Abstract
Introduction
Visual assessment of the placenta in antenatal magnetic resonance imaging is important to confirm healthy appearances or to identify pathology complicating fetal anomaly or maternal disease.
Methods
We assessed the placenta in a large cohort of 228 women with low and high risk pregnancies across gestation. All women gave written informed consent and were imaged using either a 3T Philips Achieva or 1.5T Philips Ingenia scanner. Images were acquired with a T2-weighted single shot turbo spin echo sequence of the whole uterus (thereby including placenta) for anatomical information.
Results
A structured approach to visual assessment of the placenta on T2-weighted imaging has been provided including determination of key anatomical landmarks to aid orientation, placental shape, signal intensity, lobularity and granularity. Transient factors affecting imaging are shown including the effect of fetal movement, gross fetal motion and contractions. Placental appearances across gestation in low risk pregnancies are shown and compared to pregnancies complicated by preeclampsia and chronic hypertension. The utility of other magnetic resonance techniques (T2* mapping as an indirect marker for quantifying oxygenation) and histological assessment alongside visual assessment of placental T2-weighted imaging are demonstrated.
Discussion
A systematic approach with qualitative descriptors for placental visual assessment using T2-weighted imaging allows confirmation of normal placental development and can detect placental abnormalities in pregnancy complications. T2-weighted imaging can be visually assessed alongside functional imaging (such as T2* maps) in order to further probe the visual characteristics seen.
Keywords: Placenta, magnetic resonance imaging (MRI), T2 weighted imaging
Introduction
To date, the main clinical role for placental magnetic resonance imaging has been to assess the abnormally invasive placenta [1]. For the clinician performing antenatal fetal magnetic resonance imaging, there are relatively few published data to assist identification of placental appearances in healthy pregnancies across gestation, or pregnancies with complications such as hypertensive disease, fetal growth restriction, twin pregnancies and preterm premature rupture of membranes (PPROM).
An assessment of the placenta could be performed as part of any antenatal magnetic resonance examination regardless of clinical indication, as part of a comprehensive evaluation of the feto-placental unit. Placental assessment may aid in confirmation of normal development or provide additional information not revealed by routine ultrasound scan. Examples include succenturiate lobes, the presence of which would aid delivery management as there is an increased risk of retaining placental tissue within the uterus and thus there would be a low threshold for surgical intervention if the placenta was incomplete to reduce post partum maternal haemorrhage. Appearances not consistent with normal placental maturation may prompt closer monitoring of fetal growth or maternal hypertension. The presence of placental abnormalities in association with a fetal chromosomal defect is well recognised [2]. An ability to assess the placenta adds clinical value to the fetal magnetic resonance examination and may assist clinical management, in addition to indicating whether placental dysfunction may underlie or accompany specific fetal anomalies [3].
Magnetic resonance imaging has several advantages over the current visualisation of the placenta in vivo in comparison with ultrasound. Magnetic resonance imaging has superior contrast, with a larger field of view with scope for quantitative structural and functional analysis. Magnetic resonance imaging is more user independent and robust to factors such as oligohydramnios, fetal position and maternal body mass index compared to ultrasound imaging if acquisition is performed in a consistent manner and with knowledge of different factors, including magnet strength, positioning of the woman in the scanner, sequence parameters and planes of acquisition, which can influence visual appearances and alter quantification. When images are optimally acquired with a standardised approach, visual analysis can be rapid, simple and easy to adopt in a clinical environment. It is also possible to use automatic processing pipelines to assist in quantitative image analysis (for example in segmentation, T2* mapping and diffusion mapping) thus speeding up analysis and minimising operator variability.
There is a paucity in the literature of placental visual descriptors using magnetic resonance imaging. This may be due to the limited availability of antenatal magnetic resonance imaging and current clinical indications are mostly centred around fetal anomalies. Healthy uncomplicated pregnancies are not routinely imaged and thus research studies are required to examine healthy pregnancies in large cohort studies. The placenta is normally discoid in shape, approximately 20cm in diameter and centrally 1.5–2.5cm thick at term [4] with tapered edges. A grading system, analogous to the Grannum grading system [5], based on signal intensity of T2-weighted imaging at 1.5 Tesla has been proposed previously [6] but its application to pregnancy complications and adverse pregnancy outcomes warrant further investigation. Recent published research studies using more advanced sequences such as placental T2* and diffusion acquired at both 1.5 and 3 Tesla provide quantifed data on the placenta in health and disease but often without detailed visual description.
The aim of this project was to provide a systematic approach to visual assessment of the typical placenta using data collected from uncomplicated pregnancies, acquired through T2-weighted imaging, at both 1.5 and 3 Tesla. Placental imaging of the abnormally invasive placenta was not the focus of this study. We additionally aimed to provide further information on visual appearances using more advanced MR sequences (T2* mapping) and with pregnancy complications, and a linked online placental atlas.
Methods
All women included in this study gave written informed consent (Ethics numbers 07/H0707/105 16/LO1573 14/LO1169 14/LO1806 17/LO0282 19/SS0032 19/LO0852). In the majority of women placental imaging was performed solely for research; for the reminder of the women, there was a clinical indication to obtain the MRI and women consented to additional research sequences. Low risk pregnancies fulfilled the following criteria: no diagnosis of a hypertensive disorder at enrolment and until delivery, no significant past medical history, no pregnancy complications (including gestational diabetes mellitus), delivery at term with birthweight between the third and 97th centile (calculated using International Fetal and Newborn Growth Consortium for the 21st Century version 1.3.5)[7]. High risk pregnancies included women with a hypertensive disorder of pregnancy, those who had an abnormality detected on routine clinical anomaly ultrasound screening and those who delivered preterm. Exclusion criteria were as follows for all women: under 16 years of age, contraindication for magnetic resonance imaging (e.g. implanted electric and electronic devices), intracranial metal clips, metallic bodies in the eyes, surgery in the preceding 12 weeks and claustrophobia.
Women were imaged using either a 3T Philips Achieva or 1.5T Philips Ingenia scanner. Women were imaged supine with a short period of lying left lateral prior to slowly rotating to supine position for imaging. All women were imaged with the presence and support of either a midwife or obstetrician. A blood pressure was taken every 10 minutes during imaging with concomitant heart rate and oxygen saturation monitoring.
