Abstract
Background
Inappropriate macrophages phenotype transition contributes to the development of ulcerative colitis, and the poly (ethylene glycol)-block-poly (d, l-lactic acid) (PEG-PLA) nanoparticles delivery system can be utilized to improve the cryptotanshinone (CTS)-based therapy.
Methods
We used a single emulsification method to prepare CTS-encapsulated nanoparticles (NPCTS). The therapeutic efficacy of NPCTS was evaluated in dextran sulfate sodium (DSS)-induced colitis mice. Then the proportion of total macrophages and M2-like macrophages were assayed with flow cytometry, and the relative content of pro-inflammatory cytokines in the colon was detected with Western blot. Bone-marrow-derived macrophages (BMDMs) were induced into M1-like macrophages, which were further incubated with NPCTS to repolarize into M2 subtype.
Results
Cryptotanshinone could induce the transition of M1 subtype to M2 subtype as indicated by up-regulated expression of arginase 1 (ARG1), interleukin (IL)-10, and CD206. In vivo, orally administrated NPCTS accumulated in the colon-infiltrated macrophages in colitis mice. It further revealed that NPCTS significantly alleviated colitis symptoms as indicated by increased body weight and colon length, decreased tumor necrosis factor (TNF)-α, IL-1β, and IL-6 content in the colon, and diminished total macrophage proportion (CD45+CD11b+F4/80+) and up-regulated M2 proportion (CD45+CD11b+F4/80+CD206hi).
Conclusion
Oral administration of NPCTS could ameliorate ulcerative colitis with the conversion of M1-like macrophages to M2-like macrophages.
Keywords: Cryptotanshinone, PEG-PLA, Ulcerative colitis, Macrophage
Introduction
As one of the two primary forms of inflammatory bowel diseases (IBD), ulcerative colitis is characterized by the chronic and spontaneous recurrence of inflammation in the inner surface of the human rectum and colon with increasing prevalence.4, 10, 13 It is worth noting that about 20% of ulcerative colitis patients will develop colitis-associated colon cancer (CAC),12, 19 which ranks third in terms of incidence, but second in terms of mortality as estimated globally in 2020.23 Although 5-aminosalicylic acid, corticosteroids, and immune-suppressants are recommended to treat human ulcerative colitis, the treatment benefit is not satisfying due to the long-term medication and limited efficacy. It is urgent to develop a more promising solution.
Although the precise chronic inflammatory mechanism leading to ulcerative colitis is still unclear, inappropriate macrophage phenotype transition to integrate signals from the environment may contribute to the pathogenesis.8, 9, 15 Tissue-resident macrophages are essential for intestinal homeostasis.20 In contrast, tissue-infiltrating macrophages may enter the mucosa through the broken intestinal barrier to mediate the inflammatory pathology.20 Some studies have shown that most macrophages infiltrated are pro-inflammatory M1-like macrophages, while the proportion of M2-like macrophages that can repair damaged tissues is relatively tiny. The impaired transition from pro-inflammatory responses to anti-inflammatory responses may promote IBD development and delay the resolution.11, 18 Previous studies suggest that polarizing M1-like macrophages into M2-like macrophages may be an effective treatment strategy for colitis.16, 17
As a natural compound derived from the roots of Salvia miltiorrhiza Bunge (Danshen), cryptotanshinone (CTS) could significantly inhibit the phosphorylation and dimerization of signal transducer and activator of transcription 3 (STAT3),22 which has been commonly utilized in traditional medicine to treat circulatory disorders, hepatitis, and chronic renal failure.1, 14 Due to the complex physical environment within the gastrointestinal tract, oral administration is limited in the therapeutic efficiency to deliver therapeutics to the site of infection. Nanoparticle-based pharmaceuticals delivery can promote the drug loading specifically to accumulate at the inflammatory sites due to the increased permeability of such tissues. Whether CTS-encapsulated nanoparticles (NPCTS) could be utilized in colitis to alter the macrophage phenotype transition is investigated.
