Abstract
Background
Non‐specific low back pain (LBP) is a common condition. It is reported to be a major health and socioeconomic problem associated with work absenteeism, disability and high costs for patients and society. Exercise is a modestly effective treatment for chronic LBP. However, current evidence suggests that no single form of exercise is superior to another. Among the most commonly used exercise interventions is motor control exercise (MCE). MCE intervention focuses on the activation of the deep trunk muscles and targets the restoration of control and co‐ordination of these muscles, progressing to more complex and functional tasks integrating the activation of deep and global trunk muscles. While there are previous systematic reviews of the effectiveness of MCE, recently published trials justify an updated systematic review.
Objectives
To evaluate the effectiveness of MCE in patients with chronic non‐specific LBP.
Search methods
We conducted electronic searches in CENTRAL, MEDLINE, EMBASE, five other databases and two trials registers from their inception up to April 2015. We also performed citation tracking and searched the reference lists of reviews and eligible trials.
Selection criteria
We included randomised controlled trials (RCTs) that examined the effectiveness of MCE in patients with chronic non‐specific LBP. We included trials comparing MCE with no treatment, another treatment or that added MCE as a supplement to other interventions. Primary outcomes were pain intensity and disability. We considered function, quality of life, return to work or recurrence as secondary outcomes. All outcomes must have been measured with a valid and reliable instrument.
Data collection and analysis
Two independent review authors screened the search results, assessed risk of bias and extracted the data. A third independent review author resolved any disagreement. We assessed risk of bias using the Cochrane Back and Neck (CBN) Review Group expanded 12‐item criteria. We extracted mean scores, standard deviations and sample sizes from the included trials, and if this information was not provided we calculated or estimated them using methods recommended in the Cochrane Handbook. We also contacted the authors of the trials for any missing or unclear information. We considered the following time points: short‐term (less than three months after randomisation); intermediate (at least three months but less than 12 months after randomisation); and long‐term (12 months or more after randomisation) follow‐up. We assessed heterogeneity by visual inspection of the forest plots, and by calculating the Chi2 test and the I2 statistic. We combined results in a meta‐analysis expressed as mean difference (MD) and 95% confidence interval (CI). We assessed the overall quality of the evidence using the GRADE approach.
Main results
We included 29 trials (n = 2431) in this review. The study sample sizes ranged from 20 to 323 participants. We considered a total of 76.6% of the included trials to have a low risk of bias, representing 86% of all participants. There is low to high quality evidence that MCE is not clinically more effective than other exercises for all follow‐up periods and outcomes tested. When compared with minimal intervention, there is low to moderate quality evidence that MCE is effective for improving pain at short, intermediate and long‐term follow‐up with medium effect sizes (long‐term, MD –12.97; 95% CI –18.51 to –7.42). There was also a clinically important difference for the outcomes function and global impression of recovery compared with minimal intervention. There is moderate to high quality evidence that there is no clinically important difference between MCE and manual therapy for all follow‐up periods and outcomes tested. Finally, there is very low to low quality evidence that MCE is clinically more effective than exercise and electrophysical agents (EPA) for pain, disability, global impression of recovery and quality of life with medium to large effect sizes (pain at short term, MD –30.18; 95% CI –35.32 to –25.05). Minor or no adverse events were reported in the included trials.
Authors' conclusions
There is very low to moderate quality evidence that MCE has a clinically important effect compared with a minimal intervention for chronic low back pain. There is very low to low quality evidence that MCE has a clinically important effect compared with exercise plus EPA. There is moderate to high quality evidence that MCE provides similar outcomes to manual therapies and low to moderate quality evidence that it provides similar outcomes to other forms of exercises. Given the evidence that MCE is not superior to other forms of exercise, the choice of exercise for chronic LBP should probably depend on patient or therapist preferences, therapist training, costs and safety.
Plain language summary
Motor control exercise for chronic non‐specific low‐back pain
Review question
To evaluate the effectiveness of motor control exercise (MCE) in patients with chronic non‐specific low back pain (LBP).
Background
Motor control exercise is a popular form of exercise that aims to restore co‐ordinated and efficient use of the muscles that control and support the spine. Patients are initially guided by a therapist to practise normal use of the muscles during simple tasks. As the patient's skill increases the exercises are progressed to more complex and functional tasks involving the muscles of the trunk and limbs.
Search date
The evidence is current to April 2015.
Study characteristics
In total, 2431 participants were enrolled in 29 trials. The study sample sizes ranged from 20 to 323 participants, and most of them were middle‐aged people recruited from primary or tertiary care. The duration of the treatment programmes ranged from 20 days to 12 weeks, and the number of treatment sessions ranged from one to five sessions per week. Sixteen trials compared MCE with other types of exercises, seven trials compared MCE with minimal intervention, five trials compared MCE with manual therapy, three trials compared MCE with a combination of exercise and electrophysical agents, and one trial compared MCE with telerehabilitation based on home exercises.
Key results and quality of evidence
MCE probably provides better improvements in pain, function and global impression of recovery than minimal intervention at all follow‐up periods. MCE may provide slightly better improvements than exercise and electrophysical agents for pain, disability, global impression of recovery and the physical component of quality of life in the short and intermediate term. There is probably little or no difference between MCE and manual therapy for all outcomes and follow‐up periods. Little or no difference is observed between MCE and other forms of exercise. Given the minimal evidence that MCE is superior to other forms of exercise, the choice of exercise for chronic LBP should probably depend on patient or therapist preferences, therapist training, costs and safety.
Summary of findings
Background
Low back pain (LBP) is one of the most common conditions worldwide. It has been reported as a major health and socioeconomic problem associated with work absenteeism, disability and high costs for patients, governments and health insurance companies (Airaksinen 2006; Dagenais 2008). Despite its high prevalence, the source of pain is not established in the majority of cases and the term 'non‐specific LBP' is widely used (Hancock 2007; Niemisto 2004; Niemisto 2005; Panjabi 2003).
One proposed mechanism for non‐specific LBP is lack of stability of the spine (Panjabi 1992; Panjabi 2003; Panjabi 2006). Previous studies have demonstrated that patients with LBP may have impairments in the control of the deep trunk muscles (e.g. transversus abdominis and multifidus) responsible for maintaining the co‐ordination and stability of the spine (Hodges 1997; Hodges 1998; Moseley 2002a). Based on this principle, motor control exercise (MCE) was developed with the aim of restoring the co‐ordination, control and capacity of the trunk muscles (Hodges 2003). The intervention involves the training of isolated contraction of the deep trunk muscles, with further integration of these muscles into more complex static, dynamic and functional tasks (Ferreira 2007; O'Sullivan 1997). The intervention also includes the co‐ordination and optimal control of the global trunk muscles (Costa 2009; Macedo 2012).
The effectiveness of MCE has been tested in randomised controlled trials and summarised in systematic reviews (Bystrom 2013; Costa 2009; Ferreira 2007; Lomond 2015; Macedo 2012; Rasmussen‐Barr 2009; Wang 2012a). Our aim was to perform the first Cochrane systematic review on this topic in order to provide accurate and robust information on the effectiveness of MCE for chronic non‐specific LBP, as compared to no intervention or other types of interventions.
Description of the condition
LBP is defined as pain and discomfort located below the ribs and above the gluteal crease, with or without referred leg pain (Airaksinen 2006; van Tulder 2006). Non‐specific LBP has been reported as the most common type of LBP and is defined as LBP not attributed to a recognisable or specific pathology, such as nerve root compromise or serious spinal pathology (i.e. fracture, cancer and inflammatory diseases) (Airaksinen 2006; van Tulder 2006). Chronic LBP is usually defined as an episode of LBP lasting for 12 weeks or longer (Airaksinen 2006). Patients with acute non‐specific LBP demonstrate a favourable improvement rate within the first six weeks (Menezes Costa 2012); however, approximately 40% of patients will develop chronic LBP (Menezes Costa 2009).
Description of the intervention
MCE is based on the theory that the stability and control of the spine are altered in patients with LBP (Hodges 1996). The intervention focuses on the activation of the deep trunk muscles, targeting the restoration of control and co‐ordination of these muscles, which involves the training of pre‐activation of the deep trunk muscles with progression toward more complex and functional tasks integrating the activation of deep and global trunk muscles (O'Sullivan 1997). MCE is usually delivered in 1:1 supervised treatment sessions, and sometimes involves ultrasound imaging, the use of pressure biofeedback units or palpation to provide feedback on the activation of trunk muscles (Macedo 2012; Teyhen 2005).
During the intervention, patients are taught how to contract trunk muscles in a specific manner (Costa 2009; Ferreira 2007), and progress until they are able to maintain isolated contractions of the target muscles while maintaining normal respiration. Over‐activation of the superficial trunk muscles is also identified and corrected as part of the intervention. The advanced stage of the treatment includes the progression of the exercises toward more functional activities (Costa 2009), starting with static activities and progressing to dynamic and more complex tasks. During this process, the recruitment of the trunk muscles, posture, movement patterns and breathing are assessed and corrected.
MCE is a complex intervention; however, reports of randomised controlled trials do not always completely follow all the principles previously described in their interventions (Macedo 2009). Trials often include the training or control of the co‐ordination of deep muscles in the intervention but do not always take into consideration the principles of motor learning or the progression to more functional activities (Macedo 2012). For this reason, the intervention can also be described as specific stabilisation exercises, and not necessarily MCE.
How the intervention might work
Previous studies have demonstrated that patients with LBP may have a delayed onset of activity of the deep trunk muscles in dynamic tasks that challenge the control of the spine (Hodges 1998; Hodges 1999). Morphologically, a lower cross‐sectional area and a larger percentage of intramuscular fat in the multifidus muscle were found in patients with LBP compared with asymptomatic controls in cross‐sectional studies (Alaranta 1993; Hides 1994). Moreover, it was found that patients with low back pain tend to increase spinal stiffness to compensate for the lack of control of the spine by increasing the activity of the superficial muscles (van Dieen 2003). MCE uses the motor learning approach to optimise control of the spine by rehabilitating the posture, movement and the co‐ordination of the deep muscles of the spine (Richardson 2004). Thus, by correcting the co‐ordination and control of the spine, this intervention may be able to decrease pain as well as symptoms associated with LBP.
Why it is important to do this review
The number of studies on MCE has increased as well as its popularity and use in clinical practice. There are recent published trials that have not been included in other reviews (e.g. Moon 2013; Rabin 2014). Further, the systematic reviews available on this topic are out of date, did not perform meta‐analysis or did not include an evaluation of the strength of the evidence, such as the GRADE approach. Thus, a well‐conducted Cochrane systematic review with meta‐analysis is important to better inform clinicians, patients and policy makers about the effectiveness of MCE in patients with chronic non‐specific LBP.
Objectives
To evaluate the effectiveness of MCE in patients with chronic non‐specific LBP.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials. We did not consider trials with quasi‐random allocation procedures for this review.
Types of participants
We included studies if they explicitly reported that a criterion for entry was chronic (> 12 weeks) non‐specific LBP (with or without leg pain) or recurrent LBP. We excluded studies that included individuals with specific conditions such as disc herniation, spinal stenosis, cancer etc. We included studies evaluating adults of either gender. We planned a secondary analysis of patients with chronic and recurrent LBP if we were able to retrieve information on whether patients had chronic LBP (first time onset) versus recurrent LBP (defined as pain lasting at least 24 hours, following a 30‐day pain‐free period following a previous episode).
We included trials with a mixed population in relation to type and duration of back pain only if separate data for each group were provided or if the majority of patients had chronic LBP (> 75%). In cases where articles did not include enough information to classify patients as having non‐specific LBP or the duration of the pain, we contacted the authors to clarify. If no response was received within one month, with bi‐weekly emails, we excluded and adequately referenced the study.
Types of interventions
We included trials comparing MCE with placebo, no treatment, another active treatment, or when MCE was added as a supplement to other interventions. When MCE was used in addition to other treatments, it had to represent at least 50% of the total treatment programme to be included.
We considered trials to have evaluated MCE if the exercise treatment was described as motor control or specific stabilisation exercise, and/or the trial described exercise aiming to activate, train or restore the function of specific muscles of the spine, such as multifidus and transversus abdominis. We considered specific stabilisation exercises and exercises aiming to activate, train, or restore the stabilisation or co‐ordination of specific deep muscles because these principles integrate the MCE intervention. As reports of trials do not always take into consideration the principles of motor learning, the intervention is often described as specific stabilisation exercises, instead of MCE. Articles were not included if generalised (whole body) stability exercises without consideration of specific muscle activity were performed.
A Cochrane review of Pilates was recently published (Yamato 2015), therefore we excluded trials evaluating Pilates from this review although principles of Pilates may overlap with the principles of a motor control intervention.
Types of outcome measures
Primary outcomes were pain intensity and disability and the secondary outcomes were function, quality of life, global impression of recovery, return to work, adverse events and recurrence. All outcomes must have been measured with a valid and reliable instrument.
Search methods for identification of studies
Electronic searches
We performed a computerised electronic search to identify relevant articles in the following databases up to April 2015:
Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 3);
MEDLINE (OvidSP, 1946 to March Week 5 2015);
MEDLINE In‐Process & Other Non‐Indexed Citations (OvidSP, 1 April 2015);
EMBASE (OvidSP, 1980 to 2015 Week 13);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1981 to April 2015);
Allied and Complementary Medicine (AMED) (OvidSP, 1985 to March 2015);
SPORTDiscus (EBSCO, 1800 to April 2015);
Physiotherapy Evidence Database (PEDro);
Latin American and Caribbean Health Sciences Literature (LILACS);
ClinicalTrials.gov;
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP);
PubMed.
We conducted searches in 2012 and 2014. For the 2015 update, we added a search of MEDLINE In‐Process & Other Non‐Indexed Citations and PubMed, using the strategy by Duffy 2014, to capture studies not yet in MEDLINE.
We used the search strategies developed by the Cochrane Back and Neck Review Group. We did not restrict the searches or inclusion criteria to any specific language. The search strategy for each database is presented in Appendix 1.
Searching other resources
We performed citation tracking using Web of Science (Thomson Reuters) and also performed a manual search of the reference lists of previous reviews and the eligible trials.
Data collection and analysis
Selection of studies
Two independent review authors (LGM and LC or BTS and TPY) screened all search results for potentially eligible studies. A third independent review author (RO or CM) resolved any disagreement about inclusion of trials, quality assessment and data extraction. For non‐English language manuscripts, we identified a native speaker within local universities to assist with the translation. We performed pilot testing of the assessment of risk of bias and the extraction of data with two studies as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data extraction and management
We extracted data from each included study using a standardised extraction form. Two independent review authors (BTS and TPY) extracted all data. We resolved disagreements through discussion or arbitration by a third review author (CM). We extracted mean scores, standard deviations and sample sizes from the studies. When this information was not provided in the trial, we calculated or estimated the values using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also extracted information about characteristics of participants, treatments provided, co‐interventions, duration of the treatment, outcome measures and risk of bias criteria from the studies.
Assessment of risk of bias in included studies
We assessed risk of bias using the Cochrane Back and Neck Review Group expanded 12‐item criteria (Appendix 2) (Furlan 2009; Higgins 2011). We assessed the risk of bias of a trial as 'low risk' (at least six of the 12 criteria met) to 'high risk' (fewer than six criteria met). Two independent review authors (BTS and TPY) extracted all data. We resolved disagreements through discussion or arbitration by a third review author (CM). For the purpose of this review, we did not consider the assessor blinded when patients were not blinded, since the patient is considered to be the outcome assessor for patient‐reported outcomes such as pain, disability or function.
Measures of treatment effect
We expressed pooled effects of continuous variables as mean differences if the same outcomes were used. If continuous outcomes measures were different between studies, we also expressed pooled effects with mean differences (MD), but we first converted the different outcome measures to a common 0 to 100 scale. We used risk ratios (RR) and odds ratios (OR) with 95% confidence interval (CI) to calculate treatment effects of dichotomous variables. We converted ordinal variables if present to dichotomous variables for the purpose of the analysis. For the measurement of effect sizes, we defined three levels: small effect size (MD < 10% of the scale), medium effect size (MD 10% to 20% of the scale) or large effect size (MD > 20% of the scale) (Rubinstein 2012). A clinically important effect was considered when the magnitude of the effect size was at least medium (>10% of the scale).
Unit of analysis issues
If trials were sufficiently homogenous we conducted a meta‐analysis for the time points: short (less than three months after randomisation), intermediate (at least three months but less than 12 months after randomisation) and long‐term (12 months or more after randomisation) follow‐up. When there were multiple time points that fell within the same category we used the one that was closest to the end of the treatment, six months and 12 months. Finally, we used intention‐to‐treat analysis preferably over per‐protocol or as‐treated analysis.
Dealing with missing data
When not enough information was provided in the trial to evaluate treatment effects, we contacted authors to provide the required information. We estimated data from graphs and figures in cases where this information was not presented in tables or text. If any information regarding standard deviations is missing, we calculated them from confidence intervals (if available) of the same study. Finally, if no measure of variability was presented anywhere in the text, we estimated the standard deviation from the most similar trial, taking patient profile and the risk of bias of individual studies into consideration.
Assessment of heterogeneity
We performed a visual inspection of the forest plot looking at the overlap of the confidence intervals to evaluate heterogeneity. Furthermore, we calculated the Chi2 test and I2 statistic as recommended by theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We defined substantial heterogeneity as I2 > 50%, and described results in the text qualitatively and did not pool them. When I2 values were slightly higher than 50% but we identified no clear heterogeneity by visual inspection, we combined the results into a meta‐analysis using a random‐effects model and downgraded the evidence for inconsistency in the quality of evidence assessment.
Data synthesis
Regardless of whether there were sufficient data available to use quantitative analyses to summarise the data, we assessed the overall quality of the evidence for each outcome. To accomplish this, we used the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted in the updated CBN method guidelines (Furlan 2015). The quality of the evidence was based upon five main domains and for each domain that was not met we reduced the quality by one level from high quality to moderate, low or very low quality. The five domains are: 1) study design and risk of bias (downgraded if > 25% of the participants were from studies with a high risk of bias); 2) inconsistency of results (downgraded if significant heterogeneity was presented by visual inspection or I² > 50%); 3) indirectness (generalisability of the findings; downgraded if > 50% of the participants were outside the target group); 4) imprecision (downgraded if fewer than 400 participants were included in the comparison for continuous data and there were fewer than 300 events for dichotomous data (Mueller 2007)); and 5) other (for example publication bias). We considered single studies with fewer than 400 participants for continuous outcomes (or fewer than 300 participants for dichotomous outcomes) inconsistent and imprecise, providing 'low quality evidence', which could be downgraded to 'very low quality evidence' if there were further limitations on the quality of evidence (Rubinstein 2012). We described the quality of the evidence as follows (Balshem 2011):
High quality evidence: there are consistent findings among at least 75% of RCTs with no limitations of the study design, consistent, direct and precise data and no known or suspected publication biases. We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality evidence: one of the domains is not met. We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality evidence: two of the domains are not met. Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality evidence: three of the domains are not met. We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
No evidence: no RCTs were identified that addressed this outcome.
We also presented the results using the 'Summary of findings' tables following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the GRADE guidelines (Guyatt 2013). We selected the primary outcomes (pain and disability) and adverse events to include in the main findings of this review.
Sensitivity analysis
We planned to perform a secondary analysis to evaluate separately the studies using a more strict definition of MCE (Macedo 2012): "Motor control exercises utilise the principles of motor learning to retrain control of the trunk muscles, posture, and movement pattern…". There are three essential components to be considered as motor control (must have all three to be considered in this definition): (1) assessment of the individual participant's motor control impairment; (2) an assessment of the postures, movements patterns and muscle activation associated with symptoms and implementation of a retraining programme designed to improve activity of muscles assessed to have poor control; and (3) the use of one or more principles of motor learning (e.g. feedback, segmentation, simplification). We also performed sensitivity analyses to assess the influence of the methodological quality (i.e. trials fulfilling six or more risk of bias criteria) on the overall estimates of effectiveness for the primary outcomes. We conducted the sensitivity analysis for the comparisons that included trials with high risk of bias.
Results
Description of studies
see: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The search retrieved 2055 records of trials, of which we selected 181 for full‐text assessment and 29 trials (33 records) fulfilled the inclusion criteria (total sample = 2431 participants). Figure 1 shows the flowchart of the inclusion process of this review.
1.
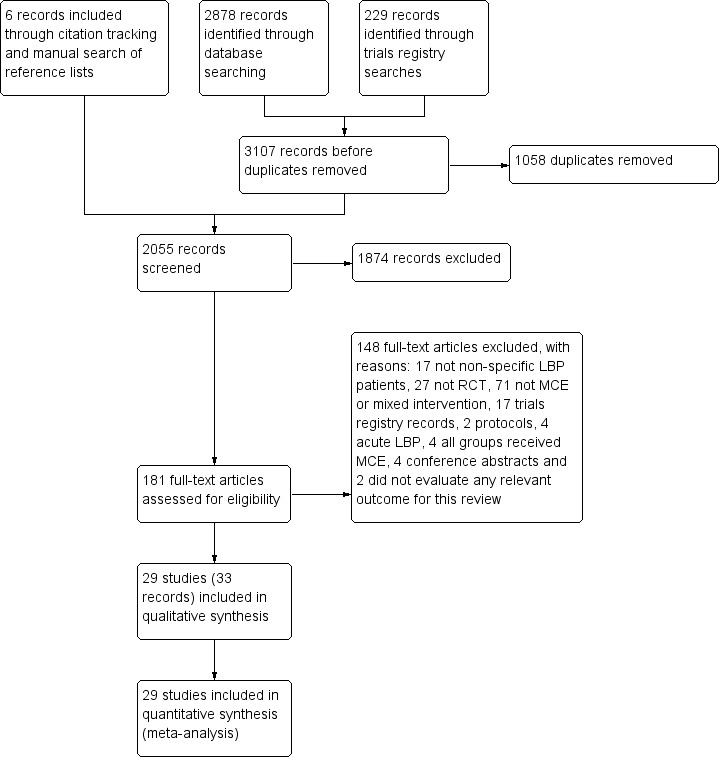
Study flow diagram.
The searches for ongoing and unpublished trials retrieved 17 registered trials and 2 published protocols. One registered trial and one protocol were from the same trial (ISRCTN80064281; Saner 2011). Four registered trials were for trials already included in this review (Akbari 2008; Franca 2010; Lomond 2015; Unsgaard‐Tondel 2010), one was ineligible as both groups received MCE (NCT01061632), three were not considered as MCE (ACTRN12609000293268; NCT00624533; ISRCTN80064281), and one included a mix of MCE and manual therapy in the intervention group and was not included (ACTRN12609000334202). We contacted the authors of eight trials but they did not yet have any results or published material (ACTRN12609000343202; ACTRN12611000971932; Magalhaes 2013;NCT02398760; NCT02170753; NCT02374970; NCT02221609; NCT02200913), and for one trial the authors did not reply to our email contact attempts for more information (NCT02112760).
The 29 trials included in this review were conducted in 16 different countries: four trials were conducted in Iran (Akbari 2008; Hemmati 2011; Hosseinifar 2013; Javadian 2012), four in the United Kingdom (Cairns 2006; Critchley 2007; Goldby 2006; Koumantakis 2005), three in India (Inani 2013; Kumar 2009; Kumar 2010), three in Australia (Costa 2009; Ferreira 2007; Macedo 2012), one in Norway (Unsgaard‐Tondel 2010), two in Sweden (Rasmussen‐Barr 2003; Rasmussen‐Barr 2009), one in Brazil (Franca 2010), two in Korea (Moon 2013; Rhee 2012), one in Thailand (Puntumetakul 2013), two in the USA (Miller 2005; Lomond 2015), and one in each of Ireland (Shaughnessy 2004), Serbia (Stankovic 2012), Taiwan (Tsauo 2009), Kingdom of South Arabia (Kachanathu 2012), Turkey (Alp 2014), and Israel (Rabin 2014). All trials were published in English.
Included studies
In total, 2431 participants were enrolled in 29 trials (33 records). The study sample sizes ranged from 20 to 323 participants (median (interquartile range ‐ IQR) = 42 (77.0)). From the 33 reports, we included 29 trials in this review as three studies had three publications with the same participant data, so we used the main trial publication (Unsgaard‐Tondel 2010). Another study was an interim report of a subset of participants as confirmed by the authors, and so we only included the article reporting the results from all participants (Puntumetakul 2013). Finally, one report was a duplicate publication of an earlier trial report (Franca 2010), so we only included the first publication.
Three trials in this review reported implausibly small values for standard deviations that were markedly different to the values reported in other trials (Inani 2013; Javadian 2012; Kachanathu 2012). As there were insufficient data in the reports to calculate the standard deviations we attempted to contact the authors, but were unsuccessful. We therefore elected to estimate the standard deviation for these trials from the median of the reported standard deviations for the trials included in the same comparisons. In addition, one trial reported results as median and range (Alp 2014); thus we considered the median as mean and estimated the standard deviation from the P values, difference between groups, or range.
Types of studies
In total, 16 trials compared MCE with other types of exercises that included: general or conventional exercises (Akbari 2008; Cairns 2006; Critchley 2007; Ferreira 2007; Inani 2013; Javadian 2012; Koumantakis 2005; Unsgaard‐Tondel 2010); stretching and/or strengthening exercises (Franca 2010; Kachanathu 2012; Stankovic 2012); McKenzie (Hosseinifar 2013; Miller 2005); lumbar dynamic exercises (Moon 2013); graded activity (Macedo 2012); and movement system impairment treatment (Lomond 2015). Seven trials compared MCE with minimal intervention, which included a placebo physiotherapy intervention (Costa 2009), education or advice (Goldby 2006; Rasmussen‐Barr 2009; Rhee 2012), and no treatment (Hemmati 2011; Shaughnessy 2004; Tsauo 2009). Five trials compared MCE with manual therapy (Critchley 2007; Ferreira 2007; Goldby 2006; Rabin 2014; Rasmussen‐Barr 2003); and three trials compared MCE with a combination of exercise and electrophysical agents (EPA) that included the use of ultrasound, short‐wave diathermy and strengthening exercises in two trials (Kumar 2009; Kumar 2010), and heat and active stretching in other trial (Puntumetakul 2013). One trial compared MCE with telerehabilitation based on home exercises with phone calls twice a week (Alp 2014). Three trials had multiple arms (Critchley 2007; Ferreira 2007; Goldby 2006), and we included both arms since it was for different comparisons.
Study population
Most participants in the included trials were middle‐aged (median (IQR) = 40.9 (11.2) years), ranging from 20.8 to 54.8 years and recruited from primary or tertiary care with chronic LBP (LBP persisting for 12 weeks or more). Two trials also included patients with recurrent LBP (Koumantakis 2005; Rasmussen‐Barr 2003). One trial only included patients with clinical instability as indicated by the instability catch sign (Puntumetakul 2013), one trial only included patients with an aberrant trunk movement pattern (Javadian 2012), one trial only included patients with mechanically induced LBP (Rasmussen‐Barr 2009), one only included a population of professional fast bowlers (Kachanathu 2012), and one only included male hockey players (Kumar 2009).
Technique: number and duration of treatments
The duration of the treatment programmes ranged from 20 days to 12 weeks (median (IQR) = 8 (2.0) weeks), with a median of 12 sessions (IQR: 6.0), ranging from one to five sessions per week during the treatment programmes. The shortest session duration was 20 minutes and the longest was 90 minutes (median (IQR) = 45 (30) minutes). One trial did not provide information about the programme duration, sessions or frequency (Javadian 2012).
Primary outcomes
Pain intensity: all included trials measured pain intensity, except for one (Shaughnessy 2004). Pain was measured with a visual analogue scale (VAS) or numerical rating scale (NRS) in all trials. We converted all pain outcomes to a 0 to 100‐point scale.
Disability: 13 trials measured disability using the Oswestry Disability Index (Franca 2010; Hemmati 2011; Inani 2013; Javadian 2012; Kachanathu 2012; Lomond 2015; Moon 2013; Rasmussen‐Barr 2003; Rasmussen‐Barr 2009; Rhee 2012; Stankovic 2012; Tsauo 2009; Unsgaard‐Tondel 2010). Nine trials used the Roland Morris Disability Questionnaire (Alp 2014; Cairns 2006; Costa 2009; Critchley 2007; Ferreira 2007; Koumantakis 2005; Macedo 2012; Puntumetakul 2013; Shaughnessy 2004), one trial used the Functional Rating Index Questionnaire (FRI) (Hosseinifar 2013), and one used the modified Oswestry Disability Index (MODI) (Rabin 2014). We converted all disability outcomes to a 0 to 100‐point scale.
Secondary outcomes
Function: four trials measured function (Costa 2009; Ferreira 2007; Macedo 2012; Miller 2005). Three trials used the Patient Specific Functional Scale (Costa 2009; Ferreira 2007; Macedo 2012), and one trial used the Functional Status Questionnaire (Miller 2005).
Global impression of recovery: four trials measured global impression of recovery using the Global Perceived Effect Scale (Costa 2009; Ferreira 2007; Macedo 2012; Moon 2013). We used this scale without conversion. The Global Perceived Effect Scale is an 11‐point scale ranging from ‐5 ("vastly worse") to 0 ("no change") and to +5 ("completely recovered").
Quality of life: seven trials measured quality of life. Five trials used the SF‐36 questionnaire (Alp 2014; Cairns 2006; Macedo 2012; Puntumetakul 2013; Rasmussen‐Barr 2009); one used The Nottingham Health Profile (Goldby 2006), and the other one used the EQ‐5D (EuroQoL) questionnaire (Critchley 2007). However, only the overall score, for both the Nottingham Health Profile and the EQ‐5D (EuroQoL) questionnaire was provided, which is not comparable with the physical and mental component of the SF‐36, which were the only domains included in this comparison.
Adverse events: seven trials attempted to evaluate adverse events (Costa 2009; Critchley 2007; Ferreira 2007; Franca 2010; Kumar 2009; Macedo 2012; Unsgaard‐Tondel 2010).
Follow‐up
Twenty trials included measurements of at least one outcome for short‐term follow‐up, ranging from 4 to 10 weeks. Fourteen trials measured intermediate follow‐up, from three to six months; and nine trials measured long‐term follow‐up, which varied from 12 to 36 months. Only five trials included measures of short, intermediate and long‐term follow‐up (Costa 2009; Ferreira 2007; Macedo 2012; Rasmussen‐Barr 2003; Rasmussen‐Barr 2009).
Excluded studies
In total, we excluded 148 studies throughout the full‐text analysis. A total of 71 studies were not MCE or were a mix of interventions (Ali 2006; Ammar 2011; Andrusaitis 2011; Aasa 2015; Bentsen 1997; Bi 2013; Bronfort 1996; Bronfort 2011; Brooks 2012; Brox 2003; Byuon 2012; Cairns 2003; Chan 2011; Cho 2014; Chung 2013; Descarreaux 2002; Donzelli 2006; Dufour 2010; Durante 2010; Dvorak 2011; Faas 1993; Faas 1995; Freitas 2008; Gagnon 2005; Gatti 2011; Hagen 2010; Hansen 1993; Harkapaa 1989; Harts 2008; Helewa 1999; Helmhout 2004; Henchoz 2010; Hunter 2012; Hwang 2013; Jang 2013; Johannsen 1995; Johnson 2007; Jones 2007; Kaapa 2006; Kline 2013; Kofotolis 2008; Koldas 2008; Kumar 2011; Lie 1999; Long 2004; Mannion 1999; Mannion 2009; Mannion 2012; Marshall 2008; Mohseni‐Bandpei 2011; Moseley 2002b; Nelson 1995; Niemisto 2003; Niemisto 2004; Niemisto 2005; Oguzhan 2011; Riipinen 2005; Rydeard 2006; Saner 2015; Shnayderman 2013; Smith 2011; Suni 2006; Torstensen 1998; Wang 2012b; Willemink 2012; Williamson 2008; Xueqiang 2012; Yelland 2004; Yoo 2012; You 2014; Zhang 2015), 27 were not RCTs (Allison 2012; Appling 2009; Barbosa 2013; Buchbinder 2002; Croft 1999; Dehner 2009; Gustafsson 2008; Harringe 2007; Hides 2008; Hurwitz 2005; Karimi 2009; Kumar 2012; Kuukkanen 1996; Magnusson 2008; Mannion 2009; Mannion 2012; Monteiro 2009; Moussouli 2014; Navalgund 2009; Nelson‐Wong 2009; Norris 2008; Ota 2011; Pereira 2010; Smeets 2009; Sokunbi 2008; Streicher 2014; Yang 2010), 17 did not include non‐specific LBP patients (Aggarwal 2010; Belcher 1998; Bilgin 2013; Bordiak 2012; Childs 2009; Childs 2010; Ewert 2009; George 2011; Guven 2003; Kladny 2003; Lee 2015; Monticone 2004; O'Sullivan 1997; Shakeri 2013; Shnayderman 2012; Stuge 2004; Teyhen 2010), four included patients with acute LBP (Aluko 2013; Brennan 2006; Hides 1996; Hides 2001), in four all groups received MCE (Ahmed 2014; Lewis 2005; Moseley 2003; Trampas 2014), three were conference abstracts for which repeated attempts to contact the authors were unsuccessful (Bayraktar 2013; Carmo 2013; Meira 2013), one was a conference abstract from a study already included in this review (Alp 2014), and two studies did not evaluate any relevant outcome for this review (Earde 2014; Javadian 2015). Finally, 19 were registered trials or protocols already discussed.
Risk of bias in included studies
We considered a total of 76.6% of the included trials to have a low risk of bias, representing 86% of all participants (n = 2088). Overall risk of bias scores varied from 3 to 11, from a total of 12 points with a mean (SD) of 6.8 (1.93). Figure 2 and Figure 3 show the results of the risk of bias analysis for the individual trials.
2.
