy platelets also did not significantly impact platelet activation (Fig. 4H, I), what contradicts findings of Hottz et al. [19]. We presume that this can be caused by the fact that Hottz et al. were considering percent of CD41/CD63 positive platelets [19], while we were considering the platelet population in general. Pelzl et al. have recently shown that incubation of healthy donor platelets with COVID-19 patients' serum cause procoagulant platelet formation in an Akt-dependent manner [57]. This observation seemingly contradicts our findings on incubation of healthy donor platelets with COVID-19 patient's plasma (Fig. 4H, I). However, this disagreement could be explained by traces of active coagulation factors present in blood serum in comparison to blood plasma [58] used in our study.
Platelet count and quality in patients with severe inflammation were not sensitive to LMWH therapy (Fig. 3A–E). Combined with the fact that in our patients platelet malfunctioning also correlated with NETosis (Fig. 5F, G), these findings further confirm that immunothrombosis contributes to the platelet dysfunction in COVID-19 [10], [28]. In line with the work of Bye et al., SARS-CoV-2 IgG can cause platelet hyperactivity [59], what supports our findings that recombinant COVID-19 plasma reduces positive impact of LMWH therapy (Fig. 3A–E). Our findings on the LMWH efficacy emphasize the significance of the inflammation management in order to obtain positive effects of the anticoagulant therapy.
Finally, to establish the role of plasma coagulation in vitro we have tested conditions for COVID-19-like phenotype. Previously it has been shown that platelet refractoriness could originate from GPVI [60] or GPIb [61] shedding. In our setting, we did not see the effect of variation of the collagen concentration or whole blood perfusion through the VWF covered microslide (Fig. 6A–D). On the other hand, platelet interaction with pre-formed on collagen thrombi (Fig. 6С, D), variation of thrombin receptor activation or pre-incubation of platelets with sub-nanomolar concentrations of thrombin resulted in the COVID-19-like platelet responses to stimulation (Fig. 6E–H).
Most of the discussed mechanisms are initiated by endothelial dysfunction, which is among the most significant drivers of micro-thrombosis in COVID-19 patients [2], [10]. Thereby, endothelial function in COVID-19 patients should be monitored alongside platelet function. On the other hand, increased platelet size is the sign of increased platelet production, but it is not known whether platelet production itself is altered in COVID-19. Comparison of the ECMO impact on platelets of patients with COVID-19 and other ARDS-causing diseases also should be of essential importance. Finally, platelet pre-activation in circulation can cause platelet desialylation, which will result in platelet clearance through the spleen or liver, which can contribute to thrombocytopenia [62]. Assessment of these phenomena would allow us to finally define the mechanism and the role of platelets in COVID-19.
CRediT authorship contribution statement
A.A.M. performed flow cytometry, aggregation and NETosis assays, analyzed experimental and clinical data and wrote the paper; A.E.B. performed flow cytometry, aggregation and NETosis assays; M.G.S. performed flow cytometry and aggregation studies and analyzed experimental data; O.I.A. performed in vitro flow cytometry studies and analyzed clinical data; A.S.G., D.V.K. and V.A.Y. performed and analyzed NETosis assays and edited the paper; A.V.B. analyzed RNA presence in the patient PRP; A.A.B. analyzed clinical data; S.S.K., E.V.F. and S.V.T. analyzed clinical and experimental data; S.A.R. and A.G.R. analyzed clinical data and edited the paper; M.A.P. and F.I.A. planned experimental research, analyzed the data and edited the paper; A.N.S. supervised and planned the project development, analyzed data and edited the paper. All authors have read and agreed to the published version of the manuscript. The authors declare no conflict of interests.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Dr. Tatiana Vuimo, Mr. Fedor Balabin, Dr. Ekaterina Koltsova (all CTP PCP RAS, Moscow), Ms. Lidya Nekrasova and Ms. Irina Dzumaniyazova (all MSU, Moscow) for the experimental studies on the blood plasma coagulation of the patients. The work was supported by the RFBR grant 20-04-60505; Russian Presidential Scholarship SP-2675.2019.4; a grant from the endowment foundation “Science for Children” and Lomonosov Moscow State University Digital Medicine School. Thrombodynamics experiments were supported by Russian Science Foundation, grant 21-45-00012.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2022.01.013.
