Abstract
Accurate and rapid detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is significant for early tracing, isolation, and treatment of infected individuals, which will efficiently prevent large-scale transmission of coronavirus disease 2019 (COVID-19). Here, two kinds of test strips for receptor binding domain (RBD) and N antigens of SARS-CoV-2 are established with high sensitivity and specificity, in which AIE luminogens (AIEgens) are utilized as reporters. Because of the high brightness and resistance to quenching in aqueous solution, the limit of detection can be as low as 6.9 ng/mL for RBD protein and 7.2 ng/mL for N protein. As an antigen collector, an N95 mask equipped with a test strip with an excellent enrichment effect would efficiently simplify the sampling procedures. Compared with a test strip based on Au nanoparticles or fluorescein isothiocyanate (FITC), the AIEgen-based test strip shows high anti-interference capacity in complex biosamples. Therefore, an AIEgen-based test strip assay could be built as a promising platform for emergency use during the pandemic.
Keywords: lateral flow test strip, aggregation-induced emission, SARS-CoV-2, COVID-19, wearable N95 mask
Graphical abstract

Zhang et al. report a general test strip platform based on AIE luminogens as a reporter for SARS-CoV-2 antigen detection. The assay is a rapid, scale-up detection tool for SARS-CoV-2 screening with high sensitivity and specificity and good reproducibility without the need for expensive machines or highly trained personnel.
Introduction
A new and highly pathogenic coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the ongoing global pandemic of coronavirus disease 2019 (COVID-19).1 , 2 Because of its rapid human-to-human transmission, as of October 28, 2021, over 0.24 billion infected individuals and approximately 5 million deaths worldwide have been reported, collapsing many health care systems and disrupting the normal modern social order on a large scale3, 4, 5 without any decreasing trends. Although vaccines, powerful weapons against SARS-CoV-2, have been developed, they are affected by rapid and serious mutations of the RNA virus,6 and it takes a long time to achieve universal immunization. To avoid large-scale spread of the virus, prompt and accurate diagnosis is still a powerful tool for tracing and isolating infected individuals.7
SARS-CoV-2 is a positive-sense, single-stranded RNA virus with approximate 30,000 bases8 , 9 and four structural proteins, known as spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins.10 The S protein is assembled as a trimer containing approximately 3,900 amino acid residues and has a most crucial role in viral attachment, fusion, and entry.11 , 12 The receptor binding domain (RBD) of the S protein mediates binding with angiotensin-converting enzyme (ACE2), which is composed of approximate 300 amino acid residues.13 The N protein, as the central component of the virion, binds to viral RNA to package the RNA into a ribonucleoprotein complex.8 Other functions of the N protein have also been discovered, such as mRNA transcription and replication,14 , 15 cytoskeleton organization, and immune regulation and response.16, 17, 18
Quantitative reverse-transcriptase PCR (qRT-PCR), the proven clinical gold-standard diagnostic test for SARS-CoV-2 infection,19 has been used widely for screening and offers tremendously reliable reference data for policymakers. With the development of technology, RNA isolation and purification steps have been bypassed when detecting SARS-CoV-2 via qRT-PCR; however, because of temperature cycling, the PCR technique is still a time-consuming and laboratory-based protocol for virus detection.20 Therefore, alternative approaches are urgently needed that can rapidly provide an accurate diagnosis at the point of use.19
Various detection methods have been developed for SARS-CoV-2. A promising strategy is a near-infrared fluorescence-activated molecular probe that could be employed to detect SARS-CoV-2 protease in living mice.21 Viral antigen-based detection could be developed as a complementary screening strategy for early diagnosis of SARS-CoV-2 infection, because N protein, as the predominantly expressed structural protein, has been identified as one of the best early diagnostic targets in SARS-CoV, broke out in 2003, and could be detected before the antibody appears in serum.22, 23, 24 The RBD protein is located on the surface of the virus leading to directly test without virus lysis,25 so it would be a suitable diagnostic epitope for simplifying pretreatment procedures.
Typical AIE luminogens (AIEgens) are often equipped with peripheral phenyl rings leading to 3D twisting of the molecular structure, which greatly reduces intermolecular interactions such as π-π stacking when aggregated and greatly decreases the nonradiative decay of excited states.26, 27, 28 According to the Jablonski diagram, it is competitive between the radiation and nonradiation energy dissipation pathways.29 The emergence of AIEgens offers an opportunity to maximize the absorbed energy flowing to the fluorescence emission pathway.30 AIEgens are desirable organic fluorophores with high brightness and resistance to quenching in aqueous solution.
