Abstract
Introduction
In vivo, breast cancer cells spend on average 3–7 days adhered to the endothelial cells inside the vascular lumen before entering the brain. IL-1β is one of the highly upregulated molecules in brain-seeking triple negative breast cancer (TNBC) cells. In this study, the effect of IL-1β on the blood–brain barrier (BBB) and astrocytes and its role in transmigration of TNBC cells were evaluated.
Methods
The effect of IL-1β on transendothelial electrical resistance, gene and protein expression of human induced pluripotent stem cell-derived brain-specific microvascular endothelial-like cells (iBMECs) was studied. Transport of IL-1β across the iBMEC layer was investigated and the effect of IL-1β treatment of astrocytes on their cytokine and chemokine secretome was evaluated with a cytokine membrane array. Using BBB-on-a-chip devices, transmigration of MDA-MB-231 cells and their brain-seeking variant (231BR) across the iBMECs was studied, and the effect of an IL-1β neutralizing antibody on TNBC cell transmigration was investigated.
Results
We showed that IL-1β reduces BBB integrity and induces endothelial-to-mesenchymal transition in iBMECs. IL-1β crosses the iBMEC layer and induces secretion of multiple chemokines by astrocytes, which can enhance TNBC cell transmigration across the BBB. Transmigration assays in a BBB-on-a-chip device showed that 231BR cells have a higher rate of transmigration across the iBMECs compared to MDA-MB-231 cells, and IL-1β pretreatment of BBB-on-a-chip devices increases the number of transmigrated MDA-MB-231 cells. Finally, we demonstrated that neutralizing IL-1β reduces the rate of 231BR cell transmigration.
Conclusion
IL-1β plays a significant role in transmigration of brain-seeking TNBC cells across the BBB.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-021-00710-y.
Keywords: Brain metastasis, Breast cancer, Blood–brain barrier, Astrocytes, Chemokines, BBB-on-a-chip, Pluripotent stem cells, Endothelial-to-mesenchymal transition
Introduction
Metastasis, one of the key hallmarks of cancer, is responsible for more than 90% of human cancer deaths.22,28 It is estimated that in the United States 170,000 new cases of metastatic brain tumors are diagnosed every year, while the estimated number of new cases of primary brain tumors is around 20,000.50,56,60 Comprising 24% of brain metastasis cases, breast cancer is among the most common primary tumors that metastasizes to the brain.56 As advances in imaging technologies and improved therapeutics have resulted in longer survival time, the incidence of secondary brain tumors in breast cancer patients is increasing.56 The blood–brain barrier (BBB), a highly selective interface between the central nervous system and the circulatory system, tightly controls the transport of substances into the brain. Consequently, most anti-cancer drugs are ineffective for brain tumors as they are unable to cross the BBB.28 However, breast cancer cells are able to extravasate across the BBB to enter the brain during metastasis.38 Thus, targeting the extravasation step is a potential approach to prevent the formation of brain metastases. Understanding the mechanisms of cancer cell extravasation across the BBB is crucial for designing preventive therapies that inhibit brain metastasis.
A study of initial events in breast cancer extravasation into the brain in a mouse model revealed that the majority of breast cancer cells spend 3-7 days inside the capillary lumen before entering the brain.38 The long interaction time between breast cancer cells and the brain-specific microvascular endothelial cells (BMECs) comprising the BBB might result in phenotypic alterations in endothelial cells. BMECs possess an elaborate network of tight junctions and adherens junctions, which impedes paracellular passage of cells and large molecules across the BBB.63 Cancer cells that are able to successfully extravasate into the brain need to overcome the BBB by transmigrating through intercellular junctions (paracellular pathway) or through a single endothelial cell (transcellular pathway).17 Additionally, astrocytes, the most abundant cells in the central nervous system,42 are located in close proximity to the brain microvasculature and can modulate BBB integrity via paracrine signaling. As highly secretory cells, astrocytes become activated in pathological conditions and secrete various factors that can result in BBB damage or repair depending on the pathology.62,71 Interestingly, an animal study showed that during the initial stages of cancer cell extravasation across the BBB, astrocyte activation was detected while cancer cells were arrested in the capillaries prior to transmigration.38 Thus, reactive astrocytes could play a significant role in extravasation of cancer cells into the brain, which needs to be studied in more detail.
Both paracellular and transcellular pathways have been reported to be used by cancer cells to cross the BBB,16,24,26,51,72 but the molecular mechanisms that facilitate extravasation are not fully understood. Importantly, some types of cancer such as liver, pancreatic, and prostate cancer rarely metastasize to the brain,11,13,32 while lung and breast cancer commonly form brain metastases.47 Among primary breast cancers, HER2-overexpressing and triple negative breast cancer (TNBC) cells are the most common brain metastasis-forming subtypes.25 Previous studies showed cancer cell lines exhibit different extravasation potential based on their organ of origin and molecular subtypes.38,76 Additionally, generation of brain metastatic derivatives of existing cell lines through multiple rounds of in vivo selection have provided invaluable resources for studying molecular mechanisms of breast cancer extravasation into the brain. In these models, the brain-seeking variants of the parental cell lines exhibit significantly higher brain, but not bone and lung, metastasis.10
A recent review article by Fares et al. summarizes the genes that have been reported to play a role in extravasation of cancer cells, including breast cancer, across the BBB.17 ST6GALNAC5 overexpression in brain-seeking TNBC cells was shown to enhance adhesion of cancer cells to the brain endothelial cells.10 Angiopoietin-2 and TNF-α have been also been reported to be involved in extravasation of breast cancer cells into the brain via BBB impairment.6,74 Another study indicated a direct relationship between brain metastasis and c-Met pathway activation in breast cancer patients.75 Importantly, activation of this pathway results in upregulation of IL-1β in breast cancer cells. Furthermore, TNBC cells exhibit higher expression of c-Met compared to other subtypes of breast cancer, suggesting a potential role for IL-1β in TNBC extravasation across the BBB.75 Therefore, studying the effect of IL-1β on brain endothelial cells, specifically in extended times after treatment, could improve our understanding of the endothelial cell alterations during extravasation.
Previous studies on the effect of IL-1β on formation of brain metastases from breast cancer cells have focused on colonization rather than the extravasation step.73,75 IL-1β is a well-studied pro-inflammatory cytokine that is involved in numerous pathophysiological phenomena including central nervous diseases.27,35,58 One effect of IL-1β on brain endothelium is upregulation of cell adhesion molecules such as ICAM-1, VCAM-1, and E-selectin, which enhance the adhesion of both cancer cells and immune cells to the endothelial layer.48 IL-1β treatment of brain endothelial cells also results in reduced barrier tightness and increased permeability.5,9,48 Previously, treatment of endothelial cells of other tissues with IL-1β has been reported to induce endothelial-to-mesenchymal transition (EndoMT).12,40,54 EndoMT is the transient acquisition of a mesenchymal phenotype by endothelial cells, which can be caused by different molecular stimuli.20 Importantly, EndoMT is proposed to be tissue-specific, so based on the tissue in which endothelial cells reside, the response to EndoMT-inducing factors might be substantially different.19 Thus, use of brain-specific endothelial cells for studies concerning EndoMT in the brain is crucial. TGF-β1, a known EndoMT inducer, has been implicated in extravasation of melanoma cells to the brain, but breast cancer cells are thought to utilize a different mechanism to cross the BBB.31 The effect of synergistic treatment with IL-1β and TGF-β1 on inducing EndoMT in the hCMEC/D3 cell line has been investigated, suggesting that IL-1β exacerbates the effect of TGF-β1.14 However, the ability of IL-1β to induce EndoMT in BMECs in the absence of other stimuli has not been fully investigated.
Induced pluripotent stem cell-derived brain-specific microvascular endothelial-like cells (iBMECs) express the crucial markers of BMECs and possess superior barrier properties compared to existing cell lines and primary cells owing to the expression of essential junctional proteins of the BBB.36 Due to the tight barrier formed by iBMECs, they are an appropriate platform to study the transmigration of TNBC cells across the BBB in vitro and to investigate the effect of IL-1β on BMEC barrier integrity. However, iBMECs have not been used before to study breast cancer cell transmigration across the BBB. To enable such trans-endothelial migration studies, we recently reported a 3-D BBB-on-a-chip device that is composed of iBMECs and astrocytes and supports cell transmigration studies across the BBB in a physiologically relevant platform.45
In this study, we first investigated the effect of IL-1β, which was highly upregulated in brain-seeking TNBC cells, on iBMEC function and showed that IL-1β reduces the barrier tightness, as measured by transendothelial electrical resistance (TEER), and induces EndoMT in iBMECs after long-term (3 day) treatment. We also demonstrated that IL-1β is able to cross the iBMEC monolayer via receptor-mediated transcytosis, which results in exposure of astrocytes to IL-1β. Astrocytes that are exposed to IL-1β secrete a mixture of various chemokines and cytokines, which could further attract cancer cells into the brain. We then confirmed that IL-1β upregulation in brain-seeking TNBC cells increases their ability to transmigrate across the iBMEC monolayer, and the presence of astrocytes results in an increase in transmigrated 231BR cells in a BBB-on-a-chip system. Finally, we showed that the use of a neutralizing monoclonal IL-1β antibody could reduce the extravasation potential of brain-seeking TNBC cells.
