Abstract
Autophagy is a catabolic process that captures cellular waste and degrades them in the lysosome. The main function of autophagy is quality control of cytosolic proteins and organelles, and intracellular recycling of nutrients in order to maintain cellular homeostasis. Autophagy is upregulated in many cancers to promote cell survival, proliferation and metastasis. Both cell-autonomous autophagy (also known as tumor autophagy) and non-cell autonomous autophagy (also known as host autophagy) supports tumorigenesis through different mechanisms, including inhibition of p53 activation, sustaining redox homeostasis, maintenance of essential amino acids levels in order to support energy production and biosynthesis, and inhibition of anti-tumor immune responses. Therefore, autophagy may serve as a tumor-specific vulnerability and targeting autophagy could be a novel strategy in cancer treatment.
Keywords: Autophagy, Cancer, Metastasis, p53, Immune Response, Cancer Metabolism, Cancer treatment
1. Introduction
Macroautophagy (herein referred to as autophagy) is a major catabolic pathway for the delivery of dysfunctional or unnecessary cellular components to the lysosome for degradation and subsequent reuse, a process which is conserved from yeast to mammals [1]. Autophagy is essential for cellular homeostasis, and abnormal autophagy is associated with many human diseases [2]. We will focus on the role of autophagy in cancer, and in particular, we will elucidate how autophagy regulates the immune response and metabolism to support tumor growth and survival.
2. Autophagy Regulation and Machinery
Autophagy is induced by environmental stressors such as nutrient deprivation. It is negatively regulated by the Mammalian Target of Rapamycin (mTOR), a nutrient sensor for the cell, and positively regulated by the AMP-activated protein kinase (AMPK), a master regulator of energy metabolism (Fig. 1) [3].
Figure 1. Autophagy regulatory pathways.
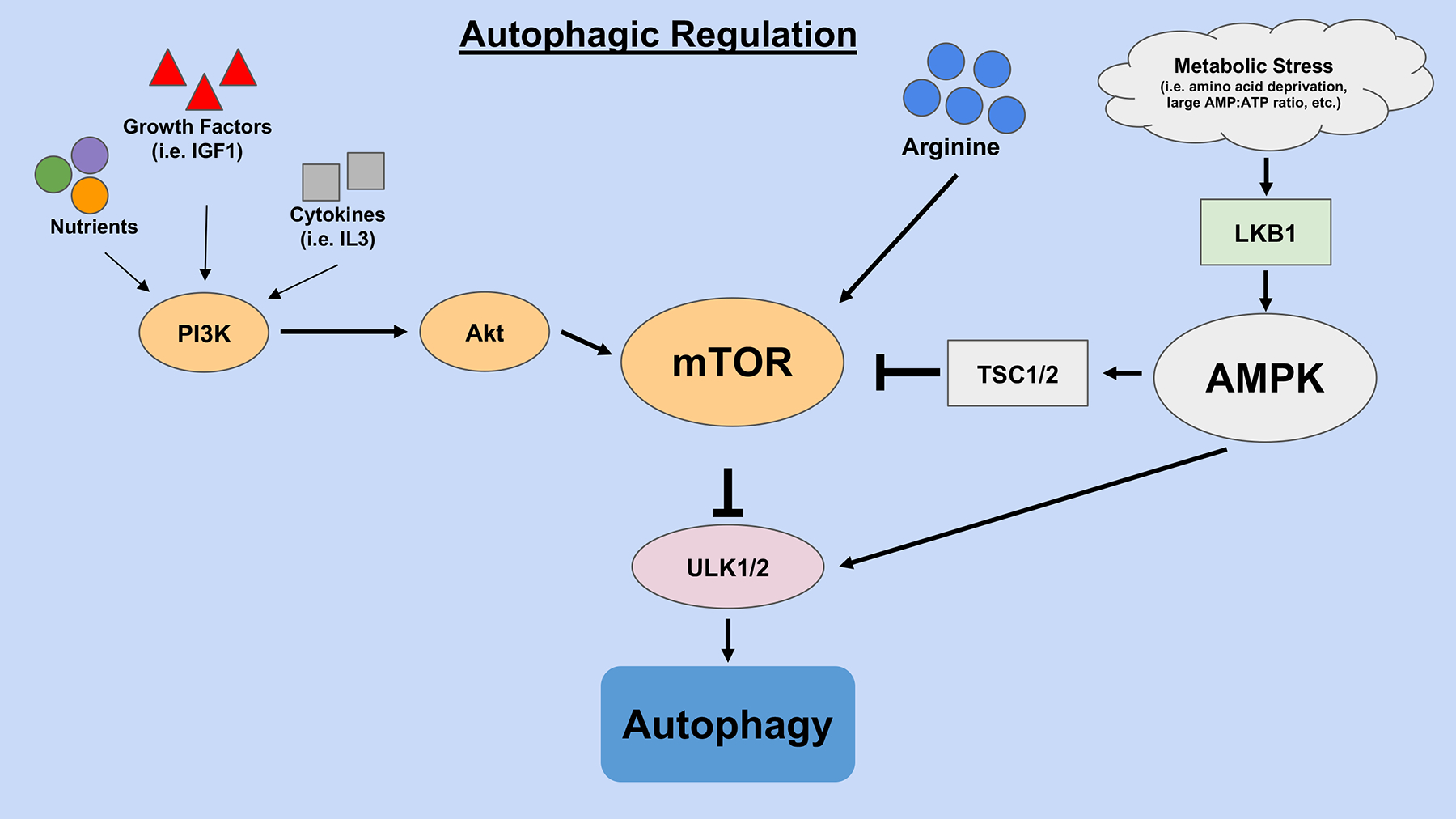
The PI3K/AKT/mTOR pathway, activated by nutrients and growth factors, leads to downregulation of autophagy. On the other hand, the LKB1/AMPK/ULK pathway leads to upregulation of autophagy due to metabolic stressors.
2.1. The PI3K/AKT/mTOR Pathway
Phosphoinositide 3-kinases (PI3Ks) are a class of enzymes that generate secondary messengers known as 3′ phosphoinositide lipids [4]. PI3K enzymes are usually activated by metabotropic G-protein coupled receptors in response to intracellular growth factors, such as insulin-like growth factor-1 (IGF1) or interleukin 3 (IL-3) [5]. These 3’ phosphoinositide lipids are then able to activate Protein kinase B (PKB), also known as Akt, which phosphorylates downstream targets and activates mTOR pathways. More specifically, in nutrient rich conditions or stimulation by growth factors, Akt activates mTOR Complex 1 (mTORC1), suppressing autophagy by phosphorylation-dependent inhibition of Unc-51 like-autophagy activating kinase 1/2 (ULK1/2) and the Vacuolar protein sorting 34 (Vps34) complex [6].
2.2. The AMPK/ULK Pathway
AMPK is activated during energy crisis, such as during amino acid depletion or glucose deprivation, which leads to a large AMP:ATP ratio within the cell. More specifically, AMPK is allosterically activated by AMP and ADP, while competitively inhibited by ATP. AMPK, activated during low-charged cellular energy states, can then phosphorylate ULK1/2, two crucial mammalian enzymes responsible for stimulating autophagy. Activated AMPK can also coincidentally inhibit mTOR through stimulation of the tuberous sclerosis complex 1/2 (TSC1/2). Hence, AMPK possesses a unique dual ability to robustly activate autophagy within the cell in response to metabolic stress [3].
2.3. Autophagy Machinery
The process of autophagy consists of multiple distinct steps and is mediated by several autophagy-related (Atg) genes (Fig. 2). The preliminary step in the formation of autophagy vesicles is the generation of the ULK complex. The ULK complex consists of a ULK family kinase, the focal adhesion kinase interacting protein of 200 kDa (FIP200), and autophagy-related gene 13 (Atg13) [7]. This complex is doubly regulated by two important sensors of cellular stress, mTOR and AMPK. Thus, the ULK complex acts as the main “gatekeeping” protein that regulates whether or not the autophagic vesicles form.
Figure 2. Autophagy machinery.
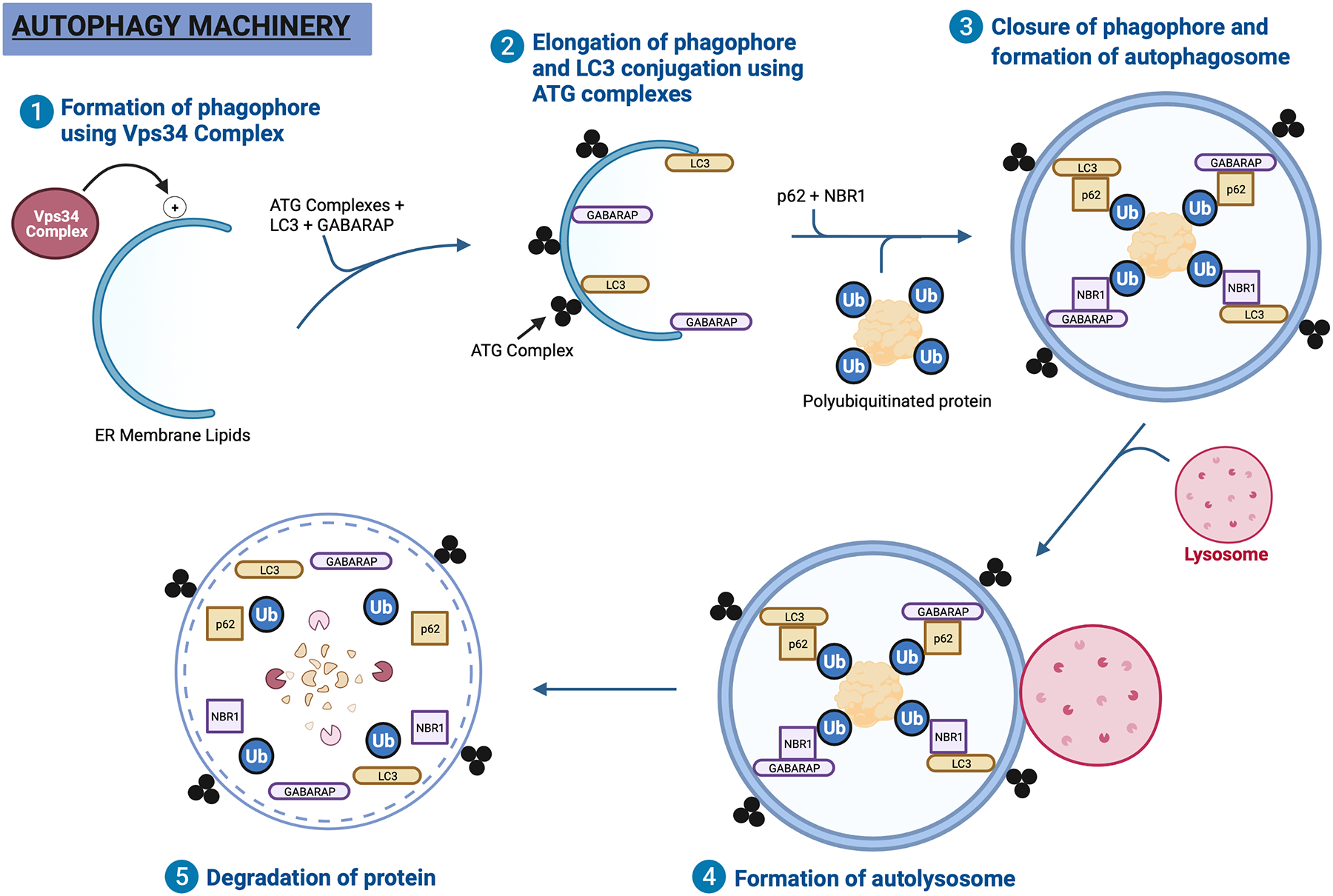
Step 1, the phagophore is synthesized from ER membrane lipids upon activation by the Vps34 Complex. Step 2, the phagophore is elongated with the help of structural proteins such as LC3 and GABARAP. These Atg8 family of proteins are attached to the phagophore with the help of ATG complexes. Step 3, p62 and NBR1 serve as cargo receptor proteins that carry polyubiquitinated proteins destined for degradation to the phagophore. Once the phagophore closes itself around the protein, it is known as an autophagosome. Step 4, a neighboring lysosome then fuses with the autophagosome to create and autolysosome. Step 5, the polyubiquitinated protein is degraded using lysosomal enzymes.
The first and second step of autophagy activation is broadly known as the “elongation step” and involves the preparation of autophagic vesicle membranes. The ULK complex first activates the Vps34 complex, which is composed of Vps34 (a class III PI3K) and Beclin-1 (BECN1) [7]. Using the endoplasmic reticulum as a base, the Vps34 complex is able to form the autophagic vesicle membrane lipids. These vesicle membranes, known as a “phagophore”, generate a spherical vesicle. These lipids are then conjugated to microtubule-associated protein 1A/1B light chain 3B (LC3), a microtubule-associated protein that serves a structural purpose in vesicle formation [7]. Additionally, LC3 serves a functional role as a docking site for cargo receptor proteins carrying polyubiquitinated organelles or proteins.
There are two ubiquitin-like systems used during phagophore elongation and maturation. The first is Atg5-Atg12 conjugation, which is mediated by E1-like activating enzyme Atg7. The Atg5-Atg12 forms a complex with Atg16 to promote LC3 cleavage by ATG4B as well as LC3-phosphatidylethanolamine (LC3-PE) conjugation (the second ubiquitin-like system), which is also mediated by Atg7 [8]. It is believed that curvature of the phagophore membrane is driven by the binding of damaged cargo to receptors lined along the lipid membrane. Eventually, the phagophore completely wraps around the cargo to form a double-membrane “autophagosome” [9].
Cargo receptor proteins play important roles during autophagy. p62, also known as sequestosome1, is a cargo receptor protein that recognizes polyubiquitinated proteins and binds them with autophagosomes. Since most organelles targeted for autophagy are tagged with ubiquitin, p62 plays a crucial role in facilitating degradation of these organelles within autophagosomes [10]. Neighbor of BRCA1 gene 1 protein (NBR1) is another cargo receptor protein that binds polyubiquitinated proteins to autophagosomes. Both p62 and NBR1 contain an LIR motif that binds to the Atg8 family of proteins (such as GABARAP and LC3) found on autophagosomes [11]. Although p62 and NBR1 act similarly, having different cargo receptor proteins provides a high degree of selectivity in the autophagic process. In other words, having different cargo receptor proteins enables damaged organelles or protein aggregates to preferentially bind to their specific cargo receptor protein.
