Abstract
It is generally believed that the efficacy of cochlear implants is partly dependent on the condition of the stimulated neural population. Cochlear pathology is likely to affect the manner in which neurons respond to electrical stimulation, potentially resulting in differences in perception of electrical stimuli across cochlear implant recipients and across the electrode array in individual cochlear implant users. Several psychophysical and electrophysiological measures have been shown to predict cochlear health in animals and were used to assess conditions near individual stimulation sites in humans. In this study we examined the relationship between psychophysical strength-duration functions and spiral ganglion neuron density in two groups of guinea pigs with cochlear implants who had minimally-overlapping cochlear health profiles. One group was implanted in a hearing ear (N = 10) and the other group was deafened by cochlear perfusion of neomycin, inoculated with an adeno-associated viral vector with an Ntf3-gene insert (AAV.Ntf3) and implanted (N = 14). Psychophysically measured strength-duration functions for both monopolar and tripolar electrode configurations were then compared for the two treatment groups. Results were also compared to their histological outcomes. Overall, there were considerable differences between the two treatment groups in terms of their psychophysical performance as well as the relation between their functional performance and histological data. Animals in the neomycin-deafened, neurotrophin-treated, and implanted group (NNI) exhibited steeper strength-duration function slopes; slopes were positively correlated with SGN density (steeper slopes in animals that had higher SGN densities). In comparison, the implanted hearing (IH) group had shallower slopes and there was no relation between slopes and spiral ganglion density. Across all animals, slopes were negatively correlated with ensemble spontaneous activity levels (shallower slopes with higher ensemble spontaneous activity levels). We hypothesize that differences in strength-duration function slopes between the two treatment groups were related to the condition of the inner hair cells, which generate spontaneous activity that could affect the across-fiber synchrony and/or the size of the population of neural elements responding to electrical stimulation. In addition, it is likely that spiral ganglion neuron peripheral processes were present in the IH group, which could affect membrane properties of the stimulated neurons. Results suggest that the two treatment groups exhibited distinct patterns of variation in conditions near the stimulating electrodes that altered detection thresholds. Overall, the results of this study suggest a complex relationship between psychophysical detection thresholds for cochlear implant stimulation and nerve survival in the implanted cochlea. This relationship seems to depend on the characteristics of the electrical stimulus, the electrode configuration, and other biological features of the implanted cochlea such as the condition of the inner hair cells and the peripheral processes.
Keywords: Cochlear implant, cochlear health, electrode configuration, spiral ganglion neurons, inner hair cells, strength-duration functions
1. Introduction
Long-term functional and structural alterations occur in the auditory system in conjunction with sensorineural hearing loss (Leake and Hradek, 1988; Ryugo et al., 1998; Shepherd and Hardie, 2001; Lee et al., 2003; Shepherd et al., 2004; Butler and Lomber, 2013). Animal studies have demonstrated considerable cochlear hair cell and/or auditory nerve fiber degeneration. Both acute and progressive changes have been reported in the spiral ganglion neurons (SGNs); these include demyelination of the soma with axonal preservation, loss or demyelination of the peripheral processes, and loss of the SGNs (Spoendlin, 1975; Nadol, 1997; Shepherd and Hardie, 2001). Additionally, the central axons of the auditory nerve can be susceptible to atrophic changes with prolonged auditory deprivation (Leake and Hradek, 1988; Nadol, 1997; Wise et al., 2017).
The cochlear implant (CI) is an effective management approach for bilateral sensorineural loss and is considered as one of the greatest achievements in modern medicine. Cochlear health is believed to be crucial for the implant function. Specifically, functionality of the CI is based on the assumption that there should be healthy and functional populations of SGNs near the electrode contacts in order to carry the electrical stimulation and information effectively to higher processing centers in the auditory pathway (Pfingst et al., 1981, Shepherd and Javel, 1997). Degenerative changes in the organ of Corti can result in variable degrees of neural preservation across the implanted electrode array leading to differences in sensitivity to electrical stimulation such that not all sites are equally optimal for electrical stimulation.
Earlier studies from our laboratories and others demonstrated that functional measures vary appreciably across sites of stimulation providing converging evidence for the effect of local conditions near the stimulating electrodes on the implant function (Pfingst and Xu, 2004; Pfingst et al., 2004; 2008; Bierer, 2007; Garadat and Pfingst, 2011; Cosentino et al., 2016; Carlyon et al., 2018). It has been proposed that across-site differences can be minimized by either improving the biology of the inner ear through novel surgical and tissue engineering techniques or by modifying the strategies for electrical stimulation of the sub-optimal sites (Pfingst and Xu, 2004; Bierer and Faulkner, 2010; Garadat et al., 2012; Zhou and Pfingst, 2012; Pfingst et al., 2015; Goehring et al., 2019). The former approach has not yet been achieved in human patients; whereas the latter is more readily feasible but requires sensitive measures of variations in cochlear health. Extensive efforts have been made to estimate variations in cochlear health in the vicinity of the implanted electrodes using various behavioral and electrophysiological noninvasive measures (e.g. Bierer, 2007; Ramekers et al., 2014; Pfingst et al., 2015; Abbas et al., 2017; Jahn and Arenberg, 2019; Mesnildrey et al., 2020; Schvartz-Leyzac et al., 2020). Yet, the underlying mechanisms for variability in implant function in relation to local irregularities are not completely understood.
One potential measure that can be used to assess cochlear health is the response threshold to changes in pulse phase duration. The relation between threshold current and phase duration is often referred to as a strength-duration function which is considered as a measure of the excitation properties of neural tissue (Abbas and Miller, 2004). Several studies have demonstrated that strength-duration functions can be predictive of variations in cochlear health (Miller et al., 1995a, b; Prado-Guitierrez et al., 2006; Ramekers et al., 2014). The characteristics of strength-duration functions can additionally be affected by degenerative changes in the auditory nerve fibers. For example, it has been shown that loss of the myelin sheath can result in increased membrane capacitance (Tasaki, 1955) and that loss of the peripheral processes can shift the site of action potential initiation to more central axonal regions of the cell that exhibit larger diameter and heavier myelination (Javel and Shepherd, 2000). These structural changes can lead to complex alterations in the nerve excitability which can be reflected by the properties of strength-duration functions.
There is also evidence that sensitivity to phase duration can be altered by mode of stimulation such that monopolar (MP) stimulation can result in steeper slopes than are obtained for tripolar (TP) stimulation (Smith and Finley, 1997; Miller et al., 1999; Chatterjee et al., 2006; Chatterjee and Kulkarni, 2014). Generally, factors underlying differences in phase duration sensitivity in relation to mode of stimulation are not well understood. However, it has been hypothesized that by narrowing the spread of the electrical field as in TP stimulation, there is a stronger contribution from surviving peripheral processes and alternatively by broadening the electrical field as in MP stimulation, there is more contribution from distant central axons thus shifting the site of excitation to the more distant central processes (Miller et al., 2003; Chatterjee and Kulkarni, 2014).
The purpose of the current study was to determine whether psychophysical strength-duration functions can serve as a measure to assess cochlear health. Effects of variations in cochlear health and electrode configuration (MP, TP) on sensitivity to changes in pulse phase duration were examined in two groups of cochlear implanted guinea pigs with different cochlear-health profiles. Slopes of strength-duration functions were compared for the two treatment groups in relation to their SGN density and ensemble spontaneous activity (ESA) data using both electrode configurations. It was hypothesized that the two treatment groups would exhibit marked differences in their strength-duration functions depending on the electrode configuration and their cochlear health.
2. Materials and Methods
2.1. Overview
Data reported in this manuscript were obtained as part of a series of studies conducted in psychophysically trained guinea pigs over periods ranging from 7 to 26 months after implantation. The general strategy in these studies was to train and implant animals, follow functional measures over time until stable, then run a variety of experiments with different measures, and finally, euthanize the animals and perform histological analyses. In this study, data from fourteen animals was reported in earlier experiments (Pfingst et al., 2017; Swiderski et al., 2020; Schvartz-Leyzac et al., 2019). Animals were adult male guinea pigs bred and maintained by the Unit for Laboratory Animal Medicine at the University of Michigan. The animal use protocol was reviewed and approved by the University of Michigan Institutional Animal Care and Use Committee. Veterinary care and animal husbandry were provided by the Unit for Laboratory Animal Medicine in facilities certified by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
All Animals were trained using positive reinforcement operant conditioning techniques to perform a stimulus-detection task. Once training was achieved, animals were implanted in one ear with an animal CI electrode array obtained from Cochlear™. Following implantation, psychophysical detection thresholds for electrical stimulation were followed over time until ears stabilized from surgical trauma and treatment (stabilization criteria were described in Kang et al., 2010). Once stabilization was achieved, the phase duration data were collected. Following completion of all data collection, ESA data were measured, and animals euthanized and processed for histological analysis.
2.2. Subjects and treatment groups
Twenty-four specific pathogen-free pigmented guinea pigs weighing between 1 and 1.5 kg at the time of implantation served as subjects in these experiments. All animals were maintained on a healthy calorie-restricted diet to facilitate operant psychophysical training and testing using food reinforcement. Animals had free access to water, but were fed only once a day, after each test session in order to keep them motivated to complete the required behavioral task. Animals were trained to perform a detection threshold task with acoustic stimuli. Once trained, they were deafened in one ear in order to control for the assessment of any residual hearing in the treatment ear under free-field conditions. Baseline psychophysical acoustic thresholds were then collected for the contralateral ear. After baseline acoustic thresholds were measured for all animals, they were placed in one of two experimental groups.
