Abstract
In this study test characteristics of three newly developed enzyme-linked immunosorbent assays (ELISAs) for Salmonella enterica subsp. enterica serovar Dublin were evaluated and compared with two agglutination tests. The ELISAs involved were an indirect ELISA with serovar Dublin lipopolysaccharide (LPS ELISA), an indirect ELISA with serovar Dublin flagellar antigen (GP ELISA), and a double-antibody sandwich blocking ELISA that uses monoclonal antibodies against S. enterica subsp. enterica serovar Enteritidis flagellin (GM-DAS ELISA). The agglutination tests involved were two routine serum agglutination tests with either somatic (O) or flagellar (H) antigen. Diagnostic specificity of the three ELISAs was determined using 840 serum samples from seven dairy herds without any history of serovar Dublin infection. Cutoff values at a titer of 100, 100, and 10, respectively, for the LPS ELISA, GP ELISA, and GM-DAS blocking ELISA resulted in a specificity of 99.3, 100, and 100%, respectively. Using these cutoff values the LPS ELISA, GP ELISA, and GM-DAS ELISA were able to detect, respectively, 30, 46, and 38% of 50 fecal culture-positive animals from 13 herds with a recent serovar Dublin infection. With the same cutoff values, active carriers (n = 18) were detected for 94.4% with the LPS ELISA and for 100% with the GP and GM-DAS ELISAs. Kappa values determined on the results of all tests from 8 of the 13 serovar Dublin-infected herds and the 7 control herds demonstrated a good correlation between the results of all ELISAs and the H-agglutination test. The results of the O-agglutination test failed to correlate with those of the other tests. Using a set of sera from 170 aborting cows (with 25 abortions due to serovar Dublin), test results of the ELISAs and the H-agglutination test were comparable. The H-agglutination test may be used successfully for single sample testing, especially to diagnose abortion due to serovar Dublin. It is concluded that the ELISAs are useful diagnostic tools in serovar Dublin control programs and that they are preferred to agglutination tests for reasons of automation and costs.
Worldwide, Salmonella enterica subsp. enterica serovar Dublin causes infections in cattle, usually with serious clinical disease (19). Control of serovar Dublin on serovar Dublin-infected farms is difficult, partly due to the ability of Salmonella to survive in the environment and certainly due to the occurrence of carrier animals. Detection and subsequent culling of carrier animals is thought to be crucial for control of serovar Dublin in persistently infected herds (7, 10, 13, 15, 16). Active carriers excrete serovar Dublin for many months or even for their lifetime in feces and/or milk. They can be detected easily by bacteriological examination. Excretion of serovar Dublin by latent carriers is unpredictable. The use of bacteriological examination for the detection of these animals is consequently limited (7, 12). Serology can improve the identification of active and latent carriers, even in herds with a vaccination program (7, 10). Serological detection of carriers is based on the persistence of antibody titers in blood or milk. However, there are also limitations for using serology in detecting carriers and transiently infected animals (6, 11), such as the existence of persistently seronegative carriers (6) and the inability of young animals to produce antibodies against lipopolysaccharide (LPS) of serovar Dublin (14, 22). Moreover, serological tests can differ in sensitivity. It is, for instance, reported that agglutination tests are less sensitive than enzyme-linked immunosorbent assays (ELISAs) (1). Many serological tests have been described, such as agglutination tests for serovar Dublin based on somatic (O) or flagellar (H) antigen (13, 21), and ELISAs for serovar Dublin based on LPS antigen (1, 5, 16). Studies of ELISAs for serovar Dublin, based on flagellar antigen, are not known.
Recently, two ELISAs based on flagellar antigen and one ELISA based on LPS antigen became available for evaluation in bovines. The aim of this study was to compare the different ELISAs and two conventional agglutination tests with each other and with bacteriological examination in serovar Dublin-infected and control animals.
MATERIALS AND METHODS
Study design. (i) Infected and control farms.
The study was performed on 13 farms with recent history of clinical salmonellosis due to serovar Dublin. Diagnosis on all farms was confirmed by isolation of serovar Dublin from one or more different samples of diseased animals. The period between the onset of clinical symptoms and first sampling moment of all animals on a farm was shorter than 6 months for 11 of the 13 farms. Clinical symptoms were seen mainly within the group of young calves. Farms on which animals were infected with serovar Dublin were sampled four times, with intervals of 6 months. One farm was not sampled at the third and fourth sampling moment because of lack of motivation of the farmer. Each sampling consisted of a blood sample and a fecal sample of all animals present. The mean number of animals per farm for the first sampling moment was 135 (ranging from 66 to 389). The total number of animals on serovar Dublin-infected farms at the first sampling moment was 1,763. The seven control farms were located on two isles in the northern region of The Netherlands. The selected isles had no history of bovine salmonellosis. Control farms were sampled once. Sampling consisted of a blood sample and a fecal sample of all animals. The mean number of animals per farm was 120 (ranging from 96 to 142). The total number of animals present on the control farms was 840.
