Abstract
Microorganisms, especially gram-negative bacteria, are considered to play a role in the etiology of certain corneal infiltrative events (CIEs) observed during soft contact lens wear. This study explored the possibility of microbial colonization of soft contact lenses as a risk factor leading to CIEs. In a clinical trial conducted from March 1993 to January 1996, 330 subjects wore disposable soft contact lenses on a 6-night extended-wear and disposal schedule. During this period, 4,321 lenses (118 during CIEs; 4,203 during asymptomatic lens wear) were recovered aseptically and analyzed for microbial colonization. A greater percentage of lenses were free from microbial colonization during asymptomatic wear than during CIEs (42 versus 23%; P < 0.0001). The incidence of gram-positive bacteria, gram-negative bacteria and fungi was greater during CIEs than during asymptomatic lens wear (P < 0.05). During asymptomatic lens wear, gram-positive bacteria were isolated most frequently and were usually normal external ocular microbiota. Of the gram-positive bacteria, the incidence of Streptococcus pneumoniae was greater during CIE than during asymptomatic wear (7.6 versus 0.6%; P < 0.0001). While gram-negative bacteria were seen in few cases during asymptomatic wear, their incidence during CIE in comparison to asymptomatic wear was substantial and significant (23.7 versus 3.8%; P < 0.0001). Also, the level of colonization was high. Of CIEs, events of microbial keratitis, contact lens acute red eye, and asymptomatic infiltrative keratitis were associated with lens colonization with gram-negative bacteria or S. pneumoniae. Colonization of soft contact lenses with pathogenic bacteria, especially gram-negative bacteria and S. pneumoniae, appears to be a significant risk factor leading to CIE.
Soft contact lenses worn on an extended-wear (EW) schedule are associated with infective and infiltrative conditions of the cornea. Infectious keratitis with contact lens wear is caused predominantly by gram-negative bacteria, with Pseudomonas aeruginosa being the leading cause of lens-related infections (17). In many events the offending microorganism is isolated from the corneal scrape and also from the contact lens (6, 12). Also, during contact lens acute red eye (CLARE) and corneal infiltrative events (CIEs), significant numbers of gram-negative bacteria such as P. aeruginosa, Serratia marcescens, and Haemophilus influenzae have been isolated from the contact lens (4, 11). This suggests that microbial colonization of contact lenses is a probable risk factor for such infiltrative events.
Studies on microbial colonization of contact lenses have focused mainly on colonization during normal lens wear. Lenses are colonized mainly by low numbers of external ocular microbiota. Coagulase-negative staphylococci followed by Corynebacterium spp. and Bacillus spp. are the most frequently isolated microbes, and pathogenic microorganisms such as gram-negative bacteria are found in only a small percentage of samples (2, 3, 5, 9).
To date, microbial colonization of contact lenses during normal lens wear and during CIEs for a single population has not been studied. We examined the microbial colonization of lenses during asymptomatic lens wear and during CIEs in a group of subjects using disposable soft contact lenses on an EW schedule. This project aimed to determine (i) whether the type of microorganisms found during an adverse event were different to those seen during asymptomatic lens wear and (ii) whether the level of colonization on the lenses (number of CFU) was different during a CIE than in asymptomatic lens wear. The information is useful in determining whether microbial colonization is involved in the etiology of contact lens-induced corneal infiltration.
MATERIALS AND METHODS
Study population.
Three hundred thirty subjects with no prior lens wear experience wore disposable soft contact lenses on a 6-night EW and replacement schedule in a prospective trial conducted at the L. V. Prasad Eye Institute, Hyderabad, India. The study was conducted from March 1993 to January 1996. All subjects were aged between 16 and 39 years, had visual acuity of 6/9 or better with correction, were free of ocular or systemic disease and had signed an informed consent.
Study procedure.
Subjects were fitted with one of two different marketed disposable hydrogel lenses in each eye (Food and Drug Administration group I, low water content, nonionic hydrogel; Food and Drug Administration group IV, high water content, ionic hydrogel). Lens wear began on a daily wear basis for a minimum of 2 weeks to ensure adaptation to lens wear. Following this, EW on a 6-night wear schedule was commenced. Subjects were examined at 24 h and at 1 week to assess the ocular response to EW. Thereafter they were seen at 1 month of EW and then at three monthly intervals.
