Abstract
Background
Inflammation is one of major contributors of diabetic osteoporosis. Here, we combined adipose tissues derived mesenchymal stem cells (AD-MSCs)-derived exosomes and microRNA-146a (miR-146a) to develop more effective anti-inflammation strategy in osteoclasts.
Methods
miR-146a was overexpressed in AD-MSCs and miR-146a exosomes (miR-146a-Exo) were isolated and characterized. Cellular and animal diabetic osteoporosis models were created to evaluate the anti-inflammation effect of miR-146a-Exo by using ELISA, qRT-PCR, MTT, bone resorption assay, Western blot, and bone mineral content and density analysis in vitro and in vivo.
Results
miR-146a-Exo administration presented the most potent effect on inhibition of pro-inflammatory cytokines production in high glucose-treated osteoclasts, restraint bone resorption, and restoration of the bone loss in streptozotocin-induced diabetic osteoporosis rats. Mechanistically, miR-146a-Exo suppressed the expression of TNF-α, IL-18, and IL-1β, induced the inactivation of inflammasome, and finally reduced bone resorption and recovered bone loss.
Conclusion
Combination of AD-MSCs-Exo and miR-146a more effectively exert the anti-inflammation effect in osteoclasts, providing a potential drug for the treatment of diabetic osteoporosis.
Keywords: Diabetic osteoporosis, miR-146a, MSCs, Exosomes, Inflammasome
Introduction
Diabetic osteoporosis, a metabolic bone disease, is characterized by reduction of bone mineral density (BMD), destruction of bone microstructural, and high risk of fractures.1,2 The incidence of diabetes mellitus (DM) is increasing due to the high-sugar and -fat diet of modern people and the aging of world population.3,4 As one of the major complications of DM, the prevalence of diabetic osteoporosis also has a significant increasing trend.5 Moreover, the osteoporosis incidence in diabetic patients is 60% that is dramatically higher than that in non-diabetic patients.1 Hence, more and more scholars have begun to study diabetic bone metabolism and its mechanisms to prevent and treat diabetic osteoporosis and fractures.
Although the pathogenetic mechanisms of diabetic osteoporosis are still unclear, several factors have been suggested to be implicated in its development and progression. Hyperglycemia stimulates the generation of advanced glycation end products (AGEs) that is significantly elevated in diabetic patients, and the accumulation of AGEs is able to up-regulate metabolic gene expression and induce osteoclast differentiation, which cause reduction of bone density and decrease bone formation, finally lead to osteoporosis.6,7 In addition, oxidative stress trigged by the excessive production and accumulation of oxygen reactive species (ROS) is reported to be involve in the formation of osteoblasts and osteoclasts and development of osteoporosis.8,9 Lots of diabetic patients encounter ROS elevation and oxidative stress that contribute to the progression of diabetes and initiation of osteoporosis.10 Recent studies demonstrated that the increased expression and secretion of pro-inflammatory cytokines, such as TNF-α and IL-1β, could enhance osteoclastogenesis, promote bone resorption, and lead to osteoporosis.11,12
Mesenchymal stem cells (MSCs) derived exosomes (MSCs-Exo) have been reported to have promising anti-inflammatory properties. Tofiño-Vian et al. demonstrated extracellular vesicles from adipose tissues derived MSCs (AD-MSCs) could significantly reduce the inflammatory mediators and inhibit the activity of nitric oxide synthase in osteoarthritic chondrocytes.13 Yun et al. reported that dietary bovine milk-derived exosomes could improve bone health in glucocorticoid-induced osteoporosis mice.14 Our latest study also revealed the anti-diabetic osteoporosis role of AD-MSCs-derived exosomes which is mediated by inhibiting the secretion of pro-inflammatory cytokines and inactivation of NLRP3 in osteoclasts. The miR-146a was one of exosomal microRNAs that played an important role in the anti-inflammatory effect of exosomes.15,16 Boldin et al. demonstrated the miR-146a was a negative feedback regulator of innate immune response.17
Although microRNAs present promising anti-inflammatory functions, they are easily degraded by RNase in plasma, which limits the study and application of microRNA. Therefore, in order to more effectively apply MSCs-Exo and miR-146a to the treatment of diabetic osteoporosis, we created miR-146a overexpressed AD-MSCs (miR-146a-Exo) to explore its effect on the activation of osteoclast inflammation and verified whether miR-146a-Exo can be used as a potential drug for the treatment of diabetic osteoporosis in vitro and in vivo.
