Abstract
Purpose
Male ageing is often associated with defective sperm DNA remodeling mechanisms that result in poorly packaged chromatin and a decreased ability to repair DNA strand breaks. However, the impact of advanced paternal age on DNA fragmentation remains inconclusive. The aim of the present systematic review was to investigate the impact of advancing paternal age (APA) on DNA fragmentation.
Materials and Methods
We conducted a thorough search of listed publications in Scopus, PubMed, and EMBASE, in accordance with the PRISMA guidelines.
Results
We identified 3,120 articles, of which nineteen were selected for qualitative analysis, resulting in a sample of 40,668 men. Of the 19 articles evaluating the impact of APA on DFI% (DNA fragmentation Index) included, 4 were on Normozoospermic and subfertile men, 3 on normozoospermic, Oligoasthenoteratozoospermic and Teratozoospermic, 6 on fertile and infertile men, 4 on just infertile men, and 2 evaluated a general population. Seventeen of the ninrnteen studies demonstrated APA's effect and impact on DFI%.
Conclusions
Although there was no universal definition for APA, the present review suggests that older age is associated with increased DFI. In elderly men with normal semen parameters, further studies should be performed to assess the clinical implications of DFI, as a conventional semen analysis can often fail to detect an etiology for infertility.
Keywords: Aging, DNA fragmentation, Paternal age, Sperm parameters
INTRODUCTION
The average age at which couples first reproduce has increased significantly in recent decades, with the mean age now at around 30 years in many countries [1,2,3]. Since the 1980s, United States birth rates have increased 40% for men 35 to 49 years old and have subsequently decreased 20% for men less than 30 years old [2]. Increased life expectancy, modern societal expectations pressures, and advanced age of marriage has resulted in the tendency for couples to delay parenthood. The increased accessibility to assisted reproductive technology (ART) has increased the chance of older couples to conceive children, hence increasing the average paternal age at first childbirth. While increasing maternal age is well established as a risk factor for adverse reproductive outcome and offspring fitness, the influence of paternal age on sperm parameters and fecundity is unclear [4,5].
There's a preponderance of evidence reporting an age-related decline in semen volume, motility, and proportion of morphologically normal sperm [3,6,7,8,9,10,11,12,13]. Additionally, compared with fertile men, infertile men exhibit poor sperm chromatin integrity and in vivo fertilizing capability. One study which examined 277 normozoospermic men identified a significantly higher DNA fragmentation Index (DFI) percentage in older (>40 years) men compared to that of younger men [14]. Conversely, Winkle et al [15], found no significant associations with male age, DNA fragmentation, and semen parameters. The mechanisms responsible for age-dependent patterns of DNA fragmentation are not fully understood, but oxidative stress and inefficient apoptosis are thought to be important contributors [16,17].
One intrinsic difficulty with attempting to summarize data for advanced paternal age (APA) is that there is no clearly accepted universal definition of APA. Given that the current population mean for paternal age is 27, the most frequently utilized criterion for APA is more than 40 years of age [18]. While the general consensus is that APA tends to be associated with a decline in semen quality, as well as an increase in DNA fragmentation [10,16], the suggested data appears to be mixed. Further complicating the study is the lack of a universally accepted definition for an abnormal DNA fragmentation threshold, and standard assays. Therefore, we carried out a systematic review to better elucidate the effect of APA on DNA fragmentation by evaluating both age and DNA fragmentation as continuous variables, rather than using cut-offs. Utilizing data from 19 articles (40,668 subjects) we conducted a systematic review to summarize the impact of increasing paternal age on DNA fragmentation.
MATERIALS AND METHODS
1. Methods
A prospective systematic literature review, inclusion and exclusion criteria, and outcome measurement were prepared a priori according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Supplement File) [19]. This systematic review was accepted in the International Prospective Register of Systematic Reviews (CRD42020191371) before the commencement of the study, which ensured the transparency of the review process and originality of this study.
2. Data sources and search strategy
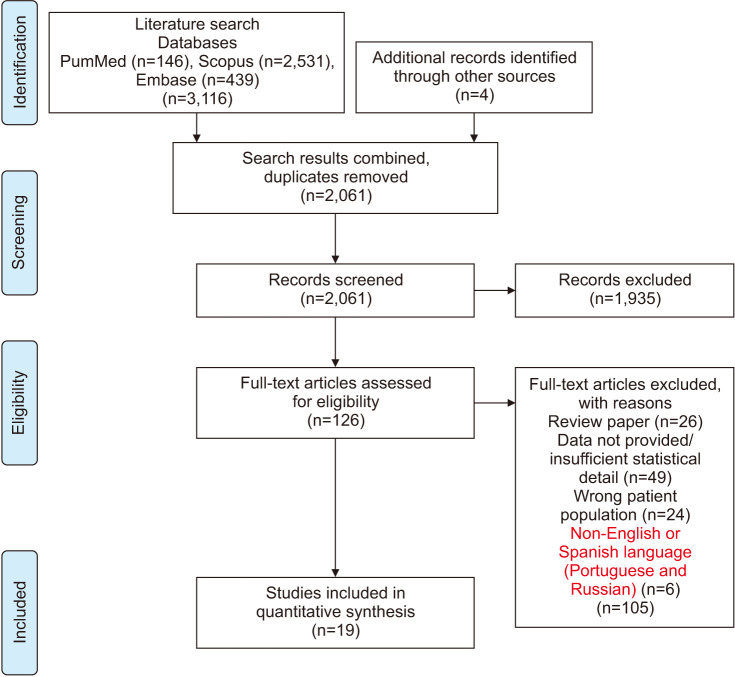
A systematic search of Scopus, PubMed, and Embase electronic databases was conducted to identify the relevant studies from inception up until March 2020. We conducted the search by using the search string in [All field] setting: “((((((age OR aging)) AND ((sperm OR semen))) AND ((male))) AND ((fertility OR fertile))) AND ((DFI OR DNA fragmentation))”. Additional bibliography lists of retrieved original articles and review papers were manually searched for additional relevant references. We utilized the preferred reporting items for systematic review and meta-analysis checklist (PRISMA) while conducting this study (Fig. 1).
