Abstract
Succinic acid widely exists in foods and is used as a food additive. Succinate not only serves as an energy substrate, but also induces protein succinylation. Histone succinylation activates gene transcription. The brown adipose tissue (BAT) is critical for prevention of obesity and metabolic dysfunction, and the fetal stage is pivotal for BAT development. Up to now, the role of maternal succinate supplementation on fetal BAT development and offspring BAT function remains unexamined. To test, female C57BL/6J mice (2-month-old) were separated into two groups, received with or without 0.5% succinic acid in drinking water during gestation and lactation. After weaning, female offspring were challenged with high fat diet (HFD) for 12 weeks. Newborn, female weanling, and HFD female offspring mice were analyzed. For neonatal and weaning mice, the BAT weight relative to the whole body weight was significantly increased in the succinate group. The expression of PGC-1α, a key transcription co-activator promoting mitochondrial biogenesis, was elevated in BAT of female neonatal and offspring born to succinate-fed dams. Consistently, maternal succinate supplementation enhanced thermogenesis and the expression of thermogenic genes in offspring BAT. Additionally, maternal succinate supplementation protected female offspring against HFD-induced obesity. Furthermore, in C3H10T1/2 cells, succinate supplementation promoted PGC-1α expression and brown adipogenesis. Mechanistically, succinate supplementation increased permissive histone succinylation and H3K4me3 modification in the Ppargc1a promoter, which correlated with the higher expression of Ppargc1a. In conclusion, maternal succinate supplementation during pregnancy and lactation enhanced fetal BAT development and offspring BAT thermogenesis, which prevented HFD-induced obesity and metabolism dysfunction in offspring.
Keywords: Brown adipose tissue, Maternal, Metabolism, Offspring, Succinate
1. Introduction
Succinic acid is a natural four-carbon dicarboxylic acid, and succinate is an anion form of succinic acid. Succinic acid is enriched in produce with high mitochondrial contents such as broccoli, sugar beets, rhubarb etc [1]. In addition, microbial fermentation can generate succinic acid. Thus, it is also abundant in fermented foods, such as cheese, wine and sauerkraut [1]. The concentration of succinic acid in wine ranges from 0.5 to 1.5 g/L, with a maximal content up to 3 g/L [2]. In addition, as a weak acid, succinic acid is often used as an acidic regulator in the manufacture of foods and beverages [3].
In vivo, succinate is one of intermediates of the tricarboxylic acid (TCA) cycle. In mitochondria, succinyl-CoA synthetase catalyzes the generation of succinate from succinyl-CoA. Subsequently, succinate is oxidized to fumarate by succinate dehydrogenase (SDH) [4]. After its formation in the matrix of mitochondria, succinate can be transferred to intermembrane space mediated by SLC25A10, a succinate transporter in the inner mitochondrial membrane [5]. Then, porins in the outer mitochondrial membrane facilitate the release of succinate into cytosol [5]. For the plasma membrane, SLC13 family proteins serve as transporters of succinate, which exist in numerous tissues [6]. Circulatory succinate binds to its receptor, SUCNR1 (also called GPR91), which is widely expressed, including adipocytes, hepatic stellate cells, immature dendritic cells, and others [7, 8].
As a Gαi-protein coupled receptor, activation of SUCNR1 inhibits protein kinase A but activates p38 pathway signaling [9–11]. Also, it can modulate the intracellular calcium concentration via PLCβ [12]. In addition, succinylation is a novel posttranslational modification but its biological function remains poorly understood [13, 14]. Succinylation was found widely in nucleosomes [15], and succinyl-lysine marks are positively associated with the euchromatin marker, histone 3 lysine 4 trimethylation (H3K4me3), but negatively correlated with the heterochromatin marker, H3K27me3 [15], facilitating gene expression.
Brown adipose tissue (BAT) contains abundant mitochondria and is a key tissue for thermogenesis and energy expenditure [16]. The discovery of widespread existence of BAT in human adults underscores its importance in obesity prevention [17, 18]. During BAT development, a cascade of BAT specific genes is expressed, which are associated with increased H3K4me3 modification in their promoters [19]. The PPARγ coactivator-1α (Ppargc1a for gene, and PGC-1α for protein) is a master regulator of mitochondrial biogenesis and thermogenesis in BAT. As a transcriptional activator, PGC-1α interacts with peroxisome proliferator-activated receptor gamma (PPAR-γ), stimulating the expression of gene participating in the brown adipocyte differentiation [20]. In addition, PGC-1α regulates nuclear respiratory factors (NRFs), which mediates the synthesis of electron transfer chain (ETC) subunits and mitochondrial DNA (mtDNA) replication [21]. Thus, PGC-1α is a key regulator of BAT development and subsequent BAT thermogenesis.
Interestingly, when mice were treated with high fat diet (HFD), succinate supplement reduces HFD-induced obesity and enhances thermogenesis in BAT which is independent of adrenergic signaling [22]. In addition, dietary succinate supplementation ameliorates hyperglycemia in ob/ob mice [23]. Considering the fetal stage is the main stage for BAT development [24], maternal nutrition profoundly affects fetal BAT development, which has long-term effects on BAT thermogenic function and metabolic health in offspring [25, 26]. Thus, we hypothesized that dietary succinate promotes fetal BAT development which is correlated with enhanced succinylation and H3K4me3 modification in the Ppargc1a promoter, protecting offspring mice from HFD-induced obesity.
2. Materials and Methods
2.1. In vivo study using C57BL/6 mice
All animal experimental and care procedures were carried out according to the guidelines approved by the Institute of Animal Use and Care Committee (IAUCC) at Washington State University. Twenty female C57BL/6 J mice at age of 2 months with similar weight were selected and assigned randomly to two groups (n = 10 per group). All mice were mated with 2 months old C57BL/6 J male mice. Each mouse was housed individually in an environmentally controlled room (23 ± 2°C, 12 h −12 h light - dark cycle). After mating, the appearance of vagina plug was designated as 0 day of gestation. Pregnant mice were fed with ad libitum access to chow diet (10% energy from fat, D12450H; Research Diets, New Brunswick, USA). Pregnant mice received either drinking water (CON) or drinking water added 0.5% succinic acid (SUCC, w/v) (ACROS, A0394488) until weaning (21d after birth). In previous studies, the succinate supplementation was in a range from 0.5% - 2% [22, 27]. The water was changed every other day. Weight gain, food intake and water consumption were monitored. After birth, the litter sizes were normalized to 5 – 6. Litters were removed from the study when the litter sizes were less than 5. On postnatal day 21, one female offspring per litter was harvested. BAT, iWAT and blood were collected. One piece of adipose tissue per mouse was snap-frozen in liquid nitrogen, and another piece was fixed in 4% paraformaldehyde for embedding. The remaining female mice were challenged with high fat diet (HFD, 60% energy from fat, D12492; Research Diets, New Brunswick, USA) to mimic the obesogenic environment of western societies. Food intake and body weight were recorded. After 12 weeks, whole body metabolic analysis and glucose tolerance test were performed. Then the adult mice were euthanized, and BAT, iWAT, eWAT and blood sample were collected.
