Abstract
Objectives
This study is to explore the clinical significance of folate receptor‐positive circulating tumor cells (FR+CTC) in the early diagnosis and disease progress in patients with breast cancer.
Methods
Folate receptor‐positive circulating tumor cells was enriched from peripheral blood of the patients with immunomagnetic separation method and quantitated by folate receptor on the CTC with the ligand‐targeted PCR.
Results
The levels of FR+CTC were significantly higher in breast cancer patients compared with healthy controls. Detective rate of FR+CTC was decreased in 19 of 27 patients underwent the surgery in 2 weeks post‐operation compared with pre‐operation; statistical analysis showed the difference was significant. We also found that the combination of FR+CTC, CEA, CA125, and CA153 can significantly improve the diagnostic efficiency for breast cancer.
Conclusions
This study showed the detective rate of FR+CTC is significantly increased in the patients with breast cancer, and the detective level is associated with disease progress.
Keywords: biomarkers, breast cancer, diagnosis, folate receptor‐positive circulating tumor cells
In this paper, FR+CTC was detected in peripheral blood of the patients with breast cancer with the ligand‐targeted PCR. The levels of FR+CTC were significantly higher in breast cancer patients compared to healthy controls and the FR+CTC level were significantly higher in the distant metastasis and high TNM stage. The level of FR+CTC was decreased underwent the surgery in 2 weeks post‐operation compared to pre‐operation. The detection sensitivity and specificity of FR+CTC was higher diagnostic efficacy than other biomarkers, such as CEA, CA125, and CA153. This study showed that may be used as a potential biomarker for auxiliary diagnosis and early detection particularly combined with other biomarkers assay in breast cancer.

1. INTRODUCTION
The prognosis of breast cancer is dramatically improved owing to progress on the early diagnosis and effective therapy, and the survivor number is higher than any other types of cancers. 1 However, the number of new breast cancer cases reached 2.26 million in 2020, accounted for 11.7% of all new tumor cases, which exceeded the number of new cases of lung cancer for the first time. 2 Also, the survival rate of breast cancer all over the world was unsatisfactory. This is because most of the patients have no typical symptoms in the early stage, and the patients usually have advanced or even metastatic tumors at the time of diagnosis. 3 Therefore, searching for new diagnostic markers has a great significance for the early diagnosis and the early detection of metastasis and recurrence of breast cancer.
Circulating tumor cells (CTCs) spread from the original tumor sites to distant locations through the circulatory system and retain the essential characteristics of the primary tumor. 4 CTC leads to distant metastasis of tumors and poor prognosis of patients; and it has crucial value in the early diagnosis, efficacy evaluation, and prognosis estimation of breast cancer patients. 5 CTC can be detected in the peripheral blood of cancer patients but rarely in healthy people. It is estimated that there were 1–10/ml CTC in whole blood of patients with metastatic tumors. 6 As a liquid biopsy, CTC technology has been included in the National Comprehensive Cancer Network guidelines and also defined as a prognostic factor for breast cancer by the American Joint Committee on Cancer. 7
Until now, more than 50 analytical methods have been developed for the identification, counting, and even molecular characterization of CTCs. The analytical methods are divided into two categories: one was based on the surface marker of the cells, and another was based on the size and density of the cells. The CellSearch® system is the most commonly used system for separation, enrichment, and counting of CTCs in breast cancer, and it is the only technology approved by the United States FDA for clinical use in breast cancer, prostate cancer, and colorectal cancer. The CTC methodologies from all the company including the CellSearch® system are based on detection of the epithelial cell adhesion molecule (EpCAM) on the CTC, which have a significant defect that the expression of the epithelial marker is downregulated or lost during EMT. 8
Folate receptor (FR) is a glycosylated phosphatidylinositol‐coupled protein with high affinity and endocytosis and transport capacity for its natural ligand folic acid. It is a cell surface protein that has been proven to be an ideal tumor marker such as the non‐small cell lung cancer. Tumor cells transport folate through folate receptors to synthesize thymine. There are 500,000 folate receptors on each tumor cell. Except for macrophages, most normal tissues express folate receptors at negligible levels. 9 However, activated macrophages are almost undetectable in blood samples from healthy donors or patients with benign diseases. 10 Studies have shown that FR is highly expressed in a variety of cancers, including breast cancer, and the high expression level of FR is related to the poor prognosis of breast cancer. 11
In this study, we, for the first time, used the FR to detect CTC in breast cancer and evaluated the clinical significance of FR+CTC in the early diagnosis and efficiency in monitoring progress of breast cancer. Besides, we also compared the detection rate of CTC and traditional tumor markers in patients with breast cancer, which reveal a potential application on the early diagnosis and the disease progress monitoring.
