Abstract
Background
The Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study was launched in 2013 to evaluate the complex interactions of genetic and environmental factors in multifactorial diseases. The present study describes the maternal baseline profile and perinatal data of participating mothers and infants.
Methods
Expectant mothers living in Miyagi Prefecture were recruited from obstetric facilities or affiliated centers between 2013 and 2017. Three sets of self-administered questionnaires were collected, and the medical records were reviewed to obtain precise information about each antenatal visit and each delivery. Biospecimens, including blood, urine, umbilical cord blood, and breast milk, were collected for the study biobank. The baseline maternal sociodemographic characteristics, results of screening tests, and obstetric outcomes were analyzed according to the maternal age group.
Results
A total of 23,406 pregnancies involving 23,730 fetuses resulted in 23,143 live births. Younger maternal participants had a tendency toward a higher incidence of threatened abortion and threatened premature labor, while older age groups exhibited a significantly higher rate of low lying placenta, placenta previa, gestational diabetes, and hypertensive disorders of pregnancy.
Conclusions
The present study clearly shows the distribution of maternal baseline characteristics and the range of perinatal outcomes according to maternal age group. This cohort study can provide strategic information for creating breakthroughs in the pathophysiology of perinatal, developmental, and noncommunicable diseases by collaborative data visiting or sharing.
Key words: baseline profile, perinatal outcome, birth cohort, developmental origins of health and disease
INTRODUCTION
Noncommunicable diseases (NCDs) are defined as chronic diseases caused by the interaction of genetic, physiologic, environmental, and behavioral factors.1,2 Accumulating evidence for the developmental origins of health and disease (DOHaD)3,4 suggests that the intrauterine environment plays an important role of the onset of NCDs, mainly through effects on the maternal or placental factors. Affected fetuses develop NCDs later in life, including developmental, psychologic, cardiovascular, endocrinologic, and respiratory disorders.1,2 To uncover the etiology and pathophysiology of these NCDs, several large-scale birth-cohort studies have been established.5–10 Recent cohort studies have been focused on improving statistical power by maximizing the number of participants or by adding familial information from several generations.11,12
In 2011, the Great East Japan Earthquake (GEJE) and Tsunami destroyed wide areas of the Tohoku region, including Miyagi, Iwate, and Fukushima prefectures. Nine years have passed since the earthquake, and the official toll is 19,689 people confirmed dead and 2,563 still missing.13 Perinatal medical support systems were also affected by the GEJE,14 leading to concerns about short- and long-term health problems in mothers and infants.15,16 The Tohoku Medical Megabank Project (TMM), established by the Tohoku Medical Megabank Organization (ToMMo) and the Iwate Tohoku Medical Megabank Organization, was started in February 2012 to provide maximal efforts to reconstruct damaged health care services in areas severely affected by the GEJE.17,18 The TMM Birth and Three-Generation Cohort Study (BirThree Cohort Study) was launched in 2013 to evaluate the complex interactions of genetic and environmental factors using information about in utero and subsequent pediatric exposures, and about maternal, pediatric, and family outcomes by evaluating a birth cohort and members of three generations.19 The detailed study design has been reported elsewhere19; briefly, expectant mothers were recruited from obstetric facilities, with subsequent recruitment of their family members.
Advanced maternal age at delivery has been raised as one of serious issues in perinatal medicine. In the last decades, rate of pregnancies with advanced age has increased steadily in developed countries.20 Recent reports suggested that advanced maternal age was related to obstetric outcomes such as preeclampsia, gestational diabetes, placenta previa and fetal growth restriction.21 However, landscape obstetric outcomes in large scale cohort studies have not been precisely demonstrated, especially in Japan.
We aim to analyze the maternal baseline information and perinatal outcomes in mothers and newborns by maternal age group. The present paper would provide strategic information for future integrative association studies on a variety of perinatal, developmental, health, and disease issues, including NCDs.
METHODS
Study participants
The detailed protocol of the TMM BirThree Cohort Study has been reported elsewhere.19 A sample size of approximately 70,000 participants was calculated based on the requirements for a multipurpose research platform that included genetic analysis,17 the number of births in Miyagi Prefecture, the estimated number of incident disease cases, feasibility and costs.19 In the TMM BirThree Cohort Study, all obstetric facilities in the prefecture were asked to participate for the study. And seven community support centers, for the voluntary admission-type recruitment and health assessment of participants, were newly established to cover wide areas of inland and coast side of Miyagi Prefecture.17 And pregnant women were recruited in an early stage of pregnancy from approximately 50 obstetric clinics and hospitals, and from 7 community support centers in Miyagi Prefecture, between 2013 to 2017. Several women were recruited during multiple pregnancies across the study period, as previously reported.19 Certified genome medical research coordinators (GMRCs) provided precise information on the TMM BirThree Cohort Study to potential participants and obtained written informed consent from each participant.22
Assessments during the prenatal period
Self-administered questionnaires were designed by referring to ongoing cohort studies.19 Participants were provided with three questionnaires: at enrolment, during midpregnancy, and 1 month after delivery.19 The first questionnaire included baseline and family information, reproductive and medical history, medication history, and assessments of smoking and alcohol consumption, physical activity, sleep, employment history, mental health, and eating habits and nutrition. The second questionnaire included assessments of the participants’ living environment, social connections, socioeconomic status, and personality. The third questionnaire included assessments of mental health and information about the newborn, including its living environment.19 Questionnaires were also administered to family participants, including fathers, grandparents, great-grandparents, and siblings of the included infants.
Medical records
Medical and health care information was collected by transcribing medical records from obstetric clinics and hospitals. In Japan, health checkups for pregnant women are supported by the government, and 14 perinatal visits are designated to check maternal body weight, blood pressure, and urinalysis, and to conduct necessary screening for blood type, irregular antibodies, infection, cervical cancer, and anemia, and to assess the fetal heart rate.23 Fetal ultrasound examination included measurement of the biparietal diameter, abdominal circumference (or fetal trunk area), femur length, estimated fetal body weight, and amniotic fluid index (or vertical pocket depth). The records from each antenatal visit and the birth information were collected by GMRCs and transferred to the TMM integrated database with strict security precautions.24
Maternal baseline profiles were taken from the medical records or questionnaire data and included maternal age, parity, prepregnancy body mass index (BMI), smoking status, alcohol consumption, any fertility treatment for the current pregnancy, educational background, occupation, and annual household income. Information on obstetric complications were collected from the medical records and included hyperemesis, threatened abortion, threatened premature labor, placenta previa, hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), placental abruption, nonreassuring fetal status (NRFS), fetal growth restriction (FGR), intrauterine fetal demise (IUFD), premature rupture of membranes (PROM), chorioamnionitis, oligohydramnios, polyhydramnios, atonic bleeding, and the hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. The chorionicity of multiple gestations was also recorded. Clinical diagnosis was transcribed from medical record data and doctor’s diagnosis was conducted according to the Japan Society of Obstetrics and Gynecology Guideline.25
Birth information, transcribed from medical record data, included the mode of delivery, blood loss within 2 hours after delivery, status of labor induction, and history of blood transfusion. The birth profile of newborns included the gestational age at delivery, sex, birth weight, birth height, head circumference, chest circumference, placental weight, Apgar scores, pH of the umbilical artery (UApH), and admission to the neonatal intensive care unit (NICU). Medical record data and questionnaire data both collected for each participant and stored separately. When the medical record data and questionnaire data did not match, data cleaning committee in our organization would add flags on each data and decided appropriate data for the analysis.
