Abstract
The lipooligosaccharide (LOS) of Haemophilus somnus undergoes antigenic phase variation, which may facilitate evasion from the bovine host immune response and/or colonization and dissemination. However, LOS antigenic diversity in H. somnus has not been adequately investigated. In this study, monoclonal antibodies (MAbs) specific to various LOS epitopes were used to investigate antigenic variation and stability in LOS from H. somnus strains and phase variants. Clinical isolates of H. somnus exhibited intrastrain, as well as interstrain, antigenic heterogeneity in LOS when probed with MAbs to outer core oligosaccharide epitopes in an enzyme-linked immunosorbent assay (ELISA). However, epitopes reactive with MAbs directed predominately to the inner core heptose region were highly conserved. At least one epitope, which was expressed in few strains, was identified. One LOS component affected by phase variation was identified as phosphorylcholine (PCho), which is linked to the primary glucose residue. Inhibition ELISA, immunoblotting, and electrospray-mass spectrometry were used to confirm that MAb 5F5.9 recognized PCho. LOS reactivity with MAb 5F5.9 was associated with loss of most of the outer core oligosaccharide, indicating that reactivity with PCho was affected by phase variation of the glycose residues in this region. Our results indicate that outer core epitopes of H. somnus LOS exhibit a high degree of random, phase-variable antigenic heterogeneity and that such heterogeneity must be considered in the design of vaccines and diagnostic tests.
Haemophilus somnus is a gram-negative coccobacillus that colonizes the mucosal surfaces of cattle, but it may also cause multisystemic diseases such as pneumonia, thrombotic meningoencephalitis, septicemia, abortion, myocarditis, and arthritis (8, 16, 18, 25). Whole-cell, killed vaccines are commercially available, but they do not offer adequate protection against systemic diseases (18, 33). The lack of adequate protection by presently available vaccines is due, in part, to insufficient understanding of the virulence factors and host immune response during the disease process. Furthermore, the role of individual surface components in the protective immune response is not well understood. The oligosaccharide of H. somnus lipooligosaccharide (LOS), like that of other Haemophilus and Neisseria spp., can be divided into two regions: an inner core region consisting of 3-deoxy-d-manno-2-octulosonic acid (KDO) and heptose and an outer core region consisting of glucose, galactose, and hexosamine. Some of the outer core glycoses may be modified by phosphoethanolamine (PEtn) or phosphorylcholine (PCho). The LOS of H. somnus is known to undergo antigenic phase variation in vitro and in vivo, and that clearance of respiratory infection is associated with humoral recognition of most of the antigenic variants that can develop (8, 13, 21). Therefore, characterizing H. somnus LOS epitopes, as well as identifying the diversity and stability of these epitopes, may provide insight into the role of this important component in pathogenesis and new approaches toward vaccination.
Control of H. somnus disease also requires early and accurate diagnosis, as well as identification of carrier animals. Identification of the immune status of individual animals and herd immunity is particularly important in management practices to control H. somnus diseases. Epidemiological studies on H. somnus are hindered by the lack of an adequate antigenic typing system. Polyclonal sera, raised against H. somnus whole cells, have been used in assays such as bacterial agglutination, complement fixation, and enzyme-linked immunosorbent assay (ELISA) in attempts to establish a typing scheme for H. somnus (16). In one study, 46 American and Swiss H. somnus isolates could be placed into four serotypes using cross-adsorbed polyclonal antisera to H. somnus whole cells in tube agglutination tests (5), suggesting a high degree of antigenic similarity among strains (15, 16, 34). These results are in contrast to the high rate of antigenic phase variation previously observed in H. somnus LOS (21, 22). A more specific analysis of H. somnus LOS epitopes, which requires the use of monoclonal antibodies (MAbs) to LOS, is therefore needed.
In this study we tested the reactivity of 5 LOS MAbs in a whole-cell ELISA with 44 strains and phase variants of H. somnus. There was substantial interstrain and intrastrain antigenic heterogeneity in the LOS of these isolates, and MAb reactivity with epitopes such as PCho and outer core oligosaccharide glycoses was unstable due to phase variation. These results indicate that antigenic reagents containing H. somnus LOS are unsuitable for use in typing systems and that further investigation of the role of antibodies to LOS in the protective immune response is required.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The sources, derivation, and associated disease or isolation site of the 44 H. somnus strains and phase variants used in this study are shown in Table 1. Escherichia coli J5, a rough lipopolysaccharide mutant, was used as a negative control. Growth of H. somnus on Colombia blood agar plates or in supplemented brain heart infusion (BHI) broth has been previously described (20). For some studies, broth-grown bacteria were washed once in phosphate-buffered saline (PBS), pH 7.4, and stored in 1% buffered formalin as a preservative. The cells were diluted in PBS to an absorbance of 0.70 at 550 nm for use in ELISA.
TABLE 1.
H. somnus strains used in this studya
| Strain(s) | Disease or isolation site | Source or reference |
|---|---|---|
| 1p, 24p, 127p | Prepuce | 7 |
| 1225 | Prepuce | V. Fussing, NVL, Copenhagen, Denmark |
| 649 | Abortion | 7 |
| 8025 | TME | Vet. Diag. Lab, Iowa State University |
| 2336 | Pneumonia | 7 |
| 738 | Clonal isolate of 2336 | 12 |
| 768, 797, 803, 807, 808, 813 | Phase variants of 738 | 21 |
| 93, 6110, 6489, 6651, 6711, 6728, 6743, 6791, 6948, 7082, 7226, 7323, 7460, 7684, 7704, 7735, 7754, 7768, 7816, 7112, 7484 | Pneumonia | A. Potter, VIDO, University of Saskatchewan, Canada |
| 7291, 7509, 7606, 7653, 7757, 7807, 7809, 7929, 7286 | TME | A. Potter |
TME, thrombotic meningoencephalitis; NVL, National Veterinary Laboratory; Vet. Diag. Lab, Veterinary Diagnostic Laboratory; VIDO, Veterinary Infectious Disease Organization.
