Abstract
Various adenovirus (AdV) vector systems have proven to be lucrative options for gene delivery. They can serve as potential vaccine candidates for prevention of several common infectious diseases and hold the promise for gene therapy, especially for cancer. Several AdV vector-based therapies are currently at various stages of clinical trials worldwide, which make an immense interest of both the clinicians and researchers. Since these vectors are easy to manipulate, have broad tropism, and have the capability to yield high titers, this delivery system has a wide range of applications for different clinical settings. This chapter emphasizes on some of the current usages of AdV vectors and their production methods.
Keywords: Adenovirus vector, gene delivery system, gene therapy, vector design, vector production, recombinant vaccines
1. Introduction
Adenoviruses (AdVs) are non-enveloped double-stranded DNA viruses containing genomes of approximately 34-44 kilobase pairs (kbp). Initially, a human AdV was first isolated in 1953 from the adenoid tissues [1] and hence was named AdV [2]. They are known to cause inapparent or symptomatic infections of the upper or lower respiratory tract, gastrointestinal tract or eyes, which are usually self-limiting in healthy individuals. Although AdVs were known for a long time, their therapeutic potential as a gene delivery vehicle was realized only with the advent of recombinant DNA technology. With continual advancement in the biology of AdV, it became the first viral gene delivery vector to be used in humans. More than 60 types of human AdVs have been described, of which the vector backbone of human AdV type C5 has been used extensively for gene delivery [3].
1.2. AdV vectors: pros and cons
AdV vectors have several advantages which make them ideal for gene delivery. The AdV biology has been deciphered, which makes the molecular manipulation of its genome easier. Moreover, several AdVs have low or no virulence in humans and have high transduction efficiency for both replicating as well as non-replicating cell types. The vector can also be grown and purified in very high titers and large quantities at a reasonable cost. Furthermore, AdV vectors possess the minimal risk of insertional mutagenesis because of their inability to integrate into the host genome. The transient transgene expression by AdV vectors have been harnessed for oncolytic therapy and also for expression of vaccine antigens [4-9].
However, the transient nature of transgene expression by AdV vectors sometimes limits their use where continuous transgene expression is necessary for a desired therapeutic effect. Apart from this limitation, AdV vectors are known to activate innate immunity, which can lead to severe toxicity at a very high vector dose. One such evidence is the death of a patient enrolled in the ornithine transcarbamylase (OTC) deficiency clinical trial due to high vector dosage leading to multiple organs failure [10].
Due to the high prevalence of human AdVs, nearly 80% of human population is exposed to one or more AdV types multiple times in their lives [11-13], thereby developing AdV neutralizing antibodies popularly known as ‘pre-existing vector immunity’ [14]. The issue of pre-existing vector immunity can be rectified to some extent by increasing the vector dosage without increasing toxicity [13,15,16]. Alternatively, pre-existing vector immunity can also be circumvented using nonhuman AdV vectors and heterologous prime-boost approaches. Innovation in vector engineering strategies and the use of different immunosuppressive agents can also be used to overcome some of these limitations in the existing vector systems [17-19].
1.3. Nonhuman AdV vectors
Nonhuman AdV vector systems based on bovine AdV, simian AdV, porcine AdV, ovine AdV, canine AdV, avian AdV and murine AdV [20,21] were developed in search of safe and efficient gene delivery vehicles to overcome the shortcomings of human AdV vector systems, especially the concern of pre-existing vector immunity. For example, bovine AdV vectors are not neutralized by human AdV-specific neutralizing antibodies, and the prevalence of bovine AdV cross-neutralizing antibodies was not detected in human serum samples [22,23]. Moreover, various nonhuman AdV vectors use different receptors for internalization thereby broadening the range of cell types that can be targeted [24,25].
1.4. AdV vectors: usage and current status
Initially, when the therapeutic potential of AdV vectors was realized, they were evaluated for a broad range of medical conditions including genetic diseases and metabolic disorders. However, soon it was realized that transgene expression is usually for a short duration [26,27]. This limits the use of AdV vectors to conditions where transient transgene expression is required for the desired effects, such as recombinant vaccines and cancer therapeutics.
1.4.1. AdV vector-based vaccines for infectious diseases
With the advancement in the field of viral vectored vaccines, various AdV vectors have been tested both in pre-clinical as well as clinical studies [28,6,29] for different infectious diseases. This is due to the fact that AdV vector-based vaccines induce a balanced humoral and cell-mediated immune (CMI) responses [30,31] by stimulating innate immunity through pathways that are both Toll-like receptor (TLR)-dependent and TLR-independent [32,33]. AdV vectors expressing different antigens of influenza virus have been tested in different animal models and have shown high protection efficiency against homologous and heterologous influenza viruses [34-39]. A vaccine construct expressing hemagglutinin (HA) of an H5N1 influenza virus provided cross-protection in mice following challenge with different strains of highly pathogenic H5N1 influenza viruses [40]. Similarly, in a clinical trial, immunization with an AdV vector encoding the HA gene of influenza virus increased hemagglutination inhibition (HI) titers in more than 75% of the participants [41].
With time, several modifications were incorporated in the AdV vector system to overcome the existing limitations of pre-existing vector immunity and inadequate antigen-specific immunogenicity. For this purpose, other AdV types from both human and nonhuman AdVs have been evaluated. An AdV35 vector-based HIV vaccine was assessed in a clinical trial and was found very effective and safe [42]. AdV26, another less common type of human AdV, has been recently evaluated for Ebola vaccine in a clinical trial and it elicited a favorable antibody response [43]. In addition to less prevalent human AdVs, several nonhuman AdVs, in particular chimpanzee AdV (ChAdV) vectors have shown very encouraging results in clinical trials for malaria [44], leishmanial [45] and Ebola [46]. Recently, ChAdV vector, ChAd3-EBOZ, encoding for the Ebola G glycoprotein gene of the Zaire strain showed robust antibody and T cell responses in Phase I and II clinical trials [47]. Another approach which has been adopted to improve transgene immunogenicity is the use of single cycle AdV vectors having the deletion of pIIIa protein-coding gene. A single cycle AdV vector encoding influenza HA was assessed for immunogenicity in both cotton rats and hamsters leading to enhanced immune responses at a low dosage [48].