Following these steps, organelle degradation occurs when the autophagosomes fuse with lysosomes to form the “autolysosome”. The autolysosome contains the lysosomal enzymes and acidic lumen necessary for protein degradation [9]. The acidic inner environment that is generated by this process causes destruction of the inner membrane of the autolysosome but not the outer membrane. This allows the autolysosome to still retain its shape and function [9]. Once degradation is complete, the broken-down contents are released and recycled for subsequent use by the cell [7].
3. Autophagy Function
Autophagy is mainly responsible for the breakdown and recycling of long-lived cell components and cytosolic organelles and is present in a wide range of organisms and cell types. Its functions are broad but mainly include organelle and protein turnover, tissue remodeling, and survival during starvation (Fig. 3) [12, 13].
Figure 3. Common functions of autophagy.
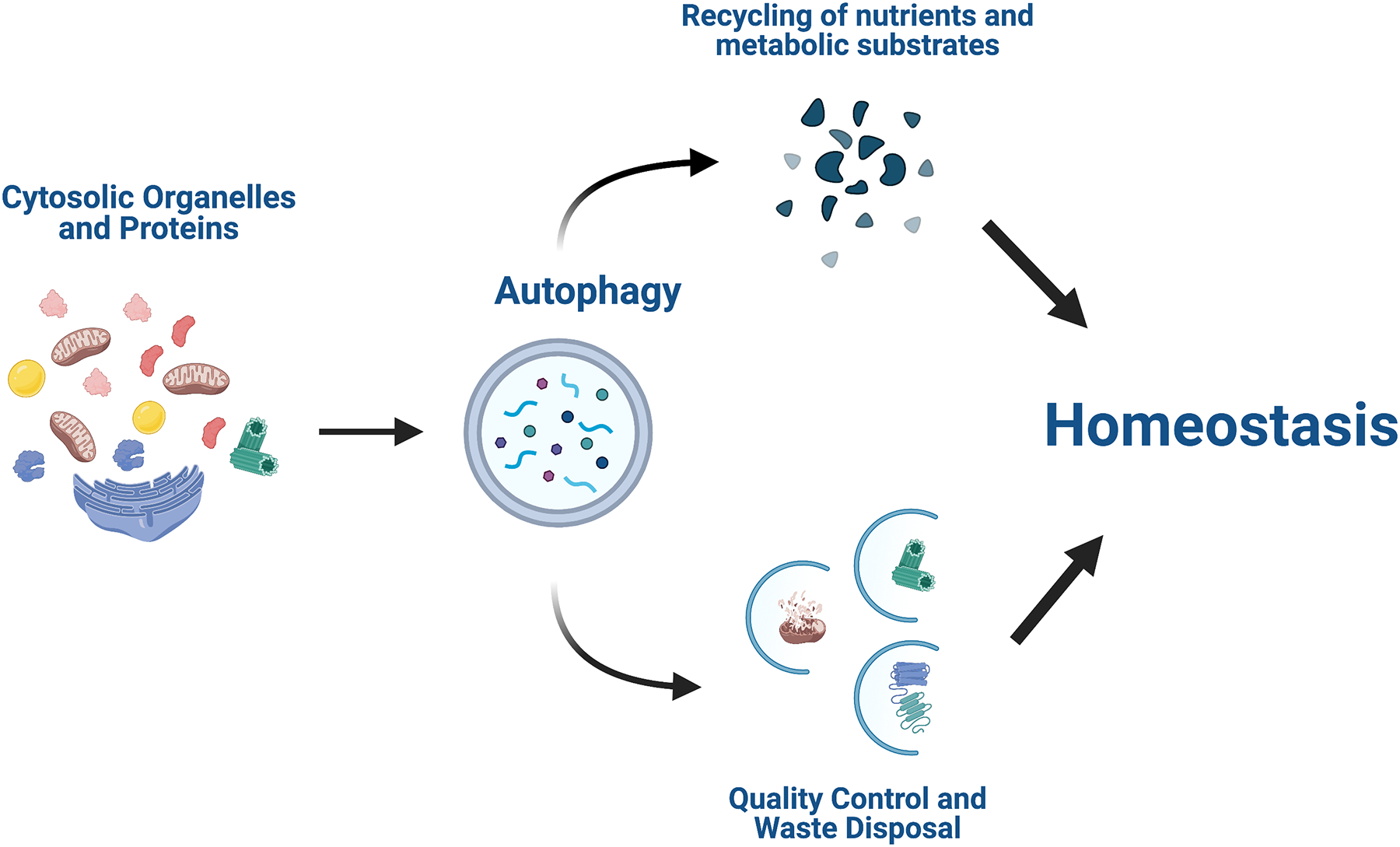
Autophagy degrades damaged or dysfunctional cytosolic organelles and proteins, serving as a quality control and waste disposal mechanism for the cell. Autophagy-mediated degradation also serves as a way for the cell to recycle nutrients and other substrates for use in biosynthesis and energy production - especially during times of stress or starvation.
3.1. Protein and Organelle Quality Control
One of the major functions of autophagy is to remove and degrade damaged cytosolic components such as protein aggregates and organelles, which enables the cell to maintain quality control of cellular contents [14, 15]. Elimination of toxic protein aggregates or damaged organelles by autophagy can prevent reactive oxygen species (ROS) production, making autophagy essential for sustaining genomic stability [16]. Mitophagy is another important type of cargo-specific autophagy in which damaged and dysfunctional mitochondria are selectively captured in autophagosomes for degradation to ensure mitochondrial quality and prevent buildup of defective mitochondria [17, 18]. Both non-selective and selective mechanisms of autophagy are responsible for the turnover and quality control of several other organelles as well, including endoplasmic reticulum, ribosomes, centrosomes, and lipid droplets [19–23].
3.2. Intracellular Nutrient Recycling
Autophagy plays an essential role in recycling cellular components to maintain metabolism, especially during metabolic stress such as starvation. Autophagy can breakdown cytosolic components into amino acids, nucleic acids, sugars, and fatty acids, which can then be recycled into carbon metabolism for energy homeostasis and biosynthesis [15, 24]. Autophagy is also responsible for maintaining amino acid levels during the neonatal starvation period in mice [25, 26], and is required to maintain glucose homeostasis for adult mice to survive fasting conditions [27]. Autophagy also promotes survival in cells undergoing high levels of metabolic stress, such as rapidly proliferating tumor cells [28–30]. Thus, autophagy provides metabolic substrates required for the growth and survival of both healthy cells and cancer cells [29, 31–33].
3.3. Non-Canonical Roles of Autophagy Proteins
The proteins involved in autophagy pathways also play non-canonical roles in other cellular contexts. Beginning with LC3, there are two different LC3 family members that play a role in cancer cells. LC3C plays a tumor suppressive role through regulation of Met/hepatocyte growth factor receptor tyrosine kinases (Met/HGF RTKs). Met/HGF RTKs are implicated in the increased migratory invasive responses of cancer cells [34]. However, complex formation with LC3C selectively degrades these Met/HGF RTKs through autophagic pathways. Therefore, LC3C plays an important role in reducing tumor metastasis. In fact, decreased LC3C expression in cancer cells causes a loss of autophagic degradation of Met/HGF RTKs, resulting in increased cancer cell invasion and metastatic progression [34, 35]. On the other hand, LC3B is believed to promote tumor growth by initializing a survival role for cancer cells once they reach metastatic competence. Tumor microarrays of various cancer types, such as beast carcinomas and melanomas, have shown to stain positively for LC3B in moderate to high levels [36]. An explanation for this paradoxical role of LC3 is that LC3C reduces metastasis through selective degradation of Met/HGF RTKs, while LC3B promotes metastasis via eliciting autophagy in response to environmental stressors [36].
ATG proteins also play non-canonical functions in pathogen replication and immune response. For example, the Atg5-Atg12 conjugate is crucially involved in phagophore elongation and maturation. However, studies show that the Atg5-Atg12 conjugate can also suppress innate immune responses by downregulating type I IFN production. This is accomplished since the Atg5-Atg12 conjugate can inhibit the function of cytoplasmic RNA helicases responsible for neutralizing virus-derived immunostimulatory RNA structures (isRNA), such as double-stranded RNA or 5’-triphosphorylated RNA [37]. In fact, mouse embryonic fibroblasts deficient in Atg5 and/or Atg12 have shown hyperproduction of type I IFNs in response to isRNA structures, affirming the Atg5-Atg12 conjugate’s role in suppressing innate immune responses to viral replication [38]. In response to mouse norovirus (MNV) infection, instead of inducing autophagy, IFN-gamma activated phagocytes recruited Atg5-Atg12-Atg16L1 conjugate to suppress expression of MNV polymerases, thereby inhibiting the viral replication complex [39, 40]. Other studies have shown that decreasing levels of Atg13 and FIP200 have led to reduced viral replication of encephalomyocarditis virus, two picornaviruses, and coxsackievirus (CV) B3, although an exact mechanism of action remains to be elucidated [41]. In addition, FIP200 suppresses immune checkpoint therapy responses in breast cancers by limiting AZI2/TBK1/IRF signaling [42]. Finally, the Atg8-related family of proteins, such as Atg8 in yeast and LC3 in mammals, also play non-canonical roles in antiviral immune signaling. More specifically, IFN production in human peripheral blood mononuclear cells (PBMCs) is dependent on Toll-like receptor 9 (TLR9) trafficking into a specialized interferon signaling compartment via a LC3-associated phagocytosis (LAP) process [43, 44].
4. Autophagy, the Immune System, and Cancer
4.1. Autophagy in Immune Cell Differentiation
Findings have established a key role of autophagy in cellular homeostasis and survival, including in immune cells. Monocytes circulating in the blood typically undergo apoptosis in the absence of stimulation or differentiation. However, in the presence of activating signals such as inflammation, they become viable, migrate to tissues, and differentiate into macrophages. Autophagy activation is observed in monocytes survival and differentiation induced by Granulocyte-macrophage colony-stimulating factor (GM-CSF) [45]. Inhibition of autophagy with 3-methyladenine (3-MA), chloroquine (CQ), or BECN1 knockdown in monocytes reduces survival, increases apoptosis, and blocks macrophagic differentiation and acquisition of phagocytic functions even when treated with stimulatory factors [45–47]. Inhibition of the P2RY6-PRKAA1-ULK1 pathway blocked both macrophagic differentiation and autophagy in stimulated cells, suggesting a mechanism by which differentiation signals induce autophagy [48]. Thus, autophagy is essential for proper differentiation of monocytes into macrophages.
4.2. Autophagy in Pathogen Clearance
Within the immune system, one of the main routes of pathogen removal from tissues is phagocytosis and degradation. However, several intracellular pathogens have adapted mechanisms to evade destruction such as blocking lysosomal degradation or migrating out of phagocytic vesicles into the cytosol [46]. When typical phagocytic pathways fail to clear pathogens, autophagy’s function in degradation of cytosolic cellular components makes it well-suited to target pathogens that escape phagocytic vesicles and replicate in the cytoplasm [49]. Autophagy also enhances phagocytic pathways to clear pathogens [50–52]. LC3-associated phagocytosis (LAP) is a noncanonical form of autophagy where the core autophagy pathway component LC3 conjugates to phagosomes [53]. LAP plays an important role in regulating inflammatory responses and clearing cell debris, and it is required for clearance of Aspergillus fumigatus infection [50, 52]. LAP is also involved in the elimination of apoptotic cells by macrophages in a process known as efferocytosis to dampen proinflammatory responses [50, 52]. Interferon-stimulated gene 15 (ISG15) recruits LC3 to phagosomes for the IFN-gamma mediated restriction of Toxoplasma gondii infection, which involves engulfment of the parasite through LAP. This shows that ISG15 serves as one of the functional links connecting LAP and phagocytosis in host defense, suggesting its importance for future studies [54].
4.3. Autophagy Suppresses Inflammation
Autophagy is a potent anti-inflammatory process that inhibits inflammasome activation and modulates type I interferon responses [55, 56]. The interaction between autophagy and inflammation is complex and mediated by a number of mechanisms. Autophagy degrades proinflammatory signaling molecules and regulates inflammatory responses from innate immune cells [57]. Autophagy also inhibits inflammation through regulation of inflammasome complexes, which are required to process and activate proinflammatory signals such as procaspase 1 or pro-IL-1β [58]. Additionally. autophagy is a key regulator of RIP homotypic interaction motif (RHIM) domain proteins, which are molecules important for inflammatory signaling and cell death. Indeed, defective autophagy causes a decrease in turnover in RHIM domain proteins in cells, leading to an increase in necroptosis and inflammatory signaling [59]. Defective mitochondrial function is associated with accumulation of ROS inside the cell as well as inflammasome activation, suggesting that impaired mitochondrial quality control as a consequence of autophagy inhibition could be another mechanism connecting autophagy and inflammasome activation [60].
Autophagy has the paradoxical effects of promoting tumor survival while also suppressing initiation of new tumors [61]. A typical inflammatory response contributes to increased cell proliferation, cell survival, cell migration, and angiogenesis, which are critical in wound responses [62]. However, the upregulation of these processes in conjunction with the metabolic stress and DNA damage resulting from production of ROS that occurs in persistent and chronic inflammation contribute to tumorigenesis [62–66]. Therefore, suppression of inflammation is a mechanism by which autophagy inhibits tumor initiation. This effect is contrast to autophagy’s protective role in existing tumors and demonstrates that the role of autophagy in cancer is context-dependent.
4.4. Non-Cell Autonomous Autophagy Inhibits Type I and II IFN Response
Non-cell autonomous autophagy outside of the tumor microenvironment can inhibit an anti-tumor immune response [67]. In particular, autophagy-mediated “hepatic autophagy immune tolerance” is sufficient to inhibit anti-tumor T-cell responses against tumor growth. Systemic or liver-specific Atg7 or Atg5 ablation results in mitochondrial DNA (mtDNA) release, upregulating IFN type I and II responses and antigen presentation. This increased immune response leads to reduced tumor growth, which was restored by the loss of IFNγ or STING [67]. Overall, non-cell autonomous autophagy promotes tumor growth by reducing IFN levels and regulating the anti-tumor immune response.