Materials and Methods
Dextran Sulfate Sodium (DSS)-Induced Colitis
Specific pathogen-free C57BL/6 mice (male, 8-week-old, 20 ± 2 g) were ordered from Peking Vital River Laboratory Animal Ltd. (Beijing, China) and received 4% DSS (MP Biomedicals, USA) in autoclaved drinking water for 7 days. A comprehensive evaluation of body weight and the length of the colon were performed. All experimental procedures were performed in accordance with the guidelines approved by the Animal Ethics Committee of Daqing Oilfield General Hospital.
Isolation and In Vitro Stimulation of Bone Marrow-Derived Macrophages
Bone marrow cells derived from C57BL/6 mice hind femurs and tibiae were cultured in Dulbecco's Modified Eagle Medium (10% fetal bovine serum, 10 ng/mL macrophage colony-stimulating factor) for 7 days to get matured bone marrow-derived macrophages (BMDMs) as previously reported,26 which was further co-stimulated with 10 ng/mL lipopolysaccharides (LPS, St. Louis, MO, USA) and 10 ng/mL interferon (IFN)-γ (PeproTech, Bedford, MA, USA) for 24 hrs to induce polarized M1-like macrophages. The phenotype transition of M1 to M2 was induced by CTS (10 μM) incubation for another 24 h. F4/80-PerCP-Cy5.5, CD45-FITC, and CD206-BV510 antibodies (eBioscience, San Diego, CA, USA) were utilized to detect the proportion of macrophages. Flow cytometric analysis was performed with FlowJo 7.6 software (Treestar, Woodburn, OR, USA).
Quantitative Real-Time PCR
TRIzol reagent (Invitrogen, Waltham, MA, USA) was utilized to extract total RNA from BMDMs, which was further converted into cDNA with High-Capacity cDNA Reverse Transcription kits (Applied Biosystems, Foster City, CA, USA). FastStart Universal SYBR Green Master Mix (Roche, Penzberg, Upper Bavaria, Germany) was applied to detect the amplification in an ABI STEPONE real-time PCR System (Applied Biosystems). The relative expression was quantitated with the 2−∆∆Ct method. Primer3 designed primers were synthesized from Sangon Biotech (B661302, Shanghai, China), and the sequences were list as follow: IL-1β, forward primer 5′-TGGACCTTCCAGGATGAGGACA-3′, 5′-GTTCATCTCGGAGCCTGTAGTG-3′; iNOS, forward primer 5′-TGCCACGGACGAGACGGATAG-3′, reverse primer 5′-CTCTTCAAGCACCTCCAGGAACG-3′; TNF-α, forward primer 5′-GGTGCCTATGTCTCAGCCTCTT-3′, reverse primer 5′-GCCATAGAACTGATGAGAGGGAG-3′; Arg-1, forward primer 5′-CATTGGCTTGCGAGACGTAGAC-3′, reverse primer 5′-GCTGAAGGTCTCTTCCATCACC-3′; IL-10, forward primer 5′-CGGGAAGACAATAACTGCACCC-3′, reverse primer 5′-CGGTTAGCAGTATGTTGTCCAGC-3′; CD206, forward primer 5′-GTTCACCTGGAGTGATGGTTCTC-3′, reverse primer 5′-AGGACATGCCAGGGTCACCTTT-3′.
Preparation, Characterization, and Cellular Internalization of Nanoparticles
NPCTS were prepared based on the single emulsification technique as previously reported.25 In brief, PEG5000-b-PLA11,000 (10.0 mg) and CTS or RhoB-Chol (0.1 mg), with or without lipids (1 mg), were dissolved in 200 µL ethyl acetate, which was further emulsified with 1 mL deionized water by sonication for 2 min at 80 W. Ethyl acetate was final removed by a rotary evaporator and non-emulsified chemical was removed by centrifugation at 3000 rpm for 10 min. The morphology and size distribution were detected via Malvern Zetasiser Nano ZS90 dynamic light scattering (DLS) and JEOL JEM2010 transmission electron microscopy (TEM) instruments. Six hours after NPCTS oral gavage, the mice were sacrificed to collect the colon tissues, and IVIS in vivo imaging system (Perkin Elmer; Waltham, MA, USA) was used to detect the accumulation of NPCTS.
