Abstract
Tropical theileriosis is a lymphoproliferative disease caused by the intracellular schizonts of Theileria annulata, an apicomplexan parasite. It causes severe infection in cattle and the untreated cattle would possibly die within 3–4 weeks of infection. The chemotherapy for this disease is largely dependent on the use of hydroxynaphthoquinone, namely buparvaquone. There have been reports recently of the development of resistance against this drug in T. annulata. Hence, identification of new drug molecule(s) or repurposing of existing drug molecule(s) against T. annulata is quite important. Here, we present the screening of 400 compounds included in the open-access Pathogen box from Medicine for Malaria Venture (MMV) to discover the novel compounds with potential inhibitory activity against T. annulata infected bovine leucocytes. We identified two compounds, MMV000062 and MMV560185, with IC50 values of 2.97 μM and 3.07 μM, respectively. MMV000062 and MMV560185 were found non-toxic to BoMac cells with CC50 values 34 μM and > 100 μM, respectively. The therapeutic indices of these compounds, MMV000062 and MMV560185, were calculated as more than 33 and 11, respectively. Further, it was observed that the parasite-infected cells under long-term culture were unable to recover with these compounds. We further deciphered that MMV560185 kills the infected cell by activation of TNFR-1 mediated extrinsic pathway of the apoptosis. The phenotypic characteristics of apoptosis were confirmed by Transmission Electron Microscopy. Our results suggest that it may be possible to develop MMV560185 further for chemotherapeutics of tropical theilerosis.
Keywords: Theileria annulata, Pathogen box, Anti-theilerial, Drug, Therapeutics, Medicine for Malaria Venture (MMV)
Graphical abstract

1. Introduction
Theileria parasites belong to the phylum Apicomplexa that also includes other parasites such as Plasmodium, Babesia, Toxoplasma, and Cryptosporidium. These parasites are largely responsible for the major parasitic diseases in human and animals (Morrison, 2009; Seeber and Steinfelder, 2016). Theileria infection in ruminants onsets with the transmission of sporozoites stage of the parasite by the ticks during the blood meal. The sporozoites preferentially invade the B cells and monocytes of the cattle (Spooner et al., 1989) leading to lympho-proliferation (Irvin, 1985). The disease caused by Theileria parva is commonly known as East coast fever while Theileria annulata causes Tropical theileriosis. Tropical theileriosis, transmitted by the tick of Hyalomma anatolicum and other Hyalomma species, is one of the most important tick-borne diseases in Asia, North Africa, and Southern Europe that causes huge economic losses (Noval et al., 1992). Bovine cells infected with Theileria parasite share several hallmarks of cancer such as deregulation of energetic metabolism, resistance to apoptosis, uncontrolled proliferation, and acquiring an invasive phenotype (Tretina et al., 2015). The untreated animal would die within 3–4 weeks post-infection in the acute disease conditions.
The commonly used strategies for the treatment and control of theileriosis are (i) targeting the parasite by vaccination of animal, (ii) treating infected animal by chemotherapy, and (iii) targeting vector by the use of chemical acaricides. One of the strategies of vaccination is ‘infection and treatment’ wherein cattle are immunized with live sporozoites and simultaneously treated with oxytetracycline resulting in effective immunization of cattle (Brown et al., 1977). Another strategy for vaccination is the use of T. annulata schizont cell culture as live attenuated vaccines (Brown, 1990). The vaccinated cattle are protected; however, it is worrisome that some cattle remain as carriers of the disease even after vaccination (Singh et al., 2001). Chemotherapy is also an adequate method of treatment for animals infected with tropical theileriosis. In vitro screening for anti-theilerial drugs showed that buparvaquone (hydroxynaphthoquinone), and ciprofloxacin are promising in controlling the proliferation of Theileria infected host cells (Guergnon et al., 2003b; Lizundia et al., 2009; McHardy et al., 1976). Further, parvaquone and buparvaquone (BPQ) are very effective in controlling the disease in vivo (Hashemi-Fesharki, 1991; McHardy et al., 1985; Sharma and Mishra, 1990). Presently, buparvaquone is the commonly used drug in field for the treatment of theileriosis. The present regime of treatment of tropical theileriosis needs to be looked all over again as (i) different tick species are gaining resistance towards chemical acaricides (Abbas et al., 2014), and (ii) Theileria parasites have started developing resistance towards buparvaquone (Mhadhbi et al., 2010). The failure of BPQ has been associated with mutation in the Theileria cytochrome b encoding gene or Theileria prolyl isomerase (TaPin1) (Chatanga et al., 2019; Marsolier et al., 2015; Mhadhbi et al., 2015; Sharifiyazdi et al., 2012).
The reports of BPQ resistance in cattle advocate the urgency to identify new drugs to target T. annulata parasite. There have been efforts to develop new drugs for other tropical diseases such as malaria. The Medicine for Malaria Venture (MMV) has been developing new anti-malarials and provides the ‘Malaria Box’ which is a collection of 400 diverse compounds with activity against the blood-stage of Plasmodium falciparum. MMV also promotes drug discovery for neglected diseases by providing open access of these drug-like compounds to the scientific community through the Malaria box (Van Voorhis et al., 2016) and Pathogen box (which also consists of 400 diverse, drug-like molecules active against neglected tropical diseases). Previously, the malaria box compounds have been shown to possess active compounds against various infectious agents like Toxoplasma gondii (Boyom et al., 2014), Plasmodium falciparum (Bowman et al., 2014), Schistosoma mansoni (Ingram-Sieber et al., 2014), Cryptosporidium parvum (Bessoff et al., 2014), Piroplasm (Babesia bovis, Babesia bigemina, Babesia caballi and Theileria equi) (Nugraha et al., 2019). The in vitro screening of Malaria box compounds against T. annulata identified MMV666022 and MMV666054 as active compounds (Hostettler et al., 2016). The recent screening of the Pathogen box compounds against T. parva identified the compounds MMV008212 and MMV688372 as promising leads (Nyagwange et al., 2019).
We screened the compounds of the Pathogen box against a T. annulata infected field isolate (Ana2014 cells) in the present study (Araveti and Srivastava, 2019). We tested the cytotoxicity of these compounds on BoMac cell line. We identified two compounds, MMV000062 and MMV560185, with in vitro therapeutic indices more than 33 and 11.07 respectively, as novel candidates to kill T. annulata infected bovine leucocytes. Furthermore, MMV560185 was found to specifically induce an extrinsic pathway of apoptosis in the parasitized cell.
2. Materials and methods
2.1. Chemicals
Culture media RPMI-1640, Penicillin-Streptomycin, Dulbecco's Modified Eagle's Medium (DMEM), were purchased from Gibco, life technologies. Fetal Bovine Serum (FBS) was purchased from Hyclone, GE Healthcare. MTT (Methylthiazolyldiphenyl-tetrazolium bromide), L-glutamine, HEPES and DMSO were purchased from Sigma. Ficoll-Paque plus was purchased from GE Healthcare. Annexin V and TUNEL assay kits were purchased from BD pharmingen. MycoAlert™ Mycoplasma detection kit was procured from Lonza. Antibodies against caspase 3, PARP, pBad, Bcl2, TNF-R1, TRADD, LC3A/B were purchased from Cell Signalling Technology. Antibodies against beta-tubulin were obtained from Santa Cruz Biotechnology. Antibodies against caspase 8 were purchased from BD Pharmingen. The Pathogen box was obtained from the Medicines for Malaria Venture (MMV) foundation (Geneva, Switzerland). All information about compounds and plate mapping is available online [(https://www.mmv.org/mmv-open) and (http://www.pathogenbox.org/about-pathogen-box/supporting-information)] (MMV2019). The compounds were diluted in the culture medium to 10 μM concentration in accordance with MMV instructions.