At 3T Philips Achieva, a long echo time (180ms) T2-weighted single shot turbo spin echo sequence of the whole uterus (thereby including placenta) was acquired in maternal coronal and sagittal planes with repetition time (TR) = 16s, SENSitivity Encoding (SENSE) = 2.5 and partial Fourier 0.625. In-plane resolution was 1.5 mm x 1.5mm, slice thickness 2.5mm with an overlap of 0.5mm. The field of view was 300 × 360 × [100–200] mm (coronal) and 300 × 300 × 340 mm (sagittal) in the foothead (FH) x right-left (RL) x anterior-posterior (AP) directions respectively. For T2* mapping, a multi-echo gradient echo echo planar imaging at 3mm3 resolution was performed with free breathing and took less than one minute, with the whole placenta covered within 60 slices (5 echo times: 13.81ms/70.40ms/127.00ms/183.60ms/240.2ms, TR=3s, SENSE=3, halfscan=0.6).The acquisition and processing pipeline for T2* mapping has been published and is freely available [8].
At 1.5T Philips Ingenia, imaging parameters were adapted to the longer T2* at this field strength and the different acoustic properties of the scanner resulting in a resolution of 2.5mm isotropic, FOV of 380×380×340mm, 5 echo times (11.376ms/57.313ms/103.249ms/149.186ms/195.122ms), no SENSE, halfscan=0.
Results
Following a review of 228 magnetic resonance imaging examinations (135 in low risk pregnancies (median gestational age at imaging 29.1, interquartile range 25.7–33.7) and 93 high risk pregnancies (median gestational age at imaging 29.9, interquartile range 25.3–33.6)), we describe a systematic approach (Table 1) to visual assessment of the placenta using multiple examples. 197 (86%) magnetic resonance imaging examinations were performed solely for research. In 31 examinations (14%), there was a clinical indication for magnetic resonance imaging and these women consented to additional research sequences.
Table 1:
Parameters and possible findings in visual assessment of placental antenatal magnetic resonance imaging
| Parameter assessed | Possible findings |
|---|---|
| Placental Site | Anterior, posterior, lateral, fundal High, low lying, praevia (Figure 5) Extra lobes |
| Placental Shape | Discoid, bilobed, succenturiate (Figure 3) |
| Umbilical cord appearances and insertion site | Vasa praevia, two vessel umbilical cord |
| Amniotic fluid volume | Oligohyramnios, anhydramnios, polyhydramnios The amniotic fluid index can be calculated using the sagittal plane and four quadrant deepest pools, measured in a way similar to that used in ultrasound. |
| Placental surface | Contraction (Figure 3) |
| Placental parenchyma | Lobularity, size and number of lobules Granularity. High granularity is defined as the presence of both high and low signal intensity within individual lobules. Presence of substantial low signal intensity areas Maturation appropriate for gestational age (Figure 2) |