Immunofluorescence and H&E Staining
Excised colon tissues (10 mm away from the caecum) were weighed and fixed with 4% paraformaldehyde for 4 h and flash frozen in Optimal Cutting Temperature compound (OCT) for immunofluorescence detection with Alexa Fluor 488 for the cytoskeleton or embedded in paraffin for hematoxylin and eosin stain (H&E). Sections were sequently cut at 5-µm thickness with a Leica CM1950 cryostat and stained.
Western Blotting
The colon homogenate was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes, which were further incubated with primary antibodies against IL-1β, TNF-α, and IL-6 (Santa Cruz, Dallas, TX, USA) at a 1:1000 dilution at 4 °C overnight. Then, the membranes were incubated with a peroxidase-conjugated secondary antibody (Sigma-Aldrich) at a 1:1000 dilution for 2 h at room temperature and developed with an ECL system (GE Healthcare Life Sciences, Chalfont, UK). The relative intensity of the interest bands was calculated by correcting for β-actin (Santa Cruz) with NIH-Image J1.51.
Statistical Analysis
Student t-test, one-way or two-way ANOVA analysis with a post hoc test was applied for the data analysis. The significance level was set as p-value < 0.05.
Results
CTS Repolarizes Macrophage Towards the M2 Subtype In Vitro
The chemical structure of CTS was shown in Fig. 1a, and the culture procedure was shown in Fig. 1b. In brief, hind femurs and tibiae-derived BMDMs (M0) were stimulated with LPS and IFN-γ to induce the differentiation of M1-like macrophages, which were further stimulated with CTS to assay the transition on macrophage phenotype. Genetic expression of M1 and M2 markers were quantified by qRT-PCR in BMDMs. As expected, the relative mRNA expression of M1 polarization-relevant IL-1β, inducible nitric oxide (iNOS), and TNF-α were all significantly decreased in BMDMs after CTS incubation (Fig. 1c), while M2 polarization-relevant Arg1, IL-10, and CD206 showed dramatically up-regulated expression (Fig. 1d). All of these demonstrated that CTS could repolarize M1-like macrophages towards the M2 subtype in vitro.
Figure 1.
Cryptotanshinone (CTS) repolarizes macrophage towards the M2 subtype in vitro. (a) Chemical structure of cryptotanshinone (CTS). (b) Murine bone-marrow-derived macrophages (BMDMs) were treated with lipopolysaccharide (LPS, 100 ng/mL) plus interferon (IFN)-γ (20 ng/mL) to induce an M1-like polarization state, the phenotype was examined after further treated with CTS (10 μM) for 24 h. (c) Expressions of IL-1β, iNOS, and TNF-α in CTS-treated M1-like macrophages were examined via real-time PCR. (d) Expressions of Arg1, IL-10, and CD206 in CTS-treated M1-like macrophages were examined via real-time PCR. Data were shown as means ± SD, **p < 0.01, ***p < 0.001 (vs control group).
