Abstract
Background
Following traumatic brain injury (TBI) there is an increased prevalence of depression compared to the general population. It is unknown whether non‐pharmacological interventions for depression are effective for people with TBI.
Objectives
To investigate the effectiveness of non‐pharmacological interventions for depression in adults and children with TBI at reducing the diagnosis and severity of symptoms of depression.
Search methods
We ran the most recent search on 11 February 2015. We searched the Cochrane Injuries Group Specialised Register, The Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), three other databases and clinical trials registers. Relevant conference proceedings and journals were handsearched, as were the reference lists of identified studies.
Selection criteria
Randomised controlled trials (RCTs) of non‐pharmacological interventions for depression in adults and children who had a TBI.
Data collection and analysis
Two authors independently selected trials from the search results, then assessed risk of bias and extracted data from the included trials. The authors contacted trial investigators to obtain missing information. We rated the overall quality of the evidence of the primary outcomes using the GRADE approach.
Main results
Six studies met the inclusion criteria, with a total of 334 adult participants. We identified no studies that included children as participants. All studies were affected by high risk of bias due to a lack of blinding of participants and personnel; five studies were affected by high risk of bias for lack of blinding of outcome assessors. There was high or unclear risk of biases affecting some studies across all the Cochrane risk of bias measures.
Three studies compared a psychological intervention (either cognitive behaviour therapy or mindfulness‐based cognitive therapy) with a control intervention. Data regarding depression symptom outcome measures were combined in a meta‐analysis, but did not find an effect in favour of treatment (SMD ‐0.14; 95% CI ‐0.47 to 0.19; Z = 0.83; P = 0.41). The other comparisons comprised of single studies of depression symptoms and compared; cognitive behaviour therapy versus supportive psychotherapy (SMD ‐0.09; 95% CI ‐0.65 to 0.48; Z = 0.30; P = 0.77); repetitive transcranial magnetic stimulation plus tricyclic antidepressant (rTMS + TCA) versus tricyclic antidepressant alone (SMD ‐0.84; 95% CI ‐1.36 to ‐0.32; Z = 3;19, P = 0.001); and a supervised exercise program versus exercise as usual (SMD ‐0.43; 95% CI ‐0.88 to 0.03; Z = 1.84; P = 0.07). There was very‐low quality evidence, small effect sizes and wide variability of results, suggesting that no comparisons showed a reliable effect for any intervention.
Only one study mentioned minor, transient adverse events from repetitive transcranial magnetic stimulation.
Authors' conclusions
The review did not find compelling evidence in favour of any intervention. Future studies should focus on participants with a diagnosed TBI and include only participants who have a diagnosis of depression, or who record scores above a clinical cutoff on a depression measure. There is a need for additional RCTs that include a comparison between an intervention and a control that replicates the effect of the attention given to participants during an active treatment.
Plain language summary
Non‐drug treatments for depression in children and adults who have had a traumatic brain injury
Review question
We reviewed the evidence about the effect of non‐drug treatments for depression after traumatic brain injury (TBI), to determine whether these treatments are better than no intervention, or better than drug‐based treatments, at reducing the symptoms or diagnosis of depression. We searched for evidence about the relative effectiveness of different types of treatments, and whether the treatments had any harmful or negative effects.
Background
Depression is more common in people who have had a TBI. Depression increases the risk of suicide and is a factor that limits recovery from TBI. There are many non‐drug treatments for depression. This review aimed to determine the effects of non‐drug interventions for people with TBI.
Search date
The review authors searched for randomised studies that had been published up to February 2015.
Study characteristics
We found six studies, with a total of 334 adult participants. We found no studies that included people younger than 18 years of age. Four studies investigated psychological interventions. One study investigated an exercise intervention, and another investigated repetitive transcranial magnetic stimulation (rTMS).
Key results
Three studies compared a psychological therapy (cognitive behaviour therapy or mindfulness‐based cognitive therapy) with a no‐treatment control intervention. When the data for these studies were combined, there was no reliable effect in support of psychological therapy. One study compared cognitive behavioural therapy with another psychological intervention (supportive psychotherapy), and did not find an effect in favour of either intervention. One study compared a supervised exercise programme with exercise as usual, but did not find a effect in favour of either intervention. One study compared rTMS plus an antidepressant medication with the antidepressant medication alone. Because the quality of the evidence was very low, it was not possible to draw the conclusion that the addition of rTMS improved outcomes. Only one study, of rTMS, reported any harmful effects and these were relatively minor and resolved quickly.
Quality of the evidence
The quality of the evidence was rated very low. All studies were at high risk of bias in some ways, and therefore it was not possible to draw conclusions in support of any intervention. There was a high degree of variability in the main results, which meant we could have little confidence in the findings. Some studies had major methodological flaws.
Conclusions
It is not possible to recommend any particular treatment based on the current evidence. The review authors have made some recommendations to improve the quality of the evidence in future studies.
Summary of findings
Background
Description of the condition
Major depression is defined by at least one episode of either depressed mood or loss of interest and pleasure in usual activities (or both) consistently for at least a two‐week period. During depressive episodes there can be a loss of appetite, weight (or both), insomnia, psychomotor agitation or retardation, low energy, fatigue (or both), feelings of worthlessness, inappropriate guilt (or both), difficulty concentrating, indecisiveness, and in more severe cases, persistent thoughts of death or suicide. Depression can affect children, adolescents, and adults, and can be associated with somatic complaints, psychotic symptoms, such as delusions, or both (APA 2000). In addition, depressive symptoms, such as depressed mood or poor motivation, may co‐occur with other mental conditions (e.g. adjustment disorder), or may be present in the absence of a diagnosable condition (NICE 2009).
Traumatic brain injury (TBI) is a heterogenous condition that can affect people of any age. The common factor in all presentations is that damage to the brain occurs because of external forces, such as direct impact, rapid acceleration or deceleration, a penetrating injury, or blast waves from an explosion. These external forces can vary greatly along parameters of intensity, location, direction, and duration and determine the nature of the injury (Maas 2008). The immediate impact of the trauma leads to a disruption in the neurological function of the brain in any of the following ways: i) loss of consciousness, ii) loss of memory for events immediately before or after the injury, iii) a change in mental state at the time of the injury, or iv) permanent or transient focal neurological deficits (Kay 1993).
Traumatic brain injury is associated with a combination of temporary or permanent changes in cognitive abilities, emotional regulation, and behavioural control (Maas 2008). Traumatic brain injury can vary in severity and is classified as mild, moderate, severe, or extremely severe. It can also result in physical impairments and functional disabilities.
Following TBI, there is an increased occurrence of depression compared with the general population. Bombadier 2010 found that 53.1% of a hospital sample met the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) diagnostic criteria for major depressive disorder in a 12‐month period after suffering TBI. This is in contrast to a general population survey which found that the 12‐month prevalence of all mood disorders was 6.2% (Slade 2009).
In a prospective study, it was found that the prevalence of moderate to severe symptoms of depression ranged from 31% at one month, to 17% at three to five years post‐injury (Dikmen 2004). There was little relationship between brain injury severity and symptoms of depression. When people with TBI were rated by their relatives, a similar frequency of depression was found (Ciurli 2011). Compared with the general population, there is an increased risk of emotional disorders In children and adolescents following TBI, with a recent study finding that half of a sample of eight‐ to 15‐year olds presented with symptoms of an internalising disorder, and that as a group, they displayed elevated scores on ratings of anxiety, depression, and social withdrawal (Poggi 2005).
Depression is a relevant condition to investigate because it represents a significant risk factor for mortality through suicide. Simpson 2002 found that in a community sample of brain injured outpatients in Australia, 18% had made a suicide attempt since their injury, and 35% had clinically significant levels of suicidality. Furthermore, Simpson 2002 found that post‐injury factors had greater significance than pre‐injury emotional disturbance (including previous suicide attempts) in predicting suicidality post‐injury, so it was changes associated with TBI that had led to increased suicide risk.
Description of the intervention
Interventions for depression can be pharmacological, non‐pharmacological, or a combination (NICE 2009). Because there is already a Cochrane review in preparation which focuses on pharmacological interventions (Vattakatuchery 2013), this review will focus on non‐pharmacological interventions. These are predominantly psychological interventions, but also include medical, physical, or other interventions. Psychological interventions include those that are behavioural, cognitive, or a combination (cognitive‐behavioural therapy (CBT)). There are extensions of CBT which are referred to as 'third‐wave' interventions; these include mindfulness, acceptance, and commitment therapy (ACT), and dialectical behaviour therapy (DBT). There are also the separate schools of humanistic, interpersonal, and psychodynamic psychotherapies.
Non‐pharmacological medical interventions include electro‐convulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), neurosurgical interventions, and biofeedback. Physical interventions include exercise programmes and other physical activation strategies. There are also complementary and alternative medicine (CAM) interventions, which include the administration of herbal supplements, traditional Chinese medicine, homeopathy, acupuncture, and other interventions.
How the intervention might work
Non‐pharmacological interventions might work in a variety of ways, which reflect the heterogeneity of the interventions.
Psychological interventions, such as CBT, might work by training people with depression in strategies to manage their symptoms, such as learning to identify and challenge patterns of negative thinking. Psychological interventions may work in the TBI population similarly to the non‐brain injured population and other clinical groups that have cognitive impairments or reduced ability to concentrate, remember or solve problems, such as children, people with intellectual disabilities, or people with other types of acquired brain injuries such as stroke.
Medical interventions, such as TMS, might work by exciting or inhibiting cortical areas of the brain in order to manipulate mood. Physical interventions, such as exercise programmes, might work because of various reasons, for example, depression is often associated with inactivity, and exercise helps to increase activity levels and self‐efficacy, and distract from negative thoughts. If successful, these treatments reduce the severity of depression symptoms and the rate of diagnosis of a major depressive disorder.
For the non‐brain injured population, there is varying evidence in support of non‐pharmacological interventions for depression. There is a series of Cochrane reviews that have either been recently published, or are in the protocol stage, that examine the effectiveness of specific psychological interventions in comparison with 'treatments as usual', or examine the relative effectiveness of treatments in comparison with other treatments. As an example, Churchill 2013 examined 'third wave' cognitive and behavioural therapies versus treatment as usual for depression, and found that these treatments were effective on a short‐term basis, albeit there was insignificant evidence to state whether these treatments were any more or less effective than other psychological therapies (Hunot 2013). The same group has evaluated behavioural therapies and found that they were as effective as other treatments, albeit with a lack of high‐quality evidence (Shinohara 2013). The same group has completed a Cochrane review that compared the effectiveness of psychological therapies versus antidepressant medication, alone and in combination, for depression in children and adolescents; however, there were no clear findings, suggesting that either mode of therapy, or a combination of both, is preferable (Cox 2012). And finally, the comparison between behavioural therapies and treatment as usual by the same team, is in the protocol stage (Caldwell 2010). Other reviews by the same group that are in the protocol stage relate to: cognitive‐behavioural therapies (Churchill 2010a; Hunot 2010), humanistic therapies (Churchill 2010b; Davies 2010), interpersonal, cognitive‐analytic, and other integrative therapies (Churchill 2010c; Hunot 2010a), and psychodynamic therapies (Churchill 2010e; Moore 2010).
Aside from psychological interventions, other modes of intervention examined by previous Cochrane reviews show that there is a lack of evidence in support of acupuncture (Smith 2010), or transcranial magnetic stimulation (Rodriguez‐Martin 2001), and moderate support for light therapy (Tuunainen 2004), music therapy (Maratos 2008), and relaxation (Jorm 2008). A recent Cochrane review found a small effect in support of physical exercise interventions when compared with a no‐treatment control, and no significant difference between psychological or pharmacological interventions and physical exercise in treating depression (Cooney 2013). Leiknes 2011 is currently investigating the benefits and harms of electroconvulsive therapy (ECT) for depression.
For children and adolescents, two previous Cochrane reviews found some evidence that indicated limited support for family therapy (Henken 2007), and exercise (Larun 2006), in the prevention and treatment of depression .
Why it is important to do this review
As discussed above, the TBI population has a higher prevalence of depression in comparison with the general population (e.g. Deb 1999). Depression and anxiety might be factors that limit recovery from TBI (Whitnall 2006). Depression is one of the risk factors for increased risk of suicide after TBI (Simpson 2002).
Although depression is a significant problem following TBI, it is unknown whether non‐pharmacological interventions are effective in the TBI population. In particular, people with TBI often have impairments of cognition, behavioural or emotional control, which affect the suitability of interventions that were developed for non‐brain injured populations.
This review sought to determine the effectiveness of non‐pharmacological interventions for depression when applied to the TBI population. Where interventions are successful, it is important to understand how these interventions were applied and what modifications were necessary for this population with cognitive impairments.
Objectives
-
To determine whether non‐pharmacological interventions (either with or without combined pharmacological interventions) for depression following TBI in adults and children are superior to:
no intervention;
pharmacological intervention alone.
To compare the effectiveness of different types of non‐pharmacological interventions for depression following TBI in adults and children.
To investigate the occurrence of adverse effects as a consequence of non‐pharmacological interventions in order to assist practitioners in identifying appropriate interventions.
To describe how interventions were adapted and modified to suit this population.
Methods
Criteria for considering studies for this review
Types of studies
This review was restricted to randomised controlled trials (RCTs).
Types of participants
We included studies of adults or children (or both) who had a TBI and were diagnosed with a depressive condition, or had clinically significant depressive symptoms.
For the purposes of this review, we searched for studies of participants with a history of TBI who had brain damage due to external forces, such as direct impact, either rapid acceleration or deceleration, a penetrating injury, or blast waves from an explosion. We included studies with mixed samples of participants (such as people with non‐traumatically acquired brain injuries) if there were data available which allowed separate analysis of participants with TBI.
For the purposes of this review, we searched for studies of participants with depression who either:
fulfilled the diagnostic criteria for an applicable mood disorder as stated by a well‐established diagnostic system such as the DSM‐IV‐TR (APA 2000), or the International Classification of Diseases (ICD‐10; WHO 1992). The applicable diagnoses were major depressive episode, major depressive disorder, dysthymic disorder, mood disorder due to a general medical condition with depressive features, or adjustment disorder with depressed mood; or
presented with clinically significant depressive symptoms as indicated by subjective report (self‐ or other‐rated) or by observational methods, using standardised measures.
We included studies with participants who had co‐morbid psychological conditions, such as anxiety disorders or substance abuse disorders, but we excluded studies with participants with bipolar disorders.
Types of interventions
We included any form of intervention which was non‐pharmacological, which aimed to reduce depressive symptoms or resolve the presence of a diagnosable depressive disorder. Interventions might have been psychological, physical or medical (e.g. electro‐convulsive therapy). We had planned to compare the types of interventions against each other, against no intervention, or against other control interventions, such as placebo, usual care, or a control group receiving comparable attention to the intervention group.
There were no restrictions on duration or frequency of intervention. We included studies that focused on the presence of depressive disorders or the symptoms of depression. We included studies where participants were concurrently prescribed medications that may have affected depressive symptoms, such as antidepressants or stimulants, provided that medication was not the sole intervention.
Types of outcome measures
Primary outcomes
Our primary outcome was:
the presence or remission of depressive disorders, as determined by the use of accepted diagnostic criteria (e.g. DSM‐IV or ICD‐10), by the use of a standardised structured interview based on such criteria (e.g. Structured Clinical Interview for the DSM Disorders), or the results of validated self‐ or observer‐rated questionnaires of depressive symptoms.
Secondary outcomes
Where information was available, secondary outcome measures included:
neuropsychological functioning, psychosocial adjustment, everyday functioning, quality of life, and participation;
medication usage, healthcare service usage;
treatment compliance, as indicated by the proportion of withdrawals from intervention;
the occurrence of suicide or self harm; or
any adverse effects of the intervention.
The information size required to reliably detect a treatment effect was calculated using a power analysis for a single RCT. The analysis was based on the assumption the RCT would report a continuous outcome; the measure chosen as a representative outcome measure was the Hamilton Scale for Depression (HAM‐D; Hamilton 1960). A four‐point change on the HAM‐D was regarded as clinically significant. We calculated the sample size for a single RCT with 90% power at the 5% significance level as 38 people per group, or 76 in total for a treatment versus control RCT.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date, or publication status.
