Abstract
Patients with prostate cancer (PCa) on androgen-deprivation therapy (ADT) are at high risk of osteoporosis and fragility fractures. We aimed to provide some practical insights into the delivery of optimal bone health care for PCa patients, particularly those on ADT. An interdisciplinary group of experts, including urologists and rheumatologists developed recommendations based on their expertise, current evidence and guidelines. The multidisciplinary group's main recommendations are: fragility fracture risk should be assessed in all PCa patient, especially, in those under ADT. FRAX® tool may be incorporated into clinical practice to identify patients at high risk of fracture. Bone mineral density (BMD) should be measured routinely by dual energy X-ray absorptiometry in all patients scheduled for or on ADT. Thoracic and lumbar spine X-ray may be performed at the initial evaluation of patients with the diagnosis of osteoporosis and in case of suspected clinical vertebral fracture. Basic laboratory tests are recommended to exclude secondary osteoporosis. Treatment with bisphosphonates or denosumab should be considered in patients on ADT with fragility fracture, osteoporosis (BMD T-score ≤−2.5), or high risk of fracture according to FRAX®. Referral to a bone metabolism specialist should be contemplated in some cases. The recommendations provided in this document, tailored for clinicians treating PCa patients, may be of help to identify and treat patients at high risk of fracture.
Keywords: Androgen antagonists, Denosumab, Diphosphonates, Osteoporosis, Prostatic neoplasms
INTRODUCTION
Patients with prostate cancer (PCa) are at high risk of osteoporosis and fragility fractures [1], as a result long-term preservation of bone health remains a clinical challenge. Bone health may be jeopardized by different therapies throughout patients' life, including radiotherapy, glucocorticoids and the long-term use of androgen-deprivation therapy (ADT) [1]. ADT, one of the cornerstones of the PCa treatment, is strongly associated with high bone turnover rate that may result in reduction of bone mineral density (BMD) and increased risk of fracture, leading to higher morbidity and mortality of the patients, and a high economic burden [2].
Although preserving bone health should be a crucial goal for all physicians involved in the management of PCa patients, actually this is not a very widespread practice, partly explained because osteoporosis is underdiagnosed in men [3].
The purpose of this document is to provide some practical insights into the delivery of optimal bone health care for PCa patients, particularly those on ADT, which are at high risk of osteoporosis and fragility fractures. An interdisciplinary group of Spanish experts give some recommendations for clinical practice on identifying PCa patients at high risk of fracture through FRAX®, BMD and spine X-ray; selecting the most suitable laboratory tests; and selecting the best pharmacological and non-pharmacological treatment.
Skeletal-related events associated to metastatic bone disease are not considered in this document.
METHODS
An interdisciplinary group of experts, including urologists (AB, JMuñoz-Rodríguez, JMorote), and rheumatologists (EC, MC, JG), aimed to provide in this publication some practical insights and recommendations on how to deliver optimal bone health care for PCa patients, particularly those starting or already on ADT. The recommendations are based on current evidence, different national and international guidelines and their own expertise, and are tailored specifically for clinicians who treat PCa patients with hormonal therapies.
OSTEOPOROSIS AND FRAGILITY FRACTURES
Osteoporosis is a diffuse skeletal disease characterized by low bone strength, which leads to a higher risk of fractures, also known as fragility fractures [4]. The concept of “bone strength” comprises elements related with bone density and bone quality [4].
A fragility fracture normally appears spontaneously or as a consequence of a low energy trauma, which normally would not break a healthy bone [5]. Although osteoporosis is asymptomatic, it may have serious clinical consequences because of possible fragility fractures, which could cause a severe impact on the patient's life. The most frequent fractures are vertebral, hip, forearm and shoulder. Amongst older patients vertebral and hip fractures have higher incidence, causing an increase in morbidity and mortality. Around 20% of patients die in the first year after a hip fracture [6].
Fragility fractures also represent a huge social cost. In Spain (47 million of people), the estimated cost related to fragility fractures is more than 4 billion Euros per year [7].
BMD can be measured by dual energy X-ray absorptiometry (DXA) at lumbar spine, femoral neck and total hip. BMD can be categorized according the T-score (difference between the patient BMD and the young male BMD expressed in standard deviation) as [4]:
• T-score ≥−1 standard deviation (SD): normal
• T-score between −1 and −2.5 SD: osteopenia
• T-score ≤−2.5 SD: osteoporosis
In absence of other osteopathy, osteoporosis, in a clinical setting, can be considered in men older than 50 years according to the following definitions [4]:
• Lumbar spine, femoral neck or total hip T-score ≤-2.5 SD
• Fragility hip fracture, regardless of BMD
• Fragility vertebral, shoulder or pelvic fracture in patients withlow BMD (T-score <−1.0 SD)
OSTEOPOROSIS AND FRAGILITY FRACTURES IN PROSTATE CANCER PATIENTS
A study performed in 618 men with newly diagnosed PCa (mean age 73 years) showed that 41% had osteoporosis, 39% osteopenia and only 20% normal BMD [8]. Many therapies used in PCa patients may lead to an even more accelerated bone loss. In a meta-analysis of 14 trials, men who received ADT had a 23% increase of overall fracture risk compared with men with PCa who did not undergo ADT [9]. Bone loss is higher (5%–10%) during the first year of ADT, and continues gradually throughout the ADT duration [10,11]. One study with 390 men with PCa (mean age 68.9 years) showed that the prevalence of osteoporosis was 35% in hormone-naïve patients, 43% after 2 years of ADT, 59.5% after 6 years, and 81% after 10 or more years of ADT [12].
