Abstract
Objective
Most patients infected with the novel coronavirus (SARS‐CoV‐2), as the causative agent of COVID‐19 disease, show mild symptoms, but some of them develop severe illness. The purpose of this study was to analyze the blood markers of COVID‐19 patients and to investigate the correlation between serum inflammatory cytokines and the disease severity.
Methods
In this prospective cross‐sectional study, 50 patients with COVID‐19 and 20 patients without COVID‐19 were enrolled. According to ICU admission criteria, patients were divided into two groups of non‐severe and severe. Differences in the serum levels of C‐reactive protein (CRP), IL‐6, and TNF‐α, as well as erythrocyte sedimentation rate (ESR), lymphocytes (LYM) count, and neutrophils (NEU) count between the two groups were determined and analyzed.
Results
Out of the 50 patients with COVID‐19, 14 were diagnosed as severe cases. There was no significant difference between the two groups of COVID‐19 patients in terms of gender and age. Blood tests of COVID‐19 patients showed a significant decrease and increase in NEU and LYM counts, respectively. There were significant differences in the serum levels of IL‐6, TNF‐α, and CRP between the severe and non‐severe groups, which were higher in the severe group.
Also, there was a significant correlation between the disease severity and CRP with ESR (r = 0.79), CRP with IL‐6 (r = 0.74), LYM with NEU (r = −0.97), and ESR with TNF‐α (r = 0.7).
Conclusion
The findings of this study, as the first study in Iran, suggest that the levels of IL‐6, TNF‐α, ESR, and CRP could be used to predict the severity of COVID‐19 disease.
Keywords: COVID‐19, CRP, cytokines, IL‐6, SARS‐CoV‐2, TNF‐α
Serum inflammatory parameters were analyzed in patients with severe and non‐severe COVID‐19. The serum levels of LYM, CRP, IL‐6, TNF‐α and ESR in severe patients were significantly higher than in non‐severe patients. Measurement of inflammatory markers might help clinicians monitor and evaluate the severity and prognosis of COVID‐19.
1. INTRODUCTION
The novel coronavirus (SARS‐CoV‐2), as the causative agent of COVID‐19 disease (coronavirus disease 2019), was first isolated on January 7, 2020, by the Chinese authorities. 1 The disease rapidly spread throughout the world and was declared as a public health emergency of international concern by the World Health Organization (WHO) on January 30, 2020. 2 Generally, patients with critical and severe COVID‐19 are treated in the intensive care unit (ICU), while patients with non‐severe disease are hospitalized in a usual isolation room. 1
Studies have shown that clinical hematology parameters, such as complete blood count (CBC), play an important role in the early diagnosis of acute pulmonary diseases in patients in the triage stage during outbreaks. 2 In addition, C‐reactive protein (CRP) level could be used in the early pneumonia diagnosis 3 because it increases in 75%–93% of COVID‐19 cases and therefore could be used along with other biomarkers such as lymphocyte count to assess lung lesions and the severity of COVID‐19 disease. 4
Another parameter is ESR which is widely used as an inexpensive and available test in routine laboratory patient workup, regardless of clinical questions. 5 Several cytokine species are widely used as imaging biomarkers to describe the immune system function, predict diseases, and monitor their evolution and progression. 6
Different types of pro‐inflammatory cytokines (IL‐1, IL‐2, IL‐6, IL‐8, and TNF‐α), produced primarily by activated macrophages, are involved in upregulating inflammatory immune responses. 7 Like in other viral infections, NF‐μB activation via the MyD88 pathway plays a major role in the progression of COVID‐19 infection through stimulation of several pro‐inflammatory cytokines, including interleukin‐6 (IL‐6) and tumor necrosis factor alpha (TNFα). 8 TNF‐α is a pleiotropic cytokine which is produced by activated macrophages and monocytes and regulates a variety of physiological and pathological processes. 9 The expression of IL‐6 and TNF‐α increases and decreases during the process of illness and recovery. 10
In COVID‐19 patients with severe symptoms and poor prognosis, IL‐6, and TNF‐α rapidly increase, while in patients with milder symptoms, these cytokines are reduced to lower levels. 11 Moreover, some reports describing the immunological profile of COVID‐19 patients have suggested that hyper‐activation of the humoral immune system, including the secretion of interleukin (IL)‐6, is a critical mediator for respiratory failure and multi‐organ dysfunction. 12
During the COVID‐19 pandemic, different countries have investigated and applied various approaches to the diagnosis and prognosis of COVID‐19 patients. According to previous studies on viral pneumonia as well as current clinical experience with severe COVID‐19, the storm of inflammatory factors may be the main reason for rapid disease progression and poor treatment response. 13 , 14 Therefore, in this study, serum inflammatory parameters were analyzed in patients with severe and non‐severe COVID‐19 to explore potential markers that allow to precisely monitor the disease progression.