2.2. Glucose tolerance test and insulin concentration measurement
Before measurement, mice were fasted overnight and injected with 1g/kg D-Glucose intraperitoneally. At 0, 15, 30, 60 and 120 min after injection, blood samples were collected from tail tips. The glucose concentration was determined by a glucometer (Bayer Contour, Tarrytown, NY, USA). The insulin concentration was measured by a commercial kit (10111301, Mercodia, NC, USA).
2.3. Metabolic analysis
Metabolic analysis was conducted using an Oxymax indirect open-circuit calorimetry system (Columbus Instruments, Columbus, OH, USA). Oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RER) and heat production were recorded. The whole system was kept in a constant environment (23 ± 2°C, 12 h light - 12 h dark cycle). Mice had free access to food and water.
2.4. Acute cold tolerance test
Mice were housed at 4 °C for 3 days with access to food and water ab libitum. The dorsal interscapular temperature was measured using E6 Thermal Imaging Infrared Camera (FLIR, Oregon, USA).
2.5. Histological analysis
Tissues were fixed in 4% paraformaldehyde at room temperature (RT) for 24 h. Then fixed tissues were rinsed with tap water. Subsequently, samples were dehydrated using a series of ethanol and xylene solution and then embedded in paraffin. Sections (5 μm) were obtained using a microtome (Leica, Wetzlar, Germany), deparaffinized, and subjected to Hematoxylin and Eosin staining (H&E). The size and number of adipocytes were assayed using Image J software (National Institute of Health, Baltimore, MD). Four images of each section and five sections at a constant interval (25 μm) per animal were used.
2.6. C3H10T1/2 cell culture
C3H10T1/2 was used to determine the effect of succinate on brown adipogenesis. When cells reached about 80% confluence, they were treated with brown adipogenic differentiation medium, containing Dulbecco’s modified Eagle’s medium (DMEM, Sigma, D5546), 10% FBS (FBS, Gibco #10,439,001), 1 μg/mL insulin (Sigma, I3536), 0.5 mM of 3-isobutyl-1-methylxanthine (IBMX, Sigma, I5878), 1 μM dexamethasone (DEX, Sigma, D4902) 1 nM 3,3’,5-Triiodo-L-thyronine (T3, Sigma, T2877), 12.5 μM Indomethacin (Sigma, 1341001), plus 0 mM, 4 mM, 8 mM, 16 mM dimethyl-succinate (Sigma, S0755). After two days of differentiation, the medium was changed to maintenance medium, including 10% FBS (FBS, Gibco #10,439,001), 1 μg/mL insulin (Sigma, I3536), 1 nM 3,3’,5-Triiodo-L-thyronine (T3, Sigma, T2877) plus 0 mM, 4 mM, 8 mM, and 16 mM dimethyl-succinate (Sigma, S0755). The maintenance medium was changed every two days. The cells were harvested at 0, 2 and 8 days.
2.7. Oil Red O staining
According to the previous protocol [28], differentiated cells were fixed in 4% paraformaldehyde for 10 min at RT. Then cells were rinsed by dH2O and isopropanol. Next, cells were stained with Oil Red O (C26H24N4O, Sigma, O1391) for 10 min at RT. Cells were rinsed with dH2O to remove excessive dye. After capturing images, isopropanol was added to each well for extraction of Oil Red O, and the absorbance at 518 nm was measured using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, VT, USA).
2.8. Quantitative real-time PCR
Total RNA was isolated utilizing Trizol reagent (Sigma, St. Louis, MO) and treated with deoxyribonuclease. The reverse transcription and synthesis of cDNA were conducted using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Quantitative Real-time PCR (qRT-PCR) was performed on CFX ConnectTM Real-Time PCR detection system (Bio-Rad), using a SYBR Green RT-PCR kit (Bio-Rad, Hercules, CA, USA). 18S was used as the reference gene to normalize mRNA expression levels. Relative mRNA expression was calculated using a 2-ΔΔCt method [29].
2.9. Immunoblotting analysis
Immunoblotting analyses were performed using the Odyssey Infrared Image System (LICOR Biosciences, Lincoln, NE, USA). The primary antibodies were used as follows: anti-PGC1α (Proteintech, 20658–1-AP); anti-UCP-1 (Proteintech, 23673–1-AP); and anti-succinyllysine (PTM Biolab, PTM-401), as well as antibodies from Cell Signaling Technology (Danvers, MA, USA): anti-p38 (No. 9212S); anti-Phospho-p38 (No. 9211S); anti-Cytochrome c (No. 4280); anti-trimethyl-H3K4 (No. 9727); β-tubulin (No. 2146); and regular rabbit IgG (No. 2729).
2.10. Chromatin Immunoprecipitation- qPCR Assay
Chromatin immunoprecipitation qPCR (ChIP-qPCR) was conducted according to a previous report with some adjustments [25]. Briefly, harvested cells were fixed with 1% formaldehyde for 10 min at RT. To stop cross-linking, 125 mM Glycine was added with shaking for 10 min at RT. Cells were collected by centrifuging at 1,000 × G for 5 min, 4 °C. The pellet was washed with PBS twice and lysed with a lysis buffer (1% SDS, 10 mM Tris-HCl, pH 8.0, 10 mM EDTA) supplemented with a protein inhibitor cocktail (Sigma, P2714). Then, the mixture was sonicated to produce 200 – 400 bp chromatin segments. After centrifugation, the supernatant was pre-blocked with ChIP-grade Pierce™ magnetic protein A/G (Thermo Scientific, Waltham, MA), incubated with anti-H3K4me3 antibody, anti-succinyllysine antibody or regular rabbit IgG overnight at 4 °C. Next, magnetic beads were collected and washed with a low salt washing buffer (0.10% SDS, 1.0% Triton X-100, 2.0 mM EDTA, 20 mM Tris-HCl, 150 mM NaCl) twice, a high salt buffer (0.10% SDS, 1.0% Triton X-100, 2.0 mM EDTA, 20 mM Tris-HCl, 500 mM NaCl) twice, and a LiCl wash buffer (1.0% NP-40, 1.0% Deoxycholic acid sodium, 1.0 mM EDTA, 10 mM Tris-HCl, 0.25 M LiCl) twice. After that, 5 M NaCl was added to the magnetic beads and incubated in boiling water for 10 min. Then the recovered solution was treated with RNase A and proteinase K for 1 h at 60 °C respectively. The DNA was extracted with a mixture of Phenol: Chloroform: Isoamyl alcohol (25:24:1) (ACROS, A0417977). Finally, the DNA was washed with 75% ethanol and dried at RT. Obtained DNA was used for qRT-PCR. The primer listed in Table 1.