Appendix A. Supplementary data
Supplementary Figures S1-S12 and Tables S1-S2
References
- 1.Leentjens J., van Haaps T.F., Wessels P.F., Schutgens R.E.G., Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents—lessons after 1 year. Lancet Haematol. 2021;8:e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021:1–11. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan,China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W., Pan J.Y. Anatomical and pathological observation and analysis of SARS and COVID-19: microthrombosis is the main cause of death. Biol.Proced.Online. 2021;23:4. doi: 10.1186/s12575-021-00142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.S Subrahmanian A Borczuk S Salvatore K-M Fung JT Merrill J Laurence , et al. Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. Journal of Thrombosis and Haemostasis n.d.;n/a. doi:10.1111/jth.15451. [DOI] [PMC free article] [PubMed]
- 7.Mokhtari T., Hassani F., Ghaffari N., Ebrahimi B., Yarahmadi A., Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J. Mol. Histol. 2020:1–16. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alon R., Sportiello M., Kozlovski S., Kumar A., Reilly E.C., Zarbock A., et al. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat. Rev. Immunol. 2020:1–16. doi: 10.1038/s41577-020-00470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An O.I., Martyanov A.A., Stepanyan M.G., Boldova A.E., Rumyantsev S.A., Panteleev M.A., et al. Platelets in COVID-19: “innocent by-standers” or active participants? Pediatr.Hematol./Oncol.Immunopathol. 2021;20:184–191. 10.24287/1726-1708-2021-20-1-184-191 [Google Scholar]
- 12.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct.Target.Ther. 2020;5:1–13. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behnood Bikdeli, Madhavan Mahesh V., David Jimenez, Taylor Chuich, Isaac Dreyfus, Elissa Driggin. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kow C.S., Hasan S.S. Use of antiplatelet drugs and the risk of mortality in patients with COVID-19: a meta-analysis. J. Thromb. Thrombolysis. 2021 doi: 10.1007/s11239-021-02436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JH Chow Y Yin DP Yamane D Davison R Keneally K Hawkins , et al. Association of Pre-Hospital Antiplatelet Therapy with Survival in Patients Hospitalized with COVID-19: A Propensity Score-Matched Analysis. Journal of Thrombosis and Haemostasis n.d.;n/a. doi:10.1111/jth.15517. [DOI] [PMC free article] [PubMed]
- 16.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C.J., et al. Platelet gene expression and function in COVID-19 patients. Blood. 2020 doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., et al. Platelets can contain SARS-CoV-2 RNA and are hyperactivated in COVID-19. MedRxiv. 2020 doi: 10.1101/2020.06.23.20137596. 2020.06.23.20137596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taus F., Salvagno G., Canè S., Fava C., Mazzaferri F., Carrara E., et al. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40:2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolla R., Puricelli C., Bertoni A., Boggio E., Gigliotti C.L., Chiocchetti A., et al. Platelets: “multiple choice” effectors in the immune response and their implication in COVID-19 thromboinflammatory process. Int. J. Lab. Hematol. 2021 doi: 10.1111/ijlh.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wool G.D., Miller J.L. The impact of COVID-19 disease on platelets and coagulation. PAT. 2020:1–13. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rampotas A., Pavord S. Platelet aggregates, a marker of severe COVID-19 disease. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206933. [DOI] [PubMed] [Google Scholar]
- 24.Stepanyan M., Martyanov A., An O., Boldova A., Roumiantsev S., Rumyantsev A., et al. A strong correlation exists between platelet consumption and platelet hyperactivation in COVID-19 patients. Pilot study of the patient cohort from CCH RAS Hospital (Troitsk) Syst.Biol.Physiol.Rep. 2021;1:1. 10.52455/sbpr.01.202102011 [Google Scholar]
- 25.M Koupenova HA Corkrey O Vitseva K Tanriverdi M Somasundaran P Liu , et al. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circulation Research n.d.;0. doi:10.1161/CIRCRESAHA.121.319117. [DOI] [PMC free article] [PubMed]
- 26.Barberis E., Timo S., Amede E., Vanella V.V., Puricelli C., Cappellano G., et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21228623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell R.A., Boilard E., Rondina M.T. Is there a role for the ACE2 receptor in SARS-CoV-2 interactions with platelets? J. Thromb. Haemost. 2021;19:46–50. doi: 10.1111/jth.15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217 doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolai L., Leunig A., Brambs S., Kaiser R., Joppich M., Hoffknecht M.-L., et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J. Thromb. Haemost. 2021;19:574–581. doi: 10.1111/jth.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl. Sci. 2021;6:202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouck E.G., Denorme F., Holle L.A., Middelton E.A., Blair A.M., de Laat B., et al. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler. Thromb. Vasc. Biol. 2021;41:401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lax S., Rayes J., Wichaiyo S., Haining E.J., Lowe K., Grygielska B., et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313:L1016–L1029. doi: 10.1152/ajplung.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ignatova A.A., Ponomarenko E.A., Polokhov D.M., Suntsova E.V., Zharkov P.A., Fedorova D.V., et al. Flow cytometry for pediatric platelets. Platelets. 2019;30:428–437. doi: 10.1080/09537104.2018.1513473. [DOI] [PubMed] [Google Scholar]
- 34.K Ikemura M Barouqa K Fedorov M Kushnir H Billett M Reyes-Gil . Artificial Intelligence to Identify Neutrophil Extracellular Traps in Peripehral Blood Smears. ISTH Congress Abstracts, n.d.