Both groups were implanted in the remaining ear, but with two different protocols. The first group consisted of ten animals that were implanted in the non-deafened ear (hearing ear). Animals in this group demonstrated residual hearing and high SGN survival in the area of the implant and/or apical to it. The second group consisted of fourteen animals that received a cochlear injection of 5% w/v neomycin sulfate solution, to deafen the treatment ear, followed by cochlear inoculation with an adeno-associated or adeno- virus with a neurotrophin gene insert (AAV.Ntf3, AAV.BDNF, AAV.Ntf3/BDNF or Ad.BDNF + dexamethasone) and then a cochlear implant. The neurotrophin inoculation typically resulted in reduced degeneration of SGN neurons in the deafened ears (Pfingst et al., 2017) and also regrowth of peripheral neurites into the basilar membrane area (see Shibata et al., 2011; Budenz et al., 2012); this in theory would minimize the secondary SGN degeneration process that occurs in neomycin-deafened guinea pigs at a much more rapid rate than that in humans (Nadol et al., 2001; Fransson & Ulfendahl, 2017) which would allow us to assess the functional effect of SGN in the absence of IHCs. For simplicity, we will hereafter refer to the first group that received the implant in a hearing ear as the implanted-hearing (IH) group and the second group that was injected with neomycin, inoculated with viral-mediated neurotrophin, and implanted as the neomycin + neurotrophin + implant (NNI) group.
2.3. Deafening, inoculation, and implantation procedures
All surgical techniques were performed under ketamine (40 mg/kg) and xylazine (10 mg/kg) anesthesia; these were previously described in detail (see Pfingst et al., 2017) and are summarized here. In general, the cochlea was accessed by exposing the temporal bone through a post-auricular incision followed by opening the bulla. For ears that were deafened, neomycin sulfate solution (10 μL, 5% w/v) in sterile water was administered to contralateral non-implanted ears slowly through the round window with a syringe and needle, and to implanted treated ears through a small hand drilled cochleostomy in the basal turn of the cochlea with a cannulated syringe and infusion pump at a rate of 5 μl/min. For animals receiving neurotrophin gene therapy, ears were inoculated, 20 minutes after deafening, through the same small cochleostomy with 5 μl neurotrophin using a new cannula and infusion pump at a rate of 1 μl/min. In this NNI group, half the animals were implanted 30 minutes after neurotrophin inoculation and half were implanted 2 – 4 weeks later. Delayed implant insertions were an attempt to improve neurotrophin uptake through decreased displacement of the neurotrophin solution at the time of inoculation surgery. It is important to note that these group differences in time interval between inoculation and implantation did not affect any of the physiological or psychophysical data reported in this study.
Prior to cochlear implantation, an inverted bolt, which we call the “anchor bolt”, was secured to the skull on the bregma with three screws and methyl methacrylate; this anchor bolt was used to secure the implant connector to the skull and as a ground reference for the ESA measurements. Following placement of the anchor bolt, all animals were implanted unilaterally by inserting the implant into the scala tympani through a cochleostomy created (IH group) or enlarged (NNI group) with a diamond bur. Implants (purchased from Cochlear™) consisted of eight banded (N = 14) or half-banded (N = 10) electrodes surrounding a silicone rubber carrier and spaced at approximately 0.75 mm center to center. Implant insertion depths ranged from 5 – 6 electrodes in the basal turn (banded) to all 8 electrodes throughout the first turn (half-banded). In these experiments, the primary electrode for stimulation and recording was located anywhere from 2.2 – 6.1 mm (banded averaged 2.9 mm and half-banded averaged 5.1 mm) apical to the cochleostomy. Specifically, the second most apical electrode was the default primary intracochlear electrode. If that electrode failed the third or the fourth electrode was used; this was used only in two subjects. All electrodes were implanted in the basal turn of the scala tympani.
2.4. Psychophysical procedures
These procedures were previously described in detail (see Pfingst et al., 2017) and are summarized here. All psychophysical testing was completed in custom built wire-mesh test cages located inside sound-attenuating chambers manufactured by Acoustic Systems (Model RF Shielded Animal Test Chambers), Industrial Acoustics Corporation (Model 1201-A) and Tracoustics (Models 240-B and 240-C). The cages were equipped with a response button, feeder, water, and restraint system. Animals were trained to respond to both acoustic and electrical stimuli using a positive reinforcement-operant conditioning paradigm. Stimulus generation and delivery for all psychophysical testing were controlled by a locally written program on personal computers.
To initiate a trial, animals were trained to depress a button for a variable observation period of 1 to 6 sec and to release it once an acoustic signal was detected. The training stimulus was a 2-kHz tone presented in 200-ms bursts separated by 100-ms intervals. Animals were rewarded with a food pellet for only correct responses (releases of the button within 1 sec of stimulus onset). Once animals responded reliably at a moderate intensity level, they were trained to respond to lower stimulus levels. The psychometric functions were obtained using 10 to 20 trials per stimulus level; these stimuli were presented in a random order. In addition, a sham trial with an inaudible stimulus was presented to determine the guess rate; data was only accepted if this trial was ≤20 %. Threshold was defined as the level at which the subject responded correctly on 50% of the trials; three measurements were obtained for each condition in order to increase test-retest reliability. Acoustic psychophysical threshold measurements were conducted periodically throughout the experiment for all implanted animals with residual acoustic hearing using pure tone stimuli at 50, 100, 250, 500, 1 k, 2 k, 8 k, 16 k, and 24 kHz.
After implantation, animals were tested with electrical stimulation using the same procedure. Electrical thresholds for 25-μs 500-pps 200-ms-duration biphasic pulse trains were followed overtime until post-surgical conditions in the cochlea and perception with the implant stabilized (Pfingst et al., 2015). After stabilization, the phase duration data were collected for MP and TP functions. To obtain each animal’s strength-duration functions, psychophysical thresholds were measured for 200-msec trains of negative-leading biphasic pulses at 100 pps using both MP and TP configurations. Phase durations ranged from 25 to 5000 μs. Three repeated measures were obtained for each stimulus and the average of these three thresholds is reported in this paper. Electrical stimuli were delivered by a custom-built voltage controlled constant current source.
2.5. Measurement of spontaneous activity
To estimate the level of spontaneous activity in the auditory nerve, ESA was recorded and analyzed as previously reported by Dolan et al. (1990). Animals were tested at specified time intervals post implantation. ESA measurements described in this paper were obtained on the same day or slightly before the day the animals were euthanized for histology. Measurements were made under ketamine (40 mg/kg) and xylazine (10 mg/kg) in a sound-attenuating booth (Acoustic Systems model AS02893). The electrical potentials were recorded from the primary intracochlear electrode ground to the anchor bolt.
The recorded electrical potentials were filtered from 0.3 to 3 kHz, amplified with a gain of 10,000, and transmitted to a spectrum analyzer with a sample rate of 256 kHz (SR760, Stanford Research Systems, Sunnyvale, CA, USA). The frequency span of the SR760 was from zero to 12.5 kHz which resulted in an analysis time record of 32- ms. Each record was analyzed with a fast Fourier transform (FFT) using Blackman-Harris windowing. The resultant FFT had a frequency resolution of 31.25 Hz; one hundred fifty FFTs were acquired and averaged (linear, RMS averaging). Response levels (dB re 100 μV peak) were obtained for each animal for the frequency range from 593.75 to 1312.5 Hz and an across frequency average was calculated to facilitate comparison across animals with varying cochlear health. The presence of spontaneous neural activity was indicated by a spectral peak typically centered around 900 Hz. Waveforms from the SR760 were retrieved, stored, and processed using a local MATLAB™ script.
2.6. Histological procedures
Following the completion of psychophysical and ESA data collection guinea pigs were euthanized with a 1-mL intraperitoneal injection of sodium pentobarbital (Vortech Pharmaceuticals, Dearborn, MI, USA) and perfused intravascularly with either 4% paraformaldehyde (N = 16) or 2 % glutaraldehyde in 3 % cacodylate buffer (N = 8). Temporal bones of the implanted ears were extracted with the implant in place.
For 10 of the animals, cochleae were decalcified in 5% EDTA with 0.25% glutaraldehyde until the bone was sufficiently soft for sectioning and the CI electrodes became visible through the bone. Then, the cochleae were marked in the lateral wall at the location of the primary intracochlear electrode, the implant was gently extracted, and the cochleae were embedded in JB-4 resin (Electron Microscopy Sciences, Hatfield, PA, USA). For 14 animals, cochleae were dissected for whole mounts. Without prior decalcification, bone was carefully removed from the lateral wall of the cochlea and the location of the primary electrode was noted before removal of the implant and the organ of Corti. For 11 of these animals, the modiolus was also removed, decalcified in 5% EDTA with 0.25% glutaraldehyde, and embedded in JB-4 resin. Of the 24 animals, only 18 had usable histological data for both SGN density and IHC assessment (IH = 7; NNI = 11).
Embedded tissues were sectioned at 3 μm with a glass knife in the peri-midmodiolar plain at the location of the primary intracochlear electrode which provided views of the six profiles of Rosenthal’s canal that were labeled “A” through “F” as shown in the left panel of Fig. 3. Five sections were selected for SGN counting with the first section being closest to the mark or noted distance of the primary electrode. If that section was damaged, the next closest section was used. In order to prevent double counting, four other sections were additionally selected at intervals separated by a minimum of six sections (for further details see Kang et al., 2010 and Pfingst et al., 2017). The five selected sections were stained with toluidine blue and the SGN cell bodies in profiles A, B, C, and D were counted if they had a 12–25 μm diameter and a nucleus diameter of 5–9 μm. SGN survival was estimated from the spiral ganglion packing density which was derived by dividing the number of cells counted by the cross-sectional area of Rosenthal’s canal (cells/mm2) which was calculated using an ImageJ computerized image-analysis system. IHC survival was assessed in peri-midmodiolar sections and counted in whole mount tissues which are described more thoroughly in Pfingst et al. 2017 and Schvartz-Leyzac et al. 2019 respectively.
