Abstract
Chalcogen-containing carboranes have been known for several decades and possess stable exopolyhedral B(9)-Se and B(9)-Te σ bonds despite the electron-donating ability of the B(9) vertex. While these molecules are known, little has been done to thoroughly evaluate their electrophilic and nucleophilic behavior. Herein, we report an assessment of the electrophilic reactivity of meta-carboranyl selenyl (II), tellurenyl (II), and tellurenyl (IV) chlorides and establish their reactivity pattern with Grignard reagents, alkenes, alkynes, enolates, and electron-rich arenes. These electrophilic reactions afford unique electron-rich B-Y-C (Y = Se, Te) bonding motifs not commonly found before. Furthermore, we show that meta-carboranyl selenolate, and even meta-carboranyl tellurolate, can be competent nucleophiles and participate in nucleophilic aromatic substitution reactions. Arene substitution chemistry is shown to be further extended to electron-rich species via palladium mediated cross-coupling chemistry.
Keywords: carborane, boron cluster, chalcogen, organoselenium, organotellurium
Graphical Abstract (TOC)

TOC Synopsis:
Electrophilic and nucleophilic reactivity of selenium- and tellurium-containing meta-carboranes is reported in this study. The newly synthesized compounds feature exopolyhedral B(9)-Se-C and B(9)-Te-C bonding motifs with electron-rich Se and Te sites present in the molecules. All compounds have been thoroughly characterized by heteronuclear NMR spectroscopy with key intermediates structurally characterized by single crystal X-ray diffraction.
Introduction
Organoselenium and organotellurium compounds containing carbon-selenium and -tellurium bonds have been widely studied for the past decades and have recently found a variety of uses in nearly all aspects of chemical research, including: organic synthesis,1a–f organometallics,1g–i self-assembled and applied materials,1j–n photochemistry,1o–s and chemical biology.1t–w In contrast to organochalcogen compounds, the reactivity of boronochalcogens containing boron-selenium and -tellurium bonds is significantly less established. Of currently known molecules containing boron-selenium or -tellurium bonds, a large portion consist of tricoordinate mononuclear boron centers (Figure 1A).2a–i
Figure 1:

A. Literature examples of tricoordinate boron centers containing a boron-chalcogen single bond or double bond. B. Literature examples of tetracoordinate boron centers containing boron-chalcogen single bonds. C. Extent of previous studies regarding the reactivity of B-Se and B-Te containing carboranes. Nucleophilic reactivity has been shown between carboranyl chalcogenolates (Y = Se, Te) and electrophilic reactivity has been shown with carboranyl selenyl (II) chlorides, though not with tellurenyl (II) or (IV) chlorides.
Boron sites in these molecules contain a lowest unoccupied molecular orbital (LUMO) capable of accepting some electron density from the bound Se or Te atom, resulting in a shortening of the boron chalcogen bond. Additionally, several examples containing tetracoordinate boron centers2i–r exist as well (Figure 1B). In these cases, tricoordinate BR3 (R: C, F, Cl, Br, I, H) are typically stabilized by coordination of chalcogen-based ligands where lone pair electrons on the chalcogen are shared with the vacant boron-centered p-orbital. Lesser known molecular platforms that can support boron-selenium and -tellurium bonding interactions, are boron-rich clusters.3 Among these, icosahedral carboranes (C2B10H12) in particular have afforded a unique and stable scaffold for the study of compounds containing boron chalcogen bonds.4 Similar in size to adamantane, this cluster type exists in three isomeric forms (ortho-, meta-, para-).5 In all cases, electrons responsible for the cluster bonding are delocalized in three dimensions. Given the presence of the carbon vertices and the above delocalization, the resulting asymmetry in the ortho- and meta-isomers causes boron vertices most distal from the carbon sites to exhibit strong electron-donating character through induction, similar in magnitude to tertiary alkyl groups.6 Furthermore, electron delocalization in the cluster results in an inaccessible LUMO, affording B-Y single bond character.
Carboranes functionalized with exopolyhedral chalcogens (Se, Te) at these electron-rich boron-vertices were first reported in the early 1980’s,4d–f but surprisingly little has been established in terms of understanding the reactivity of these compounds. In previous reports, Zakharkin and coworkers have shown examples of nucleophilic reactivity between caborane-based selenolates or tellurolates with alkyl halides, as well as examples of electrophilic reactivity between carborane-based selenyl (II) reagents and Grignard reagents with no reported electrophilic reactivity of the tellurenyl (II) or tellurenyl (IV) congeners (Figure 1C).4d–f Notably, the reported compounds have only been characterized by melting point and elemental analysis with no rigorously reported NMR spectroscopy or structural studies. In this work, we report a reactivity map for B(9) functionalized meta-carborane, appended with selenium- and tellurium-based functional groups (Figure 2). Specifically, we show that B(9)-bound meta-carboranyl selenyl (II), tellurenyl (II), and tellurenyl (IV) chlorides participate in electrophilic substitution reactions reminiscent to the established reactivity of analogous carbon-based electrophilic chalcogen reagents.1a Furthermore, we show the ability of B(9)-bound meta-carboranyl selenolates and tellurolates to participate in nucleophilic aromatic substitution reactions as well as the ability of the corresponding tellurol congener to undergo palladium mediated cross-coupling with an aromatic electrophile. For all compounds, we provide full heteronuclear NMR characterization (1H, 13C, 11B, 19F, 77Se, and/or 125Te) in addition to single-crystal X-ray structural characterization for key intermediates studied in this work (Figure 2). Our findings reveal that the electrophilic and nucleophilic reactivity of selenium- and tellurium-containing meta-carboranes is largely analogous to carbon-based reagents.
Figure 2:

This work, overview of compounds synthesized by the electrophilic and nucleophilic reactions of selenium and tellurium-containing meta-carboranes.
Results and Discussion
To ascertain the electrophilic reactivity of these compounds, we first sought to prepare the 9,9’-meta-carboranyl diselenide (1A) and ditelluride (1B) using modified procedures from previous reports (Figure 3A, SI sec. 3).4d–f,6b
Figure 3:
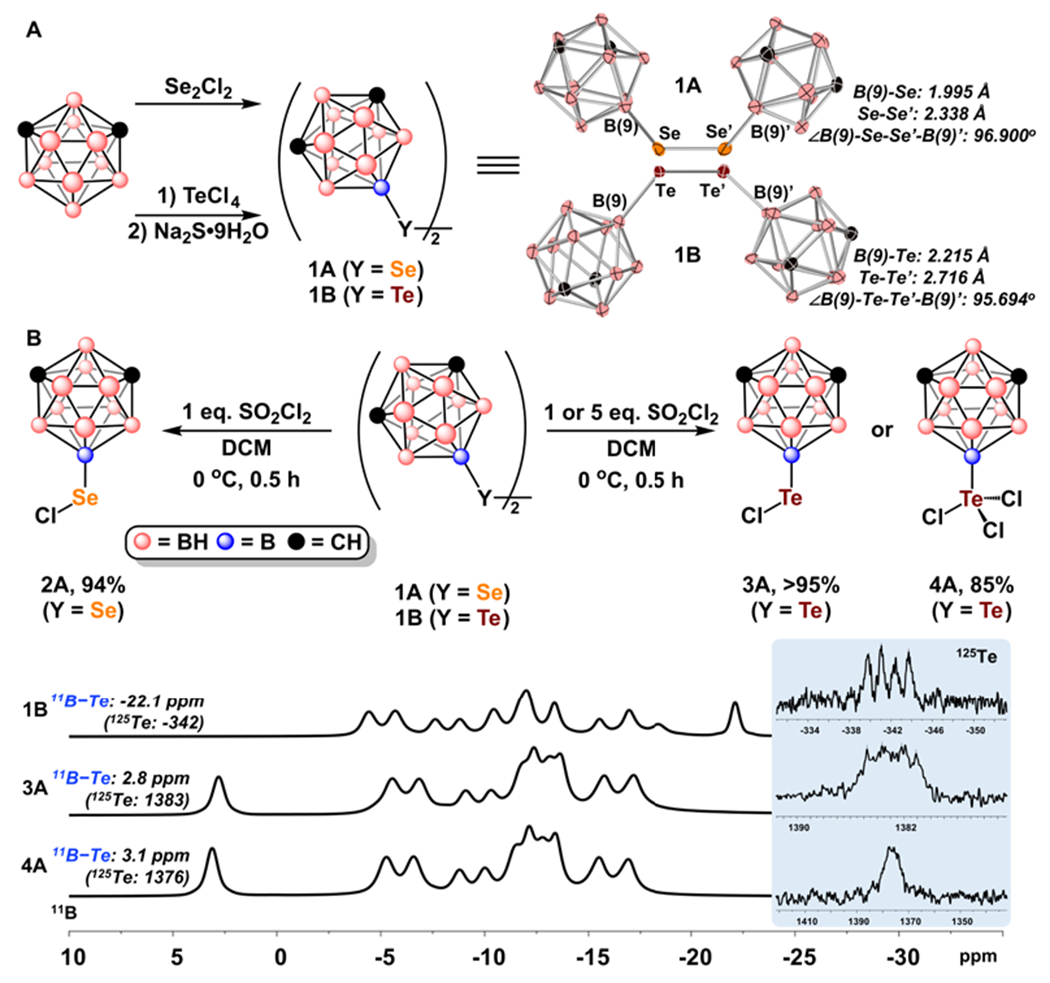
A. Synthesis of dichalcogenides 1A and 1B including their respective crystallographically derived structures. Thermal ellipsoids are drawn at 50% probability, hydrogens are omitted for clarity. B. Synthesis of electrophilic selenyl (II), tellurenyl (II), and tellurenyl (IV) reagents 2A, 3A, and 4A from carboranyl dichalcogenides. Comparison of 11B and 125Te NMR for compounds 1B, 3A, and 4A.
