Abstract
The effect of modification of sodium alginate on the beads properties was investigated and compared with unmodified alginate beads. The effect of storage conditions on the shelf life of the beads was determined. The modification of sodium alginate was achieved with octenyl succinic anhydride (OSA). Antioxidant activity and total phenolic compounds of the jujube extract was evaluated. Some physical and structural properties yield and encapsulation efficiency of the beads were measured. The encapsulation yield of the beads was significantly affected by modification of sodium alginate. The highest yield was observed in the alginate stored at ambient temperature on the day 14th which was 35.21%. The average beads size of alginate was 5.10 and 4.68 mm in modified and unmodified alginate beads, respectively. Modification also had a significant effect on the hardness of the beads. Higher Tg which ensures good product stability in thermal process was clearly seen in the modified beads maintained at ambient temperature. Fourier Transform Infrared Spectroscopy (FT-IR) revealed the existence of OH groups in the extract-loaded beads. These findings have important implications on designing preservation and delivery systems of soluble bioactive compounds of jujube extract to apply in development of new functional foods and drinks.
Keywords: Encapsulation, Jujube extract, Modified calcium alginate, Bead, Octenyl succinic anhydride
Graphical abstract
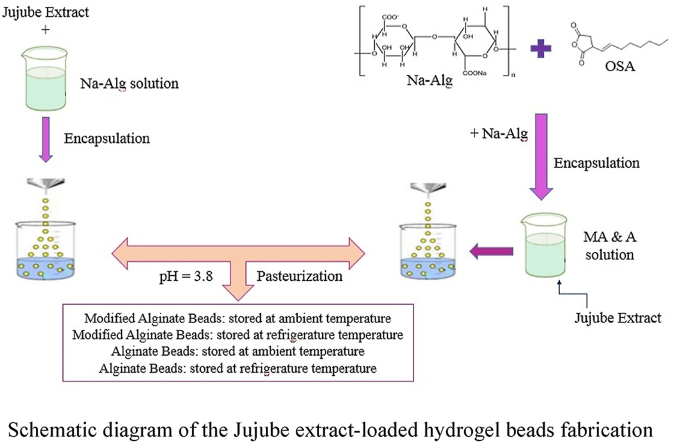
Schematic diagram of the Jujube extract-loaded hydrogel beads fabrication.
Highlights
-
•
Calcium alginate was modified with octenyl succinic anhydride (OSA) to make beads.
-
•
Both modified alginate and unmodified alginate beads were loaded with jujube extract (JE).
-
•
The encapsulation efficiency of jujube extract of the beads was improved.
-
•
Incorporation of OSA to alginate increased beads strength and preserved jujube extract effectively.
1. Introduction
One of the reasons for the rapid growth of natural products market is the growing consumer knowledge of food safety. The bioactive compounds of herbal plants including polyphenols, antioxidants and nutraceutical compounds have functional properties such as anti-carcinogenic, anti-inflammatory and anti-oxidative stress (Owen et al., 2000; Kris-Etherton et al., 2004). In recent decades, some species of the popular herbal plants in Iran have attracted much attention from industrial sectors. A number of them have a limited market and their consumption is traditional. One of these plants is jujube fruit, which is traditionally used as a drug compound and more as nuts. Proper harvesting and innovative processing of jujube are the leading attempts to introduce it as a functional and nutraceutical product to the global market.
Jujube (Ziziphus spp.) belongs to the Rhamnaceae family, and is a known medicinal plant. Jujube extensively is grown in the world (San and Yildirim, 2010)and Iran is one of the main producers of jujube (Golmohammadi, 2013). It is widely used in functional foods such as jams, candy, beverages, cakes, jelly, etc. Jujube fruit is used as antitussive, blood pressure reducer, anti-diabetes, aphrodisiac, anticancer, antifungal, antibacterial, anti-inflammatory, antispastic, cardiotonic, antioxidant, and wound healing. Pharmacological results have indicated that flavonoids, high polyphenolic and antioxidant capacity are the main active ingredients in jujube fruit (Nazni and Mythili, 2013; Sun et al., 2011; San and Yildirim, 2010; Najafabadi et al., 2017). Encapsulation and other delivery systems can be applied to protect functional ingredients of jujube fruit such as bioactive compounds due to their sensitivity to environmental and destructive factors. Extrusion is one of the most economical methods commonly used to produce macro-sized beads (Gombotz and Wee, 1998). In this method a biopolymer solution is injected into ionic solution and hydrogel formation occurs (Zhang et al., 2016). Alginate is one of the most commonly used natural biopolymer among the hydrocolloids for this purpose. It is a copolymer of 1–4 linked-β-D-mannuronate and α-L-guluronate homo polymeric blocks (Kahya and Erim, 2019). It is derived from brown algae and is a biodegradable, compatible, low price, non-toxicity, capability to absorb large amount of water, thermally and chemically stable (Chan et al., 2010) and is suitable biopolymer for a delivery matrix because gel beads can be formed very easily in ionic aqueous solution (ionic cross-linking with multivalent metal ions solution) at room temperature (Chen and Subirade, 2006; Kikuchi et al., 1999). Alginate is used for encapsulation because of it is a food grade biopolymer (Vandenberg et al., 2001). The egg-box model is the mechanism of alginate gelation. Since alginate has two different chains, so the carboxyl groups coordinate the metal ions between them (Kahya and Erim, 2019).
Some gums are water-soluble and absorb water molecules and some of the extracts are water-soluble as well. Usually, some of the encapsulated compounds are released into the aquatic environment after being encapsulated. For this reason, modification of the gum structure and adding hydrophobic groups to it, improves the stability of the beads and better preserves the compounds. Alkenyl succinic acid is a derivative of succinic acid as dicarboxylic anhydride. According to the US Food and Drug Administration, only octenyl succinic anhydride among the various alkenyl succinic anhydrides has the potential for food applications. During the esterification reaction, hydrophobic groups bond with hydrocolloid hydrophilic groups. These changes can cause stabilization, hydrocolloid condensation, emulsifying properties and improve the functional properties of the gel. In fact, hydrocolloids become amphiphilic and act as a surfactant (Mohammadi et al., 2016).
Various properties including antioxidant activity, organic acids, and phenolic profiles of 10 species of Ziziphus Jujuba Mill has been reported in the literatures. Zizyphus jujube Jiaxianmuzao is known as an important source of natural antioxidants. The optimization of extraction and nanocapsulation of jujube pulp and seed to increase its antioxidant activity has also been reported (Han et al., 2015). Researchers improved the formulation of controlled delivery of caffeine in alginate hydrogel beads with pectin, carrageenan, chitosan and psyllium for encapsulating caffeine. The high concentration of sodium alginate (3%) to produce hydrogel beads in combination with psyllium or chitosan coatings showed the best carrier systems for inactivating caffeine (Belscak-Cvitanovic et al., 2015). Another researcher examined the emulsifying properties of modified zedo gum with octenyl succinic anhydride (OSA). The use of OSA is one of the ways to esterification of gums to make functional applications (Mohammadi et al., 2016). Sugar beet pectin (SBP) was esterified using octenyl succinic anhydride (OSA) by researchers. The results indicated that emulsions stabilized by OS-SBP with a low degree of substitution (DS) possessed a smaller oil droplet size and improved storage stability compared with those of the emulsion generated using SBP and OS-SBP with a DS of 1.8 would be a strong emulsifying agent in acidic environments (Chen et al., 2015).
In the present work, the encapsulation of jujube extract by alginate and modified alginate with OSA has been studied. The objective of this work was to fabricate and evaluate the stability and structural properties of alginate and modified alginate gel beads and the possible interaction between the matrix materials and jujube active compounds. The effect of encapsulation on antioxidant activity and protection of bioactive compounds was also evaluated.