CTS-Encapsulated Nanoparticles Accumulate in the Colon-Infiltrated Macrophages in DSS-Induced Colitis Mice
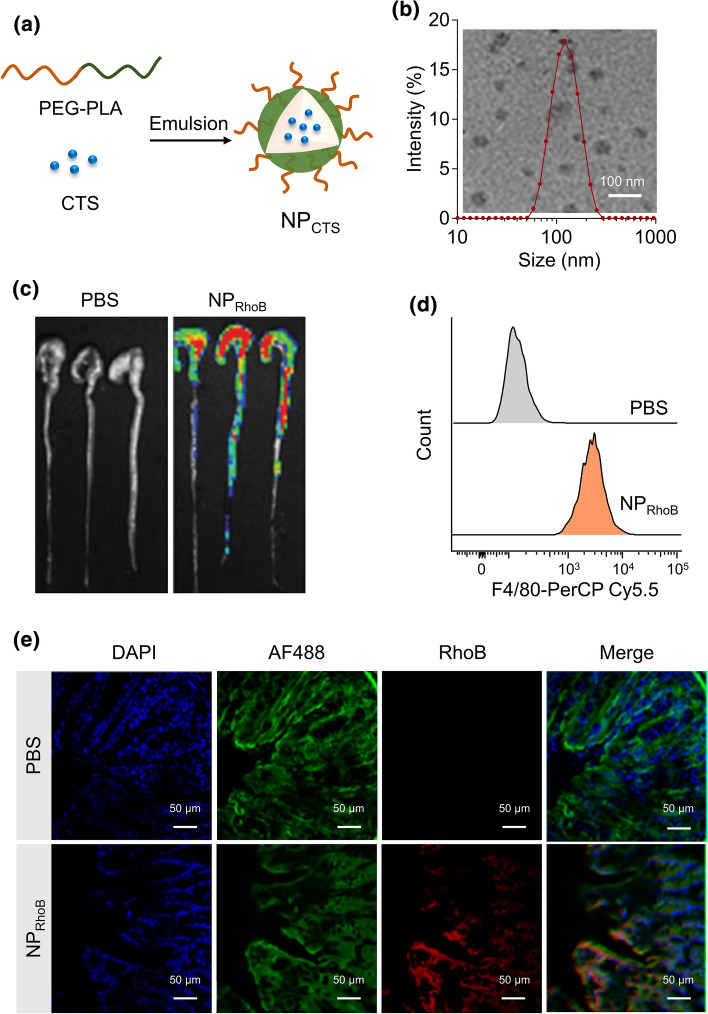
Schematic illustration of the procedure of CTS co-assembled with polymer PEG-b-PLA to form CTS-loaded nanoparticles (NPCTS) (Fig. 2a). The average size of NPCTS was around 100 nm, as detected by DLS and TEM (Fig. 2b). The accumulation of RhoB-Chol labeled nanoparticles in colon tissues after oral gavage was detected by the IVIS Lumina imaging system (Fig. 2c), which had also been testified by the fluorescence detection in the localized epithelial cells layer in the slices of colons (Fig. 2e). The single epithelial cells were isolated, and the fluorescence of RhoB-Chol in F4/80-PerCP-Cy5.5 macrophages was detected by flow cytometry, and the results indicated that NPCTS could be internalized by macrophage in the inflammatory colitis (Fig. 2d).
Figure 2.
CTS-encapsulated nanoparticles accumulate in the colon-infiltrated macrophages in DSS-induced colitis mice. (a) CTS-loaded nanoparticles were prepared after co-assemble polymer PEG-b-PLA with CTS using the single emulsification method. (b) The morphology and size distribution were detected via transmission electron microscopy (TEM) and dynamic light scattering (DLS). (c) Distribution of RhoB-labeled nanoparticles (150 μg) in the colon of dextran sulfate sodium salt (DSS)-induced colitis mice were imaged by IVIS after 6 h post oral gavage. (d) Distribution of RhoB-Chol-loaded nanoparticles in the colons-infiltrating macrophages in colitis mice. (e) Distribution of RhoB-Chol-loaded nanoparticles in the colons of colitis mice.
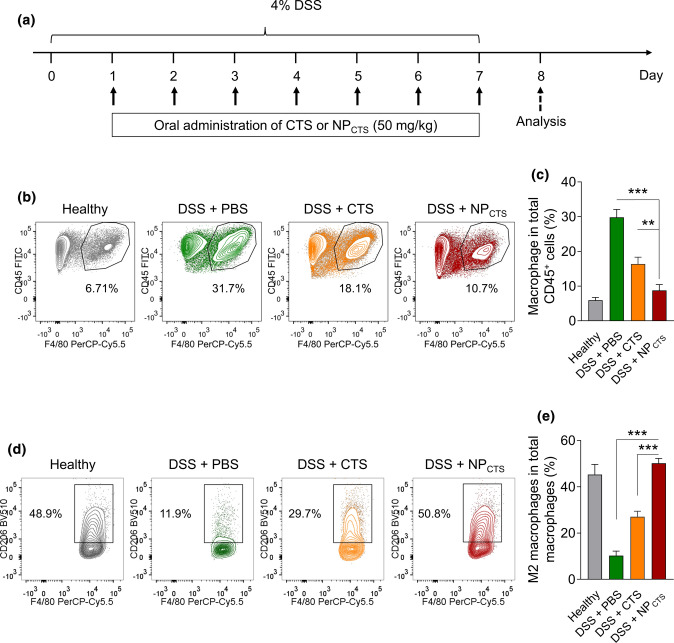
Oral Administration of NPCTS Repolarizes Macrophage Towards the M2 Subtype In Vivo
As shown in Fig. 3a, mice were fed with 4% DSS in drinking water to induce colitis for 7 days, and at the same time, NPCTS was administrated to investigate the curative effect. The proportion of infiltrating CD45+CD11b+F4/80+ macrophages in the colon was detected by flow cytometry. Experimental data showed that CTS could decrease the proportion of infiltrating macrophages in colitis tissue, which could be further significantly diminished by NPCTS treatment (Figs. 3b and 3c). It was further revealed that NPCTS treatment could dramatically up-regulate the proportion of M2 macrophage (CD45+CD11b+F4/80+CD206hi) in colitis (Figs. 3d and 3e). All in all, these results indicated that NPCTS could diminish the total proportion of macrophage with up-regulated M2 phenotype transformation.