Materials and Methods
Gene Set Enrichment Analysis
The existing GEO dataset GSE12237 was analyzed using bioinformatics array research tool (BART)3 in R, and extracted data were used to plot the graphs. A list of the human secretome reported by Uhlen et al.67 was used to select the secreted proteins from the differentially expressed genes. In addition, a list of 230 cytokines reported by Al-Yahya et al.2 was utilized to identify the differentially expressed cytokines in brain-seeking TNBC cells.
Culture of Astrocytes and Cancer Cells
Primary human astrocytes (from cerebral cortex) were purchased from ScienCell. Astrocytes were expanded and maintained on plates coated with gelatin (EmbryoMax, Millipore Sigma) in astrocyte growth medium (Cell Applications). Accutase was used for the detachment and dissociation of astrocytes. Low passage astrocytes (p4-p6) were used for all experiments in this study. MCF-7 (ATCC), MDA-MB-231 (ATCC) and 231BR (kindly provided by Dr. Lonnie Shea, University of Michigan) cell lines were maintained in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Millipore Sigma) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% penicillin-streptomycin (Thermo Fisher Scientific). Trypsin-EDTA (0.25%, Thermo Fisher Scientific) was used to detach cells from tissue culture surfaces for routine passaging.
iBMEC Differentiation
iBMECs were differentiated from the IMR-90-4 (WiCell) human induced pluripotent stem cell (hiPSC) line using a slightly modified version of the protocol described by Stebbins et al.64 In summary, on day 6 of differentiation, the medium was switched to endothelial cell medium (EC medium) supplemented with 20 ng/mL basic fibroblast growth factor (bFGF) (PeproTech), and 10 μM retinoic acid (Millipore Sigma). The EC medium consists of human endothelial serum free medium (Thermo Fisher Scientific) supplemented with 1% FBS. On day 8, cells were subcultured on collagen IV-coated surfaces (0.8 mg/mL) for selective purification of iBMECs. For BBB-on-a-chip devices, the purified endothelial cells were then subcultured again at a density of 500,000 cells per device in BBB-on-a-chip devices coated with 0.8 mg/mL collagen IV (Millipore Sigma). Fibronectin was not used in this study due to its reported role in inducing an inflammatory phenotype in endothelial cells.1,30 iBMECs on inserts (12-well plate format) were cultured for one day in EC medium supplemented with bFGF and retinoic acid. The next day, the medium was changed to EC medium without bFGF and retinoic acid, and for coculture systems, inserts were moved to 12-well plates that were already seeded with astrocytes at 90% confluency in 1.5 mL of EC medium.
IL-1β Treatment of iBMECs
Recombinant human IL-1β protein (Abcam) was reconstituted as directed by the supplier and diluted to 200 ng/mL in EC medium. Next, one-tenth of the iBMEC supernatant medium was replaced with the 200 ng/mL IL-1β solution to reach a final concentration of 20 ng/mL. The untreated samples received EC medium. For the long-term treatments, 20 ng/mL IL-1β was added daily to the iBMECs.
Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of IL-1β in Cancer Cell Conditioned Medium
Cancer cells were seeded onto 6-well plates at a density of 500,000 per well and were incubated overnight. The next day, the medium was changed to high glucose DMEM without serum, and cells were incubated for an additional 24 h. Then, conditioned medium was collected from each well and centrifuged (10 min, 700 rcf, 4 °C) to remove cell debris. Supernatant from each sample was aliquoted and stored at – 80 °C. The Legend Max Human IL-1β ELISA kit (BioLegend) was used to measure the concentration of IL-1β in the samples.
IL-1β Transcytosis Across the iBMECs
iBMECs were seeded onto cell culture inserts to form a tight barrier. Four days after initial seeding, TEER was measured to ensure the presence of a cellular barrier before conducting IL-1β transport studies. First, human IL1R1 or human IL1R2 blocking antibodies (R&D Systems) were added to the apical side of the cell culture inserts at a final concentration of 5 μg/mL and incubated at 37 °C for 30 min. Next, 20 ng/mL IL-1β was added to the apical side, and the inserts were incubated for 2 h. Samples were collected from the basolateral side and stored at – 80 °C until an ELISA assay was conducted to quantify IL-1β concentration.
Membrane Cytokine Array
A membrane-based human cytokine antibody array (80 targets, Abcam) was used to compare the level of cytokines and chemokines secreted by astrocytes treated with IL-1β and control samples. Astrocytes were seeded onto 6-well plates and cultured until around 90% confluency. Next, the medium was changed to serum-free astrocyte basal medium (Cell Applications) with or without 20 ng/mL IL-1β, and cells were incubated for 24 h. 1.5 mL of the medium was collected and briefly centrifuged (10 min, 700 rcf, 4 °C) to remove any cell debris. The supernatant of the centrifuged samples was used in the cytokine array assay following the manufacturer’s protocol. The arrays were imaged with a ChemiDoc Imager (Bio-Rad), and pixel intensity was quantified using Bio-Rad Image Lab Software (version 5.2.1). The quantified intensities were normalized to the positive control regions of the membrane as recommended by the manufacturer.
Gene Expression Analysis
RNA was extracted using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions, with lysate homogenization performed using QIAshredder columns (Qiagen). Residual DNA was removed using a RNase-free DNase Set (Qiagen). Purified RNA was reverse-transcribed into cDNA using the OmniScript Reverse Transcriptase Kit (Qiagen) and Oligo(dT)20 Primers (Life Technologies). PrimePCR SYBR Green Assays (Bio-Rad) and iTaq Universal SYBR Green Supermix (Bio-Rad) were used to conduct quantitative PCR. Table S1 shows the list of the primers used in this study. Cycle threshold (Ct) values determined by the BioRad CFX Connect Real-Time PCR Detection System were utilized to quantify relative mRNA expression levels using the ΔΔCt method, with GAPDH as the housekeeping gene.
BBB-on-a-Chip Fabrication
BBB-on-a-chip devices were fabricated using standard soft lithography as described previously.45 In brief, a polyethylene terephthalate (PET) membrane with 8.0 μm pore diameter was sandwiched between two layers of patterned polydimethylsiloxane (PDMS). The assembled device was bonded to a No. 1 microscope coverslip by oxygen plasma treatment. The top (apical) channel of the device was coated with 0.8 mg/mL collagen IV (Millipore Sigma) for at least 4 h. The collagen IV solution was removed and devices were left in a laminar flow hood for 20 min before introducing 5 mg/mL collagen type I with or without astrocytes to the hydrogel channel. After adding collagen I, devices were incubated for 30 min at 37 °C to cross-link the hydrogel. Afterwards, EC medium was added to the medium channels adjacent to the hydrogel channel, purified iBMECs were introduced to the apical channel (approximately 500,000 cells per device), and devices were incubated for an additional hour. The unattached iBMECs were subsequently removed from the apical channel, and EC medium in the reservoirs was replaced with fresh medium. After one day of culture in the devices, formation of a tight barrier (TEER > 900 Ω cm2) was confirmed using impedance spectroscopy, as previously reported,45 and the devices were ready for transmigration assays.
Immunocytochemistry of iBMECs, Astrocytes, and TNBC Cells
The primary antibodies, secondary antibodies, and dilution factors used in this study are listed in Supplementary Tables S2 and S3. iBMECs cultured on glass-bottom petri dishes or cell culture inserts and TNBC cells cultured on well plates were fixed for 15 min in 4% paraformaldehyde (PFA) and permeabilized with 0.3% Triton X-100 in DPBS for another 10 min. Next, cells were blocked for 1 hour in 10% normal goat serum (NGS) at room temperature. Subsequently, cells were incubated overnight at 4 °C or for 1 hour at room temperature with primary antibodies diluted in 10% NGS. After aspirating primary antibody solutions, cells were washed three times with DPBS prior to adding secondary antibodies (1:200 dilution in 10% NGS solution). Samples were washed three times with DPBS and stained with DAPI. Texas Red-conjugated Wheat Germ Agglutinin (WGA) staining was conducted on iBMECs, which were fixed with 4% PFA and stained with DARC (ACKR1) antibody without permeabilization, following the manufacturer’s instructions. All steps for cell staining in the BBB-on-a-chip devices were conducted using the pipette tip reservoirs connected to the medium channels of the devices with the same immunostaining steps described for cells on dishes and inserts.