Fragility fractures in PCa patients can occur in many sites, but are particularly frequent in the thoracic and lumbar vertebrae below T4, distal forearm, ribs, and hip [13], and they correlate with poor survival outcomes [14]. Although the incidence of hip fractures is lower in men, their mortality rate is higher [15]. Data from large registries indicate that there is a higher risk of death following a hip fracture for PCa patients on ADT than for PCa patients not on ADT or PCa-free men, particularly in the first year [16]. PCa patients on ADT with a hip fracture may be 2.44 times more likely to die compared with the cohort of all PCa patients [16]. Furthermore, fragility fractures significantly affect the patients' quality of life and health status, and have a considerable impact on healthcare resources [1]. Assessing the fracture risk before prescribing long-term ADT together with close monitoring of bone health during this treatment may reduce the risk of fracture and improve patients' quality of life and survival [1].
MECHANISMS OF BONE LOSS IN PROSTATE CANCER PATIENTS
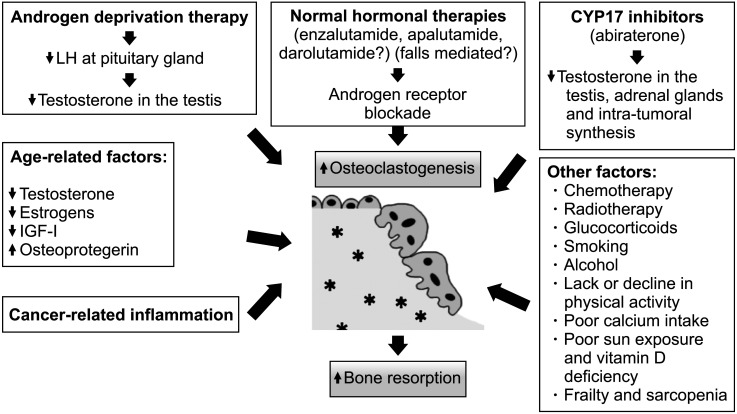
Skeletal system integrity is maintained by a dynamic complex process named remodeling or bone turnover, which is regulated by three major types of bone cells: bone-forming osteoblasts, bone-resorbing osteoclasts, and mechanosensor/mediator osteocytes [17]. Some of these cells express androgen receptors (ARs) and estrogen receptors [17]. AR signaling in osteoblasts is responsible for the protective effects of androgens on trabecular bone mass. AR activation leads to a decrease in osteoclasts and bone resorption. Estrogens in men are produced via aromatization of androgens, and their activation in mesenchymal cells protects against endocortical resorption [14]. Current evidence suggests that estrogens play a much more significant role in regulating bone metabolism in men than testosterone [14].
The critical driving force for PCa is the AR-regulated gene expression that is initiated by the binding of androgen to AR [18]. ADT is the backbone of systemic therapy for men with PCa. Almost one-half of patients receive ADT treatment during their disease course [19]. ADT in PCa patients lowers serum testosterone to castration levels (<5% of the normal range) and serum estradiol levels to <20% of the normal range, inducing a high bone turnover and, eventually, an increased bone loss rate [20]. This increase in bone turnover is often associated with an accelerated microarchitectural bone damage that is usually not reversible [1]. Both, bone loss and microarchitectural bone damage explain, in part, the high risk of fracture of these patients [1].
Although ADT is the mainstay therapy for PCa, after the initial response to this therapy most PCa will inevitably shift from castration-sensitive PCa to castration-resistant PCa (CRPC) [18]. Additional second-line AR antagonists hormonal treatments (enzalutamide, apalutamide, or darolutamide), CYP17A inhibition to further decrease androgen biosynthesis with abiraterone acetate and/or chemotherapy are frequently the 3 therapeutic mainstays during the CRPC stage [21]. A group of patients with substantial risk of osteoporosis and osteoporotic fractures are those with CRPC in the absence of clinically detectable metastatic disease, This group is referred to as non-metastatic CRPC [22], and represents about 2%–8% of the total PCa population [23]. Prescription of novel and more potent AR inhibitors in this population, in addition to prior long-term use of ADT, results in longer exposure times and consequently higher risk of osteoporotic fractures [22].
All these novel hormonal treatments such as abiraterone/prednisone, enzalutamide or apalutamide, although they are not clearly associated with bone loss, seem to induce a higher risk of falls [24,25,26]. Enzalutamide and apalutamide inhibit the AR, potentially blocking the bone-protective effects of androgens, and are associated with adverse effects related to the central nervous system that may lead to falls and subsequent traumatic fractures [24,25]. By contrast, darolutamide was not associated with a higher incidence of falls in a pivotal trial [27], maybe due to its lower blood–brain barrier penetration and less central nervous system-related adverse-events, including falls. Nevertheless, long-term studies are lacking and further analysis would be needed to investigate its impact on bone health. These new antihormonal therapies are now being utilized earlier in the treatment of PCa. Therefore, patients with PCa are receiving more potent treatments and longer durations of intensified ADT, which increase further their risk of bone loss and fractures [22].
Other PCa treatments including chemotherapy or glucocorticoids, which are used in addition to background ADT for the treatment of advanced PCa, also have a negative impact on bone health [14].
Beside treatments, many other factors may be associated with bone loss in PCa patients, such as age. Most men diagnosed with PCa are over 65 years, and aging is clearly associated with gradual bone loss in men [28,29]. With age, there is also a decrease in hormone levels, including testosterone and estrogens. In addition, levels of sex hormone binding globulin increase with age, decreasing free or bioavailable testosterone [29]. Furthermore, males with low body mass index also have decreased estrogen levels. Apart from sexual hormones, other hormones and proteins are implicated in bone turnover change with age. An increase in insulin-like growth factor-1 levels and a reduction in osteoprotegerin levels in serum may be associated to age-related osteoporosis [29].
Additionally, many PCa patients may have some modifiable risk factors that can affect BMD, such as smoking, alcohol, lower weight or low body mass index, physical/functional limitations, weight loss, prolonged corticosteroid use, low dietary calcium intake, or low vitamin D levels [29,30].
Finally, PCa disease itself, mainly mediated by local and systemic inflammation, may promote increased bone loss through altered systemic bone remodeling, increased bone resorption, and impaired bone formation [10].