The study on serovar Dublin-infected and control farms was carried out between September 1994 and September 1996.
(ii) Carriers.
Fecal culture-positive animals were sampled repeatedly in order to identify active carriers. Active carriers were defined as animals with at least three successive serovar Dublin-positive fecal cultures with a sampling interval of at least 14 days, according to the definition of Field (2). Eighteen active carriers were identified. In 13 of these active carriers the period between successive positive fecal cultures was at least 28 days. Nine of the 18 selected carriers had a history of clinical symptoms due to serovar Dublin. Most of the animals had high fever and diarrhea during the infection period (seven animals). Four animals had an abortion with (two animals) or without (two animals) other symptoms. At least five animals were treated because of clinical symptoms. The mean age of the active carriers was about 3 years and 3 months. Five animals were younger than 1 year.
Five fecal culture-positive animals, from two farms, were purchased for more-detailed studies on fecal shedding and serology and for postmortem examination. Two animals from farm A and one animal from farm B were active carriers according to the definition of Field (2). The other two animals from farm B were on two occasions fecal culture-positive for serovar Dublin, with an interval of 7 months, and could not be classified as active carriers. At the farm, these animals were housed in the same group as the active carrier from farm B. After purchase the five fecal culture-positive animals were housed separately from each other to prevent cross-infection. The animals were sampled two times per week for bacteriological examination of feces and milk (if lactating), and once a week for serological monitoring by the three ELISAs. After the study the animals were necropsied.
(iii) Aborting cows.
Aborted fetuses were collected from material that was sent to the Animal Health Service in Drachten, The Netherlands, for regular diagnostic reasons. Involved farmers were contacted and asked for a blood sample of the aborting cow. In total 170 aborted fetuses with corresponding blood samples of their dams were collected. The farmers were also asked for the salmonellosis history of the herd over the last 2 years. The study was carried out between September 1996 and April 1997.
Bacteriological examination.
Fecal samples were examined for the presence of Salmonella by direct inoculation onto brilliant green agar (Oxoid CM 263), using a 10-μl loop, and by inoculation of 10 g of feces into 100 ml of brilliant green selenite broth. After incubation for 18 to 24 h at 37°C, 10 μl of selenite broth was inoculated onto brilliant green agar. Brilliant green agar plates were incubated for 18 to 24 h at 37°C and read for the presence of suspected Salmonella colonies. Subcultures were made and typed by standard biochemical tests and by slide agglutination using specific antisera. Bacteriological examination of aborted fetuses was done by standard bacteriological techniques on abomasal contents, liver, and different organs and, if present, the placenta. A sample was considered positive if at least one of the colonies was identified as serovar Dublin.
ELISAs and agglutination tests.
The ELISAs used in this study were the same as or modifications of ELISAs described earlier for poultry (18). Details of the buffers and substrate used, the length of the incubation steps, the conjugation of monoclonal antibodies (MAbs) to horseradish peroxidase (HRPO) are described by van Zijderveld et al. (17). Optimal concentrations of antigens and HRPO conjugates were determined by checkerboard titrations. Appropriate controls were included on each test plate.
(i) Indirect ELISA with LPS from serovar Dublin (LPS ELISA).
The wells of microdilution plates were each coated with 100 μl of a solution of serovar Dublin LPS containing 5 μg of LPS per ml. LPS was prepared from serovar Dublin strain ID-Sd2 by the hot water-phenol method of Westphal and Jann as described by Helander (4). Serum samples were added in serial twofold dilutions starting with a dilution of 1:25. After incubation for 1 h at 37°C, plates were washed and the conjugate, an HRPO-labeled MAb against bovine immunoglobulin G1 (ID 15.8.1a), was added. After incubation for 1 h at 37°C, plates were washed and the substrate solution with 5-aminosalicylic acid as chromogen was added, and the plates were read after 2 h at room temperature. Titers were expressed as the reciprocal of the highest dilution or its logarithm, yielding an A450 that was 50% of the absorbance value obtained with the positive control serum.
(ii) Indirect ELISA with purified serovar Dublin flagellin (GP ELISA).