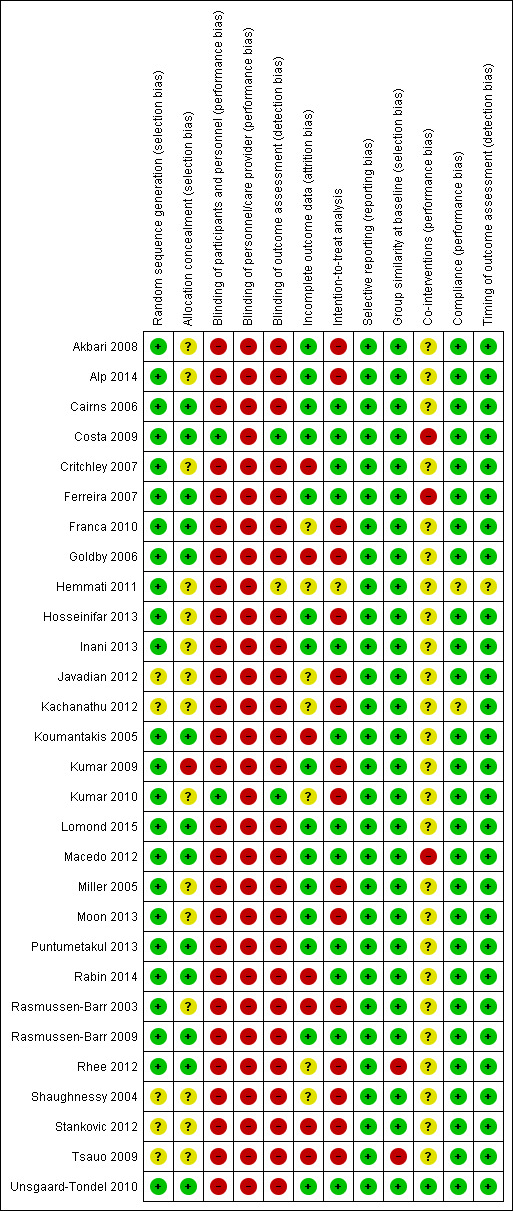
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
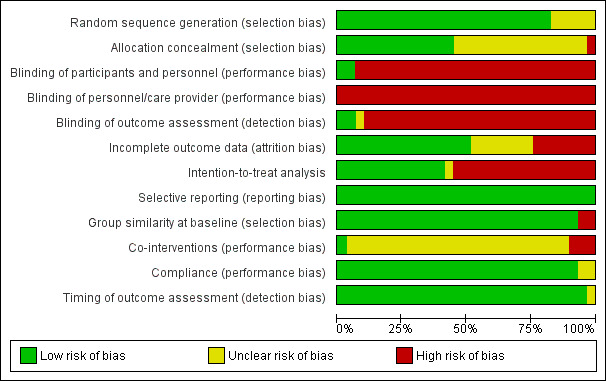
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Twenty‐four trials met the criteria for adequate randomisation and 13 for allocation concealment. In five trials the information about the randomisation procedure was unclear or we did not find sufficient data to judge (Javadian 2012; Kachanathu 2012; Shaughnessy 2004; Stankovic 2012; Tsauo 2009). Fifteen trials did not provide enough information regarding the allocation procedures (Akbari 2008; Alp 2014; Critchley 2007; Hemmati 2011; Hosseinifar 2013; Inani 2013; Javadian 2012; Kachanathu 2012; Kumar 2010; Miller 2005; Moon 2013; Rasmussen‐Barr 2003; Shaughnessy 2004; Stankovic 2012; Tsauo 2009).
Blinding
One trial blinded patients by providing a placebo treatment, and then the outcome assessor was also considered blinded (Costa 2009). One trial reported that patients were blinded to the intervention, so the assessor was also considered as blinded (Kumar 2010). A total of 14 trials attempted to blind the outcome assessor (Akbari 2008; Alp 2014; Cairns 2006; Critchley 2007; Ferreira 2007; Franca 2010; Goldby 2006; Hosseinifar 2013; Lomond 2015; Macedo 2012; Moon 2013; Puntumetakul 2013; Rabin 2014; Tsauo 2009); however, as the patients were not blinded we did not consider the assessors blinded as specified beforehand. We also assumed that blinding of therapists was not possible for the intervention evaluated and none of the included trials claimed that the care providers were blinded.
Incomplete outcome data
In total, 15 trials provided adequate information about missing data and kept this below 20% for short and intermediate‐term, or 30% for long‐term outcomes (Akbari 2008; Alp 2014; Cairns 2006; Costa 2009; Ferreira 2007; Hosseinifar 2013; Inani 2013; Kumar 2009; Lomond 2015; Macedo 2012; Miller 2005; Moon 2013; Puntumetakul 2013; Rasmussen‐Barr 2009; Unsgaard‐Tondel 2010). Seven trials did not provide sufficient information about missing data (Franca 2010; Hemmati 2011; Javadian 2012; Kachanathu 2012; Kumar 2010; Rhee 2012; Shaughnessy 2004). One trial exceeded 20% of withdrawals (Rabin 2014), and six trials exceeded 30% of withdrawals (Critchley 2007; Goldby 2006; Koumantakis 2005; Rasmussen‐Barr 2003; Stankovic 2012; Tsauo 2009).
Selective reporting
Published protocols or registered trials were available for eight trials in this review (Akbari 2008; Costa 2009; Critchley 2007; Ferreira 2007; Franca 2010; Lomond 2015; Macedo 2012; Unsgaard‐Tondel 2010). Two trials, also published their protocols (Costa 2009; Macedo 2012). We considered trials in which it was not possible to find any registry record or publicly available report, but for which it was clear that all expected outcomes were included or were reported in a pre‐specified way, to have fulfilled this criterion. We considered all included trials at low risk of bias for this criterion.
Other potential sources of bias
Publication bias: the examination of publication bias with funnel plots was possible for only one comparison, MCE versus other exercises, for pain and disability. We did not assess publication bias for other comparisons because too few studies were included. Figure 4 and Figure 5 show the funnel plots for the outcomes, pain and disability, respectively. For both outcomes, it appears that small trials with larger effect sizes favouring MCE are published whilst trials favouring the control group are missing. This might indicate publication bias.
4.
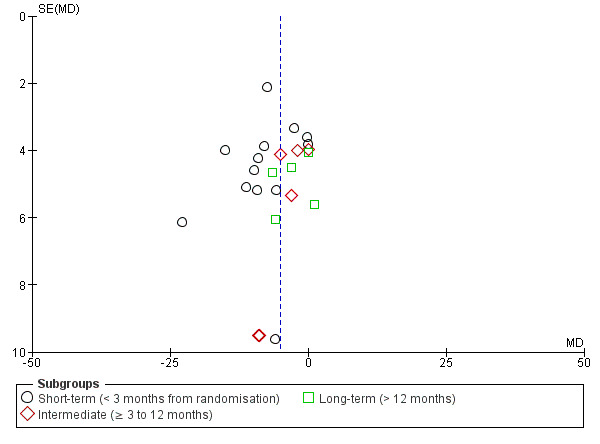
Funnel plot of comparison: motor control exercise versus other exercises, outcome: Pain.
5.
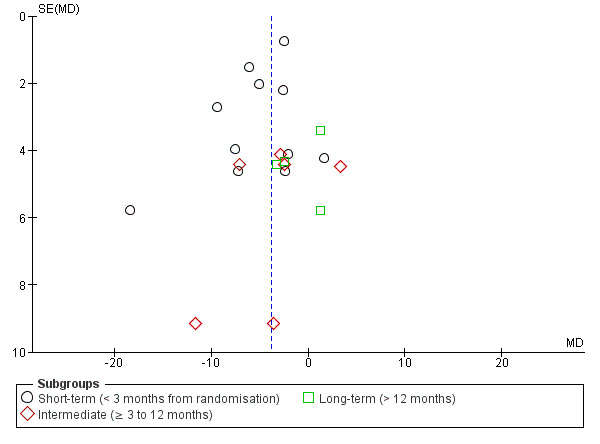
Funnel plot of comparison: Motor control exercise versus other exercises, outcome: Disability.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
for the main comparison.
| Motor control exercise compared with other exercises for chronic low back pain | |||||
|
Patient or population: patients with non‐specific chronic low back pain Settings: primary or tertiary care Intervention: motor control exercise Comparison: other exercises | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Other exercises | Motor control exercise | ||||
|
Pain VAS (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean pain ranged across control groups from 10.5 to 48 points |
The mean pain in the intervention groups was
7.43 points lower (10.47 to 4.40 lower) |
872 participants (13 studies) |
⊕⊕⊝⊝ low1,2 | — |
|
Pain VAS (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean pain ranged across control groups from 17.8 to 48 points |
The mean pain in the intervention groups was
4.88 points lower (8.14 to 1.62 lower) |
588 participants (6 studies) |
⊕⊕⊕⊕ high | — |
|
Pain VAS (0 to 100) Long‐term follow‐up (> 12 months from randomisation) |
The mean pain ranged across control groups from 26.6 to 52 points |
The mean pain in the intervention groups was
2.69 points lower (6.90 lower to 1.53 higher) |
643 participants (5 studies) |
⊕⊕⊕⊕ high | — |
|
Disability Multiple scales (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean disability ranged across control groups from 11 to 40.4 points |
The mean disability in the intervention groups was
4.84 points lower (7.02 to 2.65 lower) |
794 participants (11 studies) |
⊕⊝⊝⊝ low1,2 | — |
|
Disability Multiple scales (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean disability ranged across control groups from 8 to 42.1 points |
The mean disability in the intervention groups was
4.17 points lower (8.12 to 0.23 lower) |
588 participants (6 studies) |
⊕⊕⊕⊕ high | — |
|
Disability Multiple scales (0 to 100) Long‐term follow‐up (> 12 months from randomisation) |
The mean disability ranged across control groups from 27.1 to 40 points |
The mean disability in the intervention groups was
0.71 points lower (4.87 lower to 3.45 higher) |
570 participants (4 studies) |
⊕⊕⊕⊕ high | — |
| Adverse events | See comment | See comment | — | See comment | 2 trials reported mild adverse events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to risk of bias (> 25% of the participants from trials with a high risk of bias).
2Downgraded due to publication bias.
2.
| Motor control exercise compared with manual therapy for chronic low back pain | |||||
|
Patient or population: patients with non‐specific chronic low back pain Settings: primary or tertiary care Intervention: motor control exercise Comparison: manual therapy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Manual therapy | Motor control exercise | ||||
|
Pain VAS (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean pain ranged across control groups from 27.2 to 41 points |
The mean pain in the intervention groups was
4.36 points lower (9.52 lower to 0.81 higher) |
282 participants (3 studies) | ⊕⊕⊕⊝ moderate1 | — |
|
Pain VAS (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean pain ranged across control groups from 26.7 to 43 points |
The mean pain in the intervention groups was
7.05 points lower (14.20 lower to 0.11 higher) |
485 participants (4 studies) | ⊕⊕⊕⊝ moderate2 | — |
|
Pain VAS (0 to 100) Long‐term follow‐up (> 12 months from randomisation) |
The mean pain ranged across control groups from 26.2 to 49 points |
The mean pain in the intervention groups was
3.67 points lower (9.28 lower to 1.94 higher) |
406 participants (4 studies) | ⊕⊕⊕⊕ high | — |
|
Disability Multiple scales (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean disability ranged across control groups from 14 to 32.9 points |
The mean disability in the intervention groups was
2.79 points lower (6.60 lower to 1.02 higher) |
282 participants (3 studies) | ⊕⊕⊕⊝ moderate1 | — |
|
Disability Multiple scales (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean disability ranged across control groups from 14 to 33.3 points |
The mean disability in the intervention groups was
3.28 points lower (6.97 lower to 0.40 higher) |
485 participants (4 studies) | ⊕⊕⊕⊕ high | — |
|
Disability Multiple scales (0 to 100) Long‐term follow‐up (> 12 months from randomisation) |
The mean disability ranged across control groups from 14.3 to 38.3 points |
The mean disability in the intervention groups was
3.40 points lower (7.87 lower to 1.07 higher) |
406 participants (4 studies) | ⊕⊕⊕⊕ high | — |
| Adverse events | See comment | See comment | — | See comment | None of the included trials reported any relevant adverse events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High quality We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to imprecision.
2Downgraded due to inconsistency.
3.
| Motor control exercise compared with minimal intervention for chronic low back pain | |||||
|
Patient or population: patients with non‐specific chronic low back pain Settings: primary or tertiary care Intervention: motor control exercise Comparison: minimal intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Minimal intervention | Motor control exercise | ||||
|
Pain VAS (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean pain ranged across control groups from 9.4 to 56 points |
The mean pain in the intervention groups was
10.01 points lower (15.67 to 4.35 lower) |
291 participants (4 studies) | ⊕⊕⊕⊝ moderate1 | — |
|
Pain VAS (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean pain ranged across control groups from 30.3 to 56 points |
The mean pain in the intervention groups was
12.61 points lower (20.53 to 4.69 lower) |
348 participants (4 studies) | ⊕⊕⊝⊝ low1,2 | — |
|
Pain VAS (0 to 100) Long‐term follow‐up (> 12 months from randomisation) |
The mean pain ranged across control groups from 26.6 to 50.9 points |
The mean pain in the intervention groups was
12.97 points lower (18.51 to 7.42 lower) |
279 participants (3 studies) | ⊕⊕⊕⊝ moderate1 | — |
|
Disability Multiple scales (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean disability ranged across control groups from 17.5 to 49.6 points |
The mean disability in the intervention groups was
8.63 points lower (14.78 to 2.47 lower) |
332 participants (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | — |
|
Disability Multiple scales (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean disability ranged across control groups from 0.1 to 50.8 points |
The mean disability in the intervention groups was
5.47 points lower (9.17 to 1.77 lower) |
348 participants (4 studies) | ⊕⊕⊕⊝ moderate1 | — |
|
Disability Multiple scales (0 to 100) Long‐term follow‐up (> 12 months from randomisation) |
The mean disability ranged across control groups from 14.9 to 51.3 points |
The mean disability in the intervention groups was
5.96 points lower (9.81 to 2.11 lower) |
279 participants (3 studies) | ⊕⊕⊕⊝ moderate1 | — |
| Adverse events | See comment | See comment | — | See comment | One trial reported mild adverse events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High quality We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to imprecision.
2Downgraded due to inconsistency.
3Downgraded due to risk of bias (> 25% of the participants from trials with a high risk of bias).
4.
| Motor control exercise compared with a combination of exercise and electrophysical agents (EPA) for chronic low back pain | |||||
|
Patient or population: patients with non‐specific chronic low back pain Settings: primary or tertiary care Intervention: motor control exercise Comparison: exercise and EPA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Exercise and EPA | Motor control exercise | ||||
|
Pain VAS (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean pain ranged across control groups from 43.3 to 57.1 points |
The mean pain in the intervention groups was
30.18 points lower (35.32 to 25.05 lower) |
68 participants (2 studies) | ⊕⊕⊝⊝ low1,2 | — |
|
Pain VAS (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean pain ranged across control groups from 28.7 to 58.1 points |
The mean pain in the intervention groups was
19.39 points lower (36.83 to 1.96 lower) |
179 participants (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | — |
|
Disability Multiple scales (0 to 100) Short‐term follow‐up (< 3 months from randomisation) |
The mean disability in the control group was 34.54 points |
The mean disability in the intervention group was
20.83 points lower (28.07 to 13.59 lower) |
38 participants (1 study) |
⊕⊝⊝⊝ very low1,2,3 | — |
|
Disability Multiple scales (0 to 100) Intermediate follow‐up (> 3 months and < 12 months) |
The mean disability in the control group was 26.79 points |
The mean disability in the intervention group was
11.50 points lower (20.69 to 2.31 lower) |
38 participants (1 study) |
⊕⊕⊝⊝ low1,3 | — |
| Adverse events | See comment | See comment | — | See comment | None of the included trials reported any relevant adverse events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EPA: electrophysical agents; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High quality We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to imprecision.
2Downgraded due to indirectness.
3Downgraded due to inconsistency.
Effect of motor control exercise versus other exercises
See:Table 1.
Primary outcomes
In total, we included 16 trials in this comparison (Akbari 2008; Cairns 2006; Critchley 2007; Inani 2013; Ferreira 2007; Franca 2010; Hosseinifar 2013; Javadian 2012; Kachanathu 2012; Koumantakis 2005; Lomond 2015; Macedo 2012; Miller 2005; Moon 2013; Stankovic 2012; Unsgaard‐Tondel 2010); three of them were at high risk of bias (n = 220) (Javadian 2012; Kachanathu 2012; Stankovic 2012). For the outcome pain, there is low quality evidence (downgraded due to risk of bias and publication bias) that there is a small, but not clinically important, effect of motor control exercise (MCE) for reducing pain at short‐term (mean difference (MD) –7.53; 95% confidence interval (CI) ‐10.54 to ‐4.52; P value < 0.001, 13 trials) compared with other exercises, and high quality evidence that there is no clinically important difference for pain at intermediate (MD –2.98; 95% CI –6.96 to 0.99, six trials) and long‐term follow‐up (MD –2.69; 95% CI –6.90 to 1.53, five trials) (Analysis 1.1).
1.1. Analysis.
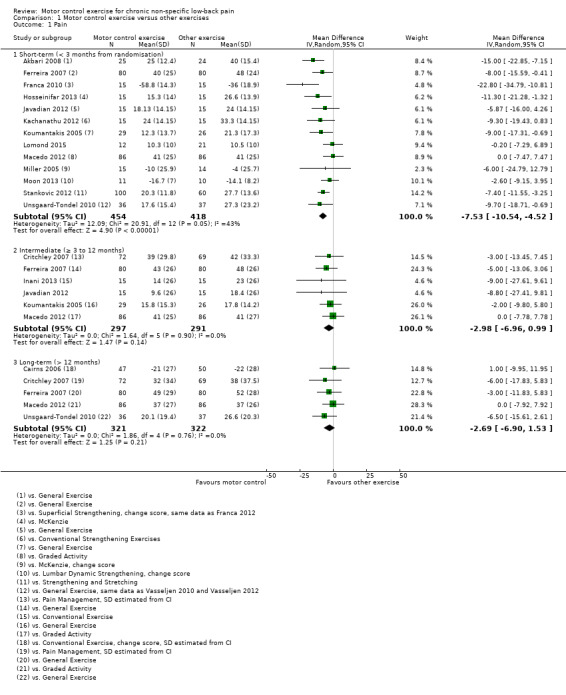
Comparison 1 Motor control exercise versus other exercises, Outcome 1 Pain.
For disability, there is low quality evidence (downgraded due to risk of bias and publication bias) that there is a small, but not clinically important, effect on improving disability at short‐term follow‐up (MD –4.82; 95% CI ‐6.95 to ‐2.68; P value < 0.001, 11 trials), and high quality evidence for no clinically important difference at intermediate (MD –2.88; 95% CI –6.92 to 1.15, 10 trials) and long‐term follow‐up (MD –0.71; 95% CI –4.87 to 3.45, four trials) (Analysis 1.2).
1.2. Analysis.
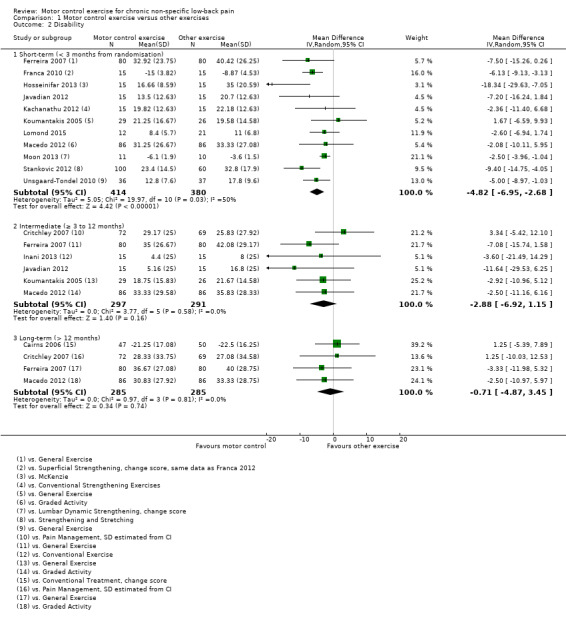
Comparison 1 Motor control exercise versus other exercises, Outcome 2 Disability.
Secondary outcomes
For the outcome function, there is moderate quality evidence (downgraded due to imprecision) that there is a small, but not clinically important, effect of MCE for improving function at short‐term follow‐up (MD 7.29; 95% CI 1.53 to 13.04, P value = 0.01, three trials); however we cannot discard an important effect for function as the CI includes a clinically important value. For intermediate term follow‐up, there is moderate quality evidence (downgraded due to imprecision) that there is no clinically important difference between MCE and other exercises (MD 0.31; 95% CI –0.83 to 1.44, two trials) and low quality evidence (downgraded due to imprecision and inconsistency) for long‐term follow‐up (MD 0.52; 95% CI –1.36 to 2.41, two trials) (Analysis 1.3).
1.3. Analysis.
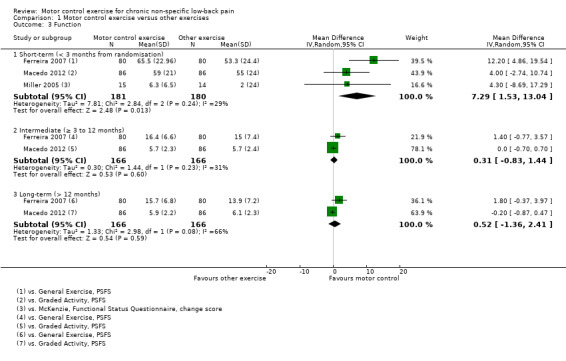
Comparison 1 Motor control exercise versus other exercises, Outcome 3 Function.
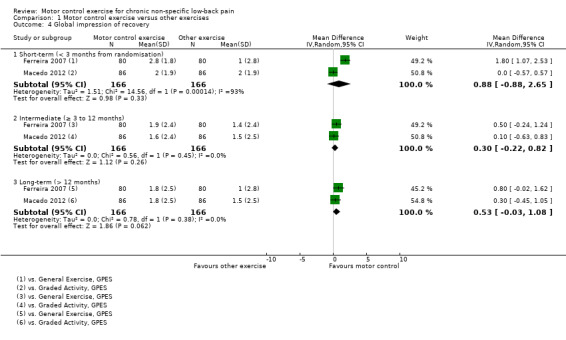
For global impression of recovery, there is moderate quality evidence (downgraded due to imprecision) that there is no clinically important difference at intermediate (MD 0.30; 95% CI –0.22 to 0.82, two trials) and long‐term follow‐up (MD 0.53; 95% CI –0.03 to 1.08, two trials) (Analysis 1.4). We did not pool results for short‐term follow‐up due to high heterogeneity; however, the two studies included in this comparison did not report a clinically important difference.
1.4. Analysis.
Comparison 1 Motor control exercise versus other exercises, Outcome 4 Global impression of recovery.
For the physical component of quality of life, there is low quality evidence (downgraded due to inconsistency and imprecision) that there is no clinically important difference at short (MD 0.00; 95% CI –3.80 to 3.80, one trial) and intermediate‐term follow‐up (MD 1.40; 95% CI –2.61 to 5.41, one trial), and there is moderate quality evidence (downgraded due to imprecision) that there is no clinically important difference at long‐term follow‐up (MD 0.08; 95% CI –3.14 to 3.30, two trials) (Analysis 1.5). For the mental health component of quality of life, there is low quality evidence (downgraded due to inconsistency and imprecision) that there is no clinically important difference at short (MD 0.20; 95% CI –3.39 to 3.79, one trial) and intermediate‐term follow‐up (MD –2.00; 95% CI –5.32 to 1.32, one trial), and moderate quality evidence of a non‐significant effect at long‐term follow‐up (MD –0.75; 95% CI –3.33 to 1.83, two trials) (Analysis 1.6)..
1.5. Analysis.
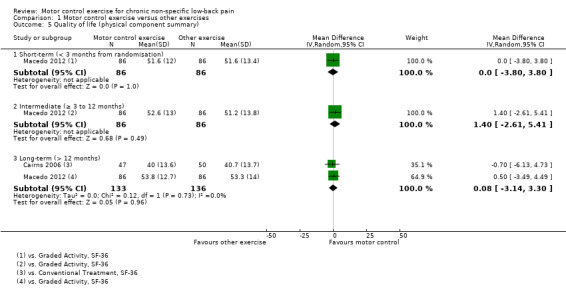
Comparison 1 Motor control exercise versus other exercises, Outcome 5 Quality of life (physical component summary).
1.6. Analysis.
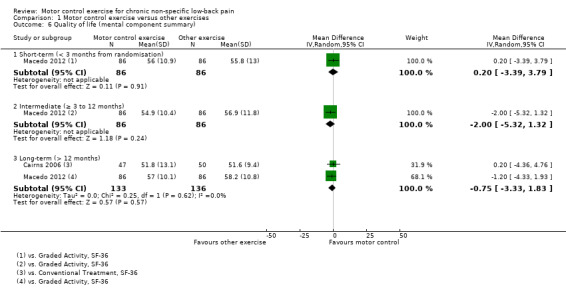
Comparison 1 Motor control exercise versus other exercises, Outcome 6 Quality of life (mental component summary).
Five trials attempted to evaluate adverse events for this comparison (Critchley 2007; Ferreira 2007; Franca 2010; Macedo 2012; Unsgaard‐Tondel 2010). Three trials did not report any adverse events related to the intervention or control groups (Critchley 2007; Ferreira 2007; Franca 2010). One trial reported mild adverse events for 19 participants in the MCE group and 17 participants in the graded activity group (Macedo 2012). Another trial reported one adverse event in the MCE group, which was a withdrawal from the study (Unsgaard‐Tondel 2010).
Sensitivity analysis
The pooled effect sizes for low risk of bias trials were of similar magnitude to the main comparison for pain and disability. The estimates of this sensitivity analysis seem to be precise and consistent since the confidence intervals around the estimates are narrow and no clear heterogeneity was present. Overall, the inclusion of high risk of bias studies in the analyses does not appear to overestimate the effect of MCE versus other exercise.
Effect of motor control exercise versus manual therapy
See:Table 2.
Primary outcomes
We included a total of five trials with low risk of bias in the meta‐analysis (Critchley 2007; Ferreira 2007; Goldby 2006; Rabin 2014; Rasmussen‐Barr 2003). For the outcome pain, there is moderate quality evidence (downgraded due to imprecision) that there is no clinically important effect of MCE compared to manual therapy at short (MD –4.36; 95% CI –9.52 to 0.81; P value = 0.10, three trials) and intermediate‐term follow‐up (MD –7.05; 95% CI –14.20 to 0.11; P value = 0.05, four trials), and there is high quality evidence for long‐term follow‐up (MD –3.67; 95% CI –9.28 to 1.94, four trials) (Analysis 2.1). We cannot discard an important effect for pain at intermediate term as the confidence interval includes a clinically important effect.
2.1. Analysis.
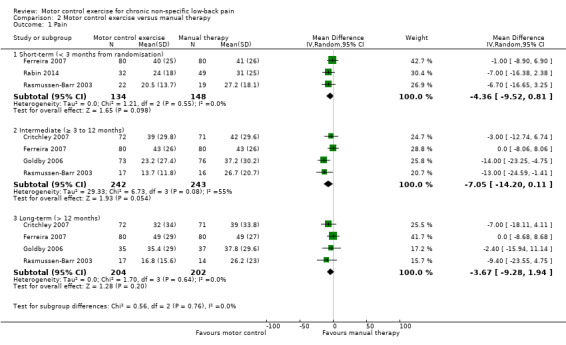
Comparison 2 Motor control exercise versus manual therapy, Outcome 1 Pain.
For disability, there is moderate quality evidence (downgraded due to imprecision) that there is no clinically important difference at short‐term follow‐up (MD –2.79; 95% CI –6.60 to 1.02, three trials), and high quality evidence for intermediate (MD –3.28; 95% CI –6.97 to 0.40, four trials) and long‐term follow‐up (MD –3.40; 95% CI –7.87 to 1.07, four trials) (Analysis 2.2).
2.2. Analysis.
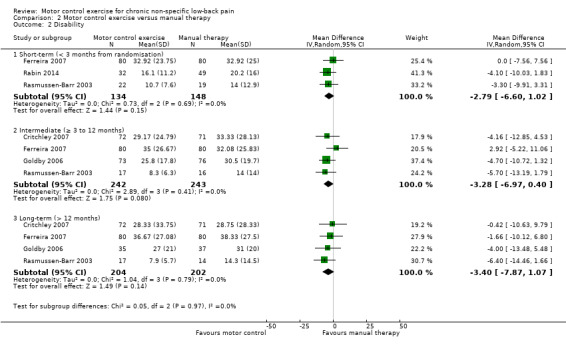
Comparison 2 Motor control exercise versus manual therapy, Outcome 2 Disability.
Secondary outcomes
Based on low quality evidence (downgraded due to inconsistency and imprecision) from one trial, there is no clinically important difference between MCE and manual therapy for the outcome function (short‐term, MD 0.20; 95% CI –1.82 to 2.22; intermediate‐term, MD –0.90; 95% CI –3.01 to 1.21; long‐term, MD 0.50; 95% CI –1.61 to 2.61) (Analysis 2.3), and global impression of recovery (short‐term, MD 0.50; 95% CI –0.12 to 1.12; intermediate‐term, MD 0.20; 95% CI –0.58 to 0.98; long‐term, MD 0.60; 95% CI –0.24 to 1.44) (Analysis 2.4). Two trials attempted to evaluate adverse events for this comparison, but none were reported (Critchley 2007; Ferreira 2007).
2.3. Analysis.
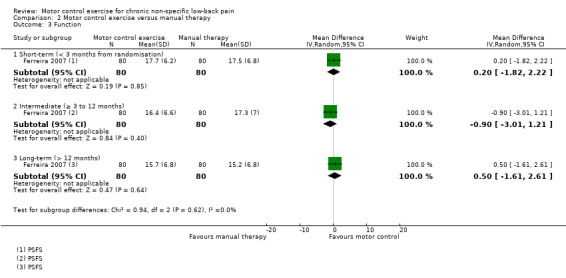
Comparison 2 Motor control exercise versus manual therapy, Outcome 3 Function.
2.4. Analysis.
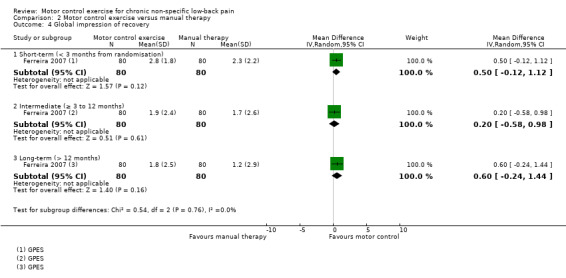
Comparison 2 Motor control exercise versus manual therapy, Outcome 4 Global impression of recovery.
Sensitivity analysis
The pooled effect sizes for low risk of bias trials were similar to the main comparison for pain and disability in that there is no difference in treatment estimates between MCE and manual therapy. The estimates of this sensitivity analysis seem precise and consistent; therefore the inclusion of high risk of bias studies in this analysis does not appear to overestimate the effects of MCE.
Effect of motor control exercise versus minimal intervention
See:Table 3.
Primary outcomes
We included seven trials in the meta‐analysis for this comparison (Costa 2009; Goldby 2006; Hemmati 2011; Rasmussen‐Barr 2009; Rhee 2012; Shaughnessy 2004; Tsauo 2009); two of them (n = 66) with a high risk of bias (Shaughnessy 2004; Tsauo 2009). For the outcome pain, there is moderate quality evidence (downgraded due to imprecision) that there is a clinically important effect of MCE for reducing pain with medium effect size at short‐term (MD –10.01; 95% CI –15.67 to –4.35; P value < 0.001, four trials) and long‐term follow‐up (MD –12.97; 95% CI –18.51 to –7.42; P value < 0.001, three trials). There is low quality evidence (downgraded due to inconsistency and imprecision) for a clinically important effect in favour of MCE in the intermediate term, with a medium effect size (MD –12.61; 95% CI –20.53 to –4.69; P value = 0.002, four trials) (Analysis 3.1).
3.1. Analysis.
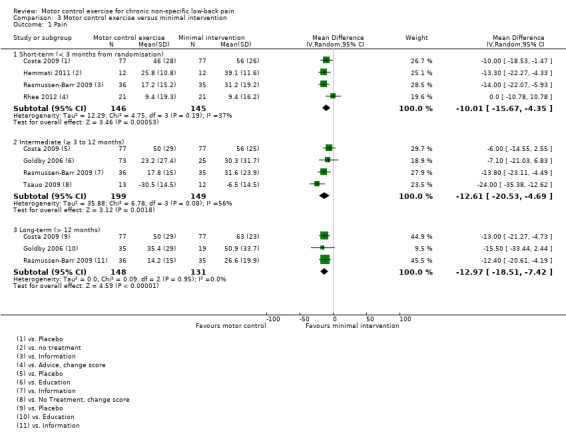
Comparison 3 Motor control exercise versus minimal intervention, Outcome 1 Pain.
For disability, there is very low quality evidence (downgraded due to risk of bias, inconsistency and imprecision) that there is a small, but not clinically important, effect on MCE for improving disability at short‐term follow‐up (MD –8.63; 95% CI –14.78 to –2.47; P value < 0.01, five trials); however we cannot discard an important effect as the confidence interval includes a clinically important effect.There is moderate quality evidence (downgraded due to imprecision) that there is no clinically important effect at intermediate (MD –5.47; 95% CI –9.17 to –1.77; P value = 0.004, four trials) and long‐term follow‐up (MD –5.96; 95% CI –9.81 to –2.11; P value = 0.002, three trials), with small effect sizes (Analysis 3.2).
3.2. Analysis.
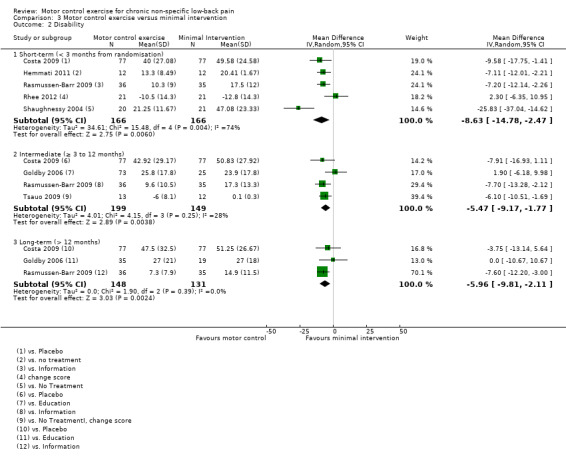
Comparison 3 Motor control exercise versus minimal intervention, Outcome 2 Disability.