Here, based on the AIEgen-based lateral flow test strip, we developed a rapid and sensitive assay strategy for detection of the RBD protein and N protein from SARS-CoV-2. As a proof of concept, the AIEgen-based lateral flow test strip assay was first carried out for C-reactive protein (CRP), and the results demonstrated that it could accurately detect CRP with a linear relationship from 0.001–20 μg/mL and an ultralow limit of detection (LOD) of 5.5 ng/mL. Subsequently, two kinds of AIEgen-based lateral flow test strips were fabricated to detect the RBD protein and N protein of SARS-CoV-2. Both of them presented excellent performance with ultralow LODs of 6.9 ng/mL for the RBD protein and 7.2 ng/mL for the N protein. Within 20 min, the test results could be obtained with high sensitivity and specificity; this could serve as an alternative detection tool for SARS-CoV-2 screening, similar to qRT-PCR. We also assembled the test strip on a remodeled N95 mask, which was utilized as a collector to enrich the droplet containing virions or nanoparticles. These results demonstrated an efficient enrichment effect for AIE 2 nanoparticles (NPs). With this collection strategy, the AIEgen-based test strip was able to detect low concentrations of antigens because of its ultrahigh sensitivity and strong anti-interference ability in complex biosamples.
Results and discussion
Synthesis and characterization of AIEgens
Five rationally designed AIE fluorescence molecules equipped with carboxyl groups for linking to the antibodies were synthesized via Knoevenagel condensation between the aldehyde group and the methylene at rhodanine-3-acetic acid31 , 32 (Figure 1A, Schemes S1–S4), whose structures were characterized by 1H-NMR, 13C-NMR and high resolution mass spectrum (HRMS) (Figures S15–S23). The ultraviolet-visible (UV-vis) absorption spectra of five AIEgens were investigated, as shown in Figure 1B, and the absorption peak values ranged from 438–504 nm. The fluorescence (FL) emission spectra of five AIEgens were subsequently measured. There was almost no FL emission or weak emission in DMSO solution, which indicated that the active dynamics of the phenyl ring rotors dissipate energy through nonradiative pathways for the reduced FL emissions. To demonstrate their AIE characteristics, FL spectra were also recorded in DMSO/toluene mixtures with different toluene fractions. When the toluene fraction was less than 70%, there was weak emission. However, by increasing the toluene fraction, the FL intensity of five AIEgens increased dramatically with obvious emission peaks in the range of 550–632 nm, which demonstrated typical AIE properties (Figures 1C–1G). Notably, the αAIE values (the ratio of the maximal FL intensity in the DMSO/toluene mixture [90% toluene] to that in pure DMSO) were 29.4, 155.6, 2.8, 61.4, and 6.5 for AIEs 1, 2, 3, 4, and 5, respectively (Figure 1H), which revealed that AIE 2 had the strongest AIE effect. The relative FL quantum yields (QYs) of AIEs 1, 2, 3, 4, and 5 in the DMSO/toluene mixture (99% toluene) were 8.56%, 73.2%, 5.41%, 57.7%, and 10.2%, respectively (Figure 1I; Figures S1–S6), which were measured by referring to 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran (DCM) as the standard with a 43% QY in MeOH. Furthermore, when utilized as reporters in testing, the photostability of AIEgens would be significant. Therefore, a photostability experiment was carried out with Rose Bengal as a control. As shown in Figure S7, under white light irradiation for 10 min, AIEs 1–5 were very stable, with a negligible decrease in UV absorbance, whereas Rose Bengal was decomposed, which indicated the excellent photostability of AIEs 1–5.
Figure 1.
Design and spectroscopic properties of AIEs 1–5
(A) Chemical structures of AIEs 1–5.
(B) Normalized UV spectra of AIEs 1–5 in DMSO.
(C–G) FL spectra of AIEs 1–5 (10 μM) in toluene (Tol)/DMSO mixtures with different fractions of Tol.
(H) Plot of I/I0 versus Tol fraction. I0 and I are the maximum PL intensities of AIEs 1–5 (10 μM) in pure DMSO and Tol/DMSO mixtures, respectively.
(I) The relative QY of AIEs 1–5, with DCM as the standard reference with a QY of 43% in MeOH.
(J) Electron density distribution of frontier orbitals of AIE 2 and optimized molecular structure.
Density functional theory (DFT) calculations (Figure 1J; Figures S8–S11) revealed that the electron density of the highest occupied molecular orbital (HOMO) was primarily distributed on the triphenylamine (TPA) segments, which are electron-rich groups, and the electron density of the lowest unoccupied molecular orbital (LUMO) was mainly distributed on the rhodanine group, which is an electron-poor moiety. As shown in Figure S12, the ΔEst values were calculated to be 0.0738, 0.4417, 0.0474, 0.4755, and 0.7164 eV for AIEs 1–5, respectively. Because of the highest QY and αAIE values among the five AIEgens, AIE 2 was selected as the indicator to label the antibodies for detecting the recombinant antigens of SARS-CoV-2.