Biospecimens
Biospecimens were collected for the TMM biobank.26 Blood and urine samples were collected at same time as the three questionnaires were administered. Additionally, umbilical cord blood was collected at birth, and breast milk was collected at the routine visit 1 month after delivery. Family members were also asked to provide blood and urine samples. Laboratory data were obtained from the medical records and including screening syphilis testing (rapid plasma reagin [RPR], treponema pallidum hemagglutination [TPHA]), and testing for hepatitis B virus (HBV), hepatitis C virus (HCV), HIV, rubella, human t-cell leukemia virus type 1, toxoplasmosis, and herpes simplex virus (HSV). As previously reported, bioresources were anonymized and transferred to the TMM biobank under an extremely high level of security.24,26
Ethical issues
As previously reported, the protocol for the TMM BirThree Cohort Study was approved by the Ethics Committee of ToMMo (2013-1-103-1).19 The present study was conducted in accordance with the Declaration of Helsinki27 and the Ethical Guidelines for Human Genome/Gene Analysis Research.28 Broad and continuing consent was required for participation in the cohort.17 Written informed consent was obtained from all participants as described above. For participants lacking the ability to understand the study protocol, informed consent from the participant’s guardian was obtained.
Statistical analysis
We analyzed the characteristics of mothers and newborns by maternal age group: ≤24 years of age, 25–29 years of age, 30–34 years of age, 35–39 years of age, and ≥40 years of age. Maternal characteristics, results of maternal infection screening tests, and maternal and birth outcomes stratified by maternal age were compared using Cochran-Armitage trend test and linear regression analysis, as appropriate.
All data fixed in our database as of February 1, 2019, were used in the present study.
RESULTS
The final number of participants in the BirThree Cohort Study was 22,493 mothers, 23,143 newborns, 9,459 fathers, 5,941 grandparents of mothers, 2,117 grandparents of fathers, and 1,553 other family members. Selected perinatal baseline data were reported separately.19 Maternal baseline characteristics by age group are shown in Table 1. Of the 23,406 pregnancies, 9.4% were in women ≤24 years of age, 27.5% were in women 25–29 years of age, 35.8% were in women 30–34 years of age, 21.5% were in women 35–39 years of age, and 5.9% were in women ≥40 years of age. There was a high prevalence of prepregnancy BMI <18.5 kg/m2 group in younger participants (P < 0.0001). Some women required fertility treatment to achieve the current pregnancy; ovulation induction was used in 1.2%, artificial insemination in 0.9%, in vitro fertilization (IVF) in 2.9%, and intracytoplasmic sperm injection (ICSI) in 1.2%. The older age groups tended to use more AIH, IVF, and ICSI (P < 0.0001). Higher education levels (≥13 years) were noted in 66.9% of participants, and 73.3% had an occupation. The annual household income was 2 to 4 million Japanese yen per year in 32.6%, and 4 to 6 million in 32.5%.
Table 1. Maternal baseline characteristics.
| Variables | n a | Total | Age at enrollment, years | P for trend | ||||
|
| ||||||||
| ≤24 | 25–29 | 30–34 | 35–39 | ≥40 | ||||
|
| ||||||||
| % | % | % | % | % | % | |||
| Number | 23,406 | 2,192 | 6,427 | 8,370 | 5,027 | 1,390 | ||
| Parity | 23,068 | |||||||
| 0 | 46.9 | 70.9 | 55.3 | 41.4 | 35.9 | 42.3 | <0.0001 | |
| 1 | 35.4 | 25.0 | 32.5 | 38.3 | 39.0 | 33.7 | <0.0001 | |
| ≥2 | 17.7 | 4.1 | 12.2 | 20.3 | 25.2 | 23.9 | <0.0001 | |
| Prepregnancy BMI, kg/m2 | 23,406 | |||||||
| <18.5 | 20.1 | 28.4 | 21.1 | 17.9 | 16.0 | 29.9 | <0.0001 | |
| 18.5 to <25 | 68.8 | 63.3 | 68.4 | 71.0 | 70.7 | 58.9 | 0.25 | |
| 25 to <30 | 8.3 | 6.3 | 7.8 | 8.0 | 10.4 | 8.1 | <0.0001 | |
| ≥30 | 2.8 | 2.0 | 2.6 | 3.1 | 2.9 | 3.1 | 0.022 | |
| Smoking status | 22,129 | |||||||
| Never smoker | 59.8 | 54.9 | 62.0 | 61.5 | 56.1 | 60.0 | 0.12 | |
| Past smoker before pregnancy | 23.5 | 15.0 | 19.2 | 24.2 | 30.4 | 26.9 | <0.0001 | |
| Past smoker after pregnancy | 14.2 | 26.0 | 16.3 | 12.0 | 11.3 | 10.3 | <0.0001 | |
| Current smoker | 2.5 | 4.1 | 2.4 | 2.4 | 2.2 | 2.8 | 0.0056 | |
| Alcohol consumption | 22,151 | |||||||
| Current drinker | 19.4 | 14.0 | 18.7 | 20.1 | 21.4 | 19.6 | <0.0001 | |
| Past drinker | 34.6 | 34.5 | 34.7 | 34.0 | 34.4 | 39.9 | 0.16 | |
| Never drinker | 40.3 | 45.5 | 41.3 | 40.0 | 38.5 | 34.4 | <0.0001 | |
| Constititionally never drinker | 5.8 | 5.9 | 5.4 | 5.9 | 5.8 | 6.1 | 0.53 | |
| Fertility treatmentb | 23,083 | |||||||
| Natural pregnancy | 93.4 | 99.5 | 97.5 | 94.0 | 87.8 | 78.0 | <0.0001 | |
| Ovulation Induction | 1.2 | 0.2 | 1.2 | 1.4 | 1.2 | 0.8 | 0.060 | |
| AIH | 0.9 | 0.0 | 0.3 | 1.0 | 2.0 | 1.3 | <0.0001 | |
| IVF | 2.9 | 0.0 | 0.5 | 2.3 | 6.1 | 12.9 | <0.0001 | |
| ICSI | 1.2 | 0.0 | 0.3 | 0.9 | 2.4 | 5.7 | <0.0001 | |
| Educational background | 13,721 | |||||||
| Elementary/junior high school | 2.6 | 9.6 | 2.7 | 2.3 | 1.2 | 1.7 | <0.0001 | |