Factors associated with bone loss in PCa patients are summarized in Fig. 1.
Fig. 1. Factors associated to bone loss in men with prostate cancer. LH: luteinizing hormone, IGF-I: insulin-like growth factor-I.
FRACTURE RISK ASSESSMENT IN PROSTATE CANCER PATIENTS
The risk of fragility fractures should be assessed in all PCa patients, in particular, in those starting or receiving ADT [20]. Several tools have been developed to estimate fracture risk over time considering clinical risk factors with or without BMD measurements [4].
1. FRAX® tool
The FRAX® (Fracture Risk Assessment) tool (Centre for Metabolic Bone Diseases, University of Sheffield, Shefflied, UK) is the most popular and widely used tool to assess fracture risk [31]. Risk factors included in this tool are shown in Table 1.
Table 1. Risk factors included in FRAX® tool to calculate the absolute 10-year risk of hip fracture and major osteoporotic fractures.
| Continuous risk factors | Dichotomic risk factors |
|---|---|
| Age | Sex |
| Body mass index | Previous fracture |
| Femoral neck BMD (optional)a | Parent fractured hip |
| Current Smoking | |
| Glucocorticoids use | |
| Rheumatoid arthritis | |
| Secondary osteoporosisb | |
| Alcohol intake (three or more drinks per day) |
BMD: bone mineral density.
aAlthough femoral neck BMD is optional, this item increases the accuracy of FRAX® in predicting the absolute 10-year fracture risk.
bTreatment with androgen-deprivation therapy, as a cause of hypogonadism, must be included as a cause of secondary osteoporosis.
The FRAX® tool provides a 10-year probability of a major osteoporotic fracture (clinical vertebral, forearm, proximal humerus, and hip) or a hip fracture. Femoral neck BMD is an optional item in this tool and provides further accuracy of fracture risk [4,31]. Although the FRAX® tool considers some causes of secondary osteoporosis to calculate fracture risk, it's important to highlight that previous, current or scheduled use of ADT is not considered [20].
Fracture risk has a high variability worldwide, so FRAX® must be used considering the country of the patient, in order to adjust the risk to the appropriate fracture epidemiology and mortality [32]. Some guidelines establish determined FRAX® thresholds to define high risk of fracture and pharmacological treatment recommendation to prevent fractures. Examples include a ten-year probability of a major fracture of 20% in US or Canada, or 15% in Sweden or Japan [32]. In Spain, these thresholds are ≥7.5% (with BMD) or ≥10% (without BMD) for major fracture, and ≥3% for hip fracture [4]. Although the FRAX® tool has not been fully validated in the Spanish population, with the currently available evidence, some scientific societies such as the Spanish Society of Rheumatology recommend the incorporation of FRAX® into clinical practice to identify patients at high risk of fragility fracture [4].
To our knowledge there are no specific FRAX® thresholds for PCa patients on ADT. However, FRAX® can provide a useful estimation of baseline fracture risk of these patients for other causes beyond ADT.
In our opinion, FRAX® tool may be incorporated into clinical practice for PCa patients on ADT to identify patients at highfracture risk. The threshold defining high fracture risk may be different in each country (i.e., >3% for hip fracture, and >7.5%–10% for major fracture in Spain; or >3% for hip fracture, and >20% for major fracture in USA).
2. Bone densitometry
Bone densitometry by DXA is the gold standard method for measuring BMD in clinical practice. It is a technique with very low radiation exposure [10].
It is used to diagnose osteoporosis and assess an individual's risk of developing fragile fractures [4]. The International Society for Clinical Densitometry (ISCD) recommends that BMD should be measured in lumbar spine (ideally L1-L4), total hip and femoral neck. It's important to note that lumbar spine BMD could be artefactually increased in individuals with spine degenerative conditions, which often occur in older patients [33]. BMD of the distal radiousshould be assessed in cases where lumbar spine and/or hip cannot be measured (e.g., obese patient who exceeds weight limit of table) or is unreliable (e.g., patient with lumbar laminectomy) [34].
Recommendations supporting the need for DXA in PCa patients differ amongst different scientific societies:
• The Spanish Society of Rheumatology 2019 Recommendations on Osteoporosis recommend measuring BMD in the population with risk factors for fragility fractures such as males treated with ADT for PCa [4]. These recommendations are in line with the French 2019 recommendations for osteoporosis prevention and treatment in patients with PCa treated by ADT, which recommend routine BMD measurement in patients scheduled for or receiving ADT [33].
• The European Society for Medical Oncology (ESMO) 2020 clinical practice guidelines for diagnosis, treatment and follow-up of PCa also consider that men starting long-term ADT should be monitored with DXA [1].
• The International Osteoporosis Foundation (IOF) 2017 guidelines state that osteoporosis and fragility fracture risk should be evaluated by DXA in every PCa patient, and especially, in the ones startint or receiving ADT [20].
• The 2020 guidelines for PCa issued by the National Comprehensive Cancer Network (NCCN) recommend obtaining a baseline DXA before starting therapy in men at increased risk for fracture based on FRAX® screening [35].
• The American Society of Clinical Oncology (ASCO) 2019 guidelines for management of osteoporosis in survivors of adult cancers with non-metastatic disease [10] consider that patients with non-metastatic cancer with one or more risk factors for osteoporotic fracture should be offered BMD testing with DXA; and in PCa patients ADT induced hypogonadism should be considered a risk factor [10].
Similarly, recommendations on how often to monitor BMD with DXA after initiating ADT are not consistent amongst guidelines [10,20,35].
