Abstract
Purpose
Preliminary data suggest that remdesivir may influence the course of COVID-19 according to the duration of pre-admission symptoms. We aim to evaluate whether early use of remdesivir is associated with a reduced COVID-19 progression in a homogeneous cohort of patients with mild to moderate COVID-19.
Methods
This prospective, observational study included patients with COVID-19 pneumonia treated with remdesivir at the University Hospital of Pisa (Italy) from September 2020 to January 2021. According to national recommendations, remdesivir was prescribed in patients with pneumonia who required oxygen supplementation by nasal cannula or mask but without the need for high-flow nasal cannula, non-invasive or invasive mechanical ventilation and had symptoms from no more than 10 days. Patients who received early (≤5 days from onset of symptoms) versus late (>5 days from onset of symptoms) remdesivir were compared. The primary outcome was a composite of high-flow nasal cannula, non-invasive or invasive mechanical ventilation, or death. A multivariate logistic regression analysis was performed to identify factors independently associated with the composite endpoint.
Findings
Among 312 consecutive patients with COVID-19 pneumonia who received remdesivir, 90 (28.8%) received early remdesivir, whereas 222 (71.2%) received late remdesivir. Twenty-nine patients (32.2%) in the early-remdesivir group versus 104 patients (46.8%) in the late-remdesivir group met the primary end point (P = 0.018). On multivariate analysis, a history of dyspnea at home (odds ratio = 2.53; 95% CI, 1.55–4.12; P < 0.001) was the strongest factor independently associated with the progression to severe COVID-19, whereas early-remdesivir use was a protective factor (odds ratio = 0.49; 95% CI, 0.27–0.87; P = 0.015). The delayed admission to the hospital was associated with a delayed administration of remdesivir.
Implications
The early use of remdesivir (<5 days from symptoms onset) may reduce COVID-19 progression. The identification of patients who need early hospitalization and early remdesivir may provide clinical benefit in patients with COVID-19.
Keywords: COVID-19, COVID-19 progression, early treatment, remdesivir, SARS CoV-2
Introduction
The treatment options for patients with COVID-19 have markedly changed since the beginning of the pandemic, and the management of patients needing hospitalization is still being studied.1 Immunomodulatory therapy with monoclonal antibodies,2 corticosteroids,3 tocilizumab,4 and baricitinib5 was found to reduce mortality in the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial in the so-called hyperinflammatory phase of infection. Conversely, during the initial phase of infection, when viral replication predominates, the only antiviral drug approved for clinical use is remdesivir. Remdesivir has been approved by the Italian Medicines Agency for the treatment of hospitalized patients requiring supplemental oxygen within 10 days of symptoms onset.6 Patients who need high-flow nasal cannula (HFNC), non-invasive ventilation invasive mechanical ventilatory support, or extracorporeal membrane oxygenation (ECMO) are excluded.6 This restriction to remdesivir use is based on the results of published randomized clinical trials.7, 8, 9 However, although it is known that remdesivir decreases the duration of symptoms, it is still questionable whether remdesivir reduces the mortality of patients infected with COVID-19. In fact, the World Health Organization COVID-19 Solidarity Trial for COVID-19 Treatments (SOLIDARITY) did not find a reduction of mortality in patients treated with remdesivir.8 Instead, the Adaptive COVID-19 Treatment Trial (ACTT) found a significant benefit of remdesivir therapy compared with placebo on clinical recovery and mortality in the subgroup of patients requiring low-flow oxygen therapy.9 These controversial results could be explained by the different timing of administration of this antiviral and need to be more investigated. The aim of this study was to evaluate whether early treatment with remdesivir compared with delayed use is associated with improved clinical outcome.
Participants and Methods
Study Design
This prospective, observational study included patients with SARS-CoV-2 infection confirmed by real-time reverse-transcription polymerase chain reaction on nasopharyngeal throat swabs admitted to the University Hospital of Pisa (Italy) during the second wave of the COVID-19 pandemic (September 2020 to January 2021) who received remdesivir. All patients received the same standard of care treatment according to national and local guidelines.5 , 6 , 10 In our hospital, a panel of experts elaborated on a guide for the management of patients with COVID-19, which was regularly updated according to any new release from scientific literature (Supplemental Table I). Patients were prospectively followed up until discharge or death. Data concerning demographic characteristics (age and sex), comorbidities, history of COVID-19 symptoms at home, clinical presentation and laboratory findings on admission at the emergency department (ED), COVID-19 treatment, and outcome were prospectively collected. Noninvasive or invasive ventilatory support was defined as the need for HFNC, noninvasive ventilatory support, or invasive mechanical ventilatory support. The study was conducted according to the principles stated in the Declaration of Helsinki and approved by the local ethical committee of the Area Vasta Nord Ovest of Tuscany region. All patients provided written informed consent. The data sets generated during and/or analyzed during the study are available from the corresponding author on reasonable request.