2. Materials & methods
2.1. Materials
Sodium alginate (molecular weight: 250000 Da) was purchased from Sigma Aldrich (USA), 2-Octenylsuccinic anhydride, mixture of cis and trans were purchased from Sigma Aldrich (97%, Germany), Calcium chloride dehydrate powder (CaCl2.2H2O) was used as gelling ions (Merck, Germany) and ethanol from Merck (Darmstadt, Germany). All reagents were used as received without further purification and were analytical grade. Jujube fruit was supplied by local market (Bojnourd, khorasan razavi, Iran).
2.2. Preparation of jujube and its extract
2.2.1. Preparation of jujube
The first, the surface of fresh jujube fruits was washed with distilled water and were dried at room temperature for 15 days in the dark and transferred to the laboratory. After removing the nucleus, they were powdered with an electric milling machine and passed through the mesh 60. Then they were dried in hot oven at 40 °C for 8 h to remove the residual moisture. Jujube powder was kept in the dark refrigerator until use (Han et al., 2015; Prachayasittikul et al., 2008).
2.2.2. Alcoholic extract
50 gr jujube powder was weighted in a dark vessel and 200 ml ethanol/water solvent (80:20) was added and mixed. The mixture was placed on a shaker at 180 rpm (37 °C) for 1 h. The extract was then centrifuged at 1000 rpm for 2 min at 4 °C and filtered using watman Paper No. 1 (Prachayasittikul et al., 2008).
2.2.3. Aqueous extract
500 ml distilled water was added to 50 gr pre-jujube pomace (the residue of the jujube extracted with ethanol solvent of the previous step). The mixture was then shaken gently on a shaker at 180 rpm (20 °C) for 24 h. Then extract was filtered by watman Paper No.1. The extract was concentrated by rotary evaporator (Heidolph, Laborota 4003, Germany) under vacuum at 40 °C. Finally, transparent extract was placed in dark and colored vials and kept at refrigerated temperature until use. Extraction of the extract was done in two stages due to some phenolic compounds of plants and fruits are dissolved in water and some in ethanol, so to extract all these compounds, the extraction was done in two steps and finally the two extracts were easily mixed and dissolved together.
2.3. Extraction efficiency
After mixing the plant with the solvents as described in the previous sections and extracting the plant extract, the solvents of the extracts were evaporated by rotary evaporator and the extracts were mixed together and weighed by a digital scale. The extract efficiency was measured using the following Eq. (1) (Rostami and Gharibzahedi, 2016).
| Eq. (one) |
2.4. Measurement of DPPH antioxidant activity
Antioxidant power or scavenging of DPPH radicals was carried out according to a method with slight correction (Kamiloglu et al., 2009). 2.0 ml of different concentrations of extract from the jujube was added to 1.0 ml solution of DPPH radicals (0.1 mM, in 96% ethanol) and allowed to react at room temperature. The mixture was shaken vigorously and allowed to stand for 30 min. The absorbance of samples was measured at 517 nm with a UV-VIS spectrophotometer and important point is lower absorbance of the jujube samples showed higher free radicals scavenging activity. A standard curve according to ascorbic acid with its concentrations (0.05, 0.075, 0.1, 0.15, 0.25, 0.35, 0.5 and 1 mg/ml) was prepared. The DPPH radical scavenging effect was calculated according to the following Eq. (2):
| Eq. (two) |
where ACX0 is absorbance of the control DPPH jujube extract solution at 0 min, and ACXt is absorbance in the presence of test samples (different concentrations) at 30 min. Also a standard diagram was plotted by plotting the activity of free radicals' ascorbic acid against its concentration (0.05–1 mg/ml). The results are expressed as AEAC/100 g DW.
2.5. Preparation of alginate/jujube extract beads
A specific amount of Sodium alginate powder was dispersed in deionized water and stirred for 2 h at a temperature of 40 °C overnight to obtain a homogeneous solution (Na-Alg). Jujube extract was added to sodium alginate solution. For preventing of degradation phenolic compounds in extract, it kept under minimum light exposure. For formation of beads, sodium alginate solution was dropped into CaCl2 solution (5% w/v) using a syringe pump through a 0.5 cm gauge needle to obtain spherical beads (Kahya and Erim, 2019; Lupo et al., 2015).
2.6. Modification of sodium alginate with octenyl succinic anhydride
The esterification was done according to a modified method (Mohammadi et al., 2016). For sodium alginate esterification with OSA, the gum powder was weighed and dispersed in distilled water (1:25 w/v) and stirred at 60 °C for 1 h to hydrate. The pH was adjusted to 8.6 by the addition of alkaline solution (0.5 M NaOH). The OSA was added as 15% of gum (by dry weight) and the dispersion was diluted with pure ethanol at various temperatures (40–60 °C) while stirring continuously. Then reaction time was adjusted 90 min and when it completed, the dispersion was neutralized to pH 6.5 using hydrochloric acid (1 N, HCl), then washed (twice with 80% ethanol) and centrifuged (3000 rpm for 2 min). The isolated sections were dried (24 h at 45 °C), milled and kept for future required experiments.
2.7. Preparation of alginate/modified alginate/jujube extract beads
Sodium alginate powder and modified sodium alginate (ratio 1:1) were dispersed in deionized water and stirred at a temperature of 40 °C overnight on a magnetic stirrer. Extract was prepared and added to the gum solution. It was prepared at minimal exposure to light to minimize damage to the polyphenols. Biopolymer solution was extruded into a CaCl2 solution by a syringe pump through a 0.5 cm nozzle to form the beads (Wichchukit et al., 2013). The beads waited for some hours in the ionic solution (Kaygusuz and Erim, 2013). Then, the beads were collected and taken into distilled water to remove residual ions from the beads (Kahya and Erim, 2019; Lupo et al., 2015).
2.8. pH adjustment and pasteurization and storage conditions
The beads were distributed into the glass vials containing distilled water after washing beads. The pH was adjusted to 3.8 using HCl (0.1 M) and then pasteurized at 72 °C for 10 min. Eventually, two types of beads divided into two portions and maintained in the refrigerator and ambient temperature for two weeks. This duration was on the assumption of the shelf life of fresh beverages.
2.9. Beads experiments
2.9.1. Determination of size and morphology
The diameter of 10 beads obtained from each treatment were taken at random, and measured with an electronic digital caliper (Titan, China) in order to record their diameters (Lupo et al., 2015).
2.9.2. Measurement of total phenolic compounds
Total phenol was obtained by Folin-Ciocalteu method (Chen et al., 2008). The first, 20 μl sample was mixed with 1.6 ml distilled water and 100 ml folin solution (1:10 v/v). It was left for 5 min then300 μl of 7.5% sodium carbonate solution was added and stirred. It was then stored for 2 h at room temperature. For the control, 10 μl of distilled water was added instead of 20 μl sample. The absorbance was read by a Cary 300 spectrophotometer (Varian, Mulgrave, Victoria, Australia) at 765 nm. The standard curve of Gallic acid was used to determine the actual content of total phenol (0, 50, 100, 150, 250, 500 and 1000 mg/l Gallic acid). By placing the sample absorption rate in the following Eq. (3) derived from the standard curve, the values were calculated in milligrams per liter instead of y on a standard basis (Gallic acid).
| y = 0.0005x + 0.0714 | Eq. (three) |
Total phenol of the wall compound (sodium alginate) was not brought in the calculations.