Materials and Methods
AD-MSCs Isolation and In Vitro Transduction
MSCs were isolated from the epididymal adipose tissues of healthy wild type male rats, and AD-MSCs were identified as described previously.13,18 Rat miR-146a fragments were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and sub-cloned into Ubi-MCS-SV40 lentiviral vector (GeneChem, Shanghai, China). Control or miR-146a lentiviral vector were co-transfected with packing plasmids, pHelper1.0 and pHelper2.0, into HEK293T cells (ATCC, Manassas, USA) to produce the corresponding lentivirus. When AD-MSCs reached 50%-60% confluence, the in vitro transduction was performed by using the above control or miR-146a lentivirus as described previously,19 to generate control and miR-146a overexpressing AD-MSC lines, AD-MSC+vector and AD-MSC+miR146a. Successfully transfected cells were isolated and expanded for the exosomes harvest.
Isolation and Characterization of AD-MSC-Exosomes
Conditional medium (CM) of AD-MSC, or AD-MSC+vector, or AD-MSC+miR146a cells was collected 2-day after culture for the exosome isolation as described previously.13 The exosomes from AD-MSC, AD-MSC+vector, and AD-MSC+miR-146a cells were named as Exo, vector-Exo, and miR-146a-Exo, which were dissolved in phosphate-buffered saline (PBS). Exosomes visualization and quantification were conducted through nanoparticle tracking analysis as described previously.20 The sucrose gradient fractions of AD-MSCs-exosomes was isolated by using Beckman TL-100 ultracentrifugation at 200,000 g for 24 hours and fixed in 2% paraformaldehyde plus 3% glutaraldehyde cacodylate buffer. Fixed exosomes were stained by 2% uranyl acetate in continuous carbon grid and imaged by using cm12 transmission electron microscopy (Philips, Amsterdam, Netherlands). Surface protein markers of exosomes, Hsp70, Tsg101 and CD9, were detected by western blotting using ExoAb antibody kit (System Biosciences, Palo Alto, USA) following the manufacturer’s protocol.
Osteoclasts Culture and Treatment
Eight-week-old male rat was used to collect bone marrow cells (BMCs). The whole BMCs were flushed from long bones and plated in 10-cm plates with MEM medium with 10% FBS. After 12 hours incubation at 37°C with 5% CO2, the non-adherent cells were harvested and placed into a new dish with normal MEM culture medium plus 30 ng/ml rat macrophage colony-stimulating factor (M-CSF) for 2 days to generate bone marrow derived monocytes (BMMs). With stimulation of TNFSF11R (R&D, Minneapolis, USA), the large spread multinucleated cells were observed, rat osteoclasts were formed after 7 days of culture. Leukocyte acid phosphatase kit (Sigma) was used to perform tartrate-resistant acid phosphatase (TRAP) staining and identify the osteoclasts following the manufacturers’ instruction. For the inflammatory response test, osteoclasts were cultured in 6-well plate and incubated with 35 mM high glucose (HG) for 36 hours. Added 1 µl exosomes (Exo, vector-Exo, or miR-146a-Exo, 1.0 µg/µl) to each well (1 ml medium) and incubated for 72 hours, PBS was used as negative control.
MTT Assay
MTT cell proliferation and cytotoxicity assay kit (Beyotime, Shanghai, China) was used to determine cell viability of the treated osteoclasts with different conditions, following the protocol provided by the manufacturer.
Bone Resorption Assay
Bone resorption assessment of osteoclast was performed as described previously.21 In brief, MCSF (25 ng/ml) and mRANKL (30 ng/ml) were added into the above HG treated osteoclasts to induce differentiation. After 5 days of differentiation, cells were digested with trypsin to make cell suspension, and then re-plated on bovine bone slices in 24-well plate (50000 cells/well), medium alone as Sham control. Cells were cultured with MCSF (25 ng/ml) and mRANKL (30 ng/ml) for another 48 hours, the number of mature cells was determined by TRAP staining. After scraping osteoclasts, bone slices were stained with 1% toluidine solution (g/ml). Widefield microscope was used to acquire images and Fiji software was applied to perform quantitative analysis. The normalization and calculation of bone resorption were performed as described previously.22 Five different samples pools were analyzed in a duplicate manner.
Diabetic Osteoporosis Rat Model Establishment, Treatment, and Bone Mineral Density (BMD) Analysis
Eight-week-old Sprague-Dawley male rats treated with streptozotocin (STZ, Sigma, St. Louis, MO) were used to establish diabetic osteoporosis model in this study. Rats were housed at 24°C with unlimited filtered tap water and chow pellets in the 12 hours light/dark cycle. Single dose of STZ (60 mg/kg) was injected intraperitoneally to induce diabetes of rats as described previously.23 Diabetic status of these rats was monitored by blood glucose measurement biweekly. All animal studies were approved by the ethical committee of Cangzhou Central Hospital.