As a general rule, BMD must be considered in all PCa patients starting ADT, and should not be repeated more often than once annually [10]. In high-risk patients placed on antiresorptives for osteoporosis treatment, guidelines consider that BMD should be monitored using DXA in 18 to 24 months intervals [10,20], althoughmonitoring after 1 year [35] or after the first 3 to 5 years [33] has also been suggested. Patients on ADT at low risk for fracture (T-score >−1, and therefore not receiving antiresorptives) may be monitored by DXA every 18 to 24 months [20]. Osteopenic patients (T-score between −1 and −2.5) taking ADT without antiresorptives, BMD may be assessed every year to detect significant changes that could change of fracture risk classification [20].
3. Vertebral fracture assessment
Clinical guidelines recommend assessing prevalent vertebral fractures in patients on ADT [20]. Although thoracic and lumbar spine X-ray is the most frequently used tool [4], some DXA equipment incorporates the Vertebral Fracture Assessment (VFA) technology, which is also useful to identify vertebral fractures, with a lower radiation [20].
A diagnosis of vertebral fracture is made when there is loss of height in the anterior, middle, or posterior portion of the vertebral body >20%. When in doubt, additional views or studies are recommended for confirmation.
Osteoporotic vertebral fractures can be graded according to height loss as mild (up to 20%–25%), moderate (25%–40%) or severe (>40%) [36]. X-ray or VFA interpretation requires some experience to avoid diagnostic errors, like diagnosing other vertebral deformities as vertebral fractures.
The 2019 Spanish Society of Rheumatology Osteoporosis Recommendations considers that thoracic and lumbar spine X-rays are helpful during the first assessment of every individual with the diagnosis of osteoporosis, or in those cases where there is an important reduction of height or kyphosis, or clinical features of vertebral fracture, such as newly emerging back pain of traumatic or non-traumatic origin [4].
4. Trabecular bone score
Using data from the DXA, the Trabecular Bone Score (TBS) can be calculated to analyze the bone texture and obtain bone microarchitecture related parameters [4]. Low TBS values are associated with an increased risk of fracture, regardless of BMD, and the introduction of the TBS in the FRAX® algorithm provides a more accurate absolute fracture risk prediction [4]. Despite all these potential benefits from the assessment of fracture risk, guidelines suggest that further research is still necessary to recommend TBS in clinical practice [4,37].
5. Laboratory
Other secondary causes of osteoporosis and other bone diseases differential diagnosis should be assessed through basic laboratory tests in all patients with PCa receiving ADT with osteoporosis or fragility fractures.
Several scientific societies recommend the following blood and urine tests to rule out secondary causes of osteoporosis: complete hemogram, erythrocyte sedimentation rate, transaminases, creatinine, albumin, calcium, phosphate, total alkaline phosphatase, 25-hydroxyvitamin D, serum protein electrophoresis and 24-urine calcium [4,33]. Additional tests may be necessary in some cases to rule out specific conditions associated with osteoporosis such as hyperthyroidism, hyperparathyroidism, mastocytosis, celiac disease, or Cushing syndrome [4].
6. Bone turnover markers
Although bone turnover markers (BTMs) can offer prognostic information on fracture risk and provide information about osteoporosis treatment efficacy [4,38], no data are available on the usefulness of BTM in patients with osteoporosis induced by ADT. Consequently, BMT testing is not generally recommended in this population [33].
PREVENTION AND TREATMENT OF OSTEOPOROSIS IN PROSTATE CANCER PATIENTS
Patients with PCa, particularly those starting or receiving ADT, should follow some general steps for primary prevention of osteoporosis and fractures. The first step would be to keep a healthy lifestyle, in accordance to the following recommendations [4,10]:
• A healthy diet with sufficient amount of proteins (0.8 gr/kg of body weight), calcium, vegetables, and fruits should be adopted to cover nutritional demands. An adequate intake of calcium may be around 1,000 to 1,200 mg/day.
• Stop smoking and limit alcohol consumption to fewer than 3 units/day.
• Have a regular and prudent exposure to sunlight.
• Engage into physical activity combining different exercises, such as balance training, stretching, endurance and resistance and/or progressive strengthening exercises [39].
1. Calcium and vitamin D
A daily intake of 1,000 to 1,200 mg of calcium, mainly from diet, is recommended in all patients for osteoporosis prevention [4]. Aliments that are particularly high in calcium include dairy products, such as milk, yogurt and cheese. Milk and yogurt can provide between 100 and 180 mg per 100 g. Nuts and seeds are also rich in calcium, especially almonds, sesame and chia that can provide between 250 to 600 mg of calcium per 100 g. Some green vegetables such as broccoli or watercress provide between 100 and 150 mg of calcium per 100 g [40].
It is advisable to check daily calcium intake using self-reported questionnaires in order to provide proper supplements if needed or receive suitable nutritional advice [20]. The IOF and the SEIOMM (Sociedad Española de Investigación Ósea y del Metabolismo Mineral) provide online tools to estimate daily calcium intake from diet [41,42].
Regarding vitamin D, guidelines suggest an intake of at least 800 to 1,000 IU/day [10,20]. The main natural source of vitamin D is its synthesis by the skin after sunlight exposure. Very few aliments contain vitamin D, and most of them contain oily fish such as sardines, salmon, and mackerel [43].
25-hydroxyvitamin D serum levels, an appropriate marker of vitamin D status [43], and should be kept at least above 30 ng/mL [20,33]. It is advisable to monitor 25-hydroxyvitamin D levels in all patients with PCa on ADT and treat all patients with vitamin D insufficiency or deficiency. SEIOMM also provide an online tool to assess the risk of vitamin D deficiency [42].
Notably, there are two exogenous vitamin D supplementation options most used in a clinical setting: cholecalciferol (vitamin D3) and calcifediol (25-hydroxyvitamin D), which are available only in some countries, like Spain [44].
Vitamin D supplementation regimen will depend on 25-hydroxyvitamin D serum levels and the vitamin D metabolite used for treatment. A regimen approach is detailed on Table 2.