| High school | 30.2 | 57.1 | 33.1 | 26.4 | 25.4 | 26.5 | <0.0001 | |
| Vocational college | 26.7 | 19.3 | 28.1 | 28.1 | 25.6 | 25.4 | 0.42 | |
| Junior College and Technical College | 11.9 | 5.3 | 8.8 | 10.1 | 17.8 | 18.5 | <0.0001 | |
| University | 26.2 | 8.2 | 26.1 | 29.7 | 26.6 | 25.1 | <0.0001 | |
| Graduate School | 2.1 | 0.1 | 1.1 | 2.5 | 3.1 | 2.7 | <0.0001 | |
| Other | 0.2 | 0.4 | 0.2 | 0.2 | 0.3 | 0.1 | 0.76 | |
| Occupation | 18,885 | |||||||
| Housewife or unemployed | 26.2 | 23.2 | 23.8 | 26.7 | 28.6 | 32.0 | <0.0001 | |
| Employed | 73.3 | 73.4 | 76.1 | 73.2 | 71.2 | 68.0 | <0.0001 | |
| Student | 0.4 | 3.4 | 0.1 | 0.1 | 0.2 | 0.0 | <0.0001 | |
| Household income, million Japanese yen/year | 20,579 | |||||||
| <2 | 4.2 | 11.8 | 4.5 | 3.0 | 3.0 | 3.3 | <0.0001 | |
| 2 to <4 | 32.6 | 51.3 | 39.1 | 29.7 | 24.7 | 21.0 | <0.0001 | |
| 4 to <6 | 32.5 | 24.4 | 32.4 | 34.9 | 32.7 | 27.6 | 0.0036 | |
| 6 to <8 | 18.4 | 7.2 | 15.3 | 19.8 | 22.6 | 26.6 | <0.0001 | |
| 8 to <10 | 7.4 | 2.5 | 5.5 | 8.0 | 9.6 | 11.6 | <0.0001 | |
| ≥10 | 4.9 | 2.9 | 3.2 | 4.6 | 7.4 | 9.9 | <0.0001 | |
AIH, artificial insemination with husband’s semen; BMI, body mass index; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization (conventional).
aMissing values were excluded.
bMultiple choice allowed and excluded in this table.
Table 2 shows the results of pregnancy-related infectious disease screening. The rates of positive results were: RPR, 0.27%; TPHA, 0.23%; HBV, 0.26%; HCV, 0.22%; HIV, 0.1%; rubella X256, 5.41%, rubella, X512 1.5%, rubella X1024, 0.1%; and HITLV-1, 0.34%. Some study participants opted to be tested for HSV and toxoplasmosis, with positive rates of 3.4% and 8.1%, respectively. Older participants exhibited a higher rate of HBs Ag positive and Rubella Ab ≥X 64 (P < 0.0001).
Table 2. Maternal infectious disease screening.
| Variables | n | Total | Age at enrollment, years | P for trend | ||||||||||
|
| ||||||||||||||
| ≤24 | 25–29 | 30–34 | 35–39 | ≥40 | ||||||||||
|
| ||||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||
| RPR | 19,062 | |||||||||||||
| + | 51 | 0.27 | 1 | 0.06 | 12 | 0.23 | 19 | 0.28 | 16 | 0.38 | 3 | 0.31 | 0.040 | |
| − | 19,011 | 99.7 | 1,808 | 99.9 | 5,172 | 99.8 | 6,863 | 99.7 | 4,199 | 99.6 | 969 | 99.7 | ||
| TPHA | 19,071 | |||||||||||||
| + | 44 | 0.23 | 6 | 0.32 | 10 | 0.19 | 17 | 0.25 | 7 | 0.17 | 4 | 0.44 | 0.65 | |
| − | 19,027 | 99.7 | 1,846 | 99.7 | 5,247 | 99.81 | 6,869 | 99.75 | 4,153 | 99.83 | 912 | 99.56 | ||
| HBs Ag | 21,188 | |||||||||||||
| + | 56 | 0.26 | 0 | 0 | 8 | 0.14 | 19 | 0.25 | 20 | 0.44 | 9 | 0.88 | <0.0001 | |
| − | 21,132 | 99.7 | 2,051 | 100.0 | 5,869 | 99.9 | 7,638 | 99.8 | 4,565 | 99.6 | 1,009 | 99.1 | ||
| HCV Ab | 20,813 | |||||||||||||
| + | 46 | 0.22 | 2 | 0.10 | 15 | 0.26 | 13 | 0.17 | 14 | 0.31 | 2 | 0.20 | 0.36 | |
| − | 20,767 | 99.8 | 2,025 | 99.9 | 5,759 | 99.7 | 7,478 | 99.8 | 4,507 | 99.7 | 998 | 99.8 | ||
| HIV Ab | 21,005 | |||||||||||||
| + | 21 | 0.10 | 3 | 0.15 | 7 | 0.12 | 5 | 0.07 | 4 | 0.09 | 2 | 0.20 | 0.72 | |
| − | 20,984 | 99.9 | 2,034 | 99.9 | 5,829 | 99.9 | 7,558 | 99.9 | 4,569 | 99.9 | 994 | 99.8 | ||
| Rubella Ab | 15,646 | |||||||||||||
| <X 8 | 251 | 1.60 | 49 | 3.09 | 107 | 2.40 | 70 | 1.24 | 20 | 0.61 | 5 | 0.72 | <0.0001 | |
| X 8 | 1,980 | 12.65 | 406 | 25.57 | 808 | 18.14 | 446 | 7.92 | 258 | 7.86 | 62 | 8.99 | <0.0001 | |
| X 16 | 2,635 | 16.84 | 374 | 23.55 | 932 | 20.92 | 764 | 13.57 | 467 | 14.22 | 98 | 14.20 | <0.0001 | |
| X 32 | 3,870 | 24.73 | 387 | 24.37 | 1,175 | 26.37 | 1,362 | 24.19 | 797 | 24.28 | 149 | 21.29 | 0.030 | |
| X 64 | 3,634 | 23.23 | 259 | 16.31 | 853 | 19.15 | 1,525 | 27.09 | 821 | 25.01 | 176 | 25.51 | <0.0001 | |
| X 128 | 2,178 | 13.92 | 88 | 5.54 | 408 | 9.16 | 950 | 16.87 | 602 | 18.34 | 130 | 18.84 | <0.0001 | |
| X 256 | 846 | 5.41 | 17 | 1.07 | 134 | 3.01 | 401 | 7.12 | 244 | 7.43 | 50 | 7.25 | <0.0001 | |
| X 512 | 234 | 1.50 | 8 | 0.50 | 34 | 0.76 | 104 | 1.85 | 69 | 2.10 | 19 | 2.75 | <0.0001 | |
| X 1024 | 18 | 0.1 | 0 | 0.0 | 4 | 0.1 | 8 | 0.1 | 5 | 0.2 | 1 | 0.1 | 0.14 | |
| HTLVAb | 19,423 | |||||||||||||
| + | 67 | 0.34 | 4 | 0.21 | 16 | 0.30 | 32 | 0.46 | 11 | 0.26 | 4 | 0.43 | 0.51 | |
| − | 19,356 | 99.7 | 1,867 | 99.8 | 5,387 | 99.7 | 6,964 | 99.5 | 4,212 | 99.7 | 926 | 99.6 | ||
| HSV | 529 | |||||||||||||
| + | 18 | 3.40 | 0 | 0 | 4 | 2.55 | 6 | 3.21 | 4 | 4.44 | 4 | 16.00 | 0.0023 | |
| − | 511 | 96.6 | 70 | 100.0 | 153 | 97.5 | 181 | 96.8 | 86 | 95.6 | 21 | 84.0 | ||
| Toxoplasmosis | 5,020 | |||||||||||||
| + | 407 | 8.11 | 33 | 6.69 | 112 | 7.63 | 159 | 8.81 | 80 | 7.77 | 23 | 10.13 | 0.18 | |
| − | 4,613 | 91.9 | 460 | 93.3 | 1,355 | 92.4 | 1,645 | 91.2 | 949 | 92.2 | 204 | 89.9 | ||
HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C antibody; HIV Ab, HIV antibody; HSV, herpes simplex virus; HTLV-1Ab, human t-cell leukemia virus type 1; RPR, rapid plasma regain test; Rubella Ab, Rubella antibody; TPHA, Treponema pallidum hemagglutination test.
