Summary
Background
HIV self-testing (HIVST) can overcome barriers to HIV testing, but its potential as an HIV prevention strategy for women in sub-Saharan Africa has not been assessed. We examined whether sustained provision of self-tests to women promotes testing among sexual partners and reduces HIV risk.
Methods
We conducted a pair-matched cluster-randomized trial in 66 community clusters in Siaya County, Kenya. Within clusters, we recruited HIV-negative women aged ≥18 years with ≥2 partners in the past month. In each of the 33 cluster pairs, we randomly assigned clusters to an intervention and comparison group. In intervention clusters, we provided participants multiple self-tests at regular intervals and encouraged secondary distribution of self-tests to sexual partners. In comparison clusters, we provided participants referral cards for facility-based testing. Follow-up visits and HIV testing occurred at 6-month intervals for up to 24 months. The primary outcome of HIV incidence among all participants who contributed at least one HIV test was analyzed using discrete-time mixed effects models. This trial is registered with ClinicalTrials.gov, NCT03135067.
Findings
Between June 4, 2017 and August 31, 2018, we enrolled 2,090 participants (1033 in the 33 intervention clusters and 1057 in the 33 comparison clusters). Participants’ median age was 25 years and 66.6% reported sex work as an income source. Participant follow-up ended on March 25, 2020, with median follow-up duration of 17.6 months. HIV incidence was not significantly different between the intervention and comparison groups (1.2 vs. 1.0 per 100 person-year, HR 1.2; 95% CI 0.6-2.3; p=0.64). Social harms related to study participation occurred in three participants (two in the intervention group and one in the comparison group).
Interpretation
Sustained provision of multiple self-tests to high-risk women in Kenya facilitated secondary distribution of self-tests to sexual partners, but did not affect HIV incidence.
Funding
National Institute of Mental Health, Center for Health Incentives and Behavioral Economics, National Institute of Allergies and Infectious Diseases through the University of Pennsylvania Center for AIDS Research.
Background
The major expansion in access to HIV services in eastern and southern Africa over the past decade has led to considerable progress in combatting the region’s HIV epidemic. The region as a whole experienced a 38% reduction in new HIV infections and 49% reduction in AIDS-related deaths between 2010-19.1 Despite this progress, utilization of HIV services is lower among men than women and this results in worse health outcomes for men as well as missed opportunities for antiretroviral-based prevention.2 HIV incidence also remains very high among populations like female sex workers (FSW) and adolescent girls and young women.3 Increasing the coverage of HIV testing among men remains vital for both improving men’s health outcomes and reducing HIV risk among women. Furthermore, identifying new prevention options for women at high risk of HIV infection is an important priority even as pre-exposure prophylaxis (PrEP) becomes more widely available.
Recent studies of the universal test and treat approach as well as the experience of several countries in sub-Saharan Africa (SSA) have shown that multiple community-based testing strategies are needed to achieve high levels of HIV status awareness.4-8 They have also highlighted a need for targeted testing approaches to reach those who remain unaware of their status or have a high risk of HIV infection. HIV self-testing (HIVST) is a promising approach in this regard, with high acceptability among diverse populations, including men.4,9-11 Many countries have expanded access to self-tests and an important priority now is to identify optimal self-test distribution strategies that reach individuals who do not test regularly. One such strategy is the provision of multiple self-tests to women so they can voluntarily initiate male partner testing and couples testing. Studies of this secondary distribution strategy in SSA have found it to be acceptable, feasible, and safe.12-15 Additionally, randomized trials in Kenya, Malawi, and South Africa have shown that one-time provision of multiple self-tests to pregnant women, postpartum women, and young women results in considerably higher male partner testing that conventional strategies like invitations for clinic-based testing.14,16-18 Among FSW and women who report multiple partners, however, there have been relatively few evaluations of the secondary distribution approach. Moreover, sustained access to multiple self-tests in these populations has not been studied.
For HIV-negative women who reside in high prevalence settings and have a high risk of HIV infection due to multiple partnerships and transactional sex, providing sustained access to self-tests may convey important HIV prevention benefits. Through secondary distribution of self-tests to male partners, women may become more aware of the HIV status of their current and potential sexual partners. Prevention benefits for women could result from changes in sexual behavior stemming from learning a partner’s HIV status as well as ART linkage among male partners who learn they are HIV-positive. While many studies of HIV testing services (HTS) have found that receipt of an HIV-negative diagnosis does not result in sexual behavior change,19 sustained access to self-tests may promote sexual behavior change because it enables women to frequently learn not only their own status but also that of their sexual partners. A cohort study in Kenya and qualitative research suggests, for example, that the secondary distribution approach enabled women engaged in transactional sex to make better-informed decisions about sexual behavior, including condom use and relationship decisions.12,13 However, no studies have assessed whether sustained access to self-tests for women at high risk of HIV infection conveys prevention benefits to them.
In high HIV prevalence settings like western Kenya, where commercial and transactional sex is a source of new infections and HIV incidence remains above UNAIDS HIV elimination targets, there is a need for increased testing among men and additional HIV prevention options for women. We conducted a randomized trial among HIV-negative women at high risk of HIV infection to test the hypothesis that sustained access to HIV self-tests for secondary distribution to partners would reduce the number of new HIV infections and increase male partner and couples testing rates.
Methods
Study design and participants
The Jikinge (Kiswahili for “protect yourself”) study was a pair-matched cluster-randomized trial conducted in rural and peri-urban communities in Siaya County, Kenya from 2017 through 2020 (ClinicalTrials.gov NCT03135067). HIV prevalence in Siaya County is among the highest in Kenya, at 15.3%, and several studies have reported high prevalence of transactional sex among men and women.20-23 Randomization units were communities with a high prevalence of transactional sex, including beach communities along Lake Victoria and inland communities with hotspots for transactional sex such as bars and hotels.
After comprehensive mapping of beach communities, bars, and hotels in Siaya County we selected 66 community clusters. We consolidated nearby beach communities or nearby hotspots into single clusters. Clusters were pair-matched on the basis of spatial proximity and population size since beach communities and inland communities had different characteristics (including HIV prevalence, access to HIV services, and prevalence of venue-based commercial sex work) and there was also substantial heterogeneity in the population size of study clusters.