At each visit, prior to ocular examination, contact lenses were removed from eyes using aseptic procedures (with sterile gloves), placed in a vial with 2 ml of sterile phosphate-buffered saline, and immediately transported to the laboratory where they were stored at 4°C until processing. Lenses were processed every 3 h. A detailed evaluation of the patients' eyes was made using a Zeiss slit-lamp biomicroscope. A detailed history was conducted, which included questions on general health (e.g., any illness or respiratory tract infections since the last visit).
Adverse events.
All subjects were advised to present at the clinic for any events they considered were unusual for lens wear. At the clinic, a brief examination was conducted using the slit-lamp biomicroscope to ascertain the nature of the event. Contact with the eye or lid margins and use of bright lights was avoided during this procedure. In the event of an adverse reaction, contact lenses were removed from the eyes using aseptic procedures and sent for microbiological analysis.
Microbiological analysis of contact lenses.
Lenses were vortexed (1 min) in the transport medium (2 ml of phosphate-buffered saline), removed aseptically, and cultured using an agar sandwich technique. A description of this technique is given in detail elsewhere (11). The agar plates (chocolate) were incubated under aerobic, 5% CO2 and anaerobic conditions for 48 h. Also, a Sabouraud agar plate was maintained at room temperature for 7 days.
The number of CFU on all agar plates was recorded at 48 h, anaerobic plates were reincubated for up to 5 days at 35°C, and Sabouraud agar plates were reincubated for 5 days at ambient temperature. Colonies were enumerated, and bacteria were identified by Gram stain. Further identification was conducted using a combination of API strips (bioMerieux, Marcy l'Etoile, France) and biochemical tests.
Data analysis.
The incidence of each microbial species isolated during asymptomatic lens wear in comparison to that during CIE was determined using Fisher's exact test. The level of significance was maintained at P < 0.05. The level of colonization was recorded as CFU per lens and reported as median colonization. The range of colonization is also given.
RESULTS
Number of contact lenses.
A total of 4,321 contact lenses were sampled from the 330 subjects during the EW phase of the study. The duration in EW for these subjects was 12.8 ± 10 months and ranged from 1 night to 42 months of EW. The number of lens sampling occasions per subject was 17 ± 3 and ranged from 2 to 45 samples (includes both eyes). Of these, 118 lenses were recovered during CIEs and the remaining 4,203 lenses were recovered during asymptomatic lens wear.
Microbial colonization of contact lenses.
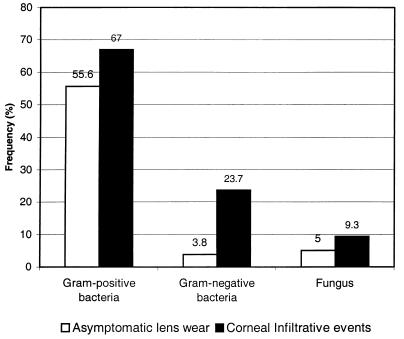
The frequency of negative cultures (no microorganisms on lenses) was significantly greater during asymptomatic lens wear in comparison to CIE (Fig. 1) (42 versus 23%; P < 0.0001). In contrast, the frequency of isolation of gram-positive bacteria, gram-negative bacteria, and fungi was significantly greater at CIE in comparison to asymptomatic lens wear (P = 0.0036, P < 0.0001, and P < 0.0215, respectively).
FIG. 1.
Frequencies of bacteria and fungi during asymptomatic lens wear and CIEs.
Gram-positive bacteria.
Table 1 lists the incidence and level of colonization of gram-positive bacteria isolated during asymptomatic lens wear in comparison to CIE.
TABLE 1.