Figure 3.
Oral administration of NPCTS repolarizes macrophage towards the M2 subtype in vivo. (a) Timeline of CTS or NPCTS-mediated treatment of DSS-induced colitis mice. (b–c) Flow cytometry demonstrating the percentages of CD45+CD11b+F4/80+ macrophages in colon tissues after treatment. (d–e) Flow cytometry demonstrating the percentages of CD45+CD11b+F4/80+CD206hi M2-like macrophages in colon tissues after treatment. Data were shown as means ± SD, **p < 0.01, ***p < 0.001.
Therapeutic Effect of CTS or NPCTS on DSS-Induced Colitis Mice
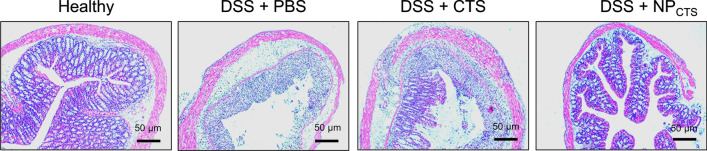
The relative expressions of IL-1β, IL-6, and TNF-α in the colon homogenate were examined via Western blot (Fig. 4a). It revealed that both CTS or NPCTS could down-regulate the relative content of TNF-α (Fig. 4b), IL-6 (Fig. 4c), and IL-1β (Fig. 4d) in DSS-induced colitis mice, and NPCTS could further diminish the content of the above cytokines in the colon when compared with CTS treatment. It was further revealed that both CTS and NPCTS could increase the length of the colon (Fig. 4e) and the body weight (Fig. 4f), and NPCTS showed the additional treatment benefit when compared with CTS with more regular mucosal structure and less infiltrated mononuclear cells (Fig. 5). These results demonstrated the treatment advantage of NPCTS compared with CTS with diminished cytokines expression and less severe symptoms.
Figure 4.
The therapeutic effect of CTS or NPCTS on DSS-induced colitis in mice. (a) Expressions of IL-1β, IL-6, and TNF-α in the colon homogenate from different groups were examined via western blot. Expressions of TNF-α (b), IL-6 (c), and IL-1β (d) in the colon homogenate from different groups were examined via real-time PCR. (e) The length of colon after 7 days of treatment. (f) Changes in body weight. Data were shown as means ± SD, *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 5.
Protection of mucosal structure. After 7 days of treatment, the colons were collected and H&E stained.
Discussion
Macrophages are locally enriched and polarized into M1 subtype to drive chronic and relapsing inflammation in ulcerative colitis, while M2 subtype macrophages may have inflammation resolution roles.3, 5, 29 Therefore, targeting the paradigm of macrophage phenotypes may be a novel treatment option to alter the disease status. In the present study, we firstly demonstrate that CTS remarkably attenuates the clinical symptoms of DSS-induced colitis in mice. Mechanistic investigations show that CTS converts inflammatory M1-like macrophages to anti-inflammatory M2-like macrophages. More importantly, the nanoparticle-based delivery system can deliver CTS to macrophages in the inflammatory site and get better treatment effects than CTS treatment in vivo. Our investigation demonstrates the potential of NPCTS in reprogramming macrophage polarization, which may be considered a promising therapeutic method for treating ulcerative colitis.