Within each cluster, we prepared a list of women aged ≥18 years who were potentially eligible. In beach clusters, we did this by conducting a census of female residents while in hotspot-based clusters, we relied on a roster of FSW receiving key population services as well as other women referred by peer educators. We then randomly selected women and screened them for eligibility. Because we sought to enroll women who were at high risk of HIV infection, criteria for eligibility included self-reporting ≥2 male partners in the past four weeks and testing HIV-negative, as per rapid HIV antibody testing that we did in accordance with Kenya Ministry of Health guidelines. Additional eligibility criteria included owning or having access to a mobile phone, intending to remain in the study area for the next 24 months, and not being enrolled in another HIV prevention study. Eligible participants were enrolled after providing written informed consent in their preferred language (English, Swahili or Dholuo).
The study protocol (supplement) was approved by the ethics review committee at Maseno University and institutional review board at the University of Pennsylvania. The study was also reviewed by a Data Safety Monitoring Board (DSMB) on four occasions.
Randomization and masking
Within each of the 33 cluster pairs, we randomly assigned clusters to an intervention group in which participants received sustained access to HIV self-tests for secondary distribution and to a comparison group in which participants received basic encouragement to seek clinic-based HTS. We performed randomization with a computer-generated algorithm. We used a cluster randomized trial design to reduce the likelihood of that participants in the intervention group would share self-tests with participants in the comparison group. The study was not blinded.
Procedures
Following enrolment, we administered a baseline questionnaire to collect information on participants’ demographic and socio-economic characteristics, sexual behavior, knowledge of primary and non-primary partners’ HIV status, and experience of intimate partner violence (IPV). In the intervention group, participants initially received 5 oral fluid-based rapid HIV self-tests along with training on their correct use (OraQuick Rapid HIV-1/2 antibody tests, OraSure Technologies). Since the intervention had several purposes including promoting male partner testing, enabling participants to learn their partners’ HIV status, and facilitating safer sexual behavior, we encouraged participants to use the tests themselves and to offer tests to their primary sexual partner and to any other sexual partners with whom unprotected sex was likely. We also counselled participants on how to discuss HIVST with sexual partners and on the importance of using their discretion when deciding whether to offer a self-test to a sexual partner. Self-test kits included written and pictorial use instructions in the three languages, including information on interpretation of results and a list of nearby clinics where users could confirm their test result and seek post-test services.
In the comparison group, participants received up to 10 referral cards listing names and locations of nearby public sector clinics with HTS. We encouraged participants to offer these referral cards to their sexual partners and encourage those partners to seek HIV testing services.
At 3 months and at 6-monthly study visits, study staff asked participants about their experience using self-tests (intervention group) or HTS referral cards (comparison group). They also gave participants an opportunity to receive additional self-tests (intervention group) or HTS referral cards (comparison group) for the purpose of partner or couples testing. Participants in both study groups were also asked to contact study staff by phone to request additional self-tests (intervention group) or HTS referral cards (comparison group) during interim periods. Participants in the intervention group were encouraged to request additional self-tests only if they intended to use the tests themselves or with current and potential partners. The actual number provided also depended on their self-reported number of partners and the number of previously provided self-tests that they reported having used or distributed. Self-tests and HTS referral cards were provided at a central location in each study cluster, and the number of additional self-tests provided typically ranged from 1-3 additional tests.
We conducted follow-up visits at 6-monthly intervals for a total of 18-24 months, depending on the date when the participant enrolled. At each follow-up visit, we performed rapid HIV antibody testing and provided the results to participants. We also administered questionnaires to collect information on participants’ self-reported use and distribution of self-tests and HTS referral cards, own testing behavior and partners’ testing behavior, sexual behavior, and IPV. We also asked participants about own testing, partner testing, and sexual behavior in their three most recent transactional sex encounters. Participants who missed a 6-monthly follow-up study visit were offered rapid HIV antibody testing at their next 3-monthly contact (i.e., at 9, 15, or 21 months).
A dried blood sample was collected at enrollment. If a participant tested positive at 6 months, we used this sample to conduct PCR-based HIV testing to confirm negative HIV-status at enrollment. Participants with a positive result were excluded from analysis.
Outcomes
The pre-specified primary outcome was HIV incidence, which was determined at each follow-up visit. Key secondary outcomes assessed at each follow-up visit included self-reported primary partner testing in the past 6 months (participant reported her primary partner had an HIV test), couples testing with the primary partner in the past 6 months (participant reported that she and her primary partner had an HIV test at the same time), partner testing in the most recent transactional sex encounter, couples testing in the most recent transactional sex encounter, and intimate partner violence in the past 6 months. Transactional sex encounters were defined as those in which participants reported receiving money, goods, or services in exchange for sex. Another pre-specified outcome we assessed whether participants ever declined to have sex with a partner who refused an offer to test for HIV or who tested HIV-positive, and whether they ever chose to use a condom with a partner who refused to test for HIV or who tested HIV-positive. In addition, we examined the total number of male partners that participants reported as having obtained an HIV-positive test result while participating in the study. All these secondary outcomes were pre-specified. Finally, although it was not pre-specified, we examined self-reported PrEP use at each follow-up visit since PrEP implementation began during the study and our intervention could have affected PrEP use through its effects on knowledge of partners’ HIV status.
Statistical analysis
We estimated that a sample size of 1,980 women from 66 clusters with follow-up duration of 18 months would provide 80% power to detect a 52.2% reduction in HIV incidence due to the intervention. We assumed HIV incidence of 4.5 per 100 person-years in the comparison group given the underlying HIV prevalence, HIV incidence among women who engage in transactional sex, and the high incidence reported in some other studies, a coefficient of variation (k) of 0.4, and 10% loss to follow-up over 18 months. These power calculations were based on a generalized estimating equation (GEE) model based on the primary outcome of HIV infection at 18 months.
Cluster- and participant-level baseline characteristics, including demographic and socio-economic characteristics, HIV testing behaviors, and sexual behaviors were described by study group using proportions (categorical variables), medians and interquartile ranges or means and standard deviations (continuous variables). We used a similar approach to describe characteristics of participants with and without complete primary outcome data.
For the primary outcome, we compared the risk of HIV seroconversion between study groups using a discrete-time (interval of 6 months) mixed effects survival model with a complementary log-log link function24 and fixed effects for the matched cluster pairs. This model replaced the initially planned GEE with a single primary endpoint, as we were able to ascertain HIV status more frequently than initially planned. Participants were censored at death or at last contact if they withdrew from the study or were lost to follow-up. We report hazard ratios (HRs) for both unadjusted and adjusted analyses, controlling for age, education, and marital status at baseline. Those with missing baseline covariates (4 participants) were excluded from the adjusted model.
