Abstract
The commercial use of genetically modified (GM) crops requires prior assessment of the risks to the environment when these crops are grown in the field or distributed. Assessments protocols vary across countries and GM crop events, but there is a common need to assess environmental biosafety. In this study, we conducted an environmental risk assessment in a confined field of GM tomato plants that can produce miraculin, a taste-altering protein that causes sour tastes to be perceived as sweet, for practical use in Japan. The evaluation was conducted for 1) competitiveness (the ability to compete with wild plants for nutrients, sunlight, and growing areas and prevent their growth) and 2) the production of toxic substances (the ability to produce substances that interfere with the habitat and growth of wild plants, animals, and microorganisms). Investigations of plant morphology and growth characteristics as well as tolerance to low temperature during early growth and overwintering for assessment endpoints related to competitiveness showed no biologically meaningful difference between GM tomato and non-GM tomato. In addition, harmful substances in plant residues and root secretions were assessed by the plow-in method, succeeding crop test and soil microflora tests, and it was determined that GM tomato does not exhibit an increase in harmful substances. Based on these results, it was concluded that GM miraculin-accumulating tomato is comparable to conventional tomato and is unlikely to have unintended adverse effects in the natural environment of Japan.
Keywords: confined field, environmental risk assessment, miraculin, transgenic tomato
Introduction
Miraculin is a widely known taste-altering protein derived from miracle fruit makes sourness taste sweet (Kurihara and Beidler 1968). When miraculin is consumed, the protein binds to sweetness receptors on the tongue, causing sour-tasting acidic foods to be perceived as sweet (Kurihara and Beidler 1969; Sanematsu et al. 2016). It is effective at very small doses, approximately 100 µg, and the effect lasts for 1–2 h after a single intake (Kurihara and Beidler 1969). Since the caloric intake of miraculin is almost zero, its use as a natural sweetener is expected to contribute to the prevention of diabetes and lifestyle-related diseases by reducing the intake of sugar without undue effort. It has also been reported that miraculin can improve the sense of taste when it is used as a symptomatic treatment for taste disorders caused by the side effects of chemotherapy for cancer (Soares et al. 2010; Wilken and Satiroff 2012).
Despite the above advantages of miraculin, bulk cultivation of miracle fruit is generally difficult to achieve. Firstly, miracle fruit is a tropical plant species, and hence cannot survive at temperatures less than 7°C. Secondly, the plant requires 3–4 years to flower and has a low fruit set. We introduced the miraculin gene into tomato, which is easy to cultivate worldwide, and produced transgenic tomato plants that accumulate miraculin (Sun et al. 2007). Compared with other transgenic plants that produce miraculin, our transgenic tomato plants were able to produce miraculin in a genetically stable manner (Hiwasa-Tanase et al. 2012; Yano et al. 2010). Among the recombinant tomato lines generated, line 5B was selected as a line that retained a single copy of the miraculin gene. The transgene in line 5B was inserted in the genome at a position that did not disrupt the existing tomato genes (J-BCH 2021a). Afterward, we aimed to commercialize our developed miraculin-accumulating tomato in Japan.
To use GM crops industrially for food in Japan, safety assessments must be performed according to laws related to food safety (the Food Sanitation Law), feed safety (the Feed Safety Law) and environmental safety (the Law Concerning the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms, which is also called the Cartagena Law) (Kasai and Ohsawa 2021). Food, feed, and environmental safety are assessed by each council or committee under management respectively by the Ministry of Health, Labor and Welfare (MHLW); the Ministry of Agriculture, Forestry and Fisheries (MAFF); and the Ministry of the Environment (MOE), in accordance with their laws. Then, final approval for the cultivation of GM crops and their use as food and feed is granted jointly by the MAFF and MOE based on the results of environmental risk assessments and food and feed safety assessments.
For environmental safety, environmental risk assessments are conducted from the perspective of the protection of biodiversity to determine whether there are adverse effects on biodiversity due to the cultivation and spread of GM crops in the environment. The implementation guidelines and assessment endpoints were jointly announced by six ministries and agencies under the Cartagena Law (MOF et al. 2003). According to the guidelines, the potential risks of a GM crop to biodiversity in Japan are mainly evaluated with regard to (1) competitiveness, which involves the ability to compete with wild plants in terms of nutrients, sunlight, and growing area and to interfere with their growth; (2) the production of harmful substances, which involves the ability to produce substances that interfere with the habitat or growth of wild plants, animals, or microorganisms; and (3) crossability, which involves the ability to interbreed with closely related wild organisms and then to replace and reduce wild relatives with hybrid plants. The standard of these assessments is the familiarity between GM crops and conventional species.
Application for cultivation approval generally involves a two-step approval process. The first step is to apply for approval to conduct confined field trials. The application is performed after test cultivation in a laboratory or in a specific netted greenhouse with measures taken to prevent spread, followed by an environmental risk assessment based on the collected data. The second step is to apply for approval for cultivation in general fields or distribution as food and feed. The purpose of confined field trials is to compare the results obtained in controlled environments such as a laboratory or specific netted greenhouses with the results obtained from trials in confined fields, which are under realistic conditions (Raybould 2007). It is important to determine whether the cultivation of GM crops is generating unintended adverse changes in relation to the endpoints of environmental risk assessments (Nakai et al. 2015; Raybould 2007). It is also important to conduct tests that are possible only under realistic conditions, such as wintering ability, weediness and competitiveness. In many countries worldwide, environmental risk assessments are usually conducted in multiple locations over multiple years to account for differences in growth due to soil type, weather, and other environmental conditions. However, unlike other major GM crops, it is expected that the practical production of miraculin-accumulating tomato will be performed exclusively through nutriculture in greenhouses. This means that the effect of different environmental conditions on growth is extremely small. Therefore, in this study, an environmental risk assessment was conducted through one confined field trial in two different seasons. Miraculin-accumulating GM tomato line 5B was approved by the MAFF and MOE for cultivation in a confined field from June 2018 to March 2020 (J-BCH 2021b).
There have been 175 regulatory approvals of GM crop species, mainly corn, soybean, canola and cotton, for environmental safety in Japan (J-BCH 2021b). In most of these studies, the details of the procedures and the results of the experiments conducted for environmental risk assessment in confined field trials are usually not disclosed. Although a few reports have described the details of confined field trials in soybean (Matsushita et al. 2020), cotton (Asanuma et al. 2017) and oilseed rape (Asanuma et al. 2011), they are atypical. In addition, to date, there is no regulatory approval for the cultivation of GM tomato plants in Japan.
In the present study, we performed a confined field trial for a miraculin-accumulating GM tomato line and investigated the plant morphology, growth characteristics, cold tolerance at the early stage of growth, overwintering ability and allelopathic effect as parameters of competitiveness as well as production of harmful substances. The overall aim was to compare our GM tomato with conventional tomato and to assess whether the environmental risks to biodiversity were comparable. A method of evaluating GM crops for environmental risk assessment needs to be established for each crop. Therefore, information on a variety of crops is needed, and the information in this paper contributes to similar regulatory science efforts.