This ELISA was essentially the same as the LPS ELISA, except that plates were coated with 100 μl of a solution of purified serovar Dublin flagellin containing 5 μg of flagellin per ml. Highly motile serovar Dublin strain ID-Sd2 was obtained after several passages on heart infusion swarm agar (0.5% agar). The strain was then inoculated into 4-liter quantities of the medium described by Ibrahim et al. (8). After incubation for 12 to 16 h at 37°C, Salmonella cells were harvested, checked for the presence of serovar Dublin flagella, and resuspended in saline solution. The isolation and purification of flagellin were essentially the same as those described by Ibrahim et al. (8). The purity of the flagellin preparation was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described before by van Zijderveld et al. (18). Titers were expressed as the reciprocal of the highest dilution or its logarithm, yielding an A450 that was 50% of the absorbance value obtained with the positive control serum.
(iii) GM-DAS ELISA.
This ELISA is a double-antibody sandwich blocking ELISA as described before by van Zijderveld et al. (18). Briefly, microdilution plates were coated with MAb gm-5 directed against a g epitope of the serovar Enteritidis flagellin, which is also present on serovar Dublin flagellin. A crude antigen preparation containing native flagella of serovar Enteritidis was added, and after washing serum samples were added in twofold dilutions starting at a dilution of 1:10. After incubation for 1 h at 37°C and washing, an HRPO-conjugated MAb against another g epitope was added. Titers were expressed as the reciprocal of the highest dilution or its logarithm, yielding an A450 that was at least 50% of the absorbance value obtained by the negative control serum.
(iv) Serum agglutination tests.
Serum agglutination tests were performed in tubes according to standard procedures. Antigens used were a serovar Dublin somatic (O) antigen and a serovar Dublin flagellar (H) antigen, prepared essentially the same as described in the OIE Manual of Standards (10a). The antigens were washed once, stored as stock suspension at 4°C, and just before use were diluted from the stock suspension to an optical density at 600 nm of approximately 0.425. Serum samples were tested in twofold dilutions starting with a dilution of 1:20. Titers were expressed as the reciprocal of the highest dilution yielding at least 50% agglutination. Positive and negative controls were included in each test run.
Analysis and analytical methods.
Data on fecal culture-positive animals at serovar Dublin-infected farms and data on animals at control farms were used to estimate specificity and sensitivity of the tests. Specificity and sensitivity were evaluated at several cutoff values of the ELISAs. Two most appropriate cutoff values were determined for the three ELISAs: a low cutoff value and high cutoff value. Differences in results among the three ELISAs and their combinations were tested using McNemar's chi-square test for paired data (3).
The proportion of positive serum samples was compared between serovar Dublin-infected farms that remained fecal culture positive after the initial serovar Dublin infection (seven farms) and farms without fecal culture-positive animals after the initial infection (five farms), using the three ELISAs and determined cutoff values. Differences were tested using Pearson's chi-square test (3). Data on the 5 serovar Dublin-infected farms that had no fecal culture-positive animals after the initial outbreak, were further used to study the course of titers in initially seropositive animals. An animal was considered initially seropositive in an ELISA when the titer at the first sampling moment was equal to or above the low cutoff value of that ELISA.
Data on active carriers were used to estimate test results of the ELISAs in this category of infected animals.
Data on control farms and the first sampling moment on eight serovar Dublin-infected farms (915 animals) were used to calculate kappa values in order to determine agreement between ELISAs and agglutination tests (3). Standard cutoff values were used for the routine O- and H-agglutination test: a low cutoff value of 40 and a high cutoff value of 80 for both tests.
Data on aborting cows were used to estimate test results of the ELISAs and agglutination tests within these animals. The proportion of positive serum samples was compared between the two groups of sera from aborting cows, using Pearson's chi-square test (3).
Specificity was defined as the proportion of serum test-negative samples (below cutoff value) among the fecal culture-negative animals of control farms. Sensitivity was defined as the proportion of serum test-positive samples (equal to or above the cutoff value) among the fecal culture-positive animals where serum and fecal samples were taken at the same point in time (9). An animal was regarded as seropositive if the titer was equal to or above the given cutoff value. An animal was regarded as seronegative if the titer was lower than the given cutoff value. Combinations of tests were evaluated in parallel (any positive test result was regarded as a positive test combination), thereby attempting to increase the number of seropositive animals. Statistical significance was defined at P = 0.05.
RESULTS
Test characteristics of ELISAs on serovar Dublin-infected and control farms.
Results of the ELISAs with 840 animals on the seven control farms are presented in Table 1. All animals were fecal culture-negative for serovar Dublin at the time of blood sampling. With a specificity of >99% a low and high cutoff value was selected per test. The low cutoff values for the LPS ELISA, GP ELISA, and GM-DAS ELISAs were determined at a titer of 100, 100, and 10, respectively. The high cutoff values for the same ELISAs were determined at a titer of 200, 200, and 20, respectively.
TABLE 1.
