Abstract
Alpinia zerumbet (Pers.) B.L. Burtt and R.M. Smith belongs to the Alpinia genus in the Zingiberaceae family. In East Asia, Alpinia zerumbet has been widely used as food and traditional medicine. Previously, we identified proanthocyanidins (PACs), an anti-plant-virus molecule in A. zerumbet, using Nicotiana benthamiana and tomato mosaic virus (ToMV). Here, we found that PACs from A. zerumbet, apple, and green tea effectively inhibited ToMV infection. Additionally, the PACs from A. zerumbet exhibited greater antiviral activity than those from apple and green tea. The PACs from A. zerumbet also effectively inactivated influenza A virus and porcine epidemic diarrhea virus (PEDV), which acts as a surrogate for human coronaviruses, in a dose-dependent manner. The results from the cytopathic effect assays indicated that 0.1 mg/ml PACs from A. zerumbet decreased the titer of influenza A virus and PEDV by >3 log. These findings suggested that the direct treatment of viruses with PACs from A. zerumbet before inoculation reduced viral activity; thus, PACs might inhibit infections by an influenza virus, coronaviruses, and plant viruses.
Keywords: Alpinia zerumbet, antiviral activity, coronavirus, influenza virus, tomato mosaic virus
Proanthocyanidins (PACs), also known as condensed tannins, are oligomers and polymers of monomeric flavan-3-ols, such as (+)-catechin and (−)-epicatechin, linked through a specific single (B linkage) and double (A linkage) bonds produced by the flavonoid biosynthetic pathway (Beecher 2004; Dixon et al. 2005). The PACs, abundant in the fruits, bark, seeds, flowers, leaves, and nuts of many plants, exhibit antioxidant activity (Koga et al. 1999) and protect plants from microbial pathogens, insect pests, and herbivores (Barbehenn and Constabel 2011; Bernays 1978; de Colmenares et al. 1998; Scalbert 1991).
Previously, we found that Alpinia zerumbet extracts contained proanthocyanidins (AzPACs) with high antiviral activity against tomato mosaic virus (ToMV) infection of Nicotiana benthamiana of the Solanaceae family (Hatanaka et al. 2021; Narusaka et al. 2020). Alpinia zerumbet (Pers.) B.L. Burtt and R.M. Smith, commonly known as shell ginger, is a member of the Alpinia genus in the Zingiberaceae family (Teschke and Xuan 2018). Native to subtropical and tropical regions in East Asia, including the Nansei islands surrounded by the Pacific Ocean and the East China Sea in Japan, A. zerumbet is also known as an aromatic and medicinal herb plant. A. zerumbet leaves and extracts are used to produce essential oils, herbal tea, flavor noodles, cosmetics, and traditional folk medicine for their anti-inflammatory, antioxidant, bacteriostatic, and fungistatic properties (Elzaawely et al. 2007a, b; Tu and Tawata 2015; Yonaha et al. 2013; Zoghbi et al. 1999). AzPACs are abundant in A. zerumbet extracts. Additionally, the AzPACs, comprising mostly epicatechin units linked through a single B-type linkage, exhibit more than 40 degrees of polymerization. However, AzPACs have not been characterized; thus, this study examined their ability to inhibit plant and animal viruses.
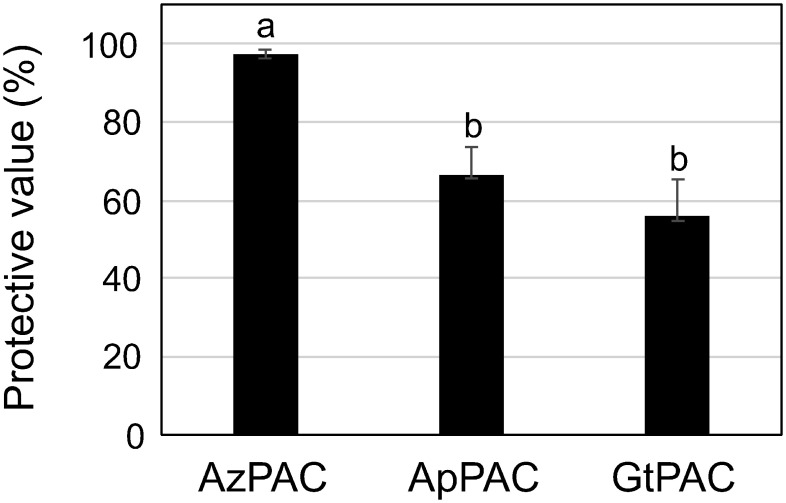
The antiviral assay (Narusaka et al. 2020) was performed using the AzPACs, and PACs were purified from apple juice (ApPAC) and green tea (GtPAC) (Hatanaka et al. 2021), which had approximate molecular weights of 14,700, 8,400, and 1,200–1,900, respectively (Supplementary Figure S1). Our results showed that treatments with the PACs effectively protected N. benthamiana leaves against ToMV-GFP infection compared with the control (Figure 1). Additionally, AzPAC demonstrated greater antiviral activity than GtPAC and ApPAC.
