Abstract
Rice is an important staple crop and fungal blast disease destroys about 10–30% of its global produce, annually. Although genetic manipulation has largely been employed in crop-improvement programmes and agricultural biotechnology, the ease of transformation of several recalcitrant indica cultivars continues to be a challenge. HR-12 and CO-39 are two indica cultivars that are commonly used in breeding programmes, but are susceptible to biotic threats like fungal blast and sheath blight disease. Here in this study, we have optimised a rapid and reproducible transformation protocol for the said cultivars, having compared both the tissue-culture and in-planta methods of transformation. Murashige & Skoog basal media supplemented with maltose and 2.5 mg l−1 2,4-D induced efficient callogenesis in HR-12, while maltose with 3 mg l−1 2,4-D gave optimum results in case of CO-39. The media containing 0.5 mg l−1 NAA, 3 mg l−1 BAP, and 1 mg l−1 kinetin yielded a maximum regeneration efficiency of 62% and 65% in HR-12 and CO-39, respectively. The studies with Agrobacterium tumefaciens, LBA4404 strain harbouring pCAMBIA1303 suggested that although these cultivars demonstrated successful gene-transfer, they failed to regenerate efficiently, post-transformation. Alternatively, our modified in-planta piercing and vacuum infiltration-based protocol resulted in 33–35% transformation efficiency in less than half the time required for tissue-culture based transformation method. As per our knowledge, it is among the highest obtained from existing piercing-based direct transformation protocols in rice, and can also be implemented in genetically manipulating other recalcitrant varieties of rice.
Keywords: Agrobacterium, fungal-blast, Indica rice, in-planta transformation, regeneration
Introduction
Fungal blast, bacterial blight and sheath blight are three major diseases limiting rice production (Chen et al. 2009). Annually, it causes 10–30% of crop loss across 13 countries, that could feed 60 million people. An unprecedented outbreak of rice blast was observed on a newly released cultivar BRS Colosso in Brazil, in the rice-growing season 2004–2005 (Prabhu et al. 2009). The devastating wheat blast fungus caused havoc in Bangladesh during 2016 and it cannot be ruled out that any day it would pose an epidemic threat on to the rice (Bibi et al. 2013) and wheat fields. The genetic constitution of blast fungus is highly variable and hence often the initially resistant hybrid cultivars become susceptible eventually. Hence, there is a need for a more robust method of developing resistance in these cultivars through genetic transformation. HR-12 is a long-grained, naturally cold tolerant indica rice cultivar, that has been used as donor parent in breeding programmes for developing cold tolerant varieties that are moderately resistant to sheath blight. CO-39 is a short-duration rice variety that has been repeatedly crossed for pyramiding R-genes to develop near isogenic differentially blast resistant lines (Yanoria et al. 2011). However, to our knowledge, no prominent efforts have been made for developing blast resistant transgenics using HR-12 and CO-39 (Mahesh et al. 2016) that are highly susceptible Indian rice varieties (Yadav et al. 2019; Yashaswini et al. 2017).
Genetic transformation is not only used for developing biotic and abiotic stress-tolerant cultivars but also in improvement of the qualitative, nutritional and agronomic traits of different rice genotypes. Despite various methods for transgenic rice development such as protoplast transformation via electroporation or Polyethylene Glycol (PEG)-mediated method and microprojectile bombardment, genetic transformation by Agrobacterium tumefaciens is one of the most renowned techniques for its high efficiency and accuracy of transformation events (Chen et al. 2009). Though, genetic transformation by Agrobacterium tumefaciens is a prominent tool for improvement of rice cultivars, genetic transformation of mature seed derived embryogenic calli of most of the indica cultivars remain recalcitrant to the transformation. Most of the successful studies on transgenic rice are limited to japonica varieties along with a few successful indica cultivars like IR-64, IR-72 (Datta et al. 2001), White Ponni, Pusa Basmati I (Sridevi et al. 2003, 2005), Basmati 370 (Maqbool and Christou 1999). Although some transformation and regeneration optmisation studies have been successful using some of the indica cultivars (Khanna and Raina 1999; Kumar et al. 2005; Lin and Zhang 2005; Zaidi et al. 2006), few studies state that indica cultivars are sensitive to tissue culture and the stressed calli after transformation often resulted in poor or no regeneration (Lin and Zhang 2005). Henceforth there have been endeavours to establish modified transformation and regeneration protocol for different recalcitrant indica cultivars with different traits (Hiei and Komari 2006; Kumar et al. 2005; Sahoo et al. 2011; Shri et al. 2013; Sivakumar et al. 2010). However, it takes a lot of time to obtain the transgenic lines following different steps of plant tissue culture. As an alternative, in-planta transformation method was developed to address the regeneration efficiency, involving various techniques like the floral drop, floral dip, spray method, sonication, Agrobacterium-infiltration, and vacuum infiltration (Ratanasut et al. 2017). In-planta piercing and vacuum infiltration protocol were developed for some indica varieties using ATMT (Lin et al. 2009).
The purpose of this study was to address the challenge of genetically manipulating the said varieties for trait improvement and optimize an efficient genetic transformation method for the development of transgenic rice lines. Here we have optimised tissue culture media for callus induction and regeneration, and compared the transformation frequencies of the cultivars using both tissue culture-based and piercing-based in-planta transformation strategies. In the first method, the molecular analysis of the transformed calli and their regeneration efficiency was determined to deduce the efficiency of generating transgenic events. The transformation efficiency was determined from molecular analysis of T0 and T1 plants, following antibiotic marker-based screening, in case of in-planta transformation.
Materials and methods
Plant material, strain and vector
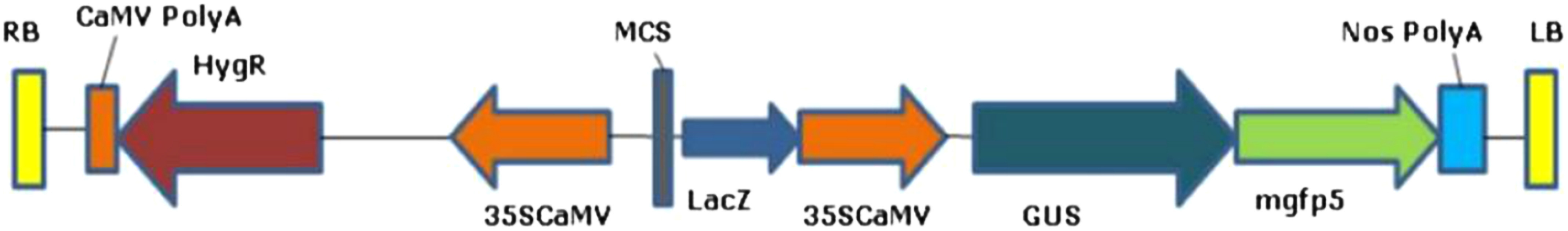
CO-39 and HR-12 seeds were obtained from Regional Rice Research Station, Chinsurah, West Bengal and the seeds were maintained under dry and cool conditions in the lab. The LBA4404 strain of Agrobacterium harbouring pCAMBIA1303 vector was used for transformation. The vector having a HPT marker for eukaryotic selection, reporters GUS, and GFP fused under the 35S CaMV promoter and NOS terminator (Figure 1), was transformed into Agrobacterium by tri-parental mating using a helper strain pRK2013.
Figure 1. Schematic representation of pCAMBIA1303. The T-DNA portion was used to transform the Agrobacterium strain LBA4404 by tri-parental mating. It has a HygR or HPT and two reporter genes mGFP and GUS under 35SCaMV promoter and NOS terminator.