Fig. 1. Flow diagram of search and selection strategy in a systematic review on the impact of advancing paternal age on sperm DNA fragmentation Index.
3. Study selection: inclusion and exclusion criteria
Inclusion criteria were original research articles in English and Spanish language addressing the relationship between paternal age, semen parameters, and DNA fragmentation. The search was restricted to studies in humans. Considering the type of participants, studies that assessed DNA fragmentation in fertile men with normal semen analysis in addition to men with a diagnosis of infertility or subfertility were considered, from oligozoospermia (when total sperm count was less than 15 million per mL) to severe oligospermia (when total sperm count was below 5 million per mL). Men who had an underlying varicocele or conditions such as diabetes and obesity were included. This analysis included prospective or retrospective comparative studies which examined the association between age and DNA fragmentation as measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labelling (TUNEL), the sperm chromatin structure assay (SCSA), the single-cell gel electrophoresis (Comet) assay, and the sperm chromatin dispersion (SCD) test.
Azoospermic men were not considered eligible and reports including testicular DNA fragmentation were excluded. Articles that were not in English or Spanish language were excluded. Due to the influence of the abstinence period on DNA fragmentation, we excluded articles that did not clearly state the abstinence period. Men who underwent interventions (e.g., chemotherapy, radiotherapy, antioxidants, etc.) were excluded from the analysis. Men who were diagnosed with a malignancy and performed a semen analysis before treatment were excluded. Additionally, case reports, editorials, review articles, articles for which the full text could not be found, animal experimental studies, and articles for which the data were not extractable were excluded. Lastly, articles studying the influence of factors such as senescence, environmental pollutants, and cryptorchidism were excluded.
4. Data extraction
Two researchers (D.G and J.B) performed the systematic review and independently extracted data from all selected articles. The opinion of a third observer (J.O) was sought to gain consensus, in the event of any discordance on selecting studies. Extracted data included on study design, publication year, population characteristics, inclusion and exclusion criteria, population, DNA fragmentation assay, main outcomes and conclusions, adjusted results, and statistical methods. The primary outcome of the study was DNA fragmentation evaluation, and volume, concentration, motility, progressive motility, and vitality were considered secondary outcomes.
5. Quality assessment
Each study was scored for their relevance and methodological quality by using the QUADAS 2 (Quality Assessment of Diagnostic Accuracy Studies 2) checklist [20]. Furthermore the following characteristics of the studies were taken into consideration: study population and DNA fragmentation assay.
RESULTS
1. Eligible studies of systematic review
The systematic search retrieved a total of 3,120 articles: 3,116 were identified utilizing the search strategy and four additional articles were identified by manually searching relevant references. After removing duplicates, we were left with 2,061 potentially relevant articles (Fig. 1). The screening for study inclusion was performed in two stages: titles and abstracts were screened in the first stage, and full manuscripts of the articles identified as relevant in the initial screening were retrieved and read in detail for the second stage. After first stage screening, 1,935 articles were excluded, and 126 articles were identified to assess the full text for eligibility. After this second stage of screening, 105 articles were excluded for reasons shown in (Supplement Table).
2. Characteristics of included studies and comparison of outcomes
The main characteristics of the present systematic review included 19 studies as summarized in Table 1. There were four studies that examined the impact of APA on DNA fragmentation between Normozoospermic and subfertile males [21,22,23,24]. Three of the four studies showed a significant difference (p<0.01) in favor of APA increasing DNA fragmentation, even among normozoospermic males [21,22,24]. All six studies examining DNA fragmentation between fertile and infertile males with proven primary or secondary infertility, which reported a significant difference in favor of increasing DNA fragmentation with APA [25,26,27,28,29,30]. Four studies examined males with proven primary or secondary infertility [31,32,33,34]. Two studies examined the effect of APA on DNA fragmentation within a general population of healthy males [35,36]. Two studies examined DNA fragmentation between normozoospermic and oligoasthenoteratozoospermic males [37,38] and one study examined teratozoospermic males [39]. Six of the nineteen studies showed a significant difference (p<0.05) in favor of APA decreasing sperm motility [22,24,27,29,34,35]. Moreover, in this review three studies examined and noted the incidence of varicoceles, which there was no demonstrated effect of varicocele presence on DNA fragmentation, irrespective of APA [25,31,33]. Five studies showed a significant difference in favor of APA decreasing semen volume [23,34,35,38,39] and two studies demonstrating APA decreasing concentration [34,38]. Out of the 19 studies, 2 failed to show an association with APA and DNA fragmentation [23,32].