For the primary outcome, we pre-specified three subgroup analyses based on the following individual- or cluster-level baseline characteristics: younger (≤24 years) versus older (>24 years) age, those reporting/not reporting sex work as an income source, and beach versus hotspot-based clusters. Not all these models converged, as some subgroups had few events. Thus we simply added each of these terms individually to the main model to assess the association of these factors with the outcome.
The secondary outcomes of primary partner and couples testing with the primary partner, partner and couples testing in the most recent transactional sex encounter, declining sex with a partner who tested HIV-positive or refused an offer to test, choosing to use a condom with a partner who tested HIV-positive or refused an offer to test, IPV, and PrEP use were analyzed longitudinally using Poisson models with random effects for participants nested within random effects for clusters. All outcomes were binary and are modelled as risk ratios (RRs) using a log link and robust standard errors. Each model included indicators for the matched cluster pairs, the intervention group, the follow-up visit (6, 12, 18, 24 months), and an interaction between the intervention group and follow-up visit.
The total number of HIV-positive sexual partners identified by each participant was summed across all follow-up visits and modelled as an incidence rate ratio (IRR) for the intervention versus comparison group using Poisson regression with cluster robust standard errors to account for overdispersion, and fixed effects for the matched cluster pairs. The log of time on-study from enrollment to the final 6-month follow-up was included as an offset.
The Type I error rate was 0.05, all tests were two-sided, and hypothesis tests were based on Wald tests. For longitudinal secondary outcomes, hypothesis tests were adjusted for multiple comparisons using a Holm-Bonferroni correction. Hazard, risk, and incidence rate ratios, as appropriate, are reported along with 95% confidence intervals (CI). All analyses were conducted using Stata (version 15.1 and 17.0; College Station, TX, USA) and R (version 3.5.2).
Role of the funding source
The funder of the study had no role in study design, data collection, analysis, and interpretation, or writing of the manuscript. The authors designed the study, gathered the data, performed all analyses, prepared the manuscript, and were responsible for the decision to submit for publication.
Results
Between June 4, 2017 and August 31, 2018, we screened 3179 women for eligibility and enrolled and randomized 2102 participants from 66 study clusters (16 beach clusters and 50 hotspot clusters) (Figure 1). A total of 1077 were not enrolled for various reasons. An additional 10 records (5 individuals) were withdrawn post-enrollment because of an administrative problem allowing these individuals to enroll in the study twice, and 2 participants were ineligible due to subsequent determination of HIV-positivity at baseline. Of the 2090 participants in the final study cohort, 1033 were in the intervention group and 1057 were in the comparison group. We enrolled between 25 and 40 participants in each cluster.
Figure 1.
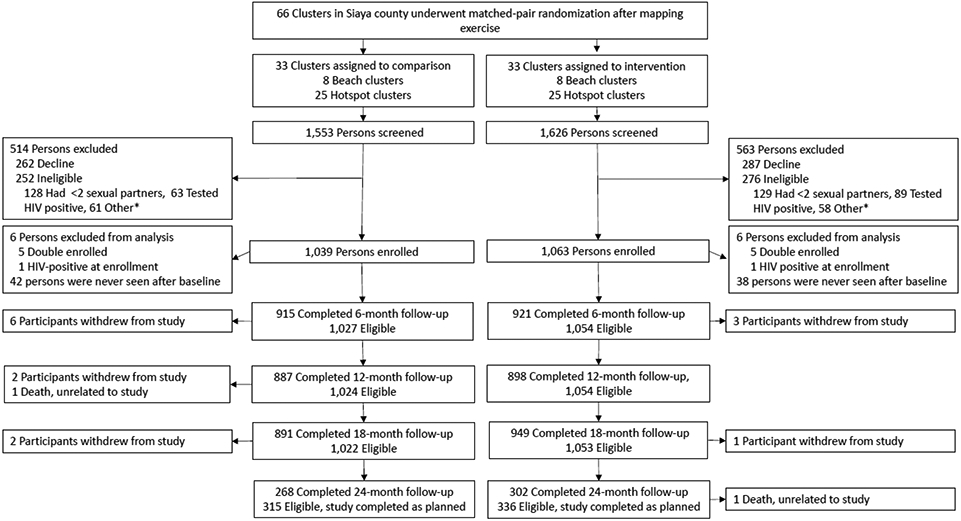
Trial profile
Did not plan to stay in study area; Lived in study area <6 months; <18 years old; Not current resident of study area; Enrolled in another HIV prevention study, Refuses HIV test; No access to phone
Follow-up visits concluded on March 25, 2020. A total of 2010 (96.2%) participants were tested for HIV at least once during study follow-up, 1019 (96.4%) and 991 (95.9%) in the intervention and comparison groups, respectively (appendix p.1). Similarly, 2002 (95.8%) participants completed at least one 6-month survey, including 1017 (96.2%) in the intervention group and 985 (95.4%) in the comparison group. The median follow-up duration for participants was 17.6 months and the total person-years of follow-up was 3150. Retention at 18 months was 90.1% (N=949) and 87.2% (N=891) in the intervention and comparison groups, respectively. Among the 651 participants followed for 24 months, retention was 89.9% (N=302) and 85.1% (N=268) in the intervention and comparison groups, respectively. Baseline characteristics were generally similar for participants measured at their last eligible follow-up or seroconversion versus those who missed their last follow-up or all follow-ups, although those with missing data were younger and more likely to be single (appendix p.2).
Baseline characteristics of participants in the two study groups were similar. Participants’ median age was 25 years (IQR 22-31) and 64.5% (N=1,345) were married (Table 1). The median number of sexual partners in the past month was 2 (IQR 2-3). Sex work was the primary source of income for 322 (15.4%) participants and a secondary source of income for an additional 1,068 (51.2%) participants. While 90.4% (N=1,885) of participants had been tested for HIV in the past year, only 58.1% (N=1,160) reported that their primary partner had been tested for HIV in the past year. Very few participants (1.5%, N=31) had used an HIV self-test previously.
Table 1.