Electronic searches
The Cochrane Injuries Group Trials Search Co‐ordinator searched the following:
Cochrane Injuries Group Specialised Register (February 2015);
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2015, issue 1);
Database of Abstract of Reviews of Effects (DARE; The Cochrane Library 2015, issue 1);
MEDLINE (OvidSP; 1946 to February 2015);
Embase (OvidSP; 1974 to February 2015);
CINAHL Plus (EBSCO; 1937 to February 2015);
PsycINFO (OvidSP; 1806 to February 2015);
PsycBITE (OvidSP; 1806 to May 2012).
Search strategies are listed in Appendix 1.
Searching other resources
The authors searched the following online trials registers to February 2015:
Current controlled trials (www.controlled‐trials.com);
Clinicaltrials.gov (www.clinicaltrials.gov);
Trials Central (www.trialscentral.org).
We checked reference lists of included studies and previously published reviews for additional material. We also contacted authors and experts in the field to identify additional studies.
We handsearched the following journals and conference proceedings: Brain Injury (1992 to February 2015); Brain Impairment (2000 to February 2015); Archives of Physical Medicine and Rehabilitation (1992 to February 2015); Neuropsychological Rehabilitation (1992 to February 2015); the Journal of Affective Disorders (1992 to February 2015); and the World Federation for Neuro‐Rehabilitation Congress proceedings (2000 to February 2015).
Data collection and analysis
We collated the search results using EndNote bibliographic software and removed duplicates before two review authors began the screening process.
Selection of studies
Two review authors (PG and RT) independently inspected all citations identified by the search. They assessed the titles and abstracts to determine whether each article met the predetermined criteria. Where there was inadequate information contained in the abstract and title, they inspected the full article.
They obtained and independently assessed the identified articles to determine whether they met the review criteria. Inter‐rater reliability for the study selection was kappa = 0.93 (percent agreement = 99.6%), which reflects 'excellent' agreement (Higgins 2011). On studies where there was disagreement, they held discussions to reach a consensus. They tracked identified studies using an electronic reference management system (EndNote).
When we found articles in languages other than English, we arranged translation of the paper to assess the eligibility, rate the quality, and extract the data for the trial (where necessary).
Data extraction and management
We used a specific data extraction form for this review. Two review authors independently extracted data from identified trials and compared the results. When there was doubt or disagreement, they held discussions to reach a consensus. Where there was information missing from a trial, we contacted the original investigators.
Assessment of risk of bias in included studies
Two authors (PG and RT) independently assessed the studies for methodological quality using the Cochrane 'Risk of bias' tool, which examines bias in studies using the following criteria (Higgins 2011).
Random sequence generation: was the method used to generate allocation adequate to ensure randomisation?
Allocation concealment: was allocation to groups adequately concealed in order to prevent prediction of allocation?
Blinding of participants and personnel: were the participants and personnel delivering the intervention aware of the intervention group to which participants were allocated?
Blinding of outcome assessment: were outcome assessors aware of the group to which the participants had been allocated?
Incomplete outcome data: were sufficient data available to draw reliable and meaningful conclusions?
Selective reporting: were the reports of the study free of bias in the way in which results were reported?
Other sources of bias: were there any other apparent sources of bias?
For each study selected, they provided detailed text and graphic description of the risk of bias, and provided an interpretation based on available information on whether the study was of low, high or unclear risk of bias for each criterion. Where there was disagreement in judgements of bias, they discussed this and reached a consensus. Where information was unavailable to make a judgement, we contacted the study authors and sought further information.
Measures of treatment effect
Continuous data
In studies where the outcome measures related to the severity of depressive symptoms, we expected that these would be continuous outcomes. We calculated the standardised mean difference (SMD) and the 95% confidence interval (CI) for continuous data where comparable measurement scales were used (e.g. Beck Depression Inventory, Hospital Anxiety and Depression Scale, etc.).
Dichotomous data
In studies where the outcome measures related to the participants' diagnostic status, we expected dichotomous outcomes. We had planned to analyse these outcomes by calculating the risk ratio (RR), which allows for easier communication of treatment effect and is more consistent across clinical populations than other measures of treatment effect.
Unit of analysis issues
We found substantial heterogeneity in the nature of the studies included. The possibilities we anticipated were: multiple intervention groups, the use of alternative designs, such as cross‐over studies, repeated observation of participants in the case of long‐term follow‐up, and variability in the dependent measures used.
Multiple intervention groups
We had planned to combine groups to allow pair‐wise comparison of groups, as recommended by Higgins 2011. If this was not possible, we had planned to select one pair of interventions that were comparable with other selected studies and exclude other interventions.
Cross‐over studies
Cross‐over studies can be confounded by carry‐over effects in the group receiving the intervention first. In studies where this was apparent, we only included data from the first intervention period.
If the results from the experimental and control interventions approximated those of parallel studies, we had planned to analyse the data as if they were pair‐wise comparisons. While this method of analysis is not ideal, Higgins 2011 indicates that this is likely to lead to a lower weighting of these studies in meta‐analysis, due to wider confidence intervals.
Dealing with missing data
Where possible, we attempted to identify where data were missing and ascertain the missing values. We searched for registered protocols of selected studies and then contacted the original investigators to determine whether all data had been published.
Assessment of heterogeneity
It was anticipated that there would be heterogeneity due to differences in participant characteristics, clinical outcome measures, or the range of interventions for depression, including psychological, physical and non‐pharmacological medical interventions, as well as sub‐types within these categories. We assessed the selected trials for the type of intervention used, and grouped trials accordingly. We had planned to assess heterogeneity using the visual inspection method and the I² statistic. According to section 9.5.2 of the Cochrane Handbook of Systematic Reviews of Intervention s, the I² statistic can be classified as representing either moderate (30% to 60%), substantial (50% to 90%) or considerable (75% to 100%) heterogeneity (Higgins 2011). For the purpose of this review, we did not pool the data if the I² statistic was greater than 75%.
Assessment of reporting biases
There was a risk of reporting bias because not all studies would necessarily be published in sources that were easily identifiable (Higgins 2011). By searching a broad range of sources, including multiple databases, trials registries, and grey literature, the authors attempted to reduce this risk. When we identified registered trials that had not yet been published, we contacted the investigators to seek further information and data. If sufficient trials had been identified, we had planned to undertake a funnel plot analysis to predict the likelihood of unpublished studies, and the impact this could have on the findings of meta‐analyses.
Data synthesis
If multiple trials were identified that were clinically homogenous (for example, all psychological interventions), in which outcomes had been measured in similar ways, and for which data were available, we had planned to perform meta‐analyses using the inverse‐variance method. The inverse‐variance method can be applied to either dichotomous or continuous data.
Subgroup analysis and investigation of heterogeneity
If there had been a sufficient number of studies available, we had planned to perform the following subgroup analyses:
injury severity (mild versus moderate‐to‐severe TBI);
age group;
time post‐injury (acute versus long‐term);
categories of intervention (for example, psychological versus physical or medical) and sub‐types of interventions (for example, behavioural therapy versus psychodynamic therapy); and
baseline severity of depression.
We had planned to apply a random‐effects model, because it was expected that the included studies would use a variety of intervention delivery methods, which were expected to have variable treatment effects.
Sensitivity analysis
We had expected that the included studies would vary in their methodological quality and risks of bias. If there had been sufficient studies, we had planned to repeat the meta‐analyses, excluding studies which had a high or unclear risk of bias for allocation concealment.
Results
Description of studies
Results of the search
The most recent search was run on 11 February 2015; the search process is displayed in Figure 1. Two authors (PG and RT) individually searched the titles and abstracts of all of these records and identified 28 articles that warranted further investigation. Twenty‐five of these were excluded, leaving three studies that were eligible for inclusion in the review. In addition, one author (PG) conducted a handsearch of five specified journals and proceedings of one conference (the conference proceedings for another could not be located). The handsearch involved review of the titles of 14,073 articles and further investigation of the abstracts where the title appeared relevant. Aside from studies already identified in the database search, the handsearch did not identify any further studies for investigation.
1.
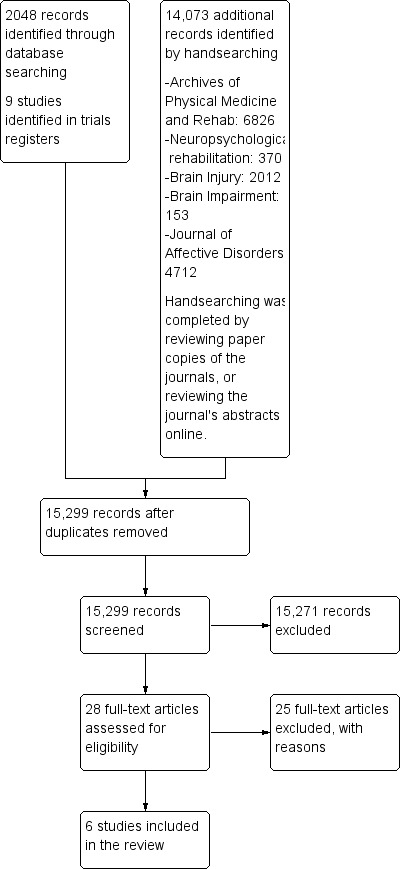
Study flow diagram.
One author (PG) also conducted a search of trials registry databases, which yielded six studies for further investigation. Of these, three were excluded and three RCTs fulfilled the inclusion criteria (Ashman 2014; Bedard 2013; Fann 2015). In addition, four relevant studies are in progress, and are described in the table of Ongoing studies.
Included studies
The included studies examined the following comparisons:
Cognitive behavioural therapy (CBT), or a variant of CBT, versus a waiting list control (Bedard 2013; Fann 2014; Simpson 2011)
CBT versus supportive psychotherapy (SPT; Ashman 2014)
Repetitve transcranial magnetic stimulation (rTMS) combined with oral tricyclic anti‐depressant (TCA) medication versus oral TCA alone (He 2004)
Supervised exercise program versus exercise as usual (Hoffman 2010)
Of the six studies that were included, one was conducted in China (He 2004), three in the USA (Ashman 2014; Fann 2015; Hoffman 2010), one in Canada (Bedard 2013), and one in Australia (Simpson 2011). All of the included studies investigated intervention effects in adults. None of the included studies related to people under the age of 18 years.
This study compared two popular modes of psychological therapy: CBT and supportive psychotherapy (SPT). Participants engaged in up to 16 therapy sessions on a twice‐weekly or weekly basis over a three‐month period. Seventy‐seven participants were allocated to treatment and 43 participants completed the study. Participants who dropped out before the intervention tended to have lower educational attainment and lower income. At baseline, all participants met the inclusion criteria for depression, either by diagnosis or clinical cutoff on a self‐report measure (BDI‐II score of 20 or higher). All participants had a confirmed history of TBI. The mean age was 47 for both groups, with an average time since injury of 7.8 years for the CBT group and 13.2 for the SPT group. There were more women than men in both groups (CBT group 64% female and SPT group 54% female). The primary outcome measure was diagnosis of depression as measured by the Structured Clinical Interview for the DSM‐IV (SCID). There are some missing data for some outcomes and so the number of included participants is different for each outcome measure.
This study examined the benefit of mindfulness‐based cognitive therapy (MBCT) in comparison with wait‐list control. All participants met the criteria for depressive symptoms (BDI‐II score of 16 or higher) and were engaged in a multi‐centre trial of weekly group therapy over a 10‐week period. All participants had a history of TBI. One hundred and five participants were allocated to an intervention. While assignment was randomised, there were five participants who were allocated to the intervention in order to increase participation at one of the treatment centres. Of the 105 participants randomised, 76 completed the study. The MBCT intervention group had an average age of 47.1 and was 50% female, while the average age of the wait‐list control group was 46.8 and was 40% female. The primary outcome measure was the Beck Depression Inventory (BDI‐II). There are some missing data for some outcomes and so the number of included participants is different for each outcome measure.
This study compared CBT delivered either in person, by telephone, or usual care. Participants were recruited at multiple sites and were included if they had a documented history of TBI, a confirmed diagnosis of major depressive disorder (MDD) on the SCID, and symptom severity was above the clinical cutoff on the Patient Health Questionnaire (PHQ‐9). Choice‐stratification randomisation gave participants two sets of options to which they could be randomly allocated: the in‐person intervention (CBT‐IP) or usual care, or the telephone intervention (CBT‐T) or usual care. In this way, the authors were able to ensure random allocation and also provide a treatment intervention that suited each participant. One hundred participants were allocated to either CBT‐IP (N = 18), CBT‐T (N = 40), or usual care (UC, N = 42). The CBT intervention was based on a protocol specifically designed for delivery by telephone over eight weeks. This program was expanded to 12 weeks and adapted for the TBI population by presenting material in smaller portions, more slowly and with greater repetition. In many instances, support people were involved in the treatment sessions. The mean age was 45.4 for the CBT groups and 46.3 for UC. Forty‐one percent of the CBT groups and 31% of the usual care groups were female. Mean number of years since injury was 2.84 for the CBT groups and 2.58 for UC. The primary outcome measures were the clinician‐administered Hamilton Depression scale (HAM‐D; Hamilton 1960), and the self‐administered Symptom Checklist‐20 (SCL‐20).
This study examined the effect of a non‐pharmacological, medical intervention (rTMS) in addition to a pharmacological intervention (TCA). Study participants had a TBI that was confirmed through CT or MRI scans and were included in the study when their score on the HAM‐D was eight or higher. Sixty‐four patients from a hospital neurosurgery and rehabilitation department met the inclusion criteria. Thirty‐two people (15 female) were allocated to the intervention group (rTMS plus TCA) and 32 people (15 female) were allocated to the control group (TCA alone); one control group participant was lost to follow up. The intervention group underwent rTMS on 10 days over a 12‐day period. The mean (SD) age for the intervention group was 37.2 (9.98) years, and 37.4 (10.6) years for the control group. Primary outcome measures were the HAM‐D, the Mini‐Mental State Examination (MMSE), and plasma monoamine neurotransmitter concentrations, specifically 5‐hydroxytryptamine (5‐HT) and noradrenaline (NA).
This study examined the benefit of a supervised exercise program to improve mood following TBI. Participants were recruited from the practices of medical and allied health professionals, and the local media. In order to be included, participants must have had a history of TBI of at least six months, and not more than five years prior to enrolment, and scored five or more on the Patient Health Questionnaire‐9 (PHQ‐9). This study excluded people with active suicidal ideation.
Over a 10‐week period, the intervention group underwent a weekly exercise session with a personal trainer plus a home‐based exercise program that participants were encouraged to complete four times a week. The control group was instructed to exercise as normal and were followed up at the conclusion of 10 weeks. Forty people were allocated to the intervention (25 female) and 40 were allocated to the control intervention (20 female), with 39 completing the intervention and 37 completing the control interventions. The mean age of the intervention group was 39.7 years; the mean age of the control group was 37.1. The primary outcome measure was the score on the Beck Depression Inventory (BDI‐II).
This study examined an intervention specifically for suicide prevention. After consultation with the primary author, it was determined that the study sample consisted of people with depression following TBI, who had presented with the symptom of suicidal ideation or a history of suicide attempts. The study included patients recruited from a hospital‐based brain injury community outreach program with TBI, who scored in the moderate or severe range on the Beck Hopelessness Scale (BHS), presented with suicidal ideation, or both. As such, the study met the inclusion criteria by specifying a cutoff on a clinical measure of depression. Subjects were randomised to either an active intervention (N = 8; male/female ratio unknown), or a wait‐list control group (N = 9). The intervention was 10 weekly two‐hour CBT groups for the treatment of hopelessness, and was structured according to a treatment manual entitled 'Window to Hope'. The mean (SD) age of participants was 39.4 (12.4) years for the intervention group and 44.1 (11.7) years for the control group. The mean time (SD) post‐injury was 6.3 (6.8) years for the intervention group and 7.6 (4.6) years for the control group. The median duration of post‐traumatic amnesia (PTA) was 10 days for the intervention group and 21 days for the control group.
The primary outcome measure was the Beck Hopelessness Scale (BHS). Secondary outcomes measures were the Beck Scale for Suicidal Ideation (BSS), the Hospital Anxiety and Depression Scale (HADS), the Herth Hope Index, the Rosenberg Self‐Esteem Scale and the Social Problem‐Solving Inventory‐Revised (SPSI‐R).