Optimisation of tissue culture media
Mature seeds of CO-39 and HR-12 were manually dehusked and surface sterilised with 70% ethanol for 1 min. The seeds were then rinsed with 0.1% Carbendazim (Sigma-Aldrich, St. Louis, MO, USA), an anti-fungal agent for 20 min. Further, the seeds were washed with sterile water thrice for 2 min each and then rinsed with sodium hypochlorite solution (4%) and 0.25% Tween 20 (Emplura, Merck, Mumbai, India) for half an hour, with intermittent shaking. The sterilised seeds were washed, blotted onto sterile Whatman paper, and cultured on MS media (Holme et al. 1997) supplemented with a range of 2,4-D concentrations, viz. 2 mg l−1, 2.5 mg l−1, 3 mg l−1, carbon source like sucrose or maltose, nitrogen source like proline and casein hydrolysate, and sorbitol. The CIM was classified into six types based on the composition (Table 1). All the media were prepared with a final pH of 5.8, autoclaved at 121°C, and 15 psi, for 20 min. The seeds were placed horizontally onto the media and incubated in dark at (25±2)°C under aseptic conditions. The growth parameters of the calli like fresh weight and diameter were monitored at different time points over a 24 days’ time frame. An optimum media was formulated for each variety based on the callus induction frequency, which was calculated as follows:
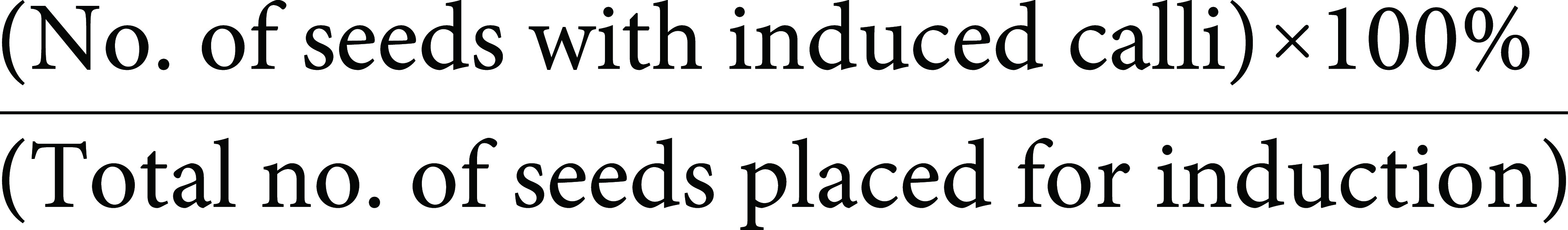 |
(1) |
NAA (0.5–1.5 mg l−1), BAP (1–3 mg l−1) and Kinetin (1–3 mg l−1) were supplemented in the background of the optimised callus induction media (Table 1) for regeneration of the healthy calli. Healthy friable calli were first incubated in dark phase on RM for 4 days followed by 10–15 days of incubation under light, on RM with 8 g/l agarose. The microcalli were cultured under 16 : 8 photoperiod on fresh RM for 10 days, at (25±2)°C. The calli with emergent nascent shoots were transferred to a rooting medium containing half-strength MS and 2 g l−1 phytagel. At this stage, the percentage regeneration frequencies for HR-12 and CO-39 were calculated as follows:
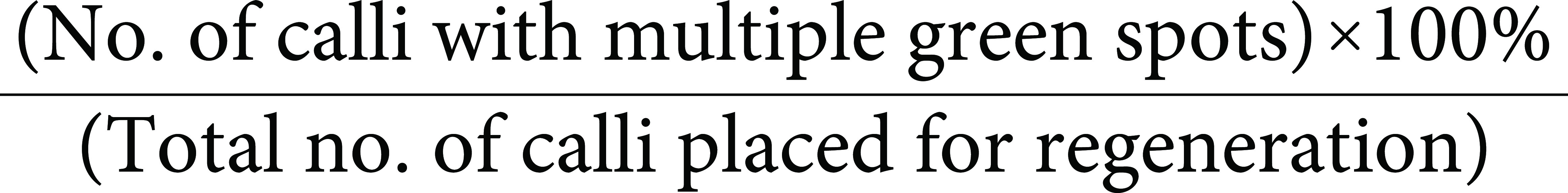 |
(2) |
Table 1. Different categories of culture media based on their combinations, proportions and concentrations of phytohormones and antibiotics.
| Media | Composition | |
|---|---|---|
| CIMa | MS media 4.4 g l−1, Casein hydrolysate 200 mg l−1, Proline 400 mg l−1, Sucrose 40 g l−1, Phytagel 3 g l−1, pH-5.8 | |
| CIMb | MS media 4.4 g l−1, Casein hydrolysate 200 mg l−1, Proline 400 mg l−1, Maltose 40 g l−1, Phytagel 3 g l−1, pH-5.8 | |
| CIMI | CIMa+2,4-D 2 mg/l | |
| CIMII | CIMa+2,4-D 2.5 mg/l | |
| CIMIII | CIMa+2,4-D 3 mg/l | |
| CIMIV | CIMb+2,4-D 2 mg/l | |
| CIMV | CIMb+2,4-D 2.5 mg/l | |
| CIMVI | CIMb+2,4-D 3 mg/l | |
| RMI | MS media 4.4 g l−1, Casein hydrolysate 200 mg l−1, Proline 400 mg l−1, Maltose 40 g l−1, Sorbitol 1%, NAA 0.5 mg l−1, BAP 3 mg l−1, Agarose 10 or 8 g l−1, pH-5.8 | |
| RMII | MS media 4.4 g l−1, Casein hydrolysate 200 mg l−1, Proline 400 mg l−1, Maltose 40 g l−1, Sorbitol 1%, BAP 3 mg l−1, Kinetin 1 mg l−1, Agarose 10 or 8 g/l, pH-5.8 | |
| RMIII | MS media 4.4 g l−1, Casein hydrolysate 200 mg l−1, Proline 400 mg l−1, Maltose 40 g l−1, Sorbitol 1%, NAA 0.5 mg l−1, Kinetin 1 mg l−1, Agarose 10 or 8 g/l, pH-5.8 | |
| RMIV | MS media 4.4 g l−1, Casein hydrolysate 200 mg l−1, Proline 400 mg l−1, Maltose 40 g l−1, Sorbitol 1%, NAA 0.5 mg l−1, BAP 3 mg l−1, Kinetin 1 mg l−1, Agarose 10 or 8 g/l, pH-5.8 | |
| Rooting media | MS media 2.2 g l−1, Maltose 10 g/l, Phytagel 2.5 g/l, pH-5.8. | |
| SM | CO-39 | HR-12 |
| 1st cycle | CIM VI, 30 mg l−1 Hygromycin, 175 mg l−1 Cefotaxime, 175 mg l−1 Carbenicillin | CIM V, 30 mg l−1 Hygromycin, 175 mg l−1 Cefotaxime, 175 mg l−1 Carbenicillin |
| 2nd cycle | CIM VI, 40 mg l−1 Hygromycin, 150 mg l−1 Cefotaxime, 150 mg l−1 Carbenicillin | CIM V, 40 mg l−1 Hygromycin, 150 mg l−1 Cefotaxime, 150 mg l−1 Carbenicillin |
| 3rd cycle | CIM VI, 50 mg l−1 Hygromycin, 250 mg l−1 Cefotaxime | CIM V, 50 mg l−1 Hygromycin, 250 mg l−1 Cefotaxime |
Agrobacterium-mediated In-vitro transformation and In-planta transformation by piercing and vacuum infiltration
The Agrobacterium strain carrying pCAMBIA1303, was streaked and then grown in Luria Bertani medium supplemented with 100 mg l−1 Rifampicin and 50 mg l−1 Kanamycin, at 28°C. A secondary culture was grown in YEM media and was adjusted to a final OD of 0.6. The healthy mature calli were infected with this Agrobacterium culture which had been induced with 10 µM acetosyringone (Sigma-Aldrich, St. Louis, MO, USA), prior to infection for 30 min. They were then co-cultivated at (28±2)°C on CCM media (Table 1), supplemented with 150 µM of acetosyringone. The infection and co-cultivation time were optimised and the infected calli were washed with sterile water containing 150 mg l−1 Cefotaxime (HiMedia, Mumbai, India) and 150 mg l−1 carbenicillin (HiMedia, Mumbai, India) to remove the Agrobacterium and selected on SM media (Table 1). At this stage, the incubation was done in three cycles of 10 days each and in each cycle, the Hygromycin B gold (InvivoGen, San Diego, CA, USA) selection pressure was gradually increased. The microcalli developing on the parent calli were separated gently in the later phases of selection. The proliferating calli were taken forward for regeneration on the optimised RM, under similar culture conditions as stated before. For the in-planta transformation, the Agrobacterium harbouring the binary vector pCAMBIA1303 was streaked and allowed to grow on Luria Bertani plates supplemented with Rifampicin and Kanamycin as mentioned earlier. After 2 days, the biomass was scraped and dissolved in 150 ml of half-strength MS media and the O.D was adjusted to 0.8. The husked seeds were surface-sterilised as mentioned before and placed on moist sterile filter paper, for 2 days, in dark at 25°C. The mature swollen and white embryo was pierced with common syringe needle of 0.5 mm diameter, immersed into the Agrobacterium culture and subjected to vacuum infiltration (Lin et al. 2009). The infected seeds were allowed to germinate on sterile moist vermiculite for 5–7 days and washed with previously mentioned Agrobacterium-specific antibiotics, before planting them into potting mix. The young plants were first grown in a controlled environment and after 13 days, the T0 plants were shifted to greenhouse for acclimatisation. All the steps until acclimatisation were done under aseptic environment to prevent contamination.