Purification and O-deacylation of LOS.
H. somnus LOS was purified by enzyme digestion-hot aqueous phenol extraction for use in ELISA and mass spectrometry analysis (9, 23), or by micro-phenol extraction for Western blotting (17). For preparation of LOS expressing PCho, a population of cells as homogeneous as possible was desired. A MAb 5F5.9-reactive colony of strain 738 was subcultured, and the colony blot was repeated. From this second colony blot, a strongly reactive colony (738-P) was subcultured, expanded onto 10 Columbia blood agar plates, and incubated overnight at 37°C in a candle extinction jar. The cells were washed off the plates with PBS, and washed once in PBS. A diluted aliquot of these cells was used in a confirmatory colony blot with 5F5.9 (>95% reactive), and the remainder was lyophilized. LOS was extracted from 100 mg of lyophilized cells, suspended in 2 ml of distilled water, and stirred at 65 to 70°C. Two ml of 90% phenol was added, and the mixture was stirred for 30 min. The mixture was cooled on ice, centrifuged at 5,000 × g for 30 min, and the upper aqueous phase was aspirated. A second extraction was performed as described above, and the combined aqueous phases were dialyzed and lyophilized. The LOS (5 to 10 mg) was O-deacylated as previously described (9), washed twice with cold acetone, then redissolved in water and lyophilized.
MAbs.
The source and specificity of MAbs 3F11, 6B4, 5F5.9, 5D7, MAHD7, and others used in this study are described in Table 2. MAb 5D7 was produced by immunization of A/J mice with H. somnus cells coated with LOS prepared by a modification of a method previously described (26). Briefly, 10 mg of lyophilized H. somnus strain 649 cells were suspended in 5 mls of PBS. One milliliter of a 1-mg/ml solution of homologous LOS was added, and the mixture was incubated at 60°C for 20 min, with occasional vortexing. The mixture was then dried in a rotary evaporator at 60°C, and the temperature gradually decreased manually 5°C/h to 30°C over a 6-h period. The LOS-coated material was then suspended at 10 mg/ml in PBS for immunization of young adult female A/J mice. The mice were injected intraperitoneally with LOS-coated cells mixed 1:1 with Freund's complete adjuvant (Sigma Chemical Co., St. Louis, Mo.) in a total volume of 0.1 ml. Antibody titers were determined 10 days after immunization by serial dilution of serum in an ELISA against homologous LOS. A second intraperitoneal immunization of LOS-coated cells mixed 1:1 in Freund's incomplete adjuvant was administered to responsive mice (optical density at 405 nm [OD405] > 1.0 at a dilution of 1:5,000) 4 weeks after the first immunization. At the same time 50 μl of the LOS-coated cell suspension (in PBS) was administered by intrasplenic injection. The mice were euthanized, and the spleens were harvested for fusion 4 days after the second immunization. Antibody-secreting hybridoma cells were produced by standard methods (10) at the Lymphocyte Culture Facility (University of Virginia, Charlottesville, Va.) using Sp2/O myeloma cells. Cell lines producing MAbs reactive with LOS were cloned twice by limiting dilution.
TABLE 2.
Specificities of MAbs used in this study
| MAb | Made to: | Specificity | Isotype | Source (reference) |
|---|---|---|---|---|
| 5D7 | H. somnus | NDa | IgG3 | This work |
| 5F5.9 | Haemophilus agyptius | PCho | IgG3 | A. Lesse |
| 59.6C5 | S. pneumoniae | PCho | IgG3 | D. Briles (4) |
| TEPC-15 | Natural murine myeloma cell line | PCho | IgA | M. Potter |
| 3F11 | N. gonorrhoeae | Galβ(1-4)GlcNAc | IgM | M. Apicella (3) |
| 6B4 | N. gonorrhoeae | Galβ(1-4)GlcNAcβ(1-3)Galβ(1-4)Glc | IgM | M. Apicella (3) |
| MAHD7b | H. ducreyi | Glcβ(1-4)Hepα(1-)Kdo(P)- | ND | A. Ahmed (1, 2) |
| α↑(1-3) | ||||
| Hepα(1-2)Hepα |
ND, not determined.
Suggested specificity given for this MAb.
ELISA.
Wells of Immunlon-4 ELISA plates (Dynatech Laboratories, Chantilly, Va.) were coated in replicates of four with 10 μl of 1% formalin-treated whole cells diluted to an absorbance at 550 nm (A550) of 0.70. Ninety microliters of PBS was then added to each well, and the plates were covered and incubated overnight at 4°C. Nonspecific binding was blocked with 1% nonfat skim milk in PBS for 1 h at room temperature, followed by incubation with the previously determined optimal dilution of MAb for 18 h at 4°C (see below). A 1:5,000 dilution of horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) and IgM (heavy plus light chains) antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.) was added and incubated for 1 h at room temperature. After each incubation the plates were washed five times with PBS containing 0.05% Tween 20. The color reaction was developed with ABTS [2,2′-azino-di(3-ethyl-benzthiazoline sulfonate)] peroxidase substrate (Kirkegaard and Perry Laboratories Inc., Gaithersburg, Md.) for 30 min. and then stopped by the addition of 1% sodium dodecyl sulfate in PBS. The A405 was measured with a microtiter plate reader (Molecular Devices Corp., Menlo Park, Calif.).
To determine the optimum MAb dilution for ELISA reactivity with all strains, serial twofold dilutions of MAbs 3F11, 6B4, MAHD7, 5F5.9, and 5D7 were tested with 10 strains each, and the strain that generated the highest A405 was used to construct a titration curve with that MAb. The optimal dilution of each MAb was considered the greatest dilution resulting in maximum A405 (top of the linear portion of the titration curve) and was used in the ELISA with whole cells of 44 H. somnus strains and phase variants.