Excluded studies
Twenty‐five studies were identified but excluded for at least one of the following reasons: the inclusion criteria did not specify either a diagnosis of depression or a clinical cutoff on a depression scale (21 studies); the intervention was not for depression (12 studies); the sample included people with non‐traumatic brain injuries, participants with TBI could not be clearly identified from the published article and it was not feasible to contact the authors about extracting individual data for people with TBI because the studies were conducted a long time ago (six studies); the intervention was found to be pharmacological (one study); and the study was not a RCT (one study).
Most excluded studies reported intervention outcomes for adults; two studies reported treatment outcomes for children (Wade 2006), or adolescents (Wade 2008).
Risk of bias in included studies
The included studies were assessed using the Cochrane 'Risk of Bias' tool, according to chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data were extracted from the included studies in order to classify low, high or unclear risk for the following criteria; allocation sequence was randomised, allocation to groups was concealed, blinding of participants and personnel, blinding of outcome assessment, attrition of participants to final outcome collection, selective reporting of outcomes and other potential biases. A summary of the risk of bias is described in Figure 2 and Figure 3.
2.
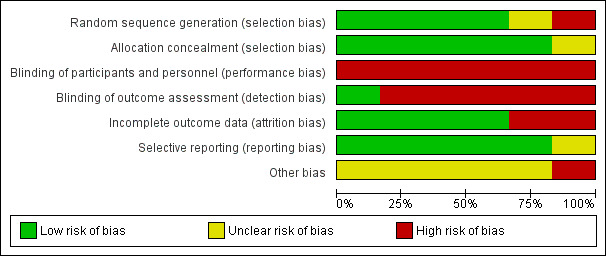
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Six studies are included in this review.
3.
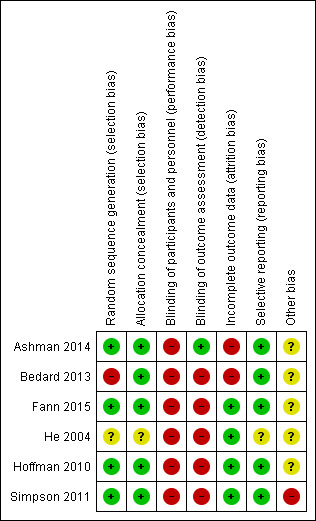
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Selection biases may affect the way in which participants are allocated to groups and may lead to systematic variances in the nature of the participant groups. Selection biases relate to the sequence in which participants were allocated to groups (sequence generation) and also the awareness of the group that participants may be allocated to (allocation concealment). Some studies are not truly random because they may employ a non‐random selection sequence (such as, allocation by month of birth) which introduces the possibility of bias in the study findings. Where participants or personnel might be aware of the allocation sequence this might influence participants' inclusion in the study.
In He 2004, the risk of bias for random allocation was unclear. The allocation sequence was determined before allocation to groups, however there is insufficient detail to determine how the allocation sequence was determined and whether a truly random sequence was generated. In Ashman 2014, Simpson 2011 and Hoffman 2010 there was low risk of selection bias as the authors employed a computer generated sequence determined prior to allocation. Fann 2015 employed choice‐stratified randomisation, which was assessed as low risk of bias.
For Bedard 2013, randomisation was conducted by a statistician who was independent of the clinicians and site investigators. The statistician used a minimisation procedure to ensure balance at baseline between the groups on a key outcome measure (BDI‐II). These measures point to a low risk of selection bias. However, five participants at one site were allocated to the intervention intervention because there were low participant numbers at that site rather than being randomly allocated to intervention; therefore, the study was reclassified at a high risk of bias on this criterion.
Blinding
Blinding refers to the processes that the study authors implemented in order to prevent participants finding out to which intervention they had been allocated (performance bias) and to prevent personnel conducting outcome assessments from detecting to which intervention participants had been allocated (detection bias).
Five studies demonstrated high risk of performance bias (Bedard 2013; Fann 2015; He 2004; Hoffman 2010; Simpson 2011). This was because in each study the intervention was compared with a control that involved little or no intervention. In these studies, the intervention required subjects to attend for a specific treatment, whereas control participants were instructed to continue on with their lives as usual.
In Ashman 2014, there was less risk of performance bias since participants from each intervention received a similar level of clinician attention. However, it was not possible for the personnel providing the intervention to be blind to the intervention, and there is also the risk that if participants from each intervention were to compare their treatment they would find them to be distinct, therefore this was also assessed as high risk of bias.
Only one study demonstrated low risk of detection bias, since the primary outcome measure was a diagnostic assessment conducted by an independent clinician (Ashman 2014). In four other studies (Fann 2015; He 2004; Hoffman 2010; Simpson 2011), there was an attempt to minimise detection bias by using different personnel to conduct the outcome assessments. In Simpson 2011, participants were requested not to disclose their group allocation to the outcome assessor. Nevertheless, all studies except Ashman 2014 relied upon primary outcome measures which were either self‐report scales or had a heavy component of self‐report (such as the HAM‐D in Fann 2015) and as such must be considered at high risk of bias.
Incomplete outcome data
Attrition bias refers to the potential confounding influence of substantial dropout from the study. Often this is because of systemic issues within the study, such as a particularly demanding treatment intervention.
Four studies were rated as low risk for attrition bias as there was minimal dropout (Fann 2015; He 2004; Hoffman 2010; Simpson 2011). For these four studies, of the 261 participants randomised, outcome data were collected on 241 (92%). Two studies were rated as high risk for attrition bias due to substantial dropout (Ashman 2014; Bedard 2013).
Selective reporting
Selective reporting refers to bias that can be introduced when the study authors fail to report all the outcomes that they intended to collect. This is more often true of findings that are not statistically significant. In order to be classified as low risk on this criterion there must be an a priori study protocol available (Higgins 2011). He 2004 was classified as unclear risk due to a lack of information that could identify a priori the outcome measures (e.g. a protocol for the study that pre‐dated the publication). The other five studies were classified as low risk. For four studies, there were registered trial protocols available which indicated that the primary outcome measures that were planned were in fact used (Ashman 2014; Bedard 2013; Fann 2015; Simpson 2011). In the case of Hoffman 2010, personal communication with the authors confirmed that all outcomes were reported in the final publication.
Other potential sources of bias
A potential source of bias affecting Simpson 2011 is the small sample size of N = 17 (intervention group, N = 8 and control group, N = 9). The baseline characteristics of the groups were not significantly different according to statistical tests, however, there was a clinically meaningful difference between the groups relating to the mean duration of post‐traumatic amnesia (intervention group, PTA = 10 days and control group, PTA = 21 days), which is a key clinical indicator of the severity of TBI. The authors reported that the data pertaining to PTA and time since injury were not normally distributed between the groups and this could have biased the findings.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. CBT compared to wait‐list control for post‐TBI depression.
| CBT compared to wait‐list control for post‐TBI depression | ||||||
| Patient or population: Post‐TBI depression Settings: Community setting Intervention: CBT Comparison: Wait‐list control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| wait‐list control | CBT | |||||
| Depression scales (BDI‐II, HAM‐D and HADS); higher score means more depressed | The mean depression score in the control groups was 15.364 | The mean depression score in the intervention groups was 0.14 standard deviation lower (0.47 lower to 0.19 higher) | SMD ‐0.14 (‐0.47 to 0.19) | 146 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Of these three studies, there is variability in the quality of the evidence as it relates to risks of bias. Bedard 2013 had serious risk of bias as it related to random sequence generation (selection bias) and incomplete outcome data (attrition bias). Simpson 2011 suffered from other risks of bias due to a very small sample size. All three studies (including Fann 2015) were subject to biases that are virtually unavoidable when attempting an RCT on this topic. All studies suffered from lack of blinding as it relates to participants and personnel (performance bias) and blinding of outcome assessment (detection bias).
2Small effect sizes. Two studies slightly favour CBT (Bedard 2013; Fann 2015). One study slightly favours control (Simpson 2011).
3The 95% confidence interval of the outcome is very broad and ranges from a moderate effect in favour of CBT to a small effect against CBT.
4 The assumed risk was calculated by adding the means of the scores of the control groups and dividing by the number of studies in the analysis.
Summary of findings 2. CBT compared to Supportive Psychotherapy for Post‐TBI Depression.
| CBT compared to Supportive Psychotherapy for Post‐TBI Depression | ||||||
| Patient or population: Post‐TBI Depression Settings: Community setting Intervention: CBT Comparison: Supportive Psychotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Supportive Psychotherapy | CBT | |||||
| Beck Depression Inventory (BDI); higher score means more depressed | The mean BDI score in the control group was 20.43 | The mean BDI in the intervention group was 0.09 standard deviations lower (0.65 lower to 0.48 higher) | SMD ‐0.09 (‐0.65 to 0.48) | 48 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Very high dropout rate (attrition bias). As with other studies in this field, blinding of participants and personnel was not achieved (performance bias).
2Very wide 95% confidence interval.
3The assumed risk is the mean score of the control group.
Summary of findings 3. Repetitive transcranial magnetic stimulation (rTMS) compared to rTMS plus Tricyclic Anti‐depressant for Post‐TBI Depression.
| Repetitive transcranial magnetic stimulation (rTMS) compared to rTMS plus Tricyclic Anti‐depressant for Post‐TBI Depression | ||||||
| Patient or population: Post‐TBI Depression Settings: People receiving care through a hospital neurology department (not specified whether in‐patient or out‐patient) Intervention: Repetitive transcranial magnetic stimulation (rTMS) Comparison: rTMS plus Tricyclic Antidepressant | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| rTMS plus Tricyclic Anti‐depressant | Repetitive transcranial magnetic stimulation (rTMS) | |||||
| Hamilton Rating Scale for Depression (HAM‐D); higher score means more depressed | The mean HAM‐D score in the control group was 6.3 | The mean HAM‐D in the intervention group was 0.84 standard deviations lower (1.36 lower to 0.32 lower) | SMD ‐0.84 (‐1.36 to ‐0.32) | 63 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1High or unclear risk relating to selection, performance, detection, reporting and other biases.
2Very wide 95% confidence interval.
3The assumed risk is the mean score of the control group.
Summary of findings 4. Supervised exercised compared to Exercise as usual for Post‐TBI Depression.
| Supervised exercised compared to Exercise as usual for Post‐TBI Depression | ||||||
| Patient or population: Post‐TBI Depression Settings: Community setting Intervention: Supervised exercises Comparison: Exercise as usual | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise as usual | Supervised exercised | |||||
| Beck Depression Inventory (BDI); higher score means more depression | The mean BDI score in the control group was 16.4.3 | The mean BDI in the intervention group was 0.43 standard deviations lower (0.88 lower to 0.03 higher) | SMD ‐0.43 (‐0.88 to 0.03) | 77 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Study subject to risk of biases consistent with the highest quality studies in this population. High risk of bias relates to lack of blinding of participants and personnel (performance bias) and lack of blinding of outcome assessors (detection bias).
2Very wide 95% confidence interval.
3The assumed risk is the mean score of the control group.
Comparison one: cognitive‐behavioural therapy (CBT) or variant of CBT versus waiting list²
1.1 Depression diagnosis (ITT analysis)
One study (100 participants) compared CBT with waiting list for the outcome depression diagnosis (Fann 2015). The intention‐to‐treat (ITT) analysis included 58 CBT participants and 42 controls, with a depression diagnosis of 34% for CBT versus 52% for controls (RR 0.66; 95% CI 0.42 to 1.04; Z = 1.79; P = 0.07; Analysis 1.1) at the end of the intervention period. After the eight‐week follow‐up period, depression diagnosis was 40% for CBT versus 45% for controls (RR 0.88; 95% CI 0.55 to 1.39; Z = ‐0.56; P = 0.58; Analysis 1.2).
1.1. Analysis.

Comparison 1 CBT versus control, Outcome 1 Major depressive disorder (MDD) on the structured clinical interview for depression (SCID) scale.
1.2. Analysis.
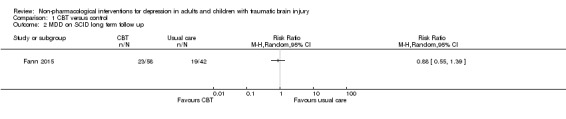
Comparison 1 CBT versus control, Outcome 2 MDD on SCID long term follow up.
1.2 Reduction in depression symptoms
Three studies (146 participants) compared CBT, or a variant of CBT, with a no‐treatment control and were combined in a meta‐analysis in which the most commonly used depression measure was chosen as the outcome (BDI‐II, HAM‐D and HADS depression scales; Bedard 2013; Fann 2015; Simpson 2011). The I² statistic was applied and demonstrated minimal statistical heterogeneity (I² = 0%; Chi² = 1.56; df = 2; P = 0.46), which confirmed the appropriateness of performing a meta‐analysis (Analysis 1.3). The standardised mean difference (SMD) was ‐0.14 (95% CI ‐0.47 to 0.19; Z = 0.83; P = 0.41), indicating no difference was attributable to the intervention when outcomes were measured at the end of the interventions. The quality of the evidence was very‐low, indicating that we are uncertain this estimate represents a true treatment effect. The studies also reported long‐term follow‐up data, collected at either two or three months after completion of the intervention; the SMD was ‐0.02 (95% CI ‐0.33 to 0.29; Z = 0.12; P = 0.91; Analysis 1.4), indicating no effect of treatment.
1.3. Analysis.
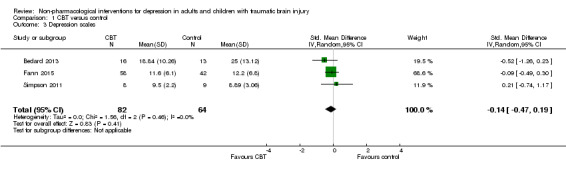
Comparison 1 CBT versus control, Outcome 3 Depression scales.
1.4. Analysis.
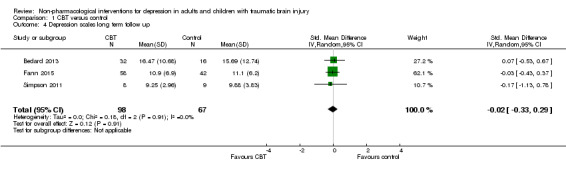
Comparison 1 CBT versus control, Outcome 4 Depression scales long term follow up.
1.3 Secondary outcomes
All studies that compared CBT or a variant of CBT with a waiting list assessed outcomes with additional depression measures. Two studies used a version of the Symptom Checklist (SCL) as a secondary measure of depression symptoms; these studies were combined for meta‐analysis (Bedard 2013; Fann 2015; N = 175). There was minimal heterogeneity (I² = 0%; Chi² = 0.01; df = 1; P = 0.90), with no difference between CBT and waiting list groups. The SMD was ‐0.15 (95% CI ‐0.45 to 0.15; Z = 1.0; P = 0.32; Analysis 1.5). In a separate analysis, Fann 2015 found that participants who completed at least eight of 12 CBT sessions had improved SCL‐20 scores when compared with the control group at the end of treatment (treatment effect 0.43; 95% CI 0.10 to 0.76; P = 0.011). This study conducted follow‐up eight weeks after the completion of the intervention, and found that the benefit did not continue (no effect on the SCL‐20; SMD 0.01; 95% CI ‐0.38 to 0.41; Z = 0.06; P = 0.95; Analysis 1.6).
1.5. Analysis.
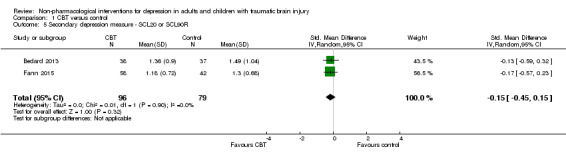
Comparison 1 CBT versus control, Outcome 5 Secondary depression measure ‐ SCL20 or SCL90R.
1.6. Analysis.
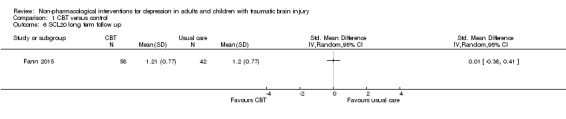
Comparison 1 CBT versus control, Outcome 6 SCL20 long term follow up.