2. STUDY DESIGN AND SETTING
2.1. Participants
COVID‐19 disease was diagnosed in patients based on the WHO interim guideline as described previously. In Loghman hospital in Tehran (Iran), a total of 50 patients who were diagnosed with COVID‐19 disease from March 26 to April 22/ 2020 were enrolled in this study. In addition, 20 patients without COVID‐19 disease were also enrolled as control. COVID‐19 patients were divided into two groups based on their disease severity, including the severe and non‐severe groups. The study protocol was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
2.2. Data gathering
Patients’ demographic data were gathered, including age, gender, and clinical data. Blood samples were taken from each participant, and the following blood tests were conducted on the samples, including lymphocyte count (LYM), neutrophil count (NEU), and erythrocyte sedimentation rate (ESR). In addition, C‐reactive protein (CRP) was measured using CRP immunoturbidimetric PARS Azmon kit and a HITACH 7600–020 automatic biochemical analyzer.
2.3. Determination of serum TNF and IL‐6 level
Anti‐TNF‐α and IL‐6 drug are option of possible therapeutic strategy for COVID‐19 patients. None of the patients used these drugs. Serum TNF‐α and IL‐6 levels in blood samples were calculated by ELISA kits (eBioscience) according to the manufacturer's instructions. The limit of detection of TNF‐α and IL‐6 was 5 and 4 pg/ml.
2.4. Statistical analysis
The statistical analysis was carried out using Graphpad software Version 8. Using chi‐square test, categorical variables were analyzed. Data with a normal distribution were summarized as mean ± SD and analyzed by ANOVA test. Using Spearman's rank correlation coefficient, the relationship between variables was calculated. A p value of <0.05 was considered as statistically significant.
3. RESULTS
3.1. Characteristics of the admitted cases
A total of 50 COVID‐19 positive (case group) and 20 COVID‐19 negative cases (control group) with a mean age of 42.7 ± 12.4 years (range: 19–78) were included in this study. The highest proportion of patients belonged to the age group of 30–49 years (40.2%). Also, 40 (57.1%) cases were male, and the rest were female. COVID‐19 positive cases included 14 (28%) patients with severe disease, admitted in ICU, and 36 (73%) patients with non‐severe disease, admitted in the emergency department.
3.2. Laboratory parameters
Table 1 compares patients’ laboratory parameters between negative and positive COVID‐19 cases. The serum levels of IL‐6, TNF‐α, and CRP in positive patients were significantly higher than in the negative cases.
TABLE 1.