4.5. Autophagy Inhibits Antigen Presentation
Antigen presentation is a critical process responsible for the body’s adaptive immune response against cancer growth. The primary responsibility of T cells, both CD8+ cytotoxic (CTLs) and CD4+ helper (Th cells), is to recognize foreign protein antigens docked on major histocompatibility complexes (MHCs) on the surface of malignant cells [68]. However, cancers can evade such immunity by immunodominance, display of immune checkpoints, or immunoediting for loss of specific tumor antigens [69].
The proteasome plays an essential role in immune surveillance mechanisms by generating peptides from intracellular antigens that are then “presented” to T cells [70]. Autophagy has also been identified as a route to display antigens on MHC class II molecules to CD4+ T cells, and is implicated in MHC class I cross-presentation of tumor antigen and the activation of CD8+ T cells [71, 72]. The expression of MHC I is commonly low in pancreatic ductal adenocarcinoma (PDAC), leading to defective antigen presentation that prevents T cell-mediated tumor killing. MHC I molecules are selectively targeted for lysosomal degradation by an autophagy-dependent mechanism that involves NBR1 [72]. Autophagy inhibition by genetically knocking-out essential autophagy genes or pharmacologically using autophagy-inhibitors such as CQ results in enhanced antigen peptide presentation, higher levels of T cells producing pro-inflammatory cytokines, such as IFNγ and tumor necrosis factor (TNF), along with overall lower metastatic burden [72]. An effective strategy of synergizing autophagy-inhibitor drugs with dual immune checkpoint blockade (ICB) therapy (anti-PD1 and anti-CTLA4 monoclonal antibodies) shows great promise at stemming PDAC metastasis [72]. Similarly, autophagy inhibits MHC I presentation in Kras-mutant, Lkb1-deficient (KL) lung cancer cells. Upon treatment with autophagy inhibitors, such as CQ or ULK1 inhibitors MRT68921, KL cells show restored sensitivity to host immune responses due to enhanced immunoproteasome activity and restoration of antigen processing [73]. As a result, autophagy inhibition increases the sensitivity of KL tumors to anti-PD-1 therapy [73].
T cell immunoglobulin and mucin domain protein-4 (TIM-4), which is expressed on myeloid cells, regulates T cell homeostasis, thereby impacting host immunity within the tumor microenvironment. TIM-4 is induced on tumor-infiltrating myeloid cells by tumor-derived danger-associated molecular patterns (DAMPs), which subsequently interacts with AMPKɑ1 and activates autophagy. Autophagy activation impedes tumor-antigen presentation, leading to impaired antitumor immunity [3]. Therefore, targeting the TIM-4-AMPKα1 interaction could be a unique strategy for augmenting anti-tumor T-cell responses in tumor microenvironment.
Besides regulating antigen presentation in tumor cells, autophagy supports proper antigen phagocytosis and presentation to MHC class II via modulation of CD36 in dendritic cells [74]. Moreover, autophagy is involved in B cell polarization and presentation of particulate antigens [75].
4.6. Autophagy Negatively Regulates Cytotoxic T Lymphocytes
IFNγ resistance is a conserved tumor cell-autonomous mechanism of CTL evasion in cancer. Autophagy within various cancer cells, such as renal and breast carcinomas, acts as a cell-autonomous negative regulator of CD8+ T cell metabolism and anti-tumor immunity [76, 77]. A combination of mapping cytokine- and CTL-based genetic interactions and in vivo CRISPR screens identified that autophagy is a conserved mediator of both evasion of CTLs by cancer cells and resistance to cytotoxicity induced by the cytokines IFNγ and TNF. Such regulation occurs through the autophagy–NF-κβ axis. Both genetic ablation (by Atg12 deletion) and pharmacological inhibition (using the VPS34 inhibitor Autophinib) to block autophagy sensitized cancer cells to TNF-induced CTL-mediated cell death [77]. Ablation of FIP200 in mice resulted in defective autophagy, increased production of chemokines in tumor cells, increased infiltration of effector T cells in the tumor microenvironment, and reduced mammary tumorigenesis [78]. Moreover, a non-canonical autophagy function of FIP200 is responsible for limiting T-cell recruitment and activation of the TBK1-IFN signaling axis [42].
4.7. Autophagy Supports Treg Lineage Stability and Survival Fitness, while Preventing Treg Infiltration into Tumors
Regulatory T cells (Tregs) are characterized by the expression of the master transcription factor forkhead box protein p3 (FoxP3). FoxP3 expression in Tregs is crucial for reinforcing Treg functional integrity and lineage stability [79]. In tumor immunity, FoxP3+ Tregs can promote the development and progression of tumors by preventing effective anti-tumor immune responses in tumor-bearing hosts [80]. Autophagy acts as a central and intrinsic regulator of Treg cell maintenance and immune homeostasis. Deletion of Atg7 or Atg5 specifically in Treg cells results in increased apoptosis and impaired lineage stability characterized by the loss of FoxP3 expression. Thus, autophagy in Tregs acts to support their functionality and stability. Mechanistically, autophagy protects Treg cell stability by restraining mTORC1-dependent c-Myc expression and function [79]. Treg infiltration in tumors has been associated with adverse prognosis of cancer patients. In FoxP3CreAtg7fl/fl mice inoculated with MC38 colon adenocarcinoma cells, tumor growth was severely inhibited [79, 81] .
While autophagy ablation within Tregs leads to their impaired lineage stability, autophagy inhibited in certain cancers leads to a distinct immune response. In a genetically engineered mouse model (GEMM) for Kras-driven non-small cell lung cancer (NSCLC), conditional deletion of Atg5 in tumors markedly increased numbers of FoxP3+ Tregs infiltrating the lung tumor [82]. It has also been shown that late metastatic lung cancers treated with autophagy inhibitors such as CQ triggers upregulation of CD4+, Foxp3+ tumor infiltrating lymphocytes in late metastatic lung cancer tissues, leading to the induction of chemosensitization to carboplatin, immune activation and cell cycle arrest [83]. Thus, data from these studies point to the role that autophagy could prevent FoxP3+ Treg lymphocyte infiltration in tumor microenvironment.
Tumor cells deploy multiple mechanisms to escape from immunosurveillance. Autophagy promotes tumor immune tolerance and tumor growth via hepatic autophagy mediated suppression of STING and IFNγ activation, reduction of antigen presentation, inhibition of cytotoxic T cell infiltration and enforcing functional integrity of Tregs. However, autophagy ablation in certain cancers/settings have also shown to increase Treg infiltration, which could increase the sensitivity to chemotherapy (Fig. 4).
Figure 4. Autophagy modulates immune response for tumor progression.
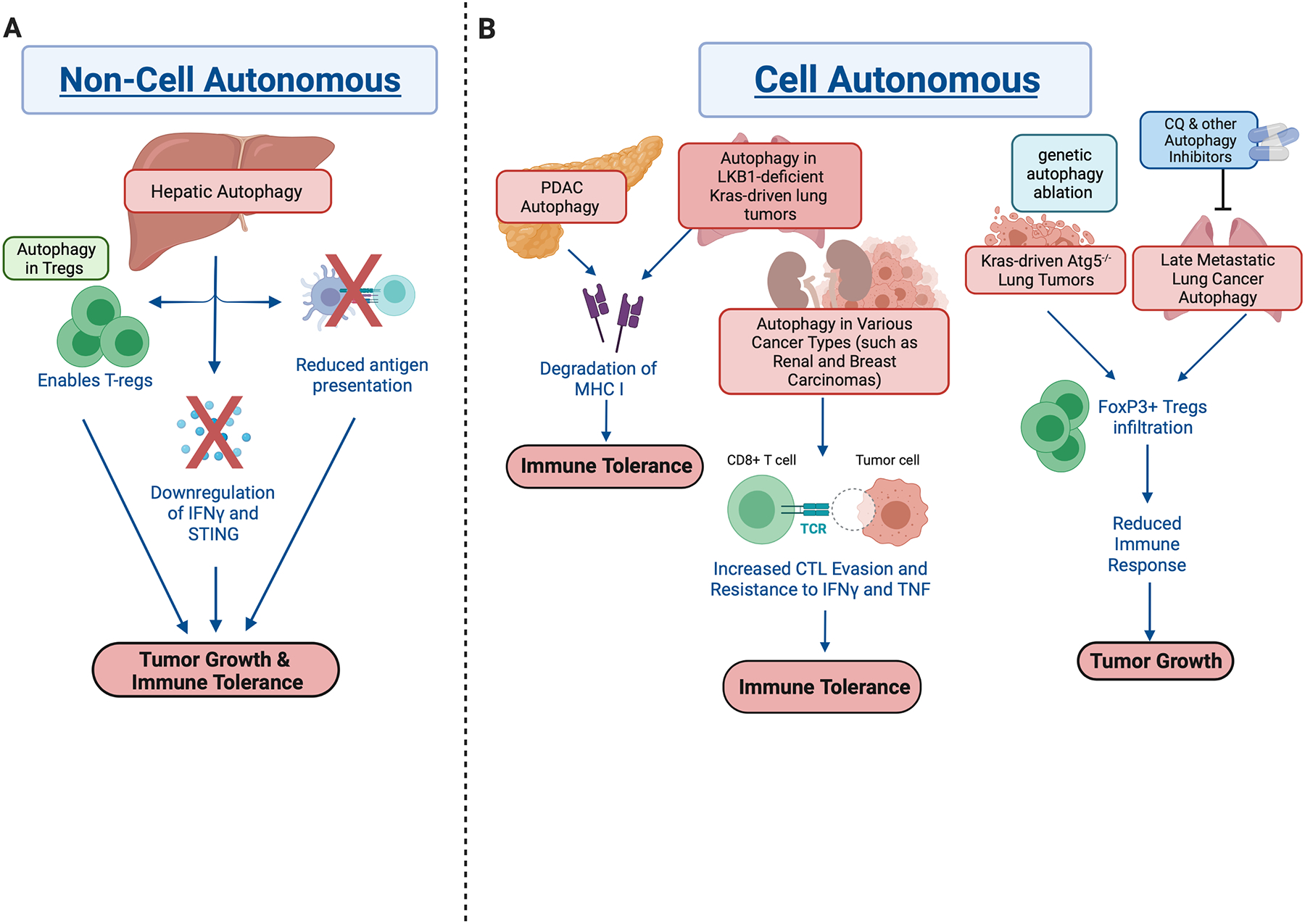
A. Non-cell autonomous autophagy supports immune evasion for tumor growth. Hepatic autophagy suppresses STING and IFNγ activation to prevent tumor killing by T cells [67].
B. Cell autonomous autophagy promotes tumor growth via: 1) reduction of antigen presentation [71, 72] and 2) inhibition of cytotoxic T cell infiltration into the tumor [76, 77]. In certain types of lung cancers, 3) ablation of autophagy has been shown to activate regulatory T cells, thus promoting tumorigenesis [82, 83].
5. Autophagy and Cancer Metabolism
Cancer cells alter their metabolism to meet metabolic demands of high proliferation, malignance, and metastasis. Recent studies using genetically engineered mouse models (GEMMs) demonstrate that autophagy supports different types of tumor growth through distinct mechanisms, including both cell autonomous (tumor cell-intrinsic) and non-cell autonomous (host) autophagy [28, 30, 84–88] (Fig. 5).
Figure 5. Autophagy Supports Cancer Cell Metabolism.
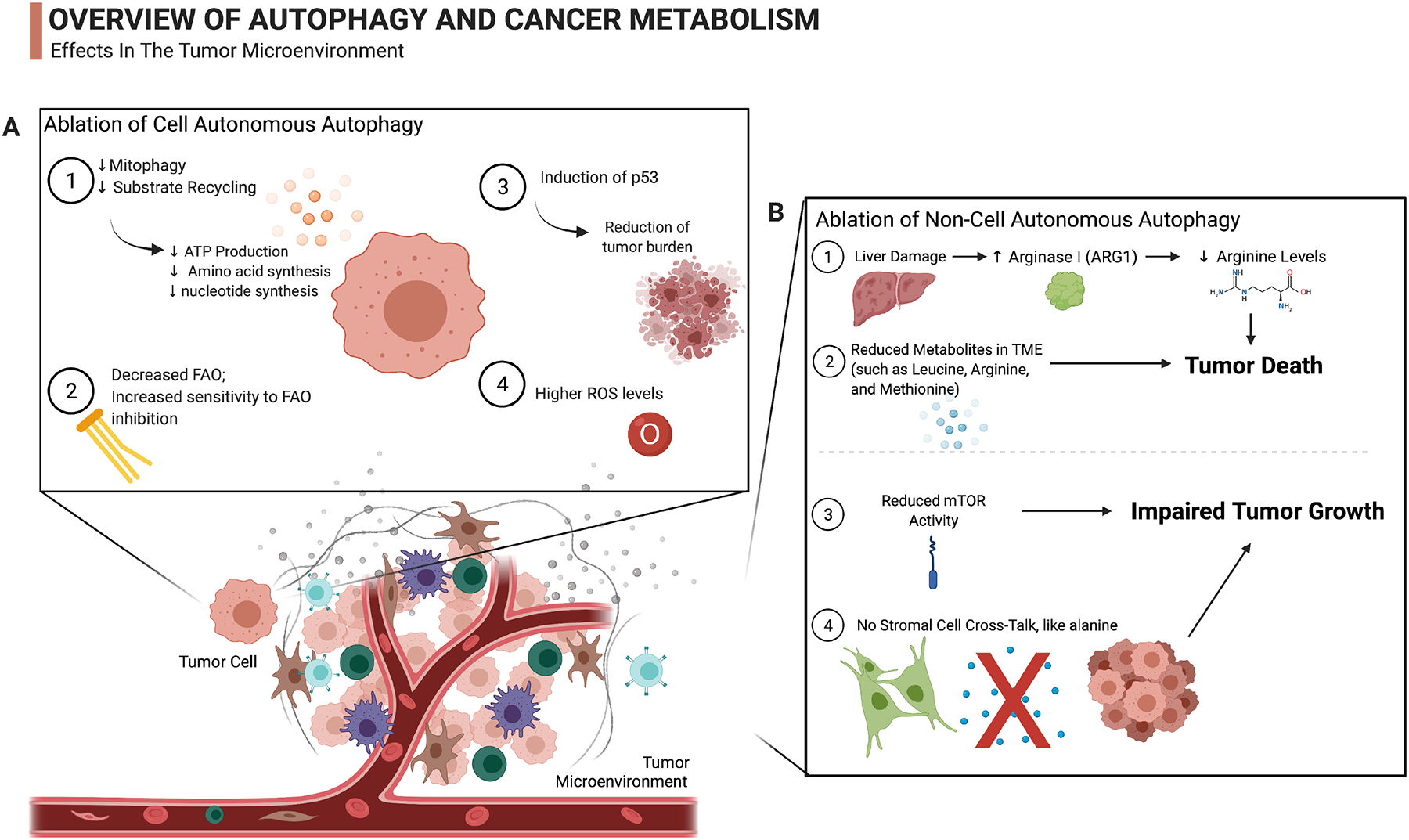
A. Ablation of cell autonomous autophagy in tumor cells leads to a variety of outcomes: 1) lowered levels of amino acids, ATP production, and nucleotides synthesis due to reduced substrate recycling [29, 89, 90], 2) defective FAO and increased sensitivity to FAO inhibition [28, 30], 3) p53 induction [28, 95–97], and 4) higher basal ROS levels [29].
