Abstract
Oxidative stress is a condition due to an imbalance between the concentrations of oxidants and antioxidants, and it is a well-recognized contributor in several male infertility conditions. Varicocele, a common vascular condition, may cause male infertility due to hyperthermia, hypoxia and/or exposure to toxic adrenal and renal metabolites. In this review, the mechanisms by which oxidative stress can affect cellular integrity and functions are described, along with molecular markers of cellular oxidative damage, and the most commonly performed techniques for their detection in seminal fluid. Moreover, we focus on the role of oxidative stress in the pathophysiology of varicocele based on recently published evidence from omics based studies, such as proteomics and genomics. Finally, we discuss strategies for the management of oxidative stress and the clinical guidelines for testing oxidative stress-related sperm DNA fragmentation in this group of patients.
Keywords: DNA damage, Genomics, Male infertility, Oxidative stress, Proteomics, Reactive oxygen species
INTRODUCTION
Unicellular prokaryote life evolved in a mildly reducing environment dominated by carbon dioxide and nitrogen, based on anaerobic respiration. Oxygen is produced as a by-product of anaerobic respiration, which resulted in the transformation of the biosphere to an oxidizing environment toxic to this early life [1,2]. This oxygenation of the biosphere, termed the Great Oxidation Event, lead to the development of aerobic respiration and mitochondria as an evolutionary adaption to the toxic oxygen-rich environment [3]. Mitochondria emerged as a critical cellular organelle, significantly increasing energy production as well as being involved in the regulation of cellular activities. This subsequently enabled the evolution of multicellular eukaryote life [4].
The mitochondria produce adenosine triphosphate (ATP) through oxidative phosphorylation. ATP production is mediated via the electron transfer chain, in which electrons are passed through a series of 5 protein complexes in the mitochondrial inner membrane [4]. However, a leakage of electrons from protein complexes I and III leads to the production of potentially toxic reactive oxygen species (ROS), specifically superoxide (.O2−). .O2 is rapidly metabolized into hydrogen peroxide (H2O2) in the mitochondria by superoxide dismutase (SOD) −1 and −2, and then undergoes reduction to produce hydroxyl radicles (.OH−) [5,6].
Since the discovery of ROS over 100 years ago, they have been found to act physiologically as signalling molecules through adaptive phosphorylation/dephosphorylation (redox) switches [6,7]. These redox switches regulate numerous physiological functions, including important roles in immune regulation, the inflammatory response, growth factor signalling response, ion transport, apoptosis, and regulation of genetic expression [8]. ROS are also important mediators in male reproductive physiology, involved in spermatogenesis, chromatin condensation, spermatozoa maturation in the epididymis, hyperactivation, capacitation, acrosome reactions, and sperm-zona binding in fertilization [9,10,11]. In seminal fluid, ROS are generated predominantly from spermatozoa and seminal leukocytes, while prostatic and seminal fluids contribute to generate antioxidants and antioxidant co-factors [9,10,12,13].
ROS are highly reactive, and excessive ROS can damage lipids, proteins, and carbohydrates. This results in damage to cellular structures, enzymes, organelles and DNA, and activates cellular apoptosis [6,14]. The potentially toxic nature of ROS resulted in the evolution of endogenous antioxidant enzymes to neutralise their high reactivity. Important endogenous enzymatic antioxidants include catalase (CAT), glutathione peroxidase (GPx), SOD1 (containing copper and zinc), SOD2 (containing manganese) and extracellular SOD3 (also containing copper and zinc), peroxiredoxins, and thioredoxins [15,16,17,18]. Important exogenous non-enzymatic antioxidants obtained predominantly as nutritional micronutrients include β-carotenes, ascorbic acid, tocopherols, glutamine, and co-factors such as copper, selenium, zinc, and manganese [19].
OXIDATIVE STRESS
An imbalance in a ratio between ROS and total antioxidant capacity (TAC) gives rise to oxidative or reductive stress. Specifically, oxidative stress is defined as an increased ROS:TAC ratio due to increased ROS and/or reduced TAC (Fig. 1) [6,7].
Fig. 1. The imbalance between reactive oxygen species and the antioxidant defence system results in oxidative stress, with consequent cellular damage.
The impact of oxidative stress results in cellular injury similar to the general adaption syndrome response to cellular stressors [6,7]. This cellular damage can accumulate over time, mediating the ageing process and the pathogenesis of non-communicable chronic pathologies such as obesity, type 2 diabetes mellitus, cardiovascular disease, various malignancies (including prostate), and Alzheimer's disease [8].
Oxidative stress is also a significant contributor to male infertility [20]. Important causes of oxidative stress in male reproduction include leukocytospermia, varicocele, testicular heat stress, obesity, and type 2 diabetes mellitus. Additional causes associated with male reproductive oxidative stress include a sedentary lifestyle as well as excessive exercise, consumption of tobacco, alcohol, cannabis, and amphetamines, nutritional deficiencies, exposure to endocrine disrupting chemicals through pesticide residues in food and water, and environmental pollutants and toxins [21,22,23,24,25,26,27].
Oxidative stress in the male reproductive tract results in abnormal spermatogenesis and damage to spermatozoa through lipid peroxidation, denaturing of proteins, and DNA damage [26]. This results in reduced sperm concentration, total and progressive sperm motility, hyperactivation, and percentage of normal morphological forms. There is also impaired chromatin condensation and sperm DNA integrity, mitochondrial dysfunction with a reduced mitochondrial membrane potential (MMP), and reduced capacitation and acrosome reaction, negatively affecting oocyte binding and fertilization [10,28,29,30].
MECHANISMS AND MOLECULAR MARKERS OF OXIDATIVE DAMAGE IN MALE FERTILITY
1. Lipid peroxidation
ROS can damage lipids through lipid peroxidation, which is a molecular chain reaction targeting double-bonds in fatty acids that produces higher reactive and mutagenic molecules such as aldehydes [14,31]. Mutagenic products of lipid peroxidation include malondialdehyde (MDA), an important indirect molecular marker of oxidative stress [32,33].
Sperm cell membranes have a higher percentage of omega 3 polyunsaturated fatty acids (PUFAs) compared to somatic cells. With the increased number of double bonds that are more prone to oxidation, sperm cells are more susceptible to oxidative stress through lipid peroxidation than other cells [14,28]. Additionally, sperm cells have a large surface area with relatively little cytoplasm, making them also more susceptible to ROS overcoming antioxidant defenses [34]. Spermatozoa can remove excessive toxic peroxides from the cell membrane; however, excessive lipid peroxidation results in mitochondrial membrane damage, dysfunction and reduced ATP production [14,31]. The damage to double bonds in the PUFA reduces the fluidity of the sperm membrane, which reduces capacitation, acrosome reaction and sperm-oocyte fusion [14,31,34].
2. Chromatin damage and sperm DNA fragmentation
Sperm DNA is vulnerable to oxidative stress and is a significant mediator of ROS-induced infertility [34,35]. ROS damage DNA directly, or indirectly via the mutagenic products of lipid peroxidation such as MDA [32,33]. An important molecular marker and indirect assessment of DNA damage due to oxidative stress is 8-hydroxy-2′-deoxyguanosine (8-OHdG) [34]. DNA damage results in poor sperm maturation, sperm DNA fragmentation (SDF) and increased apoptotic rate in germ cells and spermatozoa [34,36,37]. Furthermore, reduced genomic fusion following penetration of the oocyte, increased risk for spontaneous abortions and recurrent pregnancy loss are observed when sperm DNA is damaged. This damage can also be transmitted to the offspring, increasing the risk for numerous developmental disorders [34,36,37].
3. Mitochondrial dysfunction
Mitochondria are an important source of endogenous ROS, and mitochondrial dysfunction results in excessive ROS production which further damages mitochondria [38]. Mitochondrial dysfunction results in minor variations in MMP, which exacerbates ROS production, reduces ATP generation and further increases mitochondrial damage [23,39]. Similar to oxidative stress, mitochondrial dysfunction increases with age and is increasingly found as a common feature of a wide range of age-related non-communicable chronic pathologies (such as obesity and its associated metabolic co-morbidities, including type-2 diabetes mellitus, cardiovascular disease, malignancy) as well as neuro-degenerative diseases such as Alzheimer's disease [23,39,40].
4. Apoptosis
Apoptosis, a process known commonly as programmed cell death, occurs through characteristic morphological changes and energy-dependent molecular cascades [41,42]. As with redox biology, apoptosis is usually an important physiological process in fetal development, cellular turnover, and immune development. However, increased or decreased apoptotic rate is observed in many pathological processes, including ischemic injury, neuro-degeneration, autoimmunity, and malignancy [41].