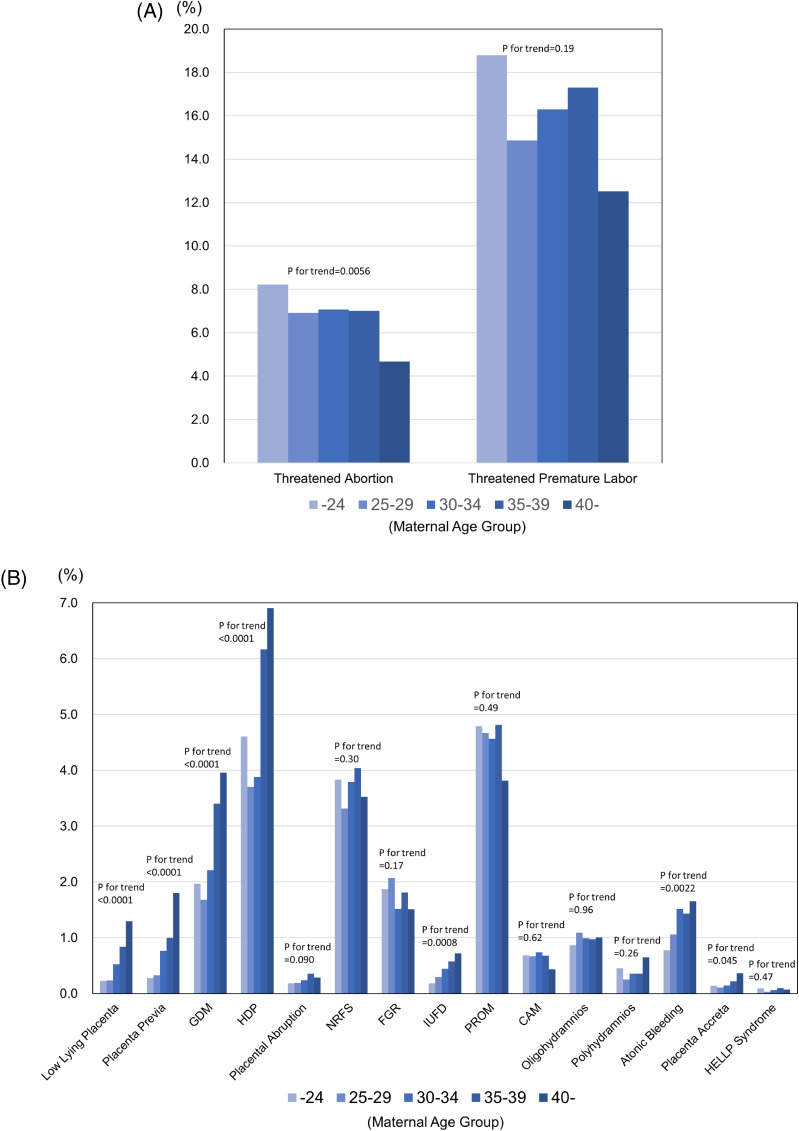
Figure 1 shows the prevalence of major pregnancy-related complications. Common obstetric complications were threatened premature labor (16.1%), threatened abortion (7.0%), HDP (4.6%), NRFS (3.7%), and FGR (1.8%). Younger participants tended to have a higher incidence of threatened abortion and threatened premature labor (Figure 1A), while older age groups had a significantly higher rate of low lying placenta, PP, GDM, and HDP (P < 0.0001) (Figure 1B).
Figure 1. Prevalence of pregnancy-related complications A) by age group (n = 23,406) and B) by age group (n = 23,406). CAM, chorioamnionitis; FGR, fetal growth restriction; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; IUFD, intrauterine fetal demise; NRFS, nonreassuring fetal status; PROM, premature rupture of membranes; HELLP, hemolysis, elevated liver enzymes, and low platelet count.
