Abstract
Background
The aim of this study was to evaluate the predictive value of the hematological parameters in the identification of human cytomegalovirus (CMV) infection in infants less than 3 months.
Methods
A single‐center, observational study of infants with CMV infection was conducted retrospectively. Routine blood parameters were analyzed in CMV‐infected infants and controls with no differences of birthweight, sex, gestational age at birth, and date of admission. Furthermore, receiver‐operating curve was used to assess the predictive value of the hematological parameters for CMV infection.
Results
One hundred ninety cases with CMV infection were studied retrospectively. Compared with the control group, there were significant differences in the white blood cell count, neutrophil count, lymphocyte count, platelet count, hemoglobin, neutrophil‐to‐lymphocyte (NLR), platelet‐to‐lymphocyte (PLR), and lymphocyte‐to‐monocyte (LMR) for the patients with CMV infection (all p < 0.001). The best predicted values for CMV infection based on the area under the curve (AUC) were NLR and PLR with the optimal cut‐off value of 0.28 and 65.36. NLR‐PLR score of 0, 1, or 2 based on an elevated NLR (>0.28), an elevated PLR (>65.36), or both. NLR‐PLR score for CMV infection prediction yielded higher AUC values than NLR or PLR alone (0.760 vs. 0.689, 0.689; p < 0.001).
Conclusions
The NLR combined with PLR is potentially useful as a predictor of CMV infection in infants less than 3 months.
Keywords: cytomegalovirus, infection, inflammation, neutrophil‐to‐lymphocyte, platelet‐to‐lymphocyte
NLR‐PLR score for CMV infection prediction yielded higher AUC values than NLR or PLR alone (0.760 vs. 0.689, 0.689; p < 0.001). Therefore, the NLR combined with PLR is potentially useful as a predictor of CMV infection in infants less than 3 months.
1. INTRODUCTION
Congenital cytomegalovirus (CMV) infection is a huge public health problem all over the world, 1 which can result in severe long‐term sequelae. Furthermore, as many as 85%–90% of CMV infection is clinically inapparent and cannot be identified in the neonatal period, even though, about 10%–15% will develop late‐onset hearing loss and other developmental disorders. 2 , 3 Postnatal CMV screening is encouraged gradually. 4 However, there is no consensus about targeted and universal screening for congenital CMV infection.
In China, neonates are not routinely screened for CMV infection. CMV is identified by polymerase chain reaction (PCR) or culture. However, CMV detection is not available for some Chinese hospitals. Therefore, although the CMV infection is common in China, part of infants infected with CMV are missed diagnosis.
Routine blood test is the most common test performed in pediatric clinics. Markers of systemic inflammation, such as the neutrophil‐to‐lymphocyte (NLR) and platelet‐to‐lymphocyte (PLR), are generally appealing to clinicians, as these laboratory data are routinely collected prior to PCR or culture and are therefore readily available. NLR is a novel marker of inflammation, which has been shown to be correlated with thyroid conditions, type 2 diabetes mellitus (DM), irritable bowel syndrome, malignant conditions, ulcerative colitis, and cardiac conditions. 5 , 6 , 7 , 8 , 9 , 10 On the other hand, platelet‐related markers are also considered as a novel inflammatory markers. PLR is one of these novel indices and associated with inflammation in type 2 DM, malignant conditions, and thyroid conditions. 8 , 11 , 12
Thus, we studied the hematological parameters differences of the patients with CMV infection or without CMV infection, which might provide convenient indicators for CMV infection and then reduce the misdiagnosis of CMV infection.
2. MATERIALS AND METHODS
2.1. Study population
The infants aged 7 days to 3 months diagnosed with CMV infection being treated at the Division of Neonatology, Children's Hospital, Zhejiang University School of Medicine from January to December 2019 were included. Medical records of patients were retrospectively reviewed. This study targeted the previously healthy and immunocompetent children. Patients with primary immunodeficiency, leukemia, inherited bone marrow failure syndrome, or acquired immune deficiency syndrome were excluded. Furthermore, the exclusion criteria included: repeat hospitalizations, bacterial infections, such as sepsis, urinary tract infections and meningitis, necrotic enterocolitis, fungal pneumonia. CMV infection was defined as positive CMV‐DNA in urine by RT‐PCR. A control infant without CMV infection was selected retrospectively from the remaining infants, matched for sex, age, and birthweight. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and has been approved by the Ethics Review Committee of the Children's Hospital of Zhejiang University School of Medicine. Individual consent for this retrospective analysis was waived.