Apoptosis is mediated through two major molecular pathways that are interlinked through the mitochondria, namely the intrinsic and extrinsic pathways [41,42]. An additional identified pathway for apoptosis is the perforin/granzyme pathway that can cause apoptosis through granzyme B or granzyme A from T-cell mediated cytotoxicity [41]. All pathways culminate in the activation of a common terminal mediator, caspase-3, leading to protein cross-linking, cytoskeletal and nuclear degradation, SDF, intracellular apoptotic body formation, expression of phagocytic receptors and ultimately the phagocytosis of the cell [41].
The intrinsic pathway is initiated based on intracellular detection of irreparable DNA damage or intracellular viral particles [43]. This is triggered by the mitochondria, and is associated with increased membrane permeability, cytochrome c release and activation of caspases [44,45]. In sperm, this pathway can only be activated with inhibition of phosphatidylinositol 3-kinase (PI3K), which is in turn an inhibitor of intrinsic apoptosis [46]. The intrinsic pathway results in increased ROS production in the mitochondria that mediates annexin binding to the cell surface, and DNA damage [45,46]. Extrinsic apoptosis is triggered by the extracellular ligand binding to the Fas ‘death-receptor’ and other tumor necrosis factor family receptors, leading to activation of caspase-8 and caspase-3 [42]. These signals are most commonly observed via leukocytes such as natural killer cells and CD8+ T-lymphocytes [42,43].
Apoptosis is an important regulatory mechanism in germ cells during spermatogenesis, whereas infertile males have increased apoptosis in testicular biopsy [47]. Increased apoptosis in the male reproductive tract, germ cells and spermatozoa can be induced in case of leukocytospermia, varicocele, obesity, type 2 diabetes mellitus, poor lifestyle factors, and exposure to environmental toxins and radiation [48,49,50]. Molecular markers of apoptosis associated with increased ROS in seminal fluid includes caspases 3 and 9, and cytochrome c [47].
PATHOPHYSIOLOGY OF OXIDATIVE STRESS IN VARICOCELE
1. Overview of varicocele
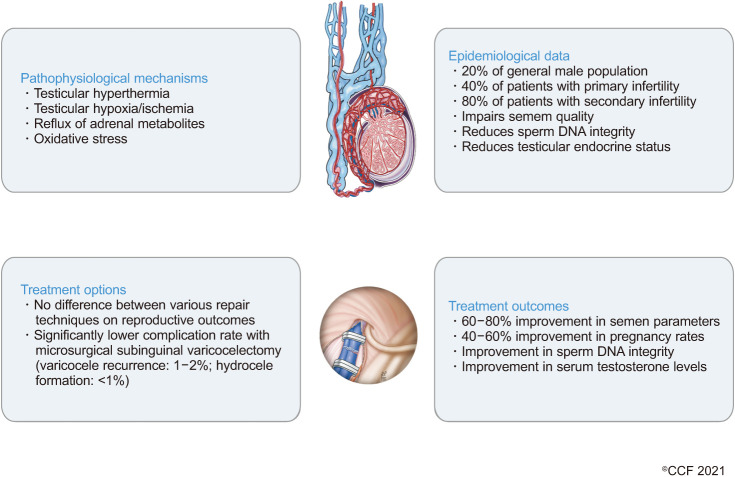
Varicocele is a vascular disease characterized by the abnormal dilatation, elongation and tortuosity of the pampiniform plexus veins of the spermatic cord. Approximately 20% of the adult and adolescent male population presents with varicocele globally, with varicocele being responsible for up to 40% and 80% of cases of primary and secondary infertility, respectively [51]. Fig. 2 further summarizes the pathophysiological mechanisms, epidemiological data, and treatment options and outcomes in varicocele.
Fig. 2. Schematic summary of pathophysiological mechanisms in varicocele condition, along with epidemiological data, treatment options, and outcomes.
Around 90% of varicocele occurs on one side (unilateral) and 10% on both testes (bilateral) [52,53,54,55]. However, varicocele on the left testes is more common due to the anatomy of the venous system, where the left testicular vein enters the left renal vein at a 90° angle. This results in a more turbulent flow and back pressure than the testicular vein on the right side, which enters the inferior vena casa obliquely [56].
Varicocele is clinically assessed by physical examination and palpation and classified following the established criteria. According to the Dubin and Amelar classification, subclinical varicocele is not identifiable during the physical examination [57,58,59]. Clinical varicocele is classified into grade 1 (palpable during the Valsalva maneuver), grade 2 (palpable at the rest), and grade 3 (visible and palpable at the rest) [60].
2. Varicocele and male infertility
An association between varicocele and reduced semen parameters and testicular size has been reported in the literature, with an improvement of semen parameters and pregnancy rates after varicocelectomy [61]. A large observational study conducted on more than 9,000 infertile men reported a 25.4% incidence of varicocele, with the affected population showing reduced total sperm counts and testosterone levels, as well as reduced testicular size when compared with those without varicocele [54]. On the contrary, men with normal semen parameters showed a significantly lower incidence (11.7%) of varicocele [54]. Moreover, a meta-analysis of 10 studies (n=783 patients with varicocele, n=449 healthy controls) reported a negative association between varicocele and sperm count, motility and normal morphology [62]. Despite the association between varicocele and poor semen quality, approximately 80% of men with varicocele are fertile and have normal fecundity [63]. The reasons why some patients with varicocele are infertile, whereas the majority of them are not, remain unclear.
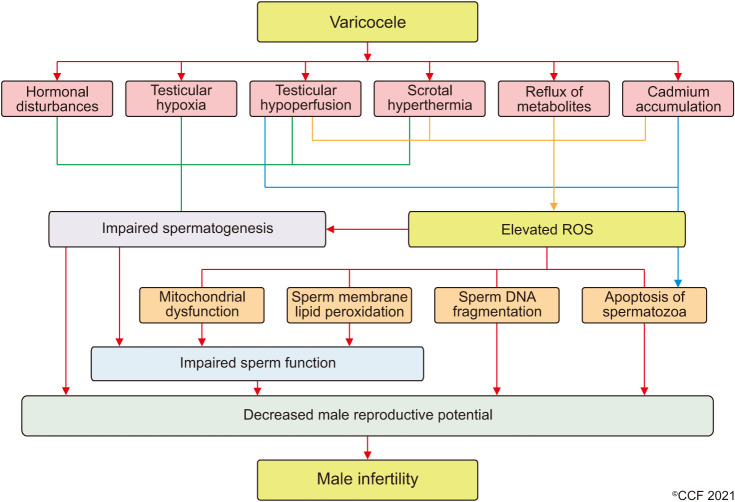
Varicocele can cause male infertility through several mechanisms, including scrotal hyperthermia, hormonal disturbances, testicular hypoperfusion, and hypoxia as well as the backflow of toxic metabolites, which are potential mediators of varicocele-related infertility (Fig. 3) [64]. The pathologic enlargement of the pampiniform venous plexus causes venous stasis and retrograde flow which affects the heat exchange system in the testes and results in elevated local temperatures [65,66]. High temperatures dramatically alter protein synthesis and structure, with repercussions on their activity [67,68,69]. Varicocele patients show reduced expression of PI3K, an important regulator of physiological processes such as capacitation, acrosome reaction, and fertilization [70] while studies report a reduced expression of heat shock protein 5 (HSPA5) [71] and 2 (HSPA2) [72], suggesting an increased susceptibility of testes to elevated temperatures. Furthermore, vasoconstriction of pre-capillary arterioles, a compensatory mechanism to maintain the physiological intratesticular pressure, leads to hypoperfusion and the generation of a hypoxic microenvironment in the testis [73]. A higher expression of hypoxia-inducible factor 1α has been reported in internal spermatic veins resected by varicocele patients [74]. This factor, expressed in hypoxic conditions, promotes germ cell apoptosis in the experimentally-induced varicocele rats, hence contributing to male infertility [75].
Fig. 3. Pathophysiology of varicocele. ROS: reactive oxygen species.
3. Varicocele and oxidative stress: insights from proteomics studies
Oxidative stress is the central mediator of testicular damage in varicocele. The exposure to heat, hypoxia and toxic adrenal and renal metabolites stimulates the generation of ROS. Although ROS are essential mediators of physiological processes such as capacitation, acrosome reaction, hyperactivation, and fertilization, their concentration must be maintained within physiological ranges by the antioxidant systems, as increased ROS can result in DNA damage, protein oxidation and lipid peroxidation [11]. In varicocele patients, reduced semen quality has been reported, correlating with higher values of oxidation-reduction potential (ORP), a measure of redox balance in seminal plasma [9]. A meta-analysis published in 2006 reported higher ROS levels in varicocele patients along with lower TAC in comparison with healthy controls [76]. The comparison of the sperm proteome of varicocele and healthy men showed a decreased expression of SOD1 enzyme and glutathione S-transferases in varicocele patients [71,77,78], suggesting an imbalance in the pro- and antioxidant systems in sperm. However, oxidative stress has also been detected in normozoospermic men with varicocele [79], therefore other mechanisms may exist that either protect germ cells from oxidation or exacerbate ROS harmful effects.
