Abstract
The part of the brain most important in regulating sleep duration is the hypothalamus. Certain groups of hypothalamic neurons and adjacent groups of basal forebrain neurons produce the neuro-transmitter γ-aminobutyric acid (GABA). Projections of these GABA neurons inhibit the firing of cells involved in wakefulness. Several groups of neurons have been shown to be inhibited by this action—including neurons containing histamine, norepinephrine, serotonin, hypocretin, and glutamate—and this inhibition promotes sleep. Hypocretin (also called orexin) was discovered in 1998, and its role in sleep and narcolepsy was identified in 2001. Other as-yet undiscovered transmitters are undoubtedly involved in sleep control. The transmitters discussed in this article have been the most thoroughly studied, and many aspects of the role of each of these transmitters in relation to sleep are reasonably well understood.
The most rostral neurons in the brain with a major role in sleep control are γ-aminobutyric acid (GABA)-ergic cells located in the basal forebrain and in the anterior hypothalamus. These GABAergic cells are unique: while most neurons tend to have minimal activity during non–rapid eye movement (NREM) sleep, these cells are more active during NREM sleep than they are in rapid eye movement (REM) sleep or in waking.1–5 They also increase discharge rates with sleep onset and continue to release GABA at a high level while sleep continues. In some cases, GABA neurons continue firing during REM sleep. In other cases, neurons are active in relation to NREM sleep in particular.
GABAergic cells induce sleep by inhibiting cells that are involved in arousal functions. Cholinergic neurons in the basal forebrain are directly inhibited by GABAergic sleep-active neurons (Figure 1), and since the cholinergic system is one of the main forebrain arousal systems of the brain, the inhibition produced by this activity deactivates the cortex.
Figure 1.
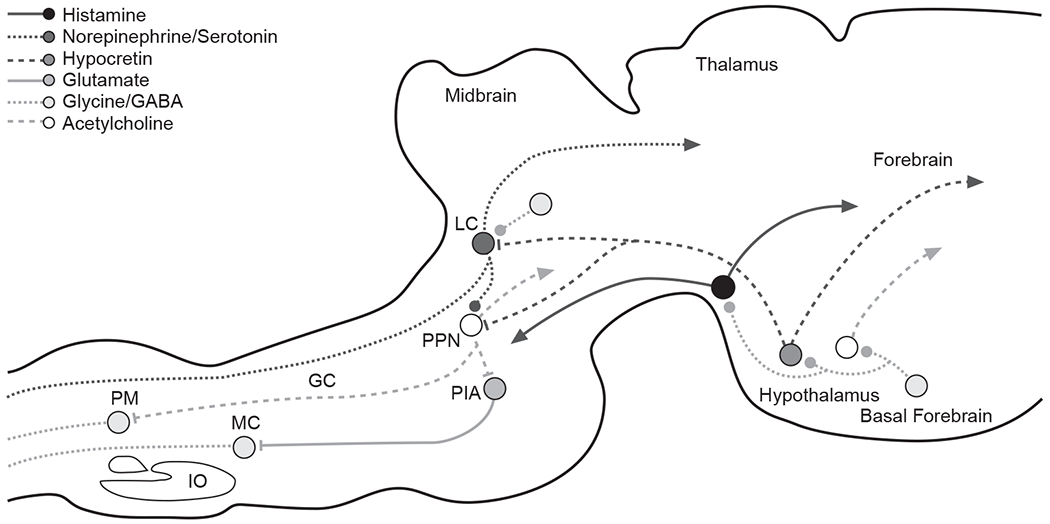
Synaptic Relationships Underlying the Loss of Consciousness and Correlated Loss of Muscle Tone in Normal Sleepa
aThis simplified drawing of a sagittal section of a cat’s brain shows some of the major connections that play an important role in sleep control. It also illustrates the complexity of the synaptic relationships underlying the loss of consciousness and correlated loss of muscle tone that characterize normal sleep. Systemic drug application can affect sleep through actions on any one of these synapses as well as on many other synapses not illustrated. Lines ending in solid dots indicate inhibitory output. Lines ending in arrows indicate excitatory output.