Analytically pure dichalcogenides 1A and 1B were isolated via silica gel column chromatography as air stable red-orange and dark red solids, respectively (SI sec. 3). The isolated dichalcogenides were characterized by heteronuclear NMR spectroscopy (1H, 13C, 11B, 77Se, and/or 125Te), revealing diagnostic resonances consistent with the proposed structural formulations.4d–f,6b Single crystals suitable for X-ray crystallography of both dichalcogenides were subsequently grown from layered solutions of dichloromethane and hexanes. The crystallographically derived structures of 1A and 1B further corroborate the presence of exopolyhedral B-Y bonds (1.995 Å (Y=Se, 1A) and 2.215 Å (Y=Te, 1B) in length respectively) located at the B(9) position of meta-carborane (Figure 3A). The measured Y-Y dichalcogenide bond lengths and torsional angles, 2.338 Å/96.900° (Y=Se, 1A) and 2.716 Å/95.694 (Y=Te, 1B) are similar in length to other crystallographically characterized dichalcogenides in addition to torsional angles greater that 90° being consistent with more sterically hindered dichalcogenides.7
1A was then subjected to chlorination by treatment with SO2Cl2 in anhydrous dichloromethane at 0 °C. After stirring the mixture for 30 minutes all volatiles were removed, and NMR spectroscopy revealed full consumption of 1A as determined by diagnostic downfield shifts in both the 11B and 77Se resonances attributed to the exopolyhedral boron-selenium bond. The observed downfield shifts in signal resonances are consistent with an increase in the oxidation state of the bound selenium and confirms the formation of 2A (Figure 3B, SI sec. 4). By applying similar chlorination procedures to 1B with varying equivalencies of SO2Cl2, isolation of tellurenyl (II) chloride (3A) and tellurenyl (IV) trichloride (4A) was accomplished (Figure 3B, SI sec. 4). Like with 2A, NMR spectroscopy (11B, 125Te) of 3A and 4A revealed significant downfield shifts in the 11B and 125Te resonances attributed to the corresponding boron and tellurium nuclei within the exopolyhedral boron-tellurium bond (see Figure 3B for a comparison of 11B and 125Te NMR spectra for compounds 1B, 3A, and 4A) and are consistent with an increase in oxidation state at tellurium. While the majority of carbon-based selenyl and tellurenyl chlorides are sensitive to moisture, resulting in selenininc or tellurenic acids,1a compounds 2A, 3A, and 4A exhibit improved stability and do not show any signs of decomposition when exposed to an atmosphere of laboratory air on a time scale of several months.
The anticipated electrophilicity of 2A prompted us to explore its reactivity with several common nucleophiles. Thus, 2A was treated with an excess of phenylmagnesium bromide in anhydrous diethyl ether at room temperature. After stirring for 16 hours, analysis of the reaction mixture by GC-MS indicated quantitative formation of phenyl selenide (2B). The resulting product was then isolated in 91% yield after purification via silica gel column chromatography and characterized by heteronuclear NMR spectroscopy (Figure 4, SI sec. 5a). Characterization of 2B is in agreement with previously reported data of meta-carboranyl phenyl selenide obtained via an independent chemical route,8 and confirms the electrophilic behavior of 2A. Considering the successful outcome of the reaction between 2A and a model Grignard reagent, we wanted to assess whether less reactive carbon-based nucleophiles would still undergo transformations with 2A. Previously, researchers have established the reactivity of selenyl chlorides with unsaturated hydrocarbons and enolates.1a As such, norbornene, phenylacetylene, and cycloheptanone were chosen as model compounds potentially susceptible to electrophilic substitution by 2A.
Figure 4:

Reactions of 2A with common carbon-based nucleophiles. aReaction was performed in anhydrous diethyl ether under an inert atmosphere at r.t.. bReaction was performed in anhydrous dichloromethane at r.t.. cReaction was performed in anhydrous toluene with 2 eq. of AlCl3 at 50 °C. See SI for full experimental details. Thermal ellipsoids are drawn at 50% probability, hydrogens are omitted for clarity.
A solution of 2A in anhydrous dichloromethane was treated with norbornene and the progress of the reaction was monitored by GC-MS. After stirring the mixture overnight, GC-MS indicated the presence of two isomers (2C and 2C’, m/z: 352.10, SI sec. 5b,e) approximately in a 1:2 ratio consistent with the anticipated reaction between the electrophilic RSe-Cl fragment in 2A and the C=C in norbornene, forming a distribution of endo and exo isomers. Separation of the two isomers via silica gel column chromatography proved difficult and thus the products were isolated as a mixture in 47% yield and characterized by heteronuclear NMR spectroscopy. While 13C, 11B, and 77Se NMR spectroscopic data of the isomeric mixture provided little insight on the relationship between 2C and 2C’, they confirmed the formation of the anticipated selenium-carbon bond as indicated by the upfield shift in the resonances associated with the exopolyhedral boron-selenium bond. 600 MHz and 400 MHz 1H NMR spectroscopy was used in an attempt to resolve proton resonances in the alkyl region that would correlate to protons bound to the selenium-bound and chlorine-bound carbons of the norbornene (SI sec. 5d). While there was still difficulty resolving 1H resonances for individual isomers, the presence of more than just two isomers was evident due to there being multiple unique resonances that integrated together as a single proton, in contrast to the isomer distribution observed by GC-MS. To further understand the substitutional isomers present in the purified product, single crystals suitable for X-ray crystallography were grown from a solution of the isomer mixture in dichloromethane layered with hexanes. The crystallographically derived structure indicated the co-crystallization of enantiomeric 2C’, revealing the (R,R) and (S,S) exo-products (Figure 4), further confirming the formation of the desired selenium-carbon bond. The measured B(9)-Se and Se-C(1) bond lengths, 1.999 Å and 2.010(R,R)/1.968(S,S) Å respectively, are typical of other crystallographically measured boron-selenium and selenium-carbon bonds.9 Furthermore, when compared more closely to crystallographically studied compounds, the exopolyhedral B-Se bond present in 2C is notably longer than tri-coordinate boron-selenium bonds (average: 1.940 Å)9 that would expectedly have partial double bond character due to the unoccupied p-orbital located on a tricoordinate boron center. The length of the exopolyhedral B-Se bond in 2C more closely parallels reported tetra-coordinate boron-selenium bonds (average: 2.093 Å)9 with a more pronounced single bond character. All characterization of 2C and 2C’ suggests the formation of both possible diastereomeric products (endo and exo), resulting from the addition of 2A across the double bond within norbornene in addition to their corresponding enantiomers (R,R and S,S), producing four isomers in total. When subjecting 2A to similar reaction conditions in the presence of phenylacetylene or cycloheptanone, compounds 2D and 2E (SI sec. 5c), were both coincidentally isolated in 54% yield (Figure 4). 1H NMR spectroscopy of purified 2D revealed the exclusive formation of a single isomer due to presence of only one olefinic 1H resonance. Single crystals of 2D suitable for X-ray crystallography were then grown from a cold (0 °C) solution of dichloromethane layered with hexanes. The crystallographically derived structure revealed the formation of the E-1-SeR-2-Cl-2-Ph isomer (Figure 4), having resulted from Markovnikov, anti-addition of 2A across the carbon-carbon triple bond in phenylacetylene. The formation of the thermodynamically-favored Markovnikov products from the addition of RSeCl reagents across unsaturated hydrocarbons is expected for this type of process.1a In contrast to products 2C and 2D, 2E does not incorporate the chloride of the electrophilic reagent, but still forms the expected selenium-carbon bond as indicated by 11B and 77Se NMR spectroscopy (Figure 4).