B. Ablation of non-cell autonomous autophagy causes various outcomes in the host and the TME: 1) Ablation of host autophagy or liver autophagy causes the release of Arginase I from hepatocytes, thereby reducing circulating arginine that is essential for tumor growth [31], 2) Ablation of systemic autophagy also leads to deduced metabolites in the TME, leading to tumor death [31], 3) Ablation of systemic autophagy is associated with reduced mTOR activity, impairing tumor growth [27, 31], and 4) ablation of autophagy in stromal cells of PDAC restrains the supply of metabolites to tumor cells [33].
Figure Template Taken from BioRender.com [169].
Tumor Cell-Intrinsic Autophagy Supports Tumor Growth
5.1. Autophagy Maintains Energy Homeostasis.
Autophagy is known to promote cell survival through a number of cell autonomous mechanisms such as mitigating metabolic stress, in part by recycling intracellular components to provide substrates for metabolic and biosynthetic pathways [29, 33]. Pulse-chase studies with isotope-labeled nutrients revealed that autophagy is essential to maintain levels of amino acids when cancer cells are starved [29]. This “autophagy addiction” may arise out of the role of autophagy in sustaining mitochondrial metabolism, which is critical for cancer cell survival and tumorigenesis, through mitophagy and recycling of substrates for the TCA cycle [89, 90]. Autophagy ablation significantly reduced mitochondrial respiration and ATP production during starvation, leading to fatal nucleotide depletion, which was rescued by exogenous glutamine supplementation [29]. This suggests that glutamine recycled through autophagy is essential to maintain proper mitochondrial function for energy production and homeostasis. Autophagy defective tumor cells also accumulate structurally abnormal mitochondria and exhibit other defects in mitochondrial respiration [84]. Autophagy is thus required for proper mitochondrial function and tumor cell metabolism under conditions of metabolic stress.
5.2. Autophagy Regulates Lipid Metabolism
Fatty acids are essential metabolic intermediates for several biosynthetic pathways, and in cancer cells continuous fatty acid synthesis is required for proliferation and maintaining metabolism [91]. Autophagy deficient Kras-mutant p53-deficient (KP) lung tumors show increased lipid droplet (LD) accumulation due to defective lipophagy and impaired fatty acid oxidation (FAO) [28]. However, in Kras-driven Lkb1-deficient (KL) lung tumors, autophagy ablation upregulates lipolysis, and autophagy deficient cancer cells rely more on FAO to survive starvation compared to autophagy intact cells. This has shown to lead to excessive FAO and energy crisis from depletion of LDs [30]. Despite exhibiting paradoxical responses to autophagy deficiency, both autophagy-deficient KP and KL tumor derived cell lines are significantly more sensitive to FAO inhibition during starvation as well as starvation-induced cell death compared with autophagy competent cells [28, 30]. Interestingly, inactivation of autophagy by FIP200 ablation reduced fatty acid release from LDs, resulting in decreased mTORC1 hyperactivation in TSC-deficient neural stem cells and a reversal of neural defects [92].
5.3. Autophagy Inhibits p53-mediated Tumor Suppression
p53 is a well-known tumor suppressive gene that inhibits tumor growth through several mechanisms, and there is growing evidence for tumor promoting interactions between p53 and autophagy. Under nutrient deprivation, AMPK activation induces p53 activation [93]. Subsequently, induction of p53 activates the transcription of genes involved in autophagy activation or induces autophagy via transcriptional activation of damage-regulated autophagy modulator (DRAM-1) in human and mouse cell lines [94]. On the other hand, in various mouse models, p53 induction was observed when autophagy was ablated, suggesting that autophagy also regulates p53 and may serve as a resistance mechanism against p53 activators [95].
In normal cells, autophagy protects tissues from damage resulting from p53 overactivation, but in tumor cells this same function prevents p53-induced apoptosis and promotes tumor cell survival. p53 induction was observed in Atg7-deficient Kras-driven lung tumors, accompanied by accumulation of defective mitochondria, reduction of tumor burden and suppression of cell proliferation. This suppression was partially relieved by the deletion of p53 [28]. This is supported by similar findings in models of PALB2-associated breast cancer, where autophagy ablation reduced tumorigenesis in wild type models but not in models with p53 conditionally deleted [96]. Thus, autophagy promotes tumorigenesis by inhibiting p53-mediated tumor suppression [97].
This conclusion is complicated by findings that in p53-deficient mice containing oncogenic KRAS, autophagy inhibition accelerates pancreatic tumor growth, suggesting that the role of autophagy in pancreatic tumor progression is context dependent on the status of p53 in tumor cells [98]. On the other hand, another study showed contradictory findings in which autophagy deletion in the setting of p53 loss impaired pancreatic tumor progression [99]. These studies utilized different models of p53 deletion which could have contributed to conflicting results. Overall, the interactions between autophagy and p53 are complex and further investigation can elucidate the role of these interactions in various cancers.
5.4. Autophagy Attenuates Oxidative Stress
ROS are constantly generated in the cell from a number of processes including cellular respiration, and excessive levels of ROS result in oxidative stress that can interfere with vital functions [100]. Due to differences in metabolism from normal cells, including increased mitochondrial dysfunction, cancer cells have elevated levels of ROS, which if unmitigated can lead to damage to cellular components and induce cell death [101, 102]. Autophagy is activated in response to ROS production and oxidative stress, suggesting that it plays an antioxidant role, particularly in tumor cells [103–105]. Indeed, Atg7 deficient tumor cells showed higher basal ROS levels in nutrient rich conditions and even higher levels in starvation conditions, which was attenuated by glutamine supplementation [29]. Mitochondrial dysfunction leads to elevated ROS production, so autophagy’s role in organelle quality control and clearance of dysfunctional mitochondria is also important to mitigate oxidative stress [106, 107]. Thus, autophagy is required to sustain redox balance, maintain mitochondrial function, and suppress ROS production during metabolic stress, and these are all functions that promote tumor cell survival.
5.5. Autophagy Prevents Genomic Instability
While autophagy may play a tumor-promoting role as a survival pathway for tumor cells, it also acts as a suppressor of tumor initiation. The metabolic stress caused by starvation or other stressors can induce DNA damage and chromosomal instability. Autophagy-competent cells can mitigate this metabolic stress and limit genomic instability [108]. However, in autophagy-deficient cells, an accumulation of oncogenic mutations resulting from metabolic stress can serve to promote chromosomal abnormalities and tumorigenesis [16]. Treatment of cells with an autophagy inhibitor led to elevated levels of micronuclei, a sensitive indicator of genomic instability and DNA damage [109]. Hence, autophagy can suppresses tumor initiation by suppressing known inducers of genomic instability and tumor initiation, such as reactive oxygen species (ROS), tissue damage, and inflammation [15].
Several nontraditional autophagy functions represent mechanisms by which autophagy plays an important role in maintaining genomic stability. Through noncanonical piecemeal microautophagy (PMN), cells can specifically remove parts of the nuclei containing damaged DNA to mitigate effects of oxidative stress [105]. Autophagy is also linked to the DNA damage response (DDR) in a number of ways, such as induction of AMPK by DDR, suggesting that autophagy plays a role in maintaining genomic stability through DNA repair [110, 111]. Autophagy is required for the selective degradation of RNA granules containing localized retrotransposon replication intermediates. Retrotransposons are a major source of genetic variation chromosomal instability, and mice lacking essential autophagy genes accumulate both retrotransposon RNA and genomic insertions [112]. Appropriative activation of RhoA GTPase is essential in proper cytokinesis at the end of mitosis, and autophagy deficient cells demonstrate cytokinesis failure, chromosomal instability, and multi-nucleation [113].
Host Autophagy Supports Tumor Growth
5.6. Autophagy Maintains Circulating Arginine
Tumor survival heavily relies on a variety of circulating nutrients present in the host bloodstream. Besides tumor-intrinsic autophagy, systemic host autophagy is indispensable for tumorigenesis [27, 31]. Systemic ablation of autophagy leads to the depletion of a number of amino acids and other essential metabolites in serum, including leucine, arginine and methionine, some of which are essential for mTORC1 activation [27, 31, 95, 114–116]. Indeed, acute, systemic deletion of Atg7 or Atg5 impaired tumor growth, which was accompanied by reduced mTOR activity [27, 31]. Among the altered circulating metabolites caused by the loss of autophagy, reduction of serum arginine levels is the most striking [27, 31]. “Arginine-auxotroph” tumors are particularly sensitive to depleting arginine levels and are characterized by their lack of expression for argininosuccinate synthase, which furthers their dependence on environmental arginine [31]. Systemic or liver specific autophagy ablation leads to liver damage, which triggers the release of arginine-degrading enzyme arginase I (ARG1) from the liver into the serum and causes the degradation of arginine to ornithine. Dietary supplementation of arginine to the autophagy deficient host or liver-specific autophagy deficient host partially restored levels of circulating arginine and rescued tumor growth [31].
5.7. Autophagy Provides Nourishment in the Tumor Microenvironment
Not only do tumor cells rely on host metabolites for nutrition, but the tumor microenvironment also serves as a potent source of nourishment for cancer cells. Tumor microenvironment is composed of endothelial cells, fibroblasts, inflammatory cells, extracellular matrix, cytokines, and growth factors, which support commencement and progression of many types of tumors. Pancreatic cancer has a robust stromal component that damages vasculature, thereby creating a nutrient poor environment. Acute, systemic expression of a dominant-negative ATG4b in mice led to regression of KRAS-driven pancreatic cancer, which is caused by the elimination of autophagy-dependent metabolic cross-talk between tumor cells and nearby stromal cells [33]. Autophagy activation in stromal-associated pancreatic stellate cells promotes the secretion of alanine and other amino acids into the tumor microenvironment to provide substrates to neighboring PDAC cells for lipid and protein biosynthesis [32, 33].
The tumor microenvironment is also associated with an increase in macrophage levels, possibly due to immune responses that aim to clear dying tumor cells [33]. The inflammatory effects of these macrophages can be suppressed via activation of LAP in the myeloid compartment of the tumor microenvironment, which additionally aims to suppress surrounding T lymphocytes [117]. Inhibition of LAP consequently provides a potential avenue for augmenting the anti-tumor effects of T lymphocytes.
The tumor microenvironment also provides exogenous signals to promote tumor autophagy during times of starvation. For RAS-driven Drosophila melanogaster cancer models, the release of TNF or IL-6 by starving tumor cells into the microenvironment acted as exogenous signals to induce autophagy, both in the microenvironment and distal tissues. Normal epithelial cells are engaged as an active part of the microenvironment by providing amino acids, supporting tumor proliferation [118]. Moreover, allografted tumor implants placed into autophagy-deficient and control models demonstrated poorer tumor growth in autophagy-deficient models, regardless of where in the body the tumor was implanted. Tumor growth can subsequently be rescued if the tumor implants are transplanted into a model where autophagy is sustained [118]. Thus, systemic autophagy plays a major role in determining growth, regardless of tumor location or primary tumor microenvironment.
6. Autophagy and Metastasis
While autophagy is known to promote tumor cell survival through a number of mechanisms, the role of autophagy in cancer metastasis is also being examined in depth. Emerging evidence indicates that autophagy is upregulated during tumor metastasis and suggests that autophagy promotes several key processes during metastasis. In general, autophagy’s importance in cellular survival in response to metabolic stress makes it well-suited to promote metastasis, where tumor cells must survive and adapt to altered extracellular conditions [119]. The transition of epithelial to more mobile and invasive mesenchymal cells through the epithelial-mesenchymal transition (EMT) often occurs during embryonic development, wound healing, and the early stages of metastasis [120]. Induction of autophagy promotes EMT through TGF-beta dependent signaling, while ablation of autophagy diminishes EMT and cancer cell invasiveness- which can be rescued through addition of recombinant TGF-beta [121]. A recent study suggests that autophagy may promote EMT through myeloid cell activity, a hypothesis supported by findings that myeloid-specific autophagy ablation decreased tumor cell invasiveness, suppressed EMT, and reduced TGF-beta production [122]. In addition to directly promoting tumor metastasis and invasiveness, autophagy plays a key role in enabling malignant cancer cells to enter a reversible dormant state and then reactivate at a later time [123]. These cells are often resistant to cancer treatments which tend to target actively proliferating tumor cells. Through a number of mechanisms, autophagy promotes not only cancer cell survival but also metastasis and treatment resistance [124].
Though autophagy seems to promote cancer metastasis through a number of mechanisms, several studies show that autophagy suppresses growth of migratory tumor cells into lethal macrometastases [125]. In a GEMM with transplanted tumor cells, the size of metastatic lesions was significantly increased in mice with defective autophagy compared to controls. Interestingly, results from the study indicate that autophagy only meaningfully suppressed metastasis in the initial stages of outgrowth [126]. Surprisingly, pharmacological inhibition of autophagy in CQ does not result in enhanced tumor metastasis. This may be explained by the fact that in CQ treatment, autophagosomes still form since core autophagy machinery is functional, but after CQ treatment NBR1 remains sequestered in undigested autophagosomes [127]. This hypothesis is supported by the finding that preventing cytoplasmic accumulation of NBR1 in autophagy-deficient tumor cells reversed increases in metastasis [126, 127]. Autophagy is a pleiotropic cellular process with a number of diverse and seemingly contradictory effects, and its elucidation can aid in the development of new and more effective therapeutics.