Fig. 3:
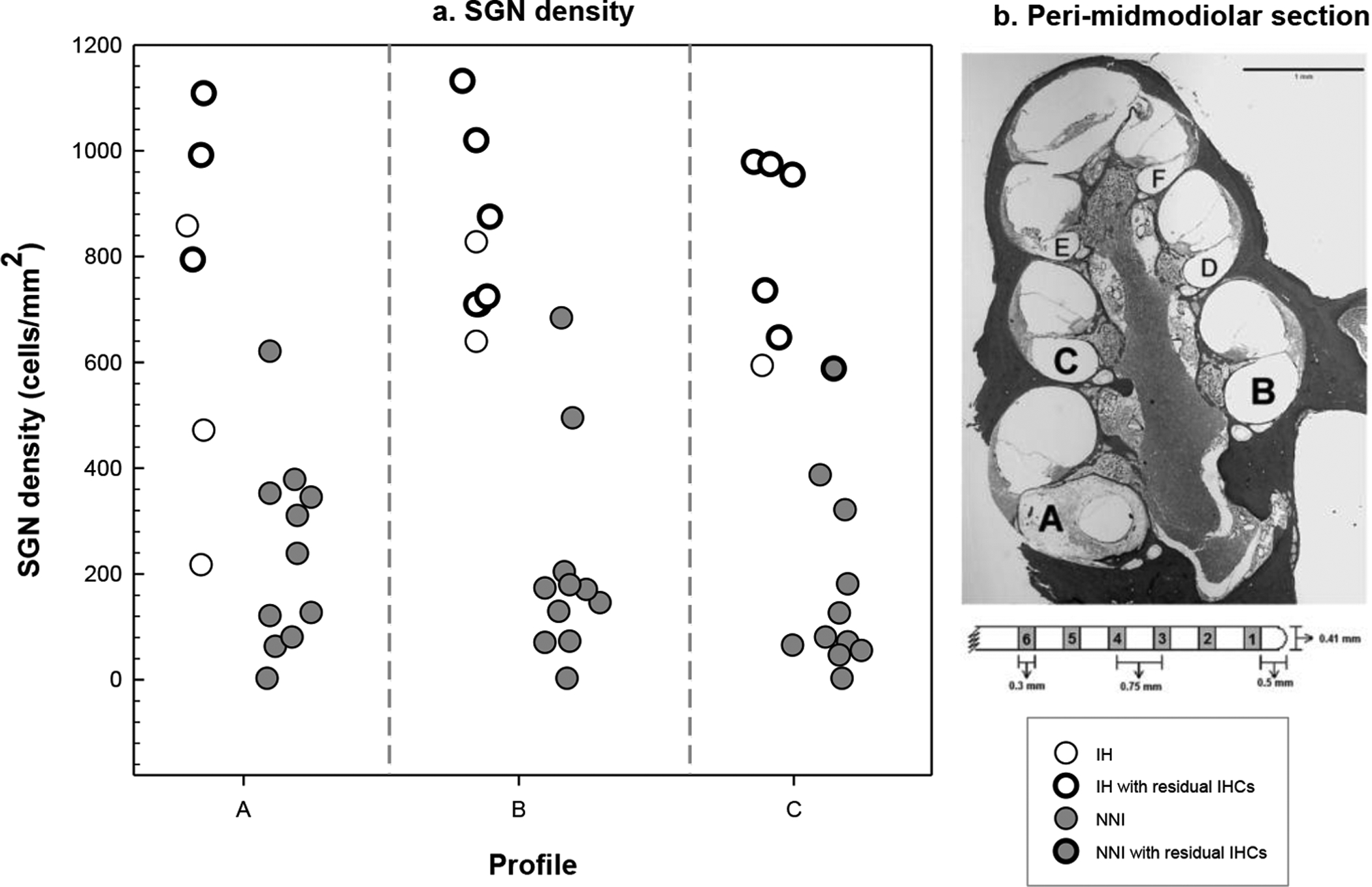
a) Density of SGN (profiles A, B, and C) are displayed in the left panel for the two groups. Unfilled symbols represent SGN density for the IH group (N = 7) and filled symbols represent those for the NNI group (N = 11). Bold symbols represent animals with residual IHC survival. Symbols are jittered across the x-axis to avoid overlap. b) The right panel displays a peri-midmodiolar section of a guinea pig cochlea demonstrating the labeling of Rosenthal’s canal for reporting histological results (Pfingst et al., 2017). The profiles are labeled as A through F in basal to apical direction. An eight-electrode array was used. The primary electrode for stimulation was electrode 2. This section was taken at the location of the primary electrode used for stimulation which was located in the vicinity of profile A for banded electrodes and profile B for half-banded electrodes. In this particular example, the implant location can be seen from the circular tract left in the tissue in profile A.
3. Results
As a general approach, psychophysical and histological data were compared for the two treatment groups (IH and NNI). In presenting the results of this study, we first compared treatment-group differences on measures of strength-duration functions, then we showed the results of their histological analyses, and finally we examined the relation of strength-duration functions to hair cells and SGN survival.
3.1. Thresholds
In this study, thresholds were not related to the implant insertion depth (p>0.05). Given this finding, all threshold data were then subjected to a mixed-design analysis of variance (ANOVA) with two within-subject factors: electrode configuration (MP, TP) and phase duration (25, 50, 75, 150, 300, 625, 1250, 2500, 5000 μs) and one between-subject factor: treatment group (IH, NNI). Mauchly’s test indicated that the assumption of sphericity had been violated for the within-subject variable of phase duration (χ2(35) = 281.078, p< 0.001) and for the interaction of electrode configuration by phase duration (χ2(35) = 170.427, p< 0.001). Therefore, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = 0.19 for duration and 0.29 for configuration by duration). In general, electrode configuration (F(1, 22) = 152.818, p < 0.0001, ηp2 = 0.874), stimulus phase duration (F(1.5, 34.2) = 378.935, p < 0.0001, ηp2 = 0.945), and treatment group (F(1, 22) = 11.627, p < 0.005, ηp2 = 0.346) all significantly affected psychophysical detection thresholds for electrical stimulation, and there were interactions among these variables. A summary of these results is shown in Fig. 1 and Table 1. As shown in the left panel of Fig. 1, results overall demonstrated differences between MP and TP electrode configurations with lower thresholds for MP configuration (−45.2±0.9) than those for TP configuration (−31.4±1.1). MP thresholds decreased more rapidly with increasing phase duration leading to large differences at long duration; this was evidenced by the significant interaction between electrode configuration and phase duration (F(2.333, 51.326) = 78.856, p < 0.0001, ηp2 = 0.782).
Fig. 1:
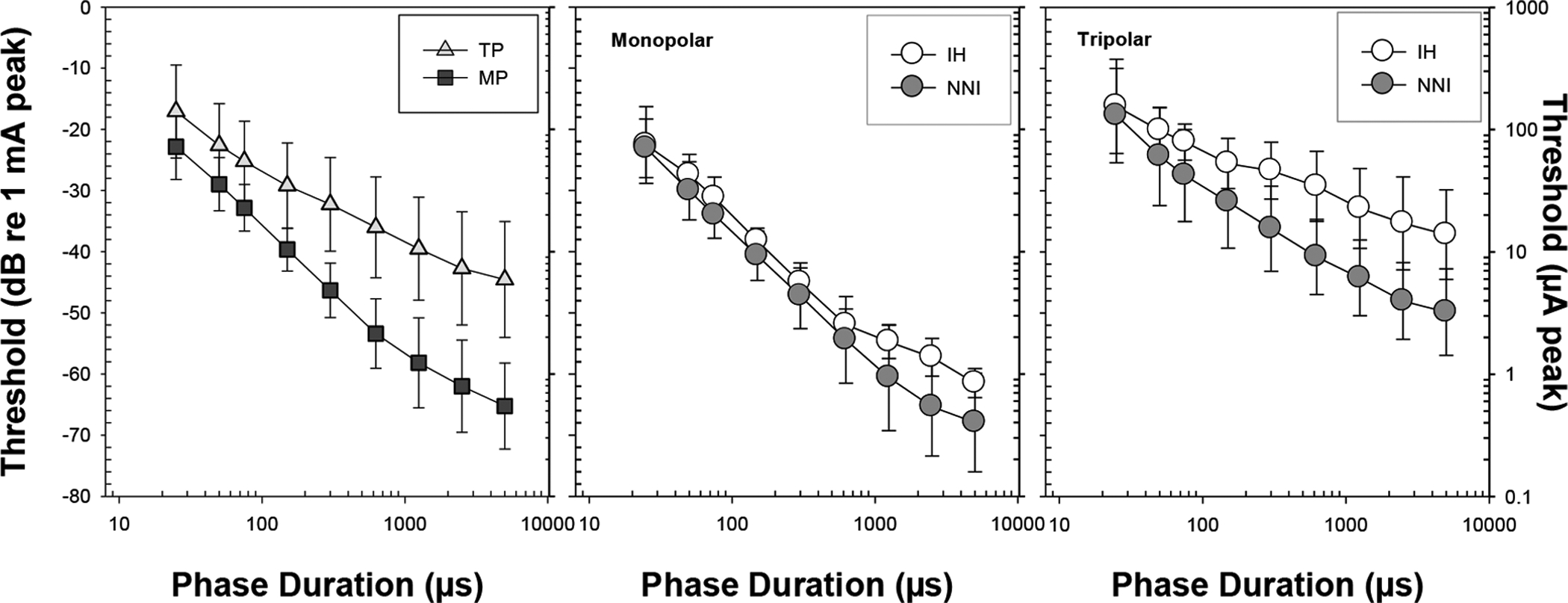
Average thresholds for all animals for the different phase durations (±1SD) are shown for each electrode configuration (left panel) with the main effect of treatment group collapsed. Group average thresholds (±1SD) are shown for MP (middle panel) and TP (right panel) electrode configurations; the open symbols represent the IH group (n=10) and the filled symbols represent the NNI treatment group (n=14).
Table 1.