Frequencies of isolation of gram-positive bacteria during asymptomatic lens wear and CIEs
| Microorganism | Isolation of bacterium during:
|
P | |||
|---|---|---|---|---|---|
| Asymptomatic lens wear (n = 4,203)
|
CIE (n = 118)
|
||||
| Incidence (%) | Median CFU (range) | Incidence (%) | Median CFU (range) | ||
| S. epidermidis | 41.4 | 4 (1–1,800) | 40.7 | 5 (1–620) | 0.924 |
| Corynebacterium sp. | 9.1 | 2 (1–1,300) | 12.7 | 2 (1–68) | 0.192 |
| Micrococcus sp. | 8.4 | 3 (1–740) | 6.8 | 5 (1–15) | 0.734 |
| S. aureus | 6.3 | 3 (1–615) | 9.3 | 3 (1–398) | 0.180 |
| Bacillus sp. | 4.7 | 2 (1–500) | 8.5 | 4 (1–500) | 0.075 |
| S. pneumoniae | 0.6 | 143 (2–1,800) | 7.6 | 48 (3–525) | <0.0001a |
| Staphylococcus sp. | 6.0 | 2 (1–500) | 4.2 | 2 (2–33) | 0.554 |
| Propionibacterium sp. | 0.9 | 15 (2–1,500) | 1.7 | 10 (1,500) | 0.309 |
| Propionibacterium acnes | 0.5 | 5 (2–375) | 1.7 | 20 (100) | 0.138 |
| A. viridans | 0.1 | 2 (2) | 0 | 1.000 | |
| Nocardia sp. | 0.1 | 3 (1–3) | 0 | 1.000 | |
| Streptomyces sp. | 0.1 | 5 (1–30) | 0 | 1.000 | |
| Alpha-hemolytic streptococcus | 0.5 | 4 (1–500) | 0.9 | 2 | 0.425 |
| Peptostreptococcus sp. | 0.1 | 3 (2–5) | 0 | 1.000 | |
Mann-Whitney U test, 0.677.
During asymptomatic lens wear, Staphylococcus epidermidis, Corynebacterium spp., Micrococcus spp., and Staphylococcus aureus were the most frequently identified bacteria and Streptococcus spp., Peptostreptococcus spp., Aerococcus viridans, and Nocardia spp. were the least common. The median level of colonization was ≤15 CFU per lens for all gram-positive bacteria except Streptococcus pneumoniae, which had a median level of colonization of 143 CFU per lens.
During CIEs S. epidermidis, Corynebacterium spp., S. aureus, Bacillus spp., and S. pneumoniae were most frequently identified. The frequency of isolation of S. pneumoniae was significantly greater at CIEs in comparison to asymptomatic lens wear (7.6 versus 0.6%; P < 0.0001). As with asymptomatic lens wear, the median colonization or CFU was low, ≤15 CFU for all bacteria except for S. pneumoniae, which had a median level of colonization of 48 CFU per lens. The median S. pneumoniae levels of colonization during asymptomatic lens wear and during CIEs were not statistically different (P = 0.677 [Mann-Whitney U test]).
Gram-negative bacteria.
During asymptomatic lens wear, the frequency of isolation of gram-negative bacteria was less than or equal to 1.0% for any species. Pseudomonas spp., followed by Acinetobacter calcoaceticus and H. influenzae, was most frequently isolated (Table 2). Many of the gram-negative bacteria isolated had median colonizations greater than 10 CFU per lens, including Aeromonas hydrophila, Escherichia coli, Haemophilus spp., H. influenzae, Pseudomonas spp., and Citrobacter spp.
TABLE 2.
Frequencies of isolation of gram-negative bacteria during asymptomatic lens wear and CIEs
| Microorganism | Isolation of bacterium during:
|
P | |||
|---|---|---|---|---|---|
| Asymptomatic lens wear (n = 4,203)
|
CIE (n = 118)
|
||||
| Incidence (%) | Median CFU (range) | Incidence (%) | Median CFU (range) | ||
| Pseudomonas sp. | 1.0 | 18 (1–2,575) | 2.5 | 320 (8–1,800) | 0.111 |
| Haemophilus influenzae | 0.6 | 68 (2–1,800) | 9.3 | 200 (4–1,308) | <0.0001b |
| Acinetobacter calcoaceticus | 0.6 | 8 (1–580) | 1.7 | 6 (1–10) | 0.148 |
| Moraxella sp. | 0.4 | 5 (1,800) | 0.9 | 4 | 0.376 |
| Gram-negative bacillusa | 0.2 | 10 (1–1,000) | 0 | 1.000 | |
| Acinetobacter sp. | 0.2 | 3 (1–13) | 0 | 1.000 | |
| Klebsiella pneumoniae | 0.2 | 5 (2–20) | 0.9 | 1,800 | 0.199 |
| Pseudomonas aeruginosa | 0.2 | 7 (5–18) | 1.7 | 1,800 (1,800) | 0.0142e |
| Enterobacter sp. | 0.2 | 3 (1–312) | 0.9 | 20 | 0.272 |
| Aeromonas hydrophila | 0.1 | 503 (4–1,133) | 0 | 1.000 | |
| Escherichia coli | 0.1 | 18 (2–300) | 0.9 | 900 | 0.176 |
| Flavobacterium sp. | 0.1 | 2 (4) | 0 | 1.000 | |
| Haemophilus sp. | 0.1 | 887 (2–1800) | 2.5 | 12 (5–903) | 0.001d |
| Methylobacterium sp. | 0.1 | 1 | 0 | 1.000 | |
| Neisseria sp. | 0.1 | 2 (1–5) | 0 | 1.000 | |
| Citrobacter sp. | 0.1 | 305 (2–348) | 0.9 | 365 | 0.105 |
| Serratia marcescens | 0.1 | 1 (6) | 0 | 1.000 | |
| Haemophilus parainfluenzae | —f | 2 | 3.4 | 704 (30–1,303) | <0.0001c |
With the biochemical techniques adopted in the study, these bacilli could not be identified further.