A proteomic approach has been used to investigate the role of key proteins and the altered molecular mechanisms in male infertility-associated conditions, including varicocele. Mass spectrometry and in silico analysis revealed that sperm proteins related to spermatogenesis, sperm motility, and metabolism are significantly under-expressed in varicocele patients, leading to male infertility [64,73,74,75,76,77,78,79,80,81,82,83]. Particularly, proteins related to mitochondrial structure, electron transport chain and mitochondrial metabolism were under-expressed in varicocele patients, stressing the role of mitochondrial dysfunctions in varicocele etiology [84].
Varicocele patients also showed an altered seminal plasma proteomic profile associated to the oxidative stress response, with an increased generation of ROS and pro-oxidant associated proteins. An up-regulation of antioxidant systems was also seen, possibly as a compensatory mechanism [85,86]. Specifically, an in silico analysis predicted the establishment of reductive stress in the testicular microenvironment of unilateral varicocele patients due to the up-regulation of antioxidant systems and mitochondrial dysregulation at the level of the electron transport chain, the main pathway involved in ROS generation [87].
A total number of 64 and 31 proteins were reported to be uniquely expressed in bilateral and unilateral varicocele patients, respectively [88], reflecting the severity of varicocele and its impact on seminal parameters. Moreover, 253 proteins were differentially expressed between unilateral and bilateral varicocele patients, mainly involved in reproductive sperm functions such as motility, capacitation, hyperactivation, acrosome reactions, and fertilization, with bilateral patients having more altered protein pathways [88]. More studies conducted on larger population are needed to confirm the proteomics finding and to validate protein biomarkers in clinical practice. However, the outcomes of investigations regarding the seminal proteomics profile in varicocele patients are very promising, as they may influence the choice of treatment and help in the identification of those patients who could specifically benefit from varicocelectomy.
4. Genetic polymorphisms and epigenetic regulation in varicocele associated with increased susceptibility to DNA damage
Several studies have reported an association between infertility in varicocele patients and specific polymorphisms in genes involved in oxidative stress regulation. For instance, polymorphisms in the antioxidant glutathione S-transferase (GST) genes can result in non-functional enzymes. This has been correlated with a worse response in terms of motile sperm concentration after varicocelectomy, whereas the wild-type allele showed a significantly better response [78]. Similarly, specific genotypes in nitric oxide synthase (NOS3) gene have been reported in higher frequency in varicocele patients and associated with a variable reduction of MDA after varicocele repair [89]. However, it is important to highlight that genetic association studies require a large number of patients to be analysed in order to reach an appropriate level of statistical significance clinically. In fact, a meta-analysis conducted in 2015 including 497 varicocele patients failed to identify any significant association between specific GST null genotypes and the varicocele condition [90]. Another candidate gene whose polymorphism has been associated to varicocele is acid phosphatase locus 1 (ACP1). ACP1 negatively regulates spermatogenesis through the modulation of platelet-derived growth factors enzymatic activity [91]. Hence, polymorphisms resulting in increased ACP1 activity are reportedly higher in varicocele patients with reduced sperm count and abnormal morphology [92].
Methylenetetrahydrofolate reductase is involved in epigenetic regulation during spermatogenesis [93]. Studies investigating its role in male infertility reported that the A1298C polymorphism results in reduced enzymatic activity [94], lower semen quality [95,96], and a 2.3-fold higher risk of developing varicocele when it is present in homozygosis [97]. Epigenetic regulation is particularly important during spermatogenesis [98], as altered methylation profile has been reported in male infertility [99,100]. Two studies have independently observed a lower percentage of global sperm DNA methylation in varicocele patients, in comparison with fertile controls, along with altered sperm DNA integrity [99,101]. Increased sperm DNA damage may also be due to polymorphisms in protamine genes. Gene variants resulting in increased protamine deficiency have been reported in higher percentage in varicocele patients in association with impaired semen parameters [102].
CLINICAL DETERMINATION OF OXIDATIVE STRESS IN SEMEN ANALYSIS
ROS can be measured in semen samples by chemiluminescence, flow cytometry, or nitroblue tetrazolium (NBT). This is of great importance, as the role of oxidative stress has been widely described as contributing to varicocele disease [64,103,104]. Further direct and indirect markers of oxidative stress are reported below, and include MDA assessment, lipid peroxidation, and ORP.
1. Chemiluminescence
Direct assessment of ROS in the semen can be done by chemiluminescence assay, which is a phenomenon where light is emitted as a consequence of chemical reactions [105,106]. Common probes used in this technique include lucigenin and luminol. They are reduced or oxidized to generate a dioxetane or endoperoxide, respectively, which undergo prompt decomposition, generating light [106,107]. The photon emission is detected by means of a luminometer. This consists of a series of photodiodes and photomultiplier tubes for the capture of the signal, and a signal processing software for data analysis. Results are expressed as relative light units (RLUs). The probe is chosen mainly based on the type of ROS to be investigated. Lucigenin is selected to measure extracellular O2−, while luminol can be used to measure both intra- and extracellular ROS. A cut-off value of <102.2 RLU/s/106 sperm/mL was proposed to differentiate the infertile population from fertile controls [108].
2. Flow cytometry detection of intracellular reactive oxygen species
The use of a flow cytometer allows the investigation of a large number of cells in a limited amount of time. Furthermore, it can profile cells by detecting the light scatter or the fluorescence emitted by dyes or monoclonal antibodies used to stain/bind selected molecules [109]. Intracellular ROS analysis is conducted by using two dyes, which are 2′,7′-dichlorofluorescin diacetate (DCFDA), and dihydroethidium (DHE). The former reveals intracellular peroxyl, peroxynitrite, alkoxyl, NO2., carbonate (CO3.−) and OH. radicals, while the latter identifies intracellular O2−.. The principle of the assay is based on ROS-mediated dye oxidation, which generates a fluorescence compound. DCFDA is oxidized in 2′,7′-dichlorofluorescein, which emits a green-colored fluorescence after binding to DNA, while DHE is converted into ethidium bromide, which emits a red fluorescence [109]. The intensity of the fluorescence depends on the intracellular ROS concentration.
3. Nitroblue tetrazolium
Intracytoplasmic ROS can be detected by the NBT assay. This compound is reduced by superoxide radicals and forms formazan crystals [110]. These crystals are purple-blue colored, and can be detected microscopically or spectrophotometrically [111]. Results are expressed as µg formazan/107 sperm. A cut-off of 24 µg formazan/107 sperm was suggested to differentiate male patients based on their fertility status [110].
4. Thiobarbituric acid-reactive substance assay
MDA is the final by-product of lipid peroxidation, and it is measured using the thiobarbituric acid-reactive substance (TBARS) assay [112,113]. The reaction between thiobarbituric acid and MDA generates an adduct, which can be measured either colorimetrically by using a spectrophotometer or fluorometrically. Results are expressed as nmol MDA/107 sperm. It was shown that infertile males with varicocele harbour higher levels of seminal H2O2 and MDA compared to their fertile counterparts [114].
5. Oxidation-reduction potential
ORP evaluation considers the concentration of both oxidants and antioxidants, providing a global overview on the redox status of a semen sample. This is possible by measuring the electron transfer during reductive reactions from antioxidants to oxidants [115]. In a multi-center study including 2,092 patients from 9 countries, ORP measurement was shown to discriminate between normal and abnormal semen samples, with a cut-off value of 1.34 mV/106 sperm/mL [116]. Varicocele patients with abnormal semen quality reported higher ORP than healthy controls [117].
6. Sperm DNA fragmentation testing in varicocele
In their guidelines, Agarwal et al (2020) [35], and Esteves et al (2021) [118] shared common ground in acknowledging the validity and reliability of SDF testing in varicocele patients, using any of the available assays, such as terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), Comet, sperm chromatin structure assay (SCSA), and sperm chromatin dispersion (SCD). Both guidelines also offered a cut-off of 20% to differentiate between fertile and infertile males and recommended SDF testing in case of unexplained or idiopathic male infertility, recurrent pregnancy loss, clinical varicocele, lifestyle risk factors (such as smoking, alcohol, obesity, and environmental exposures), and in the failure of assisted reproductive technology (ART) [35,118,119].
Agarwal et al (2021) [119], have recently stated that oral antioxidants, lifestyle modifications, recurrent ejaculation and controlling infections and inflammation can lower SDF. Additionally, men with high SDF and concurrent varicocele should consider undergoing varicocelectomy, while men with persistently high SDF are advised to perform intracytoplasmic sperm injection (ICSI) with sperm processing and preparation or to use testicular sperm in ICSI in the context of recurrent pregnancy loss with high SDF [119].