Materials and methods
Since there are no closely related wild species of tomato in Japan, evaluation of crossability was not required for our GM tomato. Therefore, an environmental risk assessment in this study was performed with respect to the competitiveness and production of harmful substances. The content and methods of the survey were in accordance with appropriate guidelines (MAFF and MOE 2007).
Plant morphology and growth characteristics
The study was conducted on tomato plants grown in a confined field at the T-PIRC Gene Research Center, University of Tsukuba, from 2018–2019. Seeds of a non-GM tomato plant (Solanum lycopersicum L. cv. Moneymaker) and a GM miraculin-accumulating tomato plant (line 5B, T6 generation, cv. Moneymaker) were planted on August 8, 2018, for fall-winter cultivation and on March 4, 2019, for spring-summer cultivation in sterilized soil (Kumiai Horticultural Seedling Medium, Genkikun No. 1). The seedlings were first grown in a cultivation room (25°C, 16 h light/8 h darkness) and then transferred to a plastic greenhouse established in a confined field on September 5, 2018, for fall-winter cultivation and again on April 8, 2019, for spring-summer cultivation; the seedlings were transplanted into 6 l pots filled with red ball earth below potting media (Kumiai Horticultural Seedling Medium, Genkikun No. 1). Normally, farmers plant tomato directly in the field or use nutriculture. We are planning to grow miraculin-accumulating tomato exclusively for nutriculture in greenhouses. For this reason, we used the pot cultivation method, as this method is closer to actual cultivation methods. The axillary buds were removed, and one stem per plant was used in this experiment.
Characteristic surveys were conducted in accordance with the Standards for the Characteristics of Tomato Varieties Registered (Ministry of Agriculture, Forestry and Fisheries, Test Guideline for Plant Varieties). Shape characteristics (length and width of the largest compound leaf and foliole) and days until flowering were investigated during the flowering period of the second and third bunches (2–3 months after sowing), and fruit characteristics (number of fruits, fruit weight, fruit lateral diameter, fruit vertical diameter, and number of seeds) were investigated during the harvest period of the first to third bunches (3–4 months after sowing). Days until flowering (flowering speed) was evaluated as the number of days between flowering on the second flower of the second cluster and the second flower of the third cluster. The survey was essentially conducted on the second or third flower, but for those that deviated, the survey was conducted on the fourth or fifth flower. The average internode length from the first to the fourth fruit cluster was also measured. Leaf surveys were conducted on one leaf per individual plant, and fruit characterization surveys were conducted on three to five fruits per individual. Two to three fully developed flowers per individual plant were selected for floral morphology assessment, and the number of petals, flower diameter and flower color were evaluated. Flower color was assessed according to the number of the RHS color chart (Sixth Edition, Royal Horticultural Society). The inflorescence was also evaluated.
To study pollen, three flowers were collected from each plant, and the stamens and pistils were removed together with tweezers and placed in a 1.5-ml Eppendorf tube, for three flowers. A total of 200 µl of Alexander staining solution (Alexander 1969) was added to the Eppendorf tube, and the contents were mixed by crushing the anthers with the tip of a pipette. After incubating in the dark overnight at room temperature, the pollen count and pollen size were measured under a microscope. The pollen count was determined by measuring the pollen density using a cell counter (Biomedical Sciences), and the pollen diameter was measured for 5 randomly selected pollen samples per plant under a microscope. Circular pollen that was stained red was considered normal pollen, pollen that was not stained red and was deformed was considered abnormal pollen, and the percentage of normal pollen was determined.
Cold tolerance tests at the early stage of growth
Seeds of the tomato cultivar Moneymaker, a non-GM tomato and a GM tomato that accumulates miraculin (5B, T6 generation) were sown on October 17, 2018, in 4×4 groups of pots containing sterilized soil (Kumiai Horticultural Nursery Medium, Genkikun No. 1). Seedlings were grown in a cultivation room at the T-PIRC Gene Research Center at the University of Tsukuba (25°C, 16 h light/8 h darkness). Approximately 2 weeks after sowing, the plant height and stem diameter were measured, and cold treatment (5°C, 16 h light/8 h darkness) was applied in an artificial growth chamber (TOMY CF-305) for 8 of 12 plants. Each of the remaining four plants was grown under nursery conditions (25°C, 16 h light/8 h darkness). Surveys were conducted once a week to measure the plant height and diameter of the stems, and images of the plants were taken. The survey of the plants without cold treatment was terminated on November 23, 2018 (20 days after cold treatment), when cultivation in the groups of pots reached the shelf limit. On December 18, the seedlings grown at low temperature for 46 days were moved to a rack with bird netting installed in a confined field. During the field test, images were periodically taken under low-temperature conditions during winter.
Overwintering ability test
Seeds of Moneymaker, a non-GM tomato and a GM tomato that accumulates miraculin (line 5B, T6 generation) were sown on October 19, 2018 in sterilized soil (Kumiai Horticultural Seedling Medium, Genkikun No. 1). The seedlings were grown in a cultivation room (25°C, 16 h light/8 h darkness). On November 22, 2018, the seedlings were transferred to a plastic greenhouse established in a confined field and planted in 6 l pots filled with red ball earth at the bottom and in media (Kumiai Horticultural Nursery Medium, Genkikun No. 1). The plants were cultivated in a plastic greenhouse for approximately two weeks and moved outside the greenhouse on December 7, 2018. Bird netting was installed to cover the entire plants to prevent damage by wild bird feeding.
Production of harmful substances
Effects of soil mixed with GM tomato residues on plant growth (plow-in method)
The tests with plow-in method were conducted as described in previous reports, with some modifications (Ko et al. 2019; Shiomi et al. 1992; Yu et al. 2013). Approximately 50 g of leaves (fresh weight) was collected from 5–6 individuals of non-GM and GM tomato plants grown in a confined field in 2018–2019 and air-dried at 60°C for several days until the material was dry. Afterward, the leaves were crushed using a mortar and pestle. Sixteen lettuce seeds (Lactuca sativa L. cv. Cisco) were then sown in pots containing a mixture of 4 g of powdered tissue and 200 g of fresh, sterilized soil (Kumiai Horticultural Nursery Medium, Genkikun No. 1). The number of seeds that germinated after one week was counted, and the germination rate was calculated for each soil. The stem length and root length of the seedlings were also measured.
Effects of soil grown with GM tomato on plant growth (succeeding crop test)
Succeeding crop tests were conducted as described in previous reports, with some modifications (Shiomi et al. 1992; Tran et al. 2018). After cultivation, soil was collected from the pots of eight (2019) or thirteen (2018) non-GM and GM tomato plants and added to 4×4 groups of pots. Sixteen lettuce seeds (Lactuca sativa L. cv. Cisco) were then sown into the pots. The number of seeds that germinated after one week was counted, and the germination rate of each soil was calculated. The stem length and root length of the seedlings were also measured.