Secondary outcomes
There is low quality evidence (downgraded due to inconsistency and imprecision) based on one trial that there is a clinically important effect of MCE for improving function with medium effect size (short‐term, MD 1.10; 95% CI 0.36 to 1.84, P value = 0.004; intermediate‐term, MD 1.00; 95% CI 0.16 to 1.84, P value = 0.02; long‐term, MD 1.50; 95% CI 0.68 to 2.32, P value < 0.001) (Analysis 3.3), and global impression of recovery with medium effect size (short‐term, MD 1.30; 95% CI 0.30 to 2.30, P value = 0.01; intermediate‐term, MD 1.20; 95% CI 0.31 to 2.09, P value = 0.008; long‐term, MD 1.50; 95% CI 0.61 to 2.39, P value < 0.001) (Analysis 3.4). One trial reported that five patients (three from the MCE group and two from the minimal intervention group) had mild adverse effects during the study (all temporary exacerbations of pain) (Costa 2009). None of the patients withdrew from the trial due to adverse events.
3.3. Analysis.
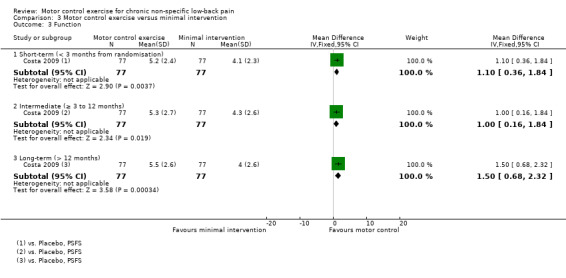
Comparison 3 Motor control exercise versus minimal intervention, Outcome 3 Function.
3.4. Analysis.
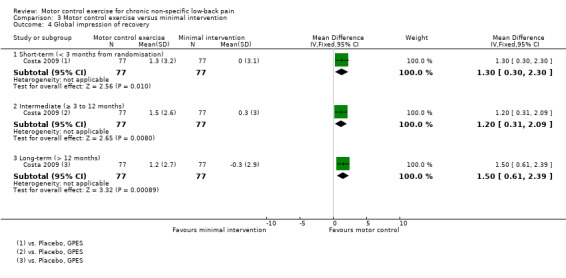
Comparison 3 Motor control exercise versus minimal intervention, Outcome 4 Global impression of recovery.
Sensitivity analysis
The pooled effect sizes for low risk of bias trials were of similar magnitude to those in the main comparison for pain in the short and intermediate term. For disability, the effect estimate for short‐term follow‐up was of similar magnitude. The effect estimate for intermediate‐term follow‐up was no longer statistically significant; however, it may be explained by the reduced precision since fewer trials were included. Overall, inclusion of high risk of bias trials in this comparison did not appear to result in a bias due to effect overestimation.
Effect of motor control exercise versus combination of exercise and electrophysical agents (EPA)
See:Table 4.
Primary outcomes
We included three trials with low risk of bias in the meta‐analysis for this comparison (Kumar 2009; Kumar 2010; Puntumetakul 2013). The treatment programme included in this comparison comprises a combination of ultrasound, short‐wave diathermy and lumbar strengthening exercises in two trials (Kumar 2009; Kumar 2010), and active trunk stretching exercises plus heat application in another trial (Puntumetakul 2013).
There is low quality evidence (downgraded due to imprecision and indirectness) that there is a clinically important effect of MCE for reducing pain at short‐term follow‐up compared with exercise and electrophysical agents (EPA), with a large effect size (MD –30.18; 95% CI –35.32 to –25.05, P value < 0.001, two trials) (Analysis 4.1). We did not pool results for intermediate‐term follow‐up due to high heterogeneity, but these are presented descriptively. Two studies reported a clinically important difference in favour of MCE compared with exercise and EPA, with very low quality evidence for this comparison (downgraded due to inconsistency, imprecision and indirectness).
4.1. Analysis.
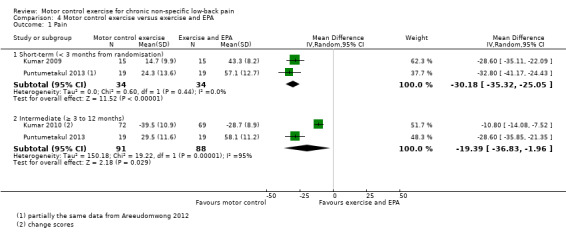
Comparison 4 Motor control exercise versus exercise and EPA, Outcome 1 Pain.
For disability, based on one trial and very low quality evidence (downgraded due to imprecision, indirectness and inconsistency) there is a clinically important effect in favour of MCE at short‐term follow‐up with large effect size (MD –20.83; 95% CI –28.07 to –13.59, P value < 0.001) and there is low quality evidence (downgraded due to inconsistency and imprecision) for an intermediate‐term effect, with medium effect size (MD –11.50; 95% CI –20.69 to –2.31, P value = 0.01, one trial) (Analysis 4.2).
4.2. Analysis.
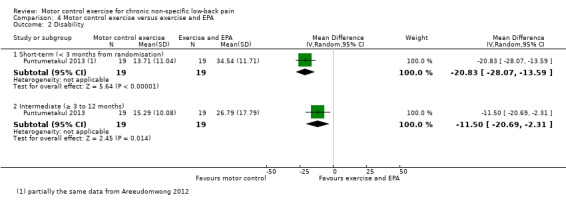
Comparison 4 Motor control exercise versus exercise and EPA, Outcome 2 Disability.
Secondary outcomes
For global impression of recovery, there is low quality evidence (downgraded due to inconsistency and imprecision) of a clinically important effect in favour of MCE at short‐term (MD 1.85; 95% CI 1.09 to 2.61, P value < 0.001, one trial) and intermediate‐term follow‐up (MD 1.67; 95% CI 0.89 to 2.45, P value < 0.001, one trial), with medium effect sizes (Analysis 4.3). For the physical component of quality of life, there is low quality evidence (downgraded due to inconsistency and imprecision) that there is a small, but not clinically important, effect at short‐term (MD 8.40; 95% CI 2.68 to 14.12, P value < 0.01, one trial) and intermediate‐term follow‐up (MD 8.0; 95% CI 2.25 to 13.75, P value < 0.01, one trial); however we cannot discard an important effect as the CI includes a clinically important effect (Analysis 4.4). For the mental component of quality of life, there is low quality evidence (downgraded due to inconsistency and imprecision) that there is no clinically important difference at short‐term (MD 2.48; 95% CI –2.17 to 7.13, one trial) and intermediate‐term follow‐up (MD 1.64; 95% CI –2.95 to 6.23, one trial) (Analysis 4.5). One trial attempted to evaluate adverse events, but none were reported (Kumar 2009).
4.3. Analysis.
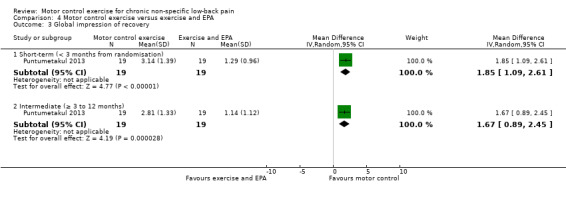
Comparison 4 Motor control exercise versus exercise and EPA, Outcome 3 Global impression of recovery.
4.4. Analysis.
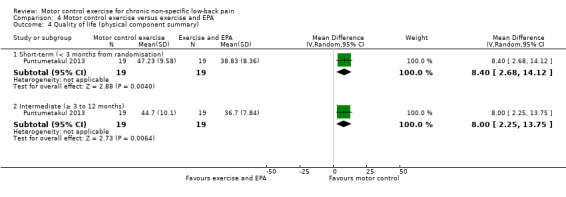
Comparison 4 Motor control exercise versus exercise and EPA, Outcome 4 Quality of life (physical component summary).
4.5. Analysis.
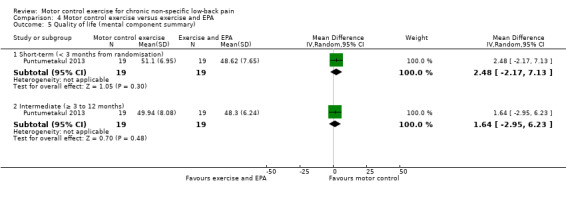
Comparison 4 Motor control exercise versus exercise and EPA, Outcome 5 Quality of life (mental component summary).
Effect of motor control exercise versus telerehabilitation
One trial with low risk of bias compared MCE with telerehabilitation, which included home exercises with phone calls twice a week for six weeks (Alp 2014). Based on very low quality evidence, there is no clinically important difference between MCE and telerehabilitation for intermediate term pain (MD ‐10.00; 95% CI ‐32.35 to 12.35), disability (MD 12.50; 95% CI ‐16.38 to 41.38) and the metal and physical component of quality of life (MD 0.00; 95% CI ‐9.05 to 9.05, and MD ‐5.00; 95% CI ‐16.32 to 6.32, respectively).
Discussion
Summary of main results
In general, for the outcomes pain and disability there is low quality evidence that there is a small, but not clinically important, effect of motor control exercise (MCE) compared to other exercises in the short term and high quality evidence that there is no clinically important difference for intermediate and long term follow‐ups. There is low to moderate quality evidence that there is a clinically important effect of MCE for reducing pain compared with minimal intervention at all follow‐up periods, and there is very low to moderate quality evidence that there is a small, but not clinically important, effect of MCE compared with minimal intervention for all follow‐up periods. There is moderate to high quality evidence of no clinically important difference in the effect of MCE compared to manual therapy at all follow‐up periods for pain and disability. There is very low to low quality evidence that there is a clinically important difference between MCE and electrophysical agents (EPA) in the short and intermediate term for pain and disability. MCE showed a clinically important effect when compared with minimal intervention and exercise and EPA for the other secondary outcomes investigated, except for the mental component of quality of life for exercise and EPA. There was no clinically important difference in the effect of MCE compared with other exercises and manual therapy for the secondary outcomes. Additionally, all the results were consistent with a sensitivity analysis of high quality trials, which suggests that low quality trials did not overestimate the effects of MCE.
These results are unexpected to some extent because we did not expect that the effect of MCE versus exercise and EPA would be much greater than MCE versus minimal intervention. One explanation may be that the combination of exercise and EPA is harmful, which seems unlikely. It is perhaps more likely that these results might be explained by the small sample sizes and limitations in the trials' designs for this comparison since, according to GRADE, very low to low quality evidence indicates that the true effect may be or is very likely to be substantially different from the estimate of the effect. Additionally, it was unclear how much care was taken in implementing both the comparison treatments and MCE in the included studies, since most of the treatment protocols were very briefly described. This also prevents us from performing a sensitivity analysis between stricter and broader definitions of MCE and other interventions.
Overall completeness and applicability of evidence
The studies included in this review were undertaken in 16 different countries from Oceania, South America, Europe and Asia. Most participants were middle‐aged adults recruited from primary or tertiary care with non‐specific chronic LBP. Two studies included participants with recurrent LBP (Koumantakis 2005; Rasmussen‐Barr 2003). The treatment was delivered by an experienced physiotherapist in more than 80% of the trials. There was small variability in the population included, but we do not believe that it would affect the generalisability of the findings. One study included a sample of hockey players (Kumar 2009), one study included only patients with clinical instability (Puntumetakul 2013), one study included fast bowlers (Kachanathu 2012), and one study included only patients with aberrant movement pattern (Javadian 2012).
Quality of the evidence
In this review, we classified most studies included as having low risk of bias although half of the studies scored between 6 and 7, which is just over the limit previously defined. To explore any potential bias from low quality studies we performed a sensitivity analysis with trials classified as high quality and it seems that the inclusion of low quality trials did not introduce bias due to over‐estimation or under‐estimation of the effect estimates. Regarding the quality of the extracted data, we extracted final scores or change scores, depending on which form was available. We also calculated change scores when groups were different at baseline. The assessment of the evidence through GRADE varied from very low to high quality, and the most downgraded points were due to inconsistency and imprecision, that is related to high heterogeneity and insufficient pooled sample size. Although there were concerns about the quality of evidence for some outcomes, we are confident of our findings for the primary outcomes as for most comparisons we had at least moderate quality evidence.
Potential biases in the review process
A limitation of this review is the presence of publication bias in the comparisons assessed with funnel plots. However, for most comparisons it was not possible to assess publication bias using the funnel plots as too few studies were included; thus we did not include or downgrade publication bias with GRADE for these comparisons. We do not have data from three conference abstracts as all attempts to contact the authors regarding the full‐text article were unsuccessful; thus this may also potentially indicate publication bias.
Agreements and disagreements with other studies or reviews
In this review, we did not find a clinically important effect for MCE compared with other exercises, which is consistent with previous version of this review (Macedo 2009) and the most recent systematic review on the topic (Bystrom 2013) that reported a small effect size, which was not considered clinically important in this review. For disability, we found a small effect size but not clinically important in the short term similar to that reported by a previous review (Wang 2012a). The recent review Bystrom 2013 reported a statistically significant effect on disability favouring MCE for all time periods compared to general exercise. This small divergence from our results may be explained because this previous review only included general exercise in this comparison, while we considered all types of exercises other than MCE.
For the comparison of MCE with manual therapy, we did not find any clinically important differences for pain and disability although most treatment effects were in favour of MCE, which is partially consistent with the review of Bystrom 2013 that did not find differences for pain but reported an effect of MCE for disability with a small effect size. Moreover, the previous version of this review reported a small effect size of MCE for pain and disability in the intermediate term (Macedo 2009).
When comparing MCE with minimal intervention, we found a clinically important effect in favour of MCE for pain for all time periods, with medium effect sizes, which is consistent with the findings of Bystrom 2013, and the previous version of this review (Macedo 2009). For the comparison of MCE with exercise and EPA, one previous review included a similar comparison, named as multimodal physical therapy (Bystrom 2013). Our results were consistent in reporting a clinically important effect in favour of MCE for pain and disability in the short and intermediate term, although based on low or very low quality evidence in this review.
The slight discrepancy of results compared to other reviews may be explained by the number of trials included in these previous reviews. The previous reviews, Bystrom 2013, Macedo 2009 and Wang 2012a, included 16, 14 and five trials respectively, while we were able to include 29 trials in our review, with a total of 2431 participants.
Authors' conclusions
Implications for practice.
Although the quality of evidence varied among the outcomes and time period investigated, our findings demonstrate that there is low to moderate quality evidence that motor control exercise (MCE) is more effective than a minimal intervention for chronic low back pain. There is very low to low quality evidence that MCE is more effective than exercise plus EPA. We are uncertain about the effectiveness of MCE compared to exercise and EPA as we considered the quality of the evidence low or very low. We did not find a clinically important difference between MCE and manual therapy for any of the outcomes investigated, with moderate to high quality evidence. There is low quality evidence that there is no clinically important difference between MCE and other forms of exercise in terms of pain and disability in the short term. As MCE appears to be a safe form of exercise and none of the other types of exercise stands out, the choice of exercise for chronic low back pain should depend on patient or therapist preferences, therapist training, costs and safety.
Implications for research.
Future randomised controlled trials in chronic non‐specific low back pain should include more complete descriptions of the exercise interventions so that interpretation of the results would be more transparent. We strongly recommend that future trials have adequate sample size as most of the trials in this review are considered small (fewer than 50 participants). Trials including cost‐effectiveness analysis and long‐term outcomes are also needed in this area. The effectiveness of motor control exercise should be also tested in target groups, such as subgroups of patients more likely to respond to this treatment approach (Macedo 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 28 November 2016 | Amended | Broken link fixed. |
| 26 January 2016 | Amended | Updated author affiliation. |
Acknowledgements
The authors would like to thank the institutions providing funding to the authors of this review. Dr Luciana G Macedo is supported by the Canadian Institutes of Health Research and the Alberta Innovates Health Solutions. Prof Chris Maher is supported by the National Health and Medical Research Council of Australia. Bruno T Saragiotto is supported by CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil; and Tiê P Yamato is supported by CAPES (Coordenacção de Aperfeiçoamento de Pessoal de Nível Superior), Brazil. An earlier version of this review has been published previously (Macedo 2009), and it was updated and converted to a Cochrane review.
Appendices
Appendix 1. Database search strategy
MEDLINE
Last searched 2 April 2015.
randomized controlled trial.pt.
controlled clinical trial.pt.
clinical trial.pt.
exp clinical trial/
Random Allocation/
Double‐Blind Method/
Single‐Blind Method/
Comparative Study/
evaluation studies/
Follow‐Up Studies/
cross‐over studies/
Research Design/
Placebos/
(clinic$ adj25 trial$).tw.
((single$ or double$ or treble$ or triple$) adj (mask$ or blind$)).tw.
(control$ or prospective$ or volunteer$).tw.
(latin adj square).tw.
placebo$.tw.
random$.tw.
or/1‐19
(animals not (humans and animals)).sh.
20 not 21
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
exp sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
low back pain.mp.
or/23‐33
22 and 34
exp Exercise/
exercise$.mp.
train$.mp.
36 or 37 or 38
specific.mp.
stabili$.mp.
segment$.mp.
multifidus.mp.
transversus.mp.
motor control.mp.
or/40‐45
39 and 46
35 and 47
limit 48 to yr=2014‐2015
limit 48 to ed=20140516‐20150402
49 or 50
MEDLINE In‐Process & Other Non‐Indexed Citations
Searched 2 April 2015.
randomized controlled trial.pt.
controlled clinical trial.pt.
clinical trial.pt.
exp clinical trial/
Random Allocation/
Double‐Blind Method/
Single‐Blind Method/
Comparative Study/
evaluation studies/
Follow‐Up Studies/
cross‐over studies/
Research Design/
Placebos/
(clinic$ adj25 trial$).tw.
((single$ or double$ or treble$ or triple$) adj (mask$ or blind$)).tw.
(control$ or prospective$ or volunteer$).tw.
(latin adj square).tw.
placebo$.tw.
random$.tw.
or/1‐19
(animals not (humans and animals)).sh.
20 not 21
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
exp sciatic neuropathy/
spondylosis.ti,ab.
lumbago.ti,ab.
low back pain.mp.
or/23‐33
22 and 34
exp Exercise/
exercise$.mp.
train$.mp.
36 or 37 or 38
specific.mp.
stabili$.mp.
segment$.mp.
multifidus.mp.
transversus.mp.
motor control.mp.
or/40‐45
39 and 46
35 and 47
EMBASE
Last searched 2 April 2015.
randomi#ed controlled trial.mp.
clinical trial/
double blind.mp.
single blind.mp.
placebo/
Controlled Study/
Randomized Controlled Trial/
Double Blind Procedure/
Single Blind Procedure/
crossover procedure/
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
(versus or vs).mp.
(clinic$ adj2 trial$).tw.
or/1‐14
limit 15 to human
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
low back pain.mp.
or/17‐27
16 and 28
exp exercise/
exercise$.mp.
train$.mp.
30 or 31 or 32
motor control.mp.
stabili$.mp.
segment$.mp.
multifidus.mp.
transversus.mp.
or/34‐38
33 and 39
29 and 40
limit 41 to yr=2014‐2015
limit 41 to em=201419‐201513
42 or 43
CENTRAL
Last searched 2 April 2015.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor: [Low Back Pain] explode all trees
#5 lumbar next pain OR coccyx OR coccydynia OR sciatica OR spondylosis
#6 MeSH descriptor: [Sciatica] explode all trees
#7 MeSH descriptor: [Spine] explode all trees
#8 MeSH descriptor: [Spinal Diseases] explode all trees
#9 lumbago OR discitis OR disc near degeneration OR disc near prolapse OR disc near herniation
#10 spinal fusion
#11 spinal neoplasms
#12 facet near joints
#13 MeSH descriptor: [Intervertebral Disk] explode all trees
#14 postlaminectomy
#15 arachnoiditis
#16 failed near back
#17 MeSH descriptor: [Cauda Equina] explode all trees
#18 lumbar near vertebra*
#19 spinal near stenosis
#20 slipped near (disc* or disk*)
#21 degenerat* near (disc* or disk*)
#22 stenosis near (spine or root or spinal)
#23 displace* near (disc* or disk*)
#24 prolap* near (disc* or disk*)
#25 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 MeSH descriptor: [Exercise] explode all trees
#27 exercis*
#28 train*
#29 #26 or #27 or #28
#30 motor control
#31 transversus
#32 multifidus
#33 segment*
#34 stabili*
#35 #31 or #32 or #33 or #34
#36 #25 and #29 and #35 Publication Year from 2014 to 2015, in Trials
CINAHL
Last searched 2 April 2015.
S62 S61 Limiters ‐ Published Date: 20130501‐20150431
S61 S49 AND S56 AND S60
S60 S57 OR S58 OR S59
S59 "train*"
S58 "exercise*"
S57 (MH "Exercise+")
S56 S50 OR S51 OR S52 OR S53 OR S54 OR S55
S55 specific W2 stabili?ation
S54 "stabili?ation"
S53 "multifidus"
S52 (MH "Multifidus Muscles")
S51 "transversus"
S50 "motor control"
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago" 33
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia"
S39 "coccyx"
S38 "sciatica"
S37 (MH "Sciatica")
S36 (MH "Coccyx")
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain 282
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind 94
S5 single‐blind 6,829
S4 double‐blind 24,437
S3 clinical W3 trial 14,324
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
AMED
Last searched 2 April 2015.
randomized controlled trial.pt.
controlled clinical trial.pt.
clinical trial.pt.
exp clinical trials/
random allocation/
double blind method/
single blind method/
comparative study/
follow up studies/
research design/
placebos/
(clinic$ adj25 trial$).tw.
((single$ or double$ or treble$ or triple$) adj (mask$ or blind$)).tw.
(control$ or prospective$ or volunteer$).tw.
(latin adj square).tw.
placebo$.tw.
random$.tw.
or/1‐17
(animals not (humans and animals)).sh.
18 not 19
dorsalgia.mp.
exp backache/
sciatica/
(lumbar adj pain).ti,ab.
sciatica.mp.
spondylosis.mp.
coccyx.mp.
lumbago.mp.
low back pain.mp.
or/21‐29
20 and 30
exercise/
exercise$.mp.
train$.mp.
or/32‐34
specific.mp.
stabili$.mp. [mp=abstract, heading words, title]
segment$.mp.
multifidus.mp.
transversus.mp.
motor control.mp.
or/36‐41
35 and 42
31 and 43
limit 44 to yr=2014‐2015
SPORTDiscus
Last searched 2 April 2015.
S28 S27 Limiters ‐ Published Date: 20140501‐20150431
S27 S16 AND S20 AND S26
S26 S21 OR S22 OR S23 OR S24 OR S25
S25 specific W2 stabili?ation
S24 stabili?ation
S23 multifidus
S22 transversus
S21 motor control
S20 S17 OR S18 OR S19
S19 train*
S18 exercise*
S17 DE "EXERCISE" or DE "BACK exercises" or DE "EXERCISE therapy" or DE "PHYSICAL education & training" or DE "PHYSICAL fitness"
S16 S10 AND S15
S15 S11 OR S12 OR S13 OR S14
S14 DE "LUMBAR vertebrae" or DE "LUMBOSACRAL region"
S13 DE "SCIATICA"
S12 low back pain
S11 DE "BACKACHE"
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 single blind
S8 random allocation
S7 SU randomized controlled trial
S6 SU clinical trials
S5 clinical trials
S4 placebo
S3 controlled clinical trial
S2 double blind
S1 randomi?ed controlled trial
PEDro
Last searched 2 April 2015.
Abstract & Title: Exercise
AND
Problem: pain
AND
Body Part: lumbar spine, sacro‐iliac joint or pelvis
AND
Method: clinical trial
New records added since: 15/05/2014
LILACS
Last searched 2 April 2015.
back pain AND exercise, all indexes on the homepage
Filter: Type of study: clinical Trial OR guidelines
dor lombar AND exercicio, all indexes on the homepage
Filter: Type of study: clinical trial OR guidelines
ClinicalTrials.gov
Last searched 2 April 2015.
Condition: back pain
Intervention: exercise
received on or after 05/15/2014
WHO ICTRP
Last searched 2 April 2015.
Condition: back pain
Intervention: exercise
Date of registration is between 15/05/2014‐02/04/2015
PubMed
Searched 2 April 2015.
((dorsalgia OR back pain OR backache OR lumbar pain OR coccydynia OR sciatica OR lumbago OR spondylosis) AND ((exercise* OR train*) AND (specific* OR stabili* OR segment* OR multifidus OR transverses OR motor control)) AND (pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb]))
From 2014/05/01 to 2015/12/31
Appendix 2. Criteria for assessing risk of bias for internal validity
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
for patient‐reported outcomes in which the patient was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if dropouts are very large, imputation using even 'acceptable' methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and dropouts should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s) and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of patients with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat‐analysis
There is low risk of bias if all randomised patients were reported/analysed in the group to which they were allocated by randomisation.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Data and analyses
Comparison 1. Motor control exercise versus other exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (< 3 months from randomisation) | 13 | 872 | Mean Difference (IV, Random, 95% CI) | ‐7.53 [‐10.54, ‐4.52] |
| 1.2 Intermediate (≥ 3 to 12 months) | 6 | 588 | Mean Difference (IV, Random, 95% CI) | ‐2.98 [‐6.96, 0.99] |
| 1.3 Long‐term (> 12 months) | 5 | 643 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐6.90, 1.53] |
| 2 Disability | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3 months from randomisation) | 11 | 794 | Mean Difference (IV, Random, 95% CI) | ‐4.82 [‐6.95, ‐2.68] |
| 2.2 Intermediate (≥ 3 to 12 months) | 6 | 588 | Mean Difference (IV, Random, 95% CI) | ‐2.88 [‐6.92, 1.15] |
| 2.3 Long‐term (> 12 months) | 4 | 570 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐4.87, 3.45] |
| 3 Function | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term (< 3 months from randomisation) | 3 | 361 | Mean Difference (IV, Random, 95% CI) | 7.29 [1.53, 13.04] |
| 3.2 Intermediate (≥ 3 to 12 months) | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.83, 1.44] |
| 3.3 Long‐term (> 12 months) | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐1.36, 2.41] |
| 4 Global impression of recovery | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Short‐term (< 3 months from randomisation) | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐0.88, 2.65] |
| 4.2 Intermediate (≥ 3 to 12 months) | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.22, 0.82] |
| 4.3 Long‐term (> 12 months) | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.03, 1.08] |
| 5 Quality of life (physical component summary) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term (< 3 months from randomisation) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.80, 3.80] |
| 5.2 Intermediate (≥ 3 to 12 months) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐2.61, 5.41] |
| 5.3 Long‐term (> 12 months) | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐3.14, 3.30] |
| 6 Quality of life (mental component summary) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Short‐term (< 3 months from randomisation) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐3.39, 3.79] |
| 6.2 Intermediate (≥ 3 to 12 months) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐5.32, 1.32] |
| 6.3 Long‐term (> 12 months) | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐3.33, 1.83] |
Comparison 2. Motor control exercise versus manual therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (< 3 months from randomisation) | 3 | 282 | Mean Difference (IV, Random, 95% CI) | ‐4.36 [‐9.52, 0.81] |
| 1.2 Intermediate (≥ 3 to 12 months) | 4 | 485 | Mean Difference (IV, Random, 95% CI) | ‐7.05 [‐14.20, 0.11] |
| 1.3 Long‐term (> 12 months) | 4 | 406 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐9.28, 1.94] |
| 2 Disability | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3 months from randomisation) | 3 | 282 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐6.60, 1.02] |
| 2.2 Intermediate (≥ 3 to 12 months) | 4 | 485 | Mean Difference (IV, Random, 95% CI) | ‐3.28 [‐6.97, 0.40] |
| 2.3 Long‐term (> 12 months) | 4 | 406 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.87, 1.07] |
| 3 Function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term (< 3 months from randomisation) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.82, 2.22] |
| 3.2 Intermediate (≥ 3 to 12 months) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.01, 1.21] |
| 3.3 Long‐term (> 12 months) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐1.61, 2.61] |
| 4 Global impression of recovery | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Short‐term (< 3 months from randomisation) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.12, 1.12] |
| 4.2 Intermediate (≥ 3 to 12 months) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.58, 0.98] |
| 4.3 Long‐term (> 12 months) | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.24, 1.44] |
Comparison 3. Motor control exercise versus minimal intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (< 3 months from randomisation) | 4 | 291 | Mean Difference (IV, Random, 95% CI) | ‐10.01 [‐15.67, ‐4.35] |
| 1.2 Intermediate (≥ 3 to 12 months) | 4 | 348 | Mean Difference (IV, Random, 95% CI) | ‐12.61 [‐20.53, ‐4.69] |
| 1.3 Long‐term (> 12 months) | 3 | 279 | Mean Difference (IV, Random, 95% CI) | ‐12.97 [‐18.51, ‐7.42] |
| 2 Disability | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3 months from randomisation) | 5 | 332 | Mean Difference (IV, Random, 95% CI) | ‐8.63 [‐14.78, ‐2.47] |
| 2.2 Intermediate (≥ 3 to 12 months) | 4 | 348 | Mean Difference (IV, Random, 95% CI) | ‐5.47 [‐9.17, ‐1.77] |
| 2.3 Long‐term (> 12 months) | 3 | 279 | Mean Difference (IV, Random, 95% CI) | ‐5.96 [‐9.81, ‐2.11] |
| 3 Function | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short‐term (< 3 months from randomisation) | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.36, 1.84] |
| 3.2 Intermediate (≥ 3 to 12 months) | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.16, 1.84] |
| 3.3 Long‐term (> 12 months) | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.68, 2.32] |
| 4 Global impression of recovery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term (< 3 months from randomisation) | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.3 [0.30, 2.30] |
| 4.2 Intermediate (≥ 3 to 12 months) | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.31, 2.09] |
| 4.3 Long‐term (> 12 months) | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.61, 2.39] |
Comparison 4. Motor control exercise versus exercise and EPA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (< 3 months from randomisation) | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐30.18 [‐35.32, ‐25.05] |
| 1.2 Intermediate (≥ 3 to 12 months) | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐19.39 [‐36.83, ‐1.96] |
| 2 Disability | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3 months from randomisation) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐20.83 [‐28.07, ‐13.59] |
| 2.2 Intermediate (≥ 3 to 12 months) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐11.5 [‐20.69, ‐2.31] |
| 3 Global impression of recovery | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term (< 3 months from randomisation) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 1.85 [1.09, 2.61] |
| 3.2 Intermediate (≥ 3 to 12 months) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 1.67 [0.89, 2.45] |
| 4 Quality of life (physical component summary) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Short‐term (< 3 months from randomisation) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 8.40 [2.68, 14.12] |
| 4.2 Intermediate (≥ 3 to 12 months) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 8.0 [2.25, 13.75] |
| 5 Quality of life (mental component summary) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term (< 3 months from randomisation) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 2.48 [‐2.17, 7.13] |
| 5.2 Intermediate (≥ 3 to 12 months) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 1.64 [‐2.95, 6.23] |
Comparison 5. Motor control exercise versus telerehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Intermediate (≥ 3 to 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Disability | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Intermediate (≥ 3 to 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (mental component summary) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Intermediate (≥ 3 to 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life (physical component summary) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Intermediate (≥ 3 to 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
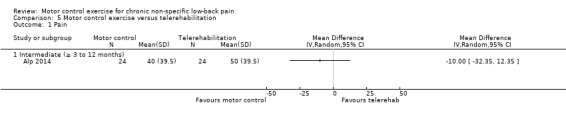
Comparison 5 Motor control exercise versus telerehabilitation, Outcome 1 Pain.
5.2. Analysis.
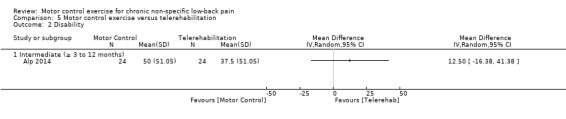
Comparison 5 Motor control exercise versus telerehabilitation, Outcome 2 Disability.
5.3. Analysis.
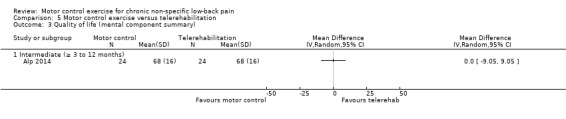
Comparison 5 Motor control exercise versus telerehabilitation, Outcome 3 Quality of life (mental component summary).
5.4. Analysis.
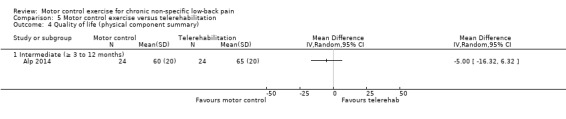
Comparison 5 Motor control exercise versus telerehabilitation, Outcome 4 Quality of life (physical component summary).
Comparison 6. Sensitivity analysis 1: motor control exercise versus other exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 High quality (≥ 6 'Risk of bias' items) ‐ Short‐term (< 3 months from randomisation) | 10 | 652 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐11.97, ‐3.63] |
| 1.2 High quality (≥ 6 'Risk of bias' items) ‐ Intermediate (≥ 3 to 12 months) | 5 | 558 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐6.65, 1.59] |
| 2 Disability | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 High quality (≥ 6 'Risk of bias' items) ‐ Short‐term (< 3 months from randomisation) | 8 | 574 | Mean Difference (IV, Random, 95% CI) | ‐4.27 [‐6.58, ‐1.96] |
| 2.2 High quality (≥ 6 'Risk of bias' items) ‐ Intermediate (≥ 3 to 12 months) | 5 | 558 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐6.37, 1.09] |
6.1. Analysis.
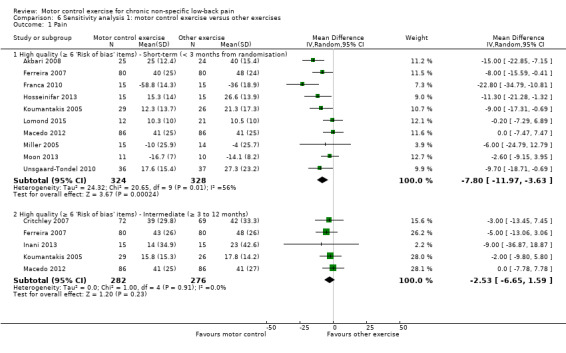
Comparison 6 Sensitivity analysis 1: motor control exercise versus other exercises, Outcome 1 Pain.