Figure 1. Effects of foliar application of proanthocyanidins on GFP-tagged tomato mosaic virus (ToMV) virions. Nicotiana benthamiana plants were treated with water and 1,000 ppm of proanthocyanidins from Alpinia zerumbet (AzPAC), apple (ApPAC), and green tea (GtPAC) and then inoculated with GFP-tagged ToMV 3 days after treatment. The number of GFP spots formed on the inoculated leaves was counted at 3 days post-inoculation, and the protected value of each application was calculated. Bars indicate the standard error (SE). The experiment was independently performed twice (n>3 per experiment). The differences between the control and treated plants were statistically significant (p<0.01). Different letters represent a statistically significant difference (p<0.05).
During the development of antiviral drugs, medicinal plants have been explored as alternative sources of agents against human and animal viruses (Kwon et al. 2010; Sawamura et al. 2010; Shoji et al. 2017; Watanabe et al. 2011). For example, the influenza virus and coronaviruses are enveloped RNA viruses that can cause outbreaks and even pandemics; particularly, the H1N1 influenza virus and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have caused pandemics (Nicholson et al. 2003; Wölfel et al. 2020). Here, we examined whether the influenza A virus and porcine epidemic diarrhea virus (PEDV), which acts as a surrogate for human coronaviruses, could be inactivated by exposure to PACs.
The effect of PACs on influenza and PEDV infections, which was an indicator of the antiviral activity of the PACs, was investigated using a cytopathic effect (CPE) assay. The treatments with the PACs dose-dependently inhibited CPE in the cells infected with influenza A virus compared with those in the control (Figure 2; Supplementary Figure S2). Additionally, AzPAC showed greater antiviral activity against influenza A virus than GtPAC and ApPAC; 0.1 mg/ml AzPACs decreased the titer of influenza A virus by >3 logs. Then, the CPE assay with PEDV was used to examine whether AzPACs could prevent PEDV infection (Table 1). AzPACs remarkably inhibited CPE compared with the control; AzPACs at 0.1 mg/ml decreased the PEDV titer by >4 logs.