Evaluation of sensitivity and specificity of three ELISAs on animals at 13 serovar Dublin-infected and 8 control dairy farms
| ELISA | Cutoff value | serovar Dublin-infected farms
|
Control farms (fecal culture-negative animals [n = 840])
|
||||
|---|---|---|---|---|---|---|---|
| All animals (n = 1,763)
|
Fecal culture-positive animalsa(n = 50)
|
||||||
| No.b | %b | No.b | %b | No.c | %c | ||
| LPS | 25 | 837 | 47.5 | 31 | 62.0 | 702 | 83.6 |
| 50 | 311 | 17.6 | 22 | 44.0 | 805 | 95.8 | |
| 100 | 144 | 8.2 | 16 | 32.0 | 834 | 99.3 | |
| 200 | 81 | 4.6 | 13 | 26.0 | 839 | 99.9 | |
| 400 | 49 | 2.8 | 10 | 20.0 | 840 | 100.0 | |
| 800 | 25 | 1.4 | 7 | 14.0 | 840 | 100.0 | |
| 1,600 | 14 | 0.8 | 6 | 12.0 | 840 | 100.0 | |
| 3,200 | 7 | 0.4 | 3 | 6.0 | 840 | 100.0 | |
| GP | 25 | 1136 | 64.4 | 36 | 72.0 | 669 | 79.6 |
| 50 | 391 | 22.2 | 26 | 52.0 | 827 | 98.5 | |
| 100 | 175 | 9.9 | 23 | 46.0 | 840 | 100.0 | |
| 200 | 102 | 5.8 | 17 | 34.0 | 840 | 100.0 | |
| 400 | 64 | 3.6 | 12 | 24.0 | 840 | 100.0 | |
| 800 | 41 | 2.3 | 12 | 24.0 | 840 | 100.0 | |
| 1,600 | 25 | 1.4 | 9 | 18.0 | 840 | 100.0 | |
| 3,200 | 9 | 0.5 | 4 | 8.0 | 840 | 100.0 | |
| GM-DAS | 10 | 135 | 7.9 | 19 | 38.0 | 840 | 100.0 |
| 20 | 92 | 5.2 | 18 | 36.0 | 840 | 100.0 | |
| 40 | 61 | 3.5 | 15 | 30.0 | 840 | 100.0 | |
| 80 | 46 | 2.6 | 12 | 24.0 | 840 | 100.0 | |
| 160 | 26 | 1.5 | 7 | 14.0 | 840 | 100.0 | |
| 320 | 12 | 0.7 | 4 | 8.0 | 840 | 100.0 | |
| 640 | 7 | 0.4 | 3 | 6.0 | 840 | 100.0 | |
Presented serology is from the moment feces were culture positive for serovar Dublin for the first time.
Positive at the cutoff titer indicated or at a higher titer.
Negative at a titer lower than the indicated cutoff titer.
The percentage of seropositive animals, for two groups of animals on 13 serovar Dublin-infected farms, is given in Table 1. At time of first sampling and with the low cutoff value, the percentage of seropositive animals for the LPS, GP, and GM-DAS ELISA was 8.2, 9.9, and 7.9, respectively. Calves tested negative up to 2 months of age, using the high cutoff value of the LPS ELISA. The seroprevalence determined with the high cutoff value of the GP and GM-DAS ELISA for these calves was 11.9 and 13.6%, respectively. At the four successive sampling moments, seven, five, three, and two farms had at least one serovar Dublin culture-positive animal, respectively. The interval between successive sampling moments was 6 months. The total number of fecal culture-positive animals for serovar Dublin was, for the successive sampling moments, 38, 21, 4, and 2 animals, respectively. Eight culture-positive animals on three farms were culled at the initiative of the farmer. Five of these animals could be classified as active carriers. In total 50 animals were found fecal culture positive for serovar Dublin at one or more sampling moments. Using the low cutoff value, determined on the control farms, the percentage of seropositive animals among the 50 serovar Dublin culture-positive animals by the LPS, GP, and GM-DAS ELISA was 32.0, 46.0, and 38.0, respectively. The percentage of seropositive animals by the LPS ELISA was lower than that by the GP ELISA (P < 0.01). Combining the GP ELISA and LPS ELISA, using the low cutoff values, did not increase the percentage of seropositive animals (P = 1.00); neither did a combination of the GP and GM-DAS ELISAs (P = 0.84).
The percentage of seropositive animals, at successive sampling moments for 12 serovar Dublin-infected farms, is given in Table 2, comparing seven farms that remained fecal culture positive after the initial serovar Dublin infection with five farms without any fecal culture-positive animal after the initial infection. There was little difference in percentage seropositive animals between the two groups of farms at the second and third sampling moment. However, at the fourth sampling moment, a clear difference was found. The greatest differences between the two groups of farms were found using the LPS ELISA or GP ELISA.
TABLE 2.