Preparation of AIEgen-labeled antibody
With the brightest AIE 2 in hand, the detection antibodies were labeled through a condensation reaction between the carboxyl group of AIE-COOH and the amino groups of antibodies via the NHS active ester method. Here, AIE 2 was activated by N-hydroxysuccinimide (NHS) and 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) to form the active ester in dry DMSO at room temperature, which was directly employed to label antibodies without any further purification (Figure 2A) and could be stored at −20°C for several weeks. The antibody was dissolved in PBS solution followed by addition of active ester stock solution, and the mixture was vortexed for 30 min to form the AIEgen-labeled antibody (Figure 2B). Finally, to reduce nonspecific binding of the AIEgens with the nitrocellulose (NC) membrane, excess NHS active esters were blocked by blocking buffer (10% BSA in PBS, 0.01 M, pH 7.4). Labeling similarity with the UV and FL spectra of the detection antibodies to that of AIE 2 was demonstrated (Figures 2C and 2D).
Figure 2.
Preparation of AIEgen-labeled detection antibodies
(A) Schematic of the preparation of NHS active ester.
(B) Schematic of antibody labeling with AIE 2.
(C) Normalized UV spectra of detection antibodies of CRP, RBD protein, and N protein in PBS.
(D) Normalized FL spectra of detection antibodies of CRP, RBD protein, and N protein in PBS.
Fabrication of the AIEgen-based lateral flow test strip
The designed test strip comprises a polyvinyl chloride (PVC) backing card, sample pad, NC membrane, and absorption pad (Figure 3A, top). The sandwich structure of the detection antibody/antigen/capture antibody intelligently explained the detection mechanism of the test strip (Figure 3A, center and bottom). The mixture of antigens, detection antibody, and PBS solution was first loaded onto the sample pad, and, under capillary forces, the solution along with the detection antibody/antigen binary complexes was absorbed and migrated along the strip,33 which was subsequently trapped by the capture antibodies pre-coated on the test line of the NC membrane. The FL signal was read out under irradiation with a 365-nm UV lamp. As the solution continued to run, free detection antibodies moved farther and were captured by the secondary antibody to form the control line. If there was no target antigen in the sample, free detection antibodies, which would be absent from the interaction between antigens and capture antibodies, would run past the test line, resulting in the only the control line forming from the reaction between detection antibodies and secondary antibodies.
Figure 3.
Testing mechanism and result determination
(A) Schematic of the configuration and the detection mechanism of AIEgen-based test strips.
(B) Determination of test results.
An illustration of the assay results is presented in Figure 3B. A result was considered negative when only the control line appeared (Figure 3B, left) and positive when both FL signals appeared on the test line and control line (Figure 3B, center). The invalid test results were identified as no line or only one line located in the test zone (Figure 3B, right).
Sensitivity and specificity of the AIEgen-based lateral flow test strip assay for CRP
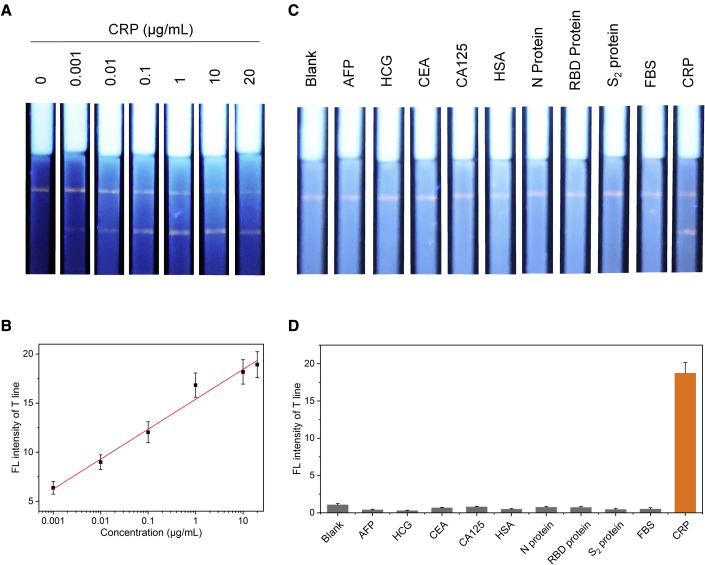
CRP is identified as an acute inflammatory response biomarker in the clinic, and after effective treatment, the CRP concentration decreases;34, 35, 36 the detection methods have been well established based on a lateral flow test strip.37 This was the first study to apply AIEgens as indicators in a test strip assay. First, the detection sensitivity of the AIEgen-based lateral flow test strip assay for CRP was investigated as a proof of concept. One microliter of CRP solution at different concentrations (0, 0.001, 0.01, 0.1, 1, 10, and 20 μg/mL) was mixed with 1 μL of detection antibody solution and then incubated at 37°C for 5 min for sufficient immunoreaction between the antigen and detection antibody. The complex was mixed into 60 μL PBS (0.01 M, pH 7.4, containing 1% BSA and 1% Tween 20) in a 96-well plate, and the CPR test strips were inserted into the solution for 4 min. Without CRP, a negative result was observed; however, a positive result with two lines on the test strip was presented when CRP was present in the sample (Figure 4A), which could be quantified by the FL intensity of the test line. A linear relationship was obtained between 1 ng/mL and 20 μg/mL with a linear correlation coefficient (R2) of 0.99 (Figure 4B), which led to an LOD of 5.5 ng/mL. The specificity of the AIEgen-based lateral flow test strip assay for CRP detection was also evaluated. Samples containing alpha fetoprotein (AFP), human chorionic gonadotropin (HCG), carcinoembryonic antigen (CEA), CA125, human serum albumin (HSA), N protein, RBD protein, S2 protein, and fetal bovine serum (FBS) were used to detect nonspecific interference. Two FL signals were clearly present on the test and control lines only when testing the CRP antigen, and a nonspecific FL signal was hardly detected for other samples (Figure 4C). The FL intensity of the test line for CRP was much higher than those of other samples (Figure 4D), which indicated that the testing strategy dominated the excellent specificity toward the target antigen. Based on the testing results, the strategy has tremendous advantages, such as speed, portability, cost-effectiveness, ease of use, ultrahigh sensitivity, and specificity for CRP testing.