Antibiotic leaf painting assay of T0 and T1 plants, GUS histochemical staining and fluorescence microscopy
T0 and T1 plants were primarily screened based on their resistance towards the selective antibiotic Hygromycin. About 2 cm pieces of flag leaves from each T0 and T1 plant across all the independent events were excised, washed, and soaked in the selection solution having 1 mg l−1 6-BAP and 50 mg l−1 hygromycin (Wang et al. 1997). The putatively transformed plants were scored based on the degree of necrosis observed 3 days and 7 days post-treatment. The leaves that were largely green even after 7 days were considered to be resistant to hygromycin. In case of in-vitro transformation, after the co-cultivation and first round of selection, some of the Hygromycin-resistant calli were subjected to a preliminary β-glucuronidase assay to decipher the transformation frequency. They were placed on selection media containing freshly prepared staining solution of 1 mM X-gluc (5-bromo, 4-chloro, 3-indolyl-D-glucuronide) along with carbenicillin and hygromycin (Chakraborty et al. 2016). The calli that showed bluish spots, were sectioned and visualised for expression of GFP under a Zeiss Axio Imager ZI microscope, along with the control untransformed calli. Images (10× magnified) in bright field and FITC filters were captured using the inbuilt AxioCam MRm, and processed with ZEN 2 light blue edition software. On the other hand, the transformation frequency of in-planta transformation was determined by GUS assay of leaves from Hygromycin resistant T0 and T1 plants. The tender leaves of a month old T1 plants were cut into small pieces, fixated and stained with the X-gluc (Sigma-Aldrich, St. Louis, MO, USA) containing staining solution, using vacuum infiltration (Jefferson et al. 1987). The stained leaves were then placed in a 24 well culture plate, and washed with 70% and 90% ethanol respectively, to remove extra stain and bleach out the chlorophyll. The calli and leaves that showed bluish staining were considered to be positively transformed, with tissues exhibiting GUS expression.
Genomic DNA extraction, Polymerase Chain Reaction, DOT Blot
The putatively transformed calli and leaves from T0 and T1 plants were collected, washed with distilled water, dried, and broken into smaller pieces. The tissue were frozen into liquid nitrogen for a few mins and homogenised with 200 µl of chromosomal DNA Extraction Buffer (DEB) (200 mM Tris-Cl, pH-7.5; 250 mM NaCl; 25 mM EDTA pH-8.0 and 0.5% SDS) followed by the genomic DNA isolation (Sabnam and Barman 2017). For each event, 0.1 mg of genomc DNA was denatured at 94°C for 5 min and amplified (30 s at 94°C, 30 s at 60°C, 50 s at 72°C) using GUS, HPT and GFP specific primers (Supplementary Table S2) with a final extension at 72°C for 5 min. The amplified products were separated on 2% agarose gel, at 70 V, for 30 min. The primers used in this study are given in Supplementary Table S1. Approximately 2–3 µg of genomic DNA isolated from the T1 transgenic and non-transgenic lines were denatured individually with 4 M NaOH and applied as dots within 0.5 cm2 boxes (Chakraborty et al. 2016) drawn on a positively charged Nylon membrane (BrightStar-Plus, ThermoFisher Scientific, USA). The blotting, pre-hybridisation and post-hybridisation washing were carried out as per Sambrook et al. A 300 bp region of GUS was amplified, purified, and used as a probe. The labelling of the probe, hybridization, and chemiluminescent detections were done as per instructions given by the manufacturers of AlkPhos Direct Labelling and Detection System with CDP-Star (Amersham, GE Healthcare, Buckinghamshire, UK).
Statistical and segregation analysis
The experiments were validated for statistically significant outcomes using GraphPad Prism 8.0.1 Two-way ANOVA test with Tukey’s correction, at 95% confidence level. The observations with their standard deviations were considered to be significant if p<0.05. In the case of media optimisations, the changes in outcomes were analysed for significance and the differences between various treatments were represented by *, when p<0.0332; **, when p<0.0021; ***, when p<0.0002 and ****, when p<0.0001. The segregation analyses of T1 transgenic lines were done based on 50 mg l−1 hygromycin selection of seed germination. The seeds that did not carry the construct were screened out due to their inability to germinate as compared to the transgenic progenies.