Effect of GM tomato on soil microorganisms
Post-cultivation soil (30 g) was collected from the pots of five (2019) or six (2018) non-GM and GM tomato plants and put in a sterile 500 ml flask containing 270 ml of 15 mM phosphate buffer (pH 7.0). After stirring (100–150 rpm) for 10 min at room temperature, 5 ml of the contents of the 500 ml flask was added to a sterile flask containing 45 ml of 15 mM phosphate buffer (pH 7.0), yielding a 102-fold dilution. The dilutions were subsequently made into 102- to 106-fold dilutions. Each diluted solution (0.1 ml) was added to the agar medium of a 9 cm diameter petri dish. For fungal investigation, OGYE agar medium (5 g l−1 bacto yeast extract, 20 g l−1 glucose, 0.1 mg l−1 biotin, 12 g l−1 agar, 50 mg l−1 oxytetracycline (pH 7.0)) was used, and solutions of 102- to 104-fold dilutions were applied to three petri dishes each (total of 9 petri dishes per individual). For bacterial and actinomycete investigations, PTYG agar medium (0.25 g l−1 bacto pepton, 0.25 g l−1 bacto trypton, 0.5 g l−1 bacto yeast extract, 30 mg l−1 MgSO4·7H2O, 3.5 mg l−1 CaCl2·2H2O, 15 g l−1 agar) was used, and solutions of 104- to 106-fold dilutions were added to three petri dishes each (total of 9 petri dishes per individual). Petri dishes coated with the diluted solution were incubated at 20°C. Colonies were measured after 4 days of incubation on OGYE plates and after 7 days on PTYG plates. Thirty grams of cultivated soil collected at the same time was air-dried at 60°C for 48 h to measure the moisture content, and the number of bacteria in the media were calculated per gram of dry soil.
Results and discussion
The environmental risk assessment is essentially done by comparing the GM lines with non-GM conventional varieties and assessing their familiarity. Therefore, in this study, an evaluation was performed by comparing the non-GM tomato cultivar Moneymaker, which is a GM tomato host, with GM tomato.
Plant morphology and growth characteristics
Among the investigated traits, the width of the largest compound leaf of the GM tomato plants was significantly smaller than that of the non-GM tomato plants (Table 1). However, there were no significant differences in any of the other traits. These results indicated that the morphological characteristics of the GM tomato were nearly the same as those of the non-GM tomato. In addition, the significantly shorter width of the compound leaves of the GM tomato plants indicates that, compared with non-GM tomato plants, these plants have a lower degree of shading of neighboring plants, which means that they have a weaker effect of interfering with the growth of other plants. Taken together, these results indicated that, compared with non-GM tomato, GM tomato does not have an increased impact on biodiversity.
Table 1. Characteristics of the plant morphology of non-GM and GM tomato.
| Unit | September–December 2018 | April–July 2019 | p | |||
|---|---|---|---|---|---|---|
| Non-GM | GM (5B) | Non-GM | GM (5B) | |||
| Largest compound leaf | length (cm) | 46.9±0.6 (n=24) | 47.9±0.4 (n=24) | 48.3±0.5 (n=24) | 44.4±0.6 (n=24) | 0.534 |
| width (cm) | 41.7±0.9 (n=24) | 37.2±1.0 (n=24) | 39.1±0.9 (n=24) | 34.2±0.8 (n=24) | <0.001* | |
| Foliole | length (cm) | 21.7±0.5 (n=24) | 21.8±0.3 (n=24) | 20.2±0.4 (n=24) | 18.1±0.5 (n=24) | 0.536 |
| width (cm) | 10.5±0.3 (n=24) | 11.1±0.4 (n=24) | 9.5±0.4 (n=24) | 9.4±0.4 (n=24) | 0.561 | |
| Flowering speed1 | (days) | 7.6±0.4 (n=22) | 5.9±0.5 (n=21) | 6.8±0.3 (n=24) | 6.2±0.3 (n=24) | 0.121 |
| Fruit weight | (g) | 100.5±4.0 (n=100) | 95.6±3.6 (n=115) | 114.5±8.8 (n=114) | 104.7±2.5 (n=106) | 0.166 |
| Fruit diameter | (mm) | 58.0±0.8 (n=100) | 56.4±0.8 (n=115) | 60.4±0.6 (n=114) | 59.6±0.8 (n=106) | 0.117 |
| Longitudinal diameter of fruit | (mm) | 51.8±0.8 (n=100) | 51.2±0.7 (n=115) | 53.1±0.5 (n=114) | 54.2±0.5 (n=106) | 0.813 |
| Seeds number | (seeds fruit−1) | 43.5±3.8 (n=100) | 44.4±3.4 (n=115) | 58.8±3.6 (n=114) | 71.1±4.3 (n=106) | 0.455 |
| Average internodal length2 | (cm) | 14.1±0.4 (n=21) | 14.5±0.4 (n=21) | 19.9±0.5 (n=22) | 20.3±0.9 (n=18) | 0.341 |
1) Flowering speed: The number of days between flowering of the second flower of the second cluster and the second flower of the third cluster. Essentially, the second or third flower was used. 2) The length from the first to the fourth flower clusters was divided by three. The values are the means±SEs. Statistical analysis was performed with a generalized linear mixed model, with genotype as a fixed effect and year of cultivation as a variable effect. The p values indicate the effect of genotype. * indicates a significant difference between non-GM and GM (5B) tomato for each measurement at p<0.01.
No statistically significant differences were observed in any of the floral morphology traits (Supplementary Table S1). The inflorescence type of both non-GM and GM tomato was a scorpioid cyme monochasium on a single cluster and a compound type of monochasium on a double cluster (data not shown). The flower color of both types of tomato plants was 5A, 6A, 6B, or 7A (brilliant greenish yellow or brilliant yellow) in the yellow group, and there were no significant differences between the non-GM and GM tomato plants (Figure 1). These results suggested that the transgene did not affect the inflorescence, flower morphology or color.
Figure 1. Flower color of non-GM and GM tomato plants. Flowers of tomato cultivated in a confined field in 2018 are shown. Flower color was assessed by the number of the RHS color chart (Sixth Edition, Royal Horticultural Society).
As shown in Table 2, there was no statistically significant difference in the normal pollen count or normal pollen percentage between the non-GM and GM tomato. In addition, no significant difference in pollen size was observed between these groups. Together, these results suggested that the transgene had no effect on pollen formation.
Table 2. Characteristics of pollen morphology and maturation for non-GM and GM tomato.
| Unit | September–December 2018 | April–July 2019 | p | |||
|---|---|---|---|---|---|---|
| Non-GM | GM (5B) | Non-GM | GM (5B) | |||
| Normal pollen count | (pollens µl−1) | 3,860±199 (n=12) | 3,432±149 (n=12) | 3,235±221 (n=9) | 3,452±288 (n=9) | 0.724 |
| Normal pollen rate | (%) | 95.2±0.5 (n=12) | 95.2±0.4 (n=12) | 88.0±2.8 (n=9) | 87.2±1.9 (n=9) | 0.814 |
| Pollen diameter | (µm) | 21.2±0.2 (n=30) | 21.3±0.1 (n=30) | 18.8±0.2 (n=35) | 18.7±0.2 (n=35) | 0.871 |
The values are the means±SEs. Statistical analysis was performed with a generalized linear mixed model, with genotype as a fixed effect and year of cultivation as a variable effect. The p values indicate the effect of genotype.