6.2. Analysis.
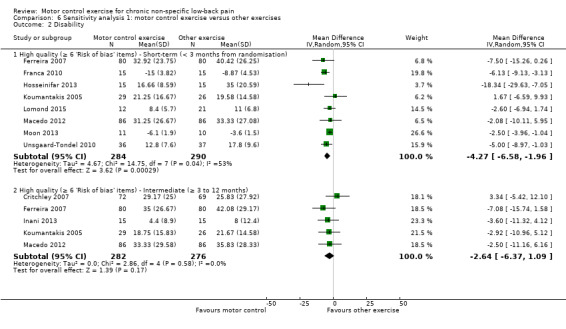
Comparison 6 Sensitivity analysis 1: motor control exercise versus other exercises, Outcome 2 Disability.
Comparison 7. Sensitivity analysis 2: motor control exercise versus minimal intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 High quality (≥ 6 'Risk of bias' items) ‐ Short‐term (< 3 months from randomisation) | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐12.11 [‐17.98, ‐6.25] |
| 1.2 High quality (≥ 6 'Risk of bias' items) ‐ Intermediate (≥ 3 to 12 months) | 3 | 323 | Mean Difference (IV, Random, 95% CI) | ‐9.15 [‐14.89, ‐3.41] |
| 2 Disability | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 High quality (≥ 6 'Risk of bias' items) ‐ Short‐term (< 3 months from randomisation) | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐7.84 [‐12.07, ‐3.61] |
| 2.2 High quality (≥ 6 'Risk of bias' items) ‐ Intermediate (≥ 3 to 12 months) | 3 | 323 | Mean Difference (IV, Random, 95% CI) | ‐4.82 [‐10.96, 1.32] |
7.1. Analysis.
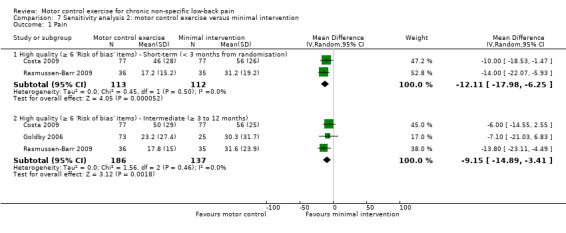
Comparison 7 Sensitivity analysis 2: motor control exercise versus minimal intervention, Outcome 1 Pain.
7.2. Analysis.
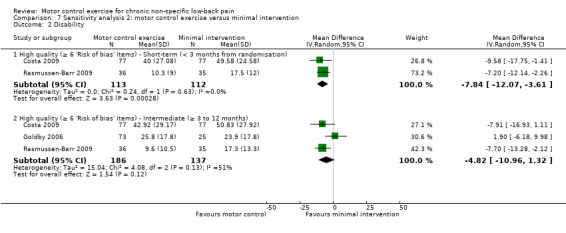
Comparison 7 Sensitivity analysis 2: motor control exercise versus minimal intervention, Outcome 2 Disability.
Comparison 8. Sensitivity analysis 3: motor control exercise versus manual therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 High quality (≥ 6 'Risk of bias' items) ‐ Short‐term (< 3 months from randomisation) | 2 | 241 | Mean Difference (IV, Random, 95% CI) | ‐3.49 [‐9.54, 2.55] |
| 1.2 High quality (≥ 6 'Risk of bias' items) ‐ Intermediate (≥ 3 to 12 months) | 3 | 452 | Mean Difference (IV, Random, 95% CI) | ‐5.51 [‐13.94, 2.92] |
| 1.3 High quality (≥ 6 'Risk of bias' items) ‐ Long‐term (> 12 months) | 3 | 375 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐8.71, 3.50] |
| 2 Disability | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 High quality (≥ 6 'Risk of bias' items) ‐ Short‐term (< 3 months from randomisation) | 2 | 241 | Mean Difference (IV, Fixed, 95% CI) | ‐2.54 [‐7.20, 2.13] |
| 2.2 High quality (≥ 6 'Risk of bias' items) ‐ Intermediate (≥ 3 to 12 months) | 3 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐2.51 [‐6.74, 1.72] |
| 2.3 High quality (≥ 6 'Risk of bias' items) ‐ Long‐term (> 12 months) | 3 | 375 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐7.44, 3.30] |
8.1. Analysis.
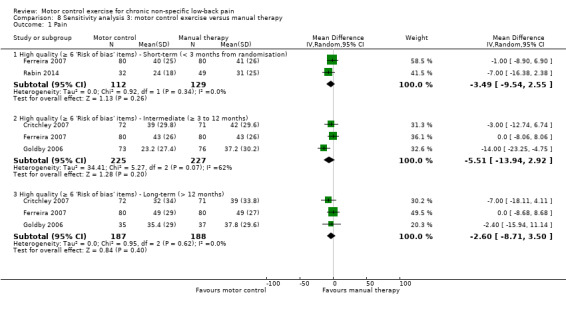
Comparison 8 Sensitivity analysis 3: motor control exercise versus manual therapy, Outcome 1 Pain.
8.2. Analysis.
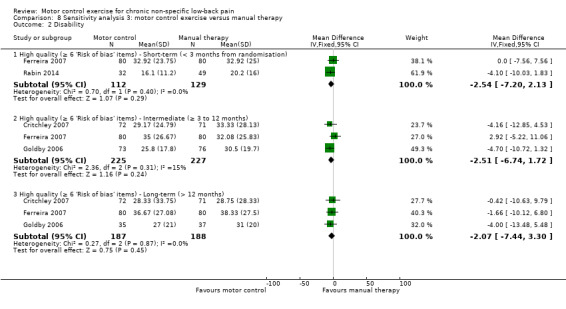
Comparison 8 Sensitivity analysis 3: motor control exercise versus manual therapy, Outcome 2 Disability.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akbari 2008.
| Methods | Randomised controlled trial | |
| Participants | 58 participants included (29 motor control, 29 general exercise) Inclusion criteria: patients were selected based on the following inclusion criteria: non‐specific LBP with or without leg pain of at least 3 months duration, aged greater than 18 and less than 80 years, suitable for motor control exercise based on clinical assessment. The patients must also have sufficient knowledge of the Persian language to understand instructions Exclusion criteria: patients were excluded if they had suspected or confirmed serious spinal pathology, suspected or confirmed pregnancy, nerve root compromise (2 of strength, reflex or sensation affected for same nerve root), spinal surgery, and any of the contraindications to exercise listed on page 42 of the ACSM guidelines. Specific spinal pathology or contraindication to treatment may be suspected based on the results of the screening questionnaires |
|
| Interventions | 16 individually supervised half‐hour sessions of an exercise programme, of 8 weeks duration, 2 sessions per week, was performed for both groups in Razmejo‐Moghadam Physiotherapy Clinic Motor control exercise: low‐load activation of the local stabilising muscles was initially administered, isometrically and in minimally loaded positions (4‐point kneeling, supine lying, sitting, standing). Patients were taught how to contract these muscles independently from the superficial trunk muscles. Progressively, the holding time was increased to the point where patients were able to perform 10 contractions with 10‐second holds, during normal respiration (weeks 1 and 2). The clinical measure used to ensure correct activation of the TA was to observe a slight drawing in manoeuvre of the lower part of the anterior abdominal wall below the umbilical level, consistent with the action of this muscle. In addition, a bulging action of the multifidus muscle should have been felt under the physical therapist's fingers when they were placed on either side of the spinous processes of the L4 and L5 vertebral levels, directly over the belly of this muscle General exercise: this exercise activates paravertebral and abdominal muscles. This exercise imposes extra loading on the spinal tissues, therefore the general exercise was selected on the basis of maximising the contraction benefit/spinal loading ratio, according to the recommendations provided from recent experimental studies |
|
| Outcomes | Pain (NRS 0 to 10) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised through a physical therapist generated random number sequence" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | High risk | Did not analyse all patients randomised |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | "Sixteen individually supervised half‐hour sessions exercise program which lasted 8 weeks and twice per week was performed for both groups" |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Alp 2014.
| Methods | Randomised controlled trial | |
| Participants | 48 participants (24 stabilisation exercise, 24 home‐based exercise) Inclusion criteria: the inclusion criterion was that the patient should have chronic low back pain lasting for a minimum of 6 months leading to disability. Patients were diagnosed with a physical examination, laboratory analysis and imaging techniques, such as X‐ray or MRI scans Exclusion criteria: the patients were excluded if they were found to have active peripheral arthritis, spinal surgery or failed back surgery, new motor or neurologic deficit, systemic infection, cardiovascular/pulmonary disorder with contraindication to exercise, red flags suggesting spinal pathology, pregnancy or unwillingness to do exercise, recent spinal stabilisation, or therapeutic treatment in the last 6 weeks |
|
| Interventions |
Stabilisation exercise (SE): patients in the SE group (n = 24) joined a supervised (physiotherapist) group exercise programme 3 times a week and for a duration of 6 weeks. The lumbar stabilisation exercise programme consisted of warming (5 minutes), stretching (5 minutes), stabilisation exercises for the multifidus/transversus abdominis muscles (30 minutes) and cooling (5 minutes), for a total of 45 to 60 minutes a day Home‐based exercise (HE): patients in the HE group (n = 24) were instructed to do lumbar isometric and lumbar flexion‐extension exercises, 1 x 20 repetitions a day for 6 weeks (standardised home‐based exercise programme for LBP patients given in the outpatient unit), and their adherence to the programme was checked by telephone calls twice a week |
|
| Outcomes | Pain intensity: NRS (0 to 10 scale) Disability: the Roland Morris Disability Questionnaire (RMDQ) ‐ 24 items Quality of life: SF‐36 (Physical and Mental components) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomization was performed using a computer‐generated table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in the baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Cairns 2006.
| Methods | Randomised controlled trial | |
| Participants | 97 patients between 18 and 60 years old were included (47 spinal stabilisation, 50 conventional treatment) Inclusion criteria: patients with LBP, with or without radiating leg pain, aged between 18 and 60 years, who had had a minimum of 1 previous episode of LBP necessitating alteration in normal activities or for which medical care/intervention had been sought Exclusion criteria: evidence of cauda equina compression, non‐mechanical LBP, clinical presentations suggestive of acute objective motor radiculopathy or nerve root compression, with new or progressive neurologic loss, abdominal surgery within the last 12 months, any spinal surgery, systemic illness, neurologic or muscular degenerative disorder, pregnancy or less than 1 year postpartum, psychologic distress (Distress Risk Assessment Method Distressed Depressed or Distressed Somatic) |
|
| Interventions | Patients received a maximum of 12 treatment sessions over 12 weeks. No restriction was placed on prescribed or over‐the‐counter medication. The same physiotherapists delivered both interventions. Details of the content and number of sessions were recorded. Hydrotherapy, back school or other group therapy was prohibited. There were 10 senior physiotherapists, with a minimum of 4 years since qualification and 3 years specialisation in musculoskeletal care, that delivered both treatment packages. To achieve appropriate expertise, all were experienced in stabilisation training, having undertaken recognised postgraduate training courses, and 3 training days as part of the trial, including the use of diagnostic ultrasound to identify correct muscular activation patterns. Both groups received standardised educational information based on the best available evidence regarding continuing normal activities and avoiding rest (The Back Book). The 2 groups received manual and exercise treatments currently used within UK clinical practice. The protocol allowed treatment to be adapted to individual patient needs, with therapists able to select from a range of techniques. Specific spinal stabilisation exercise: endurance training for the deep abdominal and back extensor muscles was the predominant component of this treatment group. A treatment manual for clinicians outlined appropriate exercise progression, but treatment was individualised at the discretion of the clinician. A patient booklet was developed to emphasise the specific nature of the exercises, outlining anatomy and function of the muscles and the concept of endurance training. Diagnostic ultrasound was available at the discretion of the treating clinician for patients in the stabilisation groups if needed. The majority of patients received manual therapy, such as Maitland mobilisations, exercise and advice, with little use of electrotherapy or mechanical lumbar traction. Conventional treatment: exercises using low load, high repetition muscle activity were excluded. All participating departments had adopted an active approach to back pain management, with encouragement to remain active and the minimal use of more “passive” forms of treatment. |
|
| Outcomes | Disability (the Roland Morris Disability Questionnaire) Pain (11‐point numerical rating scale) Quality of life (Short‐Form 36 (SF‐ 36)) Functional disability (Oswestry Disability Index 0% to 100%) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An adaptive stratified randomisation procedure was used incorporating minimisation, using laterality of symptoms, total duration of symptoms (more than or less than 5 years), and Roland Morris Disability Questionnaire score (0–12 or 13). Participants' characteristics were assessed against these categories. If the specified category had uneven numbers in each treatment arm, allocation balanced the distribution. If the category was empty or had even numbers in each treatment arm, a coin flip by an independent observer determined patient allocation" |
| Allocation concealment (selection bias) | Low risk | "Patients were naive to allocation, and therapists had no influence over the randomisation process and treatment allocation, and follow‐up consisted of patient‐completed measures only" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | "Following a random 20% check of the data for accuracy, both per‐protocol and intention‐to‐treat analyses were undertaken" |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Costa 2009.
| Methods | Randomised controlled trial | |
| Participants | 154 patients (77 exercise group, 77 placebo group) Inclusion criteria: participants had to have non‐specific low back pain (defined as pain and discomfort) localised below the costal margin and above the inferior gluteal folds, with or without referred leg pain of at least 3 months' duration; be currently seeking care for low back pain; be aged between 18 and 80 years; comprehend English; and expect to continue residing in the study region for the study duration. In addition, potential participants underwent a simple trunk muscle test to determine that motor control exercise treatment was indicated. Exclusion criteria: suspected or confirmed spinal pathology (e.g. tumour, infection, fracture, inflammatory disease), pregnancy, nerve root compromise, previous spinal surgery, major surgery scheduled during treatment or follow‐up period, and presence of any contraindication to exercise, ultrasound or shortwave therapy |
|
| Interventions | Participants in each group received 12 half‐hour treatments over an 8‐week period (2 sessions per week in the 1st month and 1 session per week in the 2nd month). Both interventions were provided by 3 senior physical therapists who received training from experts in motor control exercise and placebo interventions. This training included a 1‐day workshop prior to the commencement of the study and 3 half day follow‐up sessions during the trial period. Random audits and regular meetings provided by the same experts were conducted during the trial to monitor delivery of interventions. No deviations from the treatment protocol were observed during the audits. Exercise group: at the 1st session, participants were comprehensively assessed by the physical therapist, who prescribed exercises that were individualised based on the participant's presentation. The exercises were designed to improve function of specific muscles of the low back region and control of posture and movement. The motor control exercise programme involved 2 stages. Each participant was progressed through the stages according to specific criteria that should be met in each stage. The 2 stages and their main objectives were: Stage 1 ‐ Train co‐ordinated activity of the trunk muscles, including independent activation of the deeper muscles (including transversus abdominis and multifidus) and reduce overactivity of specific superficial muscles in an individualised manner. Stage 2 ‐ Implement precision of the desired co‐ordination and train these skills in static tasks and incorporate them into dynamic tasks and functional positions. Stage 1 of the exercise programme involved retraining of the multifidus and transversus abdominis muscles. These exercises were supplemented with exercises for the pelvic floor muscles, breathing control, and control of spinal posture and movement. The specific muscles that were trained depended on the initial assessment. Participants were taught how to contract these muscles independently from the superficial trunk muscles. Physical therapists used real‐time ultrasound biofeedback to enhance learning of the tasks. The exercises were progressed until the patient was able to maintain isolated contractions of the target muscles for 10 repetitions of 10 seconds each while maintaining normal respiration. When this level of competence was achieved, patients were considered ready to progress to stage 2. Stage 2 of the exercise programme involved increasing the complexity of the exercise by progressing through a range of functional tasks and exercises targeting co‐ordination of trunk and limb movement, maintenance of optimal trunk stability, and improvement of posture and movement patterns. Participants required the ongoing support of a trained physical therapist to ensure correct performance of the exercises. The participants were instructed to perform a daily set of home exercises. These exercises were performed at the same level and in the same position as those demonstrated during the treatment session. Session 12 was a discharge session in which the patient's progress was reviewed and exercises were prescribed to be continued at home. Placebo group: the placebo treatment was designed to be structurally equivalent to the active intervention, providing similar contact time with the physical therapist. The placebo intervention consisted of 20 minutes of detuned shortwave diathermy and 5 minutes of detuned ultrasound for 12 sessions over an 8‐week period. This form of placebo was used because the detuned machines do not provide a specific treatment effect, but it has been established in previous trials that participants view this intervention as credible. To ensure the perceived credibility of the placebo intervention, physical therapists followed the usual clinical routine for the delivery of the active form of these 2 treatments (i.e. by checking for contraindications, monitoring changes in symptoms, adjusting the detuned devices and appearing to progress the treatment). Each placebo treatment session lasted 30 minutes to match the duration of active treatment sessions. |
|
| Outcomes | Primary outcomes: pain intensity over the previous week (measured with a 0 to 10 numeric rating scale (NRS), activity (measured with the 0 to 10 Patient‐Specific Functional Scale (PSFS)), and global impression of recovery (measured with the 5 to ‐5 Global Perceived Effect Scale (GPE)). Secondary outcomes: pain intensity over the previous week, activity (measured with the PSFS), patient's global impression of recovery measured at 6 and 12 months, and activity limitation (measured with the 0 to 24 Roland‐Morris Disability Questionnaire (RMDQ)) at 2, 6 and 12 months. | |
| Notes | The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN012605000262606), and the protocol was published (Maher 2005) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was computer‐generated by one of the investigators who was not involved in recruitment of participants. The sequence was blocked (block sizes of 4, 6, and 8, in random order)" |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed in sequentially numbered, sealed, opaque envelopes". Eligible patients were allocated to treatment groups by the physical therapist who opened the next‐numbered envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A careful explanation was provided to patients to ensure they remained blinded to treatment allocation" |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | "The nature of the interventions precluded blinding of the treatment provider" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was considered blinded as patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | "The statistical analysis was performed on an intention‐to‐treat basis" |
| Selective reporting (reporting bias) | Low risk | "The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN012605000262606), and the protocol was published (Maher 2005)" |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | High risk | "Ten patients from the exercise group and 14 patients from the placebo group reported use of co‐interventions during the study period" |
| Compliance (performance bias) | Low risk | "Participants in each group received 12 half‐hour treatments over an 8‐week period (2 sessions per week in the first month and 1 session per week in the second month)" |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Critchley 2007.
| Methods | Randomised controlled trial | |
| Participants | 212 participants included (71 individual physiotherapy, 72 spinal stabilisation, 69 pain management) Inclusion criteria: LBP for more than 12 weeks duration, with or without leg symptoms or neurologic signs; being 18 years of age or older; adequate command of English; ability to give informed consent; and ability to attend classes Exclusion criteria: people were excluded if they had previous spinal surgery, physiotherapy for low back pain in the last 6 months, medical conditions such as rheumatological diseases, or other disabilities rendering them unsuitable for group treatment of low back pain |
|
| Interventions | All treating physiotherapists had at least 2 years clinical experience and were briefed about and had agreed to treat according to the trial protocol. The hospitals had their own common internal teaching programme for the leaders of the pain management programme and spinal stabilisation training, but otherwise treating physiotherapists had no extra training for the trial. Spinal stabilisation: the spinal stabilisation physiotherapy consisted of individual transversus abdominis and lumbar multifidus muscle training followed by group exercises that challenged spinal stability. Exercises were tailored to assessment findings and progressed within participants' ability to maintain a stable and minimally painful spine. The exercise programme aimed to improve trunk muscle motor control to provide dynamic segmental stability for the lumbar spine Individual physiotherapy: In the individual physiotherapy protocol, patients were assessed and treated according to assessment findings. Treatment consisted of a combination of joint mobilisations, joint manipulation and massage. Exercises were taught individually to be performed at home and included specific trunk muscle retraining, stretches and general spinal mobility. Patients usually also received back care advice. Up to 12 sessions of around 30 minutes each were permitted in the protocol and according to departmental policy Pain management: the pain management programme consisted of a combination of structured back pain education with group general strengthening, stretching and light aerobic exercises progressed according to pacing principles. The programme consisted of a maximum of 8 sessions of 90 minutes supervised by a senior physiotherapist and physiotherapy assistant |
|
| Outcomes | Disability (Roland Morris Disability Questionnaire) Pain (NRS 0 to 10) Quality of life (EQ‐5D) |
|
| Notes | Registration number: ISRCTN56323917 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before the trial started, the randomisation protocol was computer‐generated and held by a trials unit independent of and distant from the trial setting" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "As this was a pragmatic trial evaluating clinical practices, masking of participants or clinicians was neither possible nor desirable" |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | "As this was a pragmatic trial evaluating clinical practices, masking of participants or clinicians was neither possible nor desirable" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "We randomised 212 participants between March 2002 and September 2003, reassessed 169 (80%) participants at 6 months, 154 (73%) at 12 months and 160 (75%) at 18 months following baseline" |
| Intention‐to‐treat analysis | Low risk | "Clinical outcomes were analysed on both intention to treat and complete case bases according to a previously prepared data analysis plan" |
| Selective reporting (reporting bias) | Low risk | The trial was prospectively registered (ISRCTN56323917) |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Ferreira 2007.
| Methods | Randomised controlled trial | |
| Participants | 240 participants (80 general exercise, 80 motor control, 80 spinal manipulative) Inclusion criteria: to be eligible for inclusion patients had to have non‐specific low back pain for at least 3 months, be aged between 18 and 80 years, and give written informed voluntary consent. Patients who reported osteoarthritis or disc lesions (prolapse, protrusion or herniation without neurological compromise) with or without leg pain were eligible to participate in the study. Exclusion criteria: participants were excluded prior to randomisation if they had neurological signs, specific spinal pathology (e.g. malignancy, or inflammatory joint or bone disease) or if they had undergone back surgery |
|
| Interventions | Participants attended for up to 12 treatment sessions over an 8‐week period Motor control: participants allocated to the motor control exercise group were prescribed exercises aimed at improving function of specific trunk muscles thought to control inter‐segmental movement of the spine, including transversus abdominis, multifidus, the diaphragm and pelvic floor muscles. Each participant was trained by a physical therapist to recruit the deep muscles of the spine and reduce activity of other muscles. Initially participants were taught how to contract the transversus abdominis and multifidus muscles in isolation from the more superficial trunk muscles, but in conjunction with the pelvic floor muscles. Ultrasonography was used to provide feedback about muscle recruitment, except where the therapist judged that ultrasound feedback would not be useful (for example, if the patient was too obese). The difficulty of the tasks was progressed by incorporating more functional positions and training the co‐ordination of all trunk muscles during functional tasks in a manner that was tailored to the individual patient's presentation. General exercise: a physical therapist carried out an initial assessment of each participant allocated to the general exercise group to determine how physically active the participant was, how troublesome the back problem was and the ability of the participant to perform the exercises. Participants were then taught the exercises and advised of the intensity at which they should exercise. The exercises were performed under supervision of a physical therapist in classes of up to 8 people with each class lasting approximately 1 hour. The intensity of the exercises was progressed over the 12 treatments with participants being encouraged to improve their own performance rather than competing with other members of the class. Spinal manipulative: Participants allocated to the spinal manipulative therapy group were treated with joint mobilisation or manipulation techniques applied to the spine or pelvis. The particular dose and techniques were at the discretion of the treating physical therapist, based on each participant's physical examination findings. Participants in this group were not given exercises or a home exercise programme, and they were advised to avoid pain‐aggravating activities. Manipulative therapy was discontinued if the participant completely recovered before the 12 sessions were completed, as is standard clinical practice. |
|
| Outcomes | Function (PSFS) Global Perceived Effect (GPES) Pain (NRS 0 to 10) Disability (RMDQ) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was by a random sequence of randomly permuted blocks of sizes 6, 9 and 15" |
| Allocation concealment (selection bias) | Low risk | "The randomisation schedule was known only to one investigator who was not involved in recruiting participants, and it was concealed from patients and the other investigators using consecutively numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of 240 participants, 93% were followed up at 8 weeks and 88% were followed up at 6 and 12 months" |
| Intention‐to‐treat analysis | Low risk | "Analysis was by intention‐to‐treat in the sense that data were analysed for all randomised subjects for whom follow‐up data were available" |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered with the Australian Clinical trials Registry (ACTRN012605000053628) |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | High risk | "Alternatively, because we did not control treatment after the first eight weeks, it could be that participants in the general exercise group subsequently sought effective". |
| Compliance (performance bias) | Low risk | Compliance was considered similar for the 3 groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Franca 2010.
| Methods | Randomised controlled trial | |
| Participants | 30 participants (15 segmental stabilisation, 15 stretching) Inclusion criteria: low back pain for more than 3 months (pain felt between T12 and the gluteal fold), patients willing and able to participate in an exercise programme safely and without cognitive impairments that would limit their participation Exclusion criteria: exclusion criteria were history of back surgery, rheumatologic disorders, spine infections and spine exercise training in the 3 months before the onset of the study |
|
| Interventions | Interventions were conducted over 6 weeks, twice per week, each session lasting 30 minutes. Sessions were supervised by the investigator, and participants were instructed to report any adverse event, whether it was related to the exercises or not. Groups were instructed not to participate in any other physical programme during the study and not to exercise while at home. Three series of 15 repetitions were done for each exercise. Segmental stabilisation: in the segmental stabilisation (SS) group, exercises focused on the transversus (TrA) and lumbar multifidus (LM) muscles according to the protocol proposed by Richardson et al. Exercises for the TrA in 4 point kneeling, exercises for the TrA in dorsal decubitus with flexed knees; exercises for the LM in ventral decubitus; co‐contraction of the TrA and LM in upright position Superficial stretching: in the superficial strengthening (ST) group, exercises focused on the rectus abdominis (RA), abdominus obliquus internus (OI), abdominus obliquus externus (OE), and erector spinae (ES). Strengthening of the rectus abdominis (RA), external and internal obliquus (EO and IO) and erector spinae (ES). Exercises for the RA in dorsal decubitus with flexed knees: trunk flexion, exercises for the RA, IO and EO in dorsal decubitus and flexed knees: trunk flexion and rotation, exercises for the RA in dorsal decubitus and semi‐flexed knees: hip flexion, exercises for the ES in ventral decubitus: trunk extension. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | ODI: 0% to 100% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomised by means of opaque envelopes to one of two treatment groups" |
| Allocation concealment (selection bias) | Low risk | "They were randomised by means of opaque envelopes to one of two treatment groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Goldby 2006.
| Methods | Randomised controlled trial | |
| Participants | 323 participants included Inclusion criteria: all patients with chronic low back disorder referred to St. George Hospital physiotherapy departments (2 sites) by his/her general practitioner or hospital consultants were informed of the trial. All participants had chronic low back disorder, with the current episode lasting for a minimum of 12 weeks, were aged between 18 and 65 years, and able to read and write English. Exclusion criteria: all patients with non‐mechanical low back pain were excluded. Some participants with mechanical pathology were excluded where evidence indicated that they would benefit from alternative treatment, or if they had conditions that could skew the data. Spinal stenosis, spondylolisthesis grades III or IV, or recent fractures. Significant or worsening signs of neurologic deficit. Evidence of inflammatory joint disease. Lower limb pathology likely to influence leg pain intensity. Present or past history of metastatic disease. Medically unsuitable for participation in the exercise class. Chronic pain syndrome or a history of ≥ 2 operative interventions for low back pain. History of anxiety neurosis. Pregnancy. Participants were withdrawn if they withdrew consent or had exclusion criteria develop while participating. |
|
| Interventions | There were 18 physiotherapists who performed the physiotherapy treatments. All had a minimum of 5 years postgraduate experience in the treatment of chronic low back disorders, and 60% held masters' level qualifications in musculoskeletal physiotherapy. All physiotherapists were instructed by the researcher in the prescription of exercises to facilitate the stabilising muscles and the spinal stabilisation programme. The Back School was provided for all groups and consisted of 1 group specific 3‐hour question and answer session. The class covered anatomy, biomechanics and lifting, pathologies, and advice on education, exercise and general fitness Spinal stabilisation: spinal stabilisation rehabilitation programme was developed aiming to rehabilitate the neural control and active subsystems of the lumbar spine's stabilising system. The 10‐week course was formulated in conjunction with clinical experts and the available literature. It consisted of a functionally progressive exercise class that emphasised the selective retraining of the transversus abdominis, multifidus, the pelvic floor and diaphragm muscles, while inhibiting global muscle substitution mechanisms. A video illustrating the effect of the muscles on the stability of the spine was shown at the beginning and end of each class, between which the patients exercised at facilitation stations. Each station consisted of exercises, which had been verified by the author using ultrasonography to determine the action produced, that facilitated the contraction of the stabilising muscles. The same 2 physiotherapists staffed each class, and a maximum of 12 patients attended at any time. After attending 10 x 1‐hour classes, the patients were discharged. All participants were booked to attend the Back School. Manual therapy: in group B, the physiotherapists continued to treat the patient according to the diagnosis and clinical reasoning. They were not permitted to prescribe any exercises for the transversus abdominis, multifidus, diaphragm or pelvic floor muscles. Nor were they allowed to prescribe any electrophysical methods. Any other form of exercise or manual procedure within the remit of musculoskeletal physiotherapy was allowed. Patients were discharged at the discretion of the physiotherapist or to a maximum number of 10 interventions. All patients were booked to attend the Back School Education: after being informed of the patient's allocation, the physiotherapist explained the contents of the educational booklet "Back in Action" to the patient. Patients were then discharged and booked to attend the Back School. Because patients in all groups attended the Back School, the independent variable in group C became the "Back in Action" booklet. This booklet has shown the ineffectiveness in the treatment of patients with chronic low back disorder and, as such, it formed the basis for establishing group C as the control group. |
|
| Outcomes | Pain (NRS 0 to 100) Disability (Oswestry Disability Index (ODI)) Quality of life (NHP) |
|
| Notes | NHP: Nottingham Health Profile | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to 1 of the groups using a stratification procedure"... "Using the computer package Clinstat,12 blocks of random numbers were created" |
| Allocation concealment (selection bias) | Low risk | "The research assistant collected the data related to the dependent variables and informed the researcher of the details required to allocate randomly the subject. At all times, the research assistant remained blind to the patients' group allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Of subjects, 10% were lost to follow‐up between the 3 and 12‐month stage, and 50% between the 12 and 24‐month stage" |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Hemmati 2011.
| Methods | Randomised controlled trial | |
| Participants | 24 women were included (12 experimental and 12 control). All participants were from a convenience sample of friends and family. | |
| Interventions | Motor control exercise versus no treatment | |
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | Data were extracted by a Persian translator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate random sequence generation used |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no mention of loss to follow‐up or how many were included at the follow‐up |
| Intention‐to‐treat analysis | Unclear risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not mentioned |
| Compliance (performance bias) | Unclear risk | Not mentioned |
| Timing of outcome assessment (detection bias) | Unclear risk | Not mentioned |
Hosseinifar 2013.
| Methods | Randomised controlled trial | |
| Participants | 37 participants (18 stabilisation exercises, 19 McKenzie exercises) Inclusion criteria: age between 18–50 years, CLBP in the area between the costal margin and buttocks, with or without reference to the lower extremity (no radicular pain) that lasted more than 3 months Exclusion criteria: patients were excluded if they had a history of recent fracture, trauma or previous surgery in the lumbar region; had spondylolysis or spondylolisthesis, spinal stenosis, neurological disorders, systemic diseases, cardiovascular diseases, diseases; were pregnant; were receiving concomitant treatment, with physical therapy modalities; or were receiving other therapies simultaneously |
|
| Interventions | The training programme consisted of 18 sessions of supervised individual training for both groups, with the sessions performed 3 times per week for 6 weeks. Each training session lasted an hour and was performed at the Physiotherapy Clinic in the School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran, between 2011 and 2012 Stabilisation exercises: (1 hour, 18 sessions, 3 times per week, 6 weeks). For warming up and before performing specific exercises, participants pedalled a stationary bike for 5 minutes and then did stretching exercises for 10 minutes). Stabilisation exercises were divided into 6 levels from easy to difficult. At the end of each training level, participants performed each exercise 10 times for 10 seconds with low intensity). The stabilisation exercises were performed in 6 steps: 1) segmental control exercises (SCE) with emphasis on training the of isolated contraction of the TrA, MF and pelvic floor muscles; 2) SCE with emphasis on co‐contractions of the TrA, MF and pelvic floor muscles in the prone, supine and 4‐point kneeling positions; 3) closed kinematic chain SCE; 4) development of SCE into the low load apply by adding leverage of the limbs during open chain exercises; 5) development of SCE in functional situations; and 6) co‐contraction of the TrA and MF muscles during application of an external load, complication of movements, increased load with the lumbar spine in the correct position, addition of a co‐contraction pattern to light aerobic activities such as walking, and activities that have already exacerbated the symptoms) McKenzie exercises: (1 hour, 18 sessions, 3 times per week, 6 weeks). During the treatment session, between 80 and 100 repetitions of the selected exercises were carried out in the McKenzie group. In the McKenzie group, 6 exercises were used: 4 extension‐type exercises and 2 flexion‐type and 2 flexion‐type exercises. The extension‐type exercises were performed in prone and standing positions, and the flexion‐type exercises were performed in the supine and sitting positions. The final position of each exercise was maintained for 10 seconds. |
|
| Outcomes | Pain (NRS 0 to 100) Disability (Functional Rating Index (FRI)) |
|
| Notes | FRI questionnaire: 10 sections ‐ each section was rated using a 5‐point scale (0: without pain; 4: maximum pain). Total score: sum of all sections | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...the participants were selected through a simple non‐probability sampling method and were randomly divided into two equal groups using sequences of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Inani 2013.