Table 1:
Primers used in this study
| Cidea | F | GCTCCCCGTAATTCCCACTG |
| R | GACAGAACCACACCCTGACA | |
| CytoC | F | ACACTGTGGAAAAGGGAGGC |
| R | GCACTGGTTAACCCAAGCAA | |
| Elovl 3 | F | GATGGTTCTGGGCACCATCTT |
| R | CGTTGTTGTGTGGCATCCTT | |
| Ppargc1α | F | CCCTGCCATTGTTAAGACC |
| R | TGCTGCTGTTCCTGTTTTC | |
| PPAR-γ | F | AGCTCCAAGAATACCAAAGTGCGAT |
| R | AGGTTCTTCATGAGGCCTGTTGTAGA | |
| UCP-1 | F | ACTGCCACACCTCCAGTCATT |
| R | CTTTGCCTCACTCAGGATTGG | |
| 18S | F | GTAACCCGTTGAACCCCATT |
| R | CCATCCAATCGGTAGTAGCG | |
| Ppargc1α (−1kb) | F | GCCTGGAAGGGTTAAGTCTG |
| R | CAATGAGGGGTAATGCAGGT | |
| Ppargc1α (−0.1kb) | F | GACGTCAGGAGTTTGTGCAG |
| R | GACGCCAGTCAAGCTTTTTC | |
| Ppargc1α (1kb) | F | ACACAAGCAGTTTCCCCGTA |
| R | GGGTCCATCTCACCAGAGTC | |
| Ppargc1α (2kb) | F | TCAGCGTCCAGCCTTAGATT |
| R | ATGGAAGCTGCCCACACTAC | |
| Ppargc1α (3kb) | F | TCTCCCCTCATCTCTGTGCT |
| R | GTAACAATCCCAAGGCTCCA |
2.11. Statistical analysis
Data are presented as means ± SEM. All data were analyzed using GraphPad Prism (San Diego, CA, USA) with Student’s T-test for two groups. A one-way ANOVA and Dunnett’s multiple comparison were used to compare multiple groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Maternal phenotypic changes due to succinate supplementation
Succinate supplementation did not affect either the initial body weight before treatment or final body weight at the end of lactation (Fig. 1A), nor the ratio of BAT and iWAT mass to body weight (Fig. 1B). However, the ratio of eWAT to body weight was decreased in the SUCC group (Fig. 1B). Administration of succinate had no effect on food and water intake (Fig. 1C and 1D), nor blood glucose and insulin levels at weaning (Fig. 1E and 1F).
Fig. 1.
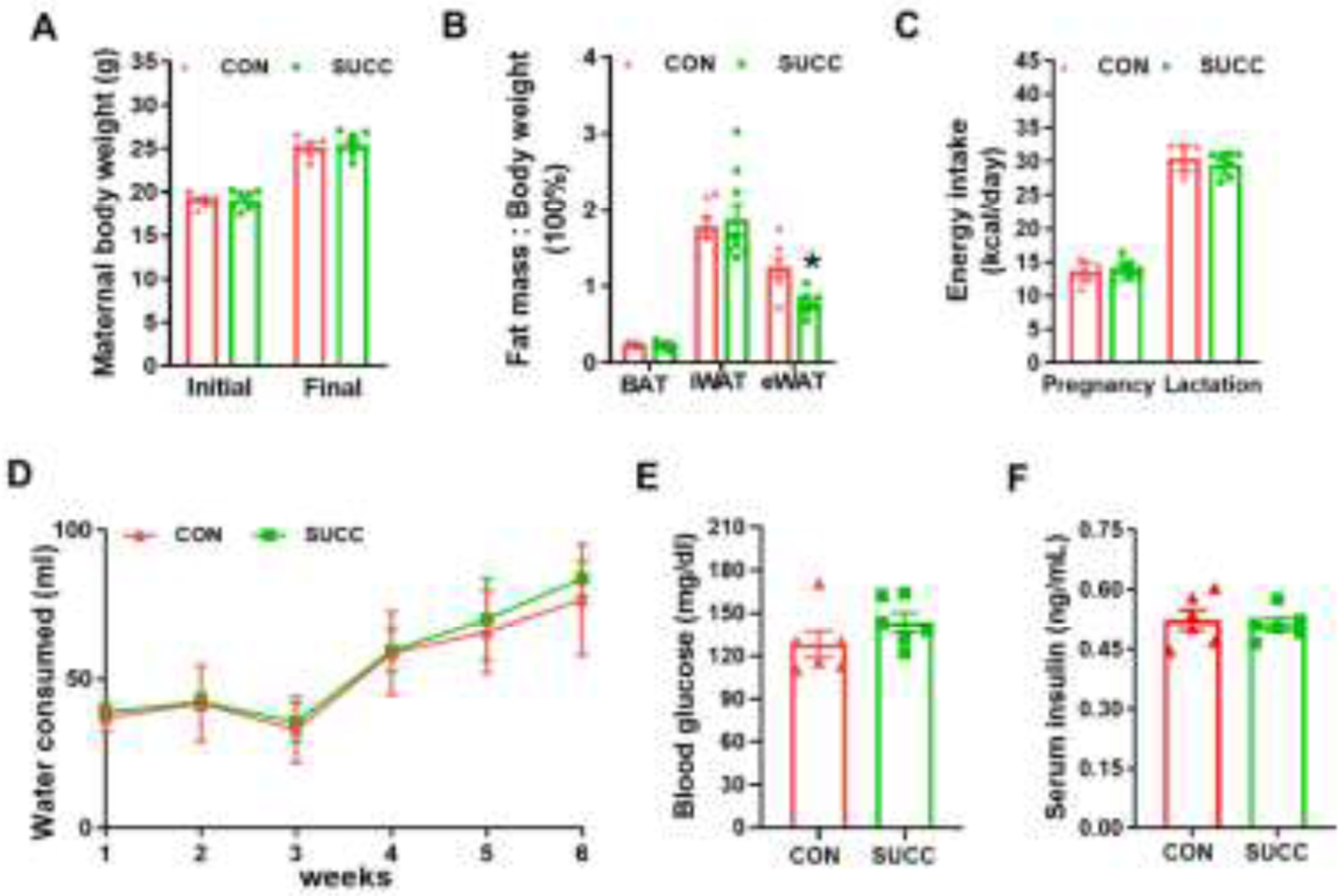
Maternal phenotypic changes due to succinate supplementation
A, Initial and final (at weaning) maternal body weight. B, Fat index (the ratio of fat mass to the whole body weight) of BAT, iWAT and eWAT. C - D, Maternal energy intake (C) and water consumption (D) during pregnancy and lactation. E - F, Blood glucose (E) and insulin concentrations (F) at the end of lactation. *P < 0.05. Data are shown as means ± SEM (n = 6). Abbreviations: BAT, brown adipose tissue; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue; SUCC, succinic acid.
3.2. Succinate supplementation during gestation and lactation increases BAT mass and enhances brown adipogenesis in neonates and weaning female offspring
At birth, no difference was observed in neonatal body weight (Fig. 2A). However, SUCC neonates had greater BAT mass (Fig. 2B, 2C). Consistently, maternal succinate supplementation increased the surface temperature of neonates (Fig. 2D, 2E). In addition, H&E staining showed BAT of SUCC offspring had denser structure (Fig. 2F). Consistent with increased surface temperature, SUCC neonates had higher expression of Uncoupling protein 1 (Ucp-1) and tended to have higher expression of Ppargc1a (P = 0.06) and Cytochrome C (Cyto C, P = 0.08). No difference was detected in Cell death-inducing DFFA-like effector A (Cidea), Elongation of very long-chain fatty acids protein 3 (Elovl3) and Peroxisome proliferator-activated receptor gamma (PPAR γ) and Prdm16 (Fig. 2G). Moreover, SUCC neonates had increased phospho-p38 (P-p38) content. Also, the PGC-1α and UCP-1 protein levels in the SUCC group tended to be higher (P = 0.09, and P = 0.06, respectively), but no difference was observed in the contents of p38 and PRDM16 (Fig. 2H and 2I).
Fig. 2.