Study Outcomes
The primary objective of the study was to evaluate the effect of the early treatment with remdesivir (exposure variable) in preventing progression to severe COVID-19 pneumonia. In particular, the research question was to evaluate whether early compared with late treatment with remdesivir is effective in reducing the risk of progression to severe COVID-19, defined as a composite of need of HFNC, noninvasive or invasive mechanical ventilation, or death (main outcome measure).
Treatment Exposure
Intravenous remdesivir was administered (200 mg on the first day and 100 mg once daily from day 2 to day 5), according to the Italian Medicines Agency criteria. Inclusion criteria included radiologic confirmed pneumonia, symptom onset within 10 days, and need of supplemental low-flow oxygen. Exclusion criteria were requirement of HFNV, invasive mechanical ventilatory support, vasoactive drugs, or ECMO. Contraindications included aspartate amino transferase (and alanine amino transferase ≥5 times the normal range values and a glomerular filtration rate <30 m/min.
Patients who received early treatment with remdesivir were defined as those who had initiated use of remdesivir intravenously within the first 5 days from the onset of symptoms. The treatment exposure period was calculated from symptom onset (and not from hospital admission) to mitigate selection bias caused by heterogeneity of treatment initiation days among patients. Patients who had initiated use of remdesivir after 5 days from symptoms onset were included in the control group (late remdesivir group).
Statistical Analysis
According to the study outcome, a comparison between patients who met the primary end point and those who did not was performed. Patients who did not receive remdesivir (according to exclusion criteria), those who received a do not resuscitate order, and those who died within the first 24 hours of hospital admission were excluded from the analysis.
Continuous variables are reported as mean (SD) and medians (interquartile ranges [IQRs]) according to their distribution. The normality of distributions was assessed by the Kolmogorov-Smirnov test. Continuous variables were compared with the t test or the Mann-Whitney U test, as appropriate. Categorical data were expressed as frequency distributions, and the χ2 test or Fisher exact test was used to determine whether differences existed between groups.
A multivariate analysis to identify factors independently associated with the primary end point was performed. Multivariate analysis using logistic regression prediction models was constructed using a forward stepwise procedure, entering all variables with univariate P < 0.05 and those deemed clinically significant. The final multivariate model was chosen according to the Akaike information criterion and to parsimony and clinical interpretability of data. Odds ratio (ORs) (95% CIs) were calculated. Kaplan-Meier curves were built to compare time to disease progression between patients in the early-remdesivir group versus those in the late-remdesivir group. Nonparametric (log-rank) tests were used to compare event-free survival functions in the 2 study groups. A subgroup analysis was also performed to evaluate differences in the primary end point in patients admitted to the hospital within 5 days of onset of symptoms.
P < 0.05 was considered statistically significant. The statistical analysis was conducted using IBM SPSS Statistics software, version 23.0 (IBM Corp., Armonk, New York).
Results
A total of 312 consecutive patients with COVID-19 pneumonia who received remdesivir were included in the study. Median age was 69 years (IQR, 58–81 years). A total of 142 patients (45.5%) were admitted to the hospital within 5 days from onset of symptoms. Ninety patients (28.8%) received remdesivir within <5 days, whereas 222 (71.2%) received remdesivir 5 days after symptom onset. Patients who received early remdesivir were older and more frequently affected by chronic pulmonary disease, chronic heart disease, and cerebrovascular disease (Table O). Conversely, patients who received remdesivir after 5 days from onset of symptoms more frequently had a history of fever and cough at home (Table I ). The 2 study groups were comparable in term of concomitant treatments for COVID-19: the whole population received intravenous corticosteroid therapy (6 mg/d of dexamethasone) and prophylactic dose of low-molecular-weight heparin.
Table I.