| Methods | Randomised controlled trial | |
| Participants | 30 participants. The inclusion criteria were: age group 20 to 50 years, both sexes, diagnosed with non‐specific low back pain. | |
| Interventions |
Core stabilisation exercise: the treatment was given in 4 rehabilitation phases. Phase–I: Activation phase: here patient was taught to cognitively perform skilled activation of deep muscles i.e. transversus abdominis (TA) and lumbar multifidus (LM) alone while relaxing the superficial muscle. Phase–II: Skill precision. Once the patients could perform independent contraction of local muscles (TA/LM) the next phase was to improve precision of task. Phase–III: Superficial and deep muscle co‐activation. Here, the participants were asked to co‐ordinate the activity of deep and superficial muscles without the global muscle taking over. Fitness activities were given with closed and open chain activities. Closed chain segmental control stage involves following procedure: (1) Training individual part of the anti‐gravity weight bearing holding postures. (2) Weight bearing exercises in flexed postures. Open chain segmental control involves the exercises like leg standing with hip flexion, extension and abduction, adduction in position such as lying, side‐lying, sitting or standing. Phase‐IV: Functional re‐education. It was subject‐specific training. The exercises in the experimental group were as follows. Before starting with actual core exercises patients were asked to do warm up by performing stretching exercises of tight muscles, isometric exercises of abdominals and back extensors of spine for 10 times each: ‐ Transversus abdominis (TA) and lumbar multifidus (LM) exercises: according to 4 rehabilitation phases above explained were progressed. Few exercises which activate other core muscles like quadratus lumborum, psoas major, lumbar portions of longissimus, Iliocostalis responsible for dynamic stability of spine were given. ‐ Slow curl‐ups: responsible for co‐activation of transversus abdominis and rectus abdominis. Patient in supine position, with hand under lumbar region (to help to stabilise the pelvis and preserve the neutral spine posture) and with one leg bent and other leg straight to assist in pelvic lumbar stabilisation; then the patient was instructed to raise the head and shoulder off the ground – Sit ups: activates the psoas muscle. Patient in supine position with knee bent, head and shoulders are raised off the ground with hand under the head – Oblique plank/side bridge: activates the quadratus lumborum and oblique muscle. Patient is side‐lying with knee bent/straight, then rising horizontally with support on elbows and knee/legs – Bird‐dog exercises: activates back extensors (longissimus, Iliocostalis and multifidus) Patient in quadruped position with elbow locked straight and head in neutral position. Patient pulls in the belly button and lifts one leg off the floor so that the limb is in line with the trunk and then the opposite side arm is lifted off the ground Conventional exercise: the exercises in the control group were as follows: (1) Stretching exercises: the muscles that were found to be tight during assessment were given static stretching exercises. (2) Isometric exercises of spine: – Hollowing in abdominals: participant supine position, lying with knee bent. Patients were asked to slowly draw in the abdomen towards the spine (press down on towel placed under lumber curve) without the movement of the trunk – Isometric for back extensors: participant in supine position with arm at side. Participant was instructed to arch the back by pressing against the mat with the back of neck and sacrum. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A total number of 30 subjects were divided in 2 groups (15 in each group) with simple random sampling method" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | All patients randomised were analysed according to Figure 3 of the study |
| Selective reporting (reporting bias) | Low risk | No protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Javadian 2012.
| Methods | Randomised controlled trial | |
| Participants | 30 participants included Inclusion criteria: patients had to have at least 3 months low back pain and show one of the trunk aberrant movement patterns (painful arc during flexion and return from flexion, Gowern's sign and instability catch). Also they exhibited negative straight leg rising and a positive prone instability test. Exclusion criteria: patients with vertebral fracture, disc herniation, acute low back pain, systemic diseases, osteoarthritis, spondylolisthesis and spondylolysis, leg length discrepancy, history of spinal surgery, pregnancy and any low back pain with known causes were excluded |
|
| Interventions | The participants were evaluated 3 times; before treatment, at 8 weeks and 3 months after the last treatment session. Stabilisation exercise (SE): specific exercises programme in the experimental group included all routine exercises and SE. These exercises containing bracing and hollowing exercises in supine, bridging, kneeling, sitting and standing positions. SE exercises were conducted in dynamic situations including associate movements of extremities, on the Swiss ball and the wobble board in the advanced phase. The duration of exercise therapy was the same for the 2 groups. General exercise: the control group was treated under routine exercise only. The treatment sessions were divided into warm up exercises and specific training. Warm up lasted 15 minutes, which included cycling and the stretching of trunk, hip adductor, hip abductor and hamstring and gastrocnemius and soleus muscles in both groups. The specific exercises in the control group were routine exercises, including: single and double leg knee to chest, bridging, bridging and interval lower limb raising, supine cycling, heel slide, leg slide and lower abdominal crunch in the supine position. Exercises were done in all 4 positions with intermittent rising of upper and lower limbs cross rising of the upper and lower limbs and finally bridging in the side lying position. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information regarding this issue |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | "The duration of exercise therapy was the same for the two groups" |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time. "The participants were evaluated three times; before treatment, at 8 weeks and 3 months after the last treatment session". |
Kachanathu 2012.
| Methods | Randomised controlled trial | |
| Participants | 30 professional fast bowlers with chronic LBP were screened at sports complexes around Riyadh, Kingdom of Saudi Arabia Inclusion criteria: non‐specific LBP with or without referred pain (of a non‐radicular nature) of at least 3 months with physiotherapy scheduled to start; average pain intensity over the last 2 weeks ≥ 3 and ≤ 8 on a 0 to 10 visual analogue scale; minimal to moderate disability score (0% to 40%) on the Oswestry Low Back Pain Disability Questionnaire (OLBPDO) Exclusion criteria: abdominal surgery within the past 12 months, or a history of spinal or limb surgery; systemic illness; neurological or muscular degenerative disorders; peripheral vascular disease; participants with body mass index of more than 27; participants with central nervous system impairments; respiratory or cardiovascular impairment affecting the perturbation trial; and prior participation in a programme of spine segmental stabilisation exercises. |
|
| Interventions | Both groups of patients were given back ergonomics care lessons, and a model demonstration of safe lifting techniques in back care classes during the 1st week of the intervention. As commonly prescribed in Indian settings, 10 minutes of moist heat was also given to both groups at the end of each session. An exercise session lasted approximately 45 minutes. Core stabilisation: (45 minutes, 14 sessions, 4 days per week, 8 weeks). A basic outline of the various exercises for local and global muscles and the differences in their function was given before the start of the programme. In group A, participants initially received 14 guided training sessions each lasting 45 minutes, which emphasised core muscle co‐contraction, 4 days a week. The 8‐week treatment protocol was divided into 3 phases. Each exercise was performed in 3 sets of 5 repetitions with 5 seconds hold time and 10 seconds rest between each repetition and a minutes rest in between each set. In the 1st phase of the training, attention was focused on facilitating isolated local muscle activity with emphasis on continuation of normal breathing. Subsequently, the hold time and the number of repetitions were increased, and participants were trained to maintain these contractions in various postures (4‐point kneeling, supine, prone, sitting and standing). Once an accurate and sustained contraction of the local muscles was achieved in different postures (10% to 15% MVC, 10 contractions with 10‐second holds), the exercises progressed to the second phase, which involved applying low load to the muscles through controlled movements of the upper and lower extremities. The main aim during the 3rd phase was to integrate these low grade static contractions with normal static and dynamic functional tasks so that these contractions became habitual. Conventional regimen: group B performed basic conventional physiotherapy strengthening exercises. The rate of perceived exertion was used to monitor the level of exertion during strengthening exercises, and it ranged from 6 to 9, 10 to 15 and 16 to 20 in the respective phases. Based on physical examination and the clinical judgement of treating therapist, 83% of the participants received a hyperextension exercise programme as the main mode of treatment, and 17% of participants received a flexion exercise programme as the main mode of treatment. Progression of patients in both groups was decided by the treating physiotherapist. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information regarding this issue |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Unclear risk | Not clearly described |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Koumantakis 2005.
| Methods | Randomised controlled trial | |
| Participants | 55 participants (29 stabilisation + general exercise, 26 general exercise). Patients were recruited from the orthopaedic clinic of a local hospital and several general practitioners' practices. Patients took part in the study after informed consent had been obtained. The rights of human participants were protected at all times. Inclusion criteria: patients were eligible for the study if they had a history of recurrent LBP (repeated episodes of pain in past year collectively lasting for less than 6 months) of a non‐specific nature, defined as back pain complaints occurring without identifiable specific anatomical or neuro‐physiological causative factors. To establish this, all patients included in the trial had a prior clinical examination by their physician, including a radiograph or a magnetic resonance imaging scan. Exclusion criteria: patients with previous spinal surgery, "red flags" (i.e. serious spinal pathology or nerve root pain signs) as outlined in the Clinical Standards Advisory Group (CSAG) report for back pain, or signs and symptoms of instability (radiological diagnosis of spondylolysis or spondylolisthesis corresponding to a symptomatic spinal level; "catching", "locking", "giving way" or "a feeling of instability" in one direction or multiple directions of spinal movements) were excluded. |
|
| Interventions | The same frequency (twice per week), programme duration (8 weeks) and class duration (45 to 60 minutes per session) were provided for both groups. All participants received an information booklet (The Back Book) providing the latest scientific facts on LBP management at the beginning of the programme. The main aim of this booklet is to change patient beliefs and behaviours regarding back pain. The clinical physical therapist who administered the exercise sessions monitored class adherence, and participants were required to keep an exercise diary monitoring home adherence. The number of sessions in class environment and at home was recorded. Stabilisation + general exercise: 8 weeks, 2 times per week, 45 to 60 minutes per session. Briefly, low‐load activation of the local stabilising muscles was initially administered, with no movement (isometric) and in minimally loaded positions (4‐point kneeling, supine lying, sitting, standing). Progressively, the holding time and then the number of contractions were increased in those positions up to 10 contraction repetitions,10‐second duration each (weeks 1 and 2). The clinical measure used to ensure correct activation of the transversus abdominis muscle was to observe a slight drawing‐in manoeuvre of the lower part of the anterior abdominal wall below the umbilical level, consistent with the action of this muscle. In addition, a bulging action of the multifidus muscle should have been felt under the clinical physical therapist's fingers when they were placed on either side of the spinous processes of the L4 and L5 vertebral levels, directly over the belly of this muscle. Various facilitation techniques were used throughout the programme to draw participants' attention to the specific nature of the desired muscle contractions (tactile and pressure cues over areas of the specific muscles, auditory cues to enhance their contraction, use of contraction of the pelvic‐floor muscles). Furthermore, participants were made aware of and were told to avoid several incorrect muscle activation ("substitution") strategies, where a movement muscle takes over the control of movement from the stabilising muscles (too much effort causing unwanted spasms in the movement muscles or spinal movement at the initial stages were discouraged). Integration with dynamic function (activities that required spinal or limb movements) through incorporation of the stabilising muscles' co‐contraction into light functional tasks was advised as soon as (1) the specific pattern of co‐activation was achieved in the minimally loading positions and (2) the participants could comfortably perform 10 contraction repetitions x 10 seconds duration each (weeks 3 to 5). Heavier‐load functional tasks, with exercises similar to those performed by the participants who performed general exercise only, were progressively introduced in the last 3 weeks of the programme. General exercise: 8 weeks, 2 times per week, 45 to 60 minutes per session. For the participants who performed general exercise only, exercises activating the extensor (paraspinals) and flexor (abdominals) muscle groups were administered. Muscle contraction occurring with exercise imposes extra loading on the spinal tissues, therefore the general exercises were selected on the basis of maximising the contraction benefit/spinal loading ratio, according to recommendations provided from recent experimental studies. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Roland Morris Disability Questionnaire (RMDQ)) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Following completion of all pre‐intervention assessments, subjects were randomly assigned to 1 of the 2 intervention groups via a computer generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | "Randomisation codes were kept in sealed envelopes with consecutive numbering" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "From the 55 randomly assigned subjects, 10 dropped out of the program (n=5 per group)..." |
| Intention‐to‐treat analysis | Low risk | "All analyses were performed primarily according to the "intention‐to‐treat" (ITT) principle, with all subjects randomly assigned for intervention analysed in their assigned groups" |
| Selective reporting (reporting bias) | Low risk | No protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Kumar 2009.
| Methods | Randomised controlled trial | |
| Participants | 30 hockey players included Inclusion criteria: male hockey players from Sports Authority of India (SAI), Lucknow, aged 18 to 28 years, who were diagnosed clinically by a physician with no neurological involvement but having symptomatic (overuse, overload or overstretching) non‐specific subacute or chronic low back pain Exclusion criteria: no neurological involvement |
|
| Interventions | After group allocations, respective participants were treated either with conventional or dynamic muscular stabilisation treatment. Both the treatments were given as individual treatment by the same physiotherapist with the same intensity and capacity on alternate day for 35 days. The duration of each individual treatment session was about 40 minutes per day. The participants were not allowed to receive any other treatment, including pain killers. Dynamic muscular stabilisation treatment (DMST): in DMST, muscles with direct attachment to the lumbar spinal segment stabilise the joints "neutral zone" and prevent excessive deflection. Exercise is given in 4 stages in the following order: (i) 1st week: isolation and facilitation of target muscles. Verbal instruction such as drawing in and hollowing the lower abdomen, drawing the naval up and in toward the spine, or feeling the muscle tighten at the waist. From the beginning the patient learns to breathe normally while activating or holding the muscular contraction. The patient is in supine hook lying position and instructed to perform abdominal hollowing (in which the patient is instructed to make the lower abdomen cave in) or abdominal bracing (in which the patient is instructed to contract the abdominals by actively flaring out laterally in the region of the waist just above the iliac crest). (ii) 2nd week: training of trunk stabilisation under static conditions of increased load. The patient's position and concentration pattern are the same as the first week; the individual is then asked to hold the position while load is added via the weight of the lower limbs being moved passively into a loaded position. (iii) 3rd week: development of trunk stabilisation during slow controlled movement of the lumbar spine. Once stability is trained through static procedure, the movement of the trunk will optimise the activation of the supporting muscle. The first step is to produce and explore lumbopelvic movement and learn abdominal hollowing or bracing in a variety of positions: sitting, quadruped, standing, supine, kneeling and inclination by degree to control loading. (iv) 4th and 5th weeks: lumbar stabilisation during high‐speed and skilled movement. High‐speed phasic exercises are recommended to the patient along with abdominal hollowing or bracing in a variety of positions. Conventional treatment: ultrasound, short‐wave diathermy (SWD) and lumber strengthening exercises. Ultrasound (US): for the purpose of this study as a treatment for a chronic condition, a frequency of 1 MHz was used rather than 3 MHz, which penetrates least and is absorbed superficially. Continuous pattern ultrasound is recommended for use in chronic conditions at intensity 1.2 W/cm2 for a period of 8 minutes for 18 sittings in 18 alternate days. Ultrasound equipment was used from Medichem Electronics, which has international standard certification. Short‐Wave Diathermy. SWD is a deep heating modality used in relieving pain. It is also used to enhance flexibility and blood flow and reduce inflammation. Short‐wave forms are used for selected patients without neurological lesion. Continuous mode of SWD is used for 15 minutes with 18 sittings in 18 alternate days. The SWD was used from Medichem Electronics, which has international standard certification. Lumbar strengthening exercises. The uses of lumbar strengthening exercises (LSE) are well documented, including spinal extension exercises and trunk extensor muscles exercises. LSEs were done for 10 repetitions each exercise per sitting on alternate days. |
|
| Outcomes | Pain (NRS 0 to 10) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned equally into 2 groups by a lottery method |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Kumar 2010.
| Methods | Randomised controlled trial | |
| Participants | A total of 141 (male/female) LBP patients from Department of Physical Medicine and Rehabilitation, CSM Medical University, Lucknow, aged 20 to 40 years who were diagnosed clinically by a physician with no neurological involvement, non‐specific, sub‐acute or chronic low back pain were included in this study Exclusion criteria: neurological involvement |
|
| Interventions | After group allocations, respective participants were treated either with conventional (CONV) or dynamic muscular stabilisation treatment (DMST) in a single‐blind manner (i.e. patients were not aware of the treatment groups). Both the treatments were given as individual treatments by the same physiotherapist with the same intensity and capacity on 20 regular days and followed up for 180 days. Follow‐up was started after 20 days of regular exercises at OPD, which was ended after 6 months from the 0 day. During follow‐up, participants had an appointment periodically with the investigator at 15‐day intervals for review of exercises. The duration of each individual treatment session was about 40 minutes per day. The participants were not allowed to get any other treatment options including the pain killers. Dynamic muscular stabilisation treatment (DMST): in DMST, muscles with direct attachment to the lumbar spinal segment stabilise the joint's 'neutral zone' and prevent excessive deflection. Details of the DMST exercise programme are described elsewhere. Conventional treatment: consisted of ultrasound (1 MHz continuous at an intensity of 1.2 W per cm square for 5 minutes). Short‐wave diathermy (continuous mode of SWD for 15 minutes) and the lumbar strengthening exercises (10 repetitions each of prone lying leg elevation, prone lying chest elevation and supine lying bridging). Participants received 20 sitting in 20 regular days. Ultrasound and short‐wave diathermy equipment from Medichem Electronics were used in the study, which has international standard certification |
|
| Outcomes | Pain (NRS 0 to 10) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomised equally in two groups by lottery method" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this topic |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...patients were not aware of the treatments groups..." |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Considered, as the participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Lomond 2015.
| Methods | Randomised controlled trial | |
| Participants | 33 participants Inclusion criteria: participants who were admitted to the study were between 21 and 55 years old, had a history of chronic LBP (> 12 months) with or without recurrences, could stand and walk independently, had an ODI score of 19% or more and/or a score of less than 8 on at least one activity from the Patient Specific Functional Scale, could understand English and were currently employed or actively engaged in daily activities Exclusion criteria: exclusion criteria included structural spinal deformity, spinal fracture, osteoporosis, systemic disease processes, disc herniation, previous spinal surgery, pregnancy or less than 6 months of postpartum or postweaning, magnified symptom behaviour and a body mass index (BMI) of greater than 30 |
|
| Interventions |
Stabilisation intervention (STB): the STB protocol focused on 3 components of spinal stability: (1) motor control of the deep trunk muscles, (2) strengthening of the flexor, extensor and oblique trunk muscles by focusing on repeated submaximal efforts to mimic the function of these muscles in spine STB, and (3) an education booklet that describes proper body mechanics of the spine during activities of daily living Movement system impairment (MSI): the MSI protocol to focus on (1) education regarding how the subject's lumbopelvic movement patterns and postures repeated daily might accelerate lumbar tissue stress as well as education about positions or postures to control symptoms, (2) exercises to modify the subject's specific trunk movements and postures in particular directions that were pain‐free, and (3) functional activity modifications (based on their Patient Specific Functional Scale) to change the subject's trunk movement and alignment patterns. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | ODI: 100% The study was prospectively registered (NCT01362049) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization with centralized allocation concealment was used to randomise subjects into each treatment" |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomization with centralized allocation concealment was used to randomise subjects into each treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | According to Figure 2, patients were analysed in the group to which they were allocated by randomisation |
| Selective reporting (reporting bias) | Low risk | "This study was prospectively registered (NCT01362049)" |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Macedo 2012.
| Methods | Randomised controlled trial | |
| Participants | 172 participants (86 motor control, 86 graded activity). Participants were recruited to the trial by general practitioners in Sydney and Brisbane or drawn from the waiting list of an outpatient physical therapy department from a public hospital in Sydney. Inclusion criteria: chronic non‐specific low back pain (> 3 months duration) with or without leg pain, currently seeking care for low back pain, between 18 and 80 years of age, English speaker (to allow response to the questionnaires and communication with the physical therapist), clinical assessment indicated that the patient was suitable for active exercises expected to continue residing in the Sydney or Brisbane region for the study duration, had a score of moderate or greater on question 7 ("How much bodily pain have you had during the past week?") or question 8 ("During the past week, how much did pain interfere with your normal work, including both work outside the home and housework?") of the 36‐Item Short‐Form Health Survey questionnaire (SF‐36). Exclusion criteria: known or suspected serious pathology such as nerve root compromise (at least 2 of the following signs: weakness, reflex changes or sensation loss, associated with the same spinal nerve), previous spinal surgery or scheduled for surgery during trial period, comorbid health conditions that would prevent active participation in exercise programmes. They used a "red flag" checklist to screen for serious pathology and the Physical Activity Readiness Questionnaire from the American College of Sports Medicine guidelines to screen for comorbid health conditions that would prevent safe participation in exercise. |
|
| Interventions | Participants in each group were to receive 14 individually supervised sessions of approximately 1 hour. The treatment consisted of 12 initial treatment sessions over an 8‐week period and 2 booster sessions at 4 and 10 months following randomisation. The initial 12 sessions were conducted twice a week for the first 4 weeks and once a week for the following 4 weeks. The treatment sessions were designed to become less frequent and promote independence. In order to facilitate adherence to treatment sessions and to be consistent with clinical practice, if patients could not complete the initial 12 treatment sessions within the first 8 weeks, they received an extension of another 4 weeks to complete the 12 treatment sessions. Patients included in both exercise programmes were advised to do home exercises for at least half an hour per week in the first month and 1 hour per week in the 2nd month. The type of home exercises, intensity and number of sessions per day were at the discretion of the physical therapist. Trial interventions were provided by 10 physical therapists with at least 2 years of clinical experience who received training in motor control exercises and graded activity. Therefore, all therapists provided both interventions. The training included a 2‐day workshop for the motor control exercises and a series of evening interactive seminars for graded activity, both administered by recognised experts in the field. The same experts performed audits of trial treatment of most of the treating physical therapists to evaluate and encourage compliance with the treatment protocols. Although they did not have the specific data necessary to evaluate the physical therapists' compliance with the treatment protocols, their audits revealed that most physical therapists followed the treatment protocols and there was no evidence of cross‐contamination. The physical therapists worked at private clinical practices or at the university clinic. Motor control: 14 sessions, 1 hour, 8 weeks (4 weeks ‐ 2 times per week/4 weeks ‐ once a week). A primary goal of the exercise was to enable the patient to regain control and co‐ordination of the spine and pelvis using principles of motor learning such as segmentation and simplification. The intervention was based on assessment of the individual participant's motor control impairments and treatment goals (set collaboratively with the therapist). The first stage of the treatment involved assessment of the postures, movement patterns and muscle activation associated with symptoms and implementation of a retraining programme designed to improve activity of muscles assessed to have poor control (commonly, but not limited to, the deeper muscles such as transversus abdominis, multifidus, pelvic floor and diaphragm) and reducing activity of any muscle identified to be overactive, commonly the large, more superficial trunk muscles such as the obliquus externus abdominis. Participants were taught how to contract trunk muscles in a specific manner and progress until they were able to maintain isolated contractions of the target muscles for 10 repetitions of 10 seconds each while maintaining normal respiration. Feedback such as palpation and real‐time ultrasound images were available to enhance learning of the tasks. During this stage, additional exercises for breathing control, posture of the spine, and lower limb and trunk movement were performed. The 2nd stage of the treatment involved the progression of the exercises toward more functional activities, first using static and then dynamic tasks. Throughout this process, the recruitment of the trunk muscles, posture, movement pattern and breathing were assessed and corrected. In contrast to the graded activity programme, motor control exercise was guided by pain, and exercises were mostly pain‐free. Graded activity: 14 sessions, 1 hour, 8 weeks (4 weeks ‐ 2 times per week/4 weeks ‐ once a week). A primary goal of the programme was to increase activity tolerance by performing individualised and submaximal exercises, in addition to ignoring illness behaviours and reinforcing wellness behaviours. The programme was based on activities that each participant identified as problematic and that he or she could not perform or had difficulty performing because of back pain. The activities in the programme were progressed in a time‐contingent manner (rather than a traditional pain‐contingent manner) from the baseline‐assessed ability to a target goal set jointly by participant and therapist. Participants received daily quotas and were instructed to only perform the agreed amount, not less or more, even when they felt they were capable of doing more. Cognitive‐behavioural principles were used to help the participants overcome the natural anxiety associated with pain and activities. The physical therapists used positive reinforcement, explained pain mechanisms, and addressed negative behaviours and pain‐related anxiety. A plan for managing relapses was developed by the therapists and participants. |
|
| Outcomes | Pain (NRS 0 to 10) Function (Patient Specific Function Scale (PSFS)) Global Perceived Effect (GPES) Quality of life (SF‐36) Disability (Roland Morris Disability Questionnaire (RMDQ)) |
|
| Notes | Clinical trial registration: ACTRN12607000432415 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was computer generated by an investigator not involved in recruitment or treatment allocation" |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed in sequentially numbered, sealed, opaque envelopes by an investigator not involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | "Clinicians could not be blinded to the interventions" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | "The statistical analyses were performed using SPSS version 16.0 for Windows (SPSS Inc, Chicago, Illinois) and STATA version 9.0 (Stata‐Corp LP, College Station, Texas) (linear mixed models) on an intention‐to‐treat basis" |
| Selective reporting (reporting bias) | Low risk | "This trial was prospectively registered (ACTRN12607000432415), and the protocol has previously been published" |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | High risk | "Ten, five, and eight participants in the graded activity group and 6, 17, and 9 participants in the motor control exercise group reported receiving co‐interventions in addition to the trial treatment at the 2‐, 6‐, and 12‐month follow‐ups, respectively" |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Miller 2005.
| Methods | Randomised controlled trial | |
| Participants | The sample population for this study was composed of individuals with chronic LBP who were referred to an outpatient physical therapy clinic in western New York State. All potential participants for this study were examined and referred to physical therapy by a physician prior to consideration for participation. Inclusion criteria: participation in the study was based on the following criteria: participants had to have been experiencing chronic LBP for greater than 7 weeks since the initial onset, as defined by the Quebec Task Force, at the time of acceptance to the study Exclusion criteria: patients were also excluded from the study if they were under 18, pregnant, received Workman's Compensation benefits, had litigation pending associated with their present injury, had undergone more than one lumbar surgery, had been diagnosed with a psychological illness, were unable to understand English, or had been diagnosed with a systemic inflammatory disease (e.g. lupus, rheumatoid arthritis, ankylosing spondylitis, etc.) |
|
| Interventions | Participants in both groups received a physical therapy examination that assessed their baseline strength, range of motion, response to repeated lumbar movements, passive intervertebral motion, straight leg raising and neurological status. The examination was conducted prior to instruction in the preceding interventions. Treatment schedules were based on the physical therapist's recommendation and the patient's availability. Patients in both groups were asked to perform approximately 10 to 15 minutes of home exercises that were prescribed according to the treatment group to which they were assigned. Specific spine‐stabilising exercise: 6 weeks, once a week. These exercises focused on strengthening the lumbar multifidus and transversus abdominis muscles through performance of a lower abdominal contraction. In performing this exercise, the patient moved the umbilicus towards the spine while the spine was maintained in a neutral alignment. To assist in the facilitating a contraction of these muscles, a pressure gauge was placed under the low back (Stabiliser, Chattanooga Pacific Pty. Ltd., Brisbane, Australia) to serve as a biofeedback mechanism. Both verbal and tactile cues were used to insure that the patient was not substituting contractions of the rectus abdominus, external oblique or diaphragm muscles for the transversus abdominis. Once the patient was able to initiate an isolated co‐contraction of these muscles, they were progressed to holding the contraction while performing a progressive exercise programme consisting of movements of the extremities in multiple positions and during functional tasks such as sitting, standing and walking McKenzie: 6 weeks, once a week. Participants assigned to this group received treatment based on their history and response to the repeated movement examination. Following the completion of the McKenzie exam, patients with mechanical LBP were assigned to one of 4 syndrome classifications (postural, derangement, dysfunction, other). Depending on the classification, a treatment programme was prescribed that may have included posture correction, performance of end‐range repeated movements of the spine, or the use of manual techniques designed to reduce and/or abolish the patient's signs and symptoms. |
|
| Outcomes | Function (Functional Status Questionnaire) Pain (NRS 0 to 10) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done using a random number generator to assign each subject a number" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The examiners were not blinded during data collection" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 30 subjects, 29 completed the study. One subject withdrew from the study following the initial examination with no specific reason provided". |
| Intention‐to‐treat analysis | High risk | No mention of any attempts to blind the participants |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Statistical analyses revealed no differences between groups for subject characteristics or baseline data" |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was 6 weeks, once a week for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Moon 2013.
| Methods | Randomised controlled trial | |
| Participants | 24 participants (12 lumbar stabilisation exercise group, 12 lumbar dynamic strengthening exercise group) Inclusion criteria: patients complaining of non‐specific LBP without any structural or neuropsychological cause, for more than 3 months, were recruited from the rehabilitation outpatient clinic Exclusion criteria: history of neurological, infectious and systemic diseases, including cerebrovascular disease, spinal cord disease, spondylitis, cancer, rheumatologic disorders and other chronic diseases that cause long‐term immobilisation. Patients who had undergone prior surgery for back pain, patients who were prescribed exercise therapy in the past, patients who seemed to have radicular pain due to nerve root involvement on physical examination and patients with structural lesions, such as spondylolisthesis, vertebral bone fracture, scoliosis and kyphosis on X‐ray, were also excluded. |
|
| Interventions | Each exercise session lasted 60 minutes and was performed 2 days per week, for 8 weeks. All patients in both groups performed warm‐up stretching exercises for 15 minutes before the main exercises and cool down exercises for 10 minutes after each session. All exercises were performed in the treatment room under the supervision of a physical therapist with technical knowledge. The therapist put each patient into the appropriate position to achieve the correct posture and muscle contraction. For all exercises in both groups, the final static position was held for 10 seconds, and each exercise was performed for 10 repetitions. There was a pause of 3 seconds between repetitions and a 60‐second rest between each exercise. Exercise intensity (holding time and number of repetitions) was increased gradually, based on the tolerance of each patient. Lumbar stabilisation exercise group: 1 hour, 2 days per week, 8 weeks. Lumbar stabilisation exercises consisted of 16 exercises, which aimed to strengthen the deep lumbar stabilising muscles: the transversus abdominis, lumbar multifidi and internal obliques. All 16 stabilisation exercises were performed once, consecutively, and in the same order. Before each exercise, the physical therapist gave detailed verbal explanation and visual instructions (pictures), regarding the start and end positions. All exercises were conducted according to the following specific principles: breathe in and out, gently and slowly draw in your lower abdomen below your umbilicus without moving your upper stomach, back or pelvis"; resulting in a situation referred to as hollowing. Participants practised "hollowing" with a therapist providing verbal instruction and tactile feedback until they were able to perform the manoeuvre in a satisfactory manner. In addition, a "bulging" of the multifidus muscle should have been felt by the therapist when the fingers were placed on either side of the spinous processes of the L4 and L5 vertebrae, directly over the belly of this muscle. These feedback techniques provided by precise palpation of the appropriate muscles, ensure effective muscle activation. Lumbar dynamic strengthening exercise group: 1 hour, 2 days per week, 8 weeks. Conventional lumbar dynamic strengthening exercises consisted of 14 exercises, which activated the extensor (erector spinae) and flexor (rectus abdominis) muscle groups |
|
| Outcomes | Pain (NRS 0 to 100) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were enrolled in the study and randomly assigned to one of the two groups, a lumbar stabilisation exercise group (n=12) and a conventional lumbar dynamic exercise group (n=12) by a computer‐generated random number sequence" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding the allocation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Puntumetakul 2013.
| Methods | Randomised controlled trial | |
| Participants | 42 participants (21 core stabilisation exercise, 21 conventional group) Patients aged 20 to 60 years who had had LBP for 3 months or longer were recruited from the Orthopaedic Outpatient Department, the Physical Medicine and Rehabilitation Outpatient Department, and the Physical Therapy Department of Srinagarind Hospital, Khon Kaen Province, Thailand. They underwent a history‐taking interview and a physical examination by an orthopaedic surgeon who was unaware of the treatment procedure. Anteroposterior, lateral and flexion‐extension radiographs were used to exclude other spinal conditions. An instability catch sign was performed to confirm the diagnosis of clinical lumbar stability. A positive instability catch sign was defined as a sudden painful snap when a patient extends his/her back from the trunk forward‐bending position into the upright position. To be eligible, patients must have a pain intensity of at least 5 out of 10, based on a numeric rating scale during instability catch sign, and have a positive sign in one of the following provocation tests: painful catch, apprehension sign, or prone instability test. |
|
| Interventions | Eligible participants were enrolled in the study and randomly assigned to one of the 2 groups: CSE and conventional group (CG). Lasting approximately 20 minutes, all training sessions of both groups took place at the Physical Therapy Laboratory twice a week for 10 weeks. Exercises were demonstrated and supervised by a research assistant blinded to the outcome assessment. Core stabilisation exercise: 20 minutes, 2 times per week, 10 weeks. The 10‐week exercise programme was divided into 3 phases. The first phase, weeks 1 and 2, focused on correctly isolating low‐load activation of the transversus abdominis (TrA) and lumbar multifidus (LM) muscles. Then, co‐activation of TrA and LM were taught. A pressure biofeedback device (Chattanooga Australia Pty Ltd, Brisbane, QLD, Australia) and electromyography biofeedback (MP100, BIOPAC Systems Inc., Goleta, CA, USA) were used to provide feedback and facilitate correct performance during training. The 2nd phase, weeks 3 to 7, started as soon as individuals could accurately control the TrA and LM muscles. The exercises progressed to the application of low load to the muscles through controlled movements of the upper and lower extremities. The last phase, weeks 8 to 10, aimed to integrate this co‐activation into functional tasks. The participants were trained to maintain co‐activation of TrA and LM during walking and 2 chosen tasks previously known to aggravate pain. Conventional group: 20 minutes, 2 times per week, 10 weeks. This group performed active trunk stretching exercises, which are the standard treatment for LBP in Thailand. The exercises consisted of 10 repetitions of an alternating single knee to chest; as well as a lateral trunk‐bending in standing. Each exercise was to be held for 10 seconds. Immediately after the exercises, a Hydrocollator (60°C) (Enraf‐Nonius Medical Equipment Company Ltd., Bangkok, Thailand), was placed over the lumbar area in the supine position for 15 minutes. Both groups were required to practise the demonstrated exercises at home on a daily basis. To monitor their compliance, they were asked to record this in their logbook. In addition, every week, the same physical therapist made a phone call to participants in both groups to motivate them to continue their home exercises. After 10 weeks of training, all participants were asked to completely stop their exercise. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Roland Morris Disability Questionnaire (RMDQ)) Patient Satisfaction (GPES) Quality of life ‐ physical and mental components (SF‐36) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were enrolled in the study and randomly assigned to one of the two groups: CSE and conventional group by a block randomisation with block sizes of two, four, and six" |
| Allocation concealment (selection bias) | Low risk | "Randomisation results were concealed in sealed and opaque envelopes with consecutive numbering" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Although the loss to follow‐up was two participants for each group at 10 weeks of intervention, it was within the 15% attrition rate" |
| Intention‐to‐treat analysis | Low risk | "All analyses were performed on the basis of intention‐to‐treat with the last observation carried forward" |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not reported |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Rabin 2014.