Percentage of seropositive animals at successive sampling moments with 6-month intervals on 12 serovar Dublin-infected farms
| ELISA | Cutoff value | Serovar Dublin-infected farms (n = 5) with negative fecal cultures at 4 sampling moments (575 animals)
|
Serovar Dublin-infected farms (n = 7) with one or more positive fecal cultures at 1, 2, 3, or 4 sampling moments (1,092 animals)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| LPS | Low | 9.4 | 2.8 | 5.6 | 1.4 | 8.0 | 4.9* | 6.8 | 6.9† |
| High | 5.7 | 1.6 | 0.8 | 0 | 4.1 | 2.0 | 1.9 | 2.5† | |
| GP | Low | 10.8 | 3.3 | 9.7 | 1.7 | 9.6 | 9.9† | 7.2 | 9.7† |
| High | 6.7 | 1.8 | 2.4 | 0 | 5.1 | 4.1* | 2.0 | 3.3† | |
| GM-DAS | Low | 7.9 | 6.5 | 2.1 | 1.7 | 8.2 | 6.8 | 4.0* | 3.9* |
| High | 5.9 | 3.9 | 1.4 | 1.3 | 5.2 | 3.5 | 2.4 | 1.7 | |
The significance of the differences was calculated (Pearson's chi-square) between the two groups of farms using the test result of the same ELISA at the same sampling moment. ∗, P < 0.05; † P < 0.01).
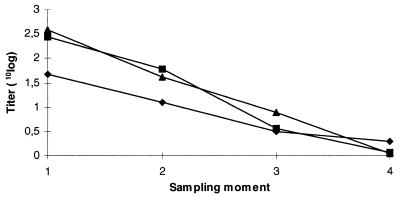
The results of the course of mean titers are shown in Fig. 1. There was a mean decrease of about one log10 of the titer in the period between two sampling moments (6 months). The percentage animals with a decline between the first and second sampling moment of less than one log10 of the titer, was 80, 78, and 73 for the LPS, GP, and GM-DAS ELISA, respectively. Differences in course of mean titers between ELISAs were small.
FIG. 1.
Course of mean titer of the LPS (▴), GP (■), and GM-DAS (⧫) ELISA in initially seropositive animals (n = 74) on five serovar Dublin-infected farms without any positive fecal culture at four sampling moments (6-month intervals).
Active carriers.
The percentage of serovar Dublin seropositive active carriers, using the low cutoff value, was 94.4% for the LPS ELISA and 100% for the GP and GM-DAS ELISAs. Using the high cutoff value, 94.4% of the active carriers tested seropositive in all three ELISAs.
After purchase of the five fecal culture-positive animals the three active carriers remained fecal culture-positive. The other two animals remained serovar Dublin culture negative from 1 week after purchase. Test results of the five animals are shown in Table 3. The three active carriers remained seropositive by all three ELISAs, using the high cutoff values. The other two animals (2205 and 2492) had no serum response in the GM-DAS ELISA and, only in the first month after purchase, a low serum response with the GP and LPS ELISA. After 8 months these two animals were necropsied. Necropsy revealed no abnormalities nor any serovar Dublin-positive culture of gut contents, lymph nodes, and internal organs. The three active carriers were necropsied after 17 months. Necropsy of animal 2201 revealed an extensive pyelonephritis of the left kidney and a serovar Dublin-positive culture of the gut content, mesenteric lymph nodes, and left kidney. Necropsy of the other two active carriers (3858 and 3863) revealed typical liver fluke-infested livers and a serovar Dublin-positive culture of the gut content, mesenteric lymph nodes, portal lymph nodes, liver tissue (10,000 to 100,000 CFU/g) and gallbladder (10,000,000 CFU/ml).
TABLE 3.
Test results with sera and feces of three active and two passive carriers of serovar Dublin after they were housed individually
| Farm | Animal | ELISA titer range
|
Bacteriological examination
|
|||
|---|---|---|---|---|---|---|
| LPS | GP | GM-DAS | Feces | Milk | ||
| A | 3858 | 400–6,400 | 400–3,200 | 40–80 | +++a | +−e |
| 3863 | 800–3,200 | 800–3,200 | 80–160 | +++a | +−e | |
| B | 2201 | 400–6,400 | 400–1,600 | 20–80 | +++b | —f |
| 2205 | <100–400 | <100–100 | <10 | −−−c | — | |
| 2492 | <100–100 | <100–100 | <10 | +−−d | — | |
10,000 to 10,000,000 CFU/g of feces.
1 to 1,000,000 CFU/g of feces.
No positive fecal culture.
Intermittent positive fecal culture (<10,000 CFU/g of feces) during the first 3 weeks of individual housing.
Intermittent positive culture (<10,000 CFU/ml of milk).