Similar investigations of growth and morphological characteristics related to plants, flowers and pollen conducted in a specific netted greenhouse did not show any significant differences in any of the parameters (J-BCH 2021a). The results of both the specific netted greenhouse and the confined field agree with our conclusion that the morphology and growth characteristics of GM tomato do not enhance their competitiveness compared with those of non-GM tomato.
Cold tolerance tests at the early stage of growth
Since tomato is a summer crop species, it is necessary to investigate the growth of young seedlings under winter-season conditions in Japan (MAFF and MOE 2007). After one week of cultivation under low temperature in an artificial growth chamber, anthocyanin accumulation due to cold stress was observed in the stems and undersides of the leaves (Figure 2B). The accumulation level of anthocyanins was more significant in non-GM tomato than in GM tomato. However, the accumulation level gradually increased in the GM tomato, and anthocyanins accumulated in nearly the whole stem and in the abaxial side of the leaves on day 42 under low temperature. In contrast, anthocyanin accumulation in non-GM tomato decreased as the anthocyanins approached the growing point. Plant height was mostly stagnant until day 20 in the low-temperature treatment compared with the noncold treatment but gradually increased from day 28 (Figure 2). From the beginning of the low-temperature treatment to day 14, the height of the GM tomato plants was significantly greater than that of the non-GM tomato plants, but from day 35, the height of the non-GM tomato plants was significantly greater than that of the GM tomato plants (Figure 2 and Supplementary Figure S1). The stem diameter was always thicker for non-GM tomato than for GM tomato during the experiment. The growth of stems slowed as a result of the low temperature for both non-GM and GM tomato plants compared to noncold-treated tomato plants, but the growth did not stop.
Figure 2. Effect of low temperature on early growth of tomato seedlings. (A) Day 0 (2 weeks after sowing), (B) day 7, (C) day 14, (D) day 20, (E) day 28, (F) day 35, and (G) day 42 of the low-temperature treatment (5°C, 16 h light/8 h darkness) in an artificial climate chamber. Scale bars shows 5 cm.

The results above indicated that cultivation at low temperature in the early stage of growth resulted in suppressed growth for both tomato types, but the effect was more marked for the GM tomato plants than for the non-GM tomato plants. An accumulation of anthocyanins in leaf and stem tissues at low temperature was also observed in GM tomato, although the accumulation was delayed by one to two weeks compared to that in non-GM tomato. Gene expression of enzymes important for anthocyanin biosynthesis has been found to increase during the latter half of tomato cold acclimation, resulting in an increase in anthocyanins (Barrero-Gil et al. 2016). This means that GM tomato plants were also cold acclimatized. In GM tomato, the 35S promoter constantly produces miraculin throughout the growth period in all tissues (J-BCH 2021a; Kim et al. 2010; Sun et al. 2007). It is possible, therefore, that the delayed response to low temperature may be related to an adjustment in the metabolic balance or load of miraculin production. The possible effect of miraculin on the cold response is also discussed. The physiological reasons for the presence of miraculin in miracle fruit are unclear; nonetheless, on the basis of sequence similarity, miraculin is classified as a member of the Kunitz-type soybean trypsin inhibitor family (Takai et al. 2013; Theerasilp et al. 1989). Kunitz-type inhibitors of soybean act as plant defense mechanisms, inhibiting the enzymatic activity of digestive proteases that are important for the survival of pests and pathogens (Selvakumar et al. 2011). The miraculin-like proteins of tomato (Brenner et al. 1998), rough lemon (Tsukuda et al. 2006), and coffee (Mondego et al. 2011), which belong to the same family as miraculin, have trypsin inhibitor activity and have been reported to be involved in defensive functions to protect plants from pests and injury stress. However, miraculin does not exhibit trypsin inhibitor activity (Takai et al. 2013). Therefore, it is unlikely to have the same defensive function as miraculin-like proteins. Reactive oxygen species (ROS) that accumulate in cells under various stresses affect the expression of cold-regulated genes (Chinnusamy et al. 2007). Although miraculin may not have a defensive function, it may be possible that miraculin itself acts as a stress signal, resulting in a chronically stressed state and accumulation of ROS, which in turn affects the cold stress response. However, further research is needed to verify this hypothesis. GM tomato in the early stages of growth showed lower tolerance to high temperature than non-GM tomato did, as their growth points died by day 28 of the 35°C high-temperature treatment, whereas all the growing points of the non-GM tomato plants survived (data not shown). Based on these results, it was suggested that GM tomato in the early stage of growth was less adaptable to environmental stresses than non-GM tomato.
In the indoor tests, neither type of plant died during the experiment. In other words, the results suggested that they had acclimated to low temperatures. The nursery plants grown in an artificial growth chamber at 5°C for approximately 6 weeks in the above experiment were moved outdoors to evaluate their tolerance to low temperatures. The average temperature in the confined field throughout the test period was 5.6°C, which was comparable to the conditions of the indoor cultivation, but the minimum temperature was approximately 0°C on many days, with the lowest temperature of –1.2°C being recorded one day. On days 2 and 5 of cultivation, the leaves of every plant withered from the bottom; on day 8, the stems were bent, and the growing points had died (Supplementary Figure S2). On day 2 of cultivation, the GM tomato plants seemed to be more affected by low temperature, but on days 5 and 8, there was no difference between the plant types.
These results revealed that both non-GM and GM tomato plants in the early stage of growth have difficulty growing outdoors in winter when the minimum temperature is below 0°C. Taken together with the indoor test results, these results suggested that although the acclimation ability of non-GM tomato was better than that of GM tomato under low-temperature conditions where they could acclimate, there was no difference in low-temperature tolerance in environments where the minimum temperature was below 0°C.
Overwintering ability test
In this study, we examined the potential of GM tomato to overwinter under field conditions. The tomato plants were moved outdoors on December 7, 2018, and three days later, both the non-GM and GM tomato plants appeared wilted (Figure 3). At this stage, the lateral shoots remained alive, but 11 days later, on December 18, the stems had completely turned brown and died. During the test period, the average temperature was 4.6°C, and the minimum temperature was −5.3°C. There were 8 days when the minimum temperature was below 0°C until the end of the test, and frost columns were also observed in the field. Tomato can survive at temperatures in the range of 5 to 40°C but require minimum temperatures above 18°C for vegetative growth, are they very sensitive to temperatures below 10°C and can freeze to death at 1–2°C (Lyons 1973; OECD 2017; Tomato Dictionary 2015). Moreover, tomato does not tolerate frost (OECD 2017). Tomato plants can be acclimatized to low temperatures to a certain extent, but even cold-acclimated tomato plants are still sensitive to freezing temperatures (Barrero-Gil et al. 2016). Since the minimum temperature was below 0°C, the tomato plants were considered to have not survived. According to the above results, this GM tomato line did not have overwintering ability, nor did the non-GM tomato.