Mann-Whitney U test, 0.468.
Mann-Whitney U test, 0.4.
Mann-Whitney U test, 0.571.
Mann-Whitney U test, 0.095.
—, n = 1.
During CIEs, H. influenzae, Haemophilus parainfluenzae, Haemophilus spp., and Pseudomonas spp. were most frequently isolated, and the incidence of these bacteria was significantly greater at a CIE than during asymptomatic lens wear (P ≤ 0.001). While the median CFU of these bacteria on the lens during CIE was high in comparison to asymptomatic lens wear, the differences in CFU were not significant.
Fungi.
Table 3 lists the incidence and level of colonization in CFU of fungi isolated during asymptomatic lens wear versus CIE.
TABLE 3.
Frequencies of isolation of fungi during asymptomatic lens wear and CIEs
| Microorganism | Isolation of fungus during:
|
P | |||
|---|---|---|---|---|---|
| Asymptomatic lens wear (n = 4,203)
|
CIE (n = 118)
|
||||
| Incidence (%) | Median CFU (range) | Incidence (%) | Median CFU (range) | ||
| Cladosporium sp. | 1.6 | 5 (1–600) | 2.5 | 5 (3–10) | 0.444 |
| Aspergillus flavus | 0.7 | 5 (1–700) | 3.4 | 3 (1–5) | 0.011a |
| Aspergillus niger | 0.7 | 5 (1–5) | 0.9 | 5 | 0.574 |
| Unidentified dematiaceous fungus | 0.3 | 5 (1–6) | 0 | 1.000 | |
| Unidentified hyaline fungus | 0.3 | 5 (2–300) | 0.9 | 7 | 0.340 |
| Aspergillus fumigatus | 0.3 | 5 (2–5) | 1.7 | 5 (5) | 0.047b |
| Curvularia sp. | 0.2 | 3 (1–1,000) | 0 | 1.000 | |
| Aspergillus terreus | 0.1 | 2 (1–5) | 0.9 | 1 | 0.129 |
| Alternaria sp. | 0.1 | 5 (1–5) | 0 | 1.000 | |
| Bipolaris spicifera | 0.1 | 3 (1–5) | 0 | 1.000 | |
| Candida tropicalis | 0.1 | 2 (16) | 0 | 1.000 | |
| Candida sp. | 0.1 | 6 (1–16) | 0.9 | 12 | 0.153 |
| Fusarium sp. | 0.1 | 5 (5–6) | 0 | 1.000 | |
| Mucor sp. | 0.1 | 1 (5) | 0 | 1.000 | |
| Paecilomyces sp. | 0.1 | 3 (3) | 0 | 1.000 | |
| Penicillium sp. | 0.1 | 2 (1–35) | 0 | 1.000 | |
| Rhizopus sp. | 0.1 | 5 (5) | 1.7 | 13 (5–21) | 0.004 |
| Saccharomyces cerevisiae | 0.1 | 3 (5) | 0 | 1.000 | |
| Aspergillus sp. | 0.1 | 5 (1–5) | 0.9 | 5 | 0.176 |
| Humicola sp. | 0.1 | 3 (1–5) | 0 | 1.000 | |
Mann-Whitney U test, 0.407.