Fann 2015 also analysed outcomes for secondary measures of depression. These included the inventories of symptom improvement, as measured by the Patient Global Impression (PGI), and satisfaction with depression care. There was a difference on the PGI, with more participants in the CBT group rating their depression symptoms as 'much or very improved' (RR 0.67; 95% CI 0.47 to 0.96; Z = 2.18; P = 0.03; Analysis 1.7), but this was not maintained at long‐term follow‐up (RR 0.75; 95% CI 0.54 to 1.05; Z = 1.68; P = 0.09; Analysis 1.8). Similarly, at the end of treatment, there was a statistically significant difference on a Likert rating scale of satisfaction, with CBT participants three times more likely to report that they were 'moderately or very satisfied' with their depression care than participants assigned to usual care (RR 0.35; 95% CI 0.22 to 0.55; Z = 4.60; P < 0.0001; Analysis 1.9).
1.7. Analysis.

Comparison 1 CBT versus control, Outcome 7 Secondary depression measure ‐ PGI.
1.8. Analysis.

Comparison 1 CBT versus control, Outcome 8 PGI long term follow up.
1.9. Analysis.
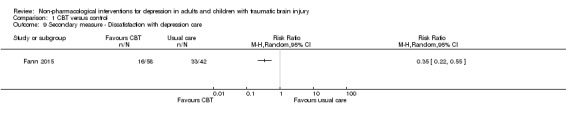
Comparison 1 CBT versus control, Outcome 9 Secondary measure ‐ Dissatisfaction with depression care.
Bedard 2013 used the Patient Health Questionnaire (PHQ‐9) as a secondary measure of depression. There was no difference on outcome between participants receiving Mindfulness‐based CBT and those on the waiting list (SMD ‐0.41; 95% CI ‐0.87 to 0.05; Z = 1.76; P = 0.08; Analysis 1.10).
1.10. Analysis.

Comparison 1 CBT versus control, Outcome 10 Secondary depression measure ‐ PHQ.
Simpson 2011 measured hopelessness, suicidality and self‐esteem at the end of treatment. There was a difference of one point on the Beck Hopelessness Scale (BHS), SMD ‐1.04 (95% CI ‐2.07 to ‐0.01; Z = 1.98; P = 0.05; Analysis 1.11). There was no difference between treatment groups on the Beck Scale for Suicidal Ideation (BSS), SMD ‐0.49 (95% CI ‐1.46 to 0.48; Z = 0.98; P = 0.33; Analysis 1.12). There was no difference between treatment groups on the Rosenberg Self‐Esteem Scale (SMD 0.00; 95% CI ‐0.95 to 0.95; Z = 0.00; P = 1.0; Analysis 1.13).
1.11. Analysis.
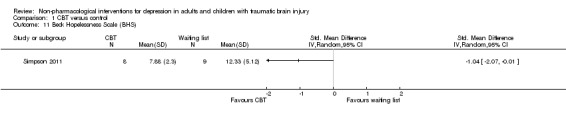
Comparison 1 CBT versus control, Outcome 11 Beck Hopelessness Scale (BHS).
1.12. Analysis.
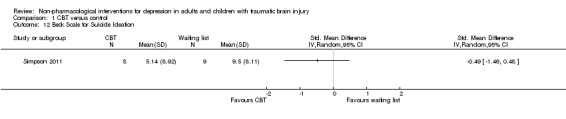
Comparison 1 CBT versus control, Outcome 12 Beck Scale for Suicide Ideation.
1.13. Analysis.
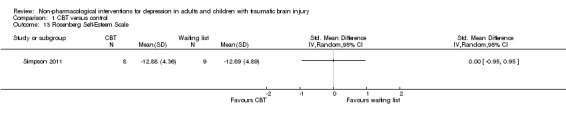
Comparison 1 CBT versus control, Outcome 13 Rosenberg Self‐Esteem Scale.
1.4 Treatment compliance, withdrawals from study (dropouts)
One hundred and twenty‐three people were allocated to a CBT or variant intervention and 98 completed the study (79%). Ninety‐nine people were allocated to a waiting‐list control group and 83 completed outcome measures (84%). This was subjected to an ITT analysis which demonstrated low heterogeneity (I² = 35%; Chi² = 1.55; df = 1; P = 0.21). There was no difference in withdrawals from the study between the CBT and waiting list groups (RR 1.20; 95% CI 0.57 to 2.54; Z = 0.49; P = 0.63; Analysis 1.14).
1.14. Analysis.
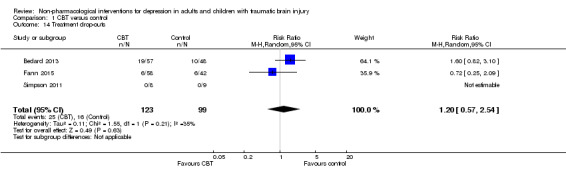
Comparison 1 CBT versus control, Outcome 14 Treatment drop‐outs.
1.5 Any adverse effects
No adverse effects were reported.
Comparison two: CBT versus Supportive Psychotherapy (SPT)
The only study of this comparison was Ashman 2014.
2.1 Depression diagnosis (ITT analysis)
Ashman 2014 found that following the intervention, 64% of the CBT group and 84% of the SPT group still had a diagnosis of major depressive disorder; the difference in remission was not statistically significant (RR 0.76; 95% CI 0.58 to 1.00; Z = 1.96; P = 0.05; Analysis 2.1).
2.1. Analysis.

Comparison 2 CBT versus SPT, Outcome 1 MDD present on SCID following intervention.
2.2 Reduction in depression symptoms
There was no difference between treatment groups in BDI‐II score (SMD ‐0.09; 95% CI ‐0.65 to 0.48; Z = 0.30; P = 0.77; Analysis 2.2). The combined‐groups sample demonstrated a modest mean reduction in BDI‐II score regardless of group allocation (F (1, 47) = 9.63, p = 0.003). The quality of the evidence was very‐low, indicating that we are uncertain this estimate represents the true treatment effect.
2.2. Analysis.
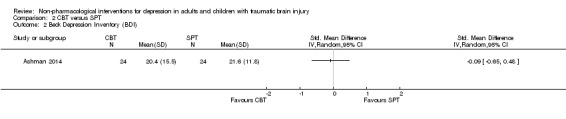
Comparison 2 CBT versus SPT, Outcome 2 Beck Depression Inventory (BDI).
2.3 Secondary outcomes
There was no difference in the Life 3 Quality of Life inventory between participants who received CBT or SPT (SMD ‐0.06; 95% CI ‐0.52 to 0.39; Z = 0.27; P = 0.78; Analysis 2.3).
2.3. Analysis.
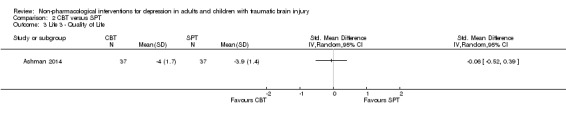
Comparison 2 CBT versus SPT, Outcome 3 Life 3 ‐ Quality of Life.
2.4 Treatment compliance, withdrawals from study (dropouts)
Seventy‐seven participants were allocated to treatment by Ashman 2014 but only 43 participants completed a treatment. There was no difference in treatment completion between CBT and SPT (RR 0.97; 95% CI 0.59 to 1.61; Z = ‐0.10; P = 0.92; Analysis 2.4).
2.4. Analysis.
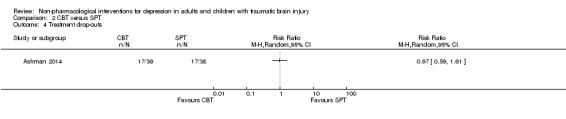
Comparison 2 CBT versus SPT, Outcome 4 Treatment drop‐outs.
2.5 Any adverse effects
No adverse effects were reported.
Comparison three: repetitive transcranial magnetic stimulation (rTMS) plus tricyclic antidepressant (TCA) versus TCA alone
The only study of this comparison was He 2004.
3.1 Remission of depression diagnosis (ITT analysis)
ITT analysis was not reported.
3.2 Reduction in depression symptoms
He 2004 compared the effect of rTMS plus TCA to TCA alone. The main outcome measure was the Hamilton Depression scale (HAM‐D). A four‐point change on the HAM‐D is regarded as clinically significant. There was a clinically irrelevant difference in favour of rTMS plus TCA (SMD ‐0.84; 95% CI ‐1.36 to ‐0.32; Z = 3.19; P = 0.001; Analysis 3.1). The quality of the evidence was very‐low, indicating that we are uncertain this estimate represents the true treatment effect.
3.1. Analysis.

Comparison 3 Transcranial magnetic stimulation plus TCA versus TCA alone, Outcome 1 Hamilton Rating Scale for Depression (HAM‐D).
3.3 Secondary outcomes
He 2004 included the Mini Mental State Exam (MMSE) score as a secondary outcome measure and found a statistically significant change in favour of the rTMS plus TCA intervention, but the change was not clinically relevant (SMD ‐0.99; 95% CI ‐1.51 to ‐0.46; Z = 3.69; P = 0.0002; Analysis 3.1). A change of at least 1.5 points on the MMSE is considered clinically significant.
He 2004 included serotonin levels as a secondary outcome measure and found no difference between groups (SMD ‐0.19; 95% CI ‐0.68 to 0.31; Z = 0.75; P = 0.45; Analysis 3.3). Another secondary outcome measure was noradrenaline levels, which were slightly higher in the rTMS plus TCA group (SMD 1.31; 95% CI 0.76 to 1.86; Z = 4.69; P < 0.0001; Analysis 3.4).
3.3. Analysis.
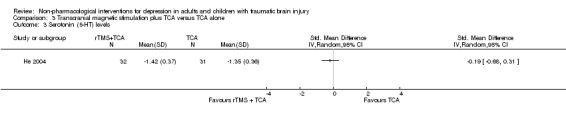
Comparison 3 Transcranial magnetic stimulation plus TCA versus TCA alone, Outcome 3 Serotonin (5‐HT) levels.
3.4. Analysis.
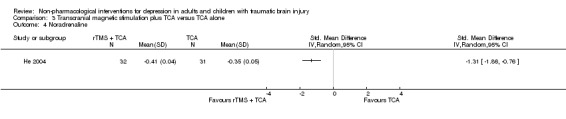
Comparison 3 Transcranial magnetic stimulation plus TCA versus TCA alone, Outcome 4 Noradrenaline.
3.4 Treatment compliance, withdrawals from study (dropouts)
Sixty‐four participants were enrolled in He 2004. There were no withdrawals from the intervention group and only one participant withdrew from the control group (RR 0.33; 95% CI 0.01 to 7.89; Z = ‐0.68; P = 0.49; Analysis 3.5).
3.5. Analysis.
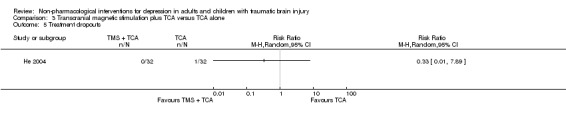
Comparison 3 Transcranial magnetic stimulation plus TCA versus TCA alone, Outcome 5 Treatment dropouts.
3.5 Adverse effects
Two participants reported transient tinnitus, but this did not affect participation and in each case there was spontaneous remission.
Comparison four: supervised exercise versus exercise as usual
There was one study of this comparison (Hoffman 2010).
4.1 Remission of depression diagnosis (ITT analysis)
Diagnostic status was not examined.
4.2 Reduction in depression symptoms
The primary outcome measure in Hoffman 2010 was the Beck Depression Inventory (BDI). There was no difference on the BDI score between groups (SMD ‐0.43; 95% CI ‐0.88 to 0.03; Z = 1.84; P = 0.07; Analysis 4.1). Hoffman 2010 noted that the groups were not equivalent at baseline for the main outcome measure. The quality of the evidence was rated as moderate, and it is likely that further research would have an impact on our confidence in the estimate.
4.1. Analysis.
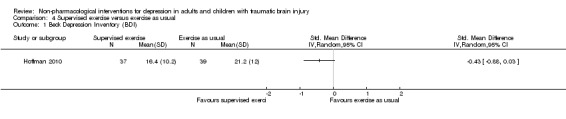
Comparison 4 Supervised exercise versus exercise as usual, Outcome 1 Beck Depression Inventory (BDI).
4.3 Secondary measures
Hoffman 2010 collected a variety of secondary outcomes, however did not provide variability data, which precluded independent analyses. They reported a reduction in pain on the Brief Pain Inventory (P= 0.03) and a reduction in pain interference (P= 0.02). No differences were found for measures of head injury symptoms, perceived quality of life, sleep, general health status, heart rate, or ability to walk. One of the secondary outcomes collected was frequency of exercising. During the 10‐week course, participants in the intervention group increased their frequency of exercise from a mean of 1.28 days per week to 3.68, whereas the control participants increased from 1.47 to 2.05 days per week. The duration of exercise increased accordingly: in the intervention group from a mean of 58 minutes to 143 minutes per week; and in the control group from a mean of 66 minutes to 252 minutes per week.
4.4 Treatment compliance, withdrawals from the study (dropouts)
Eighty‐four participants were enrolled in the Hoffman 2010 study and 76 completed the outcome assessments. There was no difference in completion of treatment between treatment groups (RR 1.67; 95% CI 0.43 to 6.53; Z = 0.73; P = 0.46; Analysis 4.2).
4.2. Analysis.

Comparison 4 Supervised exercise versus exercise as usual, Outcome 2 Treatment dropouts.
4.5 Adverse effects
Hoffman 2010 did not report on adverse effects, but did comment that exercise has relatively few adverse effects compared to pharmacological interventions.
Discussion
Summary of main results
The aim of this review was to investigate the effectiveness of non‐pharmacological interventions for depression in adults and children following traumatic brain injury (TBI). Following an exhaustive search process, six studies were identified that met strict criteria for inclusion, including three that were completed recently in 2013 and 2014. We identified no studies that investigated an intervention for children or adolescents, and so it is not possible to comment on the efficacy of any intervention for people under the age of 18.
The primary objective was to determine whether non‐pharmacological interventions (either with or without pharmacological interventions) for depression in adults and children following TBI were superior to (a) no intervention or (b) pharmacological intervention alone. Four studies compared an intervention with no intervention or treatment as usual. Three of these investigated a psychological intervention that was either cognitive‐behavioural therapy (CBT; Fann 2015; Simpson 2011), or mindfulness‐based cognitive therapy (Bedard 2013). The quality of evidence in support of psychological interventions was very low due to methodological limitations, small effect sizes and very wide confidence intervals of effect size. One study investigated an exercise intervention (Hoffman 2010). While there was an effect in favour of the intervention, the experimental groups were not equivalent at baseline and no conclusion could be drawn about the effects of exercise as an intervention for mood. One study investigated a combination of a non‐pharmacological intervention (repetitive transcranial magnetic stimulation (rTMS)) and a pharmacological intervention (tricyclic antidepressant (TCA)) compared with a pharmacological intervention (TCA alone; He 2004). This study did find an effect in favour of the combined intervention, however, the quality of the evidence was judged to be very low.
Prior to 2013, there was a paucity of high quality evidence related to the benefit of psychological interventions for depression following TBI. The results of our meta‐analysis did not support the effectiveness of psychological interventions compared with no treatment. The studies showed that many participants improved without intervention, and there was a lack of evidence to indicate the reasons that some individuals responded to treatment but others did not.
Ashman 2014 was the only study that compared two active psychological interventions (CBT versus supportive psychotherapy (SPT)), and did not provide evidence in support of one intervention above the other. In addition, the dropout rate from the psychological intervention was high, suggesting that the treatment as delivered was not practical for a large proportion of people with TBI.
Overall completeness and applicability of evidence
The second stated objective of the review was to compare the effectiveness of different types of non‐pharmacological interventions for depression in adults and children following TBI. The six included studies described five different interventions, three psychological (CBT, mindfulness‐based cognitive therapy (MBCT), and SPT) and two physical interventions (rTMS and supervised exercise). Only one of these studies compared two active non‐pharmacological interventions and found no difference between CBT and another psychological intervention, SPT (Ashman 2014). Three of the studies investigating a psychological intervention were published in the two years prior to the completion of this review; prior to that, there was a lack of research on arguably the most commonly applied class of non‐pharmacological interventions. With the addition of these three studies, and ongoing research on this topic, we are encouraged that current research activities will clarify the true effects of available treatments.