2.9.3. Encapsulation efficiency (EE)
The encapsulation efficiency (EE) of the beads was calculated according to the method proposed by Lupo et al. (2015) and Rocha-Selmi et al. (2013) with slight modification as shown in Eq. (1). In order to estimate the amount of total phenol entrapped in the beads, 100 mg bead was dispersed in 20 ml of 0.1 M phosphate buffer (pH = 7.4) at 37 °C for 18 h using a homogenizer and kept for 30 min in an ultrasound bath. Then, the amount of total phenol (from a uniform solution) was measured by a Cary 300 spectrophotometer (Varian, Mulgrave, Victoria, Australia) at 765 nm (Eq. (4))
| Eq. (four) |
where EE is encapsulation efficiency, TPB is total phenol content of the beads dissolved in phosphate buffer solution and TPX is the total phenol content of the initial extract. All measurements were carried out in triplicate.
2.9.4. Encapsulation yield
The encapsulation yield was calculated according to Eq. (5) (Rocha-Selmi et al., 2013).
| Eq. (five) |
where EY is encapsulation yield, WB is the weight of beads obtained in each test and WS is the weight of solids materials used in each test.
2.9.5. Hardness of the beads
Instrumental analysis of the beads was carried out using a General Texture Analyzer (GTA) (Brookfield Engineering Laboratories Inc., Middleboro, MA, USA). Each measurement was performed at room temperature on 10 beads of each treatment placed on a fixed bottom of the disc under the probe. The specimens were compressed with a 50 N load cell. The probe speed was adjusted at 10 mm min−1 the distance of compression was1 mm. Hardness (N) was determined according to (Belscak-Cvitanovic et al., 2015; Tsai et al., 2017) with partial correction.
2.9.6. Scanning electron microscopy (SEM)
Scanning electron microscope (SEM; LEO 440i; LEO Electron Microscopy Ltd, Cambridge, UK) at an accelerating voltage of 10 kV was used to study surface structure of the beads (Tsai et al., 2017). The beads were freeze dried and fixed on the stub with the assistance of double-sided sticky tape and coated with gold by sputter coater before SEM test.
2.9.7. Fourier-transform infrared spectroscopy (FT-IR)
Fourier-transform infrared (FTIR) spectra of freeze-dried beads were recorded in KBr pellets by FTIR spectroscopy (Nexus 870; Nicolet, WI, USA). The spectrum was scanned in transmission mode in a wave number range from 400 to 4000 cm−1 and the resolution was 4 cm−1 (Belscak-Cvitanovic et al., 2015; Mendes et al., 2016).
2.9.8. Differential scanning calorimetry (DSC)
Differential Scanning Calorimeter (DSC 131, Setaram Instrumentation, France) was used to analyze thermal properties of the beads. Samples were accurately weighed (16 mg) in aluminum pans and hermetically sealed. The DSC thermograms were recorded at the heating rate of 10 °C/min from 30 °C to 300 °C. Nitrogen fuel flow was held at 10 ml/min. A blank aluminum pan was used as a reference standard and the software program Data Processing Module version 1.54 f (Setaram, France) was used for data analysis (Lupo et al., 2015).
2.10. Statistical analysis
All statistical analysis of the results was performed using SPSS 26 (SPSS Inc., Chicago, IL, USA) statistics software. The results were analyzed using three repetitions (Technical replicates) and Duncan's multiple range test was used to obtain significant differences (P < 0.05) between treatments. Also, S test was used to explain significant differences (P < 0.05) between two treatments.
3. Results and discussion
3.1. Extraction efficiency
As tabulated in Table 1, the extraction was carried out by two solvents to extract all polar and non-polar phenolic compounds in two steps. An 80% ethanol was used first with extraction efficiency of 79.08% and then extraction was performed on the pre-extracted pulp with 50.44% efficiency. Due to the high polarity of water, it extracts many compounds but not all phenolic compounds. Organic solvents are usually more efficient in extracting phenolic compounds. These results are in agreement with the findings of other researchers (Rezaei et al., 2013; Tuncel and Yılmaz, 2015).
Table 1.
Extraction efficiency of jujube fruit.
| Efficiency of alcoholic extract (%) | Efficiency of aqueous extract (%) |
|---|---|
| 79.08 ± 0.12a | 50.44 ± 0.15b |
Values are mean ± SD from triplicate determinations; Different superscript letters indicate statistically significant differences among the means (P ˂ 0.05).
3.2. Measurement of antioxidant activity (DPPH)
As shown in Table 2, the concentration of 1 mg/ml of extract had the highest antioxidant and inhibitory activity. Due to the direct relationship between phenolic compounds and antioxidant activity, high content of phenolic compounds could be expected. This extract had a high inhibitory effect and these findings are in agreement with those obtained by other researchers (Choi et al., 2012; Gao, Wu, Wang, Xu, & Du, 2012; Yu et al., 2012; Wang et al., 2016; Ji, X, Liu, Ullah, & Wang, 2018). It can obviously be resulted that the use of this fruit as a source of bioactive compounds and natural antioxidants is effective in promoting the quality and development of functional foods and nutraceuticals. Extract inhibitory percentage was compared with ascorbic acid standard and results indicated significant difference at 5% level (P ˂ 0.05). IC50 (Half maximal Inhibitory Concentration) was also measured and the IC50 of ascorbic acid was approximately three times that of the extract (Table 2, Table 3).
Table 2.
DPPH antioxidant activity of extract and ascorbic acid.
| Extract concentrations (mg/ml) | Extract inhibition (%) | Ascorbic acid Inhibition (%) |
|---|---|---|
| 0.05 | 73.68 ± 0.02h | 90.92 ± 0.03h |
| 0.075 | 74.83 ± 0.03f | 92.17 ± 0.03g |
| 0.1 | 74.73 ± 0.03g | 92.31 ± 0.05f |
| 0.15 | 77.03 ± 0.03e | 92.41 ± 0.06e |
| 0.25 | 79.02 ± 0.02d | 93.18 ± 0.02d |
| 0.35 | 80.80 ± 0.05c | 94.02 ± 0.04c |
| 0.5 | 83.60 ± 0.04b | 94.85 ± 0.01b |
| 1 | 88.10 ± 0.03a | 96.89 ± 0.02a |
Values are mean ± SD from triplicate determinations; Different superscript letters indicate statistically significant differences among the means in each column (P ˂ 0.05).
Table 3.
IC 50 of the extract and ascorbic acid.
| Treatments | IC 50 (mg/gr) |
|---|---|
| Extract | 323.848 ± 0.002b |
| Ascorbic acid | 862.639 ± 0.001a |
Values are mean ± SD from triplicate determinations; Different superscript letters indicate statistically significant differences among the means (P ˂ 0.05).
3.3. Determination of beads size
It can be seen in Table 4, that the diameter of modified alginate beads was significantly higher than unmodified. The diameter of the beads maintained at refrigerated temperature was higher than that of ambient temperature. This may be due to the effect of low temperature on reducing the diffusion rate of the core material through the beads. OSA contributes to the strength of the beads structure. This compound contributes to the coherence of the alginate capsules by forming a covalent bond between the atoms. In fact, this compound can increase the hydrophobic property by incorporating hydrophobic groups into the alginate structure and assist the viscosity of the polymer solution to form particles. Hence, the modified alginate with OSA remarkably increased the mean size of beads. The diameter of the beads also decreased due to environmental parameters and the loss of aqueous content during storage. These findings are in agreement with a study which reported the effect of some parameters on gelling mechanism and changing the size of capsules (Lupo et al., 2015), and other research which reported that a filler occupying the interstitial spaces of capsules can affect the size of capsules (Chan et al., 2010).
Table 4.