Antigenic grouping.
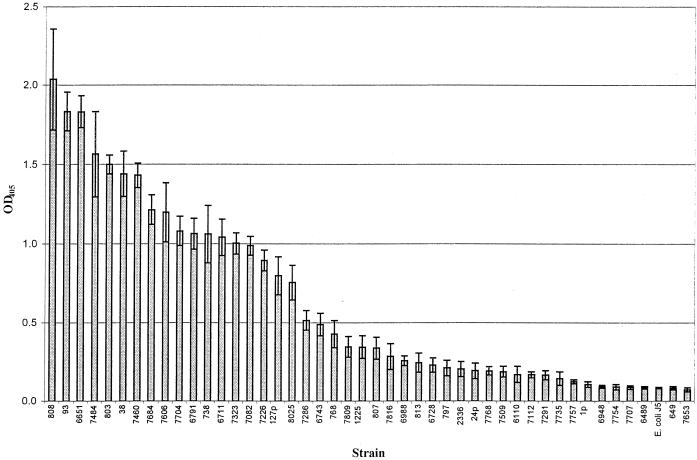
H. somnus strains were assigned to antigenic groups based on the relative reactivity of each strain with a given MAb compared to the maximum A405 obtained with one of the 44 strains tested (referred to as the positive control strain). The formula used for relative percent binding of each strain-MAb combination was as follows: A405 of test strain ÷ maximum A405 of the positive control strain × 100. For instance, the relative percent binding of strain 6743 with MAb 5F5.9 was as follows: 0.5 ÷ 2.03 × 100, where 0.5 was the absorbance obtained with strain 6743 and MAb 5F5.9 and 2.03 was the absorbance obtained with strain 803 and MAb 5F5.9 (derived from Fig. 1). Semiquantitative reactivity was further assigned as follows: −, +, ++, or +++, representing <15%, ≥15 to 50%, >50 to 90%, or >90% of maximum reactivity, respectively.
FIG. 1.
Reactivity of MAb 5F5.9 with strains of H. somnus by ELISA. Error bars, ±2 standard deviations from the mean of the results from four replicate experiments. E. coli J5 is the negative control.
Inhibition ELISA.
The titer of MAbs 5F5.9 and 5D7 for use in inhibition studies was first determined by ELISA. Twofold serial dilutions of antibody in PBS were tested against the broadly reactive H. somnus strain 93 LOS at a concentration of 1 μg/well. The dilution of each MAb used in the inhibition ELISA corresponded to approximately 75% of maximum A405 determined from the titration curve. Microcentrifuge tubes were filled with 900 μl of diluted MAb and 100 μl of serial twofold dilutions of inhibitor, beginning at 100 μg/ml in PBS. The mixture was incubated for 1 h at room temperature with agitation and then used in an ELISA with strain 93 LOS-coated wells in replicates of five. The negative control consisted of 900 μl of diluted MAb and 100 μl of PBS. The positive inhibition control consisted of 900 μl of the diluted MAb mixed with 100 μl of a 1-mg/ml suspension of H. somnus strain 93 LOS. Inhibitors tested included galactose (Gal), glucose (Glc), Galβ(1-4)Glc, Glcβ(1-4)Glc, Galβ(1-4)GlcNAc, Galβ(1-3)GlcNAcβ(1-3)Galβ(1-4)Glc, PEtn, and PCho.
Mass spectrometry Analysis.
Samples were analyzed on a VG Quattro triple quadrupole mass spectrometer (Fisons Instruments) with an electrospray ion source. Deacylated samples were dissolved in an aqueous solvent containing 50% acetonitrile and 0.1% formic acid. The electrospray tip voltage was 2.5 kV and the mass spectrometer was scanned at an m/z of from 150 to 2,500 with a scan time of 10 s (9).
SDS-PAGE and immunoblotting.
LOS sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as previously described (19). Gels were stained with ammoniacal silver following periodate oxidation (36). Western blotting was performed by transfer of LOS bands to a Westran polyvinylidene difluoride (PVDF) membrane (Schleicher & Schuell, Keene, N.H.) using a Transblot apparatus (Bio-Rad Laboratories, Richmond, Calif.) at 24 volts for 45 min (21). Detection of LOS bands on the membrane with the selected MAb was done by standard methods (14). Colony immunoblotting was done as previously described (22).
Cluster analysis.
The arithmetic mean and standard deviation of the ELISA A405 values from four replicates of each of the 44 H. somnus strains tested was used for cluster analysis by average linkage using Euclidian distance to determine dissimilarity. Statistical analysis software for Windows (SPSS Inc., Chicago, Ill.) was used for these calculations.
Digital images and LOS structure.
A Fujix 505 digital camera (Fuji Photo Film USA, Elmsford, N.Y.) or Microteck ScanMaker III digital scanner (Microtek Lab, Redondo Beach, Calif.) was used to record digital images. Adobe Photoshop v. 5.0 (Adobe Systems, San Jose, Calif.) was used to compile the images. Structural representation of H. somnus strain 738 LOS was performed using ChemSketch v. 4.0 (Advanced Chemistry Development Inc., Toronto, Canada).
RESULTS
Reactivity of MAbs with H. somnus.