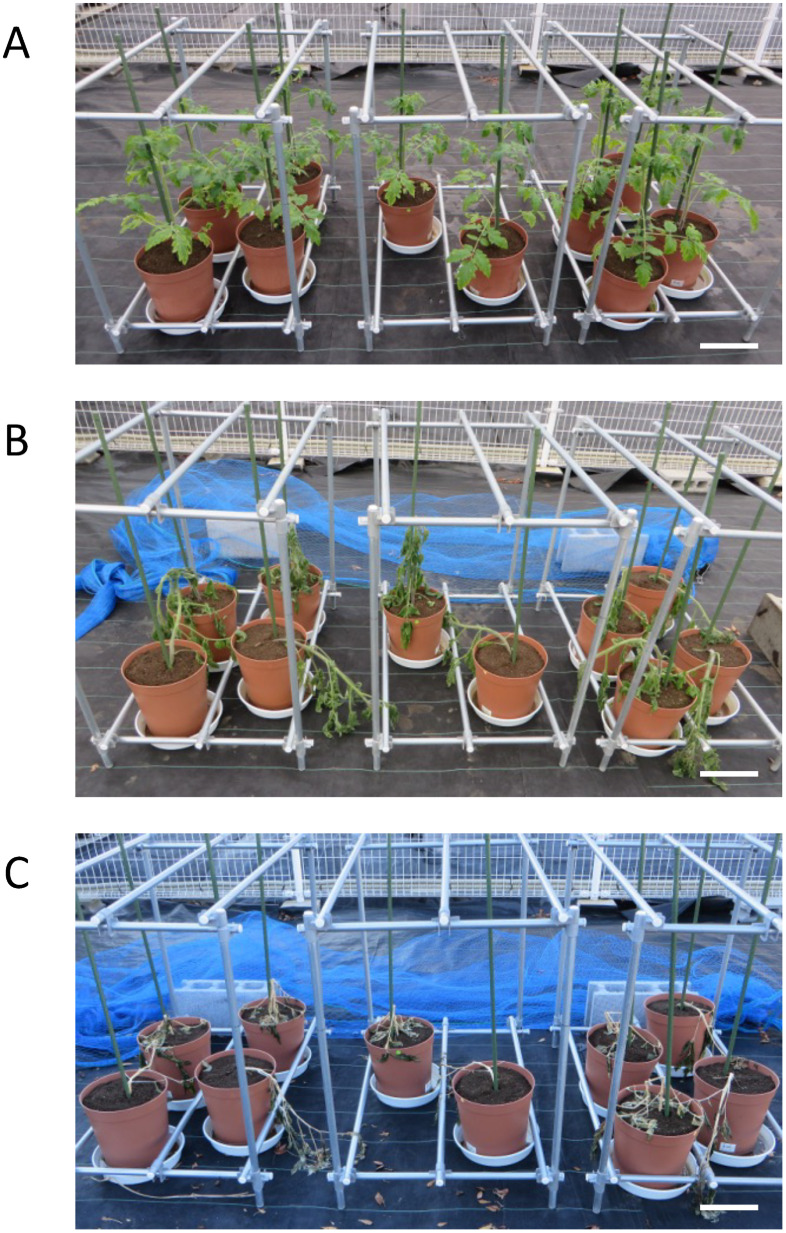
Figure 3. Conditions of tomato plants cultivated in the winter in a confined field. (A) December 7, 2018 (day 0), (B) December 10, 2018 (day 3), (C) December 18, 2018 (day 11). After sowing, the seedlings were grown for one month in a cultivation room and then transferred to a plastic greenhouse in a confined field. Two weeks after planting, the plants were moved outside of the plastic greenhouse for the experiment. Scale bars shows 20 cm.
Plant growth on soil mixed with GM tomato residues (plow-in method)
The plow-in method was used to evaluate the effects of substances in the leaves and other parts of GM plants after they died on other plants. In the experiment using leaves of plants under fall-winter cultivation in 2018, statistically significant differences in the hypocotyl length of lettuce seedlings were observed between the non-GM and GM tomato treatments (Table 3). However, there was no significant difference in lettuce root length. In the experiment using leaves from plants under spring-summer cultivation in 2019, there was no statistically significant difference in either hypocotyl length or root length of lettuce seedlings. Similarly, there was no statistically significant difference in the germination rate of lettuce seeds between the 2018 and 2019 trials.
Table 3. Effects of soil mixed with GM tomato residue on lettuce growth.
| September–December 2018 | p | April–July 2019 | p | ||||
|---|---|---|---|---|---|---|---|
| Non-GM | GM (5B) | Non-GM | GM (5B) | ||||
| Hypocotyl | (cm) | 0.71±0.03 (n=71) a | 0.80±0.02 (n=75) | 0.013* | 1.00±0.03 (n=83) | 0.99±0.04 (n=85) | 0.875 |
| Root | (cm) | 2.78±0.11 (n=72) | 2.93±0.09 (n=75) | 0.295 | 1.59±0.07 (n=83) | 1.70±0.08 (n=85) | 0.316 |
| Germination rate | (%) | 90.0±4.2 (n=5) b | 93.8±2.8 (n=5) | 0.481 | 86.5±3.0 (n=6) | 88.5±3.0 (n=6) | 0.632 |
The values are the means±SEs. a: The number of lettuce seedlings that germinated and used for the measurements. b: The number of pots whose soil was mixed with each plant residue. Sixteen lettuce seeds were sown in each pot, and the germination rate was determined. Statistical analysis was performed by the F-test followed by Student’s t-test for equal variances and Welch’s t-test for unequal variances. * indicates a significant difference between the non-GM and the GM tomato at p<0.05.
In the 2018 trial, the hypocotyl length of lettuce seedlings in soil mixed with GM tomato leaves was significantly longer than in soil mixed with non-GM tomato leaves. However, this result does not indicate that our GM tomato has increased production of harmful substances. Instead, it leads to the conclusion that residues of the GM tomato had little or no effect on both the germination of lettuce seeds and growth of young seedlings. Tomatine is one of the major harmful substances in tomato plants, especially in the leaves (Friedman 2002). Therefore, the present study suggests that harmful substances such as tomatine do not over accumulate in our GM tomato. Separately, we found no significant difference between the tomatine content in red ripe fruits of GM and non-GM tomato (data not shown), which may further support the present conclusion.
With respect to our experiment using the sandwich method (Fujii et al. 2003; Itani et al. 1998) conducted during cultivation in a specific netted greenhouse, there was no significant difference in the hypocotyl length of lettuce on agar media embedded with leaves of GM or non-GM tomato (J-BCH 2021a). In contrast, the root length of lettuce in media including the young leaves of GM tomato plants was significantly shorter than that of in media including the young leaves of non-GM plants. However, there was no significant difference in the root length of lettuce in media including the leaves of Momotaro, which is a Japanese domestic cultivar, grown in the same way, indicating that the significant difference in the root length in the media including young leaves compared with that of non-GM was within the range of differences between tomato varieties.