The third stated objective of the review was to investigate the occurrence of adverse effects as a consequence of non‐pharmacological interventions in order to assist practitioners in identifying appropriate interventions. Only one study reported adverse effects, and these were reported as minimal (He 2004). Two participants reported tinnitus (ringing in the ears) that spontaneously resolved. Repetitive transcranial magnetic stimulation (rTMS) has had proven efficacy in the non‐brain injured population, but it has not been investigated in the TBI population because of concern about possible adverse effects, particularly increased risk of seizures (Fitzgerald 2011). Studies of other interventions did not comment on adverse effects.
The fourth stated objective of the review was to describe how interventions were adapted and modified to suit this population. In the case of two studies, it is not clear if the intervention was adapted or modified specifically for the population of people with TBI (He 2004; Hoffman 2010). Ashman 2014, Bedard 2013, Fann 2015 and Simpson 2011 used CBT programs that were adapted for people with TBI. Common adaptations included providing additional sessions, reducing and repeating the session content, and providing a workbook that accompanied the treatment sessions in order to aid memory. Other modifications included the addition of Motivational Interviewing and problem‐solving for TBI‐specific symptoms at the outset of the intervention.
Quality of the evidence
Each selected study was reviewed for quality using the Cochrane 'Risk of bias' tool. All studies were judged to be at high risk of bias due to a lack of blinding of participants and personnel. This could have introduced bias because some participants were aware that they were receiving an active intervention, while others received no additional treatment. Knowledge that they were receiving an active intervention may have biased their scores on self‐rated outcome questionnaires. This also introduced a high risk of detection bias (blinding of outcome assessment) for all studies that relied on these as the primary outcome measures. The exception was Ashman 2014, which used diagnostic status on an independent, blinded, clinician‐rated interview as the primary outcome measure. Fann 2015 also applied this as a secondary outcome.
Given the nature of the interventions, it is not necessarily possible to arrange blinding of participants, however, it is possible to deliver control interventions which appear to the participants to be active treatment. For instance, He 2004 could have created a sham rTMS intervention that involved fitting the equipment onto the control participants' heads, but not turning it on. In another study of CBT, a social contact intervention (a social activity group) served as a control intervention, which appeared to the participants to be active treatment (McDonald 2008). Hoffman 2010 suggested that a social contact intervention could have been employed as a control intervention for their study of supervised exercise.
Participation was a source of bias for the psychological intervention studies. Ashman 2014 and Bedard 2013 were both affected by substantial dropout (attrition bias). Fann 2015 reported a much lower dropout rate, however it was noted that 43% of patients contacted declined to participate in the study. Simpson 2011 was limited by small sample size, and this may have influenced the equivalence of groups, due to possible heterogeneity of participants.
Potential biases in the review process
Prior to conducting the review, a preliminary search identified 19 studies of non‐pharmacological interventions which used a depression scale as an outcome measure. In most cases, these studies did not specify a diagnosis of depression or a cut‐off score on a depression scale, as an inclusion criteria. Many of these studies sought to treat more general concepts, such as 'quality of life' or 'psychological well‐being'. In reviewing these studies, it was clear that they had failed to adequately address the question of whether the treatment had been effective for depression, because the researchers did not study a sample of participants who were depressed. Therefore, the authors of this review made the decision to exclude studies where a diagnosis of depression, or score on a depression measure, was not specified as an inclusion criterion. In doing so, this introduced a potential source of bias, because it restricted the studies that could be included, some of which were of clinical interest. Alternatively, the authors of this review felt that studies that had depression diagnosis or symptoms as an inclusion criterion were more likely to show a treatment effect, and were more clinically relevant, because they more closely represented patients seen in clinical practice.
There were several studies identified for possible inclusion that had mixed samples that included people with diagnoses other than TBI. In these studies, it was likely that many of the participants had TBI and would have met the inclusion criteria for depression, however, because it was not possible to identify separate outcome data for these particular individuals, the studies could not be considered (e.g. Teasdale 1995). Similarly, studies that did not purport to treat depression specifically were excluded, therefore, some interventions devised for other clinical problems, which may be of benefit for depression, were not able to be considered in this review.
At the protocol stage, the sources of studies were specified. At this stage, key decisions were made about which sources to search, and it is possible that key sources were missed. In relation to the electronic database search, the sources were recommended by the Cochrane Injuries Group, and the search strategy was developed by the Trials Search Co‐ordinator. The authors of this review specified additional sources to search. It is unlikely that key sources for research on TBI were missed because the literature on this topic tends to be published in key journals. However, in the case of depression, the sources for literature on affective disorders are published more widely, and it is more likely that if studies were missed, it would be in this literature.
The review authors set out to identify key conferences that would represent research in both TBI and depression. Although it was possible to search the proceedings of international conferences relating to TBI (Special Interest Group in Neuropsychological Rehabilitation of the World Federation for Neuro‐Rehabilitation, 2000 to present and the International Brain Injury Association (IBIA), 1992 to present), the proceedings of the World Congress of Behavioural and Cognitive Therapies (1993 to present) were unavailable because they were not published in a central journal and the authors could not locate paper copies of the proceedings through personal contacts. Therefore an identified source of studies was not searched.
Agreements and disagreements with other studies or reviews
There have been several other review papers that relate to treatment of depression following TBI. These include literature reviews and clinician guidelines for the treatment of depression following TBI (e.g. Alderfer 2005), or mild TBI (Silver 2009), and a literature review examining the efficacy of CBT as a treatment for depression following TBI and other acquired brain impairments (Khan‐Bourne 2003). There were some systematic reviews that had a similar objective to this review (Fann 2009; Guillamondegui 2011; Rapoport 2012; Rosenthal 1998; Waldron 2013), two reviews that were limited to depression following mild TBI (Bay 2009; Barker‐Collo 2013), and another that reviewed psychological interventions across a range of interventions affecting people with mild TBI (Snell 2009). These systematic reviews are discussed in chronological order.
At the time of publication of this review, the authors found no RCTs of any type of intervention for depression following TBI. This is consistent with the current review, in which all of the identified studies were published from 2004 onwards.
This review engaged in a widespread search of databases, similar to a Cochrane review. It was far more inclusive than the current review, and included any peer‐reviewed study of pharmacological and non‐pharmacological interventions, where depression or depressive symptoms were primary or secondary outcomes. As such, it was not restricted to RCTs and as a consequence, it included a greater number of studies. Of the 16 studies included, six were non‐pharmacological physical interventions, and 10 were psychotherapeutically‐based interventions. It did not include the studies included in the current review since most were published following its publication (Ashman 2014; Bedard 2013; Fann 2015; Hoffman 2010; Simpson 2011), and one was not written in English (He 2004). Fann 2009 noted that none of the studies identified in their systematic review used diagnosis of depression as an inclusion criterion, and of the eight studies of psychological interventions, none specifically set out to treat depression.
This review was conducted by the Vanderbilt Evidence‐based Practice Center, USA and systematically reviewed literature pertaining to TBI and depression including epidemiology, assessment and diagnosis, the course of the condition, and intervention options. This review employed strict selection criteria, which included limiting searches to studies with 50 participants or more. As a consequence, Guillamondegui 2011 identified only two studies of pharmacological interventions, and none of non‐pharmacological interventions. The search included studies from 1966 up to May 2010 and was also limited to English‐language articles, therefore missing the studies identified in the current review. The authors concluded that no evidence was available to guide treatment choices after TBI.
Rapoport 2012 sought to provide an 'up‐to‐date selective review' of the current epidemiology, risk factors, and management strategies of major depression following TBI. The search was limited to articles published from 2006 to 2011 that were available on the MEDLINE database. The review included studies that were not RCTs, and studies of mixed acquired brain injury, not just TBI samples. Rapoport 2012 found three studies investigating physical exercise interventions (including Hoffman 2010 identified in the current review), and three pertaining to CBT. Rapoport 2012 concluded that the evidence regarding interventions was inconclusive, and although CBT and exercise interventions showed promise, those studies were subject to bias due to the inclusion criteria not specifying either a diagnosis of depression or the existence of clinically significant depressive symptoms. The advice to clinicians was to follow best practice guidelines for treating major depression in the general population.
Waldron 2013 reviewed outcomes for CBT interventions for anxiety and depression following acquired brain injury (including non‐traumatic injuries such as cerebrovascular events, hypoxic or neurotoxic injuries). The review authors did not limit the search to RCTs. Describing their study selection criteria as 'relaxed', the authors sought to assemble a broad spread of research data that related to the efficacy of CBT. Therefore, Waldron 2013 includes 24 studies of various designs, including 12 studies of single‐case designs, two of uncontrolled group studies and 10 RCTs of varying quality. They applied the PEDro methodological rating scale to the studies and found that the quality of the studies ranged from very low (2/10) to acceptable (7/10), with the acknowledgement that it is difficult to achieve several items on the PEDro scale, such as blinding of participants and therapists, due to the nature of the studies. Seven of 24 included studies identified mood as an outcome. Waldron 2013 combined many of these studies in a meta‐analysis, despite the variety of clinical problems targeted and interventions applied, concluding that CBT had demonstrated efficacy for the clinical problem it sought to address (e.g. anger management), but these effects did not generalise to other clinical problems such as depression, unless that was specifically targeted. When depression was the primary focus of the intervention, CBT showed large effect sizes, albeit these conclusions were based on uncontrolled studies.
This review included English‐language studies of any intervention for depression following mild TBI. Some of the papers included had mixed samples and the authors were able to access separate data for participants with mild TBI. Barker‐Collo 2013 included all study designs and identified 13 studies of mixed design, with five non‐pharmacological interventions (CBT, group education and support, and magnetic field stimulation). Five studies compared an intervention with a control group and eight studies did not, relying on pre‐post comparisons. Meta‐analyses were conducted which found significant treatment effects in support of the intervention. Meta‐analysis of the pre‐post studies found a treatment effect of 1.89 (95% CI ‐ 1.20 to 2.58; P< 0.001). Meta‐analysis of controlled studies (of which only one was a comparison of a non‐pharmacological intervention) found a much more modest treatment effect of 0.46 (95% CI ‐0.44 to 1.36; P < 0.001) in favour of the control group. The disparity in findings between controlled and uncontrolled studies is highly relevant and is consistent with the findings of the current review, which identified several studies in which the control group demonstrated improvement throughout the course of data collection.
In conclusion, this review is the only review of RCTs yet published, which focuses specifically on non‐pharmacological interventions for people with TBI who demonstrated symptoms, or had a diagnosis, of depression. The findings of the current review are consistent with previous reviews, albeit the inclusion criteria for this review was stricter, and the range of sources searched was wider. Previous reviews identified a multitude of studies, most of which were of lower quality (with the exception of Hoffman 2010), and were therefore excluded in the current review. Because of the reliance on higher quality evidence, the authors of this review have more confidence in the findings of this review than any previous review.
Authors' conclusions
Implications for practice.
The review did not find compelling evidence in support of any particular intervention that would inform clinical practice. The identified studies did find that some participants responded to interventions, whereas without an intervention, some control‐group participants experienced reduction in depression symptoms or remission of diagnoses. It is important when considering an intervention for depression following traumatic brain injury, that clinicians think carefully about what outcomes would be personally meaningful to the patient, their families and other supporters. It is important at the outset to establish the desired outcomes and how these would be measured, and to set up systems so that progress can be evaluated throughout. In this way, despite the absence of a treatment of choice, at least the clinician can be informed whether the patient is improving, and might be able to determine which components of treatment are beneficial for that patient.
Implications for research.
This review has important implications for studies of non‐pharmacological interventions for depression following TBI. Primarily, it is critically important that researchers carefully consider the selection criteria for participants. Most of the studies that were identified but not included in the review were rejected either because the selection criteria did not specify a diagnosis of a depressive disorder, there was no cut‐off score applied to a clinical measure of depression, or both. A lack of selection criteria that specify the presence of depression is problematic, because it is likely that these studies included participants who were not depressed and therefore would not be expected to show substantial improvement on depression measures. In the clinical setting, it is unlikely they would be offered treatment and therefore their participation in clinical research is of questionable value for addressing the issue of effective treatments for depression after TBI. Therefore, it is recommended that selection of participants is based on their diagnostic status as specified by a recognised diagnostic manual (e.g. DSM‐IV; APA 2000). If diagnostic status is not specified as an inclusion criterion, then at the very least, the inclusion criteria should include a clinical cutoff on a recognised measure of depression. Where self‐rating scales are used, authors should give careful consideration to using a scale that has widespread use in the general population, and has been proven valid in TBI, such as the Beck Depression Inventory (BDI: Green 2001; Sliwinski 1998), or the Depression Anxiety and Stress Scales (DASS; Ownsworth 2008). It is recommended that self‐rating scales are used as secondary outcomes to clinician‐rated scales, such as the SCID (e.g. Ashman 2014). Because it is very difficult to blind participants to the intervention, it is likely that self‐rating scales will reflect subjective impressions of the benefit (or otherwise) of interventions.
Some studies were investigated but excluded on the basis that there were mixed samples of TBI and other non‐traumatic brain injuries, and separate data were not available for TBI participants. Although non‐TBI participants might have been similar to TBI participants in age and demographic factors, they were not directly comparable in terms of their underlying pathology, cognition, behaviour, physical symptoms or adjustment to impairment. Finally, another common reason for exclusion of studies was that the intervention did not target depression specifically, but rather more general concepts, such as 'emotional distress'. As has been discussed, some interventions (particularly CBT) tend to be effective for specific clinical problems and therefore, it is not advisable to set out to treat a broadly‐defined clinical presentation, because it appears to weaken the effect of the intervention. An example of this was Simpson 2011, who set out to target hopelessness in relation to suicidality. On the measure of hopelessness, Simpson 2011 found a positive effect in favour of CBT, however, this was not found on a secondary measure of depression.
When designing studies, researchers should give careful consideration to the nature of the intervention given to the control group. In all of the selected studies, there was a lack of an alternative placebo intervention, and therefore intervention participants were unable to be blinded to the intervention they received. Ashman 2014 compared two active psychological interventions that comprised a similar level of therapist contact (i.e. treatment done), and did not find a difference on the main clinician‐rated outcome. Other RCTs have been able to include both a wait‐list control intervention and a 'sham' treatment intervention so that the impact of the attention of personnel on addressing the clinical problem could be evaluated (e.g. McDonald 2008).
At present, there is a growing pool of intervention studies for depression following TBI. The treatment that showed the larger treatment effect was rTMS plus TCA (He 2004), but there is a need for replication of the He 2004 study, with the addition of a more objective clinician‐rated measure and long‐term follow‐up data. In addition, it would be possible to compare the intervention with a placebo control intervention. An earlier Cochrane review of rTMS reporting the use of a 'sham' TMS intervention amongst the selected studies (Rodriguez‐Martin 2001).
The recent studies of psychological interventions found a high percentage of recovery for control participants (Ashman 2014; Bedard 2013; Fann 2015). A criticism of the group designs (including RCTs) is that while an intervention group may or may not respond as a whole to an intervention, this masks interesting individual responses to the intervention. Group studies do not explain why some individuals will respond while others may not. There is concern that structured, manualised treatments that are investigated in group studies, do not adequately reflect interventions in the 'real world', which are usually tailored to the individual case. In the case of an intervention such as CBT, there are various components that are part of the intervention, but group studies do not distinguish which components of the intervention might be the most effective. The RCTs of psychological interventions were subject to bias due to issues with participation, including a high dropout rate (Ashman 2014; Bedard 2013), a small sample size (Simpson 2011), or an adequate sample size, but a very high refusal rate for referrals to the study (Fann 2015). This suggests that there are many drawbacks to attempting to evaluate a psychological treatment with an RCT design, and that alternative treatment designs, such as a well designed, single case experiment, might provide more useful information about the effectiveness of a particular psychological treatment.
Acknowledgements
Marisa Chau, Australian Cochrane Centre, Monash University, for her assistance in translating a Chinese language paper.
Sharon Cramer and Matthew Page, Australian Cochrane Centre, Monash University, for their guidance and support during the introductory and review completion workshops.
Ulli Rosenkoetter, Rehabilitation Studies Unit, Medicine, The University of Sydney, for her assistance in translating a German language paper.
All the personnel at the Injuries Group in London.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
At the time of running the search we could not access PsycBITE and for that reason we ran only one search in this database in 2012.