1.4.2. Oncolytic AdV vector-based therapies for cancer
The oncolytic nature of AdV has been utilized to combat various forms of cancer. To achieve effective oncolysis, the virus should infect and replicate within the cancer cells. Most of human AdVs require Coxsackievirus and Adenovirus Receptor (CAR) for virus internalization, but in many forms of cancer, there is a marked downregulation or complete absence of CAR [49], the reason for a marked reduction in cell transduction with many AdV vectors. To increase the interaction between the virus and cancer cell surface molecules, an introduction of a motif, like RGD, in the knob region of AdV fiber improves the interaction with integrins which are expressed on the cancer cell surface [50]. In some cases, a complete swapping of fiber is done for its preferred interaction with a cell surface molecule such as desmoglein 2, which is expressed in large number on cancer cells [51-54]. It seems very assuring that only tumor cells can be lysed by these oncolytic AdV vectors since their replication competency is dependent on the presence of a specific tumor antigen. A successful oncolytic AdV therapy also requires some other vector modifications to overcome the immunological as well as structural barriers of the tumor microenvironment. Oncolytic AdV vector expressing relaxin facilitates better vector spread in the dense extracellular matrix (ECM) [55]. VCN-01 is another armed oncolytic AdV vector which expresses hyaluronidase and is currently being tested in Phase 1 clinical trials [56]. Recently, oncolytic AdV vector ONCOS 102 expressing GM-CSF demonstrated a potent therapeutic effect with minimal side effects [57]. Many other molecules like interferon alpha, tumor necrosis factor alpha, and other interleukins are also being investigated as delivery molecules with oncolytic AdV vectors. It seems that targeting the tumor microenvironment, in addition to the tumor cell lysis, is a better approach for cancer therapeutics using oncolytic AdV vectors.
1.5. AdV vector types
Several changes have been made in the AdV vector design methodology to improve vector recovery, transgene expression, and safety. AdV vectors can be broadly classified into three types based on the deletions of the viral genes.
1.5.1. First and second generation AdV vectors
First generation AdV vectors contain the deletion of the early (E) region 1 (E1) or E1 & E3 regions of the viral genome. Deletion of the E1 region results in a replication-incompetent vector, and it also serves the purpose of increasing the capacity of the foreign gene cassette for insertion [58]. E1-deleted AdV vectors can only be grown in a cell line (e.g., HEK 293) that constitutively expresses E1 proteins [59]. However, anchorage-dependent cell lines can be used only for the small-scale production of vector preparations. To achieve scalability and batch-to-batch consistency, a suspension cell culture bioreactor system is used with a variant of HEK 293 cell line capable of growing cultures in suspension without serum. A bioreactor with 10,000 L capacity is projected to yield 109 −1010 viral particle/milliliter (VP/mL) [60]. However, the usage of HEK 293 cells can result in the production of contaminating replication-competent AdV due to homologous recombination. The PER.C6 [61] and SL0036 cell lines [62] have been developed with a minimal E1 region to eliminate the possibility of homologous recombination. The major drawback of the first generation AdV vector system is high immunogenicity in the host, which raises safety concern in situations where a very high vector dose is required for desired effects.
Second generation AdV vectors were created to minimize the shortcomings of first-generation vectors. Second generation AdV vectors were designed with deletion of two more gene regions, E2 and/or E4, along with E1 and E3 deletions. The idea was to reduce the vector immunogenicity by minimizing the leaky expression of viral genes [63]. Apart from this, the deletion/s also increase/s the transgene carrying capacity of the vector. However, these multiple regions deleted vectors require an appropriate complimentary cell line for their propagation.
1.5.2. Third generation AdV vectors
Third generation AdV vectors include the helper-dependent vectors, also known as gutless vectors, which are designed by removing all the AdV genes. These vectors retain only the AdV packaging signal along with the inverted terminal repeat (ITR) sequences of the viral genome [64-67]. Due to the complete absence of protein-coding regions in the helper-dependent vectors, a significant reduction in vector immunogenicity with improved safety occurs when these vectors are used in patients. Moreover, the transgene carrying capacity of helper-dependent vector system can be up to 36 kbp. Production of these vectors requires a helper virus – a first generation empty AdV vector. Initially, the low yield of helper-dependent vector and the contamination with the helper virus were two major concerns with this vector system [64]. Both of these concerns were addressed by replacing the helper virus with the AdV vector in which the packaging sequences are flanked with a site-specific recombination sequence, e.g., loxP. The helper-dependent vector is grown with this novel helper vector in a cell line that expresses an appropriate recombinase, e.g., Cre recombinase for loxP sites [68,67]. The loxP-Cre recombinase or an equivalent system will results in the generation of novel helper virus genomes without the packaging sequences thereby allowing efficient packaging of the helper-dependent vector genomes. Third generation AdV vectors have shown promising results in different animal models with minimal adverse effects [69].
1.6. Construction of AdV vectors
Several techniques were developed to construct AdV vectors which can be broadly divided into two approaches: 1) direct insertion of the foreign gene into the viral genomic DNA, in a plasmid form, using unique restriction enzymes [70,30]; and 2) recombination between two plasmids through homologous recombination either in bacteria or in a permissive cell line [71-75]. The two plasmids system includes a genomic plasmid that contains nearly the complete AdV genome with appropriate deletion/s, and a shuttle plasmid carrying the foreign gene cassette and AdV sequences that are essential for homologous recombination and generation of an infectious AdV vector. There are two commonly used recombination techniques for generating AdV vectors: 1) Homologous recombination in bacteria, and 2) Cre/lox recombination in mammalian cells. The detailed protocols to create and purify AdV vectors using these two recombination approaches are described below.
2. Materials
2.1. Generation of AdV vector by homologous recombination in bacteria
Genomic plasmid (pAdV-ΔE1E3)
Shuttle plasmid containing the desired transgene (pAdV-shuttle-T)
E. coli BJ5183 strain: kept in aliquots at −80°C
E. coli DH5α strain: kept in aliquots at −80°C
BIO-RAD Gene Pulser II Electroporation System
0.2 cm electroporation cuvette
Lysogeny broth or Luria-Bertani (LB) broth and LB agar
Ampicillin or carbenicillin 50mg/mL
Miniprep plasmid kit (Mini Plus Plasmid DNA 250 Prp. #GF2002)
Enzymes- PacI, HindIII, and Antarctic phosphatase
1% TAE agarose (50 × 1% TAE: Tris 242 g, glacial acetic acid 57.1 mL, 0.5 M EDTA, pH 8.0 100 mL, and MilliQ water to adjust the volume to 1 L)
GENECLEAN® III Kit (MP Biomedicals)
Maxi Fast Ion Plasmid Kit (IBI Scientific)
Minimum essential medium (MEM)
Opti-MEM (Gibco)
Fetal bovine serum (FBS)
Lipofectamine 2000 (Invitrogen)
Appropriate cell line (i.e. HEK 293) that supports the replication of the desired AdV vector
2.2. Generation of AdV vector using Cre/loxP recombination system
Genomic plasmid pAdVΔψ,E1,E3/loxP
Shuttle plasmid pAdV-shuttle/loxP containing a transgene (pAdV-shuttle/loxP/T)
E. coli DH5α strain: kept in aliquots at −80°C
Lysogeny broth or Luria-Bertani (LB) broth and LB agar.