2. MATERIALS AND METHODS
2.1. Patients
This study enrolled 60 breast cancer female patients and 13 volunteers from January 2019 to January 2021, Renmin Hospital of Wuhan University. The diagnosis for the patients was confirmed by cytology or histopathology, and none of the patients had received anti‐tumor therapy or had another tumor before enrolling in the study. Breast cancer stages were determined following the American Joint Committee on Cancer's (AJCC) Breast Cancer Staging Manual (8th edition). 12 The peripheral blood was collected for isolation of CTC on the diagnosis in all patients of the cohort. The 27 patients underwent radical mastectomy, and the peripheral blood was also collected in 2 weeks after the operation. The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (WDRY2020‐K078), and informed consent was obtained from patients or their families.
2.2. CTCs detection
Circulating tumor cells was isolated with folate receptor‐positive (FR+) CTC isolation method using CytoploRare® kit (GenoSaber Biotech Co., Ltd.). 13 The 3 ml peripheral blood was collected in ethylene diamine tetra acetic acid (EDTA) anticoagulation tubes and stored at 4°C. FR+CTC analysis was performed within 24 h after collection based on the manufacturer's instructions. The method includes negative enrichment of FR+circulating tumor cells with immunomagnetic beads to deplete leukocytes first, then CTC level was quantitated by ligand‐targeted PCR. First, leukocytes and mononuclear macrophages were removed by anti‐CD45 and anti‐CD14 magnetic beads. Then, the cells were activated and washed to free up the folate binding site. After blocking the nonspecific nucleic acid binding site, CTC was incubated with a proprietary probe consisting of a conjugate of folate and a synthetic oligonucleotide, and then the excess unbound probe is washed away. The bound probes are finally eluted and stored at 4°C. The real‐time PCR system was used to run fluorescent quantitative PCR to amplify and quantify the signal from the detection probe. 12 FR+CTCs level was expressed as arbitrarily defined "FR units" (Fu), defined as the number of FR‐positive CTC detected in 3 ml of blood. A series of standards containing oligonucleotides (10−14 to 10−19 M, equivalent to 2 to 2 × 105 CTC units/3 ml) is used to calibrate FR+CTC units. The carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), and cancer antigen 153 (CA153) were measured by chemiluminescent immunoassay.
2.3. Statistical analysis
Independent sample t test or paired samples t test was used for the comparison between two groups under different conditions. ANOVA analysis of variance was used for comparison between multiple groups. All tests are two‐sided probabilities, and the differences with p value <0.05 were considered statistically significant. FR+CTC levels are expressed in terms of median and interquartile range (IQR). The significant differences were assessed by the chi‐square test (for categorical variables) or Student's t test and nonparametric rank‐sum tests (depends on whether the continuous variables conform to the normal distribution). The receiver operating characteristic (ROC) analysis was used to evaluate the diagnostic efficiency, and the area under the curve (AUC) of each indicator is calculated, Youden index was used to determine the optimal cut‐off threshold. The above statistical analyses were performed by SPSS 22.0 and GraphPad Prism 6.0 (GraphPad Software).