Beyond reactions of 2A with alkenes, alkynes, and enolates, we also explored its ability to participate in electrophilic aromatic substitution (SEAr) with toluene. 2A was treated with an excess of anhydrous toluene and aluminum chloride to catalyze the transformation at 50 °C, with the reaction progress monitored by GC-MS. After stirring the mixture overnight, GC-MS indicated the formation of three isomers consistent (Figure 4) with SEAr occurring between toluene and 2A. The crude products were then purified via silica gel column chromatography, affording a mixture of aryl selenide isomers (2F) in 21% isolated yield. In addition to 11B and 77Se NMR spectroscopy revealing the formation of the desired aryl selenide, 1H NMR spectroscopy also indicates the para tolyl isomer as the major component in the isomeric mixture (SI sec. 5c), consistent for SEAr mechanisms with toluene. Resolving all three isomers by 1H NMR spectroscopy proved to be quite difficult not only due to the low abundance of the ortho- and meta-isomers, but also the prevalence of 1H resonances attributed to the carborane B-H vertices in the alkyl region.
In order to deconvolute the 1H NMR and obtain a more accurate ratio of isomer, the SEAr between 2A and toluene was repeated with perdeuterated toluene (SI sec. 5g). 2H NMR spectroscopy of the isolated product clearly revealed the distribution (para:ortho/meta) of isomers to be 89:11, in agreement with the distribution determined by GC-MS.
With the electrophilicity of 2A established, we chose next to explore that of 3A. First, the reaction of 3A with phenylmagnesium bromide was assessed to confirm the electrophilic character of 3A. After addition of the Grignard reagent to form the desired phenyl telluride (3B), the product was isolated via silica gel column chromatography in 75% yield (SI sec. 6a). Importantly, the NMR characterization of 3B agrees with previously reported characterization by our group of meta-carboranyl phenyltelluride obtained via an independent chemical route (Figure 5A).8 We then attempted the reaction of 3A with phenylacetylene to determine its ability to react with unsaturated hydrocarbons. 3A was suspended in anhydrous dichloromethane before the addition of phenylacetylene. After stirring the suspension at room temperature for 16 hours, 11B NMR spectroscopy of the reaction mixture revealed only the presence of 3A and 1B as a decomposition product (Figure 5A, SI sec. 6b). In an attempt to improve conversion to the desired telluride, the reaction was attempted in chloroform at reflux temperature. However, after stirring the reaction mixture for 8 hours, conversion to the desired telluride was lower than anticipated (SI sec. 6c) and contained significant quantities of starting material (3A) and 1B as indicated by 11B NMR spectroscopy. We attribute this general decrease in reactivity to a combination of both the inherently lower electronegativity of tellurium and the electron donating ability of meta-carborane at the B(9) position,6 resulting in the diminished electrophilicity of 3A.
Figure 5:

A. Reaction of 3A with phenylmagnesium bromide in anhydrous Et2O and phenylacetylene in various solvents. B. Reaction of 4A with phenylacetylene, including in situ 11B and 125Te NMR characterization of reaction intermediates, 4B* and 4B*’.
To test our hypothesis, we attempted the reaction between 4A and phenylacetylene in refluxing chloroform. In contrast to the selectivity of organic selenyl (II) chlorides and 2A to form products with unsaturated hydrocarbons resulting from Markovnikov anti-addition, the preferred substitution mechanisms of organic tellurenyl (IV) trichlorides are much more difficult to predict, due to the possible formation of four-centered intermediates (Markovnikov, syn in nonpolar solvents), three-centered intermediates (Markovnikov, anti), or radical-based intermediates.1a,10 Notably, the reactions of tellurenyl (IV) trichlorides with phenylacetylene have typically afforded Markovnikov syn-addition products, consistent with the in situ formation of a four-centered intermediate, and selectivity for syn over anti-addition predicated on the polarity of solvent used for the reaction medium.
To assess the reactivity of 4A, it was first suspended in chloroform followed by the addition of phenylacetylene. After stirring the white suspension for 6 hours at reflux (65 °C), a clear, yellow solution remained (SI sec. 7a). In situ 11B NMR spectroscopy of the reaction mixture revealed full consumption of 4A and a diagnostic upfield shift in the resonance attributed to the exopolyhedral boron-tellurium bond (Figure 5B). This change in resonance chemical shift is consistent with the presence of the key dichlorotelluride intermediate (4B*), and suggests the formation of the desired tellurium-carbon bond. However, in contrast to the formation of the selenium-containing congener (2D), in situ 125Te NMR spectroscopy revealed the presence of two tellurium-containing isomers with similar chemical shifts approximately in a 2:1 ratio, attributed to 4B* and 4B*’ Z/E isomers. To further understand the reaction mechanism being employed by 4A in the reaction with phenylacetylene, a series of control reactions were performed to rule out the possible intermediates previously shown to be accessible with tellurenyl (IV) trichlorides vide supra (SI sec. 7b,c)1a,10 and probed via in situ 125Te NMR spectroscopy. When the reaction was performed in the presence of a radical inhibitor, no significant change in the distribution of 4B* and 4B*’ was observed, likely ruling out the possibility of a radical-mediated substitution mechanism. However, when the reaction was performed in toluene, a relatively non-polar solvent, 4B* was formed exclusively with no measurable amount of 4B*’ by 125Te NMR. This selectivity, as influenced by solvent polarity, is reminiscent to the behavior of four-centered tellurenyl (IV) trichloride intermediates, resulting in selective Markovnikov syn addition (4B*) of 4A to phenylacetylene when conducting the reaction in toluene and a mixture of four-centered and three-centered tellurenyl (IV) trichloride intermediates forming syn (4B*) and anti-addition (4B*’) products when in chloroform. The isomeric mixture of 4B* and 4B*’ was then reduced to the desired telluride (4B and 4B’) by treatment with an aqueous solution of sodium thiosulfate and the progress of the reduction was monitored by TLC. Once the reduction was complete, 4B and 4B’ were isolated from the crude reaction mixture via silica gel column chromatography as a mixture of Z/E isomers in 70% yield and characterized by heteronuclear NMR spectroscopy to confirm the formation of the desired tellurium-carbon bond. 1H and 125Te NMR spectroscopy revealed the isolation of two distinct isomers, 4B and 4B’ in approximately a 2:1 ratio (Figure 5B, SI sec. 7a). Two broad quartet resonances in the 1H aromatic region (7.45 and 7.07 ppm) are attributed to the olefinic 1H’s and are used to determine the isomer distribution. Furthermore, 125Te NMR spectroscopy revealed two 125Te resonances at −10 and −15 ppm, with approximate relative intensities of 1:2, respectively, and agrees with the distribution observed by 1H NMR (Figure 5B, see SI sec. 7a). This additional isomer is likely attributed to the formation of E-1-TeR-2-Ph-2-Cl (R: meta-carboranyl) as the minor product that was enabled by the increased polarity of the reaction solvent. This minor product is produced from a portion of 4A reacting with phenylacetylene through a three-centered intermediate, similar to the substitution mechanism employed by 2A.
In summary, the assessment of electrophilic behavior for meta-carboranyl selenyl (II), tellurenyl (II), and tellurenyl (IV) chlorides reveal that their reactivity is reminiscent to carbon-based reagents. 2A reacts with Grignard reagents, alkenes, alkynes, enolates, and electron-rich aromatics to form products that would generally be expected for organic selenyl chlorides. Despite, the electron-donating ability of the B(9) position to which the selenyl (II) chloride in 2A is appended to, no deleterious effects to the overall electrophilic reactivity of 2A are observed. In contrast, the electrophilic reactivity of the tellurenyl (II) chloride 3A is significantly dampened, only showing good reactivity with Grignard reagents. To enhance the electrophilic reactivity, the tellurenyl (IV) chloride 4A was studied. An increase in oxidation state at tellurium significantly enhanced the electrophilic reactivity causing it to react more readily with terminal alkynes. The regioselective and stereoselective behavior of 4A when reacting with terminal alkynes in various solvents closely parallels the behavior of organic tellurenyl (IV) chlorides, forming either the syn or anti-addition products as a function of solvent polarity.