BIO-RAD Gene Pulser II Electroporation System
0.2 cm electroporation cuvette
Miniprep plasmid kit (Mini Plus Plasmid DNA 250 Prp. #GF2002)
Ampicillin 50mg/mL
GENECLEAN® III Kit (MP Biomedicals)
Maxi Fast Ion Plasmid Kit (IBI Scientific)
1% TAE agarose
CaCl2 2.5 M
Hepes buffer saline (HBS): Hepes 5g/L, NaCl 8g/L, KCl 0.37 g/L, Na2HPO4.2H2O 0.125 g/L, glucose 1 g/L; final pH 7.1
Carrier DNA: salmon sperm DNA (SSDNA) 1mg/mL
Minimum essential medium (MEM)
Fetal bovine serum (FBS)
293Cre4 cell line or any other appropriate cell line
2.3. Purification of AdV vectors
Minimum Essential Medium (MEM) Eagle (Corning).
Fetal bovine serum (FBS)
Gentamicin 50 mg/ml
AdV vector permissive cell line, e.g., HEK 293 for human AdV vectors
10 × Phosphate buffer saline (PBS) pH 7.4: NaCl 80 g, KCl 2 g, Anhydrous Na2HPO4 14.4 g, KH2PO4 2.4 g, MilliQ H2O 900 mL
PBS++: PBS containing 0.1% MgCl2 & 0.1% CaCl2
Sterile cell lifter (scraper)
500 mL centrifuge bottles
0.1M Tris, pH 8.0
Sterile 50 mL centrifuge tubes
5 % Sodium deoxycholate solution
Tissue homogenizer (THb Handheld Tissue Homogenizer, Omni International, Inc #10046-846)
Saturated cesium chloride (CsC1) solution in 0.01M Tris and 0.001M EDTA
Beckman Swinging rotors, SW 40 Ti and SW 55 Ti
Beckman Coulter polyallomer tubes size 13 mL (#331374) and size 5 mL (#326819)
Plastic syringes (3 mL) and 18 G needles
Dialysis tube (Thermo Scientific™ SnakeSkin™ 3.5K MWCO Dialysis Tubing #PI68035)
Sterile glycerol.
3. Methods
3.1. Generation of AdV vector by homologous recombination in bacteria
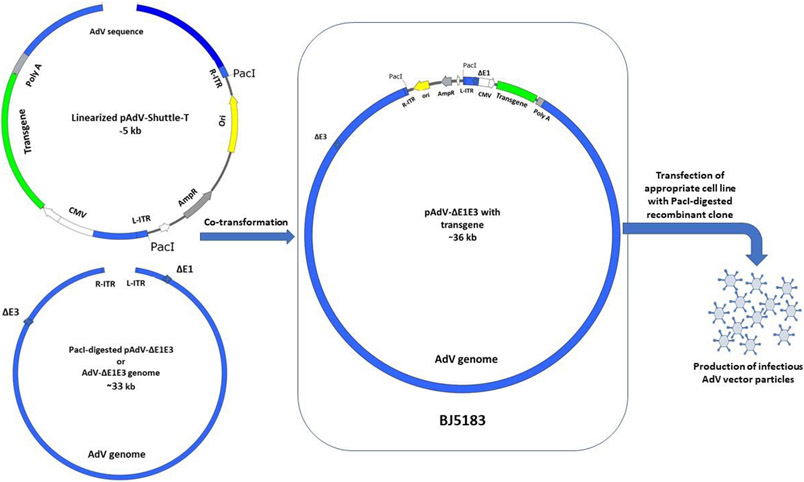
For the construction of first-generation AdV vectors, the genomic plasmid, pAdV-ΔE1E3, comprises the entire AdV genome flanked with a unique restriction enzyme (e.g., PacI) and also contains appropriate E1 and E3 deletions (Fig. 1). The shuttle plasmid, pAdV-shuttle-T, contains both ends of the AdV genome. The E1 deletion replaced with an eukaryotic promoter (e.g., human cytomegalovirus (HCMV) promoter), multiple cloning sites (MCS) for foreign gene insertion, and a polyadenylation (poly A) signal (e.g., bovine growth hormone (BGH) polyA) followed by the AdV sequences for the protein IX gene for homologous recombination (Fig. 1). The shuttle plasmid also contains a unique restriction enzyme site (e.g., PacI) at both ends of AdV sequences, and another unique restriction enzyme site (e.g., HindIII) in between the left and right AdV genome sequences. The genomic DNA is released from pAdV-ΔE1E3 by PacI digestion followed by gel purification. Alternatively, genomic DNA extracted from a purified preparation of AdV-ΔE1E3 (empty vector) can be used. The HindIII-linearized shuttle plasmid containing a transgene (pAdV-shuttle-T) and the AdV genomic DNA are used for co-transformation of Escherichia coli (E. coli) BJ5183. This bacterial strain is recBC sbc positive and thus is capable of homologous recombination (Note 1). The desired recombination will produce pAdV-ΔE1E3/T plasmid carrying the AdV genome with a foreign insert in the E1 region (Fig. 1). The transfection of an appropriate cell line (HEK 293) with PacI-digested pAdV-ΔE1E3/T will result in the generation of infectious AdV vector carrying the desired transgene.
Fig 1. Generation of AdV vector using homologous recombination in bacteria.
After insertion of the desired transgene into AdV vector shuttle plasmid (pAdV-shuttle-T), the pAdV-shuttle-T is linearized by restriction digestion between the right and left ends of AdV of genome. AdV vector genomic plasmid with deletion in E1 and E3 sequences (pAdV-ΔE1E3) is digested to release AdV-ΔE1E3 sequence from the plasmid backbone. The linearized pAdV-shuttle-T and AdV-ΔE1E3 co-transformation into BJ5183 will result in combination between the homologous sequences and create recombinant plasmid clones contain the whole sequence of AdV-ΔE1E3 with the transgene (pAdV-ΔE1E3-transgene) inside an expression cassette. Transfection of a cell line expressing E1 proteins of AdV with pAdV-ΔE1E3-transgene digested with appropriate restriction enzyme (i.e. PacI) to remove the plasmid backbone, will develop AdV vector expressing the inserted transgene protein or peptide.
AdV, adenovirus; R-ITR, right inverted terminal repeats of adenovirus genome; L-ITR, left inverted terminal repeats of adenovirus genome; CMV, cytomegalovirus promoter; PolyA, polyadenylation signal; AmpR, ampicillin resistance gene; Ori, plasmid bacterial origin of replication; ΔE1, Adenovirus genome without the E1 region; ΔE3, Adenovirus genome without the E1 region; BJ5183, E.coli bacterial strain for homologous recombination.
3.1.2. Preparation of plasmid DNA for bacterial transformation
Digest 5 μg of genomic plasmid pAdV-ΔE1E3 with PacI to release AdV-ΔE1E3/T sequences from the plasmid backbone.
Digest 5 μg of the pAdV-shuttle-T plasmid containing the transgene with HindIII to linearize and then dephosphorylate it using Antarctic phosphatase (Note 2).
Purify AdV-ΔE1E3 and the linearized pAdV-shuttle-T by running into 1% TAE agarose gel, collecting the appropriate bands and then recovering the DNA using the GENECLEAN III Kit following the manufacturer’s instructions (Note 3).