Table 1. Characteristics of studies included into systematic review.
| Reference (author, year, nationality) | Population characteristic | Sample size | Age range (y) | Sperm DFI % | Method used | Volume (mL) | Concentration (×106/mL) | % Motility | Progressive motility (%) | Vitality (%) | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colasante et al, 2019, Brazil [21] | NORMO and subfertile | 3,124 | 5 IQR (2–10) | TUNEL | 3±1 | 60±10 | |||||
| 775 | <35 | r=-0.17a | - | - | - | - | - | p<0.001 | |||
| 1,110 | 36–40 | r=-0.16a | - | - | - | - | - | p<0.001 | |||
| 1,239 | >41 | r=-0.18a | - | - | - | - | - | p<0.001 | |||
| Moskovtsev et al, 2006, USA [22] | NORMO and subfertile | 1,125 | SCSA | - | - | ||||||
| 57 | <30 | 15.2±8.4a | 3.2±1.6 | 55.5±49.2 | 35.3±13.7a | - | 76.7±7.9a | ap<0.001when compared to >45 | |||
| 386 | 30–34 | 19.4±12.1a | 3.3±1.5 | 57.6±57.4 | 33.5±17.1a | - | 71.7±11.5a | ||||
| 406 | 35–40 | 20.1±10.9a | 3.2±1.5 | 60.7±59.4 | 33.4±16.7a | - | 71.5±11.9a | ||||
| 187 | 40–44 | 26.4±16.0a | 2.9±1.4 | 61.9±62.1 | 29.8±17.7 | - | 65.5±16.8 | ||||
| 89 | >45 | 32.0±17.1 | 2.9±1.8 | 65.5±65.7 | 24.9±15.3 | - | 62.5±14.6 | ||||
| Brahem et al, 2011, Tunisia [23] | NORMO and subfertile | 190 | TUNEL | ||||||||
| 10 | 20–29 NORMO | 10.5±1.4 | 2.8±0.9 | 95.7±22.9 | 53.7±4.4 | - | - | NS | |||
| 16 | 30–39 NORMO | 10.3±4.3 | 2.8±0.9 | 93.0±29.4 | 51.3±6.8 | - | - | r=-0.094 | |||
| 14 | 40–49 NORMO | 9.9±4.0 | 2.4±0.3b | 100±46.2 | 48.1±5.1 | - | - | p=0.516 | |||
| 10 | 50–70 NORMO | 9.0±5.6 | 2.0±0.1b | 99.1±31.3 | 47.5±3.5 | - | - | ||||
| 11 | 20–29 Subfertile |
26.2±13.4 | 3.2±1.6 3.3±1.5 |
62.0±40.9 | 26.3±17.0 | - | - | NS r=0.08 p>0.05 |
|||
| 79 | 30–39 Subfertile | 27.6±15.8 | 3.3±1.5 | 76.0±59.8 | 29.0±15.8 | - | - | p>0.05 | |||
| 40 | 40–49 Subfertile | 30.4±16.8 | 2.0±1.3b | 97.9±86.1 | 25.5±14.2 | - | - | ||||
| 10 | 50–70 Subfertile | 31.6±18.0 | 2.4±1.1 | 98.1±61.3 | 22.0±17.4 | - | - | ||||
| Guo et al, 2020, China [24] | NORMO and subfertile | 654 | SCD | ||||||||
| 71 | <30 NORMO | 13.0±7.4 | - | 75.7±42.9 | 68.5±11.6 | 61.7±10.9 | - | p=0.006 DFI & age for NORMO | |||
| 83 | 30–35 NORMO | 14.0±8.6 | 81.0±51.3 | 63.3±15.5 | 56.6±14.3 | - | |||||
| 71 | >35 NORMO | 17.5±10.2a | - | 82.2±44.3 | 62.5±12.6a | 55.6±11.7 | - | ||||
| 135 | <30 Subfertile | 14.5±11.4 | 58.3±51.4 | 52.8±21.6 | 46.9±20.0 | - | p=0.001 DFI & age for subfertile | ||||
| 124 | 30–35 Subfertile | 16.0±11.0 | - | 58.6±46.5 | 47.0±21.2 | 41.0±19.8a | - | ||||
| 170 | >35 Subfertile | 19.8±13.9a | - | 65.5±64.2 | 45.7±20.5a | 39.5±18.8a | - | ||||
| Petersen et al, 2018, Brazil [25] | Fertile & infertile | 2,178 | TUNEL | 2.7±1.4 | 74.6±61.6 | 63.7±15.7 | 56.9±16.2 | 65.0±14.5 | |||
| 852 | <35 | 14.7±8.3a | - | - | - | - | - | r=0.10 | |||
| 1,014 | 36–44 | 15.9±8.7 | - | - | - | - | - | p=0.002 | |||
| 312 | >45 | 16.2±8.4a | - | - | - | - | - | ||||
| Kaarouch et al, 2018, Morocco [26] | Fertile & infertile | 83 | TUNEL | ||||||||
| 42 | <40 | 25 | 2.6±1.1 | 24.3±2.9 | 33 | - | 60 | p<0.05 | |||
| 41 | >40 | 41b | 2.4±1.5 | 18.4±2.6 | 26 | - | 55 | ||||
| Cohen-Bacrie et al, 2009, France [27] | Fertile & infertile | 1,653 | TUNEL | ||||||||