Results and discussions
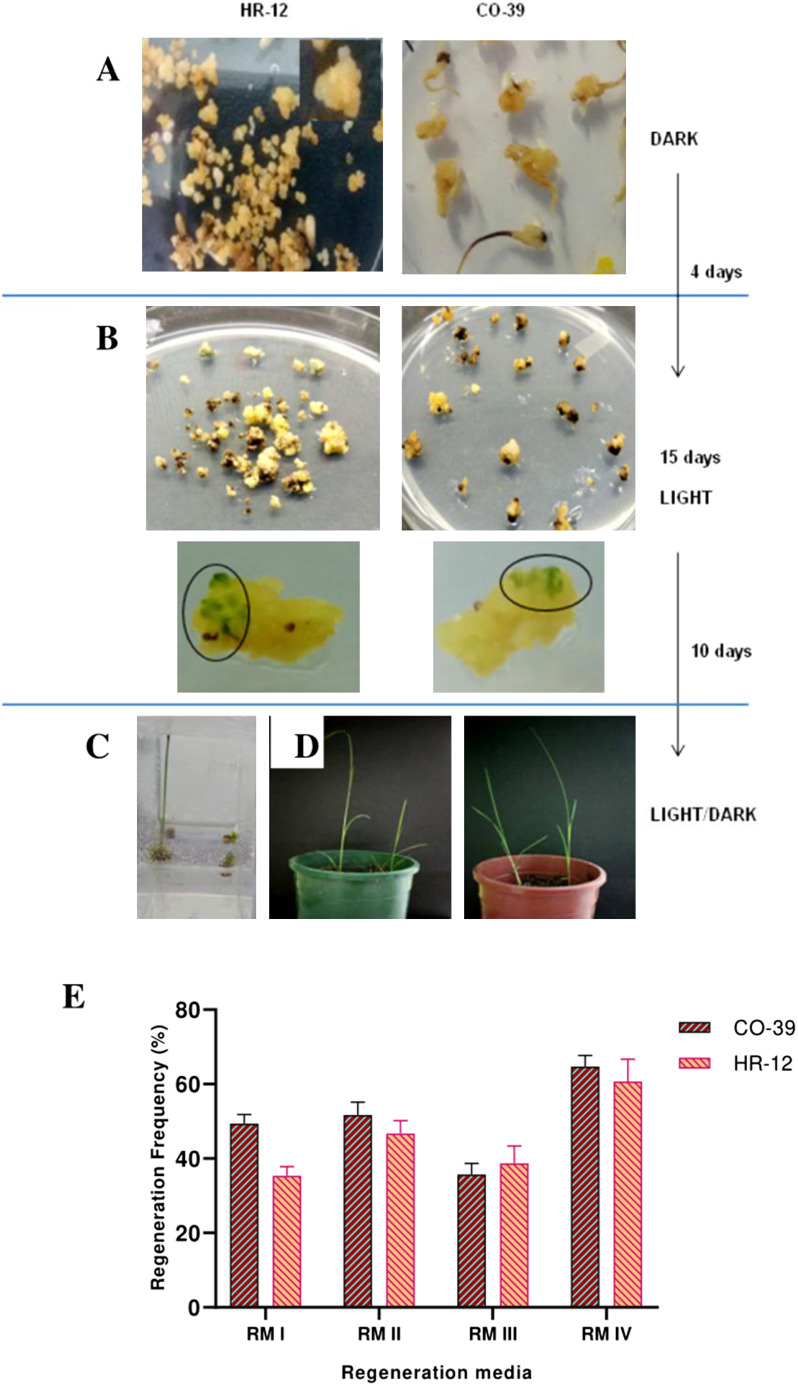
The embryogenic callogenesis and regeneration potential largely depend on the genotype and quality of explant (Holme and Petersen 1996; Khatun et al. 2003), nutrient profile (Chowdhry et al. 1993; Holme et al. 1997; Huang and Liu 2002; Khaleda et al. 2006) in the media used for culturing and culture conditions (Al-Khayri et al. 1996). In order to optimise the CIM, first the carbon source and concentration of 2,4-D were optimised for callus induction. The growth of the calli in terms of dry weight and diameter were monitored across a 21 days’ time frame. The different types and stages of scutellum derived callogenesis at different time points have been shown in Figure 2A and Supplementary Figure S1. Our data showed that for HR-12, the callus induction media that was supplemented with maltose and 2.5 mg l−1 2,4-D, gave rise to calli that weighed around 165 mg with 6.8 mm diameter (Figure 2B), whereas, the one with sucrose produced brownish calli that weighed 125 mg with 5.5 mm diameter (Figure 2B, D). On the other hand, for CO-39, on average 9.6 mm calli weighing 175 mg were obtained using maltose and 3 mg l−1 2,4-D, as compared to 6.3 mm calli weighing approximately 160 mg as in case of sucrose (Figure 2C). CIM V gave the maximum of 86.32% scutellar callus induction frequency for HR-12, while CIM IV was the best for CO-39, yielding 95.86% (Figure 2E). Based on our findings we conclude that for the said varieties, maltose is the preferred carbon source over sucrose for callogenesis. When the regenerability of the proliferating calli was checked, the highest regeneration frequency found for HR-12 and CO-39 were 62% and 65%, respectively, in the media containing all the three phytohormones viz. 0.5 mg l−1 NAA, 3 mg l−1 BAP, and 1 mg l−1 kinetin, as compared to the other combinations that showed a regeneration frequency ranging between 50–58% (Figure 3A, B). We found that, besides healthy and properly stored explants, the selection of healthy, friable, embryogenic calli is also critical for successful regeneration. It is already known that non-embryogenic and brownish calli that produce high levels of phenolic compounds are not appropriate for regeneraton (Bano et al. 2005; Yunita et al. 2014). The first dark phase and reduction in agarose concentration in the second light phase promoted somatic embryogenesis and multiple green spot formation, which are precursors for shoot generation (Sahoo and Tuteja 2012; Sahoo et al. 2011). A half-strength of MS medium and a lesser concentration of phytagel was maintained to facilitate better proliferation of roots (Ramesh et al. 2009).
Figure 2. Different stages of scutellum derived callogenesis, growth parameters of calli and callus induction frequency in CIM supplemented with 2 g/L, 2.5 g/L and 3 g/L 2,4-D and different Carbon sources. A. The development of scutellar calli from HR-12 (top) and CO-39 (bottom) seeds at different timepoints. B. The diameter (left) and the average fresh weight (right) of HR-12 calli. C. The diameter (left) and the average fresh weight (right) of CO-39 calli. D. Whitish friable calli formed in the presence of Maltose (left) and callus browning in the presence of Sucrose (right). E. Percentage callus induction frequency obtained in different CIMs. Each biological replicate had three technical replicates, which were again maintained in triplicates. The standard deviations for each group have been represented with their corresponding error bars and * are indicating significant differences in comparison with various treatments, where, calculations were done considering N=3, at 95% confidence level. (* is p<.0332, ** is p<.0021, *** is p<.0002 and **** is p<0.0001; Two-way ANOVA test).