2.2. Detection of urine CMV‐DNA using RT‐PCR
Urine samples were collected for DNA extraction. CMV‐DNA was evaluated by RT‐PCR according to the instructions. The kit was produced by Da An Gene Co. The Real‐Time System was produced by the company ABI 7500 in USA.
2.3. Detection of routine blood parameters
A routine analyzer (XN‐2800, SYSMEX) was used for routine blood tests. The white blood cell (WBC) count, neutrophil (NEU) count, lymphocyte (LYM) count, monocyte (MON) count, platelet (PLT) count, and hemoglobin (HB) were recorded. Additionally, the NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte count, while the lymphocyte‐to‐monocyte (LMR) was calculated by dividing the absolute lymphocyte count by the absolute monocyte count on routine blood tests.
2.4. Statistical analysis
Analysis was performed with SPSS software version 23.0. The Kolmogorov‐Smirnov normality test was run for checking the distribution of hematological parameters and the Levene statistic test was used to test the homogeneity of variances. One‐way analysis of variance test and t‐test were conducted for comparison of normally distributed variables. The results of normally distributed variables are presented as the mean ± standard deviation (SD). Kruskal‐Wallis H test in conjunction with the Mann‐Whitney U‐test was used for comparison of non‐normally distrusted hematological parameters. Furthermore, receiver‐operating curve (ROC) was used to assess the predictive value of the hematological parameters for CMV infection. Descriptive statistics were performed to determine the patients’ features. p‐value <0.05 was considered significant.
3. RESULTS
3.1. Patient characteristics
In the present study, 257 infants with CMV infection were admitted at our department in 2019. Sixty‐seven infants were excluded, because of the following conditions: older than 3 months, younger than 7 days, repeat hospitalizations, sepsis, urinary tract infection, umbilical abscess, peri‐umbilical cellulitis, lacrimal abscess, intracranial infection, necrotic enterocolitis, and fungal pneumonia. Finally, 190 infants with CMV infection were enrolled. Meanwhile, 190 infants without CMV infection were selected retrospectively from the remaining infants, matched for sex, age, and birthweight (Figure 1). The patients’ characteristics are shown in Table 1. There are no statistically significant differences in age, sex, birthweight, and premature/mature distributions between two groups.
FIGURE 1.
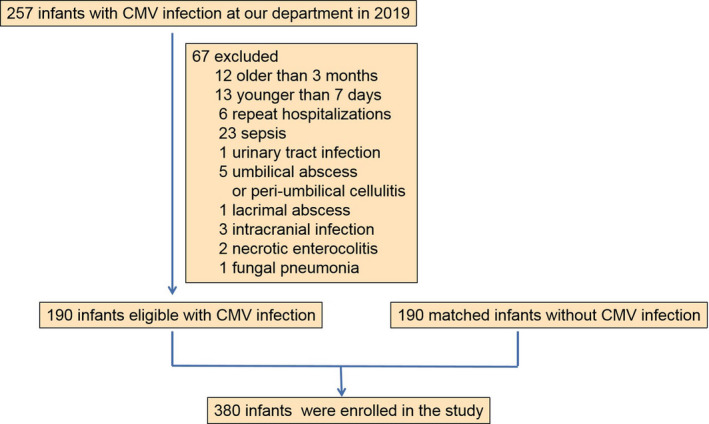
Flow chart of infants selected for the study
TABLE 1.
Clinical characteristics of infants with or without CMV infection
| CMV infection group (n = 190) | Control group (n = 190) | p | |
|---|---|---|---|
| Age (days) | 36 | 34 | 0.140 |
| Birthweight (g) | 2931 | 2975 | 0.562 |
| Gender | 0.672 | ||
| Males | 117 | 121 | |
| Females | 73 | 69 | |
| Gestation | 0.909 | ||
| Mature | 138 | 137 | |
| Premature | 52 | 53 | |
Abbreviation: CMV, cytomegalovirus.