Summary of statistical results.
| Variable | Significance | Finding |
|---|---|---|
| Phase duration | P< 0.0001 | Increasing phase duration leads to lower thresholds |
| Electrode configuration (TP vs MP) | P< 0.0001 | Thresholds are lower for MP than for TP stimulation |
| Treatment group (IH vs NNI) | p< 0.005 | Overall thresholds were higher for IH animals than for NNI animals |
| Phase duration and treatment group | p< 0.001 | Effect of treatment on thresholds was more robust using long phase durations. |
| Electrode configuration and treatment group | p= 0.048 | Group threshold differences were more robust using TP electrode configuration |
| Electrode configuration, phase duration, and treatment group | p< 0.001 | Effect of treatment on psychophysical thresholds was greater for TP stimulation using longer phase durations |
Additionally, there were differences in thresholds between the two treatment groups that were dependent on electrode configuration (Fig. 1, middle and right panels). Group differences were more evident using TP electrode configuration but were less noticeable when MP configuration was used. This was suggested by the significant interaction between electrode configuration and treatment group (F(1, 22) = 4.388, p= 0.048, ηp2= 0.166). Post hoc independent t-tests revealed that there were larger group differences between the IH group (−27.3±8.7) and the NNI group (−35.6±12.5) using TP stimulation (p<0.0001). In comparison, differences between the IH group (−43.3±13.7) and the NNI group ((−47.03±16.5) were not significant in the MP configuration (p=0.078). Interestingly, these group differences were also dependent on phase duration as suggested by a significant three-way interaction among the three factors (F(8, 22) = 3.803, p< 0.001, ηp2 = 0.147). Post hoc t-tests with Bonferroni’s correction for multiple comparisons revealed that group differences in TP configuration were only significant (p<0.03) for phase durations ≥300 μs. Overall, these results suggest that differences in the treatment groups were only revealed when focused stimulation and long phase durations were used.
3.2. Strength-Duration Function Slopes
Analyses further showed that electrode configuration and treatment group also affected strength-duration function slopes (shown in Fig. 2). In this study, slopes of the strength-duration functions in dB/doubling of phase duration were calculated using the least-squares linear regressions. Data were subjected to a one-way repeated measure ANOVA with electrode configuration as the within-subject factor and treatment group (IH, NNI) as the between-subject factor. As shown in Fig. 2 (left panel), results indicated that main effect of within subject factor was significant (F(1, 22) = 103.625, p< 0.0001) such that slopes were steeper for MP configuration (−7±1.4 dB/ doubling) than those obtained for TP configuration (−4.4±1.6 dB/ doubling). Additionally, main effect of between-subject factor was significant (F(1, 22) = 8.6, p< 0.01); specifically, slopes were steeper for the NNI group (−6.3±0.31 dB/ doubling) than those obtained in the IH group (−4.9±0.37 dB/ doubling); these differences can be seen in Fig. 2 (right panel).
Fig. 2:
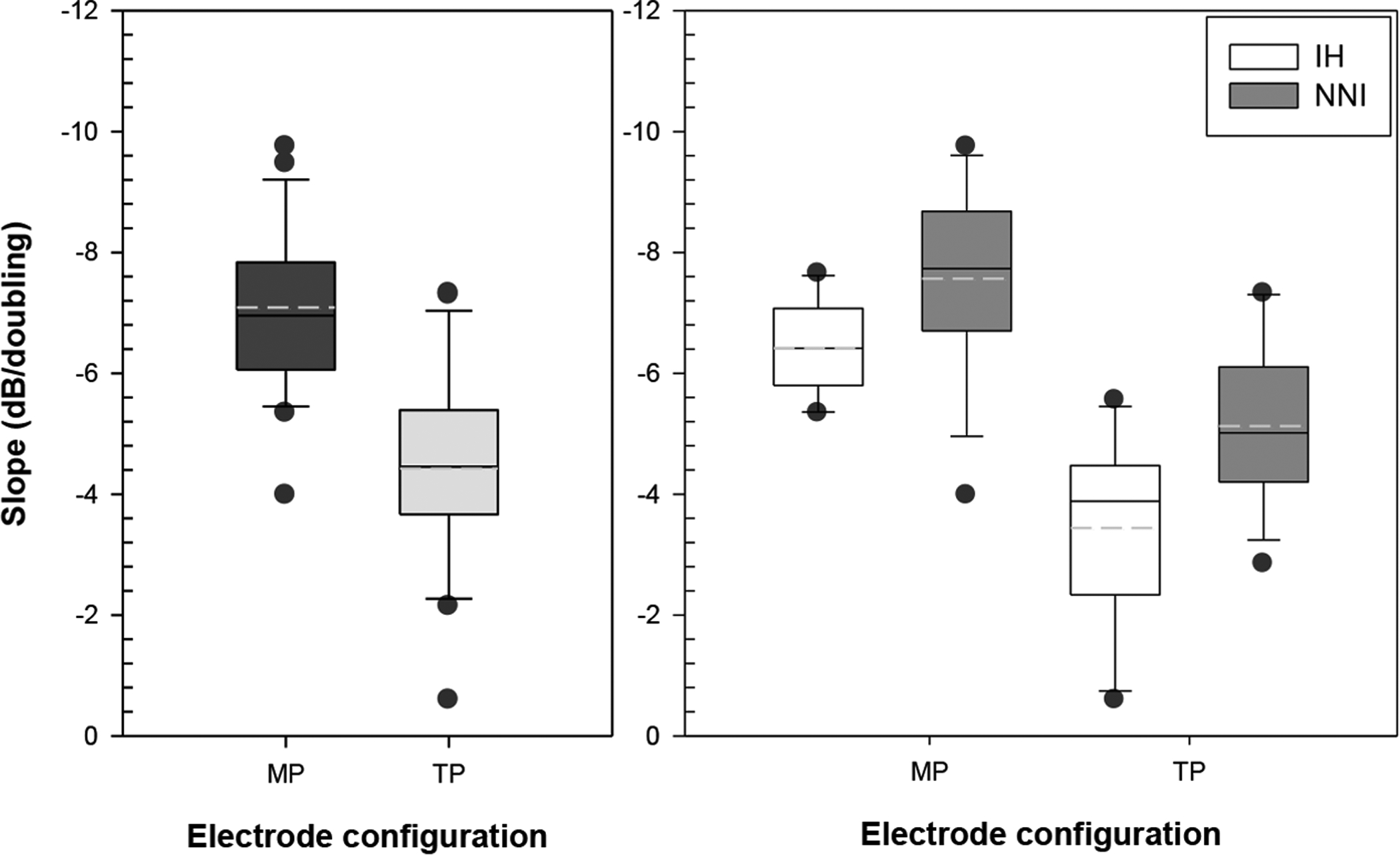
Slopes for the MP (dark bar) and TP (gray bar) electrode configuration are shown in the left panel. In the right panel, differences between treatment groups as a function of electrode configuration are shown; IH group are represented by white bars and the NNI group are represented by gray bars. In these graphs, the solid lines in the box represent the median and the dashed lines represent the mean. The upper and lower limits of the box plots represent estimates of the 75th and 25th percentiles and the upper and lower whiskers represents estimates of the 90th and 10th percentiles, respectively. The dark circles represent measures that fell outside the 90th and 10th percentiles.
3.3. Histology and Ensemble Spontaneous Activity
SGN densities and IHC assessments were only available for a subset of animals (IH = 7, NNI = 11). SGN densities for these 18 animals are shown in the left panel of Fig. 3. SGN densities in each of the three basal-half turns of the cochlea are shown (one animal in the IH group only has SGN data for profile B due to the other profiles being damaged during processing). The three analyzed profiles are labeled in Fig. 3 (right panel) where profile A represents the region closest to the implant (i.e., the location of the primary stimulating and recording electrode) and profiles B and C are apical to the implant (Pfingst et al., 2017). Data were subjected to one-way repeated measure ANOVA with SGN density within each profile (A, B, and C) as the within-subject factor and treatment-group (IH, NNI) as the between-subject factor. Results demonstrated that the SGN density was not significantly different across the three profiles (p> 0.05). However, main effect of treatment group was significant (F (1, 16) = 49.3, p < 0.0001) suggesting that nerve-survival patterns for the two groups were different as a result of the treatment approach with higher SGN density in the IH than the NNI group.
Survival of IHCs is shown in the left panel of Fig. 3 as bold symbols. The percentage of animals in the IH group who demonstrated IHC survival was about 50% in profile “A”, 71% in profile “B”, and 83% in profile “C”; generally, all animals in the IH group exhibited IHC survival in at least one profile. In comparison, only one of 11 animals in the NNI group (9%) had IHC survival, and that subject had IHCs only in profile “C”. Overall, this suggests that there was greater survival and preservation of hair cells in the hearing animals. To examine the effect of hair cells on nerve activity, spontaneous activity, presumably generated by the hair cells, was compared for the two treatment groups. ESA recordings were available for all 24 animals; spectra of ESA recordings are shown in Fig. 4. ESA activity, as evidenced by a spectral peak between 900 and 1000 Hz, in the auditory nerve was present in nine animals in the IH group and was absent in thirteen animals in the NNI group. Peak response levels averaged across the spectral range (593.75 – 1312.5 Hz) were higher for animals in the IH group (−36.7 dB re 100 μV ±4.1) than those obtained for the NNI group (−43.5 dB re 100 μV ±2.4); these differences were statistically significant (t (22) = 5.175, p< 0.0001).