| Cervix | Distance of most distal placental margin from inner cervical os Cervical length, cervical funnelling (cervical widening at the internal cervical os, best demonstrated in the maternal sagittal plane (Figure 5)). |
Whole uterus imaging
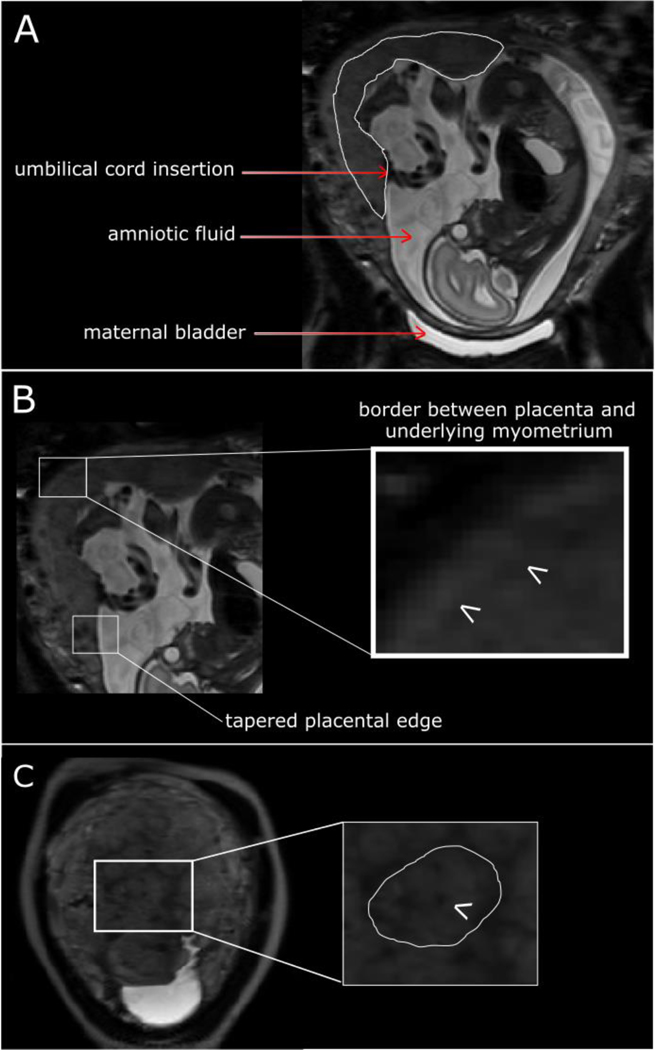
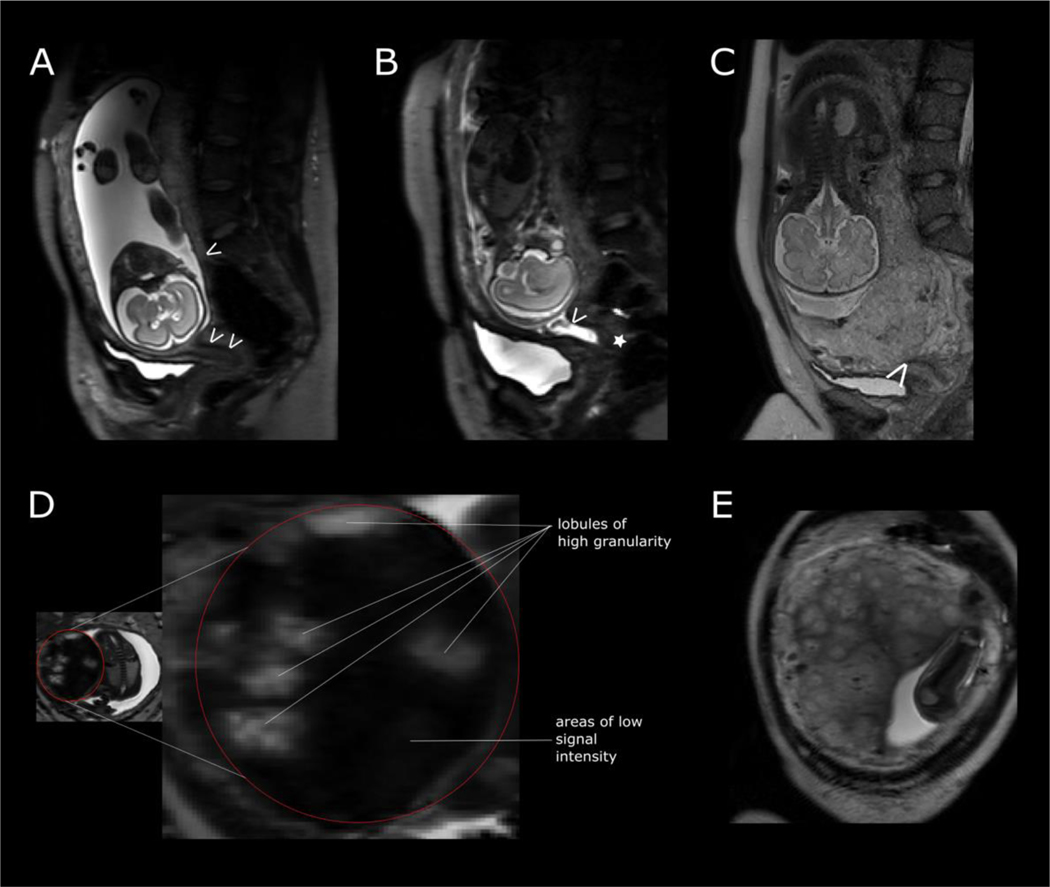
Inspection of T2-weighted imaging acquired through the entire uterus in the maternal coronal and/or sagittal plane, enables easy assessment of the placenta site and size, umbilical cord appearances, length and insertion site, amniotic fluid volume and fetal position. The umbilical cord insertion site can be seen if surrounded by sufficient amniotic fluid to provide contrast, which is more likely at early gestations (Figure 1A), and furthermore influenced by the angle of cord insertion. The interface between the basal plate of the placenta and underlying myometrium can usually be distinguished as a change in signal intensity and is best determined when the image plane is perpendicular to the uterine surface (Figure 1B). It may however be difficult to determine along the entire length depending on intrinsic signal intensity of the placenta and plane of imaging, where the plane provides a sagittal view of the placenta. Determining the length of the fetal (chorionic) surface of the placenta is easiest in the sagittal view allowing the tapered edges to be detected (Figure 1B). This may be difficult and alternative planes and sequences that provide slightly different tissue contrast may be helpful, particularly if determining the distance of the placental edge from the internal cervical os, in low lying placentas.
Figure 1:
T2-weighted imaging in maternal coronal plane at 25 weeks’ gestation in an uncomplicated pregnancy. (A) Placenta outlined in white. (B) Illustrating tapered placental edge and border between placenta and underlying myometrium. (C) Arrow indicates dot of low signal intensity of spiral artery and the circumference of a lobule is outlined in white.
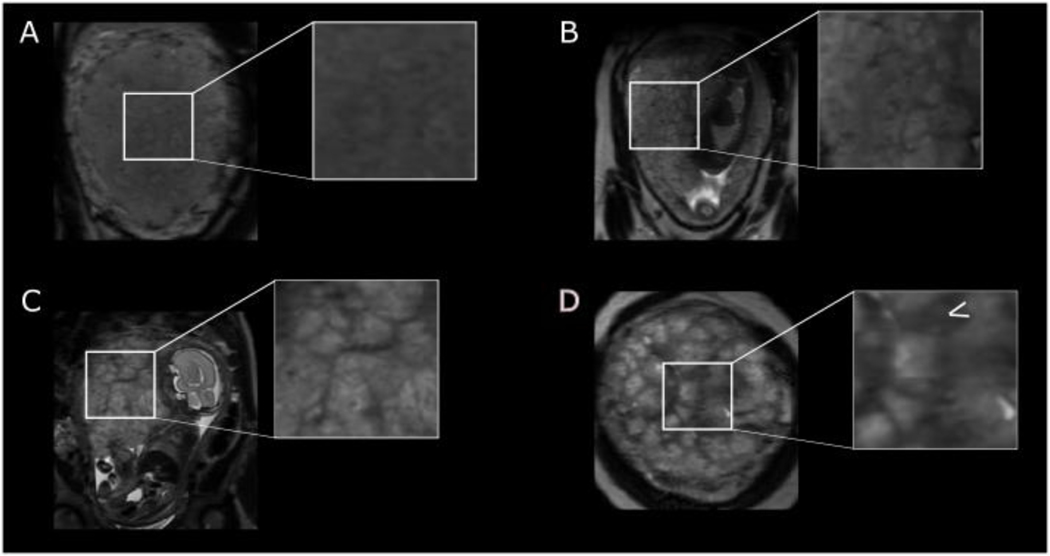
Assessment of placental parenchyma
On T2-weighted magnetic resonance imaging, placental signal intensity is relatively homogenous in the first and second trimesters but towards term becomes lobulated by the presence of low signal septa and develops a more widespread granular appearance (Figure 2, Supplementary Figure S1). The septa traverse the entire thickness of the placenta in the sagittal plane, especially at advancing gestations when their low signal becomes more marked. In a mature placenta at later gesations, there are between 15–25 lobules that may vary in size, approximately 2–3 cm in diameter, with some containing a visible central area of low signal intensity, likely representing a spiral artery (Figure 1C).
Figure 2:
T2-weighted imaging in uncomplicated pregnancies at (A) 24 weeks’ gestation with a homogenous parenchyma (B) 28 weeks’ gestation (C) 34 weeks’ gestation with lobularity becoming more apparent and (D) 37 weeks’ gestation with a heterogenous parenchyma and arrow indicating low signal septa.