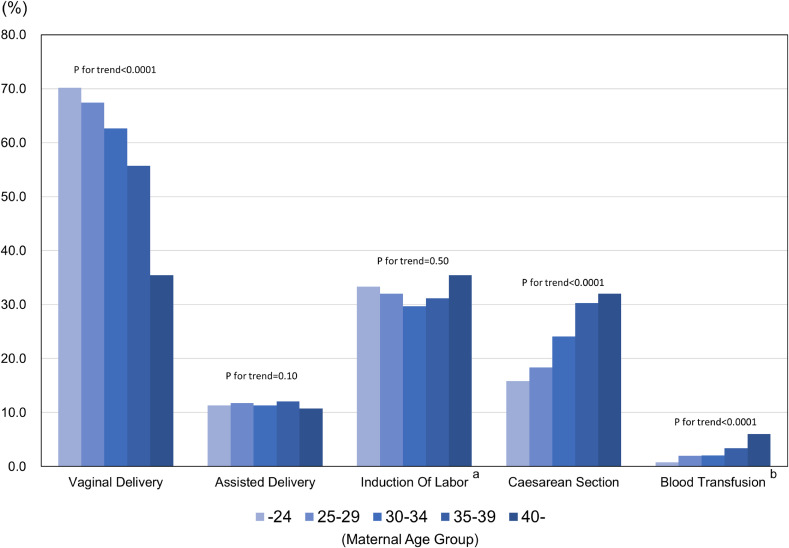
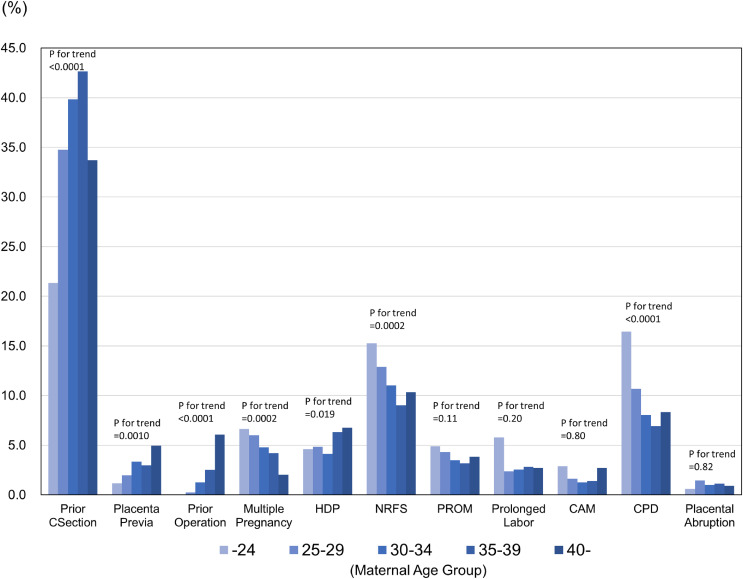
Delivery information according to age group is shown in Figure 2. The incidence of each mode of delivery was: spontaneous vaginal delivery, 61.5%; assisted delivery, 11.5%; and cesarean delivery, 23.5%. Older age groups exhibited a lower rate of vaginal delivery and a higher rate of cesarean section and blood transfusion (P < 0.0001). The indications for cesarean delivery were: prior cesarean delivery, 37.8%; NRFS, 11.1%; cephalopelvic disproportion, 8.8%; HDP, 5.1%; multiple gestation, 4.8%; PROM, 3.7%; placenta previa, 2.9%; prolonged labor, 2.8%; prior uterine surgery, 1.7%; chorioamnionitis, 1.6%; and placental abruption, 1.1% (Figure 3). Prior Cesarean section and operation were observed more often in the older age groups, whereas cephalopelvic disproportion were observed more often in younger age group (P < 0.0001).
Figure 2. Delivery information according to age group (n = 23,406). aInformation on induction of labor was collected from 18,456 medical records; bInformation on blood transfusion was collected from 7,337 medical records.
Figure 3. Indications for cesarean delivery according to age group (n = 5,506). CAM, chorioamnionitis; CPD, cephalopelvic disproportion; HDP, hypertensive disorders of pregnancy; NRFS, non-reassuring fetal status; PROM, premature rupture of membranes.
Table 3 shows the birth information for 22,561 singleton liveborn infants. The mean gestational age at delivery was 38.7 (standard deviation [SD], 1.8) weeks. The proportion of premature delivery was 6.5% and post-term delivery was 0.1%. The mean birth weight was 3,024.1 (SD, 435) g and the proportion of low birth weight was 8.5%. The proportion of Apgar scores <7 was 10.4% at 1 minute and 10.4% at 5 minutes. The mean UApH was 7.31 (SD, 0.15), the proportion of UApH <7.2 was 6.51%, and the proportion <7.0 was 0.08%. Admission to the NICU was required in 5.14% of singleton newborns. Older age groups exhibited a higher rate of premature labor and NICU admission (P < 0.0001).
Table 3. Birth information from 22,561 singleton liveborn infants.
| Variables | Total | Maternal age at enrollment, years | P for trend | |||||||||||||||||
|
| ||||||||||||||||||||
| ≤24 | 25–29 | 30–34 | 35–39 | ≥40 | ||||||||||||||||
| n | (%, mean) | (SD) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||||||||
| Gestational age at delivery, weeks | <37 | 1,474 | 6.5 | 121 | 5.7 | 340 | 5.4 | 488 | 6.0 | 367 | 7.5 | 158 | 13.7 | <0.0001 | ||||||
| 37–41 | 21,060 | 93.3 | 2,010 | 94.1 | 5,895 | 94.5 | 7,660 | 93.9 | 4,496 | 92.4 | 999 | 86.3 | <0.0001 | |||||||
| >42 | 27 | 0.1 | 4 | 0.2 | 6 | 0.1 | 12 | 0.1 | 5 | 0.1 | . | 0.36 | ||||||||
| Mean, SD | 22,309 | 38.7 | 1.8 | 2,107 | 39.0 | 1.6 | 6,181 | 38.9 | 1.7 | 8,097 | 38.7 | 1.7 | 4,842 | 38.5 | 1.9 | 1,082 | 38.3 | 2.1 | <0.0001 | |
| Birth weight | Mean, SD | 22,311 | 3,024.4 | 435.0 | 2,108 | 3,037.1 | 415.1 | 6,180 | 3,032.7 | 412.5 | 8,100 | 3,023.7 | 431.9 | 4,841 | 3,016.7 | 462.5 | 1,082 | 2,991.7 | 489.0 | 0.028 |
| Length | Mean, SD | 22,039 | 49.3 | 3.8 | 2,075 | 49.4 | 2.2 | 6,085 | 49.4 | 2.4 | 8,006 | 49.3 | 5.5 | 4,802 | 49.2 | 2.7 | 1,071 | 49.0 | 3.0 | 0.0002 |
| Head circumference | Mean, SD | 22,019 | 33.4 | 2.5 | 2,075 | 33.2 | 1.5 | 6,082 | 33.3 | 1.5 | 8,000 | 33.4 | 3.5 | 4,791 | 33.5 | 1.6 | 1,071 | 33.5 | 1.8 | <0.0001 |
| Chest circumference | Mean, SD | 21,984 | 31.8 | 2.8 | 2,070 | 31.8 | 1.8 | 6,074 | 31.9 | 1.8 | 7,989 | 31.9 | 3.8 | 4,781 | 31.9 | 2.0 | 1,070 | 31.8 | 2.4 | 0.78 |
| Sex | Male | 11,553 | 51.8 | 1,123 | 53.2 | 3,242 | 52.4 | 4,197 | 51.8 | 2,465 | 50.9 | 526 | 48.6 | 0.0052 | ||||||
| Female | 10,764 | 48.2 | 985 | 46.7 | 2,942 | 47.6 | 3,905 | 48.2 | 2,376 | 49.1 | 556 | 51.4 | 0.0050 | |||||||
| Unknown | 6 | 0.0 | 1 | 0.0 | 2 | 0.0 | 1 | 0.0 | 2 | 0.0 | . | 0.66 | ||||||||
| Placental weight | Mean, SD | 20,058 | 562.6 | 156.1 | 1,935 | 572.1 | 242.7 | 5,451 | 560.8 | 124.9 | 7,213 | 561.5 | 159.0 | 4,456 | 563.2 | 139.6 | 1,003 | 558.6 | 142.9 | 0.33 |
| Apgar score <7 at 1 min | + | 2,348 | 10.4 | 165 | 7.7 | 710 | 11.4 | 894 | 11.0 | 425 | 8.7 | 154 | 13.3 | 0.40 | ||||||
| − | 20,213 | 89.6 | 1,970 | 92.3 | 5,531 | 88.6 | 7,266 | 89.0 | 4,443 | 91.3 | 1,003 | 86.7 | ||||||||
| Apgar score <7 at 5 min | + | 2,343 | 10.4 | 181 | 8.5 | 748 | 12.0 | 905 | 11.1 | 367 | 7.5 | 142 | 12.3 | 0.016 | ||||||
| − | 20,218 | 89.6 | 1,954 | 91.5 | 5,493 | 88.0 | 7,255 | 88.9 | 4,501 | 92.5 | 1,015 | 87.7 | ||||||||