Mean diameter and total phenol of the beads and encapsulation efficiency of the extract.
| Treatments/Time | Analysis | Day 1 | Day 7 | Day 14 |
|---|---|---|---|---|
| Alginate beads stored at ambient temperature (20̊ C) | Size (beads diameter) (mm) | 4.84 ± 0.04f | 4.56 ± 0.01h | 4.32 ± 0.01i |
| Total phenol of beads (mg/l) | 98.2 ± 0.02j | 137.4 ± 0.03f | 141.4 ± 0.02d | |
| Encapsulation Efficiency (%) | 6.70 ± 0.01k | 21.18 ± 0.01g | 25.85 ± 0.04c | |
| Alginate beads stored at refrigerated temperature (4 ° C) | Size (beads diameter) (mm) | 5.10 ± 0.02c | 4.72 ± 0.04g | 4.54 ± 0.01h |
| Total phenol of beads (mg/l) | 97 ± 0.01k | 133.2 ± 0.02h | 135.2 ± 0.01g | |
| Encapsulation Efficiency (%) | 6.62 ± 0.04k | 20.53 ± 0.03h | 24.71 ± 0.04d | |
| Alginate- modified alginate beads stored at ambient temperature | Size (beads diameter) (mm) | 5.15 ± 0.03b | 5.02 ± 0.02d | 4.89 ± 0.04e |
| Total phenol of beads (mg/l) | 133.2 ± 0.02h | 149.2 ± 0.02c | 192.6 ± 0.02a | |
| Encapsulation Efficiency (%) | 9.10 ± 0.01i | 23 ± 0.03e | 35.21 ± 0.03a | |
| Alginate- modified alginate beads stored at refrigerated temperature | Size (beads diameter) (mm) | 5.24 ± 0.02a | 5.20 ± 0.03a | 5.15 ± 0.03b |
| Total phenol of beads (mg/l) | 105 ± 0.01i | 139.4 ± 0.04e | 151.6 ± 0.02b | |
| Encapsulation Efficiency (%) | 7.17 ± 0.03j | 21.49 ± 0.18f | 27.71 ± 0.04b |
Values are mean ± SD from triplicate determinations; Different superscript letters indicate statistically significant differences among the means for each experiment (P ˂ 0.05).
3.4. Measurement of total phenolic compounds
As tabulated in Table 5, total phenol content of jujube extract during the storage time decreased by approximately one-third on the 14th day. The adverse effect of environmental parameters on these compounds such as ambient light, oxygen in the head space of the extract storage medium and storage time on the reduction of some phenolic compounds has been reported by several authors (Gao, Wu, Wang et al., 2012; Ji et al., 2018; Wang et al., 2016). Some researchers extracted phenolic compounds using different solvents (Mokrani and Madani, 2016). In one study done on phytochemical compounds and antioxidant capacity of bean seeds, the polarity of extracting solvents affected the extraction performance of these compounds, therefore, the use of both polar and non-polar solvents was more (Nawaz et al., 2020).
Table 5.
Total phenol content of jujube extract.
| Storage time | Total phenol (mg/l) |
|---|---|
| Day 1 | 1463.6 ± 0.06a |
| Day 7 | 648.6 ± 0.11b |
| Day 14 | 547 ± 0.10c |
Values are mean ± SD from triplicate determinations; Different superscript letters indicate statistically significant differences among the means (P ˂ 0.05).
The results of total phenol of beads in Table 4 reveal a significant difference between all treatments on specific days (P˂0.05) and the highest and lowest total phenol content belonged to the modified alginate beads maintained at ambient temperature and the alginate beads maintained at refrigerated temperature, respectively. As previously mentioned, modification provides the strongest structure of gum with addition of carboxyl groups. There are many reasons to justify the changes of total phenol of the extracts of the beads. Firstly, heat treatment, which may occur to pasteurize beverages enriched with the beads, affects the structure and pores of Ca-alginate beads and promote the release of some part of the encapsulated extract in the aqueous medium. It is noteworthy that Ca-alginate bead is capable to absorb water. Therefore, the extract released in the aqueous medium can be partly reabsorbed by Ca-alginate beads and can increase the content of total phenol which is also observed on the days 7th and 14th. Secondly, due to the presence of pores in the beads wall, some of the extract may be released into the aqueous medium, while total phenol value was measured just in the beads. Waste of some parts of the extract in the encapsulation process and washing is also possible. Moreover, polyphenols are usually attached to other compounds with various chemical bonds. These compounds can be affected by various factors such as temperature and detach polyphenols from these junctions. Therefore, the content of polyphenols in the medium may increase due to their increased bioavailability and their presence is clearly detectable by diagnostic devices. Hence, sometimes bioavailability limitations may prevent them from being detected in the analysis. Nanoencapsulation of jujube for enhancing antioxidant activity has been investigated by Han et al. (2015) and similar results observed. Another research also confirmed this finding (Yu et al., 2012). These researches showed environmental factors can effect on phenolics release which can be dependent to pH, temperature and etc. and Fick's diffusion is the main mechanism of release.
3.5. Encapsulation efficiency and yield
The outcomes of the extract encapsulation efficiency (EE) measurements are shown in Table 4. A significant difference (P˂0.05) between the modified and unmodified treatments was observed and the highest was in modified alginate maintained at ambient temperature. Some of the values obtained in this study were greater than those obtained by other researchers which may be due to the application of higher concentration of sodium alginate in our research, which protects the extract and reduces its flow from the beads. Modified alginate in some samples has improved the stability of the beads structure due to covalent bonds between atoms induced by OSA (Comunian et al., 2013; Rocha-Selmi et al., 2013). The encapsulation efficiency of extract was dependent to the coating structure and the type of junctions established in the beads structure. Similar studies have reported that the method of encapsulation, cross linking, type and coating materials are effective on the encapsulation efficiency (Amiryousefi et al., 2016; Belscak-Cvitanovic et al., 2015; Bourbon et al., 2016). Many studies have reported low encapsulation efficiency of soluble extract in water due to diffusion of core materials to the solution (Chan et al., 2010; Kulkarni et al., 2000; Moses et al., 2000). The efficiency of the modified alginate beads was more than that of other investigations. However, similar results about alginate beads in other investigations were observed that were about 14% and 7.4–26.7% (Chan et al., 2010; Kulkarni et al., 2000). Samples maintained at ambient temperature appear to be more efficient. Increased efficiency was also observed during the storage time of the beads for the reasons previously stated.
Encapsulation Yield (EY) of the extract was 28–34% (Table 6). The results revealed that there was a significantly difference between the treatment. The highest yield was for the modified alginate beads maintained at ambient temperature (Comunian et al., 2013; Rocha-Selmi et al., 2013). The modification of alginate by OSA improved the stability of the bead structure, leading to better protection of encapsulated compounds.
Table 6.
Encapsulation yield and hardness of beads.
| Treatments | Encapsulation Yield (%) | Hardness (N) |
|---|---|---|
| Alginate beads stored at ambient temperature | 28.37 ± 0.03c | 0.19 ± 0.02c |
| Alginate beads stored at refrigerated temperature | 28.24 ± 0.01d | 0.18 ± 0.02c |
| Modified alginate beads stored at ambient temperature | 34.16 ± 0.02a | 2 ± 0.02a |
| Modified alginate beads stored at refrigerated temperature | 29.27 ± 0.02b | 0.91 ± 0.02b |
Values are mean ± SD from triplicate determinations; Different superscript letters indicate statistically significant differences among the means in each column (P ˂ 0.05).