2.2. Maintenance of Theileria parasite and cell cultures
Theileria annulata infected bovine cells (Ana2014 cells) were cultured in the laboratory conditions as described previously (Araveti and Srivastava, 2019). The Ana2014 cells were cultured in complete RPMI media-1640 (supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 25 mM HEPES, 0.1% antibiotic (100 IU/ml penicillin and 0.1 mg/ml streptomycin) at 37 °C with 5% CO2 in a humidified incubator. BoMac cell line, a bovine macrophage line immortalized by the simian virus 40 (SV 40) large T antigen, was cultured as described previously (Stabel and Stabel, 1995). Cells were counted using a hemocytometer slide counting chamber with trypan blue. The parasites were routinely tested for Mycoplasma contamination and found negative using MycoAlert™ Mycoplasma Detection Kit.
2.3. Polymerase chain reaction
Total RNA was extracted from the Ana2014 cells using NucleoSpin® RNA Plus kit (TaKaRa Bio.) following the instructions provided by the manufacturer. The cDNA preparation was carried out with 1 μg of total RNA using PrimeScript™ 1st strand cDNA synthesis kit (TaKaRa Bio.) following the manufacturer's instructions. The cDNA was used to perform PCR using Q5® High-Fidelity DNA polymerase (New England Biolabs Inc.) with the specific primers of bovine CD markers CD5, CD11a and CD14 as described previously (Modirrousta et al., 2019).
2.4. Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay: screening of the pathogen box compounds
The effect of each drug on the growth of Ana2014 cells was tested using MTT assay following the standard protocol. Briefly, Ana2014 cells were seeded on to a 96-well plate with 2 × 104 cells per well in 100 μl complete RPMI 1640 media and incubated at 37 °C with 5% CO2 in a humidified incubator. All Pathogen box compounds were initially tested against Ana2014 cells at 10 μM concentration diluted in complete RPMI 1640 media. The specific drugs were added to specific wells after 4 h of incubation and further incubated for 24 and 48 h. 0.1 percent DMSO was used for solvent control. The cell viability was determined by MTT assay using a standard protocol with some modifications. Briefly, after incubation, cells were centrifuged at 400 g for 5 min and 50 μl of supernatant was removed. 50 μl MTT (1 mg/ml) was added to each well in a manner that the final concentration became 0.5 mg/ml per well. The cells were incubated for 4 h at 37 °C with 5% CO2 and then 150 μl DMSO was added. The plates were incubated for 30 min for the colour to develop. The colorimetric reading was recorded at 570 nm using the multimode reader.
2.5. Cytotoxicity and therapeutic/specificity index (SI) estimation
Cytotoxicity of each drug showing activity at 10 μM concentration was tested on BoMac cell line. BoMac cells were seeded at 0.3 × 104 cell/well onto a 96-well plate with 100 μl of respective media and grown initially for 12 h. The cells were exposed to specific compounds for 24/48 h at 37 °C with 5% CO2. Cell viability was estimated using the standard MTT assay. The cytotoxicity concentration (CC50) of each drug was calculated.
Therapeutic index was calculated for each drug as the ratio between cytotoxic and anti-parasitic activities according to the following formula: TI = CC50BoMac/IC50Ana2014.
2.6. In silico analysis of cytotoxicity prediction
The cytotoxicity of the identified compounds was predicted using lazar (lazy structure-activity relationships, https://lazar.in-silico.ch/predict), a modular framework for predictive toxicology (Maunz et al., 2013). Lazar predicts the in silico cytotoxicity of molecules using the SMILES notation of each compound. We retrieved data, including carcinogenicity in rodents, mutagenicity in Salmonella enterica serovar Typhi, acute toxicity for the fathead minnow, blood-brain barrier penetration and the maximum recommended daily dose in humans, using lazar tool.
2.7. Structural similarity prediction
The structures of active molecules were compared using atom pair fingerprints (APfp) (Carhart et al., 1985; O'Boyle and Sayle, 2016). Seven compounds, buparvaquone (MMV689480, commonly used anti-theilerial drug), two compounds from this study and four compounds from previous studies showing the best activity against T. annulata or T. parva, were used to map the molecular structure for the absence or presence of similar molecular fragments using APfp. The Tanimoto coefficient (Tc) was used to measure the structural similarity between the compounds (Bajusz et al., 2015). It works with the following formula: SA,B = a/(a + b − c), where S represents the similarity between two molecules, A and B, a represents the number of 1 bit in molecule A, b represents the number of 1 bit in molecule B, and c represents the number of common bits. Chemmine server was used to generate the SDF file of all the selected seven molecules. These seven molecules were then analysed using ChemmineR package (Cao et al., 2008). Clustering analysis was done using the R base stats package, and gplots package was used for drawing the plots.
2.8. Annexin V-FITC/propidium iodide (PI) and TUNEL assay
Annexin V-FITC/Propidium Iodide (PI) assay was performed using the BD Pharmingen kit. Briefly, Theileria infected cells were treated with MMV000062 and MMV560185 each at half maximal inhibitory concentration (IC50). Rifampicin (10 μM) was taken as a negative control while curcumin (20 μM) was taken as a positive control. Rifampicin and curcumin treated cells were analysed after 24 h while MMV000062 and MMV560185 treated cells were analysed after 48 h. For analysis of apoptosis, 1 × 106 cells from each conditions were washed twice with 1X PBS followed by centrifuging at 400 g for 5 min at room temperature. Subsequently cells were re-suspended in 100 μl of binding buffer containing 5 μl of Annexin V-FITC and 5 μl of PI. The cells were incubated for 15 min at room temperature in dark and analysed by Flow cytometer.
Cells were labelled by catalytically incorporating FITC-dUTP at the 3- hydroxyl ends of the fragmented DNA by terminal deoxynucleotidyl transferase enzyme using APO-DIRECT KIT (BD pharmingen) for Terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick-end labelling (TUNEL) assay. Staining of the cells was performed as per the instructions provided in the kit.
For the Annexin V-FITC/Propidium Iodide and TUNEL assay both, a total of 10,000 events were acquired on the flow cytometer (BD LSRFortessa, BD Bioscience) and analysed using FlowJo software.
2.9. Western blotting
Ana2014 cells were treated with MMV560185 at IC50 concentration. After 60 h, equal number of cells from treated and untreated conditions were taken and cell lysates were prepared as described previously (Araveti and Srivastava, 2019). Briefly, cell pellets were lysed in RIPA buffer (50 mM Tris-HCl pH-7.4, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 50 mM sodium fluoride, 0.2 mM sodium orthovanadate, 1 mM PMSF, 1 mM leupeptin). Supernatant was collected after centrifuging at 13000 g for 10 min at 4 °C. The cell lysates thus obtained were denatured at 95 °C for 5 min by adding laemmli buffer and were resolved by SDS-PAGE and electro-transferred onto PVDF membrane. Blocking was performed using 5% non-fat milk powder in TBST for 1 h at room temperature. After blocking, the membrane was incubated with primary antibody at 4 °C for overnight. The membranes were washed thrice with TBST, 5 min each. After washes, the membranes were incubated with respective secondary antibodies for 1 h at room temperature. Again membranes were washed as mentioned above. Finally, the chemiluminescent signals were captured using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) in ChemiDoc™ imaging system (BIO-RAD). ImageJ software was used for densitometry analysis.