Four days after the STZ treatment, rats were divided in four groups and injected 1.6 mg/kg exosomes (Exo, vector-Exo, or miR-146a-Exo) every two days through the tail vein, PBS injected rats were used as negative control, and non-STZ treated rats were used as Sham. Bone mineral content and bone mineral density were measured by using dual-energy X-ray absorptiometry (Hologic, Marlborough, USA) as previously described.24 Both femur and tibia were harvested and weighted at the end of the experiment.
Immunoblotting Analysis
Bone tissues from different diabetic osteoporosis rat groups were homogenized and lysed by RIPA lysis buffer (Beyotime, Shanghai, China) with fresh protease inhibitor cocktails. Target proteins expression level was determined by Western blot as previously described.22 Primary antibody against of Hsp70 (#ab2787, 1:1500), Tsg101 (#ab125011, 1:1000), and CD9 (#ab92726, 1:1000) were obtained from Abcam (Cambridge, UK). TNF-α (#3707, 1:1000) and GAPDH (#5174, 1:2000) were purchased from Cell Signaling Technology (Danvers, USA). Primary antibody against of IL-18 (# PA5-79477, 1:1000) and IL-1β (sc-12742, 1:1000) were ordered from Thermo Fisher Scientific (Waltham, USA) and Santa Cruz Biotechnology (Dallas, USA), respectively.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA of treated osteoclasts cells was extracted by using the TRIzol™ Reagent (Invitrogen, Carlsbad, USA), following the manufacturer’s instructions. The complementary DNA (cDNA) was synthesized by using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV-RT, Promega Corporation, Madison, USA). LightCycler® 96 Real-Time PCR Cycler system (F. Hoffmann-La Roche AG, Basel, Switzerland) was used for qRT-PCR reaction of TBF-α, IL-1β, IL-18, and MiR-146. The expression of target genes was normalized by GAPDH and U6, respectively, and fold-change was calculated by using 2−ΔΔCT method. All Primers used in this study were listed in Table 1.
Table 1.
Primers used in this study.
| Name | Sequence |
|---|---|
| MiR-146a |
Sense: 5′- GGCTGAGAACTGAATTCCA-3′ Antisense: 5′-CAGTGCAGGGTCCGAGGTAT-3′ |
| U6 |
Sense: 5′- CTCGCTTCGGCAGCACATA-3′ Antisense: 5′-CGAATTTGCGTGTCATCCT-3′ |
| TNF-α |
Sense: 5′- CACCACGCTCTTCTGTCTACTG -3′ Antisense: 5′-GCTACGGGCTTGTCACTCG -3′ |
| IL-1β |
Sense: 5- GTGGCAGCTACCTATGTCTTGC-3 Antisense: 5′- CCACTTGTTGGCTTATGTTCTGT-3′ |
| IL-18 |
Sense: 5’ -CGCAGTAATACGGAGCATAAATGAC-3’ Antisense:5; -GGTAGACATCCTTCCATCCTTCAC-3’ |
| GAPDH |
Sense: 5′-GTCGGTGTGAACGGATTTG-3′ Antisense: 5′-TCCCATTCTCAGCCTTGAC-3’ |
ELISA
Conditional medium of osteoclasts was harvested 72 hours after exosomes (Exo, vector-Exo, or miR-146a-Exo) treatment for the pro-inflammatory cytokines, IL-1β, IL-18, and TNF-α measurement. Rat serum was collected at the end of treatment for the measurement of the above pro-inflammatory cytokines. The conditional medium of bone resorption test was harvested to measure the concentration of cathepsin K, an important osteoclast marker. The concentration of the above factors was detected by using ELISA kits following the manufacturer’s instructions. IL-1β, IL-18, and TNF-α were determined by using IL-1β cell lysates rat ELISA kit (# ERIL1B), IL-18 rat ELISA kit (# KRC2341), and TNF alpha Rat ELISA Kit (# BMS622) ordering from Thermo Fisher Scientific Inc. (Waltham, USA). Deoxypyridinoline in the urine was measured by using the Rat DPD / Deoxypyridinoline ELISA Kit (RTFI00737, AssayGenie, Dublin, Ireland). Cathepsin K was determined by using rat CTSK/Cathepsin K Sandwich ELISA kit (LS-F4597-1, LSBio, Seattle, USA).
Statistical Analysis
Prism 8.0 software were used to carry out statistical analyses of this study. One- or two-way analysis of variance (ANOVA) methods with a post hoc test was used to analyze the differences between groups. All data were represented mean ± standard deviation (SD). P value less than 0.05 (*P < 0.05) was presented comparing to control or Sham groups.