In clinical practice, the decision to undergo varicocele repair may be uncertain, and hence, the role of SDF testing is rather helpful in supporting the decision of repair, especially in the subset of patients with low grade varicocele and abnormal semen parameters or patients with grade 2 or 3 varicocele and normal conventional semen parameters [120,121].
MANAGEMENT OF OXIDATIVE STRESS IN VARICOCELE
Varicocele can be treated by varicocelectomy or angiographic embolization. Also, the effects of varicocele on sperm parameters and sperm functions can be managed by antioxidant supplementation. ART is also an option for management of varicocele, although outcomes may be improved with surgical and/or antioxidant treatment [122]. In the American Society for Reproductive Medicine (ASRM)'s 2015 guidelines, it was recommended that varicocele repair and the use of antioxidants may both be valuable methods in reducing SDF [123]. The European Association of Urology (EAU) also acknowledged the benefits of varicocele repair on SDF, recommending varicocele repair in males with elevated SDF and/or unexplained infertility [124]. As varicocele is closely mediated by oxidative stress, there is significant interest in antioxidants-based treatment as part of management considerations [125].
1. Varicocele repair
Varicocele repair can either follow a micro-surgical technique or non-microsurgical alternatives. The approaches include open retroperitoneal ligation (Palomo technique), inguinal, subinguinal, laparoscopic, and embolization [122]. Although it remains unclear if varicocele repair improves natural pregnancy and live birth rates, the evidence suggests that this management approach can improve sperm parameters (sperm concentration, motility, and morphology), seminal oxidative stress and SDF [126,127,128,129]. Varicocele repair may also improve serum testosterone concentration [130]. Although the positive impact on semen quality is reported for all surgical techniques, microsurgical repair appears to potentially be the most beneficial approach [126,131]. Further, varicocele repair is increasingly demonstrated to have positive outcomes on oxidative stress markers and sperm parameters in male fertility due to underlying varicocele [132].
Chen et al (2008) [133] showed that varicocelectomy improves sperm parameters alongside a reduction in 8-OHdG and increases thiols and ascorbic acid (vitamin C) in seminal fluid 6 months post-surgery. Their findings were supported in a retrospective study, where varicocelectomy led to a reduction in oxidative stress by reducing seminal nitric oxide, hexanoyl-lysine, 8-OHdG, SDF and SOD concentrations post-surgery, alongside reduced sperm apoptosis [134]. Varicocelectomy was further reported to significantly reduce seminal MDA, H2O2, NO, and vitamin E, and increase seminal SOD, CAT, GPx, and vitamin C levels at 3 months post-surgery [135]. An increase in seminal TAC and decrease in seminal ROS with improved SDF were also reported after inguinal varicocelectomy with loupe magnification [136]. In a small preliminary study, Dada et al (2010) [137], reported a reduction in seminal ROS determined by chemiluminescence at 1 month following varicocelectomy, with a slower reduction in SDF over 3 to 6 months. Ni et al (2016) [104] reported a significant reduction in seminal MDA 3 and 6 months following microsurgical retroperitoneal high ligation procedure. In males with unilateral (left-side) varicocele who underwent varicocelectomy, a reduction of testicular 4-hydroxy-2-nonenal modified proteins (markers of oxidative stress) was associated with significantly improved sperm motility [138]. The percentage of sperm with oxidative stress, assessed by sperm DCFH-DA staining, is also reportedly improved 3 months after varicocelectomy [101]. Additionally, spermatic vein ligation has been reported to increase TAC at 3 and 6 months following the procedure, particularly in patients with grade II and III varicocele [139]. Varicocele ligation has also been reported to improve seminal ROS, semen parameters, and SDF in association with an increased chance of successful spontaneous pregnancy, intrauterine insemination, in vitro fertilization, and ICSI [140].
However, these results are not consistent. Mancini et al (2004) [141] reported no change in TAC levels at 10 and 24 months post-varicocelectomy, although the data suggested having a positive impact on fine regulation of TAC in relation to an improvement in sperm motility. Further contradictory data was reported in a prospective study investigating varicocelectomy in adolescents, where there was no post-operative change in TBARS, although there was improvement in SDF and sperm mitochondrial activity [142].
Varicocelectomy has been reported in a meta-analysis including 12 studies to significantly ameliorate SDF observed in varicocele patients [143]. This was supported by an additional systematic review and meta-analysis involving 19 varicocele studies, demonstrating a significant reduction in the SDF mean difference (−8.3%) after varicocele repair compared to preoperative measurements [144]. However, a study by García-Peiró et al [145] showed that improvement in chromatin integrity post-varicocele repair was only detected in the group of men with clinical varicocele, with no effect of subclinical varicocele repair seen on SDF.
2. Antioxidant supplementation
The use of antioxidants in male infertility has received significant attention due the central role of oxidative stress in many causes of male infertility [25]. Antioxidants are also reported to be used extensively by urologists and reproductive medicine specialists globally [146]. Common antioxidants used in clinical practice include vitamin A (carotenes), vitamin C (ascorbic acid), vitamin E (tocopherols), carnitine, N-acetyl-cysteine (NAC) and Co-Enzyme Q10 (CoQ10), as well as antioxidant co-factors including selenium, zinc, and folic acid [25,147]. However, the use of antioxidants remains controversial, particularly in the absence of various guidelines and the determination of appropriate outcomes in clinical trials [25,148,149,150,151].
As oxidative stress is also closely associated with varicocele pathogenesis, there is significant interest in the use of antioxidants in the management of varicocele as a sole therapy or alongside varicocele repair [122]. In fact, antioxidants for varicocele in clinical practice is reportedly recommended by 39.9% of reproductive medicine specialists [146]. Antioxidants may also be a non-invasive alternative to consider prior to surgery or more expensive ART management [122]. However, despite the role of oxidative stress as a contributing factor in varicocele-associated male infertility and the potential use of antioxidants, no validated published guidelines are currently available about the use of antioxidant supplementation for the management of male infertility [25,148,149,150,151].
A combination therapy for ‘spermatogenesis stimulation’ that included clomiphene citrate, vitamin A, vitamin E, selenium, L-carnitine, and pentoxifylline reportedly improved sperm parameters in varicocele males [152]. However, the authors reported a more significant impact on sperm concentration and pregnancy outcomes from microsurgical varicocelectomy compared to the spermatogenesis stimulation group [152]. In grade I varicocele, the combination of vitamins B9, B12, C, E, L-Carnitine, CoQ10, zinc, and selenium daily for three months improved sperm concentration, motility, vitality, morphology, and SDF [153]. CoQ10 has also been reported to improve semen parameters and seminal TAC in men with low grade varicocele over a 12-week duration [154]. In a cohort of males with ongoing oligospermia following varicocele embolization, treatment with NAC, and a combination micronutrients did not improve semen parameters, nor increase spontaneous pregnancies, compared to control [155]. The intervention of pentoxifylline combined with zinc and folic acid improved morphology in males with varicocele-induced infertility [156] Supplementation with zinc sulphate in infertile males with or without varicocelectomy reported a significant increase in sperm motility at 2 months post therapy, concluding that zinc sulphate may be beneficial particularly in patients with low seminal zinc concentrations [157].
Antioxidants combined with varicocelectomy may provide additional benefits compared to surgery alone [158]. Supplementation with vitamin C for 6 months following varicocelectomy improved sperm morphology and motility, but not sperm count, compared to post-surgical varicocele group [159]. NAC was found to improve SDF and pregnancy rates post-varicocelectomy compared to no additional intervention [160]. A combination of folic acid and zinc sulphate combined with varicocelectomy improved seminal parameters and serum inhibin-B levels, compared to surgery alone, or the provision of zinc sulphate or folic acid alone [161]. However, L-carnitine added to inguinal varicocelectomy for 6 months did not show any additional benefits on sperm parameters and SDF compared to surgery and placebo control patients [162], nor did the addition of vitamin E in the context of varicocelectomy in a pre- and postoperative setting at 3, 6 and, 12 months respectively) [163].
Animal models may provide further potential insight for investigation in human males with varicocele. In a rat model of induced varicocele, vitamin B was found to be superior to vitamin E in improving varicocele-induced sperm parameters, SDF, and lipid peroxidation [164]. In rats with varicocele-induced damage, the combination of vitamin E with dexamethasone resulted in significant improvement (p<0.05) in GPx and SOD with concomitant down regulation in MDA levels [165]. The extractions of the medicinal herbs Melissa officinalis and Pilea microphylla have also been found to reduce the negative impact of varicocele on sperm parameters and SDF in experimental rats through antioxidant activity [166,167]. Lycopene, a carotenoid primarily derived from tomato, has been found to improve oxidative stress through reduced seminal MDA, with improvements in serum testosterone, testicular weight, SDF and sperm Bcl-2 and BAX expression in a rat model [168].