The results of the specific netted greenhouse did not match the results of the confined field trials, but the conclusion coincided in that there was no increase in harmful substances in the plant residues of GM tomato compared to common tomato cultivars.
Plant growth on soil pre-cultivated with GM tomato (succeeding crop test)
Succeeding crop tests were used to evaluate the effects of secretions from the roots of GM plants on other plants. The root length of lettuce seedlings was significantly shorter in soils pre-cultivated with GM tomato than soils pre-cultivated with non-GM tomato, regardless of the growing season (Table 4). On the other hand, there were no significant differences in the germination rate of lettuce seeds or their hypocotyl length. Tomato plants have an inherent allelopathic effect, and it was reported that lettuce germination and seedling growth are inhibited when grown near tomato (Kim and Kil 2001). This inhibitory effect could be attributed to tomatine, one of the allelopathic compounds secreted from tomato roots (Rial et al. 2018), and whose phytotoxic effect towards lettuce and two weeds (Echinochloa crusgalli and Lolium perenne) has been clearly demonstrated. Indeed, the growth of lettuce hypocotyls was significantly suppressed in soils pre-cultivated with either non-GM and GM tomato plants compared with fresh soil (Supplementary Table S2). However, lettuce grown in fresh soil had shorter roots than those grown in soils pre-cultivated with GM and non-GM tomato plants. This finding is inconsistent with previous reports that lettuce root growth is more sensitive than the hypocotyl to the inhibitory effects of tomatine (Rial et al. 2018). While fresh soil is free of contaminants such as plant roots, it has smaller pores, making it more difficult for roots of subsequent plants to physically grow compared with plants in pre-cultivated soil. Asanuma et al. (2017) also pointed out that the roots of test plants are susceptible to external factors and thus, they eliminated soil pores by using a 5-mm mesh screen to remove plant residues and make the soil particle size uniform. In the present study, we removed the major root residues visually. Therefore, root contamination of the soil and the difference in soil particle size may affect the root length of lettuce. To evaluate the harmful substances secreted from the roots of GM plants based on the length of the test plant roots, it is essential to eliminate physical differences in the soil as much as possible.
Table 4. Effects of soil in which GM tomato was grown on lettuce growth.
| September–December 2018 | p | April–July 2019 | p | ||||
|---|---|---|---|---|---|---|---|
| Non-GM | GM (5B) | Non-GM | GM (5B) | ||||
| Hypocotyl | (cm) | 0.84±0.03 (n=85)a | 0.83±0.04 (n=55) | 0.904 | 1.18±0.03 (n=116) | 1.16±0.03 (n=115) | 0.615 |
| Root | (cm) | 3.73±0.19 (n=85) | 3.01±0.24 (n=55) | 0.020* | 4.56±0.12 (n=116) | 3.95±0.11 (n=113) | <0.001** |
| Germination rate | (%) | 40.9±10.6 (n=13)b | 26.4±10.1 (n=13) | 0.336 | 90.6±4.1 (n=8) | 89.8±3.5 (n=8) | 0.887 |
The values are the means±SEs. a: The number of lettuce seedlings that germinated and were used for the measurements. b: The number of pots filled with soil after tomato cultivation. Sixteen lettuce seeds were sown in each pot, and their germination rates were determined. * indicates a significant difference between the non-GM and the GM tomato at p<0.05 according to Student’s t-test. ** indicates a significant difference between non-GM and GM individual tomato plants at p<0.01 according to Student's t-test.
Lettuce root lengths were longer in soils in which GM and non-GM tomato plants were grown than in fresh soils, while hypocotyl growth was significantly reduced in both tomato plant types compared with that in fresh soils. This means that both tomato plants have allelopathic effects. In addition, the root length does not reflect the impact of allelopathic effects on this experiment. Moreover, the hypocotyl lengths were not significantly different between non-GM and GM tomato, suggesting that this allelopathic effect was not enhanced in GM tomato plants. Neither root length nor hypocotyl length of lettuce were significantly different in the succeeding crop test where soils in which GM and non-GM tomato plants were grown in a specific netted greenhouse, which is consistent with the present results showing no increase in allelopathic effects (J-BCH 2021a).
Effects of GM tomato on soil microorganisms
Various metabolites are secreted from plant roots into the rhizosphere, which is the zone of soil closest to the roots (Hartmann et al. 2008; van Dam and Bouwmeester 2016). Plants use these secreted metabolites to improve the soil conditions and contribute to interactions such as symbiosis, attraction, and repulsion with soil microorganisms (Guerrieri et al. 2019; Haichar et al. 2014; Massalha et al. 2017; Parniske 2008). As stated earlier, tomatine is one of the major inhibitory compounds secreted from tomato roots and it displays toxicity to a broad range of fungi and bacteria (Nakayasu et al. 2021; Rial et al. 2018; Sandrock and VanEtten 1998).
To evaluate the environmental risk of the root secretions of GM tomato, we investigated the effect on soil microorganisms by dilution plating, which is widely used for the assessment of GM plants in Japan (Ko et al. 2019; Shiomi et al. 1992; Tran et al. 2018; Yu et al. 2013). There were no significant differences in any type of soil microbial count between soils cultivated with non-GM tomato and GM tomato in 2018 (Table 5). There were also no statistically significant differences in fungal or bacterial counts in cultivated soils in 2019. On the other hand, the number of actinomycetes was significantly higher in the soils cultivated with GM tomato than in those cultivated with non-GM tomato. This result indicates that, in comparison with non-GM tomato, GM tomato does not secrete substances that reduce the number of soil microorganisms.
Table 5. Effects of GM tomato on soil microorganisms.
| Unit | September–December 2018 | p | April–July 2019 | p | |||
|---|---|---|---|---|---|---|---|
| Non-GM | GM (5B) | Non-GM | GM (5B) | ||||
| Fungus | (×105 CFU g−1) | 3.58±0.77 | 2.59±0.52 | 0.317 | 0.19±0.02 | 0.37±0.08 | 0.078 |
| Bacteria | (×107 CFU g−1) | 2.20±0.10 | 1.99±0.17 | 0.322 | 2.53±0.41 | 2.22±0.30 | 0.556 |
| Actinomycete | (×106 CFU g−1) | 1.39±0.27 | 1.29±0.24 | 0.796 | 0.70±0.07 | 1.54±0.18 | 0.007* |
CFU: colony forming unit. The values (means±SEs) are indicated as CFUs per dried soil (n=5–6). Statistical analysis was performed by the F-test followed by Student’s t-test for equal variances and Welch’s t-test for unequal variances. * indicates a significant difference between non-GM and GM (5B) tomato for each measurement at p<0.01.
In the cultivation tests conducted in a specific netted greenhouse, the soil of GM tomato had significantly fewer actinomycetes during fall-winter cultivation and significantly more fungi during spring-summer cultivation (J-BCH 2021a). These results were not consistent between the two cultivation tests conducted or with the results of the present tests in the confined field. Unlike direct planting in the field, all the trials were performed in pots, which may have contributed to the high variability. However, since no consistent effects on soil microorganisms were identified, which did not lead to the conclusion that GM tomato secrete more harmful substances than non-GM tomato. In other words, the results suggest that there was no increase in harmful substances like tomatine affecting soil microorganisms in GM tomato.