Baseline characteristics
| N (%) | |||
|---|---|---|---|
| Cluster-level | Overall N = 66 |
Intervention N = 33 |
Comparison N = 33 |
| Sub-county | |||
| Bondo | 18 (27.3) | 9 (27.3) | 9 (27.3) |
| Rarieda | 12 (18.2) | 6 (18.2) | 6 (18.2) |
| Siaya | 36 (54.5) | 18 (54.5) | 18 (54.5) |
| Cluster type | |||
| Beach | 16 (24.2) | 8 (24.2) | 8 (24.2) |
| Hotspot | 50 (75.8) | 25 (75.8) | 25 (75.8) |
| Participant-level | Overalla N = 2,086 |
Interventiona N = 1,054 |
Comparisona N = 1,032 |
| Demographics | |||
| Age, median (IQR) | 25 (22-31) | 26 (22-31) | 25 (22-31) |
| Education | |||
| Some Primary or less | 665 (31.9) | 343 (32.5) | 322 (31.2) |
| Primary | 631 (30.3) | 310 (29.4) | 321 (31.1) |
| Some Secondary | 389 (18.6) | 189 (17.9) | 200 (19.4) |
| Secondary/High School or more | 401 (19.2) | 212 (20.1) | 189 (18.3) |
| Marital status | |||
| Married | 1,345 (64.5) | 657 (62.3) | 688 (66.7) |
| In a relationship, but not married | 198 (9.5) | 111 (10.5) | 87 (8.4) |
| Single | 387 (18.5) | 205 (19.5) | 182 (17.6) |
| Divorced or Widowed | 156 (7.5) | 81 (7.7) | 75 (7.3) |
| Primary source of income | |||
| Sales and service | 688 (33.0) | 348 (33.0) | 340 (32.9) |
| Sex work | 322 (15.4) | 153 (14.5) | 169 (16.4) |
| Unskilled manual labor | 241 (11.6) | 146 (13.9) | 95 (9.2) |
| Fishing/fish trade | 210 (10.1) | 108 (10.2) | 102 (9.9) |
| Skilled manual labor | 157 (7.5) | 80 (7.6) | 77 (7.5) |
| Agriculture | 116 (5.6) | 56 (5.3) | 60 (5.8) |
| Informal/seasonal work | 64 (3.1) | 28 (2.7) | 36 (3.5) |
| Domestic work | 40 (1.9) | 19 (1.8) | 21 (2.0) |
| Professional/Salary work | 33 (1.6) | 15 (1.4) | 18 (1.7) |
| Student | 69 (3.3) | 36 (3.4) | 33 (3.2) |
| Unemployed | 140 (6.7) | 62 (5.9) | 78 (7.6) |
| Other | 3 (0.1) | 2 (0.2) | 1 (0.1) |
| Don’t Know/Refused | 3 (0.1) | 1 (0.1) | 2 (0.2) |
| Sex work is another source of incomeb | 1,068 (51.2) | 594 (56.4) | 474 (45.9) |
| Typical one-month income in U.S. $, median (IQR) | 30 (20, 60) | 30 (20, 60) | 30 (18, 55) |
| Household size, mean (range) | 5.0 (1-16) | 4.9 (1-16) | 5.0 (1-13) |
| Number of people participant currently supports, median (IQR) | 3 (1, 4) | 3 (1, 4) | 3 (1, 4) |
| Has regularly eaten at least two meals a day in the past month | 1,979 (94.9) | 999 (94.8) | 980 (95.0) |
| Health and health behaviors | |||
| Drank alcohol at all in the past 12 months | 461 (22.1) | 242 (23.0) | 219 (21.3) |
| Moderately severe or severe depression (PHQ-9)c | 38 (1.8) | 27 (2.6) | 11 (1.1) |
| Age at first sexual encounter, mean (SD) | 16 (2.2) | 16 (2.3) | 16 (2.2) |
| Used condom during last sexual encounter | 750 (35.9) | 388 (36.8) | 362 (35.0) |
| Diagnosed with an STI in the past 6 mo. | 25 (1.2) | 15 (1.4) | 10 (1.0) |
| Uses PrEP | 35 (1.7) | 18 (1.7) | 17 (1.7) |
| Ever used an HIV self-test prior to Jikinge study | 31 (1.5) | 13 (1.2) | 18 (1.7) |
| Sexual behavior and IPV | |||
| Number of sexual partners in the past month, median (IQR) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| Have had a primary partner in past month | 1,997 (95.7) | 1,010 (95.8) | 987 (95.6) |
| Number of non-primary sexual partners in the past month, median (IQR) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Ever engaged in transactional sex | 1,980 (95.9) | 998 (94.7) | 982 (95.2) |
| Number of transactional sex partners in the past month, median (IQR) | 2 (1, 2) | 2 (1, 2) | 2 (1, 2) |
| Experienced intimate partner violence in the past 12 months | 1,052 (50.4) | 533 (50.6) | 519 (50.3) |
| HIV testing | |||
| Participant tested for HIV in the past 12 months | 1,885 (90.4) | 956 (90.7) | 929 (90.0) |
| Participant’s primary partner tested for HIV in the past 12 monthsd | 1,160 (58.1) | 563 (55.7) | 597 (60.5) |
Data are n (%), median (IQR), mean (SD), or mean (range). Percentages may not sum to 100 due to rounding. PHQ-9=Patient Health Questionnaire-9.
Four baseline surveys were lost after participant enrollment. Three from the Intervention arm, and one from the Comparison arm.
Among individuals whose primary source of income was not sex work. Overall N = 1,068; Intervention N = 594; Comparison N = 474.
Modified version of the PHQ-9. Among those who completed modified PHQ-9 questionnaire: Overall N = 2,070; Intervention N = 1,044; Comparison N = 1,026.
Among individuals who reported having a primary partner. Overall N = 1,997; Intervention N = 1,010; Comparison N = 987.