With the electrophilicity of 2A, 3A, and 4A established, we proceeded to expand the nucleophilic substitution chemistry available with selenium and tellurium-containing meta-carboranes. The ability of meta-carborane-based selenolates and tellurolates to participate in SN2 substitution mechanisms with alkyl halides is well established.4d,f However, their ability to participate in SNAr substitution mechanisms is not known. To further understand the nucleophilic behavior of boron-bound selenium and tellurium-containing carboranes, we prepared the 9-meta-carboranyl selenol (5A) and tellurol (6A). We envisioned that these compounds, upon deprotonation, would act as precursors to the corresponding nucleophilic chalcogenolates. Selenol (5A), was synthesized according to previously reported methods and its spectroscopic characterization is in agreement to the proposed formulation (SI sec. 8a).4d,6b While the boron-bound tellurol (6A) has not been reported previously, we were able to successfully synthesize 6A using modified reduction procedures (SI sec. 9a). 6A is isolated in 67% yield as a colorless, odorless, air sensitive solid that nevertheless can be handled in air for short periods of time (~10 minutes) without significant decomposition. In contrast to all other known carborane chalcogenols, 6A is light sensitive and reverts to the ditelluride (1B) when exposed to ambient light, even when stored in a nitrogen-filled glovebox.
The 1H and 125Te resonances measured by NMR spectroscopy, −7.15 ppm and −596.5 ppm respectively, are indicative of the exceedingly electron-rich environment experienced by the tellurol from the B(9) meta-carboranyl susbstituent and are consistent with other reported sterically hindered electron-rich tellurols.11 This is the first reported synthesis of an isolable carboranyl tellurol, and is a rare example of an isolable tellurol.
SNAr with the meta-carboranyl chalcogenolates was first attempted with 5A by deprotonating the selenol with Cs2CO3 in dimethylformamide (SI sec. 8b). Perfluorotoluene was then introduced to the mixture as the electrophilic substrate to initiate SNAr with the in situ generated selenolate. After stirring the mixture overnight, GC-MS indicated full conversion to a single isomer with an m/z (223.20) consistent with the formation of the desired aryl selenide (5B). The compound was subsequently purified from the crude reaction mixture via silica gel column chromatography in 61% isolated yield and characterized by NMR spectroscopy. 19F NMR revealed a diagnostic resonance pattern consistent with mono-substituted perfluorotoluene in the para-position and is in agreement with the proposed structure formulation. Furthermore, 77Se NMR showed a downfield shift in the resonance attributed to the exopolyhedral boron-selenium bond found in 5B, and is consistent with the formation of an aryl selenide (Figure 6A). SNAr was then attempted with the tellurol (6A) using similar conditions, though taking additional precautions to limit exposure to light and oxygen (SI sec. 9b). Following similar isolation procedures to 5B, the desired aryl telluride (6B) was isolated in small yield (13%), sufficient for full characterization by NMR spectroscopy. Due to the general instability of the in situ generated tellurolate under the reaction conditions, ditelluride (1B) was a major byproduct formed during the reaction between 6A and perfluorotoluene (SI sec. 9d).
Figure 6:

A. SNAr of 5A and 6A with perfluorotoluene. 77Se and 125Te NMR of 5B and 6B. B. Reaction of 6A with palladium oxidative addition complex.
To study the reactivity of 6A further with more electron-rich aryl-based electrophiles, we attempted arylation with a stochiometric palladium-based oxidative addition complex. These oxidative addition complexes are typically used stoichiometrically for the arylation of sensitive substrates due to their high degree of selectivity for chalcogenols and ease of preparation.12 A solution of 6A in dichloromethane was prepared in a dark, nitrogen filled glovebox without the addition of base to avoid incompatibilities of the tellurolate with the oxidative addition complex (SI sec. 9c). Subsequently, [4-tolyl-PdRuPhos][OTf] (Figure 6B) was added and the reaction progress was monitored by GC-MS. After 30 minutes, GC-MS indicated the formation of a compound with an m/z of 363.20, suggesting the formation of the desired tolyl telluride (6C). 6C was isolated from the crude reaction mixture via silica gel column chromatography in 56% yield and characterized by NMR spectroscopy. 125Te NMR revealed a resonance with a chemical shift of 46.5 ppm, similar to that of 3A (Figure 5, 6B). Overall, these studies indicate that B(9) meta-carboranyl selenolate and tellurolate can be competent nucleophiles and are able to participate in SNAr and Pd-mediated arylation processes, leading to the formation of selenoether and telluroether moieties with B-Y-C connectivity.
Conclusions
In conclusion, B(9)-connected meta-carboranyl selenyl and tellurenyl reagents have been shown to participate in electrophilic substitution reactions with unsaturated hydrocarbons, including alkenes, alkynes, enolates, and aromatic substrates; reminiscent to other electrophilic organochalcogen compounds. We further show the first examples of nucleophilic aromatic substitution with carborane selenolates and tellurolates as well as the first use of palladium-based oxidative addition complexes for the arylation of a free tellurol. All formed products contained the unique B-Se-C or B-Te-C bonding motifs and are stable in air despite the exceedingly electron-rich environment experienced by either the selenium or tellurium nucleus as suggested by the 77Se and 125Te NMR spectroscopic experiments. The reactivity map developed in this work serves as an expansion of available modification reactions for carboranes and other polyhedral boron clusters containing C- or B-connected exopolyhedral heteroatom substituents,13 as well as benchmarks similarities and differences in terms of reactivity and stability with the fundamental chemistry of electron-rich chalcogen-containing molecules.1a,10,11,14
Supplementary Material
ACKNOWLEDGMENTS
A. M. S. thanks NIGMS (R35GM124746) for supporting this work. H. A. M. is a recipient of the UCLA Dissertation Year Fellowship. F. A. is a recipient of Arthur Furst Summer Undergraduate Research Fellowship and Raymond and Dorothy Wilson Fellowship. A. M. S. is a Research Corporation for Science Advancement (RCSA) Cottrell Scholar and a Dreyfus Foundation Camille Dreyfus Teacher Scholar.
Footnotes
Crystallographic data are available from the Cambridge Crystallographic Data Centre, under reference numbers CCDC 2105009 (1A), 2105007 (1B), 2105010 (2C), and 2105008 (2D).
The following files are available free of charge.
Full synthetic procedures, spectroscopic data (PDF).
The authors declare no competing financial interests.