The PLA-PEG drug delivery systems can promote the macrophage-specific target effect.28 Macrophages can entrap PLA through the opsonization process, and PEG can add stealth features and biocompatibility of the emulsification,7 which has also been testified by the RhoB-labeled nanoparticles detection in the infiltrating macrophage in our colitis mice. All of these findings indicate that NPCTS has increased drug uptake by macrophages, and the intracellular mechanism may mediate the reprogramming of macrophage polarization.
Mechanically, JAK2/STAT3 inhibitor AG490 can inhibit bisphenol F-promoted macrophage M1 polarization.21 In comparison, IL-6/STAT3 signaling inhibition with anti-IL-6-treatment can turn macrophages into M1-like phenotype in hepatocellular carcinoma.27 Although the molecular mechanism is not clear, our investigation indicates that CTS-mediated STAT3 inhibition might promote the transition from M1-like macrophages to M2-like macrophages. Our results suggest that the phenotype transition might depend on the context environment.
Some limitations should be noted here. The precious phenotype of targeted macrophages is not deciphered in this investigation. A previous study demonstrates that Ly6Chi monocyte precursors can differentiate into both the resident and pro-inflammatory macrophages in the colon.2 Whether CTS could induce the differentiation of M0 macrophages into M2 macrophages needs further investigation. CTS can inhibit PI3K mediated macrophage migration induced by complement 5a6 and NF-κB and MAPK signaling pathways promoted inflammatory cytokines secretion in RAW264.7 macrophages.24 Whether such mechanisms also contribute to the alleviation of colitis needs further detailed analysis.
All in all, our investigation reveals that CTS-encapsulated PEG-PLA nanoparticles could be utilized to alter the macrophage phenotype transition and the pathology of colitis.
Conclusions
Oral administration of NPCTS could be considered as a novel therapeutic option to ameliorate ulcerative colitis.
Acknowledgments
Conflict of interest
Li Zhang, Longfei Yu and Yueguang Wei declare that they have no conflict of interest.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Daqing Oilfield General Hospital. No human studies were carried out by the authors for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Zhang and Longfei Yu have contributed equally to this work.
References
- 1.Ashrafizadeh M, Zarrabi A, Orouei S, Saberifar S, Salami S, Hushmandi K, Najafi M. Recent advances and future directions in anti-tumor activity of cryptotanshinone: a mechanistic review. Phytother. Res. 2021;35(1):155–179. doi: 10.1002/ptr.6815. [DOI] [PubMed] [Google Scholar]
- 2.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6(3):498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang JT. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020;383(27):2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 5.Domínguez Conde C, Teichmann SA. Deciphering immunity at high plexity and resolution. Nat. Rev. Immunol. 2020;20(2):77–78. doi: 10.1038/s41577-019-0254-0. [DOI] [PubMed] [Google Scholar]
- 6.Don MJ, Liao JF, Lin LY, Chiou WF. Cryptotanshinone inhibits chemotactic migration in macrophages through negative regulation of the PI3K signaling pathway. Br. J. Pharmacol. 2007;151(5):638–646. doi: 10.1038/sj.bjp.0707271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacalone G, Tsapis N, Mousnier L, Chacun H, Fattal E. PLA-PEG nanoparticles improve the anti-inflammatory effect of rosiglitazone on macrophages by enhancing drug uptake compared to free rosiglitazone. Materials (Basel, Switzerland) 2018;11(10):1845. doi: 10.3390/ma11101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gren ST, Grip O. Role of monocytes and intestinal macrophages in Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 2016;22(8):1992–1998. doi: 10.1097/MIB.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 9.He W, Kapate N, Shields CWT, Mitragotri S. Drug delivery to macrophages: A review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 2020;165:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Herrlinger KR, Stange EF. 25 years of biologicals in IBD: what’s all the hype about? J. Intern. Med. 2021;290:806–825. doi: 10.1111/joim.13345. [DOI] [PubMed] [Google Scholar]