Gender and serum inflammatory parameters in patients with and without COVID‐19 disease
| Characteristic | Control*, N = 201 | Case, N = 50¶ | p‐Value* |
|---|---|---|---|
| Severity | 0.8 | ||
| Emergency | 16 (80%) | 36 (73%) | |
| ICU | 4 (20%) | 14 (28%) | |
| TNF | 23 (20, 27) | 66 (45, 82) | <0.001 |
| ESR | 8 (7, 10) | 36 (20, 46) | <0.001 |
| IL−6 | 12 (10, 13) | 20 (15, 27) | <0.001 |
| CRP | 4.25 (4.47, 5.30) | 8.70 (6.75, 9.70) | <0.001 |
| NEU | 55 (49, 57) | 60 (57, 66) | <0.001 |
| LYN | 44 (41, 51) | 37 (31, 41) | <0.001 |
| Sex | >0.9 | ||
| Female | 9 (45%) | 21 (42%) | |
| Male | 11 (55%) | 29 (58%) | |
| Age | 46 (40, 51) | 40 (31, 52) | 0.3 |
Table 2 compares patients’ laboratory parameters between non‐severe and severe COVID‐19 patients. There was no significant difference in terms of gender and age between the two groups. Laboratory studies showed that 4 (28.6%) severe and 4 (5%) non‐severe cases had neutropenia, and 9 (64.3%) severe and 24 (29%) non‐severe cases had lymphopenia. There was no significant difference in the mean number of WBC and LYM between severe and non‐severe COVID‐19 patients (Table 2). In 11 (78.6%) severe and 16 (19%) non‐severe cases, the erythrocyte sedimentation rate (ESR) was high, whereas the level of ESR was significantly higher in severe patients than in non‐severe patients.
TABLE 2.
Gender and serum inflammatory parameters in patients with severe and non‐severe COVID‐19
| Characteristic | Positive Covid−19 (Case) |
Control N = 20¶ |
p‐value* | |
|---|---|---|---|---|
| Emergency, N = 36¶ | ICU, N = 14¶ | |||
| TNF | 44 (25, 76) | 54 (28, 64) | 23 (20, 27) | <0.001 |
| ESR | 20 (9, 42) | 31 (14, 40) | 8 (7, 10) | <0.001 |
| IL−6 | 15 (11, 24) | 18 (13, 26) | 12 (10, 13) | 0.002 |
| CRP | 6.80 (5.05, 8.90) | 8.90 (6.70, 9.70) | 4.25 (4.47, 5.30) | 0.12 |
| NEU | 60 (52, 64) | 59 (57, 61) | 55 (49, 57) | 0.9 |
| LYN | 39 (32, 44) | 40 (35, 41) | 44 (41, 51) | 0.7 |
| Sex | 0.4 | |||
| Female | 16 (44%) | 6 (52.9%) | 9 (45%) | |
| Male | 20 (56%) | 8 (57.1%) | 11 (55%) | |
| Age | 44 (36, 53) | 38 (29, 47) | 46 (40, 51) | 0.2 |
¶Statistics presented: n (%); Median (IQR).
*Statistical tests performed: Fisher's exact test; ANOVA test; chi‐square test of independence.
aData were expressed as ± SD for quantitative measures and also both number and percentage.
bComparison using chi‐square test. Intensive Care Unit(ICU), neutrophils (NEU), lymphocyte (LYM), C‐reactive protein (CRP), ESR (Erythrocyte sedimentation rate), and TNF‐α (tumor necrosis factor alpha).
In addition, IL‐6 levels were also found to be lower than 7.4 pg/ml in non‐severe patients and higher than 8.2 pg/ml in severe patients. The serum levels of IL‐6, TNF‐α, and ESR in severe patients were significantly higher than in non‐severe patients (Table 2).
The results of the correlation analysis between the disease severity and serum laboratory parameters are shown in Figure 1. According to the results, the correlation between IL‐6, TNFα, and CRP levels along with other factors and the disease severity was significant. There was a significant correlation between the disease severity and CRP with ESR (r = 0.79), CRP with IL‐6 (r = 0.74), LYM with NEU (r = −0.97), and ESR with TNF‐α (r = 0.7).
FIGURE 1.