Results
Isolation and Identification of miR-146a-Exo
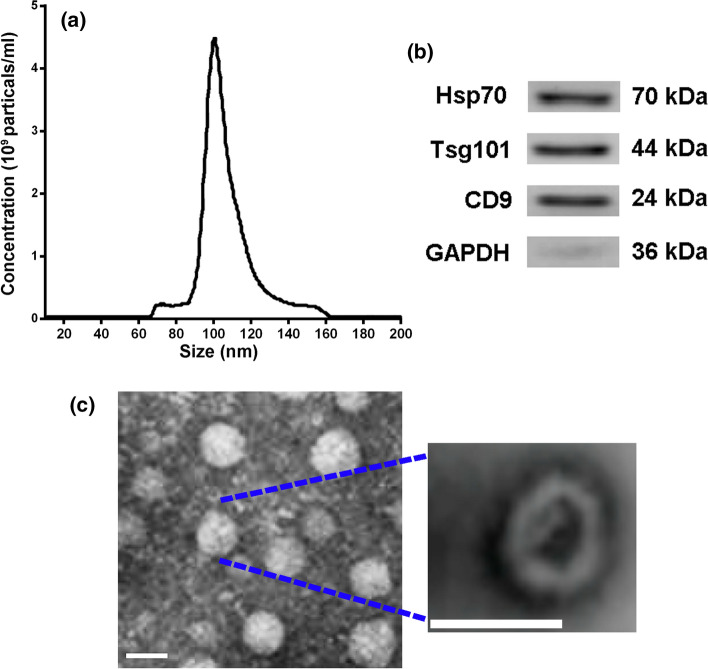
MSCs were isolated from epididymal adipose tissues and in vitro transduced with control or miR-146a lentivirus to generate miR-146 overexpressed AD-MSCs (miR-146a-MSC). Exosomes were extracted and purified from the conditional medium of MSC, vector-MSC, and miR-146a-MSC, and their size and concentration were determined by using nanoparticle tracking analysis. The result showed that the presence of exosomes peaked at 100 nm with 4.5 × 109 particles/ml (Figure 1a). Exosomal surface markers Hsp70, Tsg101 and CD9 were detected using Western blot, which confirmed the expression of these markers in the isolated exosomes (Figure 1b). We further profiled the morphology of the isolated exosomes by using transmission electron microscopy, and found exosomes were spherical body with a diameter of 50-100 nm (Figure 1c).
Figure 1.
Confirmation and characterization of AD-MSC-derived exosomes. (a) Size of the particles in the isolated exosome mixture obtained through nanoparticle tracking analysis. (b) Western blot analysis confirmed the expression of indicated exosomal surface markers in the isolated exosome mixture. (c) Transmission electron microscopy revealed exosome morphology. Scale bar = 100 nm.
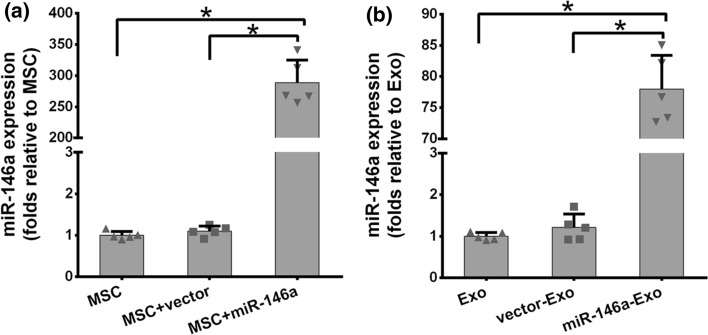
Next, we examined the expression level of miR-146a in three AD-MSC cell lines and the exosomes secreted by these cells through qRT-PCR. As shown in Figure 2a, the expression of miR-146a were comparable in MSCs and vector transduced MSCs (MSC+vector), however, there was almost 300-fold increase in miR-146a transduced MSCs (MSC+miR-146a). Similarly, miR-146a expression level was significantly higher in MSC+miR-146a than that in MSC and MSC+vector cells (Figure 2b). These results indicated the exosomes were isolated from MSC+miR-146a, which successfully overexpressed miR-146a as predicted.
Figure 2.
miR-146a levels in cultured MSCs (a) and the corresponding exosomes (b). *P < 0.05 between the indicated groups. Results are expressed as mean ± SD from three separate experiments (n = 5).