Junction Analysis
Junction Analysis Program (JAnaP)21 was downloaded from https://github.com/StrokaLab/JAnaP and run following the author’s instructions. In brief, confocal images of iBMECs double-stained for ZO-1 and claudin-5 after 6 h of IL-1β treatment were used for junction integrity analysis. ZO-1 images were used for determining the cell–cell junctions (“waypointing”), and the results were projected onto claudin-5 images. Threshold values of 5 were used to analyze both claudin-5 and ZO-1 images. For each group (control and IL-1β treated), 46 cells from 4 different images were analyzed.
TEER Measurements
Using the EVOM2 voltohmmeter with STX2 chopstick electrodes (World Precision Instruments), TEER was evaluated in iBMECs cultured on inserts with or without astrocytes. An insert coated with the collagen IV, but without cells, was used to subtract medium and membrane effects on TEER. The measured TEER values at each time point were divided by the initial TEER value of each insert to obtain the normalized TEER.
Transmigration Assay
The plasma membrane of MDA-MB-231 and 231BR cells was labeled with PKH26 Red Fluorescent Cell Linker (Millipore Sigma), which is a stable dye for long-term tracking of cells, following the manufacturer’s instructions. Labeled cells were seeded in the top channel of BBB-on-a-chip devices at a concentration of 20,000 cells per device and incubated for one hour to adhere to the iBMEC layer. The unattached cancer cells were removed from the device and fresh medium was added to the top channel. Devices were incubated under static conditions with replacement of medium every day. After approximately 72 h, cells in the device were fixed and prepared for immunocytochemistry. For the IL-1β treatment group, the medium in top channel was replaced with 20 ng/mL IL-1β and incubated for 1 hour prior to adding the cancer cells in fresh EC medium to the top channel.
For transmigration studies in cell culture inserts, iBMECs were seeded onto inserts with 8.0 μm pore sizes and cultured for 3 days. One challenge with culture of iBMECs on 8.0 μm pore size inserts is migration of iBMECs across the membrane and formation of a double-layer of iBMECs in some regions. In order to address this issue, any migrated iBMECs were removed from the bottom side of the membrane using a cell scraper prior to cancer cell seeding. Cancer cells were seeded onto the inserts at a density of 80,000 cells per insert. Cells were incubated for 3 days and then fixed and stained for nuclei.
In order to study the effect of IL-1β blocking monoclonal antibody on transmigration of 231BR cells, the top channel of microfluidic devices was incubated with 5 μg/mL IL-1β blocking antibody prior to introduction of 231BR cells. The EC medium used for medium changes in BBB-on-a-chip devices was supplemented with 5 μg/mL human IL-1β blocking antibody (see Table S2 for antibody details). The devices were incubated for 72 h in the presence of IL-1β blocking antibody prior to fixation and imaging.
Fluorescence imaging of the BBB-on-a-chip devices and inserts that were used for transmigration assays was performed on a Nikon TiE stand with an A1Rsi confocal scanhead, powered by NIS-Elements confocal software (Nikon, Japan). A 10x objective with numerical aperture of 0.45 was used for confocal imaging. Excitation for fluorescence images were performed sequentially via 405, 488, 561, and 637 nm lasers. NIS-Elements software was used for image visualization. The number of cancer cells that transmigrated across the iBMECs was counted using Fiji (ImageJ). Three images per device and three devices per each condition were analyzed. Some devices were stained for GFAP (astrocyte marker) or ZO-1 (tight junction protein) in order to visualize astrocytes and iBMECs, respectively.
Statistical Analysis
All data are presented as the mean ± standard deviation. To compare null hypotheses between two groups for data with less than 5 samples, the unpaired Student’s t test was used. For data with more than 5 samples, the Kolmogorov–Smirnov test was used to determine the normality of data. For non-normal data, the Mann–Whitney test was performed to evaluate the P values. Multiple groups were compared by ordinary one-way ANOVA and Tukey’s multiple comparison tests. The number of data points, P values, and statistical tests used are stated in the figure captions. P values smaller than 0.05 are considered statistically significant. Unless otherwise stated within the figure caption, data are representative of three independent experiments (biological replicates) each with three technical replicates per condition.
Results
IL-1β is Upregulated in Brain-Seeking TNBC Cells
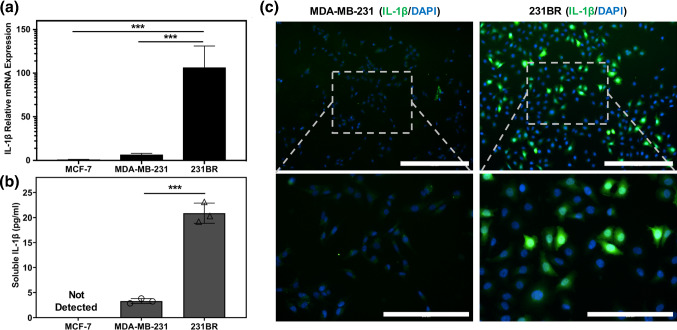
Analysis of the existing GSE12237 dataset suggested multiple genes for secreted proteins were differentially expressed in the brain-seeking variants of both MDA-MB-231 and CN34 breast cancer cells (231BR and CN34BR, respectively) compared to parental cells (Fig. S1a, b). Some of the identified genes such as MMP1 and Serpins (SERPINI1 and SERPINE2) have been previously reported by others to play a role in cancer metastasis to the brain.23,69 Next, to identify the upregulated cytokines in brain seeking TNBC cells, the expression levels of 230 cytokines in the GSE12237 dataset were analyzed. IL-1β gene expression (IL1B) was identified as the only cytokine that was highly upregulated in both 231BR and CN34BR cells compared to their parental cells. In order to confirm IL-1β upregulation in 231BR cells compared to MDA-MB-231 cells, we conducted qRT-PCR and ELISA assays to quantify the gene expression and cytokine secretion of IL-1β in these cells compared to non-invasive MCF-7 cells. Our results indicated significant upregulation of the IL1B gene in 231BR cells compared to both MDA-MB-231 and MCF-7 cells (Fig. 1a). In addition, ELISA assays confirmed differences in IL-1β cytokine levels in the conditioned media of 231BR and MDA-MB-231 cells, which followed the same trend as the gene expression data (Fig. 1b). IL-1β was not detected in MCF-7 conditioned medium via the ELISA kit used in this study. Immunostaining of MDA-MB-231 and 231BR cells also indicated significant upregulation of IL-1β in a subset of 231BR cells compared to MDA-MB-231 cells (Fig. 1c).
Figure 1.
IL-1β expression is highly upregulated in brain-seeking TNBC cells. (a) Gene expression analysis of IL1B in MCF-7, MDA-MB-231, and 231BR cells, showing higher expression in 231BR cells compared to MDA-MB-231 and MCF-7 cells. ***p < 0.001, calculated by ordinary one-way ANOVA with Tukey's multiple comparisons test. (b) ELISA assay quantification of soluble IL-1β secreted by MCF-7, MDA-MB-231, and 231BR cells. ***p < 0.001 (unpaired t test). Each data point represents the concentration of IL-1β in a technical replicate. Results are representative of two independent experiments. Error bars represent standard deviation in (a, b). (c) Immunocytochemistry of IL-1β showing substantially higher expression in a subset of 231BR cells compared to MDA-MB-231 cells. Lower row images are higher magnifications of the indicated rectangular region in the top row images. Scale bars indicate 400 μm in the top row images and 200 μm in the bottom row images.
