Abstract
We report IgG anti-spike levels up to 3 months after vaccination with 2 doses of BNT162b2 mRNA vaccine in healthcare workers (HCW). The antibody response was significantly stronger in previously infected vaccinated HCW compared to uninfected HCW, and stronger after vaccination compared to (mostly) mild natural infection.
Keywords: SARS-CoV-2, COVID-19, COVID-19 testing, Immunoassay, Spike, IgG, Vaccination
The Pfizer-BioNtech mRNA vaccine BNT162b2 against the severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) has proven to be effective against Coronavirus Disease 2019 (COVID-19) in large clinical trials as well as in real-life settings (Polack et al., 2020; Dagan et al., 2021). Data from the phase 1 clinical trial of the BNT162b2 vaccine suggest that this vaccine induces higher levels of antibodies compared to natural infection (Sahin et al., 2020). Recent studies on the short-term antibody response up to 3 weeks after the second dose of the BNT162b2 vaccine showed that previously infected individuals have higher levels of anti-spike antibodies compared to COVID-19 naive individuals (Ebinger et al., 2021; Favresse et al., 2021).
This study was approved by the local ethics committee at the University Hospitals Leuven (S64152). After obtaining informed consent, we collected serum samples from a cohort of 150 healthcare workers (HCW) who received two doses of the BN162b2 vaccine with a 21 day interval between both doses at 4 different time points: before the first dose (“baseline”, day 0, n = 150), 3 weeks after the first dose (“3 weeks”, 20-22 days, n = 150), 3 weeks after the second dose (“6 weeks”, 40-45 days, n = 150) and three months after the first dose (“3 months”, 89-105 days, n = 139). The study cohort included 24 previously infected HCW (Table 1 ).
Table 1.
Patient demographics and IgG anti-S results.
| Cohort HCW vaccination | Comparator groups | ||||
|---|---|---|---|---|---|
| COVID-19 Naive | Prior COVID-19a | Hosp. 6 weeks | Hosp. 3 months | HCW 3 months | |
| Number of individuals | 126 | 24 | 64 | 42 | 112 |
| Median age (years [range]) | 49.5 (39.3-60) | 54 (40.3-57.5) | 62.5 (56.8-68) | 65 (54.5-72.8) | 47.6 (35.0-55.5) |
| Male/Female (% men) | 35/91 (28%) | 2/22 (8%) | 39/25 (61%) | 25/17 (60%) | 14/98 (13%) |
| Severity (%) | |||||
| Asymptomatic | NA | 9 (38%) | NA | NA | 8 (7%) |
| Mild | NA | 11 (46%) | NA | NA | 98 (88%) |
| Moderate | NA | 4 (17%) | 28 (44%) | 15 (28%) | 6 (5%) |
| Severe/critical | NA | 0 (0%) | 36 (56%) | 38 (72%) | 0 (0%) |
| Median IgG anti-S level (AU/mL) (P25-P75) | |||||
| Baseline | 2.4 (1.1-4.8) | 287 (104-629)c | NA | NA | NA |
| 3 weeks | 559 (244-1133) | 24444 (15697-33073) c | NA | NA | NA |
| 6 weeks | 9887 (5599-16189) | 32538 (24061-42078) c | 5941 (2369-10470)d | NA | NA |
| 3 months | 2942 (1642-5016)b | 15633 (9355-21382) ,b,c | NA | 3542 (606-5859) d | 624 (296-1149)e |
| High probability neutralizing antibodies (≥4160 AU/mL) (%) | |||||
| Baseline | 0 (0%) | 0 (0%) | NA | NA | NA |
| 3 weeks | 1 (1%) | 23 (96%)c | NA | NA | NA |
| 6 weeks | 108 (86%) | 24 (100%) | 46 (72%)d | NA | NA |
| 3 months | 37 (32%)b | 20 (91%)b,c | NA | 23 (55%)e | 4 (4%)e |
HCW = health care workers; Hosp. = hospitalized COVID-19 patients; NA = not applicable; AU = arbitrairy units.
Median time between positive PCR and administration of the first dose was 274 days (range:39-301).
Eleven participants were not tested at 3 months (9 COVID-19 naive and 2 prior COVID-19).
P < 0.01 prior COVID-19 vs COVID-19 naïve.
P < 0.01 vs prior COVID-19, but not vs COVID-19 naive at 6 weeks.
P < 0.01 vs all at 3 months.
Antibody levels after vaccination were compared with levels in HCW 3 months after positive PCR (median 86 days, range 60-109) (n = 112), and hospitalized COVID-19 patients at 6 weeks (median 44 days, range 30-59) (n = 64) and 3 months (median 85 days, range 75-105) (n = 42) after positive PCR, from previous studies (Van Elslande et al., 2021a, 2021b) (Table 1).