REFERENCES
- (1).(a) Rappaport Z The Chemistry of Organic Selenium and Tellurium Compounds; John Wiley & Sons: New York, 2013; Vol. 4 [DOI] [Google Scholar]; (b) Block E; Glass RS; Gruhn N; Jin J; Lorance E; Zakai UI; Zhang S-Z Chemistry of Mixed Sulfur-, Selenium-, or Tellurium- and Silicon, or Tin-Containing Heterocycles. Phosphorus, Sulfur, and Silicon and the Related Elements 2008. 183, 4, 856–862. [DOI] [Google Scholar]; (c) Kumar S; Helt J-CP; Autschbach J; Detty MR A New Reaction of Organoselenium Compounds: Alkyl Transfer from Diorganoselenium(IV) Dibromides to Give Alkenoic Acids to Give γ- and δ-Lactones. Organometallics 2009, 28, 12, 3426–3436. [DOI] [Google Scholar]; (d) Evans DH; Gruhn NE; Jin J; Li B; Lorance E; Okumara N; Macías-Ruvalcaba NA; Zakai UI; Zhang S-Z; Block E; Glass RS Electrochemical and Chemical Oxidation of Dithia-, Diselena-, Ditellura-, Selenathia-, and Tellurathiamesocycles and the Stability of Oxidized Species. J. Org. Chem 2010, 74, 6, 1997–2009. [DOI] [Google Scholar]; (e) Garrett GE; Gibson GL; Straus RN; Seferos DS; Taylor MS Chalcogen Bonding in Solution: Interactions of Benzotelluradiazoles with Anionic and Uncharged Lewis Bases. J. Am. Chem. Soc 2015, 137, 12, 4126–4133.25781631 [DOI] [Google Scholar]; (f) Zhou B; Gabbai FP Lewis Acidic Telluronium Cations: Enhanced Chalcogen-Bond Donor Properties and Application to Transfer Hydrogenation Catalysis. Organometallics 2021. DOI: 10.1021/cas.organomet.1c00279. [DOI] [Google Scholar]; (g) Lin T-P; Gabbai FP Two-Electron Redox Chemistry at the Dinuclear Core of a TePt Platform: Chlorine Photoreductive Elimination and Isolation of a TeVPtI Complex. J. Am. Chem. Soc 2012, 134, 29, 12230–12238.22708610 [DOI] [Google Scholar]; (h) Lin T-P; Gabbai FP Telluronium Ions as σ-Acceptor Ligands. Angew. Chem. Int. Ed 2013, 52, 14, 3864–3868. [DOI] [Google Scholar]; (i) Yang H; Lin T-P; Gabbai FP Telluroether to Telluroxide Conversion in the Coordination Sphere of a Metal: Oxidation-Induced Umpolung of a Te-Au Bond. Organometallics 2014, 33, 17, 4368–4373. [DOI] [Google Scholar]; (j) Kryman MW; Nasca JN; Watson DF; Detty MR Selenorhodamine Dye-Sensitized Solar Cells: Influence of Structure and Surface-Anchoring Mode on Aggregation, Persistence, and Photochemical Performance. Langmuir 2016, 32, 6, 1521–1532.26791741 [DOI] [Google Scholar]; (k) Ye S; Jansaz L; Zajaczkowski W; Manion JG; Mondal A; Marszalek T; Andrienko D; Müllen K; Pisula W; Seferos DS Self-Organization and Charge Transport Properties of Selenium and Tellurium Analogues of Polythiophene. Macromol. Rapid Commun 2019, 40, 1, 1800596. [DOI] [Google Scholar]; (l) Scholes DT; Yee PY; McKeown GR; Li S; Kang H; Lindemuth JR; Xia X; King SC; Seferos DS; Tolbert SH; Schwartz BJ Designing Conjugated Polymers for Molecular Doping: The Roles of Crystallinity, Swelling, and Conductivity in Sequentially-Doped Selenophene-Based Copolymers. Chem. Mater 2019, 31, 1, 73–82. [DOI] [Google Scholar]; (m) Manion JG; Panchuk JR; Seferos DS Applying Heteroatom Substitution in Organic Photovoltaics. Chem. Rec 2019, 19, 6, 1113–1122.30793821 [DOI] [Google Scholar]; (n) Hicks GEJ; Jarrett-Wilkins CN; Panchuk JR; Manion JG; Seferos DS Oxidation promoted self-assembly of π-conjugated polymers. Chem. Sci. 2020, 11, 6383.34094104 [DOI] [Google Scholar]; (o) Kryman MW; Schamerhorn GA; Yung K; Sathyamoorthy B; Sukumaran DK; Ohulchanskyy TY; Benedeict JB; Detty MR Organotellurium Fluorescence Probes for Redox Reactions: 9-Aryl-3,6-diaminotelluroxanthylium Dyes and Their Telluroxides. Organometallics 2013, 32, 15, 4321–4333. [DOI] [Google Scholar]; (p) Stockett MH; Kjær C; Linder MK; Detty MR; Nielsen SB Luminescence spectroscopy of chalcogen substituted rhodamine cation in vacuo. Photochem. Photobiol. Sci 2017, 16, 779–784.28352922 [DOI] [Google Scholar]; (q) Carrera EI; Seferos DS Ring Opening of π-delocalized 2,5-Diphenyltelurophene by Chemical or Self-Sensitized Aerobic Photooxidation. Organometallics 2017, 36, 14, 2612–2621. [DOI] [Google Scholar]; (r) Lutkus LV; Rettig ID; Davies KS; Hill JE; Lohman JE; Eskew MW; Detty MR; McCormick TM Photocatalytic Aerobic Thiol Oxidation with a Self-Sensitized Tellurorhodamine Chromophore. Organometallics 2017, 36, 14, 2588–2596. [DOI] [Google Scholar]; (s) Drummond BH; Hoover GC; Gillet AJ; Aizawa N; Myers WK; McAllister BT; Jones STE; Pu Y-J; Credgington D; Seferos DS Selenium Susbtitution Enhances Reverse Intersystem Crossing in a Delayed Fluorescence Emitter. J. Phys. Chem. C 2020, 124, 6364–6370. [DOI] [Google Scholar]; (t) Cao W; McCallum NC; Ni QZ; Li W; Boyce H; Mao H; Zhou X; Sun H; Thompson MP; Battistela C; Wasielewski MR; Dhinojwala A; Shawkey MD; Burkhart MD; Wang Z; Gianneschi NC Selenomelanin: An Abiotic Selenium Analogue of Pheomelanin. J. Am. Chem. Soc 2020, 142, 29, 12802–12810.32638590 [DOI] [Google Scholar]; (u) Glass RB; Berry MJ; Block E; Boakye HT; Carlson BA; Gailer J; George GN; Gladyshev VN; Hatfield DL; Jacobsen NE; Johnson S; Kahakachchi C; Kamiński R; Manley SA; Mix H; Pickering IJ; Prenner EJ; Saira K; Skowrońska A; Tyson JF; Uden PC; Wu Q; Xu X-M; Yamdagni R; Zhang Y Insights into the Chemical Biology of Selenium. Phosphorus, Sulfur, and Silicon and the Related Elements 2008, 183, 4, 924–930. [DOI] [Google Scholar]; (v) Block E; Booker SJ; Flores-Penalba S; George G; Gundala G; Landgraf BJ; Liu J; Lodge SN; Pushie MJ; Rozovsky S; Vattekkatte A; Yaghi R; Zeng H Trifluoroselenomethionine – a New Non-Natural Amino Acid. ChemBioChem 2016, 17, 18, 1738–1751.27383291 [DOI] [Google Scholar]; (w) Zhao Z; Shimon D; Metanis N Chemoselective Copper-Mediated Modification of Selenosysteines in Peptides and Proteins. J. Am. Chem. Soc 2021, 143, 12817–12824.34346673 [DOI] [Google Scholar]
- (2).(a) Wentz KE; Molino A; Weisflog SL; Kaur A; Dickie DA; Wilson DD; Gilliard RJ Stabilization of the Elusive 9-Carbene-9-Borafluorene Monoanion. Angew. Chem. Int. Ed 2021, 60, 23, 13065–13072. [DOI] [PubMed] [Google Scholar]; (b) Clive DLJ; Menchen SM Conversion of Aldehydes and Ketones into Selenoacetals: Use of Tris(phenylseleno)borane and Tris(methylseleno)borane. J. Org. Chem 1979, 44, 24, 4279–4285. [DOI] [PubMed] [Google Scholar]; (c) Dolati H; Denker L; Trzaskowski B; Frank R Superseeding β-Diketiminato Ligands: An Amido Imidazoline-2-Imine Ligand Stabilizes the Exhaustive Series of B=X Boranes (X=O, S, Se, Te). Angew. Chem. Int. Ed 2020, 60, 9, 4633–4639. [DOI] [PubMed] [Google Scholar]; (d) Köster R; Seidel G; Yalpani M; Siebert W; Gangus B 1,5-Cyclooctanediboryl Selenides. Inorganic Synthesis 1992, 29, 70–77. [DOI] [PubMed] [Google Scholar]; (e) Männig D; Narula CK; Nöth H; Wietelmann U Beiträge zur Chemie des Bors, 159. [2 + 2]-Cycloadditionen von (tert-Butylimino)(2,2,6,6-tetramethylpiperdino)boran mit Kohlenstoffdichalkogeniden. Chem. Ber 1985, 118, 9, 3748–3758. [DOI] [PubMed] [Google Scholar]; (f) Yalpani M; Köster R; Boese R Nitrogen Base – Dialkyl-1,2,4,3,5-triselenadiborolanes. Chem. Ber 1990, 123, 4, 707–712. [DOI] [PubMed] [Google Scholar]; (g) Köuster R; Seidel G; Schüuβler W; Wrackmeyer B Die ersten Organobor-Tellur-Verbindungen. Chem. Ber 1995, 128, 1, 87–89. [DOI] [PubMed] [Google Scholar]; (h) Liu S; Légaré M-A; Auerhammer D; Hofmann A; Braunschweig H The First Boron-Tellurium Double Bond: Direct Insertion of Heavy Chalcogens into a Mn=B Double Bond. Angew. Chem. Int. Ed 2017, 56, 49, 