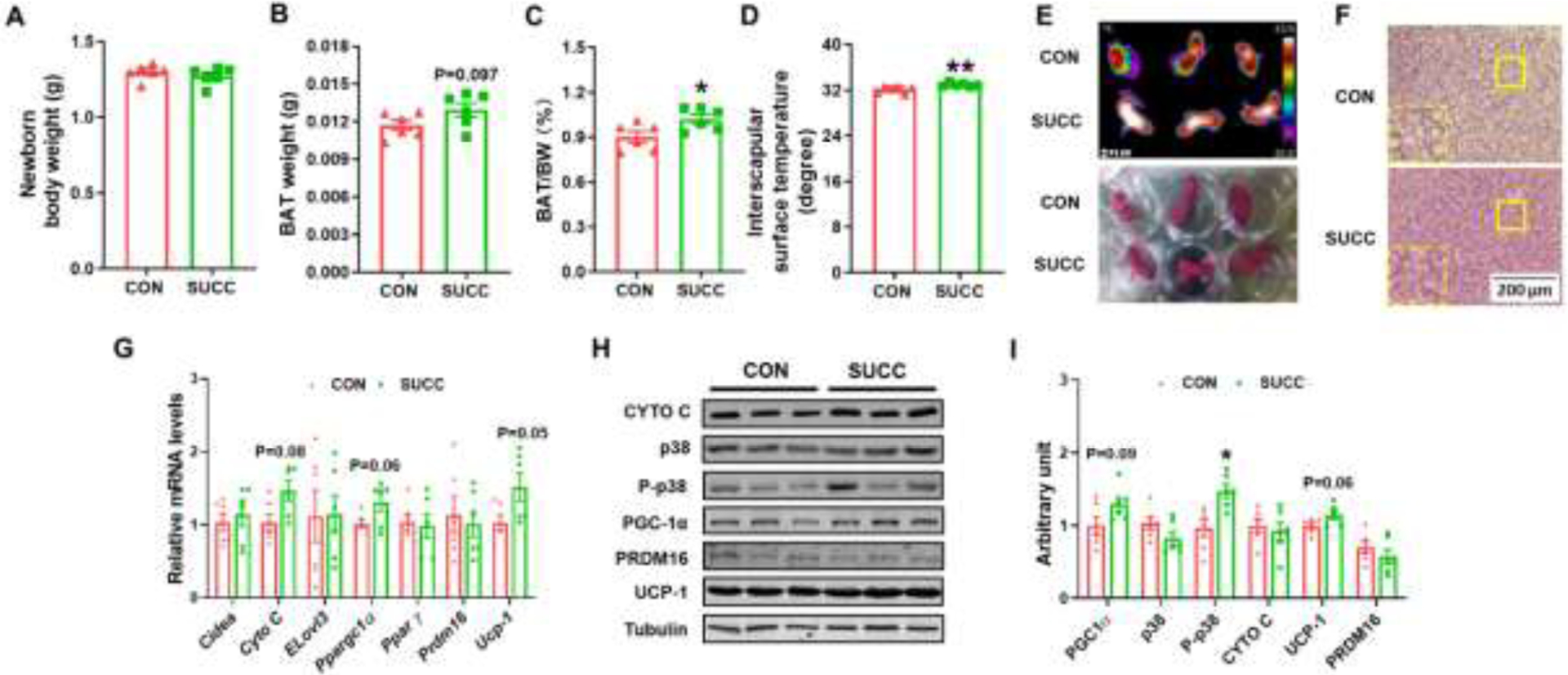
Succinate supplementation during gestation and lactation increases BAT mass and enhances brown adipogenesis in neonates
A, Body weight. B, BAT mass. C, Fat index (the ratio of fat mass to the whole body weight) of BAT. D, Interscapular surface temperature. E, Infrared (up) and photographic (down) images of CON and SUCC offspring. F, Representative H&E staining for BAT sections. G: mRNA expression of brown adipocyte markers in BAT. H - I, Immunoblotting analysis for brown adipocyte markers and p38. *P < 0.05. Data are shown as means ± SEM (n = 6). Abbreviations: Cidea, Cell death-inducing DFFA-like effector A; Cyto C, Cytochrome C; Elovl3, Elongation of very long-chain fatty acids protein 3; H&E, Hematoxylin and Eosin; p38, p38 MAPK; P-p38, phosphorylation-p38; PPAR γ, Peroxisome proliferator-activated receptor gamma; UCP-1, uncoupling protein 1; for other abbreviations, see legend of Fig. 1.
Maternal succinate supplementation did not affect body weight gain of offspring during lactation (Fig 3A). Of interest, administration of succinate elevated the BAT mass at weaning (Fig. 3B and 3C). Maternal succinate supplementation also increased serum succinate concentration (Fig. 3D). Although there was no difference in blood glucose and serum insulin contents (Fig. 3E and 3F), the surface temperature was higher in the SUCC group after cold challenge (Fig. 3G and 3H), consistent with data from neonates. To determine the structure of adipocytes in BAT and iWAT, we performed H&E staining. The BAT of SUCC weanlings was denser and the adipocyte sizes of iWAT were smaller in weanlings from SUCC dams (Fig. 3I and 3J). In addition, the gene expression of Ppargc1a was also higher in the SUCC group, and Cyto C (P = 0.06) and Ucp-1 (P = 0.09) expression showed a tendency of increase (Fig. 3K). Although maternal succinate feeding did not change the p38 level, the P-p38 was enhanced compared to CON group (Fig. 3L and 3M). Also, the offspring from SUCC dam had higher content of PGC-1α (Fig. 3L and 3M). No difference was observed in the protein level of PRDM16 (Fig. 3L and 3M). The enrichment of H3K4me3 and succinyllysine in the Ppargc1a promoter were measured. H3K4me3 and succinyllysine were enriched at 1KB upstream of the Ppargc1a promoter in the BAT of weaning mice born to SUCC dam (Fig. 3N and O).
Fig. 3.