| Methods | Randomised controlled trial | |
| Participants | 105 participants (48 lumbar stabilisation exercise, 57 manual therapy) 105 patients diagnosed with LBP and referred to physical therapy at 1 of 5 outpatient clinics of Clalit Health Services in the Tel‐Aviv metropolitan area, Israel, were recruited for this study. Participants were included if they were 18 to 60 years of age, had a primary complaint of LBP with or without associated leg symptoms (pain, paraesthesia), and had a minimum score of 24% on the Hebrew version of the modified Oswestry Disability Index (ODI) outcome measure. |
|
| Interventions | 16 physical therapists were involved in the study. 11 therapists, with between 4 and 12 years of experience in outpatient physical therapy patient care, provided treatment, and 5 therapists, with between 13 to 25 years of experience, performed baseline and follow‐up evaluations. Prior to beginning the study, all participating therapists underwent 2 x 4‐hour sessions in which the rationale and protocol of the study were presented and the examination and treatment procedures were demonstrated and practised. Therapists had to pass a written examination of the study procedures prior to data collection. Finally, each therapist received a manual describing treatment and evaluation procedures, based on the therapist's role in the study (treatment or evaluation). Therapists involved in treating patients were unaware of the concept of the clinical prediction rule throughout the study, to avoid bias from this knowledge during treatment. All treating therapists provided both treatments of the study (lumbar stabilisation exercise and manual therapy). Patients in both groups received 11 treatment sessions over an 8‐week period. Each patient was seen twice a week during the first 4 weeks, then once a week for 3 additional weeks. A 12th session (usually on the 8th week) consisted of a final evaluation. The total number of sessions (12) matched the maximum number of physical therapy visits allowed annually per condition under the policy of the Clalit Health Services health maintenance organisation, which covered all patients participating in the study. Patients in both groups were prescribed a home exercise programme consistent with their treatment group; however, no attempt was made to monitor patients' compliance with the home exercise programme. Lumbar stabilisation exercise (LSE): 11 sessions (plus 1 final evaluation), 8 weeks (first 4 weeks: 2 times per week/last 3 weeks: 1 time per week). Patients were initially educated about the structure and function of the trunk musculature, as well as common impairments in these muscles among patients with LBP. Patients were then taught to perform an isolated contraction of the transversus abdominis and lumbar multifidus through an abdominal drawing‐in manoeuvre (ADIM) in the quadruped, standing and supine positions. Once a proper ADIM was achieved (most likely by the 2nd or 3rd visit), additional loads were placed on the spine through various upper extremity, lower extremity and trunk movement patterns. Exercises were performed in the quadruped, side‐lying, supine and standing positions, with the goal of recruiting a variety of trunk muscles. In each position, exercises were ordered by their level of difficulty, and patients progressed from one exercise to the next after satisfying specific predetermined criteria. In the 7th treatment session, functional movement patterns were incorporated into the training programme while performing an ADIM and maintaining a neutral lumbar spine. Manual therapy (MT): 11 sessions, 8 weeks (first 4 weeks: 2 times per week/last 3 weeks: 1 time per week). The MT intervention included several thrust and nonthrust manipulative techniques directed at the lumbar spine that have been used previously with some degree of success in various groups with LBP. In addition, manual stretching of several hip and thigh muscles was performed, as flexibility of the lower extremity is purported to protect the spine from excessive strain. Finally, active range of motion and stretching exercises were added to the programme, as these are commonly prescribed in combination with MT to maintain or improve the mobility gains resulting from the manual procedures. The exercises included were selected to minimise trunk muscle activation and to avoid duplication between the 2 interventions. All manual procedures and exercises were prescribed based on the clinical judgement of the treating therapist; however, each session could include up to 3 manual techniques, 1 of which had to be a thrust manipulative technique directed at the lumbar spine, and an additional technique that had to include a manual stretch of a lower extremity muscle. The 3rd technique, as well as the complementary range of motion/flexibility exercises, was given at the discretion of the treating therapist. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Modified Oswestry Disability Questionnaire 0 to 100) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on a computer‐generated list of random numbers, stratified by CPR status to ensure that adequate numbers of patients with a positive and a negative CPR status would be included in each intervention group" |
| Allocation concealment (selection bias) | Low risk | "The list was kept by a research assistant who was not involved in patient recruitment, examination, or treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Sixteen patients did not complete the LSE intervention, and 8 patients did not complete the MT intervention". (81/105 ‐ more than 20% dropouts) |
| Intention‐to‐treat analysis | Low risk | The analysis was carried out by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Rasmussen‐Barr 2003.
| Methods | Randomised controlled trial | |
| Participants | 47 participants included. Patients with low back pain (subacute, chronic or recurrent) seeking care at a physiotherapy clinic in Stockholm in 1999–2000 were asked to participate in the study. 47 patients (12 men, 35 women) volunteered to take part. They received verbal and written information about the trial. Inclusion criteria: men and women aged 18 to 60 years with LBP (pain > 6 weeks) with or without radiation to the knee and pain provoked by provocation tests of lower lumbar segments | |
| Interventions |
Stabilising training (ST): 6 weeks, once a week, 45 minutes. The ST‐group patients (n = 22) underwent a 6‐week treatment programme, meeting individually with a physiotherapist once a week for 45 minutes. The patients were told how to activate and control their deep abdominal and lumbar multifidus muscles. The first phase was cognitive and the patients were taught how these muscles act as stabilisers for the lumbar spine. The importance of re‐learning motor control of these muscles was underlined. The patients were taught how to activate the deep abdominal muscles together with relaxed breathing in different positions (e.g. supine crooked‐lying, 4‐point kneeling, prone, sitting and standing). The activation of lumbar multifidus together with the deep abdominal muscles was also trained.The physiotherapist monitored the patient by palpating the lower abdominal quadrant for deep tensioning of the abdominal muscles and by palpating the lumbar multifidus at the painful level. A bio‐pressure unit (Chattanooga Pacific P/L, Australia) was used in the learning process. The exercises were gradually developed by applying low load to the muscles through the limbs in different positions. The patients were instructed in how to use contraction of the muscles during activities of daily living and in situations that set off pain. They were encouraged to perform a training programme, designed to take 10 to 15 minutes, at home each day. They kept a training diary to control compliance. During each session the physiotherapist monitored how well the patient was able to control the muscle activity and to perform the exercises. The patients were also taught basic ergonomics. Manual therapy (MT): 6 weeks, once a week, 45 minutes. The MT‐group patients (n = 20) underwent a 6‐week programme, being treated individually once a week by a physiotherapist for 45 minutes. Manual techniques were used, based on findings from the physical examination. They could include a combination of muscle stretching, segmental traction and soft tissue mobilisation and, if needed, mobilisation of stiff thoracic and upper lumbar segments. No manipulation was done. The patients were encouraged to go on with their usual activities or exercises (not controlled). None of these exercises included specific stabilising exercises. The patients were also taught basic ergonomics. |
|
| Outcomes | Pain (VAS 0 to 100) Disability (Oswestry LBP Questionnaire) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The first woman and first man included in the study were randomized to one of the groups by lot (25 ST cards and 25 MT cards in a box). The men and the women were then separately and consistently randomized to either group. At randomization the patients were assigned a unique code". |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Forty‐one patients completed the 6‐week intervention. Thirty‐three patients replied to the 3‐month follow‐up questionnaire and thirty‐one to the 12‐ month follow‐up (Table 1)". |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | "At baseline there was no significant difference between the groups regarding age, gender, sick leave, pain duration, medication, exercise habits or earlier treatment (Table 2)" |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Rasmussen‐Barr 2009.
| Methods | Randomised controlled trial | |
| Participants | 71 patients included (36 men, 35 women) Participants with LBP seeking care at a primary health care setting, a private physiotherapy clinic, between August 2003 and May 2004 were considered for inclusion. Of the participants, 23% were referred from general practitioners, and the rest sought care by own initiative or by recommendations. Inclusion criteria: men and women aged 18 to 60 years, still at work despite ongoing recurrent LBP (8 weeks) but with at least 1 pain‐free period during the previous year. LBP was defined as pain, ache or discomfort, localised below the costal margin and above the inferior gluteal folds without referred leg pain. The participants had mechanically induced LBP with pain on active movement (e.g. extension, flexion and lateral flexion), paravertebral tenderness and a positive springing test of at least 1 lumbar segment. The clinical tests used have previously been tested for good inter‐examiner reliability. |
|
| Interventions | The initial clinical examination lasted for 60 minutes for all included participants. All participants were informed on inclusion that physical activity is beneficial for LBP, but not what activity is best. All participants received information on the importance of continuing normal activities and basic advice on e.g. lifting, resting and sitting. The treatment period was 8 weeks; the participants in the exercise group were individually supervised by a PT weekly for 45 minutes and the participants in the reference group met the PT for 45 minutes the first (week 1) and last week (week 8). Exercise group: 8 weeks, once a week, 45 minutes. The PT individually supervised and used clinical judgement in the progression of the graded stabilising exercises. First, the participants were informed of how the stabilising muscles act, as hypothesised, in healthy people and in those with LBP. The PT demonstrated how the muscles act as stabilisers. It was explained that the "deep inner muscle corset" (i.e. the local muscle system) and the "outer corset" (i.e. the global muscle system) are both important for maintaining good functional stability of the spine. The importance of relearning activation of the deep inner corset (i.e. transversus abdominis and the deep multifidus) was emphasised. To avoid recurrent LBP periods, the importance of contracting the stabilising muscles in activities of daily life, especially those that set off pain, was underlined. The progression of the exercises was based on the patients' pain level and observed movement control and quality. In contrast to strength training, the programme used low‐load endurance exercises. The first stage consisted of specific exercises to address the stabilising muscles, after the protocol described by Richardson et al, with instructions to gently draw in the anterolateral abdominal wall (i.e. transversus abdominis isolated from the other abdominal muscles) together with a tightening of the MF in different non‐postural positions, together with relaxed breathing. A bio‐pressure unit was used in the learning process (Stabiliser; Chattanooga Group, Hixon, TM). In the subsequent phase, the programme gradually progressed to performing the exercises posturally more upright and to functionally loaded positions/exercises. Exercises with moderate resistance via pulleys in standing and seated positions were performed to increase the demand on the stabilising muscle system and to train the "local" and "global" muscle system together. A natural spine position both during the exercises and in daily life was emphasised, avoiding pain‐generating postures. The patients were encouraged to perform the low‐load exercises at home every day. The home‐training programme was designed to take approximately 15 minutes, and has previously been reported on. The participants were instructed to maintain the programme indefinitely to avoid recurrence of pain. It was emphasised that although adherence with a home‐training programme is important, the most important thing is to incorporate activation of the stabilising muscles in daily life. Reference group: 8 weeks, once a week, 45 minutes. The participants in the reference group (n = 35) were informed of the benefits of daily walks as physical activity. They were instructed to take a 30‐minute walk every day. The walk might be divided into 2 parts of 15 minutes. They were instructed to walk at the fastest pace that was convenient and did not set off pain. If their pain persisted or increased they should slow down. They should continue with other usual activities. They were also given general home exercises but with no follow‐up instructions. The daily walks taken were recorded in a diary, which was returned to the PT at the last visit. The participants were informed that if the pain increased or if they had any questions they were free to call their PT. |
|
| Outcomes | Disability (Oswestry Disability Index (ODI)) Pain (VAS 0 to 100) Quality of life (SF‐36) |
|
| Notes | "Foundation funds were received in support of this work from the Capio Research Foundation and the Ann‐Mari and Ragnar Hemborg Foundation. These funding organisations had no authority over or input into any part of the study. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A fixed allocation randomization procedure guaranteed equal numbers of patients of each sex in each group: the first woman and the first man were allocated by lot to either the exercise group or the reference group" |
| Allocation concealment (selection bias) | Low risk | "The assignments were presented in sealed, sequentially numbered envelopes, and the assignment list was maintained by the clinic's secretarial staff" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | "An intention‐to‐treat procedure was followed (last‐observation carried‐forward)" |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | "Clinical and demographic characteristics were similar between the 2 groups (Table 1)" |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered similar for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Rhee 2012.
| Methods | Randomised controlled trial | |
| Participants | 21 patients included Inclusion criteria: participants were recruited from the greater city Seoul, Korea. Participants who expressed interest in the study became eligible for the study. Those participants who met study inclusion criteria received information regarding the purpose and methods of the study and signed a copy of the Institutional Review Board approved consent form. In this study, patients with recurrent LBP were defined as those who met study inclusion criteria and experienced a disturbing impairment or abnormality in the functioning of the low back. The patients with recurrent LBP were defined as those who previously experienced at least 1 episode of work‐related back pain. Current diagnoses and prior injury data were based on both a physician's history and physical exam results, which were obtained from the patients' records. Participants were eligible to participate if they: 1) were 21 years of age or older, 2) had at least 1 episode of work‐related back pain without referred pain into the lower extremities, and 3) indicated a willingness to participate in a daily exercise programme and in supervised exercise sessions 5 times a week for 4 weeks during the intervention period. Exclusion criteria: participants were excluded from participation if they: 1) had a diagnosed mental illness that might interfere with the study protocol, 2) had difficulty in understanding written/spoken English that precluded them from completing questionnaires, 3) had overt neurological signs (sensory deficits or motor paralysis), or 4) were pregnant |
|
| Interventions |
Spinal stabilisation exercise (SSE): 3 times per week, 4 weeks (5 times per week ‐ home exercise). The SSE protocol was designed to improve spinal stabilisation through core muscle strengthening rather than to improve spinal stabilisation through low back muscles endurance or strengthening. The SSE group performed specific localised exercises aimed at restoring the stabilising protective function of the spinal muscles around the spinal joint. As applied by several authors, the exercises were designed specifically to activate and train the isometric holding function of the spinal muscle at the affected vertebral segment (in co‐contraction with the transversus abdominis muscle); this rehabilitation approach is described in detail. Patients from the SSE group were seen 3 times per week, but performed the exercises 5 times per week at home. In addition to performing home exercises, the patients performed the 20‐minute exercise session in the lab (supervised by the research co‐ordinator) 3 times per week for 4 weeks to ensure that the exercises were being performed correctly. Patients kept an exercise log, and phone calls were made to ensure compliance with the exercise protocol. Control group (advice only): the control group received a hard copy of medical management techniques, which included advice regarding bed rest, absence from work, prescription medications and resuming normal activity as tolerated |
|
| Outcomes | Pain (VAS 0 to 100) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation list was provided, with patients having an equal chance of being allocated to the intervention or control group. The coordinator ensured anonymity of allocation with respect to randomisation" |
| Allocation concealment (selection bias) | Low risk | "The randomisation schedule was prepared prior to the beginning of the trial, and the coordinator was given a sealed envelope for each patient before the assessment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding dropouts |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | High risk | We did not consider the groups to be similar at baseline regarding the outcomes included in this review |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was acceptable for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Shaughnessy 2004.
| Methods | Randomised controlled trial | |
| Participants | 41 participants. Patients presenting to the orthopaedic clinics of Merlin Park Hospital, Galway, Ireland, who were given a referral for physiotherapy and met the inclusion criteria were included in the trial. Inclusion criteria: the study required that the participants were aged 20 to 60 years, complained of LBP for a minimum of 12 weeks and possessed the ability to give informed consent, understand instructions and co‐operate with treatment Exclusion criteria: participants were excluded if their LBP was as a result of systemic or structural pathology, were diagnosed with inflammatory joint disease or displayed overt neurological signs |
|
| Interventions |
Treatment group: 10 exercise training sessions, 10 weeks (week 1: 2 times, 1 hour/week 2: 2 times, 30 minutes weeks 3 to 10: 1 time, 30 minutes). Participants in the treatment group underwent a standardised treatment schedule of 10 exercise‐training sessions over 10 weeks. This consisted of 2 x 1‐hour sessions during week 1, 2 x 30‐minute sessions during week 2, 1 x 30‐minute session during each of weeks 3 to 6, and 1 x 30‐minute session during week 8 and 10. Treatment was delivered by a chartered physiotherapist and involved exercise therapy sessions aimed at training core stability muscles. The training programme was carried out in the following manner: (1) Participants were trained how to activate their transversus abdominus and multifidus muscles. Facilitation strategies utilised by the physiotherapist included visualisation techniques, verbal instruction, manual palpation and education using illustrations. Strength of contraction, monitored using a pressure biofeedback unit, was restricted to low levels compared to maximum voluntary contraction. (2) Training commenced in a low‐load non‐functional position (prone lying, 4‐point kneeling, supine lying with flexed knees). Substitution strategies such as raising the rib cage, external oblique over activity or breath holding were avoided. (3) Holding time for exercises was gradually increased to the point where participants were able to perform 10 contractions with 10‐second holds. (4) Once participants were able to perform sustained contractions in low‐load postures, the regime was progressed by adding leverage through limb movement. (5) Participants performed a daily maintenance exercise programme at home in between exercise sessions with the physiotherapist. Participants' performance of this programme was facilitated by means of written material (created using PhysioTools software). Control group: control participants received no intervention. Following a period of 10 weeks, participants completed follow‐up testing on all questionnaires. Control participants received standard physiotherapy intervention once their follow‐up testing was completed. |
|
| Outcomes | Disability (Oswestry Disability Index (ODI)) Disability (RMDQ) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information regarding this issue |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | We considered the compliance acceptable for both groups |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Stankovic 2012.
| Methods | Randomised controlled trial | |
| Participants | 160 patients (100 specific lumbar stabilisation, 60 control) Inclusion criteria: low back pain that lasted more than 12 weeks, varying in intensity and irradiation, from mild to very strong, Functional limitations in performing certain activities of everyday living: dressing, lifting heavy objects, walking, running, sitting, standing, sleeping, etc. Exclusion criteria: proven acute radiculopathy or severe pain below the knee (clinical examination and interview), inability to perform isometric muscle contractions or to be exposed to medium level of physical exertion due to some internal illness (cardiovascular, pulmonary, systemic etc.), some neurological illness (stroke, polyneuropathy), lack of understanding of the study (dementia, language problems), drug or alcohol abuse |
|
| Interventions | All participants had a total of 20 therapeutic treatments, for 4 weeks (5 days per week). Each treatment lasted 30 minutes. All data were collected before and after the therapy. Specific lumbar stabilisation: 20 sessions, 4 weeks, 5 days/week, 30 minutes. The Study Group (SG) had a combined exercise programme that included spinal segmental stabilisation exercises. The programme consisted of 15 exercises, designed to combine isometric contraction of stabilising muscles of the lower back, abdominal wall and the pelvic floor, with aerobic set of exercises for CLBP. Each session began in a standing position. After several relaxation and breathing exercises, the patients were given instructions about how to form a stabilising corset by joint isometric contraction of the multifidus and transversus abdominis muscles. The verification of the achieved stabilisation was carried out by the therapist and the patients themselves, palpating the contracted muscles. The participants learned how to maintain and properly quantify achieved contractions while doing simple exercises. After the initial stabilisation training, the patients were ready to begin with strengthening and stretching aerobic exercises. The programme was performed in standing, sitting, kneeling and lying positions. During the exercises, the patients were trying to keep their trunk and pelvic girdle inactive. The programme consisted of different sets of exercises such as: pelvic elevation (bridging), abdominal training (curl‐ups), mixed extension/flexion stretch of the spinal column (cat‐camel), hook‐lying (posterior pelvic inclination), etc. They also included exercises on unstable support (Swiss Ball), in order to improve proprioception, co‐ordination and balance. Control (exercise): 20 sessions, 4 weeks, 5 days/week, 30 minutes. Control group (CG) consisted of treatment carried out according to traditional Regan‐Michelle's protocol, strengthening and stretching aerobic exercises, without pelvic immobilisation and core stabilisation. The programme was designed to activate the large muscle groups in the superficial layer of the lower back and abdomen in order to improve overall muscle strength and endurance. |
|
| Outcomes | Disability (Oswestry Disability Index (ODI)) Pain (NRS 0 to 10). |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information regarding this issue |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | According to Figure 1 the dropout rate exceeded 20% |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | "All subjects had a total of 20 therapeutic treatments, for 4 weeks (5 days per week). Each treatment lasted 30 minutes" |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Tsauo 2009.
| Methods | Randomised controlled trial | |
| Participants | 37 participants ‐ functional training group (20), control group (17) Inclusion criteria: patients with non‐specific LBP for at least 3 months, with activity limitation in work or home maintenance, were recruited for this study Exclusion criteria: they were screened by a physiatrist to rule out LBP with specific origins, such as sciatica from a herniated disc or radiculopathy |
|
| Interventions | Patients in both groups might maintain their current rehabilitation programme, and patients in the training group underwent an additional training programme. The current treatments for participants in both groups were almost the same because they were recruited from the same department. Functional training group: maximum of 3 hours per day, 2 to 3 months of training. The strengthening programme was focused on trunk stabilisation training for the superficial and deep trunk muscles and the extremities. Trunk stabilisation training was executed with a stabiliser (Chattanooga Group, USA) for biofeedback initially. After patients could control their trunk muscles (transversus abdominis and multifidus) effectively in both static and dynamic conditions, movements of the extremities with graded increments of range and weight were added. Core muscle contraction was further incorporated into the training activities to simulate patients' life needs. Programmes of work/activity simulation training, such as push, pull and lift with or without weights, were determined according to the testing results of the FCE and the patient's activity requirement. Patients could terminate the programme if they felt uncomfortable during training. The training intensity and time increased to a maximum of 3 hours per day as the patients' endurance improved. In all, patients would spend 100 hours over a period of 2 to 3 months in training. Control group: no treatment |
|
| Outcomes | Disability (Oswestry Disability Index (ODI)) Pain (0‐20) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information regarding this issue |
| Allocation concealment (selection bias) | Unclear risk | Not enough information regarding this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not considered as patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Seven patients in the training group did not have enough time to complete the training programme. Five patients in the control group were not willing to receive the second evaluation after 3 months. The presented results are the data of the remaining 25 patients, 13 in the training group and 12 in the control group" |
| Intention‐to‐treat analysis | High risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No previous protocol or trial registration, but it was clear that the published report included all expected outcomes |
| Group similarity at baseline (selection bias) | High risk | We did not consider the groups to be similar at baseline regarding the outcomes included in this review |
| Co‐interventions (performance bias) | Unclear risk | Not described |
| Compliance (performance bias) | Low risk | Compliance was considered acceptable |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
Unsgaard‐Tondel 2010.
| Methods | Randomised controlled trial | |
| Participants | Participants included were 19 to 60 years of age with chronic non‐specific LBP of at least 3 months' duration and with pain at presentation between 2 and 10 on the numeric pain rating scale (NPRS) (0 to 10). Participants were recruited from general practitioners or physical therapists (29/109) and by announcement to employees at a large local hospital (80/109) in Norway. Exclusion criteria: participants were excluded for the following reasons: previous back surgery, radiating pain below the knee or neurologic signs from nerve root compression, systemic or widespread pain, overweight preventing ultrasound imaging, pregnancy, diagnosed psychiatric disease, sick leave for more than 1 year, recipient of disability benefits, unresolved social security or insurance problems or insufficient language capabilities |
|
| Interventions | The participants in all treatment groups attended treatment once a week for 8 weeks. The attendance at weekly treatment sessions was recorded, but adherence to home exercise was not recorded. All participants were encouraged to stay active in their daily life, as recommended by systematic reviews on advice for management of LBP. In addition, all participants received a booklet with general information on LBP provided by the Norwegian Network of Back Pain that also emphasised benefits of varied physical activity for non‐specific LBP. Participants were not allowed to receive other treatment for LBP during the intervention period. The physical therapists were experienced in the exercise methods applied. Participants in all treatment groups received home exercises for flexibility when considered necessary. Motor control: once a week, 8 weeks, 40 minutes. The motor control treatments lasted 40 minutes and took place in an outpatient clinic. The low‐load motor control exercises were individualised and taught by a specially trained physical therapist according to a protocol on therapeutic exercise for lumbopelvic stabilisation and ultrasound imaging. Ultrasound imaging was used as both a teaching tool and an evaluation tool (separate substudy). Patients with motor control deficits may benefit from visual feedback of muscle function from ultrasound imaging. Ultrasound imaging is increasingly used among clinicians to retrain motor control in the deep abdominal muscles. The low‐load motor control exercises focused on isolated control and activity of the transversus abdominus during the abdominal drawing‐in manoeuvre (ADIM). The aim of the ADIM was to voluntary activate transversus abdominis (TrA) thickening and lateral slide while the internal oblique and external oblique abdominal muscles remained relatively unchanged. The exercises were executed with low effort and with relaxed respiration and were continuously monitored by direct observation of respiration and by real‐time b‐mode ultrasound imaging of superficial and deep muscle activity. Activity in the abdominal muscles was visualised on the ultrasound screen for each participant and used for feedback in all treatment sessions. Participants also were instructed in pelvic floor and multifidus muscle contractions. Furthermore, a goal was to obtain controlled co‐contraction of the TrA, the deep fibres of the multifidus muscle, and the pelvic floor muscles while keeping other muscles relaxed. Participants who achieved isolated activity of the TrA in the supine position progressed to activation of the TrA similarly in sitting and standing positions. Toward the end of the intervention period, the participants were instructed to incorporate the ADIM into activities of daily living. Written instruction to carry out the ADIM at home was provided, and participants were encouraged to perform 10 pain‐free contractions 2 to 3 times per day, holding each contraction for 10 seconds. Sling exercise: once a week, 8 weeks. The participants in the sling exercise intervention group were instructed individually by a specially trained physical therapist. The exercises were chosen from a predefined set of back exercises in slings on the basis of an assessment of each participant's ability to keep the lumbar spine stable in the neutral position through a range of leg and arm positions and movements. The sling method for dosing lumbopelvic exercises has been assessed in combination with other treatment modalities in earlier studies. Unloading elastic bands were attached to the pelvis to help participants maintain the neutral spine position at all times and for exercises to progress without pain. Exercise progression was achieved by gradually reducing the elastic band support. The supported position where the participants could no longer maintain the neutral spine position was used as the baseline for further exercise progression. By placing the participants in demanding but pain‐free positions and asking them to hold the spine in neutral, the aim was to activate the deep and superficial stabilising trunk muscles (local and global muscles). When weakness, pain, fatigue or asymmetry was identified, this position served as starting point for training and further progression. The number of repetitions and sets was individually adjusted according to pain and fatigue. The sling exercises were performed for 40 minutes once a week in a physical therapy clinic. General exercise: once a week, 8 weeks. The general exercise intervention received general trunk strengthening and stretching exercises, as recommended in the management of non‐specific LBP. Exercises were instructed by a physical therapist and performed in small groups of 2 to 8 people. Exercises performed were, for instance, trunk extension, flexion and rotation with resistance and stretching of trunk and extremity muscles. The exercises were performed for 1 hour weekly in a local fitness centre with a traditional resistance apparatus and with 10 repetitions in 3 sets. The exercise instructor supervised each participant and individually directed and adapted the exercise performance when needed. |
|
| Outcomes | Pain (NRS 0 to 10) Disability (Oswestry Disability Index (ODI)) |
|
| Notes | The study was registered in ClinicalTrials.gov with identifier NCT00201513 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was administered by an independent study secretary via telephone. The secretary consecutively reported group allocation for included participants from a list of random numbers between 0 and 1 that were computationally generated" |
| Allocation concealment (selection bias) | Low risk | "The randomization was administered by an independent study secretary via telephone" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of any attempts to blind the participants |
| Blinding of personnel/care provider (performance bias) All outcomes | High risk | No mention of any attempts to blind the care provider |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of any attempts to blind the assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage of withdrawals and dropouts was within the acceptable rate |
| Intention‐to‐treat analysis | Low risk | "All participants were included in the statistical analyses, independent of completion" |
| Selective reporting (reporting bias) | Low risk | The study was registered in ClinicalTrials.gov with identifier NCT00201513 |
| Group similarity at baseline (selection bias) | Low risk | Patients did not differ in their baseline characteristics |
| Co‐interventions (performance bias) | Low risk | "Participants were not allowed to receive other treatment for LBP during the intervention period" |
| Compliance (performance bias) | Low risk | "The participants in all treatment groups attended treatment once a week for 8 weeks" |
| Timing of outcome assessment (detection bias) | Low risk | All important outcome assessments for both groups were measured at the same time |
ACSM: American College of Sports Medicine ADIM: abdominal drawing‐in manoeuvre CLBP: chronic low back pain CSE: core stabilisation exercise DMST: dynamic muscular stabilisation treatment FCE: functional capacity evaluation FRI: Functional Rating Index GPES: Global Perceived Effect HE: home‐based exercise LBP: low back pain LSE: lumbar strengthening/stabilisation exercise LM: lumbar multifidus MF: multifidus MRI: magnetic resonance imaging MSI: movement system impairment MVC: maximum voluntary contraction NHP: Nottingham Health Profile NRS: numerical rating scale ODI: Oswestry Disability Index OLBPDO: Oswestry Low Back Pain Disability Questionnaire PSFS: Patient Specific Functional Scale PT: physical therapist RMDQ: Roland Morris Disability Questionnaire SCE: segmental control exercise SE: stabilisation exercise STB: stabilisation intervention SWD: short‐wave diathermy TA: transversus abdominis TrA: transversus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aasa 2015 | Not MCE or mixed intervention |
| ACTRN12609000293268 | Not MCE or mixed intervention |
| ACTRN12609000334202 | Not MCE or mixed intervention |
| Aggarwal 2010 | Not non‐specific LBP patients |
| Ahmed 2014 | All groups received MCE |
| Ali 2006 | Not MCE or mixed intervention |
| Allison 2012 | Not a RCT |
| Aluko 2013 | Acute LBP |
| Ammar 2011 | Not MCE or mixed intervention |
| Andrusaitis 2011 | Not MCE or mixed intervention |
| Appling 2009 | Not a RCT |
| Barbosa 2013 | Not a RCT |
| Belcher 1998 | Not non‐specific LBP patients |
| Bentsen 1997 | Not MCE or mixed intervention |
| Bi 2013 | Not MCE or mixed intervention |
| Bilgin 2013 | Not non‐specific LBP patients. |
| Bordiak 2012 | Not non‐specific LBP patients |
| Brennan 2006 | Acute LBP |
| Bronfort 1996 | Not MCE or mixed intervention |
| Bronfort 2011 | Not MCE or mixed intervention |
| Brooks 2012 | Not MCE or mixed intervention |
| Brox 2003 | Not MCE or mixed intervention |
| Buchbinder 2002 | Not a RCT |
| Byuon 2012 | Not MCE or mixed intervention |
| Cairns 2003 | Not MCE or mixed intervention |
| Chan 2011 | Not MCE or mixed intervention |
| Childs 2009 | Not non‐specific LBP patients |
| Childs 2010 | Not non‐specific LBP patients |
| Cho 2014 | Not MCE or mixed intervention |
| Chung 2013 | Not MCE or mixed intervention |
| Croft 1999 | Not a RCT |
| Dehner 2009 | Not a RCT |
| Descarreaux 2002 | Not MCE or mixed intervention |
| Donzelli 2006 | Not MCE or mixed intervention |
| Dufour 2010 | Not MCE or mixed intervention |
| Durante 2010 | Not MCE or mixed intervention |
| Dvorak 2011 | Not MCE or mixed intervention |
| Earde 2014 | Did not evaluate any relevant outcome for this review |
| Ewert 2009 | Not non‐specific LBP patients |
| Faas 1993 | Not MCE or mixed intervention |
| Faas 1995 | Not MCE or mixed intervention |
| Freitas 2008 | Not MCE or mixed intervention |
| Gagnon 2005 | Not MCE or mixed intervention |
| Gatti 2011 | Not MCE or mixed intervention |
| George 2011 | Not non‐specific LBP patients |
| Gustafsson 2008 | Not a RCT |
| Guven 2003 | Not non‐specific LBP patients |
| Hagen 2010 | Not MCE or mixed intervention |
| Hansen 1993 | Not MCE or mixed intervention |
| Harkapaa 1989 | Not MCE or mixed intervention |
| Harringe 2007 | Not a RCT |
| Harts 2008 | Not MCE or mixed intervention |
| Helewa 1999 | Not MCE or mixed intervention |
| Helmhout 2004 | Not MCE or mixed intervention |
| Henchoz 2010 | Not MCE or mixed intervention |
| Hides 1996 | Patients with acute low back pain |
| Hides 2001 | Patients with acute low back pain |
| Hides 2008 | Not a RCT |
| Hunter 2012 | Not MCE or mixed intervention |
| Hurwitz 2005 | Not a RCT |
| Hwang 2013 | Not MCE or mixed intervention |
| ISRCTN80064281 | Not MCE or mixed intervention |
| Jang 2013 | Not MCE or mixed intervention |
| Javadian 2015 | Did not evaluate any relevant outcome for this review |
| Johannsen 1995 | Not MCE or mixed intervention |
| Johnson 2007 | Not MCE or mixed intervention |
| Jones 2007 | Not MCE or mixed intervention |
| Kaapa 2006 | Not MCE or mixed intervention |
| Karimi 2009 | Not MCE or mixed intervention |
| Kladny 2003 | Not non‐specific LBP patients |
| Kline 2013 | Not MCE or mixed intervention |
| Kofotolis 2008 | Not MCE or mixed intervention |
| Koldas 2008 | Not MCE or mixed intervention |
| Kumar 2011 | Not MCE or mixed intervention |
| Kumar 2012 | Not a RCT |
| Kuukkanen 1996 | Not a RCT |
| Lee 2015 | Not non‐specific LBP patients |
| Lewis 2005 | All groups received MCE |
| Lie 1999 | Not MCE or mixed intervention |
| Long 2004 | Not MCE or mixed intervention |
| Magnusson 2008 | Not a RCT |
| Mannion 1999 | Not MCE or mixed intervention |
| Mannion 2009 | Not a RCT |
| Mannion 2012 | Not a RCT |
| Marshall 2008 | Not MCE or mixed intervention |
| Mohseni‐Bandpei 2011 | Not MCE or mixed intervention |
| Monteiro 2009 | Not a RCT |
| Monticone 2004 | Not non‐specific LBP patients |
| Moseley 2002b | Not MCE or mixed intervention |
| Moseley 2003 | All groups received MCE |
| Moussouli 2014 | Not a RCT |
| Navalgund 2009 | Not a RCT |
| NCT00624533 | Not MCE or mixed intervention |
| NCT01061632 | All groups received MCE |
| Nelson 1995 | Not MCE or mixed intervention |
| Nelson‐Wong 2009 | Not a RCT |
| Niemisto 2003 | Not MCE or mixed intervention |
| Niemisto 2004 | Not MCE or mixed intervention |
| Niemisto 2005 | Not MCE or mixed intervention |
| Norris 2008 | Not a RCT |
| O'Sullivan 1997 | Not non‐specific LBP patients |
| Oguzhan 2011 | Not MCE or mixed intervention |
| Ota 2011 | Not a RCT |
| Pereira 2010 | Not a RCT |
| Riipinen 2005 | Not MCE or mixed intervention |
| Rydeard 2006 | Not MCE or mixed intervention |
| Saner 2011 | Not MCE or mixed intervention |
| Saner 2015 | Not MCE or mixed intervention |
| Shakeri 2013 | Not non‐specific LBP patients |
| Shnayderman 2012 | Not non‐specific LBP patients |
| Shnayderman 2013 | Not MCE or mixed intervention |
| Smeets 2009 | Not a RCT |
| Smith 2011 | Not MCE or mixed intervention |
| Sokunbi 2008 | Not MCE or mixed intervention |
| Streicher 2014 | Not a RCT |
| Stuge 2004 | Not non‐specific LBP patients |
| Suni 2006 | Not MCE or mixed intervention |
| Teyhen 2010 | Not non‐specific LBP patients |
| Torstensen 1998 | Not MCE or mixed intervention |
| Trampas 2014 | All groups received MCE |
| Wang 2012b | Not MCE or mixed intervention |
| Willemink 2012 | Not MCE or mixed intervention |
| Williamson 2008 | Not MCE or mixed intervention |
| Xueqiang 2012 | Not MCE or mixed intervention |
| Yang 2010 | Not a RCT |
| Yelland 2004 | Not MCE or mixed intervention |
| Yoo 2012 | Not MCE or mixed intervention |
| You 2014 | Not MCE or mixed intervention |
| Zhang 2015 | Not MCE or mixed intervention |
LBP: low back pain MCE: motor control exercise RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Bayraktar 2013.