Figure 2. Inactivation of influenza A virus by different concentrations of proanthocyanidins (PACs) from Alpinia zerumbet, apple, and green tea after 30 min. PACs were mixed with or without influenza A H1N1 virus (A/PR/8/34) and added to MDCK cells. After incubation for 4–7 days, a cytopathic effect was observed under an inverted microscope. The 50% endpoint dilution (TCID50/ml) was calculated using the Reed and Muench (1938) method.
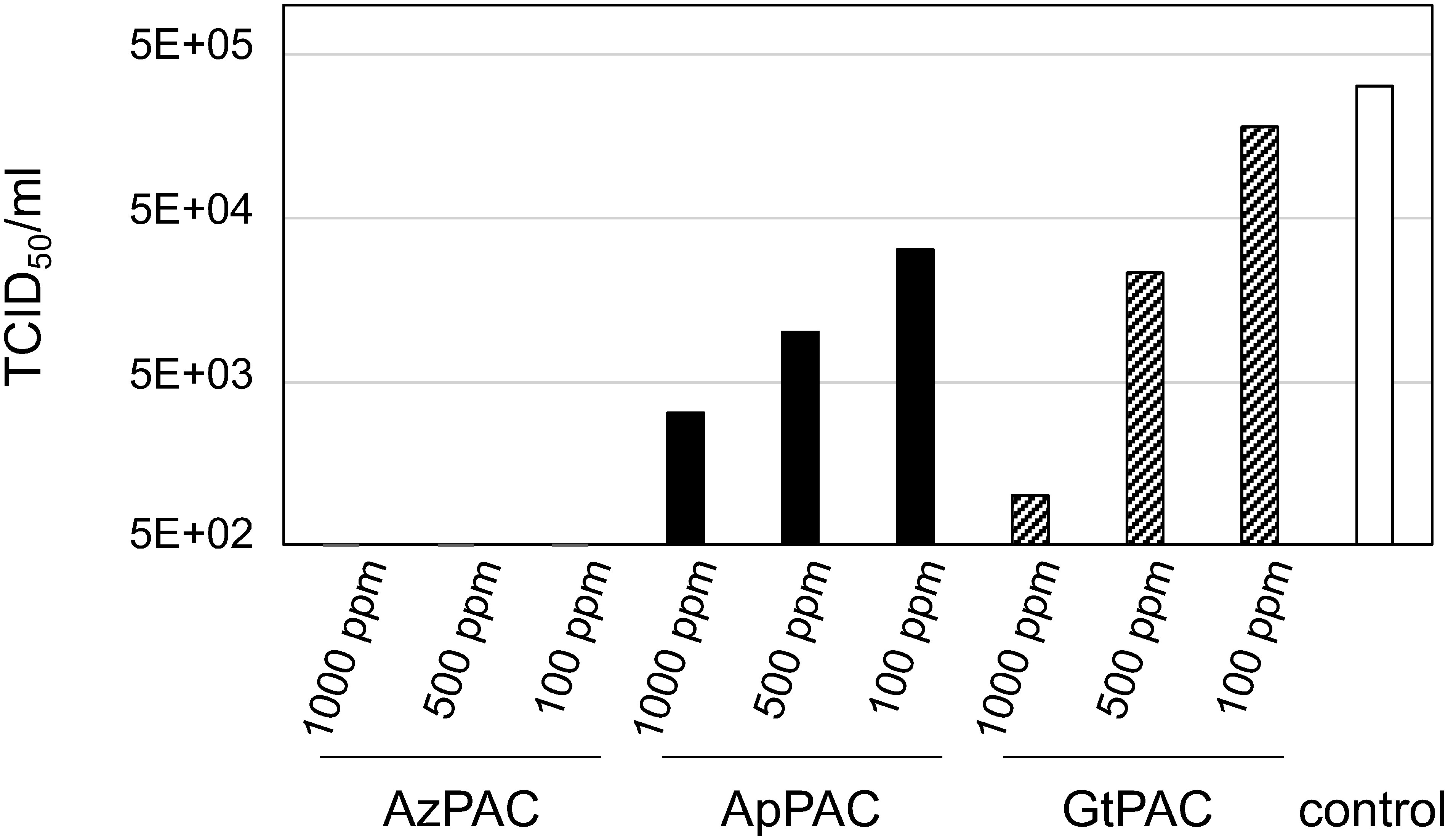
Table 1. Inactivation of porcine epidemic diarrhea virus (PEDV) by different concentrations of proanthocyanidins from Alpinia zerumbet after 30 min.
| Concentrations of proanthocyanidin (ppm) | Contact time (min) | |
|---|---|---|
| 0 | 30 | |
| 0 (control) | 106.5* | 106.5 |
| 100 | <102.5 | |
| 500 | <102.5 | |
* The 50% endpoint dilution (TCID50/ml) was calculated by the Reed and Muench (1938) method.
In conclusion, PACs, especially AzPACs, effectively inactivated influenza A virus and PEDV in vitro in a dose-dependent manner along with inhibiting ToMV infection. The findings suggested AzPACs had broad-spectrum antiviral activities against various viruses. The PACs are polyphenolic compounds in A. zerumbet extracts and likely contribute to most of their biological effects. PACs also have beneficial effects on human health and activities against infectious diseases. PAC-containing plant materials have been used as food, and PACs are safer than other chemicals. Thus, AzPAC is a potential lead compound for developing novel drugs or agricultural chemicals. Future research characterizing the anti-virus mechanisms of PACs will provide further insight into the use of antiviral plant agents as medicine and agricultural chemicals.
Acknowledgments
We thank Dr. Masayuki Ishikawa (National Institute of Agrobiological Sciences) for the pTLBN.G3. We thank Yuriko Imai, Chizuru Namba, Yuko Ihara, Akie Yamamoto, and Shoko Nieda of RIBS for technical assistance. The CPE assays for influenza A virus were performed by FALCO biosystems Ltd. and Protectea Ltd. The CPE assay for PEDV was performed by Shokukanken Inc. The authors would like to thank Enago (www.enago.jp) for the English language review. This work was supported by the research program on development of innovative technology grants from the Project of the Bio-oriented Technology Research Advancement Institution (BRAIN) to T.H. and Y.N.