Mann-Whitney U test, 0.641.
The incidence and median level of colonization of fungi was low during both asymptomatic lens wear and CIE. While the incidence of Aspergillus flavus, Aspergillus fumigatus, and Rhizopus spp. was significantly greater at CIEs, the level of colonization was low and there were no differences in the median levels of colonization in comparison to asymptomatic lens wear.
CIE.
The type of CIE seen during the study period and the number of lens samples retrieved for each of the events were as follows: microbial keratitis (MK) (1 sample), contact lens acute red eye (CLARE) (35 samples), contact lens peripheral ulcers (CLPU) (34 samples), infiltrative keratitis (IK) (16 samples), and asymptomatic infiltrative keratitis (AIK) (32 samples). A detailed description of the signs and symptoms of each of these events is presented in detail elsewhere (10).
MK.
Only one event of MK was seen. Significant levels of P. aeruginosa (1,800 CFU) were isolated from the lens, and the organism was also isolated from the corneal scrape. No other organisms were seen on the lens.
CLARE.
Of the 35 lens samples from CLARE events, 17 (49%) had lenses colonized by gram-negative bacteria. The most frequent of these were H. influenzae (22.9%), H. parainfluenzae (8.6%), and Haemophilus spp (5.7%). A majority of lenses had colonization exceeding 300 CFU. S. pneumoniae was isolated from three samples (8.6%), and normal ocular microbiota were isolated from 12 samples (34%). No microorganisms were isolated from lenses in three samples (8.6%).
CLPU.
Of the 34 lens samples from cases of CLPU, 4 lenses were found to be colonized with gram-negative bacteria. The level of colonization was low except for one sample with 320 CFU of Pseudomonas spp. isolated from the lens. Gram-positive ocular microbiota were isolated from lenses in 18 samples (53%). The level of colonization was low and not significantly higher than during asymptomatic wear. No microorganisms were isolated from lenses in 12 events (35%).
IK.
No microorganisms were isolated from lenses in 4 of the 16 (25%) events of IK. H. influenzae was isolated from one sample at 4 CFU per lens. Lenses from most of the remaining cases were colonized with low numbers (<100 CFU) of gram-positive ocular bacteria.
AIK.
No microorganisms were isolated from 8 of the 32 lens samples from cases of AIK (25%). Five lenses (16%) were colonized with S. pneumoniae, and seven (22%) were colonized with gram-negative bacteria. Of the lenses colonized with gram-negative bacteria, two were heavily colonized (1,800 CFU each) with P. aeruginosa and Pseudomonas spp., respectively. It is interesting that in spite of the heavy colonization of the lenses, the event remained asymptomatic. Some of the other organisms isolated were H. influenzae, H. parainfluenzae, and Haemophilus spp. Three events (9%) had lenses colonized with low levels of fungi, and the remaining events (28%) had lenses colonized with low numbers of gram-positive ocular microbiota.
DISCUSSION
We report the type and level of microbial colonization of contact lenses during asymptomatic EW in comparison to CIE in a single population of extended soft-lens wearers. During asymptomatic EW, 42% of contact lenses were not colonized by any microorganism despite being worn continuously for 6 nights. In contrast, only 22% of the lenses recovered at the time of an infiltrative event were not colonized. This indicates that microbial colonization of lenses is likely to play a role in the etiology of many CIE.
The results on the frequency of isolation of negative lens cultures during asymptomatic EW concur with the frequency of negative cultures from other reports in the literature. Gopinathan et al. reported the microbial colonization levels for a set of 50 subjects monitored for a period of 18 months and found no microorganisms on 46 to 47% of the lenses (2). In another study, 62% of contact lenses used on a daily-wear basis were not colonized with any microorganisms (3).
During asymptomatic EW, lenses were predominantly colonized with gram-positive bacteria such as S. epidermidis, Corynebacterium spp., Micrococcus spp., S. aureus, Staphylococcus spp., and Bacillus spp. These organisms constitute normal ocular microbiota (1, 8, 13, 15, 16). The median number of normal ocular bacteria did not exceed 5 CFU/lens except for Propionibacterium spp. These results are in agreement with work published by Hart et al. who found counts of fewer than 10 CFUs for 89% of soft lenses worn either on a daily wear or EW schedule (3). Similarly, another study showed that the level of colonization in CFU for Staphylococci spp. isolated from lens cultures, upper bulbar conjunctiva, and lower lid margin is on average less than 3 CFU for each site (7). The reason why resident ocular biota are found on lenses in such low numbers is unclear.