Figure 4.
Sensitivity and specificity of the CPR test strip
(A) Images of the test strips under 365-nm light irradiation for 0, 0.001, 0.01, 0.1, 1, 10, and 20 μg/mL CRP in PBS.
(B) Plot of the FL intensities on the test line, measured by ImageJ software, versus the CRP concentration. The error bars can be defined as the SD calculated by testing the FL intensities of five parallel samples for each sample.
(C) Images of the test strips under 365-nm light irradiation after reaction with samples containing different antigens (AFP, HCG, CEA, CA125, has, N protein, RBD protein, S2 protein, FBS, and CRP at 1 μg/mL).
(D) Quantification of the FL intensities on the test line in (C). The error bars can be defined as the SD calculated by testing the FL intensities of five parallel samples for each sample.
Sensitivity and specificity of the AIEgen-based lateral flow test strip assay for RBD protein
Because of its superior properties, the AIEgen-based lateral flow test strip assay could be expanded to detect other antigens as a reliable and general platform. SARS-CoV-2 infects host cells via the RBD of the S protein, which mediates binding with ACE2 to attack respiratory cells.13 The RBD is present on the outside of the virus. Hence, it would be suitable for a delicate testing strategy for the SARS-CoV-2 RBD whereby all virions could be detected directly without a virus lysis procedure.
Before testing the antigens, the incubation time and incubation temperature of the detection antibody and RBD protein were optimized through orthogonal experimental design, as shown in Figure S13. Under different temperatures (10°C–30°C), the incubation time ranged from 1–5 min. Test strip images were obtained and quantified, which indicated that there was no obvious distinction among the 25 groups. This result demonstrated that the immunoreaction between the detection antibody and antigen was extremely efficient and fast under various working conditions, which was a significant prerequisite for building this kind of assay based on test strips.
As illustrated in Figure 5A, mixtures of 1 μL solution containing RBD protein at different concentrations and 1 μL detection antibody solution were incubated at 37°C for 5 min. The mixtures were added to 60 μL PBS (0.01 M, pH 7.4, containing 1% BSA and 1% Tween 20) in 96-well plates, and the RBD protein test strips were inserted for 4 min. Only the control line was observed without RBD protein, and two lines were displayed in the presence of RBD protein (Figure 5B). This test can be quantified by measuring the brightness of the test line. A linear relationship was obtained in the range of 10 ng/mL to 20 μg/mL with an R2 of 0.99 (Figure 5B). The LOD was determined to be 6.9 ng/mL. In addition, negative results for AFP, CEA, N protein, S2 protein, FBS, and CRP detection indicated the excellent specificity of the test strip (Figure S14).
Figure 5.
Detection of RBD protein and N protein
(A) Images of the test strips under 365-nm light irradiation for 0, 0.001, 0.01, 0.1, 1, 10, and 20 μg/mL RBD protein in PBS.
(B) Plot of the FL intensities on the test line, measured by ImageJ software, versus the RBD protein concentration. The error bars can be defined as the SD calculated by testing the FL intensities of five parallel samples for each sample.
(C) Images of the test strips under 365-nm light irradiation for 0, 0.001, 0.01, 0.1, 1, 10, and 20 μg/mL N protein in PBS.
(D) Plot of the FL intensities on the test line, measured by ImageJ software, versus the N protein concentration. The error bars can be defined as the SD calculated by testing the FL intensities of five parallel samples for each sample.
(E) Images of the test strips under 365-nm light irradiation after reaction with samples containing different antigens (AFP, HCG, CEA, CA125, HSA, S2 protein, RBD protein, FBS, CRP, and N protein at 1 μg/mL).