| UmA pH | Mean, SD | 17,226 | 7.31 | 0.15 | 1,696 | 7.30 | 0.17 | 4,689 | 7.31 | 0.12 | 6,128 | 7.31 | 0.15 | 3,811 | 7.31 | 0.08 | 902 | 7.29 | 0.34 | 0.47 |
| <7.00 | 45 | 0.26 | 6 | 0.36 | 12 | 0.26 | 20 | 0.33 | 3 | 0.08 | 4 | 0.44 | 0.35 | |||||||
| 7.00 to <7.20 |
1,095 | 6.36 | 118 | 6.96 | 313 | 6.68 | 360 | 5.87 | 245 | 6.43 | 59 | 6.54 | 0.38 | |||||||
| ≥7.20 | 16,086 | 93.38 | 1,572 | 92.69 | 4,364 | 93.07 | 5,748 | 93.80 | 3,563 | 93.49 | 839 | 93.02 | 0.29 | |||||||
| NICU admission | + | 1,159 | 5.1 | 96 | 4.5 | 265 | 4.2 | 409 | 5.0 | 300 | 6.2 | 89 | 7.7 | <0.0001 | ||||||
| − | 21,402 | 94.9 | 2,039 | 95.5 | 5,976 | 95.8 | 7,751 | 95.0 | 4,568 | 93.8 | 1,068 | 92.3 | ||||||||
NICU, Neonatal intensive care unit; SD, standard deviation; UmA, umbilical artery.
Table 4 shows the birth outcomes of 542 twin liveborn infants. A dichorionic diamniotic (DD) pregnancy was noted in 63.1%, monochorionic diamniotic (MD) in 35.8%, and monochorionic monoamniotic (MM) in 1.1%. The mean gestational age at delivery was 35.2 (SD, 2.6) weeks. Premature delivery was noted in 68.3%. The mean birthweight was 2,190.1 (SD, 474.7) g, and the proportion of low birth weight was 76.0%. The proportion of Apgar scores <7 was 12.9% at 1 minute and 9.2% at 5 minutes. The proportion of infants with UApH <7.2 was 5.0%, and 56.1% of twin newborns were admitted to the NICU.
Table 4. Birth outcomes in 542 twin liveborn infants.
| Variables | Total | Maternal age at enrollment, years | P for trend | ||||||||||||||||||
|
| |||||||||||||||||||||
| ≤24 | 25–29 | 30–34 | 35–39 | ≥40 | |||||||||||||||||
| n | n | (%, mean) | (SD) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||||||||
| Chorionicity | DD | 542 | 342 | 63.1 | 26 | 56.5 | 82 | 56.2 | 124 | 63.3 | 98 | 73.1 | 12 | 60.0 | 0.011 | ||||||
| MD | 194 | 35.8 | 18 | 39.1 | 64 | 43.8 | 70 | 35.7 | 34 | 25.4 | 8 | 40.0 | 0.015 | ||||||||
| MM | 6 | 1.1 | 2 | 4.4 | 0 | 0.0 | 2 | 1.0 | 2 | 1.5 | 0 | 0.0 | 0.59 | ||||||||
| Gestational age at delivery, weeks | <37 | 542 | 370 | 68.3 | 36 | 78.3 | 98 | 67.1 | 136 | 69.4 | 88 | 65.7 | 12 | 60.0 | 0.18 | ||||||
| 37–41 | 172 | 31.7 | 10 | 21.7 | 48 | 32.9 | 60 | 30.6 | 46 | 34.3 | 8 | 40.0 | 0.18 | ||||||||
| Mean, SD | 542 | 35.2 | 2.6 | 46 | 34.2 | 3.5 | 146 | 35.5 | 2.0 | 196 | 35.0 | 2.8 | 134 | 35.3 | 2.5 | 20 | 35.8 | 1.6 | 0.0032 | ||
| Birth weight | Mean, SD | 542 | 2,190.9 | 474.7 | 46 | 2,057.4 | 571.3 | 146 | 2,199.4 | 398.5 | 196 | 2,180.9 | 501.5 | 134 | 2,224.7 | 479.9 | 20 | 2,305.8 | 413.3 | 0.032 | |
| Length | Mean, SD | 524 | 45.0 | 3.9 | 44 | 44.1 | 4.8 | 138 | 44.9 | 3.3 | 192 | 44.9 | 4.1 | 130 | 45.4 | 3.6 | 20 | 46.5 | 3.3 | 0.0041 | |
| Head circumference | Mean, SD | 520 | 31.7 | 2.2 | 44 | 31.0 | 3.1 | 144 | 31.7 | 2.0 | 195 | 31.5 | 2.6 | 130 | 32.0 | 1.8 | 20 | 32.1 | 1.8 | 0.018 | |
| Chest circumference | Mean, SD | 518 | 28.5 | 2.7 | 44 | 27.4 | 3.2 | 136 | 28.5 | 2.3 | 190 | 28.4 | 3.0 | 128 | 28.9 | 2.3 | 20 | 28.7 | 2.0 | 0.048 | |
| Sex | Male | 542 | 272 | 50.2 | 21 | 45.7 | 74 | 50.7 | 99 | 50.5 | 71 | 53.0 | 7 | 35.0 | 0.92 | ||||||
| Female | 270 | 49.8 | 25 | 54.3 | 72 | 49.3 | 97 | 49.5 | 63 | 47.0 | 13 | 65.0 | 0.92 | ||||||||
| Placental weight | Mean, SD | 484 | 913.2 | 320.9 | 40 | 988.5 | 265.9 | 122 | 923.5 | 325.6 | 178 | 867.6 | 291.9 | 124 | 930.0 | 353.0 | 20 | 1,002.4 | 393.8 | 0.80 | |
| Apgar score <7 at 1 min | + | 542 | 70 | 12.9 | 8 | 17.4 | 19 | 13.0 | 24 | 19 | 14.2 | . | 0.32 | ||||||||
| − | 472 | 87.1 | 38 | 82.6 | 127 | 87.0 | 172 | 87.8 | 115 | 85.8 | 20 | 100.0 | |||||||||
| Apgar score <7 at 5 min | + | 542 | 50 | 9.2 | 5 | 10.9 | 16 | 11.0 | 15 | 7.7 | 14 | 10.4 | . | 0.36 | |||||||
| − | 492 | 90.8 | 41 | 89.1 | 130 | 89.0 | 181 | 92.3 | 120 | 89.6 | 20 | 100.0 | |||||||||
| UmA pH | Mean, SD | 449 | 7.34 | 0.05 | 39 | 7.34 | 0.04 | 119 | 7.34 | 0.04 | 165 | 7.34 | 0.05 | 106 | 7.33 | 0.04 | 20 | 7.32 | 0.05 | 0.017 | |
| <7.00 | 458 | ||||||||||||||||||||
| 7.00 to <7.20 |
4 | 0.87 | 0 | 0.00 | 1 | 0.81 | 2 | 1.19 | 0 | 0.00 | 1 | 5.00 | 0.47 | ||||||||
| ≥7.20 | 454 | 99.13 | 39 | 100.00 | 123 | 99.19 | 166 | 98.81 | 107 | 100.00 | 19 | 95.00 | 0.47 | ||||||||
| NICU admission | + | 542 | 304 | 56.1 | 32 | 69.6 | 90 | 61.6 | 104 | 53.1 | 67 | 50.0 | 11 | 55.0 | 0.011 | ||||||
| − | 238 | 43.9 | 14 | 30.4 | 56 | 38.4 | 92 | 46.9 | 67 | 50.0 | 9 | 45.0 | |||||||||
DD, dichorionic diamniotic; MD, monochorionic diamniotic; MM, monochorionic monoamniotic; NICU, neonatal intensive care unit; SD, standard deviation; UmA, umbilical artery.