3. RESULTS
3.1. Patient characteristics
This study was enrolled 60 breast cancer patients with a mean age of 52 years (range, 27–75 years) and 32 healthy controls with an age of 52 (range, 40–66 years). All participants were women. The clinical characteristics of patients, including age, gender, tumor size (calculated with the maximum diameter), metastasis status, TNM stage, and the expression level of tumor markers are displayed in Table 1. There were no significant differences between breast cancer patients and healthy controls in age and gender. The patients were classified based on tumor size, ie, maximum diameter of the tumor, in which 17 patients had tumors with the size ≥3 cm in diameter, 43 patients <3 cm; also, 8 patients had distant metastases, and no metastases were found in the remaining 52 patients. The expression of tumor biomarkers (CEA, CA125, and CA153) was also examined in this cohort, and they showed the statistical difference between the breast cancer patients and the control group.
TABLE 1.
Clinical feature of the participants in the cohort
| Variable |
Breast cancer patients (n = 60) |
Control (n = 32) |
p |
|---|---|---|---|
| Age | |||
| ≤45 years | 17 (28.3%) | 6 (18.8%) | >0.05 a |
| >45 years | 43 (71.7%) | 26 (81.3%) | |
| Gender | |||
| Female | 60 (100%) | 32 (100%) | — |
| Tumor size (maximum diameter) | |||
| ≥3 cm | 17 (28.3%) | >0.05 b | |
| <3 cm | 43 (71.7%) | ||
| Metastasis status | |||
| Yes | 8 (13.3%) | <0.05 b | |
| No | 52 (86.7%) | ||
| TNM stage | |||
| Ⅰ | 17 (28.3%) | >0.05 b | |
| Ⅱ | 17 (28.3%) | ||
| Ⅲ | 18 (30.0%) | ||
| Ⅳ | 8 (13.3%) | ||
| CEA | 1.58 ± 2.04 | 0.87 ± 0.55 | >0.05 a |
| CA125 | 14.25 ± 14.82 | 7.91 ± 3.11 | >0.05 a |
| CA153 | 12.87 ± 16.00 | 5.61 ± 4.83 | <0.05 a |
Abbreviations: CA125, carbohydrate antigen 125; CA153, carbohydrate antigen 153; CEA, carcinoembryonic antigen.
Denoted the statistical difference between the breast cancer patients and the control group.
Denoted the differences among the subgroups in the breast cancer patients.
3.2. Association of FR+CTC levels with clinical traits
In order to explore the association of CTC cells with clinical feature (metastasis, stage, survival, etc.), FR+CTC was negatively enriched through deleting leukocytes in the peripheral blood and quantitatively detected by ligand‐targeted PCR with commercially available kit in the cohort. FR+CTCs level was expressed as arbitrarily defined "FR units" (Fu) as described in method section. The levels of FR+CTC in breast cancer patients and healthy people were 10.95 (7.28–14.15) FU/3 ml and 6.93 (5.46–8.19) FU/3 ml, respectively, which is significantly higher in breast cancer patients compared with healthy controls (p < 0.05) (Figure 1). The association of the FR+CTC levels with clinical features was further explored. No difference of the FR+CTC level was observed in the patients with age ≥45 years vs. <45 years and tumor size ≥3 cm vs. <3 cm (Figure 2A,B). The FR+CTC level was significantly higher in the patients with distant metastasis 10.3 (6.95–13.71) compared to that without metastasis 14.28 (9.83–34.06) (p < 0.05, Figure 2C). Also, no significant difference was observed in FR+CTC level in the four TNM stages, which may be due to the small sample size (Figure 2D), but the FR+CTC level was significantly higher with high TNM stage (stage III + IV, 11.3 (9.1–14.48)) than that with low‐stage tumors (stage I + II, 8.1 (5.5–13.2)) (p < 0.05, Figure 2E).
FIGURE 1.