3.1.3. Transformation of E. coli BJ5183 for homologous recombination and selection of positive clones
Mix the gel purified AdV-ΔE1E3 DNA (insert) to the linearized dephosphorylated pAdV-shuttle-T DNA (vector) at a 2:1 molar ratio (insert:vector). For example, 0.1 μg linearized and dephosphorylated pAdV-shuttle-T DNA should be combined with 1 μg of Ad-ΔE1E3 DNA.
- Co-transform the E. coli BJ5183 with the insert, and vector DNA mixture using the blow described electro-transformation protocol:
- Thaw a BJ5183 bacterial stock at room temperature and place the tube on ice.
- In a cold 1.5 mL polypropylene tube, mix 50 μl of BJ5183 bacteria with 1-4 μl of the insert and vector DNA mixture.
- Mix well and let sit on ice ~1 minute.
- Set the Gene Pulser apparatus at 25μlF, 2.25kV, and pulse controller to 200 Ω.
- Transfer the mixture of bacteria and DNA to a cold, 0.2 cm electroporation cuvette, and shake the suspension to the bottom of the cuvette.
- Place the cuvette in a shielded safety chamber, slide it into the chamber until the cuvette is seated between the electrode contacts at the base of the chamber.
- Pulse once at the above settings. This setting will produce a pulse with a time constant of 4.5 to 5 msec. The field strength will be 12.5 kV/cm.
- Remove the cuvette from the chamber and immediately add 1 ml of cold LB medium to the cuvette and resuspend the transformed BJ5183 bacteria with a Pasteur pipette. The rapid addition of LB medium after the pulse is essential for maximizing the recovery of transformants.
- Transfer the transformed bacteria from the cuvette to a 15 mL tube and incubate for 15 min at 37°C in a shaking incubator.
- Centrifuge the LB medium containing bacteria at 1,000×g for 5 min, discard 750 μL of the supernatant and then resuspend the bacterial pellet in the remaining 250 μL of LB medium.
- Spread the transformed bacteria onto LB agar plates containing ampicillin or carbenicillin as a selection antibiotic and incubate the plates at 37°C for 18 h.
Using standard culturing techniques, pick small colonies and grow them in LB broth containing the appropriate antibiotic at 37°C overnight (Note 4).
Extract DNA from miniprep cultures using the plasmid isolation kit according to the manufacturer’s instructions. Digest the miniprep DNA samples with appropriate restriction enzyme/s to confirm the positive recombinant clones.
- Since E. coli BJ5183 is not a high copy number strain, one or more positive recombinant DNA clones should be used to electro-transform E. coli Dh5α (as described above) to obtain a high yield of recombinant plasmid DNA.
- Following electro-transformation, incubate the transformed bacteria at 37°C for 1 hour for the plasmid containing the ampicillin-resistant gene or 2 hours for the plasmid containing the kanamycin-resistant gene. The tubes should be shaking at 225 rpm during this incubation to improve the recovery of transformants
- Spread suitable volume (50 μl) of the LB containing transformed Dh5α on LB agar containing the selective antibiotic (ampicillin). Incubate overnight at 37°C for growing transformed colonies.
- Pick up 5 colonies and incubate them separately in 2.5 mL of LB medium containing ampicillin overnight in a shaking incubator.
- Extract the plasmid DNA using a miniprep kit. Digest the extracted DNA with appropriate restriction enzyme/s and run on the agarose gel to confirm the constructs.
- Use the transformed bacteria representing the right constructs and grow them for large-scale plasmid DNA preparations in 200 mL of LB medium with an antibiotic in a 1L flasks. Grow the clones in a 37°C shaking incubator for overnight (16 h).
Extract the plasmid DNA using a maxiprep kit. Determine the plasmid DNA concentration spectrophotometrically at 260nm.
3.1.4. Generation of infectious AdV vector containing the desired transgene by transfection of an appropriate cell line with the recombinant DNA clones
Digest 50-100 μg of recombinant DNA clones with PacI to release the AdV genome containing the transgene cassette.
Clean the digested DNA by phenol/chloroform/isoamyl alcohol (25:24:1) solution, precipitate the DNA with absolute alcohol, and resuspend it in sterile water (Note 5).
Grow the appropriate cells (HEK 293) in 4.5 mL of complete growth medium [minimum essential medium (MEM) supplemented with 10% fetal bovine serum and gentamycin (50 μg/ml)] overnight in four 60 mm tissue culture plates. Make sure the cells will not attain more than 70% confluency at the time of transfection.
Change the media to Opti-MEM (4.5 mL/plate) 2 h before transfection and keep the plates in a CO2 incubator at 37°C.
In four 1.5 mL sterile tubes, add 5 μg of PacI-digested recombinant plasmid DNA and 150 μL Opti-MEM into each tube.
In another four 1.5 mL sterile tubes, dilute 15 μL of lipofectamine 2000 into 150 μL Opti-MEM in each tube.
Incubate all tubes at room temperature for 20-30 min.
From each tube containing Opti-MEM/DNA, add the diluted DNA drop by drop into one of the tubes containing Opti-MEM/lipofectamine. Repeat this process to all four tubes.
Incubate the four tubes containing DNA/lipofectamine mixture at room temperature for 20 min.
Increase the volume of DNA/lipofectamine mixture in each tube to 500 μL with Opti-MEM.
Add the content of each tube (500 μL) drop by drop onto the cells of one of the 60-mm plates. Repeat this process for all four plates.
Incubate the four plates in a CO2 incubator at 37°C for 5-6 h, change the medium to MEM containing 5% fetal bovine serum and continue the incubation. Change the medium every 3-4 days.
The visible cytopathic effects (CPE) due to the generation of infectious AdV vector will be observed any time after 10 days post-transfection (Note 6).
Once the CPE spread to approximately 50% of the cells in the culture plate, collect the cells with the medium in a sterile 15 mL centrifuge tube. A cell lifter can be used to remove the cells from the plate.
Centrifuge at 2000 ×g for 10 min at 4 °C to pellet the infected cells, discard the medium and keep approximately 1 mL of the medium to resuspend the pelleted cells.
Freeze and thaw the cell suspension 3 times. Centrifuge at 2000 ×g for 10 min at 4 °C to pellet the cell debris and collect the supernatant. Use 0.5 mL of the supernatant to infect a 100 mm culture plate containing appropriate cells (e.g., HEK 293) at approximately 75% confluency.
Incubate the plate in a CO2 incubator at 37°C for approximately 42-48 h. Collect the infected cells when CPE is about 80-90%. Follow the freezing and thawing process again and this time infect 150 mm culture plate containing appropriate cells (e.g., HEK 293) at about 90% confluency. Incubate the plate in a CO2 incubator at 37°C for approximately 48 h.
Collect the infected cells when CPE is approximately 90-100%, centrifuge at 2000 ×g for 10 min at 4°C to pellet the infected cells and discard the supernatant. Resuspend the infected cell pellet in 1 mL of PBS++ containing 10% glycerol. Freeze and thaw 3 times and store this crude virus stock at −80°C. This virus stock is used to make a large stock of the crude virus, which eventually will be used for growing a large scale of AdV vector for purification (Note 7).