Table 2. Suggested regimen approach for vitamin D supplementation in patients with prostate cancer under androgen deprivation therapy.
| Vitamin D deficiency (25-hydroxyvitamin D <20 ng/mL) | |
| • Cholecalciferol 50,000 IU weekly for 8 weeks | |
| • Calcifediol 16,000 IU (0.266 mg) weekly for 4 weeks | |
| • This regimen should be followed by the maintenance dose. | |
| Vitamin D insufficiency (25-hydroxyvitamin D=20–30 ng/mL) | |
| • Cholecalciferol 25,000–50,000 IU weekly for 6 weeks | |
| • Calcifediol 16,000 IU (0.266 mg) weekly for 3 weeks | |
| • This regimen should be followed by the maintenance dose. | |
| Maintenance dose of vitamin D supplementation in patients with optimal 25-hydroxyvitamin D levels (30–50 ng/mL) | |
| • Cholecalciferol 25,000–50,000 IU monthly (800–1,666 IU per day) | |
| • Calcifediol 16,000 IU (0.266 mg) every 3–4 weeks | |
2. Antiresorptive therapy
Antiresorptive therapy with bisphosphonates or denosumab should be considered in patients with higher risk of fractures.
Denosumab (60 mg s.c. every 6 months) is a human monoclonal antibody that specifically inhibits RANKL with a potent bone turnover inhibition. It is licensed by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) to treat bone loss related to hormone ablation in males with PCa and high fracture risk [45,46].
Zoledronic acid (5 mg i.v. annually) is an intravenous bisphosphonate with a potent resorptive inhibition. It is licensed by FDA and EMA for the treatment of osteoporosis in adult men at increased risk of fracture, including those with a recent low energy hip fracture [47,48], so it could be used in PCa patients at high risk of fracture (Table 3).
Table 3. Antiresorptive agents for the treatment of osteoporosis in men with prostate cancer.
| Drug | Indication approved for male osteoporosis | Dosage | Recommendations for administration | Contraindications | |
|---|---|---|---|---|---|
| U.S. Food and Drug Administration | European Medicines Agency | ||||
| Alendronate [4,35] | Yes | No | 70 mg orally once a week | At least 30 minutes before the first food, or drink of the day with a glass of plain water; do not lie down for at least 30 minutes after taken | Gastroesophageal refluxbreakr/>
Inability to stand/sit upright for at least 30 minutes Hypocalcemia Renal impairment (CrCl <35 mL/min) Scheduled dental procedures |
| Risedronate [4,35,49] | Yes | Yes (only 35 mg weekly formulation) |
35 mg orally once a week 75 mg orally on two consecutive days each month 150 mg orally once a month |
At least 30 minutes before the first food, or drink of the day with a glass of plain water; do not lie down for at least 30 minutes after taken | Gastroesophageal reflux Inability to stand/sit upright for at least 30 minutes Hypocalcemia Renal impairment (CrCl <35 mL/min) Scheduled dental procedures |
| Risedronate delayed-release tablets [4,35] | No | No | 35 mg orally once a week | Immediately following breakfast | Hypocalcemia Renal impairment (CrCl <35 mL/min) Scheduled dental procedures |
| Zoledronic acid [47,48] | Yes | Yes | 5 mg intravenous once a year | Infusion given intravenously over no less than 15 minutes | Hypocalcemia Renal impairment (CrCl <35 mL/min) Scheduled dental procedures |
| Denosumab [45,46] | Yesa | Yesa | 60 mg subcutaneous every 6 month | Subcutaneous injection in the upper arm, upper thigh, or abdomen | Hypocalcemia Scheduled dental procedures |
CrCl: creatinine clearance.
aDenosumab is the only antiresorptive agent with specific indication for the treatment of bone loss associated with hormone ablation in men with prostate cancer at increased risk of fractures.
The results from randomized clinical trials in patients with non-metastatic PCa on ADT have shown that treatment with both zoledronic acid and denosumab are associated with an increase in BMD. No data on fracture risk reduction are available with zoledronic acid, but denosumab 60 mg every 6 months has been demonstrated to reduce the risk of fracture in PCa patients on ADT [33].
Oral bisphosphonates have proven to be also effective in increasing BMD and reducing the risk of fracture in men, with a good safety profile. According to some guidelines both alendronate and risedronate are recommended for PCa patients [4,35], although only risedronate (35 mg orally once a week) is licensed by the EMA for osteoporosis in men (Table 3) [49].
In a recent systematic review and network meta-analysis in men with PCa receiving ADT for more than 6 months, oral or intravenous bisphosphonates and denosumab appeared to be effective in reducing the rate of bone loss, without evidence that one drug was superior to another [50]. Hence, there are no uniform criteria in guidelines regarding the indications of osteoporosis treatment in PCa patients on ADT [4,10,20,35].
However, it seems reasonable to consider antiresorptive treatment with bisphosphonates (oral or intravenous) or denosumab in PCa patients on ADT with any of the following conditions:
• Previous or current fragility fractures, regardless of BMD.
• Osteoporosis by DXA (T-score ≤-2.5 SD in the lumbar spine, femoral neck or total hip).
• High fracture risk according to country-specific FRAX® threshold (i.e., >3% for hip fracture, and >7.5%–10% for major fracture in Spain).
Selection of oral bisphosphonates, zoledronic acid or denosumab will depend on patient characteristics and availability of these medications in each clinical setting.
Although oral bisphosphonates can be used as the first option for many patients, zoledronic acid or denosumab may be a better option for some patients:
• Alendronate or risedronate: first option.
• Zoledronic acid: non-adherence or intolerance to oral bisphosphonates, in a hospital setting.
• Denosumab: non-adherence or intolerance to oral bisphosphonates, any contraindication to oral or intravenous bisphosphonates, as chronic kidney disease with glomerular filtration rate <35 mL/min; and very low BMD (T-score <-3.5).
Although the risk of hypocalcaemia with antiresorptives is low, it is important to maintain a positive calcium balance and vitamin D intake specially with zoledronic acid or denosumab [20].