7. Autophagy and Cancer Treatment
Given the significant role autophagy plays in tumor progression and the effects of its ablation on tumor survival, autophagy has been explored as a key target for cancer treatments (Fig. 6). The modulation of autophagy can enhance patient outcomes when combined with current treatments [128].
Figure 6. Autophagy in cancer treatment.
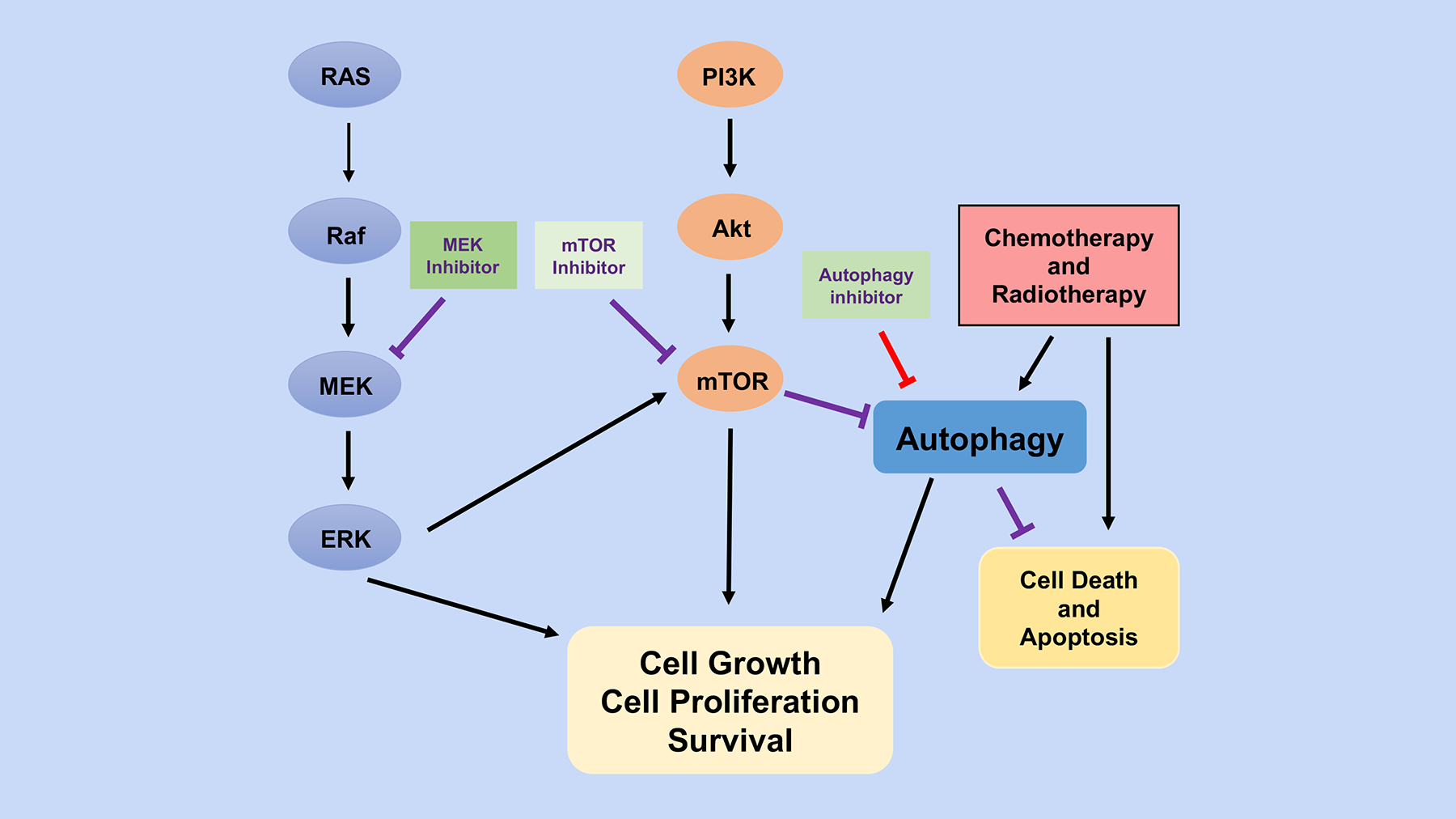
Autophagy inhibition in combination with other cancer treatments can potentiate overall treatment effectiveness and overcome resistance. One common cancer treatment involves the use of RAS/RAF/MEK/ERK pathway inhibitors, but tumor cells can adapt resistance to this therapeutic strategy. ERK inhibition can partially downregulate mTOR activity, leading to de-repression and activation of protective autophagy, which may partially explain tumor adaptation to ERK pathway inhibitors such MEK inhibitors. Combining an ERK inhibitor with both autophagy inhibition and mTOR inhibition can therefore overcome resistance pathways and increase overall response to these treatments.
7.1. Autophagy Inhibition combined with MAPK/ERK Inhibitors
RAS proteins are central mediators downstream of growth factor receptor signaling and are critical for cell proliferation, survival, and differentiation. In addition, RAS mutations are frequently detected in many types of cancers [129, 130]. Therefore, targeting components of the Ras-mediated MAPK cascade, also known as the RAF/MEK/ERK pathway, is an attractive strategy in the development of novel therapeutic approaches to treat RAS-driven cancers [131]. With a near 100% KRAS mutation frequency, PDAC is considered the most RAS-addicted of all cancers [132]. In particular, the RAF/MEK/ERK pathway is central to the initiation and maintenance of PDAC [133]. Attempts have been made to treat PDAC through the use of ERK/MAPK pathway inhibitors. However, such treatments generally fail to produce durable responses in patients [134], indicating that cancer cells develop inhibitor bypass mechanisms that limit the effectiveness of this approach.
A combinatorial siRNA approach to simultaneously target multiple genes in KRAS-driven cells showed that targeting BRAF and CRAF in combination with Atg7 is a promising therapeutic approach for RAS-driven cancers [135]. One possible reason for this is that the inhibition of the RAF/MEK/ERK pathway activates autophagy as a survival response to ERK inhibition, which is supported by findings that genetic or pharmacological inhibition of autophagy enhanced the anti-tumor activity of ERK inhibition in KRAS-driven PDAC [133, 136, 137]. ERK pathway inhibition is also associated with decreased mTORC1 signaling, and since mTOR activity regulates autophagy activation, inhibition of ERK leads to a de-repression of autophagy [138, 139]. This is consistent with findings that ERK inhibition elicits autophagy as a protective survival mechanism and may explain resistance to ERK inhibition based treatment [136]
Inhibition of MEK and autophagy had anti-proliferative effects against PDAC cells both in vitro and in mice xenografted tumors [138]. This anti-proliferative effect of combined ERK pathway and autophagy inhibition persisted for other cancer types including melanoma and colorectal cancer [136, 140]. Combinational treatment of MEK inhibitor Trametinib with autophagy inhibitor hydroxychloroquine (HCQ) in a human patient with PDAC even resulted in a partial but nonetheless striking disease response [136, 137].
Pediatric central nervous system (CNS) tumor cells with BRAF(V600E) (but not wild-type) cells display high rates of induced autophagy, are sensitive to pharmacologic and genetic autophagy inhibition, and show synergy when the clinically used autophagy inhibitor CQ was combined with the RAF inhibitor Vemurafenib or standard chemotherapeutics [141]. A patient with BRAF-mutant brain cancer who had progressed while on anti-BRAF therapy experienced more than 2.5 years of disease regression on a combination of CQ plus a BRAF-targeted therapy[141]. Hence, autophagy inhibition in combination with or RAF/MEK/ERK pathway inhibitors presents promising possibilities for future therapies.
While autophagy inhibition in combination with ERK inhibition has shown promising results for PDAC patients, other cancers mediated by RAS activation have also shown sensitivity to this combination therapy [136, 137, 140]. Therefore, it would be worth determining if all RAS-driven cancers are susceptible to this therapeutic modality. Furthermore, studies should be conducted to determine whether the effectiveness of autophagy inhibition is specific to RAS-driven cancers or if it can be effective for other cancer types as well.
7.2. Autophagy inhibition combined with mTOR Inhibitors
mTOR regulates cell growth, proliferation, and survival via mTORC1 and mTORC2, which are often aberrantly activated in cancers. mTOR-based targeting strategies exhibit potent anti-cancer effects in many preclinical models. However, development of resistance can occur, which limits its success in the clinic [142, 143]. There are multiple mechanisms underlying resistance to mTOR inhibitors, including the rewiring of metabolic pathways [143]. Autophagy activation is tightly regulated by mTOR, so induction of autophagy by mTOR inhibitors may lead to the drug resistance and survival of cancer cells. Therefore, studies of mTOR inhibitors combined with autophagy inhibitor HCQ have been tested in several different cancer types. Myeloma cells treated with both cyclophosphamide and rapamycin have shown to “doubly” induce autophagy-dependence, providing a more robust platform for HCQ to work on [144]. Moreover, multiple patients experienced prolonged stable disease and less adverse growth rates; however, it is not to the degree that would provide any meaningful effect on patient outcomes [144]. In patients with clear-cell renal carcinoma (ccRCC), treatment with the mTOR inhibitor Everolimus along with HCQ was seen to prolong stable disease [145]. mTOR inhibitor Temsirolimus, when combined with HCQ, has been proven to be safe and tolerable in a phase I clinical trial of patients with either advanced solid malignant tumors or metastatic melanoma. In fact, a median of 3.5 months of PFS was achieved in approximately 68% of the melanoma patients [146]. A HCQ dose of 600mg twice daily in combination with a Temsirolimus dose of 25mg weekly was ultimately recommended as approximately 67% of solid tumor patients and 74% of melanoma patients achieved stable disease with this dosing regimen [146].
7.3. Autophagy Inhibition combined with Immunotherapy
Recent studies have demonstrated that both tumor-intrinsic autophagy and host autophagy play essential role for tumor cells to escape immunosurveillance [67, 72]. Moreover, HCQ synergizes with ICB therapy to inhibit pancreatic tumor growth [72]. Host autophagy inhibition also promotes inflammation in the tumor microenvironment to enhance immune surveillance [67]. A noncanonical function of the essential autophagy gene FIP200 was found to limit T-cell recruitment in mammary tumors and promote tumor progression. When combined with immune checkpoint inhibitors, ablation of FIP200 enhanced efficacy of this therapy and slowed tumor growth [42]. Based on these animal experiments, studies combining autophagy inhibitors and immune checkpoint inhibitors may hold promise for human trials.
7.4. Autophagy Inhibition combined with Other Therapies
Many forms of cancer therapy such as chemotherapy and radiotherapy rely on inducing stress in cancer cells in order to cause tumor death. However, the protective role of autophagy under stress conditions mitigates these effects and can contribute to therapy resistance. Autophagy is upregulated in response to many chemotherapeutic drugs, suggesting that autophagy inhibition in combination with chemotherapy may yield more effective treatments than chemotherapy alone [147, 148]. Indeed, studies combining autophagy inhibition with chemotherapy agents have shown promising results [149]. A novel inhibitor of autophagy acting on ULK1 sensitized NSCLC cells to cisplatin and suppressed cancer cell growth [150]. Promising results have also been shown in human clinical trials utilizing an autophagy inhibition and chemotherapy combination [149]. A phase Ib/II single-arm study for metastatic NSCLC utilized carboplatin and paclitaxel chemotherapies combined with HCQ. Not only was the treatment found to be generally safe and tolerable, but patients’ ORR and PFS showed marked improvement [151].
Radiation therapy often works by inducing DNA damage in cancer cells, causing them to undergo cell death. However, autophagy may serve as a resistance mechanism against this kind of therapy by removing damaged DNA and promoting DNA repair, preventing cell death [152]. Therefore, autophagy inhibition may also potentiate radiotherapy, and indeed combining autophagy inhibitors with radiation has shown improved anti-tumor effects [149]. Treatment with chloroquine sensitized bladder cancer cells to radiation, elevating apoptosis markers, inhibiting proliferation, and impeding tumorigenesis of xenograft tumors [153]. A study on whole brain radiation therapy combined with chloroquine showed that treatment was well-tolerated and suggested a survival benefit compared to radiotherapy alone [154]. Autophagy inhibition may play role as a treatment modulator even for radiotherapy.
7.5. Developing New Autophagy Inhibitors
So far, CQ and its derivative HCQ are the only FDA approved autophagy inhibitors for clinical usage. Indeed, besides blocking the fusion of autophagosomes with lysosomes using CQ and HCQ, autophagy processes can be targeted at multiple steps, leading to other autophagy inhibition strategies that are currently under investigation, including Vps34 inhibitors, ULK1 inhibitors, and Palmitoyl-Protein Thioesterase1 (PPT1) inhibitors. ULK1 inhibitors, such as SBI-0206965, aim to suppress ULK1’s function in phosphorylating enzymes required for autophagy initiation [155]. Vps34 inhibitors, such as SAR405, aim to inhibit Vps34’s role in vesicle trafficking and autophagosome formation [156]. Finally, PPT1 inhibitors such as Lys05 aim to deacidify autophagic lysosomes by inhibiting the action of the lysosomal enzyme PPT1 [157].