Abbreviations: GABA = γ-aminobutyric acid, GC = nucleus gigantocellularis, IO = inferior olive, LC = locus ceruleus, MC = nucleus magnocellularis, PIA = pontine inhibitory area, PM = nucleus paramedianus, PPN = pedunculopontine nucleus.
HISTAMINE
The histaminergic cells, which are located in the posterior hypothalamus, play a major role in the maintenance of wakefulness.6,7 It has long been known that lesions in the posterior hypothalamus produce a comatose-like continuous sleepiness,7 just as it has also been shown that lesions of the basal forebrain and anterior hypothalamus—the sleep-active cell group—produce a persistent insomnia (for review, see Szymusiak8). One could say that humans have a sleep center in the anterior hypothalamus and basal forebrain and a wake center in the posterior hypothalamus. Further, histaminergic cells in the posterior hypothalamus are strongly and directly inhibited by the GABAergic neurons.6,7,9 Therefore, the GABAergic neurons not only turn off the cholinergic cells, but they also turn off the histaminergic cells. Activity in the histaminergic cells appears to be tightly linked to wakefulness,6 and their inactivity, caused by GABAergic cells, appears to be tightly linked to sleepiness,7 as evidenced by the general observation that antihistamine medications that cross the blood-brain barrier make people drowsy.6
NOREPINEPHRINE
Norepinephrine cells are mostly localized to the locus ceruleus of the pons. Like histaminergic cells, the norepinephrine cells are inactive during REM sleep. However, there is one important difference between the activity of norepinephrine and histamine cells: only the norepinephrine cells become inactive during cataplexy, which is an episodic loss of muscle tone while awake and occurs in patients with narcolepsy. Neuronal recording studies6 suggest that the normal cessation of activity of norepinephrine cells during sleep may be related to the loss of muscle tone during sleep, while the normal cessation of activity of histamine cells during sleep may be directly related to the loss of consciousness during sleep. Several studies support the concept that activity in histaminic cell groups is strongly linked to forebrain arousal,6,10,11 whereas norepinephrine and serotonin cell groups are associated with the regulation of muscle tone and perhaps motor activity.6,12–15
SEROTONIN
The next cell group in this caudal progression contains serotonin and is located in the raphe nuclei (a midline system extending from the midbrain to the medulla). These serotonin cells, like the histamine and norepinephrine cells, are inactive in sleep (most completely in REM sleep), and they may have a role in maintaining arousal and regulating muscle tone and in regulating some of the phasic events of REM sleep.6,12,16 If these cells are destroyed, these phasic events are released from inhibition. The tonic activity of these serotonin cells during waking would tend to suppress phasic events, and their inactivity during REM sleep allows high voltage electrical activity (called ponto-geniculo-occipital [PGO] spikes) to propagate from the pons to the thalamus and cortex, releasing associated eye movements and twitches.1
Serotonin, histamine, and norepinephrine cells normally turn off during REM sleep (i.e., they are normally silent during REM sleep and active in waking) because they are inhibited by GABAergic neurons. GABA is the most common inhibitory transmitter in the brain. The fact that under some conditions, such as in cataplexy, these 3 cell groups do not cease activity together shows that they can be controlled individually by various GABAergic cell populations.6,9 GABA applied to the serotonin and norepinephrine cell groups triggers REM sleep,9,17,18 demonstrating that the cessation of activity in these brain stem cell groups is important in the control of REM sleep.