| Methods | — |
| Participants | 38 participants included |
| Interventions | Core Stabilization or Water Specific Therapy |
| Outcomes | Pain, static endurance of trunk muscles, functional status and quality of life before and after the treatment |
| Notes | We attempted to contact authors for more information, but they did not reply |
Carmo 2013.
| Methods | — |
| Participants | 10 participants with non‐specific chronic low back pain |
| Interventions | Strengthening exercise or trunk stabilizing exercise |
| Outcomes | Pain, quality of life and disability before and after the treatment |
| Notes | We attempted to contact authors for more information, but they did not reply |
Meira 2013.
| Methods | — |
| Participants | 26 workers complaining of chronic low back pain presenting current pain and limitation of movement during work activities |
| Interventions | Functional reeducation program associated with back school |
| Outcomes | Pain and disability |
| Notes | We attempted to contact authors for more information, but they did not reply |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000343202.
| Trial name or title | Effects of specific physiotherapy treatment and advice versus advice alone on pain and function for people with subacute reducible discogenic low back pain: a randomised controlled trial |
| Methods | RCT |
| Participants | Patients with reducible discogenic low back pain |
| Interventions | Intervention group: 10 sessions of specific physiotherapy management over 10 weeks, involving 30‐minute sessions. Frequency of sessions will be individually tailored based on the individual's progress. Generally, participants will initially attend 2 sessions per week for the first fortnight and then will attend the final 6 sessions spread out over the final 8 weeks. Management will include specific lumbar spine directional preference exercises, taping of the lumbar spine, a gradually progressed core stability exercise programme and condition specific advice and education. Control group: 2 sessions of physiotherapy advice over a 10‐week period (involving one 30‐minute session directly following randomisation and another 30‐minute session 5 weeks following randomisation). The sessions will involve condition‐specific advice regarding the prognosis and self management of the condition. |
| Outcomes | Primary outcomes: back‐specific function (Oswestry Low Back Pain Disability Questionnaire), back pain intensity on a 0 to 10 numerical rating scale, leg pain intensity on a 0 to 10 numerical rating scale |
| Starting date | 1 May 2009 |
| Contact information | Luke Surkitt |
| Notes | — |
ACTRN12611000971932.
| Trial name or title | A comparison of mechanical diagnosis & therapy and motor control exercises on the thickness of the trunk muscle in patients with chronic low back pain |
| Methods | RCT |
| Participants | Patients with chronic low back pain |
| Interventions | Arm 1: mechanical diagnosis and therapy, more commonly known as the McKenzie method. This is a system of exercises and manual therapy that uses repeated end range lumbar spine movements that promote a reduction in pain from its most distal point on the limb towards the centre of the back. The exercises are done in a specific direction (for example, lumbar extension), which has been determined by a trained therapist during the patient assessment. Following the consultation the patient is asked to perform these exercises at home and implement postural correction 5 to 6 times per day for a few minutes each time. The patient is required to attend for regular reviews as clinically warranted. The patient may attend for a maximum of 12 treatments over an 8‐week period. Each session may last up to 30 minutes. Arm 2: motor control exercises. These are exercises that are specifically designed to improve the co‐ordination of the trunk muscles. They are given under the guidance of a physiotherapist who has been trained in their implementation. They are initially done in the lying position and as able are progressed to be done as sitting, standing and functional activities. Patients will receive what is clinically required as decided by the therapist. Patients may receive up to 12 sessions over an 8‐week period. Each session may last up to 30 minutes. Patients will be given a home exercise programme to practise at home for 30 minutes each day. |
| Outcomes | Primary outcomes: thickness of the trunk muscles analysed from images obtained by real time ultrasound scans |
| Starting date | 29 April 2011 |
| Contact information | Mark Halliday |
| Notes | — |
Magalhaes 2013.
| Trial name or title | Efficacy of graded activity versus supervised exercises in patients with chronic non‐specific low back pain: protocol of a randomised controlled trial |
| Methods | RCT |
| Participants | Patients with non‐specific CLBP |
| Interventions | Patients in the supervised exercise group will perform stretching, strengthening and motor control exercises. For the graded activity group, we will follow the protocols described by Macedo et al and Smeets et al, which are based on individualised, progressive and sub‐maximal exercises aiming to improve physical fitness and stimulate changes in behaviour and attitudes due to pain. Positive reinforcement will be provided during the sessions ("you are doing great", "congratulations", "keep up with the good work", "you can make it"), with the aim of maintaining the motivation. In the beginning of the treatment, patients will select 1 or 2 activities considered difficult to them and receive guidance concerning them throughout the treatment, with the establishment of weekly goals. Participants will also receive an educational material (based on "Back Book"), with the purpose of providing important information about how to care for the spine. Weekly reading goals of the educational material will also be defined and the topics will be discussed at the end of each week. |
| Outcomes | Primary outcomes: pain (NRS and McGill Questionnaire) and function disability (RMDQ) |
| Starting date | Not reported |
| Contact information | Mauricio Oliveira Magalhaes |
| Notes | — |
NCT02112760.
| Trial name or title | Specific stabilisation exercise with ultrasound feedback for patient with recurrent low back pain |
| Methods | RCT |
| Participants | Patients with recurrent low back pain for at least the past year |
| Interventions | Not reported |
| Outcomes | Primary outcomes: increased change of sliding of transversus abdominis muscle and change of thickness on multifidus muscle in asymptomatic and low back pain group |
| Starting date | June 2011 |
| Contact information | Shwu‐Fen Wang |
| Notes | — |
NCT02170753.
| Trial name or title | Regional manual therapy and motor control exercise for chronic low back pain |
| Methods | RCT |
| Participants | Patients with low back pain |
| Interventions | The experimental group will receive regional thoracic, pelvic and hip manual therapy and a standard physical therapy approach including motor control exercise and local lumbar spine manual therapy. The control group will receive standard physical therapy including motor control exercise and local lumbar spine manual therapy. |
| Outcomes | Primary outcomes: change in disability level, subjective report of the participant's average level of perceived disability with functional tasks due to LBP as measured by the Modified Oswestry Low Back Pain Disability Questionnaire (ODQ) |
| Starting date | June 2014 |
| Contact information | Jason A Zafereo |
| Notes | — |
NCT02200913.
| Trial name or title | Effects of core stabilization exercise on balance |
| Methods | RCT |
| Participants | Participants with lumbar spinal instability |
| Interventions | Experimental: core stabilisation exercise Control: general trunk strengthening exercise |
| Outcomes | Primary outcome: centre of pressure |
| Starting date | July 2014 |
| Contact information | Wantanee Yodchaisarn |
| Notes | — |
NCT02221609.
| Trial name or title | Movement system impairment based classification versus general exercise for chronic non‐specific low back pain: a randomised controlled trial |
| Methods | RCT |
| Participants | Participants with chronic non‐specific LBP |
| Interventions | Experimental: treatment based on movement system impairment‐based model Active comparator: general exercise |
| Outcomes | Primary outcome: pain intensity |
| Starting date | October 2014 |
| Contact information | Leonardo Costa |
| Notes | — |
NCT02374970.
| Trial name or title | Transversus abdominis muscular training and chronic low back pain |
| Methods | RCT |
| Participants | Participants with chronic low back pain |
| Interventions | Experimental: actual lumbar stability exercises involving co‐contraction of the transversus abdominis and protocolised physiotherapy Active comparator: protocolised physiotherapy treatment: therapeutic exercises and thermotherapy |
| Outcomes | Primary outcome: change in transversus abdominis muscle thickness |
| Starting date | Feb 2015 |
| Contact information | Edurne Villar‐Mateo |
| Notes | — |
NCT02398760.
| Trial name or title | Relationship between clinical tests and clinical outcomes after motor control exercises intervention |
| Methods | RCT |
| Participants | Participants with non‐specific chronic low back pain |
| Interventions | Motor control exercise Control group |
| Outcomes | Primary outcome: pain and disability |
| Starting date | July 2014 |
| Contact information | Ruben FN Filho |
| Notes | — |
Data from the intervention groups were directly transcribed from the original article.
CLBP: chronic low back pain LBP: low back pain NRS: numerical rating scale RCT: randomised controlled trial RMDQ: Roland Morris Disability Questionnaire
Differences between protocol and review
No previous protocol published as an earlier version of this review was published previously (Macedo 2009).
Contributions of authors
Bruno T Saragiotto, Tiê P Yamato, Luciana G Macedo, Leonardo OP Costa, Luciola C Menezes Costa and Chris Maher selected the studies for inclusion. Bruno Tirotti Saragiotto and Tiê Parma Yamato assessed risk of bias, extracted the data and analysed the data. All other authors contributed to writing and editing the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Bruno Saragiotto has no conflicts of interest.
Professor Prof Chris Maher is author of two included studies (Costa 2009; Macedo 2012), and participated in the inclusion process of trials in this review.
Tie Yamato has no conflicts of interest.
Dr Leonardo Costa is author of one study included in this review (Costa 2009), and participated in the inclusion process of trials in this review.
Dr Luciola Menezes Costa has no conflicts of interest.
Prof Raymond Ostelo has no conflicts of interest.
Dr Luciana Macedo is author of one study included in this review (Macedo 2012), and participated in the inclusion process of trials in this review.
Edited (no change to conclusions)
References
References to studies included in this review
Akbari 2008 {published data only}
- Akbari A, Khorashadizadeha S, Abdi G. The effect of motor control exercise versus general exercise on lumbar local stabilizing muscles thickness: randomized controlled trial of patients with chronic low back pain. Journal of Back and Musculoskeletal Rehabilitation 2008;21:105‐12. [Google Scholar]
- NCT00555802. The effect of motor control exercise versus general exercise on lumbar local stabilizing muscles thickness. https://clinicaltrials.gov/ct2/show/NCT00555802 (accessed 25 June 2015).
Alp 2014 {published data only}
- Alp A, Mengi G, Atik T, Mert M, Avsarotlu H. The evaluation of the efficacy of core stabilization exercises on female patients with chronic low back pain [Kronik bel atrili kadin hastalarda core‐stabilizasyon egzersizi etkinlitinin deterlendirilmesi]. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi. 2011; Vol. 56:249.
- Alp A, Mengi G, Avsaroglu AH, Mert M, Sigirli D. Efficacy of core‐stabilization exercise and its comparison with home‐based conventional exercise in low back pain patients [Bel Aðrýlý Hastalarda Core Stabilizasyon Egzersizi Etkinliði ve Konvansiyonel EvEgzersiz Programý ile Karþýlaþtýrýlmasý]. Turkish Journal of Physical Medicine and Rehabilitation 2014;60:S36‐S42. [Google Scholar]
Cairns 2006 {published data only}
- Cairns MC, Foster NE, Wright C. Randomized controlled trial of specific spinal stabilization exercises and conventional physiotherapy for recurrent low back pain. Spine 2006;31:E670‐81. [DOI] [PubMed] [Google Scholar]
Costa 2009 {published data only}
- ACTRN012605000262606. The effect of motor control exercise versus placebo in patients with chronic low back pain. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=012605000262606 (accessed 25 June 2015). [DOI] [PMC free article] [PubMed]
- Costa LOP, Maher CG, Latimer J, Hodges PW, Herbert RD, Refshauge KM, et al. Motor control exercise for chronic low back pain: a randomized placebo‐controlled trial. Physical Therapy 2009;89:1275‐86. [DOI] [PubMed] [Google Scholar]
- Maher CG, Latimer J, Hodges PW, Refshauge KM, Moseley L, Herbert RD, et al. The effect of motor control exercise versus placebo in patients with chronic low back pain. BMC Musculoskeletal Disorders 2005;6:54. [DOI: 10.1186/1471-2474-6-54] [DOI] [PMC free article] [PubMed] [Google Scholar]
Critchley 2007 {published data only}
- Critchley DJ, Ratcliffe J, Noonan S, Jones RH, Hurley MV. Effectiveness and cost‐effectiveness of three types of physiotherapy used to reduce chronic low back pain disability: a pragmatic randomized trial with economic evaluation. Spine 2007;32:1474‐81. [DOI] [PubMed] [Google Scholar]
- ISRCTN56323917. A comparison of the effectiveness of three physiotherapy regimes commonly used to reduce disability in patients with chronic low back pain. http://www.isrctn.com/ISRCTN56323917 (accessed 25 June 2015).
Ferreira 2007 {published data only}
- ACTRN12605000053628. A comparison of the effects of spinal manipulative therapy versus exercise on pain and disability in subjects with chronic LBP. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83 (accessed 25 June 2015).
- Ferreira ML, Ferreira PH, Latimer J, Herbert RD, Hodges PW, Jennings MD, et al. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: a randomized trial. Pain 2007;131:31‐7. [DOI] [PubMed] [Google Scholar]
Franca 2010 {published data only}
- Franca FR, Burke TN, Caffaro RR, Ramos LA, Marques AP. Effects of muscular stretching and segmental stabilization on functional disability and pain in patients with chronic low back pain: a randomized controlled trial. Journal of Manipulative and Physiological Therapeutics 2012;35:279‐85. [DOI] [PubMed] [Google Scholar]
- Franca FR, Burke TN, Hanada ES, Marques AP. Segmental stabilization and muscular strengthening in chronic low back pain ‐ a comparative study. Clinics 2010;65:1013‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01124201. Lumbar stabilization, strengthening and stretching in chronic low back pain. https://clinicaltrials.gov/ct2/show/NCT01124201 (accessed 25 June 2015).
Goldby 2006 {published data only}
- Goldby LJ, Moore AP, Doust J, Trew ME. A randomized controlled trial investigating the efficiency of musculoskeletal physiotherapy on chronic low back disorder. Spine 2006;31:1083‐93. [DOI] [PubMed] [Google Scholar]
Hemmati 2011 {published data only}
- Hemmati S, Rajabi R, Karimi N, Jahandideh AA. Effects of consecutive supervised core stability training on pain and disability in women with nonspecific chronic low back pain [Persian]. Koomesh 2011;12:244‐52. [Google Scholar]
Hosseinifar 2013 {published data only}
- Hosseinifar M, Akbari M, Behtash H, Amiri M, Sarrafzadeh J. The effects of stabilization and McKenzie exercises on transverse abdominis and multifidus muscle thickness, pain, and disability: a randomized controlled trial in nonspecific chronic low back pain. Journal of Physical Therapy Science 2013;25:1541‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inani 2013 {published data only}
- Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non‐specific low back pain: a randomized clinical trial. Journal of Back and Musculoskeletal Rehabilitation 2013;26:37‐43. [DOI] [PubMed] [Google Scholar]
Javadian 2012 {published data only}
- Javadian Y, Behtash H, Akbari M, Taghipour‐Darzi M, Zekavat H. The effects of stabilizing exercises on pain and disability of patients with lumbar segmental instability. Journal of Back and Musculoskeletal Rehabilitation 2012;25:149‐55. [DOI] [PubMed] [Google Scholar]
Kachanathu 2012 {published data only}
- Kachanathu SJ, Zakaria AR, Sahni A, Jaiswal P. Chronic low back pain in fast bowlers a comparative study of core spinal stabilization and conventional exercises. Journal of Physical Therapy Science 2012;24:821‐5. [Google Scholar]
Koumantakis 2005 {published data only}
- Koumantakis GA, Watson PJ, Oldham JA. Trunk muscle stabilization training plus general exercise versus general exercise only: randomized controlled trial of patients with recurrent low back pain. Physical Therapy 2005;85:209‐25. [PubMed] [Google Scholar]
Kumar 2009 {published data only}
- Kumar S, Sharma VP, Negi MPS. Efficacy of dynamic muscular stabilization techniques (DMST) over conventional techniques in rehabilitation of chronic low back pain. Journal of Strength and Conditioning Research 2009;23:2651‐9. [DOI] [PubMed] [Google Scholar]
Kumar 2010 {published data only}
- Kumar S, Sharma VP, Shukla R, Dev R. Comparative efficacy of two multimodal treatments on male and female sub‐groups with low back pain (part II). Journal of Back and Musculoskeletal Rehabilitation 2010;23:1‐9. [DOI] [PubMed] [Google Scholar]
Lomond 2015 {published data only}
- Lomond KV, Jacobs JV, Hitt JR, DeSarno MJ, Bunn JY, Henry SM. Effects of low back pain stabilization or movement system impairment treatments on voluntary postural adjustments: a randomized controlled trial. The Spine Journal 2015;15:596‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01362049. Mechanisms of specific trunk exercises in low back pain. https://clinicaltrials.gov/ct2/show/NCT01362049 (accessed 25 June 2015).
Macedo 2012 {published data only}
- ACTRN12607000432415. A comparison of the effects of motor control exercises and graded activity on pain and disability in subjects with chronic non specific low back pain. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82219 (accessed 25 June 2015).
- Macedo LG, Latimer J, Maher CG, Hodges PW, McAuley JH, Nicholas MK, et al. Effect of motor control exercises versus graded activity in patients with chronic nonspecific low back pain: a randomized controlled trial. Physical Therapy 2012;93:1‐15. [DOI] [PubMed] [Google Scholar]
- Macedo LG, Latimer J, Maher CG, Hodges PW, Nicholas M, Tonkin L, et al. Motor control or graded activity exercises for chronic low back pain? A randomised controlled trial. BMC Musculoskeletal Disorders 2008;5(9):65. [DOI: 10.1186/1471-2474-9-65.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller ER, Schenk RJ, Karnes JL, Rousselle JG. A comparison of the McKenzie approach to a specific spine stabilization program for chronic low back pain. Journal of Manual & Manipulative Therapy 2005;13:103‐12. [Google Scholar]
Moon 2013 {published data only}
- Moon HJ, Choi KH, Kim DH, Kim HJ, Cho YK, Lee KH, et al. Effect of lumbar stabilization and dynamic lumbar strengthening exercises in patients with chronic low back pain. Annals of Rehabilitation Medicine 2013;37:110‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puntumetakul 2013 {published data only}
- Areeudomwong P, Puntumetakul R, Jirarattanaphochai K, Wanpen S, Kanpittaya J, Chatchawan U, et al. Core stabilization exercise improves pain intensity, functional disability and trunk muscle activity of patients with clinical lumbar instability: a pilot randomized controlled study. Journal of Physical Therapy Science 2012;24:1007‐12. [Google Scholar]
- Puntumetakul R, Areeudomwong P, Emasithi A, Yamauchi J. Effect of 10‐week core stabilization exercise training and detraining on pain‐related outcomes in patients with clinical lumbar instability. Patient Preference and Adherence 2013;7:1189‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabin 2014 {published data only}
- Rabin A, Shashua A, Pizem K, Dickstein R, Dar G. A clinical prediction rule to identify patients with low back pain who are likely to experience short‐term success following lumbar stabilization exercises: a randomized controlled validation study. Journal of Orthopaedic & Sports Physical Therapy 2014;44:6‐B13. [DOI] [PubMed] [Google Scholar]
Rasmussen‐Barr 2003 {published data only}
- Rasmussen‐Barr E, Nilsson‐Wikmarn L, Arvidsson I. Stabilizing training compared with manual treatment in sub‐acute and chronic low‐back pain. Manual Therapy 2003;8:233‐41. [DOI] [PubMed] [Google Scholar]
Rasmussen‐Barr 2009 {published data only}
- Rasmussen‐Barr E, Ang B, Arvidsson I, Nilsson‐Wikmar L. Graded exercise for recurrent low‐back pain: a randomized, controlled trial with 6‐, 12‐, and 36‐month follow‐ups. Spine 2009;34:221‐8. [DOI] [PubMed] [Google Scholar]
Rhee 2012 {published data only}
- Rhee HS, Kim YH, Sung PS. A randomized controlled trial to determine the effect of spinal stabilization exercise intervention based on pain level and standing balance differences in patients with low back pain. Medical Science Monitor 2012;18:CR174‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaughnessy 2004 {published data only}
- Shaughnessy M, Caulfield B. A pilot study to investigate the effect of lumbar stabilisation exercise training on functional ability and quality of life in patients with chronic low back pain. International Journal of Rehabilitation Research 2004;27:297‐301. [DOI] [PubMed] [Google Scholar]
Stankovic 2012 {published data only}
- Stankovic A, Lazovic M, Kocic M, Dimitrijevic L, Stankovic I, Zlatanovic D, et al. Lumbar stabilization exercises in addition to strengthening and stretching exercises reduce pain and increase function In patients with chronic low back pain: randomized clinical open‐label study. Turkish Journal of Physical Medicine and Rehabilitation 2012;58:177‐83. [Google Scholar]
Tsauo 2009 {published data only}
- Tsauo JY, Chen WH, Liang HW, Jang Y. The effectiveness of a functional training programme for patients with chronic low back pain – a pilot study. Disability and Rehabilitation 2009;31:1100‐6. [DOI] [PubMed] [Google Scholar]
Unsgaard‐Tondel 2010 {published data only}
- NCT00201513. Anticipatory muscle control and effect of stabilizing exercises in patients with subacute and chronic low back pain. https://clinicaltrials.gov/ct2/show/NCT00201513 (accessed 25 June 2015).
- Unsgaard‐Tondel M, Fladmark AM, Salvesen O, Vasseljen O. Motor control exercises, sling exercises, and general exercises for patients with chronic low back pain: a randomized controlled trial with 1‐year follow‐up. Physical Therapy 2010;90:1426‐40. [DOI] [PubMed] [Google Scholar]
- Vasseljen O, Fladmark AM. Abdominal muscle contraction thickness and function after specific and general exercises: a randomized controlled trial in chronic low back pain patients. Manual Therapy 2010;15:482‐9. [DOI] [PubMed] [Google Scholar]
- Vasseljen O, Unsgaard‐Tondel M, Westad C, Mork PJ. Effect of core stability exercises on feed‐forward activation of deep abdominal muscles in chronic low back pain. Spine 2012;37:1101‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aasa 2015 {published data only}
- Aasa B, Berglund L, Michaelson P, Aasa U. Individualized low‐load motor control exercises and education versus a high‐load lifting exercise and education to improve activity, pain intensity, and physical performance in patients with low back pain: a randomized controlled trial. Journal of Orthopaedic & Sports Physical Therapy 2015;45:77‐85. [DOI] [PubMed] [Google Scholar]
ACTRN12609000293268 {published data only}
- ACTRN12609000293268. Effects of advice versus physiotherapy functional restoration on pain and function for people with multi‐factorial persistent low back pain: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83875 (accessed 25 June 2015).
ACTRN12609000334202 {published data only}
- ACTRN12609000334202. Effects of physiotherapy manual therapy on pain and function for people with sub acute low back pain with or without leg pain: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000334202 (accessed 25 June 2015).