—, no milk production (yearlings).
Comparison of ELISAs and agglutination tests.
Differences between ELISAs and agglutination tests were compared by calculating kappa values (Table 4). Agreement between tests was best for GP ELISA, GM-DAS ELISA, and the H-agglutination test. There was a poor agreement between the O-agglutination test and the other tests.
TABLE 4.
Comparison between three ELISAs and two agglutination tests for serovar Dublin detection using eight serovar Dublin-infected farms and seven control farms
| Test | Cutoff value | Kappa value
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| GP ELISA
|
LPS ELISA
|
H-agglutination
|
O-agglutination
|
||||||
| 100 | 200 | 100 | 200 | 40 | 80 | 40 | 80 | ||
| GM-DAS ELISA | 10 | 0.52 | 0.67 | 0.40 | 0.46 | 0.56 | 0.63 | 0.18 | 0.02 |
| 20 | 0.48 | 0.70 | 0.44 | 0.74 | 0.50 | 0.60 | 0.13 | 0 | |
| GP ELISA | 100 | 0.51 | 0.48 | 0.50 | 0.48 | 0.17 | 0.02 | ||
| 200 | 0.45 | 0.46 | 0.51 | 0.57 | 0.15 | 0 | |||
| LPS ELISA | 100 | 0.40 | 0.40 | 0.30 | 0.17 | ||||
| 200 | 0.37 | 0.40 | 0.20 | 0.05 | |||||
| H-agglutination | 40 | 0.33 | 0.15 | ||||||
| 80 | 0.24 | 0.04 | |||||||
Aborting cows.
Test results for sera of 170 aborting cows were compared with results of bacteriological examination of involved aborted fetuses. In 25 cases serovar Dublin could be isolated. In 11 of these cases there was a known history of salmonellosis on the farm. From 145 aborted fetuses serovar Dublin could not be isolated by bacteriological examination. For 55 of these cases there was a known history of salmonellosis of the farm. The percentage of seropositive animals for serovar Dublin, using different tests, is shown in Table 5. The percentage of seropositive animals by the O-agglutination test was substantially lower among the cows that aborted due to serovar Dublin, using the high cutoff value (P < 0.01). The sensitivity of the combination of H-agglutination test and GP ELISA was 9.5% higher (95% confidence interval [CI], 0 to 23.2%) and the specificity was 9.4% lower (95% CI, 4.5 to 14.4%) than the sensitivity and specificity of the H-agglutination test alone, using the low cutoff values. Using the high cutoff values and the same combination of tests, sensitivity was 4.8% higher (95% CI, 0 to 14.7%) and specificity was 2.2% lower (95% CI, 0 to 4.6%). There was no further positive effect on test characteristics by other combinations of tests or by using more than two test combinations. Limited data indicated a further improvement of sensitivity if blood was sampled more than 4 days after abortion (data not shown).
TABLE 5.
Percentage of animals seropositive for serovar Dublin among 170 aborting cows, using various tests and cutoff values
| Serovar Dublin status of aborted fetus | Cutoff value | ELISA
|
Agglutination test
|
|||
|---|---|---|---|---|---|---|
| LPS | GP | GM-DAS | O | H | ||
| Culture positive (n = 25) | Low | 76.0 | 68.0 | 64.0 | 66.7 | 76.2 |
| High | 56.0a | 60.0a | 64.0a | 23.8 | 62.9a | |
| Culture negative (n = 145) | Low | 15.3b | 11.0b | 11.7b | 3.1 | 2.9 |
| High | 4.9c | 3.4 | 4.8c | 0.8 | 0.7 | |
Different (P < 0.01) from seroprevalence with O-agglutination test in same row.
Different (P < 0.01) from seroprevalence with H- and O-agglutination test in same row.
Different (P < 0.05) from seroprevalence with H- and O-agglutination test in same row.
The percentage of seropositive animals, among cows that aborted for reasons other than serovar Dublin, was higher by the ELISAs than by the agglutination tests (P < 0.05).
DISCUSSION
In this study test characteristics of three ELISAs were evaluated in comparison with the results of bacteriological examination of individual animals. The sensitivity of the ELISAs was low in detecting animals with a positive fecal culture for serovar Dublin, except for the active carriers. There can be several explanations for this low sensitivity. First, not all animals may have a humoral immune response after a serovar Dublin infection (20). Second, the time interval between infection and blood sampling was probably too short for some animals to result in a measurable immune response. Third, young calves may not be capable of producing or may have a delayed response in producing antibodies against LPS antigen. This age-related response was seen for very young calves after experimental infections (F. G. van Zijderveld, unpublished observation) and with calves up to 3 months after immunization with killed Salmonella bacterin (14, 22). This argument is especially important for the LPS ELISA and O-agglutination test. Furthermore, in our study most clinical symptoms on the serovar Dublin-infected farms were seen among young calves.