3.2. Routine blood parameters in patients with and without CMV infection
Compared with the control group, the infants with CMV infection had lower NEU counts, PLT counts and HB values, and higher WBC counts and LYM counts. The NLR and PLR in the CMV infection group were significantly lower than that of control group, while the LMR in the CMV infection group was higher than that of control group (Table 2). Additionally, no significant difference of MON count was observed between two groups.
TABLE 2.
Hematological parameters of the CMV infection group and control group
| Blood routine test | CMV infection group (n = 190) | Control group (n = 190) | p |
|---|---|---|---|
| WBC (109/L) | 10.95 ± 4.38 | 9.77 ± 3.18 | 0.003 |
| NEU (109/L) | 2.30 ± 1.78 | 2.79 ± 1.61 | 0.005 |
| LYM (109/L) | 6.92 ± 3.09 | 5.27 ± 1.72 | <0.0001 |
| MON (109/L) | 1.31 ± 0.63 | 1.27 ± 0.56 | 0.483 |
| PLT (109/L) | 324.35 ± 125.49 | 380.93 ± 119.35 | <0.0001 |
| HB (g/dl) | 11.40 ± 2.17 | 12.00 ± 2.22 | 0.009 |
| NLR | 0.37 ± 0.31 | 0.56 ± 0.34 | <0.0001 |
| PLR | 54.69 ± 29.57 | 79.03 ± 34.68 | <0.0001 |
| LMR | 5.98 ± 2.85 | 4.79 ± 2.91 | <0.0001 |
Abbreviations: CMV, cytomegalovirus; HB, hemoglobin; LMR, lymphocyte‐to‐monocyte; LYM, lymphocyte; MON, monocyte; NEU, neutrophil; NLR, neutrophil‐to‐lymphocyte; PLR, platelet‐to‐lymphocyte; PLT, platelet; WBC, white blood cell count.
p‐value <0.05 marked in bold font shows statistical significance.
3.3. The predictive value of NEU, LYM, NLR, PLR, and LMR in CMV infection screening
To further evaluate the ability of the hematological parameters to predict the CMV infection, receiver‐operating characteristic (ROC) curve analyses were used. The results showed that the areas under the curve (AUC) for NEU, LYM, NLR, PLR, and LMR were 0.631, 0.674, 0.729, 0.728, and 0.65, respectively, and the optimal cut‐off value was 2.01 (109/L), 5.61 (109/L), 0.28, 65.36, and 0.54, corresponding to the maximum joint sensitivity and specificity, respectively (Figure 2).
FIGURE 2.

ROC curves of the NLR, PLR, NEU, LYM, and LMR for predicting the CMV infection
Given the better ability of NLR and PLR for predicting CMV infection, we further analyzed the ability of NLR combined with PLR for predicting the CMV infection. Patients were assigned an NLR‐PLR score of 0, 1, or 2 based on an elevated NLR (>0.28), an elevated PLR (>65.36), or both, as follows: patients with elevated NLR and PLR were assigned a score of 2, and patients with either or neither were assigned a score of 1 or 0, respectively. NLR‐PLR score for CMV infection prediction yielded higher AUC values than NLR or PLR alone (0.760 vs. 0.689, 0.689; p < 0.001) (Table 3) (Figure 3).
TABLE 3.
Areas under the ROC curves of NLR, PLR, and NLR‐PLR score for predicting CMV infection
| Variables | Area under the ROC curve (95% CI) | p |
|---|---|---|
| NLR (≤0.28/>0.28) | 0.689 (0.636–0.743) | <0.001 |
| PLR (≤65.36/>65.36) | 0.689 (0.636–0.743) | <0.001 |
| NLR‐PLR score (0/1/2) | 0.760 (0.712–0.809) | <0.001 |
Abbreviations: CMV, cytomegalovirus; NLR, neutrophil‐to‐lymphocyte; PLR, platelet‐to‐lymphocyte; ROC, receiver‐operating characteristic.
p‐value <0.05 marked in bold font shows statistical significance.