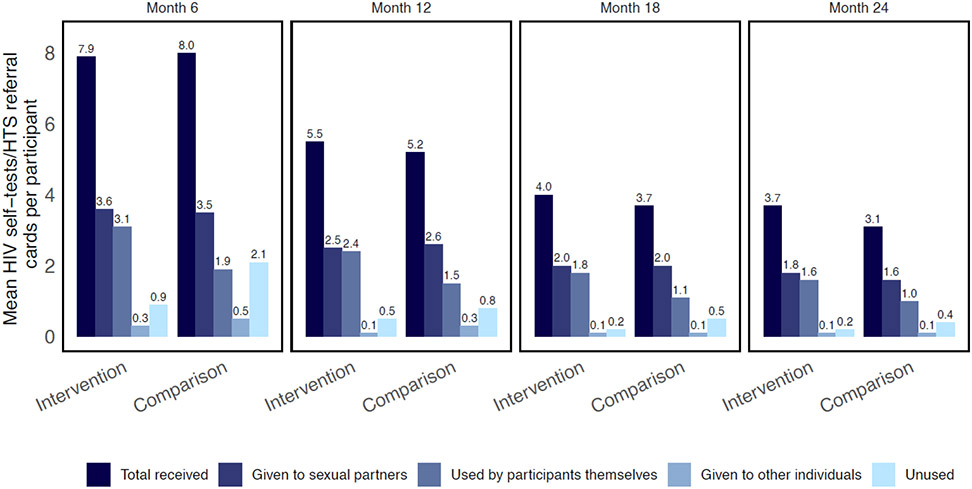
Participants in the intervention group reported receiving an average of 16.8 self-tests during the study, while participants in the comparison group reported receiving an average of 16.3 HTS referral cards (Fig. 2; appendix p.5). The number of self-tests and HTS referral cards given to participants declined over time. On average, participants in the intervention group reported using 42.3% (N=7,205) of self-tests themselves and offering 47.0% (N=8,032) to their partners. Participants offered only a small percentage of self-tests to individuals other than their sexual partners (2.4%, N=419) and similarly, only a small percentage (8.4%, N=1,470) were not used at all. Including the study-administered rapid tests at 6-monthly intervals, participants in the intervention group had around 10.1 HIV tests while on the study, while participants in the comparison group had 5.5 tests (appendix p. 6).
Figure 2.
Number of HIV self-tests and HIV testing services referral cards received, used, and distributed
A total of 34 incident HIV infections were observed: 19 (55.9%) in the intervention group and 15 (44.1%) in the comparison group (Table 2). Overall HIV incidence was lower than anticipated, at 1.08 per 100 person-years: 1.18 per 100 person-years in the intervention group and 0.98 per 100 person-years in the comparison group. The risk of HIV acquisition in the intervention group was not significantly different from the comparison group (HR 1.2, 95% CI 0.6-2.3, p=0.64, Table 2). Adjustment for baseline age, education, and marital status had little impact on the estimate (HR 1.2, 95% CI 0.6-2.3, p=0.65). HIV incidence was similar by study group among participants aged 18-24 years, those who reported income from sex work at baseline, or by residence in beach clusters or hotspot-based clusters (appendix p.7).
Table 2.
Effect of HIV self-testing intervention on HIV incidence
| Seroconversions | Person- years of follow-up |
HIV incidence per 100 person- years of follow-up |
Unadjusted Hazard Ratio (95% CI) |
Adjusted Hazard Ratio (95% CI) |
|
|---|---|---|---|---|---|
| Intervention (N=1,019) | 19 | 1,615 | 1.18 | 1.2 (0.6-2.3) p=0.64 |
1.2 (0.6-2.3) p=0.65 |
| Comparison (N=991) | 15 | 1,535 | 0.98 | 1.0 (ref) | 1.0 (ref) |
Note: A total of 2,010 participants (96%) were included in the unadjusted survival analysis from 2,090 who were randomized. 2,006 (96%) were included in the adjusted survival analysis, which also controlled for baseline age, education, and marital status.
HIV testing by the primary partner of participants was more likely due to the intervention (Table 3). At 6 months, 87.5% (N=806) of participants in the intervention group reported their primary sexual partner completed an HIV test in the past 6 months, compared to only 48.3% (N=442) in the comparison group (RR 1.8, 95% CI 1.6-2.1, p<0.0001). The intervention group also had significantly higher primary partner testing in the past 6 months at 12, 18, and 24 months. At 6 months, couples testing with the primary partner was higher in the intervention group (80.3%, N=740) than the comparison group (32.9%, N=301) (RR 2.5, 95% CI 2.1-2.9, p<0.0001). The intervention group also had significantly higher rates of couples testing in the past 6 months at 12, 18, and 24 months.
Table 3.
Effect of HIV self-testing intervention on secondary outcomes at each 6-month follow-up visit
| 6 months | 12 months | 18 months | 24 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Group | Rate % (N) |
RR (95% CI) |
p value | Rate % (N) |
RR (95% CI) |
p value | Rate % (N) |
RR (95% CI) |
p value | Rate % (N) |
RR (95% CI) |
p value |
| Primarypartner tested for HIV | Int | 87.5 (806) | 1.8 (1.6, 2.1) | 81.2 (729) | 1.7 (1.5, 1.9) | 77.8 (738) | 2.0 (1.8, 2.2) | 74.9 (227) | 2.0 (1.7, 2.3) | ||||
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||||
| Cmp | 48.3 (442) | 1.0 (ref) | 48.6 (431) | 1.0 (ref) | 39.8 (355) | 1.0 (ref) | 39.0 (104) | 1.0 (ref) | |||||
| Couples testing with primary partner | Int | 80.3 (740) | 2.5 (2.1, 2.9) | 75.3 (676) | 2.2 (1.9, 2.5) | 34.7 (308) | 2.7 (2.4, 3.1) | 71.6 (217) | 2.8 (2.3, 3.4) | ||||
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||||
| Cmp | 32.9 (301) | 1.0 (ref) | 34.7 (308) | 1.0 (ref) | 26.7 (238) | 1.0 (ref) | 26.2 (70) | 1.0 (ref) | |||||
| Most recent transactional sex partner tested for HIV | Int | 58.0 (534) | 1.3 (1.1, 1.4) | 52.3 (470) | 1.2 (1.1, 1.3) | 45.5 (432) | 1.3 (1.2, 1.5) | 38.4 (116) | 1.7 (1.4, 2.1) | ||||
| <0.0001 | <0.0014 | <0.0001 | <0.0001 | ||||||||||
| Cmp | 46.3 (424) | 1.0 (ref) | 44.2 (392) | 1.0 (ref) | 34.9 (311) | 1.0 (ref) | 22.8 (61) | 1.0 (ref) | |||||
| Couples testing with most recent transactional sex partner | Int | 50.7 (467) | 1.9 (1.6, 2.1) | 46.5 (418) | 1.5 (1.3, 1.7) | 40.9 (388) | 1.6 (1.4, 1.8) | 36.1 (109) | 2.6 (2.0, 3.4) | ||||
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||||
| Cmp | 27.4 (251) | 1.0 (ref) | 31.1 (3276) | 1.0 (ref) | 26.0 (232) | 1.0 (ref) | 14.2 (38) | 1.0 (ref) | |||||
| Declined sex with≥1 partner(s) after partner(s) refused self-test/HTS referral card, or tested HIV-positive | Int | 14.2 (131) | 2.0 (1.5, 2.7) | 5.1 (46) | 0.9 (0.6, 1.2) | 4.5 (43) | 1.2 (0.7, 1.9) | 2.3 (7) | 0.8 (0.2, 3.1) | ||||