For downstream processing, please refer to section 3.3.
3.2. Generation of AdV vector using Cre/loxP recombination system
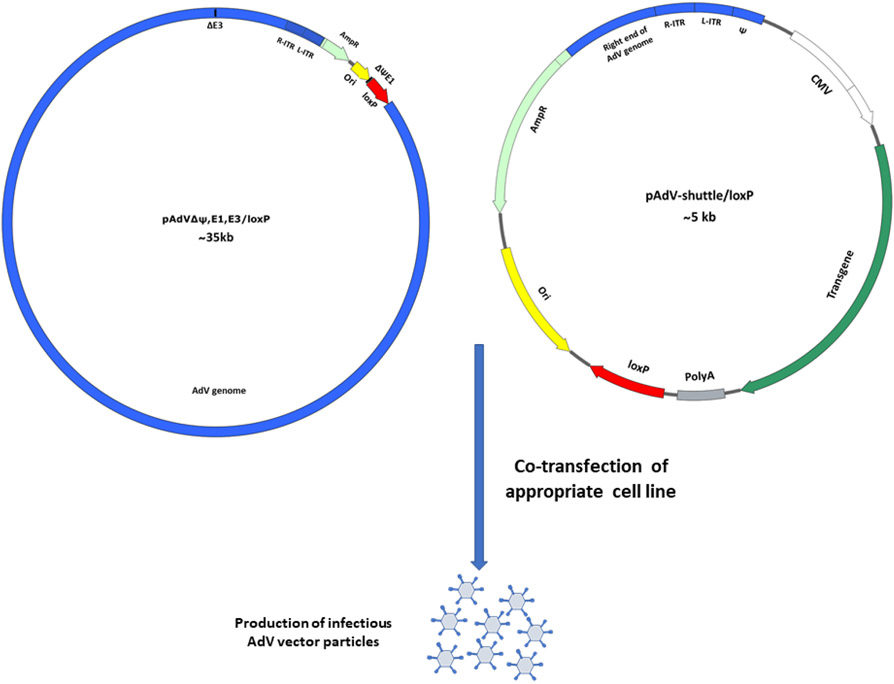
In this method, the genomic plasmid pAdVΔψ,E1,E3/loxP contains the entire AdV genome except for the packaging signal (ψ) sequences, E1, and E3 regions, and a loxP site is added just after the E1 deletion for recombination (Fig. 2). The shuttle plasmid, pAdV-shuttle/loxP, carries both ends of the AdV genome, the ψ sequences, the E1 region deletion is replaced with the HCMV promoter, multiple cloning sites, and the BGH poly A (Fig. 2). Just after the BGH ploy A, a loxP site is added. To obtain human AdV vectors by Cre/loxP recombination, HEK 293 cell line carrying the Cre recombinase gene (293Cre4) can be transfected with the genomic plasmid and the shuttle plasmid containing a transgene cassette [76,75].
Fig. 2. Generation of AdV vector using Cre/loxP recombination system.
Co-transfection of pAdVΔψ,E1,E3/loxP and pAdVshuttle/loxP (AdV vector shuttle plasmid for cre/loxP recombinase system) containing the desired transgene, into a cell line expressing Cre recombinase protein and E1 proteins of AdV, AdV vector expressing the inserted transgene protein or peptide will be generated.
AdV, adenovirus; R-ITR, right inverted terminal repeats of adenovirus genome; L-ITR, left inverted terminal repeats of adenovirus genome; CMV, cytomegalovirus promoter; PolyA, polyadenylation signal; AmpR, ampicillin resistance gene; Ori, plasmid bacterial origin of replication; ΔE1, Adenovirus genome without the E1 region; ΔE3, Adenovirus genome without the E1 region; ψ, AdV packaging signal.
3.2.1. Co-transfection of 293Cre4 to recover infectious AdV vector
Purify the genomic (pAdVΔψ,E1,E3/loxP) and shuttle plasmid containing the transgene (pAdV-shuttle/loxP/T) by maxiprep using a kit.
Grow 293Cre4 (or appropriate cells) overnight in 60 mm tissue culture plates making sure that the cells will attain confluency of approximately 70-90% at the time of transfection.
Change the medium with 4.5 mL of MEM containing 10% FBS 2 h before transfection.
2 ml of HBS containing 20 μg SSDNA (10μg/mL HBS) is sheared by vortex for 1 min, and 0.5 mL of this solution is aliquoted into four 1.5 mL sterile tubes.
Add 10μg of pAdVΔψ,E1,E3/loxP and 5μg of pAdV-shuttle/loxP/T to each 1.5 mL tube from step 4. Incubate HBS and plasmid DNA mixture for 30-45 min at room temperature (Note 8).
Add 25 μL of 2.5 M CaCl2 into each 1.5 mL tube and incubated them for 20-30 min at room temperature (Note 9).
Add the content of each 1.5 mL tube drop by drop onto 293Cre4 cells in one of the 60-mm plates and repeat the same procedures in the other 3 plates.
Incubate the plates at 37°C in a CO2 incubator for 4-5 h, and then change the medium with 5 mL of MEM containing 5% FBS.
The media should be changed every 3-4 days, and the CPE depicting the generation of infectious AdV vector should appear any time after 7 days post-transfection.
After the spread of the CPE to at least 50% of the plate, collect the cells and the medium and prepare the crude virus stock as mentioned above (section 3.1.4).
For downstream processing, please refer to section 3.3.
3.3. Purification of AdV vectors
Purified stocks of AdV vectors are required for all preclinical and clinical studies to evaluate the efficacy of the desired AdV vectors. Cesium chloride (CsCl) density-gradient centrifugation technique is the most common and reliable method for AdV vector purification for preclinical studies in animal models.
3.3.1. Purification of a large stock of AdV vector
Split HEK 293 cells into thirty 150 mm tissue culture plates in MEM containing 10% FCS and incubate them overnight at 37°C in a CO2 incubator (Note 10).
When the cells are approximately 90% confluent (usually after 24 h incubation), infect the cells at a multiplicity of infection (MOI) of 5 plaque forming units (PFU) of the desired AdV vector after diluting the crude vector stock in PBS++ (Note 11).
For infection, remove the medium from each plate and add 3 mL of PBS++ containing the appropriate amount of vector.
Incubate the infected plates at 37°C in a CO2 incubator for 30 minutes and then add the 25 mL of maintenance medium (MEM + 2% FCS) per plate.
Incubate the infected plates at 37°C in a CO2 incubator until complete CPE is observed. The CPE is characterized by cell rounding and detachment and is usually achieved within 48 h post-infection.
Harvest the infected cells and the culture medium using a cell lifter and collect into a sterile 500 mL centrifuge bottle. Centrifuge the contents at 2000 ×g for 10 min at 4°C to pellet the infected cells. Discard the supernatant in another container and autoclave the discarded supernatant.
Resuspend the pellet in 15 mL of 0.1 M Tris, pH 8.0 solution and transfer it to a 50 mL tube.