Antibodies were measured on Abbott Architect (Abbott, Lake Forest Illinois) with the chemiluminiscence IgG II Quant (anti-S) assay using the manufacturer's cut-off for positivity of 50 AU/mL. As a surrogate for the presence of high neutralizing antibody titer, we determined the proportion of patients with IgG anti-S levels above 4.160 AU/mL (“high titer”). This antibody level has been correlated with a 95% probability for the presence of a neutralizing antibody titer (PRNT ID50) of at least 1:250) (Ebinger et al., 2021). See the online data supplement for more information.
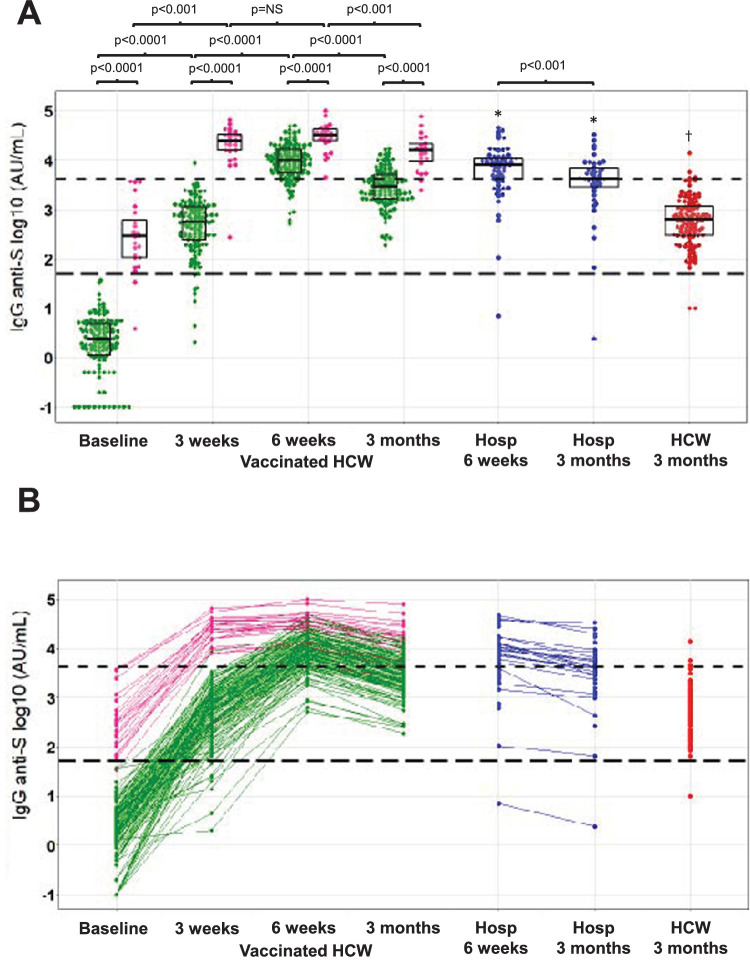
IgG anti-S levels were significantly higher in vaccinated HCW with prior COVID-19 compared to COVID-19 naive HCW at each time point (Fig. 1 , P < 0.001). Previously infected HCW had higher IgG anti-S 3 weeks after the first dose compared to uninfected HCW 3 weeks after the second dose (P < 0.001). Antibody levels rose significantly after the first and second dose in naive HCW (P < 0.001 each, Fig. 1). In previously infected HCW, however, there was a significant rise after the first dose, but the rise after the second dose did not reach significance (P = 0.14). After 3 months, antibody titers decreased significantly compared to 6 weeks, but remained significantly higher compared to 3 weeks, in both previousy infected and uninfected HCW (P < 0.001).
Fig. 1.
IgG anti-spike antibody response after vaccination and after natural infection. (A) Anti-S antibody titers in vaccinated HCW with (pink) and without (light green) a prior SARS-CoV-2 infection (time points indicating time after first vaccine dose), hospitalized COVID-19 patients (blue) and non-vaccinated HCW after natural infection (red) (time points indicating time after first positive SARS-Cov-2 PCR). The boxplots indicate the median, 25th and 75th percentile. (B) dynamic evolution of anti-S antibody levels. The manufacturer's cut-off for positivity of 50 AU/mL (1.7 on the log10 scale) and the cut-off for high probability of neutralizing antibody titer at 4160 AU/mL (3.6 on the log10 scale) are depicted as long dashed and short dashed horizontal black lines, respectively. *P < 0.001 compared to vaccinated HCW with prior COVID-19 at the same time point, †P < 0.001 vs all groups at 3 months. (Color version of figure is available online.)