Although the use of antioxidants for varicocele-induced infertility is seemingly common, there remains insufficient evidence for their clinical use. High quality studies are too limited to clearly recommend antioxidant use in varicocele, as well as the type or combination of antioxidants, dosage and duration, and potential complications including reductive stress [25]. However, the current literature available warrants further clinical investigations for antioxidants in varicocele, with or without combination varicocele repair.
3. Antioxidant paradox, reductive stress, and possible risks
The antioxidant paradox is the observational finding encountered after using high doses of dietary antioxidants to counteract the deleterious effects of oxygen radicals, yet ending up with a modest therapeutic and preventive outcome [169]. This may be due to an incomplete understanding of the complex role of antioxidants in health and disease progression, and that the endogenous antioxidant defence systems are complex and seemingly unaffected by high dose exogenous antioxidant therapy [169]. Additionally, the excessive use of antioxidants may lead to reductive stress, which occurs when the redox levels are shifted towards reduced states [170]. Reductive stress can lead to mitochondrial dysfunction, endothelial cell proliferation, and blood-brain-barrier dysfunction [171,172]. Hence, the trend of antioxidant utilization in current practice is rather empirical, and care should be practiced while using such agents, to prevent inadvertent reductive stress [171].
FUTURE PERSPECTIVES
Recently, there has been more attention diverted towards the identification of molecular factors which are involved in male infertility-related disorders, by conducting proteomics and bioinformatics studies. This may help in clarifying why some varicocele patients are fertile or infertile, by further highlighting the cellular pathways and post-translational modifications and the subsequent role of proteins. Importantly, further clinical investigations examining the impact of varicocele repair and/or the use of antioxidants on seminal oxidative stress in these patients is required. For the use of antioxidants, investigations are needed to determine the clinical indications for antioxidant use, the type or combination of antioxidants, the dosage and duration of treatment.
CONCLUSIONS
Redox biology is important in the (patho)physiology of male reproduction and infertility. Oxidative stress is a well-defined mechanism associated with male infertility in numerous etiologies and risk factors, including varicocele. Varicocele, a common etiology of male infertility, is mediated by increased local temperature, hypoxia, exposure to toxic adrenal and renal metabolites as well as oxidative stress, resulting in impaired spermatogenesis and sperm parameters, including increased SDF. Varicocele repair and/or the use of antioxidants may improve oxidative stress in the male reproductive system. Advanced proteomic and genomic studies have provided interesting insights into the role of oxidative stress in varicocele-mediated infertility; however, molecular mechanisms associated with varicocele disease needs to be further investigated.
ACKNOWLEDGEMENTS
Authors are thankful to the artists from the Cleveland Clinic's Center for Medical Art & Photography for their help with the illustrations. Also, authors sincerely thank Prof. Ralf Henkel, Dr. Damayanthi Durairajanayagam, Dr. Rupin Shah, Prof. Ramadan Saleh, and Prof. Taha Abo-Almagd Abdel-Meguid for their support in reviewing this manuscript.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: RF, KL, AA.
- Writing — original draft: all the authors.
- Writing — review & editing: all the authors.
References
- 1.Crowe SA, Døssing LN, Beukes NJ, Bau M, Kruger SJ, Frei R, et al. Atmospheric oxygenation three billion years ago. Nature. 2013;501:535–538. doi: 10.1038/nature12426. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton TL, Bryant DA, Macalady JL. The role of biology in planetary evolution: cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ Microbiol. 2016;18:325–340. doi: 10.1111/1462-2920.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anbar AD. Oceans. Elements and evolution. Science. 2008;322:1481–1483. doi: 10.1126/science.1163100. [DOI] [PubMed] [Google Scholar]
- 4.Brochier-Armanet C, Talla E, Gribaldo S. The multiple evolutionary histories of dioxygen reductases: Implications for the origin and evolution of aerobic respiration. Mol Biol Evol. 2009;26:285–297. doi: 10.1093/molbev/msn246. [DOI] [PubMed] [Google Scholar]
- 5.Hayyan M, Hashim MA, AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell CN. Reactive oxygen species: finding the right balance. Circ Res. 2008;103:571–572. doi: 10.1161/CIRCRESAHA.108.184325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016;8:302–318. doi: 10.1177/1756287216652779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016;106:566–73.e10. doi: 10.1016/j.fertnstert.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Baskaran S, Finelli R, Agarwal A, Henkel R. Reactive oxygen species in male reproduction: a boon or a bane? Andrologia. 2021;53:e13577. doi: 10.1111/and.13577. [DOI] [PubMed] [Google Scholar]
- 12.Baker MA, Aitken RJ. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol. 2005;3:67. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 14.Tafuri S, Ciani F, Iorio EL, Esposito L, Cocchia N. In: New Discoveries in embryology. Wu B, editor. London: IntechOpen; 2015. Reactive oxygen species (ROS) and male fertility. [Google Scholar]
- 15.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 16.Sheweita SA, Tilmisany AM, Al-Sawaf H. Mechanisms of male infertility: role of antioxidants. Curr Drug Metab. 2005;6:495–501. doi: 10.2174/138920005774330594. [DOI] [PubMed] [Google Scholar]
- 17.Altobelli GG, Van Noorden S, Balato A, Cimini V. Copper/Zinc superoxide dismutase in human skin: current knowledge. Front Med (Lausanne) 2020;7:183. doi: 10.3389/fmed.2020.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ściskalska M, Ołdakowska M, Marek G, Milnerowicz H. Changes in the activity and concentration of superoxide dismutase isoenzymes (Cu/Zn SOD, MnSOD) in the blood of healthy subjects and patients with acute pancreatitis. Antioxidants (Basel) 2020;9:948. doi: 10.3390/antiox9100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micheli L, Cerretani D, Collodel G, Menchiari A, Moltoni L, Fiaschi AI, et al. Evaluation of enzymatic and non-enzymatic antioxidants in seminal plasma of men with genitourinary infections, varicocele and idiopathic infertility. Andrology. 2016;4:456–464. doi: 10.1111/andr.12181. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leisegang K. In: Oxidants, antioxidants and impact of the oxidative status in male reproduction. Henkel R, Samanta L, Agarwal A, editors. London: Elsevier; 2018. Malnutrition and obesity; pp. 117–134. [Google Scholar]
- 22.Leisegang K, Henkel R. In: Male infertility: contemporary clinical approaches, andrology, ART and antioxidants. Parekattil SJ, Esteves SC, Agarwal A, editors. Cham: Springer; 2020. Environmental factors; pp. 437–453. [Google Scholar]
- 23.Leisegang K, Henkel R, Agarwal A. Redox regulation of fertility in aging male and the role of antioxidants: a savior or stressor. Curr Pharm Des. 2017;23:4438–4450. doi: 10.2174/1381612822666161019150241. [DOI] [PubMed] [Google Scholar]
- 24.Leisegang K, Dutta S. Do lifestyle practices impede male fertility? Andrologia. 2021;53:e13595. doi: 10.1111/and.13595. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner Selvam MK, et al. Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. World J Mens Health. 2021;39:233–290. doi: 10.5534/wjmh.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed. 2016;14:231–240. [PMC free article] [PubMed] [Google Scholar]
- 27.Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 28.Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17:87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panner Selvam MK, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic Biol Med. 2020;152:375–385. doi: 10.1016/j.freeradbiomed.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A, Leisegang K, Sengupta P. In: Pathology: oxidative stress and dietary antioxidants. Preedy VR, editor. London: Elsevier; 2020. Oxidative stress in pathologies of male reproductive disorders; pp. 15–27. [Google Scholar]
- 31.Moazamian R, Polhemus A, Connaughton H, Fraser B, Whiting S, Gharagozloo P, et al. Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod. 2015;21:502–515. doi: 10.1093/molehr/gav014. [DOI] [PubMed] [Google Scholar]
- 32.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57(5 Suppl):779S–785S. doi: 10.1093/ajcn/57.5.779S. discussion 785S-6S. [DOI] [PubMed] [Google Scholar]
- 33.Łuczaj W, Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol Biol Lett. 2003;8:391–413. [PubMed] [Google Scholar]