Physiological factors affecting T2-weighted imaging
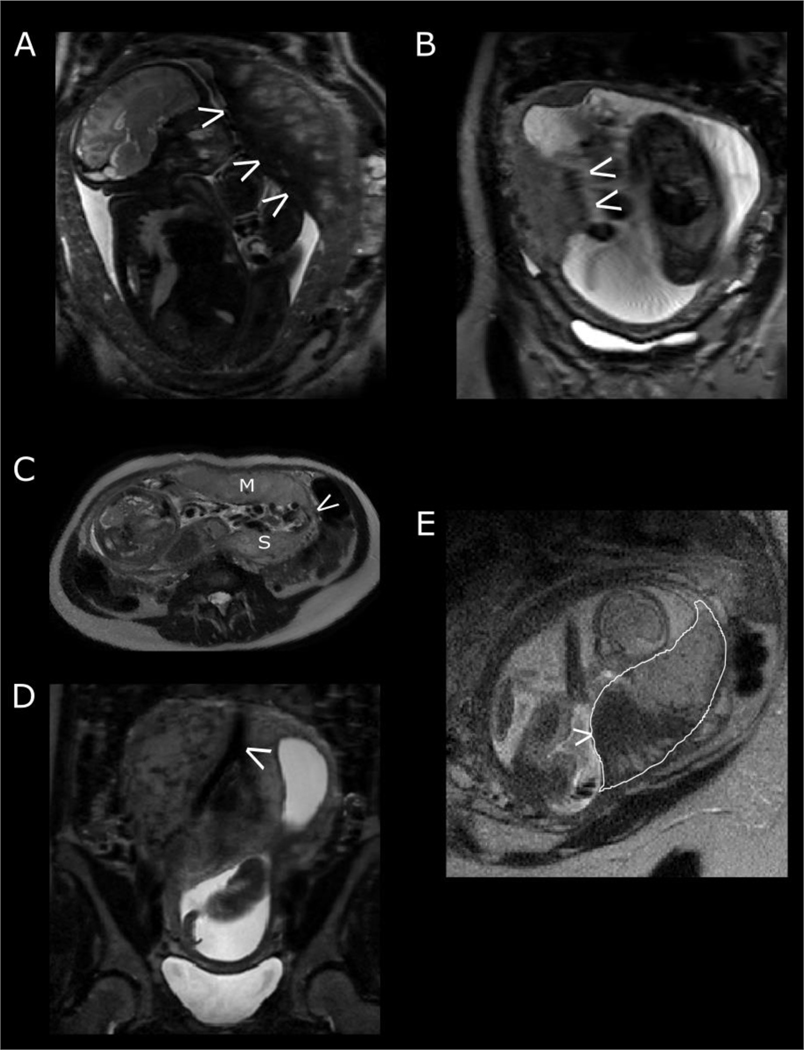
Fetal movement may compress the placenta and cause a local drop in signal intensity (Figure 3A) while gross motion may result in a transient blurring of the image (Figure 3B). The fetal or chorionic placental surface may appear undulating, giving the appearance of a varying thickness throughout the placenta, often secondary to local fetal movement against the placenta (Figure 3B).
Figure 3:
Physiological factors affecting T2-weighted imaging. (A) fetal compression against the placenta resulting in a reduction in signal intensity. (B) artefact exacerbated by maternal respiratory motion. The fetal side of the placenta does not appear to have a distinct border. (C) Placenta with succenturiate lobe. M represents the main placental disc, S the smaller succenturiate lobe and the arrow pointing to vessels joining the two together. (D) the maternal aorta bifurcating into the right and left common iliac arteries. (E) The effect of a contraction on placental signal intensity. The placenta is outlined in white. Note the large low signal intensity area (arrow) and that the peripheral placenta is thicker than expected with less tapering at the edges.
The placenta is seen as an intermediate and relatively homogenous signal intensity structure adjacent to the uterine myometrium with variable location. In comparison to the normal discoid placental shape, variations in shape that should be documented include bilobed and succenturiate lobe, the former consisting of two equal lobes while the latter describes a smaller accessory lobe(s) separate to the main disc of the placenta with communicating vessels running between the two lobes. These communicating vessels are protected only by placental membranes. Viewing images in all three maternal planes (coronal, sagittal and axial) enables assessment of the entire placenta and ensures that succenturiate (extra) lobes are not missed (Figure 3C). The presence of a succenturiate lobe increases the risk of maternal postpartum haemorrhage as delivery of the placenta may be incomplete, retaining placental tissue within the uterus. It is important to note the placental location in relation to the cervix as connecting vessels between the succenturiate lobe and main body of the placenta may traverse over the internal cervical os (termed vasa praevia). Connecting vessels may be challenging to identify on magnetic resonance imaging and therefore supporting transvaginal ultrasound imaging is required. A diagnosis of vasa praevia has clinical implications for the timing and mode of delivery, usually necessitating caesarean section as rupture of these vessels during labour or when the membranes rupture risks life-threatening fetal haemorrhage.
To assess posterior placentas, the maternal sagittal and axial imaging planes are also helpful in excluding partial volume effects from the maternal aorta and branching common iliac arteries which may be mistaken for large vessels coursing through the placental parenchyma in the maternal coronal plane. (Figure 3D).
Uterine contractions in pregnancy occur as a normal phenomenon (typically known as Braxton-Hicks contractions during the antenatal period). These are typically irregular tightenings in the second and third trimester and are more evident with increasing parity. On T2-weighted imaging, this may cause a change in placental shape and placental signal intensity (Figure 3E). The placenta may appear thicker than expected at the periphery rather than showing the usual tapered edges. The signal intensity throughout the placental parenchyma may show substantial areas of low signal intensity that appear and disappear during the examination. These contractions may not always be appreciated by the woman. A contraction may also transiently alter the site of the placenta, particularly in earlier pregnancy when relatively low lying, placing it near to or over the internal os. This needs to be differentiated from a persistently low lying placenta.