2.10. Long-term culture of Ana2014 cells with selected compounds
Ana2014 cells were seeded in 6-well plate with 2 × 105 cells/ml with complete RPMI 1640 media and incubated for 4 h at 37 °C with 5% CO2 in a humidified incubator. After 4 h of incubation, specific drugs at their respective IC50 value and buparvaquone (0.612 μM), were added to specific wells and further incubated at 37 °C with 5% CO2. Each compound was diluted in complete RPMI 1640 media. After every 48 h (2 days) till 288 h (12 days) the cells were stained with trypan blue and the viability of the cells upon treatment was estimated by counting dead cells using hemocytometer. Old media was replaced with fresh media every 48 h in each well with respective compounds.
2.11. Reactive oxygen species (ROS) measurement
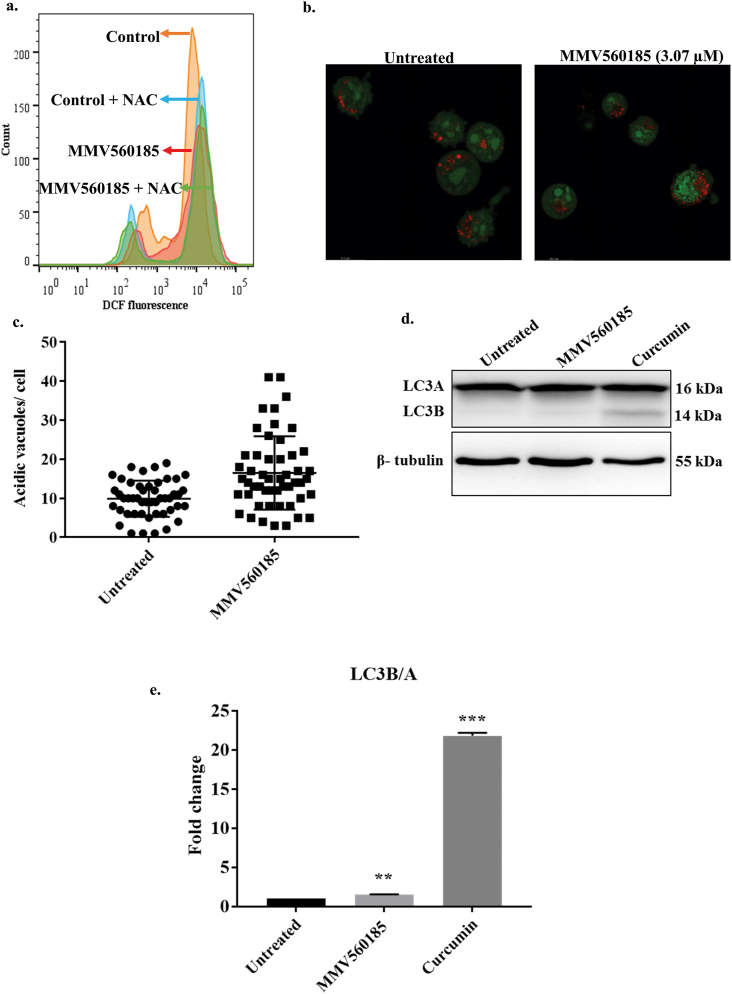
The generation of Reactive Oxygen Species (ROS) was monitored using 2′,7′ –Dichlorofluorescin diacetate (DCF-DA). Ana2014 cells were treated for 2 h with 10 mM NAC (N-acetyl cysteine) and then with 3.07 μM of MMV560185. After incubation of cells for 48 h, cells were washed twice with PBS and incubated with 20 μM DCF-DA in PBS at 37 °C, 5% CO2 in dark for 20 min. The levels of ROS were measured using a flow cytometer, BD LSRFortessa (BD Biosciences).
2.12. Acridine orange staining
Ana2014 cells were treated with 3.07 μM of MMV560185 for 48 h. After washing once with PBS, cells were re-suspended in PBS with 5 μg/ml acridine orange. After incubation at 37 °C for 15 min in dark, cells were mounted on poly-lysine coated slides. The images were captured using confocal microscope (Leica SP8, Leica Microsystems) and processed using LAS X software.
2.13. Transmission electron microscopy
Ana2014 cells were treated with MMV560185 at IC50 concentration (3.07 μM). Sixty hours post-treatment the treated cells were fixed with 2.5% glutaraldehyde for 2 h and washed thrice with deionized distilled water. The untreated cells were taken as a control. The cells were fixed with 2% osmium tetroxide for 2 h then thrice washed with deionized distilled water. The washed cells were treated with 2% uranyl acetate for an hour and again washed thrice with deionized distilled water. After dehydrating with series of graded alcohol the cells were then treated twice with propylene oxide for 20 min. The cells were embedded in the epoxy embedded medium kit (Sigma) following protocol of the manufacturer and incubated at 70 °C–80 °C for 72 h for complete polymerization. The ultra-thin (30 nm) sections were made with diamond knife on an ultramicrotome (Leica UC7). The sections were mounted on a copper grid and stained with 2% uranyl acetate and counterstained with Reynold's lead citrate. Transmission electron microscope (JEM 1400, 120 kV, Jeol, Japan) was used for capturing images (available at the central instrumentation facility of NIAB Hyderabad).
2.14. Statistical analysis
The screening of the compounds of MMV Pathogen box was performed thrice in triplicates. All other experiments were performed triplicates. GraphPad Prism 6.0 or Microsoft Excel were used for calculating IC50 and CC50 values. t-test was used for calculating statistical significance.
3. Results
3.1. Ana2014 cell line is a mixed population of B cells and monocytes/macrophages
Previously, we established the Ana2014 cell line using the protocol described earlier (Hulliger, 1965; Viseras et al., 1997). The Ana2014 cell line is a T. annulata infected bovine leucocytes which was confirmed in our previous study (Araveti and Srivastava, 2019). In this study, we further characterized this cell line. T. annulata parasite is reported to infect CD5+ B cells, CD11a and CD14 monocytes/macrophages (Moreau et al., 1999; Sager et al., 1997). Hence, we tested the transcription of CD5, CD11a and CD14 in Ana2014 cells. We observed transcript of CD5 and CD14 markers in the Ana2014 cells (Supplementary Fig. 1). This suggests that Ana2014 cell line has a mixed population of B cells and monocytes/macrophages.
3.2. Identification of active compounds from the pathogen box against Theileria annulata infected bovine leucocytes
Ana2014 cells (Araveti and Srivastava, 2019) were individually treated with all the 400 compounds present in the Pathogen box at 10 μM concentration for initial screening. Cell growth inhibition was measured with respect to the control (untreated) cells by MTT assay. A total of 15 compounds showed more than 80% growth inhibition after 24 h post treatment (Table S1) while 13 other compounds showed more than 60% inhibition compared with the control (data not shown). We further incubated Ana2014 cells with these 13 compounds for 48 h to observe their effect on Ana2014 cells after increasing incubation time. Five out of these 13 compounds showed more than 80% cell growth inhibition in 48 h of incubation (Table S2). Based on these observations, we shortlisted these 20 compounds (Tables S1 and S2) for testing their cytotoxicity. The physical properties of these 20 compounds are presented in Tables S1 and S2. Buparvaquone, a drug known to kill Theileria parasite, is one of the compounds present in the Pathogen box. We observed that buparvaquone could inhibit cell growth by 14.6% only after 24 h. Hence, it was not considered for further experiments.