On the infected farms, the serum response for serovar Dublin was low based on the results of the ELISAs. This may be partly explained by the moment of first blood sampling. The first moment of sampling for most farms was about 4 to 6 months after the initial infection of the herd, and there may have been a substantial decrease in antibody titer for transiently infected animals in a period of 6 months (Fig. 1). The definition of active carrier is important for the evaluation of the ELISAs in detecting these animals and probably also for the detection of latent carriers. The sensitivity of the three ELISAs for detection of active carriers, according to the definition of Field (2), was promising. Using the low cutoff value, 94.4% of the active carriers were detected by the LPS ELISA and 100% of the active carriers were detected by the GP and GM-DAS ELISAs. The three active carriers from the detailed study could also be detected using higher cutoff values for the ELISAs. It is not known from this study if the test results with the active carriers of serovar Dublin can be extrapolated to the serology of latent carriers of serovar Dublin. The detailed study with the five culture-positive animals clearly shows the difference between active and passive carriers as earlier mentioned by Field (2) and Richardson (12). Two animals stopped shedding serovar Dublin after they were housed separately from the active carrier. Necropsy of these animals revealed no abnormalities nor any serovar Dublin-positive culture. These animals can be classified as passive carriers during the time they were housed together with the active carrier (12).
The course of mean titers on serovar Dublin-infected farms without active carriers (Fig. 1) revealed a period of about 1.5 years before most of the serum responses were below the low cutoff value of the tests. This is important for the detection of persistent seropositive animals. It is concluded, in contrast with other observations (16), that a period of 2 or 3 months between two sampling moments is probably to short to discriminate between carriers and transient infected animals.
The specificity of the agglutination tests on sera of aborting cows was higher than that for the ELISAs (Table 5). A reason might be the occurrence of “incomplete” (nonagglutinating) antibodies in formerly infected animals, because sera of the aborted cows were mainly received from regions where serovar Dublin infections are endemic. Sensitivity of the H-agglutination test was higher than the sensitivity of the O-agglutination test in the sera of the aborting cows. This is in agreement with other work on diagnosis of serovar Dublin, using vaccinated animals (21) or carrier cows (12). The lower test results of the O-agglutination test on serovar Dublin-infected farms, also according to the lower kappa values (Table 4), is probably due to the inability of young animals to form antibodies against LPS (14, 22).
Estimation of the costs of the tests was not part of this study, but for reasons of automation ELISAs are favorable for testing large numbers of sera. With individual samples agglutination tests are cheaper than ELISAs.
It is concluded that test characteristics of the three ELISAs are comparable in detecting animals which are fecal culture positive for serovar Dublin and in detecting active carriers. ELISAs based on flagellar antigen are preferred for estimation of the seroprevalence. Test characteristics of ELISAs and agglutination tests are comparable except for the O-agglutination test. Agglutination tests gave fewer false-positive results with blood samples of aborting cows. The H-agglutination test may be used successfully for single-sample testing, especially to diagnose abortion due to serovar Dublin. ELISAs are more suitable for use on a large scale than agglutination tests, for reasons of automation and costs.
Test characteristics in this study were primarily based on results with individual animals, which were not in the acute phase of the serovar Dublin infection. Further research is therefore needed concerning the characteristics of the ELISAs on a herd level and the diagnostic possibilities of the ELISAs in the acute phase of the infection.
ACKNOWLEDGMENT
This work was supported by the Ministry of Agriculture, Nature Management, and Fisheries, grant KWA/DS/07/94/0050.