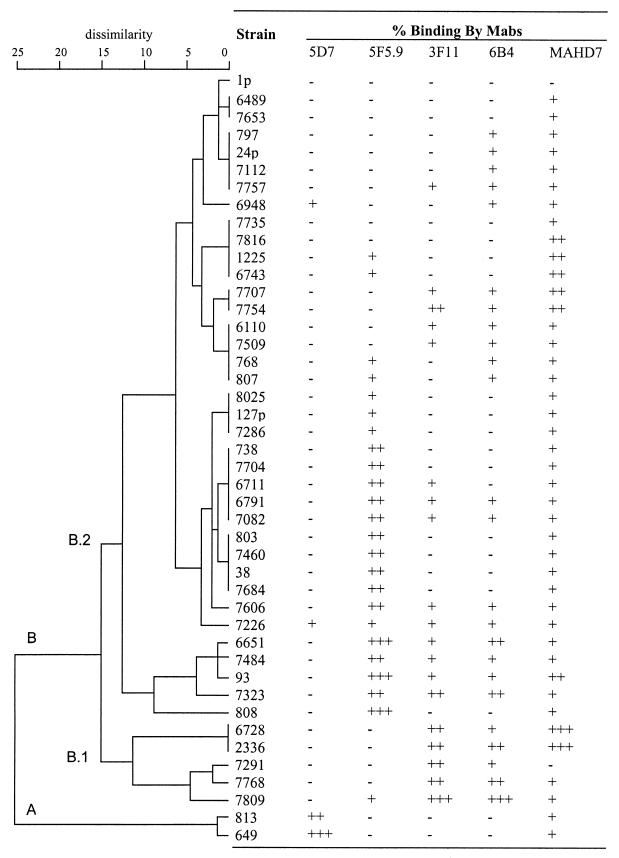
The reactivity of MAb 5F5.9 with 44 H. somnus strains or LOS phase variants is shown in Fig. 1, which is a representation of the analysis with MAbs 5F5.9, 5D7, 3F11, 6B4, and MAHD7. A dendrogram demonstrating the heterogeneity of LOS epitopes between strains and LOS phase variants and the semiquantitative relative reactivity by each of these MAbs is shown in Fig. 2. Based on cluster analysis using the absorbance means and standard deviations for the 44 H. somnus strains, two very dissimilar groups (A [n = 2] and B [n = 42]) were identified. The asymmetry of this grouping was due to the high relative reactivity of anti-H. somnus LOS MAb 5D7 with only two strains, and the lack of reactivity of these strains with other MAbs. In total only 9% of the strains reacted with MAb 5D7. This result was not unexpected, since MAb 5D7 was made to H. somnus strain 649, which did not react with any of the other MAbs directed to outer core epitopes. The specificity of MAb 5D7 has not yet been determined. Group B was further divided into groups B.1 and B.2. Subgroups of B.1 and B.2 were also identified, but they are not labeled in the dendrogram. Random LOS phase variation resulted in strains changing their associated antigenic group following in vitro or in vivo passage. Therefore, the usefulness of further division of these groups would be limited.
FIG. 2.
Dendrogram obtained from cluster analysis of all H. somnus strains (four replicate experiments) and the relative percent binding by ELISA with each corresponding MAb. Semiquantitative binding was determined as previously described (29). Symbols: −, +, ++, and +++ represent <15%, ≥15 to 50%, >50 to 90%, and >90% of maximum optical density, respectively.
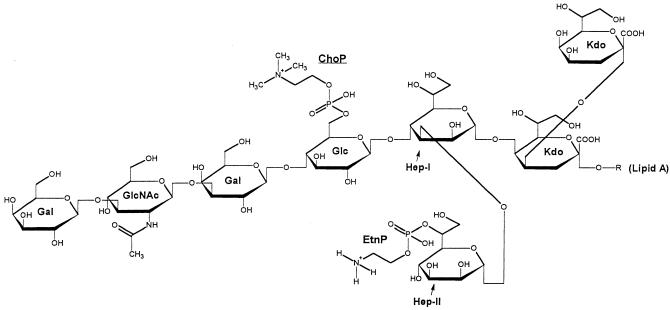
MAbs 3F11 and 6B4, which were made to Neisseria gonorrhoeae LOS, are specific for Galβ(1-4)GlcNAc and Galβ(1-4)GlcNAcβ(1-3)Galβ(1-4)Glc (28, 41, 42) and cross-reacted with 43 and 55% of the H. somnus strains, respectively. MAb 6B4 reacted with six strains not reactive with MAb 3F11 in this study. MAbs 3F11 and 6B4, which react with the lacto-N-neotetraose component on N. gonorrhoeae LOS, reacted with only the largest molecular size LOS bands by Western blotting (22; data not shown). Furthermore, structural analysis indicated that the nonreducing end of H. somnus strain 738 LOS contains a Galβ(1-3)GlcNAcβ(1-3)Galβ(1-4)Glc component, and therefore these MAbs most likely react with outer core epitopes of H. somnus LOS (9) (Fig. 3). Variable reactivity with these MAbs illustrated the high degree of antigenic heterogeneity in this region among the 44 strains tested. MAb 5F5.9 also reacted with 55% of the H. somnus strains examined but not always with the same strains as MAbs 3F11 and 6B4.
FIG. 3.
Modified Haworth projection of the predominant structure of H. somnus strain 738 LOS (9). All outer core hexoses are beta-D oriented and both inner core l-glycero-d-manno heptoses are l-α-d oriented. Abbreviations: Gal, galactose; GlcNAc, N-acetyl-glucosamine; Glc, glucose; Hep, heptose; Kdo, 3-deoxy-d-manno-2-octulosonic acid.
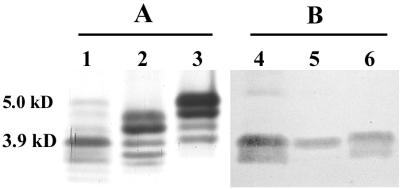
MAb MAHD7, which is directed to the inner core heptose region of Haemophilus ducreyi (2), was reactive with all but 2 of the 44 H. somnus strains tested (95.5%), indicating this region is relatively conserved in H. somnus (Table 1). The degree of reactivity of LOS phase variants of strain 738 with MAb MAHD7 was similar. However, strain 2336, from which strain 738 was derived, was more reactive with MAb MAHD7 than the phase variants of strain 738. MAb 5D7 reacted with low-molecular-size bands (3.9 to 3.3 kDa) of strains 649, 6948, and 7226 LOS in a Western blot (Fig. 4B). This MAb was not inhibited by either PCho or PEtn (data not shown), but its specificity was not further characterized.