HYPOCRETIN
The most recently discovered transmitter related to sleep control is located in a cell group in the hypothalamus, situated between the rostral region, where there are sleep-active neurons, and the caudal regions, where there are histamine wake neurons. These neurons contain a peptide called hypocretin, also called orexin. They were first connected with sleep when it was found that the loss of these neurons is linked to human narcolepsy.19–23 Most narcoleptics with cataplexy exhibit about a 90% decrease in the number of hypocretin cells.21 In contrast, other degenerative diseases of the central nervous system cause no appreciable loss of these cells. For example, hypocretin cells were present in almost normal numbers in the one postmortem Alzheimer’s patient examined21; whereas in postmortem brains of people with narcolepsy, even in young postmortem narcoleptic brains, this cell population was depleted, and hypocretin levels in the cerebrospinal fluid were low.21,24 Although the cause of cell loss in narcolepsy is not well established, it may be a consequence of autoimmune attack. Mechanical damage to the hypothalamus that affects the hypocretin system also causes symptoms of narcolepsy.25 The hypocretin system appears to drive many of the other arousal systems—there are strong hypocretin projections to the histamine, norepinephrine, and serotonin neurons.
GLUTAMATE
An unusual relationship appears to exist between hypocretin neurons and amino acids. Hypocretin can cause the release of the amino acid glutamate.25–28 Applying hypocretin to trigeminal motor neurons causes excitation, but only in the presence of glutamate. If glutamate receptors are blocked, hypocretin does not activate the motoneurons.29
Similarly, in other systems and sometimes in the same system, hypocretin releases GABA. For example, in the locus ceruleus, hypocretin releases both glutamate and GABA, which results in a simultaneous excitation and inhibition that may tend to stabilize the electrical polarization of the membranes.25 In the absence of hypocretin, physiologic and behavioral instability occurs. Narcolepsy, for instance, appears to be the result of an unstable arousal system that causes individuals to be sleepy during the day yet sleep poorly at night. This instability is associated with cataplexy in waking. Conversely, the normal suppression of muscle tone during REM sleep tends to be disrupted in narcoleptics by periods without muscle tone suppression. This overt motor activity during REM sleep is called REM sleep behavior disorder and frequently accompanies narcolepsy.30 The instability of the arousal and motor control systems in narcolepsy appears to be a function of the loss of the dual action of hypocretin on excitatory and inhibitory neurotransmitters.
CONCLUSION
Until recently, sleep experts assumed that the transmitters histamine, norepinephrine, and serotonin worked together to regulate arousal and became inactive during REM sleep to keep the body from acting out dreams. In fact, each of these neurotransmitters plays a distinct role in the sleep-wake cycle. Histamine has a major role in the control of arousal and a limited direct role in muscle tone control, whereas norepinephrine and serotonin affect both muscle tone and arousal but are not as tightly linked to the maintenance of the waking state as is histamine.
Suppression of muscle tone at the motor neuronal level in REM and NREM sleep has been the focus of recent investigation. Obstructive sleep apnea, for example, is a sleep disorder that is triggered by the suppression of tone in muscles that normally hold the airway open. Conversely, in REM sleep behavior disorder, there is not enough suppression of muscle tone and people act out their dreams.31 Other parasomnias, such as nocturnal bruxism, result from a hypofunction of motor inhibition systems during sleep.
Motor neurons are inhibited in REM sleep by glycine.32 Recent studies6,9,13,33 have shown that there is a concurrent GABA inhibition and withdrawal of serotonin and norepinephrine facilitatory input onto motoneurons in REM sleep.
The discovery of hypocretin and the subsequent inquiry into its clinical and biological significance has substantially advanced our understanding of narcolepsy. Loss of hypocretin in the hypothalamus is linked to narcolepsy and cataplexy. The precise role of hypocretin in motor activity and arousal, however, has not yet been elucidated, and further inquiry into its function may lead to its use in treating narcolepsy34 as well as other motivated behaviors.
Acknowledgments
This article is derived from the teleconference “Differential Diagnosis and Management of Daytime Sleepiness and Nighttime Wakefulness,” which was held April 5, 7, and 22, 2004, and supported by an unrestricted educational grant from Cephalon, Inc.