(F) Quantification of the FL intensities on the test line in (E). The error bars can be defined as the SD calculated by testing the FL intensities of five parallel samples for each sample.
Sensitivity and specificity of the AIEgen-based lateral flow test strip assay for N protein
The N protein not only plays a vital role in transcription and replication of SARS-CoV-2 but is also expressed abundantly; it can be detected after just 1 day of infection.38 It would provide rapid, higher sensitivity when used as a target protein.39
The detection sensitivity of the AIEgen-based lateral flow test strip assay for the N protein from SARS-CoV-2 was investigated. One microliter of N protein in PBS solution at different concentrations (0, 0.001, 0.01, 0.1, 1, 10, and 20 μg/mL) was mixed with 1 μL of detection antibody solution and then incubated at 37°C for 5 min. The mixture was added to 60 μL PBS (0.01 M, pH 7.4, containing 1% BSA and 1% Tween 20) in 96-well plates, and the N protein test strips were inserted for 4 min. Under UV lamp irradiation (365 nm), two bright FL signals were observed in the presence of N protein and only one without N protein (Figure 5C). This test could be quantified by measuring the FL intensity of the test line. A linear relationship was obtained between 1 ng/mL and 20 μg/mL of N protein with a linear correlation coefficient (R2) of 0.98 (Figure 5D), and the LOD was as low as 7.2 ng/mL.
As shown in Figure 5E, the specificity was also evaluated via samples containing AFP, HCG, CEA, CA125, HSA, S2 protein, RBD protein, FBS, and CRP, which clearly showed that only the N protein was specifically bound on the test line, and almost no nonspecific signal was detected for the others. Consequently, the FL intensity of the N protein on the test line was much higher than that of other samples (Figure 5F), which indicated the ultrahigh specificity of the AIEgen-based lateral flow test strip for the N protein.
The strategy was applied successfully to quantitative detection of CRP, N protein, and RBD protein. Our results demonstrated the ability of the AIEgen-based lateral flow test strip assay to detect the target antigens in PBS with high sensitivity and specificity.
A remodeled N95 face mask as a virion collector with high enrichment capacity
Masks, as personal protective equipment (PPE), have huge potential for blocking transmission of pathogens from human to human. The N95 face mask, which is so named because it blocked 95% of very small (0.3 μm) particles during testing,40 was designed to minimize facial seal leakage because of a tight fit and to prevent inhalation of small airborne particles.41 The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have recommended that physicians on the front lines wear the N95 face mask.42 For affected individuals, the virus could be restricted by the N95 face mask, which was also identified as a collector to enrich the particles, such as AIE NPs, viruses, or antigens, for our design.
As a proof of concept, we designed a respiratory tract model, and three holes were drilled at the location of the nose and mouth on the front side as the air outlet (Figure 6A) and one on the bottom as the air inlet (Figure 6B), which could simulate respiration. The test strip was located on the mask. The sample pad, which could adsorb the analytes, was just located on the breather valve pore to seal, as shown in Figures 6C and 6D. The weight of the N95 mask with the test strip is only approximate 10 g, making it easy to wear. To test the enrichment effect, AIE 2 was coprecipitated with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) as a matrix to formulate AIE 2 NPs. Dynamic light scattering (DLS) measurements indicated that AIE 2 NPs had an average hydrodynamic diameter of ∼100 nm (Figure 6F), which was close to the results seen by transmission electron microscopy (TEM) (Figure 6F, inset), and TEM also revealed that AIE 2 NPs had a uniform spherical morphology. Furthermore, the stability of AIE 2 NPs in PBS buffer was also measured by monitoring the size variation using DLS. The average diameter remained nearly the same, and no precipitation was observed after storage at room temperature for 7 days (Figure 6G).
Figure 6.
Design and preparation of the antigen collector by attaching a test strip to an N95 mask
(A and B) Pictures of the respiratory tract model: front (A) and bottom (B).
(C and D) Pictures of the remodeled N95 mask equipped with test strips: front (C) and side (D).
(E) Image of an atomizer spraying AIE 2 NPs PBS solution into the respiratory tract model.
(F) Hydrodynamic diameter distribution, measured by DLS, in PBS buffer (pH 7.4) and transmission electron microscopy (TEM) image (inset) of AIE 2 NPs. Scale bar in the TEM image, 300 nm.
(G) The hydrodynamic diameter of AIE 2 NPs as a function of storage time. The error bars can be defined as the SD calculated by testing the hydrodynamic diameter of three parallel samples for each sample.
The NPs solution was sprayed into the respiratory tract model through an atomizer at 0.5 mL/min inspiratory flow for 5 min (Figure 6E). Then mask images were obtained through the in vivoimaging system (IVIS), as shown in Figure 7A, which indicated that AIE 2 NPs accumulated efficiently around the breather valve pore compared with the mask without NPs enrichment. The test strips were also imaged under IVIS, and it was obvious that AIE 2 NPs were attached to the sample pad (Figure 7B). The respiratory tract model could cause droplets to accumulate efficiently on the sample pad.