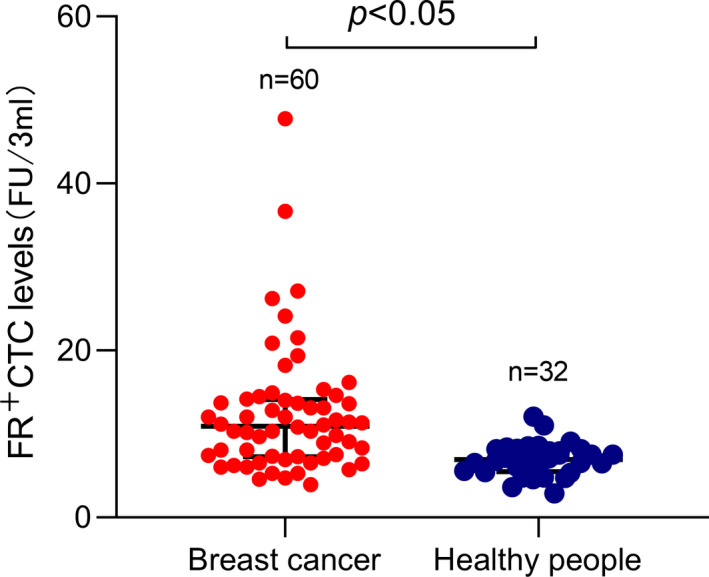
Folate receptor‐positive circulating tumor cells (FR+CTC) levels in breast cancer patients and healthy people. The box‐and‐whisker plot showing the median and IQR of FR+CTC levels in breast cancer patients and healthy people. The average FR+CTC level of 60 patients was 12.38 FU per 3 ml, and 32 healthy people was 6.93 FU per 3 ml
FIGURE 2.
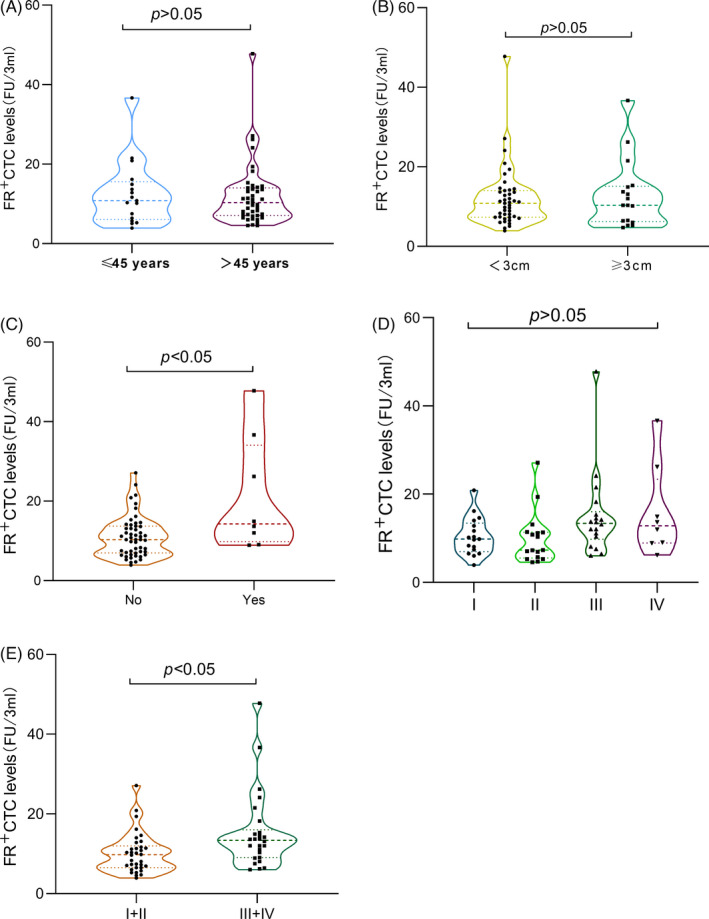
Folate receptor‐positive circulating tumor cells (FR+CTC) levels and clinical traits. The violin plot showing the median and IQR of FR+CTC levels in stratified analysis of breast cancer patients. Every points denoted every participant. (A) Comparison of CTC levels in patients with ≤45 years and >45 years. (B) Comparison of CTC levels in patients of tumor maximum diameter ≥3 cm and <3 cm. (C) Comparison of CTC levels in patients without metastasis and with distant metastasis. (D) Comparison of CTC levels in patients of TNM stage (I, II, III, and IV). (E) Comparison of CTC levels in patients of TNM stage (I and II, III and IV)
3.3. FR+CTC levels in paired samples from pre‐operation and post‐operation
In the cohort, 27 of 60 patients underwent the surgery, and the FR+CTC level was compared 2 weeks post‐operation vs. pre‐operation. Results showed that the FR+CTC level in 19 patients decreased after the operation, and statistical analysis showed the difference in FR+CTC was significant (p < 0.05 (Figure 3).