7.6. Identifying Potential Biomarkers for Autophagy Inhibition
RAS activation promotes tumor cell reliance on autophagy [90, 158, 159]. Studies from pre-clinical mouse models and clinical trials demonstrated autophagy inhibition could be effective in treating pancreatic cancer when combined with MEK inhibition or ICB therapy [72, 136, 137]. Similar to PDAC, KRAS mutations are frequently detected in NSCLC patients and autophagy is essential for Kras-driven lung tumor growth [27, 28, 30]. LKB1, a metabolic sensor and master modulator of AMPK, is the third most mutated gene detected in NSCLC. Interestingly, ablation of the LKB1 causes Kras-driven NSCLC to become much more sensitive to autophagy inhibition compared with LKB1 WT lung tumors [30]. Autophagy temporally compensates for acute systemic LKB1 loss for adult mouse survival [95]. In the case of Kras- driven lung tumors, studies suggest that the loss of LKB1 leads to the reduced metabolic plasticity in response to metabolic stress during tumorigenesis, which is further exaggerated by autophagy ablation, leading to energy crises in the tumor cell [30]. LKB1 deficiencies are also correlated with elevated tumor mutational burdens (TMBs) in NSCLCs. However, NSCLC patients bearing co-mutations of LKB1 and KRAS are resistant to immunotherapy [160]. Recent studies found that, compared to LKB1 WT lung tumors, LKB1-deficient cancers treated with autophagy inhibitors show higher sensitivity to immune checkpoint blockade anti-PD-1 treatment [73]. As a result, LKB1 mutations could consequently be explored as a predictive biomarker for the treatment of lung cancers with autophagy inhibition. However, the potential of LKB1 biomarkers still requires further investigation.
8. Addressing Arguments that Autophagy is Anti-Tumorigenic
While the above studies certainly point to the autophagy-mediated tumor promotion and promising effects of autophagy inhibition in cancer treatment, this contention is certainly up for debate. Other studies report that autophagy can actually be anti-tumorigenic and thus oppose inhibiting autophagy in cancer lines [161–163]. For example, one study has shown that immunosurveillance against transplanted carcinomas can be improved by pharmacologically inducing autophagy under specific caloric restrictions [164]. The role of autophagy in cancer and tumor progression seems paradoxical due to apparently contradictory effects of autophagy dysfunction. It has been shown that autophagy serves as a survival mechanism for tumor cells in response to metabolic stress, but there is growing evidence for autophagy as a tumor suppression mechanism. This evidence includes findings that deletion of essential autophagy genes suppressed tumor initiation in mouse models of breast cancer and that defects in autophagy resulting from allelic loss of Beclin1 promote tumorigenesis and are commonly observed in multiple human cancers [161, 162]. However, this evidence is complicated by studies that demonstrate that loss of BECN1 in breast cancer does not occur independently of deletion of BRCA1, a known tumor suppressor gene [163, 165]. This suggests that BRCA1 is the main driver or tumorigenesis in these cases [61]. Indeed, core autophagy machinery and genes are generally not mutated across most cancers [166].
Another argument against autophagy inhibition is tolerability. Some studies state that human cancer lines grown in vitro remain unaffected and show no size reduction when Atg genes are deleted [167]. However, it has been shown that autophagy-ablated tumors indeed display growth defects and can be restored upon nutrient-rich conditions despite being sensitive to starvation [28, 30]. Additionally, the in vitro environment may lack crucial anti-tumor effects that are prominent within the in vivo environment, such as immune responses, stromal cells in tumor microenvironment, or development of resistance mechanisms. Indeed, by claiming to observe tumor reduction in mice which have compromised immune systems [27, 67, 72], critics argue that immunocompromised mice models neglect the realistic activation of the host immune system compared to autochthonous models [167]. However, the use of nude mice is critical to investigate tumor progression dependency on oncogenic signaling. Additionally, nude mice do not necessarily neglect in vivo host immune responses, as activation of innate and adaptive immune responses are defining characteristics of autophagy-deficient tumor cells [64].
An additional argument proposes that it is only host autophagy that promotes tumor growth, not tumor cell-autonomous autophagy. Proponents of this argument conclude that inhibition of autophagy in cancer cells therefore is a fruitless endeavor [168]. However, extensive data supports the importance of tumor-intrinsic autophagy, in addition to host autophagy, in promoting cancer energy homeostasis, metabolism, attenuation of oxidative stress and immune tolerance [29, 30, 72, 73, 103–105].
9. Concluding Remarks
This review seeks to provide a broad overview of the interactions of autophagy and cancer as well as examine potential clinical applications of findings in this area. Cell-autonomous autophagy supports tumor growth via intracellular recycling to maintain essential nutrients, removing toxicity caused by the accumulation of protein aggregates and damaged organelles, inhibiting p53 function, and preventing cell surface expression of MHC-I for antigen presentation to escape immunosurveillance. Host autophagy support tumor growth by sustaining necessary levels of essential amino acids and metabolites for tumor cell metabolism and inhibiting innate and adaptive anti-tumor immune responses. Given its role in cell metabolism and immune responses, autophagy inhibition can potentially be combined with MAPK/ERK inhibitors, mTOR inhibitors or immunotherapy as novel cancer therapies. Autophagy and its role in cancer continues to be uncovered, and further research in this area can reveal new ways to target various kinds of cancer and disease.
Acknowledgments:
This work was supported by supported by NIH grant R01CA237347-01A1, ACS grant 134036-RSG-19-165-01-TBG, the GO2 Foundation for Lung Cancer, Rutgers Busch Biomedical Grant, the New Jersey Health Foundation, Rutgers and Princeton Collaboration Seed Funding Pilot Award and Seed Funding for Large Multi-PI Awards to J.Y.G; NJCCR Pre-doctoral Fellowship Award, Steven A. Cox Foundation Award and Mistletoe Research Fellowship.to V.B.
Abbreviations:
- 3-MA
3-methyladenine
- AMPK
AMP-activated protein kinase
- ARG1
arginine-degrading enzyme arginase I
- ATG
autophagy-related gene
- BECN1
beclin-1
- ccRCC
clear-cell renal carcinoma
- CNS
central nervous system
- CQ
chloroquine
- CTL
cytotoxic T lymphocyte
- CV
coxsackievirus
- DAMP
danger-associated molecular patterns
- DDR
DNA damage response
- DRAM
damage-regulated autophagy modulator
- EMT
epithelial-mesenchymal transition
- FAO
fatty acid oxidation
- FIP200
focal adhesion kinase interacting protein of 200kDa
- GEMM
genetically engineered mouse model
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HCQ
hydroxychloroquine
- HGF
hepatocyte growth factor
- ICB
immune checkpoint blockade
- IGF1
insulin-like growth factor-1
- IL-3
interleukin 3
- ISG15
interferon-stimulated gene 15
- isRNA
immunostimulatory RNA
- KL
Kras-mutant LKB1-deficient
- KP
Kras-mutant p53-deficient
- LAP
LC3-associated phagocytosis
- LC3
microtubule-associated protein 1A/1B light chain 3B
- LC3-PE
LC3-phosphatidylethanolamine
- LD
lipid droplet
- LIR
LC3-interaction region
- MHC
major histocompatibility complex
- MNV
mouse norovirus
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of Rapamycin
- mTORC1
mTOR complex 1
- NBR1
neighbor of BRCA1 gene 1 protein
- NSCLC
non-small cell lung cancer
- PBMC
peripheral blood mononuclear cell
- PDAC
pancreatic ductal adenocarcinoma
- PI3K
phosphoinositide 3-kinase
- PKB
protein kinase B
- PMN
piecemeal microautophagy
- PPT1
palmitoyl-protein thioesterase1
- RHIM
RIP homotypic interaction motif
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- TIM-4
T cell immunoglobulin and mucin domain protein-4
- TLR
toll-like receptor
- TSC1/2
tuberous sclerosis complex 1/2
- ULK1/2
Unc-51 like autophagy activating kinase 1/2
- Vps34
vacuolar protein sorting 34 complex
Footnotes
Potential Conflicts of Interest
We have no conflict of interest to declare.
References
- 1.Glick D, Barth S & Macleod KF (2010) Autophagy: cellular and molecular mechanisms, J Pathol. 221, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang P & Mizushima N (2014) Autophagy and human diseases, Cell Res. 24, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Takeya M, Viollet B, Yagita H & Jinushi M (2013) TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance, Immunity. 39, 1070–81. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G, Liu J & Zhang J (2020) Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy, Appl Microbiol Biotechnol. 104, 575–587. [DOI] [PubMed] [Google Scholar]
- 5.Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J & Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration, Cell Signal. 26, 2694–701. [DOI] [PubMed] [Google Scholar]
- 6.Kim YC & Guan KL (2015) mTOR: a pharmacologic target for autophagy regulation, J Clin Invest. 125, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaravadi RK, Kimmelman AC & Debnath J (2019) Targeting Autophagy in Cancer: Recent Advances and Future Directions, Cancer Discov. 9, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T & Ohsumi Y (2000) A ubiquitin-like system mediates protein lipidation, Nature. 408, 488–92. [DOI] [PubMed] [Google Scholar]
- 9.Parzych KR & Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation, Antioxid Redox Signal. 20, 460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twitty CG, Jensen SM, Hu HM & Fox BA (2011) Tumor-derived autophagosome vaccine: induction of cross-protective immune responses against short-lived proteins through a p62-dependent mechanism, Clin Cancer Res. 17, 6467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamark T, Kirkin V, Dikic I & Johansen T (2009) NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets, Cell Cycle. 8, 1986–90. [DOI] [PubMed] [Google Scholar]
- 12.Levine B & Kroemer G (2019) Biological Functions of Autophagy Genes: A Disease Perspective, Cell. 176, 11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine B & Klionsky DJ (2004) Development by Self-Digestion: Molecular Mechanisms and Biological Functions of Autophagy, Developmental Cell. 6, 463–477. [DOI] [PubMed] [Google Scholar]
- 14.Klionsky DJ & Codogno P (2013) The Mechanism and Physiological Function of Macroautophagy, Journal of Innate Immunity. 5, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poillet-Perez L & White E (2019) Role of tumor and host autophagy in cancer metabolism, Genes Dev. 33, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S & White E (2007) Autophagy suppresses tumor progression by limiting chromosomal instability, Genes Dev. 21, 1367–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I, Rodriguez-Enriquez S & Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy, Arch Biochem Biophys. 462, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youle RJ & Narendra DP (2011) Mechanisms of mitophagy, Nat Rev Mol Cell Biol. 12, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holdgaard SG, Cianfanelli V, Pupo E, Lambrughi M, Lubas M, Nielsen JC, Eibes S, Maiani E, Harder LM, Wesch N, Foged MM, Maeda K, Nazio F, de la Ballina LR, Dötsch V, Brech A, Frankel LB, Jäättelä M, Locatelli F, Barisic M, Andersen JS, Bekker-Jensen S, Lund AH, Rogov VV, Papaleo E, Lanzetti L, De Zio D & Cecconi F (2019) Selective autophagy maintains centrosome integrity and accurate mitosis by turnover of centriolar satellites, Nat Commun. 10, 4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft C, Deplazes A, Sohrmann M & Peter M (2008) Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease, Nat Cell Biol. 10, 602–10. [DOI] [PubMed] [Google Scholar]
- 21.Li W, He P, Huang Y, Li Y-F, Lu J, Li M, Kurihara H, Luo Z, Meng T, Onishi M, Ma C, Jiang L, Hu Y, Gong Q, Zhu D, Xu Y, Liu R, Liu L, Yi C, Zhu Y, Ma N, Okamoto K, Xie Z, Liu J, He R-R & Feng D (2021) Selective autophagy of intracellular organelles: recent research advances, Theranostics. 11, 222–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson S (2020) Emerging Principles of Selective ER Autophagy, J Mol Biol. 432, 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zechner R, Madeo F & Kratky D (2017) Cytosolic lipolysis and lipophagy: two sides of the same coin, Nature Reviews Molecular Cell Biology. 18, 671–684. [DOI] [PubMed] [Google Scholar]
- 24.Florey O & Overholtzer M (2019) Macropinocytosis and autophagy crosstalk in nutrient scavenging, Philosophical Transactions of the Royal Society B: Biological Sciences. 374, 20180154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K & Chiba T (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice, Journal of Cell Biology. 169, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T & Mizushima N (2004) The role of autophagy during the early neonatal starvation period, Nature. 432, 1032–1036. [DOI] [PubMed] [Google Scholar]
- 27.Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD & White E (2014) Autophagy is required for glucose homeostasis and lung tumor maintenance, Cancer Discov. 4, 914–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, Snyder E, Santanam U, Dipaola RS, Jacks T, Rabinowitz JD & White E (2013) Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis, Genes Dev. 27, 1447–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo JY, Teng X, Laddha SV, Ma S, Van Nostrand SC, Yang Y, Khor S, Chan CS, Rabinowitz JD & White E (2016) Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells, Genes Dev. 30, 1704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt V, Khayati K, Hu ZS, Lee A, Kamran W, Su X & Guo JY (2019) Autophagy modulates lipid metabolism to maintain metabolic flexibility for Lkb1-deficient Kras-driven lung tumorigenesis, Genes Dev. 33, 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poillet-Perez L, Xie X, Zhan L, Yang Y, Sharp DW, Hu ZS, Su X, Maganti A, Jiang C, Lu W, Zheng H, Bosenberg MW, Mehnert JM, Guo JY, Lattime E, Rabinowitz JD & White E (2018) Autophagy maintains tumour growth through circulating arginine, Nature. 563, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA & Kimmelman AC (2016) Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion, Nature. 536, 479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, Deng J, Hai J, Yang S, Wong K-K & Kimmelman AC (2018) Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms, Cancer Discovery. 8, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell ES, Coelho PP, Ratcliffe CDH, Rajadurai CV, Peschard P, Vaillancourt R, Zuo D & Park M (2019) LC3C-Mediated Autophagy Selectively Regulates the Met RTK and HGF-Stimulated Migration and Invasion, Cell Rep. 29, 4053–4068 e6. [DOI] [PubMed] [Google Scholar]