FIGURE 3.
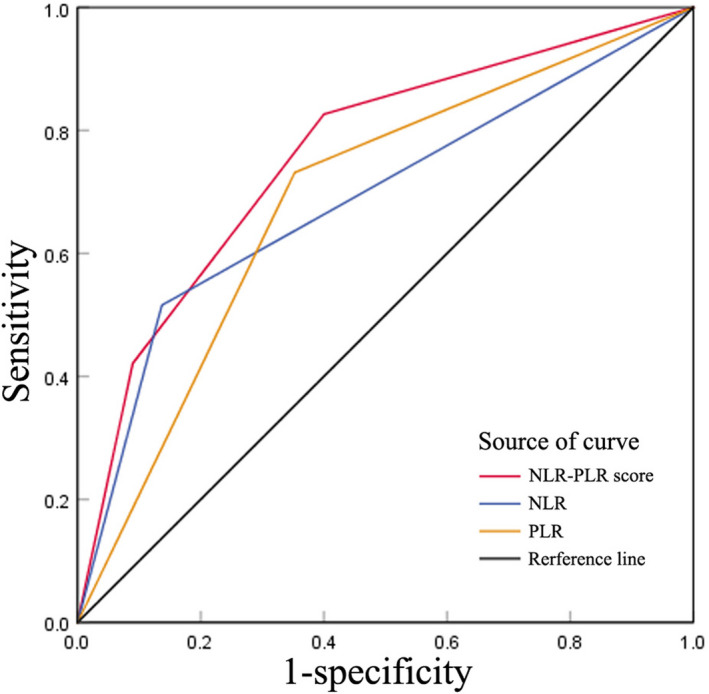
Comparison of area under the receiver‐operating characteristic curve (AUC) in different inflammation‐based scores. NLR‐PLR score for CMV infection prediction yielded higher AUC values than NLR or PLR alone
4. DISCUSSION
In the current study, we found that the NEU, NLR, and PLR were significantly lower in infants with CMV infection than that in patients without CMV infection, while higher WBC counts and LYM counts were observed in patients with CMV infection. Furthermore, the best predicted values for CMV infection based on the AUC were NLR and PLR with the optimal cut‐off value of 0.28 and 65.36. Interestingly, NLR‐PLR score for CMV infection prediction yielded higher AUC values than NLR or PLR alone (0.760 vs. 0.689, 0.689; p < 0.001).
Congenital CMV infection is prevalent in China. Maternal CMV seroprevalence is reported to be higher than 90%. The rate of congenital CMV infection is about 0.7% among all live births. 13 , 14 CMV can affect multiple organs with a variety of manifestations, such as intrauterine growth retardation, microcephaly, brain damage, hearing damage, retinitis, and hepatosplenomegaly. 15 , 16 CMV has been associated with myelosuppression, including granulocytopenia, thrombocytopenia, and anemia. 17 , 18 , 19 Traditionally, CMV infection is identified by PCR or culture, which is not available for some Chinese hospitals. Therefore, it is necessary to search for potential diagnostic indicators that are available for the community hospitals.
Recently, the NLR was shown to be closely associated with lots of diseases, such as systemic inflammation, cardiovascular diseases, malignant diseases. 10 , 20 , 21 NLR is an important marker affecting tumor metastasis and prognosis. 22 Additionally, a retrospective study of bacteremia found that NLR greater than 7 was an independent risk factor for increased mortality. 23 NLR is also investigated as useful predictors for the viral infection. The NLR is sensitive for the detection of influenza virus infection. 24 Meanwhile, higher white blood cells, neutrophil, and NLR were found in COVID‐19 cases as compared to healthy controls. 25 NLR is considered independent biomarker for indicating poor clinical outcomes in COVID‐19 patients, which may reduce the overall mortality of COVID‐19 patients. 26 In the present study, a significant difference of the NLR was observed in the CMV infection group. Based on the dates, the NLR is a good index for the prediction of CMV infection with an AUC of 0.729.