REFERENCES
- 1.Carlsson H E, Lindberg A A, Hammarstom S. Titration of antibodies to Salmonella O antigens by enzyme-linked immunosorbent assay. Infect Immun. 1972;6:703–708. doi: 10.1128/iai.6.5.703-708.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field H I. A survey of bovine salmonellosis in mid and west Wales. Vet J. 1948;104:251–266. doi: 10.1016/s0372-5545(17)30203-1. [DOI] [PubMed] [Google Scholar]
- 3.Fleiss J L. Statistical methods for rates and proportions. New York, N.Y: John Wiley & Sons; 1981. [Google Scholar]
- 4.Helander I M. Isolation and electrophoretic analysis of bacterial lipopolysaccharides. In: Korhonen T K, Dawes E A, Mäkelä P H, editors. Enterobacterial surface antigens: methods for molecular characterization. Amsterdam, The Netherlands: Elsevier; 1985. pp. 263–274. [Google Scholar]
- 5.Hoorfar J, Feld N C, Schirmer A L, Bitsch V, Lind P. Serodiagnosis of Salmonella dublin infection in Danish dairy herds using O-antigen based ELISA. Can J Vet Res. 1994;58:268–274. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoorfar J, Wedderkopp A, Lind P. Comparison between persisting anti-lipopolysacharide antibodies and culture at postmortem in Salmonella-infected cattle herds. Vet Microbiol. 1996;50:81–94. doi: 10.1016/0378-1135(95)00199-9. [DOI] [PubMed] [Google Scholar]
- 7.House J K, Smith B P, Dilling G W, Roden L D. Enzyme-linked immunosorbent assay for serologic detection of Salmonella dublin carriers on a large dairy. Am J Vet Res. 1993;54:1391–1399. [PubMed] [Google Scholar]
- 8.Ibrahim G F, Fleet G F, Lyons M J, Walker R A. Methods for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin S W, Meek A H, Willeberg P. Veterinary epidemiology: principles and methods. Ames: Iowa State University Press; 1987. [Google Scholar]
- 10.Nielsen B B, Vestergaard E M. Proceedings. International symposium Salmonella and salmonellosis. Ploufragan, France: Zoopôle; 1992. Use of ELISA in the eradication of Salmonella dublin infection; pp. 220–224. [Google Scholar]
- 10a.Office International des Epizooties. OIE manual of standards for diagnostic tests and vaccines. Paris, France: Office International des Epizooties; 1996. [DOI] [PubMed] [Google Scholar]
- 11.Richardson A. Serological responses of Salmonella dublin carrier cows. Br Vet J. 1973;129:liii–liv. doi: 10.1016/s0007-1935(17)36457-6. [DOI] [PubMed] [Google Scholar]
- 12.Richardson A R. The transmission of Salmonella dublin to calves from adult carrier cows. Vet Rec. 1973;92:112–115. doi: 10.1136/vr.92.5.112. [DOI] [PubMed] [Google Scholar]
- 13.Robertsson J A. Humoral antibody responses to experimental and spontaneous Salmonella infections in cattle measured by ELISA. Zentbl Vet Med. 1984;31:367–380. doi: 10.1111/j.1439-0450.1984.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 14.Roden D L, Smith B P, Spier S J, Dilling G W. Effect of calf age and Salmonella bacterin type on ability to produce immunoglobulins directed against Salmonella whole cells or lipopolysaccharide. Am J Vet Res. 1992;53:1895–1899. [PubMed] [Google Scholar]
- 15.Smith B P, Oliver D G, Singh P, Dilling G, Marvin P A, Ram B P, Jang L S, Sharkov N, Orsborn J S, Jackett K. Detection of Salmonella dublin mammary gland infection in carrier cows, using an enzyme-linked immunosorbent assay for antibody in milk or serum. Am J Vet Res. 1993;50:1352–1360. [PubMed] [Google Scholar]
- 16.Spier S J, Smith B P, Cullor J W, Dilling J S, Pfaff L D. Use of Elisa for detection of immunoglobulins G and M that recognize Salmonella dublin lipopolysaccharide for prediction of carrier status in cattle. Am J Vet Res. 1990;51:1900–1904. [PubMed] [Google Scholar]
- 17.van Zijderveld F G, Westenbrink F, Anakotta J, Brouwers R A M, van Zijderveld A M. Characterization of the F41 fimbrial antigen of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1989;57:1192–1199. doi: 10.1128/iai.57.4.1192-1199.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Zijderveld F G, van Zijderveld-van Bemmel A M, Anakotta J. Comparison of four different enzyme-linked immunosorbent assays for serological diagnosis of Salmonella enteritidis infection in experimentally infected chickens. J Clin Microbiol. 1992;30:2560–2566. doi: 10.1128/jcm.30.10.2560-2566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser I J R, Veen M, van der Giesen J W B. Salmonella dublin infections in dairy cattle, a review. Tijdschr Diergeneeskd. 1992;117:730–735. [PubMed] [Google Scholar]
- 20.World Health Organization. Salmonellosis control: the role of animal and product hygiene. WHO technical report series no. 774. Geneva, Switzerland: World Health Organization; 1988. [PubMed] [Google Scholar]
- 21.Wray C, Morris J A, Sojka W J. A comparison of indirect haemagglutination tests and serum agglutination tests for the serological diagnosis of Salmonella dublin infection in cattle. Br Vet J. 1975;131:727–737. doi: 10.1016/s0007-1935(17)35145-x. [DOI] [PubMed] [Google Scholar]
- 22.Wray C, Sojka W J. The serological responses of calves to live Salmonella dublin vaccine—a comparison of different serological tests. J Biol Stand. 1984;12:277–282. doi: 10.1016/s0092-1157(84)80007-4. [DOI] [PubMed] [Google Scholar]