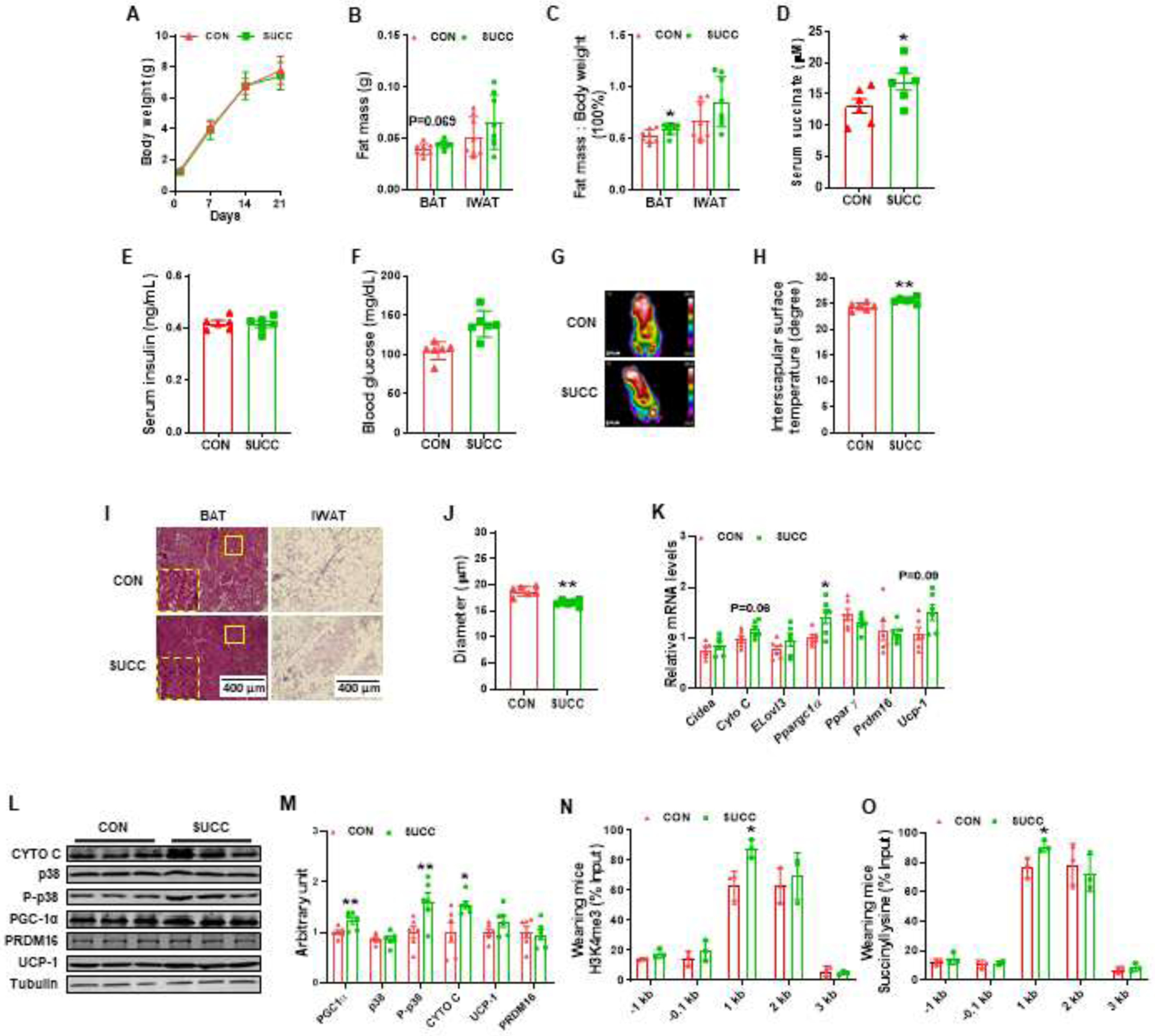
Succinate supplementation during gestation and lactation increases BAT mass and enhances brown adipogenesis in weaning female offspring
A, Body weight gain. B, BAT and iWAT mass. C, Fat index (the ratio of fat mass to the whole body weight) of BAT and iWAT. D, Succinate concentration in the plasma of weaning mice. E, serum insulin concentrations. F, Blood glucose. G - H, Infrared images (G) and interscapular surface temperature (H) of CON and SUCC offspring. I - J, Representative H&E staining for BAT and iWAT sections (I) and average adipocyte diameters (J). K, mRNA expression of brown adipocyte markers in BAT. L - M, Immunoblotting analysis for brown adipocyte markers and p38. N – O, The enrichment of H3K4me3 (N) and succinyllysine.(O) in the Ppargc1a promoter (n = 3). Data are shown as means ± SEM *P < 0.05, **P < 0.01. Data are shown as means ± SEM (n = 6).
3.3. Maternal succinate supplementation prevents offspring from HFD-induced obesity and metabolic dysfunction
To mimic the common HFD in western societies, we treated the weaning mice with 60% kcal HFD for 12 weeks. There was no difference in food intake (Fig. 4A). However, maternal succinate feeding protected offspring from HFD-induced obesity (Fig. 4B). Accordingly, the masses of adipose tissues, including BAT, iWAT and eWAT, were lower in the SUCC group (Fig. 4C and 4D). Moreover, the glucose tolerance was improved in the offspring of dams treated with succinate (Fig. 4E), and the serum insulin was also decreased (Fig. 4F). In addition, the offspring thermogenic ability was enhanced after cold exposure when dams received succinate (Fig 4G and 4H). We also examined the adipose structure by H&E staining. Maternal succinate supplementation reduced the adipocyte sizes in iWAT and eWAT (Fig. 4I, 4J and 4K). No significant difference was observed in the expression of Cidea, Elovl3, PPAR γ and Prdm16, but maternal succinate promoted the expression of Cyto C, Ppargc1a and UCP-1 (Fig. 4L). Succinate supplementation in dams also elevated the protein levels of CYTO C, PGC1α, UCP-1, but did not affect p-38 and PRDM16 levels in female offspring (Fig. 4M and 4N).
Fig. 4.
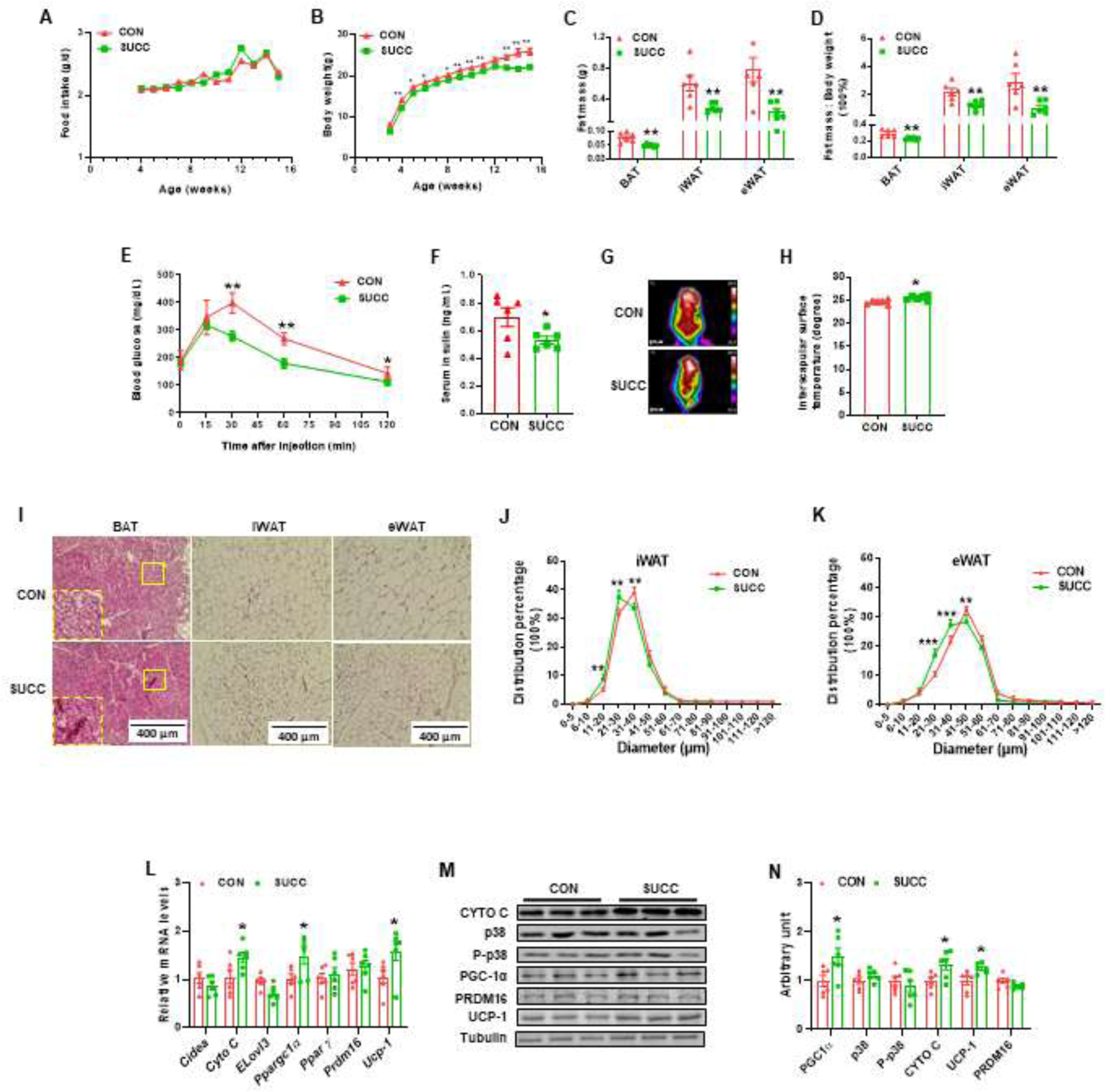
Maternal succinate supplementation prevents offspring from HFD-induced obesity and metabolic dysfunction
A, Food intake. B, Body weight gain. C, BAT and iWAT and eWAT mass. D, Fat index (the ratio of fat mass to the whole body weight) of BAT, iWAT and eWAT. E - F, Glucose tolerance test blood glucose (E) and serum insulin concentrations (F) of weaning mice challenged with HFD for 12 weeks. G - H, Infrared images (G) and interscapular surface temperature (H) of CON and SUCC offspring. I - K, Representative H&E staining for BAT, iWAT and eWAT sections (I) and percentage distribution of adipocyte diameters of iWAT (J) and eWAT (K). L, mRNA expression of brown adipocyte markers in BAT. M - N, Immunoblotting analysis for brown adipocyte markers and p38. *P < 0.05, **P < 0.01. Data are shown as means ± SEM (n = 6). Abbreviations: HFD, High fat diet.