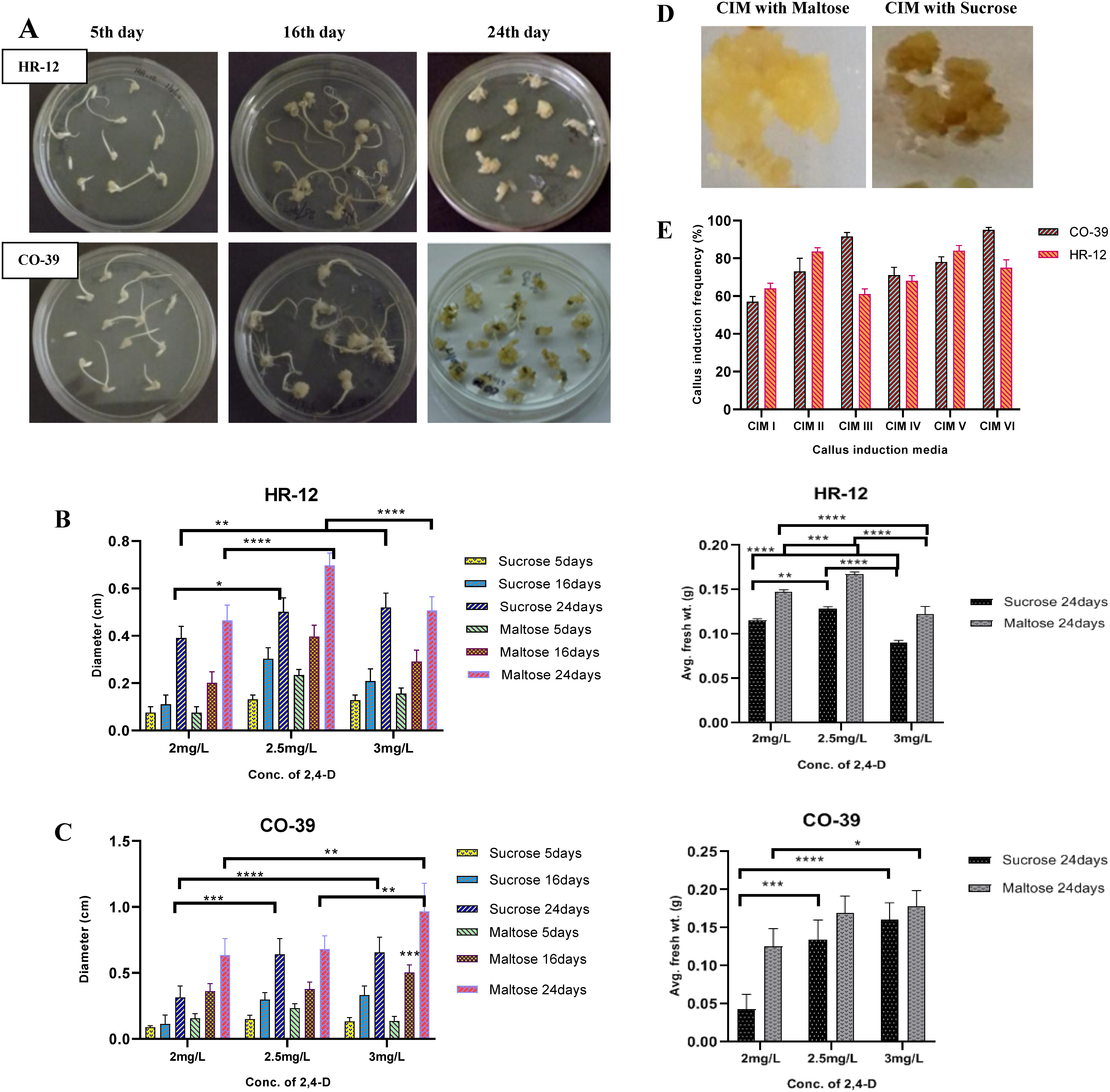
Figure 3. Different stages of development of the calli from regeneration till acclimatisation and regeneration frequency. A. Initial dark phase of regeneration on 1% agarose. B. Light phase of regeneration on 0.8% agarose and appearance of green spots on calli, the progenitors of shoots. C. Rooting of the calli with newly emerged shoots. D. Left panel shows CO-39 plantlets and the right panel shows HR-12 plantlets ready for acclimatisation. E. Regeneration frequencies of calli on different combinations of Regeneration media. Each biological replicate had three technical replicates, which were again maintained in triplicates. The ±SD for each group has been represented with their corresponding error bars.
The Acetosyringone concentration, induction of Agrobacterium, infection and co-cultivation time were optimised for in-vitro transformation, as these are crucial parameters determining its success (Declerck et al. 2002; Hiei et al. 1994; Kumria et al. 2001; Turk et al. 1991). Co-cultivation was done in dark to prevent accumulation of phenolics and degradation of light-sensitive auxins (Arezki et al. 2001). The in-vitro transformation studies showed that a 15 min infection with induced Agrobacterium strain, followed by 48 h of co-cultivation in dark, could result into 62.9% and 68% transformation efficiencies in HR-12 and CO-39, respectively (Supplementary Table S1). The histochemical, microscopic and PCR results confirmed that the transformed calli were found to express GUS and GFP (Figure 4A–C). However when the positively transformed microcalli were placed on the optimised regeneration medium, while no significant regeneration was observed in HR-12 (Supplementary Figure S2) even after two months of incubation, CO-39 showed a regeneration efficiency of as low as 10%. We observed that, even with a significant regeneration efficiency otherwise, and a good transformation potential, routine tissue culture method gave rise to very few regenerated plants, following transformation (Aldemita and Hodges 1996; Ayres and Park 1994). This kind of outcome may be attributed to the mechanical stress the calli undergo during and after transformation, which sometimes interferes with their viability (Amin et al. 2004) or regeneration potential (Lin and Zhang 2005). In order to evade the constraint of regeneration, an alternative direct transformation strategy was adopted where pricking of the swollen embryos with needle followed by vacuum infiltration ensured better penetration of the Agrobacterium, thereby increasing the chances of transformation (Lin et al. 2009). This in-planta method exhibited more promising results with better frequencies of obtaining transgenic events in relatively lesser time (Figure 5). Wounding and vacuum infiltration of the embryo is crucial to facilitate maximum Agrobacterium infection. However it can not only cause damage to the delicate structure thereby affecting its germination potential, but also can make it more prone to other fungal infections resulting into contamination. Since the problem of contamination was observed when the transformation and co-cultivation steps were done in non-sterile conditions, we maintained aseptic conditions until the acclimatisation of T0 plants. We recommend this modification, following gentle piercing (Lin et al. 2009) to ensure generation of maximum number of transgenic events. The initial screening of transformed events based on Hygromycin leaf-painting assay, followed up with histochemical GUS staining and PCR analysis for marker and reporter genes, HPT and GFP (Figure 4D–F), showed a transformation efficiency of 33% and 35% (Table 2) for HR-12 and CO-39, respectively. Taken together with these data, our DOT blot data (Figure 4G) and segregation analysis (data not shown) of T1 plants from individual T0 events indicated the successful transmission of the transgene to the subsequent generation.
Figure 4. Histochemical, microscopic, molecular analyses of T0 calli and Hygromycin leaf painting assay of independent transgenic lines. A. Transformed (T) calli checked for the GUS activity on 1 mM X-Gluc containing selection media. The bluish spots indicate the transformed tissues expressing GUS. B. GFP expression in transformed callus tissues observed under an epifluorescence microscope, at 12,000 ms exposure time. The untransformed (UT) controls showed neither GUS nor GFP activity. C. PCR confirmation of the presence of 301 bp GUS (top) and 303 bp GFP insert in independent T0 events of calli, corresponding to the 300 bp band of the reference DNA ladder (L). For Positive (P), plasmid DNA of pCAMBIA1303 was used and for negative (N), genomic DNA of UT callus was used as templates. D. Screening of T0 events based on hygromycin resistance. For each plant, a set of triplicates with three leaves on each plate was maintained. The leaves that remained largely green after 7 days of treatment, were scored as antibiotic-resistant and the leaves that looked mostly necrotic like UT leaves were considered as hygromycin sensitive. E. PCR confirmation for the presence of GUS amplicon (top) and 300 bp HPT insert (bottom) in resistant leaves, in presence of reference DNA ladder (L). F. GUS histochemical test for T1 leaves of HPT/GUS positive plants. G. DOT-BLOT image of denatured genomic DNA of positive T1 plants, developed after hybridisation with the GUS probe. Each sample was blotted in individual squares and as duplicates to avoid false positives. Denatured plasmid DNA of pCAMBIA1303 was used as (+)ve control and that of UT sample as (−)ve control.