- 11.Hine AM, Loke P. Intestinal macrophages in resolving inflammation. J. Immunol. 2019;203(3):593–599. doi: 10.4049/jimmunol.1900345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hnatyszyn A, Hryhorowicz S, Kaczmarek-Ryś M, Lis E, Słomski R, Scott RJ, Pławski A. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered. Cancer Clin. Pract. 2019;17:18. doi: 10.1186/s13053-019-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat. Rev. Dis. Primers. 2020;6(1):74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Gao C, Liu C, Liu L, Zhuang J, Yang J, Zhou C, Feng F, Sun C, Wu J. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 2021;137:111332. doi: 10.1016/j.biopha.2021.111332. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Dong Z, Liu K, Lu Y, Wu W, Qi J, Chen Z. Targeting strategies of oral nano-delivery systems for treating inflammatory bowel disease. Int. J. Pharm. 2021;600:120461. doi: 10.1016/j.ijpharm.2021.120461. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Liu D, Tan Y, Li R, Wang X, Deng F. Thalidomide attenuates colitis and is associated with the suppression of M1 macrophage polarization by targeting the transcription factor IRF5. Dig. Dis. Sci. 2021;1:1–10. doi: 10.1007/s10620-021-07067-2. [DOI] [PubMed] [Google Scholar]
- 17.Lv Q, Xing Y, Liu Y, Chen Q, Xu J, Hu L, Zhang Y. Didymin switches M1-like toward M2-like macrophage to ameliorate ulcerative colitis via fatty acid oxidation. Pharmacol. Res. 2021;169:105613. doi: 10.1016/j.phrs.2021.105613. [DOI] [PubMed] [Google Scholar]
- 18.Moreira Lopes TC, Mosser DM, Gonçalves R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 2020;69(12):1163–1172. doi: 10.1007/s00011-020-01398-y. [DOI] [PubMed] [Google Scholar]
- 19.Nebbia M, Yassin NA, Spinelli A. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2020;33(5):305–317. doi: 10.1055/s-0040-1713748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, DierckxdeCasterle I, Baekelandt V, GonzalezDominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, VandenBerghe P, Jones E, Lambrechts D, Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(2):400–415. doi: 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Shi M, Lin Z, Ye L, Chen X, Zhang W, Zhang Z, Luo F, Liu Y, Shi M. Estrogen receptor-regulated SOCS3 modulation via JAK2/STAT3 pathway is involved in BPF-induced M1 polarization of macrophages. Toxicology. 2020;433:152404. doi: 10.1016/j.tox.2020.152404. [DOI] [PubMed] [Google Scholar]
- 22.Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ, Han DC, Kwon BM. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69(1):193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- 23.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 24.Tang S, Shen XY, Huang HQ, Xu SW, Yu Y, Zhou CH, Chen SR, Le K, Wang YH, Liu PQ. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW2647 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation. 2011;34(2):111–118. doi: 10.1007/s10753-010-9214-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang JL, Gan YJ, Iqbal S, Jiang W, Yuan YY, Wang J. Delivery of tacrolimus with cationic lipid-assisted nanoparticles for ulcerative colitis therapy. Biomater. Sci. 2018;6(7):1916–1922. doi: 10.1039/C8BM00463C. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Zhang H, Sun L, Gao W, Xiong Y, Ma A, Liu X, Shen L, Li Q, Yang H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnol. 2020;18(1):38. doi: 10.1186/s12951-020-00593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S, Jian Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell Biochem. 2018;119(11):9419–9432. doi: 10.1002/jcb.27259. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Zhao Y, Hou T, Zeng H, Kalambhe D, Wang B, Shen X, Huang Y. Macrophage-based nanotherapeutic strategies in ulcerative colitis. J. Control Release. 2020;320:363–380. doi: 10.1016/j.jconrel.2020.01.047. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Yu J, Nie Y, Shi X, Liu Y, Li F, Zhang XL. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol. Invest. 2014;43(7):638–652. doi: 10.3109/08820139.2014.909456. [DOI] [PubMed] [Google Scholar]