| 688 | 36.6±6.1 | 0–20 | - | - | 32.6±49.6 | 32.3±20.7 | 74.3±13.0 | p=0.001 | |||
| 463 | 37.1±6.4 | 20–30 | - | - | 30.0±52.0 | 28.6±18.4 | 71.5±12.5 | ||||
| 286 | 38.2±6.8 | 30–40 | - | - | 23.7±40.4a | 25.3±16.8 | 68.9±12.8 | ||||
| 216 | 39.3±9.6 | >40 | - | - | 16.6±28.5a | 21.3±16.9 | 62.3±14.8 | ||||
| Evenson et al, 2020, USA [28] | Fertile & infertile | 25,262 | SCSA | - | - | - | |||||
| 2,000 | 20–29 | 12.6±9.7 | - | - | - | - | - | ||||
| 14,000 | 30–39 | 15.2±11.3 | - | - | - | - | - | ||||
| 8,000 | 40–49 | 19.5±14.0 | - | - | - | - | - | ||||
| 1,000 | 50–59 | 26.8±17.4 | - | - | - | - | - | ||||
| 262 | 60–80 | 39.3±21.7 | - | - | - | - | - | ||||
| Antonouli et al, 2019, Germany [29] | Fertile & infertile | 150 | 43.4±5.5 | r=0.23b p=0.046 | SCD | 49.5 r=0.01 p>0.05 |
54.9+19.2a r=-0.29 p=0.012 |
32.6±17.3b | - | ||
| Blachman-Braun et al, 2020, USA [30] | Fertile & infertile | 550 | 37.7±6.5 | 12.7 [7.8–20] | SCSA | 3.0±1.4 | 43.8±36.3 | 47.79±10.5 | p≤0.001 DFI & age |
||
| 38 | < 30 | 11.9±7.9 | 3.3±1.3 | 34.5±33.7 | 46.3±19.8 | - | - | ||||
| 348 | 30–40 | 13.8±9.4 | 3.2±1.5 | 44.4±41.6 | 49.2±18.3 | - | - | ||||
| 145 | 40–50 | 21.2±16.5a | 2.6±1.2 | 46.9±38.4 | 43.9±19.6 | - | - | ||||
| 19 | >50 | 29.5±16a | 2.4±1.3 | 37.5±30 | 39.3±19.9 | - | - | ||||
| Alshahrani et al, 2014, USA [31] | Infertile | 839 | 19.9 ± 15.3 | TUNEL | 3.1 ± 1.5 | - | 44.7 ± 19.7 | - | - | ||
| 69 | <30 | 16.7 ± 11.2 | 3.4 ± 1.5 | 42.0±50.5 | 44.3 ± 14.4 | - | - | p<0.005 when >40 compared to all groups | |||
| 298 | 31–40 | 19.1 ± 14.6 | 3 ± 1.4 | 36.6±39.7 | 45.2 ± 19.5 | - | - | ||||
| 367 | <40 | 18.7 ± 14. 1 | 3.1 ± 1.7 | 42.6±41.5 | 45.0 ± 18.6 | - | - | ||||
| 105 | >40 | 24.4 ± 18.5b | 3.1 ± 1.7 | 43.8±52.3 | 43.5 ± 22.9 | - | - | ||||
| Nijs et al, 2011, Belgium [32] | Infertile | 278 | NS | ||||||||
| 135 | <34 | 22.4±11.8 | 40.7±35.8 | 52.5±18.8 | - | - | r=-0.006 p=0.95 |
||||
| 96 | 35–39 | 23.35±12.3 | 40.7±34.2 | 50.2±16.9 | - | - | r=0.027 p=0.79 |
||||
| 47 | >40 | 24.5±12.6 | 41.5±31.7 | 57.38±13.0 | - | - | r=-0.149 p=0.32 |
||||
| Vagnini et al, 2007, Brazil [33] | Infertile | 508 | TUNEL | ||||||||
| 186 | <35 | 15.7±10.7b | - | - | - | - | - | p=0.034 | |||
| 140 | 36–39 | 18.2±11.3b | - | - | - | - | - | p=0.022 | |||
| 182 | >40 | 18.3±11.0 | - | - | - | - | - | p=0.93 | |||
| Lu et al, 2018, China [34] | Infertile | 1,010 | SCSA | 45.39±19.2 | |||||||
| r=0.115 p<0.001a OR: 0.865 (95% CI, 0.785–0.954) p=0.004 | r=0.145 p<0.001a OR: 0.548 (95% CI, 0.39–0.77) p=0.001 | r=-0.115 p<0.001a OR: 0.978 (95% CI, 0.962–0.994) p=0.008 | r=-0.487 p<0.001 OR: 0.985 (95% CI, 0.907–1.609) p=0.713 | 32.29±12.9 | - | ||||||
| Pino et al, 2020, Chile, [35] | General public | 2,678 | SCD | ||||||||
| 119 | 21–30 | OR: 1 | p=0.029 DFI & men over 50 | ||||||||
| 1,579 | 31–40 | OR: 1.243 (95% CI, 0.364–4.240); p=0.728 | OR: 0.821 (95% CI, 0.464–1.450); p=497 | OR: 0.987 (95% CI, 0.576–1.690); | - | OR: 3.241b (95% CI, 1.175–8.940); p=0.023 |
- | ||||
| 852 | 41–50 | OR: 1.38 (95% CI, 0.39–4.82); p=0.606 | OR: 1.332 (95% CI, 0.749–2.369); p=0.328 | OR: 1.188 (95% CI, 0.685–2.060) | - | OR: 5.243a (95% CI, 1.892–14.526); p=0.001 | - | ||||