Fig. 4:
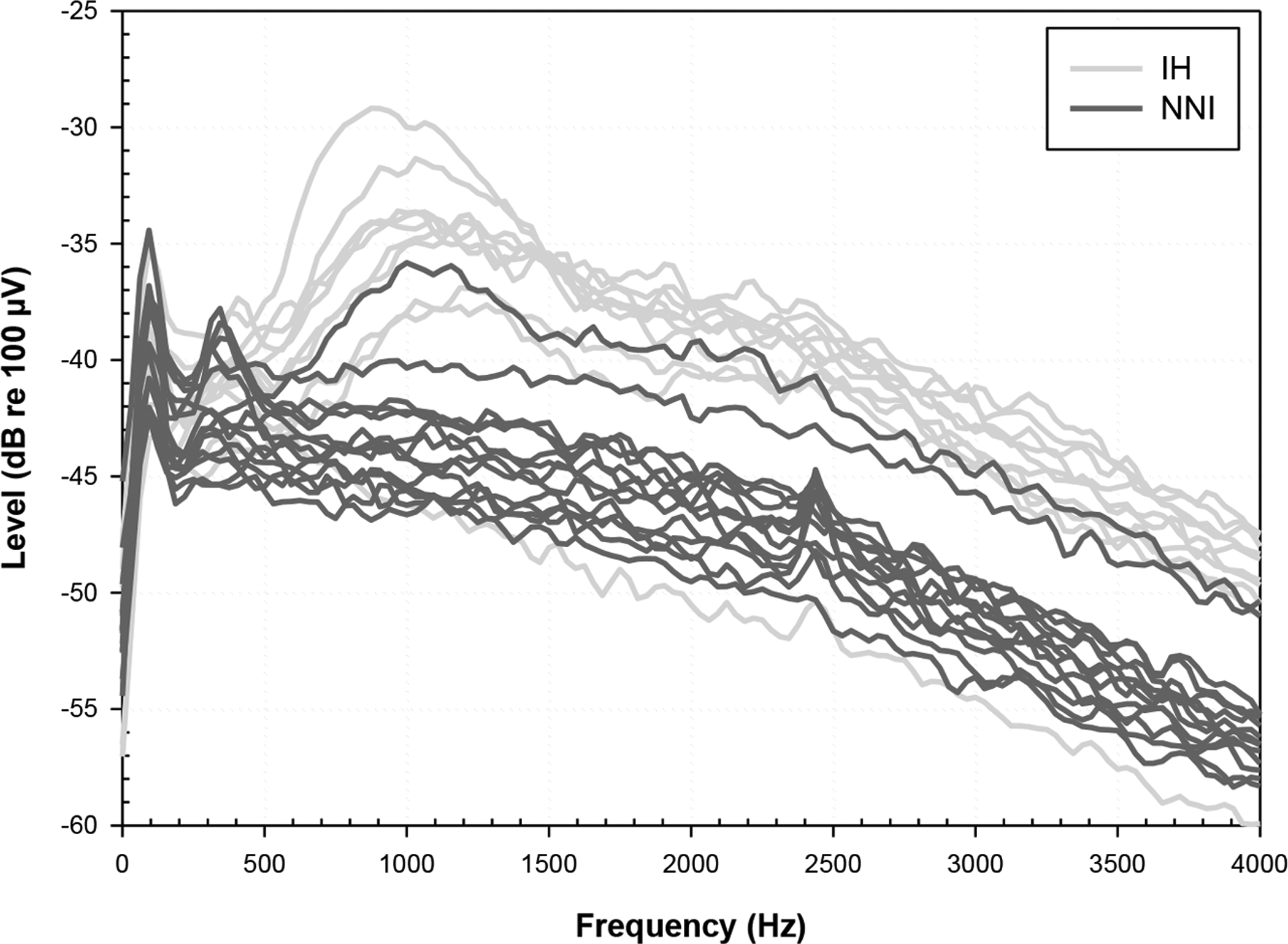
ESA recordings are displayed for the IH (N = 10; light gray traces) and NNI (N = 14; dark gray traces) groups. Spectra from the IH group, in all but one animal, demonstrate peaks between 900 and 1000 Hz suggesting spontaneous firing of the auditory nerve; the one IH animal with no peak had low IHC survival and implant issues on the day of euthanasia. Spectra from the NNI group show no peak with the exception of one animal who had residual apical IHCs.
3.4. Relationship between strength-duration functions and cochlear health
To probe differences between the two treatment groups, slopes of strength-duration functions for each animal were examined in relation to SGN densities in profile A (SGN-A; the location at which the strength duration data were collected; profile B was used in one animal due to damage of profile A) using linear regression analyses; results are shown in Fig. 5. Results demonstrated that steeper slopes were associated with higher SGN-A density for animals in the NNI group for both MP (r2=0.53, p<0.01) and TP (r2=0.43, p<0.03) configurations, but there was not a significant relationship between slope and SGN-A density for animals in the IH group. Those animals in the IH group that had low SGN density and no surviving IHC had slopes similar to NNI animals with comparable SGN densities; but IH animals with higher SGN density than NNI animals had much shallower slopes than expected based on data from the NNI animals. The majority of IH animals with high SGN densities also had surviving IHC, suggesting IHC survival may be associated with shallower slopes. Similar results were found when profiles A through B and A through C were used for comparison with the psychophysical data; however, SGN density for profile A was used in this study to be consistent with previous results from our laboratory (Kang et al., 2010; Pfingst et al., 2019).
Fig. 5:
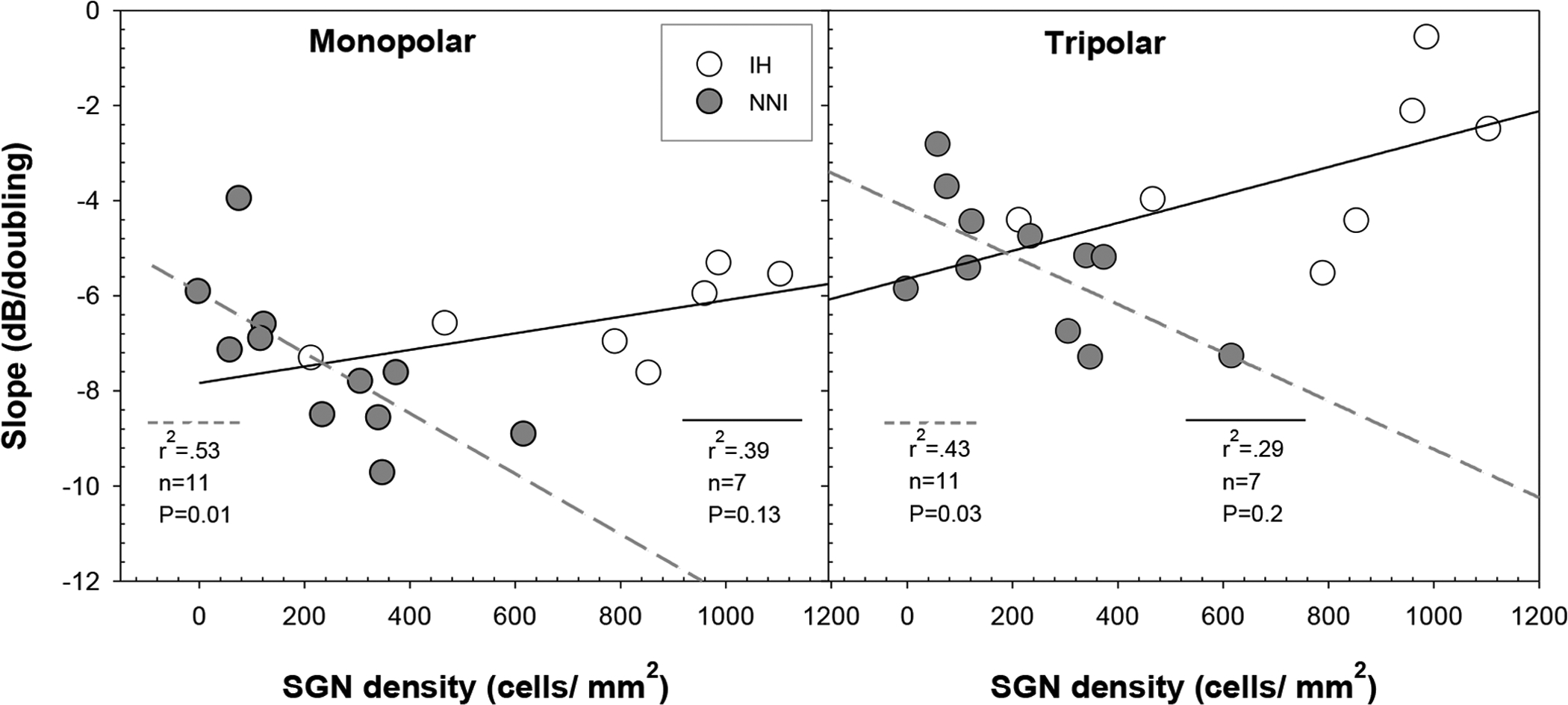
The correlation between SGN-A density (cells/mm2) and strength-duration function slopes for the IH (N = 7; unfilled symbols; solid lines) and the NNI (N = 11; filled symbols; dashed lines) groups are shown for MP (left panel) and TP (right panel) electrode configurations. The relationship between SGN-A density and strength-duration function slope was significant using both MP and TP stimulation modes in the NNI group but not in the IH group.
Because inner hair cells are expected to generate spontaneous activity in the surviving auditory nerve fibers, we evaluated the relationship between slopes of the strength-duration functions and ESA levels (Fig. 6). Results demonstrated a modest relation between slopes of strength-duration functions and spontaneous activity using TP (p=0.036) but not MP configuration. Specifically, slopes were shallower in animals with relatively higher ESA response levels and steeper in those with lower ESA levels. These results suggest that spontaneous activity generated by the hair cells affected the integration of charge as a function of phase duration.