Gestational age at the time of maternal blood collection (n = 22,356) is shown in supplementary data (eFigure 1) and gestational age at the time of cord blood collection (n = 18,874) is demonstrated in eFigure 2.
DISCUSSION
The present study describes the maternal baseline characteristics and perinatal outcomes in participants of the TMM BirThree Cohort Study.19 The TMM BirThree Cohort Study is expected to provide scientific evidence to assess risk factors for various multifactorial diseases, including NCDs, by analyzing genetic-environmental interactions. Specifically, this cohort study is accumulating genetic information from SNP arrays or whole-genome sequencing,29 epigenetic information from targeted bisulfite sequencing, and quantitative metabolome data by nuclear magnetic resonance- or mass spectrometry.30 Precise clinical information is being collected from medical records, and epidemiological data is being obtained by questionnaires and official government databases. Accordingly, this cohort study will enable a variety of genome-metabolome-wide association studies of obstetric and pediatric complications. In addition, this cohort study will reveal associations of exposures in early life with the onset of NCDs in later life, including developmental, cognitive, metabolic, and vascular disease. Finally, this cohort study will establish unexplored methods of early prevention using personalized genetic, epigenetic, medical, and epidemiologic information obtained from multiple generations. Recently, birth cohort studies have been encouraged to move forward the concept of data sharing, aiming to enable cross-comparisons or meta-analyses of integrated data at the regional, national, and global level. Our study aims to provide precise perinatal information to align with those agendas.
We found that approximately 6.6% of patients used assisted reproductive technology, comparable with nationwide Japanese data.31 These data will allow us to conduct association studies of the effect of assisted reproductive technology on developmental outcomes, from not only an epidemiologic viewpoint, but also from a molecular biological viewpoint.
Maternal infection screening tests stratified by maternal age demonstrated that an overview of series of valuable results for the birth cohort study. Further transgenerational epidemiological analysis in our cohort study might provide an informative information to develop methods for the earlier intervention. Detailed information on the rate of maternal infections that have a potential for fetal transmission will help to establish reference data for the Japanese population of women of reproductive age.
Landscape obstetric outcome data stratified by maternal age group were demonstrated in the present study. The diagnostic criteria for all major obstetric complications are standardized at the participating facilities of Tohoku University and by clinical guidelines in Japan.32 We suggest the construction of a phenotyping algorithm for subtypes of HDP, using the huge trove of outpatient data available from the BirThree cohort on blood pressure, urinalysis, and other maternal and fetal factors. Standardized phenotyping would empower the implementation of high-resolution association studies. Advanced maternal age clearly exhibited a significantly higher rate of low lying placenta, PP, GDM and HDP. These results were in agreement with previous reports,21 and biological mechanisms would be revealed by multi-omics analysis using samples from three generations in our cohort study.19
The major strength of our study is that it provides precise clinical information obtained from medical records, and that the diagnoses employ standardized criteria. Most of participants had a “Maternal and Child Health Handbook” of their own, in which they recorded their mothers pregnancy details and birth outcomes. Additionally, family information from partners, grandparents, siblings, and others was available for transgenerational analyses.19 Another strength lies in the systemic collection of biospecimens, including blood and urine samples in early and midpregnancy, umbilical cord blood, and breast milk. Blood samples were stored in the biobank as plasma, serum, and immortalized lymphocytes for future research.26 Genome analysis by Japonica array was performed in most maternal samples,29,33 allowing us to analyze GWAS for various parameters, variables, and diseases. We are eager to share our data to enable meta-analyses and international collaboration.
Several limitations should be considered in this study. Participants were recruited between 2013 and 2017 in Miyagi Prefecture, located in northern Japan. Therefore, the ability of the cohort to represent the national population could be discussed. Second, the long-term effect of the GEJE disaster in 2011 may affect baseline characteristics and epidemiologic outcomes. Extensive epidemiologic surveys and omics analyses would elucidate the mechanisms of various factors of the disaster.
In conclusion, the present study clearly and precisely demonstrates the perinatal baseline profiles in participants of the TMM BirThree Cohort Study.19 The data presented in the study are expected to provide strategic information for various types of association studies to uncover perinatal or developmental diseases, including NCDs, in later life through genomic-environmental interactions. Collaborative data visiting or sharing will bring new insight into the search for personalized early prevention and intervention strategies.
ACKNOWLEDGEMENTS
The authors would like to thank all the participants of the TMM BirThree Cohort Study and the staff of the Tohoku Medical Megabank Organization, Tohoku University, for their assistance. The full list of members is available at https://www.megabank.tohoku.ac.jp/english/a191201/. The authors would like to express our gratitude to all staff at the obstetric clinics and hospitals for their kind cooperation.
Funding: The TMM is supported by grants from the Reconstruction Agency; from the Ministry of Education, Culture, Sports, Science and Technology; and the Japan Agency for Medical Research and Development (AMED). This study was supported by AMED under grant numbers JP19km0105001, JP19km0105002.
Conflicts of interest: None declared.