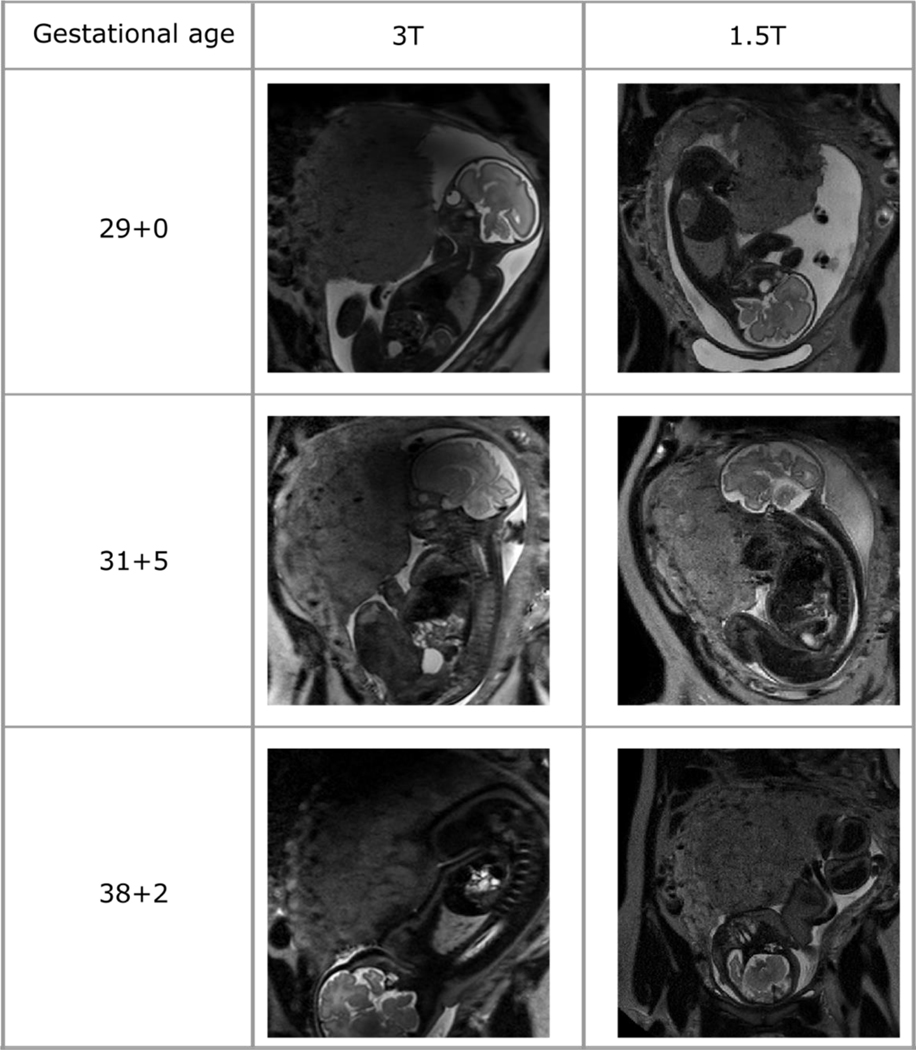
Imaging at 1.5T and 3T
A comparison of visual appearances using T2 weighted imaging at 1.5 and 3T can be seen in Figure 4 and Supplementary Figure S1. In the second trimester, the placenta homogeneity is similar at both 3T and 1.5T. The resolution and contrast also enables visualisation of central spiral arteries using both magnetic field strengths. In contrast, in the third trimester, there is a more marked difference between imaging at the two different field strengths. With advancing gestation, features including placental lobularity, heterogenous signal intensity and granularity are seen. The higher signal to noise ratio and resolution at 3T may enable more marked contrast and detail to be visualised within the placental parenchyma. Furthermore, the shorter T2 and T2* associated with higher field strength further reduces the signal especially for pathological and late gestation cases. This may in turn make it more challenging to distinguish normal form pathological placentas at late gestation at 3T compared to 1.5T.
Figure 4:
T2-weighted placental imaging in maternal coronal plane performed on the same day at two different field strengths (3T and 1.5T) for three women. A disadvantage of imaging at 3T is noted where some diffuse low signal overlying the superior aspects of the placenta can be seen, secondary to magnetic field inhomogeneity.
T2-weighted imaging with corresponding T2* mapping
T2-weighted imaging provides a context for interpretation of calculated maps, for example maps illustrating T2* values. Low signal areas between lobules on T2-weighted imaging correspond to short T2* values and thus support the suggestion that these areas represent septae (Supplementary Figure S2). This is because septae are thought to consist of slowly flowing deoxygenated blood and fibrin deposition. Within lobules, the central areas around the spiral artery have long T2* values, potentially consistent with these areas being highly oxygentated with the inflow of blood from spiral arteries. Visual appearances on T2* maps change with increasing gestational age in line with objective measures of T2* (https://www.developingbrain.co.uk/placenta-atlas/). Placental features may be transiently altered by several factors (described above) including fetal movement, gross fetal motion and uterine contractions and recognition through visual assessment of T2-weighted images is therefore vital prior to objective quantification.
Pregnancy complications (including cervical widening (funnelling), preeclampsia and chronic hypertension)
In the maternal sagittal plane, the cervix can be visulised (Figure 5A). Its tubular structure lies posterior to the maternal bladder and it has three zones of signal intensity on T2 weighted MRI. The inner endocervical canal often contains mucus, with a low signal inner stroma and a higher signal intensity outer stroma. Cervical widening (or funnelling) may be seen at the internal cervical os, accompanied by a short section of closed cervix (Figure 5B). The distance between the internal cervical os and the leading edge of the placenta is of particular clinical relevance in later gestations as a distance of less than 20mm infers a diagnosis of low-lying placenta (Figure 5C). Management is gestational age specific and requires confirmation by transvaginal ultrasound as ultrasound remains the current imaging modality for the basis of clinical management decisions. In addition, contractions may transiently alter the site of the placenta, giving an apparent low lying position.
Figure 5:
T2-weighted imaging in pregnancy complications. (A) maternal sagittal plane illustrating a normal cervical length. A single arrow at the leading edge of the placenta and double arrows demonstrating tubular structure of the cervix of normal cervical length. (B) Arrow at the internal cervical os (demonstrating cervical widening or funnelling) and a short section of closed cervix (star) in a woman at risk of preterm delivery at 23 weeks’ gestation. (C) Placenta praevia completely covering the internal cervical os (white arrow). (D) Placenta in a pregnancy complicated by preeclampsia at 28 weeks’ gestation. (E) Placenta in a pregnancy complicated by chronic hypertension at 26 weeks’ gestation.
An understanding of normal appearances to the placenta across gestation in low risk pregnancies is essential in order to detect differences from typical development in high risk pregnancies such as those with placental dysfunction, including those with hypertensive disorders of pregnancy.
In pregnancies complicated by preeclampsia, the placenta has a more marked lobularity with substantial additional areas of low signal intensity between lobules compared to low risk pregnancies (Figure 5D, Supplementary Figure S3, Supplementary Figure S4) [9]. The lobules are variable in size and exhibit high granularity in contrast to the consistent signal intensity within lobules (i.e. low granularity) of low risk pregnancies. They appear to reflect the hypothesised processes in preeclampsia development, whereby impaired remodelling of the spiral arteries allows inflow of blood at a high velocity resulting in injury to the villi. Women with early onset preeclampsia (requiring delivery before 34 weeks’ gestation) may show more substantial areas of low signal intensity with fewer lobules compared to those with late onset preeclampsia who exhibit these features to a lesser degree.
In women with chronic hypertension, there is a more varied spectrum of placental appearances compared to low risk pregnancies (Figure 5E, Supplementary Figure S5, Supplementary Figure 6). Images show either an appropriate for gestation placenta or an advanced for gestation placenta with increased lobulation, wider septa and more marked heterogeneity than expected for age. Further examples of placental T2-weighted imaging in pregnancy complications can be found at https://www.developingbrain.co.uk/placenta-atlas/, including those with fetal growth restriction, monochorionic and dichorionic diamniotic twins, preterm premature rupture of membranes (PPROM), placenta accrete spectrum disorder and fetal congenital cardiac disease.