3.6. Hardness of the beads
The results of the General Texture Analyzes (GTA) ranged from 0.18 to 2 N and revealed significant variations (p<0.05) between the hardness of different treatments. As tabulated in Table 6, alginate modification by OSA improved the strength the beads, turning it to better protecting of bioactive compounds. The results are in agreement with the results of different researches reported on the modification of different polysaccharides (Belscak-Cvitanovic et al., 2015). Mohammadi et al. (2016) pointed out that the modification of polysaccharide positively affected the textural properties of gum and reported in another research that the use of different compounds and materials can also affect the texture and structure of alginate (Lupo et al., 2015).
3.7. Morphology of the beads
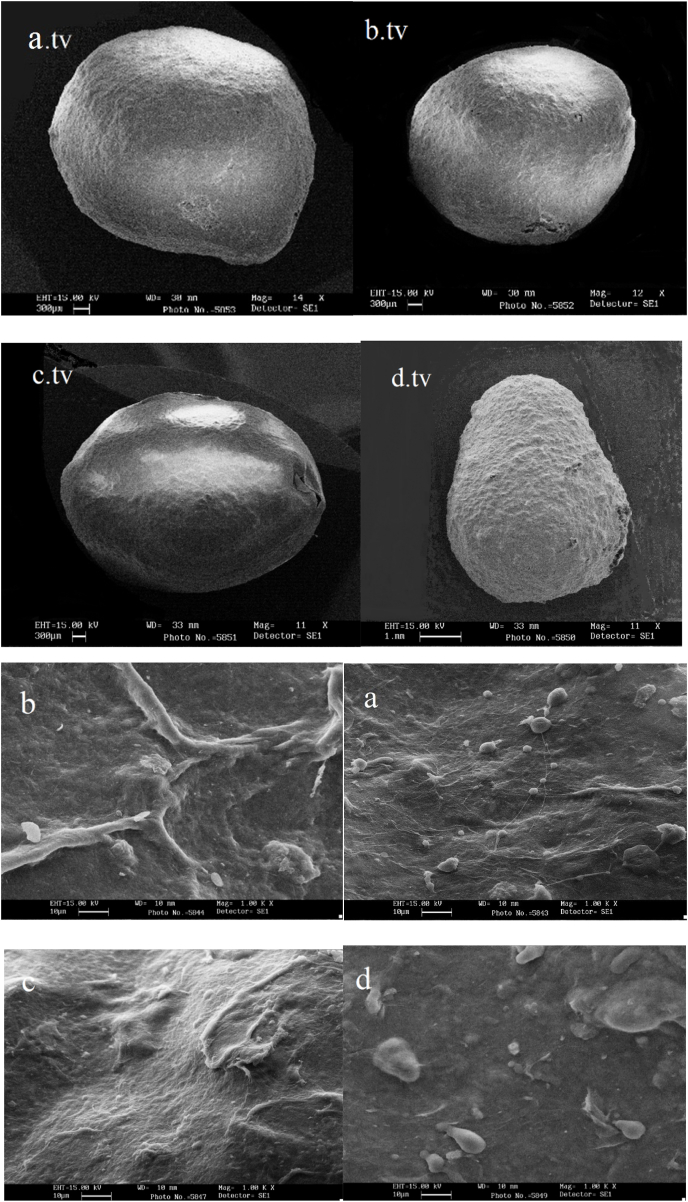
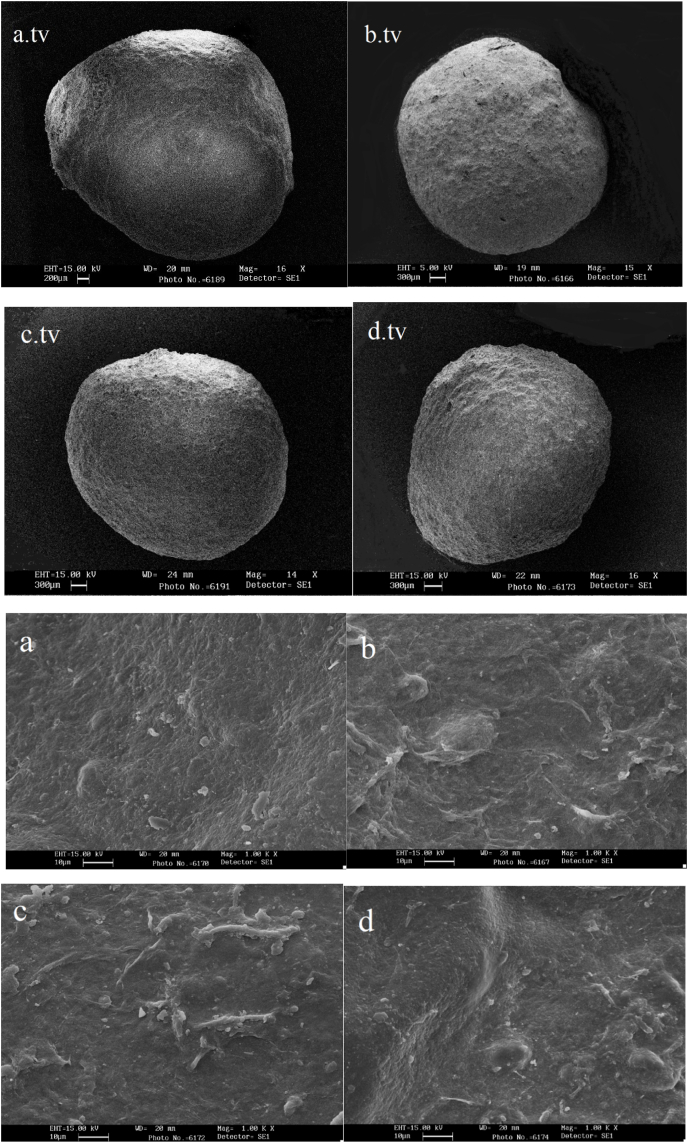
The morphology of the beads was determined by scanning electron microscope, as depicted in Fig. 1, Fig. 2. The form of the beads was typically spherical with slight surface wrinkles. The modified alginate beads were slightly elongated which may be due to the addition of OSA. It affects the viscosity of the polymer solution and is participated in the interaction of hydrophobic and hydrophilic groups when forming beads in the aqueous medium. Those with more uniform surface coverage could be related to the formation of covalent bonds between the Coo−(carboxyl) groups of OSA and hydroxyl groups of alginate.
Fig. 1.
Scanning electron microscopy images of the beads on the first day from top view (tv) and at 1000 × magnification showing: a. tv and a alginate bead stored at ambient temperature, b. tv and b alginate bead stored at refrigerated temperature, c. tv and c alginate-modified alginate bead stored at ambient temperature, d. tv and d alginate-modified alginate bead stored at refrigerated temperature.
Fig. 2.
Scanning electron microscopy images of the beads on the 14th day from top view and at 1000 × magnification showing: a. tv and a alginate bead stored at ambient temperature, b. tv and b alginate bead stored at refrigerated temperature, c. tv and c alginate-modified alginate bead stored at ambient temperature, d. tv and d alginate-modified alginate bead stored at refrigerated temperature.
The specimens stored in the refrigerator of both types of beads had a minor rougher surfaces than those stored at ambient temperature. This can be due to the influence of temperature on the gel structure of the beads and the flow of materials. Research has reported that the presence of cross links in alginate capsules outcomes a compact and more uniform structure and less wrinkling (Bagheri et al., 2014; Bourbon et al., 2016; Krasaekoopt et al., 2006; Possemiers et al., 2010; Zhou et al., 1998). The storage period also had no significant effect on the appearance of different samples.