In addition, we measured the whole-body energy expenditure. Compared with CON group, SUCC offspring showed enhanced oxygen consumption during the light phase when mice were inactive, and a trend of increase in the dark phase when mice were active (Fig. 5A and 5B). Additionally, CO2 production was higher in SUCC offspring during the light phase (Fig. 5C and 5D). Consequently, SUCC offspring had higher RER during the light phase (Fig. 5E and 5F). In addition, the SUCC group tended to have elevated heat generation during the light phase (P = 0.06) (Fig. 5G and 5H). These data showed that the basal energy consumption of SUCC mice was elevated.
Fig. 5.
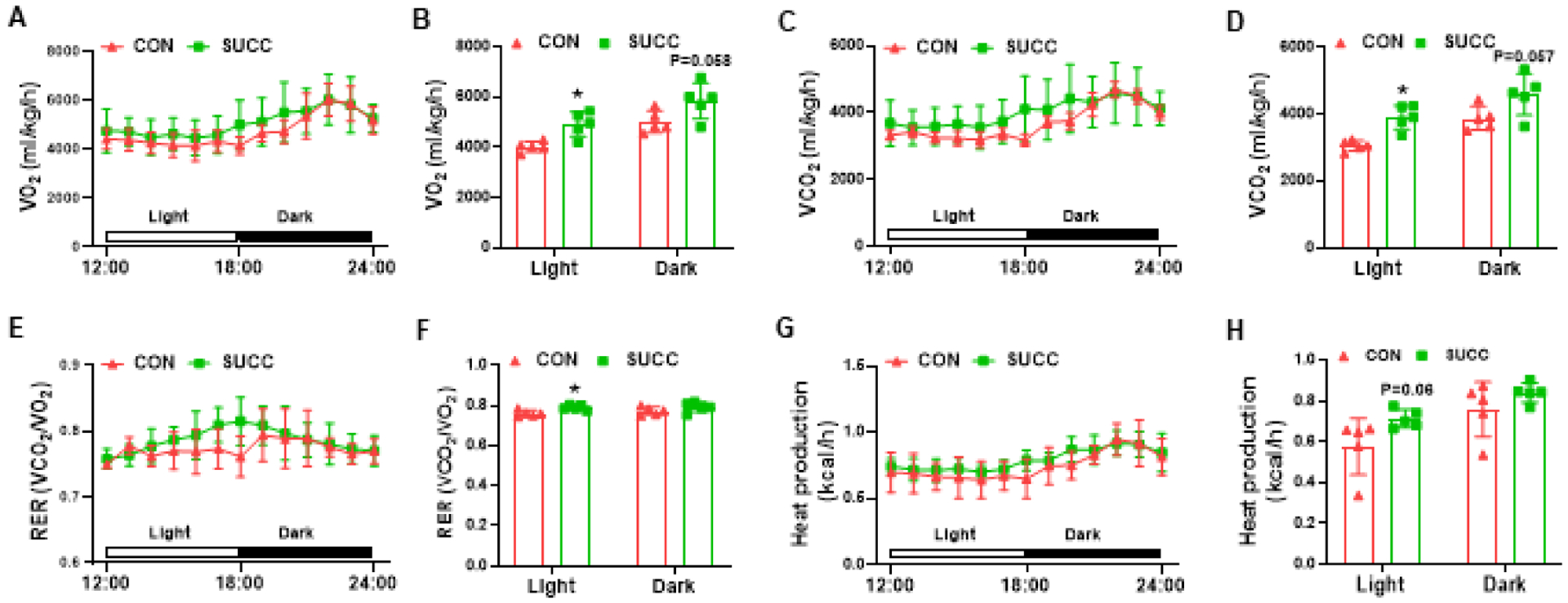
Maternal succinate supplementation enhances energy expenditure in offspring under 12-weeks of HFD challenge
A - B, Vo2 during a 6 h light - 6 h dark cycle measured in a metabolic cage (A) and the average values (B). C - D, VCO2 during a 6 h light - 6 h dark cycle (C) and the average values (D). E - F, the values of RER (ratio of VCO2 to VO2) were calculated from metabolic chamber data during a 6 h light - 6 h dark cycle. G - H, heat production during a 6 h light - 6 h dark cycle (G) and the average values (H). *P < 0.05. Data are expressed as means ± SEM (n = 6). Abbreviations: RER, respiratory exchange rate; VO2, oxygen consumption rate; VCO2, carbon dioxide production.
3.4. Succinate stimulates PGC1α expression and mitochondrial biogenesis in C3H10T1/2 cells
Because PGC1α is a key co-activator stimulating mitochondrial biogenesis, we further analyzed the direct effects of succinate in stimulating PGC1α expression. We treated C3H10T1/2 cells with a brown adipogenic cocktail supplemented with different concentrations of dimethyl-succinate, which is cell membrane permeable and converts to succinate inside cells. Based on Oil Red O staining, dimethyl-succinate addition enhanced brown adipogenic differentiation of C3H10T1/2 cells (Fig. 6A and 6B). The protein levels of CYTO C, P-p38, PGC-1α and UCP-1 tended to be higher after 8 d differentiation in cells supplemented with dimethyl-succinate (Fig. 6C and 6D). When C3H10T1/2 cells were induced brown adipogenesis, there was a tendency of elevation in the expression of Ppargc1a at day 2 (P = 0.06) and day 8 (P = 0.07) after inducing brown adipogenic differentiation in the presence of dimethyl-succinate (Fig. 6E). Because histone succinylation is positively related to histone H3K4me3 modification, a permissive epigenetic marker, we further analyzed H3K4me3 and succinyllysine enrichment in the Ppargc1a promoter. Based on ChIP-qPCR, succinate supplementation enriched H3K4me3 and succinyllysine at 1kb and 2 kb upstream of the transcription start site of the Ppargc1a promoter in C3H10T1/2 cells (Fig. 6F and 6G).