| 128 | >50 | OR: 4.58b (95% CI, 1.167–17.99) | OR: 2.20 (95% CI, 1.11–4.34); p=0.022 | OR:1.188a (95% CI, 0.685–2.060) | - | OR: 11.911a (95% CI, 4.045–35.073); p<0.0001 | - | ||||
| Wyrobek et al, 2006, USA [36] | General public | 88 | Comet SCSA | ||||||||
| 19 | 20–29 | 12.9±7.7 | - | - | - | - | - | r=0.72 | |||
| 20 | 30–39 | 16.3±9.6 | - | - | - | - | - | p<0.001 | |||
| 16 | 40–49 | 23.2±14.9a | - | - | - | - | - | ||||
| 17 | 50–59 | 35.4±18.6a | - | - | - | - | - | ||||
| 16 | 60–80 | 49.6±17.3a | - | - | - | - | - | ||||
| Das et al, 2013, Canada [37] | NORMO and OAT | 277 | SCSA | ||||||||
| 107 | <40 NORMO | 12±8 | - | 95±68 | - | 61±14 | - | p=0.008 | |||
| 41 | >40 NORMO | 17±13a | - | 99±58 | - | 58±17 | - | ||||
| 97 | <40 OAT | 12±10 | - | 35±37 | - | 30±16 | - | p=0.003 | |||
| 32 | >40 OAT | 20±18a | - | 33±37 | - | 24±14 | - | ||||
| Plastira et al, 2007, Greece [38] | NORMO and OAT | 110 | TUNEL | ||||||||
| 26 | 24–34 NORMO | 6.5±1.9 | 3.3±0.7 | 51.0±22.4 | 56.5±4.4 | - | - | r=-0.105 p=0.472 |
|||
| 23 | 35–54 NORMO | 5.9±1.7 | 3.4±0.8 | 42.0±14.0 | 54.7±4.1a | - | - | r=-0.206 p=0.155 |
|||
| 30 | 24–34 OAT | 26.3±5.3a | 3.2±1.0 | 3.3±2.3 | 23.9±9.6 | - | - | r=0.558 p<0.001 |
|||
| 31 | 35–54 OAT | 33.7±6.7b | 2.6±1.0a | 5.0±2.7 | 19.2±8.0 | - | - | r=-0.294 p=0.022 |
|||
| Rosiak-Gill et al, 2019, Poland [39] | NORMO and teratoozospermic | 336 | TUNEL | ||||||||
| 116 | <40 NORMO | 12.7±9.4 | 3.8±1.7 | 68.0±50.5 | 52.4±12.9 | - | - | p=0.005 | |||
| 44 | >40 NORMO | 17.6±10.4a | 3.3±1.8a | 72.4±46.3 | 56.2±13.6 | - | - | ||||
| 132 | <40 Teratoozo-spermic | 13.9±10.9 | 3.3±1.5 | 32.6±32.7 | 30.3±18.5 | - | - | p=0.0010 | |||
| 44 | >40 Teratoozo-spermic | 21.8±15.1a | 2.9±1.6a | 38.6±42.1 | 31.6±20.4 | - | - |
Values are presented as number only, mean±standard deviation, or median [interquartile range].
NORMO: normozoospermia, IQR: interquartile range, TUNEL: terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labelling, SCSA: sperm chromatin structure assay, NS: not significant, SCD: sperm chromatin dispersion test, DFI: DNA fragmentation Index, OR: odds ratio, CI: confidence interval, Comet: the single cell-gel electrophoresis assay, OAT: Oligoasthenoteratozoospermic.
ap<0.001, bp<0.05.
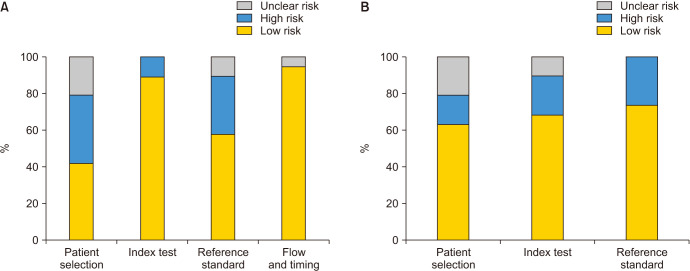
Fig. 2 and Table 2 show the scores on overall risk of bias and concerns regarding applicability in this systematic review according to QUADAS 2. For about half of the studies the patient population examined a mix of infertile and fertile patients and hence was judged to be at “high risk” of bias for QUADAS 2 domain “patient selection”. Studies were at high risk of applicability concerns in domain “reference standard” when the patient's age category threshold is not comparable to the thresholds of other studies. Overall, the domain “index test” was considered to be at “low risk” because there were 10 studies that utilized TUNEL assay [21,23,25,26,27,31,32,33,38,39], 6 studies utilizing SCSA [22,28,30,34,35,37], 1 study using Comet assay [36], and 2 studies that utilized the SCD to quantify DNA fragmentation [24,29].