3.8. Fourier-transform infrared spectroscopy (FT-IR)
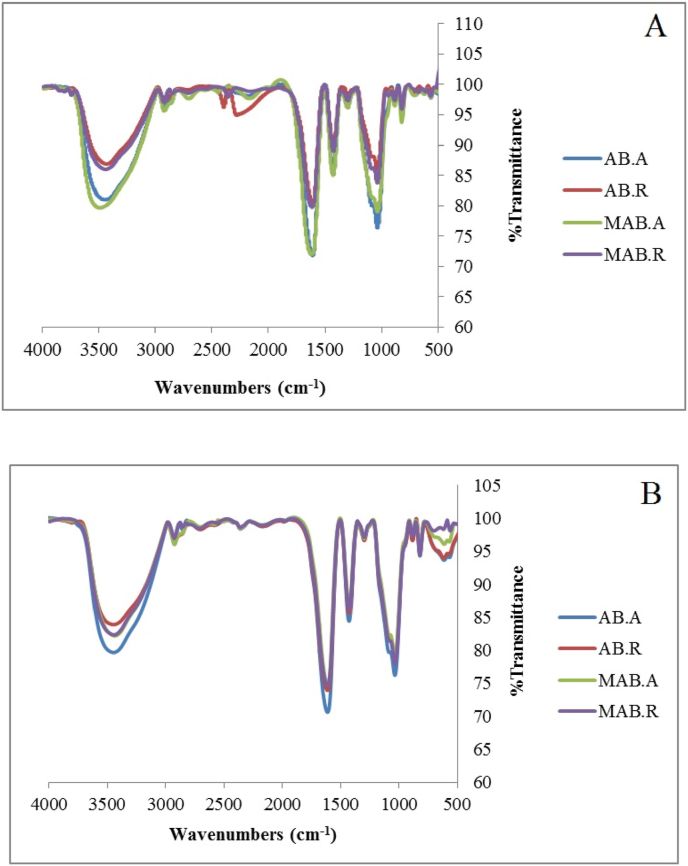
The FTIR spectra of different beads obtained to verify possible interactions induced by OSA modification (Fig. 3). In the first and 14th day spectra, a broad O–H tensile vibration appeared in the range of 3431–3492 cm−1 that are related to the presence of phenols of extract and alginate beads that is related to intermolecular hydrogen bonding, as other researchers have also reported (Belscak-Cvitanovic et al., 2015; Sartori et al., 1997).
Fig. 3.
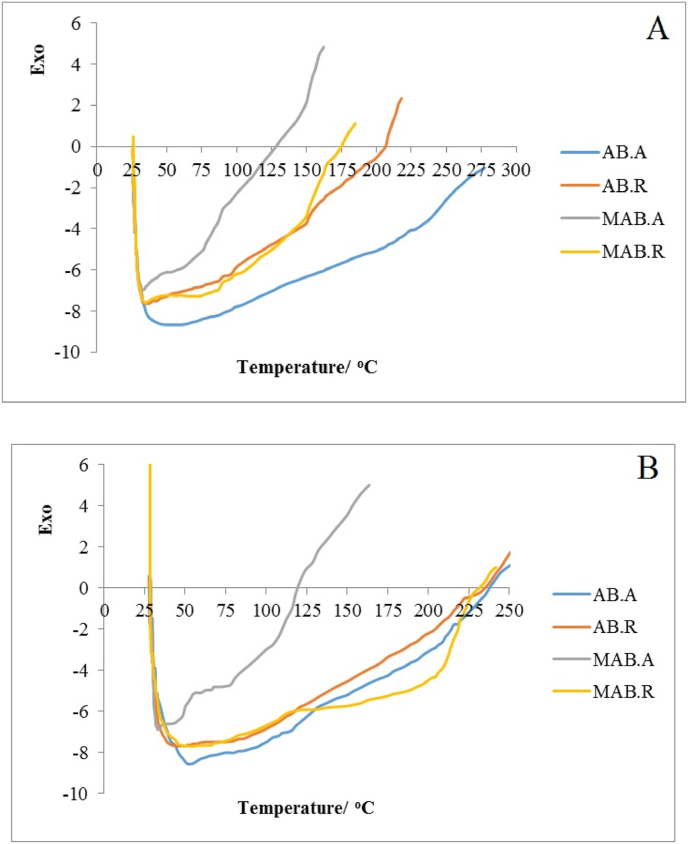
Infrared spectroscopy (FT-IR) images, on the first day (A) and 14th day (B) of storage. AB. A: Alginate beads maintained at ambient temperature, AB. R: Alginate beads maintained at refrigerator temperature, MAB. A: Modified alginate beads maintained at ambient temperature, MAB. R: Modified alginate beads maintained at refrigerator temperature.
In addition, three strong peaks at about 1600 cm−1,1425-1426-1428 cm−1 and 1033-1034-1035 cm−1were detected which correspond to the carbonyl group of carboxylic acid, the carbonyl group of amides I, and C–O ester, respectively. An absorption peaks in the regions of 2921–2924 cm−1 result from the presence of C–H groups of polysaccharide in the beads (Singh2009).
Furthermore, two weak adsorption peaks at 2705.27 cm−1 for modified alginate maintained at ambient temperature and 2707.99 cm−1 for alginate maintained at ambient temperature, seen on the right side of C–H adsorbents, correspond to C–H aldehydes.
The observed peak in the range of 1020–1070 cm−1 is related to calcium alginate which is not clearly visible due to interference with other compounds in the beads spectrum and attribute to the C–O stretching in C–O–C linkages (Pasparakis and Bouropoulos, 2006) as alginate structure of the beads.
Carbonyl groups are associated with the esterification of gum with OSA. Adsorption by carbonyl groups in the esters and in carboxylic acid reveals a new spectrum under optimum conditions but because of overlap with other carbonyl group peaks (1600 and 1400 cm−1) is not clearly seen by specific spectrum. These results are in agreement with Ribeiro et al. (2005), Bagheri et al. (2014) and Belscak-Cvitanovic et al. (2015). Since esterification of gum with OSA, leads to replacement of hydroxyl groups of gum with carbonyl groups of OSA, so formation of carbonyl groups is attributed to creation of stretching absorptions of C–O and C O groups. In alkaline systems, the modified gum with OSA is formed in the form of sodium succinic acid gum. Thus, an absorption peak between 1400 and 1600 cm−1can be created to attribute to the asymmetric stretching vibration of carboxylate ions (RCOO−) that indicates the esterification reaction and the formation of monoester and this result was in agreement with the findings of other researchers (Mohammadi et al., 2016).
3.9. Differential scanning calorimetry (DSC)
The DSC thermograms of alginate beads maintained at ambient and cold temperatures, modified alginates beads maintained at ambient and refrigerator temperatures in the first day showed the endothermic peaks at 86.70 °C, 107.49 °C, 95.32 °C and 104.04 °C respectively. The DSC thermograms of alginate beads, maintained at the same conditions, showed the endothermic peaks at 82.12 °C, 97.60 °C, 96.74 °C and 86.22 °C, in the 14th day, respectively.
According to Sarmento et al. (2006), endothermic peaks are dependent on water loss because they are associated with hydrophilic groups of polymers. Temperature change up to higher values for the extract capsules could be attributed to the interaction between the polymer and the phenolic compounds (Córdoba et al., 2013; Hambleton et al. 2009). Thus, sharper peaks can confirm the presence of an electrostatic interaction between extract and the polymer matrix that restricted the release of the extract from the beads.
The DSC thermograms of different beads are depicted in Fig. 4. As seen in the figure, in the first day, the glass transition temperature (Tg) was at 37.5, 40, 41 and 41.5 °C, for both unmodified and modified beads maintained at ambient and refrigerated conditions, respectively.