Aggarwal 2010 {published data only}
- Anoop A, Suraj K, Dharmendar K. Effect of core stabilization training on the lower back endurance in recreationally active individuals. Journal of Musculoskeletal Research 2010;13:167‐76. [Google Scholar]
Ahmed 2014 {published data only}
- Ahmed R, Shakil‐ur‐Rehman S, Sibtain F. Comparison between specific lumber mobilization and core‐stability exercises with core‐stability exercises alone in mechanical low back pain. Pakistan Journal of Medical Sciences 2014;30:157‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2006 {published data only}
- Ali TA. Stabilization Exercises for Patients with Low Back Pain [Dissertation]. Texas Woman's University, 2006. [Google Scholar]
Allison 2012 {published data only}
- Allison GT. Abdominal muscle feedforward activation in patients with chronic low back pain is largely unaffected by 8 weeks of core stability training. Journal of Physiotherapy 2012;58:200. [DOI] [PubMed] [Google Scholar]
Aluko 2013 {published data only}
- Aluko A, DeSouza L, Peacock J. The effect of core stability exercises on variations in acceleration of trunk movement, pain, and disability during an episode of acute nonspecific low back pain: a pilot clinical trial. Journal of Manipulative and Physiological Therapeutics 2013;36:497‐504. [DOI] [PubMed] [Google Scholar]
Ammar 2011 {published data only}
- Ammar TA, Mitchell K, Saleh A. Stabilization exercises in postnatal low back pain. Indian Journal of Physiotherapy and Occupational Therapy 2011;5:122‐4. [Google Scholar]
Andrusaitis 2011 {published data only}
- Andrusaitis SF, Brech GC, Vitale GF, Greve JM. Trunk stabilization among women with chronic lower back pain: a randomized, controlled, and blinded pilot study. Clinics 2011;66:1645‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Appling 2009 {published data only}
- Appling SA, Chalona A, Edwards P, Green T, Jones C, LaVigne W. Effect of a progressive core stabilization exercise program on lumbar stability and abdominal strength. Journal of Orthopaedic & Sports Physical Therapy 2009;39:A83‐4. [Google Scholar]
Barbosa 2013 {published data only}
- Barbosa AC, Martins FL, Barbosa MC, Dos Santos RT. Manipulation and selective exercises decrease pelvic anteversion and low‐back pain: a pilot study. Journal of Back & Musculoskeletal Rehabilitation 2013;26:33‐6. [DOI] [PubMed] [Google Scholar]
Belcher 1998 {published data only}
- Belcher MA. Pelvic Stabilization Exercise versus Conventional Weight Training Exercise during Resistance Training: Its Effect on the Development of Lumbar Extension Strength [Dissertation]. University of Southern Mississippi, 1998. [Google Scholar]
Bentsen 1997 {published data only}
- Bentsen H, Lindgarde F, Manthorpe R. The effect of dynamic strength back exercise and/or a home training program in 57‐year‐old women with chronic low back pain. Results of a prospective randomized study with a 3‐year follow‐up period. Spine 1997;22:1494‐500. [DOI] [PubMed] [Google Scholar]
Bi 2013 {published data only}
- Bi X, Zhao J, Zhao L, Liu Z, Zhang J, Sun D, et al. Pelvic floor muscle exercise for chronic low back pain. Journal of International Medical Research 2013;41:146‐52. [DOI] [PubMed] [Google Scholar]
Bilgin 2013 {published data only}
- Bilgin S, Temucin CM, Nurlu G, Kaya DO, Kose N, Gunduz AG. Effects of exercise and electrical stimulation on lumbar stabilization in asymptomatic subjects: a comparative study. Journal of Back and Musculoskeletal Rehabilitation 2013;26:261‐6. [DOI] [PubMed] [Google Scholar]
Bordiak 2012 {published data only}
- Bordiak FC, Silva EB. Electrical stimulation and core training on pain and range of motion in low back pain [Eletroestimulação e core training sobre dor e arco de movimento na lombalgia]. Fisioterapia em Movimento 2012;25:759‐66. [Google Scholar]
Brennan 2006 {published data only}
- Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute "nonspecific" low back pain: results of a randomized clinical trial. Spine 2006;31:623‐31. [DOI] [PubMed] [Google Scholar]
Bronfort 1996 {published data only}
- Bronfort G, Goldsmith CH, Nelson CF, Boline PD, Anderson AV. Trunk exercise combined with spinal manipulative or NSAID therapy for chronic low back pain: a randomized, observer‐blinded clinical trial. Journal of Manipulative and Physiological Therapeutics 1996;19:570‐81. [PubMed] [Google Scholar]
Bronfort 2011 {published data only}
- Bronfort G, Maiers MJ, Evans RL, Schulz CA, Bracha Y, Svendsen KH, et al. Supervised exercise, spinal manipulation, and home exercise for chronic low back pain: a randomized clinical trial. The Spine Journal 2011;11:585‐98. [DOI] [PubMed] [Google Scholar]
Brooks 2012 {published data only}
- Brooks C, Kennedy S, Marshall PWM. Specific trunk and general exercise elicit similar changes in anticipatory postural adjustments in patients with chronic low back pain: a randomized controlled trial. Spine 2012;37:E1543‐50. [DOI] [PubMed] [Google Scholar]
Brox 2003 {published data only}
- Brox JI, Sørensen R, Friis A, Nygaard Ø, Indahl A, Keller A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine 2003;28:1913‐21. [DOI] [PubMed] [Google Scholar]
Buchbinder 2002 {published data only}
- Buchbinder R, Hoving J. Specific spinal exercise substantially reduces the risk of low back pain recurrence. Australian Journal of Physiotherapy 2002;48:55. [DOI] [PubMed] [Google Scholar]
Byuon 2012 {published data only}
- Byuon S, Son H. The effects of proprioceptive neuromuscular facilitation and stabilizing exercise on trunk repositioning errors. Journal of Physical Therapy Science 2012;24:1017‐20. [Google Scholar]
Cairns 2003 {published data only}
- Cairns M. Manipulation Association of Chartered Physiotherapists (UK) Research Presentation Award: a pragmatic randomized controlled trial of stabilization exercises in the management of recurrent low back pain. Manual Therapy 2003;8:185. [Google Scholar]
Chan 2011 {published data only}
- Chan CW, Mok NW, Yeung EW. Aerobic exercise training in addition to conventional physiotherapy for chronic low back pain: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2011;92:1681‐5. [DOI] [PubMed] [Google Scholar]
Childs 2009 {published data only}
- Childs JD, Teyhen DS, Benedict TM, Morris JB, Fortenberry AD, McQueen RM, et al. Effects of sit‐up training versus core stabilization exercises on sit‐up performance. Medicine & Science in Sports & Exercise 2009;41:2072‐83. [DOI] [PubMed] [Google Scholar]
Childs 2010 {published data only}
- Childs JD, Teyhen DS, Casey PR, McCoy‐Singh KA, Feldtmann AW, Wright AC, et al. Effects of traditional sit‐up training versus core stabilization exercises on short‐term musculoskeletal injuries in us army soldiers: a cluster randomized trial. Physical Therapy 2010;90:1404‐12. [DOI] [PubMed] [Google Scholar]
Cho 2014 {published data only}
- Cho HY, Kim EH, Kim J. Effects of the CORE exercise program on pain and active range of motion in patients with chronic low back pain. Journal of Physical Therapy Science 2014;26:1237‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2013 {published data only}
- Chung S, Lee J, Yoon J. Effects of stabilization exercise using a ball on multifidus cross‐sectional area in patients with chronic low back pain. Journal of Sports Science & Medicine 2013;12:533‐41. [PMC free article] [PubMed] [Google Scholar]
Croft 1999 {published data only}
- Croft PR, Papageorgiou AC, Thomas E, Macfarlane GJ, Silman AJ. Short‐term physical risk factors for new episodes of low back pain. Prospective evidence from the South Manchester Back Pain Study. Spine 1999;24:1556‐61. [DOI] [PubMed] [Google Scholar]
Dehner 2009 {published data only}
- Dehner C, Schmelz A, Völker H, Krischak G, Kramer M. Low back pain intensity, microcirculation and muscle performance of the multifidus following back muscle strengthening in young elite oarsmen. International Sports Medicine Journal 2009;10:163‐75. [Google Scholar]
Descarreaux 2002 {published data only}
- Descarreaux M, Normand MC, Laurencelle L, Dugas C. Evaluation of a specific home exercise program for low back pain. Journal of Manipulative & Physiological Therapeutics 2002;25:497‐503. [DOI] [PubMed] [Google Scholar]
Donzelli 2006 {published data only}
- Donzelli S, Domenica E, Cova AM, Galletti R, Giunta N. Two different techniques in the rehabilitation treatment of low back pain: a randomized controlled trial. Europa Medicophysica 2006;42:205‐10. [PubMed] [Google Scholar]
Dufour 2010 {published data only}
- Dufour N, Thamsborg G, Oefeldt A, Lundsgaard C, Stender S. Treatment of chronic low back pain: a randomized, clinical trial comparing group‐based multidisciplinary biopsychosocial rehabilitation and intensive individual therapist‐assisted back muscle strengthening exercises. Spine 2010;35:469‐76. [DOI] [PubMed] [Google Scholar]
Durante 2010 {published data only}
- Durante H, Vasconcelos ECLM. Comparison between isostretching method and conventional kinesiotherapy in low back pain treatment [Comparação do método Isostretching e cinesioterapia convencional no tratamento da lombalgia]. Semina: Ciências Biológicas e da Saúde 2010;30:83‐90. [Google Scholar]
Dvorak 2011 {published data only}
- Dvorak H, Kujat C, Brumitt J. Effect of therapeutic exercise versus manual therapy on athletes with chronic low back pain. Journal of Sport Rehabilitation 2011;20:494‐504. [DOI] [PubMed] [Google Scholar]
Earde 2014 {published data only}
- Earde P, Vongsirinavarat M, Sakulsriprasert P, Vachalathiti R. Immediate effects of trunk stabilizer muscles training on muscle response time in individuals with non‐specific chronic low back pain. Journal of the Medical Association of Thailand 2014;97:S89‐S94. [PubMed] [Google Scholar]
Ewert 2009 {published data only}
- Ewert T, Limm H, Wessels T, Rackwitz B, Garnier K, Freumuth R, et al. The comparative effectiveness of a multimodal program versus exercise alone for the secondary prevention of chronic low back pain and disability. Physical Medicine and Rehabilitation (Pm&R) 2009;1:798‐808. [DOI] [PubMed] [Google Scholar]
Faas 1993 {published data only}
- Faas A, Chavannes AW, Eijk JT, Gubbels JW. A randomized, placebo‐controlled trial of exercise therapy in patients with acute low back pain. Spine 1993;18:1388‐95. [PubMed] [Google Scholar]
Faas 1995 {published data only}
- Faas A, Eijk JT, Chavannes AW, Gubbels JW. A randomized trial of exercise therapy in patients with acute low back pain. Efficacy on sickness absence. Spine 1995;20:941‐7. [DOI] [PubMed] [Google Scholar]
Freitas 2008 {published data only}
- Freitas CD, D'Andrea JM. Comparison between isokinetic dynamometer and therapeutic ball exercise in chronic low back pain of mechanical origin. Fisioterapia & Pesquisa 2008;15:380‐6. [Google Scholar]
Gagnon 2005 {published data only}
- Gagnon LH. Efficacy of Pilates Exercises as Therapeutic Intervention in Treating Patients with Low Back Pain [Dissertation]. University of Tennessee, 2005. [Google Scholar]
Gatti 2011 {published data only}
- Gatti R, Faccendini S, Tettamanti A, Barbero M, Balestri A, Calori G. Efficacy of trunk balance exercises for individuals with chronic low back pain: a randomized clinical trial. Journal of Orthopaedic & Sports Physical Therapy 2011;41:542‐52. [DOI] [PubMed] [Google Scholar]
George 2011 {published data only}
- George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, et al. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: results from the Prevention of Low Back Pain in the Military (POLM) cluster randomized trial. BMC Medicine 2011;9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustafsson 2008 {published data only}
- Gustafsson J, Nilsson‐Wikmar L. Influence of specific muscle training on pain, activity limitation and kinesiophobia in women with back pain post‐partum‐‐a 'single‐subject research design'. Physiotherapy Research International 2008;13:18‐30. [DOI] [PubMed] [Google Scholar]
Guven 2003 {published data only}
- Guven Z, Marangozoglu I, Gunduz OH. Effectiveness of lumbopelvic stabilization exercise education in patients with chronic mechanical low back pain. [Turkish] [Kronik mekanik bel agrili hastalarda lumbopelvik stabilizasyon egzersiz egitiminin etkinligi]. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2003;49:17. [Google Scholar]
Hagen 2010 {published data only}
- Hagen EM, Odelien KH, Lie SA, Eriksen HR. Adding a physical exercise programme to brief intervention for low back pain patients did not increase return to work. Scandinavian Journal of Public Health 2010;38:731‐8. [DOI] [PubMed] [Google Scholar]
Hansen 1993 {published data only}
- Hansen FR, Bendix T, Skov P, Jensen CV, Kristensen JH, Krohn L, et al. Intensive, dynamic back‐muscle exercises, conventional physiotherapy, or placebo‐control treatment of low‐back pain. A randomized, observer‐blind trial. Spine 1993;18:98‐108. [DOI] [PubMed] [Google Scholar]
Harkapaa 1989 {published data only}
- Harkapaa K, Jarvikoski A, Mellin G, Hurri H. A controlled study on the outcome of inpatient and outpatient treatment of low back pain. Part I. pain, disability, compliance, and reported treatment benefits three months after treatment. Scandinavian Journal of Rehabilitation Medicine 1989;21:81‐9. [PubMed] [Google Scholar]
Harringe 2007 {published data only}
- Harringe ML, Nordgren JS, Arvidsson I, Werner S. Low back pain in young female gymnasts and the effect of specific segmental muscle control exercises of the lumbar spine: a prospective controlled intervention study. Knee Surgery, Sports Traumatology, Arthroscopy 2007;15:1264‐71. [DOI] [PubMed] [Google Scholar]
Harts 2008 {published data only}
- Harts CC, Helmhout PH, Bie RA, StaaI JB. A high‐intensity lumbar extensor strengthening program is little better than a low‐intensity program or a waiting list control group for chronic low back pain: a randomised clinical trial. Australian Journal of Physiotherapy 2008;54:23‐31. [DOI] [PubMed] [Google Scholar]
Helewa 1999 {published data only}
- Helewa A, Goldsmith CH, Lee P, Smythe HA, Forwell L. Does strengthening the abdominal muscles prevent low back pain ‐‐ a randomized controlled trial. Journal of Rheumatology 1999;26:1808‐15. [PubMed] [Google Scholar]
Helmhout 2004 {published data only}
- Helmhout PH, Harts CC, Staal JB, Candel MJ, Bie RA. Comparison of a high‐intensity and a low‐intensity lumbar extensor training program as minimal intervention treatment in low back pain: a randomized trial. European Spine Journal 2004;13:537‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henchoz 2010 {published data only}
- Henchoz Y, Pinget C, Wasserfallen JB, Paillex R, Goumoens P, Norberg M, et al. Cost‐utility analysis of a three‐month exercise programme versus usual care following multidisciplinary rehabilitation for chronic low back pain. Journal of Rehabilitation Medicine 2010;42:846‐52. [DOI] [PubMed] [Google Scholar]
Hides 1996 {published data only}
- Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first‐episode low back pain. Spine 1996;21:2763‐9. [DOI] [PubMed] [Google Scholar]
Hides 2001 {published data only}
- Hides JA, Jull GA, Richardson CA. Long‐term effects of specific stabilizing exercises for first‐episode low back pain. Spine 2001;26:E243‐8. [DOI] [PubMed] [Google Scholar]
Hides 2008 {published data only}
- Hides JA, Stanton WR, McMahon S, Sims K, Richardson CA. Effect of stabilization training on multifidus muscle cross‐sectional area among young elite cricketers with low back pain. Journal of Orthopaedic & Sports Physical Therapy 2008;38:101‐8. [DOI] [PubMed] [Google Scholar]
Hunter 2012 {published data only}
- Hunter RF, McDonough SM, Bradbury I, Liddle SD, Walsh DM, Dhamija S, et al. Exercise and auricular acupuncture for chronic low‐back pain: a feasibility randomized‐controlled trial. Clinical Journal of Pain 2012;28:259‐67. [DOI] [PubMed] [Google Scholar]
Hurwitz 2005 {published data only}
- Hurwitz EL, Morgenstern H, Chiao C. Effects of recreational physical activity and back exercises on low back pain and psychological distress: findings from the UCLA Low Back Pain Study. American Journal of Public Health 2005;95:1817‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2013 {published data only}
- Hwang JA, Bae SH, Do Kim G, Kim KY. The effects of sensorimotor training on anticipatory postural adjustment of the trunk in chronic low back pain patients. Journal of Physical Therapy Science 2013;25:1189‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN80064281 {published data only}
- ISRCTN80064281. Movement control exercise for low back pain. http://www.isrctn.com/ISRCTN80064281 (accessed 26 June 2015). [DOI: 10.1186/ISRCTN80064281] [DOI]
Jang 2013 {published data only}
- Jang S, Lee J, Bang H. The effect of trunk control exercises performed on unstable surfaces on the spinal stability of low back pain patients. Journal of Physical Therapy Science 2013;25:459‐62. [Google Scholar]
Javadian 2015 {published data only}
- Javadian Y, Akbari M, Talebi G, Taghipour‐Darzi M, Janmohammadi N. Influence of core stability exercise on lumbar vertebral instability in patients presented with chronic low back pain: a randomized clinical trial. Caspian Journal of Internal Medicine 2015;6(2):98‐102. [PMC free article] [PubMed] [Google Scholar]
Johannsen 1995 {published data only}
- Johannsen F, Remvig L, Kryger P, Beck P, Warming S, Lybeck K, et al. Exercises for chronic low back pain: a clinical trial. Journal of Orthopaedic and Sports Physical Therapy 1995;22:52‐9. [DOI] [PubMed] [Google Scholar]
Johnson 2007 {published data only}
- Johnson RE, Jones GT, Wiles NJ, Chaddock C, Potter RG, Roberts C, et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine 2007;32:1578‐85. [DOI] [PubMed] [Google Scholar]
Jones 2007 {published data only}
- Jones M, Stratton G, Reilly T, Unnithan V. The efficacy of exercise as an intervention to treat recurrent nonspecific low back pain in adolescents. Pediatric Exercise Science 2007;19:349‐59. [DOI] [PubMed] [Google Scholar]
Kaapa 2006 {published data only}
- Kaapa EH, Frantsi K, Sarna S, Malmivaara A. Multidisciplinary group rehabilitation versus individual physiotherapy for chronic nonspecific low back pain: a randomized trial. Spine 2006;31:371‐6. [DOI] [PubMed] [Google Scholar]
Karimi 2009 {published data only}
- Karimi N, Ebrahimi I, Ezzati K, Kahrizi S, Torkaman G, Arab AM. The effects of consecutive supervised stability training on postural balance in patients with chronic low back pain. Pakistan Journal of Medical Sciences 2009;25:181. [Google Scholar]
Kladny 2003 {published data only}
- Kladny B, Fischer FC, Haase I. Evaluation of specific stabilizing exercise in the treatment of low back pain and lumbar disk disease in outpatient rehabilitation. [German]. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 2003;141:401‐5. [DOI] [PubMed] [Google Scholar]
Kline 2013 {published data only}
- Kline JB, Krauss JR, Maher SF, Qu X. Core strength training using a combination of home exercises and a dynamic sling system for the management of low back pain in pre‐professional ballet dancers: a case series. Journal of Dance Medicine & Science 2013;17:24‐33. [DOI] [PubMed] [Google Scholar]
Kofotolis 2008 {published data only}
- Kofotolis ND, Vlachopoulos SP, Kellis E. Sequentially allocated clinical trial of rhythmic stabilization exercises and TENS in women with chronic low back pain. Clinical Rehabilitation 2008;22:99‐111. [DOI] [PubMed] [Google Scholar]
Koldas 2008 {published data only}
- Koldas Dogan S, Sonel Tur B, Kurtais Y, Atay MB. Comparison of three different approaches in the treatment of chronic low back pain. Clinical Rheumatology 2008;27:873‐81. [DOI] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar SP. Efficacy of segmental stabilization exercise for lumbar segmental instability in patients with mechanical low back pain: a randomized placebo controlled crossover study. North American Journal of Medical Sciences 2011;3:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2012 {published data only}
- Kumar S, Sharma VP, Aggarwal A, Shukla R, Dev R. Effect of dynamic muscular stabilization technique on low back pain of different durations. Journal of Back & Musculoskeletal Rehabilitation 2012;25:73‐9. [DOI] [PubMed] [Google Scholar]
Kuukkanen 1996 {published data only}
- Kuukkanen T, Malkia E. Muscular performance after a 3 month progressive physical exercise program and 9 month follow‐up in subjects with low back pain. A controlled study. Scandinavian Journal of Medicine & Science in Sports 1996;6:112‐21. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee JS, Kim TH, Kim DY, Shim JH, Lim JY. Effects of selective exercise for the deep abdominal muscles and lumbar stabilization exercise on the thickness of the transversus abdominis and postural maintenance. Journal of Physical Therapy Science 2015;27:367‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2005 {published data only}
- Lewis JS, Hewitt JS, Billington L, Cole S, Byng J, Karayiannis S. A randomized clinical trial comparing two physiotherapy interventions for chronic low back pain. Spine 2005;30:711‐21. [DOI] [PubMed] [Google Scholar]
Lie 1999 {published data only}
- Lie H, Frey S. Mobilizing or stabilizing exercise in degenerative disk disease in the lumbar region? [Norwegian]. Tidsskrift for Den Norske Laegeforening 1999;119:2051‐3. [PubMed] [Google Scholar]
Long 2004 {published data only}
- Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine 2004;29:2593‐602. [DOI] [PubMed] [Google Scholar]
Magnusson 2008 {published data only}
- Magnusson ML, Chow DH, Diamandopoulos Z, Pope MH. Motor control learning in chronic low back pain. Spine 2008;33:E532‐8. [DOI] [PubMed] [Google Scholar]
Mannion 1999 {published data only}
- Mannion AF, Muntener M, Taimela S, Dvorak J. A randomized clinical trial of three active therapies for chronic low back pain. Spine 1999;24:2435‐48. [DOI] [PubMed] [Google Scholar]
Mannion 2009 {published data only}
- Mannion AF, Helbling D, Pulkovski N, Sprott H. Spinal segmental stabilisation exercises for chronic low back pain: programme adherence and its influence on clinical outcome. European Spine Journal 2009;18:1881‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannion 2012 {published data only}
- Mannion AF, Caporaso F, Pulkovski N, Sprott H. Spine stabilisation exercises in the treatment of chronic low back pain: a good clinical outcome is not associated with improved abdominal muscle function. European Spine Journal 2012;21:1301‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2008 {published data only}
- Marshall PW, Murphy BA. Muscle activation changes after exercise rehabilitation for chronic low back pain. Archives of Physical Medicine & Rehabilitation 2008;89:1305‐13. [DOI] [PubMed] [Google Scholar]
Mohseni‐Bandpei 2011 {published data only}
- Mohseni‐Bandpei MA, Rahmani N, Behtash H, Karimloo M. The effect of pelvic floor muscle exercise on women with chronic non‐specific low back pain. Journal of Bodywork & Movement Therapies 2011;15:75‐81. [DOI] [PubMed] [Google Scholar]
Monteiro 2009 {published data only}
- Monteiro FC, Kirkwood RN, Magalhaes CMB. Lumbar stabilization exercises and manual therapy for treatment of non‐specific and chronic low back pain [Exercícios de estabilizaçao lombar e terapia manual no tratamento da dor lombar crônica inespecífica]. Fisioterapia Brasil 2009;10:442‐7. [Google Scholar]
Monticone 2004 {published data only}
- Monticone M, Barbarino A, Testi C, Arzano S, Moschi A, Negrini S. Symptomatic efficacy of stabilizing treatment versus laser therapy for sub‐acute low back pain with positive tests for sacroiliac dysfunction: a randomised clinical controlled trial with 1 year follow‐up. Europa Medicophysica 2004;40:263‐8. [PubMed] [Google Scholar]
Moseley 2002b {published data only}
- Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Australian Journal of Physiotherapy 2002;48:297‐302. [DOI] [PubMed] [Google Scholar]
Moseley 2003 {published data only}
- Moseley GL. Joining forces ‐‐ combining cognition ‐‐ targeted motor control training with group or individual pain physiology education: a successful treatment for chronic low back pain. Journal of Manual & Manipulative Therapy 2003;11:88‐94. [Google Scholar]
Moussouli 2014 {published data only}
- Moussouli M, Vlachopoulos SP, Kofotolis ND, Theodorakis Y, Malliou P, Kellis E. Effects of stabilization exercises on health‐related quality of life in women with chronic low back pain. Journal of Physical Activity & Health 2014;11:1295‐303. [DOI] [PubMed] [Google Scholar]
Navalgund 2009 {published data only}
- Anand N. Evaluating the effect of a 10‐week stabilization exercise program on the postural stability and the neuromuscular control of the spine in subjects with subacute recurrent low back pain [Dissertation]. Ohio State University, 2009. [Google Scholar]
NCT00624533 {published data only}
- NCT00624533. Efficiency of GDS method for lumbar stabilization for non‐specific low back pain in primary care. https://clinicaltrials.gov/ct2/show/NCT00624533 (accessed 25 June 2015).
NCT01061632 {published data only}
- NCT01061632. High (deadlift) versus low intensity motor control exercises on low back pain. https://clinicaltrials.gov/ct2/show/NCT01061632 (accessed 25 June 2015).
Nelson 1995 {published data only}
- Nelson BW, O'Reilly E, Miller M, Hogan M, Wegner JA, Kelly C. The clinical effects of intensive, specific exercise on chronic low back pain: a controlled study of 895 consecutive patients with 1‐year follow up. Orthopedics 1995;18:971‐81. [DOI] [PubMed] [Google Scholar]
Nelson‐Wong 2009 {published data only}
- Nelson‐Wong E. Biomechanical Predictors of Functionally Induced Low Back Pain, Acute Response to Prolonged Standing Exposure, and Impact of a Stabilization‐Based Clinical Exercise Intervention [Dissertation]. University of Waterloo (Canada), 2009. [Google Scholar]
Niemisto 2003 {published data only}
- Niemisto L, Lahtinen‐Suopanki T, Rissanen P, Lindgren KA, Sarna S, Hurri H. A randomized trial of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain. Spine 2003;28:2185‐91. [DOI] [PubMed] [Google Scholar]
Niemisto 2004 {published data only}
- Niemisto L, Sarna S, Lahtinen‐Suopanki T, Lindgren KA, Hurri H. Predictive factors for 1‐year outcome of chronic low back pain following manipulation, stabilizing exercises, and physician consultation or physician consultation alone. Journal of Rehabilitation Medicine 2004;36:104‐9. [DOI] [PubMed] [Google Scholar]
Niemisto 2005 {published data only}
- Niemisto L, Rissanen P, Sarna S, Lahtinen‐Suopanki T, Lindgren KA, Hurri H. Cost‐effectiveness of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain: a prospective randomized trial with 2‐year follow‐up. Spine 2005;30:1109‐15. [DOI] [PubMed] [Google Scholar]
Norris 2008 {published data only}
- Norris C, Matthews M. The role of an integrated back stability program in patients with chronic low back pain. Complementary Therapies in Clinical Practice 2008;14:255‐63. [DOI] [PubMed] [Google Scholar]
O'Sullivan 1997 {published data only}
- O'Sullivan PB, Phyty GD, Twomey LT, Allison GT. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine 1997;22:2959‐67. [DOI] [PubMed] [Google Scholar]
Oguzhan 2011 {published data only}
- Oguzhan H, Ozyurek S, Kaya E. Effectiveness of back school program to quality of life and disability in patients with chronic low back pain. European Journal of Pain Supplements 2011, issue Conference abstract.
Ota 2011 {published data only}
- Megumi O, Koji K, Mika H, Keisuke K, Toshiki M. Effectiveness of lumbar stabilization exercises for reducing chronic low back pain and improving quality‐of‐life. Journal of Physical Therapy Science 2011;23:679‐81. [Google Scholar]
Pereira 2010 {published data only}
- Pereira NT, Ferreira LAB, Pereira WM. Effectiveness of segmental stabilization exercises on mechanical‐postural chronic low back pain. Fisioterapia em Movimento 2010;23:605‐14. [Google Scholar]
Riipinen 2005 {published data only}
- Riipinen M, Niemisto L, Lindgren KA, Hurri H. Psychosocial differences as predictors for recovery from chronic low back pain following manipulation, stabilizing exercises and physician consultation or physician consultation alone. Journal of Rehabilitation Medicine 2005;37:152‐8. [DOI] [PubMed] [Google Scholar]
Rydeard 2006 {published data only}
- Rydeard R, Leger A, Smith D. Pilates‐based therapeutic exercise: effect on subjects with nonspecific chronic low back pain and functional disability: a randomized controlled trial. Journal of Orthopaedic & Sports Physical Therapy 2006;36:472‐84. [DOI] [PubMed] [Google Scholar]
Saner 2011 {published data only}
- Saner J, Kool J, Bie RA, Sieben JM, Luomajoki H. Movement control exercise versus general exercise to reduce disability in patients with low back pain and movement control impairment. A randomised controlled trial. BMC Musculoskeletal Disorders 2011;12:207. [DOI: 10.1186/1471-2474-12-207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saner 2015 {published data only}
- Saner J, Kool J, Sieben JM, Luomajoki H, Bastiaenen CH, Bie RA. A tailored exercise program versus general exercise for a subgroup of patients with low back pain and movement control impairment: A randomised controlled trial with one‐year follow‐up. Manual Therapy 2015 [Epub ahead of print]. [DOI] [PubMed]
Shakeri 2013 {published data only}
- Shakeri H, Fathollahi Z, Karimi N, Arab A M. Effect of functional lumbar stabilization exercises on pain, disability, and kinesiophobia in women with menstrual low back pain: a preliminary trial. Journal of Chiropractic Medicine 2013;12:160‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shnayderman 2012 {published data only}
- Shnayderman I, Katz‐Leurer M. An aerobic walking programme versus muscle strengthening programme for chronic low back pain: a randomized controlled trial. Clinical Rehabilitation 2012;27:207‐14. [DOI] [PubMed] [Google Scholar]
Shnayderman 2013 {published data only}
- Shnayderman I, Katz‐Leurer M. An aerobic walking programme versus muscle strengthening programme for chronic low back pain: a randomized controlled trial. Clinical Rehabilitation 2013;27:207‐14. [DOI] [PubMed] [Google Scholar]
Smeets 2009 {published data only}
- Smeets RJEM. Do lumbar stabilising exercises reduce pain and disability in patients with recurrent low back pain?. Australian Journal of Physiotherapy 2009;55:138. [DOI] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith D, Bissell G, Bruce‐Low S, Wakefield C. The effect of lumbar extension training with and without pelvic stabilization on lumbar strength and low back pain. Journal of Back and Musculoskeletal Rehabilitation 2011;24:241‐9. [DOI] [PubMed] [Google Scholar]
Sokunbi 2008 {published data only}
- Sokunbi O, Watt P, Moore A. A randomised controlled trial (RCT) on the effects of frequency of application of spinal stabilisation exercises on multifidus cross sectional area (MFCSA) in participants with chronic low back pain. Physiotherapy Singapore 2008;11:9‐16. [Google Scholar]
Streicher 2014 {published data only}
- Streicher H, Matzold F, Hamilton C, Wagner P. Comparison of group motor control training versus individual training for people suffering from back pain. Journal of Bodywork and Movement Therapies 2014;18(3):489‐96. [DOI] [PubMed] [Google Scholar]
Stuge 2004 {published data only}
- Stuge B, Laerum E, Kirkesola G, Vollestad N. The efficacy of a treatment program focusing on specific stabilizing exercises for pelvic girdle pain after pregnancy: a randomized controlled trial. Spine 2004;29:359. [DOI] [PubMed] [Google Scholar]
Suni 2006 {published data only}
- Suni J, Rinne M, Natri A, Statistisian MP, Parkkari J, Alaranta H. Control of the lumbar neutral zone decreases low back pain and improves self‐evaluated work ability: a 12‐month randomized controlled study. Spine 2006;31:E611‐20. [DOI] [PubMed] [Google Scholar]
Teyhen 2010 {published data only}
- Teyhen DS, Usalis J, Szymanek EB, Paschall JC, Meagher MS, Harvey AD, et al. Rehabilitative ultrasound imaging assessment of the lumbar multifidus during stabilization exercises in healthy adults. Journal of Orthopaedic & Sports Physical Therapy 2010;40:A39. [Google Scholar]
Torstensen 1998 {published data only}
- Torstensen TA, Ljunggren AE, Meen HD, Odland E, Mowinckel P, Geijerstam S. Efficiency and costs of medical exercise therapy, conventional physiotherapy, and self‐exercise in patients with chronic low back pain. A pragmatic, randomized, single‐blinded, controlled trial with 1‐year follow‐up. Spine 1998;23:2616‐24. [DOI] [PubMed] [Google Scholar]
Trampas 2014 {published data only}
- Trampas A, Mpeneka A, Malliou V, Godolias G, Vlachakis P. Immediate effects of core stability exercises and clinical massage on dynamic balance performance of patients with chronic specific low back pain. Journal of Sport Rehabilitation 2014 [Epub ahead of print]. [PubMed]
Wang 2012b {published data only}
- Wang X, Zheng J, Bi X, Liu J. Effect of core stability training on patients with chronic low back pain. Health MED 2012;6:754‐9. [Google Scholar]
Willemink 2012 {published data only}
- Willemink MJ, Es HW, Helmhout PH, Diederik AL, Kelder JC, Heesewijk JP. The effects of dynamic isolated lumbar extensor training on lumbar multifidus functional cross‐sectional area and functional status of patients with chronic nonspecific low back pain. Spine 2012;37:E1651‐8. [DOI] [PubMed] [Google Scholar]
Williamson 2008 {published data only}
- Williamson W. Effect of Supervised and Directed Exercise on Low Back Pain and Functional Activity [Dissertation]. Oklahoma State University, 2008. [Google Scholar]
Xueqiang 2012 {published data only}
- Xueqiang W, Jiejiao Z, Xia B, Jing L. Effect of core stability training on patients with chronic low back pain. Health MED 2012;6:759. [Google Scholar]
Yang 2010 {published data only}
- Yang EJ, Park WB, Shin HI, Lim JY. The effect of back school integrated with core strengthening in patients with chronic low‐back pain. American Journal of Physical Medicine & Rehabilitation 2010;89:744‐54. [DOI] [PubMed] [Google Scholar]
Yelland 2004 {published data only}
- Yelland MJ, Glasziou PP, Bogduk N, Schluter PJ, McKernon M. Prolotherapy injections, saline injections, and exercises for chronic low‐back pain: a randomized trial. Spine 2004;29:9‐16. [DOI] [PubMed] [Google Scholar]
Yoo 2012 {published data only}
- Yoo YD, Lee YS. The effect of core stabilization exercises using a sling on pain and muscle strength of patients with chronic low back pain. Journal of Physical Therapy Science 2012;24:671‐4. [Google Scholar]
You 2014 {published data only}
- You JH, Kim SY, Oh DW, Chon SC. The effect of a novel core stabilization technique on managing patients with chronic low back pain: a randomized, controlled, experimenter‐blinded study. Clinical Rehabilitation 2014;28:460‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang Y, Tang S, Chen G, Liu Y. Chinese massage combined with core stability exercises for nonspecific low back pain: a randomized controlled trial. Complementary Therapies in Medicine 2015;23:1‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bayraktar 2013 {published data only}
- Bayraktar D, Guclu‐Gunduz A, Lambeck J, Yazici G, Aykol S, Demirci H. Core stability exercises: in water or on land? Comparison of the effects of two different core stabilization training. Annals of the Rheumatic Diseases 2013; Vol. 72:Conference abstract.
Carmo 2013 {published data only}
- Carmo CM, Jacob MFA, Takara KS, Santos FG, Caromano FA, Tanaka C. Trunk stabilizing exercise and strengthening exercises in patients with non‐specific chronic low back pain: a pilot blinded randomized trial. Annals of the Rheumatic Disease 2013; Vol. 71:Abstract.
Meira 2013 {published data only}
- Meira DM. Functional reeducation program associated with back school improve functional disability and pain in workers with chronic low back pain: a pilot study. Annals of the Rheumatic Diseases 2013;72:A1095. [Google Scholar]
References to ongoing studies
ACTRN12609000343202 {published data only}
- ACTRN12609000343202. Effects of specific physiotherapy treatment and advice versus advice alone on pain and function for people with sub‐acute reducible discogenic low back pain: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000343202 (accessed 25 June 2015).
ACTRN12611000971932 {published data only}
- ACTRN12611000971932. A comparison of mechanical diagnosis & therapy and motor control exercises on the thickness of the trunk muscle in patients with chronic low back pain. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000971932 (accessed 25 June 2015).
Magalhaes 2013 {published data only}
- Magalhaes MO, França FJR, Burke TN, Ramos LAV, Carvalho e Silva APMC, Almeida GPL, et al. Efficacy of graded activity versus supervised exercises in patients with chronic non‐specific low back pain: protocol of a randomised controlled trial. BMC Musculoskeletal Disorders 2013;14:36. [DOI: 10.1186/1471-2474-14-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02112760 {published data only}
- NCT02112760. Specific stabilization exercise with ultrasound feedback for patient with recurrent low back pain. https://clinicaltrials.gov/ct2/show/NCT02112760 (accessed 25 June 2015).
NCT02170753 {published data only}
- NCT02170753. Regional manual therapy and motor control exercise for chronic low back pain. https://clinicaltrials.gov/ct2/show/NCT02170753 (accessed 25 June 2015).
NCT02200913 {published data only}
- NCT02200913. Effects of core stabilization exercise on balance. https://clinicaltrials.gov/ct2/show/NCT02200913 (accessed 25 June 2015).
NCT02221609 {published data only}
- NCT02221609. Movement system impairment based classification versus general exercise for chronic non‐specific low back pain: a randomised controlled trial. https://clinicaltrials.gov/ct2/show/NCT02221609 (accessed 25 June 2015). [DOI] [PubMed]
NCT02374970 {published data only}
- NCT02374970. Transversus abdominis muscular training and chronic low back pain. https://clinicaltrials.gov/ct2/show/NCT02374970 (accessed 25 June 2015).
NCT02398760 {published data only}
- NCT02398760. Relationship between clinical tests and clinical outcomes after motor control exercises intervention. https://clinicaltrials.gov/ct2/show/NCT02398760 (accessed 25 June 2015).
Additional references
Airaksinen 2006
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber‐Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. European Spine Journal 2006;15 Suppl 2:S192‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alaranta 1993
- Alaranta H, Tallroth K, Soukka A, Heliovaara M. Fat content of lumbar extensor muscles and low back disability: a radiographic and clinical comparison. Journal of Spinal Disorders & Techniques 1993;6:137‐40. [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Boutron 2005
- Boutron I, Estellat C, Ravaud P. A review of blinding in randomized controlled trials found results inconsistent and questionable. Journal of Clinical Epidemiology 2005;58:1220‐6. [DOI] [PubMed] [Google Scholar]
Bystrom 2013
- Bystrom MG, Rasmussen‐Barr E, Grooten WJA. Motor control exercises reduces pain and disability in chronic and recurrent low back pain. Spine 2013;38:E350‐8. [DOI] [PubMed] [Google Scholar]
Dagenais 2008
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine Journal 2008;8:8‐20. [DOI] [PubMed] [Google Scholar]
Duffy 2014
- Duffy S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE In‐Process searches via OvidSP. Kleijnen Systematic Reviews Ltd, York. Poster presented at the UK InterTASC Information Specialists' Sub‐Group (ISSG) Workshop; 9 July 2014; Exeter: UK (2014) (accessed 6 August 2014). Available from: https://medicine.exeter.ac.uk/media/universityofexeter/medicalschool/research/pentag/documents/Steven_Duffy_ISSG_Exeter_2014_poster_1.pdf.
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board Cochrane Back Review Group. 2009 Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34:1929‐41. [DOI] [PubMed] [Google Scholar]
Furlan 2015
Guyatt 2013
- Guyatt GH, Thorlund K, Oxman AD, Waltera SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66:173‐83. [DOI] [PubMed] [Google Scholar]
Hancock 2007
- Hancock MJ, Maher CG, Latimer J, Spindler MF, McAuley JH, Laslett M, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. European Spine Journal 2007;16:1539‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hides 1994
- Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994;19:165‐72. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Hodges 1996
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine 1996;21:2640‐50. [DOI] [PubMed] [Google Scholar]
Hodges 1997
- Hodges PW, Richardson CA. Relationship between limb movement speed and associated contraction of the trunk muscles. Ergonomics 1997;40:1220‐30. [DOI] [PubMed] [Google Scholar]
Hodges 1998
- Hodges PW, Richardson CA. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. Journal of Spinal Disorders & Techniques 1998;11:46‐56. [PubMed] [Google Scholar]
Hodges 1999
- Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Archives of Physical Medicine and Rehabilitation 1999;80:1005‐12. [DOI] [PubMed] [Google Scholar]
Hodges 2003
- Hodges PW. Core stability exercise in chronic low back pain. Orthopedic Clinics of North America 2003;34:245‐54. [DOI] [PubMed] [Google Scholar]
Macedo 2014
- Macedo LG, Maher CG, Hancock MJ, Kamper SJ, McAuley JH, Stanton TR, et al. Predicting response to motor control exercises and graded activity for patients with low back pain: preplanned secondary analysis of a randomized controlled trial. Physical Therapy 2014;94(11):1543‐53. [DOI] [PubMed] [Google Scholar]
Menezes Costa 2009
- Menezes Costa LdaC, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, et al. Prognosis for patients with chronic low back pain: inception cohort study. BMJ 2009;339:b3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menezes Costa 2012
- Menezes Costa LdaC, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low‐back pain: a meta‐analysis. Canadian Medical Association Journal 2012;184:E613‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moseley 2002a
- Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine 2002;27:E29‐36. [DOI] [PubMed] [Google Scholar]
Mueller 2007
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Annals of Internal Medicine 2007;146:878‐81. [DOI] [PubMed] [Google Scholar]
Panjabi 1992
- Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. Journal of Spinal Disorders & Techniques 1992;5:390‐6. [DOI] [PubMed] [Google Scholar]
Panjabi 2003
- Panjabi MM. Clinical spinal instability and low back pain. Journal of Electromyography & Kinesiology 2003;13:371‐9. [DOI] [PubMed] [Google Scholar]
Panjabi 2006
- Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. European Spine Journal 2006;15:668‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2004
- Richardson C, Jull G, Hodges P, Hides J. Therapeutic Exercise for Lumbopelvic Stabilization. A Motor Control Approach for the Treatment & Prevention of Low Back Pain. Churchill Livingstone, 2004. [Google Scholar]
Rubinstein 2012
- Rubinstein SM, Terwee CB, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for acute low‐back pain. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD008880] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teyhen 2005
- Teyhen DS, Miltenberger CE, Deiters HM, Toro YM, Pulliam JN, Childs JD, et al. The use of ultrasound imaging of the abdominal drawing‐in maneuver in subjects with low back pain. Journal of Orthopaedic & Sports Physical Therapy 2005;35:346‐55. [DOI] [PubMed] [Google Scholar]
van Dieen 2003
- Dieen JH, Selen LP, Cholewicki J. Trunk muscle activation in low‐back pain patients, an analysis of the literature. Journal of Electromyography & Kinesiology 2003;13:333‐51. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
van Tulder 2006
- Tulder M, Becker A, Bekkering T, Breen A, Real MT, Hutchinson A, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. European Spine Journal 2006;15 Suppl 2:S169‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2012a
- Wang XQ, Zheng JJ, Yu ZW, Bi X, Lou SJ, Liu J, et al. A meta‐analysis of core stability exercise versus general exercise for chronic low back pain. PLoS One 2012;7:e52082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamato 2015
- Yamato TP, Maher CG, Saragiotto BT, Hancock MJ, Ostelo RWJG, Cabral CMN, et al. Pilates for low back pain. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD010265.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Macedo 2009
- Macedo LG, Maher CG, Latimer J, McAuley JH. Motor control exercise for persistent, nonspecific low back pain: a systematic review. Physical Therapy 2009;89:9‐25. [DOI] [PubMed] [Google Scholar]