Fig. 6:
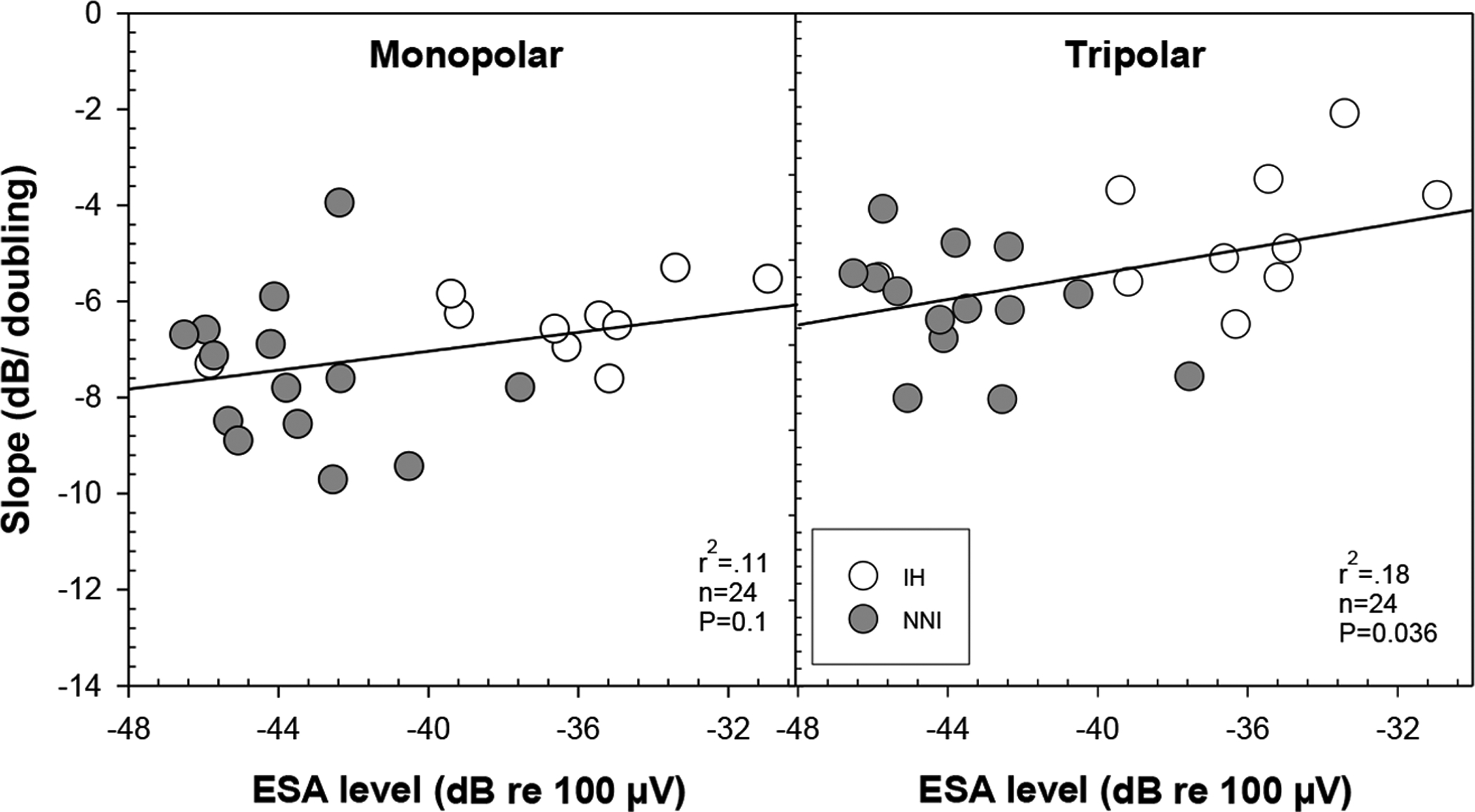
ESA levels (dB re 100 μV) were averaged over the frequency range between 593.75 to 1312.5 Hz and are plotted as a function of strength-duration function slope for both MP and TP configurations. Data for the IH (N = 10) are represented by the unfilled symbols and for the NNI (N = 14) are represented by the filled symbols. A modest but significant correlation between strength-duration function slopes and ESA levels was found for TP and but not for MP electrode configuration.
In summary, results from this study demonstrated that there were measurable differences in strength-duration functions between the two treatment groups as a function of electrode configuration. The NNI group had lower thresholds for long-phase duration pulses and steeper strength-duration function slopes than the IH group using TP configuration. These results suggest that differences across animals were related to variations in cochlear health and that focused stimulation is more sensitive to these variations.
4. Discussion
The present study examined strength-duration functions in guinea pigs with variable cochlear health conditions with the aim of understanding how the health of the implanted cochlea relates to the integration of charge in pulsatile electrical stimuli. Using electrical pulse trains with a range of phase durations, behavioral detection thresholds obtained with MP and TP configurations were compared for two treatment groups. Overall, results demonstrated considerable differences within and across treatment groups in slopes of psychophysical strength-duration functions and in survival of inner hair cells and spiral ganglion neurons. The strength-duration functions were also significantly affected by electrode configuration (MP vs TP).
4.1. Group differences in strength-duration functions
The NNI treatment group had steeper slopes of strength-duration functions (−6.3± 0.31 dB/ doubling) than those obtained for the IH group (−4.9± 0.37 dB/ doubling). Slopes of strength-duration functions are believed to be indicative of the integrative properties of the neural elements being stimulated (Pfingst et al., 1991; Smith and Finley, 1997). Generally, steeper slopes suggest a higher recruitment of activated auditory nerve fibers with increasing current. This can be attributed to a number of variables including different sites of action potential initiation, larger current spread, stronger synchronization, and better integration of charge (Navntoft et al., 2020). Therefore, group differences in slope of strength-duration functions may reflect variations in the animals’ overall sensitivity and the manner in which they respond to electrical stimulation.
The NNI treatment group had also lower thresholds than those obtained in the IH group. However, threshold differences between the two treatment groups were interestingly dependent on the pulse phase duration; this was suggested by the different effects of phase duration in the treatment groups. Specifically, thresholds were comparable for the two treatment groups when phase duration was short (< 300 μs) but improved more rapidly and were lower for the NNI group when long phase duration (≥ 300 μs) was used. This perhaps suggests that long phase duration can be more reflective of group variations in certain processes such as differences in recovery time and charge integration. First, when using a pulse with long phase duration, lower current is typically required which can result in prolonged refractory periods (Morris and Pfingst, 2000; Miller et al., 2001b; 2008; Bierer and Middlebrooks, 2002; Battmer et al., 2004; Snyder et al., 2004; Hughes and Laurello, 2017). Animals with poor SGN survival has been shown to exhibit faster recovery times than those with better cochlear health (Botros and Psarros, 2010; Ramekers et al., 2015). Hence, threshold difference between the two treatment groups could be related to differences in recovery times when a pulse with long phase duration was used.
Second, long phase duration is more likely to also reveal differences in charge integration between the two treatment groups. Neural membranes are often referred to as a “leaky integrator” such that the longer the duration of the pulse, the more charge can leak away within the duration of the current pulse; subsequently leading to an increase in the amount of current that needs to be injected in order to counteract the leaky behavior of the neural membrane (Lapicque, 1907; Miller et al., 2001a; Undurraga et al., 2013). Group differences in charge integration can presumably be attributed to the presence or absence of spontaneous activity and electrophonic responses of the auditory nerve. It has been reported that these types of fiber activities can alter the temporal coding of the electric stimuli and cause reduced fiber responsiveness in CI animals with residual hearing (Miller et al., 2006). This reduced neural responsiveness may lead to less efficient transmission of charge integration to the central nervous system in the IH animals such that to initiate a spike, they may require a higher current to compensate for membrane leakage with longer pulse durations. Consistent with this hypothesis, our IH group had shallower slopes and higher thresholds than the NNI group. On the other hand, the physiologic differences between the two treatment groups seem to be less dominant for short phase durations which is considered to be more efficient in terms of charge integration (Abbas and Miller, 2004). Collectively, these results highlight differences between the two groups in charge integration with the IH animals being less efficient.
4.2. Effect of electrode configuration
Using both modes of stimulation, general results demonstrated an inverse relation between pulse phase duration and absolute behavioral thresholds with short phase durations having higher thresholds and long phase durations having lower thresholds. This is consistent with a number of electrophysiological, psychophysical, and modeling studies that demonstrated a similar effect (van den Honert and Stypulkowski, 1984; Shannon, 1985; Parkins, 1989; Moon, et al., 1993; McKay and McDermott, 1999; Miller, et al., 1995a; 1999; Shepherd and Javel, 1999; Shepherd et al., 2001; Chatterjee and Kulkarni, 2014; Ramekers et al., 2014). Additionally, as anticipated, MP stimulation resulted in lower overall detection thresholds than those obtained with TP configuration (see Fig. 1). This effect was true for all animals in both treatment groups. Generally, less current was required to reach behavioral thresholds in the MP configuration due to the broad current spread caused by the large spatial distance between the active and return electrodes (Smith and Finley, 1997; Miller, et al., 1999; Bierer, 2007; Litvak, et al., 2007; Snyder, et al., 2008; Chatterjee and Kulkarni, 2014). The MP electrode configuration also produced more steeply sloped strength-duration functions than those obtained for TP stimulation (see Figs.1 and 2). It has been suggested that steeper slopes are associated with a larger neural population being recruited, which would be expected due to the greater spread of excitation associated with MP stimulation (Frijns, et al., 1995; Miller, et al., 1995a; 2003; Pfingst, et al., 2011; 2015; Navntoft, et al., 2020).
Group differences in behavioral thresholds (see Fig. 1, middle and right panel) were more robust when using TP than when using MP electrode configuration. This was statistically evidenced by both the significant two-way interaction between electrode configuration and treatment group and the three-way interaction between electrode configuration, phase duration, and treatment group. It has been hypothesized that site of action potential initiation is likely to be affected by the size of the electrical field such that focused stimulation can result in action potential initiation at more peripheral neural processes and broad stimulation can result in action potential initiation at more central processes (Miller et al., 2003; Chatterjee and Kulkarni, 2014). Our current results may support those earlier hypotheses suggesting that electrode configuration may reveal differences in the stimulating neural population and/or sites of excitation along the auditory nerve fibers (Chatterjee and Kulkarni, 2014). Taken together, the fact that differences between treatment groups were more pronounced using TP electrode configuration suggests that there were intrinsic variations across animals in sites of action potential initiation as a result of the treatment approach and these variations were better reflected using focused stimulation.
4.3. Effect of cochlear health
There were considerable differences between the two treatment groups in terms of their psychophysical performance and their histological analyses (see Fig. 3 and Fig. 4). Overall; steeper slopes and lower thresholds were found for the animals with the lower SGN density (NNI) than those obtained in animals with better cochlear health (IH). Yet, steeper strength-duration slopes were associated with higher SGN-A density in the NNI group but not in the IH group (see Fig. 5). These results suggest that the two groups of animals utilized different mechanisms which were likely to be related to the extent of post-deafening morphological alterations that induced functional variations in how SGNs integrate electrical charge (Colombo and Parkins, 1987; Smit et al., 2008; Resnick et al., 2018).