FIG. 4.
Silver-stained polyacrylamide gel (A) and immunoblot with MAb 5D7 (B) of H. somnus LOS extracts. Lanes 1 and 4, strain 649; 2 and 5, strain 6948; 3 and 6, strain 7226. Molecular sizes are indicated on the left (23).
Specificity of MAb 5F5.9.
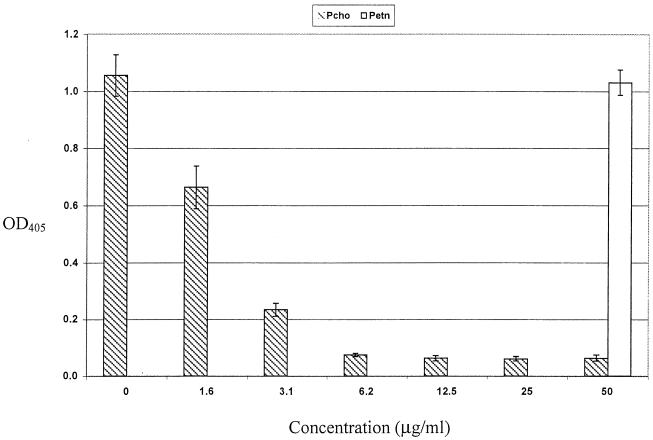
The specificity of MAb 5F5.9 was investigated by inhibition ELISA using commercially available glycoses identical in structure and linkage to glycoses present in the outer core oligosaccharide of strain 738. None of the mono-, di-, or tetrasaccharides tested inhibited binding of MAb 5F5.9 (data not shown). However, colony immunoblotting of H. somnus strains with MAb TEPC-15 (39) indicated that the LOS contained PCho, and that reactivity of this component with the MAb was phase variable. Structural analysis of strain 738 oligosaccharide later confirmed the presence of PCho attached to the primary glucose within the oligosaccharide chain (9) (Fig. 3). MAb 59.6C5, which is also directed to PCho (6), reacted with the same strains and with similar intensity as MAb 5F5.9 by ELISA, suggesting that MAb 5F5.9 was specific for PCho. This specificity was supported by inhibition of MAb 5F5.9 reactivity with H. somnus strain 93 (a strongly reactive strain) and with the use of purified PCho in an ELISA (Fig. 5). The concentration of PCho required for 50% inhibition of MAb 5F5.9 was 2.1 μg/ml, and PCho concentrations of 12.5 μg/ml and above resulted in 95% inhibition. PEtn, a structural analog of PCho, did not inhibit binding of MAb 5F5.9 at 50 μg/ml.
FIG. 5.
Inhibition ELISA of MAb 5F5.9 by PCho (hatched bars) or PEtn (open bar). H. somnus strain 93 LOS was used as the antigen. Error bars, 2 standard deviations above and below the mean data point from five replicate experiments.
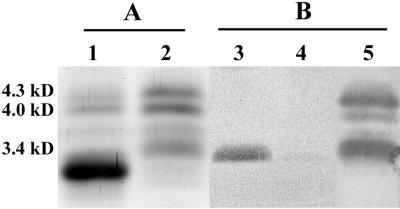
To further investigate the phase-variable nature of PCho, a colony blot of H. somnus strain 738 was performed with MAb 5F5.9. Single colonies reactive with MAb 5F5.9 were expanded, and more than 95% of colonies from these clones were reactive with MAb 5F5.9. Electrospray-mass spectrometry (ES-MS) analysis of the O-deacylated LOS obtained from a MAb 5F5.9-reactive clonal isolate (strain 738-P) indicated that the oligosaccharide of strain 738-P was more truncated than parent strain 738 (Table 3). This finding was supported by SDS-PAGE analysis, in which the majority of LOS from strain 738-P was of lower molecular size (Fig. 6A, lane 1). The 3.4-kDa band from strain 738 (lane 2) was missing in 738-P and replaced by two lower-molecular-size LOS bands. The predominant, fastest migrating of these two bands did not react in an immunoblot with MAb 5F5.9 (Fig. 6B, lane 3) consistent with the smallest glycoform observed in ES-MS m/z 2062 not containing PCho (Table 3). The less mobile, but fainter, of these two lower-molecular-size 738-P LOS bands did react with MAb 5F5.9 (Fig. 6B, lane 3), again consistent with the ES-MS data indicating that the ion at m/z 2227 did contain PCho. The relative intensities of these two bands in the SDS-PAGE also correlated well with the relative intensities deduced from the ES-MS data (Table 3). LOS from strain 738 was weakly reactive with a similarly sized MAb 5F5.9-reactive band in 738-P (Fig. 6B, lane 4) consistent with the identification of trace amounts of PCho in an ion at m/z 2227 in the LOS of strain 738 (Table 3). It is interesting to note that other PCho containing glycoforms are also present in strain 738 based on ES-MS analysis that are not reactive on the immunoblot. All these glycoforms would contain oligosaccharides bearing glycose extensions from the PCho-containing hexose moiety, and hence supports the theory that accessibility to the PCho epitope is hindered by such extensions.
TABLE 3.