miR-146a-Exo Suppress HG-Induced Pro-inflammatory Cytokines of Osteoclasts In Vitro
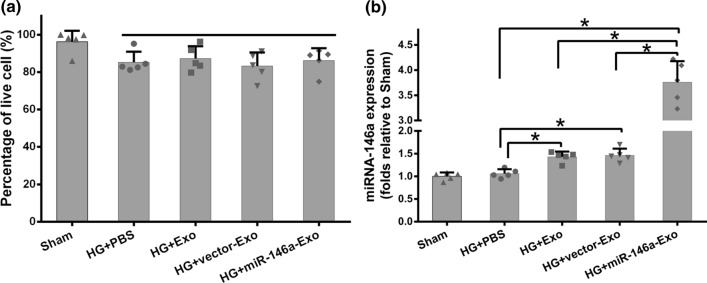
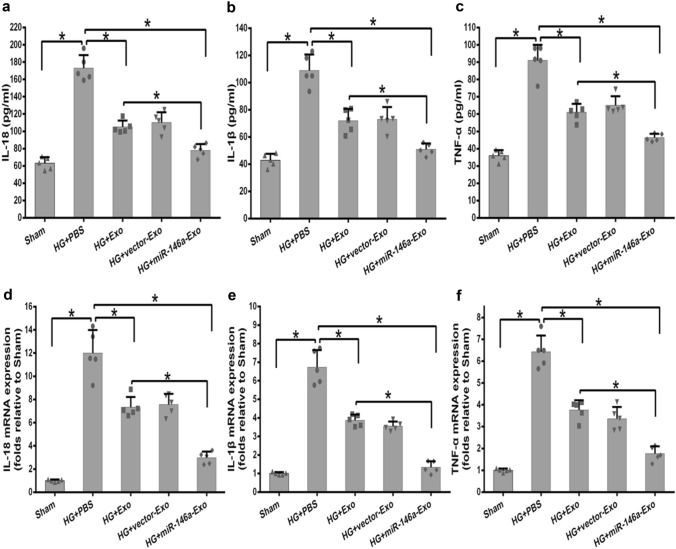
Here, we evaluated the anti-inflammation role of miR-146a-Exo using the same HG-induced inflammatory response system in vitro. MTT assay was performed firstly, and comparable viability was observed in three exosomes (Exo, vector-Exo, and miR-146a-Exo) treated osteoclasts compared to that of PBS control group (Figure 3a). The qRT-PCR was conducted to analyze miR-146a expression level in different exosomes treated groups. Interestingly, miR-146a level was significantly increased in both Exo and vector-Exo treated osteoclasts, which indicated that miR-146a is one of important natural components in AD-MSC exosomes (Figure 3b). As expected, the highest expression level of miR-146a was observed in the exosomes of miR-146a-Exo treated osteoclasts (Figure 3b). These results confirmed that the overexpression of miR-146a in MSC+miR-146a and its enrichment in exosomes. Next, we investigated the inflammatory response of HG-stimulated osteoclasts with different exosomes treatment through measuring the expression of pro-inflammatory cytokines. ELISA and qRT-PCR data showed that HG stimulation significantly increased the secretion and expression of pro-inflammatory cytokines, IL-18, IL-1β, and TNF-α (Figure 4a-4f). Exo and vector-Exo had similar inhibitory effect on the expression of pro-inflammatory cytokines compared to that of PBS group, interestingly, miR-146a-Exo treatment significantly reduced the secretion and expression of pro-inflammatory cytokines relative to both PBS and Exo controls (Figure 4a-4f). All these data suggested that HG-induced inflammatory response of osteoclasts was decreased by the exosome treatment, miR-146a overexpressed exosomes had the most potent inhibitory effect.
Figure 3.
Effects of MSCs-derived exosomes on osteoclast viability and expressions of miR-146a in osteoclast. (a) Viability of osteoclast in the presence of different Exos. (b) qRT-PCR was used to analyze the mRNA expression levels of miR-146a in osteoclasts. Data are representative of three independent experiments (n = 5). Data are presented as mean ± SD. *P < 0.05between the indicated groups.
Figure 4.
Effects of MSCs-derived exosomes on pro-inflammatory cytokines in osteoclasts. (a-c) Elisa was used to detect the concentration of IL-18 (a), IL-1β (b) and TNF-α (c) released by osteoclasts. (d-f) qRT-PCR was used to analyze the mRNA expression levels of IL-18 (d), IL-1β(e) and TNF-α(f) in osteoclasts. Data are representative of three independent experiments (n = 5). Data are presented as mean ± SD. *P < 0.05 between the indicated groups.
miR-146a-Exo Restrain Bone Resorption of Osteoclasts In Vitro
Since HG could induce bone resorption through osteoclasts, we evaluated the effects of miR-146a-Exo on mature osteoclasts resorbing bovine bone slices. Bone resorption assay showed that Exo and vector-Exo treated osteoclasts had less resorption pits than that in PBS control group (Figure 5a and 5b). And the lowest resorption pits were observed in miR-146a-Exo treated osteoclasts group, which was significantly lower than that in both PBS and Exo groups (Figure 5a and 5b). Moreover, the concertation of cathepsin K, an important marker of bone resorption, was lower in Exo and vector-Exo groups, which was further decreased in miR-146a-Exo group (Figure 5c). The above data indicated that exosomes treatment decreased the bone resorption capacity, and miR-146a overexpressed exosomes further enhanced the inhibitory effect on bone resorption of osteoclasts.
Figure 5.