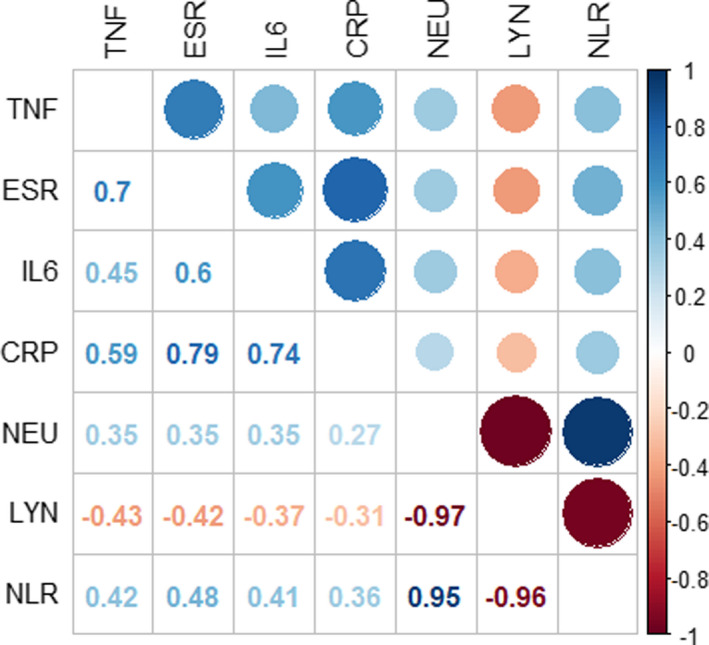
Correlation analysis between the serum laboratory parameters with severity of disease
4. DISCUSSION
COVID‐19 disease is rapidly expanding worldwide. Most infected patients have mild symptoms and a good prognosis, but some of them develop severe illness that sometimes could lead to death. To date, there is no an effective therapy for COVID‐19. 15 Therefore, it is essential to identify diagnostic markers that allow to precisely monitor the disease progression because effective early interventions are essential measures for reducing mortality. There is a great deal of evidence that suggests inflammatory responses play a critical role in the progression of COVID‐19, 16 and several markers have the potential to be used to accurately trace and predict COVID‐19 disease severity and fatality. 17 , 18 Several studies have shown increased levels of cytokines in the serum of COVID‐19 patients. 19 , 20 Also, the effectiveness of anti‐inflammatory agents in COVID‐19 therapy highlights the critical role of inflammation in the progression of COVID‐19. 21 , 22 , 23
In the present study, inflammatory markers, especially CRP, TNF‐α, and IL‐6, were found to be positively correlated with the severity of COVID‐19 by comparing these markers between the severe and non‐severe groups. Different inflammatory markers such as CRP and IL‐6 have been reported to be significantly associated with an increased risk of developing severe COVID‐19. 24 However, these results remain controversial because some other studies have reported no significant difference in the serum levels of IL‐6, ESR, and CRP between the two groups, 25 and the role of inflammatory parameters in monitoring COVID‐19 progression is still unclear. Similar to a study by Huang et al., 26 in this study, IL‐6 and TNFα levels were found to be associated with the severity of COVID‐19. Higher serum levels of pro‐inflammatory cytokines (TNF‐α and IL‐6) were found in patients with severe COVID‐19 compared to those with non‐severe disease, and these results are consistent with the results of other studies on SARS and MERS. 27 Cytokines and chemokines are believed to play an important role in immunity and immunopathology during viral infections. 27 Although there is no direct evidence for the involvement of inflammatory cytokines and chemokines in the lung pathology during COVID‐19, changes in laboratory parameters including high serum levels of cytokines and chemokines in infected patients have been reported to be associated with the disease severity and adverse outcomes, highlighting the potential function of hyper‐inflammatory reactions in COVID‐19 progression. 4 , 28
CRP is a systemic acute‐phase response marker for inflammation, infection, and tissue damage, which could be used as an inflammation indicator. 29 Previous studies have indicated that CRP levels could be used to diagnose COVID‐19 patients and predict COVID‐19 infection outcomes. 4 , 30
Unlike Gao et al., 31 in the present study, there was no statistically significant difference in CRP levels between the non‐severe and severe groups; however, CRP levels were higher in the severe group than in the non‐severe group. Also, other studies, such as Ling 3 and Chen et al. (2020), 32 have reported that CRP level is positively linked to COVID‐19 severity. ESR is another inflammatory marker, which mainly reflects changes in a variety of plasma proteins. 33 In this study, higher ESR levels were found in the severe group than in the non‐severe group, which could be attributed to the presence of more inflammation in patients with severe disease.