- 34.Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367–381. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health. 2020;38:412–471. doi: 10.5534/wjmh.200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–642. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Selvaratnam JS, Robaire B. Effects of aging and oxidative stress on spermatozoa of superoxide-dismutase 1-and catalase-null mice. Biol Reprod. 2016;95:60. doi: 10.1095/biolreprod.116.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Hekimi S. Mitochondrial dysfunction and longevity in animals: untangling the knot. Science. 2015;350:1204–1207. doi: 10.1126/science.aac4357. [DOI] [PubMed] [Google Scholar]
- 39.Bagkos G, Koufopoulos K, Piperi C. A new model for mitochondrial membrane potential production and storage. Med Hypotheses. 2014;83:175–181. doi: 10.1016/j.mehy.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hongmei Z. In: Apoptosis and medicine. Ntuli T, editor. London: IntechOpen; 2012. Extrinsic and intrinsic apoptosis signal pathway review. [Google Scholar]
- 43.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta Mol Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 45.Lucken-Ardjomande S, Martinou JC. Regulation of Bcl-2 proteins and of the permeability of the outer mitochondrial membrane. C R Biol. 2005;328:616–631. doi: 10.1016/j.crvi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436:687–698. doi: 10.1042/BJ20110114. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Sharma RK, Sikka SC, Thomas AJ, Jr, Falcone T, Agarwal A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003;80:531–535. doi: 10.1016/s0015-0282(03)00756-8. [DOI] [PubMed] [Google Scholar]
- 48.Henkel R, Leisegang K. In: Male infertility: contemporary clinical approaches, andrology, ART and antioxidants. 2nd ed. Parekattil SJ, Esteves SC, Agarwal A, editors. Cham: Springer; 2020. Origins of sperm DNA damage; pp. 361–375. [Google Scholar]
- 49.Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagn Res. 2017;11:IE01–IE05. doi: 10.7860/JCDR/2017/23927.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 51.Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology of varicocele. Asian J Androl. 2016;18:179–181. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundy SD, Sabanegh ES., Jr Varicocele management for infertility and pain: a systematic review. Arab J Urol. 2017;16:157–170. doi: 10.1016/j.aju.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–616. [PubMed] [Google Scholar]
- 54.World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–1293. [PubMed] [Google Scholar]
- 55.Baek SR, Park HJ, Park NC. Comparison of the clinical characteristics of patients with varicocele according to the presence or absence of scrotal pain. Andrologia. 2019;51:e13187. doi: 10.1111/and.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masson P, Brannigan RE. The varicocele. Urol Clin North Am. 2014;41:129–144. doi: 10.1016/j.ucl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Cocuzza M, Cocuzza MA, Bragais FM, Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics (Sao Paulo) 2008;63:395–404. doi: 10.1590/S1807-59322008000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertil Steril. 2004;81:424–429. doi: 10.1016/j.fertnstert.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Mohammed A, Chinegwundoh F. Testicular varicocele: an overview. Urol Int. 2009;82:373–379. doi: 10.1159/000218523. [DOI] [PubMed] [Google Scholar]
- 60.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–609. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 61.Miyaoka R, Esteves SC. A critical appraisal on the role of varicocele in male infertility. Adv Urol. 2012;2012:597495. doi: 10.1155/2012/597495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal A, Sharma R, Harlev A, Esteves SC. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl. 2016;18:163–170. doi: 10.4103/1008-682X.172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–690. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142:743–745. doi: 10.1016/s0022-5347(17)38874-2. [DOI] [PubMed] [Google Scholar]
- 66.Green KF, Turner TT, Howards SS. Varicocele: reversal of the testicular blood flow and temperature effects by varicocele repair. J Urol. 1984;131:1208–1211. doi: 10.1016/s0022-5347(17)50874-5. [DOI] [PubMed] [Google Scholar]
- 67.Fujisawa M, Yoshida S, Matsumoto O, Kojima K, Kamidono S. Deoxyribonucleic acid polymerase activity in the testes of infertile men with varicocele. Fertil Steril. 1988;50:795–800. doi: 10.1016/s0015-0282(16)60318-7. [DOI] [PubMed] [Google Scholar]
- 68.Fujisawa M, Yoshida S, Matsumoto O, Kojima K, Kamidono S. Decrease of topoisomerase I activity in the testes of infertile men with varicocele. Arch Androl. 1988;21:45–50. doi: 10.3109/01485018808986732. [DOI] [PubMed] [Google Scholar]
- 69.Fujisawa M, Yoshida S, Kojima K, Kamidono S. Biochemical changes in testicular varicocele. Arch Androl. 1989;22:149–159. doi: 10.3109/01485018908986765. [DOI] [PubMed] [Google Scholar]
- 70.De Amicis F, Perrotta I, Santoro M, Guido C, Morelli C, Cesario MG, et al. Human sperm anatomy: different expression and localization of phosphatidylinositol 3-kinase in normal and varicocele human spermatozoa. Ultrastruct Pathol. 2013;37:176–182. doi: 10.3109/01913123.2013.763881. [DOI] [PubMed] [Google Scholar]
- 71.Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, Sabbaghian M, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81:293–300. doi: 10.1016/j.urology.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 72.Lima SB, Cenedeze MA, Bertolla RP, Filho PA, Oehninger S, Cedenho AP. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86:1659–1663. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 73.Gat Y, Zukerman Z, Chakraborty J, Gornish M. Varicocele, hypoxia and male infertility. Fluid Mechanics analysis of the impaired testicular venous drainage system. Hum Reprod. 2005;20:2614–2619. doi: 10.1093/humrep/dei089. [DOI] [PubMed] [Google Scholar]
- 74.Lee JD, Jeng SY, Lee TH. Increased expression of hypoxia-inducible factor-1alpha in the internal spermatic vein of patients with varicocele. J Urol. 2006;175(3 Pt 1):1045–1048. doi: 10.1016/S0022-5347(05)00417-9. discussion 1048. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Sun Y, Wang L, Xu C, Yang Q, Liu B, et al. Hypoxia-induced apoptosis in the bilateral testes of rats with left-sided varicocele: a new way to think about the varicocele. J Androl. 2010;31:299–305. doi: 10.2164/jandrol.108.007153. [DOI] [PubMed] [Google Scholar]
- 76.Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12:630–633. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 77.Chen SS, Chang LS, Chen HW, Wei YH. Polymorphisms of glutathione S-transferase M1 and male infertility in Taiwanese patients with varicocele. Hum Reprod. 2002;17:718–725. doi: 10.1093/humrep/17.3.718. [DOI] [PubMed] [Google Scholar]
- 78.Ichioka K, Nagahama K, Okubo K, Soda T, Ogawa O, Nishiyama H. Genetic polymorphisms in glutathione S-transferase T1 affect the surgical outcome of varicocelectomies in infertile patients. Asian J Androl. 2009;11:333–341. doi: 10.1038/aja.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giulini S, Sblendorio V, Xella S, La Marca A, Palmieri B, Volpe A. Seminal plasma total antioxidant capacity and semen parameters in patients with varicocele. Reprod Biomed Online. 2009;18:617–621. doi: 10.1016/s1472-6483(10)60004-1. [DOI] [PubMed] [Google Scholar]
- 80.Fariello RM, Pariz JR, Spaine DM, Gozzo FC, Pilau EJ, Fraietta R, et al. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele. Hum Reprod. 2012;27:3140–3149. doi: 10.1093/humrep/des287. [DOI] [PubMed] [Google Scholar]
- 81.Del Giudice PT, da Silva BF, Lo Turco EG, Fraietta R, Spaine DM, Santos LF, et al. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil Steril. 2013;100:667–672. doi: 10.1016/j.fertnstert.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 82.Camargo M, Intasqui Lopes P, Del Giudice PT, Carvalho VM, Cardozo KH, Andreoni C, et al. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum Reprod. 2013;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 83.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, Souza GHMF, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–98. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 84.Samanta L, Agarwal A, Swain N, Sharma R, Gopalan B, Esteves SC, et al. Proteomic signatures of sperm mitochondria in varicocele: clinical use as biomarkers of varicocele associated infertility. J Urol. 2018;200:414–422. doi: 10.1016/j.juro.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Panner Selvam MK, Agarwal A, Baskaran S. Proteomic analysis of seminal plasma from bilateral varicocele patients indicates an oxidative state and increased inflammatory response. Asian J Androl. 2019;21:544–550. doi: 10.4103/aja.aja_121_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panner Selvam MK, Samanta L, Agarwal A. Functional analysis of differentially expressed acetylated spermatozoal proteins in infertile men with unilateral and bilateral varicocele. Int J Mol Sci. 2020;21:3155. doi: 10.3390/ijms21093155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swain N, Samanta L, Agarwal A, Kumar S, Dixit A, Gopalan B, et al. Aberrant upregulation of compensatory redox molecular machines may contribute to sperm dysfunction in infertile men with unilateral varicocele: a proteomic insight. Antioxid Redox Signal. 2020;32:504–521. doi: 10.1089/ars.2019.7828. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal A, Sharma R, Durairajanayagam D, Cui Z, Ayaz A, Gupta S, et al. Differential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicocele. Urology. 2015;85:580–588. doi: 10.1016/j.urology.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 89.Omar SS, Mahfouz W, Dawood W, Abo El-Wafa RAH, Ghazala RA, Zahran AM. Relation of nitric oxide synthase gene (NOS3) polymorphisms to varicocele risk and post-varicocelectomy seminal oxidative stress reduction. Andrologia. 2020;52:e13525. doi: 10.1111/and.13525. [DOI] [PubMed] [Google Scholar]