First, some of these mechanisms could be attributed to activities driven by the IHC/auditory-neuron synapse in the IH group. IHCs were preserved in all of the IH group animals compared to only one subject in the NNI group that had very apical IHCs (Fig. 3, left panel, Profile C). The presence of functional IHCs in the IH group was additionally confirmed by higher ESA level. Spontaneous activity generated by the IHCs has been generally reported to be higher in hearing animals (Miller et al., 2006; Kang et al., 2010; Pfingst et al., 2017) and lower in profoundly deafened animals (Shepherd and Javel, 1999). Alternatively, minimal or no spontaneous activity has been observed in ears with absent hair cells (Hartmann and Klinke, 1990; Lopez-Poveda et al., 1997; Spassova et al., 2004).
Current results revealed that animals with higher ESA levels had shallower strength-duration function slopes and those with lower ESA levels had steeper slopes (see Fig. 6). It is known that the spontaneous activity generated by the hair cells put auditory nerve fibers in a state of partial refractoriness causing adaptation-like changes that can make it more difficult to achieve saturated responses (Miller at al., 2006). The IHC/auditory-neuron synapse is considered one of the origins of this adaptation effect. This suggests that animals in the IH group had a greater adaptation than those in the NNI group given that IHCs were preserved in the IH group. Since this effect is bypassed in animals with no residual hearing, the mechanism responsible for auditory nerve fiber responses to electrical stimulation as such could be different in deaf cochleae (Javel, 1990; Dynes and Delgutte, 1992; Geurts and Wouters, 1999; Goutman, 2017).
Current findings indicate that functional performance in healthy cochleae is largely mediated by the properties of the IHC/auditory-neuron synapse while those with poor neural survival and absent IHCs are rather mediated by more central processes. Therefore, site of action potential initiation is probably different between the two treatment groups. With IHC survival, some peripheral processes are more likely to be preserved and serve as the site of action potential initiation. When peripheral processes serve as the site of action potential initiation, it is assumed that they are more likely to exhibit different neural membrane integrative properties than those of cell body central processes with larger diameter and heavier myelination. Alternatively, after pathologic alteration and the degeneration of peripheral processes, site of action-potential initiation shifts towards more distal central processes as reported by several physiological experiments (van den Honert and Stypulkowski, 1984; Colombo and Parkins, 1987; Javel and Shepherd, 2000; Briaire and Frijns, 2006). This may explain the relation between slopes of strength-duration function and SGN density that was observed in the NNI group but not in the IH group. Overall, we hypothesize that differences in strength-duration function slope between the two groups were mediated by variations in the conditions of IHCs and the integrative properties of the neural elements being stimulated near the implanted electrode. This relation was dependent on both the phase duration and the electrode configuration that were used.
In summary, results showed that long phase duration is more reflective of animals’ variations in sensitivity and the manner in which they respond to electric stimulation. In general, using personalized fitting strategies that target the identification of across-site differences in response to electrical stimulation and the removal of less optimal sites has been shown to improve outcome in CI recipients (Bierer and Faulkner, 2010; Garadat et al., 2012; Zhou and Pfingst, 2012). However, the extent to which long phase duration could serve as a predictive measure to estimate the variations in the local conditions near the implanted electrodes in human cochleae cannot be determined in this study. The current study used a pulse rate of 100 pps which is much slower than that implemented in the clinical CI speech processor. Therefore, future studies need to examine these effects using a higher stimulation rate comparable to that used in the clinical speech processor.
5. Conclusions
Present results demonstrated that strength-duration functions indeed served as a sensitive measure to capture differences in cochlear health in the study animals. Overall, the NNI treatment group had steeper slopes than those obtained in the IH group. Additionally, steeper strength-duration function slopes were correlated with higher SGN-A density in the NNI group but not in the IH group. Additionally, there was a modest relation between slope steepness and ESA level activities such that higher ESA levels resulted in shallower slopes. Collectively, there were considerable differences in strength-duration functions between the two treatment groups that were highlighted in this study. Overall, findings suggest that group differences in strength-duration functions may be driven by a complex interaction of a number of different variables in relation to the overall cochlear health near sites of stimulation and that stimulation with a TP electrode configuration is more sensitive to these differences than stimulation with a MP configuration.
Highlights.
Strength-duration function slopes were predictive of spiral ganglion density
Slopes were negatively correlated with ensemble spontaneous activity levels
Differences in performance were pronounced using tripolar electrode configuration
Performance was related to inner hair cell status and neural element properties
Acknowledgements
This work was supported by NIH/NIDCD grants R01 DC010786 and R01 DC015809 and a contract from MED-EL. We thank Lisa Beyer for processing the histological data and Laila Aljerdi for assistance in processing the psychophysical data.
List of abbreviations
- CI
Cochlear implant
- SGN
Spiral ganglion neuron
- TP
Tripolar
- MP
Monopolar
- IH
Implanted hearing group
- NNI
Neomycin + neurotrophin + implant group
- ESA
Ensemble spontaneous activity
- FFT
Fast Fourier transforms
- ANOVA
Analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbas P, Tejani V, Scheperle R, Brown C 2017. Using Neural Response Telemetry to Monitor Physiological Responses to Acoustic Stimulation in Hybrid Cochlear Implant Users. Ear Hear. 38, 409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas PJ, Miller CA 2004. Biophysics and physiology. In Cochlear Implants: Auditory prostheses and electric hearing, eds Zeng F-G, Popper AN and Fay RR, pp. 149–212. Springer-Verlag, New York. [Google Scholar]
- Battmer RD, Dillier N, Lai WK, et al. 2004. Evaluation of the neural response telemetry (NRT) capabilities of the nucleus research platform 8: initial results from the NRT trial. Int J Audiol. 43 Suppl 1: S10–S15. [PubMed] [Google Scholar]
- Bierer J 2007. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Amer. 121, 1642–1653. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Faulkner KF 2010. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 31, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC 2002. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 87, 478–492. [DOI] [PubMed] [Google Scholar]
- Botros A, Psarros C 2010. Neural response telemetry reconsidered: II. The influence of neural population on the ECAP recovery function and refractoriness. Ear Hear. 31, 380–391. [DOI] [PubMed] [Google Scholar]
- Briaire JJ, Frijns JH 2006. The consequences of neural degeneration regarding optimal cochlear implant position in scala tympani: a model approach. Hear Res. 214, 17–27. [DOI] [PubMed] [Google Scholar]
- Budenz CL, Pfingst BE, Raphael Y 2012. The use of neurotrophin therapy in the inner ear to augment cochlear implantation outcomes. Anat Rec 295, 1896–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler BE, Lomber SG 2013. Functional and structural changes throughout the auditory system following congenital and early-onset deafness: implications for hearing restoration. Front Syst Neurosci. 26, 7–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon R, Cosentino S, Deeks J, Parkinson W, Arenberg J 2018. Effect of Stimulus Polarity on Detection Thresholds in Cochlear Implant Users: Relationships with Average Threshold, Gap Detection, and Rate Discrimination. [DOI] [PMC free article] [PubMed]
- Chatterjee M, and Kulkarni AM 2014. Sensitivity to pulse phase duration in cochlear implant listeners: Effects of stimulation mode. J Acoust Soc Am. 136, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Galvin JJ 3rd, Fu QJ, Shannon RV 2006. Effects of stimulation mode, level and location on forward-masked excitation patterns in cochlear implant patients. J Assoc Res Otolaryngol. 7, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Parkins CW 1987. A model of electrical excitation of the mammalian auditory-nerve neuron. Hear Res. 31, 287–311. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Carlyon R, Deeks J, Parkinson W, Bierer J 2016. Rate discrimination, gap detection and ranking of temporal pitch in cochlear implant users. J Assoc Res Otolaryngol. 17, 371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan DF, Nuttall AL, Avinash G 1990. Asynchronous neural activity recorded from the round window. J Acoust Soc Am. 87, 2621–2627. [DOI] [PubMed] [Google Scholar]
- Dynes SBC, Delgutte B 1992. Phase-locking of auditory-nerve discharges to sinusoidal electric stimulation of the cochlea. Hear Res. 58, 79–90. [DOI] [PubMed] [Google Scholar]
- Fransson A, Ulfendahl M 2016. Structural changes in the inner ear over time studied in the experimentally deafened guinea pig. J Neurosci Res. 95, 869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns JHM, de Snoo SL, Schoonhoven R 1995. Potential distributions and neural excitation patterns in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res. 87,170–186. [DOI] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, and Pfingst BE 2012. Across-site patterns of modulation detection: Relation to speech recognition. J Acoust Soc Am. 131, 4030–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadat SN, Pfingst BE 2011. Relationship between gap detection thresholds and loudness in cochlear-implant users. Hear Res. 275, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts L, Wouters J 1999. Enhancing the speech envelope of continuous interleaved sampling processors for cochlear implants. J Acoust Soc Am. 105, 2476–2484. [DOI] [PubMed] [Google Scholar]
- Goehring T, Archer-Boyd A, Deeks J, Arenberg J, Carlyon R 2019. A Site-Selection Strategy Based on Polarity Sensitivity for Cochlear Implants: Effects on Spectro-Temporal Resolution and Speech Perception. J Assoc Res Otolaryngol. 20, 431–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD 2017. Mechanisms of synaptic depression at the hair cell ribbon synapse that support auditory nerve function. Proceedings of the National Academy of Sciences of the United States of America.114, 9719–9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R, Klinke R 1990. Response characteristics of nerve fibers to patterned electrical stimulation. In: Miller JM, Spelman FA, eds. Cochlear Implants: Models of the Electrically Stimulated Ear. New York: Springer-Verlag, pp. 135–160. [Google Scholar]
- Hughes ML, Laurello SA 2017. Effect of stimulus level on the temporal response properties of the auditory nerve in cochlear implants. Hear. res 351, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Arenberg J 2019. Evaluating Psychophysical Polarity Sensitivity as an Indirect Estimate of Neural Status in Cochlear Implant Listeners. J Assoc Res Otolaryngol. 20, 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javel E 1990. Acoustic and electrical encoding of temporal information. In: Miller JM, and Spelman FA (Eds.), Cochlear Implants. Models of the Electrically Stimulated Ear. Springer-Verlag, New York, pp. 247–295. [Google Scholar]