Comparison of patients who received remdesivir within 5 days of onset of symptoms and those who received remdesivir after 5 days of onset of symptoms.*
| Characteristic | Early Remdesivir n = 90 (%) |
Late Remdesivir n = 222 (%) |
P |
|---|---|---|---|
| Demographic characteristics Age, median (IQR), ys Male sex |
77 (63–85) 47 (52.2) |
67 (56–77) 150 (67.6) |
<0.001 0.011 |
| Comorbidities Chronic pulmonary disease Hypertension Chronic heart disease Diabetes mellitus Cerebrovascular disease Chronic renal disease Solid neoplasm |
15 (16.9) 41 (45.6) 38 (42.2) 26 (28.9) 19 (21.1) 6 (6.7) 12 (13.3) |
17 (7.7) 105 (47.3) 56 (25.2) 48 (21.6) 15 (6.8) 9 (4.1) 17 (7.7) |
0.017 0.780 0.003 0.172 <0.001 0.382 0.118 |
| Charlson Comorbidity Index score, median (IQR) | 1 (1–2.75) | 1 (0–2) | 0.140 |
| Symptoms before admission Temperature >37.5°C Cough Asthenia Dyspnea Diarrhea Myalgias |
45 (50) 24 (26.7) 24 (26.7) 39 (43.3) 10 (11.1) 17 (18.9) |
169 (76.1) 101 (45.5) 58 (26.1) 96 (43.2) 28 (12.6) 31 (14) |
<0.001 0.002 0.922 0.988 0.713 0.275 |
| Clinical features on admission Temperature >37.5°C P/F ratio <300 Lymphocytes <800 cells/mm3 Platelets <150 × 103/μL D-dimer >1000 ng/mL (n = 176) C-reactive protein mg/dl, median (IQR) C-reactive protein >5 mg/dL Procalcitonin, median (IQR), ng/mL LDH >250 U/L (n = 229) |
45 (50) 41 (45.6) 39 (43.3) 34 (38.2) 21/53 (39.6) 4.7 (2.2–9) 41 (45.6) 0.09 (0.07–1.9) 35/63 (55.6) |
169 (76.1–) 124 (55.9–) 104 (46.8–) 53 (24.1–) 27/123 (22) 5.6 (2.6–9.7) 125 (56.3) 0.09 (0.06–0.15) 112/166 (67.5) |
<0.001 0.099 0.573 0.013 0.016 0.098 0.085 0.685 0.093 |
| COVID-19 treatment during hospital stay Corticosteroids Low-molecular-weight heparin Plasma Baricitinib |
90 (100) 87 (96.7) 16 (17.8) 8 (8.9) |
221 (99.5) 215 (96.8) 44 (19.8) 38 (17.1) |
0.524 0.935 0.678 0.063 |
IQR = interquartile range; LDH = lactate dehydrogenase; P/F ratio = ratio of inspired oxygen to pulse saturation to fraction of inspired oxygen.
Data are presented as number (percentage) of patients unless otherwise indicated.
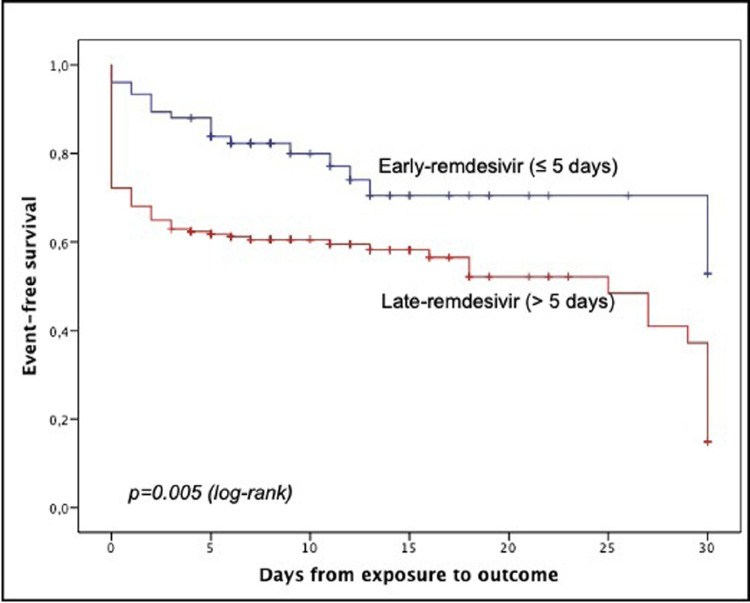
Twenty-nine patients (32.2%) in the early-remdesivir group versus 104 patients (46.8%) in the late-remdesivir group had a progression to severe COVID-19 (P = 0.018). Patients who met the primary end point versus those who did not are compared in Table II . Compared with patients who did not progress to severe COVID-19, those who met the primary end point more frequently had a history of fever and dyspnea before ED admission, a ratio of inspired oxygen to pulse saturation (Pio 2) to fraction of inspired oxygen (Fio 2) <300, and C-reactive protein level >5 mg/dL at ED admission. With regard to therapies, patients who had no disease progression were more commonly treated with