Cochrane Injuries Group Specialised Register (TBI OR "Traumatic Brain Injury") AND (depress* OR dysthmic*) Database of Abstract of Reviews of Effects (DARE) (The Cochrane Library) Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) #1MeSH descriptor Craniocerebral Trauma explode all trees #2MeSH descriptor Brain Edema explode all trees #3MeSH descriptor Glasgow Coma Scale explode all trees #4MeSH descriptor Glasgow Outcome Scale explode all trees #5MeSH descriptor Unconsciousness explode all trees #6MeSH descriptor Cerebrovascular Trauma explode all trees #7MeSH descriptor Pneumocephalus explode all trees #8MeSH descriptor Cerebral Hemorrhage, Traumatic explode all trees #9((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) NEAR/3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)) #10((head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) NEAR/3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*)) #11(Glasgow NEXT (coma or outcome) NEXT (scale* or score*)) #12"rancho los amigos scale" #13("diffuse axonal injury" or "diffuse axonal injuries") #14((brain or cerebral or intracranial) NEAR/3 (oedema or edema or swell*)) #15((unconscious* or coma* or concuss* or 'persistent vegetative state') NEAR/3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*)) #16MeSH descriptor Coma explode all trees #17(injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*) #18(#16 AND #17) #19(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #18) #20MeSH descriptor Depression, this term only #21MeSH descriptor Depressive Disorder, this term only #22MeSH descriptor Depressive Disorder, Major, this term only #23MeSH descriptor Dysthymic Disorder, this term only #24(depress* or melancholia) #25(#20 OR #21 OR #22 OR #23 OR #24) #26(#19 AND #25) MEDLINE (OvidSP) 1. exp Craniocerebral Trauma/ 2. exp Brain Edema/ 3. exp Glasgow Coma Scale/ 4. exp Glasgow Outcome Scale/ 5. exp Unconsciousness/ 6. exp Cerebrovascular Trauma/ 7. exp Pneumocephalus/ 8. exp Epilepsy, post traumatic/ 9. exp Cerebral hemorrhage, traumatic/ 10. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti. 11. ((head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*)).ti,ab. 12. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 13. "rancho los amigos scale".ti,ab. 14. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 15. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 16. ((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*)).ti,ab. 17. exp coma/ 18. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab. 19. 17 and 18 20. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 19 21. randomi?ed.ab,ti. 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. placebo.ab. 25. clinical trials as topic.sh. 26. randomly.ab. 27. trial.ti. 28. 21 or 22 or 23 or 24 or 25 or 26 or 27 29. (animals not (humans and animals)).sh. 30. 28 not 29 31. (rat* or rodent* or mouse or mice or murin* or dog* or canine* or cat* or feline* or rabbit* or pig* or porcine or swine or sheep or ovine* or guinea pig* or horse* or hamster* or goat* or chick or cattle or bovine).ti. 32. 30 not 31 33. 20 and 32 34. Depression/ 35. depressive disorder/ or depressive disorder, major/ or dysthymic disorder/ 36. (depress* or melancholia).ab,ti. 37. 34 or 35 or 36 38. 33 and 37 Embase (OvidSP) 1. exp head injury/ 2. exp brain edema/ 3. exp Glasgow coma scale/ 4. exp Glasgow outcome scale/ 5. exp unconsciousness/ 6. exp cerebrovascular accident/ 7. exp pneumocephalus/ 8. exp traumatic epilepsy/ 9. exp brain hemorrhage/ 10. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti. 11. ((head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*)).ti,ab. 12. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 13. "rancho los amigos scale".ti,ab. 14. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 15. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 16. ((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*)).ti,ab. 17. exp coma/ 18. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab. 19. 17 and 18 20. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 19 21. exp Randomized Controlled Trial/ 22. exp controlled clinical trial/ 23. randomi?ed.ab,ti. 24. placebo.ab. 25. *Clinical Trial/ 26. randomly.ab. 27. trial.ti. 28. 21 or 22 or 23 or 24 or 25 or 26 or 27 29. exp animal/ not (exp human/ and exp animal/) 30. 28 not 29 31. (rat* or rodent* or mouse or mice or murin* or dog* or canine* or cat* or feline* or rabbit* or pig* or porcine or swine or sheep or ovine* or guinea pig* or horse* or hamster* or goat* or chick or cattle or bovine).ti. 32. 30 not 31 33. 20 and 32 34. Depression/ 35. depressive disorder/ or depressive disorder, major/ or dysthymic disorder/ 36. (depress* or melancholia).ab,ti. 37. 34 or 35 or 36 38. 33 and 37 CINAHL Plus (EBSCO) S1 (MH "Clinical Trials") S2 PT clinical trial* S3 TX clinical N3 trial* S4 TI ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) or (tripl* N3 blind*) ) or TI ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) or AB ( (singl* N3 blind*) or (doubl* N3 blind*) or (trebl* N3 blind*) ) or AB ( (singl* N3 mask*) or (doubl* N3 mask*) or (trebl* N3 mask*) or (tripl* N3 mask*) ) S5 TX randomi?ed N3 control* N3 trial* S6 (MH "Placebos") S7 TX placebo* S8 (MH "Random Assignment") S9 TX random* N3 allocat* S10 MH quantitative studies S11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 S12 (MH "Head Injuries+") S13 (MH "Cerebral Edema+") S14 (MH "Glasgow Coma Scale") S15 (MH "Unconsciousness+") S16 (MH "Pneumocephalus") S17 (MH "Epilepsy, Post‐Traumatic") S18 (MH "Cerebral Hemorrhage+") S19 (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) S20 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*) S21 S19 N3 S20 S22 (head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) S23 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*) S24 S22 N3 S23 S25 "glasgow coma scale" S26 "glasgow outcome scale" S27 "rancho los amigos scale" S28 "diffuse axonal injury" or "diffuse axonal injuries" S29 (brain or cerebral or intracranial) S30 (oedema or edema or swell*) S31 S29 N3 S30 S32 (unconscious* or coma* or concuss* or 'persistent vegetative state') S33 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*) S34 S32 N3 S33 S35 (MH "Coma") S36 (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*) S37 S12 or S13 or S14 or S15 or S16 or S17 or S18 or S21 or S24 or S25 or S26 or S27 or S28 or S31 or S34 or S35 or S36 S38 (MH "Depression") S39 depress* or melancholia S40 (MH "Dysthymic Disorder") S41 "major depressive disorder" S42 S38 or S39 or S40 or S41 S43 S11 and S37 S44 S42 and S43 Limiters ‐ Exclude MEDLINE records PsycINFO (OvidSP) 1. exp Brain Damage/ 2. exp Traumatic Brain Injury/ 3. exp Epilepsy/ 4. exp Cerebral Hemorrhage/ 5. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti. 6. ((head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*)).ti,ab. 7. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 8. "rancho los amigos scale".ti,ab. 9. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 10. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 11. ((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*)).ti,ab. 12. exp Coma/ 13. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab. 14. 12 and 13 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 14 16. Depression/ 17. depressive disorder/ or depressive disorder, major/ or dysthymic disorder/ 18. (depress* or melancholia).ab,ti. 19. 16 or 17 or 18 20. 15 and 19 21. exp clinical trials/ 22. exp placebo/ 23. exp treatment effectiveness evaluation/ 24. exp mental health program evaluation/ 25. exp experimental design/ 26. exp prospective studies/ 27. clinical trial*.ab,ti. 28. controlled clinical trial.ab,ti. 29. randomi?ed controlled trial.ab,ti. 30. randomi?ed.ab,ti. 31. placebo.ab. 32. randomly.ab. 33. trial.ti. 34. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or dummy or mask*)).ab,ti. 35. ((crossover or clin* or control* or compar* or evaluat* or prospectiv*) adj3 (trial* or studi* or study)).ab,ti. 36. 21 or 22 or 23 or 24 or 25 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 37. exp animals/ 38. exp human females/ 39. exp human males/ 40. 38 or 39 41. 37 not (37 and 40) 42. 36 not 41 43. 20 and 42 PsycBite (OvidSP) depression AND “Traumatic Brain Injury”/Head Injury
Data and analyses
Comparison 1. CBT versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major depressive disorder (MDD) on the structured clinical interview for depression (SCID) scale | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 MDD on SCID long term follow up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Depression scales | 3 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.47, 0.19] |
| 4 Depression scales long term follow up | 3 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.33, 0.29] |
| 5 Secondary depression measure ‐ SCL20 or SCL90R | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.45, 0.15] |
| 6 SCL20 long term follow up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Secondary depression measure ‐ PGI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 PGI long term follow up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Secondary measure ‐ Dissatisfaction with depression care | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Secondary depression measure ‐ PHQ | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Beck Hopelessness Scale (BHS) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Beck Scale for Suicide Ideation | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Rosenberg Self‐Esteem Scale | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 Treatment drop‐outs | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.57, 2.54] |
Comparison 2. CBT versus SPT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MDD present on SCID following intervention | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Beck Depression Inventory (BDI) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Life 3 ‐ Quality of Life | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Treatment drop‐outs | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Transcranial magnetic stimulation plus TCA versus TCA alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hamilton Rating Scale for Depression (HAM‐D) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Mini Mental State Examination (MMSE) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Serotonin (5‐HT) levels | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Noradrenaline | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Treatment dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
3.2. Analysis.
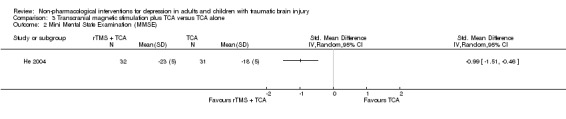
Comparison 3 Transcranial magnetic stimulation plus TCA versus TCA alone, Outcome 2 Mini Mental State Examination (MMSE).
Comparison 4. Supervised exercise versus exercise as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Beck Depression Inventory (BDI) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Treatment dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashman 2014.
| Methods | Randomised controlled trial. | |
| Participants | Seventy‐seven people who had sustained a traumatic brain injury who were living in the community. Participants were recruited from an outpatient rehabilitation service, clinic newsletter and word of mouth. Inclusion criteria: Medically documented traumatic brain injury; current DSM‐IV diagnosis of a depressive disorder or Beck Depression Inventory (BDI‐II) score greater than 20; 18 to 55 years old. Exclusion criteria: Presence or history of psychosis, substance abuse, pre‐existing neurological disorder, mental retardation, or active suicidality; currently in psychotherapy; commenced or changed antidepressant medication within the past six months. |
|
| Interventions | All participants attended 16 sessions of individual treatment over three months. Participants were allocated either for cognitive‐behavioural therapy (CBT) or supportive psychotherapy (SPT). | |
| Outcomes |
Primary outcome measure: Presence of a DSM‐IV depressive mood disorder assessed by the Structured Clinical Interview for DSM‐IV (SCID) Secondary outcome measures: Beck Depression Inventory ‐ second edition (BDI‐II) Anxiety disorder and substance abuse modules of the SCID State‐Trait Anxiety Inventory (STAI) Life‐3 Interpersonal Support Evaluation List (ISEL) Life Experiences Survey (LES) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Random number sequence was concealed in pre‐sealed individual envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to disparate experimental conditions, blinding of participants and personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome measure was a clinical scale, applied by a clinician, who was blind to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 77 participants randomised to a treatment, only 43 completed the study. Twenty‐two dropped out after baseline assessment and a further 12 participants did not complete the study. |
| Selective reporting (reporting bias) | Low risk | The published study is consistent with an earlier conference abstract (Ashman 2012) and the protocol registered on Trialscentral.org. The study was registered on clinicaltrials.gov, study ID: NCT00211835. |
| Other bias | Unclear risk | ‐ |
Bedard 2013.
| Methods | Multi‐centre randomised controlled trial, intervention and wait‐list control groups with cross‐over design. | |
| Participants | Seventy‐six people who had sustained traumatic brain injury completed the study. Recruitment sources: community clinics, media advertisements, non‐government organisations and through personal contact with rehabilitation practitioners. Inclusion criteria: Evidence of depression (score of 16 or higher on the BDI‐II); ability to read and speak English; age 18 or over; and having completed all standard treatments for the injury. Exclusion criteria: Presence of unusual psychological processes such as psychosis, suicide ideation, substance abuse or major concurrent medical illnesses. |
|
| Interventions | For intervention participants, this was a 10‐week program of weekly 90‐minute group sessions plus recommended daily meditation for 20 to 30 minutes. The treatment followed a standard manual for mindfulness‐based cognitive therapy, however, components were modified to suit people with brain injury. After the intervention group had completed treatment, the wait list group was offered treatment, the outcomes of which are reported separately. | |
| Outcomes |
Primary outcome measures: Beck Depression Inventory ‐ second edition (BDI‐II) Patient Health Questionnaire (PHQ) Symptom Checklist 90 Revised (SCL‐90R) Secondary outcome measures: Philadelphia Mindfulness Scale Toronto Mindfulness Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was conducted by a statistician who was independent of the clinicians and site investigators. The statistician used minimisation to ensure balance at baseline, between the groups, on a key outcome measure (Beck Depression Inventory). These measures present low risk of selection bias. However, five participants at one site were allocated to the intervention due to low participant numbers at that site. |
| Allocation concealment (selection bias) | Low risk | Allocation occurred off site and without the knowledge of the site investigators or group facilitators. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not possible due to one intervention being an active intervention, while the other was a passive wait‐list control. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome measures were self‐report questionnaires and therefore, subject to high risk of bias due to the participants' knowledge to which intervention they had been allocated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was substantial dropout from the study (19 of 57 participants allocated to intervention and 10 of 48 allocated to wait‐list). The higher dropout from the intervention group could have increased bias as it is possible these participants had greater symptoms of depression, the primary outcome of the study. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were stated in a study protocol registered on the Trialscentral.org website (NCT00745940). These outcomes were consistent with the published results. |
| Other bias | Unclear risk | ‐ |
Fann 2015.
| Methods | Randomised controlled trial. | |
| Participants | One hundred adults with TBI and a current diagnosis of major depressive disorder (MDD). Recruitment was conducted at community and clinical settings serving people with TBI, and through referrals from clinicians. Inclusion criteria: English‐speaking people over 18 years old, who had a documented history of mild to severe TBI, including criteria relating to Glasgow Coma Score (GCS), imaging abnormalities or duration of post‐traumatic amnesia (PTA. All participants had to meet diagnostic criteria for MDD with the use of the Structured Clinical Interview for Depression (SCID) and demonstrate symptoms of depression over the clinical cutoff on the Patient Health Questionnaire (PHQ‐9). Exclusion criteria: No stable home or access to telephone; history of diagnosis of schizophrenia; evidence of bipolar disorder, psychosis or suicidal intent, or current alcohol or drug dependence; currently receiving or planning to start evidence‐based psychotherapy for depression during the study; commencing or adjusting anti‐depressant medication during the study; or severe cognitive impairment as defined by scores below cutoff on specific neuropsychological tests. |
|
| Interventions | The intervention comprised a manualised CBT program written to be delivered by telephone. It was modified for TBI participants with an expansion in duration from eight weekly sessions to 12 and the addition of care management procedures for the life changes experienced by this population. Motivational interviewing was used to engage participants in treatment. The session material was presented in smaller portions, more slowly, and with greater repetition. Participants were provided with a workbook with in‐session materials and between‐session assignments. Two authors provided treatment and 10% of sessions were subject to fidelity checks. | |
| Outcomes |
Primary outcome measures: Hamilton Depression Rating Scale (HAMD‐17) Symptom Checklist‐20 (SCL‐20) Secondary outcome measures: MDD criteria based on the SCID Patient Global Impression (PGI) Satisfaction with Depression Care Working Alliance Inventory‐Short Form Sheehan Disability Scale MOS Short Form Health Questionnaire (SF‐36) Head Injury Symptom Checklist |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants were randomised to an intervention. In order to increase participation in the study, the authors used a choice‐stratified approach in which participants had the option of choosing to be randomised to any intervention (CBT‐T vs CBT‐IP vs usual care), or one CBT intervention (CBT‐T or CBT‐IP) vs usual care. The random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was centrally assigned following enrolment in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the interventions, it was not possible to blind participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessment was conducted over the telephone by trained study staff who were blind to randomisation status. However, most of the outcome measures rely on participant self‐report and therefore are subject to bias due to awareness of allocation. Even the HAMD, which is a clinician‐report scale, does rely upon patient self‐report for many items, and therefore cannot be considered to be an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eighty‐six percent of participants provided data at follow‐up. |
| Selective reporting (reporting bias) | Low risk | The outcome measures reported in the results section are consistent with those in the methods section. The trial protocol was registered in clinicaltrials.gov (identifier: NCT00878150). All primary outcomes and most secondary outcomes are reported in the final publication, albeit with some substitution of secondary measures prior to commencing data collection. |
| Other bias | Unclear risk | ‐ |
He 2004.