- 90.Zhu B, Yin L, Zhang JY. Glutathione S-transferase polymorphisms in varicocele patients: a meta-analysis. Genet Mol Res. 2015;14:18851–18858. doi: 10.4238/2015.December.28.34. [DOI] [PubMed] [Google Scholar]
- 91.Basciani S, Mariani S, Arizzi M, Ulisse S, Rucci N, Jannini EA, et al. Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-alpha and -beta during human testicular development and disease. J Clin Endocrinol Metab. 2002;87:2310–2319. doi: 10.1210/jcem.87.5.8476. [DOI] [PubMed] [Google Scholar]
- 92.Gentile V, Nicotra M, Scaravelli G, Antonini G, Ambrosi S, Saccucci P, et al. ACP1 genetic polymorphism and spermatic parameters in men with varicocele. Andrologia. 2014;46:147–150. doi: 10.1111/and.12059. [DOI] [PubMed] [Google Scholar]
- 93.La Salle S, Oakes CC, Neaga OR, Bourc'his D, Bestor TH, Trasler JM. Loss of spermatogonia and wide-spread DNA methylation defects in newborn male mice deficient in DNMT3L. BMC Dev Biol. 2007;7:104. doi: 10.1186/1471-213X-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwahn B, Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences. Am J Pharmacogenomics. 2001;1:189–201. doi: 10.2165/00129785-200101030-00004. [DOI] [PubMed] [Google Scholar]
- 95.Singh K, Singh SK, Sah R, Singh I, Raman R. Mutation C677T in the methylenetetrahydrofolate reductase gene is associated with male infertility in an Indian population. Int J Androl. 2005;28:115–119. doi: 10.1111/j.1365-2605.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 96.Lee HC, Jeong YM, Lee SH, Cha KY, Song SH, Kim NK, et al. Association study of four polymorphisms in three folate-related enzyme genes with non-obstructive male infertility. Hum Reprod. 2006;21:3162–3170. doi: 10.1093/humrep/del280. [DOI] [PubMed] [Google Scholar]
- 97.Ucar VB, Nami B, Acar H, Kilinç M. Is methylenetetrahydrofolate reductase (MTHFR) gene A1298C polymorphism related with varicocele risk? Andrologia. 2015;47:42–46. doi: 10.1111/and.12229. [DOI] [PubMed] [Google Scholar]
- 98.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahreinian M, Tavalaee M, Abbasi H, Kiani-Esfahani A, Shiravi AH, Nasr-Esfahani MH. DNA hypomethylation predisposes sperm to DNA damage in individuals with varicocele. Syst Biol Reprod Med. 2015;61:179–186. doi: 10.3109/19396368.2015.1020116. [DOI] [PubMed] [Google Scholar]
- 100.Hammoud SS, Purwar J, Pflueger C, Cairns BR, Carrell DT. Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil Steril. 2010;94:1728–1733. doi: 10.1016/j.fertnstert.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 101.Tavalaee M, Bahreinian M, Barekat F, Abbasi H, Nasr-Esfahani MH. Effect of varicocelectomy on sperm functional characteristics and DNA methylation. Andrologia. 2015;47:904–909. doi: 10.1111/and.12345. [DOI] [PubMed] [Google Scholar]
- 102.Nayeri M, Talebi AR, Heidari MM, Seifati SM, Tabibnejad N. Polymorphisms of sperm protamine genes and CMA3 staining in infertile men with varicocele. Rev Int Androl. 2020;18:7–13. doi: 10.1016/j.androl.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology. 2009;73:461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 104.Ni K, Steger K, Yang H, Wang H, Hu K, Zhang T, et al. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–824. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 105.Vessey W, Perez-Miranda A, Macfarquhar R, Agarwal A, Homa S. Reactive oxygen species in human semen: validation and qualification of a chemiluminescence assay. Fertil Steril. 2014;102:1576–83.e4. doi: 10.1016/j.fertnstert.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 106.Aitken RJ, Baker MA, O'Bryan M. Shedding light on chemiluminescence: the application of chemiluminescence in diagnostic andrology. J Androl. 2004;25z:455–465. doi: 10.1002/j.1939-4640.2004.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 107.Khan P, Idrees D, Moxley MA, Corbett JA, Ahmad F, von Figura G, et al. Luminol-based chemiluminescent signals: clinical and non-clinical application and future uses. Appl Biochem Biotechnol. 2014;173:333–355. doi: 10.1007/s12010-014-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agarwal A, Ahmad G, Sharma R. Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J Assist Reprod Genet. 2015;32:1721–1729. doi: 10.1007/s10815-015-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahfouz R, Sharma R, Lackner J, Aziz N, Agarwal A. Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertil Steril. 2009;92:819–827. doi: 10.1016/j.fertnstert.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 110.Tunc O, Thompson J, Tremellen K. Development of the NBT assay as a marker of sperm oxidative stress. Int J Androl. 2010;33:13–21. doi: 10.1111/j.1365-2605.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 111.Gosalvez J, Tvrda E, Agarwal A. Free radical and superoxide reactivity detection in semen quality assessment: past, present, and future. J Assist Reprod Genet. 2017;34:697–707. doi: 10.1007/s10815-017-0912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aitken RJ, Harkiss D, Buckingham DW. Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol Reprod Dev. 1993;35:302–315. doi: 10.1002/mrd.1080350313. [DOI] [PubMed] [Google Scholar]
- 113.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 114.Mostafa T, Anis T, El Nashar A, Imam H, Osman I. Seminal plasma reactive oxygen species-antioxidants relationship with varicocele grade. Andrologia. 2012;44:66–69. doi: 10.1111/j.1439-0272.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 115.Panner Selvam MK, Finelli R, Agarwal A, Henkel R. Evaluation of seminal oxidation-reduction potential in male infertility. Andrologia. 2021;53:e13610. doi: 10.1111/and.13610. [DOI] [PubMed] [Google Scholar]
- 116.Agarwal A, Panner Selvam MK, Arafa M, Okada H, Homa S, Killeen A, et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J Androl. 2019;21:565–569. doi: 10.4103/aja.aja_5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 118.Esteves SC, Zini A, Coward RM, Evenson DP, Gosálvez J, Lewis SEM, et al. Sperm DNA fragmentation testing: summary evidence and clinical practice recommendations. Andrologia. 2021;53:e13874. doi: 10.1111/and.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Agarwal A, Farkouh A, Parekh N, Zini A, Arafa M, Kandil H, et al. Sperm DNA fragmentation: a critical assessment of clinical practice guidelines. World J Mens Health. 2021 doi: 10.5534/wjmh.210056. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kandil H, Shah R. In: Varicocele and male infertility: a complete guide. Esteves SC, Cho CL, Majzoub A, Agarwal A, editors. Cham: Springer; 2019. Grades 2/3 varicocele and normal conventional semen analysis; pp. 537–543. [Google Scholar]
- 121.Cho CL, Agarwal A, Majzoub A, Esteves SC. A single cut-off value of sperm DNA fragmentation testing does not fit all. Transl Androl Urol. 2017;6(Suppl 4):S501–S503. doi: 10.21037/tau.2017.08.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su JS, Farber NJ, Vij SC. Pathophysiology and treatment options of varicocele: an overview. Andrologia. 2021;53:e13576. doi: 10.1111/and.13576. [DOI] [PubMed] [Google Scholar]
- 123.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–e25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 124.Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, et al. EAU guidelines on sexual and reproductive health [Internet] Arnhem: European Association of Urology; c2020. [cited 2021 Aug 18]. Available from: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Sexual-and-Reproductive-Health-2020.pdf. [Google Scholar]
- 125.Moazzam A. Oxidative stress induced infertility in varicocele. Andrology (Los Angel) 2016;5:1. [Google Scholar]
- 126.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, Salonia A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 127.Jensen CFS, Østergren P, Dupree JM, Ohl DA, Sønksen J, Fode M. Varicocele and male infertility. Nat Rev Urol. 2017;14:523–533. doi: 10.1038/nrurol.2017.98. [DOI] [PubMed] [Google Scholar]