| <0.0001 | 1.00 | 1.00 | 1.00 | ||||||||||
| Cmp | 7.1 (65) | 1.0 (ref) | 6.0 (53) | 1.0 (ref) | 3.8 (34) | 1.0 (ref) | 3.0 (8) | 1.0 (ref) | |||||
| Used condom with≥1 partner(s) after partner(s) refused self-test/HTS refenal card, or tested HIV-positive | Int | 11.6 (107) | 1.9 (1.4, 2.5) | 7.2 (65) | 1.4 (0.9, 2.1) | 5.3 (50) | 1.3 (0.8, 1.9) | 3.3 (10) | 0.7 (0.3, 1.6) | ||||
| 0.0001 | 0.27 | 0.60 | 0.60 | ||||||||||
| Cmp | 6.2 (57) | 1.0 (ref) | 5.2 (46) | 1.0 (ref) | 4.3 (38) | 1.0 (ref) | 4.9 (13) | 1.0 (ref) | |||||
| Any intimate partner violence (IPV) | Int | 17.3 (159) | 1.0 (0.8, 1.3) | 10.8 (97) | 1.4 (1.0, 1.9) | 6.1 (58) | 1.7 (1.0, 2.8) | 5.0 (15) | 1.0 (0.4, 2.2) | ||||
| 1.00 | 0.15 | 0.15 | 1.00 | ||||||||||
| Cmp | 16.4 (150) | 1.0 (ref) | 7.7 (68) | 1.0 (ref) | 3.5 (31) | 1.0 (ref) | 5.2 (14) | 1.0 (ref) | |||||
| Cunently on PrEP | Int | 4.1 (38) | 1.6 (0.9, 2.9) | 5.2 (47) | 1.1 (0.7, 1.6) | 3.9 (37) | 0.7 (0.5, 1.0) | 3.3 (10) | 0.8 (0.4, 1.6) | ||||
| 0.32 | 0.94 | 0.25 | 0.94 | ||||||||||
| Cmp | 2.5 (23) | 1.0 (ref) | 4.6 (41) | 1.0 (ref) | 5.4 (48) | 1.0 (ref) | 3.7 (10) | 1.0 (ref) | |||||
Note: Risk ratios and p-values are based on generalised linear mixed models, run for each secondary outcome. Models covariates included indicators for arm and follow-up time point (6, 12, 18, and 24 months), the interaction between arm and follow-up time point, and matched cluster pairs. All p-values were adjusted using the Holm-Bonferroni correction. RR=Risk ratio. Int=Intervention. Cmp=Comparison.
Focusing on participants’ most recent transactional encounter, HIV testing by the sexual partner was considerably higher in the intervention group than the comparison group (534 [58.0%] vs. 424 [46.3%] at 6 months, RR 1.3, 95% CI 1.1-1.4, p<0.0001). While partner testing in the most recent transactional sex encounter declined over time in both groups, it remained significantly higher in the intervention group at each follow-up visit. Couples testing in the most recent transactional encounter was also higher in the intervention group than the comparison group (50.7% vs. 27.4% at 6 months, N=467 vs. N=251, RR 1.9, 95% CI 1.6-2.1, p<0.0001), and this pattern was sustained at subsequent follow-up visits.
Over the study duration, participants reported that a total of 393 male sexual partners obtained an HIV-positive test result (appendix p.9). One HIV-positive partner was reported per 3.9 participants in the intervention group, as compared to one HIV-positive partner per 7.7 participants in the comparison group. Adjusting for follow-up duration, the intervention group identified 1.9 times as many HIV-positive partners as the comparison group (IRR 1.9, 95% CI 1.5-2.3, p<0.0001).
During the first six months, the intervention appeared to have influenced decision making about sexual encounters. Relative to the comparison group, the intervention group was more likely to report that there was at least one instance when they declined sex after a partner either tested HIV-positive or refused to accept an offer to test (14.2% vs. 7.0%, N=131 vs. N=65, RR 2.0, 95% CI 1.5-2.7, p<0.0001) (Table 3). The proportion of participants who reported there was at least one instance when they used condoms after a sexual partner either tested HIV-positive or refused to accept an offer to test was also higher in the intervention group, 11.6% (N = 107), than the comparison group, 6.2% (N = 57) (RR 1.9, 95% CI 1.4-2.5, p=0.0001). However, in subsequent follow-up visits, such instances occurred at similar rates in both study groups and declined in frequency. Finally, self-reported PrEP use among participants did increase over time, but it never exceeded 5% and was similar in both study groups.
At 6 months, 159 (17.3%) participants in the intervention group and 150 (16.4%) in the comparison group reported experiencing IPV in the past 6 months. These rates declined over time and there was no significant difference in IPV between study groups at each follow-up. Very few participants (N=15, 0.7%) reported that IPV was the result of study participation (appendix p.10). Social harms related to study participation occurred in 3 participants: 2 in the intervention group and 1 in the comparison group.
Discussion
In this cluster-randomized trial among women at high risk of HIV infection in Kenya, providing sustained access to HIV self-tests had no detectable effect on HIV incidence. However, the self-testing intervention enabled secondary distribution of self-tests to male partners and resulted in much higher awareness of partners’ HIV status than in the comparison group. Nearly 90% of women in the intervention group reported their primary partner tested for HIV in the past 6 months. Couples testing every 6 months and identification of HIV-positive male partners was also substantially higher due to the self-testing intervention, as was partner and couples testing in recent transactional sex encounters. This study is among the first to examine the impacts of providing women multiple self-tests for their own use and for secondary distribution to partners, over a sustained period rather a one-time basis.