| Methods | Randomised controlled trial. | |
| Participants | Sixty‐four brain injured patients were identified from the Department of Neurosurgery and Rehabilitation, Affiliated Hospital of Luzhou Medical College. Inclusion criteria: First time experiencing cranial head injury and confirmed through CT or MRI scans; score greater than 8 on the Hamilton Rating Scale for Depression (HAMD). Exclusion criteria: Aphasia, unconscious, severe dementia, drug and alcohol abuse, severe disability. |
|
| Interventions | All participants received oral tricyclic antidepressant drugs, with only the intervention group also receiving repetitive transcranial magnetic stimulation (rTMS) treatment. Consent was obtained from the patient or family members to receive the treatments. Maglite Compact magnetic stimulation was used with a coil diameter of 12 cm, maximum intensity of 1.2 T, pulse time limit of 100 μs. Quote: "Patients, in a seated position during treatment, received 60% of the maximum intensity (0.72 T), with bilateral stimulation of the frontal lobes, 30 times to each side, with a frequency of 0.5 Hz, each day consecutively for 5 d, which equals to one treatment session. Treatments were given on a 2‐day interval, with each patient receiving 4 treatment sessions." p 6045 | |
| Outcomes | Pre‐ and post‐intervention HAMD score. Pre‐ and post‐intervention Mini‐Mental State Examination (MMSE) score. Plasma monoamine neurotransmitters concentrations. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors used a predetermined list for allocation, but did not state the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation was not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants receiving the intervention were aware that they were receiving rTMS. There was no sham intervention that might prevent the control group participants from recognising that they were not getting the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different personnel, blinded to the intervention, conducted the outcome assessments, however, the primary outcome measures were self‐report scales, and therefore subject to bias since the participants were aware of the intervention to which they were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 64 participants allocated to groups, only one failed to complete data collection. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available. |
| Other bias | Unclear risk | ‐ |
Hoffman 2010.
| Methods | Randomised controlled trial. | |
| Participants | Eighty participants were recruited through posted and online advertisements in local rehabilitation clinics, newspapers, and websites. Local rehabilitation physicians and psychologists were given information and flyers for the study. Inclusion criteria: Self‐reported TBI, severe enough to have required medical evaluation or hospital admission immediately after injury; TBI from 6 months to 5 years prior to enrolment; score of 5 or more on the Patient Health Questionnaire‐9 (PHQ‐9), indicating at least a mild level of depressive symptoms; sufficient cognitive ability to maintain an exercise log and independently participate in the study, or have the involvement of a support person to facilitate involvement. Exclusion criteria: Having a medical condition that would preclude or limit exercise; current suicidal ideation with intent or plan; current pregnancy; current regular exercise program three times a week or more; any physical barrier to the use of standard aerobic exercise equipment. |
|
| Interventions | The intervention was supervised exercise training once a week in a gymnasium with a research education trainer and certified athletic trainer. Each session included; 15 minutes of education on an exercise‐related topic; 15 minutes of warm‐up exercises consisting of stretching and walking; 30 minutes of aerobic exercise. In addition the intervention included a home program, whereby each participant was asked to perform 30 minutes of aerobic exercise at least 4 times a week, in addition to the supervised exercise session. Control group participants were given instruction that they would be contacted for assessment after 10 weeks. They were given no particular instructions regarding exercise. |
|
| Outcomes |
Primary outcome measure: Beck Depression Inventory (BDI) Secondary outcome measures: Brief Pain Inventory Pittsburgh Sleep Inventory Head Injury Symptom Checklist SF‐12 Health Survey Craig Handicap Assessment and Reporting Technique ‐ Short Form (CHART‐SF) Perceived Quality of Life (PQOL) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was created using a random number generation program (personal communication with primary author). |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes to ensure blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible because study was a comparison between an active intervention (exercise program) and a wait‐list control. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was completed by a research assistant blind to group allocation (personal communication with primary author), however, the primary outcome measure was a self‐report scale and therefore subject to bias since the participants were aware of the intervention to which they were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eighty participants were randomised, with 76 completing the outcome assessment. Missing outcome data were balanced between groups, with a similar reason for missing data (participants unable to be contacted for follow‐up). |
| Selective reporting (reporting bias) | Low risk | Table 2 reports data on each measure, for each group, at each time‐point. |
| Other bias | Unclear risk | ‐ |
Simpson 2011.
| Methods | RCT with wait‐list control, cross‐over design. | |
| Participants | Seventeen patients recruited from the Liverpool (Australia) Hospital brain injury community team caseload. Inclusion: severe TBI (PTA > 1 day), aged 18 years or older, moderate to severe levels of hopelessness, suicidal ideation, or both. Exclusion: severe neuropsychological impairments in cognitive or language functions, extremely challenging behaviour that would preclude compliance with the study protocol, and non‐fluency in English. |
|
| Interventions | Cognitive‐behavioural therapy delivered via a 20‐hour manualised group‐based programme, delivered in 10 weekly 2‐hour sessions. | |
| Outcomes |
Primary outcome measures: Beck Hopelessness Scale (BHS) Beck Scale for Suicide Ideation (BSS) Hospital Anxiety and Depression Scale (HADS) Secondary outcome measures: Herth Hope Index Rosenberg Self‐Esteem Scale Social Problem Solving Inventory‐Revised (SPSI‐R) Timepoints measured: Baseline At completion of treatment (10 weeks after baseline) 3 months after completion of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation: groups of 4 participants allocated to an intervention, using a computer‐generated set of random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation to intervention conducted off‐site and allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study interventions were either an active treatment or a wait‐list control, and therefore, blinding of participants and personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessments at completion of treatment and at 3‐month follow‐up were conducted by an independent assessor who was blind to the intervention group. Participants were asked not to disclose their intervention group to the assessor. The independent assessor was asked to record any inadvertent disclosure of the participants' intervention group. However, the primary outcome measures were self‐report scales and therefore, subject to bias since the participants were aware of the intervention group to which they were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seventeen participants' were randomised to groups. Only one subject withdrew prior to the final assessment time point. |
| Selective reporting (reporting bias) | Low risk | Primary author provided the study protocol, which showed that all outcomes collected were reported. |
| Other bias | High risk | Small sample size (intervention group, N = 8 and control group, N = 9). |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anson 2006 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Bateman 2001 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| Bell 2008 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not specifically for depression. |
| Bombardier 2009 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Bradbury 2008 | Not a randomised controlled trial, but a matched controlled trial. Participants were allocated to groups by logistical considerations. Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. Sample was not limited to people with TBI, although the authors were able to provide separate data just for participants with TBI. |
| Carey 2008 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Cullen 2007 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Sample was not limited to traumatic brain injury. |
| Driver 2009 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Fleming 2009 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Sample was not limited to traumatic brain injury. |
| Geurtsen 2008 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Ghaffar 2006 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| Huckans 2010 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| Leonard 2004 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| McDonald 2008 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. Sample was not limited to people with TBI. |
| Powell 2002 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Ruff 1990 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| Smith 1994 | Sample was not limited to people with TBI. |
| Stocksmeier 1992 | Sample was not limited to people with TBI. |
| Stoll 1999 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Struchen 2011 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. |
| Sun 2008 | Pharmacological intervention. |
| Teasdale 1995 | Intervention was not for depression. Sample was not limited to people with TBI. Not a randomised controlled trial. |
| Tiersky 2005 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| Wade 2006 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
| Wade 2008 | Inclusion criteria did not specify diagnosis of depression, or clinical cutoff for a measure of depression. Intervention was not for depression. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01039857.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | This study was terminated early. The review authors are trying to obtain further information about the study. |
Characteristics of ongoing studies [ordered by study ID]
Clark 2014.
| Trial name or title | A randomised controlled trial of a modified group cognitive behavioural intervention for depressed mood following traumatic brain injury. |
| Methods | Randomised controlled trial. |
| Participants | Persons with medically documented, complicated mild, moderate, or severe TBI, who had clinically significant depressive symptoms. |
| Interventions |
Intervention: modified cognitive behavioural therapy (6 sessions). Control: support group (6 sessions). |
| Outcomes | Measures of depression, perceived stress. |
| Starting date | Not known. |
| Contact information | Allison Clark, Baylor College of Medicine, Houston, TX, USA. |
| Notes | The study author was in contact with the Injuries Group editorial team on 21 October 2015 to say that the study has been completed, and the final report has been submitted to a medical journal for publication. |
NCT00531258.
| Trial name or title | TMS in the treatment of the sequelae of traumatic brain injury. |
| Methods | Randomised controlled trial, intervention and control groups. |
| Participants | Currently recruiting adults aged 18 to 60 with a history of TBI, who meet DSM‐IV‐TR criteria for major depressive disorder and score 20 or above on the Montgomery‐Asberg Rating Scale. |
| Interventions | Repetitve transcranial magnetic stimulation (rTMS) versus sham rTMS. |
| Outcomes | Unknown |
| Starting date | October 2007 |
| Contact information | Paul Fitzgerald, paul.fitzgerald@monash.edu |
| Notes | Study identification number on clinicaltrials.gov: NCT00531258 |
NCT01691378.
| Trial name or title | Window to hope: Preliminary results from a randomised controlled trial (RCT) of a psychological treatment for hopelessness among US veterans with traumatic brain injury (TBI). |
| Methods | Randomised controlled cross‐over study. |
| Participants |
|
| Interventions | 'Window to Hope' group psychological treatment versus wait‐list control. |
| Outcomes |
Primary: Change in Beck Hopelessness Scale (BHS). Secondary: (1) Change in Beck Scale for Suicidal Ideation (BSS), (2) Change in Beck Depression Inventory (BDI‐II). |
| Starting date | January 2012 |
| Contact information | Lisa Brenner, VA Eastern Colorado Health Care System, Military Suicide Research Consortium (MSRC). |
| Notes | Study identification number on clinicaltrials.gov: NCT01691378 |
NCT02367521.
| Trial name or title | Repetitive Transcranial Magnetic Stimulation (rTMS) for the Treatment of Depression & Other Neuropsychiatric Symptoms After Traumatic Brain Injury (TBI) (rTMS TBI). |
| Methods | Randomised controlled trial. |
| Participants | TBI patients who score greater than 10 on the Hamiltion Depression Scale ‐ 17 item. |
| Interventions | Low Frequency Right sided repetitive transcranial magnetic stimulation (LFR rTMS) versus sham control. |
| Outcomes | Primary outcome: Number of participants with improvement in depressive symptoms using the HAM‐D scale, at 16 weeks follow‐up. (To determine the effectiveness of LFR rTMS for the treatment of post‐TBI depression and suicidal ideation.) Secondary outcome: Number of participants with improvement in overall functioning using the CGI scale, at 16 weeks follow‐up. (To determine the effectiveness of LFR rTMS for the treatment of post traumatic stress disorder, sleep disturbance and cognitive deficits.) |
| Starting date | March 2015 |
| Contact information | Vani Rao, MD vrao@jhmi.edu Alex Vassila avassil1@jhmi.edu |
| Notes | Sponsors and Collaborators: Johns Hopkins University, and United States Department of Defense. |
Differences between protocol and review
Search for studies: proceedings of the World Congress of Behavioral and Cognitive Therapies was not available.
Methods, Types of participants: "Where possible, the review will include tables providing categorisation by depressive conditions or symptom severity and stratification of studies by age group (child 0 to 12 years, adolescent 13 to 17 years, adult 18 to 64 years, and older adults 65 years or more)." This was not possible because the studies identified only included adults.
Contributions of authors
Paul Gertler: developed the concepts for the review, created the protocol with the assistance of the co‐authors, undertook and coordinated all aspects of the systematic review and authored the final publication. Robyn Tate: provided guidance and support in the conceptualisation of the review, provided assistance and editing in writing the protocol, culled abstracts and rated the methodological quality of the selected studies, and assisted with completion of the final publication. Ian Cameron: provided assistance in the development of the protocol, guidance during the search process and editing advice on the final publication.
Sources of support
Internal sources
-
Rehabilitation Studies Unit, Northern Clinical School, Sydney Medical School, The University of Sydney, Australia.
Infrastructure and support services
External sources
-
Australian Cochrane Centre, Australia.
Provision of introductory training and review completion workshops. Advice from Cochrane trainers and assistance in translation of an included study.
-
Cochrane Injuries Group, UK.
Provision of advice regarding trial registration. Assistance with design of the review protocol. Provision of search string for MEDLINE and translation for use in other databases. Provision of database search results abstracts and assistance locating studies that were not available online or in local libraries. Guidance during the study search phase of the review. Assistance in locating local training resources.
Declarations of interest
PG: None known.
RT: None known.
IC: None known.
New
References
References to studies included in this review
Ashman 2014 {published data only}
- Ashman T, Cantor J, Tsaousides T, Spielman L, Gordon W. Comparison of cognitive behavioral therapy and supportive psychotherapy for the treatment of depression following traumatic brain injury: a randomized controlled trial. Journal of Head Trauma Rehabilitation 2014;29:467‐78. [clinicaltrials.gov (ID: NCT00211835)] [DOI] [PubMed] [Google Scholar]
- Ashman T, Tsaousides T. Cognitive behavioral therapy for depression following traumatic brain injury: findings of a randomized controlled trial. Brain Impairment. Bergen, 2012; Vol. 13:124‐31. [DOI: 10.1017/BrImp.2012.10] [DOI]
Bedard 2013 {published data only}
- Bédard M, Felteau M, Marshall S, Cullen N, Gibbons C, Dubois S, et al. Mindfulness‐based cognitive therapy reduces symptoms of depression in people with a traumatic brain injury: results from a randomized controlled trial. Journal of Head Trauma Rehabilitation 2013;29(4):E13‐22. [Trialscentral.org (ID: NCT00745940)] [DOI] [PubMed] [Google Scholar]
Fann 2015 {published data only}
- Fann JR, Bombardier CH, Vannoy S, Dyer J, Ludman E, Dikmen S, et al. Telephone and in‐person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. Journal of Neurotrauma 2015;32(1):45‐57. [DOI: 10.1089/neu.2014.3423] [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2004 {published data only}
- He CS, Yu Q, Yang DJ, Yang M. Interventional effects of low‐frequency repetitive transcranial magnetic stimulation on patients with depression after traumatic brain injury. Chinese Journal of Clinical Rehabilitation 2004;8:6044‐5. [Google Scholar]
Hoffman 2010 {published data only}
- Hoffman JM, Bell KR, Powell JM, Behr J, Dunn EC, Dikmen S, et al. Randomized controlled trial of exercise to improve mood after traumatic brain injury. Physical Medicine and Rehabilitation 2010;2:911‐9. [DOI] [PubMed] [Google Scholar]
Simpson 2011 {published data only}
- Simpson GK, Tate RL, Whiting DL, Cotter RE. Suicide prevention after traumatic brain injury: a randomized controlled trial of a program for the psychological treatment of hopelessness. Journal of Head Trauma Rehabilitation 2011;26:290‐300. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anson 2006 {published data only}
- Anson K, Ponsford J. Evaluation of a coping skills group following traumatic brain injury. Brain Injury 2006;20:167‐78. [DOI] [PubMed] [Google Scholar]
Bateman 2001 {published data only}
- Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: A randomized controlled evaluation. Archives of Physical Medicine and Rehabilitation 2001;82:174‐82. [DOI] [PubMed] [Google Scholar]
Bell 2008 {published data only}
- Bell KR, Hoffman JM, Temkin NR, Powell JM, Fraser RT, Esselman PC, et al. The effect of telephone counselling on reducing post‐traumatic symptoms after mild traumatic brain injury: a randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79:1275‐81. [DOI] [PubMed] [Google Scholar]
Bombardier 2009 {published data only}
- Bombardier CH, Bell KR, Temkin NR, Fann JR, Hoffman J, Dikmen S. The efficacy of a scheduled telephone intervention for ameliorating depressive symptoms during the first year after traumatic brain injury. Journal of Head Trauma Rehabilitation 2009;24:230‐8. [DOI] [PubMed] [Google Scholar]
Bradbury 2008 {published data only}
Carey 2008 {published data only}
- Carey JC, Wade SL, Wolfe CR. Lessons learned: the effect of prior technology use on Web‐based interventions. Cyberpsychology & Behavior 2008;11(2):188‐95. [DOI: 10.1089/cpb.2007.0025.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullen 2007 {published data only}
- Cullen N, Chundamala J, Bayley M, Jutai J. The efficacy of acquired brain injury rehabilitation (Structured abstract). Brain Injury 2007;21:113‐32. [DOI] [PubMed] [Google Scholar]
Driver 2009 {published data only}
Fleming 2009 {published data only}
- Fleming J, Kuipers P, Foster M, Smith S, Doig E. Evaluation of an outpatient, peer group intervention for people with acquired brain injury based on the ICF 'environment' dimension. Disability and Rehabilitation 2009;31(20):1666‐75. [DOI: 10.1080/09638280902738425] [DOI] [PubMed] [Google Scholar]
Geurtsen 2008 {published data only}
- Geurtsen GJ, Martina JD, Heugten CM, Geurts AC. A prospective study to evaluate a new residential community reintegration programme for severe chronic brain injury: The Brain Integration Programme. Brain Injury 2008;22(7‐8):545‐54. [DOI] [PubMed] [Google Scholar]
Ghaffar 2006 {published data only}
- Ghaffar O, McCullagh S, Ouchterlony D, Feinstein A. Randomized treatment trial in mild traumatic brain injury. Journal of Psychosomatic Research 2006;61:153‐60. [DOI] [PubMed] [Google Scholar]
Huckans 2010 {published data only}
- Huckans M, Pavawalla S, Demadura T, Kolessar M, Seelye A, Roost N, et al. A pilot study examining effects of group‐based cognitive strategy training treatment on self‐reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. Journal of Rehabilitation Research and Development 2010;47:43‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonard 2004 {published data only}
- Leonard KN. Cognitive‐behavioral intervention in persistent postconcussion syndrome: A controlled treatment outcome study. Dissertation Abstracts International: Section B: The Sciences and Engineering. ProQuest Information & Learning. US, 2004:6332.