The MTT assay uses a readout which is a chemical reduction of tetrazolium salt, and it is widely believed to take place in respiring mitochondria. Hence, to show that the effect of the drug is specific to the parasitized cell, we tested the cytotoxicity of these selected 20 compounds on bovine macrophage cell line (BoMac) at 10 μM concentration at 24 h and 48 h. Eighteen out of these 20 compounds were found cytotoxic to either of these cell lines (Tables S1 and S2). Finally, we found two molecules, MMV000062 and MMV560185 that were not cytotoxic to BoMac cells at 10 μM concentration. In the previous study, screening of pathogen box against T. parva infected cell line identified different compounds (Nyagwange et al., 2019) (Table 2) which suggest that the two compounds identified in the present study have a very specific inhibitory effect on T. annulata infected bovine cell line. Further, we tested the effect of MMV560185 on another T. annulata infected bovine cell line (Ode cell line). We observed similar effect of MMV560185 on the Ode cell line also (Supplementary Fig. 2). Previously, both these compounds were also reported to be active against other parasites such as Trypanosoma, Plasmodium in previous studies (Table 1).
Table 2.
Summary of hit compounds identified from MMV Pathogen Box and Malaria Box against Theileria infected bovine leucocytes, along with Buparvaquone, a reference drug.
| Compounda | Structure | Mol Wt | Disease Set | ALogPb | IC50 (μM) | CC50 (μM) | TIDc (CC50/IC50) | Ref |
|---|---|---|---|---|---|---|---|---|
| MMV000062 |  |
340.43 | – | 1.75 | 2.97 | >100 | >33.67 | This study |
| MMV560185 | 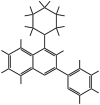 |
289.38 | – | 3.65 | 3.07 | 34 | 11.07 | This study |
| MMV688372 |  |
401.44 | T. parva Schizonts | 4.43 | 0.61 | 5.3 | 8.69 | Nyagwange et al. (2019) |
| MMV666022 | 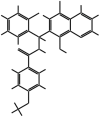 |
453.31 | T. annulata schizonts | 5.49 | 0.47 | ND | 11 | Hostettler et al. (2016) |
| MMV666054 | 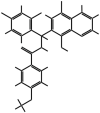 |
487.76 | T. annulata schizonts | 6.154 | 0.29 | ND | 18 | Hostettler et al. (2016) |
| MMV008212 |  |
280.32 | T. parva Schizonts | 3.59 | 0.78 | >10 | >12 | Nyagwange et al. (2019) |
| MMV689480 (Buparvaquone) |  |
326.43 | T. parva and T. annulata schizonts |
Identification code assigned by MMV (www.mmv.org).
Lipophilicity index of the compound.
Therapeutic Index of the compound.
Table 1.
Summary of the in silico toxicity of the chemical compounds with anti-theilerial activity.
| Compounda | Activity against other infectious microorganisma |
In silico toxicity featuresb |
||||
|---|---|---|---|---|---|---|
| Acute cytotoxicity (fathead minnow) (mg/liter) | Carcinogenicity (Rodent) | Mutagenicity (Salmonella typhimurium) | Blood brain barrier penetration (Human) | Maximum recommended daily dose (Human) (mg/kg body weight/day) | ||
| MMV000062 | Trypanosoma | 15.1 | non-carcinogenic | non-mutagenic | penetrating | data unavailable |
| MMV560185 | Plasmodium | 13 | carcinogenic | mutagenic | penetrating | 10 |
The IC50 values of these two compounds, MMV000062 and MMV560185, were found to be 2.97 μM and 3.07 μM, respectively. The CC50 values of MMV000062 and MMV560185 were found to be more than 100 μM and 34 μM, respectively. The therapeutic indices of MMV000062 and MMV560185 were calculated as more than 33 and 11.07 respectively. The lipophilicity indices of MMV000062 and MMV560185 are known to be 1.75 and 3.65 respectively (Tables S1 and S2). We predicted the drug likeness for MMV000062 and MMV560185 by analysing the ADME properties using the SwissADME server. The MMV00062 was predicted to be soluble in water where as MMV560185 was predicted to be moderately soluble in water (Table S3). Both the compounds followed Lipinski's rule of five with no violation. The synthetic accessibility score indicates that both the molecules could likely be chemically synthesized (Table S3).
Safety profiles of MMV560185 and MMV000062 were predicted in silico by using lazar (lazy structure-activity relationship) program (Table 1). MMV000062 was predicted to be non-carcinogenic and non-mutagenic while MMV560185 was predicted to be carcinogenic and mutagenic. However, in vivo studies would be required further to validate these predictions.
3.3. MMV560185 and MMV000062 are structurally dissimilar
The Tanimoto coefficient (Tc) was calculated on the basis of their atom pair fingerprint (APfp) to predict the similarity between the six compounds (identified previously and in this study) along with buparvaquone which could kill T. annulata infected bovine leucocytes (Table 2). Two of these compounds were identified in this study while four of them were taken based on their identification in the previous studies (Hostettler et al., 2016; Nyagwange et al., 2019) along with buparvaquone (MMV689480). Tc takes values of 0 (absence of any similarity) to 1 (100% similarity) from the least similar to the most similar molecules. The most frequent values were between 0 and 0.3 suggesting a high structural diversity among these seven molecules (Fig. 1a). The structural similarities between the seven compounds were identified by hierarchical clustering analysis (HCA) using Tc similarity matrix. The two main clusters were observed by HCA (Fig. 1a). The highest value between MMV666022 and MMV666054 by HCA was found to be 0.844. This suggests that all the molecules identified in the previous studies and this study are largely different but for MMV666022 and MMV666054 (Hostettler et al., 2016).
Fig. 1.
a. Hierarchical clustering analysis of the selected compounds. The structural similarities between seven compounds (including buparvaquone), according to their Tanimoto coefficients using ChemmineR package. b. Effect of MMV000062 and MMV560185 on long term culture of Ana2014 cells. Comparison of long term culture (14 days) of Ana2014 cells with the compounds MMV000062, MMV560185 and buparvaquone (MMV689480). Addition of MMV560185 at 12 day in the buparvaquone adapted cell line led to the death of the cells in two days.
3.4. MMV560185 and MMV000062 do not lead to adaptation in the Theileria annulata infected bovine leucocytes
Ana2014 cells (2 × 106 cells) cultured in presence of MMV560185 and MMV000062 each at their IC50 values for 12 days were unable to recover under the drug pressure. All the cells were found dead on the 6th day and the 10th day of incubation with MMV560185 and MMV000062 respectively (Fig. 1b). However, Ana2014 cells were able to recover in buparvaquone treated cells. There was an increase in the number of viable cells in Ana2014 cells after 10th day of incubation with buparvaquone. This suggests that a long term treatment with buparvaquone leads to the development of adaptation in Ana2014 cells while MMV560185 and MMV000062 lead to complete killing of the Ana2014 cells. Furthermore, we observed that MMV560185 is cytotoxic to buparvaquone adapted Ana2014 cells (Fig. 1 b). This observation suggests that the target for MMV560185 and buparvaquone are different.