The available therapeutic trials involving PCa patients lasted 6 to 36 months. Therefore, they cannot be used to decide the optimal treatment duration [33], however, 3 to 5 years of treatment seems to be effective and safe with bisphosphonates [33]. A discontinuation of bisphosphonates is reasonable because they have a prolonged skeletal retention and a residual therapeutic effect after stopping.
Regarding denosumab, there is more concern about its discontinuation, since a rebound bone turnover effect, with a rapid decrease of BMD and an increased risk of vertebral fractures has been reported [10]. To minimize this rebound effect, experts recommend the administration of potent bisphosphonates, such as alendronate or preferably zoledronic acid 6 months after the last dose of denosumab [51,52]. In this case, referral to a bone specialist should be considered to assess the need for additional antiresorptive therapy.
Bisphosphonates and denosumab may be associated with a number of adverse events (Table 3). One of the most concerning complications is osteonecrosis of the jaw (ONJ) [53]. Although this event is infrequent, especially when these drugs are used at doses for osteoporosis, patients ought to be made aware of this adverse effect. An oral health assessment at baseline and once a year during treatment is recommended in all patients starting antiresorptives [33]. Poor oral hygiene, periodontal disease, poorly fitted dentures, history of dental disease, or invasive dental procedures are risk factors that should be considered when evaluating a patient's risk of developing ONJ. All patients should be encouraged to maintain good oral hygiene, undergo routine dental check-ups, and immediately report any oral symptoms such as dental mobility, pain or swelling, non-healing of sores or discharge during treatment with bisphosphonates or denosumab. During treatment with denosumab or zoledronic acid, invasive dental procedures should be performed with caution and avoided in close proximity to the treatment administration [45,46,47,48].
Some scientific societies, as the Spanish Society of Rheumatology, have developed some recommendations for the oral health management of patients receiving bisphosphonates or denosumab [4].
CRITERIA FOR REFERRAL TO A BONE METABOLISM SPECIALIST
The following criteria for referral PCa patients to a bone metabolism specialist are suggested:
• Patients should be referred to another level of care if they are going to benefit from the referral or when their treating physician cannot or is not used to handle these types of health conditions. The training of the professional, availability of time, and organization of the work place may also condition referrals.
• In the case of any difficulties related to osteoporosis assessment or treatment, or in case of inadequate response to drug treatment.
CONCLUSIONS
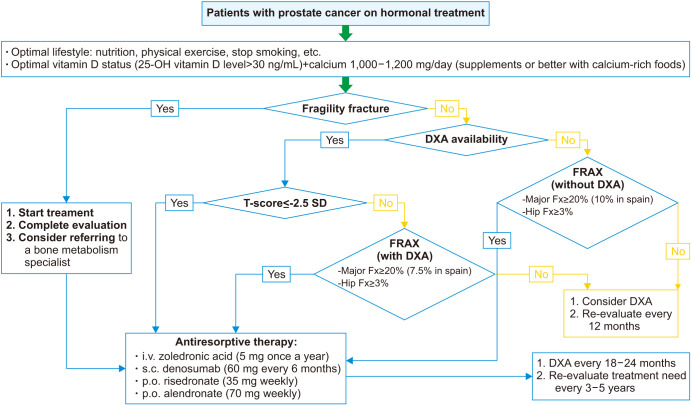
Summary of recommendations for an appropriate management of bone health in PCa patients (Fig. 2):
Fig. 2. Osteoporosis management algorithm. DXA: X-ray absorptiometry, FRAX: fracture risk assessment, Fx: Fracture.
• The risk of fragility fractures should be assessed in all PCa patients, and especially, in those starting or receiving ADT.
• FRAX® tool may be incorporated into clinical practice to identify PCa patients at higher risk of fragility fracture, according to the country-specific fracture threshold (i.e., >3% for hip fracture, and >7.5%–10% for major fracture in Spain).
• Hip and spine BMD should be measured by DXA in all patients scheduled for or on ADT. BMD monitoring with DXA should be done every 18–24 months, although in high-risk patients it could be done earlier (at 12 months) and in lower risk patients later (at 3–5 years).
• A thoracic and lumbar spine X-ray is recommended for the initial evaluation of all patients scheduled for ADT and in the case of vertebral fracture suspicion (acute back pain, significant height loss, or kyphosis) during follow-up. Baseline X-ray could be replaced by DXA for a VFA.
• The following laboratory tests are recommended to rule out other secondary causes of osteoporosis in PCa patients: complete hemogram, erythrocyte sedimentation rate, transaminases, creatinine, albumin, calcium, phosphate, total alkaline phosphatase, 25-hydroxyvitamin D, serum protein electrophoresis, and 24h-urine calcium. Only in case of clinical suspicion additional tests may be necessary to rule out other conditions associated with osteoporosis (i.e., hyperthyroidism, hyperparathyroidism, mastocytosis, celiac disease, or Cushing syndrome).
• Clinicians should encourage patients to follow a healthy lifestyle, including adequate nutrition, stop smoking, limit alcohol intake, and engage into regular physical activity.
• A daily intake of calcium between 1,000 and 1,200 mg, preferably from diet is recommended. If needed, patients should receive calcium supplements.
• A daily intake of vitamin D between 800 and 1,600 IU/day, from diet, sunlight exposure or supplements is recommended to achieve serum levels of 25-hydroxyvitamin D over 30 ng/mL.
• Treatment with bisphosphonates or denosumab may be indicated in patients on ADT with any of the following: 1) fragility fracture; 2) osteoporosis, defined as T-score ≤-2.5 SD in lumbar spine, femoral neck, or total hip, and ; 3) high risk of fracture according to the country-specific FRAX® threshold.
• In case of clinical issues or doubts related to osteoporosis assessment or treatment, the patient should be referred to a bone specialist.