15760–15763. [DOI] [PubMed] [Google Scholar]; (i) Liu S; Légaré M-A; Hofmann A; Rempel A; Hagspiel S; Braunschweig H Synthesis of unsymmetrical B2E2 and B2E3 heterocycles by borylene insertion into boradichalcogeniranes. Chem. Sci 2019, 10, 4662–4666. [DOI] [PubMed] [Google Scholar]; (j) Liu S; Légaré M-A; Hofmann A; Braunschweig H A Boradiselenirane and a Boraditellurirane: Isolable Heavy Analogs of Dioxiranes and Dithiiranes. J. Am. Chem. Soc 2018, 140, 36, 11223–11226. [DOI] [PubMed] [Google Scholar]; (k) Joseph B; Gomosta S; Prakash T; Phukan AK; Ghosh S Chalcogen Stabalized bis-Hydridoborate Complexes of Cobalt: Analogues of Tetracyclo[4.3.0.02,4.03,5]nonane. Chem. Eur. J 2020, 26, 70, 16824–16832. [DOI] [PubMed] [Google Scholar]; (l) Ramalalshmi R; Saha K; Roy DK; Varghese B; Phukan AK; Ghosh S New Routes to a Series of σ-Borane/Borate Complexes of Molybdenum and Ruthenium. Chem. Eur. J 2015, 21, 48, 17191–17195. [DOI] [PubMed] [Google Scholar]; (m) Okio CKYA; Levason W; Monzittu FM; Reid G Complexes of BX3 with EMe2 (X = F, Cl, Br, I; E = Se or Te): Synthesis, multinuclear NMR spectroscopic and structural studies. J. Organomet. Chem 2017, 848, 232–238. [DOI] [PubMed] [Google Scholar]; (n) Okio CKYA; Levason W; Monzittu FM; Reid G Systematics of boron halide complexes with dichalcogenoether ligands – Synthesis, structures, and reaction chemistry. J. Organomet. Chem 2018, 854, 140–149. [DOI] [PubMed] [Google Scholar]; (o) Tokoro Y; Nagai A, Kokado K Chujo Y Synthesis of Organoboron Quinoline-8-thiolate and Quinoline-8-selenolate Complexes and Their Incorporation into the π-Conjugated Polymer Main-Chain. Macromolecules 2009, 42, 8, 2988–2993. [DOI] [PubMed] [Google Scholar]; (p) Grotthuss E; Nawa F; Bolte M; Lerner H-W; Wagner M Chalcogen-chalcogen-bond activation by an ambiphilic, doubly reduced organoborane. Tetrahedron 2019, 75, 1, 26–30. [DOI] [PubMed] [Google Scholar]; (q) Braunschweig H; Constantinidis P; Dellermann T; Ewing WC; Fischer I; Hess M; Knight FR; Rempel A; Schneider C; Ullrich S; Vargas A; Woollins JD Highly Strained Heterocycles Constructed from Boron-Boron Multiple Bonds and Heavy Chalcogens. Angew. Chem. Int. Ed 2016, 55, 18, 5606–5609. [DOI] [PubMed] [Google Scholar]; (r) Geetharani K; Bose SK; Basak D; Suresh VM; Ghosh S A new entry into ferraborane chemistry: Synthesis and characterization of heteroferraborane complexes. Inorganica Chim. Acta 2011, 372, 42–46. [DOI] [PubMed] [Google Scholar]
- (3).(a) Chakrahari KKV; Thakur A; Mondal B; Dhayal RS; Ramkumar V; Ghosh S A close-packed boron-rich 11-vertex molybdaborane with novel geometry. J. Organomet. Chem 2012, 710, 75–79. [Google Scholar]; (b) Mondal B; Bhattacharyya M; Varghese B; Ghosh S Hypo-electronic triple-decker sandwich complexes: synthesis and structural characterization of [(Cp*Mo)2{μ-η6:η6-B4H4E-Ru(CO)3}] (E = S, Se, Te or Ru(CO)3 and Cp* = η5-C5Me5). Dalton Trans. 2016, 45, 10999–11007.27309843 [Google Scholar]; (c) Roy DK; Bose SK; Geetharani K; Chakrahari KKV; Mobin SM; Ghosh S Synthesis and Structural Characterization of New Divanada- and Diniobaboranes Containing Chalcogen Atoms. Chem. Eur. J 2012, 18, 32, 9983–9991.22782697 [Google Scholar]; (d) Chakrahari KK; Thakur A; Mondal B; Ramkumar V; Ghosh S Hypoelectronic Dimetallaheteroboranes of Group 6 Transition Metals Containing Heavier Chalcogen Elements. Inorg. Chem 2013, 52, 14, 7923–7932.23819867 [Google Scholar]; (e) Kultyshev RG; Liu S; Leung HT; Liu J; Shore SG Synthesis of Mono- and Dihalogenated Derivatives of (Me2S)2B12H10 and Palladium-Catalyzed Boron-Carbon Cross-Coupling Reactions of the Iodides with Grignard Reagents. Inorg. Chem 2003, 42, 10, 3199–3207.12739960 [Google Scholar]; (f) Paetzold P; Englert U; Hansen H-P; Meyer F; Leuschner E 11-Vertex arachno-Clusters with an NB10, SNB9, and SeNB9 Skeleton. Z. Anorg. Allg. Chem 2001, 627, 3, 498–506. [Google Scholar]; (g) di Biani FF; Laschi F; Zanello P; Ferguson G; Trotter J; O’Riordan GM; Spalding TR Synthesis, structure, spectroscopic and electrochemical study of the paramagnetic compound [2-(η7-C7H7)-7,11-F2–2,1-closo-MoTeB10H8]. J. Chem. Soc., Dalton Trans 2001, 1520–1523. [Google Scholar]; (h) Ferguson G; Gallagher JF; Kenedy JD; Kelleher A-M; Spalding TR Pentahapto-bonded gold heteroborane clusters [3-(R3P)-closo-2,1-AuTeB10H10]- and [3-(R3P)-closo-3,1,2-AuAs2B9H9]−. Dalton Trans. 2006, 2133–2139.16625258 [Google Scholar]; (i) Faridoon McGarth, M.; Spalding TR; Fontaine XLR; Kennedy JD; Thorton-Pett M Metallaheteroborane Chemistry. Part 6. Synthesis of closo-[2-(η-ligand)-1,2-TeMB10H10] Complexes with M(η-ligand) = Rh(η5-C5Me5) (1), Ru(η6-p-MeC6H4Pri) (2), Ru(η6-C6Me6) (3), and of nido-[6-(η6-C6Me6)-8-(OEt)-6-RuB9H12] (4), their Characterisation by Nuclear Magnetic Resonance Spectroscopy and, for (1) and (3), by X-Ray Crystallography. J. Chem. Soc., Dalton Trans 1990, 1819–1829. [Google Scholar]
- (4).(a) Zakharkin LI; Pisareva IV A New Simple Method for the Production and Some Conversions of B-S Bond-Containing o- and m-Carboranyl. Phosphorus and Sulfur 1984, 20, 357–370. [Google Scholar]; (b) Chen M; Zhao D; Xu J; Li C; Lu C; Yan H Electrooxidative B-H Functionalization of nido-Carboranes. Angew. Chem. Int. Ed 2021, 60, 14, 7838–7844. [Google Scholar]; (c) Chen Y; Quan Y; Xie Z 8-Aminoquinoline as a bidentate traceless directing group for Cu-catalyzed selective B(4,5)-H disulfenylation of o-carboranes. Chem. Comm 2020, 56, 12997–13000.32996955 [Google Scholar]; (d) Zakharkin LI; Pisareva IV; Antonovich VA Synthesis of Di(o- and m-Carboran-9-yl) Diselenides by Electrophilic-Substitution Reactions of o- and m-Carboranes with Selenium Chlorides Under the Action of AlCl3, and Reactions of These Products. Zhurnal Obshchei Khimii 1986, 56, 12, 2721–2728. [Google Scholar]; (e) Zakharkin LI; Pisareva IV Electrophilic Telluration of o- and m-Carboranes with TeCl4 Under the Action of AlCl3. Izvestiya Akademii Nauk, Seriya Khimicheskaya 1984, 2, 472–473. [Google Scholar]; (f) Zakharkin LI; Pisareva IV Synthesis and Some Conversions of Derivatives of o- and m-Carborane Containing a B-Te σ Bond. Izvestiya Akademii Nauk SSSR 1987, 4, 877–880. [Google Scholar]; (g) Olid D;Núñez R;Viñas C; Teixidor F Methods to produce B-C, B-P, B-N and B-S bonds in boron clusters. Chem. Soc. Rev 2013, 42, 3318–333623318646 [Google Scholar]
- (5).For a general review of carboranes, see:; Grimes RN. Carboranes, 3rd ed; Elsevier: Oxford, 2016. [Google Scholar]
- (6).(a) Hohman JN; Zhang PP; Morin EI; Han P; Kim MH; Kurland AR; McClanahan PD; Balema VP; Weiss PS Self-Assembly of Carboranethiol Isomers on Au[111]: Intermolecular Interactions Determined by Molecular Dipole Orientations. ACS Nano 2009, 3, 527. [DOI] [PubMed] [Google Scholar]; (b) Spokoyny AM; Machan CW; Clingerman DJ; Rosen MS; Wiester MJ; Kennedy RD; Stern CL; Sarjeant AA; Mirkin CA A coordination chemistry dichotomy for icosahedral carborane-based ligands. Nat. Chem 2011, 3, 590–596. [DOI] [PubMed] [Google Scholar]; (c) Serino AC; Anderson ME; Saleh LMA; Dziedzic RM; Mills H; Heidenreich LK; Spokoyny AM; Weiss PS Work Function Control of Germanium through Carborane-Carboxylic Acid Surface Passivation. ACS Appl. Mater. Interfaces 2017, 9, 34592–34596. [DOI] [PubMed] [Google Scholar]
- (7).Search was performed using Mogul 1.8.4, provided by the CCDC. Ph-Se-Se-Ph was drawn in the search window and analysis of all fragments was conducted.