Add 1.5 mL of 5% sodium deoxycholate solution, vortex for 30 seconds (the content will be more viscous due to cell lysis and the release of cellular DNA), and incubate the tube on ice for 30 min and vortex every 10 min.
Use a tissue homogenizer to homogenize the mixture 3 times at the medium speed for 10 seconds and 2 times at high speed for 5 seconds to shear the DNA. This step should be done in a hood, and the sample should be kept on ice during processing.
3.3.2. AdV vector purification by CsCl density gradient centrifugation
Add 0.58 mL saturated CsC1 solution for each 1 mL of the homogenized vector suspension (section 3.3, step 9).
Mix well and distribute the mixture into two 13 mL Beckman polyallomer tubes and spin in SW40 Ti rotor for 16 h at 4°C at 35,000 rpm.
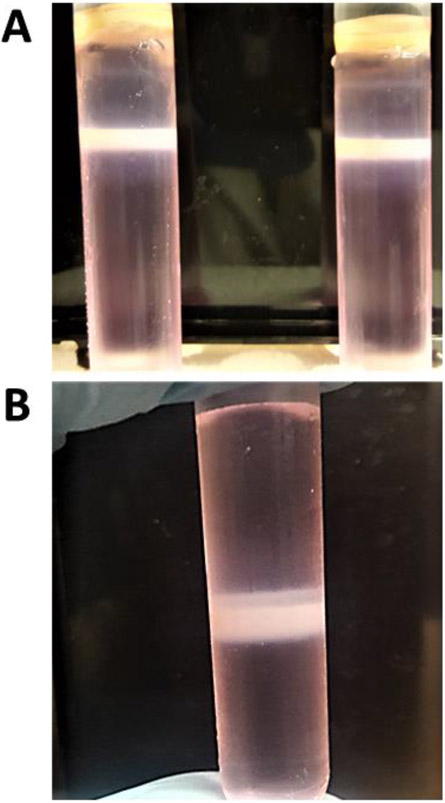
Collect the white vector bands (Fig. 3A) by piercing the tube wall just below the vector band with a needle attached to a 3 mL syringe. Pool the virus bands into a 5 mL Beckman polyallomer tube (use saturated CsC1 0.58 ml for each 1 ml of 0.1 M tris PH 8 for a balance tube) (Note 12).
Centrifuge the pooled vector in SW 55 Ti rotor at 35,000 rpm at 4°C for 16 h. Collect the white vector band from the 5 mL Beckman polyallomer tube (Fig. 3B) and keep it at 4°C till dialysis (do not keep the vector with CsCl for longer than 1 day at 4°C).
Prepare the dialysis tube by boiling in 500 mL of 1mM EDTA, pH 8.0, for 10 min. After cooling of the dialysis tube, clip the tube from one end and transfer the purified vector in CsCl solution to the tube and clip the other end.
Dialyze the vector in 1 L PBS for 2 h twice and in 1 L PBS++ for 2 h once at 4°C. After dialysis, collect the purified AdV vector from the dialysis tube into a sterile tube in a biosafety cabinet.
Add 1/10 volume of sterile glycerol in the purified AdV vector preparation, mix, make aliquots (0.5- 1 mL each), label the vials and store at −80°C.
Titration of the AdV vector can be done by the plaque assay in a suitable cell line. The vector particle count can be done by measuring OD at 260 nm using a spectrophotometer [77-79].
Fig. 3. Purification of AdV vector by CsCl density-gradient ultracentrifugation.
A) After the first round of CsCl density-gradient ultracentrifugation, the whitish color vector band is collected from two 13 mL tubes and pooled. B) The pooled vector preparation is subjected to a second round of CsCl density-gradient ultracentrifugation in a 5mL tube, and the whitish color vector band is collected for further processing.
4. Notes
AdV vector can be modified in any part of its genome by bacterial homologous recombination.
Dephosphorylation of the vector plasmid for recombination in bacteria is essential to decrease the shuttle vector colonies during selection.
If DNA fragment/s will be used for cloning or transfection, electrophoresis of DNA in an agarose gel containing ethidium bromide should be done in the dark to avoid the DNA damage by the light.
Colonies with the right recombinant plasmid are usually of small size following bacterial recombination in E. coli BJ5183.
All DNA samples for transfection should be dissolved in sterile water under sterile conditions.
The adenovirus CPE will be visible under a light microscope with rounded darker cells, which later detached from the plate and float in the medium.
A good AdV crude stock should have approximately 1010 infectious virus particles/mL and 5-10 μL of this stock will be enough to infect one 150 mm plate with 90% cell confluency to get complete CPE within 48 h.
The Cre/loxP transfection method differs from lab to lab in the ratio of the big plasmid (genomic) and the small plasmid (shuttle). We get the best results with 10 μg of the genomic plasmid + 5 μg of the shuttle plasmid.
After adding 2.5 M CaCl2 into the HBS/DNA mixture, fine precipitates are expected, making the solution somewhat whitish. If coarse white precipitates are formed, it may adversely impact the transfection efficiency.
The split ratio of HEK 293 cells is 1:3 and will take 3-4 days to be confluent. Ten 150 mm plates with confluent HEK 293 cells will be needed to seed thirty 150 mm plates, which will become 90% confluency in 3-4 days.
For infecting thirty 150 mm culture plates with the crude Adv stock, use 300-600 μL of the crude virus stock in 90 mL PBS++, mix and use 3 mL for each plate.
After ultracentrifugation of the AdV vector homogenate in CsCl density-gradient, the vector band appears as a white band in the ultracentrifuge tube. To collect the vector band, a 3 mL syringe and 18 G needle are used to puncture the tube at an oblique angle 5 mm below the band. The band will be sucked slowly into the syringe.
Acknowledgments:
This work was supported by the Public Health Service grant - AI059374 from the National Institute of Allergy and Infectious Diseases, and the Hatch and Animal Health funds.