Platelets are one of the most prevalent blood component. It has been reported that platelet contributed to the innate and adaptive immune response in various types of infection. 27 , 28 , 29 , 30 In CMV infection, platelets were found to interact with neutrophils, monocytes, and dendritic cells, suggesting an interaction with the immune systems. 28 PLR refers to the platelet‐to‐lymphocyte ratio which is more valuable in predicting various inflammations than platelet or lymphocyte counts alone. PLR, as a new inflammatory indicator, has been confirmed to be related with tumors, coronary heart disease, and connective tissue diseases. 31 , 32 , 33 Furthermore, high PLR levels indicate poorer prognosis and longer hospital stay in COVID‐19‐positive patients, while patients with severe COVID‐19 infection have greater PLR levels than those of non‐severe COVID‐19 infection. 34 In the study, the PLT and PLR were lower in the CMV infection group than the control group. The AUC of PLR for predicting CMV infection is 0.728. Furthermore, we found that the value of NLR and PLR combined was better able to predict the CMV infection which had a higher AUC (0.760) than NLR or PLR alone.
At present, the screening strategies for CMV all over the world include universal screening, targeted screening, and no screening. The universal screening for cytomegalovirus (CMV) postnatally is encouraging. 4 , 35 However, in China, CMV is not screened now. Furthermore, CMV‐DNA cannot be detected in parts of hospitals, whereas routine blood test is common, convenient, and inexpensive. Compared with simple neutrophils, lymphocytes, and platelets, NLR and PLR are more stable. CMV infection might be considered for the infants with greatly low level of NLR and PLR.
Our study had several limitations that must be considered. First, given its retrospective design, the current study was subject to possible selection bias, as well as diagnostic bias. Second, the NLR and PLR, a marker of systemic inflammation, may be affected by many conditions, including chronic inflammatory diseases, granulocyte colony‐stimulating factor administration, and other diseases. Therefore, these conditions must be accounted for in clinical practice. Finally, the present study was conducted at a single institution. The performance of multicenter studies of the markers used herein would strengthen our conclusions.
5. CONCLUSIONS
A significantly lower NLR and PLR were observed in infants (<3 months) with CMV infection. The NLR/PLR combined is potentially useful as a predictor of CMV infection in infants (<3 months) with an AUC of 0.760, which might provide information for the identification of CMV infection.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (81401235).
Zhan C, Wang W, Chen L. Predictive significance of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte for cytomegalovirus infection in infants less than 3 months: A retrospective study. J Clin Lab Anal.2022;36:e24131. doi: 10.1002/jcla.24131
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta‐analysis. Rev Med Virol. 2019;29(3):e2034. [DOI] [PubMed] [Google Scholar]
- 2. Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;4(57 suppl):S178‐S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gantt S, Bitnun A, Renaud C, Kakkar F, Vaudry W. Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr Child Health. 2017;22(2):72‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ronchi A, Shimamura M, Malhotra PS, Sánchez PJ. Encouraging postnatal cytomegalovirus (CMV) screening: the time is NOW for universal screening! Expert Rev Anti Infect Ther. 2017;15(5):417‐419. [DOI] [PubMed] [Google Scholar]
- 5. Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil‐to‐lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras (1992). 2017;63(12):1065‐1068. [DOI] [PubMed] [Google Scholar]
- 6. Bilgin S, Aktas G, Zahid Kocak M, et al. Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. Aging Male. 2020;23(5):923‐927. [DOI] [PubMed] [Google Scholar]
- 7. Güçlü M, Ağan AF. Relationship of peripheral blood neutrophil to lymphocyte ratio and irritable bowel syndrome. Turk J Med Sci. 2017;47(4):1067‐1071. [DOI] [PubMed] [Google Scholar]
- 8. Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Posul E, Yilmaz B, Aktas G, Kurt M. Does neutrophil‐to‐lymphocyte ratio predict active ulcerative colitis? Wien Klin Wochenschr. 2015;127(7–8):262‐265. [DOI] [PubMed] [Google Scholar]