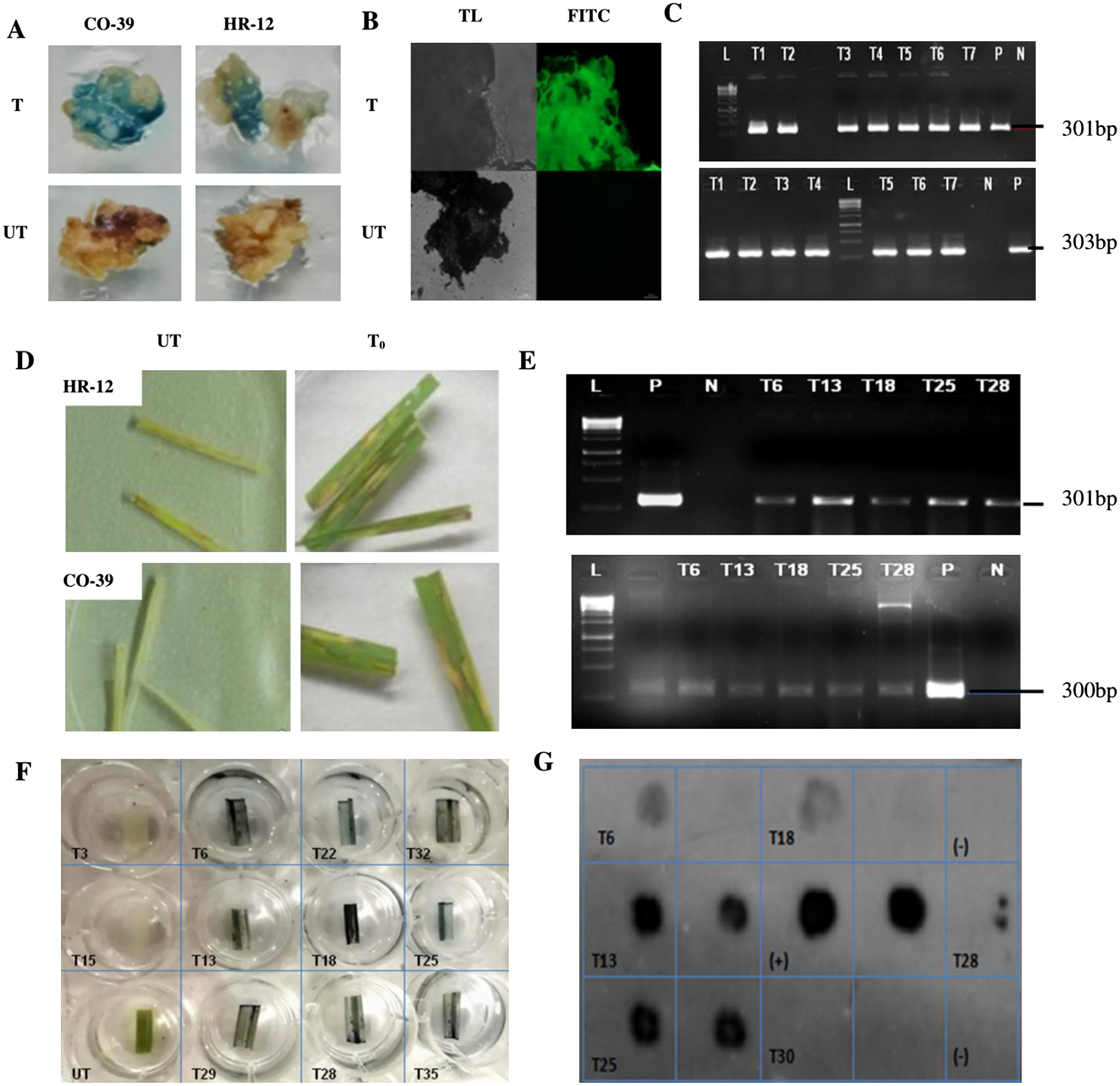
Figure 5. A brief comparative overview of the in-vitro or tissue culture-based transformation and in-planta transformation methods in the studied cultivars: steps, time involved in them, and their respective success rates. The in-vitro method showed around 63–68% transformation efficiency. HR-12 did not show any post-transformation regeneration whereas, CO-39 showed a poor regeneration frequency of 10%, thereby decreasing its success of getting positive transgenic events. The in-planta method, bypassing regeneration, showed a 33–35% success rate in HR-12 and CO-39 respectively.
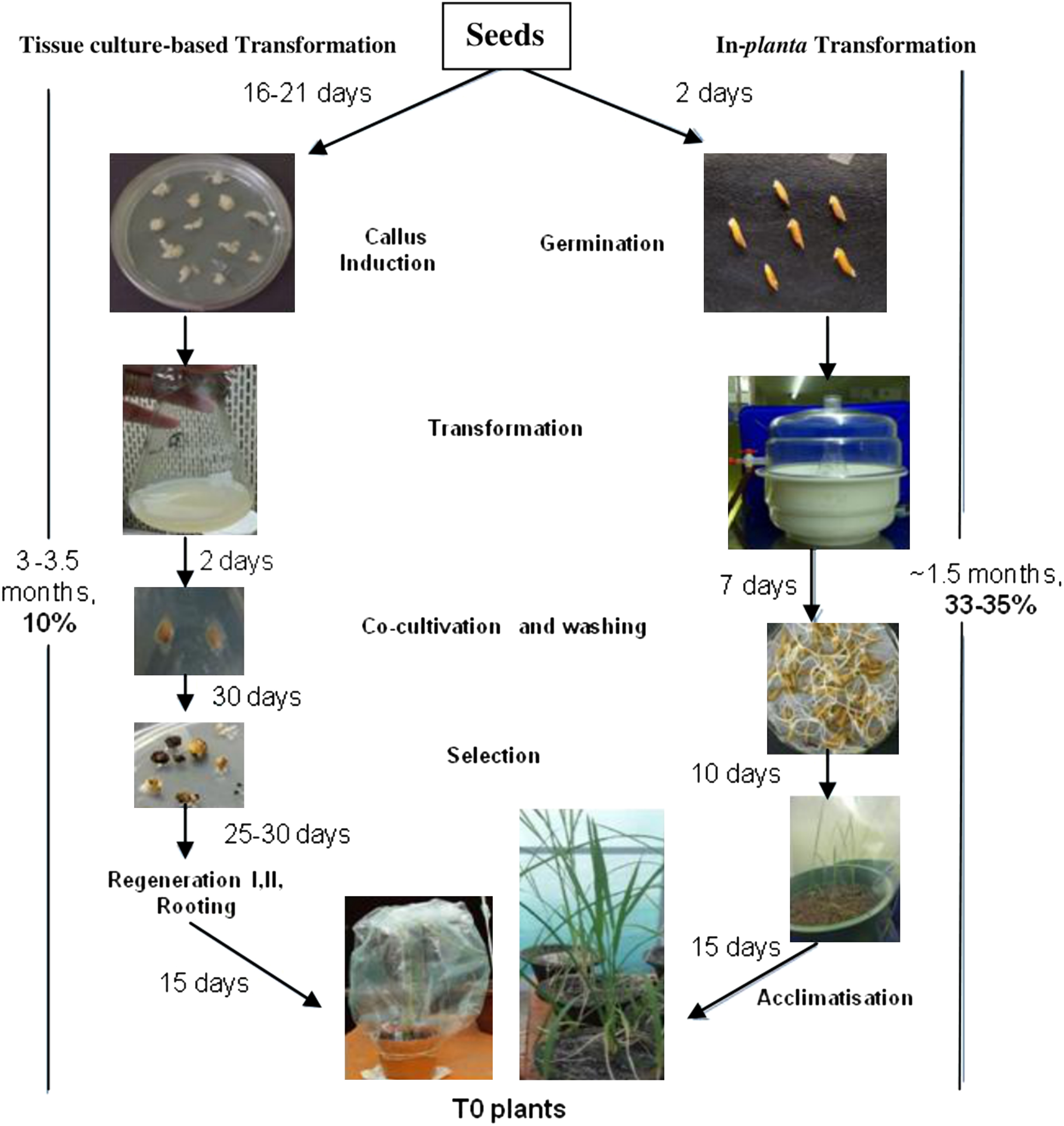
Table 2. Detailed summary of T0 and T1 plants for CO-39 and HR-12 and their respective transformation efficiencies obtained by in-planta piercing and vacuum infiltration-based transformation method.