early remdesivir. On multivariate analysis, history of dyspnea before ED admission (OR = 2.53; 95% CI, 1.55–4.12; P < 0.001), a Pio 2/Fio 2 ratio <300 (OR = 2.22; 95% CI, 1.35–3.63; P = 0.002), C-reactive protein level >5 mg/dL at admission (OR = 1.66; 95% CI, 1.01–2.72; P = 0.044), and age (OR = 1.02; 95% CI, 1.003–1.04; P = 0.025) were factors independently associated with the progression to severe COVID-19, whereas early-remdesivir administration was the only protective factor (OR = 0.49; 95% CI, 0.27–0.87; P = 0.015) (Table III ). Kaplan-Meier survival curves found a statistically significant beneficial effect of early-remdesivir compared with late-remdesivir treatment on COVID-19 progression-free survival (Figure ).
Table II.
Comparison of patients with versus without disease progression.*
| Characteristic | No Disease Progression n = 179 (%) | Disease Progression n = 133 (%) | P |
|---|---|---|---|
| Demographic characteristics Age, median (IQR), y Male sex |
67 (56–81) 112 (62.6) |
72 (61–81) 85 (63.9) |
0.038 0.808 |
| Comorbidities Chronic pulmonary disease Hypertension Chronic heart disease Diabetes mellitus Cerebrovascular disease Chronic renal disease Solid neoplasm |
12 (6.7) 74 (41.3) 59 (33) 46 (25.7) 23 (12.8) 8 (4.5) 20 (11.2) |
20 (15) 72 (54.1) 35 (26.3) 28 (21.1) 11 (8.3) 7 (5.3) 9 (6.8) |
0.015 0.025 0.206 0.340 0.199 0.746 0.185 |
| Charlson Comorbidity Index score, median (IQR) | 1 (0–3) | 1 (0–2) | 0.226 |
| Symptoms before admission Temperature >37.5°C Cough Asthenia Dyspnea Diarrhea Myalgias |
113 (63.1) 64 (35.8) 43 (24) 59 (33) 20 (11.2) 24 (13.4) |
101 (75.9) 61 (45.9) 39 (29.3) 76 (57.1) 18 (13.5) 24 (18%) |
0.016 0.071 0.293 <0.001 0.528 0.262 |
| Clinical features on admission Temperature >37.5°C P/F ratio <300 Lymphocytes <800 cells/mm3 Platelets <150 × 103/μL D-dimer >1000 ng/ml (n = 176) C-reactive protein, median (IQR), mg/dL C-reactive protein >5 mg/dL Procalcitonin, median (IQR), ng/mL LDH >250 U/L (n = 229) |
72 (40.2) 77 (43) 77 (43) 51 (28.7) 30/106 (28.3) 4.6 (2.1–8.5) 81 (45.3) 0.09 (0.06–0.15) 80/132 (60.6) |
61 (45.9) 88 (66.2) 66 (49.6) 36 (27.5) 18/70 (25.7) 6.5 (3.2–10.3) 85 (63.9) 0.1 (0.07–1.75) 67/97 (69.1) |
0.319 <0.001 0.247 0.821 0.706 <0.001 0.001 0.119 0.187 |
| COVID-19 treatment during hospital stay Early remdesivir (≤5 days from symptoms) Corticosteroids Low-molecular-weight heparin Plasma Baricitinib |
61 (34.1) 178 (99.4) 171 (95.5) 45 (25.1) 7 (3.9) |
29 (21.8) 133 (100) 131 (98.5) 15 (11.3) 39 (29.3) |
0.018 0.388 0.141 0.002 <0.001 |
IQR = interquartile range; LDH = lactate dehydrogenase; P/F ratio = ratio of inspired oxygen to pulse saturation to fraction of inspired oxygen.
Data are presented as number (percentage) of patients unless otherwise indicated.
Table III.