IL-1β Treatment Reduces BBB Tightness and Downregulates Claudin-5 and ZO-1 Expression
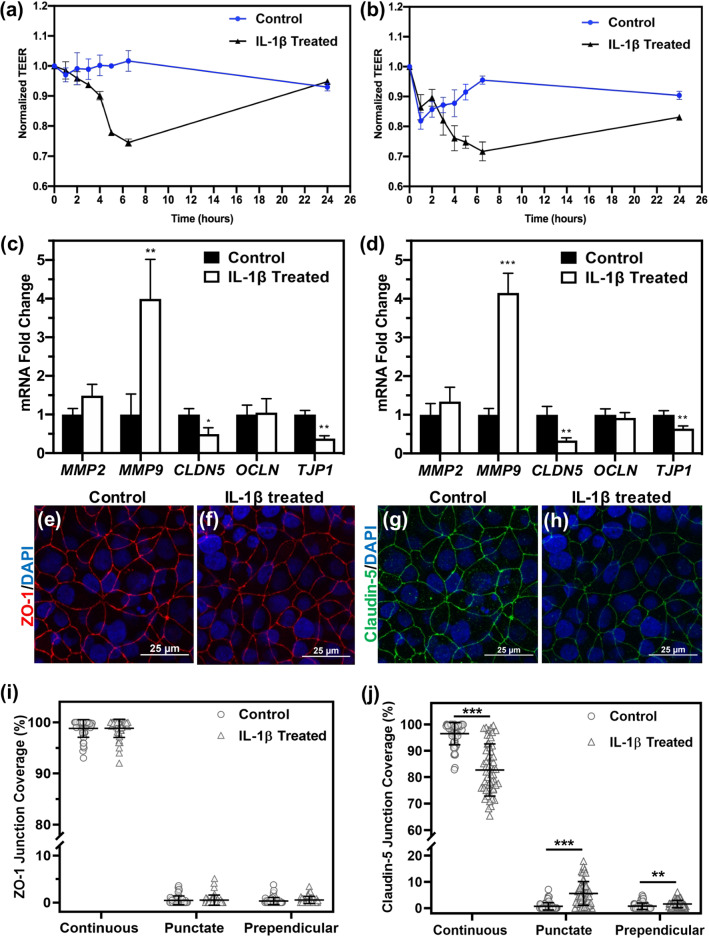
TEER measurements of iBMEC monocultures (Fig. 2a) or iBMECs cocultured with astrocytes (Fig. 2b) that were treated with 20 ng/mL IL-1β indicated a drop in TEER approximately 6 h after treatment followed by a partial or complete recovery in TEER, similar to a report by another group.48 qRT-PCR analysis of iBMECs 6 h after addition of IL-1β showed downregulation of CLDN5 (encoding claudin-5) and TJP1 (encoding ZO-1) in IL-1β treated samples, while OCLN (encoding occludin) expression was not altered (Fig 2c, d). In addition, qRT-PCR analysis of iBMECs 24 h after addition of IL-1β demonstrated that CLDN5 and MMP9 expression were not altered compared to control samples (Fig. S2), which is in agreement with the observed TEER recovery at this timepoint. Further, immunocytochemistry verified the downregulation of claudin-5 and ZO-1 in samples that were treated with IL-1β for 6 h (Figs. 2e–2h). In order to quantify the integrity of cell–cell junctions in the confocal images of claudin-5 and ZO-1 stained iBMECs, Junction Analyzer Program was used.21 Based on the results, IL-1β reduced the percentage of continuous coverage and slightly increased the percentage of punctate and discontinuous regions within the cell–cell junctions for claudin-5, but not ZO-1 (Figs. 2i and 2j). In addition to changes in expression and integrity of tight junction proteins, an increase in expression of MMP9 was observed in IL-1β treated samples (Figs. 2c and 2d). Overall, these results showed that IL-1β is able to partially disrupt the BBB by downregulating claudin-5 and ZO-1, decreasing continuous coverage of claudin-5, and enhancing MMP9 activity.
Figure 2.
IL-1β reduces barrier integrity by downregulating tight junction proteins and upregulating MMP9. (a,b) TEER measurement of iBMECs treated with 20 ng/mL IL-1β in the absence (a) or presence (b) of astrocytes, showing substantial reduction in TEER after approximately 6 h of IL-1β addition. (c, d) Gene expression analysis of iBMEC monoculture (c) and iBMECs that were cocultured with astrocytes (d) after 6 h of treatment with IL-1β (fold change compared to non-treated control samples). In IL-1β treated samples, MMP9 is upregulated, whereas TJP1 and CLDN5 are downregulated. *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired t test). (e–h) Immunocytochemistry of iBMEC monoculture showing downregulation of ZO-1 and claudin-5 in IL-1β treated samples. (e, f) show ZO-1 staining and (g, h) show claudin-5 staining. (e, g) represent control samples, while (f, h) represent IL-1β treated samples. Images are maximum intensity projections of confocal z-stacks, and scale bars indicate 25 μm. (i, j) Quantification of junction integrity showing percentage of continuous, punctate, and perpendicular junction coverage for ZO-1 (i) and claudin-5 (j). N = 46 for each condition, and each data point represents one cell. **p < 0.01 and ***p < 0.001 calculated by Mann-Whitney test.
Long-Term IL-1β Treatment Induces EndoMT in iBMECs
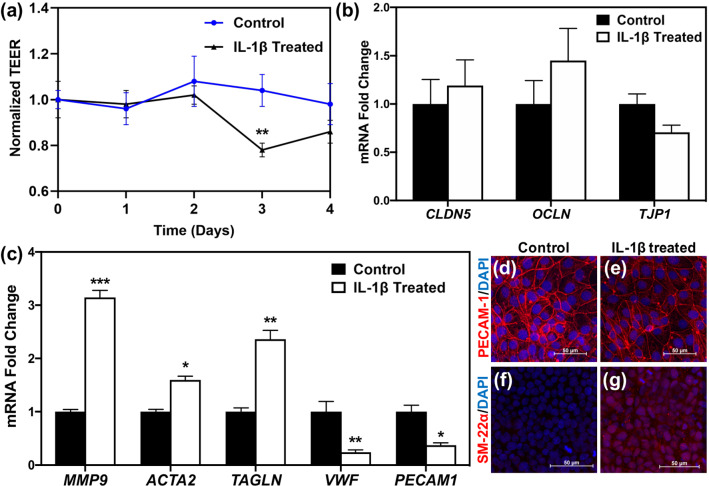
Although TEER measurements indicated partial or complete barrier recovery 24 h after initial TEER reduction, daily treatment with 20 ng/mL IL-1β revealed another drop in TEER after 3 days of IL-1β exposure (Fig. 3a). While gene expression analysis of tight junction proteins did not show statistically significant differences between control and IL-1β treated samples at day 3 (Fig. 3b), MMP9 expression remained higher in treated samples (Fig. 3c), which could result in tight junction disruption. In addition, gene expression analysis showed upregulation of mesenchymal markers (ACTA2 and TAGLN, encoding αSMA and SM-22α, respectively) and downregulation of endothelial markers (PECAM1 and VWF; Fig. 3c), suggesting EndoMT of the iBMECs occurred as a result of IL-1β treatment. Immunocytochemistry of SM22-α (mesenchymal marker) and PECAM-1 (endothelial marker) confirmed the EndoMT phenotype in IL-1β-treated iBMECs on the protein level (Figs. 3d–3g).
Figure 3.
Long-term IL-1β treatment induces EndoMT in iBMECs. (a) TEER measurements indicate a drop in iBMEC TEER after 3 days of IL-1β treatment. **p < 0.01 compared to control at day 3 (unpaired t test). (b) Gene expression levels of tight junction proteins are not altered after 3 days of treatment with IL-1β (unpaired t test). (c) Endothelial markers (VWF and PECAM1) are downregulated, whereas mesenchymal markers (ACTA2 and TAGLN) and MMP9 are upregulated as a result of IL-1β treatment for 3 days. *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired t test). For (b, c), fold change is relative to non-treated control. Error bars represent standard deviation in (a–c). (d–g) Immunocytochemistry of iBMECs treated with IL-1β for 3 days shows downregulation of PECAM-1 in treated iBMECs (e) compared to control samples (d), while SM-22α expression is higher in IL-1β treated iBMECs (g) compared to control cells (f). Images are maximum intensity projections of confocal z-stacks, and scale bars indicate 50 μm.
IL-1β crosses the BBB and activates astrocytes
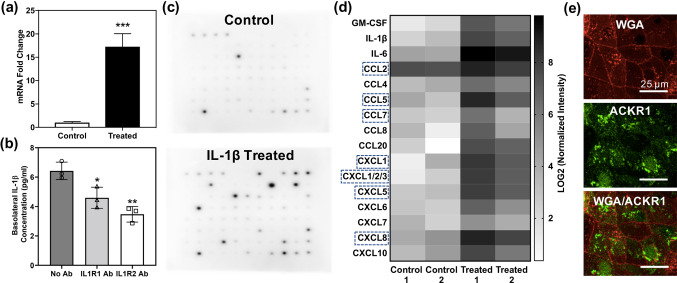
It has been previously reported that IL-1β is transported across the BBB in adult mice and ovine fetuses, possibly via a saturable transport system.41,49,57 An in vitro study also confirmed receptor-mediated transport of IL-1β across bovine brain endothelial cells.61 We observed that apical treatment of iBMECs in coculture inserts resulted in upregulation of CCL5 in the astrocytes (Fig. 4a), which commonly occurs as a result of astrocyte activation,29 suggesting potential transport of IL-1β across the iBMEC layer. ELISA assays with and without IL-1β receptor blocking were performed to determine whether receptor-mediated IL-1β transport was occurring in the iBMEC monolayer. After addition of IL-1β to the apical side of the iBMECs, it was detected in the basolateral chamber, and pre-treatment of the iBMECs with either IL1R1 or IL1R2 antibodies to block the receptors decreased the amount of IL-1β transported to the basolateral chamber (Fig. 4b), indicating receptor-mediated transport of IL-1β across the iBMEC monolayer.
Figure 4.