Figure 7.
Effect of enrichment of the collector and comparison of the AIEgen-based test strip and two other kinds of test strips
(A) Pictures of the mask before (left) and after (right) spraying the AIE 2 NPs solution.
(B) Pictures of the test strip before (left) and after (right) collecting the AIE 2 NPs solution.
(C) Testing results of three test strips based on colloidal gold (left), FITC (center), and AIEgens (right) at a 50 μg/mL N protein concentration.
(D) The results of the AIEgen-based test strip with a complex biosample containing saliva.
Generality and strong adaptive capacity of the AIEgen-based lateral flow test strip
Based on these results, two other kinds of N protein detection antibodies, which were labeled with colloidal gold and fluorescein isothiocyanate (FITC), were prepared. All three detection antibodies were incubated with N protein to form binary complexes, followed by spraying into the respiratory tract model. Then 60 μL PBS solution (0.01 M, pH 7.4, containing 1% BSA and 1% Tween 20) was dropped onto the sample pad. Four minutes later, two lines were observed only on the AIEgen-based test strip under irradiation with a 365-nm UV lamp (Figure 7C). However, there was single signal on the control line and a negligible signal on the test line for the colloidal gold-based test strip. Because of the typical aggregation-caused quench (ACQ) effect of FITC, almost all FL signals could be quenched in the aqueous system. Therefore, there was no signal on the test line or control line for the FITC-based test strip under 405-nm UV lamp irradiation. In addition, to investigate the anti-interference capability, saliva, which would mainly affect the testing result, was mixed into binary complexes in PBS buffer. To our surprise, the test effect was hardly affected by the presence of saliva (Figure 7D). The results of the AIEgen-based lateral flow test strip assay indicated high sensitivity and strong adaptive capacity to complex environments compared with the other two test strips.
AIEgens, promising indicators with superior properties, including high brightness, resistance to quenching in aqueous solution, and anti-photobleaching, were employed to establish the general lateral flow test strip assay platform. The AIEgen-based lateral flow test strip assay for CRP was carried out as a proof of concept and were used successfully to detect CRP with an LOD of 5.5 ng/mL in PBS buffer with high specificity. Subsequently, two kinds of AIEgen-based lateral flow test strips were fabricated to detect the RBD protein and N protein of SARS-CoV-2; they presented excellent testing behavior with ultralow LODs of 6.9 ng/mL for the RBD protein and 7.2 ng/mL for the N protein. The assay could be performed within 20 min with high sensitivity and specificity and good reproducibility. Without the need for expensive machines or highly trained personnel, it could be an alternative accurate, scale-up detection tool for SARS-CoV-2 screening.
A remodeled N95 mask equipped with a test strip was as a collector for virions and showed an enrichment effect on the sample pad via AIE 2 NPs testing, which will simplify sampling and pretreatment procedures. Compared with colloidal gold- and FITC-based test strips, AIEgen-based test strips had ultrahigh sensitivity and strong anti-interference ability in complex biosamples. The strategy could be expanded to detection of other biomarkers, which would be promising for clinical applications.
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dan Ding (dingd@nankai.edu.cn).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed material transfer agreement.
Fabrication of AIEgen-detection antibody conjugates
First, AIE 2 (1 mg, 1.9 μmol, 1 equiv) was activated by EDC (0.73 mg, 3.8 μmol, 2 equiv) and NHS (0.44 mg, 3.8 μmol, 2 equiv) in 500 μL DMSO with continuous shaking for 30 min at room temperature, without further purification, as a stock solution. One microliter of stock solution was dispersed in the detection antibody (2.5 μg) PBS solution (50 μL 0.01 M, pH 7.4) for approximately 1 hour at room temperature with gentle agitation. After that, the AIEgen-antibody conjugates were blocked with 100 μL of BSA PBS (0.01 M, pH 7.4) at room temperature. Finally, AIEgen-antibody conjugates were dispersed in 200 μL of PBS (0.01 M, pH 7.2) and stored at 4°C until use.
Detection of CRP, RBD protein, and N protein using the AIEgen-based lateral test strip assay
One microliter of antigen solution (CRP, RBD protein, or N protein) and 1 μL of AIEgen-detection antibody conjugate were mixed and then incubated at 37°C for 5 min. The mixture was added to 60 μL of PBS (0.01 M, pH 7.4, containing 1% BSA and 1% Tween 20) in a 96-well plate, and the CPR test strips were inserted into the liquid for 10 min. Pictures of the test strips were taken using a Fujifilm X-A7/XA7 camera under 365-nm UV light irradiation, and then the brightness of the control and test lines was quantified using ImageJ 1.52q. Five replicate measurements were carried out for each concentration.