McDonald 2008 {published data only}
- McDonald S, Tate R, Togher L, Bornhofen C, Long E, Gertler P, et al. Social skills treatment for people with severe, chronic acquired brain injuries: a multicenter trial. Archives of Physical Medicine and Rehabilitation. School of Psychology, University of New South Wales, Sydney, NSW, Australia. s.mcdonald@unsw.edu.au, 2008; Vol. 89, issue 9:1648‐59. [DOI] [PubMed]
Powell 2002 {published data only}
- Powell J, Heslin J, Greenwood R. Community based rehabilitation after severe traumatic brain injury: a randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2002; Vol. 72:193‐202. [DOI] [PMC free article] [PubMed]
Ruff 1990 {published data only}
Smith 1994 {published data only}
- Smith RB, Tiberi A, Marshall J. The use of cranial electrotherapy stimulation in the treatment of closed‐head‐injured patients. Brain Injury 1994;8(4):357‐61. [DOI] [PubMed] [Google Scholar]
Stocksmeier 1992 {published data only}
- Stocksmeier U, Eberlein M. Depressive emotional deterioration in case of brain function disorders. TW Neurologie Psychiatrie 1992;6:74‐6. [Google Scholar]
Stoll 1999 {published data only}
- Stoll JL. Effects of therapeutic massage on the psychosocial adjustment of persons with brain injury. University of Wisconsin ‐ Madison. University of Wisconsin ‐ Madison, 1999.
Struchen 2011 {published data only}
- Struchen MA, Davis LC, Bogaards JA, Hudler‐Hull T, Clark AN, Mazzei DM, et al. Making connections after brain injury: Development and evaluation of a social peer‐mentoring program for persons with traumatic brain injury. Journal of Head Trauma Rehabilitation 2011;26:4‐19. [DOI] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun M, Zhan XP, Jin CY, Shan JZ, Xu S, Wang YL. Clinical observation on treatment of post‐craniocerebral traumatic mental disorder by integrative medicine. Chinese Journal of Integrative Medicine 2008;14:137‐41. [DOI] [PubMed] [Google Scholar]
Teasdale 1995 {published data only}
Tiersky 2005 {published data only}
Wade 2006 {published data only}
- Wade SL, Michaud L, Brown TM. Putting the pieces together: Preliminary efficacy of a family problem‐solving intervention for children with traumatic brain injury. Journal of Head Trauma Rehabilitation 2006;21(1):57‐67. [DOI] [PubMed] [Google Scholar]
Wade 2008 {published data only}
- Wade SL, Walz NC, Carey JC, Williams KM. Preliminary efficacy of a Web‐based family problem‐solving treatment program for adolescents with traumatic brain injury. The Journal of Head Trauma Rehabilitation 2008;23(6):369‐77. [DOI: 10.1097/01.HTR.0000341432.67251.48] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01039857 {published data only}
- Hofer H, Grosse Holftforth M, Stalder‐Luthy F, Frischknecht E, Muri R, Znoj H. Does an integrative neuro‐psychotherapy program foster the adjustment in depressed patients with an acquired brain injury? Joint Meeting of the FESN/GNP; 2013 Sept 12‐14; Berlin Germany. Behavioural Neurology. 2013:329‐30. [NCT01039857]
References to ongoing studies
Clark 2014 {published data only}
- Clark A. A randomized controlled trial of a modified group cognitive behavioural intervention for depressed mood following traumatic brain injury. Brain Injury 2014 Conference: 10th World Congress on Brain Injury of the International Brain Injury Association; 2014 March 19 ‐ 22; San Francisco, CA. 2014.
NCT00531258 {published data only}
- NCT00531258. Transcranial magnetic stimulation in the treatment of the sequelae of closed brain injury. www.clinicaltrials.gov 19 Sept 2012.
NCT01691378 {published data only}
- Brenner L, Simpson G, Forster J, Signoracci G, Matarazzo B, Clemans T. Window to hope: Preliminary results from a randomized controlled trial (RCT) of a psychological treatment for hopelessness among US veterans with traumatic brain injury (TBI). Brain Injury 2014;28(5‐6):737. [NCT01691378] [Google Scholar]
NCT02367521 {published data only}
- NCT02367521. Repetitive Transcranial Magnetic Stimulation (rTMS) for the Treatment of Depression & Other Neuropsychiatric Symptoms After Traumatic Brain Injury (TBI) (rTMS TBI). www.clinicaltrials.gov 14 December 2015.
Additional references
Alderfer 2005
- Alderfer BS, Arciniegas DB, Silver JM. Treatment of depression following traumatic brain injury. The Journal of Head Trauma Rehabilitation 2005;20:544‐62. [DOI] [PubMed] [Google Scholar]
APA 2000
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington D.C.: APA, 2000. [Google Scholar]
Barker‐Collo 2013
- Barker‐Collo S, Starkey N, Theadom A. Treatment for depression following mild traumatic brain injury in adults: A meta‐analysis. Brain Injury 2013;27:1124‐33. [DOI] [PubMed] [Google Scholar]
Bay 2009
- Bay E. Current treatment options for depression after mild traumatic brain injury. Current Treatment Options in Neurology 2009;11:377‐82. [DOI] [PubMed] [Google Scholar]
Bombadier 2010
- Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of Major Depressive Disorder and clinical outcomes following Traumatic Brain Injury. JAMA 2010;19:1938‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caldwell 2010
- Caldwell D, Hunot V, Moore THM, Davies P, Jones H, Lewis G, et al. Behavioural therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2010a
- Churchill R, Moore THM, Caldwell D, Davies P, Jones H, Furukawa TA, et al. Cognitive behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2010b
- Churchill R, Davies P, Caldwell D, Moore THM, Jones H, Lewis G, et al. Humanistic therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008700] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Churchill 2010c
- Churchill R, Davies P, Caldwell D, Moore THM, Jones H, Lewis G, et al. Interpersonal, cognitive analytic and other integrative therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008703] [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2010e
- Churchill R, Moore THM, Davies P, Caldwell D, Jones H, Lewis G, et al. Psychodynamic therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008706] [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2013
- Churchill R, Moore THM, Furukawa TA, Caldwell DM, Davies P, Jones H, et al. 'Third wave' cognitive and behavioural therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008705.pub2] [DOI] [PubMed] [Google Scholar]
Ciurli 2011
- Ciurli P, Formisano R, Bivona U, Cantagallo A, Angelelli P. Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. Journal of Head Trauma Rehabilitation 2011;26:116‐26. [DOI] [PubMed] [Google Scholar]
Cooney 2013
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD004366.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2012
- Cox GR, Callahan P, Churchill R, Hunot V, Merry SN, Parker AG, et al. Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008324.pub2] [DOI] [PubMed] [Google Scholar]
Davies 2010
- Davies P, Hunot V, Moore THM, Caldwell D, Jones H, Lewis G, et al. Humanistic therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008701] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deb 1999
- Deb S, Lyons I, Koutzoukis C, Ali I, McCarthy G. Rate of psychiatric illness 1 year after traumatic brain injury. American Journal of Psychiatry 1999;156:374‐8. [DOI] [PubMed] [Google Scholar]
Dikmen 2004
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2004;85:1457‐64. [DOI] [PubMed] [Google Scholar]
Fann 2009
- Fann JR, Hart T, Schomer KG. Treatment of depression after traumatic brain injury: A systematic review. Journal of Neurotrauma 2009;26:2383‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2011
- Fitzgerald PB, Hoy KE, Maller JJ, Herring S, Segrave R, McQueen S, et al. Transcranial magnetic stimulation for depression after a traumatic brain injury: a case study. Journal of ECT 2011;27:38‐40. [DOI] [PubMed] [Google Scholar]
Green 2001
- Green A, Felmingham K, Baguley IJ, Slewa‐Younan S, Simpson S. The clinical utility of the Beck Depression Inventory after traumatic brain injury. Brain Injury 2001;15:1021‐8. [DOI] [PubMed] [Google Scholar]
Guillamondegui 2011
- Guillamondegui OD, Montgomery SA, Phibbs FT, McPheeters ML, Alexander PT, Jerome RN, et al. Traumatic Brain Injury and Depression. Comparative Effectiveness Review No. 25. (Prepared by the Vanderbilt Evidence‐based Practice Center under Contract No. 290‐2007‐10065‐I.) AHRQ Publication No. 11‐EHC017‐EF. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm.. Rockville, MD: Agency for Healthcare Research and Quality, April 2011. [PubMed]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henken 2007
- Henken T, Huibers MJH, Churchill R, Restifo KK, Roelofs JJ. Family therapy for depression. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006728] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunot 2010
- Hunot V, Moore THM, Caldwell D, Davies P, Jones H, Furukawa TA, et al. Cognitive behavioural therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunot 2010a
- Hunot V, Moore THM, Caldwell D, Davies P, Jones H, Lewis G, et al. Interpersonal, cognitive analytic and other integrative therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunot 2013
- Hunot V, Moore THM, Caldwell DM, Furukawa TA, Davies P, Jones H, et al. 'Third wave' cognitive and behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008704.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorm 2008
- Jorm AF, Morgan AJ, Hetrick SE. Relaxation for depression. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007142.pub2] [DOI] [PubMed] [Google Scholar]
Kay 1993
- Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, et al. Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation 1993;8:86‐7. [Google Scholar]
Khan‐Bourne 2003
- Khan‐Bourne N, Browne RG. Cognitive behaviour therapy for the treatment of depression in individuals with brain injury. Neuropsychological Rehabilitation 2003;13:89‐107. [DOI] [PubMed] [Google Scholar]
Larun 2006
- Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004691.pub2] [DOI] [PubMed] [Google Scholar]
Leiknes 2011
- Leiknes KA, Berg RC, Smedslund G, Jarosch‐von Schweder SL, Øverland S, Hammerstrøm KT, et al. Electroconvulsive therapy for depression. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD009105] [DOI] [Google Scholar]
Maas 2008
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurology 2008;7(8):728‐41. [DOI] [PubMed] [Google Scholar]
Maratos 2008
- Maratos AS, Gold C, Wang X, Crawford MJ. Music therapy for depression. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004517.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore THM, Hunot V, Davies P, Caldwell D, Jones H, Lewis G, et al. Psychodynamic therapies versus treatment as usual for depression. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008707] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence (NICE). Depression: The treatment and management of depression in adults. CG90. National Institute for Health and Clinical Excellence. London, 2009.
Ownsworth 2008
- Ownsworth T, Trudi L, Turner B, Hawkes A, Shum D. Assessing emotional status following acquired brain injury: The clinical potential of the depression, anxiety and stress scales. Brain Injury 2008;22:858‐69. [DOI] [PubMed] [Google Scholar]
Poggi 2005
- Poggi G, Liscio M, Adduci A, Galbiati S, Massimino M, Sommovigo M, et al. Psychological and adjustment problems due to acquired brain lesions in childhood: a comparison between post‐traumatic patients and brain tumour survivors. Brain Injury 2005;19(10):777‐85. [DOI] [PubMed] [Google Scholar]
Rapoport 2012
- Rapoport 2012. Depression following traumatic brain injury: Epidemiology, risk factors and management. CNS Drugs 2012;26:111‐21. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Martin 2001
- Rodriguez‐Martin JL, Barbanoj JM, Schlaepfer TE, Clos SSC, Pérez V, Kulisevsky J, et al. Transcranial magnetic stimulation for treating depression. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenthal 1998
- Rosenthal M, Christensen BK, Ross TP. Depression following traumatic brain injury. Archives of Physical Medicine and Rehabilitation 1998;79:90‐103. [DOI] [PubMed] [Google Scholar]
Shinohara 2013
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008696.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silver 2009
- Silver JM, McAllister TW, Arciniegas DB. Depression and cognitive complaints following mild traumatic brain injury. American Journal of Psychiatry 2009;166:653‐61. [DOI] [PubMed] [Google Scholar]
Simpson 2002
- Simpson G, Tate R. Suicidality after traumatic brain injury: demographic, injury and clinical correlates. Psychological Medicine 2002;32:687‐97. [DOI] [PubMed] [Google Scholar]
Slade 2009
- Slade T, Johnston A, Oakley‐Browne MA, Andrews G, Whiteford H. 2007 National Survey of Mental Health and Wellbeing: methods and key findings. Australian and New Zealand Journal of Psychiatry 2009;43:594‐605. [DOI] [PubMed] [Google Scholar]
Sliwinski 1998
- Sliwinski M, Gordon W, Bogdany J. The Beck Depression Inventory: Is it a suitable measure of depression for individuals with traumatic brain injury?. The Journal of Head Trauma Rehabilitation 1998;13:40‐6. [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith CA, Hay PP, MacPherson H. Acupuncture for depression. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD004046.pub3] [DOI] [PubMed] [Google Scholar]
Snell 2009
- Snell DL, Surgenor LJ, Hay‐Smith EJC, Siegert RJ. A systematic review of psychological treatments for mild traumatic brain injury: An update on the evidence. Journal of Clinical and Experimental Neuropsychology 2009;31:20‐38. [DOI] [PubMed] [Google Scholar]
Tuunainen 2004
- Tuunainen A, Kripke DF, Endo T. Light therapy for non‐seasonal depression. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vattakatuchery 2013
- Vattakatuchery J, Lathif N, Joy J, Cavanna A, Rickards HE. Pharmacological interventions for depression in people with traumatic brain injury. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010419] [DOI] [Google Scholar]
Waldron 2013
- Waldron B, Casserly LM, O'Sullivan C. Cognitive behavioural therapy for depression and anxiety in adults with acquired brain injury. What works for whom?. Neuropsychological Rehabilitation 2013 [Epub 2012 Nov 5];23(1):64‐101. [DOI: 10.1080/09602011.2012.724196] [DOI] [PubMed] [Google Scholar]
Whitnall 2006
- Whitnall L, McMillan TM, Murray GD, Teasdale GM. Disability in young people and adults after head injury: 5‐7 year follow up of a prospective cohort study. Journal of Neurology, Neurosurgery and Psychiatry 2006;77:640‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization (WHO). The ICD‐10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Manual. Geneva: WHO, 1992. [Google Scholar]