- 35.Bell ES, Coelho PP & Park M (2020) LC3C mediates selective autophagy of the MET RTK, inhibiting cancer cell invasion, Autophagy. 16, 959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK & Pawelek JM (2012) Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome, Clin Cancer Res. 18, 370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeshita F, Kobiyama K, Miyawaki A, Jounai N & Okuda K (2008) The non-canonical role of Atg family members as suppressors of innate antiviral immune signaling, Autophagy. 4, 67–9. [DOI] [PubMed] [Google Scholar]
- 38.Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K & Okuda K (2007) The Atg5 Atg12 conjugate associates with innate antiviral immune responses, Proc Natl Acad Sci U S A. 104, 14050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, Green KY, Lopez-Otin C, Xavier RJ, Thackray LB & Virgin HW (2012) Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein complex in antiviral activity of interferon gamma, Cell Host Microbe. 11, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solvik T & Debnath J (2016) At the crossroads of autophagy and infection: Noncanonical roles for ATG proteins in viral replication, J Cell Biol. 214, 503–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauthe M, Langereis M, Jung J, Zhou X, Jones A, Omta W, Tooze SA, Stork B, Paludan SR, Ahola T, Egan D, Behrends C, Mokry M, de Haan C, van Kuppeveld F & Reggiori F (2016) An siRNA screen for ATG protein depletion reveals the extent of the unconventional functions of the autophagy proteome in virus replication, J Cell Biol. 214, 619–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto T, Yeo SK, Hao M, Copley MR, Haas MA, Chen S & Guan JL (2020) FIP200 Suppresses Immune Checkpoint Therapy Responses in Breast Cancers by Limiting AZI2/TBK1/IRF Signaling Independent of Its Canonical Autophagy Function, Cancer Res. 80, 3580–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR & Sanjuan MA (2012) Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes, Immunity. 37, 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieto-Torres JL, Leidal AM, Debnath J & Hansen M (2021) Beyond Autophagy: The Expanding Roles of ATG8 Proteins, Trends Biochem Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Morgan MJ, Chen K, Choksi S & Liu ZG (2012) Induction of autophagy is essential for monocyte-macrophage differentiation, Blood. 119, 2895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Germic N, Frangez Z, Yousefi S & Simon H-U (2019) Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation, Cell Death Differ. 26, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacquel A, Obba S, Boyer L, Dufies M, Robert G, Gounon P, Lemichez E, Luciano F, Solary E & Auberger P (2012) Autophagy is required for CSF-1-induced macrophagic differentiation and acquisition of phagocytic functions, Blood. 119, 4527–31. [DOI] [PubMed] [Google Scholar]
- 48.Obba S, Hizir Z, Boyer L, Selimoglu-Buet D, Pfeifer A, Michel G, Hamouda M-A, Gonçalvès D, Cerezo M, Marchetti S, Rocchi S, Droin N, Cluzeau T, Robert G, Luciano F, Robaye B, Foretz M, Viollet B, Legros L, Solary E, Auberger P & Jacquel A (2015) The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML, Autophagy. 11, 1114–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T & Brumell JH (2006) Autophagy Controls Salmonella Infection in Response to Damage to the Salmonella-containing Vacuole, Journal of Biological Chemistry. 281, 11374–11383. [DOI] [PubMed] [Google Scholar]
- 50.Heckmann BL, Boada-Romero E, Cunha LD, Magne J & Green DR (2017) LC3-Associated Phagocytosis and Inflammation, J Mol Biol. 429, 3561–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levine B, Mizushima N & Virgin HW (2011) Autophagy in immunity and inflammation, Nature. 469, 323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan J-L, Tan H, Peng J, Kanneganti T-D, Virgin HW & Green DR (2015) Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins, Nature cell biology. 17, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Heckmann BL & Green DR (2019) LC3-associated phagocytosis at a glance, J Cell Sci. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhushan J, Radke JB, Perng Y-C, McAllaster M, Lenschow DJ, Virgin HW & Sibley LD (2020) ISG15 Connects Autophagy and IFN-γ-Dependent Control of Toxoplasma gondii Infection in Human Cells, mBio. 11, e00852–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deretic V, Saitoh T & Akira S (2013) Autophagy in infection, inflammation and immunity, Nat Rev Immunol. 13, 722–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White E, Karp C, Strohecker AM, Guo Y & Mathew R (2010) Role of autophagy in suppression of inflammation and cancer, Curr Opin Cell Biol. 22, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh SB, Carroll-Portillo A, Coffman C, Ritz NL & Lin HC (2020) Intestinal Alkaline Phosphatase Exerts Anti-Inflammatory Effects Against Lipopolysaccharide by Inducing Autophagy, Sci Rep. 10, 3107–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathew R, Khor S, Hackett SR, Rabinowitz JD, Perlman DH & White E (2014) Functional role of autophagy-mediated proteome remodeling in cell survival signaling and innate immunity, Mol Cell. 55, 916–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim J, Park H, Heisler J, Maculins T, Roose-Girma M, Xu M, McKenzie B, van Lookeren Campagne M, Newton K & Murthy A (2019) Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins, Elife. 8, e44452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ichinohe T, Yamazaki T, Koshiba T & Yanagi Y (2013) Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection, Proceedings of the National Academy of Sciences of the United States of America. 110, 17963–17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White E (2015) The role for autophagy in cancer, The Journal of Clinical Investigation. 125, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greten FR, Eckmann L, Greten TF, Park JM, Li Z-W, Egan LJ, Kagnoff MF & Karin M (2004) IKKβ Links Inflammation and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer, Cell. 118, 285–296. [DOI] [PubMed] [Google Scholar]
- 63.Balkwill F, Charles KA & Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease, Cancer Cell. 7, 211–217. [DOI] [PubMed] [Google Scholar]
- 64.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson DA, Jin S & White E (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis, Cancer Cell. 10, 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grivennikov SI, Greten FR & Karin M (2010) Immunity, inflammation, and cancer, Cell. 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen H-Y, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V & White E (2009) Autophagy suppresses tumorigenesis through elimination of p62, Cell. 137, 1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poillet-Perez L, Sharp DW, Yang Y, Laddha SV, Ibrahim M, Bommareddy PK, Hu ZS, Vieth J, Haas M, Bosenberg MW, Rabinowitz JD, Cao J, Guan J-L, Ganesan S, Chan CS, Mehnert JM, Lattime EC & White E (2020) Autophagy promotes growth of tumors with high mutational burden by inhibiting a T-cell immune response, Nature Cancer. 1, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abbas AK, Lichtman AH & Pillai S (2019) Basic Immunology E-Book: Functions and Disorders of the Immune System, Elsevier Health Sciences. [Google Scholar]
- 69.Schumacher TN & Schreiber RD (2015) Neoantigens in cancer immunotherapy, Science. 348, 69–74. [DOI] [PubMed] [Google Scholar]
- 70.Kloetzel PM (2001) Antigen processing by the proteasome, Nat Rev Mol Cell Biol. 2, 179–87. [DOI] [PubMed] [Google Scholar]
- 71.Crotzer VL & Blum JS (2009) Autophagy and its role in MHC-mediated antigen presentation, J Immunol. 182, 3335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, Banh RS, Paulo JA, Wen KW, Debnath J, Kim GE, Mancias JD, Fearon DT, Perera RM & Kimmelman AC (2020) Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I, Nature. 581, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng J, Thennavan A, Dolgalev I, Chen T, Li J, Marzio A, Poirier JT, Peng D, Bulatovic M, Mukhopadhyay S, Silver H, Papadopoulos E, Pyon V, Thakurdin C, Han H, Li F, Li S, Ding H, Hu H, Pan Y, Weerasekara V, Jiang B, Wang ES, Ahearn I, Philips M, Papagiannakopoulos T, Tsirigos A, Rothenberg E, Gainor J, Freeman GJ, Rudin CM, Gray NS, Hammerman PS, Pagano M, Heymach JV, Perou CM, Bardeesy N & Wong KK (2021) ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1 mutant lung cancer, Nat Cancer. 2, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh DS & Lee HK (2019) Autophagy protein ATG5 regulates CD36 expression and anti-tumor MHC class II antigen presentation in dendritic cells, Autophagy. 15, 2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arbogast F, Arnold J, Hammann P, Kuhn L, Chicher J, Murera D, Weishaar J, Muller S, Fauny JD & Gros F (2019) ATG5 is required for B cell polarization and presentation of particulate antigens, Autophagy. 15, 280–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeVorkin L, Pavey N, Carleton G, Comber A, Ho C, Lim J, McNamara E, Huang H, Kim P, Zacharias LG, Mizushima N, Saitoh T, Akira S, Beckham W, Lorzadeh A, Moksa M, Cao Q, Murthy A, Hirst M, DeBerardinis RJ & Lum JJ (2019) Autophagy Regulation of Metabolism Is Required for CD8(+) T Cell Anti-tumor Immunity, Cell Rep. 27, 502–513 e5. [DOI] [PubMed] [Google Scholar]
- 77.Lawson KA, Sousa CM, Zhang X, Kim E, Akthar R, Caumanns JJ, Yao Y, Mikolajewicz N, Ross C, Brown KR, Zid AA, Fan ZP, Hui S, Krall JA, Simons DM, Slater CJ, De Jesus V, Tang L, Singh R, Goldford JE, Martin S, Huang Q, Francis EA, Habsid A, Climie R, Tieu D, Wei J, Li R, Tong AHY, Aregger M, Chan KS, Han H, Wang X, Mero P, Brumell JH, Finelli A, Ailles L, Bader G, Smolen GA, Kingsbury GA, Hart T, Kung C & Moffat J (2020) Functional genomic landscape of cancer-intrinsic evasion of killing by T cells, Nature. 586, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei H, Wei S, Gan B, Peng X, Zou W & Guan JL (2011) Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis, Genes Dev. 25, 1510–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, Wu C, Vogel P, Neale G, Green DR & Chi H (2016) Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis, Nat Immunol. 17, 277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohue Y & Nishikawa H (2019) Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?, Cancer Sci. 110, 2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Texier L, Lineburg KE, Cao B, McDonald-Hyman C, Leveque-El Mouttie L, Nicholls J, Melino M, Nalkurthi BC, Alexander KA, Teal B, Blake SJ, Souza-Fonseca-Guimaraes F, Engwerda CR, Kuns RD, Lane SW, Teng M, Teh C, Gray D, Clouston AD, Nilsson SK, Blazar BR, Hill GR & MacDonald KP (2016) Autophagy-dependent regulatory T cells are critical for the control of graft-versus-host disease, JCI Insight. 1, e86850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, Sigl V, Aumayr K, Schmauss G, Fellner N, Handschuh S, Glosmann M, Pasierbek P, Schlederer M, Resch GP, Ma Y, Yang H, Popper H, Kenner L, Kroemer G & Penninger JM (2014) A dual role for autophagy in a murine model of lung cancer, Nat Commun. 5, 3056. [DOI] [PubMed] [Google Scholar]
- 83.Zarogoulidis P, Petanidis S, Domvri K, Kioseoglou E, Anestakis D, Freitag L, Zarogoulidis K, Hohenforst-Schmidt W & Eberhardt W (2016) Autophagy inhibition upregulates CD4(+) tumor infiltrating lymphocyte expression via miR-155 regulation and TRAIL activation, Mol Oncol. 10, 1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strohecker AM & White E (2014) Targeting mitochondrial metabolism by inhibiting autophagy in BRAF-driven cancers, Cancer discovery. 4, 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santanam U, Banach-Petrosky W, Abate-Shen C, Shen MM, White E & DiPaola RS (2016) Atg7 cooperates with Pten loss to drive prostate cancer tumor growth, Genes Dev. 30, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie T, Li S-J, Guo M-R, Wu Y, Wang H-Y, Zhang K, Zhang X, Ouyang L & Liu J (2015) Untangling knots between autophagic targets and candidate drugs, in cancer therapy, Cell Proliferation. 48, 119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo JY & White E (2016) Autophagy, Metabolism, and Cancer, Cold Spring Harb Symp Quant Biol. 81, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M & White E (2013) Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors, Cancer Discov. 3, 1272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallace DC (2012) Mitochondria and cancer, Nat Rev Cancer. 12, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo JY, Chen H-Y, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JMS, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD & White E (2011) Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis, Genes Dev. 25, 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beloribi-Djefaflia S, Vasseur S & Guillaumond F (2016) Lipid metabolic reprogramming in cancer cells, Oncogenesis. 5, e189–e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C, Haas MA, Yang F, Yeo S, Okamoto T, Chen S, Wen J, Sarma P, Plas DR & Guan J-L (2019) Autophagic lipid metabolism sustains mTORC1 activity in TSC-deficient neural stem cells, Nature metabolism. 1, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S & Levine AJ (2007) The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways, Cancer Res. 67, 3043–53. [DOI] [PubMed] [Google Scholar]
- 94.Kenzelmann Broz D, Spano Mello S, Bieging KT, Jiang D, Dusek RL, Brady CA, Sidow A & Attardi LD (2013) Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses, Genes Dev. 27, 1016–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khayati K, Bhatt V, Hu ZS, Fahumy S, Luo X & Guo JY (2020) Autophagy compensates for Lkb1 loss to maintain adult mice homeostasis and survival, Elife. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E & Xia B (2013) Autophagy Opposes p53-Mediated Tumor Barrier to Facilitate Tumorigenesis in a Model of PALB2-Associated Hereditary Breast Cancer, Cancer Discovery. 3, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White E (2016) Autophagy and p53, Cold Spring Harbor perspectives in medicine. 6, a026120–a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ & Ryan KM (2013) p53 status determines the role of autophagy in pancreatic tumour development, Nature. 504, 296–300. [DOI] [PubMed] [Google Scholar]
- 99.Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, Von Hoff DD, Maitra A & Kimmelman AC (2014) Autophagy Is Critical for Pancreatic Tumor Growth and Progression in Tumors with p53 Alterations, Cancer Discovery. 4, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hensley K, Robinson KA, Gabbita SP, Salsman S & Floyd RA (2000) Reactive oxygen species, cell signaling, and cell injury, Free Radical Biology and Medicine. 28, 1456–1462. [DOI] [PubMed] [Google Scholar]
- 101.Pelicano H, Carney D & Huang P (2004) ROS stress in cancer cells and therapeutic implications, Drug Resistance Updates. 7, 97–110. [DOI] [PubMed] [Google Scholar]
- 102.Li L, Ishdorj G & Gibson SB (2012) Reactive oxygen species regulation of autophagy in cancer: Implications for cancer treatment, Free Radical Biology and Medicine. 53, 1399–1410. [DOI] [PubMed] [Google Scholar]