- 35.Kassina D.V., Vasilenko I.A., Gur'ev A.S., Volkov A.Y., Metelin V.B. Neutrophil extracellular traps: diagnostic and prognostic value in COVID-19. Almanac Clin. Med. 2020;48:43–50. doi: 10.18786/2072-0505-2020-48-029. [DOI] [Google Scholar]
- 36.Piaton E., Fabre M., Goubin-Versini I., Bretz-Grenier M.-F., Courtade-Saïdi M., Vincent S., et al. Guidelines for May-Grünwald–Giemsa staining in haematology and non-gynaecological cytopathology: recommendations of the French Society of Clinical Cytology (SFCC) and of the French Association for Quality Assurance in Anatomic and Cytologic Pathology (AFAQAP) Cytopathology. 2016;27:359–368. doi: 10.1111/cyt.12323. [DOI] [PubMed] [Google Scholar]
- 37.Nija R.J., Sanju S., Sidharthan N., Mony U. Extracellular trap by blood cells: clinical implications. Tissue Eng. Regen. Med. 2020;17:141–153. doi: 10.1007/s13770-020-00241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nechipurenko D.Y., Receveur N., Yakimenko A.O., Shepelyuk T.O., Yakusheva A.A., Kerimov R.R., et al. Clot contraction drives the translocation of procoagulant platelets to thrombus surface. Arterioscler. Thromb. Vasc. Biol. 2019;39:37–47. doi: 10.1161/ATVBAHA.118.311390. [DOI] [PubMed] [Google Scholar]
- 39.Alexandrova V., Anisimov M., Eltsov I., Kilina A., Lopanskaia I., Makarova L., et al. Avoiding common problems with statistical analysis of biological experiments using a simple nested data simulator. Syst.Biol.Physiol.Rep. 2021;1:12. 10.52455/sbpr.01.202101013 [Google Scholar]
- 40.Baaten C.C.F.M.J., Swieringa F., Misztal T., Mastenbroek T.G., Feijge M.A.H., Bock P.E., et al. Platelet heterogeneity in activation-induced glycoprotein shedding: functional effects. Blood Adv. 2018;2:2320–2331. doi: 10.1182/bloodadvances.2017011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montague S.J., Andrews R.K., Gardiner E.E. Mechanisms of receptor shedding in platelets. Blood. 2018;132:2535–2545. doi: 10.1182/blood-2018-03-742668. [DOI] [PubMed] [Google Scholar]
- 42.Sveshnikova A.N., Balatskiy A.V., Demianova A.S., Shepelyuk T.O., Shakhidzhanov S.S., Balatskaya M.N., et al. Systems biology insights into the meaning of the platelet's dual-receptor thrombin signaling. J. Thromb. Haemost. 2016;14:2045–2057. doi: 10.1111/jth.13442. [DOI] [PubMed] [Google Scholar]
- 43.Martyanov A.A., Balabin F.A., Dunster J.L., Panteleev M.A., Gibbins J.M., Sveshnikova A.N. Control of platelet CLEC-2-mediated activation by receptor clustering and tyrosine kinase signalling. Biophys. J. 2020;0 doi: 10.1016/j.bpj.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filkova A.A., Martyanov A.A., Garzon Dasgupta A.K., Panteleev M.A., Sveshnikova A.N. Quantitative dynamics of reversible platelet aggregation: mathematical modelling and experiments. Sci. Rep. 2019;9:6217. doi: 10.1038/s41598-019-42701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzeffi M., Tanaka K. Platelets and ECMO: should we worry about count, function, or both? Intensive Care Med. 2016;42:1199–1200. doi: 10.1007/s00134-016-4360-1. [DOI] [PubMed] [Google Scholar]
- 46.Mazzeffi M., Tanaka K., Wu Y.-F., Zhang A., Kareddy N., Tadjou Tito E., et al. Platelet surface GPIbα, activated GPIIb-IIIa, and P-selectin levels in adult veno-arterial extracorporeal membrane oxygenation patients. Platelets. 2020:1–7. doi: 10.1080/09537104.2020.1856360. [DOI] [PubMed] [Google Scholar]
- 47.M Reyes-Gil D Yin K Fedorov M Barouqa K Ikemura M Kushnir , et al. Neutrophilic Extracellular Traps (NETs) Are a Subset of Smudge Cells Identifiable by Peripheral Smear Autoanalyzers. ISTH Congress Abstracts, n.d.