Fig. 6.
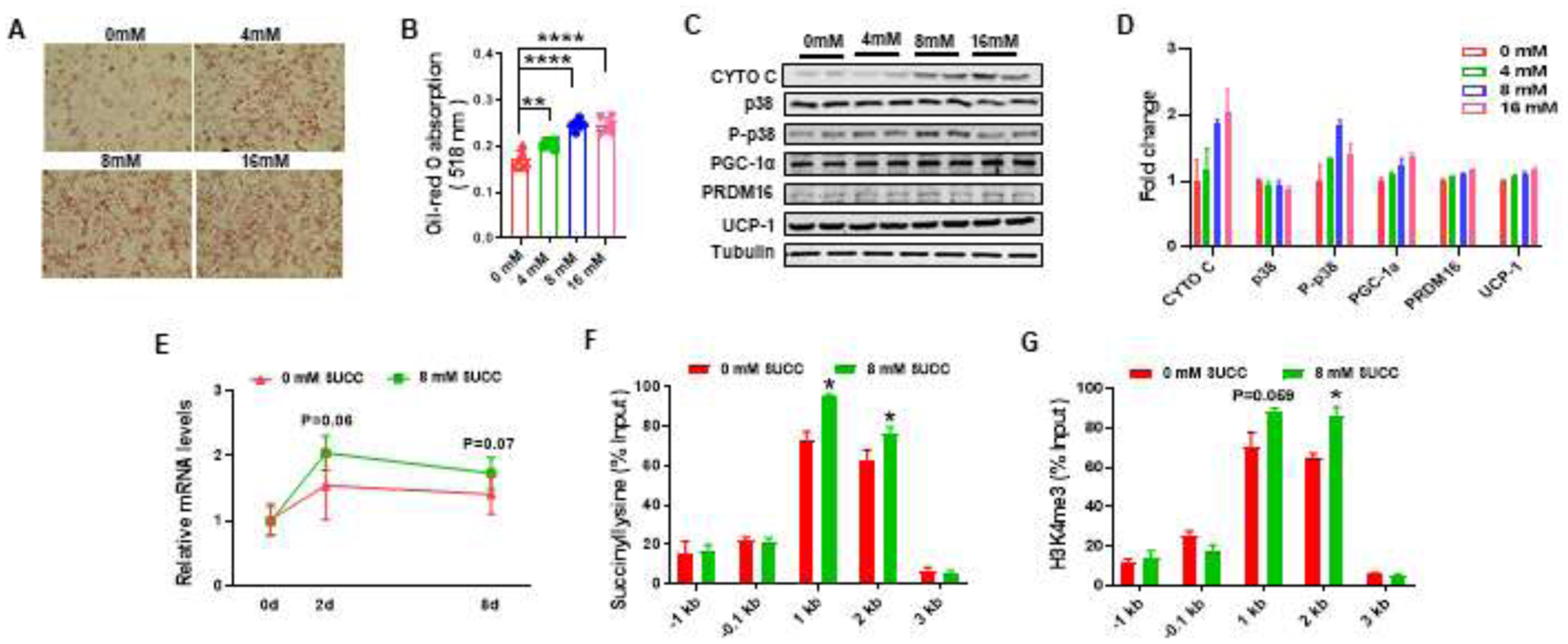
Succinate stimulates Ppargc1a expression and mitochondrial biogenesis in C3H10T1/2 cells
A - B, Oil Red O staining of C3H10T1/2 cells treated with brown adipogenic cocktail with different concentrations of dimethyl-succinate for 8 days (A), Oil Red O absorbance at 518nm (B). C - D, Immunoblotting analysis for brown adipocyte markers and p38 (C) and fold change (D). E, mRNA expression of Ppargc1a of C3H10T1/2 treated with brown adipogenesis cocktail with or without 8 mM dimethyl-succinate at day 0, 2 and 8 days. F - G, The enrichment of succinyllysine (F) and H3K4me3 (G) in the Ppargc1a promoter. *P < 0.05, **P < 0.01. ****P < 0.0001. Data are shown as means ± SEM (n = 3).
4. Discussion
Compared to white adipocytes, brown adipocytes are packed with mitochondria and also express UCP-1, which dissipates energy as heat [30]. Contrary to the common belief that BAT is only important for human neonates, new evidences show that active BAT abundantly exists in human adults [17]. Previous studies show that the fetal stage is critical for BAT development, which has long-term impacts on BAT thermogenic function in offspring [25, 26]. After birth, due to lack of shivering thermogenesis by skeletal muscle, BAT is the main site to generate heat for newborn rodents [31]. In the present study, we found that maternal succinate supplementation enhances the body temperature of female neonates, weanling and adult offspring under cold exposure, showing the enhancement of BAT function in offspring due to maternal succinate supplementation.
Previously, succinate supplementation was shown to enhance PGC-1α expression in skeletal muscle and prefrontal cortex [27, 32]. In addition, SLC13, the transporter of succinate, expresses in placenta [6]. Based on these evidences, we hypothesized that dietary succinic acid supplemented to maternal mice can be delivered to fetuses, which regulates fetal BAT development and improves long-term metabolic health of offspring by enhancing PGC-1α expression. In our study, increased PGC-1α level was observed in SUCC female offspring, including neonates, weanling and adults.
As a transcription factor, PGC-1α plays a crucial role in energy metabolism. First, PGC-1α is a key activator of mitochondrial biogenesis. PGC-1α stimulates the expression of nuclear respiratory factor 1 and 2 (NRF1 and NRF2), which regulates mtDNA replication and the expression of subunits of electron transport chain (ETC) mediators [33]. In our study, we detected that maternal succinate feeding increased the content of Cytochrome C in female offspring, a marker of mitochondrial density and one of mediators in ETC which is regulated by NRFs [34]. Moreover, PGC-1α interacts with PPARα to activate Ucp-1 expression [33]. In mitochondria, UCP-1 uncouples the proton gradient to generate heat. Accordingly, maternal succinate treatment elevated the mRNA expression and protein levels of UCP-1.
The receptor of succinate, SUCNR1, is a member of G protein-coupled receptors family [35]. Binding of succinate to SUCNR1 stimulates downstream p38 activation [9, 10]. Moreover, activated p38 phosphorylates AMP-dependent transcription factor 2 (ATF-2), leading to the recruitment of ATF-2 to cAMP response element (CRE) in the PGC-1α promoter, which stimulates Ppargc1a gene expression [36]. In the present study, we observed enhanced phosphorylation of p38 in the offspring of SUCC group, explaining the activation of PGC-1α due to succinate supplementation.