Multivariate logistic regression of factors independently associated with disease progression.*
| Factor | OR (95% CI) | P |
|---|---|---|
| Early remdesivir (≤5 days from symptoms) | 0.49 (0.27–0.87) | 0.015 |
| P/F ratio <300 on admission | 2.22 (1.35–3.63) | 0.002 |
| History of dyspnea at home | 2.53 (1.55–4.12) | <0.001 |
| Age | 1.02 (1.003–1.04) | 0.025 |
| C-reactive protein >5 mg/dL on admission | 1.66 (1.01–2.72) | 0.044 |
OR = odds ratio; P/F ratio = ratio of inspired oxygen to pulse saturation to fraction of inspired oxygen.
The multivariate model was performed using a forward stepwise procedure. Variables entered into the regression but not retained were age, chronic pulmonary disease, hypertension, temperature >37.5°C, and dyspnea before hospital admission, P/F ratio <300 on admission, and C-reactive protein >5 mg/dL (variables with statistical significance at the univariate comparison are reported in Table II).
Figure.
Kaplan-Meier analysis of disease progression between patients who received remdesivir within 5 days of symptoms onset and those who did not.
Factors associated with a delayed hospital admission are reported in Supplemental Table II. Compared with patients admitted to the hospital after 5 days from symptom onset, patients who were admitted within 5 days were older, were more frequently resident in a long-term care facility, and were more commonly affected by chronic heart disease. Conversely, those admitted later with respect to the onset of symptoms more frequently had fever and cough before hospital admission and parameters of inflammations (increased C-reactive protein and elevated D-dimer value) at ED admission. Patients admitted within the first 5 days of hospital admission more frequently received early remdesivir treatment (P < 0.001).
The subgroup analysis found that, among 142 patients admitted to the hospital within 5 days of onset of symptoms, the progression to severe COVID-19 occurred in 29 of 90 patients (32.2%) who received early remdesivir compared with 24 of 52 patients (46.2%) who received late remdesivir (P = 0.098) (Supplemental Table III). Patients with and without disease progression are compared in Table IV . The time from symptoms onset to the start of remdesivir therapy was longer in patients who had disease progression compared with those who did not (5 [IQR, 3–7] vs 4 [IQR, 2–6] days, P = 0.007).
Table IV.
Comparison of patients with versus without disease progression in the subgroup of patients admitted to the hospital within 5 days of onset of symptoms.*
| Characteristic | No Disease Progression n = 53 (%) | Disease Progression n = 89 (%) | P |
|---|---|---|---|
| Demographic characteristics Age, median (IQR), ys Male sex |
77 (66–84.5) 30 (56.6) |
72 (57.7–84) 54 (60.7) |
0.322 0.633 |
| Comorbidities Chronic pulmonary disease Hypertension Chronic heart disease Diabetes mellitus Cerebrovascular disease Chronic renal disease Solid neoplasm |
12 (22.6) 31 (58.5) 21 (39.6) 10 (18.9) 6 (11.3) 5 (9.4) 5 (9.4) |
9 (10.2) 37 (41.6) 36 (40.4) 26 (29.2) 17 (19.1) 4 (4.5) 10 (11.2) |
0.04 0.051 0.923 0.170 0.224 0.243 0.735 |
| Charlson Comorbidity Index score, median (IQR) | 1 (1–4) | 1 (0.5–2.5) | 0.201 |
| Symptoms before admission Temperature >37.5°C Cough Asthenia Dyspnea Diarrhea Myalgias |
38 (71.7) 15 (28.3) 13 (24.5) 32 (60.4) 5 (9.4) 10 (18.9) |
50 (56.2) 24 (27) 19 (21.3) 26 (19.2) 10 (11.2) 12 (13.5) |
0.065 0.863 0.661 <0.001 0.735 0.391 |
| Clinical features on admission Temperature >37.5°C P/F ratio < 300 Lymphocytes <800 cells/mm3 Platelets <150 × 103/μL D-dimer >1000 ng/ml (n = 176) C-reactive protein, median (IQR), mg/dL C-reactive protein >5 mg/dL Procalcitonin, median (IQR), ng/mL LDH >250 U/L (n = 106) |
26 (49.1) 33 (62.3) 24 (45.3) 16 (30.8) 12/31 (38.7) 6.6 (3.2–11.3) 33 (62.3) 0.09 (0.06–0.2) 26/38 (68.4) |
31 (34.8) 34 (38.2) 32 (36) 28 (31.5) 19/55 (34.5) 3.5 (1.7–5.8) 29 (32.6) 0.09 (0.06–0.2) 37/68 (54.4) |
0.094 0.005 0.271 0.932 0.699 0.005 0.001 0.786 0.159 |
| COVID-19 treatment during hospital stay Early remdesivir (<5 days from symptoms) Corticosteroids Low-molecular-weight heparin Plasma Baricitinib |
61 (68.5) 53 (100) 7 (13.2) 21 (23.6) 4 (4.5) |
29 (54.7) 89 (100) 21 (23.6) 7 (13.2) 6 (11.3) |
0.098 – 0.132 0.132 0.175 |
| Time from symptom onset to the start of remdesivir therapy, median (IQR), d | 4 (2–6) | 5 (3–7) | 0.007 |
IQR = interquartile range; LDH = lactate dehydrogenase; P/F ratio = ratio of inspired oxygen to pulse saturation to fraction of inspired oxygen.