Fig. 4.
DSC spectra of capsules on the 1stday (A) and 14th day (B) of storage; AB. A: Alginate beads maintained at ambient temperature, AB. R: Alginate beads maintained at refrigerator temperature, MAB. A: Modified alginate beads maintained at ambient temperature, MAB. R: Modified alginate beads maintained at refrigerator temperature.
In the day 14th, the alginate beads maintained at ambient temperature had the glass transition temperature (Tg) at 35.5 °C, the alginate beads stored refrigerated had a Tg of 39.5 °C, modified alginate maintained at ambient temperature and refrigerated revealed Tg at 41.5 °C and 42.5 °C, respectively. The high Tg points in the beads, especially in the modified beads is highly favorable in order to be effective in reducing the release of core compounds in thermal process. On the other hand, the specimens stored refrigerated had a higher Tg due to the effect of cold on the polymer structure. Although the effect of gum modification to increase the Tg is clearly seen in the beads maintained at ambient temperature. Some researchers reported a significant increase in the glass transition temperature of alginate capsules by adding calcium chloride as binding compounds as a result of increasing heat (Bagheri et al., 2014). In agreement with the findings of the present study, other researchers have also reported that the thermogram of cocoa extract-loaded calcium alginate capsules exhibited a double endothermic peak as a result of the cross-linking of alginate and showed a wide range of phenolic compounds present in the extract. They also proved that the glass transition temperature of the extract-loaded capsules change up to greater amounts because of intermolecular hydrogen bonding is caused by the phenolic compounds (Lupo et al., 2015).
4. Conclusions
Modification of sodium alginate by OSA was successfully implemented, followed by producing beads to encapsulate the jujube extract in order to observe possible phenolic compounds release of the beads. The encapsulation yield of the beads was significantly affected by modification of sodium alginate and proved to have the ability to protect total phenol of jujube extract. Maintaining at ambient temperature improved the efficiency of the beads and caused a significant difference due to greater bioavailability of the phenolic compounds in the extract. Moreover, the modified beads showed higher Tg, especially in the ambient temperature which is of interest in heating processes. Mean while, processing jujube extracts for usage in beverages can be an onset point for the use of this abundant Iranian fruit. Thereby, we recommend the OSA modified alginate carriers to the food and beverages industry as an innovative design for soluble compounds, to provide stronger hydrogels, increase their shelf life and protect the nutritional value in the delivery systems. These findings have important implications on designing preservation and delivery systems of soluble bioactive compounds of jujube extract to apply in development of new functional foods and drinks.
Credit author statement
Zahra Khoshdouni Farahani: Investigation, Data curation, investigation, Writing-Original draft, Methodology; Mohammad ali E. Mousavi: Methodology, Editing, Experimental guidance; Seyed Mahdi Seyedein Ardebili: Methodology, Experimental guidance; Hossein Bakhoda: Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the scientific support of the Science and Research Branch of Islamic Azad University in Tehran (Iran).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.11.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Amiryousefi M.R., Mohebbi M., Golmohammadzadeh S., Koocheki A. Encapsulation of caffeine in hydrogel colloidosome: optimization of fabrication, characterization and release kinetics evaluation. Flavour Fragrance J. 2016;31(2):163–172. [Google Scholar]
- Bagheri L., Madadlou A., Yarmand M., Mousavi M.E. Spray-dried alginate microparticles carrying caffeine-loaded and potentially bioactive nanoparticles. Food Res. Int. 2014;62:1113–1119. [Google Scholar]
- Belscak-Cvitanovic A., Komes D., Karlović S., Djaković S., Špoljarić I., Mršić G., Ježek D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015;167:378–386. doi: 10.1016/j.foodchem.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Bourbon A.I., Cerqueira M.A., Vicente A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: curcumin and caffeine as model compounds. J. Food Eng. 2016;180:110–119. [Google Scholar]
- Chan E.S., Yim Z.H., Phan S.H., Mansa R.F., Ravindra P. Encapsulation of herbal aqueous extract through absorption with ca-alginate hydrogel beads. Food Bioprod. Process. 2010;88(2–3):195–201. [Google Scholar]
- Chen H., Fu X., Luo Z.G. Esterification of sugar beet pectin using octenyl succinic anhydride and its effect as an emulsion stabilizer. Food Hydrocolloids. 2015;49:53–60. [Google Scholar]
- Chen L., Subirade M. Alginate–whey protein granular microspheres as oral delivery vehicles for bioactive compounds. Biomaterials. 2006;27(26):4646–4654. doi: 10.1016/j.biomaterials.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Chen Q., Zhao J., Liu M., Cai J., Liu J. Determination of total polyphenols content in green tea using FT-NIR spectroscopy and different PLS algorithms. J. Pharmaceut. Biomed. Anal. 2008;46(3):568–573. doi: 10.1016/j.jpba.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Kim H.Y., Seo W.T., Lee J.H., Cho K.M. Roasting enhances antioxidant effect of bitter melon (Momordica charantia L.) increasing in flavan-3-ol and phenolic acid contents. Food Sci. Biotechnol. 2012;21(1):19–26. [Google Scholar]
- Comunian T.A., Thomazini M., Alves A.J.G., de Matos Junior F.E., de Carvalho Balieiro J.C., Favaro-Trindade C.S. Microencapsulation of ascorbic acid by complex coacervation: protection and controlled release. Food Res. Int. 2013;52(1):373–379. [Google Scholar]
- Córdoba A., Deladino L., Martino M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013;95(1):315–323. doi: 10.1016/j.carbpol.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Gao Q.H., Wu C. Sen, Wang M., Xu B.N., Du L.J. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2012;60(38):9642–9648. doi: 10.1021/jf3026524. [DOI] [PubMed] [Google Scholar]
- Gao Q.H., Wu C. Sen, Yu J.G., Wang M., Ma Y.J., Li C.L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (ziziphus jujuba Mill.) selections. J. Food Sci. 2012;77(11) doi: 10.1111/j.1750-3841.2012.02946.x. [DOI] [PubMed] [Google Scholar]
- Golmohammadi F. Medicinal plant of Jujube (Ziziphus jujuba) and its indigenous knowledge and economic importance in desert regions in east of Iran: situation and problems. Tech. J. Eng. Appl. Sci. 2013;3(6):493–505. [Google Scholar]
- Gombotz W.R., Wee S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31(3):267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Hambleton A., Debeaufort F., Bonnotte A., Voilley A. Influence of alginate emulsion-based films structure on its barrier properties and on the protection of microencapsulated aroma compound. Food Hydrocolloids. 2009;23(8):2116–2124. [Google Scholar]
- Han H.J., Lee J.S., Park S.A., Ahn J.B., Lee H.G. Extraction optimization and nanoencapsulation of jujube pulp and seed for enhancing antioidant activity. Colloids Surf. B Biointerfaces. 2015;130:93–100. doi: 10.1016/j.colsurfb.2015.03.050. [DOI] [PubMed] [Google Scholar]
- Ji X., Liu F., Ullah N., Wang M. Isolation, purification, and antioxidant activities of polysaccharides from Ziziphus Jujuba cv. Muzao. Int. J. Food Prop. 2018;21(1):1–11. [Google Scholar]