Negative ion nanoES-MS data and proposed compositions of MAb 5F5.9 reactive (738-P) and non-reactive O-deacylated LPS from H. somnus strain 738a
| Strain | Molecular mass (Da)
|
Relative intensity | Proposed composition | |
|---|---|---|---|---|
| Observed | Calculated | |||
| 738 (5F5.9 negative) | 2,224.0 | 2,224.1 | 0.2 | 2Hex, 2Hep, PEtn, 2Kdo, lipid A |
| 2,226.0 | 2,227.0 | 0.2 | Hex, 2Hep, PEtn, PCho, 2Kdo, lipidA | |
| 2,386.0 | 2,386.2 | 0.2 | 3Hex, 2Hep, PEtn, 2Kdo, lipid A | |
| 2,389.0 | 2,389.1 | 0.4 | 2Hex, 2Hep, PEtn, PCho, 2Kdo, lipid A | |
| 2,590.0 | 2,589.4 | 1.0 | GlcNAc, 3Hex, 2Hep, PEtn, 2Kdo, lipid A | |
| 2,750.4 | 2,751.4 | 0.2 | GlcNAc, 4Hex, 2Hep, PEtn, 2Kdo, lipid A | |
| 2,753.4 | 2,754.5 | 0.3 | GlcNAc, 3Hex, 2Hep, PEtn, PCho, 2Kdo, lipid A | |
| 2,917.0 | 2,916.7 | 0.2 | GlcNAc, 4Hex, 2Hep, PEtn, PCho, 2Kdo, lipid A | |
| 738-P (5F5.9 positive) | 2,062.0 | 2,061.9 | 1.0 | Hex, 2Hep, PEtn, 2Kdo, lipid A |
| 2,227.4 | 2,227.0 | 0.4 | Hex, 2Hep, PEtn, PCho, 2Kdo, lipid A | |
Average mass units were used for calculation of molecular mass based on proposed composition, as follows: Hexose (Hex), 162.15; Heptose (Hep), 192.17; GlcNAc, 203.19; 3-deoxy-d-manno-octulosonic acid (Kdo), 220.18; PEtn, 123.05; PCho, 165.05. A number before an abbreviation signifies the number of components identified.
FIG. 6.
Silver-stained polyacrylamide gel (A) and immunoblot (B) analyses of LOS extracts from MAb 5F5.9-positive and -negative clonal isolates of H. somnus strain 738. Lanes: 1 and 3, 738-P LOS; 2 and 4, 738 LOS; 5, immunoblot repeated on membrane cut from lane 4 using anti-738 LOS rabbit serum as the primary antibody. Molecular sizes are indicated on the left (23).
DISCUSSION
H. somnus has been reported to have only limited antigenic diversity, even following cross-absorption of immune sera (5). Furthermore, bovine sera is commonly seropositive to H. somnus whole cells. This high degree of reactivity and homogeneity may, in part, be due to the presence of immunoglobulin binding proteins in H. somnus (40) and its common presence as a commensal of the bovine urogenital tract (16). However, reactivity to MAbs has shown that H. somnus LOS is heterogeneous, and that at least 12% of the population may undergo LOS antigenic phase variation in a particular epitope (21, 22). In this study we have used a panel of MAbs reactive with distinct epitopes or regions in some strains of H. somnus LOS to further characterize antigenic phase variation. MAb 3F11 recognizes the Galβ(1-4)GlcNAc component of lacto-N-neotetraose in the outer core of gonococcal LOS (28, 41, 42). Forty-three percent of the 44 H. somnus strains used in this study expressed an epitope reactive with MAb 3F11. MAb 6B4 has been proposed to react with the entire lacto-N-neotetraose tetrasaccharide, and as expected, reacted with most H. somnus strains reactive with MAb 3F11. Lacto-N-neotetraose is a component of the glycosphingolipid precursor of the major human blood group antigens. The presence of lacto-N-neotetraose or a similar component in H. somnus LOS suggests that H. somnus may also use molecular mimicry to avoid the host immune response, if this antigen is present on bovine glycosphingolipids. Whether expression of the 3F11 and 6B4 epitopes offers H. somnus a selective advantage in vivo has yet to be determined.
MAb MAHD7 reacted with 95.5% of H. somnus strains tested, indicating the heptose-containing inner core region recognized by this MAb was highly conserved among the strains and phase variants examined. Such conservation was not unexpected because structural analysis has indicated that the inner core of H. somnus LOS is similar in structure to that of other mucosal bacteria, particularly Neisseria spp. (9).
MAb 5D7, in contrast, was made to the LOS of an antigenically distinct strain of H. somnus and reacted with only 9% of strains in this study. The specificity of the MAb 5D7 epitope was not determined at this time. The low number of strains bound by MAb 5D7 accounted for the division of strains into two groups. Of interest was that strain 813 also reacted with MAb 5D7 but is a phase variant of group B strain 738 and was isolated from the respiratory tract of a calf 10 weeks after intrabronchial challenge with strain 738 (13). The time necessary to phase-vary from group B to group A might be a function of the dissimilarity between these groups, and this could be an exploitable factor in H. somnus epidemiology studies. Furthermore, there was a clear antigenic shift in the reactivity of parental strain 2336 and its clonal isolate 738 with MAbs 3F11 and 6B4. Structural analysis of the oligosaccharide of these variants is currently under investigation, with specific reference to the terminal Gal-GlcNAc linkage. Thus, antigens containing H. somnus LOS would not be useful in serological typing assays due to antigenic phase variation.
PCho has been identified as an epitope of H. influenzae LOS (39), as well as of H. somnus LOS (9). It is also a component of Streptococcus pneumoniae teichoic acids (30), and has been found on a 43-kDa protein in Pseudomonas aeruginosa, and on the pili of N. meningitidis and N. gonorrhoeae (37). However, in H. somnus strain 738, PCho is attached to the internal (primary) β-d-glucose residue on heptose I, with the remaining oligosaccharide chain extending from this glucose residue (9) (Fig. 3). In contrast, in Haemophilus influenzae LOS PCho has always been found attached to a terminal hexose residue (27, 31, 32). In strain 738, reactivity of PCho with MAb 5F5.9 was associated with phase-variable gain or loss of terminal outer core oligosaccharide residues. Thus, H. somnus appears to be able to use phase variation of glycose chain extension to conceal or expose PCho. We are presently investigating if the PCho epitope can phase-vary. However, strain 649 did not contain PCho on its LOS, as determined by ES-MS and nuclear magnetic resonance studies (data not shown). Strain 649 also did not react with MAb 5F5.9, and three successive colony blots were negative for reactivity with MAb 5F5.9 (data not shown). Thus, some strains of H. somnus may be incapable of adding PCho to their LOS, making such strains distinctive and useful in future studies on the role of PCho in H. somnus virulence.