ACKNOWLEDGEMENTS
The authors thank Content ed Net, Madrid, Spain and Dr. Pablo Rivas for writing and editorial assistance. Writing and editorial assistance has been provided by Content ed Net with funding by Bayer Hispania SL.
Footnotes
Conflict of Interest: Casado E has received consulting fees or speaker remuneration from Eli Lilly, Amgen, UCB, Rubió, Theramex, Gebro, Italfármaco, Gedeon-Richter, STADA, Bayer, and GP-Pharma.
Borque-Fernando A has received consulting fees or speaker remuneration from Janssen, Astellas, Bayer, Sanofi, Astra-Zeneca, MSD, Ipsen, Rovi, Opko, MDx Health, Ferrer, and Lacer.
Caamaño M has received speaker remuneration from Eli Lilly, Amgen, MSD, Gedeon-Richter, and Grünenthal Pharma.
Graña J has received speaker remuneration from Eli Lilly, Amgen, UCB, Theramex, Gebro, Italfármaco, Gedeon-Richter, and STADA.
Muñoz-Rodriguez J has received consulting fees or speaker remuneration from Amgen, Astellas, Bayer, Ipsen, and Janssen.
Morote J do not declare any conflict of interest related to this article.
- All the authors have contributed equaly in design, revision and approval of the article.
References
- 1.Santini D, Berruti A, Di Maio M, Procopio G, Bracarda S, Ibrahim T, et al. Bone health management in the continuum of prostate cancer disease: a review of the evidence with an expert panel opinion. ESMO Open. 2020;5:e000652. doi: 10.1136/esmoopen-2019-000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachner TD, Coleman R, Hadji P, Hofbauer LC. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol. 2018;6:901–910. doi: 10.1016/S2213-8587(18)30047-0. [DOI] [PubMed] [Google Scholar]
- 3.Todenhöfer T, Stenzl A, Hofbauer LC, Rachner TD. Targeting bone metabolism in patients with advanced prostate cancer: current options and controversies. Int J Endocrinol. 2015;2015:838202. doi: 10.1155/2015/838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naranjo Hernández A, Díaz Del Campo Fontecha P, Aguado Acín MP, Arboleya Rodríguez L, Casado Burgos E, Castañeda S, et al. Recommendations by the Spanish Society of Rheumatology on osteoporosis. Reumatol Clin (Engl Ed) 2019;15:188–210. doi: 10.1016/j.reuma.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Borgström F, Karlsson L, Ortsäter G, Norton N, Halbout P, Cooper C, et al. International Osteoporosis Foundation. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15:59. doi: 10.1007/s11657-020-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Osteoporosis Foundation (IOF) Facts & statistics [Internet] Nyon: IOF; [cited 2020 Sep 18]. Available from: https://www.iofbonehealth.org/facts-statistics. [Google Scholar]
- 7.International Osteoporosis Foundation (IOF) Huesos rotos, vidas rotas: guía para mejorar la atención a las fracturas por fragilidad en España [Internet] Nyon: IOF; [cited 2020 Sep 18]. Available from: https://share.osteoporosis.foundation/EU-6-Material/Reports/IOF%20Report_SPAIN_DIGITAL_SP.pdf. Spanish. [Google Scholar]
- 8.Wadhwa VK, Weston R, Mistry R, Parr NJ. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int. 2009;104:800–805. doi: 10.1111/j.1464-410X.2009.08483.x. [DOI] [PubMed] [Google Scholar]
- 9.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro CL, Van Poznak C, Lacchetti C, Kirshner J, Eastell R, Gagel R, et al. Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO clinical practice guideline. J Clin Oncol. 2019;37:2916–2946. doi: 10.1200/JCO.19.01696. [DOI] [PubMed] [Google Scholar]
- 11.Handforth C, D'Oronzo S, Coleman R, Brown J. Cancer treatment and bone health. Calcif Tissue Int. 2018;102:251–264. doi: 10.1007/s00223-017-0369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morote J, Morin JP, Orsola A, Abascal JM, Salvador C, Trilla E, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–504. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, 3rd, Lieber MM, Atkinson EJ, Achenbach SJ, Zincke H, Therneau TM, et al. Fracture risk in men with prostate cancer: a population-based study. J Bone Miner Res. 2011;26:1808–1815. doi: 10.1002/jbmr.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A M El Badri S, Salawu A, Brown JE. Bone health in men with prostate cancer: review article. Curr Osteoporos Rep. 2019;17:527–537. doi: 10.1007/s11914-019-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39:203–209. doi: 10.1093/ageing/afp221. [DOI] [PubMed] [Google Scholar]
- 16.Van Hemelrijck M, Garmo H, Michaëlsson K, Thorstenson A, Akre O, Stattin P, et al. Mortality following hip fracture in men with prostate cancer. PLoS One. 2013;8:e74492. doi: 10.1371/journal.pone.0074492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamad NV, Soelaiman IN, Chin KY. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317–1324. doi: 10.2147/CIA.S115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajaram P, Rivera A, Muthima K, Olveda N, Muchalski H, Chen QH. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi: 10.3390/molecules25102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan C, Wefel JS, Morgans AK. A review of prostate cancer treatment impact on the CNS and cognitive function. Prostate Cancer Prostatic Dis. 2020;23:207–219. doi: 10.1038/s41391-019-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cianferotti L, Bertoldo F, Carini M, Kanis JA, Lapini A, Longo N, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the International Osteoporosis Foundation. Oncotarget. 2017;8:75646–75663. doi: 10.18632/oncotarget.17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellis AE, Papatsoris AG. Perspectives on the current and emerging chemical androgen receptor antagonists for the treatment of prostate cancer. Expert Opin Pharmacother. 2019;20:163–172. doi: 10.1080/14656566.2018.1548611. [DOI] [PubMed] [Google Scholar]