Histological confirmation
Histological examination of the placenta after delivery can provide an insight into macroscopic or microscopic structural features providing correlation for magnetic resonance imaging findings. In hypertensive disorders of pregnancy, maternal vascular malperfusion lesions are often found histologically and this may account for the high granularity seen in placentas from women with preeclampsia [9]. Maternal vascular malperfusion lesions have been previously found to be highest in women with chronic hypertension and preeclampsia compared to those with gestational hypertension or no hypertensive disorder of pregnancy [10].Villous features include syncytial knots, villous agglutination, increased intervillous fibrin deposition and villous infarcts but the current resolution of in vivo magnetic resonance imaging may not be sufficient to capture these changes and the imaging correlates have not therefore been well established.
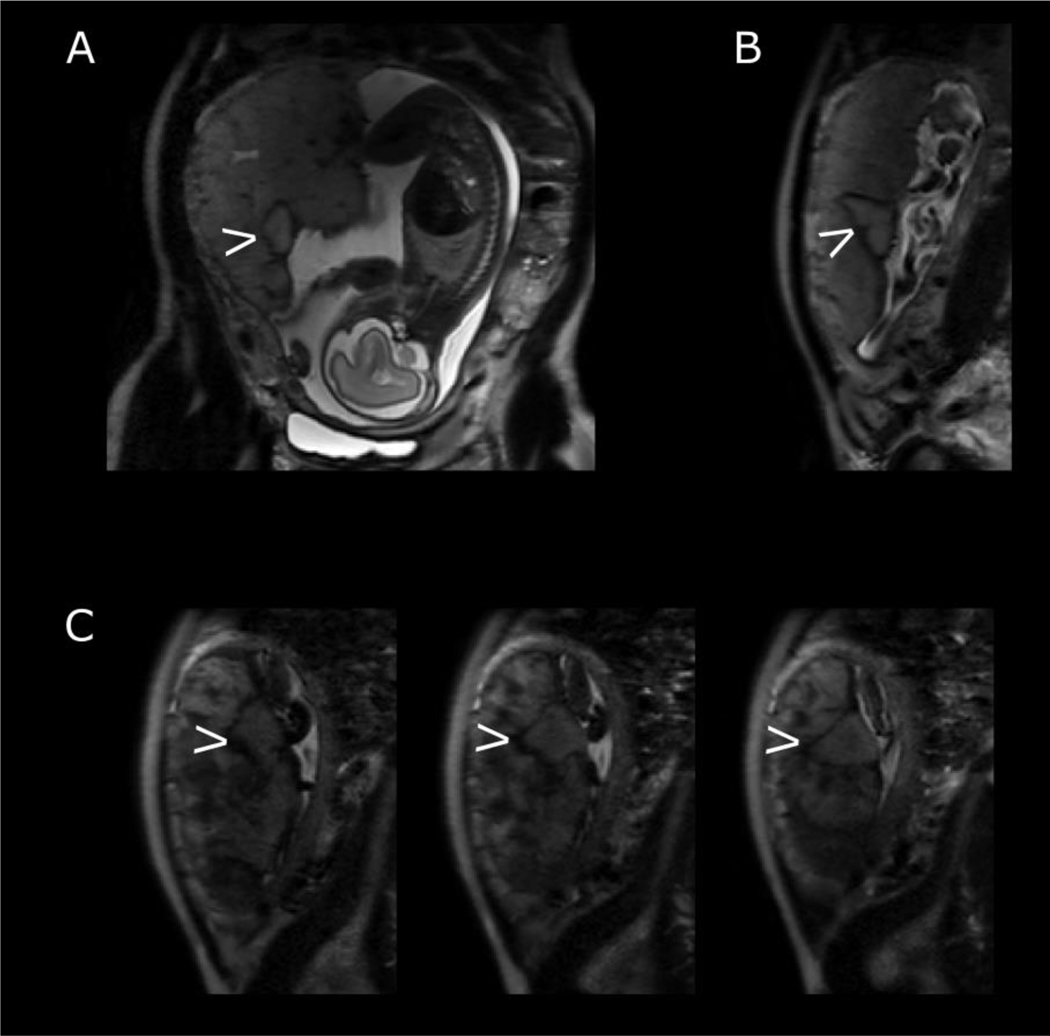
Combining imaging with histological correlation can be especially informative where large focal abnormalities are visualised on T2-weighted imaging. In Figure 6A, imaging of a low risk pregnancy at 26 weeks’ gestation shows a well circumscribed area noted because of a periphery of low signal intensity that is not seen elsewhere in the placenta. Subsequent histological examination after delivery revealed that this area corresponded to an old haematoma with fibrin deposition at the margins. Of note, this pregnancy was uneventful, delivering at term and with an infant of normal birthweight centile.
Figure 6:
Features on T2-weighted imaging confirmed by histological examination. Maternal coronal (A) and sagittal (B) planes with arrow indicating well circumscribed focal lesion with low signal periphery at 26 weeks’ gestation. This was confirmed as an evolving haematoma on histological examination after delivery. (C) Series of T2-weighted imaging in maternal sagittal plane at 30 weeks’ gestation with arrow indicating well circumscribed area below umbilical cord insertion. Histology revealed this to be an infarct.
Visualisation of the placental parenchyma may inform clinical assessment of the fetus and assist with management of the pregnancy. A similar well circumscribed area within the placental parenchyma can be seen in Figure 6B, imaged at 30 weeks’ gestation in a fetus with short femurs. The area is just below the cord insertion, potentially impeding materno-fetal exchange. The surrounding placental parenchyma is heterogenous, interspersed with areas of low signal intensity and high granularity. Histological examination revealed the circumscribed area to be an infarct with central old haemorrhage while the rest of the placenta had several foci of chronic villitis and and avascular villi. The woman was not hypertensive and delivered spontaneously at 36 weeks’ gestation with an infant with a birthweight on the 7th centile.
Discussion
This study details an approach to visual assessment of the placenta on T2-weighted imaging. Illustrative features include anatomical landmarks to aid orientation, placental shape, signal intensity, lobularity and granularity. Transient factors affecting imaging are shown including the effect of fetal movement, gross motion and contractions. Placentas in low risk pregnancies are shown and compared to placentas from pregnancies complicated by preeclampsia and chronic hypertension. The article links to an online atlas (https://www.developingbrain.co.uk/placenta-atlas/). The utility of other magnetic resonance techniques (T2* mapping) and histological assessment alongside visual assessment of placental T2-weighted imaging is highlighted.