- (8).Mills HA; Martin JL; Rheingold AL; Spokoyny AM Oxidative Generation of Boron-Centered Radicals in Carboranes. J Am. Chem. Soc 2020, 142, 10, 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Search was performed using Mogul 1.8.4 and/or ConQuest 2.0.4, provided by the CCDC. Structures containing B-Se, Se-C, and B-Se-C were analyzed.
- (10).(a) Comasseto JV; Stefani HA; Chieffi A; Zukerman-Schpector J Addition of Organotellurium Trihalides to Acetylenes. Organometallics 1991, 10, 845–846. [Google Scholar]; (b) Zukerman-Schpector J; Castellano EE; Oliva G; Comasseto JV; Stefani HA Structure of dichloro[(Z)-2-chloro-2-p-tolylvinyl](p-methoxyphenyl)tellurium(VI) Acta Cryst. 1991, C47, 960–962. [Google Scholar]; (c) Zukerman-Schpector J; Comasseto JV; Stefani HA Dichloro[(Z)-2-chloro-2-phenylvinyl](4-methoxyphenyl)tellurium(IV). Acta Cryst. 1995, C51, 861–863. [Google Scholar]; (d) Zukerman-Schpector J; Camillo RL; Comasseto JV; Santos RA; Caracelli J Trichloro[(Z)-2-chloro-1,2-diphenylvinyl]-tellurium(IV). Acta Cryst. 1999, C55, 1577–1579. [Google Scholar]; (e) Huang X; Wang Y-P Stereoselective Synthesis of (Z)- or (E)-β-Bromovinyl Tellurides and Their Application in the Synthesis of Trisubstituted Alkenes. Tet. Lett 1996, 37, 41, 7417–7420. [Google Scholar]; (f) Chauhan AKS; Bharti SN; Srivastava RC; Butcher RJ; Duthie A Stereospecific chlorotelluration of terminal acetylenes. J. Organomet. Chem 2012, 708-709, 75–81. [Google Scholar]
- (11).(a) Dabbousi BO; Bonasia PJ; Arnold J (Me3Si)3SiTeH: Preparation, Characterization, and Synthetic Utility of a Remarkably Stable Tellurol. J. Am. Chem. Soc 1991, 113, 3186–3188. [Google Scholar]; (b) Bonasia PJ; Arnold J Lithium Tri(trimethylsilyl)silyltellurolate Bis(tetrahydrofuran) and Tris(trimethylsilyl)silyltellurol. Inorg. Synth 1997, 31, 162–165. [Google Scholar]
- (12).(a) Vinogradova EV; Zhang C; Spokoyny AM; Pentelute BL; Buchwald SL Organometallic palladium reagents for cysteine bioconjugation. Nature 2015, 526, 687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Uehling MR; King RP; Krska SW; Cernak T; Buchwald SL Pharmaceutical diversification via palladium oxidative addition complexes. Science 2019, 363, 6425, 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Batsanov AS; Fox MA; Hibbert TG; Howard JAK; Kivekäs R; Laromaine A; Sillanpää R; Viñas C; Wade K Sulfur, tin and gold derivatives of 1-(2’-pyridyl)-ortho-carborane, 1-R-2-X-1,2-C2B10H10 (R = 2’-pyridyl, X = SH, SnMe3 or AuPPh3. Dalton Trans. 2004, 3822–3828. [DOI] [PubMed] [Google Scholar]; (b) Laromaine A; Teixidor F; Kivekäs R; Sillanpää R; Arca M; Lippolis V; Crespo§ F; Viñas C Synthesis, reactivity and structural studies of selenide bridged carboranyl compounds. Dalton Trans. 2006, 5240–5247. [DOI] [PubMed] [Google Scholar]; (c) Popescu AR; Teixidor F; Viñas C Metal promoted charge and hapticities of phosphines: The uniqueness of carboranylphosphines. Coord. Chem. Rev 2014, 269, 54–84. [DOI] [PubMed] [Google Scholar]; (d) Wong YO; Smith MD; Peryshkov DV Reversible water activation driven by contraction and expansion of a 12-vertex-closo-12-vertex-nido biscaborane cluster. Chem. Commun 2016, 52, 12710–12713. [DOI] [PubMed] [Google Scholar]; (e) Kleinsasser JF; Reinhart ED; Estrada J; Jordan RF; Lavallo V Ethylene Oligomerization and Polymerization by Palladium(II) Methyl Complexes Supported by Phosphines Bearing a Perchlorinated 10-Vertex closo-Carborane Anion Substituent. Organometallics 2018, 37, 24, 4773–4783. [DOI] [PubMed] [Google Scholar]; (f) Ali OM Lasseter JC; Żurawiński R; Pietrzak Pecyna, J.; Wojciechowski J; Friedli AC; Pociecha D; Kaszyński P Thermal and Photophysical Properties of Highly Quadrupolar Liquid-Crystalline Derivatives of the [closo-B12H12]2− Anion. Chem. Eur. J 2019, 25, 10, 2616–2630. [DOI] [PubMed] [Google Scholar]; (g) Yruegas S; Axtell JC; Kirlikovalli KO; Spokoyny AM; Martin CD Synthesis of 9-borafluorene analogues featuring a three-dimensional 1,1’-bis(o-carborane) backbone. Chem. Commun 2019, 55, 2892–2895. [DOI] [PubMed] [Google Scholar]; (h) Lyu H; Zhang J; Yang J; Quan Y; Xie Z Catalytic Regioselective Cage B(8)-H Arylation of o-Carboranes via “Cage-Walking” Strategy. J. Am. Chem. Soc 2019, 141, 10, 4219–4224. [DOI] [PubMed] [Google Scholar]; (i) Eleazer BJ; Smith MD; Peryshkov DV Reaction of a ruthenium B-carboranyl hydride complex and BH3(SMe2): Selective formation of a pincer-supported metallaborane LRu(B3H8). Tetrahedron 2019, 75, 11, 1471–1474. [DOI] [PubMed] [Google Scholar]; (j) Quan Y; Tang C; Xie Z Nucleophilic substitution: a facile strategy for selective B-H functionalization of carboranes. Dalton Trans. 2019, 48, 7494–7498. [DOI] [PubMed] [Google Scholar]; (k) Jiang T; Zhang K; Shen Y; Hamadaoui M; Dontha R; Liu J; Spingler B; Duttwyler S The 12-ethynylmonocarba-closo-dodecaborate anion as a versatileligand for Cu(I) alkyne and heterobimetallic Cu(I)/M(II) (M = Pd, Pt) alkynide complexes. Dalton Trans. 2019, 48, 17192–17199. [DOI] [PubMed] [Google Scholar]; (l) Fanfrlik J; Hnyk D; Hobza P Chalcogen Bonding due to the Exo-Substitution of Icosahedral Dicarbaborane. Molecules 2019, 24, 14, 2657. [DOI] [PubMed] [Google Scholar]; (m) Londesborough MGS; Macías R; Kennedy JD; Clegg W; Bould J Macropolyhedral Nickelaboranes from the Metal-Assisted Fusion of KB9H14. Inorg. Chem 2019, 58, 19, 13258–13267. [DOI] [PubMed] [Google Scholar]; (n) Fisher SP; McArthur SG; Tej V; Lee SE; Chan AL; Banda I; Gregory A; Berkley K; Tsay C; Rheingold AL; Guisado-Barrios G; Lavallo V Strongly Coordinating Ligands Form Weakly Coordinating Yet Functional Organometallic Anions. J. Am. Chem. Soc 2020, 142, 1, 251–256. [DOI] [PubMed] [Google Scholar]; (o) Keener M; Hunt C; Carroll TG; Kampel V; Dobrovetsky R; Hayton TW; Ménard G Redox-switchable carboranes for uranium capture and release. Nature 2020, 577, 652–655. [DOI] [PubMed] [Google Scholar]; (p) Kamin AA; Juhasz MA Exhaustive Cyanation of the Dodecaborate Dianion: Synthesis, Characterization, and X-ray Crystal Structure of [B12(CN)12]2−. Inorg. Chem 2020, 59, 1, 189–192. [DOI] [PubMed] [Google Scholar]; (q) Zheng F; Yui TH; Zhang J; Xie Z Synthesis and X-ray characterization of 15- and 16-vertex closo-carboranes. Nature Commun. 