Nonocular or pathogenic bacteria such as S. pneumoniae or gram-negative bacteria were isolated less frequently but at significantly high levels in comparison to ocular bacteria. The predominant organisms included S. pneumoniae, Haemophilus spp., A. hydrophila, H. influenzae, and Citrobacter spp. It is probable that, for these organisms, increased colonization is facilitated by mechanisms of adherence such as the production of a protective biofilm. For example, P. aeruginosa is known to secrete an anionic, polysaccharide matrix called biofilm on the lens surface in which the organisms are known to metabolize and reproduce (14). We believe that these pathogens are likely contaminants from other body sites such as the oropharynx or the lacrimal sac (10, 11). Fungi were infrequently isolated during asymptomatic lens wear and CIE. When fungi were present, the levels of colonization were low and are unlikely to be associated with CIE. The level of fungal colonization was shown to be slightly greater in Indian eyes in comparison to those of Australians, which may be a result of environmental differences (2).
In comparison to asymptomatic lens wear, the relatively high incidence of many nonocular and pathogenic bacteria such as H. influenzae, S. pneumoniae, H. parainfluenzae, Haemophilus spp., and P. aeruginosa found at high levels during CIE suggests a likely role for these bacteria in the development of the corneal infiltrates.
A relationship between the type of bacterial species and the type of CIE was also clear from our study. The recovery of P. aeruginosa from the lens as well as the corneal scrape of the single case of MK suggests a highly probable scenario of lens colonization with the bacteria preceding corneal infection. Similarly, a significant relationship existed between gram-negative bacteria isolated from the contact lenses and CLARE. Forty-nine percent (49%) of the lenses sampled during CLARE were colonized with large numbers of bacteria such as H. influenzae, H. parainfluenzae, and Haemophilus spp. A relationship between gram-negative-organism colonization of the lenses and infiltrates was also seen for events of AIK, where 7 of the 32 lenses were found to be colonized. Also, in an additional five events of AIK and three events of CLARE lenses were colonized with S. pneumoniae. Colonization of lenses did not appear to be associated with CLPU and IK. Previous studies have also demonstrated a link between colonization of the contact lens with gram-negative bacilli and induction of a CLARE response (4).
While MK is a direct microbial infection, other events appeared to be acute inflammatory episodes which resolved simply on discontinuation of lens wear within a short period of time (usually within days) without the need for any medical intervention. Previously we hypothesized that events such as CLARE and AIK are acute inflammatory reactions to the presence of bacterial toxins, enzymes, and by-products leaching the contact lens and provoking an infiltrative response of the cornea (10, 11).
While it is clear that pathogens such as Haemophilus and S. pneumoniae are likely etiological agents of CIE, it is interesting to observe that a small percentage of lenses were not associated with an event despite significant lens colonization with these pathogens. In a previous study, it was seen that colonized contact lenses are not the only predisposing factor for corneal infiltration (4). In this study, when contact lenses, inadvertently colonized with high levels of gram-negative bacteria, were inserted on healthy human eyes and worn overnight, only 42% of the eyes developed corneal infiltration. Factors such as the resident time of the bacteria on the lens in contact with the ocular surface, variation in the pathogenicity of the bacterial strain, and individual variation in the defense profile of the external eye could all play a role in the development of an infiltrative event in some patients and not in others.
In summary, we have shown that lens colonization during asymptomatic EW is primarily composed of resident ocular biota at low levels. In contrast, lens colonization during CIE is primarily composed of pathogenic bacteria, especially gram-negative bacteria and S. pneumoniae at high levels for events such as MK, CLARE, and AIK.
ACKNOWLEDGMENTS
This research was conducted with the support of the Hyderabad Eye Research Foundation, Hyderabad, India, and the Australian Federal Government through the Cooperative Research Centres Programme. Also, grant support from Vistakon, Johnson & Johnson is acknowledged.
We also thank Serina Stratton for her input during final stages of the preparation of the manuscript.