Footnotes
Disclosure of off-label usage: The author has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents has been presented in this article that is outside U.S. Food and Drug Administration–approved labeling.
REFERENCES
- 1.Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, et al. , eds. 3rd ed. Principles and Practices of Sleep Medicine. Philadelphia, Pa: WB Saunders Co; 2000:112–133 [Google Scholar]
- 2.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992;72:165–229 [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1981;1:876–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai K, El Mansari M, Lin JS, et al. The posterior hypothalamus in the regulation of wakefulness and paradoxical sleep. In: Mancia M, Marini G, eds. The Diencephalon and Sleep. New York, NY: Lippincott Williams & Wilkins; 1990:171–198 [Google Scholar]
- 5.Strecker RE, Nalwalk J, Dauphin LJ, et al. Extracellular histamine levels in the feline preoptic/anterior hypothalamic area during natural sleep-wakefulness and prolonged wakefulness: an in vivo microdialysis study. Neuroscience 2002;113:663–670 [DOI] [PubMed] [Google Scholar]
- 6.John J, Wu MF, Boehmer LN, et al. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 2004;42:619–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24:726–731 [DOI] [PubMed] [Google Scholar]
- 8.Szymusiak R Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep 1995;18:478–500 [DOI] [PubMed] [Google Scholar]
- 9.Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol 1996;271(6, pt 2):R1707–R1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology 1988;27:111–122 [DOI] [PubMed] [Google Scholar]
- 11.Lin JS, Sakai K, Jouvet M. Hypothalamo-preoptic histaminergic projections in sleep-wake control in the cat. Eur J Neurosci 1994; 6:818–825 [DOI] [PubMed] [Google Scholar]
- 12.Wu MF, Gulyani S, Yau E, et al. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 1999;91:1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci 2001;21: 7384–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel JM, Nienhuis R, Fahringer H, et al. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science 1991;252:1315–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel JM, Nienhuis R, Fahringer HM, et al. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J Neurosci 1992;12:1840–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu MF, John J, Boehmer LN, et al. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol 2004;554(pt 1):202–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitz D, Siegel JM. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol 1997;273:R451–R455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of the sleep/wake state. Neurosci 1997;78:795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overeem S, Scammell TE, Lammers GJ. Hypocretin/orexin and sleep: implications for the pathophysiology and diagnosis of narcolepsy. Curr Opin Neurol 2002;15:739–745 [DOI] [PubMed] [Google Scholar]
- 20.Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol 2004;29:41–59 [DOI] [PubMed] [Google Scholar]
- 21.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thannickal TC, Moore RY, Aldrich M, et al. Human narcolepsy is linked to reduced number, size and synaptic bouton density in hypocretin-2 labeled neurons. Abstr Soc Neurosci 2000;26:2061 [Google Scholar]
- 23.Thannickal TC, Siegel JM, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol 2003; 13:340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy [letter]. Lancet 2000;355:39–40 [DOI] [PubMed] [Google Scholar]
- 25.Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol 2004;55:125–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Pol AN, Gao XB, Obrietan K, et al. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 1998;18:7962–7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol 2002;545:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John J, Wu MF, Kodama T, et al. Intravenously administered hypocretin-1 alters brain amino acid release: an in vivo microdialysis study in rats. J Physiol 2003;548:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol 2003;89:2591–2600 [DOI] [PubMed] [Google Scholar]
- 30.Schenck CH, Mahowald MW. Motor dyscontrol in narcolepsy: rapid eye movement (REM) sleep without atonia and REM sleep behavior disorder. Ann Neurol 1992;32:3–10 [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 32.Chase MH, Morales FR. Control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, et al. , eds. 3rd ed. Principles and Practice of Sleep Medicine. Philadelphia, Pa: WB Saunders Co; 2000:155–168 [Google Scholar]
- 33.Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci 2003;23:1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John J, Wu MF, Siegel JM. Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res Online 2000;3:23–28 [PMC free article] [PubMed] [Google Scholar]