Discussion
We found that early treatment with remdesivir (within the first 5 days of onset of symptoms) is associated with lower risk of progression to severe disease in hospitalized patients with COVID-19 pneumonia. Notably, in our study population the concomitant treatments for COVID-19 were well standardized, and all patients received the same concomitant therapies, supporting the robustness of our observations. Our main finding was that history of dyspnea at home was the factor more strongly associated with poor outcome, whereas remdesivir within the first 5 days of onset of symptoms was associated with a 51% of reduction of disease progression.
Our study suggests 2 important observations. First, the identification of patients with COVID-19 who can benefit from early admission to the hospital has a pivotal importance, and a delayed decision to hospitalize patients at high risk of progression might preclude them from the opportunity to receive potentially life-saving treatments. We highlighted that patients admitted within the first 5 days of onset of symptoms were older, more commonly came from long-term care facilities, and more frequently had heart disease compared with patients who were admitted later. These findings underline the awareness of physicians on age, comorbidities, and frailty of the patients. However, other factors, such as the presence of fever and cough at home and inflammatory status, were underestimated and associated with a delayed decision to hospitalize the patients. The delayed hospitalization was obviously associated with late use of remdesivir and, ultimately, an increased probability of disease progression.
Second, the published randomized clinical trials on remdesivir did not provide exhaustive and concordant evidence about the efficacy of the antiviral in all patients with COVID-19.7, 8, 9 , 12 , 13 A great heterogeneity among the different studies and the different timing of remdesivir administration according to the onset of symptoms may have partially explain this discrepancy. Conversely, real-life data seem to support our observation. In fact, our study is in line with a recent study that evaluated the effect of remdesivir according to preadmission symptom duration in patients with COVID-19.11 In this study, Garcia-Vidal et al11 found that patients with ≤3 days and 4 to 6 days from symptom onset to admission had a 2.5- and 1.5-fold higher risk of death, respectively. Moreover, remdesivir was associated with 62% reduced odds of death versus standard of care: this benefit was observed in patients who were admitted within the first 6 days but not in those admitted later to the hospital.11 A recent study comparing 28.855 patients who received remdesivir with matched controls who did not found that within 2 days of hospitalization remdesivir was associated with a reduction in mortality at 14 days and 28 days.14 This benefit was confirmed also in special categories, such as patients who underwent invasive mechanical ventilatory support and ECMO.14 In line with this study, our findings provide further support that early start of remdesivir therapy may be a foundational treatment approach for COVID-19.
A Phase III randomized clinical trial that evaluated the role of remdesivir in an outpatient setting has been recently completed, and preliminary results found that remdesivir reduced the risk of hospitalizations by 87% compared with placebo in this category of patients.15 Similarly, a recent territory-wide retrospective study found that early treatment with remdesivir was associated with a lower risk of in-hospital death.16 The authors found that early initiation of 5-day remdesivir treatment within 2 days of admission was associated with significantly shorter time to clinical improvement, low viral load and positive IgG antibody, a shorter hospital length of stay, and a lower risk of in-hospital death compared with not using the antiviral drug.16 Their suggestion is to extend the use of remdesivir to patients with moderate disease but not to require oxygen therapy on admission.16 In this study, the treatment exposure period was set at 2 days from hospital admission. Conversely, we defined the early treatment with remdesivir as the receipt of remdesivir within 5 days of symptom onset. This time point may be more appropriate for the following reasons: (1) the choice of start of symptoms as day 0 may ensure more homogeneity among patients; (2) it may better reflect the initial phase of infection, where viral replication predominates; and (3) the use of remdesivir within 5 days of symptoms onset may be feasible in the clinical practice.