| Variety | CO-39 | HR-12 |
|---|---|---|
| Total no. of seeds pierced | 250 | 250 |
| No. of seeds germinated | 220 | 200 |
| HygR/Hpt II+(T0) | 107 | 83 |
| HygR/GUS+(T1) | 78 | 66 |
| Transformation efficiency (%) | 35 | 33 |
In-planta transformation has been successfully reported in different systems like Brassica (Heyser et al. 1983), maize (Bano et al. 2005), radish (Yunita et al. 2014), etc. In rice, for the first time, Supartana et al. 2005 and Lin 2009 optimised the embryo-piercing based protocol for japonica and indica varieties, respectively. Aligned with their observations, our findings suggest that with minimal requirements of chemicals, media and tissue-culture lab setup, it is possible to generate successful transformants in the same generation. T0 this end, it can be justified as a versatile and time-efficient method giving approximately 25–30% higher frequency of obtaining transgenic plants in less than half the time, as compared to in-vitro genetic modification. Our modified protocol of piercing-based Agrobacterium-mediated transformation (Lin et al. 2009) of HR-12 and CO-39 yielded a maximum of 35% transformation efficiency, which is higher than the most recent report on piercing-based in-planta transformation (Ahmed et al. 2018). Due to its high reproducibility, this method can be effectively utilised for genetic transformation experiments, especially against biotic threats like fungal blast and sheath blight pathogens, that lower the crop’s agronomic value drastically. We also believe that this protocol is quite versatile to be used for transforming other recalcitrant varieties of rice.
Acknowledgments
Conceptualization, S.R.B. and A.S.; methodology, A.S.; validation, A.S., I.S., and S.R.B.; formal analysis, A.S.; investigation, A.S., I.S.; data curation, I.S., A.S.; writing—original draft preparation, A.S.; review and editing, S.R.B.; supervision, S.R.B.; funding acquisition, S.R.B. The authors would also like to acknowledge Dr. Sudip Chattopdhyay for providing the vector pCAMBIA1303 and Dr. Rajesh Patkar for sending LBA4404 strain, as kind gifts. All authors have read and agreed to the published version of the manuscript and there are no conflicts of interest. This research was funded by the Department of Biotechnology, Govt. of India. # BT/PR1929/AGR/36/682/2011.
Abbreviations
- 2,4-D
2,4-dichlorophenoxyacetic acid
- NAA
1-napthaleneacetic Acid
- BAP
6-Benzylaminopurine
- HPT
Hygromycin Phospho-transferase
- GUS
β-glucuronidase
- GFP
Green Fluorescent Protein
- CIM
Callus Induction Media
- RM
Regeneration Media
- SM
Selection Media
- CCM
Co-cultivation Medium
- CaMV
Cauliflower Mosaic Virus
- ATMT
Agrobacterium-mediated Transformation
- DEB
DNA Extraction Buffer
- EDTA
Ethylenediaminetetraacetic acid
- PCR
Polymerase Chain Reaction
Supplementary Data
References
- Ahmed T, Biswas S, Elias SM, Rahman MS, Tuteja N, Seraj ZI (2018) In Planta transformation for conferring salt tolerance to a tissue-culture unresponsive Indica rice (Oryza sativa L.) cultivar. In Vitro Cell Dev Biol Plant 54: 154–165 [Google Scholar]
- Aldemita RR, Hodges TK (1996) Agrobacterium tumefaciens mediated transformation of Japonica and Indica rice varieties. Planta 199: 612–617 [Google Scholar]
- Al-Khayri JM, Shamblin CE, Anderson EJ (1996) Callus induction and plant regeneration of U.S. rice genotypes as affected by medium constituents. In Vitro Cell Dev Biol Plant 32: 227–232 [Google Scholar]
- Amin MA, Uddin MA, Hossain MA (2004) Regeneration study of some Indica rice cultivars followed by Agrobacterium-mediated transformation of highly regenerable cultivar BR-8. J Biol Sci 4: 207–211 [Google Scholar]
- Arezki O, Boxus P, Kevers C, Gaspar T (2001) Changes in peroxidase activity, and level of phenolic compounds during light-induced plantlet regeneration from Eucalyptus camaldulensis Dehn. nodes in vitro. Plant Growth Regul 33: 215–219 [Google Scholar]
- Ayres NM, Park WD (1994) Genetic transformation of rice. Crit Rev Plant Sci 13: 219–239 [Google Scholar]
- Bano S, Jabeen M, Rahim F, Ilahi I (2005) Callus induction and regeneration in seed explants of Rice (Oryza sativa cv. Swat-II). Pak J Bot 37: 829–836 [Google Scholar]
- Bibi N, Kai F, Shuna Y, Mi N, Mosaddek IM, Waqas M, Xuede W (2013) An efficient and highly reproducible approach for the selection of upland transgenic cotton produced by pollen tube pathway method. Aust J Crop Sci 7: 1714–1722 [Google Scholar]
- Chakraborty M, Reddy PS, Narasu ML, Krishna G, Rana D (2016) Agrobacterium-mediated genetic transformation of commercially elite rice restorer line using nptII gene as a plant selectable marker. Physiol Mol Biol Plants 22: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lin Y, Zhang Q (2009) Review and prospect of transgenic rice research. Chin Sci Bull 54: 4049–4068 [Google Scholar]
- Chowdhry CN, Tyagi AK, Maheshwari N, Maheshwari SC (1993) Effect of L-proline and L-trytophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L.cv.Pusa 169). Plant Cell Tissue Organ Cult 32: 357–361 [Google Scholar]
- Chumakov MI, Rozhok NA, Velikov VA, Tyrnov VS, Volokhina IV (2001) Agrobacterium-mediated in planta transformation of maize via pistil filaments. Russ J Genet 42: 893–897 [Google Scholar]
- Curtis IS, Nam HG (2001) Transgenic radish (Raphanus sativus L. longipinnatus Bailey) by floral-dip method: Plant development and surfactant are important in optimizing transformation efficiency. Transgenic Res 10: 363–371 [DOI] [PubMed] [Google Scholar]
- Datta K, Tu JM, Oliva N, Ona I, Velazhahan R, Mew TW, Muthukrishnan S, Datta SK (2001) Enhanced resistance to sheath blight by constitutive expression of infection–related rice chitinase in transgenic elite Indica rice cultivars. Plant Sci 160: 405–414 [DOI] [PubMed] [Google Scholar]
- Declerck S, Risede JM, Delvaux B (2002) Greenhouse response of micropropagated bananas inoculated with in-vitro monoxenically produced arbuscular mycorrhizal fungi. Sci Hortic (Amsterdam) 93: 301–309 [Google Scholar]
- Heyser JW, Dykes TA, Demott KJ, Nobors NW (1983) High frequency, long term regeneration of rice from callus culture. Plant Sci Lett 29: 175–181 [Google Scholar]
- Hiei Y, Komari T (2006) Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 85: 271–283 [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Holme IB, Krogstrup P, Hansen J (1997) Embryogenic callus formation, growth and regeneration in callus and suspension cultures of Miscanthus x ogiformis Honda Giganteus’ as affected by proline. Plant Cell Tissue Organ Cult 50: 203–210 [Google Scholar]
- Holme IB, Petersen KK (1996) Callus induction and plant regeneration from different explant types of Miscanthus×ogiformis Honda ‘Giganteus.’ Plant Cell Tissue Organ Cult 45: 43–52 [Google Scholar]