2020, 11, 5943. [DOI] [PubMed] [Google Scholar]; (r) Chan APY; Parkinson JA; Rosair GM; Welch AJ Bis(phosphine)hydridorhodacarborane erivatives of 1,1’-Bis(ortho-carborane) and Their Catalysis of Alkene Isomerization and the Hydrosilylation of Acetophenone. Inorg. Chem 2020, 59, 3, 2011–2023. [DOI] [PubMed] [Google Scholar]; (s) Couto M; Alamón C; Nievas S; Perona M; Dagrosa MA; Teixidor F; Cabral P; Viñas C; Cerecetto H Bimodal Therapeutic Agents Against Glioblastoma, One of the Most Lethal Forms of Cancer. Chem. Eur. J 2020, 26, 63, 14335–14340. [DOI] [PubMed] [Google Scholar]; (t) Zhu T-C; Xing Y-Y; Sun Y; Duttwyler S; Hong X Directed B-H functionalization of the closo-dodecaborate cluster via concerted iodination-deprotonation: reaction mechanism and origins of regioselectivity. Org. Chem. Front. 2020, 7, 3648–3655. [DOI] [PubMed] [Google Scholar]; (u) Kravchenko EA; Gippius AA; Kuznetsov NT Noncovalent Interactions in Compounds Based on Perchlorinated Boron Cluster as Monitored by 35Cl NQR (Review). Russ. J. Inorg. Chem 2020, 65, 546–566. [DOI] [PubMed] [Google Scholar]; (v) Mu X; Hopp M; Dziedzic RM; Waddington MA; Rheingold AL; Sletten EM; Axtell JC; Spokoyny AM Expanding the Scope of Palladium-Catalyzed B-N Cross-Coupling Chemistry in Carboranes. Organometallics 2020, 39, 23, 4380–4386. [DOI] [PubMed] [Google Scholar]; (w) Rončević I; Bastien G; Cvačka J; Kaleta J; Michl J CB11H10- and Related Carborenes. Inorg. Chem 2020, 59, 17, 12453–12460. [DOI] [PubMed] [Google Scholar]; (x) Hoppenz P; Els-Heindl S; Kellert M; Kuhnert R; Saretz S; Lerchen H-G; Köbberling J; Riedl B; Hey-Hawkins E; Beck-Sickinger AG A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem 2020, 85, 3, 1446–1457. [DOI] [PubMed] [Google Scholar]; (y) Kataki-Anastasakou A; Axtell JC; Hernandez S; Dziedzic RM; Balaich GJ; Rheingold AL; Spokoyny AM; Sletten EM Carborane Guests for Cucurbit[7]uril Facilitate Strong Binding and On-Demand Removal. J. Am. Chem. Soc 2020, 142, 49, 20513–20518. [DOI] [PubMed] [Google Scholar]; (z) Guo S-T; Cui P-F; Yuan R-Z; Jin G-X Transition metal-mediated B(4)-H hydroxylation/halogenation of o-carboranes bearing a 2-pyridylsulfenyl ligand. Chem. Commun 2021, 57, 2412–2415. [DOI] [PubMed] [Google Scholar]; (aa) Lee SH; Lee JH; Mun MS; Yi S; Yoo E; Hwang H; Lee KM Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores. Molecules 2021, 26, 6, 1763. [DOI] [PubMed] [Google Scholar]; (bb) Soldevilla-Sanmartín J; Ruiz E; Choquesillo-Lazarte D; Light ME; Viñas C; Teixidor F; Núñez R; Pons J; Planas JG Tuning the architectures and luminescence properties of Cu(I) compounds of phenyl and carboranyl pyrazoles: the impact of 2D versus 3D aromatic moieties in the ligand backbone. J. Mater. Chem. C 2021, 9, 7643–7657. [DOI] [PubMed] [Google Scholar]; (cc) Xiong Y; Chen D; Yao S; Zhu J; Ruzicka A; Driess M New Types of Ge2 and Ge4 Assemblies Stabalized by a Carbanionic Dicarborandiyl-Silylene Ligand. J. Am. Chem. Soc 2021, 143, 16, 6229–6237. [DOI] [PubMed] [Google Scholar]; (dd) Zhang C; Wang J; Su W; Lin Z; Ye Q Synthesis, Characterization, and Density Functional Theory Studies of Three-Dimensional Inorganic Analogues of 9,10-Diboraanthracene-A New Class of Lewis Superacids. J. Am. Chem. Soc 2021, 143, 23, 8552–8558. [DOI] [PubMed] [Google Scholar]; (ee) Murphy N; McCarthy E; Dwyer R; Farrás P Boron clusters as breast cancer therapeutics. J. Inorg. Biochem 2021, 218, 111412. [DOI] [PubMed] [Google Scholar]; (ff) Vrána J; Holub J; Samsonov MA; Růžičková Z; Cvačka J; McKee ML; Fanfrlík J; Hnyk D; Růžička A Access to cationic polyhedral carboranes via dynamic cage surgery with N-heterocyclic carbenes. Nat. Commun 2021, 12, 4971. [DOI] [PubMed] [Google Scholar]; (gg) Waddington MA; Zheng X; Stauber JM; Moully EH; Montgomery HR; Saleh LMA; Král P; Spokoyny AM An Organometallic Strategy for Cysteine Borylation. J. Am. Chem. Soc 2021, 143, 23, 8661–8668. [DOI] [PubMed] [Google Scholar]; (hh) Jaiswal K; Malik N; Tumanskii B; Ménard G; Dobrovetsky R Carborane Stablized “19-Electron” Molybdenum Metalloradical. J. Am. Chem. Soc 2021, 143, 26, 9842–9848. [DOI] [PubMed] [Google Scholar]; (ii) Gange GB; Humphries AL; Royzman DE; Smith MD; Peryshkov DV Metal-Free Bond Activation by Carboranyl Diphosphines. J. Am. Chem. Soc 2021, 143, 29, 10842–10846. [DOI] [PubMed] [Google Scholar]; (jj) Al-Joumhawy M; Marei T; Shmalko A; Cendoya P; La Borde J; Gabel D B-N Bond Formation through Palladium-Catalyzed, Microwave-Assisted Cross-Coupling of Nitrogen Compounds with Iodo-dodecaborate. Chem. Commun 2021, 57, 10007–10010. [DOI] [PubMed] [Google Scholar]
- (14).(a) Giselbrecht K; Bildstein B; Sladky F Tris(trimethylsilyl)methanetellurenyl halides (Me3Si)3CTeX (X = Cl, Br, I): synthesis of stable slkanetellurenyl halides. Chem. Ber 1989, 122, 7, 1255–1256. [Google Scholar]; (b) Ostrowski M; Wagner I; du Mont WW; Jones PG; Jeske J Tris(trimethylsilyl)methaneselenyl halides and chalcogenides. Z. Anorg. Allg. Chem 1993, 619, 10, 1693–1698. [Google Scholar]; (c) Potapov VA; Amosova SV; Petrov BV; Starkova AA; Malyushenko RN Electrophilic addition of organic selenyl chlorides and bromides to acetylene. Sulfur Lett. 1998, 21, 3, 109–114. [Google Scholar]; (d) Barrientos-Astigarraga RE; Castelani P; Sumida CY; Zukerman-Schpector J; Comasseto JV A general method of synthesis of functionalized Z-vinylic tellurides starting from β-dicarboynl compounds. Tetrahedron 2002, 58, 6, 1051–1059. [Google Scholar]; (e) Marino JP; Nguyen HN Electrotelluration: A New Approach to Tri- and Tetrasubstituted Alkenes. J. Org. Chem 2002, 67, 18, 6291–6296.12201746 [Google Scholar]; (f) Klapötke TM; Krumm B; Polborn K Isolation of a Stable Covalent Selenium Azide RSeN3. J. Am. Chem. Soc 2004, 126, 3, 710–711.14733533 [Google Scholar]; (g) Klapötke TM; Krumm B; Nöth H; Gálvez-Ruiz JC; Polborn K; Schwab I; Suter M Kinetic and Donor Stabilization of Organotellurenyl Iodides and Azides. Ionrg. Chem 2005, 44, 15, 5254–5265. [Google Scholar]; (h) Senol E; Scattolin T; Schoenebeck F Selenolation of Aryl Iodides and Bromides Enabled by a Bench-Stable PdI Dimer. Chem. Eur. J 2019, 25, 40, 9419–9422.30913326 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