References
- 1.Rowe WP, Huebner RJ, Gilmore LK, et al. (1953) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84 (3):570–573 [DOI] [PubMed] [Google Scholar]
- 2.Enders JF, Bell JA, Dingle JH, et al. (1956) Adenoviruses: group name proposed for new respiratory-tract viruses. Science (New York, NY) 124 (3212):119–120 [DOI] [PubMed] [Google Scholar]
- 3.Davison AJ, Benko M, Harrach B (2003) Genetic content and evolution of adenoviruses. J Gen Virol 84 (Pt 11):2895–2908. doi: 10.1099/vir.0.19497-0 [DOI] [PubMed] [Google Scholar]
- 4.Ewer KJ, Lambe T, Rollier CS, et al. (2016) Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol 41:47–54. doi: 10.1016/j.coi.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 5.Su C (2011) Adenovirus-Based Gene Therapy for Cancer. In: Xu K (ed) Viral Gene Therapy. InTech, Rijeka, p Ch. 06. doi: 10.5772/19757 [DOI] [Google Scholar]
- 6.Pesonen S, Kangasniemi L, Hemminki A (2011) Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm 8 (1):12–28. doi: 10.1021/mp100219n [DOI] [PubMed] [Google Scholar]
- 7.Tatsis N, Ertl HC (2004) Adenoviruses as vaccine vectors. Mol Ther 10 (4):616–629. doi: 10.1016/j.ymthe.2004.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vemula SV, Amen O, Katz JM, et al. (2013) Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res 178 (2):398–403. doi: 10.1016/j.virusres.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Tandon M, Bangari DS, et al. (2009) Adenoviral vector-based strategies for cancer therapy. Curr Drug ther 4 (2):117–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raper SE, Chirmule N, Lee FS, et al. (2003) Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80 (1-2):148–158 [DOI] [PubMed] [Google Scholar]
- 11.Harvey BG, Worgall S, Ely S, et al. (1999) Cellular immune responses of healthy individuals to intradermal administration of an E1-E3- adenovirus gene transfer vector. Hum Gene Ther 10 (17):2823–2837. doi: 10.1089/10430349950016555 [DOI] [PubMed] [Google Scholar]
- 12.Zaiss AK, Machado HB, Herschman HR (2009) The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem 108 (4):778–790. doi: 10.1002/jcb.22328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey A, Singh N, Vemula SV, et al. (2012) Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS One 7 (3):e33428. doi: 10.1371/journal.pone.0033428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mast TC, Kierstead L, Gupta SB, et al. (2010) International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28 (4):950–957. doi: 10.1016/j.vaccine.2009.10.145 [DOI] [PubMed] [Google Scholar]
- 15.Pratt WD, Wang D, Nichols DK, et al. (2010) Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol 17 (4):572–581. doi: 10.1128/cvi.00467-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wold WS, Toth K (2013) Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr Gene Ther 13 (6):421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seregin SS, Amalfitano A (2010) Improving adenovirus based gene transfer: strategies to accomplish immune evasion. Viruses 2 (9):2013–2036. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay MA, Meuse L, Gown AM, et al. (1997) Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci U S A 94 (9):4686–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelhardt JF, Ye X, Doranz B, et al. (1994) Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A 91 (13):6196–6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangari DS, Mittal SK (2006) Development of nonhuman adenoviruses as vaccine vectors. Vaccine 24 (7):849–862. doi: 10.1016/j.vaccine.2005.08.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittal SK, Ahi YS, Vemula SV (2016) 19 - Xenogenic Adenoviral Vectors A2 - Curiel, David T. In: Adenoviral Vectors for Gene Therapy (Second Edition). Academic Press, San Diego, pp 495–528. doi: 10.1016/B978-0-12-800276-6.00019-X [DOI] [Google Scholar]
- 22.Singh N, Pandey A, Jayashankar L, et al. (2008) Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther 16 (5):965–971. doi: 10.1038/mt.2008.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffatt S, Hays J, HogenEsch H, et al. (2000) Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology 272 (1):159–167. doi: 10.1006/viro.2000.0350 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Bangari DS, Sharma A, et al. (2009) Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology 392 (2):162–168. doi: 10.1016/j.virol.2009.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangari DS, Sharma A, Mittal SK (2005) Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem Biophys Res Commun 331 (4):1478–1484. doi: 10.1016/j.bbrc.2005.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrhardt A, Xu H, Kay MA (2003) Episomal Persistence of Recombinant Adenoviral Vector Genomes during the Cell Cycle In Vivo. In: J Virol, vol 77. vol 13. pp 7689–7695. doi: 10.1128/jvi.77.13.7689-7695.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiler MP, Cerullo V, Lee B (2007) Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther 7 (5):297–305 [DOI] [PubMed] [Google Scholar]
- 28.Gene Therapy Clinical Trials Worldwide. (2017. Available from; http://www.abedia.com/wiley/vectors.php). http://www.abedia.com/wiley/vectors.php.
- 29.Smaill F, Jeyanathan M, Smieja M, et al. (2013) A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med 5 (205):205ra134. doi: 10.1126/scitranslmed.3006843 [DOI] [PubMed] [Google Scholar]
- 30.Vemula SV, Mittal SK (2010) Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther 10 (10):1469–1487. doi: 10.1517/14712598.2010.519332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahi YS, Bangari DS, Mittal SK (2011) Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther 11 (4):307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Huang X, Yang Y (2007) Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol 81 (7):3170–3180. doi: 10.1128/jvi.02192-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Tandon M, Ahi YS, et al. (2010) Evaluation of Cross-Reactive Cell-Mediated Immune Responses among Human, Bovine and Porcine Adenoviruses. Gene Ther 17 (5):634–642. doi: 10.1038/gt.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan AO, Amen O, Sayedahmed EE, et al. (2017) Adenovirus vector-based multi-epitope vaccine provides partial protection against H5, H7, and H9 avian influenza viruses. PLoS One 12 (10):e0186244. doi: 10.1371/journal.pone.0186244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim EH, Han GY, Nguyen H (2017) An Adenovirus-Vectored Influenza Vaccine Induces Durable Cross-Protective Hemagglutinin Stalk Antibody Responses in Mice. Viruses 9 (8). doi: 10.3390/v9080234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamlangdee A, Kingstad-Bakke B, Anderson TK, et al. (2014) Broad protection against avian influenza virus by using a modified vaccinia Ankara virus expressing a mosaic hemagglutinin gene. J Virol 88 (22):13300–13309. doi: 10.1128/jvi.01532-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoelscher MA, Singh N, Garg S, et al. (2008) A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis 197 (8):1185–1188. doi: 10.1086/529522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao W, Liepkalns JS, Hassan AO, et al. (2016) A highly immunogenic vaccine against A/H7N9 influenza virus. Vaccine 34 (6):744–749. doi: 10.1016/j.vaccine.2015.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vries RD, Rimmelzwaan GF (2016) Viral vector-based influenza vaccines. Hum Vaccin Immunother 12 (11):2881–2901. doi: 10.1080/21645515.2016.1210729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoelscher MA, Garg S, Bangari DS, et al. (2006) Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet (London, England) 367 (9509):475–481. doi: 10.1016/s0140-6736(06)68076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Kampen KR, Shi Z, Gao P, et al. (2005) Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 23 (8):1029–1036. doi: 10.1016/j.vaccine.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 42.Fuchs JD, Bart PA, Frahm N, et al. (2015) Safety and Immunogenicity of a Recombinant Adenovirus Serotype 35-Vectored HIV-1 Vaccine in Adenovirus Serotype 5 Seronegative and Seropositive Individuals. J AIDS Clin Res 6 (5). doi: 10.4172/2155-6113.1000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milligan ID, Gibani MM, Sewell R, et al. (2016) Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA 315 (15):1610–1623. doi: 10.1001/jama.2016.4218 [DOI] [PubMed] [Google Scholar]