FIGURE 3.
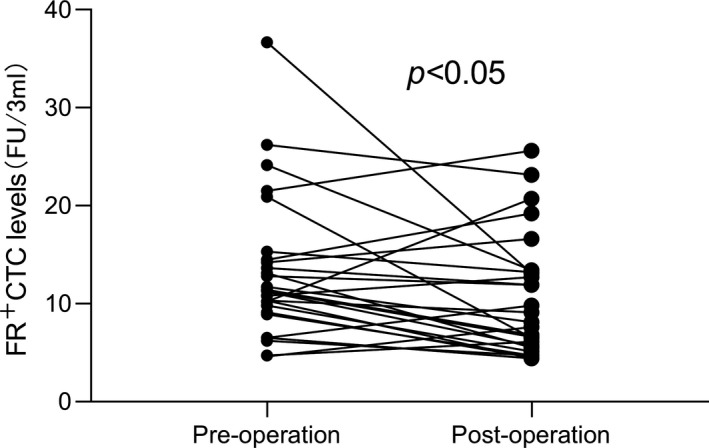
Folate receptor‐positive circulating tumor cells (FR+CTC) levels in paired samples from pre‐operation and post‐operation. In the breast cancer group, 27 patients had a preoperative FR+CTC level of 13.20 FU per 3 ml and a postoperative FR+CTC level of 10.41 FU per 3 ml. Among them, 19 patients had a downward trend
3.4. AUC for FR+CTC and tumor markers
We also used receiver operating characteristic (ROC) analysis to evaluate the diagnostic performance of FR+CTC and tumor markers. ROC curve reveals the discriminant thresholds based on the probability of positive results (sensitivity against 1 − specificity) in individual subjects. The area under the curve (AUC) is a measure of the overall accuracy of the dichotomous methods of the measurements. We compared the AUC of FR+CTC level and other breast tumor markers (CEA, CA125, and CA153). Results showed that the AUC of FR+CTC (0.778, 95% CI: 0.686–0.871) is larger than other tumor markers (Figure 4). The cut‐off value of FR+CTC for breast cancer diagnosis was 9.08 Folate Unit/3 ml, the sensitivity was 63.33%, and the specificity was93.75%. To improve the accuracy of diagnosis, we analyzed the AUC by combining FR+CTC with the tumor markers. It is worth noting that, the combination (FR+CTC, CEA, CA125, and CA153) can significantly improve the diagnostic efficiency of breast cancer (AUC = 0.8865) compared with a single tumor marker (Figure 4).
FIGURE 4.