IL-1β crosses the iBMEC layer and induces chemokine secretion by astrocytes (a) Upregulation of CCL5 in astrocytes that were cocultured with iBMECs as a result of IL-1β addition to the apical chamber. ***p < 0.001 (unpaired t test). (b) ELISA assay shows that IL-1β is transported across the iBMEC layer, and blocking IL-1β receptors (IL1R1 and IL1R2) reduces the amount of transported IL-1β. *p < 0.05 and **p < 0.01 (ordinary one-way ANOVA and Tukey's multiple comparisons test). Each data point represents the concentration of a technical replicate. The figure is representative of two independent experiments (biological replicates). Error bars represent standard deviation in (a, b). (c) Chemiluminescence images of the cytokine microarray assay conducted using the supernatant of astrocytes treated with IL-1β (control samples received no IL-1β) showing upregulation of multiple cytokines and chemokines. (d) Heat map of chemokines that are differentially expressed (fold change ≥ 2) between control and treated samples from quantification of the cytokine microarray images. Pixel intensity of each cytokine is normalized to the average pixel intensity of positive control spots on the membrane. Chemokines that bind to ACKR1 are indicated by dashed rectangles. (e) Confocal microscopy of ACKR1 (green) expression on the iBMEC cell membrane, which is stained with Texas Red-conjugated wheat germ agglutinin (red). Scale bars indicate 25 μm.
IL-1β Treated Astrocytes Secrete a Mixture of Chemokines that Could Enhance Transmigration of Cancer Cells
A membrane-based cytokine array was utilized to determine which cytokines and chemokines were secreted by astrocytes that were activated by IL-1β transported across the iBMEC monolayer (Fig. 4c), as these soluble signals could impact cancer cell extravasation. Cytokines and chemokines with more than two-fold change in expression were identified and shown in a heat map (Fig. 4d). Highly upregulated chemokines secreted by IL-1β-treated astrocytes included CCL2, CCL5, CCL7, CCL8, CXCL1, and CXCL5. Several chemokine receptors have previously been reported to be expressed by BMECs, including ACKR1.68 Immunocytochemistry showed that ACKR1 is expressed on the iBMEC plasma membrane (Fig. 4e). Since ACKR1 is able to transport various chemokines across the BBB (indicated by dashed rectangles in Fig. 4d), it could provide the cancer cells that are adhered to the apical side of iBMECs with chemokines secreted by the activated astrocytes.
MDA-MB-231 and 231BR Cells are Able to Transmigrate Across the iBMECs
In order to determine whether MDA-MB-231 and 231BR cells were able to transmigrate across the iBMECs, plasma membrane-stained cancer cells were added to the apical side of iBMECs cultured on cell culture inserts with 8.0 μm pore diameter. After 3 days, inserts were fixed and iBMECs were stained for claudin-5. Confocal microscopy demonstrated that both MDA-MB-231 and 231BR cells were able to transmigrate across the iBMECs (Fig. S3a–d). However, confocal images also revealed the formation of a double layer of iBMECs on different sides of the membrane with 8.0 μm pore diameter, making this system unsuitable for transmigration studies (Fig. S3e–f).
Astrocytes Increase Transmigration of 231BR Cells
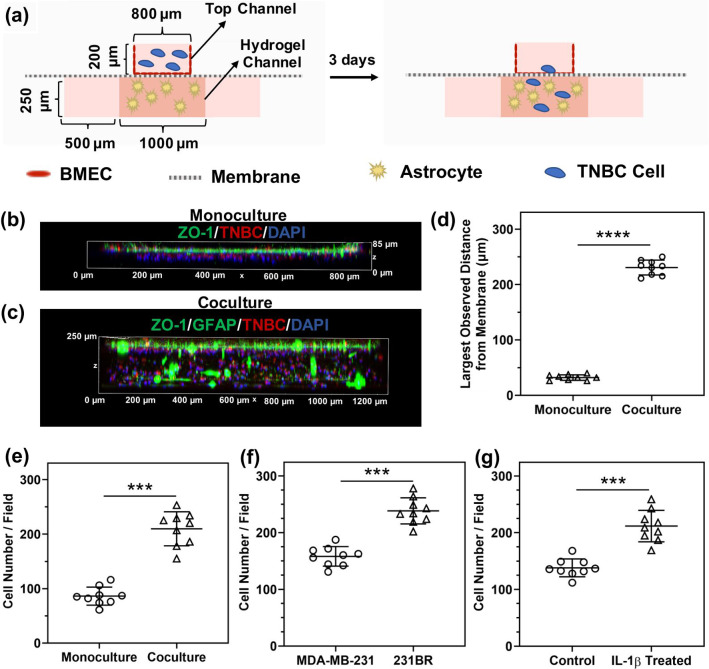
A BBB-on-a-chip platform that was fabricated with a PET membrane with 8.0 μm pores and contained a 3-D hydrogel in the basolateral channel (as described before45), was used to assess cancer cell transmigration (Fig. 5a). As previously shown, hydrogels located in the basolateral channel prevent the migration of iBMECs to the basolateral channel and the formation of a double layer.45 In addition, BBB-on-a-chip devices possess a higher surface-to-volume ratio, which facilitates paracrine signaling between different cell types present in a device. Three days after introducing cancer cells to the devices, all cells were fixed and iBMECs were stained for ZO-1. Confocal imaging of the monoculture devices showed that 231BR cells that transmigrated across the iBMEC monolayer remained close to the endothelial layer in the hydrogels that did not contain astrocytes (Figs. 5b and 5d). In the coculture devices that had astrocytes in the hydrogel channel, more 231BR cells transmigrated across the iBMEC layer, and they penetrated deeper into the hydrogel and were located throughout the basolateral channel (Figs. 5c–e). These results show that astrocytes play a critical role in extravasation of TNBC cells across the BBB.
Figure 5.
Astrocytes and IL-1β facilitate transmigration of TNBC cells across the iBMECs. (a) Cross-sectional view schematic of the BBB-on-a-chip device used for transmigration studies. (b, c) 3-D confocal images of 231BR cell transmigration across the iBMEC layer in devices with only iBMECs (no astrocytes in the basolateral hydrogel channel) (b) and devices with iBMECs cocultured with astrocytes (c). Green indicates ZO-1 (iBMECs) and GFAP (astrocytes), red indicates cancer cells, and blue indicates cell nuclei. (d) The maximum distance of a 231 BR cell from the membrane in the absence (monoculture) or presence of astrocytes (coculture), showing cancer cells penetrate deeper into the hydrogel containing astrocytes. Each data point represents the distance of the cancer cell that is located farthest from the PET membrane in each confocal image. (e) Number of 231BR cells transmigrated across the iBMEC layer in monoculture or coculture with astrocytes, showing that the presence of astrocytes enhances the number of extravasated 231BR cells. (f) Comparison of the number of transmigrated MDA-MB-231 and 231BR cells indicates a higher transmigration frequency for 231BR cells. (g) Effect of IL-1β pre-treatment of iBMECs on the transmigration rate of MDA-MB-231 cells, showing that IL-1β pre-treatment increases the number of transmigrated MDA-MB-231 cells. Studies in (f, g) were performed in BBB-on-a-chip devices with astrocytes. In (d–g), ***p < 0.001 and ****p < 0.0001 (unpaired t test). In (e–g), each data point represents the number of transmigrated cancer cells present in a captured confocal image. Three images per device and three devices per condition were used to generate the plots in (d–g). Error bars represent standard deviation in (d–g).
IL-1β Facilitates Transmigration of TNBC Cells Across the iBMECs
Using BBB-on-a-chip devices, we investigated differences in the transmigration frequency of MDA-MB-231 and 231BR cells. Our results indicated that 231BR cells, which express higher levels of IL-1β, showed a higher transmigration frequency across the iBMECs compared to MDA-MB-231 cells in BBB-on-a-chip devices containing astrocytes in the hydrogel channel (Fig. 5f). In order to confirm that IL-1β facilitates the transmigration of TNBC cells across the BBB, the apical side of the iBMEC and astrocyte coculture devices was pre-treated with IL-1β, and MDA-MB-231 cells were added to the devices after removal of the IL-1β solution. Pre-treatment with IL-1β significantly increased the number of transmigrated MDA-MB-231 cells (Fig. 5g), confirming that IL-1β promotes TNBC transmigration across the iBMECs.