- 103.Azad MB, Chen Y & Gibson SB (2008) Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment, Antioxidants & Redox Signaling. 11, 777–790. [DOI] [PubMed] [Google Scholar]
- 104.Desideri E, Filomeni G & Ciriolo MR (2012) Glutathione participates in the modulation of starvation-induced autophagy in carcinoma cells, Autophagy. 8, 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Filomeni G, De Zio D & Cecconi F (2015) Oxidative stress and autophagy: the clash between damage and metabolic needs, Cell Death & Differentiation. 22, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murphy Michael P. (2013) Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum, Cell Metabolism. 18, 145–146. [DOI] [PubMed] [Google Scholar]
- 107.Thomas RL & Gustafsson AB (2013) Mitochondrial autophagy--an essential quality control mechanism for myocardial homeostasis, Circ J. 77, 2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S & White E (2007) Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis, Genes Dev. 21, 1621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bu W, Hao X, Yang T, Wang J, Liu Q, Zhang X, Li X, Gong Y & Shao C (2020) Autophagy Contributes to the Maintenance of Genomic Integrity by Reducing Oxidative Stress, Oxidative Medicine and Cellular Longevity. 2020, 2015920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eliopoulos AG, Havaki S & Gorgoulis VG (2016) DNA Damage Response and Autophagy: A Meaningful Partnership, Frontiers in genetics. 7, 204–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ambrosio S & Majello B (2020) Autophagy Roles in Genome Maintenance, Cancers. 12, 1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo H, Chitiprolu M, Gagnon D, Meng L, Perez-Iratxeta C, Lagace D & Gibbings D (2014) Autophagy supports genomic stability by degrading retrotransposon RNA, Nat Commun. 5, 5276. [DOI] [PubMed] [Google Scholar]
- 113.Belaid A, Cerezo M, Chargui A, Corcelle-Termeau E, Pedeutour F, Giuliano S, Ilie M, Rubera I, Tauc M, Barale S, Bertolotto C, Brest P, Vouret-Craviari V, Klionsky DJ, Carle GF, Hofman P & Mograbi B (2013) Autophagy plays a critical role in the degradation of active RHOA, the control of cell cytokinesis, and genomic stability, Cancer Res. 73, 4311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS & Guan KL (2015) Metabolism. Differential regulation of mTORC1 by leucine and glutamine, Science. 347, 194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL & Sabatini DM (2015) Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1, Science. 347, 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saxton RA, Chantranupong L, Knockenhauer KE, Schwartz TU & Sabatini DM (2016) Mechanism of arginine sensing by CASTOR1 upstream of mTORC1, Nature. 536, 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cunha LD, Yang M, Carter R, Guy C, Harris L, Crawford JC, Quarato G, Boada-Romero E, Kalkavan H, Johnson MDL, Natarajan S, Turnis ME, Finkelstein D, Opferman JT, Gawad C & Green DR (2018) LC3-Associated Phagocytosis in Myeloid Cells Promotes Tumor Immune Tolerance, Cell. 175, 429–441 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katheder NS, Khezri R, O’Farrell F, Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen T, Juhasz G, Bilder D, Brech A, Stenmark H & Rusten TE (2017) Microenvironmental autophagy promotes tumour growth, Nature. 541, 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mowers EE, Sharifi MN & Macleod KF (2017) Autophagy in cancer metastasis, Oncogene. 36, 1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nieto MA, Huang RY, Jackson RA & Thiery JP (2016) Emt: 2016, Cell. 166, 21–45. [DOI] [PubMed] [Google Scholar]
- 121.Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, Song Z, Zheng Q & Xiong J (2013) Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition, Carcinogenesis. 34, 1343–51. [DOI] [PubMed] [Google Scholar]
- 122.Jinushi M, Morita T, Xu Z, Kinoshita I, Dosaka-Akita H, Yagita H & Kawakami Y (2017) Autophagy-dependent regulation of tumor metastasis by myeloid cells, PLoS One. 12, e0179357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akkoc Y, Peker N, Akcay A & Gozuacik D (2021) Autophagy and Cancer Dormancy, Front Oncol. 11, 627023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mowers EE, Sharifi MN & Macleod KF (2018) Functions of autophagy in the tumor microenvironment and cancer metastasis, FEBS J. 285, 1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marsh T, Tolani B & Debnath J (2021) The pleiotropic functions of autophagy in metastasis, J Cell Sci. 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marsh T, Kenific CM, Suresh D, Gonzalez H, Shamir ER, Mei W, Tankka A, Leidal AM, Kalavacherla S, Woo K, Werb Z & Debnath J (2020) Autophagic Degradation of NBR1 Restricts Metastatic Outgrowth during Mammary Tumor Progression, Dev Cell. 52, 591–604 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marsh T & Debnath J (2020) Autophagy suppresses breast cancer metastasis by degrading NBR1, Autophagy. 16, 1164–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dolgin E (2019) Anticancer autophagy inhibitors attract ‘resurgent’ interest, Nat Rev Drug Discov. 18, 408–410. [DOI] [PubMed] [Google Scholar]
- 129.Prior IA, Lewis PD & Mattos C (2012) A comprehensive survey of Ras mutations in cancer, Cancer Res. 72, 2457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prior IA, Hood FE & Hartley JL (2020) The Frequency of Ras Mutations in Cancer, Cancer Res. 80, 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, Ionescu C & Berindan-Neagoe I (2019) A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer, Cancers (Basel). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Waters AM & Der CJ (2018) KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer, Cold Spring Harb Perspect Med. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, Charles R-P, Rabinovich BA, Hann B, Dankort D, Spellman PT, Phillips WA, Gray JW & McMahon M (2012) A Central Role for RAF→MEK→ERK Signaling in the Genesis of Pancreatic Ductal Adenocarcinoma, Cancer Discovery. 2, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spiegel J, Cromm PM, Zimmermann G, Grossmann TN & Waldmann H (2014) Small-molecule modulation of Ras signaling, Nat Chem Biol. 10, 613–22. [DOI] [PubMed] [Google Scholar]
- 135.Lee CS, Lee LC, Yuan TL, Chakka S, Fellmann C, Lowe SW, Caplen NJ, McCormick F & Luo J (2019) MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival, Proc Natl Acad Sci U S A. 116, 4508–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap JT, Burrell LD, Lum DH, Whisenant JR, Gilcrease GW, Cavalieri CC, Rehbein KM, Cutler SL, Affolter KE, Welm AL, Welm BE, Scaife CL, Snyder EL & McMahon M (2019) Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers, Nature Medicine. 25, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mollinedo F & Gajate C (2019) Novel therapeutic approaches for pancreatic cancer by combined targeting of RAF→MEK→ERK signaling and autophagy survival response, Ann Transl Med. 7, S153–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, Tomar G, Papke B, Hobbs GA, Yan L, Hayes TK, Diehl JN, Goode GD, Chaika NV, Wang Y, Zhang G-F, Witkiewicz AK, Knudsen ES, Petricoin EF, Singh PK, Macdonald JM, Tran NL, Lyssiotis CA, Ying H, Kimmelman AC, Cox AD & Der CJ (2019) Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer, Nature Medicine. 25, 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J, Kundu M, Viollet B & Guan K-L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1, Nature cell biology. 13, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma X-H, Piao S-F, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, Lazova R, Klump V, Pawelek JM, Xu X, Xu W, Schuchter LM, Davies MA, Herlyn M, Winkler J, Koumenis C & Amaravadi RK (2014) Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma, The Journal of clinical investigation. 124, 1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, Morgan MJ, Mirsky DM, Handler MH, Foreman NK & Thorburn A (2014) Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors, Cancer Discov. 4, 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wander SA, Hennessy BT & Slingerland JM (2011) Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy, J Clin Invest. 121, 1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hua H, Kong Q, Zhang H, Wang J, Luo T & Jiang Y (2019) Targeting mTOR for cancer therapy, J Hematol Oncol. 12, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Emma CS, Richard TM, Stephen ES, Eva M, James G, Shara R-H, Byung P, Anne K, George VT, Marc L, Michael C, Jennifer P, Miranda G, Jennifer B, Edward S, Ravi A & Dan TV (2017) Double autophagy stimulation using chemotherapy and mTOR inhibition combined with hydroxychloroquine for autophagy modulation in patients with relapsed or refractory multiple myeloma, Haematologica. 102, e261–e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haas NB, Appleman LJ, Stein M, Redlinger M, Wilks M, Xu X, Onorati A, Kalavacharla A, Kim T, Zhen CJ, Kadri S, Segal JP, Gimotty PA, Davis LE & Amaravadi RK (2019) Autophagy Inhibition to Augment mTOR Inhibition: a Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma, Clin Cancer Res. 25, 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan KS, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O’Dwyer PJ & Amaravadi RK (2014) Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma, Autophagy. 10, 1391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang H & Zou Z (2020) Targeting autophagy to overcome drug resistance: further developments, Journal of Hematology & Oncology. 13, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li X, Zhou Y, Li Y, Yang L, Ma Y, Peng X, Yang S, Liu J & Li H (2019) Autophagy: A novel mechanism of chemoresistance in cancers, Biomedicine & Pharmacotherapy. 119, 109415. [DOI] [PubMed] [Google Scholar]
- 149.Pérez-Hernández M, Arias A, Martínez-García D, Pérez-Tomás R, Quesada R & Soto-Cerrato V (2019) Targeting Autophagy for Cancer Treatment and Tumor Chemosensitization, Cancers. 11, 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang F, Hu P, Yang Z, Xue C, Gong J, Sun S, Shi L, Zhang S, Li Z, Yang C, Zhang J & Xie C (2017) SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways, Oncol Rep. 37, 3449–3458. [DOI] [PubMed] [Google Scholar]
- 151.Malhotra J, Jabbour S, Orlick M, Riedlinger G, Guo Y, White E & Aisner J (2019) Phase Ib/II study of hydroxychloroquine in combination with chemotherapy in patients with metastatic non-small cell lung cancer (NSCLC), Cancer Treat Res Commun. 21, 100158. [DOI] [PubMed] [Google Scholar]
- 152.Gomes LR, Menck CFM & Leandro GS (2017) Autophagy Roles in the Modulation of DNA Repair Pathways, International journal of molecular sciences. 18, 2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang F, Tang J, Li P, Si S, Yu H, Yang X, Tao J, Lv Q, Gu M, Yang H & Wang Z (2018) Chloroquine Enhances the Radiosensitivity of Bladder Cancer Cells by Inhibiting Autophagy and Activating Apoptosis, Cell Physiol Biochem. 45, 54–66. [DOI] [PubMed] [Google Scholar]
- 154.Eldredge HB, Denittis A, Duhadaway JB, Chernick M, Metz R & Prendergast GC (2013) CONCURRENT WHOLE BRAIN RADIOTHERAPY AND SHORT-COURSE CHLOROQUINE IN PATIENTS WITH BRAIN METASTASES: A PILOT TRIAL, J Radiat Oncol. 2, 10.1007/s13566-013-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford ND & Shaw RJ (2015) Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates, Mol Cell. 59, 285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot MF, Lamberton A, Mathieu M, Bertrand T, Marquette JP, El-Ahmad Y, Filoche-Romme B, Schio L, Garcia-Echeverria C, Goulaouic H & Pasquier B (2014) A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy, Nat Chem Biol. 10, 1013–9. [DOI] [PubMed] [Google Scholar]
- 157.McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, Lynch JP, Uehara T, Sepulveda AR, Davis LE, Winkler JD & Amaravadi RK (2012) Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency, Proc Natl Acad Sci U S A. 109, 8253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM & Debnath J (2011) Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation, Mol Biol Cell. 22, 165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N & Kimmelman AC (2011) Pancreatic cancers require autophagy for tumor growth, Genes Dev. 25, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, Ali SM, Elvin JA, Singal G, Ross JS, Fabrizio D, Szabo PM, Chang H, Sasson A, Srinivasan S, Kirov S, Szustakowski J, Vitazka P, Edwards R, Bufill JA, Sharma N, Ou SI, Peled N, Spigel DR, Rizvi H, Aguilar EJ, Carter BW, Erasmus J, Halpenny DF, Plodkowski AJ, Long NM, Nishino M, Denning WL, Galan-Cobo A, Hamdi H, Hirz T, Tong P, Wang J, Rodriguez-Canales J, Villalobos PA, Parra ER, Kalhor N, Sholl LM, Sauter JL, Jungbluth AA, Mino-Kenudson M, Azimi R, Elamin YY, Zhang J, Leonardi GC, Jiang F, Wong KK, Lee JJ, Papadimitrakopoulou VA, Wistuba II, Miller VA, Frampton GM, Wolchok JD, Shaw AT, Janne PA, Stephens PJ, Rudin CM, Geese WJ, Albacker LA & Heymach JV (2018) STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma, Cancer Discov. 8, 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC & Levine B (1999) Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21, Genomics. 59, 59–65. [DOI] [PubMed] [Google Scholar]
- 162.Marsh T, Kenific CM, Suresh D, Gonzalez H, Shamir ER, Mei W, Tankka A, Leidal AM, Kalavacherla S, Woo K, Werb Z & Debnath J (2020) Autophagic Degradation of NBR1 Restricts Metastatic Outgrowth during Mammary Tumor Progression, Developmental Cell. 52, 591–604.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amaravadi R, Kimmelman AC & White E (2016) Recent insights into the function of autophagy in cancer, Genes Dev. 30, 1913–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pietrocola F, Pol J, Vacchelli E, Baracco EE, Levesque S, Castoldi F, Maiuri MC, Madeo F & Kroemer G (2016) Autophagy induction for the treatment of cancer, Autophagy. 12, 1962–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Laddha SV, Ganesan S, Chan CS & White E (2014) Mutational landscape of the essential autophagy gene BECN1 in human cancers, Mol Cancer Res. 12, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lebovitz CB, Robertson AG, Goya R, Jones SJ, Morin RD, Marra MA & Gorski SM (2015) Cross-cancer profiling of molecular alterations within the human autophagy interaction network, Autophagy. 11, 1668–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eng CH, Wang Z, Tkach D, Toral-Barza L, Ugwonali S, Liu S, Fitzgerald SL, George E, Frias E, Cochran N, De Jesus R, McAllister G, Hoffman GR, Bray K, Lemon L, Lucas J, Fantin VR, Abraham RT, Murphy LO & Nyfeler B (2016) Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy, Proc Natl Acad Sci U S A. 113, 182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nyfeler B & Eng CH (2016) Revisiting autophagy addiction of tumor cells, Autophagy. 12, 1206–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.BioRender.com (2021) Adapted from “The Tumor Microenvironment: Overview of Cancer-Associated Changes”, Retrieved from https://app.biorender.com/biorender-templates.