3.5. MMV560185 induces apoptosis Theileria annulata infected bovine leucocytes
MMV000062 and MMV560185, which were non-cytotoxic to the BoMac cells, were further analysed for their mode of action leading to the inhibition of cell growth. The Annexin V/propidium iodide staining was performed to find out if the cells were undergoing apoptosis. The Ana2014 cells were treated with MMV000062 and MMV560185 at their IC50 values and both the assays were performed after 48 h. We found that both of these compounds induced apoptosis in the Ana2014 cells (Fig. 2a and b). Curcumin treatment to Ana2014 cells was used as a positive control for Annexin V assay (Fig. 2a and b) as curcumin induces apoptosis in Ana2014 cells (Araveti and Srivastava, 2019). Rifampicin (MMV688775), a compound from the pathogen box found to have no cytotoxicity effect on Ana2014 cells, was used as a negative control for Annexin V assay (Fig. 2a and b). Further, TUNEL assay was performed to explore the ability of these two drugs to induce DNA fragmentation. The DNA fragmentation was measured by labelling the fragmented DNA with FITC-dUTP at the 3′- hydroxyl ends by enzyme terminal deoxynucleotidyltransferase. The results of the flow cytometric analysis showed no change in the DNA integrity 48 h post treatment (Fig. 2c). As caspases are the key molecules involved in the activation of apoptotic process, we thus examined the cleaved forms of caspase 3 by western blot after treating the cells with MMV560185 and MMV000062 for 60 h. We observed cleaved caspase 3 in the cells treated with MMV560185 (Fig. 2d) but not with MMV000062 (Fig. 2e). As the activation of caspase 3 is a highly important step in the apoptotic pathway and we observed that only MMV560185 could induce this pathway, we further investigated the mechanism of action of MMV560185 in Theileria infected bovine leucocytes.
Fig. 2.
MMV000062, and MMV560185 induces apoptosis in Ana2014 cells. a. Dot plot showing Annexin V FITC- PI staining of untreated, Rifampicin (10 μM), Curcumin (20 μM), MMV 560185 (3.07 μM) and MMV000062 (2.97 μM) treated Ana2014 cells, b. showing the percentage of early apoptotic, and late apoptotic cell population in three independent experiments. N = 3. Data are presented as mean ± SD. *** represents p < 0.001 calculated with an unpaired, two-tailed t-test, compared with the untreated group, c. Analysis of DNA fragmentation by compounds MMV000062 and MMV560185, using TUNEL assay, d. Western blots showing the cleavage of caspase 3 upon MMV560185 treatment to Ana2014 cells, e. Western blots showing no cleavage of caspase 3 upon MMV000062 treatment to Ana2014 cells.
3.6. MMV560185 does not induce Reactive Oxygen Species (ROS) and autophagy in the bovine leucocytes infected with Theileria annulata parasite
To analyse the level of ROS in the cells treated with MMV560185 we used the specific oxidation sensitive fluorescent dye DCFH-DA. DCFH-DA is a cell permeable dye which gets oxidized in the presence of the ROS. Oxidation of DCFH-DA by ROS leads to the formation of dichlorofluorescein (DCF), a fluorescent product, which can be monitored by FACS. We could not find any increase in the fluorescence of DCF upon treatment with MMV560185 suggesting no increase in the production of ROS in the treated cells (Fig. 3a). Next, we analysed the level of acidic vacuoles in the cells treated with MMV560185. We observed an increase in the number of acidic vacuoles in the cells treated with MMV560185 (Fig. 3b and c). The increase in the number of acidic vacuoles could be due to the formation of autophagosomes, so we further examined the effect of MMV560185 on autophagy. We estimated the levels of LC3B, a marker for autophagy, in the cells treated with MMV560185. Curcumin induces LC3B formation in Ana2014 cells (Araveti and Srivastava, 2019). So, curcumin was used as a positive control for LC3B formation. We did not observe the cleavage product of LC3, i.e., LC3B in the western blotting analysis which suggests that MMV560185 does not induce autophagy pathway (Fig. 3d and e).
Fig. 3.
MMV560185 does not induce Reactive Oxygen Species (ROS) and autophagy. a. No increase in levels of ROS detected by DCFH-DA fluorescence upon treatment of MMV560185 (3.07 μM) and both by MMV560185 (3.07 μM) and NAC treatment. b. Confocal microscopy of Ana2014 cells treated with MMV560185 (3.07 μM) shows increase in accumulation of many acridine orange (AO) positive acidic vesicles compared to untreated cells, c. Increase in number of acridine orange (AO) positive acidic vesicles Mean ± SEM of untreated 9.896 ± 0.6673, n = 48, Mean ± SEM of treated 16.51 ± 1.316, n = 51, d. Western blot analysis of Ana2014 cells treated with MMV560185 (3.07 μM) shows no cleavage of LC3A to LC3B. Curcumin that activates autophagy pathway was taken as a positive control. e. Fold change of LC3B/A in Ana2014 cells (after densitometry analysis of western blot data) treated with MMV560185 or curcumin. N = 3. Data are presented as mean ± SD. **, *** represents p < 0.01, p < 0.001 compared with the untreated group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.7. MMV560185 induces extrinsic death receptor mediated apoptosis in Theileria annulata infected bovine leucocytes
The activation of caspase 8 is a prerequisite for the activation of caspase mediated apoptotic pathway. The activation of caspase 8 leads to the cleavage of caspase 3. We also observed the cleavage product of caspase 8 in the Ana2014 cells upon MMV560185 treatment (Fig. 4a). Activated caspase 3 cleaves poly ADP ribose polymerase (PARP). So, we inspected the cleavage status of PARP after MMV560185 treatment to Ana2014 cells. We observed that the treatment with MMV560185 induced PARP cleavage in Ana2014 cells (Fig. 4a).
Fig. 4.
MMV560185 induces extrinsic death receptor mediated apoptosis in Ana2014 cells. a. Western blots showing the cleavage of caspase 8 and PARP upon MMV560185 treatment to Ana2014 cells, b. Western blots representing no change in the expression levels of Bcl2 and pBad upon MMV560185 treatment, c. Western blots showing the changes in the expression levels of TNF-R1 and TRADD upon MMV560185 treatment to Ana2014 cells, d. Fold change in the expression of TNF-R1 and TRADD upon MMV560185 treatment after densitometry analysis of western blot data. N = 3. Data are presented as mean ± SD. ** represents p < 0.01 compared with the untreated group.
BCL-2 family proteins play a key role in the intrinsic pathway of apoptosis. Thus, we examined the changes in the expression levels of Bcl2 and pBad. We found that the level of Bcl2 and pBad remained unchanged after MMV560185 treatment to Ana2014 cells (Fig. 4b), suggesting there is no activation of intrinsic pathway of apoptosis. In case of extrinsic pathway of apoptosis, TNF-R1 signaling is known to activate caspase 8 cleavage by recruiting the adaptor protein TRADD. MMV560185 treatment to Ana2014 cells led to the increased expression of both TNF-R1 and TRADD (Fig. 4c and d) which indicates that the activation of the extrinsic pathway of apoptosis leads to cell death.
Finally, to visualize the effect of MMV560185 on Ana2014 cells, we performed transmission electron microscopy. Under transmission electron microscope we observed that the control cells (untreated) had smooth surface (Fig. 5a) while the blebbing was observed on the cell surface of MMV560185 treated cells (Fig. 5b). Further, we could not observe membrane bound schizonts in the treated cells which were clearly seen in untreated cells (Fig. 5b) which provides us an evidence for the specific action of MMV560185 on the schizonts of this parasite. In summary, we propose the activation of TNR-R1 and TRADD further leading to the activation of apoptotic pathway as the mechanism of action of MMV560185 (Fig. 6).
Fig. 5.
Transmission electron microscopy: (a) TEM image of untreated and (b) treated MMV560185 (3.07 μM). HN: Host nucleus, S: Schizont, Ble: Blebbing.
Fig. 6.