Abbreviations
- ApPAC
apple juice proanthocyanidins
- AzPACs
Alpinia zerumbet extracts contained proanthocyanidins
- CPE
cytopathic effect
- DMF
dimethylformamide
- GFP
green fluorescent protein
- GtPAC
green tea proanthocyanidins
- PAC
proanthocyanidin
- PEDV
porcine epidemic diarrhea virus
- ToMV
tomato mosaic virus
Supplementary Data
References
- Barbehenn RV, Constabel CP (2011) Tannins in plant-herbivore interactions. Phytochemistry 72: 1551–1565 [DOI] [PubMed] [Google Scholar]
- Beecher GA (2004) Proanthocyanidins: Biological activities associated with human health. Pharm Biol 42(sup1): 2–20 [Google Scholar]
- Bernays EA (1978) Tannins: An alternative viewpoint. Entomol Exp Appl 24: 244–253 [Google Scholar]
- de Colmenares NG, Ramírez-Martínez JR, Aldana JO, Ramos-Niño ME, Clifford MN, Pékerar S, Méndez B (1998) Isolation, characterization and determination of biological activity of coffee proanthocyanidins. J Sci Food Agric 77: 368–372 [Google Scholar]
- Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins—a final frontier in flavonoid research? New Phytol 165: 9–28 [DOI] [PubMed] [Google Scholar]
- Elzaawely AA, Xuan TD, Koyama H, Tawata S (2007a) Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem 104: 1648–1653 [Google Scholar]
- Elzaawely AA, Xuan TD, Tawata S (2007b) Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. and their antioxidant activity. Food Chem 103: 486–494 [Google Scholar]
- Hatanaka T, Narusaka M, Uraji M, Yamaji Y, Narusaka Y (2021) Identification of an anti-plant-virus molecule in Alpinia zerumbet. Bioresour Bioprocess 8: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Moro K, Nakamori K, Yamakoshi J, Hosoyama H, Kataoka S, Ariga T (1999) Increase of antioxidative potential of rat plasma by oral administration of proanthocyanidin-rich extract from grape seeds. J Agric Food Chem 47: 1892–1897 [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Kim HH, Yoon SY, Ryu YB, Chang JS, Cho KO, Rho MC, Park SJ, Lee WS (2010) In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol J 7: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka M, Yamaji Y, Uraji M, Hatanaka T, Narusaka Y (2020) Inhibitory effects of Alpinia zerumbet extract against plant virus infection in solanaceous plants. Plant Biotechnol 37: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Wood JM, Zambon M (2003) Influenza. Lancet 362: 1733–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Hyg 27: 493–497 [Google Scholar]
- Sawamura R, Shimizu T, Sun Y, Yasukawa K, Miura M, Toriyama M, Motohashi S, Watanabe W, Konno K, Kurokawa M (2010) In vitro and in vivo anti-influenza virus activity of diarylheptanoids isolated from Alpinia officinarum. Antivir Chem Chemother 21: 33–41 [DOI] [PubMed] [Google Scholar]
- Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30: 3875–3883 [Google Scholar]
- Shoji M, Woo SY, Masuda A, Win NN, Ngwe H, Takahashi E, Kido H, Morita H, Ito T, Kuzuhara T (2017) Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn. collected in Myanmar. BMC Complement Altern Med 17: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R, Xuan TD (2018) Viewpoint: A contributory role of shell ginger (Alpinia zerumbet) for human longevity in Okinawa, Japan? Nutrients 10: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PT, Tawata S (2015) Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 20: 16723–16740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Takatsuki H, Sonoda M, Tamura S, Murakami N, Kobayashi N (2011) Anti-influenza viral effects of novel nuclear export inhibitors from Valerianae Radix and Alpinia galanga. Drug Discov Ther 5: 26–31 [DOI] [PubMed] [Google Scholar]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469 [DOI] [PubMed] [Google Scholar]
- Yonaha M, Aoki Y, Iwabuchi M, Nagahata T, Fujiwara Y (2013) The inhibitory effects of the hot-water extract of fermented shell ginger (Alpinia speciosa K. Schum.) on melanogenesis in B16 mouse melanoma cells. J Home Econ 64: 215–224 [Google Scholar]
- Zoghbi MGB, Andrade EHA, Maia JGS (1999) Volatile constituents from leaves and flowers of Alpinia speciosa K. Schum. and A. purpurata (Viell.) Schum. Flavour Fragrance J 14: 411–414 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.