Acknowledgments
This work was supported by the National Key R&D Program of China (Intergovernmental Cooperation Project, 2017YFE0132200), NSFC (51961160730, 51873092, and 81921004), the Fundamental Research Funds for the Central Universities, and the Tianjin Science Fund for Distinguished Young Scholars (19JCJQJC61200).
Author contributions
D.D., R.T.K.K., and B.Z.T. conceived the project. G.-Q.Z. conducted all experiments and analyzed data. Z.G. assisted with organic synthesis. J.Z. designed the respiratory tract model and assisted with mask imaging. H.O. provided assistance with test strips imaging. H.G. performed the DFT calculations. D.D., B.Z.T., and G.-Q.Z. wrote the paper, and all authors participated in discussions of the manuscript. D.D., R.T.K.K., and B.Z.T. supervised the whole project.
Declaration of interests
The authors declare no competing interests.
Published: January 17, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrp.2022.100740.
Supplemental information
Data and code availability
All data associated with the study are included in the article and Supplemental information.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velavan T.P., Meyer C.G. The Covid-19 epidemic. Trop. Med. Int. Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan T. Genetic diversity and evolution of SARS-Cov-2. Infect. Genet. Evol. 2020;81:104260–1042603. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun F., Ganguli A., Nguyen J., Brisbin R., Shanmugam K., Hirschberg D.L., Wheeler M.B., Bashir R., Nash D.M., Cunningham B.T. Smartphone-based multiplex 30-minute nucleic acid test of live virus from nasal swab extract. Lab. Chip. 2020;20:1621–1627. doi: 10.1039/d0lc00304b. [DOI] [PubMed] [Google Scholar]
- 6.Lauring A.S., Hodcroft E.B. Genetic variants of SARS-CoV-2-what do they mean? JAMA. 2021;325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 7.Ding X., Yin K., Li Z., Lalla R.V., Ballesteros E., Sfeir M.M., Liu C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Comm. 2020;11:4711–4720. doi: 10.1038/s41467-020-18575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y., Du N., Lei Y., Dorje S., Qi J., Luo T., Gao G.F., Song H. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. EMBO J. 2020;39:e105938. doi: 10.15252/embj.2020105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Tortorici M.A., Bosch B.-J., Frenz B., Rottier P.J.M., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620–1631. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandelli A., Monti M., Milanetti E., Armaos A., Rupert J., Zacco E., Bechara E., Ponti R.D., Tartaglia G.G. Structural analysis of SARS-CoV-2 genome and predictions of the human interactome. Nucleic Acids Res. 2020;48:2011270–2011283. doi: 10.1093/nar/gkaa864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuniga S., Cruz J.L., Sola I., Mateos-Gomez P.A., Palacio L., Enjuanes L. Coronavirus nucleocapsid protein facilitates template switching and is required for efficient transcription. J. Virol. 2010;84:2169–2175. doi: 10.1128/JVI.02011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cong Y., Ulasli M., Schepers H., Mauthe M., V’Kovski P., Kriegenburg F., Thiel V., de Haan C.A.M., Reggiori F. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J. Virol. 2020;94 doi: 10.1128/JVI.01925-19. e01925–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surjit M., Liu B., Jameel S., Chow V.T., Lal S.K. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem. J. 2004;383:13–18. doi: 10.1042/BJ20040984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surjit M., Liu B., Chow V.T., Lal S.K. The nucleocapsid protein of severe acute respiratory syndrome-coronavirus inhibits the activity of cyclincyclin-dependent kinase complex and blocks S phase progression in mammalian cells. J. Biol. Chem. 2006;281:10669–10681. doi: 10.1074/jbc.M509233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X., Pan J., Tao J., Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-b response by targeting initial step of IFN-b induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42:37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganguli A., Mostafa A., Berger J., Aydin M.Y., Sun F., Stewart de Ramireze S.A., Valera E., Cunningham B.T., King W.P., Bashir R. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:22727–22735. doi: 10.1073/pnas.2014739117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranoa D.R.E., Holland R.L., Alnaji F.G., Green K.J., Hergenrother P.J. Saliva-based molecular testing for SARS-CoV-2 that bypasses RNA extraction. bioRxiv. 2020 doi: 10.1101/2020.06.18.159434. [DOI] [Google Scholar]
- 21.Liew S., Zeng Z., Cheng P., He S., Zhang C., Pu K. Renal-clearable molecular probe for near-infrared fluorescence imaging and urinalysis of SARS-CoV-2. J. Am. Chem. Soc. 2021;143:18827–18831. doi: 10.1021/jacs.1c08017. [DOI] [PubMed] [Google Scholar]