Proposed mechanism of action of MMV560185 on Ana2014 cells. MMV560185 induces extrinsic apoptosis pathway in Ana2014 cells by inducing TNF-R1 leading to activation of TRADD. The activation of TRADD leads to cleavage of caspase 8, caspase 3 and PARP finally leading to induction of apoptosis. No induction of ROS, intrinsic pathway and autophagy was observed in Ana2014 cells upon treatment with MMV560185. Red arrows indicate no activation while green arrows indicate activation of the pathway. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Theileriosis is one of the major parasitic diseases of cattle which causes tremendous economic losses to the farmers. The present regime of treatment for tropical theileriosis includes buparvaquone. This drug is very effective against the Theileria parasite as 2.5 mg (kg bodyweight of the animal)−1 of buparvaquone is sufficient to clear the parasite in the infected cattle (McHardy et al., 1985). However, reports of development of resistance for this drug by the parasite are a matter of serious concern (Chatanga et al., 2019; Mhadhbi et al., 2010). Hence, there is both need and demand to identify newer drugs for the treatment of this disease. The Pathogen box, a follow-up project to the Malaria box and an initiative of Medicine for Malaria Venture (MMV) (MMV, 2019), comprises 400 compounds which are selected from a screen of over 6 million chemicals. The Pathogen box compounds were examined in the present study for their anti-theilerial activity in vitro.
We initially screened all these 400 compounds for the presence of compounds which could be active against T. annulata infected bovine leucocytes at 10 μM concentration in 24 h and 48 h. We found that 20 out of 400 compounds could inhibit the growth of Ana2014 cells (Theileria infected bovine leucocytes) by more than 80% either in 24 h or 48 h (Tables S1 and S2). Buparvaquone, a known anti-theilerial compound, which is present in the Pathogen box was eliminated in this initial screening. Since MTT assay is based on the readout of the chemical reduction of a tetrazolium salt, it was important to ascertain whether the cell death was due to cytotoxicity in the host cells by the tested compounds or these compounds act specifically on the parasite. We hypothesized that if these compounds would act specifically on Theileria annulata infected cells then there would not be any effect on uninfected cell lines. Hence, we examined the cytotoxic effect of the compounds (which showed activity on T. annulata infected cells) on the transformed bovine macrophage cell line, BoMac. We used BoMac cell line as T. annulata can only infect bovine B cells and monocytes/macropahges (Spooner et al., 1989). We observed that only two of these compounds, namely MMV560185 and MMV000062, showed no toxicity to BoMac cell line, with CC50 value of 34 μM and >100 μM, respectively. The therapeutic indices of these compounds were more than 33 for MMV000062 and 11.07 for MMV560185. A therapeutic index of >5 is considered to be selective for the parasitized cell (Duffy et al., 2017).
MMV000062 and MMV560185 have the lipophilicity indices (AlogP) of 1.75 and 3.65, respectively. The lipophilicity index is a good indicator of the potential of a drug to be absorbed by the cells. Thus MMV560185 is more lipophilic compared to MMV000062.
MMV000062, also known as Pentamidine, which was found to kill T. annulata infected bovine cells in this study, is a known antiprotozoal agent. It also effectively kills Crithidia oncopelti, a protozoan, in vitro and interferes with nuclear metabolism (Wallis, 1966). This drug is useful in treating leishmaniasis and trypanosomiasis as well (Lai et al., 2002; Lindsay et al., 1991). MMV560185 has been previously found to be active against P. falciparum stage V gametocytes (Duffy et al., 2017).
Four compounds, namely MMV688372, MMV008212, MMV666022 and MMV666054, have been previously identified as anti-theilerials by screening Malaria and Pathogen box against T. annulata and T. parva (Hostettler et al., 2016; Nyagwange et al., 2019). MMV688372 and MMV008212 among these were found to be effectively killing T. parva and identified by screening the Malaria box and Pathogen box respectively (Nyagwange et al., 2019). The screening of the malaria box against T. annulata led to the identification of two new compounds, namely MMV666054 and MMV008212 (Hostettler et al., 2016). These previous studies and our study taken together suggest that while T. annulata and T. parva belong to the same genus, but they are quite different in their responses to different drugs. The possible reason could be the diversity among these two Theileria parasites and the different host cells they infect. The T. annulata infects B cells and monocytes while T. parva infects T cells of the bovine (Spooner et al., 1989). Hence, they might require differential expression of genes for their survival in the host. In order to find out structurally similar compounds among these six (four from previous studies and two from our study) and buparvaquone additionally, the structures of these compounds were compared using molecular fingerprints technique (Carhart et al., 1985; O'Boyle and Sayle, 2016). The atom pair fingerprints (APfp) predict the close structure analogue more efficiently among various available methods for molecular fingerprints (Carhart et al., 1985), thus, we used this in our analysis. This analysis predicted that none of these molecules were structurally similar to MMV000062 and MMV560185. The closest two molecules were MMV666022 and MMV666054. Thus we could not find compounds (among these six recently identified compounds) that are structurally similar and can target both T. annulata and T. parva. This suggests that the mode of action of these six compounds might be different in T. annulata and T. parva.
As there are reports of the development of resistance in the parasite against buparvaquone (Chatanga et al., 2019; Mhadhbi et al., 2010), we further analysed the effect of these compounds upon the long term in vitro culture. The treatment with MMV560185 and MMV000062 led to no recovery of cells after long term treatment while the treatment with buparvaquone initially decreased cell proliferation but later the cells were able to recover from the drug treatment. Previously, it was shown that the treatment with buparvaquone initially retards the cell proliferation and later inhibits cell proliferation (Kinnaird et al., 2013). The identified compounds in this study are non-cytotoxic with no development of adaptation under tested conditions.
We analysed the ability of MMV000062 and MMV560185 to induce apoptosis and DNA fragmentation in Ana2014 cells to understand the mechanism(s) of action of these two compounds. TUNEL assay with total DNA from both parasite and the host cells (in which parasite DNA is only 1–3% of the total DNA) (Gotia et al., 2016) showed that both of these compounds does not induce DNA fragmentation in Ana2014 cells. This suggests that these compounds might interfere in some metabolic pathway of the parasitized cell as these compounds are not cytotoxic to the host cells. However, only MMV560185 was found to increase the molecular markers of apoptosis. After treatment with MMV000062 and MMV560185, we observed both early and late apoptotic cells. Early apoptotic cells are attributed with initial cell membrane permeabilization (positive for Annexin V- FITC and negative for propidium iodide) whereas late apoptotic cells are characterized with pronounced loss in the cell membrane integrity (positive for both Annexin V- FITC and propidium iodide) (Wlodkowic et al., 2011).
Furthermore, previously we found that curcumin could induce oxidative stress leading to activation of both apoptosis and autophagy in the Ana2014 cells (Araveti and Srivastava, 2019). However, we were surprised to find that although there was increase in acidic bodies but no increase in ROS, nor autophagy in Ana2014 cells treated with MMV560185.
Previously, it was shown that Theileria annulata-transformed macrophages also produce Tumor Necrosis Factor (TNF) (Brown et al., 1995; Preston et al., 1993). TNF is produced by activated macrophages and exerts its effects through two distinct receptors namely; TNFR1 and TNFR2 (Baud and Karin, 2001; Chen and Goeddel, 2002). It is known that the binding of TNF to its receptor can activate either apoptosis or proliferation. When TNF engages with TNFR1, it activates Tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD) which leads to the activation of caspase dependent apoptosis, while when TNF engages with TNFR2 it activates TNF receptor-associated factor 2 (TRAF2) which stimulates early signalling events leading to the activation of both NF-κB and AP-1 (Rothe et al., 1995). An autocrine loop which constitutively activates TNFR2 has been shown to be the activated in the Theileria-infected leucocytes (Guergnon et al., 2003a). Thus, we hypothesized that an upregulation of TNFR1 would lead to activation of apoptosis pathway. Our analysis showed that there is indeed an upregulation of TNFR1 and TRADD. Also, formation of the cleavage product of caspases in the TNFR1 signalling pathway also provided us a proof of the activation of apoptotic pathway. The apoptosis in cells is characterized by blebbing on the surface of the cells. Our TEM data provided further proof of activation of apoptosis in the MMV560185 treated cells. In this study we convincingly showed that the MMV560185 specifically kills Theileria annulata infected bovine leucocytes by inducing extrinsic pathway of apoptosis.