We hypothesized the intervention would reduce women’s risk of HIV acquisition by facilitating awareness of partners’ HIV status and safer sexual behavior. While the intervention succeeded in achieving the objectives related to testing and sexual behavior, HIV incidence was similar between study groups. The overall annual HIV incidence of 1.1% among women with multiple partners in settings with high prevalence of both commercial and transactional sex was substantially lower originally anticipated. Our screening criterion of self-reported sexual behavior (≥2 partners in the past month) may not have identified women at the highest risk of HIV infection. Other factors like the scale-up of ART, medical male circumcision, successful key populations programs that promote condom use, and more recently, the introduction of PrEP, may also explain the lower-than-expected incidence. Nonetheless, observed HIV incidence was well above HIV elimination targets and higher than HIV incidence in communities from the study region that were part of the SEARCH trial of a universal test and treat strategy.8
The lack of an intervention effect on incidence could be because the active comparison group, which received HTS referral cards and encouragement for partner testing, achieved higher partner and couples testing than would be likely in a true control group. Another explanation could be inadequate ART linkage by male partners in the intervention group who tested HIV-positive, which is vital for reducing HIV transmission risk. Other explanations include the possibility that women in both study groups were equally likely to learn (or not learn) the status of their highest-risk partners. PrEP scale-up, which began during the study period, may also have reduced the proportion of participants who could benefit from the intervention.
The HIVST intervention was highly effective in promoting partner and couples testing on a frequent basis, with primary partners and in recent transactional sex encounters. The nearly two-fold increase in partner testing rates is an encouraging finding since men who purchase sex are more likely to be HIV infected than other men, are less likely to be aware of their HIV status, and contribute to increased HIV transmission among women who sell sex and their other female partners.25-27 These findings are also broadly consistent with other studies of secondary distribution in Kenya, Malawi, and South Africa, in which provision of multiple self-tests to women on one occasion increased partner testing relative to standard approaches like encouraging clinic-based partner testing.12,14,17,18 This study builds on prior evidence by showing that sustained access to self-tests for women at high risk of HIV can facilitate frequent partner and couples testing.
We also found the intervention resulted in a higher number of HIV-positive male partners identified. Additional research with male partners reached by secondary distribution interventions is needed to determine the proportion of positive results that represent new diagnoses, and to explore care-seeking patterns among those who test HIV-positive.
This study also contributes to a larger literature on the effects of HIV testing services on sexual behavior and HIV incidence. As a recent systematic review and meta-analysis indicates, most HTS studies have found that while learning one’s own HIV status results in limited behavior change among those diagnosed HIV-negative, condom use is more likely after an HIV-positive diagnosis or following couples testing.19 Few studies have found statistically significant effects on HIV incidence. In this study, the HIVST intervention appeared to facilitate better-informed sexual decision making by women since it reduced unprotected sex with partners with unknown or HIV-positive status. Unlike most studies of HTS, any effects on sexual behavior in our study were unlikely to be driven by participants’ learning their own status (both study groups received rapid testing every 6 months). Instead, behavioral responses to the HIVST intervention were likely due to learning partners’ status or due to partners’ declining participants’ offers of self-tests. Subsequent analyses will further examine the intervention’s effect on participants’ sexual behavior with primary and non-primary partners (including transactional sex partners), thus providing a more nuanced understanding about this aspect of the intervention. To that end we are also analyzing qualitative data from a subset of participants and their partners.
This study has several limitations. First, women who did not have a desire to access self-tests or learn their partners’ HIV status may have chosen not to enroll in the study, which limits generalizability of the findings to other populations. However, we believe the results provide policy relevant insights for those who voluntarily accept or acquire self-tests as HIVST scale-up occurs. Second, while women in the intervention group identified significantly more HIV-positive male partners, these data were based on women’s self-reports and we were unable to assess whether those male partners were newly diagnosed or whether they sought confirmatory testing. However, we examined self-reported data in both study groups and do not anticipate differential reporting bias.
Our findings suggest several important priorities for future research. The first is to determine linkage to confirmatory testing under secondary distribution approaches, in which the end users of self-tests are typically not the index persons who initially obtained self-tests. Understanding how to support linkage to prevention and treatment services is a related priority. Linkage interventions that accompany secondary distribution could include small incentives for confirmatory testing or additional information provision to self-test users. Second, while our study examined the effect of women having sustained access to self-tests, subsequent work should explore optimal implementation strategies outside the research setting for regular provision self-tests to women interested in receiving them (for own use or for secondary distribution). Third, the secondary distribution approach could be adapted to other priority populations that have high HIV risk and infrequent testing.28,29
As HIVST is scaled-up in many countries, policy makers and program implementers will need to consider distribution strategies to maximize its impact. Secondary distribution of self-tests is a promising strategy to increase male partner testing rates and knowledge of HIV status between couples, including in relationships involving transactional sex. Sustained access to self-tests and secondary distribution to sexual partners, when coupled with available HIV prevention and care services, is a potentially powerful tool that can help support HIV elimination goals.
Supplementary Material
Research in context.
Evidence before the study
Among the many ways to implement HIV self-testing (HIVST), secondary distribution of self-tests by index individuals to their sexual partners is an approach that may increase testing coverage among hard-to-reach individuals who are less likely to seek facility- or community-based HIV testing services. We searched PubMed on August 27, 2021 for titles and abstracts published between Jan 1, 1980, and August 27, 2021, using the following search terms and no language restrictions: “HIV self-testing” AND “secondary distribution.” We retrieved 35 records. Three randomized trials have shown that one-time provision of 2-5 HIV self-tests to specific groups such as pregnant women or young women can increase male partner testing. However, no studies have examined the effects of providing sustained access to self-tests (rather than one-time provision of self-tests) for women who are at high risk of HIV infection. Finally, while pilot studies and qualitative studies suggest that providing women multiple self-tests may influence sexual decision making, no studies have assessed whether such an HIVST intervention can reduce women’s risk of HIV infection.
Added value of this study
This study is a cluster-randomized trial conducted among women who self-reported multiple partners and resided in settings with high prevalence of transactional sex and HIV. It is the first study to evaluate whether access to free HIV self-tests over an extended period of time enables women to become more aware of their current and potential partners’ HIV status and reduce their risk of acquiring HIV. In the intervention group that received multiple self-tests over 18-24 months, six-monthly partner and couples testing rates were very high (between 70-90%) and more than twice as high in the comparison group, where women were encouraged to refer their male partners for clinic-based HIV testing. The intervention also identified nearly twice as many HIV-positive male partners, and condom use was higher with partners who tested HIV-positive or refused an offer to test. However, the intervention had no detectable effect on annual HIV incidence, which was 1.1 per 100 person-years overall. For high prevalence settings, the study provides useful evidence on how self-tests can be utilized over time to facilitate testing among men and increase knowledge of HIV status in couples and in transactional sex relationships.