The advantages to bacteria that display PCho on their cell surface include adhesion to host epithelial cells by S. pneumoniae (11), and enhanced colonization of the nasopharynx and adherence to bronchial epithelial cells by H. influenzae (35, 39). The mechanism involved in adherence has been proposed to occur through interaction of PCho with the platelet activating factor receptor on bronchial cells (35). In addition, the expression of PCho on a terminal hexose residue enhances serum killing of H. influenzae by C-reactive protein (38). However, whether PCho is present on heptose I or heptose III determines its accessibility to binding C-reactive protein. Furthermore, the degree of serum bactericidal activity for H. influenzae mediated by C-reactive protein is also affected by which heptose residue bears the PCho-attached hexose (27). In H. somnus a similar on-off accessibility of PCho may occur through phase variation of the oligosaccharide extending from the PCho-containing hexose. The role, if any, of PCho in H. somnus colonization or virulence is unknown at this time, but steric interference of PCho may be a factor in determining if the bacterium colonizes mucosal surfaces or disseminates and causes multisystemic disease. In contrast, PCho has not been reported to be present in gram-negative bacterial lipopolysaccharides (LPS). However, the LPS of many bacterial species is involved in adherence to host cells (24).
In summary, antigenic phase variation of the outer core epitopes occurs in most H. somnus isolates recovered from infected sites. Such phase variation may complicate diagnosis and typing of H. somnus and promote evasion of the host immune response. Antigenic phase variation by PCho in strain 738 may be due to phase variation of outer core glycoses. The role(s) of LOS phase variation in colonization, adherence to epithelial cells, and dissemination will require further investigation.
ACKNOWLEDGMENTS
This work was supported by grants 94-37204-0406 and 99-35204-7670 from the United States Department of Agriculture National Research Initiative Competitive Grants Program to T.J.I. and by HATCH formula funds made available to the Virginia State Agricultural Experiment Station.
We are grateful to Lynette Corbeil for providing H. somnus strain 2336 phase variants. We also thank Michael Apicella, Alan Lesse, Teresa Lagergård, and David Briles for providing monoclonal antibodies, William Sutherland for hybridoma technology assistance and advice, and David Burt and Dan Ward for expert assistance with statistical analysis. We thank Don Krajcarski, Pierre Thibault, and Jianjun Li for mass spectrometry, and also Jennifer McQuiston, Gretchen Glindemann, and Gerald Snider for excellent technical assistance and advice.
REFERENCES
- 1.Ahmed H J, Borrelli S, Jonasson J, Eriksson L, Hanson S, Hojer B, Sunkuntu M, Musaba E, Roggen E L, Lagergård T, et al. Monoclonal antibodies against Haemophilus ducreyi lipooligosaccharide and their diagnostic usefulness. Eur J Clin Microbiol Infect Dis. 1995;14:892–898. doi: 10.1007/BF01691496. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed H J, Frisk A, Mansson J E, Schweda E K, Lagergård T. Structurally defined epitopes of Haemophilus ducreyi lipooligosaccharides recognized by monoclonal antibodies. Infect Immun. 1997;65:3151–3158. doi: 10.1128/iai.65.8.3151-3158.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apicella M A, Bennett K M, Hermerath C A, Roberts D E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981;34:751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briles D E, Forman C, Hudak S, Claflin J L. The effects of idiotype on the ability of IgG1 anti-phosphorylcholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. Eur J Immunol. 1984;14:1027–1030. doi: 10.1002/eji.1830141112. [DOI] [PubMed] [Google Scholar]
- 5.Canto G J, Biberstein E L. Serological diversity in Haemophilus somnus. J Clin Microbiol. 1982;15:1009–1015. doi: 10.1128/jcm.15.6.1009-1015.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claflin J L, Hudak S, Maddalena A. Anti-phosphocholine hybridoma antibodies. I. Direct evidence for three distinct families of antibodies in the murine response. J Exp Med. 1981;153:352–364. doi: 10.1084/jem.153.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbeil L B, Blau K, Prieur D J, Ward A C S. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985;22:192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbeil L B, Gogolewski R P, Stephens L R, Inzana T J. Haemophilus somnus: antigen analysis and immune responses. In: Donachie W, Lainson F A, Hodgson J C, editors. Haemophilus, Actinobacillus, and Pasteurella. New York, N.Y: Plenum Press; 1995. pp. 63–73. [Google Scholar]
- 9.Cox A D, Howard M D, Brisson J R, van der Zwan M, Thibault P, Perry M B, Inzana T J. Structural analysis of the phase-variable lipooligosaccharide from Haemophilus somnus strain 738. Eur J Biochem. 1998;253:507–516. doi: 10.1046/j.1432-1327.1998.2530507.x. [DOI] [PubMed] [Google Scholar]
- 10.Coyle P V, Wyatt D, McCaughey C, O'Neill H J. A simple standardised protocol for the production of monoclonal antibodies against viral and bacterial antigens. J Immunol Methods. 1992;153:81–84. doi: 10.1016/0022-1759(92)90308-g. [DOI] [PubMed] [Google Scholar]
- 11.Cundell D R, Gerard C, Idanpaan-Heikkila I, Tuomanen E I, Gerard N P. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv Exp Med Biol. 1996;416:89–94. doi: 10.1007/978-1-4899-0179-8_16. [DOI] [PubMed] [Google Scholar]
- 12.Gogolewski R P, Leathers C W, Liggitt H D, Corbeil L B. Experimental Haemophilus somnus pneumonia in calves and immunoperoxidase localization of bacteria. Vet Pathol. 1987;24:250–256. doi: 10.1177/030098588702400309. [DOI] [PubMed] [Google Scholar]