- Huang W, Liu LF (2002) Carbohydrate metabolism in rice during callus induction and shoot regeneration induced by osmotic stress. Bot Bull Acad Sin 43: 107–113 [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleda L, Al-Forkan M (2006) Stimulatory effects of casein hydrolysates and proline in in vitro callus induction and plant regeneration from five deepwater rice (Oryza sativa L.). Biotechnology (Faisalabad) 5: 379–384 [Google Scholar]
- Khanna HK, Raina SK (1999) Agrobacterium-mediated transformation of Indica rice cultivars using binary and superbinary vectors. Funct Plant Biol 26: 311–324 [Google Scholar]
- Khatun MM, Ali MH, Desamero NV (2003) Effect of genotype and culture media on callus formation and plant regeneration from mature seed scutella culture in rice. Plant Tissue Cult 13: 99–107 [Google Scholar]
- Koide Y, Ebron LA, Kato H, Tsunematsu H, Telebanco-Yanoria MJ, Kobayashi N, Yokoo M, Maruyama S, Imbe T, Fukuta Y (2011) A set of near-isogenic lines for blast resistance genes with an Indica-type rainfed lowland elite rice (Oryza sativa L.) genetic background. Field Crops Res 123: 19–27 [Google Scholar]
- Kumar KK, Maruthasalam S, Loganathan M, Sudhakar D, Balasubramanian P (2005) An improved Agrobacterium-mediated transformation protocol for recalcitrant elite Indica rice cultivars. Plant Mol Biol Report 23: 67–73 [Google Scholar]
- Kumria R, Waie B, Rajam MV (2001) Plant regeneration from transformed embryogenic callus of an elite Indica rice via Agrobacterium. Plant Cell Tissue Organ Cult 67: 63–71 [Google Scholar]
- Lin J, Zhou B, Yang Y, Mei J, Zhao X, Guo X, Hang X, Tang D, Lin X (2009) Piercing and vaccum infiltration of mature embryo: A simplified method for Agrobacterium-mediated transformation of Indica rice. Plant Cell Rep 28: 1065–1074 [DOI] [PubMed] [Google Scholar]
- Lin YJ, Zhang QF (2005) Optimising the tissue culture conditions for high efficiency transformation of Indica rice. Plant Cell Rep 23: 540–547 [DOI] [PubMed] [Google Scholar]
- Mahesh HB, Shirke MD, Singh S, Rajamani A, Hittalmani S, Wang GL, Gowda M (2016) Indica rice genome assembly, annotation and mining of blast disease resistance genes. BMC Genomics 17: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool SB, Christou P (1999) Multiple traits of agronomic importance in transgenic Indica rice plants: Analysis of transgene integration patterns, expression levels and stability. Mol Breed 5: 471–480 [Google Scholar]
- Prabhu AS, Filippi MC, Silva GB, Silva-Lobo VL, Morais OP (2009) An unanprecedented outbreak of rice blast on a newly released cultivar BRS Colosso in Brazil. In: Wang GL, Valent B (eds) Advances in Genetics, Genomics and Control of Rice Blast Disease. Springer Science, Dordrecht, Netherlands, pp 257–267
- Ramesh M, Murugiah V, Gupta AK (2009) Efficient in vitro plant regeneration via leaf base segments of Indica rice (Oryza sativa L.). Indian J Exp Biol 47: 68–74 [PubMed] [Google Scholar]
- Ratanasut K, Rod-In W, Sujipuli K (2017) In planta Agrobacterium-mediated transformation of rice. Rice Sci 24: 181–186 [Google Scholar]
- Sabnam N, Barman SR (2017) WISH, a novel CFEM GPCR is indispensable for surface sensing, asexual and pathogenic differentiation in rice blast fungus. Fungal Genet Biol 105: 37–51 [DOI] [PubMed] [Google Scholar]
- Sahoo KK, Tripathy AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) An improved protocol for efficient transformation and regeneration of diverse Indica rice cultivars. Plant Methods 7: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo RK, Tuteja N (2012) Development of Agrobacterium-mediated transformation technology for mature seed-derived callus tissues of Indica rice cultivar IR64. GM Crops Food 3: 123–128 [DOI] [PubMed] [Google Scholar]
- Shri M, Rai A, Verma PK, Misra P, Dubey S, Kumar S, Verma S, Gautam N, Tripathi RD, Trivedi PK, et al. (2013) An improved Agrobacterium-mediated transformation of recalcitrant Indica rice (Oryza sativa L.) cultivars. Protoplasma 250: 631–636 [DOI] [PubMed] [Google Scholar]
- Sivakumar P, Law YS, Ho C, Harikrishna JA (2010) High frequency plant regeneration from mature seed of elite, recalcitrant malaysian Indica rice (Oryza Sativa L.) CV. MR 219. Acta Biol Hung 61: 313–321 [DOI] [PubMed] [Google Scholar]
- Sridevi G, Dhandapani M, Veluthambi K (2005) Agrobacterium-mediated transformation of White Ponni, a non-basmati variety of Indica rice (Oryza sativa L.). Curr Sci 88: 128–132 [Google Scholar]
- Sridevi G, Sabapathi N, Meena P, Nandakumar R, Samiyappan R, Muthukrishnan S, Veluthambi K (2003) Transgenic Indica rice variety Pusa Basmati 1 constitutively expressing a rice chitinase gene exhibits enhanced resistance to Rhizoctonia solani. J Plant Biochem Biotechnol 12: 93–101 [Google Scholar]
- Supartana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M (2005) Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100: 391–397 [DOI] [PubMed] [Google Scholar]
- Turk SC, Melchers LS, den Dulk-Ras H, Regensburg-Tuïnk AJ, Hooykaas PJ (1991) Environmental conditions differentially affect vir gene induction in different Agrobacterium strains. Role of the VirA sensor protein. Plant Mol Biol 16: 1051–1059 [DOI] [PubMed] [Google Scholar]
- Wang MB, Upadhyaya NM, Brettell RIS, Waterhouse PM (1997) Intron-mediated improvement of a selectable marker gene for plant transformation using Agrobacterium tumefaciens. J Genet Breed 51: 325–334 [Google Scholar]
- Xu GS, Rao YQ, Chen Y, Zhang CY, Meng JL (2004) Genetic transformation of Brassica napus with in planta method. Zuo Wu Xue Bao 30: 1–5 [Google Scholar]
- Yadav M, Aravindan S, Ngangkham U, Raghu S, Prabhukarthikeyan SR, Keerthana U, Marndi BC, Adak T, Munda S, Deshmukh R, et al. (2019) Blast resistance in Indian rice landraces: Genetic dissection by gene specific markers. PLoS One 14: e0211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanoria MJT, Koide Y, Fukuta Y, Imbe T, Tsunematsu H, Kato H, Ebron LA, Nguyen TMN, Kobayashi N (2011) A set of near-isogenic lines of Indica-type rice variety CO39 as differential varieties for blast resistance. Mol Breed 27: 357–373 [Google Scholar]
- Yashaswini CH, Narayan RP, Pushpavati B, Srinivasa R, Seshu MM (2017) Prevalence of rice blast (Magnaporthe oryzae) incidence in South India. Bull Env Pharmacol Life Sci 6: 370–373 [Google Scholar]
- Yunita R, Khumaida N, Sopandie D, Mariska I (2014) Growth and regeneration of rice (Oryza sativa L.) callus in salt medium. Biosci Res 11: 4–9 [Google Scholar]
- Zaidi MA, Narayan M, Sardana R, Taga I, Postel S, Johns R, MacNulty M, Mottiar Y, Mao J, Loit E, et al. (2006) Optimizing tissue culture media for efficient transformation of different Indica genotypes. Agron Res (Tartu) 4: 563–575 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.