Fig. 2. Overall risk of bias in systematic review. This figure illustrates the overall risk of bias in the systematic review. (A) Proportion of studies with low, high, or unclear risk of bias (%). (B) Proportion of studies with low, high, or unclear concerns regarding applicability (%).The vertical axis represents the number of studies included. The color of the bars represents the risk of bias.
Table 2. Study characteristics according to QUADAS II recommendations to report the risk of bias for patient selection and the concerns for applicability of data collected in manuscripts eligible for the systematic review.
| Reference (author, year) | Risk of Bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection Index | Index test | Reference standard | |
| Colasante et al, 2019 [21] | Low | Low | High | Low | Low | Unclear | High |
| Moskovtsev et al, 2006 [22] | Low | Low | Low | Low | Low | Low | Low |
| Brahem et al, 2011 [23] | Low | Low | Low | Low | Low | Low | Low |
| Guo et al, 2020 [24] | Low | Low | Low | Low | Low | Low | Low |
| Petersen et al, 2018 [25] | Unclear | Low | Low | Unclear | Unclear | Unclear | Low |
| Kaarouch et al, 2018 [26] | Unclear | High | Low | Low | Unclear | Low | Low |
| Cohen-Bacrie et al, 2009 [27] | Low | Low | High | Low | Low | Low | High |
| Evenson et al, 2020 [28] | Unclear | Low | Low | Low | Unclear | Low | Low |
| Antonouli et al, 2019 [29] | Unclear | Low | High | Low | Unclear | Low | High |
| Blachman-Braun et al, 2020 [30] | Low | Low | Low | Low | Low | Low | Low |
| Alshahrani et al, 2014 [31] | High | Low | Low | Low | Low | Low | Low |
| Nijs et al, 2011 [32] | High | Low | Low | Low | Low | Low | Low |
| Vagnini et al, 2007 [33] | High | Low | Low | Low | High | Low | Low |
| Lu et al, 2018 [34] | High | Low | Unclear | Low | High | Low | Low |
| Pino et al, 2020 [35] | Low | Low | Unclear | Low | High | Low | Low |
| Wyrobek et al, 2006 [36] | Low | High/lowa | Low | Low | Low | High/lowa | Low |
| Das et al, 2013 [37] | High | High | High | Low | Low | High | High |
| Plastira et al, 2007 [38] | High | High | High | Low | Low | High | High |
| Rosiak-Gill et al, 2019 [39] | High | High | High | Low | Low | High | Low |
QUADAS: Quality Assessment of Diagnostic Accuracy Studies.
aHigh risk for Comet (the single cell gel electrophoresis assay), low risk for sperm chromatin structure assay (SCSA).
DISCUSSION
The effect of paternal age on semen quality and DNA fragmentation remains controversial. We hypothesized that APA would be associated with an increase in DNA fragmentation. We performed a systematic review comparing DNA fragmentation in different age groups among normozoospermic, subfertile, and infertile men. Our review included data on 40,668 subjects extracted from nineteen available published articles. In the majority of the articles assessed, (17/19) APA was associated with significant increase in DNA fragmentation. Conversely, two articles demonstrated that APA did not influence DNA fragmentation [23,32]. The overall quality of papers demonstrating an association were overall higher than the papers showing no association. Overall, it appears that the majority of articles utilizing SCSA and SCD assays reliably showed an association with APA and increased DNA fragmentation.
The implications of this systematic review are important because the average age of men having children has increased [2]. DNA fragmentation is not a part of a standard infertility workup and in the presence of a normal semen analysis, often no further workup is done [40,41]. However, if DNA fragmentation rates truly do increase as men age, perhaps clinicians should increase their threshold to consider this test in the older men with infertility. In addition, couples pursuing in vitro fertilization (IVF) are typically in an older age bracket, and there are several interventions which can improve DNA fragmentation and may improve IVF outcomes in men with high DNA fragmentation [42,43].
In this review, both studies that did not find an effect of APA on DNA fragmentation utilized the TUNEL assay. Several assays are currently available to assess DNA fragmentation, and these assays can be broadly categorized into two types. The first category includes assays where DNA fragmentation is quantified directly by incorporating probes at the site of damage, which detect actual DNA strand breaks. TUNEL, in situ nick translation (ISNT), and Comet assay belong to this category. Conversely, the second category includes assays such as SCD test and SCSA, which utilize the property of fragmented DNA to aid denaturation under certain conditions [44]. SCD is based on the ability of intact DNA deprived of chromatin proteins to loop around the lysed and acid treated sperm nuclear membrane carcass, thus indirectly measuring the susceptibility of DNA to denaturation. Probe incorporation in TUNEL depends on the amount of chromatin that is partially freed from the proteins protecting the DNA, thus it is possible that existing breaks are not detected due to chromatin compaction.