- 22.Hussain A, Tripathi A, Pieczonka C, Cope D, McNatty A, Logothetis C, et al. Bone health effects of androgen-deprivation therapy and androgen receptor inhibitors in patients with nonmetastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:290–300. doi: 10.1038/s41391-020-00296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liede A, Arellano J, Hechmati G, Bennett B, Wong S. International prevalence of nonmetastatic (M0) castration-resistant prostate cancer (CRPC) J Clin Oncol. 2013;31(15 Suppl):e16052 [Google Scholar]
- 24.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 25.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 27.Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 28.Boyle HJ, Alibhai S, Decoster L, Efstathiou E, Fizazi K, Mottet N, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116–136. doi: 10.1016/j.ejca.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Banu J. Causes, consequences, and treatment of osteoporosis in men. Drug Des Devel Ther. 2013;7:849–860. doi: 10.2147/DDDT.S46101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackie R. Diagnosis, assessment and management of osteoporosis. Prescriber. 2020;31:14–19. [Google Scholar]
- 31.FRAX®: fracture risk assessment tool [Internet] Sheffield: University of Sheffield; [cited 2020 Aug 8]. Available from: https://www.sheffield.ac.uk/FRAX/tool.aspx?country=4 . [Google Scholar]
- 32.Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, McCloskey EV Advisory Board of the National Osteoporosis Guideline Group. A systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11:25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briot K, Paccou J, Beuzeboc P, Bonneterre J, Bouvard B, Confavreux CB, et al. French recommendations for osteoporosis prevention and treatment in patients with prostate cancer treated by androgen deprivation. Joint Bone Spine. 2019;86:21–28. doi: 10.1016/j.jbspin.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Lewiecki EM. In: Endotext. Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. South Dartmouth (MA): MDText.com, Inc.; 2000. Osteoporosis: clinical evaluation. [Google Scholar]
- 35.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology (NCCN guidelines®). Prostate cancer. Version 2.2020 [Internet] Plymouth Meeting (PA): NCCN; c2020. [cited 2020 Aug 8]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [Google Scholar]
- 36.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 37.Dalla Volta A, Mazziotti G, Maffezzoni F, Grisanti S, Palumbo C, Pedersini R, et al. Bone mineral density and FRAX score may not predict fracture risk in patients with cancer undergoing hormone deprivation therapies. J Clin Oncol. 2020;38:3363–3366. doi: 10.1200/JCO.20.00434. [DOI] [PubMed] [Google Scholar]
- 38.Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63:464–474. doi: 10.1373/clinchem.2016.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (WHO) Global recommendations on physical activity for health [Internet] Geneva: WHO; c2010. [cited 2020 Sep 18]. Available from: https://apps.who.int/iris/handle/10665/44399 . [Google Scholar]
- 40.Cormick G, Belizán JM. Calcium intake and health. Nutrients. 2019;11:1606. doi: 10.3390/nu11071606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Osteoporosis Foundation (IOF) Calcium [Internet] Nyon: IOF; [cited 2020 Sep 18]. Available from: https://www.iofbonehealth.org/calcium-calculator. [Google Scholar]
- 42.SEIOMM. Herramientas [Internet] Madrid: Seiomm; c2016. [cited 2020 Oct 8]. Available from: https://seiomm.org/herramientas/ [Google Scholar]
- 43.Chauhan K, Shahrokhi M, Huecker MR. In: StatPearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Aeby TC, et al., editors. Treasure Island (FL): StatPearls Publishing; 2021. Vitamin D. [Google Scholar]
- 44.Sosa Henríquez M, Gómez de Tejada Romero MJ. Cholecalciferol or calcifediol in the management of vitamin D deficiency. Nutrients. 2020;12:1617. doi: 10.3390/nu12061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amgen. Prolia® (denosumab): injection, for subcutaneous use [Internet] Silver Spring (MD): U.S. Food and Drug Administration; c2020. [cited 2020 Oct 30]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125320s205lbl.pdf. [Google Scholar]
- 46.European Medicines Agency (EMA) Prolia 60 mg solution for injection in pre-filled syringe [Internet] Amsterdam: EMA; [cited 2020 Oct 30]. Available from: https://www.ema.europa.eu/en/documents/product-information/prolia-epar-product-information_en.pdf. [Google Scholar]
- 47.Novartis. Reclast® (zoledronic acid) injection [Internet] Silver Spring (MD): U.S. Food and Drug Administration; c2020. [cited 2020 Oct 30]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021817s028lbl.pdf. [Google Scholar]
- 48.European Medicines Agency (EMA) Aclasta 5 mg solution for infusion [Internet] Amsterdam: EMA; [cited 2020 Oct 30]. Available from: https://www.ema.europa.eu/en/documents/product-information/aclasta-epar-product-information_en.pdf. [Google Scholar]
- 49.Ministerio de Sanidad. Ficha técnica: actonel semanal 35 mg comprimidos recubiertos con película [Internet] Madrid: Ministerio de Sanidad; [cited 2020 Sep 18]. Available from: https://cima.aemps.es/cima/pdfs/es/ft/65167/FT_65167.pdf. Spanish. [Google Scholar]
- 50.Poon Y, Pechlivanoglou P, Alibhai SMH, Naimark D, Hoch JS, Papadimitropoulos E, et al. Systematic review and network meta-analysis on the relative efficacy of osteoporotic medications: men with prostate cancer on continuous androgen-deprivation therapy to reduce risk of fragility fractures. BJU Int. 2018;121:17–28. doi: 10.1111/bju.14015. [DOI] [PubMed] [Google Scholar]
- 51.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T. A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res. 2020;35:1207–1215. doi: 10.1002/jbmr.3962. [DOI] [PubMed] [Google Scholar]
- 53.Yarom N, Shapiro CL, Peterson DE, Van Poznak CH, Bohlke K, Ruggiero SL, et al. Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. J Clin Oncol. 2019;37:2270–2290. doi: 10.1200/JCO.19.01186. [DOI] [PubMed] [Google Scholar]