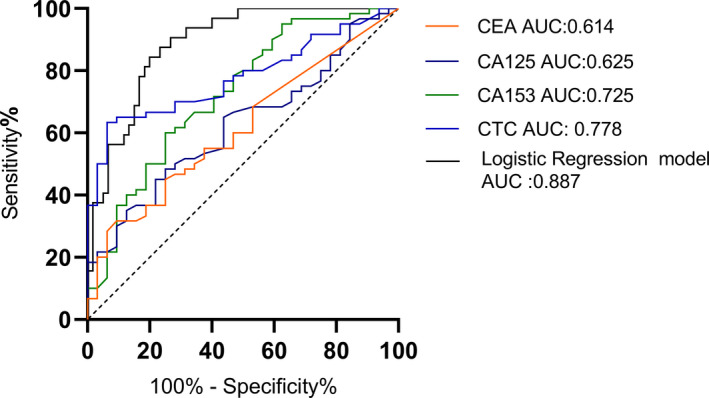
AUC of ROC analysis for folate receptor‐positive circulating tumor cells (FR+CTC), carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), and cancer antigen 153 (CA153), and that in their combination. FR+CTCs, AUC = 0.778, 95% CI = 0.6860 to 0.8707, p = 0.001; CEA, AUC = 0.614, 95% CI = 0.4974 to 0.7307, p = 0.073; CA125, AUC = 0.625, 95% CI = 0.5099 to 0.7396, p = 0.05; CA153, AUC = 0.725, 95% CI = 0.6141 to 0.8353, p = 0.004; The combined diagnosis model, AUC = 0.887, 95% CI = 0.8212 to 0.9518, p < 0.001
4. DISCUSSION
The concept of CTC that malignant cells in the blood vessels of patients with metastatic cancer were similar to the primary tumor is first proposed in 1869. 14 As CTC is an extremely rare cell in blood vessels, CTC detection is technically challenging. The technology for the detection of CTC has attracted more and more attention in the past two decades. 15 However, only one product from CellSearch® is approved by the FDA, and this product has not been widely accepted by the medical field because of its expensive equipment, high cost, low sensitivity and selectivity, and other limitations. In order to overcome these limitations, various CTC detection techniques have been developed. In this study, CTC detection is based on the principle of ligand‐targeted PCR. The detection probe is composed of folate analog and oligonucleotide chains. Folic acid analogs can bind to α‐folate receptors on CTCs, while oligonucleotide chains are used for subsequent PCR amplification. One CTC can combine tens of thousands of probes, and one probe is magnified tens of millions of times after PCR amplification. Therefore, even trace amounts of CTCs can be detected in a small amount of blood, which indicated the method has high sensitivity.
Folate receptor (FR) is a high‐affinity glycosylphosphatidyl (GPI)‐anchored membrane protein, which has 4 subtypes, namely FR alpha (FRA), beta (FRB), gamma (FRG), and delta (FRD). 16 FR‐α is selected in this kit. It is reported that the expression of FR‐ α has a specificity of tissue and tumor. It is mainly expressed in epithelial tumors, such as non‐small cell lung cancer, 13 endometrial adenocarcinoma, 17 and breast cancer. 18 FR plays a crucial role in the occurrence, development, and metastasis of cancer, and it is becoming a target for effective treatment and detection of personalized medicine. In addition, clinical research evidence from different secretory epithelial solid tumors indicates that FR may also be a negative prognostic marker associated with chemotherapy resistance in some malignant tumors. 19 In the current study, we for the first time used LT‐PCR technology to identify FR+breast tumors, which reveal the potential significance on the early diagnosis of breast cancer.
This study showed that the level of FR+CTC in breast cancer patients was significantly higher than that in healthy controls, and in the patients with distant metastasis compared to that without metastasis, also in TNM patients with high‐stage tumors than low‐stage tumors. These data reveal that FR+ CTC is closely related to the malignant degree of breast cancer, and it may be one of the independent prognostic predictors. We also found the method has very good sensitivity (63.33%) and the excellent specificity (93.75%). Also, the diagnostic efficiency of FR+CTC is higher than that of traditional tumor markers, particularly the combined diagnosis model of FR+CTC with tumor markers has achieved satisfactory diagnostic validity. Therefore, our data reveal FR+CTC has great potential value in clinical diagnosis of breast cancer.
We also observed the dynamic changes of CTC in the peripheral blood of breast cancer patients before and after surgery. Considering that the operation may lead to the massive release of CTC into the blood, resulting in a transient increase in CTC, the postoperative blood collection time is 2 weeks after surgery. The results demonstrated that the CTC of most patients showed a downward trend after surgery (n = 19), and the difference was statistically significant. This data indicated that once the primary tumor is removed, CTC shows a decrease trend, which is because CTC originates from the primary tumor. However, there are still a few patients with elevated CTC levels, and we speculate whether it is due to undetected occult lesions, which was not validated owing to the lack of follow‐up data.