Blocking IL-1β Mitigates iBMEC Disruption and Reduces Transmigration of 231BR Cells
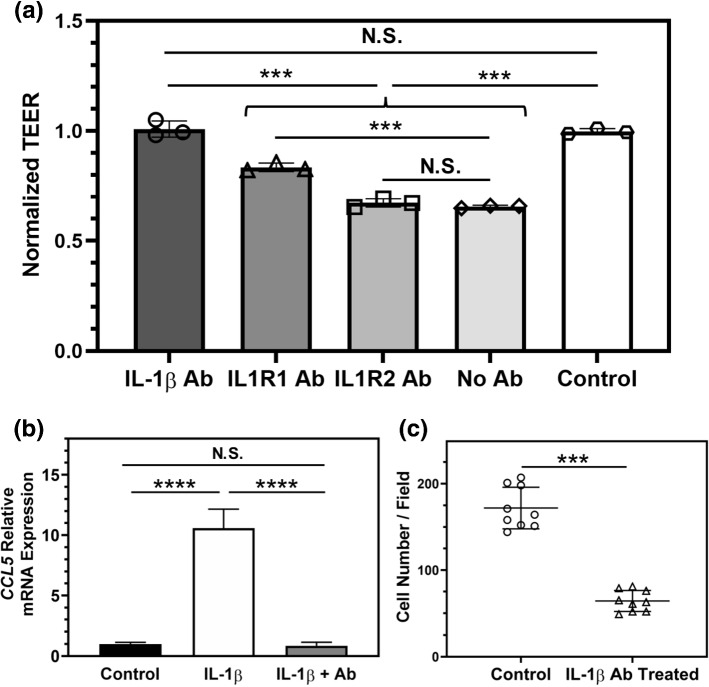
In order to determine whether antibody blocking of IL-1β or its receptors could inhibit the barrier disruption induced by IL-1β treatment of iBMECs, the apical side of cell culture inserts that were seeded with iBMECs was pretreated with antibodies against IL-1β, IL1R1, or IL1R2, and IL-1β was introduced afterwards. Our results demonstrated that the IL-1β neutralizing antibody was superior at preventing iBMEC barrier impairment compared to the antibodies targeting IL-1β receptors on the iBMECs (Fig. 6a). In addition, using the astrocyte-iBMEC coculture system we examined the effect of apical treatment with the IL-1β neutralizing antibody on chemokine secretion by astrocytes after adding IL-1β to the apical chamber. Based on our results, the IL-1β neutralizing antibody was also able to prevent the activation of astrocytes in a coculture system (Fig. 6b). In order to investigate the effect of blocking IL-1β on the rate of TNBC cell transmigration across the iBMECs, 231BR cells were introduced to the apical channel of the BBB-on-a-chip devices in the presence of IL-1β neutralizing antibody. Blocking IL-1β reduced the number of transmigrated 231BR cells, indicating a crucial role for IL-1β in transmigration of TNBC cells across the BBB (Fig. 6c).
Figure 6.
Neutralizing IL-1β reduces barrier disruption and transmigration of 231BR cells. (a) TEER measurements indicate that blocking IL-1β with a monoclonal antibody (IL-1β Ab) is most effective at preventing iBMEC barrier disruption as a result of IL-1β addition, as compared to blocking IL-1β receptors on the iBMECs using IL1R1 or IL1R2 blocking antibodies. ***p < 0.001 (ordinary one-way ANOVA and Tukey's multiple comparisons test). Control samples received no IL-1β. Each data point shows the normalized TEER of a technical replicate. Results are representative of two independent experiments. (b) Addition of the IL-1β monoclonal antibody to the apical chamber of cell culture inserts prevents activation of astrocytes on the basolateral side after apical IL-1β treatment of iBMECs for 24 h. CCL5 gene expression was used as a measure of astrocyte activation. ****p < 0.0001 (ordinary one-way ANOVA and Tukey's multiple comparisons test). Three technical replicates were used to generate the plot. Results are representative of two independent experiments. (c) Incubating 231BR cells in the BBB-on-a-chip devices with astrocytes in the presence of IL-1β monoclonal antibody in the apical channel of the devices significantly reduces the number of cancer cells that transmigrate across the iBMECs, compared to the control group that did not receive IL-1β monoclonal antibody. ***p < 0.001 (unpaired t test). Each data point represents the number of transmigrated 231BR cells across the iBMECs in a confocal image. Three images per device and three devices per condition were used to generate the plot. Error bars represent standard deviation in (a–c).
Discussion
Most of the in vitro studies thus far on transmigration of cancer cells across the BBB have been conducted using endothelial cells that fail to recapitulate the highly tight barrier observed in vivo.15,16,43 Use of an in vitro model that is able to better mimic the physiologically relevant characteristics of the human BBB could solve the poor correlation between in vitro results and in vivo findings.39 iBMECs, which are differentiated from hiPSCs, exhibit superior barrier properties compared to existing cell lines and primary cells and can be beneficial for studying changes in the BBB as a result of interaction with cancer cells. While transcriptomics studies have identified an epithelial-like gene expression pattern in these cells, we and others have shown that iBMECs express crucial endothelial markers and junctional proteins.36,37,44 Moreover, we have recently reported a BBB-on-a-chip device that can recapitulate the key features of the human BBB including barrier properties and three dimensionality.45 In this study, we investigated the transmigration of TNBC cells across the iBMECs using both cell culture inserts and BBB-on-a-chip devices.
In order to identify the molecules that are upregulated in TNBC cells that preferentially metastasize to the brain, gene expression microarray data obtained by Bos et al were analyzed.10 Our analysis indicated IL1B was one of the most highly upregulated secreted factors and the only upregulated cytokine in brain-seeking TNBC cells. Additionally, Xing et al previously reported upregulation of the c-Met pathway in TNBC cells compared to other breast cancer subtypes as well as in patient secondary brain tumors and primary tumors with brain metastasis compared to patient primary tumors exhibiting no brain metastasis.75 Their results showed that c-Met could be a potential prognostic marker to predict the formation of brain metastases in breast cancer patients. Importantly, they demonstrated that there was a positive correlation between IL-1β and c-Met expression, suggesting a possible role for IL-1β upregulation in formation of brain metastases from breast cancer.75
After confirming IL-1β upregulation in 231BR cells compared to MDA-MB-231 cells by ELISA and qRT-PCR, the effect of this proinflammatory cytokine on iBMECs was studied. Previous studies have shown that IL-1β is able to disrupt the BBB,5,7 in some cases transiently.48 However, the cells that have been utilized in these studies do not form an in vivo-like barrier. In agreement with previous studies, we observed a significant drop in iBMEC TEER as a result of IL-1β treatment. Immunocytochemistry and qRT-PCR suggested downregulation of claudin-5 (CLDN5) and ZO-1 (TJP1) mRNA levels at the time of TEER reduction in iBMECs. We also observed upregulation of MMP9, which is considered a barrier disruptive factor,53 in iBMECs. While we observed TEER recovery 24 h after IL-1β addition, continued treatment with IL-1β for 3 days caused another significant TEER reduction. Even though gene expression of tight junction proteins was not altered at this point, MMP9 was highly upregulated. Additionally, upregulation of mesenchymal markers and downregulation of endothelial markers were observed in iBMECs after 3 days of IL-1β treatment, further verifying the negative effect of IL-1β on iBMECs and its potential role in facilitating TNBC cell extravasation into the brain.
In addition to inducing EndoMT in iBMECs and damaging barrier properties, IL-1β was also able to cross the iBMEC monolayer and upregulate the secretion of chemokines and cytokines by astrocytes. Saturable transport of IL-1β across the BBB in animal models has been reported in literature.41,49,61 Since breast cancer cells spend an extended amount of time attached to the apical side of the brain endothelium during extravasation, transport of secreted cytokines such as IL-1β from the apical to basolateral side of the BBB could be a crucial factor in astrocyte activation and further recruitment of cancer cells to the brain. Importantly, activation of astrocytes prior to complete extravasation of cancer cells into the brain has been observed in animal models.38 It is also known that astrocytes treated with proinflammatory cytokines secrete various chemokines, which could play a role in attracting breast cancer cells through chemotaxis and enhancing their aggressiveness. Using an ELISA assay, we showed that IL-1β was transported across the iBMECs and blocking its receptors, IL1R1 and IL1R2, reduced the rate of transcytosis. Additionally, using a cytokine array assay we identified the chemokines that were significantly upregulated in IL-1β-treated astrocytes. Many of the upregulated cytokines, including CCL2, CCL5, CCL7, CCL8, CXCL1, and CXCL5 can induce chemotaxis and invasiveness in breast cancer cells.4,8,18,33,34,52,55,77 These secreted chemokines could be transported to the apical side of the BBB and impact the cancer cells that have not yet crossed the BBB.