Implications of all the available evidence
As HIV self-testing is scaled-up in sub-Saharan Africa, secondary distribution of self-tests should be viewed as being useful not only for increasing testing coverage among men but also for increasing women’s awareness of their partners’ HIV status and enhancing their ability to make informed decisions regarding sexual behavior. Such an intervention is particularly relevant in populations where transactional sex presents women with tradeoffs between income and risky sexual behavior, while also contributing to new HIV infections among both men and women. More broadly, HIVST and network-based approaches should be tested in other ways, such as among men and their peers. Sustained access to self-tests and secondary distribution of self-tests to sexual partners, when coupled with available HIV prevention products, is a potentially powerful tool that can help support HIV elimination goals.
Acknowledgements
This study was funded by the National Institute of Mental Health (R01MH111602), the Center for Health Incentives and Behavioral Economics, and National Institute of Allergies and Infectious Diseases through the University of Pennsylvania Center for AIDS Research (P30AI045008-23). We thank participants involved in the study, the Ministry of Health, community members and leaders in Siaya County, and research staff. We are also grateful to the Data Safety and Monitoring Board members for their feedback.
Footnotes
Declaration of interests
HT, EFB, SN report grants from National Institutes Health, during the conduct of the study. All other authors declare no competing interests.
Data sharing
Individual participant data that underlie the results reported in this Article, after deidentification, are available following publication. We will also provide the statistical code. Data are available for researchers who provide a methodologically sound proposal. The study protocol is provided in the appendix. Data and statistical code will be available beginning 3 months following article publication. Proposals should be directed to the corresponding author (hthirumu@upenn.edu).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. 2020 global AIDS update—seizing the moment—tackling entrenched inequalities to end epidemics. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2020. [Google Scholar]
- 2.UNAIDS. Blind spot: reaching out to men and boys. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2017. [Google Scholar]
- 3.Birdthistle I, Tanton C, Tomita A, et al. Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: a systematic review and meta-analysis. Lancet Glob Health 2019; 7(11): e1521–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamie G, Napierala S, Agot K, Thirumurthy H. HIV testing approaches to reach the first UNAIDS 95% target in sub-Saharan Africa. The Lancet HIV 2021; 8(4): e225–e36. [DOI] [PubMed] [Google Scholar]
- 5.Hayes RJ, Donnell D, Floyd S, et al. Effect of Universal Testing and Treatment on HIV Incidence - HPTN 071 (PopART). N Engl J Med 2019; 381(3): 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal Testing, Expanded Treatment, and Incidence of HIV Infection in Botswana. N Engl J Med 2019; 381(3): 230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. The lancet HIV 2018; 5(3): e116–e25. [DOI] [PubMed] [Google Scholar]
- 8.Havlir DV, Balzer LB, Charlebois ED, et al. HIV Testing and Treatment with the Use of a Community Health Approach in Rural Africa. N Engl J Med 2019; 381(3): 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clinical Infectious diseases : an official publication of the Infectious Diseases Society of America 2013; 57(1): 126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Consolidated Guidelines on HIV Testing Services. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 11.Choko AT, MacPherson P, Webb EL, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med 2015; 12(9): e1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. The lancet HIV 2016; 3(6): e266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maman S, Murray KR, Napierala Mavedzenge S, et al. A qualitative study of secondary distribution of HIV self-test kits by female sex workers in Kenya. PLoS One 2017; 12(3): e0174629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettifor A, Lippman SA, Kimaru L, et al. HIV self-testing among young women in rural South Africa: A randomized controlled trial comparing clinic-based HIV testing to the choice of either clinic testing or HIV self-testing with secondary distribution to peers and partners. EClinicalMedicine 2020; 21: 100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Guidelines on HIV self-testing and partner notification. Geneva: World Health Organization, 2016. [Google Scholar]
- 16.Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial. PLoS Med 2016; 13(11): e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choko AT, Corbett EL, Stallard N, et al. HIV self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: An adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med 2019; 16(1): e1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choko AT, Fielding K, Johnson CC, et al. Partner-delivered HIV self-test kits with and without financial incentives in antenatal care and index patients with HIV in Malawi: a three-arm, cluster-randomised controlled trial. Lancet Glob Health 2021; 9(7): e977–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari R, Wang J, Han H, et al. Sexual behaviour change following HIV testing services: a systematic review and meta-analysis. Journal of the International AIDS Society 2020; 23(11): e25635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camlin CS, Kwena ZA, Dworkin SL, Cohen CR, Bukusi EA. “She mixes her business”: HIV transmission and acquisition risks among female migrants in western Kenya. Social Science & Medicine 2014; 102(0): 146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassels S, Camlin CS. Geographical mobility and heterogeneity of the HIV epidemic. The lancet HIV 2016; 3(8): e339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwena Z, Mwanzo I, Shisanya C, et al. Predictors of extra-marital partnerships among women married to fishermen along Lake Victoria in Kisumu County, Kenya. PLoS One 2014; 9(4): e95298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camlin CS, Kwena ZA, Dworkin SL. Jaboya vs. jakambi: Status, negotiation, and HIV risks among female migrants in the "sex for fish" economy in Nyanza Province, Kenya. AIDS Educ Prev 2013; 25(3): 216–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC. A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications. Int Stat Rev 2017; 85(2): 185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wulandari LPL, Guy R, Kaldor J. The burden of HIV infection among men who purchase sex in low- and middle-income countries - a systematic review and meta-analysis. PLoS One 2020; 15(9): e0238639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voeten HA, Egesah OB, Ondiege MY, Varkevisser CM, Habbema JD. Clients of female sex workers in Nyanza province, Kenya: a core group in STD/HIV transmission. Sex Transm DIs 2002; 29(8): 444–52. [DOI] [PubMed] [Google Scholar]
- 27.Alary M, Lowndes CM. The central role of clients of female sex workers in the dynamics of heterosexual HIV transmission in sub-Saharan Africa. AIDS 2004; 18(6): 945–7. [DOI] [PubMed] [Google Scholar]
- 28.Choko AT, Nanfuka M, Birungi J, Taasi G, Kisembo P, Helleringer S. A pilot trial of the peer-based distribution of HIV self-test kits among fishermen in Bulisa, Uganda. PLOS ONE 2018; 13(11): e0208191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okoboi S, Lazarus O, Castelnuovo B, et al. Peer distribution of HIV self-test kits to men who have sex with men to identify undiagnosed HIV infection in Uganda: A pilot study. PLOS ONE 2020; 15(1): e0227741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.