In conclusion we identified two lead compounds after screening all the 400 molecules from the Pathogen box, namely MMV000062 and MMV560185, with their therapeutic indices more than 33 and 11, respectively. Out of these two we found that MMV560185 specifically induces extrinsic pathway of apoptosis in the parasitized cell. This molecule could be repurposed and further modified to develop more effective drug molecules against tropical theileriosis.
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgement
We are grateful to the Medicines for Malaria Venture, Geneva, Switzerland, for providing the Pathogen box. Authors are thankful to Dr. Judy Stabel (Johne's Disease Research Project, USDA-ARS-NADC) and Dr. Gordon Langley for providing BoMac and Ode cell lines respectively. AS thanks National Institute of Animal Biotechnology, Hyderabad for core grant to support this research project. MV thanks National Medicinal Plant Board, Ministry of AYUSH, New Delhi grant no. R&D/TL-01/2016-17-NMPB-IVA for providing fellowship. PBA thanks the Department of Science & Technology (DST), New Delhi for INSPIRE fellowship (IF160249) and Regional Centre for Biotechnology (RCB), Faridabad, India for pursuing Ph.D. PPK thanks the Department of Science & Technology (DST), New Delhi for INSPIRE fellowship (IF160609) and Regional Centre for Biotechnology (RCB), Faridabad, India for pursuing Ph.D. SV thanks the Science and Engineering Research Board (SERB) for providing post-doctoral fellowship (N-PDF: PDF/2016/003261). We are grateful to Mr. Shashikant Gawai, Ms. Rama Devi G and Ms. Preeti Prasanna for Confocal microscopy, FACS analysis, and TEM images respectively.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2021.12.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbas R.Z., Zaman M.A., Colwell D.D., Gilleard J., Iqbal Z. Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet. Parasitol. 2014;203:6–20. doi: 10.1016/j.vetpar.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Araveti P.B., Srivastava A. Curcumin induced oxidative stress causes autophagy and apoptosis in bovine leucocytes transformed by Theileria annulata. Cell Death Discov. 2019;5:100. doi: 10.1038/s41420-019-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajusz D., Racz A., Heberger K. Why is Tanimoto index an appropriate choice for fingerprint-based similarity calculations? J. Cheminf. 2015;7:20. doi: 10.1186/s13321-015-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V., Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Bessoff K., Spangenberg T., Foderaro J.E., Jumani R.S., Ward G.E., Huston C.D. Identification of Cryptosporidium parvum active chemical series by Repurposing the open access malaria box. Antimicrob. Agent. Chemother. 2014;58:2731–2739. doi: 10.1128/AAC.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J.D., Merino E.F., Brooks C.F., Striepen B., Carlier P.R., Cassera M.B. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the malaria box. Antimicrob. Agent. Chemother. 2014;58:811–819. doi: 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyom F.F., Fokou P.V., Tchokouaha L.R., Spangenberg T., Mfopa A.N., Kouipou R.M., Mbouna C.J., Donfack V.F., Zollo P.H. Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob. Agent. Chemother. 2014;58:5848–5854. doi: 10.1128/AAC.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.G. Control of tropical theileriosis (Theileria annulata infection) of cattle. Parassitologia. 1990;32:23–31. [PubMed] [Google Scholar]
- Brown C.G., Radley D.E., Cunningham M.P., Kirimi I.M., Morzaria S.P., Musoke A.J. Immunization against East Coast fever (Theileria parva infection of cattle) by infection and treatment: chemoprophylaxis with N-pyrrolidinomethyl tetracycline. Tropenmed. Parasitol. 1977;28:342–348. [PubMed] [Google Scholar]
- Brown D.J., Campbell J.D., Russell G.C., Hopkins J., Glass E.J. T cell activation by Theileria annulata-infected macrophages correlates with cytokine production. Clin. Exp. Immunol. 1995;102:507–514. doi: 10.1111/j.1365-2249.1995.tb03845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Charisi A., Cheng L.C., Jiang T., Girke T. ChemmineR: a compound mining framework for R. Bioinformatics. 2008;24:1733–1734. doi: 10.1093/bioinformatics/btn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart R.E., Smith D.H., Venkatraghavan R. Atom pairs as molecular features in structure-activity studies: defination and applications. J. Chem. Inf. Comput. Sci. 1985;25:64–73. [Google Scholar]
- Chatanga E., Mosssad E., Abdo Abubaker H., Amin Alnour S., Katakura K., Nakao R., Salim B. Evidence of multiple point mutations in Theileria annulata cytochrome b gene incriminated in buparvaquone treatment failure. Acta Trop. 2019;191:128–132. doi: 10.1016/j.actatropica.2018.12.041. [DOI] [PubMed] [Google Scholar]
- Chen G., Goeddel D.V. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Duffy S., Sykes M.L., Jones A.J., Shelper T.B., Simpson M., Lang R., Poulsen S.A., Sleebs B.E., Avery V.M. Screening the Medicines for malaria venture pathogen box across multiple pathogens reclassifies starting points for open-source drug discovery. Antimicrob. Agent. Chemother. 2017;61 doi: 10.1128/AAC.00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotia H.T., Munro J.B., Knowles D.P., Daubenberger C.A., Bishop R.P., Silva J.C. Absolute quantification of the host-to-parasite DNA ratio in Theileria parva-infected lymphocyte cell lines. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergnon J., Chaussepied M., Sopp P., Lizundia R., Moreau M.F., Blumen B., Werling D., Howard C.J., Langsley G. A tumour necrosis factor alpha autocrine loop contributes to proliferation and nuclear factor-kappaB activation of Theileria parva-transformed B cells. Cell Microbiol. 2003;5:709–716. doi: 10.1046/j.1462-5822.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- Guergnon J., Dessauge F., Langsley G., Garcia A. Apoptosis of Theileria-infected lymphocytes induced upon parasite death involves activation of caspases 9 and 3. Biochimie. 2003;85:771–776. doi: 10.1016/j.biochi.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hashemi-Fesharki R. Chemotherapeutic value of parvaquone and buparvaquone against Theileria annulata infection of cattle. Res. Vet. Sci. 1991;50:204–207. doi: 10.1016/0034-5288(91)90107-y. [DOI] [PubMed] [Google Scholar]
- Hostettler I., Muller J., Hemphill A. Vitro screening of the open-source Medicines for malaria venture malaria box reveals novel compounds with profound activities against Theileria annulata schizonts. Antimicrob. Agent. Chemother. 2016;60:3301–3308. doi: 10.1128/AAC.02801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger L. Cultivation of three species of Theileria in lymphoid cells in vitro. J. Protozool. 1965;12:649–655. doi: 10.1111/j.1550-7408.1965.tb03267.x. [DOI] [PubMed] [Google Scholar]
- Ingram-Sieber K., Cowan N., Panic G., Vargas M., Mansour N.R., Bickle Q.D., Wells T.N., Spangenberg T., Keiser J. Orally active antischistosomal early leads identified from the open access malaria box. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin A.D. Immunity in theileriosis. Parasitol. Today. 1985;1:124–128. doi: 10.1016/0169-4758(85)90056-0. [DOI] [PubMed] [Google Scholar]