In previously infected HCW median anti-S levels were 24.5 times higher 3 months after vaccination compared to HCW three monts after (mostly mild) natural infection. In previously uninfected vaccinated HCW, median anti-S levels were 4.6 times higher at this timepoint (P < 0.001 for both). In previously infected vaccinated HCW, we did not observe a correlation between the antibody levels and and the time interval between positive PCR and vaccination (data not shown).
When comparing with hospitalized COVID-19 patients, antibody levels were significantly higher at 6 weeks and 3 months in vaccinated HCW with prior COVID-19 but not in COVID-19 naive vaccinated HCW (Table 1). All vaccinated HCW seroconverted for IgG anti-S antibodies at 6 weeks and remained positive up to 3 months after vaccination (Supplementary Table 1). The proportion of individuals with high levels of anti-S antibodies (“high titer”, ≥4160 AU/mL), however, varied significantly between groups and changed over time (Table 1). At 3 weeks, more than 95% of vaccinated HCW with prior COVID-19 already had a high titer compared to less than 1% in COVID-19 naive HCW (P < 0.001). At 6 weeks, this proportion increased to 100% and 86%, respectively (P = 0.08). For comparison, the percentage of hospitalized COVID-19 patients with a high titer was 72% after 6 weeks (P < 0.01 vs prior COVID-19). At three months, the percentage of individuals with a high titer decreased to 91% and 32% in previously infected and uninfected vaccinated HCW, respectively (P < 0.001). For comparison, only 4% of HCW with a natural infection had a high titer 3 months after natural infection (P < 0.001 vs both). Anti-S titers decreased significantly faster between 6 weeks and 3 months in COVID-19 naive vaccinated HCW (n = 126, -66%) compared to vaccinated HCW with prior COVID-19 (n = 24, -47%) and hospitalized COVID-19 patients (n = 23, -42%) (P < 0.001 for COVID-19 naive HCW vs both).
We report a stronger antibody response after vaccination in HCW with prior COVID-19 compared to COVID-19 naive HCW. At 3 months, the proportion of participants with a high titer of anti-S suggestive of a high neutralizing antibody titer was significantly higher in previously infected HCW compared to COVID-19 naive HCW, extending the observations of Ebinger et al. who reported a stronger antibody response up to 6 weeks in HCW with prior COVID-19 (Ebinger et al., 2021).
The level of neutralizing antibody titers is linked to the amount of protection, but the exact threshold for protection against (re)infection is not yet known (Khoury et al., 2021). The fact that only 32% of COVID-19 naive vaccinated HCW had a high titer of anti-S antibodies at three months raises concerns about quick waning of protection against SARS-CoV-2 infection after vaccination, especially in previously uninfected individuals. Our observations suggest that COVID-19 naive individuals might require a third dose of the BNT162b2 vaccine at an earlier time point compared to those with prior COVID-19. These findings are consistent with other recent reports on quick waning of antibody levels after vaccination (Levin et al., 2021; Bayart et al., 2021).
Strenghts of our study are the longitudinal follow-up up to three months with only 7% dropout at three months and the comparison with antibody titers after natural infection. There are also a number of limitations to our study: the relatively small group of study participants, the use of a surrogate for the presence of neutralizing antibodies and the lack of evaluation of the cellular immune response.
Author contributions
PV and XB conceived the study. JVE and MW conducted experiments. PV, JVE and MW analyzed the data and drafted the manuscript. PV, XB, JVE, MW, LG and GVP critically reviewed the manuscript.
Acknowledgments
P. Vermeersch is a senior clinical investigator of the FWO-Vlaanderen.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of competing interests
Dr. Vermeersch reports personal fees from Roche, outside the submitted work.
Footnotes
Abbreviations: anti-S, anti-spike; COVID-19, Coronavirus Disease 19; HCW, healthcare workers; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.diagmicrobio.2022.115638.
Appendix. Supplementary materials
References
- Bayart JL, Douxfils J, Gillot C, David C, Mullier F, Elsen M, et al. Waning of IgG, total and neutralizing antibodies 6 months post-vaccination with BNT162b2 in healthcare workers. Vaccines. 2021;9:1092. doi: 10.3390/vaccines9101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N, Barda N, Kepten E, Miron O, Percheck S, Katz M, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favresse J, Bayart J-L, Mullier F, Dogné J-M, Closset M, Douxfils J. Early antibody response in healthcare professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2) Clin Microbiol Infect. 2021;27:1351.e5–1351.e7. doi: 10.1016/j.cmi.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Van Elslande J, Gruwier L, Godderis L, Vermeersch P. Estimated half-life of SARS-CoV-2 anti-spike antibodies more than double the half-life of anti-nucleocapsid antibodies in healthcare workers. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elslande J, Oyaert M, Ailliet S, Van Ranst M, Lorent N, Vande Weygaerde Y, et al. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;136 doi: 10.1016/j.jcv.2021.104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.