Our study has some limitations. First, the sample size is relatively small. However, the study population is homogeneous because all patients had the same criteria to receive remdesivir and were treated with the same standard of care defined at a national level and applied in our hospital. Second, data about the serologic status and the viral load before the start of remdesivir are lacking but may provide important information about the phase of the COVID-19 disease. Third, as for the observational nature of the study, the 2 groups of patients who received early and late remdesivir differ in terms of age and baseline comorbidities. However, because patients in the early remdesivir group were older and had more comorbidities, you can expect that these patients had a high risk of progression to severe COVID-19. To overcome this potential selection bias, we performed a multivariate analysis that considered all factors that potentially influence patients’ outcome and a subgroup analysis that evaluated the role of early remdesivir in the subgroup of patients admitted to the hospital within 5 days of onset of symptoms.
In conclusion, in our study remdesivir was useful in reducing COVID-19 disease progression when started within the first 5 days from onset of symptoms. The identification of patients who need early hospitalization, the decision of the most appropriate setting of care, and the early start of the remdesivir therapy in patients during the first phase of infection may provide clinical benefit in patients with COVID-19.
Acknowledgments
Acknowledgments
We thank the participants of the study. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Author contributions are as follows: Marco Falcone, Lorenzo Roberto Suardi, Giusy Tiseo, and Francesco Menichetti conceived of and designed the study; Lorenzo Roberto Suardi, Chiara Barbieri, Lisa Giusti, Valentina Galfo, Arianna Forniti, Claudio Caroselli, Leonardo Della Sala, Sara Tempini, and Chukwuma Okoye2 collected data; Lorenzo Roberto Suardi and Giusy Tiseo performed the statistical analysis; Marco Falcone, Lorenzo Roberto Suardi, and Giusy Tiseo drafted the manuscript; and Marco Falcone, Fabio Monzani, and Francesco Menichetti revised the manuscript. All authors contributed to the critical revision of the final manuscript.
Funding Sources
We received unconditional support from Gilead Sciences to collect and analyze data.
Conflicts of Interest
Francesco Menichetti received grants and speaker honoraria from Angelini, Shionogi, Gilead, Menarini, and Nordic Pharma. Marco Falcone has participated in advisory boards and/or received speaker honoraria from Angelini, Correvio, Merck Sharp & Dohme, Nordic Pharma, Pfizer, Astellas, Gilead, Bristol-Myers Squibb, Janssen, ViiV, bioMerieux, Biotest, Becton Dickinson, Pfizer, and Shionogi. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clinthera.2022.01.007.
Appendix. Supplementary materials
References
- 1.Mussini C, Falcone M, Nozza S, et al. Therapeutic strategies for severe COVID-19: a position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT) Clin Microbiol Infect. 2021;27:389–395. doi: 10.1016/j.cmi.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falcone M, Tiseo G, Valoriani B, et al. efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe covid-19 and role of variants of concern. Infect Dis Ther. 2021:1–10. doi: 10.1007/s40121-021-00525-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stebbing J, SánchezNievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7:eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AIFA recommendations. Medicines usable for treatment of COVID-19 disease. Remdesivirin the treatmentof adult patientswith COVID-19. Update: 24 November 2020(first publication: 18 September 2020). Available at: https://www.aifa.gov.it/documents/20142/1267737/Remdesivir_EN_24.11.2020.pdf. Last accessed on: 26th September 2021.
- 7.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for covid-19 - interim who Solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigel JH, Tomashek KM, Dodd LE, et al. ACTT-1 study group members. remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcone M, Tiseo G, Barbieri G, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021 Sep 2:dkab321. doi: 10.1093/jac/dkab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. Epub 2020 Apr 29. Erratum in: Lancet. 2020;395:1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalil AC, Patterson TF, Mehta AK, et al. ACTT-2 study group members. baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab875. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2021 Dec 22 doi: 10.1056/NEJMoa2116846. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CKH, Lau KTK, Au ICH, Xiong X, Lau EHY, Cowling BJ. Clinical improvement, outcomes, antiviral activity, and costs associated with early treatment with remdesivir for patients with COVID-19. Clin Infect Dis. 2021 Jul 15:ciab631. doi: 10.1093/cid/ciab631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.