- 48.Likic R., Kuzmanic D. Severe thrombocytopenia as a complication of acute Epstein-Barr virus infection. Wien. Klin. Wochenschr. 2004;116:47–50. doi: 10.1007/BF03040424. [DOI] [PubMed] [Google Scholar]
- 49.He J., Wei Y., Chen J., Chen F., Gao W., Lu X. Dynamic trajectory of platelet-related indicators and survival of severe COVID-19 patients. Crit. Care. 2020;24:607. doi: 10.1186/s13054-020-03339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chao Y., Rebetz J., Bläckberg A., Hovold G., Sunnerhagen T., Rasmussen M., et al. Distinct phenotypes of platelet, monocyte, and neutrophil activation occur during the acute and convalescent phase of COVID-19. Platelets. 2021:1–11. doi: 10.1080/09537104.2021.1921721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koltsova E.M., Balashova E.N., Ignatova A.A., Poletaev A.V., Polokhov D.M., Kuprash A.D., et al. Impaired platelet activity and hypercoagulation in healthy term and moderately preterm newborns during the early neonatal period. Pediatr. Res. 2019;85:63–71. doi: 10.1038/s41390-018-0184-8. [DOI] [PubMed] [Google Scholar]
- 52.Cartwright B., Bruce H.M., Kershaw G., Cai N., Othman J., Gattas D., et al. Hemostasis, coagulation and thrombin in venoarterial and venovenous extracorporeal membrane oxygenation: the HECTIC study. Sci. Rep. 2021;11:7975. doi: 10.1038/s41598-021-87026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balle C.M., Jeppesen A.N., Christensen S., Hvas A.-M. Platelet function during extracorporeal membrane oxygenation in adult patients. Front. Cardiovasc. Med. 2019;6 doi: 10.3389/fcvm.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lukito P., Wong A., Jing J., Arthur J.F., Marasco S.F., Murphy D.A., et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J. Thromb. Haemost. 2016;14:2253–2260. doi: 10.1111/jth.13497. [DOI] [PubMed] [Google Scholar]
- 55.Esper S.A., Welsby I.J., Subramaniam K., Wallisch W.J., Levy J.H., Waters J.H., et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang. 2017;112:443–452. doi: 10.1111/vox.12514. [DOI] [PubMed] [Google Scholar]
- 56.Raff L.A., Reid T.D., Johnson D., Raff E.J., Schneider A.B., Charles A.G., et al. Comparative outcomes between COVID-19 and influenza patients placed on veno-venous extracorporeal membrane oxygenation for severe ARDS. Am. J. Surg. 2021;S0002–9610(21):00233–00236. doi: 10.1016/j.amjsurg.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelzl L., Singh A., Funk J., Witzemann A., Marini I., Zlamal J., et al. Antibody-mediated procoagulant platelet formation in COVID-19 is AKT dependent. J. Thromb. Haemost. 2021 doi: 10.1111/jth.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nossel H.L. Differential consumption of coagulation factors resulting from activation of the extrinsic (tissue thromboplastin) or the intrinsic (foreign surface contact) pathways. Blood. 1967;29:331–340. doi: 10.1182/blood.V29.3.331.331. [DOI] [PubMed] [Google Scholar]
- 59.Bye A.P., Hoepel W., PhD Mitchell J.L., Jégouic S.M., Loureiro S., Sage T., et al. Aberrant glycosylation of anti-SARS-CoV-2 IgG is a pro-thrombotic stimulus for platelets. Blood. 2021 doi: 10.1182/blood.2021011871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montague S.J., Delierneux C., Lecut C., Layios N., Dinsdale R.J., Lee C.S.-M., et al. Soluble GPVI is elevated in injured patients: shedding is mediated by fibrin activation of GPVI. Blood Adv. 2018;2:240–251. doi: 10.1182/bloodadvances.2017011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W., Liang X., Syed A.K., Jessup P., Church W.R., Ware J., et al. Inhibiting GPIbα shedding preserves post-transfusion recovery and hemostatic function of platelets after prolonged storage. Arterioscler. Thromb. Vasc. Biol. 2016;36:1821–1828. doi: 10.1161/ATVBAHA.116.307639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J., van der Wal D.E., Zhu G., Xu M., Yougbare I., Ma L., et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1-S12 and Tables S1-S2