In summary, this study examined the clinical significance of FR+CTC detection in the diagnostic screening and progress monitoring of breast cancer. The detection of FR+CTCs in peripheral blood is simple, noninvasive methods with high sensitivity and specificity. It can be used as a potential biomarker for auxiliary diagnosis and early detection in breast cancer patients. Although this method has certain limitations and cannot be used as a specific tumor marker, it can provide auxiliary diagnostic help when the imaging results are not clear. However, the number of the enrolled patients in this study is small; the follow‐up data in some patients are missing, and it lacks of further prognostic survival analysis. Much bigger cohort is needed to further verify the clinical significance of this method in breast cancer and other tumors in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial, or otherwise.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (WDRY2020‐K078).
ACKNOWLEDGMENTS
This work was supported by the Special Science and Technology Cooperation Project of Ningxia Hui Autonomous Region Key R&D Program (2018BFG02008), Special Novel Coronary Pneumonia Epidemic Prevention and Control Project of Ningxia Hui Autonomous Region (2020BEG01004), and Wuhan University College of Medicine Teaching Research Project (2019013).
Wu Q, Zheng H, Gu J, et al. Detection of folate receptor‐positive circulating tumor cells as a biomarker for diagnosis, prognostication, and therapeutic monitoring in breast cancer. J Clin Lab Anal.2022;36:e24180. doi: 10.1002/jcla.24180
Qian Wu, Hongyun Zheng and Jian Gu contributed equally to this work.
Contributor Information
Hongyun Zheng, Email: shenzheng2008@163.com.
Anyu Bao, Email: baoanyu2008@163.com.
Yongqing Tong, Email: tytsing@whu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to the privacy of research participants but are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Waks AG, Winer EP. Breast cancer treatment. JAMA. 2019;321:316. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 3. Suppan C, Brcic I, Tiran V, et al. Untargeted assessment of tumor fractions in plasma for monitoring and prognostication from metastatic breast cancer patients undergoing systemic treatment. Cancers. 2019;11(8):1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31:1827‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bidard F‐C, Proudhon C, Pierga J‐Y. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10:418‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller MC, Doyle GV, Terstappen LWMM. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao Y, Fan W‐H, Duan C, et al. Enhancing the screening efficiency of breast cancer by combining conventional medical imaging examinations with circulating tumor cells. Front Oncol. 2021;11:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bankó P, Lee SY, Nagygyörgy V, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol. 2019;12(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen C, Ke J, Zhou XE, et al. Structural basis for molecular recognition of folic acid by folate receptors. Nature. 2013;500:486‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He W, Wang H, Hartmann LC, et al. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci USA. 2007;104:11760‐11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Z, Wang J, Tacha DE, et al. Folate receptor α associated with triple‐negative breast cancer and poor prognosis. Arch Pathol Lab Med. 2013;138:890‐895. [DOI] [PubMed] [Google Scholar]
- 12. Edge SB, Byrd DR. AJCC cancer staging manual.
- 13. Chen X, Zhou F, Li X, et al. Folate receptor–positive circulating tumor cell detected by LT‐PCR–based method as a diagnostic biomarker for non–small‐cell lung cancer. J Thorac Oncol. 2015;10:1163‐1171. [DOI] [PubMed] [Google Scholar]
- 14. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust. 1869;14:146. [Google Scholar]
- 15. Shen Z, Wu A, Chen X. Current detection technologies for circulating tumor cells. Chem Soc Rev. 2017;46:2038‐2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Shannessy DJ, Somers EB, Maltzman J, et al. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus. 2012;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Shannessy DJ, Somers EB, Smale R, et al. Expression of folate receptor‐α (FRA) in gynecologic malignancies and its relationship to the tumor type. Int J Gynecol Pathol. 2013;32:258‐268. [DOI] [PubMed] [Google Scholar]
- 18. O'Shannessy DJ, Somers EB, Maltzman J, et al. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus. 2012;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17:89‐95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to the privacy of research participants but are available from the corresponding author upon reasonable request.