Effects of MSCs-derived exosomes on the bone resorption capacity in osteoclasts. (a) Resorption pits induced by osteoclasts with or without exosomes. Pits as indicated by white contours were formed by osteoclasts resorbing on bovine bone slices. Scale bar = 100 μm. (b) Quantitative analysis of mean pit area (μm2) per visual field on bovine bone slices. (c) The levels of cathepsin K in the medium were detected by ELISA. Data are representative of three independent experiments (n = 5). Data are presented as mean ± SD. *P < 0.05 between the indicated groups.
miR-146a-Exo Administration Improves Diabetic Osteoporosis Through Suppressing the Activation of Inflammasome of Osteoclasts In Vivo
We further examined the anti-inflammatory effect of miR-146a-Exo in the well-established STZ-induced diabetic osteoporosis rat model. Upon STZ treatment, both bone mineral and bone mineral density were decreased with the time prolonged (Figure 6a and 6b). Exosomes administration could relieve the bone loss in STZ-induced diabetic osteoporosis rats, and miR-146a-Exo showed the strongest inhibitory effect on STZ-induced bone loss, interestingly, both bone mineral and bone mineral density of rats in STZ+miR-146a-Exo group were significantly higher than those in STZ+Exo and STZ+vector-Exo groups (Figure 6a and 6b). At the end of treatment (12-week), femur and tibia were harvested and weighted, the result showed that bone weight was recovered in exosomes treated rats, and STZ+miR-146a-Exo administration had the strongest effect on bone weight gain (Figure 6c and 6d). Urinary deoxypyridinoline, a marker of bone collage reflecting bone loss, was increased in STZ-induced diabetic osteoporosis rats, and reduced in exosomes treated rats especially in STZ+miR-146a-Exo administrated rats (Figure 7a). Similarly, the concentration of cathepsin K, marker of osteoclast reflecting activation of inflammasome, was elevated in STZ treated rats and significantly reduced in STZ+miR-146a-Exo administrated rats (Figure 7b). In bone tissues, the protein level of pro-inflammatory cytokines (TNF-α, IL-18 and IL-1β) was increased in STZ treated rats, decreased in rats of STZ+Exo and STZ+vector-Exo groups, and significantly reduced in STZ+miR-146a-Exo rats (Figure 7c and 7d). All these data indicated that miR-146a-Exo could suppress the activation of inflammasome by inhibiting HG associated inflammatory response of osteoclasts, and then improve diabetic osteoporosis in vivo.
Figure 6.
Effects of MSCs-derived exosomes on bone mineral content, bone mineral density and bone weight in diabetic osteoporosis model rats. (a-b) DXA was used to assay the bone mineral content (a) and bone mineral density (b) in diabetic osteoporosis model rats. #P < 0.05 between the STZ+Exo group (blue) and STZ+miR-146a-Exo group (green). (c-d) The weight of femur(c) and tibia (d) were measured in rats from indicated groups. *P < 0.05 between the indicated groups. Data are representative of three independent experiments (n = 5). Data are presented as mean ± SD.
Figure 7.
Effects of MSCs-derived exosomes on the indicators of osteoporosis and inflammasome-related proteins in diabetic osteoporosis model rats. (a) The levels of deoxypyridinoline in the urine were detected by ELISA. (b) The levels of cathepsin K in the serum were detected by ELISA. (c) Western blot was used to detect the expression of IL-1β, IL-18 and TNF-α in bone tissues. (d) The relative grayscale analysis of Western blot results by Fiji software. Data are representative of three independent experiments (n = 5). Data are presented as mean ± SD. *P < 0.05 between the indicated groups.
Discussion
As an important member small noncoding RNAs family, microRNAs have emerged as powerful post-transcriptional regulators of almost all biological processes.25,26 Cumulative evidence indicated that various cellular development and function of immune system are regulated by microRNA.27–29 Taganov et al. found that miR-146a was sharply up-regulated by LPS exposure and worked as an inhibitor of innate immune responses through targeting Toll-like receptor and cytokine signaling.29 However, most of microRNAs are not stable duo to the multiple pathways mediated degradation and turnover.30,31 MSCs secrete numerous factors in the form of exosomes that exert remarkable functions on the inhibition of apoptosis, anti-fibrosis, tissue regeneration, anti-inflammatory, and immunosuppressive, which provide a new way for the tissue injury and inflammation treatment.32–35 In this study, we combined the advantages of both MSC-Exo and miR-146a by generating miR-146a overexpressed AD-MSCs (miR-146a-Exo) to explore its protective effect on osteoclast inflammation and verify its potential therapeutic capacity for the diabetic osteoporosis treatment in vitro and in vivo.