- Kahya N., Erim F.B. Surfactant modified alginate composite gels for controlled release of protein drug. Carbohydr. Polym. 2019;224:115165. doi: 10.1016/j.carbpol.2019.115165. [DOI] [PubMed] [Google Scholar]
- Kamiloglu Ö., Ercisli S., Engül M., Toplu C., Serçe S. Total phenolics and antioxidant activity of jujube (Zizyphus jujube Mill.) genotypes selected from Turkey. Afr. J. Biotechnol. 2009;8(2):303–307. [Google Scholar]
- Kikuchi A., Kawabuchi M., Watanabe A., Sugihara M., Sakurai Y., Okano T. Effect of Ca2+-alginate gel dissolution on release of dextran with different molecular weights. J. Contr. Release. 1999;58(1):21–28. doi: 10.1016/s0168-3659(98)00141-2. [DOI] [PubMed] [Google Scholar]
- Krasaekoopt W., Bhandari B., Deeth H.C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT-and conventionally treated milk during storage. LWT Food Sci. Technol. 2006;39(2):177–183. [Google Scholar]
- Kris-Etherton P.M., Lichtenstein A.H., Howard B.V., Steinberg D., Witztum J.L. Antioxidant vitamin supplements and cardiovascular disease. Cités. 2004;110(5):637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- Kulkarni A., Soppimath K.S., Aminabhavi T.M., Dave A.M., Mehta M.H. Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J. Contr. Release. 2000;63(1–2):97–105. doi: 10.1016/s0168-3659(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Lupo Bryshila, Maestro A., Gutiérrez J.M., González C. Characterization of alginate beads with encapsulated cocoa extract toprepare functional food: comparison of two gelation mechanisms. Food Hydrocolloids. 2015;49:25–34. [Google Scholar]
- Mendes J., Paschoalin R.T., Carmona V.B., Neto A.R.S., Marques A.C.P., Marconcini J.M., et al. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016;137:452–458. doi: 10.1016/j.carbpol.2015.10.093. [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Abbasi S., Scanlon M.G. Development of emulsifying property in Persian gum using octenyl succinic anhydride (OSA) Int. J. Biol. Macromol. 2016;89:396–405. doi: 10.1016/j.ijbiomac.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Mokrani A., Madani K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Separ. Purif. Technol. 2016;162:68–76. [Google Scholar]
- Moses L.R., Dileep K.J., Sharma C.P. Beta cyclodextrin–insulin‐encapsulated chitosan/alginate matrix: oral delivery system. J. Appl. Polym. Sci. 2000;75(9):1089–1096. [Google Scholar]
- Najafabadi N.S., Sahari M.A., Barzegar M., Esfahani Z.H. Effect of gamma irradiation on some physicochemical properties and bioactive compounds of jujube (Ziziphus jujuba var vulgaris) fruit. Radiat. Phys. Chem. 2017;130:62–68. [Google Scholar]
- Nawaz H., Shad M.A., Rehman N., Andaleeb H., Ullah N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Brazilian J. Pharmac. Sci. 2020;56 [Google Scholar]
- Nazni P., Mythili A. Formulation and optimization of vitamin-C rich beverage prepared from ziziphus jujube. Int. J. Food Nutr. Sci. 2013;2(2):54. [Google Scholar]
- Owen R., Giacosa A., Hull W.E., Haubner R., Spiegelhalder B., Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer. 2000;36(10):1235–1247. doi: 10.1016/s0959-8049(00)00103-9. [DOI] [PubMed] [Google Scholar]
- Possemiers S., Marzorati M., Verstraete W., Van de Wiele T. Bacteria and chocolate: a successful combination for probiotic delivery. Int. J. Food Microbiol. 2010;141(1–2):97–103. doi: 10.1016/j.ijfoodmicro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Prachayasittikul S., Buraparuangsang P., Worachartcheewan A., Isarankura-Na-Ayudhya C., Ruchirawat S., Prachayasittikul V. Antimicrobial and antioxidative activities of bioactive constituents from hydnophytum formicarum jack. Molecules. 2008;13:904–921. doi: 10.3390/molecules13040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei S., Rezaei K., Haghighi M., Labbafi M. Solvent and solvent to sample ratio as main parameters in the microwave-assisted extraction of polyphenolic compounds from apple pomace. Food Sci. Biotechnol. 2013;22(5):1–6. [Google Scholar]
- Ribeiro A.J., Silva C., Ferreira D., Veiga F. Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur. J. Pharmaceut. Sci. 2005;25:31–40. doi: 10.1016/j.ejps.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rocha-Selmi G., Bozza F., Thomazini M., Bolini H.M., Fávaro-Trindade C.S. Microencapsulation of aspartame by double emulsion followed by complex coacervation to provide protection and prolong sweetness. Food Chem. 2013;139(1–4):72–78. doi: 10.1016/j.foodchem.2013.01.114. [DOI] [PubMed] [Google Scholar]
- Rostami H., Gharibzahedi S.M.T. Microwave-assisted extraction of jujube polysaccharide: optimization, purification and functional characterization. Carbohydr. Polym. 2016;143:100–107. doi: 10.1016/j.carbpol.2016.01.075. [DOI] [PubMed] [Google Scholar]
- San B., Yildirim A.N. Phenolic, alpha-tocopherol, beta-carotene and fatty acid composition of four promising jujube (Ziziphus jujuba Miller) selections. J. Food Compos. Anal. 2010;23(7):706–710. [Google Scholar]
- Sarmento B., Ferreira D., Veiga F., Ribeiro A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006;66(1):1–7. [Google Scholar]
- Sartori C., Finch D., Ralph B., Gilding K. Determination of the cation content of alginate thin films by FTi. r. spectroscopy. Polymer. 1997;38(1):43–51. [Google Scholar]
- Singh B., Sharma D.K., Gupta A. A study towards release dynamics of thiram fungicide from starch–alginate beads to control environmental and health hazards. J. Hazard Mater. 2009;161(1):208–216. doi: 10.1016/j.jhazmat.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Sun Y., Liang Z., Shan C., Viernstein H., Unger F. Comprehensive evaluation of natural antioxidants and antioxidant potentials in Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex HF Chou fruits based on geographical origin by TOPSIS method. Food Chem. 2011;124(4):1612–1619. [Google Scholar]
- Tsai F., Kitamura Y., Kokawa M. Effect of gum Arabic-modified alginate on physicochemical properties, release kinetics, and storage stability of liquid-core hydrogel beads. Carbohydr. Polym. 2017;174:1069–1077. doi: 10.1016/j.carbpol.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Tuncel N.B., Yılmaz N. Optimizing the extraction of phenolics and antioxidants from feijoa (Feijoa sellowiana, Myrtaceae) J. Food Sci. Technol. 2015;52(1):141–150. [Google Scholar]
- Vandenberg G., Drolet C., Scott S.L., De la Noüe J. Factors affecting protein release from alginate–chitosan coacervate microcapsules during production and gastric/intestinal simulation. J. Contr. Release. 2001;77(3):297–307. doi: 10.1016/s0168-3659(01)00517-x. [DOI] [PubMed] [Google Scholar]
- Wang B., Huang Q., Venkitasamy C., Chai H., Gao H., Cheng N., Pan Z. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;66:56–62. [Google Scholar]
- Wichchukit S., Oztop M.H., McCarthy M.J., McCarthy K.L. Whey protein/alginate beads as carriers of a bioactive component. Food Hydrocolloids. 2013;33(1):66–73. [Google Scholar]
- Yu Y., Zhang S., Ren Y., Li H., Zhang X., Di J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012;113(3):408–414. [Google Scholar]
- Zhang Z., Zhang R., Zou L., Chen L., Ahmed Y., Al Bishri W., McClements D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocolloids. 2016;58:160–170. [Google Scholar]
- Zhou Y., Martins E., Groboillot A., Champagne C.P., Neufeld R.J. Spectrophotometric quantification of lactic bacteria in alginate and control of cell release with chitosan coating. J. Appl. Microbiol. 1998;84(3):342–348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.