- 10. Shah N, Parikh V, Patel N, et al. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: insights from the National Health and Nutrition Examination Survey‐III. Int J Cardiol. 2014;171(3):390‐397. [DOI] [PubMed] [Google Scholar]
- 11. Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ, Savli H. Diabetes control could through platelet‐to‐lymphocyte ratio in hemograms. Rev Assoc Med Bras (1992). 2019;65(1):38‐42. [DOI] [PubMed] [Google Scholar]
- 12. Calapkulu M, Sencar ME, Sakiz D, et al. The prognostic and diagnostic use of hematological parameters in subacute thyroiditis patients. Endocrine. 2020;68(1):138‐143. [DOI] [PubMed] [Google Scholar]
- 13. Wang S, Wang T, Zhang W, et al. Cohort study on maternal cytomegalovirus seroprevalence and prevalence and clinical manifestations of congenital infection in China. Medicine (Baltimore). 2017;96(5):e6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin Q, Su J, Wu S. Cytomegalovirus infection among pregnant women in beijing: seroepidemiological survey and intrauterine transmissions. J Microbiol Biotechnol. 2017;27(5):1005‐1009. [DOI] [PubMed] [Google Scholar]
- 15. Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134:972‐982. [DOI] [PubMed] [Google Scholar]
- 16. Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17(6):e177‐e188. [DOI] [PubMed] [Google Scholar]
- 17. Cui DY, Zhang J, Zhang Q, Hao HF, Wu QQ, Sun LF. Immunological damage effects of cytomegalovirus infection on bone marrow hematopoietic cells. Zhonghua Yi Xue Za Zhi. 2019;99(22):1727‐1730. [DOI] [PubMed] [Google Scholar]
- 18. Pemde HK, Kabra SK, Agarwal R, Jain Y, Seth V. Hematological manifestations of congenital cytomegalovirus infection. Indian J Pediatr. 1995;62(4):473‐477. [DOI] [PubMed] [Google Scholar]
- 19. Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orfanu AE, Popescu C, Leuștean A, et al. The importance of haemogram parameters in the diagnosis and prognosis of septic patients. J Crit Care Med (Targu Mures). 2017;3(3):105‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu L, Saxena S, Singh RK. Neutrophils in the tumor microenvironment. Adv Exp Med Biol. 2020;1224:1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou B, Deng J, Chen L, Zheng S. Preoperative neutrophil‐to‐lymphocyte ratio and tumor‐related factors to predict lymph node metastasis in nonfunctioning pancreatic neuroendocrine tumors. Sci Rep. 2017;7(1):17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil‐lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One. 2012;7(8):e42860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu R, Chen C, Wang Q, Zhang X, Lu C, Sun Y. Routine blood parameters are helpful for early identification of influenza infection in children. BMC Infect Dis. 2020;20(1):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalid A, Ali Jaffar M, Khan T, et al. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS‐COV‐2 infected patients of Pakistan: a retrospective comparative analysis. Hematology. 2021;26(1):529‐542. [DOI] [PubMed] [Google Scholar]
- 26. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol. 2020;84:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hottz ED, Bozza FA, Bozza PT. Platelets in immune response to virus and immunopathology of viral infections. Front Med (Lausanne). 2018;5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122(2):337‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li C, Li J, Ni H. Crosstalk between platelets and microbial pathogens. Front Immunol. 2020;11:1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franchini M, Veneri D, Lippi G. Thrombocytopenia and infections. Expert Rev Hematol. 2017;10(1):99‐106. [DOI] [PubMed] [Google Scholar]
- 31. Li BO, Zhou P, Liu Y, et al. Platelet‐to‐lymphocyte ratio in advanced cancer: review and meta‐analysis. Clin Chim Acta. 2018;483:48‐56. [DOI] [PubMed] [Google Scholar]
- 32. Larmann J, Handke J, Scholz AS, et al. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non‐cardiac surgery. BMC Cardiovasc Disord. 2020;20(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet‐to‐lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39(4):345‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aktas G. Hematological predictors of novel Coronavirus infection. Rev Assoc Med Bras (1992). 2021;67(suppl 1):1‐2. [DOI] [PubMed] [Google Scholar]
- 35. Chen K, Zhong Y, Gu Y, et al. Estimated cost‐effectiveness of newborn screening for congenital cytomegalovirus infection in china using a markov model. JAMA Netw Open. 2020;3(12):e2023949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