With all systematic reviews, there are limitations that should be taken into consideration. An important weakness of most studies relating to DNA fragmentation and paternal age is that the patient populations are highly selective (i.e., infertile men). The vast majority of studies were retrospective, and therefore the possibility of confounding variables influencing the results cannot be ruled out. It is important to mention that when investigating a paternal age effect on DNA fragmentation, there may be a residual confounding by the presence of varicoceles [45]. Factors such as infertility duration, varicocele, and environmental factors were not reported in several studies. Despite these limitations, our review included data from >40,000 males, and to our knowledge, represents the first formal attempt to thoroughly assess the available data on effects of age in males attending an infertility clinic. Similarly reported by Johnson et al. [10], this review found that only two studies demonstrated the effect of APA on declining sperm concentration, while a majority of studies (6/19) demonstrated APA decreasing semen volume. This review and Johnson et al.'s review [10] supports the association of APA with DNA fragmentation. This review is unique in that we recorded the method of measurement, and were able to determine how direct vs indirect assays supported the association of APA and DNA fragmentation. After systematically collecting the information of published articles, a meta-analysis was not amenable given the heterogeneity of the reports as there was not a not consistent cut-off point defining APA among authors. Although there is no validated cut-off points to define APA based on DNA fragmentation data with fertile and infertile men as well in those with normal and abnormal semen parameters, several authors favor for using >40 years as a cut-off point to refer to APA [14,18,26,31,39]. This definition still needs to be further validated in the context of DNA fragmentation, such an analysis requires making many assumptions about the variation in both age structure and traits across different populations. Despite the limitation of the present study and need of future prospective clinical trials that help validate our observations, we believe that analyzing DNA fragmentation in men with APA starting around the age of 40 years can provide an additional tool to set expectations and counsel couples seeking fertility.
CONCLUSIONS
This study suggests a trend to support the effect of APA on DNA fragmentation. As sperm quality is a pivotal factor in fertility potential and ART outcomes, physicians should consider assessing DNA fragmentation in men around the age of 40 years. Given the significant methodological weakness and design of the included studies, future prospective studies are required to investigate the effects of aging on infertile men with normal semen parameters, as a conventional semen analysis can often fail to detect an underlying etiology for infertility.
ACKNOWLEDGEMENTS
The authors thank Dr. Manuel Molina, University of Miami School of Medicine, for his technical assistance for this study.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: DCG, RR.
- Data curation: DCG, RR, JCB, JO.
- Formal analysis: DCG, RR, JCB, RBB, SN.
- Funding acquisition: None.
- Investigation: DCG, SN.
- Methodology: DCG, RR, RBB.
- Project administration: RR, RBB.
- Resources: RR.
- Software: RBB, SN.
- Supervision: DCG, RR, JCB.
- Validation: DCG, RBB, JO, RR.
- Writing — original draft: DCG, RR, JO, JCB.
- Writing — review & editing: JCB, DCG, JO, RR.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.200195.
List of excluded full-text publications, with reasons
References
- 1.Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible? Fertil Steril. 2013;99:30–36. doi: 10.1016/j.fertnstert.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 3.Kühnert B, Nieschlag E. Reproductive functions of the ageing male. Hum Reprod Update. 2004;10:327–339. doi: 10.1093/humupd/dmh030. [DOI] [PubMed] [Google Scholar]
- 4.Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Bacrie M, De Mouzon J, Hazout A, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008;17:392–397. doi: 10.1016/s1472-6483(10)60223-4. [DOI] [PubMed] [Google Scholar]
- 5.Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod. 2008;23:538–542. doi: 10.1093/humrep/dem431. [DOI] [PubMed] [Google Scholar]
- 6.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 7.Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–1014. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 8.Berling S, Wölner-Hanssen P. No evidence of deteriorating semen quality among men in infertile relationships during the last decade: a study of males from Southern Sweden. Hum Reprod. 1997;12:1002–1005. doi: 10.1093/humrep/12.5.1002. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Lin H, Ma M, Li L, Cai M, Zhou N, et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Hum Reprod. 2009;24:459–469. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- 10.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6022 semen samples. Andrologia. 2007;39:45–50. doi: 10.1111/j.1439-0272.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz D, Mayaux MJ, Spira A, Moscato ML, Jouannet P, Czyglik F, et al. Semen characteristics as a function of age in 833 fertile men. Fertil Steril. 1983;39:530–535. doi: 10.1016/s0015-0282(16)46946-3. [DOI] [PubMed] [Google Scholar]
- 13.Rolf C, Behre HM, Nieschlag E. Reproductive parameters of older compared to younger men of infertile couples. Int J Androl. 1996;19:135–142. doi: 10.1111/j.1365-2605.1996.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 14.Das M, Al-Hathal N, San-Gabriel M, Phillips S, Kadoch IJ, Bissonnette F, et al. Infertile normozoospermic men with advanced paternal age have a high prevalence of sperm DNA damage. Hum Reprod . 2012;27(Suppl 2):ii121–ii150. doi: 10.1007/s10815-013-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkle T, Rosenbusch B, Gagsteiger F, Paiss T, Zoller N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J Assist Reprod Genet. 2009;26:41–46. doi: 10.1007/s10815-008-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online. 2003;7:65–70. doi: 10.1016/s1472-6483(10)61730-0. [DOI] [PubMed] [Google Scholar]