- 13.Gogolewski R P, Schaefer D C, Wasson S K, Corbeil R R, Corbeil L B. Pulmonary persistence of Haemophilus somnus in the presence of specific antibody. J Clin Microbiol. 1989;27:1767–1774. doi: 10.1128/jcm.27.8.1767-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock I, Poxton I. Bacterial cell surface techniques. New York, N.Y: John Wiley and Sons; 1988. [Google Scholar]
- 15.Harris F W, Janzen E D. The Haemophilus somnus disease complex (hemophilosis): a review. Can Vet J. 1989;30:816–822. [PMC free article] [PubMed] [Google Scholar]
- 16.Humphrey J D, Stephens L R. ‘Haemophilus somnus’: a Review. Vet Bull. 1983;53:987–1004. [Google Scholar]
- 17.Inzana T J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983;148:492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- 18.Inzana T J. The Haemophilus somnus complex. In: Howard J L, Smith R A, editors. Current veterinary therapy 4: food animal practice. 4th ed. Philadelphia, Pa: W.B. Saunders Co.; 1999. pp. 358–361. [Google Scholar]
- 19.Inzana T J, Apicella M A. Use of a bilayer stacking gel to improve resolution of lipopolysaccharides and lipooligosaccharides in polyacrylamide gels. Electrophoresis. 1999;20:462–465. doi: 10.1002/(SICI)1522-2683(19990301)20:3<462::AID-ELPS462>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Inzana T J, Corbeil L B. Development of a defined medium for Haemophilus somnus from cattle. Am J Vet Res. 1987;48:366–369. [PubMed] [Google Scholar]
- 21.Inzana T J, Gogolewski R P, Corbeil L B. Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage. Infect Immun. 1992;60:2943–2951. doi: 10.1128/iai.60.7.2943-2951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzana T J, Hensley J, McQuiston J, Lesse A J, Campagnari A A, Boyle S M, Apicella M A. Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus. Infect Immun. 1997;65:4675–4681. doi: 10.1128/iai.65.11.4675-4681.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inzana T J, Iritani B, Gogolewski R P, Kania S A, Corbeil L B. Purification and characterization of lipooligosaccharides from four strains of “Haemophilus somnus.”. Infect Immun. 1988;56:2830–2837. doi: 10.1128/iai.56.11.2830-2837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacques M. Role of lipo-oligosaccharides and lipopolysaccharides in bacterial adherence. Trends Microbiol. 1996;4:408–409. doi: 10.1016/0966-842X(96)10054-8. [DOI] [PubMed] [Google Scholar]
- 25.Kitching J P, Bishop G C. The Haemophilus somnus disease complex in cattle. In: Coetzer J A W, Thomson G R, Tustin R C, Kriek N P, editors. Infectious diseases of livestock: with special reference to Southern Africa. Vol. 2. New York, N.Y: Oxford University Press; 1994. pp. 1135–1142. [Google Scholar]
- 26.Luk J M, Nnalue N A, Lindberg A A. Efficient production of mouse and rat monoclonal antibodies against the O antigens of Salmonella serogroup C1, using LPS-coated bacteria as immunogen. J Immunol Methods. 1990;129:243–250. doi: 10.1016/0022-1759(90)90445-2. [DOI] [PubMed] [Google Scholar]
- 27.Lysenko E, Richards J C, Cox A D, Stewart A, Martin A, Kapoor M, Weiser J N. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 28.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunologically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandrell R, Schneider H, Apicella M, Zollinger W, Rice P A, Griffiss J M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986;54:63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 31.Risberg A, Schweda E K H, Jansson P-E. Structural studies of the cell-envelope oligosaccharide from lipopolysaccharide of Haemophilus influenzae strain Rm 118-28. Eur J Biochem. 1997;243:701–707. doi: 10.1111/j.1432-1033.1997.00701.x. [DOI] [PubMed] [Google Scholar]
- 32.Risberg A, Masoud H, Martin A, Richards J, Moxon E, Schweda E. Structural analysis of the lipopolysaccharide oligosaccharide epitopes expressed by a capsule-deficient strain of Haemophilus influenzae Rd. Eur J Biochem. 1999;261:171–180. doi: 10.1046/j.1432-1327.1999.00248.x. [DOI] [PubMed] [Google Scholar]
- 33.Stephens L R, Little P B, Humphrey J D, Wilkie B N, Barnum D A. Vaccination of cattle against experimentally induced thromboembolic meningoencephalitis with a Haemophilus somnus bacterin. Am J Vet Res. 1982;43:1339–1342. [PubMed] [Google Scholar]
- 34.Stephens L R, Little P B, Wilkie B N, Barnum D A. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981;178:378–384. [PubMed] [Google Scholar]
- 35.Swords W E, Buscher B A, Ver Steeg Ii K, Preston A, Nichols W A, Weiser J N, Gibson B W, Apicella M A. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 37.Weiser, J. N., J. B. P. Goldberg, N., and L. Wilson, Virji, M. 1998. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect. Immun. 66:4263–4267. [DOI] [PMC free article] [PubMed]
- 38.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser J N, Shchepetov M, Chong S T. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widders P R, Smith J W, Yarnall M, McGuire T C, Corbeil L B. Non-immune immunoglobulin binding by “Haemophilus somnus.”. J Med Microbiol. 1988;26:307–311. doi: 10.1099/00222615-26-4-307. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki R, Bacon B E, Nasholds W, Schneider H, Griffiss J M. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry. 1991;30:10566–10575. doi: 10.1021/bi00107a028. [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki R, Nasholds W, Schneider H, Apicella M A. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae: IgM monoclonal antibodies (3F11 and 1-1-M) recognize non-reducing termini of the LOS components. Mol Immunol. 1991;28:1233–1242. doi: 10.1016/0161-5890(91)90010-h. [DOI] [PubMed] [Google Scholar]