We have provided detailed qualitative descriptors for placental visual assessment on T2-weighted imaging amongst low risk pregnancies. We have used a consistent approach in imaging women: women were positioned in the scanner in the same way and the imaging parameters were kept consistent. Comparable imaging at two magnetic field strengths is also shown with illustrated multiple imaging examples. Application of visual placental assessment in a range of pregnancy complications (such as hypertensive disorders) and transient factors influencing T2-weighted imaging are shown in conjunction with imaging in low risk pregnancies, thus assisting visual assessment in a range of clinical contexts. T2-weighted imaging visual descriptors are assessed alongside more advance sequences of T2* mapping. Exogenous contrast is not required and therefore placental features described may be applied in cases where magnetic resonance imaging is performed for clinical indications such as fetal brain or cardiac anomaly.
In this study, we chose to image women supine rather than in left lateral tilt. Supine imaging confers an advantage in that it enables an improved proximity of the receiver coil to the large field of view given by the gravid uterus, and therefore an improvement in signal detection compared to left lateral tilt imaging. A short period of lying left lateral prior to slowly rotating to supine position for imaging can successfully shift the fetal position to minimise inferior vena cava compression [11, 12]. In anticipation of potential effects of lying supine, all women were imaged with the presence and support of either a midwife or obstetrician. In addition, women were placed in a left lateral tilt prior to rotation to supine for imaging. A blood pressure was taken every 10 minutes during imaging with concomitant heart rate and oxygen saturation monitoring.
The homogeneity of signal present with the chosen echo time at 1.5T, especially in the second trimester, may present a challenge when seeking the identification of deviations from normal development. Imaging at 3T may allow for such deviations to be more apparent even for shorter echo times and thus allow for easier identification of pathology upon visual assessment. However, the concurrent marked visual changes that occur with normal development in advancing gestationmay hinder the identification of placental parenchyma changes associated with pathology and thus imaging at 1.5T may be advantageous in the third trimester.
There is a paucity in the literature of placental visual descriptors using magnetic resonance imaging. To our knowledge, there are none described for images acquired at 3 Tesla; however, our in vivo features are consistent with those described previously at 1.5 Tesla [6]. In uncomplicated pregnancies, with advancing gestation, the placenta has previously been reported to be more heterogenous with an increasing lobularity and a reduction in the ratio of placental and amniotic fluid signal intensity on T2-weighted imaging [6]. Contrast enhanced imaging in Rhesus macques has shown local central areas within a lobule to correspond to spiral arteries [13]. These findings are consistent with our suggestion that a ‘dot’ of low signal intensity within lobules represents spiral arteries. The visual changes on T2 weighted imaging that occur with uterine contractions is supported by previous studies which have shown reduced T2* values during contractions [14] as well as global and regional changes in placental R2* [15].
Quantitative placental analysis using T2-weighted imaging have been previously reported. Placental volume and thickness have been explored in several studies, mostly in uncomplicated pregnancies and pregnancies complicated by fetal growth restriction. Longitudinal data involving women scanned at four-weekly intervals, showed a linear placental growth throughout the second and third trimester [16]. In contrast, examination of 20 pregnancies complicated by fetal growth restriction there was a reduced placental volume and a high placenta thickness to volume ratio, resulting in a more globular appearance of the placenta [17].
In vivo T2-weighted magnetic resonance imaging has provided an insight into normal placental development with a focus on gross placental structure. Placental lobules become more apparent with advancing gestation, accompanied by a loss in the homogenous signal intensity within lobules. This is supported by quantitative assessment, where T2 relaxation times have been calculated in various studies and shown to fall linearly with advancing gestation [18–20].Images presented in this study can be utilised as a reference tool when visually assessing placentas in high risk pregnancies. In addition, T2-weighted imaging may compliment obstetric ultrasound and assist in assessing gross placental structure especially given the large field of view. Aside from being performed to assess abnormal placental implantation, T2 weighted imaging has the potential to identify placental phenotypes associated with pregnancy complications such as preeclampsia. This study illustrates details from both uncomplicated and complicated pregnancies and thus provides information for future studies that may explore its application in more widespread clinical use.
Our findings are consistent with previous smaller studies exploring T2 relaxation times. In one study, 10 out of 14 compromised pregnancies (defined as those complicated by fetal growth restriction or preeclampsia) were found to have T2 relaxation times lower than the normal linear regression line with 4 lying below the 90% confidence interval [18]. Only two of these pregnancies were complicated by preeclampsia and imaging was performed beyond 35 weeks’ gestation in both, thus perhaps representing a less severe clinical phenotype of preeclampsia. A further study [19] illustrated a decreased T2 relaxation time in small for gestational age fetuses that was additionally associated with raised uterine artery pulsatility index and lower birthweight percentiles. Additionally, an examination of 20 pregnancies complicated by fetal growth restriction found that abnormal signal intensity consistent with placental pathology was found to predict fetal or neonatal death [17].
Visual assessment of T2-weighted imaging alongside corresponding T2* maps yields further understanding of factors contributing to signal intensity and allow for further quantitative analysis. In low risk pregnancies, low signal areas surrounding lobules are shown to have low T2* values, thus further strengthening the suggestion that these areas are septae. This is supported by previous studies which have also shown regional differences in T2* values within the placenta [8, 21] that may be accentuated in fetal growth restriction where a slow spread of signal intensity enhancement from a spiral artery across lobule to periphery has been shown in a naturally occurring Rhesus macaque model of fetal growth restriction [22]. Regional differences in T2* values can affect the overall placental mean T2*. This is reflected in studies that have shown a fall in mean T2* value with advancing gestation [21, 23–25]. The central spot of low signal seen in lobules on T2-weighted imaging has a corresponding high T2* value, suggesting that these spots represent the inflow of highly oxygenated blood from maternal spiral arteries. Histological comparisons with in vivo imaging show that focal abnormalities visualised correspond to a either a haematoma with fibrin deposition or infarct, while diffuse changes throughout the placental parenchyma are supported by the presence of histological maternal vascular malperfusion features in hypertensive disorders.
Future research
Future work may include quantitative placental analysis using T2-weighted imaging. Further histological analysis may elucidate factors contributing to signal intensity in T2-weighted imaging such as calcium deposition. However, this is challenging given the current extended interval between imaging and delivery in low risk pregnancies. Visual assessment in conjunction with advanced sequences yielding T2* and diffusion maps may yield further insight into the placental visual variation in T2-weighted imaging as well as the underlying mechanism in pregnancy complications and adverse pregnancy outcome.