The miR-146a is one of the major regulators in AD-MSCs exosomes on the regulation of pro-inflammatory cytokines secretion and inflammasome activation in HG-stimulated osteoclasts. Our previous study revealed that AD-MSCs-derived exosomes reduced the secretion of pro-inflammatory cytokines and suppressed NLRP3 inflammasome activation in osteoclasts (in revision). Here, we found that miR-146a is the major regulator in AD-MSCs-derived exosomes to exert the anti-inflammation effect coming from the below evidence: (1) Both Exo/vector-Exo and miR-146a-Exo significantly reduced the expression and secretion of pro-inflammatory cytokines (such as TNF-α, IL-18, and IL-1β) in osteoclasts, however, miR-146a-Exo had the most potent inhibitory effect; (2) HG significantly induced bone resorption capacity of osteoclasts,36 although Exo/vector-Exo treatment reduced bone resorption, miR-146a-Exo treatment presented the strongest inhibitory effect (Figure 5); (3) In vivo experiment showed that the most potent protective effect on bone loss (bone mineral and bone mineral density) was observed in rats with STZ+miR-146a-Exo administration. In addition, the anti-inflammation role of miR-146a has been reported in other cellular and animal models. In HG-stimulated human aortic endothelial cells (HAECs) and db/db mice, miR-146a decreased HG-induced endothelial inflammation through inhibiting the expression of interleukin-1 receptor-associated kinase 1.16 Wu et al. reported that miR-146a could attenuate experimental colitis by suppressing and IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) in inflammatory bowel disease (IBD) mouse model.37 Our studies and others suggested that miR-146a exerted potent anti-inflammation effect in various cell types and exosomes with overexpressed miR-146a was able to magnify these benefits.
As a well-known anti-inflammatory microRNA, miR-146a targets effectors in different cell types. For example, miR-146a reduced endothelial inflammation via targeting IRAK1 in human HAECs, while it attenuated IBD through inhibiting TRAF6 and IRAK1.16,37 In this study, we observed significant reduction of pro-inflammatory cytokines (such as TNF-α, IL-18, and IL-1β) expression, which indicated that miR-146a might target these genes in osteoclasts. Interestingly, miR-146a was predicted to base-pair with the TRAF6 and IRAK1 in their 3’UTR regions.29 Whether miR-146a can target TNF-α, IL-18, and IL-1β directly or through other unknown regulators in osteoclasts need to be addressed in the future study.
Conclusion
This study demonstrated that combination of AD-MSC-Exo and miR-146a could more effectively exert the anti-inflammation effect in osteoclasts. The miR-146a-Exo can be used as a potential drug for the treatment of diabetic osteoporosis.
Conflict of interest
Lei Zhang, Qinghai Wang, Hang Su, and Jiaxiang Cheng declare that they have no conflict of interest.
Ethical Approval
No human studies were carried out by the authors for this article. All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, NIH), and were approved by the ethical committee of Cangzhou Central Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamann C, Kirschner S, Gunther KP, Hofbauer LC. Bone, sweet bone–osteoporotic fractures in diabetes mellitus. Nat. Rev. Endocrinol. 2012;8(5):297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]
- 2.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 3.Cheloni R, Gandolfi SA, Signorelli C, Odone A. Global prevalence of diabetic retinopathy: protocol for a systematic review and meta-analysis. BMJ Open. 2019;9(3):e022188. doi: 10.1136/bmjopen-2018-022188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly JJ, El-Hamdouchi A, Diouf A, Monyeki A, Somda SA. Determining the worldwide prevalence of obesity. Lancet. 2018;391(10132):1773–1774. doi: 10.1016/S0140-6736(18)30794-3. [DOI] [PubMed] [Google Scholar]
- 5.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 6.Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr. Diabetes Rep. 2014;14(1):453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asadipooya K, Uy EM. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J. Endocr. Soc. 2019;3(10):1799–1818. doi: 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int. J. Mol. Sci. 2019;20(14):3576. doi: 10.3390/ijms20143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin. Cases Miner. Bone Metab. 2017;14(2):209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanazawa I. Interaction between bone and glucose metabolism [Review] Endocr. J. 2017;64(11):1043–1053. doi: 10.1507/endocrj.EJ17-0323. [DOI] [PubMed] [Google Scholar]