- 18.Toriello HV, Meck JM Professional Practice and Guidelines Committee. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 21.Colasante A, Minasi MG, Scarselli F, Casciani V, Zazzaro V, Ruberti A, et al. The aging male: relationship between male age, sperm quality and sperm DNA damage in an unselected population of 3124 men attending the fertility centre for the first time. Arch Ital Urol Androl. 2019;90:254–259. doi: 10.4081/aiua.2018.4.254. [DOI] [PubMed] [Google Scholar]
- 22.Moskovtsev SI, Willis J, Mullen JB. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril. 2006;85:496–499. doi: 10.1016/j.fertnstert.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 23.Brahem S, Mehdi M, Elghezal H, Saad A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J Assist Reprod Genet. 2011;28:425–432. doi: 10.1007/s10815-011-9537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo LY, Zhou H, Liu M, Li Q, Sun XF. Male age is more critical to sperm DNA integrity than routine semen parameters in Chinese infertile males. Andrologia. 2020;52:e13449. doi: 10.1111/and.13449. [DOI] [PubMed] [Google Scholar]
- 25.Petersen CG, Mauri AL, Vagnini LD, Renzi A, Petersen B, Mattila M, et al. The effects of male age on sperm DNA damage: an evaluation of 2,178 semen samples. JBRA Assist Reprod. 2018;22:323–330. doi: 10.5935/1518-0557.20180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaarouch I, Bouamoud N, Madkour A, Louanjli N, Saadani B, Assou S, et al. Paternal age: negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev. 2018;85:271–280. doi: 10.1002/mrd.22963. [DOI] [PubMed] [Google Scholar]
- 27.Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009;91:1801–1805. doi: 10.1016/j.fertnstert.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 28.Evenson DP, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril. 2020;114:311–320. doi: 10.1016/j.fertnstert.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Antonouli S, Papatheodorou A, Panagiotidis Y, Petousis S, Prapas N, Nottola SA, et al. The impact of sperm DNA fragmentation on ICSI outcome in cases of donated oocytes. Arch Gynecol Obstet. 2019;300:207–215. doi: 10.1007/s00404-019-05133-9. [DOI] [PubMed] [Google Scholar]
- 30.Blachman-Braun R, Best JC, Sandoval V, Lokeshwar SD, Patel P, Kohn T, et al. Sperm DNA fragmentation index and high DNA stainability do not influence pregnancy success after intracytoplasmic sperm injection. F S Rep. 2020;1:233–238. doi: 10.1016/j.xfre.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alshahrani S, Agarwal A, Assidi M, Abuzenadah AM, Durairajanayagam D, Ayaz A, et al. Infertile men older than 40 years are at higher risk of sperm DNA damage. Reprod Biol Endocrinol. 2014;12:103. doi: 10.1186/1477-7827-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijs M, De Jonge C, Cox A, Janssen M, Bosmans E, Ombelet W. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology. Andrologia. 2011;43:174–179. doi: 10.1111/j.1439-0272.2010.01040.x. [DOI] [PubMed] [Google Scholar]
- 33.Vagnini L, Baruffi RL, Mauri AL, Petersen CG, Massaro FC, Pontes A, et al. The effects of male age on sperm DNA damage in an infertile population. Reprod Biomed Online. 2007;15:514–519. doi: 10.1016/s1472-6483(10)60382-3. [DOI] [PubMed] [Google Scholar]
- 34.Lu JC, Jing J, Chen L, Ge YF, Feng RX, Liang YJ, et al. Analysis of human sperm DNA fragmentation index (DFI) related factors: a report of 1010 subfertile men in China. Reprod Biol Endocrinol. 2018;16:23. doi: 10.1186/s12958-018-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pino V, Sanz A, Valdés N, Crosby J, Mackenna A. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist Reprod. 2020;24:82–86. doi: 10.5935/1518-0557.20190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das M, Al-Hathal N, San-Gabriel M, Phillips S, Kadoch IJ, Bissonnette F, et al. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet. 2013;30:843–848. doi: 10.1007/s10815-013-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plastira K, Msaouel P, Angelopoulou R, Zanioti K, Plastiras A, Pothos A, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–443. doi: 10.1007/s10815-007-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosiak-Gill A, Gill K, Jakubik J, Fraczek M, Patorski L, Gaczarzewicz D, et al. Age-related changes in human sperm DNA integrity. Aging (Albany NY) 2019;11:5399–5411. doi: 10.18632/aging.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarow J, Sigman M, Kolettis PN, Lipshultz LR, Dale McClure R, Nangia AK, et al. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011 [Internet] Linthicum (MD): American Urological Association; c2011. [cited 2020 Dec 21]. Available from: https://www.auanet.org/education/guidelines/male-infertility-d.cfm. [Google Scholar]
- 41.Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios? Transl Androl Urol. 2016;5:935–950. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Telli O, Sarici H, Kabar M, Ozgur BC, Resorlu B, Bozkurt S. Does varicocelectomy affect DNA fragmentation in infertile patients? Indian J Urol. 2015;31:116–119. doi: 10.4103/0970-1591.152811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteves SC, Roque M, Garrido N. Use of testicular sperm for intracytoplasmic sperm injection in men with high sperm DNA fragmentation: a SWOT analysis. Asian J Androl. 2018;20:1–8. doi: 10.4103/aja.aja_7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zini A, Libman J. Sperm DNA damage: clinical significance in the era of assisted reproduction. CMAJ. 2006;175:495–500. doi: 10.1503/cmaj.060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enciso M, Muriel L, Fernández JL, Goyanes V, Segrelles E, Marcos M, et al. Infertile men with varicocele show a high relative proportion of sperm cells with intense nuclear damage level, evidenced by the sperm chromatin dispersion test. J Androl. 2006;27:106–111. doi: 10.2164/jandrol.05115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of excluded full-text publications, with reasons