To the best of our knowledge, this is the first study on the association of inflammatory markers and the severity of COVID‐19 in Iran. Inflammatory markers, especially CRP, IL‐6, and TNF‐α, were significantly correlated with the severity of COVID‐19. Measurement of inflammatory markers might help clinicians monitor and evaluate the severity and prognosis of COVID‐19. Other than studies of the of cytokine as diagnostic potential, option of possible therapeutic strategy targeting either IL‐6 or IL‐10 or both is likely to emerge through analysis of such data.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Mardani R, namavar M, ghorbi E, et al. Association between serum inflammatory parameters and the disease severity in COVID‐19 patients. J Clin Lab Anal.2022;36:e24162. doi: 10.1002/jcla.24162
Contributor Information
Nayebali Ahmadi, Email: NAYEBALIA@yahoo.com.
Seyed Dawood Mousavi‐Nasab, Email: d.mosavinasab@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545‐1546. [DOI] [PubMed] [Google Scholar]
- 2. Frater JL, Zini G, d’Onofrio G, Rogers HJ. COVID‐19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42(S1):11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ling W. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50(4):332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID‐2019 infection. Clin Chem Lab Med. 2020;58(7):1131‐1134. [DOI] [PubMed] [Google Scholar]
- 5. Lapić I, Rogić D, Plebani M. Erythrocyte sedimentation rate is associated with severe coronavirus disease 2019 (COVID‐19): a pooled analysis. Clin Chem Lab Med. 2020;58(7):1146‐1148. [DOI] [PubMed] [Google Scholar]
- 6. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W, Ye L, Ye L, et al. Up‐regulation of IL‐6 and TNF‐α induced by SARS‐coronavirus spike protein in murine macrophages via the NF‐κB pathway. Virus Res. 2007;128(1‐2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parameswaran N, Patial S. Tumor necrosis factor‐α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choy E, Rose‐John S. Interleukin‐6 as a multifunctional regulator: inflammation, immune response, and fibrosis. J Scleroderma Relat Disord. 2017;2(2_Suppl):S1‐S5. [Google Scholar]
- 11. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368(6490):473‐474. [DOI] [PubMed] [Google Scholar]
- 12. Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: a meta‐analysis. J Med Virol. 2020;92(11):2283‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID‐19: pathogenesis, cytokine storm, and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;19:66‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chakraborty I, Maity P. COVID‐19 outbreak: migration, effects on society, global environment, and prevention. Sci Total Environ. 2020;728:138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jordan RE, Adab P, Cheng K. Covid‐19: risk factors for severe disease and death. BMJ. 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Sun W, Li J, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv. 2020. 10.1101/2020.02.17.20024166 [DOI] [Google Scholar]
- 21. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): The experience of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGonagle D, Sharif K, O'Regan A, Bridgewood C. Interleukin‐6 use in COVID‐19 pneumonia‐related macrophage activation syndrome. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID‐19: a meta‐analysis. Int J Infect Dis. 2020;96:467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng F, Guo Y, Yin M, Chen X, Deng G. Association of inflammatory markers with the severity of COVID‐19. medRxiv. 2020;96(05):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Channappanavar R, Perlman S, editors. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng R, Li C, Li N, Wei L, Cui Y. The role of cytokines and chemokines in severe respiratory syncytial virus infection and subsequent asthma. Cytokine. 2011;53(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 29. Mardani R, Vasmehjani AA, Zali F, et al. Laboratory parameters in detection of COVID‐19 patients with positive RT‐PCR; a diagnostic accuracy study. Arch Acad Emerg Med. 2020;8(1):e43. [PMC free article] [PubMed] [Google Scholar]
- 30. Pepys MB, Hirschfield GM. C‐reactive protein: a critical update. J Clin Investig. 2003;111(12):1805‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol. 2020;92(7):791‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Liu H, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):203‐208. Chinese Journal of Tuberculosis and Respiratory Diseases. [DOI] [PubMed] [Google Scholar]
- 33. Tan C, Huang Y, Shi F, et al. C‐reactive protein correlates with computed tomographic findings and predicts severe COVID‐19 early. J Med Virol. 2020;92(7):856‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