REFERENCES
- 1.Cagle G D, Abshire R L. Quantitative ocular bacteriology: a method for the enumeration and identification of bacteria from the skin-lash margin and conjunctiva. Investig Ophthalmol Vis Sci. 1981;20:751–757. [PubMed] [Google Scholar]
- 2.Gopinathan U, Stapleton F, Sharma S, Willcox M D, Sweeney D F, Rao G N, Holden B A. Microbial contamination of hydrogel contact lenses. J Appl Microbiol. 1997;82:653–658. doi: 10.1111/j.1365-2672.1997.tb03598.x. [DOI] [PubMed] [Google Scholar]
- 3.Hart D E, Reindel W, Proskin H M, Mowrey-McKee M F. Microbial contamination of hydrophilic contact lenses: quantitation and identification of microorganisms associated with contact lenses while on the eye. Optom Vis Sci. 1993;70:185–191. doi: 10.1097/00006324-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Holden B A, La Hood D, Grant T, Newton-Howes J, Baleriola-Lucas C, Willcox M D, Sweeney D F. Gram-negative bacteria can induce contact lens related red eye (CLARE) responses. CLAO J. 1996;22:47–52. [PubMed] [Google Scholar]
- 5.Hovding G. The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol. 1981;59:387–401. doi: 10.1111/j.1755-3768.1981.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 6.Krachmer J H, Purcell J J., Jr Bacterial corneal ulcers in cosmetic soft contact lens wearers. Arch Ophthalmol. 1978;96:57–61. doi: 10.1001/archopht.1978.03910050021005. [DOI] [PubMed] [Google Scholar]
- 7.Leitch E C, Harmis N Y, Corrigan K M, Willcox M D P. Identification and enumeration of staphylococci from the eye during soft contact lens wear. Optom Vis Sci. 1998;75:258–265. doi: 10.1097/00006324-199804000-00022. [DOI] [PubMed] [Google Scholar]
- 8.McNatt J, Allen S D, Wilson L A, Dowell V R. Anaerobic flora of the normal human conjunctival sac. Arch Ophthalmol. 1978;96:1448–1450. doi: 10.1001/archopht.1978.03910060196020. [DOI] [PubMed] [Google Scholar]
- 9.Mowrey-McKee M F, Monnat K, Sampson H J, Smith C M, Davies G A, Mandt L, Proskin H M. Microbial contamination of hydrophilic contact lenses. Part I: quantitation of microbes on patient worn and handled lenses. CLAO J. 1992;18:89–91. [PubMed] [Google Scholar]
- 10.Sankaridurg P R, Sweeney D F, Sharma S, Gora R, Naduvilath T, Ramachandran L, Holden B A, Rao G N. Adverse events with extended wear of disposable hydrogels: results for the first thirteen months of lens wear. Ophthalmology. 1999;106:1671–1680. doi: 10.1016/S0161-6420(99)90346-9. [DOI] [PubMed] [Google Scholar]
- 11.Sankaridurg P R, Willcox M D, Sharma S, Gopinathan U, Janakiraman D, Hickson S, Vuppala N, Sweeney D F, Rao G N, Holden B A. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34:2426–2430. doi: 10.1128/jcm.34.10.2426-2431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivitz I A. Bilateral simultaneous Pseudomonas keratitis with myopic extended wear contact lenses. Ann Ophthalmol. 1987;19:204–206. [PubMed] [Google Scholar]
- 13.Singer T R, Isenberg S J, Apt L. Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br J Ophthalmol. 1988;72:448–451. doi: 10.1136/bjo.72.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slusher M M, Myrvik Q N, Lewis J C, Gristina A G. Extended-wear lenses, biofilm, and bacterial adhesion. Arch Ophthalmol. 1987;105:110–115. doi: 10.1001/archopht.1987.01060010116042. [DOI] [PubMed] [Google Scholar]
- 15.Smith C H. Bacteriology of the healthy conjunctiva. Br J Ophthalmol. 1954;38:719–726. doi: 10.1136/bjo.38.12.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stapleton F, Willcox M D P, Fleming C F, Hickson S, Sweeney D F, Holden B A. Changes to the ocular biota with time in extended and daily wear disposable contact lens use. Infect Immun. 1995;63:4501–4505. doi: 10.1128/iai.63.11.4501-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelmus K R. Review of clinical experience with microbial keratitis associated with contact lenses. CLAO J. 1987;13:211–214. [PubMed] [Google Scholar]