Conclusion
By evaluating the competitiveness and production of harmful substances, this confined field trial aimed to test the hypothesis that GM tomato is not different from conventional tomato with respect to its potential impact on domestic biodiversity. As shown by the investigation of morphological and growth characteristics, cold tolerance tests at the early stage of growth and overwintering tests, there were no biologically meaningful differences between GM tomato and non-GM tomato for assessment endpoints related to competitiveness. According to the results concerning harmful substances in plant residues and root secretions of GM tomato, there were some significant differences compared to those of non-GM tomato in the assessment endpoints related to the production of harmful substances, but they did not indicate any unintended adverse effects on the environment. Furthermore, these results were compared with those of previous tests conducted in a specific netted greenhouse, which aligned with the conclusion that this GM tomato is comparable to conventional tomato in terms of its environmental risk to biodiversity. Based on these results, we concluded that it was unlikely that the GM tomato line would increase the environmental risk in Japan.
To date, one confined field trial of GM tomato has been conducted in Japan (Shiomi et al. 1992). However, since the Cartagena Law was enforced in 2004, no confined field trials of GM tomato have been conducted, and no applications for general cultivation and distribution have been submitted. This is the first environmental risk assessment of GM tomato in Japan using a confined field conducted under the Cartagena Law and has provided knowledge on environmental risk assessments of other GM tomato plants. Additionally, the evaluation method used in this study might be able to be applied for the evaluation of future GM tomato events. In Japan, there are 175 regulatory approvals for the environmental safety of genetically modified crop species, mainly corn, soybean, rapeseed, and cotton (J-BCH 2021b). However, in most of these approvals, the procedures and details of the experimental results of the confined field trials conducted to assess environmental risks were not disclosed. In recent years, some of these product experiments have been published in scientific papers, such as those concerning soybean (Matsushita et al. 2020), oilseed rape (Asanuma et al. 2011), and cotton (Asanuma et al. 2017), but the information available to the public is limited. Therefore, we hope that the information in this paper will contribute to regulatory science efforts for other crop species, including GM tomato.
Acknowledgments
This study was supported by a joint research project with Inplanta Innovations, Inc and Program on Open Innovation Platform with Enterprise, Research Institute and Academia, Japan Science and Technology Agency (JST-OPERA, JPMJOP1851). We thank the T-PIRC Gene Research Center of the University of Tsukuba for allowing us to use their confined field.
Abbreviations
- GM
genetically modified
Supplementary Data
References
- Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Asanuma Y, Gondo T, Ishigaki G, Inoue K, Zaita N, Muguerza M, Akashi R (2017) Field trial of insect-resistant and herbicide-tolerant genetically modified cotton (Gossypium hirsutum L.) for environmental risk assessment in Japan. GM Crops Food 8: 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma Y, Jinkawa T, Tanaka H, Gondo T, Zaita N, Akashi R (2011) Assays of the production of harmful substances by genetically modified oilseed rape (Brassica napus L.) plants in accordance with regulations for evaluating the impact on biosafety in Japan. Transgenic Res 20: 91–97 [DOI] [PubMed] [Google Scholar]
- Barrero-Gil J, Huertas R, Rambla JL, Granell A, Salinas J (2016) Tomato plants increase their tolerance to low temperature in a chilling acclimation process entailing comprehensive transcriptional and metabolic adjustments. Plant Cell Environ 39: 2303–2318 [DOI] [PubMed] [Google Scholar]
- Brenner ED, Lambert KN, Kaloshian I, Williamson VM (1998) Characterization of LeMir, a root-knot nematode-induced gene in tomato with an encoded product secreted from the root. Plant Physiol 118: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12: 444–451 [DOI] [PubMed] [Google Scholar]
- Friedman M (2002) Tomato glycoalkaloids: Role in the plant and in the diet. J Agric Food Chem 50: 5751–5780 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Parez SS, Parvez MM, Ohmae Y, Iida O (2003) Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol Manage 3: 233–241 [Google Scholar]
- Guerrieri A, Dong LM, Bouwmeester HJ (2019) Role and exploitation of underground chemical signaling in plants. Pest Manag Sci 75: 2455–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haichar FZ, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77: 69–80 [Google Scholar]
- Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312: 7–14 [Google Scholar]
- Hiwasa-Tanase K, Hirai T, Kato K, Duhita N, Ezura H (2012) From miracle fruit to transgenic tomato: Mass production of the taste-modifying protein miraculin in transgenic plants. Plant Cell Rep 31: 513–525 [DOI] [PubMed] [Google Scholar]
- Itani T, Hirai K, Fujii Y, Kohda H, Tamaki M (1998) Screening for allelopathic activity among weeds and medicinal plants using the “Sandwich Methods”. J Weed Sci TechU (Zassou kenkyuu) 43: 258–266 (in Japanese) [Google Scholar]
- J-BCH (2021a) Application for approval of confined field trials of miraculin-accumulating tomato (5B). Japan Biosafety Clearing House. LMO of which type I use regulation is approved under the Cartagena protocol domestic law. https://www.biodic.go.jp/bch/download/lmo/nou%2031%20hyoukasyo%20tomato%20(5B).pdf (Accessed 6 August 2021) (in Japanese)
- J-BCH (2021b) LMO search. Japan Biosafety Clearing House. http://www.biodic.go.jp/bch/lmo/OpenList.do (Accessed 6 August 2021) (in Japanese)
- Kasai M, Ohsawa R (2021) Biotechnology and its regulatory system in Japan. In: Gujar GT, Trisyono YA, Chen M (eds) Genetically Modified Crops in Asia Pacific. CISRO Publishing, Australia, pp 215–231
- Kim YS, Kil B-S (2001) Allelopathic effects of some volatile substances from the tomato plant. J Crop Prod 4: 313–321 [Google Scholar]
- Kim YW, Kato K, Hirai T, Hiwasa-Tanase K, Ezura H (2010) Spatial and developmental profiling of miraculin accumulation in transgenic tomato fruits expressing the miraculin gene constitutively. J Agric Food Chem 58: 282–286 [DOI] [PubMed] [Google Scholar]
- Ko S-S, Liu Y-C, Chung M-C, Shih M-C, Mohammadmehdi H, Oguchi T, Watanabe KN, Yeh K (2019) Environmental biosafety assessment on transgenic Oncidium orchid modified by RNA interference of Phytoene Synthase genes. Plant Biotechnol 36: 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara K, Beidler LM (1968) Taste-modifying protein from miracle fruit. Science 161: 1241–1243 [DOI] [PubMed] [Google Scholar]