APPENDIX A. SUPPLEMENTARY DATA
The following is the supplementary data related to this article:
eFigure 1. Gestational age at the time of maternal blood collection (first time point, n = 22,449; second time point, n = 22,356)
eFigure 2. Gestational age at the time of cord blood collection (n = 18,874)
REFERENCES
- 1.Pullar J, Wickramasinghe K, Demaio AR, et al. The impact of maternal nutrition on offspring’s risk of non-communicable diseases in adulthood: a systematic review. J Glob Health. 2019;9:020405. 10.7189/jogh.09.020405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye C, Mertens T, Hirnschall G, et al. WHO and the future of disease control programmes. Lancet. 2013;381:413–418. 10.1016/S0140-6736(12)61812-1 [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. 10.1126/science.1095292 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- 5.Golding J. Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC). West Engl Med J. 1990;105:80–82. [PMC free article] [PubMed] [Google Scholar]
- 6.Jaddoe VWV, Mackenbach JP, Moll HA, et al. The Generation R Study: design and cohort profile. Eur J Epidemiol. 2006;21:475–484. 10.1007/s10654-006-9022-0 [DOI] [PubMed] [Google Scholar]
- 7.Andersen AM, Olsen J. The Danish National Birth Cohort: selected scientific contributions within perinatal epidemiology and future perspectives. Scand J Public Health. 2011;39(7 Suppl):115–120. 10.1177/1403494811407674 [DOI] [PubMed] [Google Scholar]
- 8.Wadman M. Child-study turmoil leaves bitter taste. Nature. 2012;485:287–288. 10.1038/485287a [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto T, Nitta H, Murata K, et al. ; Working Group of the Epidemiological Research for Children’s Environmental Health . Rationale and study design of the Japan Environment and Children’s Study (JECS). BMC Public Health. 2014;14:25. 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45:382–388. 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 11.Stolk RP, Rosmalen JG, Postma DS, et al. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol. 2008;23:67–74. 10.1007/s10654-007-9204-4 [DOI] [PubMed] [Google Scholar]
- 12.Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44:1172–1180. 10.1093/ije/dyu229 [DOI] [PubMed] [Google Scholar]
- 13.Fire and Disaster Management Agency of the Ministry of Internal Affairs and Communications 2019. https://www.fdma.go.jp/disaster/higashinihon/items/159.pdf; Accessed 05.07.20.
- 14.Sugawara J, Hoshiai T, Sato K, et al. Impact of the Great East Japan Earthquake on regional obstetrical care in Miyagi Prefecture. Prehosp Disaster Med. 2016;31:255–258. 10.1017/S1049023X1600025X [DOI] [PubMed] [Google Scholar]
- 15.Sugawara J, Iwama N, Hoshiai T, et al. Regional birth outcomes after the 2011 Great East Japan Earthquake and Tsunami in Miyagi Prefecture. Prehosp Disaster Med. 2018;33:215–219. 10.1017/S1049023X18000183 [DOI] [PubMed] [Google Scholar]
- 16.Nishigori H, Sugawara J, Obara T, et al. Surveys of postpartum depression in Miyagi, Japan, after the Great East Japan Earthquake. Arch Women Ment Health. 2014;17:579–581. 10.1007/s00737-014-0459-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuriyama S, Yaegashi N, Nagami F, et al. The Tohoku Medical Megabank Project: design and mission. J Epidemiol. 2016;26:493–511. 10.2188/jea.JE20150268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hozawa A, Tanno K, Nakaya N, et al. Study profile of The Tohoku Medical Megabank Community-Based Cohort Study. J Epidemiol. 2021;31:65–76. 10.2188/jea.JE20190271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama S, Metoki H, Kikuya M, et al. Cohort profile: Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study (TMM BirThree Cohort Study): rationale, progress and perspective. Int J Epidemiol. 2020;49:18–19m. 10.1093/ije/dyz169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Vital Statistics reports. Birth: Final data for 2018. Center for Disease Control and Prevention. 2019;68:13. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_13-508.pdf. [PubMed]
- 21.Kenny LC, Lavender T, McNamee R, et al. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 2013;8(2):e56583. 10.1371/journal.pone.0056583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai-Yageta M, Kawame H, Kuriyama S, et al. A training and education program for genome medical research coordinators in the Genome Cohort Study of the Tohoku Medical Megabank Organization. BMC Med Educ. 2019;19:297. 10.1186/s12909-019-1725-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health Labor and Welfare. Desirable criteria for health checkups for pregnant women. https://www.mhlw.go.jp/web/t_doc?dataId=82ab4662&dataType=0&pageNo=1; Accessed 05.07.20.
- 24.Takai-Igarashi T, Kinoshita K, Nagasaki M, et al. Security controls in an integrated biobank to protect privacy in data sharing: rationale and study design. BMC Med Inform Decis Mak. 2017;17:100. 10.1186/s12911-017-0494-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minakami H, Maeda T, Fujii T, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res. 2014;40:1469–1499. 10.1111/jog.12419 [DOI] [PubMed] [Google Scholar]
- 26.Minegishi N, Nishijima I, Nobukuni T, et al. Biobank establishment and sample management in the Tohoku Medical Megabank Project. Tohoku J Exp Med. 2019;248:45–55. 10.1620/tjem.248.45 [DOI] [PubMed] [Google Scholar]
- 27.World Medical Association. WMA Declaration of Helsinki –Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire, France: World Medical Association; 1964. https://www.wma.net/wp-content/uploads/2016/11/DoH-Oct2013-JAMA.pdf Accessed 05.07.20.
- 28.Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; Ministry of Economy, Trade and Industry. Ethical Guidelines for Human Genome/Gene Analysis Research. Tokyo: Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; Ministry of Economy, Trade and Industry; 2001.
- 29.Yasuda J, Kinoshita K, Katsuoka F, et al. Genome analyses for the Tohoku Medical Megabank Project towards establishment of personalized healthcare. J Biochem. 2019;165:139–158. 10.1093/jb/mvy096 [DOI] [PubMed] [Google Scholar]
- 30.Koshiba S, Motoike I, Saigusa D, et al. Omics research project on prospective cohort studies from the Tohoku Medical Megabank Project. Genes Cells. 2018;23:406–417. 10.1111/gtc.12588 [DOI] [PubMed] [Google Scholar]
- 31.ART registry databook. Japan Society of Obstetrics and Gynecology. (In Japanese) http://plaza.umin.ac.jp/~jsog-art/; Accessed 05.07.20.
- 32.Kawaguchi R, Matsumoto K, Akira S, et al. Guidelines for office gynecology in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2017 edition. J Obstet Gynaecol Res. 2019;45:766–786. 10.1111/jog.13831 [DOI] [PubMed] [Google Scholar]
- 33.Kawai Y, Mimori T, Kojima K, et al. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1070 Japanese individuals. J Hum Genet. 2015;60:581–587. 10.1038/jhg.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.