There are two possible routes for transport of secreted chemokines by astrocytes to the apical side of the BBB. One is through the paracellular pathway, due to damaged tight junctions as a result of upregulation of MMPs in iBMECs caused by IL-1β exposure as well as secretion of MMPs by cancer cells. Another pathway is through transport-mediated transcytosis via ACKR1 and other receptors expressed on BMECs.68 ACKR1 binds to a variety of chemokines including CCL2, CCL5, CCL7, CXCL1, CXCL2, CXCL3, CXCL5, and CXCL6 and transports them from basolateral to apical side of the BBB.46 We showed that ACKR1 is expressed in iBMECs and as a result could transport astrocyte-secreted chemokines across the BBB. It is important to note that IL-1β is not the only cytokine that can be transported from the blood to brain direction across the BBB. Other proinflammatory cytokines such as TNF-α and IL-1α, which are also secreted by TNBC cells, can also cross the BBB via saturable transport systems and potentially activate astrocytes to secrete different chemokines.49,73
Using a BBB-on-a-chip platform, we showed that the presence of astrocytes in the hydrogel on the brain side increased the total number of transmigrated TNBC cells across the iBMECs. Additionally, in devices that had astrocytes in the hydrogel channel, TNBC cells penetrated deeper into the hydrogel, while in devices without astrocytes most of the TNBC cells were located close to the iBMEC layer. A possible explanation for this outcome could be that the secretion of chemokines by the astrocytes attracts more cancer cells into the deeper regions of the hydrogel channel. It was also previously shown that astrocyte-conditioned medium enhances cancer cell invasiveness and mobility,59,70 further supporting the importance of astrocytes in cancer cell invasion. Moreover, we showed that 231BR cells transmigrate across the iBMECs at a higher rate compared to MDA-MB-231 cells. This is in agreement with previous in vitro studies using different endothelial cells and animal studies, which showed increased transmigration of 231BR cells compared to parental MDA-MB-231 cells.10,38,73 Pre-treatment of the iBMEC layer with IL-1β also increased the rate of MDA-MB-231 transmigration, suggesting IL-1β-treated iBMECs and astrocytes facilitate TNBC cell transmigration. Finally, we investigated the effect of neutralizing IL-1β with a monoclonal antibody on transmigration potential of 231BR cells. Our results showed that 231BR cells in the presence of an IL-1β monoclonal antibody exhibited lower transmigration frequency compared to control samples, suggesting the use of IL-1β blocking molecules as a potential drug for prevention of TNBC metastasis into the brain. Based on a recent study which found a bone metastasis-promoting effect of IL-1β in breast cancer, blocking IL-1β could potentially be beneficial for inhibiting metastasis to other organs as well.65,66 Taken together, our results suggest that upregulation of IL-1β in brain-seeking TNBC cells can induced changes in the BBB and astrocytes, which in turn accelerates the transmigration of TNBC cells across the BBB. These results could enable targeting of new mechanisms to inhibit the formation of brain metastases.
Conclusion
We have demonstrated that IL-1β is capable of facilitating TNBC cell transmigration across iBMECs, which exhibit in vivo-like barrier properties. IL-1β reduces barrier tightness and induces EndoMT in the iBMECs. Importantly, we showed that the presence of astrocytes in the system enhances the rate of 231BR transmigration across the iBMECs. Our results indicate that IL-1β crosses the BBB and upregulates the expression of multiple chemokines in astrocytes, which could explain the increase in the number of transmigrated cancer cells. We also demonstrated that IL-1β blocking antibodies reduce the rate of 231BR transmigration in our system. Overall, our results suggest that changes in BMECs and astrocytes as a result of IL-1β secretion by TNBC cells could pave the way for successful transmigration of cancer cells across the BBB. Future studies could focus on verifying these results in animal models and investigating the effectiveness of IL-1β blocking antibodies in preventing breast cancer metastasis to the brain.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Portions of this work were conducted in the Minnesota Nano Center, which is supported by the National Science Foundation through the National Nano Coordinated Infrastructure Network (NNCI) under Award Number ECCS-2025124. Confocal microscopy and image analysis was performed using the Nikon A1Rsi Confocal microscope and NIS-Elements software at the University Imaging Center, University of Minnesota.
Conflict of interest
All authors (PM, VVR, and SMA) declare that they have no conflict of interests.
Ethical Approval
No human or animal studies were carried out by the authors for this article.
Author Contributions
PM and SMA designed the experiments. PM and VVR performed the experiments and analyzed the data. PM and SMA wrote and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the University of Minnesota.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Yafeai Z, Yurdagul A, Peretik JM, Alfaidi M, Murphy PA, Orr AW. Endothelial FN (Fibronectin) deposition by α5β1 integrins drives atherogenic inflammation. Arterioscler. Thromb. Vasc. Biol. 2018;38:2601–2614. doi: 10.1161/ATVBAHA.118.311705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Yahya S, et al. Human Cytokinome Analysis for Interferon Response. J. Virol. 2015;89:7108–7119. doi: 10.1128/JVI.03729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral ML, Erikson GA, Shokhirev MN. BART: Bioinformatics array research tool. BMC Bioinformatics. 2018;19:1–6. doi: 10.1186/s12859-018-2308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An G, et al. Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor-associated macrophages. Oncol. Rep. 2019;42:2499–2511. doi: 10.3892/or.2019.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argaw AT, et al. IL-1β regulates blood–brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 6.Avraham HK, Jiang S, Fu Y, Nakshatri H, Ovadia H, Avraham S. Angiopoietin-2 mediates blood–brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J. Pathol. 2014;232:369–381. doi: 10.1002/path.4304. [DOI] [PubMed] [Google Scholar]
- 7.Beard RS, et al. Non-muscle Mlck is required for ß-catenin- and FoxO1-dependent downregulation of Cldn5 in IL-1ß-mediated barrier dysfunction in brain endothelial cells. J. Cell Sci. 2014;127:1840–1853. doi: 10.1242/jcs.144550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bersini S, et al. A microfluidic 3D invitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P. Interleukin-1β-induced changes in blood–brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: A magnetic resonance study. J. Neurosci. 2000;20:8153–8159. doi: 10.1523/JNEUROSCI.20-21-08153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubendorf L, et al. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri V, Zhou L, Karasek M. Inflammatory cytokines induce the transformation of human dermal microvascular endothelial cells into myofibroblasts: A potential role in skin fibrogenesis. J. Cutan. Pathol. 2007;34:146–153. doi: 10.1111/j.1600-0560.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 13.Deeb A, Haque S-U, Olowokure O. Pulmonary metastases in pancreatic cancer, is there a survival influence? J. Gastrointest. Oncol. 2015;6:E48–51. doi: 10.3978/j.issn.2078-6891.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derada Troletti, C., et al. Inflammation-induced endothelial to mesenchymal transition promotes brain endothelial cell dysfunction and occurs during multiple sclerosis pathophysiology. Cell Death Dis. 10:1–13, 2019. [DOI] [PMC free article] [PubMed]
- 15.Drolez A, et al. Selection of a relevant in vitro blood–brain barrier model to investigate Pro-Metastatic features of human breast cancer cell lines. PLoS ONE. 2016;11:1–18. doi: 10.1371/journal.pone.0151155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J, Fu BM. Quantification of Malignant Breast Cancer Cell MDA-MB-231 Transmigration Across Brain and Lung Microvascular Endothelium. Ann. Biomed. Eng. 2016;44:2189–2201. doi: 10.1007/s10439-015-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fares, J., D. Kanojia, A. Rashidi, I. Ulasov, and M.S. Lesniak. Genes that mediate metastasis across the blood–brain barrier. Trends in Cancer 6:660–676, 2020. [DOI] [PMC free article] [PubMed]
- 18.Farmaki E, Chatzistamou I, Kaza V, Kiaris H. A CCL8 gradient drives breast cancer cell dissemination. Physiol. Behav. 2017;176:139–148. doi: 10.1038/onc.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira FU, et al. Endothelial cells tissue-specific origins affects their responsiveness to TGF-β2 during endothelial-to-mesenchymal transition. Int. J. Mol. Sci. 2019;20:1–14. doi: 10.3390/ijms20030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparics, Á., L. Rosivall, I. A. Krizbai, and A. Sebe. When the endothelium scores an own goal: endothelial cells actively augment metastatic extravasation through endothelial-mesenchymal transition. Am. J. Physiol. Heart Circ. Physiol. 310(9):H1055–H1063, 2016. [DOI] [PubMed]
- 21.Gray KM, Katz DB, Brown EG, Stroka KM. Quantitative Phenotyping of cell–cell Junctions to Evaluate ZO-1 Presentation in Brain Endothelial Cells. Ann. Biomed. Eng. 2019;47:1675–1687. doi: 10.1007/s10439-019-02266-5. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan, D., and R. A. Weinberg. Hallmarks of cancer: The next generation. Cell 144:646–674, 2011. [DOI] [PubMed]