- 11.De Martinis M, Sirufo MM, Nocelli C, Fontanella L, Ginaldi L. Hyperhomocysteinemia is associated with inflammation, bone resorption, Vitamin B12 and folate deficiency and MTHFR C677T polymorphism in postmenopausal women with decreased bone mineral density. Int. J. Environ. Res. Public Health. 2020;17(12):4260. doi: 10.3390/ijerph17124260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SS, Hu Y, Xie PL, Cao J, Wang ZX, Liu JH, Yin H, Huang J, Tan YJ, Luo J, Luo MJ, Tang SY, Chen TH, Yuan LQ, Liao EY, Xu R, Liu ZZ, Chen CY, Xie H. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone Res. 2018;6:9. doi: 10.1038/s41413-018-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tofino-Vian M, Guillen MI, Perez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol. Biochem. 2018;47(1):11–25. doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
- 14.Yun B, Maburutse BE, Kang M, Park MR, Park DJ, Kim Y, Oh S. Short communication: dietary bovine milk-derived exosomes improve bone health in an osteoporosis-induced mouse model. J. Dairy Sci. 2020;103(9):7752–7760. doi: 10.3168/jds.2019-17501. [DOI] [PubMed] [Google Scholar]
- 15.Yang C, Lim W, Park J, Park S, You S, Song G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells. Mol. Hum. Reprod. 2019;25(11):755–771. doi: 10.1093/molehr/gaz054. [DOI] [PubMed] [Google Scholar]
- 16.Lo WY, Peng CT, Wang HJ. MicroRNA-146a-5p mediates high glucose-induced endothelial inflammation via targeting interleukin-1 receptor-associated kinase 1 expression. Front. Physiol. 2017;8:551. doi: 10.3389/fphys.2017.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011;208(6):1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CL, Sung PH, Chen KH, Shao PL, Yang CC, Cheng BC, Lin KC, Chen CH, Chai HT, Chang HW, Yip HK, Chen HH. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018;10(4):1053–1070. [PMC free article] [PubMed] [Google Scholar]
- 19.Vestergaard P. Bone metabolism in type 2 diabetes and role of thiazolidinediones. Curr. Opin. Endocrinol. Diabetes Obes. 2009;16(2):125–131. doi: 10.1097/MED.0b013e328325d155. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, Antounians L, Miyake H, Koike Y, Chen Y, Zani A, Sherman PM, Pierro A. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE. 2019;14(1):e0211431. doi: 10.1371/journal.pone.0211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgess D, Mazzorana M, Terrado J, Delprat C, Chamot C, Guasch RM, Perez-Roger I, Jurdic P, Machuca-Gayet I. Comparative transcriptomics reveals RhoE as a novel regulator of actin dynamics in bone-resorbing osteoclasts. Mol. Biol. Cell. 2014;25(3):380–396. doi: 10.1091/mbc.e13-07-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren ZY, Machuca-Gayet I, Domenget C, Buchet R, Wu Y, Jurdic P, Mebarek S. Azanitrile cathepsin K inhibitors: effects on cell toxicity, osteoblast-induced mineralization and osteoclast-mediated bone resorption. PLoS ONE. 2015;10(7):e0132513. doi: 10.1371/journal.pone.0132513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, Burstow D, Brown L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003;12(1):44–50. doi: 10.1046/j.1444-2892.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 24.An Y, Zhang H, Wang C, Jiao F, Xu H, Wang X, Luan W, Ma F, Ni L, Tang X, Liu M, Guo W, Yu L. Activation of ROS/MAPKs/NF-kappaB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019;33(11):12515–12527. doi: 10.1096/fj.201802805RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 2008;9(8):839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 27.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010;11(2):141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 28.Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, Suzuki M, Nagasaki M, Ohtsuki M, Ono M, Furukawa H, Nagashima M, Yoshino S, Mabuchi A, Sekine A, Saito S, Takahashi A, Tsunoda T, Nakamura Y, Yamamoto K. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat. Genet. 2003;35(4):341–348. doi: 10.1038/ng1267. [DOI] [PubMed] [Google Scholar]
- 29.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baraban JM, Shah A, Fu X. Multiple pathways mediate microRNA degradation: focus on the translin/Trax RNase complex. Adv. Pharmacol. 2018;82:1–20. doi: 10.1016/bs.apha.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Qin YW, Brewer G, Jing Q. MicroRNA degradation and turnover: regulating the regulators. Wiley Interdiscip. Rev. RNA. 2012;3(4):593–600. doi: 10.1002/wrna.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pers YM, Ruiz M, Noel D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027–2035. doi: 10.1016/j.joca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 34.Bouffi C, Bony C, Jorgensen C, Noel D. Skin fibroblasts are potent suppressors of inflammation in experimental arthritis. Ann. Rheum. Dis. 2011;70(9):1671–1676. doi: 10.1136/ard.2010.143297. [DOI] [PubMed] [Google Scholar]
- 35.Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS ONE. 2012;7(9):e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picke AK, Campbell G, Napoli N, Hofbauer LC, Rauner M. Update on the impact of type 2 diabetes mellitus on bone metabolism and material properties. Endocr. Connect. 2019;8(3):R55–R70. doi: 10.1530/EC-18-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, Dong Y, Liu Y, Nan Z, Wang Y, Yu T, Liu X. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019;68:204–212. doi: 10.1016/j.intimp.2018.12.043. [DOI] [PubMed] [Google Scholar]