Application of placental visual assessment in the context of women undergoing clinically indicated magnetic resonance imaging for fetal anomalies (such as fetal cardiac and fetal brain abnormalities) and prediction of adverse pregnancy outcomes warrants further investigation. Visual assessment may therefore provide a tool to assist in clinical management decisions such as degree of monitoring and timing of delivery.
Supplementary Material
Highlights.
We detail an approach to placental visual assessment in antenatal MRI.
Illustrative features and transient factors affecting imaging are shown.
Low risk and those with preeclampsia or chronic hypertension are included.
The utility of other MRI techniques (T2* mapping) and histology are highlighted.
The article links to an online atlas (https://www.developingbrain.co.uk/placenta-atlas/).
Acknowledgements
We thank all the women who participated in the study, their midwives and obstetricians involved in study recruitment. We thank Alexia Egloff for clinical reporting and the research radiographers.
Sources of Funding
This work is funded by the NIH Human Placenta Project grant 1U01HD087202-01, the National Institute for Health Research (NIHR) Research Professorship (Chappell; RP-2014-05-019), Tommy’s (Registered charity no. 1060508) and Holbeck Charitable Trust with support from the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z) and by the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. PTS is partly funded by King’s Health Partners Institute of Women and Children’s Health, Tommy’s (Registered charity no. 1060508) and by ARC South London (NIHR). JH is funded by the Wellcome Trust through a Sir Henry Wellcome Fellowship (201374).
Footnotes
Disclosures
The views expressed are those of the authors and not necessarily those of the UK National Health Service, the National Institute for Health Research, or the Department of Health and Social Care.
Declaration of Interest
All authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jauniaux E, et al. , Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-top Guideline No. 27a. Bjog, 2019. 126(1): p. e1–e48. [DOI] [PubMed] [Google Scholar]
- 2.Jauniaux E. and Nicolaides KH, Early ultrasound diagnosis and follow-up of molar pregnancies. Ultrasound Obstet Gynecol, 1997. 9(1): p. 17–21. [DOI] [PubMed] [Google Scholar]
- 3.Courtney JA, Cnota JF, and Jones HN, The Role of Abnormal Placentation in Congenital Heart Disease; Cause, Correlate, or Consequence? Front Physiol, 2018. 9: p. 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton GJ and Fowden AL, The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci, 2015. 370(1663): p. 20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grannum PA, Berkowitz RL, and Hobbins JC, The ultrasonic changes in the maturing placenta and their relation to fetal pulmonic maturity. Am J Obstet Gynecol, 1979. 133(8): p. 915–22. [DOI] [PubMed] [Google Scholar]
- 6.Blaicher W, et al. , Magnetic resonance imaging of the normal placenta. Eur J Radiol, 2006. 57(2): p. 256–60. [DOI] [PubMed] [Google Scholar]
- 7.Villar J, et al. , International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet, 2014. 384(9946): p. 857–68. [DOI] [PubMed] [Google Scholar]
- 8.Hutter J, et al. , Multi-modal functional MRI to explore placental function over gestation. Magn Reson Med, 2019. 81(2): p. 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho AEP, et al. , T2* Placental Magnetic Resonance Imaging in Preterm Preeclampsia: An Observational Cohort Study. Hypertension, 2020. 75(6): p. 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovo M, et al. , The relationship between hypertensive disorders in pregnancy and placental maternal and fetal vascular circulation. J Am Soc Hypertens, 2017. 11(11): p. 724–729. [DOI] [PubMed] [Google Scholar]
- 11.Hughes EJ, et al. , Magnetic Resonance Imaging quantification of venous return in pregnant women: A comparison between supine and left lateral tilt position. ISMRM 24th Annual Meeting & Exhibition, 2016. [Google Scholar]
- 12.Hughes EJ, et al. , The effect of maternal position on venous return for pregnant women during MRI. NMR Biomed, 2021. 34(4): p. e4475. [DOI] [PubMed] [Google Scholar]
- 13.Schabel MC, et al. , Functional imaging of the nonhuman primate Placenta with endogenous blood oxygen level-dependent contrast. Magn Reson Med, 2016. 76(5): p. 1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinding M, et al. , Reduced placental oxygenation during subclinical uterine contractions as assessed by BOLD MRI. Placenta, 2016. 39: p. 16–20. [DOI] [PubMed] [Google Scholar]
- 15.Abaci Turk E, et al. , Placental MRI: Effect of maternal position and uterine contractions on placental BOLD MRI measurements. Placenta, 2020. 95: p. 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langhoff L, et al. , Placental Growth during Normal Pregnancy - A Magnetic Resonance Imaging Study. Gynecol Obstet Invest, 2017. 82(5): p. 462–467. [DOI] [PubMed] [Google Scholar]
- 17.Damodaram M, et al. , Placental MRI in intrauterine fetal growth restriction. Placenta, 2010. 31(6): p. 491–8. [DOI] [PubMed] [Google Scholar]
- 18.Gowland PA, et al. , In vivo relaxation time measurements in the human placenta using echo planar imaging at 0.5 T. Magn Reson Imaging, 1998. 16(3): p. 241–7. [DOI] [PubMed] [Google Scholar]
- 19.Derwig I, et al. , Association of placental T2 relaxation times and uterine artery Doppler ultrasound measures of placental blood flow. Placenta, 2013. 34(6): p. 474–9. [DOI] [PubMed] [Google Scholar]
- 20.Wright C, et al. , Magnetic resonance imaging relaxation time measurements of the placenta at 1.5 T. Placenta, 2011. 32(12): p. 1010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutter J, et al. , T2* relaxometry to characterize normal placental development over gestation in-vivo at 3T [version 1; peer review: 1 approved, 1 approved with reservations]. Wellcome Open Research, 2019. 4(166). [Google Scholar]
- 22.Lo JO, et al. , Novel Detection of Placental Insufficiency by Magnetic Resonance Imaging in the Nonhuman Primate. Reprod Sci, 2018. 25(1): p. 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinding M, et al. , Placental magnetic resonance imaging T2* measurements in normal pregnancies and in those complicated by fetal growth restriction. Ultrasound Obstet Gynecol, 2016. 47(6): p. 748–54. [DOI] [PubMed] [Google Scholar]
- 24.Sinding M, et al. , Prediction of low birth weight: Comparison of placental T2* estimated by MRI and uterine artery pulsatility index. Placenta, 2017. 49: p. 48–54. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong T, et al. , 3D R2* mapping of the placenta during early gestation using free-breathing multiecho stack-of-radial MRI at 3T. J Magn Reson Imaging, 2019. 49(1): p. 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.