- 22.Diao B., Wen K., Zhang J., Chen J., Han C., Chen Y., Wang S., Deng G., Zhou H., Wu Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021;27:289.e1–289.e4. doi: 10.1016/j.cmi.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di B., Hao W., Gao Y., Wang M., Wang Y., Qiu L. Monoclonal antibody-based antigen capture enzyme-linked immunosorbent assay reveals high sensitivity of the nucleocapsid protein in acute-phase sera of severe acute respiratory syndrome patients. Clin. Diagn. Lab. Immunol. 2020;12:135–140. doi: 10.1128/CDLI.12.1.135-140.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F., Zhao S., Yu B., Chen Y., Wang W., Song Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J.Q., Tian F., Liu C., Liu Y., Zhao S., Fu T., Sun J.S., Tan W.H. Rapid one-step detection of viral particles using an aptamer-based thermophoretic assay. J. Am. Chem. Soc. 2021;143:7261–7266. doi: 10.1021/jacs.1c02929. [DOI] [PubMed] [Google Scholar]
- 26.Gao H., Zhang X., Chen C., Li K., Ding D. Unity makes strength: how aggregation-induced emission luminogens advance the biomedical field. Adv. Biosys. 2018;2:180074–180101. [Google Scholar]
- 27.Qi J., Chen C., Ding D., Tang B.Z. Aggregation-induced emission luminogens: union is strength, gathering illuminates healthcare. Adv. Healthc. Mater. 2018;7:1800477–1800491. doi: 10.1002/adhm.201800477. [DOI] [PubMed] [Google Scholar]
- 28.Feng G., Liu B. Aggregation-induced emission (AIE) dots: emerging theranostic nanolights. Acc. Chem. Res. 2018;51:1404–1414. doi: 10.1021/acs.accounts.8b00060. [DOI] [PubMed] [Google Scholar]
- 29.Gong Y., Tan Y., Li H., Zhang Y., Yuan W.Z., Zhang Y., Sun J., Tang B.Z. Crystallization-induced phosphorescence of benzils at room temperature. Sci. China Chem. 2013;56:1183–1186. [Google Scholar]
- 30.Ni X., Zhang X., Duan X., Zheng H.-L., Xue X.-S., Ding D. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 2019;19:318–330. doi: 10.1021/acs.nanolett.8b03936. [DOI] [PubMed] [Google Scholar]
- 31.Thamaraiselvi P., Duraipandy N., Kiran M.S., Easwaramoorthi S. Triarylamine rhodanine derivatives as red emissive sensor for discriminative detection of Ag+ and Hg2+ ions in buffer-free aqueous solutions. ACS Sustainable Chem. Eng. 2019;7:9865–9874. [Google Scholar]
- 32.Tejchman W., Orwat B., Korona-Głowniak I., Barbasz A., Kownacki I., Latacz G., Handzlik J., Zesławska E., Malm A. Highly efficient microwave synthesis of rhodanine and 2-thiohydantoin derivatives and determination of relationships between their chemical structures and antibacterial activity. RSC Adv. 2019;9:39367–39380. doi: 10.1039/c9ra08690k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei Z., Qu W., Deng Y., Chu H., Cao J., Xue F., Zheng L., El-Nezamic H.S., Wu Y., Chen W. One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A (BPA) in aqueous samples. Biosens. Bioelectron. 2013;49:457–461. doi: 10.1016/j.bios.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 35.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black S., Kushner I., Samols D. C-reactive protein. J. Biol. Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 37.Hu J., Zhang Z.-L., Wen C.-Y., Tang M., Wu L.-L., Liu C., Zhu L., Pang D.-W. Sensitive and quantitative detection of C-reaction protein based on immunofluorescent nanospheres coupled with lateral flow test strip. Anal. Chem. 2016;88:6577–6584. doi: 10.1021/acs.analchem.6b01427. [DOI] [PubMed] [Google Scholar]
- 38.Sabbih G.O., Korsah M.A., Jeevanandam J., Danquah M.K. Biophysical analysis of SARS-CoV-2 transmission and theranostic development via N protein computational characterization. Biotechnol. Prog. 2020;37:3096–3110. doi: 10.1002/btpr.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S., Shu J., Lyu A., Huang X., Zeng W., Jin T., Cui H. Label-free immunoassay for sensitive and rapid detection of the SARS-CoV-2 antigen based on functionalized magnetic nanobeads with chemiluminescence and immunoactivity. Anal. Chem. 2021;93:14238–14246. doi: 10.1021/acs.analchem.1c03208. [DOI] [PubMed] [Google Scholar]
- 40.Srivastav A.K., Saini V., Kukkar V., Rathore M.S., Khadayat S., Samuel A.J. Safeguarding from COVID-19: educating healthcare workers about the available protective equipment. J. Public Health (Berl) 2021 doi: 10.1007/s10389-021-01530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir. Viruses. 2020;14:365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg M., Garg S.M. Novel coronavirus (COVID-19): N95 mask hysteria. Int. J. Crit. Illn. Inj. Sci. 2020;10:53–55. doi: 10.4103/IJCIIS.IJCIIS_78_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with the study are included in the article and Supplemental information.