To further explore the molecular mechanism in which succinate regulates PGC-1α during the adipogenic process, C3H10T1/2 was treated with brown adipogenic cocktail supplemented with different concentrations of dimethyl-succinate, which is cell membrane permeable. Dimethyl-succinate supplementation stimulated brown adipogenesis and increased the mRNA expression of Ppargc1a. Previously, cell-permeable succinate was observed to increase the content of H3K4me3 in 293T and Hela cells [37]. In addition, succinylation is positively related with H3K4me3 marks, and negatively related with H3K27me3 marks in gene promoters [15], which is associated with Ppargc1a expression. Furthermore, knocking out of SMYD1, a histone methyltransferase, led to diminished H3K4me3 in the Ppargc1a promoter locus, which decreased the expression of Ppargc1a [38]. Accordingly, we performed ChIP-qPCR to examine enrichment of H3K4me3 and succinylation of lysine in the Ppargc1a promoter and found that dimethyl-succinate treatment increased the H3K4me3 and succinyllysine enrichment at 1k and 2k upstream of the Ppargc1a transcription start site, enabling the expression of Ppargc1a. As a key regulator of mitochondrial biogenesis and oxidative metabolism, the up-regulation of Ppargc1a explains the enhancement of offspring BAT development in SUCC mice.
In conclusion, succinate supplementation during gestation and lactation increases the expression of PGC-1α and enhances fetal BAT development, which demonstrates long-term effects on BAT thermogenesis in female offspring. Considering that succinate is widely present in foods, effectively absorbed, and succinylation is regulated by a non-enzymatic mechanism [39, 40], our findings provide an important guideline for maternal succinate intake during the pregnancy and lactation in order to improve fetal development and the metabolic health of future generations.
Funding
This work was supported by National Institutes of Health Grant R01-HD067449.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no conflict of interest.
References
- [1].Featherstone SJAccic, related processes. Microbiology P, HACCP, Ingredients. Ingredients used in the preparation of canned foods. 2015:147–211.
- [2].De Klerk J-L. Succinic acid production by wine yeasts: Stellenbosch: University of Stellenbosch; 2010. [Google Scholar]
- [3].Zeikus J, Jain M, Elankovan PJAm, biotechnology. Biotechnology of succinic acid production and markets for derived industrial products. 1999;51:545–52. [Google Scholar]
- [4].Martinez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nature communications. 2020;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Castro Fonseca M, Aguiar CJ, da Rocha Franco JA, Gingold RN, Leite MF. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun Signal. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pajor AM. Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch. 2006;451:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krzak G, Willis CM, Smith JA, Pluchino S, Peruzzotti-Jametti L. Succinate Receptor 1: An Emerging Regulator of Myeloid Cell Function in Inflammation. Trends Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- [8].Macias-Ceja DC, Ortiz-Masia D, Salvador P, Gisbert-Ferrandiz L, Hernandez C, Hausmann M, et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019;12:178–87. [DOI] [PubMed] [Google Scholar]
- [9].Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol. 2009;20:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu J, Li T, Du S, Chen Y, Wang S, Xiong F, et al. The MAPK signaling pathway mediates the GPR91-dependent release of VEGF from RGC-5 cells. Int J Mol Med. 2015;36:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ko SH, Choi GE, Oh JY, Lee HJ, Kim JS, Chae CW, et al. Succinate promotes stem cell migration through the GPR91-dependent regulation of DRP1-mediated mitochondrial fission. Scientific reports. 2017;7:12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sundstrom L, Greasley PJ, Engberg S, Wallander M, Ryberg E. Succinate receptor GPR91, a Galpha(i) coupled receptor that increases intracellular calcium concentrations through PLCbeta. FEBS letters. 2013;587:2399–404. [DOI] [PubMed] [Google Scholar]
- [13].Alleyn M, Breitzig M, Lockey R, Kolliputi N. The dawn of succinylation: a posttranslational modification. Am J Physiol Cell Physiol. 2018;314:C228–C32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Y, Gibson GE. Succinylation Links Metabolism to Protein Functions. Neurochem Res. 2019;44:2346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smestad J, Erber L, Chen Y, Maher LJ 3rd. Chromatin Succinylation Correlates with Active Gene Expression and Is Perturbed by Defective TCA Cycle Metabolism. iScience. 2018;2:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Betz MJ, Enerback S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol. 2018;14:77–87. [DOI] [PubMed] [Google Scholar]
- [17].Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, et al. Thermogenic activity of UCP-1 in human white fat-derived beige adipocytes. Molecular endocrinology. 2015;29:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:8649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nature reviews Molecular cell biology. 2016;17:480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. [DOI] [PubMed] [Google Scholar]
- [21].Gureev AP, Shaforostova EA, Popov VN. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1alpha Signaling Pathways. Front Genet. 2019;10:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell reports. 2019;26:222–35 e5. [DOI] [PubMed] [Google Scholar]
- [24].Schulz TJ, Tseng YH. Brown adipose tissue: development, metabolism and beyond. The Biochemical journal. 2013;453:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, et al. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell metabolism. 2016;24:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Son JS, Zhao L, Chen Y, Chen K, Chae SA, de Avila JM, et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv. 2020;6:eaaz0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang T, Xu YQ, Yuan YX, Xu PW, Zhang C, Li F, et al. Succinate induces skeletal muscle fiber remodeling via SUNCR1 signaling. EMBO Rep. 2019;20:e47892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhao L, Son JS, Wang B, Tian Q, Chen Y, Liu X, et al. Retinoic acid signalling in fibro/adipogenic progenitors robustly enhances muscle regeneration. EBioMedicine. 2020;60:103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- [30].Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- [31].Lidell ME. Brown Adipose Tissue in Human Infants. Handb Exp Pharmacol. 2019;251:107–23. [DOI] [PubMed] [Google Scholar]
- [32].Shakova F, Kirova Y, Germanova E, Romanova G. The Role of Ethylmethylhydroxypyridine Succinate in the Induction of PGC-1 alpha in Experimental Ischemic Damage of the Prefrontal Cortex. STROKE: LIPPINCOTT WILLIAMS & WILKINS TWO COMMERCE SQ, 2001 MARKET ST, PHILADELPHIA …; 2020. [Google Scholar]
- [33].Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–51. [DOI] [PubMed] [Google Scholar]
- [34].Virbasius JV, Virbasius CA, Scarpulla RC. Identity of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes & development. 1993;7:380–92. [DOI] [PubMed] [Google Scholar]
- [35].Gilissen J, Jouret F, Pirotte B, Hanson J. Insight into SUCNR1 (GPR91) structure and function. Pharmacol Ther. 2016;159:56–65. [DOI] [PubMed] [Google Scholar]
- [36].Krämer AI, Handschin CJIjoms. How Epigenetic Modifications Drive the Expression and Mediate the Action of PGC-1α in the Regulation of Metabolism. 2019;20:5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes & development. 2012;26:1326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Warren JS, Tracy CM, Miller MR, Makaju A, Szulik MW, Oka SI, et al. Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:E7871–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Simithy J, Sidoli S, Yuan ZF, Coradin M, Bhanu NV, Marchione DM, et al. Characterization of histone acylations links chromatin modifications with metabolism. Nature communications. 2017;8:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wagner GR, Bhatt DP, O’Connell TM, Thompson JW, Dubois LG, Backos DS, et al. A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation. Cell metabolism. 2017;25:823–37 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