- Javel E, Shepherd RK 2000. Electrical stimulation of the auditory nerve. III. Response initiation sites and temporal fine structure. Hear Res. 140, 45–76. [DOI] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su. GL, Raphael Y, Pfingst BE 2010. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 11, 245–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapicque L 1907. Recherches quantitatives sur l’excitation électrique desnerfs traitéecommeune polarisation. Journal de Physiologie et de Pathologie Générale 9, 620–635. [Google Scholar]
- Leake PA, Hradek GT 1988. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 33, 11–33. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK 2003. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 32, 229–243. [DOI] [PubMed] [Google Scholar]
- Litvak L, Spahr A, Emadi G 2007. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J. Acoust Soc Amer 122, 967–981. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, O’Mard LP, and Meddis R 1997. A revised computational inner hair cell model. in Psychophysical and Physiological Advances in Hearing: Proceedings of the 11th International Symposium on Hearing, Grantham, U.K, edited by Palmer AR, Rees A, Summerfield AQ, and Meddis R (Wurr, London, 1997), pp. 112–121. [Google Scholar]
- McKay CM, McDermott HJ 1999. The perceptual effects of current pulse duration in electrical stimulation of the auditory nerve. J Acoust Soc Am. 106, 998–1009. [DOI] [PubMed] [Google Scholar]
- Mesnildrey Q, Venail F, Carlyon R, Macherey O 2020. Polarity Sensitivity as a Potential Correlate of Neural Degeneration in Cochlear Implant Users. J Assoc Res Otolaryngol. 21, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Smith DW, Pfingst BE 1999. Across-species comparisons of psychophysical detection thresholds for electrical stimulation of the cochlea: II. Strength-duration functions for single, biphasic pulses. Hear Res. 135, 47–55. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Nourski KV, Hu N, and Robinson BK 2003. Electrode configuration influences action potential initiation site and ensemble stochastic response properties. Hear Res. 175, 200–214. [DOI] [PubMed] [Google Scholar]
- Miller CA, Woodruff KE, Pfingst BE 1995b. Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures. Hear Res. 92, 85–99. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK 2001b. Response properties of the refractory auditory nerve fiber. J Assoc Res Otolaryngol. 2, 216–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK, Nourski KV, Zhang F, Jeng FC 2006. Electrical excitation of the acoustically sensitive auditory nerve: single-fiber responses to electric pulse trains. J Assoc Res Otolaryngol. 7, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Faulkner MJ, Pfingst BE 1995a. Functional responses from guinea pigs with cochlear implants. II. Changes in electrophysiological and psychophysical measures over time. Hear Res. 92, 100–111. [DOI] [PubMed] [Google Scholar]
- Miller CA, Hu N, Zhang F, Robinson BK, Abbas PJ 2008. Changes across time in the temporal responses of auditory nerve fibers stimulated by electric pulse trains. J Assoc Res Otolaryngol. 9, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Robinson BK, Rubinstein JT, Abbas PJ, Runge-Samuelson CL 2001a. Auditory nerve responses to monophasic and biphasic electric stimuli. Hear Res. 151, 79–94 [DOI] [PubMed] [Google Scholar]
- Moon AK, Zwolan TA, and Pfingst BE 1993. Effects of phase duration on detection of electrical stimulation of the human cochlea. Hear Res. 67, 166–178. [DOI] [PubMed] [Google Scholar]
- Morris DJ, Pfingst BE 2000. Effects of electrode configuration and stimulus level on rate and level discrimination with cochlear implants. J Assoc Res Otolaryngol. 1, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol J 1997. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 117, 220–228. [DOI] [PubMed] [Google Scholar]
- Nadol JB Jr, Shiao J, Burgess B, Ketten D, Eddington D, Gantz B, Kos I, Montandon P, Coker N, Roland J Jr, Shallop JK 2001. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 110, 883–91. [DOI] [PubMed] [Google Scholar]
- Navntoft CA, Marozeau J, Barkat TR 2020. Ramped pulse shapes are more efficient for cochlear implant stimulation in an animal model. Sci Rep.10, 3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins CW 1989. Temporal response patterns of auditory nerve fibers to electrical stimulation in deafened squirrel monkeys. Hear Res. 41, 137–168. [DOI] [PubMed] [Google Scholar]
- Parkins CW, Colombo J 1987. Auditory-nerve single-neuron thresholds to electrical stimulation from scala tympani electrodes. Hear Res. 31, 267–286. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Burkholder-Juhasz RA, Xu L, Thompson CS 2008. Across-site patterns of modulation detection in listeners with cochlear implants. J Acoust Soc Am. 123, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst B, Xu L 2004. Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolaryngol. 5, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Bowling SA, Colesa DJ, et al. 2011. Cochlear infrastructure for electrical hearing. Hear Res. 281, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, et al. 2017. Neurotrophin Gene Therapy in Deafened Ears with Cochlear Implants: Long-term Effects on Nerve Survival and Functional Measures. J Assoc Res Otolaryngol. 18, 731–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, DeHaan DR, Holloway LA, 1991. Stimulus features affecting psychophysical detection thresholds for electrical stimulation of the cochlea. I: Phase duration and stimulus duration. J. Acoust Soc Am 90, 1857–1866. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D, Miller JM, Bohne BA 1981. Relation of psychophysical data to histopathology in monkeys with cochlear implants. Acta Otolaryngol (Stockh). 92,1–13. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Xu L 2004. Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolaryngol. 5, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS 2004. Across-site threshold variation in cochlear implants: relation to speech recognition. Audiol Neurotol. 9, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Zhou N, Colesa DJ, et al. 2015. Importance of cochlear health for implant function. Hear Res. 322, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK 2006. Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res. 215, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Klis SF, Grolman W 2015. Recovery characteristics of the electrically stimulated auditory nerve in deafened guinea pigs: relation to neuronal status. Hear Res. 321, 12–24. [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, Grolman W 2014. Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol. 15, 187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattay F, Lutter P, Felix H 2001. A model of the electrically excited human cochlear neuron. I. Contribution of neural substructures to the generation and propagation of spikes. Hear Res. 153, 43–63. [DOI] [PubMed] [Google Scholar]
- Resnick JM, O’Brien GE, Rubinstein JT 2018. Simulated auditory nerve axon demyelination alters sensitivity and response timing to extracellular stimulation. Hear Res. 361, 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Rosenbaum BT, Kim PJ, Niparko JK, Saada AA 1998. Single unit recordings in the auditory nerve of congenitally deaf white cats: morphological correlates in the cochlea and cochlear nucleus. J Comp Neurol. 397, 532–548. [DOI] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Colesa DJ, Buswinka CJ, Swiderski DL, Raphael Y, Pfingst BE 2019. Changes over time in the electrically evoked compound action potential (ECAP) interphase gap (IPG) effect following cochlear implantation in Guinea pigs. Hear Res. 383, 107809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac K, Holden T, Zwolan T, Arts H, Firszt J, Buswinka C, Pfingst B 2020. Effects of Electrode Location on Estimates of Neural Health in Humans with Cochlear Implants. J Assoc Res Otolaryngol. 21, 259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV 1985. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 18, 135–143. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Hardie NA, Baxi JH 2001. Electrical stimulation of the auditory nerve: Single neuron strength-duration functions in deafened animals. Ann Biomed Eng. 29, 195–201. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Hardie NA 2001. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol. 6, 305–18. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E 1997. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 108, 112–44. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E 1999. Electrical Stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res. 130, 171–188. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG 2004. Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. Eur J Neurosci. 20, 3131–3140. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Budenz CL, Bowling SA, Pfingst BE, Raphael Y 2011. Nerve maintenance and regeneration in the damaged cochlea. Hear Res. 281, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JE, Hanekom T, Hanekom JJ 2008. Predicting action potential characteristics of human auditory nerve fibres through modification of the Hodgkin-Huxley equations. S Afr J Sci. 104, 284–292. [Google Scholar]
- Smith DW, Finley CC 1997. Effects of electrode configuration on psychophysical strength-duration functions for single biphasic electrical stimuli in cats. J Acoust Soc Am. 102, 2228–2237. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC 2004. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 5, 305–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Middlebrooks JC, Bonham BH 2008. Cochlear implant electrode configuration effects on activation threshold and tonotopic selectivity. Hear Res. 235, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Avissar M, Furman AC, Crumling MA, Saunders JC, Parsons TD 2004. Evidence that rapid vesicle replenishment of the synaptic ribbon mediates recovery from short-term adaptation at the hair cell afferent synapse. J Assoc Res Otolaryngol. 5, 376–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H 1975. Retrograde degeneration of the cochlear nerve. Acta Oto-Laryngologica (Stockholm). 79, 266–275. [DOI] [PubMed] [Google Scholar]
- Swiderski DL, Colesa DJ, Hughes AP, Raphael Y, Pfingst BE 2020. Relationships between Intrascalar Tissue, Neuron Survival, and Cochlear Implant Function. J Assoc Res Otolaryngol. 4, 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stypulkowski PH, van den Honert C 1984. Physiological properties of the electrically stimulated auditory nerve. I. Compound action potential recordings. Hear Res. 14, 225–243. [DOI] [PubMed] [Google Scholar]
- Tasaki I 1955. New measurements of the capacity and the resistance of the myelin sheath and the nodal membrane of the isolated frog nerve fiber. Am J Physiol. 181, 639–650. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, Carlyon RP, Wouters J, van Wieringen A 2013. The polarity sensitivity of the electrically stimulated human auditory nerve measured at the level of the brainstem. J Assoc Res Otolaryngol. 14, 359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Honert C, and Stypulkowski PH 1984. Physiological properties of the electrically stimulated auditory nerve. II. Single fiber recordings. Hear Res. 14, 225–243. [DOI] [PubMed] [Google Scholar]
- Wise AK, Pujol R, Landry TG, Fallon JB, Shepherd RK 2017. Structural and ultrastructural changes to type I spiral ganglion neurons and schwann cells in the deafened guinea pig cochlea. J Assoc Res Otolaryngol. 18, 751–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, & Pfingst BE 2012. Psychophysically-based site selection coupled with dichotic stimulation improves speech recognition in noise with bilateral cochlear implants. J Acoust Soc Amer. 132, 994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]