- 128.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189(1 Suppl):S146–S150. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 129.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–461. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 130.Hsiao W, Rosoff JS, Pale JR, Powell JL, Goldstein M. Varicocelectomy is associated with increases in serum testosterone independent of clinical grade. Urology. 2013;81:1213–1217. doi: 10.1016/j.urology.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 131.Abdel-Meguid TA, Farsi HM, Al-Sayyad A, Tayib A, Mosli HA, Halawani AH. Effects of varicocele on serum testosterone and changes of testosterone after varicocelectomy: a prospective controlled study. Urology. 2014;84:1081–1087. doi: 10.1016/j.urology.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 132.Kroese AC, de Lange NM, Collins J, Evers JL. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 133.Chen SS, Huang WJ, Chang LS, Wei YH. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179:639–642. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 134.Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008;101:1547–1552. doi: 10.1111/j.1464-410X.2008.07517.x. [DOI] [PubMed] [Google Scholar]
- 135.Mostafa T, Anis TH, El-Nashar A, Imam H, Othman IA. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl. 2001;24:261–265. doi: 10.1046/j.1365-2605.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 136.Abdelbaki SA, Sabry JH, Al-Adl AM, Sabry HH. The impact of coexisting sperm DNA fragmentation and seminal oxidative stress on the outcome of varicocelectomy in infertile patients: a prospective controlled study. Arab J Urol. 2017;15:131–139. doi: 10.1016/j.aju.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010;132:728–730. [PMC free article] [PubMed] [Google Scholar]
- 138.Shiraishi K, Naito K. Generation of 4-hydroxy-2-nonenal modified proteins in testes predicts improvement in spermatogenesis after varicocelectomy. Fertil Steril. 2006;86:233–235. doi: 10.1016/j.fertnstert.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 139.Ozturk U, Ozdemir E, Buyukkagnici U, Dede O, Sucak A, Celen S, et al. Effect of spermatic vein ligation on seminal total antioxidant capacity in terms of varicocele grading. Andrologia. 2012;44(Suppl 1):199–204. doi: 10.1111/j.1439-0272.2011.01164.x. [DOI] [PubMed] [Google Scholar]
- 140.Baker K, McGill J, Sharma R, Agarwal A, Sabanegh E., Jr Pregnancy after varicocelectomy: impact of postoperative motility and DFI. Urology. 2013;81:760–766. doi: 10.1016/j.urology.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Mancini A, Meucci E, Milardi D, Giacchi E, Bianchi A, Pantano AL, et al. Seminal antioxidant capacity in pre-and postoperative varicocele. J Androl. 2004;25:44–49. doi: 10.1002/j.1939-4640.2004.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 142.Lacerda JI, Del Giudice PT, da Silva BF, Nichi M, Fariello RM, Fraietta R, et al. Adolescent varicocele: improved sperm function after varicocelectomy. Fertil Steril. 2011;95:994–999. doi: 10.1016/j.fertnstert.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 143.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–314. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 144.Roque M, Bedoschi G, Esteves SC. Effect of varicocele repair on sperm DNA fragmentation: a systematic review and meta-analysis. Fertil Steril. 2018;110(4 Suppl):e162 [Google Scholar]
- 145.García-Peiró A, Ribas-Maynou J, Oliver-Bonet M, Navarro J, Checa MA, Nikolaou A, et al. Multiple determinations of sperm DNA fragmentation show that varicocelectomy is not indicated for infertile patients with subclinical varicocele. Biomed Res Int. 2014;2014:181396. doi: 10.1155/2014/181396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Agarwal A, Finelli R, Selvam MKP, Leisegang K, Majzoub A, Tadros N, et al. A global survey of reproductive specialists to determine the clinical utility of oxidative stress testing and antioxidant use in male infertility. World J Mens Health. 2021;39:470–488. doi: 10.5534/wjmh.210025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011;1:CD007411. doi: 10.1002/14651858.CD007411.pub2. [DOI] [PubMed] [Google Scholar]
- 149.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;12:CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 150.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ali M, Martinez M, Parekh N. Are antioxidants a viable treatment option for male infertility? Andrologia. 2021;53:e13644. doi: 10.1111/and.13644. [DOI] [PubMed] [Google Scholar]
- 152.Gamidov CI, Ovchinnikov RI, Popova AIu, Tkhagapsoeva RA, Izhbaev SKh. [Current approach to therapy for male infertility in patients with varicocele] Ter Arkh. 2012;84(Camb):56–61. Russian. [PubMed] [Google Scholar]
- 153.Gual-Frau J, Abad C, Amengual MJ, Hannaoui N, Checa MA, Ribas-Maynou J, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225–229. doi: 10.3109/14647273.2015.1050462. [DOI] [PubMed] [Google Scholar]
- 154.Festa R, Giacchi E, Raimondo S, Tiano L, Zuccarelli P, Silvestrini A, et al. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: an open, uncontrolled pilot study. Andrologia. 2014;46:805–807. doi: 10.1111/and.12152. [DOI] [PubMed] [Google Scholar]
- 155.Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26:97–102. doi: 10.1007/s00345-007-0218-z. [DOI] [PubMed] [Google Scholar]
- 156.Oliva A, Dotta A, Multigner L. Pentoxifylline and antioxidants improve sperm quality in male patients with varicocele. Fertil Steril. 2009;91(4 Suppl):1536–1539. doi: 10.1016/j.fertnstert.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 157.Takihara H, Cosentino MJ, Cockett AT. Zinc sulfate therapy for infertile male with or without varicocelectomy. Urology. 1987;29:638–641. doi: 10.1016/0090-4295(87)90111-7. [DOI] [PubMed] [Google Scholar]
- 158.Kızılay F, Altay B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: a randomized controlled trial. Int J Impot Res. 2019;31:424–431. doi: 10.1038/s41443-018-0109-4. [DOI] [PubMed] [Google Scholar]
- 159.Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol. 2015;41:230–238. doi: 10.1590/S1677-5538.IBJU.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, Abbasi H, et al. A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril. 2016;10:120–126. doi: 10.22074/ijfs.2016.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nematollahi-Mahani SN, Azizollahi GH, Baneshi MR, Safari Z, Azizollahi S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia. 2014;46:240–245. doi: 10.1111/and.12067. [DOI] [PubMed] [Google Scholar]
- 162.Pourmand G, Movahedin M, Dehghani S, Mehrsai A, Ahmadi A, Pourhosein M, et al. Does L-carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study. Urology. 2014;84:821–825. doi: 10.1016/j.urology.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 163.Ener K, Aldemir M, Işık E, Okulu E, Özcan MF, Uğurlu M, et al. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: a randomised controlled study. Andrologia. 2016;48:829–834. doi: 10.1111/and.12521. [DOI] [PubMed] [Google Scholar]
- 164.Hassani-Bafrani H, Tavalaee M, Arbabian M, Dattilo M, Nasr-Esfahani MH. The effect of vitamin E & vitamin B on sperm function in rat varicocele model. Andrologia. 2019;51:e13429. doi: 10.1111/and.13429. [DOI] [PubMed] [Google Scholar]
- 165.Khosravanian H, Razi M, Farokhi F, Khosravanian N. Simultaneous administration of dexamethasone and vitamin E reversed experimental varicocele-induced impact in testicular tissue in rats; correlation with Hsp70-2 chaperone expression. Int Braz J Urol. 2015;41:773–790. doi: 10.1590/S1677-5538.IBJU.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rezakhaniha B, Heidari R, Abbasi M. Can Melissa officinalis improve chromatin structure and sperm parameters in a rat model of varicocele? Andrologia. 2018;50:e13058. doi: 10.1111/and.13058. [DOI] [PubMed] [Google Scholar]
- 167.Heidari R, Alizadeh R, Abbasi N, Pasbakhsh P, Hedayatpour A, Farajpour M, et al. Do pilea microphylla improve sperm DNA fragmentation and sperm parameters in varicocelized rats? Acta Med Iran. 2015;53:547–554. [PubMed] [Google Scholar]
- 168.Antonuccio P, Micali A, Puzzolo D, Romeo C, Vermiglio G, Squadrito V, et al. Nutraceutical effects of lycopene in experimental varicocele: an “in vivo” model to study male infertility. Nutrients. 2020;12:1536. doi: 10.3390/nu12051536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol. 2013;75:637–644. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: a possible cause of male infertility? Andrologia. 2019;51:e13162. doi: 10.1111/and.13162. [DOI] [PubMed] [Google Scholar]
- 171.Mentor S, Fisher D. Aggressive antioxidant reductive stress impairs brain endothelial cell angiogenesis and blood brain barrier function. Curr Neurovasc Res. 2017;14:71–81. doi: 10.2174/1567202613666161129113950. [DOI] [PubMed] [Google Scholar]
- 172.Singh F, Charles AL, Schlagowski AI, Bouitbir J, Bonifacio A, Piquard F, et al. Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis. Biochim Biophys Acta. 2015;1853:1574–1585. doi: 10.1016/j.bbamcr.2015.03.006. [DOI] [PubMed] [Google Scholar]