- 44.O'Hara GA, Duncan CJ, Ewer KJ, et al. (2012) Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 205 (5):772–781. doi: 10.1093/infdis/jir850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osman M, Mistry A, Keding A, et al. (2017) A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl Trop Dis 11 (5):e0005527. doi: 10.1371/journal.pntd.0005527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledgerwood JE, DeZure AD, Stanley DA, et al. (2017) Chimpanzee Adenovirus Vector Ebola Vaccine. N Engl J Med 376 (10):928–938. doi: 10.1056/NEJMoa1410863 [DOI] [PubMed] [Google Scholar]
- 47.Tapia MD, Sow SO, Lyke KE, et al. (2016) Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 16 (1):31–42. doi: 10.1016/s1473-3099(15)00362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crosby CM, Matchett WE, Anguiano-Zarate SS, et al. (2017) Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J Virol 91 (2). doi: 10.1128/jvi.00720-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Pong RC, Bergelson JM, et al. (1999) Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res 59 (2):325–330 [PubMed] [Google Scholar]
- 50.Shen YH, Yang F, Wang H, et al. (2016) Arg-Gly-Asp (RGD)-Modified E1A/E1B Double Mutant Adenovirus Enhances Antitumor Activity in Prostate Cancer Cells In Vitro and in Mice. PloS one 11 (1):e0147173. doi: 10.1371/journal.pone.0147173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Li ZY, Liu Y, et al. (2011) Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17 (1):96–104. doi: 10.1038/nm.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biedermann K, Vogelsang H, Becker I, et al. (2005) Desmoglein 2 is expressed abnormally rather than mutated in familial and sporadic gastric cancer. J Pathol 207 (2):199–206. doi: 10.1002/path.1821 [DOI] [PubMed] [Google Scholar]
- 53.Harada H, Iwatsuki K, Ohtsuka M, et al. (1996) Abnormal desmoglein expression by squamous cell carcinoma cells. Acta Derm Venereol 76 (6):417–420 [DOI] [PubMed] [Google Scholar]
- 54.Schmitt CJ, Franke WW, Goerdt S, et al. (2007) Homo- and heterotypic cell contacts in malignant melanoma cells and desmoglein 2 as a novel solitary surface glycoprotein. J Invest Dermatol 127 (9):2191–2206. doi: 10.1038/sj.jid.5700849 [DOI] [PubMed] [Google Scholar]
- 55.Lee SY, Park HR, Rhee J, et al. (2013) Therapeutic effect of oncolytic adenovirus expressing relaxin in radioresistant oral squamous cell carcinoma. Oncol Res 20 (9):419–425. doi: 10.3727/096504013x13657689383139 [DOI] [PubMed] [Google Scholar]
- 56.Vera B, Martinez-Velez N, Xipell E, et al. (2016) Characterization of the Antiglioma Effect of the Oncolytic Adenovirus VCN-01. PloS one 11 (1):e0147211. doi: 10.1371/journal.pone.0147211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ranki T, Pesonen S, Hemminki A, et al. (2016) Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer 4:17. doi: 10.1186/s40425-016-0121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danthinne X, Imperiale MJ (2000) Production of first generation adenovirus vectors: a review. Gene Ther 7 (20):1707–1714. doi: 10.1038/sj.gt.3301301 [DOI] [PubMed] [Google Scholar]
- 59.Graham FL, Smiley J, Russell WC, et al. (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36 (1):59–74. doi: 10.1099/0022-1317-36-1-59 [DOI] [PubMed] [Google Scholar]
- 60.Kamen A, Henry O (2004) Development and optimization of an adenovirus production process. J Gene Med 6 Suppl 1:S184–192. doi: 10.1002/jgm.503 [DOI] [PubMed] [Google Scholar]
- 61.Fallaux FJ, Bout A, van der Velde I, et al. (1998) New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 9 (13):1909–1917. doi: 10.1089/hum.1998.9.13-1909 [DOI] [PubMed] [Google Scholar]
- 62.Howe JA, Pelka P, Antelman D, et al. (2006) Matching complementing functions of transformed cells with stable expression of selected viral genes for production of E1-deleted adenovirus vectors. Virology 345 (1):220–230. doi: 10.1016/j.virol.2005.09.029 [DOI] [PubMed] [Google Scholar]
- 63.Wen S, Schneider DB, Driscoll RM, et al. (2000) Second-generation adenoviral vectors do not prevent rapid loss of transgene expression and vector DNA from the arterial wall. Arterioscler Thromb Vasc Biol 20 (6):1452–1458 [DOI] [PubMed] [Google Scholar]
- 64.Mitani K, Graham FL, Caskey CT, et al. (1995) Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc Natl Acad Sci U S A 92 (9):3854–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parks R, Evelegh C, Graham F (1999) Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther 6 (9):1565–1573. doi: 10.1038/sj.gt.3300995 [DOI] [PubMed] [Google Scholar]
- 66.Alba R, Bosch A, Chillon M (2005) Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther 12 Suppl 1:S18–27. doi: 10.1038/sj.gt.3302612 [DOI] [PubMed] [Google Scholar]
- 67.Parks R, Chen L, Anton M, et al. (1996) A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. In: Proc Natl Acad Sci U S A, vol 93. vol 24. pp 13565–13570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardy S, Kitamura M, Harris-Stansil T, et al. (1997) Construction of adenovirus vectors through Cre-lox recombination. J Virol 71 (3):1842–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vetrini F, Ng P (2010) Gene therapy with helper-dependent adenoviral vectors: current advances and future perspectives. Viruses 2 (9):1886–1917. doi: 10.3390/v2091886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stow ND (1981) Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol 37 (1):171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He TC, Zhou S, da Costa LT, et al. (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95 (5):2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chartier C, Degryse E, Gantzer M, et al. (1996) Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol 70 (7):4805–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu C, Lei X, Wang J, et al. (2011) Generation of a replication-deficient recombinant human adenovirus type 35 vector using bacteria-mediated homologous recombination. J Virol Methods 177 (1):55–63. doi: 10.1016/j.jviromet.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 74.Luo J, Deng ZL, Luo X, et al. (2007) A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2 (5):1236–1247. doi: 10.1038/nprot.2007.135 [DOI] [PubMed] [Google Scholar]
- 75.Ng P, Parks RJ, Cummings DT, et al. (1999) A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther 10 (16):2667–2672. doi: 10.1089/10430349950016708 [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Anton M, Graham FL (1996) Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat Cell Mol Genet 22 (6):477–488 [DOI] [PubMed] [Google Scholar]
- 77.Mittereder N, March KL, Trapnell BC (1996) Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 70 (11):7498–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maizel JV Jr., White DO, Scharff MD (1968) The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36 (1):115–125 [DOI] [PubMed] [Google Scholar]
- 79.Rux JJ, Burnett RM (2007) Large-Scale Purification and Crystallization of Adenovirus Hexon. In: Wold WSM, Tollefson AE (eds) Adenovirus Methods and Protocols: Volume 2: Ad Proteins, RNA Lifecycle, Host Interactions, and Phylogenetics. Humana Press, Totowa, NJ, pp 231–250. doi: 10.1007/978-1-59745-277-9_17 [DOI] [PubMed] [Google Scholar]