- 23.Harati R, Hafezi S, Mabondzo A, Tlili A. Silencing miR-202-3p increases MMP-1 and promotes a brain invasive phenotype in metastatic breast cancer cells. PLoS ONE. 2020;15:1–26. doi: 10.1371/journal.pone.0239292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haskó J, et al. Response of the neurovascular unit to brain metastatic breast cancer cells. Acta Neuropathol. Commun. 2019;7:133. doi: 10.1186/s40478-019-0788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heitz F, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur. J. Cancer. 2009;45:2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Herman H, et al. Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J. Cell. Mol. Med. 2019;23:2619–2631. doi: 10.1111/jcmm.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewett SJ, Jackman NA, Claycomb RJ. Interleukin-1β in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 2012;1:195–211. [PMC free article] [PubMed] [Google Scholar]
- 28.Kemper EM, Boogerd W, Thuis I, Beijnen JH, van Tellingen O. Modulation of the blood–brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat. Rev. 2004;30:415–423. doi: 10.1016/j.ctrv.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Kim MO, Suh HS, Brosnan CF, Lee SC. Regulation of RANTES/CCL5 expression in human astrocytes by interleukin-1 and interferon-β. J. Neurochem. 2004;90:297–308. doi: 10.1111/j.1471-4159.2004.02487.x. [DOI] [PubMed] [Google Scholar]
- 30.Klein S, et al. α5β1 Integrin activates an NF-κB-dependent program of gene expression important for angiogenesis and inflammation. Mol. Cell. Biol. 2002;22:5912–5922. doi: 10.1128/MCB.22.16.5912-5922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krizbai IA, et al. Endothelial-mesenchymal transition of brain endothelial cells: possible role during metastatic extravasation. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YT, Geer DA. Primary liver cancer: pattern of metastasis. J. Surg. Oncol. 1987;36:26–31. doi: 10.1002/jso.2930360107. [DOI] [PubMed] [Google Scholar]
- 33.Li JY, et al. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res. Treat. 2012;131:837–848. doi: 10.1007/s10549-011-1502-6. [DOI] [PubMed] [Google Scholar]
- 34.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697–28710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin C-C, Edelson BT. New insights into the role of IL-1β in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Immunol. 2017;198:4553–4560. doi: 10.4049/jimmunol.1700263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood–brain barrier model derived from stem cell sources. Sci. Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lippmann ES, Azarin SM, Palecek SP, Shusta EV. Commentary on human pluripotent stem cell-based blood–brain barrier models. Fluids Barriers CNS BioMed Central. 2020;17:4–9. doi: 10.1186/s12987-020-00222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorger M, Lee H, Forsyth JS, Felding-Habermann B. Comparison of in vitro and in vivo approaches to studying brain colonization by breast cancer cells. J. Neurooncol. 2011;104:689–696. doi: 10.1007/s11060-011-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maleszewska M, Moonen JRAJ, Huijkman N, van de Sluis B, Krenning G, Harmsen MC. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology. 2013;218:443–454. doi: 10.1016/j.imbio.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 41.McLay RN, Kastin AJ, Zadina JE. Passage of interleukin-1-beta across the blood–brain barrier is reduced in aged mice: a possible mechanism for diminished fever in aging. Neuroimmunomodulation. 2000;8:148–153. doi: 10.1159/000054275. [DOI] [PubMed] [Google Scholar]
- 42.Miller SJ. Astrocyte heterogeneity in the adult central nervous system. Front. Cell. Neurosci. 2018;12:1–6. doi: 10.3389/fncel.2018.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molnár J, et al. Transmigration characteristics of breast cancer and melanoma cells through the brain endothelium: Role of Rac and PI3K. Cell Adhes. Migr. 2016;10:269–281. doi: 10.1080/19336918.2015.1122156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motallebnejad P, Azarin SM. Chemically defined human vascular laminins for biologically relevant culture of hiPSC-derived brain microvascular endothelial cells. Fluids Barriers CNS BioMed Central. 2020;17:1–16. doi: 10.1186/s12987-020-00215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motallebnejad P, Thomas A, Swisher SL, Azarin SM. An isogenic hiPSC-derived BBB-on-a-chip. Biomicrofluidics. 2019;13:1–13. doi: 10.1063/1.5123476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nibbs RJB, Graham GJ. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 47.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: What has changed in the last 20 years? Cancer. 2011;117:2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 48.O’Carroll SJ, et al. Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflammation. 2015;12:1–18. doi: 10.1186/s12974-015-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan W, Stone KP, Hsuchou H, Manda VK, Zhang Y, Kastin AJ. Cytokine signaling modulates BBB function. Curr Pharm Des. 2014;17:3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platta CS, Khuntia D, Mehta MP, Suh JH. Current treatment strategies for brain metastasis and complications from therapeutic techniques NCF in brain metastasis. Am. J. Clin. Oncol. 2010;33:398–407. doi: 10.1097/COC.0b013e318194f744. [DOI] [PubMed] [Google Scholar]
- 51.Pranda MA, Gray KM, DeCastro AJL, Dawson GM, Jung JW, Stroka KM. Tumor cell mechanosensing during incorporation into the brain microvascular endothelium. Cell. Mol. Bioeng. 2019;12:455–480. doi: 10.1007/s12195-019-00591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajaram M, Li J, Egeblad M, Powers RS. System-wide analysis reveals a complex network of tumor-fibroblast interactions involved in tumorigenicity. PLoS Genet. 2013;9:e1003789. doi: 10.1371/journal.pgen.1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016;36:1481–1507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rieder F, et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero-Moreno R, et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat. Commun. 2019;10:4404. doi: 10.1038/s41467-019-12108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J. Neurooncol. 2016;127:407–414. doi: 10.1007/s11060-016-2075-3. [DOI] [PubMed] [Google Scholar]
- 57.Sadowska GB, et al. Interleukin-1β transfer across the blood–brain barrier in the ovine fetus. J. Cereb. Blood Flow Metab. 2015;35:1388–1395. doi: 10.1038/jcbfm.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaftel SS, Griffin WST, Kerry KM. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation. 2008;5:1–12. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shumakovich MA, Mencio CP, Siglin JS, Moriarty RA, Geller HM, Stroka KM. Astrocytes from the brain microenvironment alter migration and morphology of metastatic breast cancer cells. FASEB J. 2017;31:5049–5067. doi: 10.1096/fj.201700254R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 61.Skinner RA, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br. J. Pharmacol. 2009;156:1115–1123. doi: 10.1111/j.1476-5381.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spampinato SF, Bortolotto V, Canonico PL, Sortino MA, Grilli M. Astrocyte-derived paracrine signals: relevance for neurogenic niche regulation and blood–brain barrier integrity. Front. Pharmacol. 2019;10:1–9. doi: 10.3389/fphar.2019.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood–brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016;4:1–12. doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stebbins MJ, Wilson HK, Canfield SG, Qian T, Palecek SP, Shusta EV. Differentiation and characterization of human pluripotent stem cell-derived brain microvascular endothelial cells. Methods. 2015;101:93–102. doi: 10.1016/j.ymeth.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tulotta C, et al. Endogenous production of IL1B by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin. Cancer Res. 2019;25:2769–2782. doi: 10.1158/1078-0432.CCR-18-2202. [DOI] [PubMed] [Google Scholar]
- 66.Tulotta C, Ottewell P. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer. 2018;25:R421–R434. doi: 10.1530/ERC-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uhlén M, et al. The human secretome. Sci. Signal. 2019;12:1–9. doi: 10.1126/scisignal.aaz0274. [DOI] [PubMed] [Google Scholar]
- 68.Vacchini A, Locati M, Borroni EM. Overview and potential unifying themes of the atypical chemokine receptor family. J. Leukoc. Biol. 2016;99:883–892. doi: 10.1189/jlb.2MR1015-477R. [DOI] [PubMed] [Google Scholar]
- 69.Valiente M, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, et al. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS ONE. 2013;8:e80933. doi: 10.1371/journal.pone.0080933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wasilewski, D., Priego, N., Fustero-Torre, C., and M. Valiente. Reactive astrocytes in brain metastasis. Front. Oncol. 7:1–12, 2017. [DOI] [PMC free article] [PubMed]
- 72.Wrobel JK, Toborek M. Blood–brain barrier remodeling during brain metastasis formation. Mol. Med. 2016;22:32–40. doi: 10.2119/molmed.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing F, et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol. Med. 2013;5(3):384–396. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xing F, et al. MiR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene. 2015;34:4890–4900. doi: 10.1038/onc.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xing F, et al. Activation of the c-Met pathway mobilizes an inflammatory network in the brain microenvironment to promote brain metastasis of breast cancer. Cancer Res. 2016;76:4970–4980. doi: 10.1158/0008-5472.CAN-15-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu H, Li Z, Yu Y, Sizdahkhani S, Ho WS, Yin F. A dynamic in vivo-like organotypic blood–brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang C, et al. CXCL1 stimulates migration and invasion in ER-negative breast cancer cells via activation of the ERK/MMP2/9 signaling axis. Int. J. Oncol. 2019;55:684–696. doi: 10.3892/ijo.2019.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.