- Kurihara K, Beidler LM (1969) Mechanism of the action of taste modifying protein. Nature 222: 1176–1178 [DOI] [PubMed] [Google Scholar]
- Lyons JM (1973) Chilling injury in plants. Annu Rev Plant Physiol 24: 445–466 [Google Scholar]
- MAFF MOE (2007) Concerning the application for approval of type 1 use regulations with regard to the genetically modified plants, the production or circulation of which falls within the jurisdiction of the Minister of Agriculture, Forestry, and Fisheries. Notification No. 8999, Food Safety and Consumer Affairs Bureau, Ministry of Agriculture, Forestry, and Fisheries, Japan. https://www.maff.go.jp/j/kokuji_tuti/tuti/attach/pdf/t0000824-1.pdf (Accessed 3 June 2021) (in Japanese)
- Massalha H, Korenblum E, Tholl D, Aharoni A (2017) Small molecules below-ground: The role of specialized metabolites in the rhizosphere. Plant J 90: 788–807 [DOI] [PubMed] [Google Scholar]
- Matsushita A, Goto H, Takahashi Y, Tsuda M, Ohsawa R (2020) Consideration of familiarity accumulated in the confined field trials for environmental risk assessment of genetically modified soybean (Glycine max) in Japan. Transgenic Res 29: 229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOF MEXT, MHLW, MAFF, METI, MOE (2003) The guidance of implementation of assessment of adverse effect on biological diversity of Type 1 Use of living modified organisms (Notification Number 2 of the Ministry of Finance; Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; Ministry of Agriculture, Forestry and Fisheries; Ministry of Economy, Trade and Industry; Ministry of the Environment). https://www.maff.go.jp/j/syouan/nouan/carta/c_data/law/pdf/notice_summary.pdf (Accessed 25 August 2021) (in Japanese)
- Mondego JMC, Duarte MP, Kiyota E, Martinez L, Camargo SR, De Caroli FP, Alves BSC, Guerreiro SMC, Oliva MLV, Guerreiro-Filho O, et al. (2011) Molecular characterization of a miraculin-like gene differentially expressed during coffee development and coffee leaf miner infestation. Planta 233: 123–137 [DOI] [PubMed] [Google Scholar]
- Nakai S, Hoshikawa K, Shimono A, Ohsawa R (2015) Transportability of confined field trial data from cultivation to import countries for environmental risk assessment of genetically modified crops. Transgenic Res 24: 929–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu M, Ohno K, Takamatsu K, Aoki Y, Yamazaki S, Takase H, Shoji T, Yazaki K, Sugiyama A (2021) Tomato roots secrete tomatine to modulate the bacterial assemblage of the rhizosphere. Plant Physiol 186: 270–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2017) Safety Assessment of Transgenic Organisms in the Environment, Volume 7: OECD Consensus Documents, Harmonisation of Regulatory Oversight in Biotechnology, OECD Publishing, Paris. https://doi.org/10.1787/9789264279728-en (Accessed 6 August 2021)
- Parniske M (2008) Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat Rev Microbiol 6: 763–775 [DOI] [PubMed] [Google Scholar]
- Raybould A (2007) Ecological versus ecotoxicological methods for assessing the environmental risks of transgenic crops. Plant Sci 173: 589–602 [Google Scholar]
- Rial C, Gomez E, Varela RM, Molinillo JMG, Macias FA (2018) Ecological relevance of the major allelochemicals in Lycopersicon esculentum roots and exudates. J Agric Food Chem 66: 4638–4644 [DOI] [PubMed] [Google Scholar]
- Sandrock RW, VanEtten HD (1998) Fungal sensitivity to and enzymatic degradation of the phytoanticipin α-tomatine. Phytopathology 88: 137–143 [DOI] [PubMed] [Google Scholar]
- Sanematsu K, Kitagawa M, Yoshida R, Nirasawa S, Shigemura N, Ninomiya Y (2016) Intracellular acidification is required for full activation of the sweet taste receptor by miraculin. Sci Rep 6: 22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar P, Gahloth D, Tomar PPS, Sharma N, Sharma AK (2011) Molecular evolution of miraculin-like proteins in soybean Kunitz super-family. J Mol Evol 73: 369–379 [DOI] [PubMed] [Google Scholar]
- Shiomi M, Asakawa Y, Fukumoto F, Hamaya E, Hasebe A, Ichikawa H, Matsuda I, Muramatsu T, Okada M, Sato M, et al. (1992) Evaluation of the impact of the release of transgenic tomato plants with TMV resistance on the environment. Bull Natl Inst Agro-Environ Sci Jpn 8: 1–51 [Google Scholar]
- Soares HP, Cusnir M, Schwartz MA, Pizzolato JF, Lutzky J, Campbell RJ, Beaumont JL, Eton D, Stonick S, Lilenbaum R (2010) Treatment of taste alterations in chemotherapy patients using the “miracle fruit”: Preliminary analysis of a pilot study. J Clin Oncol 28(15_suppl): e19523 [Google Scholar]
- Sun HJ, Kataoka H, Yano M, Ezura H (2007) Genetically stable expression of functional miraculin, a new type of alternative sweetener, in transgenic tomato plants. Plant Biotechnol J 5: 768–777 [DOI] [PubMed] [Google Scholar]
- Takai A, Satoh M, Matsuyama T, Ito A, Nakata R, Aoyama T, Inoue H (2013) Secretion of miraculin through the function of a signal peptide conserved in the Kunitz-type soybean trypsin inhibitor family. FEBS Lett 587: 1767–1772 [DOI] [PubMed] [Google Scholar]
- Theerasilp S, Hitotsuya H, Nakajo S, Nakaya K, Nakamura Y, Kurihara Y (1989) Complete amino acid sequence and structure characterization of the taste-modifying protein, miraculin. J Biol Chem 264: 6655–6659 [PubMed] [Google Scholar]
- Tomato dictionary (2015) Crop Characteristics and Classification. Rural Culture Association Press, Tokyo (in Japanese)
- Tran NHT, Oguchi T, Matsunaga E, Kawaoka A, Watanabe KN, Kikuchi A (2018) Environmental risk assessment of impacts of transgenic Eucalyptus camaldulensis events highly expressing bacterial Choline Oxidase A gene. Plant Biotechnol 35: 393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda S, Gomi K, Yamamoto H, Akimitsu K (2006) Characterization of cDNAs encoding two distinct miraculin-like proteins and stress-related modulation of the corresponding mRNAs in Citrus jambhiri Lush. Plant Mol Biol 60: 125–136 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Bouwmeester HJ (2016) Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci 21: 256–265 [DOI] [PubMed] [Google Scholar]
- Wilken MK, Satiroff BA (2012) Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin J Oncol Nurs 16: E173–E177 [DOI] [PubMed] [Google Scholar]
- Yano M, Hirai T, Kato K, Hiwasa-Tanase K, Fukuda N, Ezura H (2010) Tomato is a suitable material for producing recombinant miraculin protein in genetically stable manner. Plant Sci 178: 469–473 [Google Scholar]
- Yu X, Kikuchi A, Shimazaki T, Yamada A, Ozeki Y, Matsunaga E, Ebinuma H, Watanabe KN (2013) Assessment of the salt tolerance and environmental biosafety of Eucalyptus camaldulensis harboring a mangrin transgene. J Plant Res 126: 141–150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.