- Kinnaird J.H., Weir W., Durrani Z., Pillai S.S., Baird M., Shiels B.R. A bovine lymphosarcoma cell line infected with Theileria annulata exhibits an irreversible reconfiguration of host cell gene expression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A.F.E.J., Vrede M.A., Soetosenojo R.M., Lai A.F.R.F. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int. J. Dermatol. 2002;41:796–800. doi: 10.1046/j.1365-4362.2002.01633.x. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Blagburn B.L., Hall J.E., Tidwell R.R. Activity of pentamidine and pentamidine analogs against Toxoplasma gondii in cell cultures. Antimicrob. Agent. Chemother. 1991;35:1914–1916. doi: 10.1128/aac.35.9.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizundia R., Werling D., Langsley G., Ralph S.A. Theileria apicoplast as a target for chemotherapy. Antimicrob. Agent. Chemother. 2009;53:1213–1217. doi: 10.1128/AAC.00126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolier J., Perichon M., DeBarry J.D., Villoutreix B.O., Chluba J., Lopez T., Garrido C., Zhou X.Z., Lu K.P., Fritsch L., Ait-Si-Ali S., Mhadhbi M., Medjkane S., Weitzman J.B. Theileria parasites secrete a prolyl isomerase to maintain host leukocyte transformation. Nature. 2015;520:378–382. doi: 10.1038/nature14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunz A., Gutlein M., Rautenberg M., Vorgrimmler D., Gebele D., Helma C. lazar: a modular predictive toxicology framework. Front. Pharmacol. 2013;4:38. doi: 10.3389/fphar.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy N., Haigh A.J., Dolan T.T. Chemotherapy of Theileria parva infection. Nature. 1976;261:698–699. doi: 10.1038/261698a0. [DOI] [PubMed] [Google Scholar]
- McHardy N., Wekesa L.S., Hudson A.T., Randall A.W. Antitheilerial activity of BW720C (buparvaquone): a comparison with parvaquone. Res. Vet. Sci. 1985;39:29–33. [PubMed] [Google Scholar]
- Mhadhbi M., Chaouch M., Ajroud K., Darghouth M.A., BenAbderrazak S. Sequence polymorphism of cytochrome b gene in Theileria annulata Tunisian isolates and its association with buparvaquone treatment failure. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhadhbi M., Naouach A., Boumiza A., Chaabani M.F., BenAbderazzak S., Darghouth M.A. In vivo evidence for the resistance of Theileria annulata to buparvaquone. Vet. Parasitol. 2010;169:241–247. doi: 10.1016/j.vetpar.2010.01.013. [DOI] [PubMed] [Google Scholar]
- MMV . 2019. The Pathogen Box.https://www.mmv.org/mmv-open/pathogen-box/about-pathogen-box [Google Scholar]
- Modirrousta H., Habibi G., Shayan P., Mirjalili A., Esmaeilnia K. Determination of CD markers profile of the cell line infected by S15 vaccine strain of Theileria annulata schizont using RT-PCR analysis. Arch. Razi Inst. 2019;74:433–438. doi: 10.22092/ari.2018.123081.1237. [DOI] [PubMed] [Google Scholar]
- Moreau M.F., Thibaud J.L., Miled L.B., Chaussepied M., Baumgartner M., Davis W.C., Minoprio P., Langsley G. Theileria annulata in CD5(+) macrophages and B1 B cells. Infect. Immun. 1999;67:6678–6682. doi: 10.1128/iai.67.12.6678-6682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W.I. Progress towards understanding the immunobiology of Theileria parasites. Parasitology. 2009;136:1415–1426. doi: 10.1017/S0031182009990916. [DOI] [PubMed] [Google Scholar]
- Noval R.A.I., Perry B.D., Young A. Acedemic press; London: 1992. The Epidemiology of Theilerosis in Africa. [Google Scholar]
- Nugraha A.B., Tuvshintulga B., Guswanto A., Tayebwa D.S., Rizk M.A., Gantuya S., El-Saber Batiha G., Beshbishy A.M., Sivakumar T., Yokoyama N., Igarashi I. Screening the Medicines for malaria venture pathogen box against piroplasm parasites. Int. J. Parasitol. Drugs Resist. 2019;10:84–90. doi: 10.1016/j.ijpddr.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyagwange J., Awino E., Tijhaar E., Svitek N., Pelle R., Nene V. Leveraging the Medicines for Malaria Venture malaria and pathogen boxes to discover chemical inhibitors of East Coast fever. Int. J. Parasitol. Drugs Resist. 2019;9:80–86. doi: 10.1016/j.ijpddr.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle N.M., Sayle R.A. Comparing structural fingerprints using a literature-based similarity benchmark. J. Cheminf. 2016;8:36. doi: 10.1186/s13321-016-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston P.M., Brown C.G., Entrican G., Richardson W., Boid R. Synthesis of tumour necrosis factor-alpha and interferons by mononuclear cells from Theileria annulata-infected cattle. Parasite Immunol. 1993;15:525–534. doi: 10.1111/j.1365-3024.1993.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Rothe M., Sarma V., Dixit V.M., Goeddel D.V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Sager H., Davis W.C., Dobbelaere D.A., Jungi T.W. Macrophage-parasite relationship in theileriosis. Reversible phenotypic and functional dedifferentiation of macrophages infected with Theileria annulata. J. Leukoc. Biol. 1997;61:459–468. doi: 10.1002/jlb.61.4.459. [DOI] [PubMed] [Google Scholar]
- Seeber F., Steinfelder S. Recent advances in understanding apicomplexan parasites. F1000Research. 2016;5 doi: 10.12688/f1000research.7924.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifiyazdi H., Namazi F., Oryan A., Shahriari R., Razavi M. Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet. Parasitol. 2012;187:431–435. doi: 10.1016/j.vetpar.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Sharma N.N., Mishra A.K. Treatment of bovine tropical theileriosis with buparvaquone. Trop. Anim. Health Prod. 1990;22:63–65. doi: 10.1007/BF02243504. [DOI] [PubMed] [Google Scholar]
- Singh S., Khatri N., Manuja A., Sharma R.D., Malhotra D.V., Nichani A.K. Impact of field vaccination with a Theileria annulata schizont cell culture vaccine on the epidemiology of tropical theileriosis. Vet. Parasitol. 2001;101:91–100. doi: 10.1016/s0304-4017(01)00502-7. [DOI] [PubMed] [Google Scholar]
- Spooner R.L., Innes E.A., Glass E.J., Brown C.G. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology. 1989;66:284–288. [PMC free article] [PubMed] [Google Scholar]
- Stabel J.R., Stabel T.J. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet. Immunol. Immunopathol. 1995;45:211–220. doi: 10.1016/0165-2427(94)05348-v. [DOI] [PubMed] [Google Scholar]
- Tretina K., Gotia H.T., Mann D.J., Silva J.C. Theileria-transformed bovine leukocytes have cancer hallmarks. Trends Parasitol. 2015;31:306–314. doi: 10.1016/j.pt.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W.C., Adams J.H., Adelfio R., Ahyong V., Akabas M.H., Alano P., Alday A., Aleman Resto Y., Alsibaee A., Alzualde A., Andrews K.T., Avery S.V., Avery V.M., Ayong L., Baker M., Baker S., Ben Mamoun C., Bhatia S., Bickle Q., Bounaadja L., Bowling T., Bosch J., Boucher L.E., Boyom F.F., Brea J., Brennan M., Burton A., Caffrey C.R., Camarda G., Carrasquilla M., Carter D., Belen Cassera M., Chih-Chien Cheng K., Chindaudomsate W., Chubb A., Colon B.L., Colon-Lopez D.D., Corbett Y., Crowther G.J., Cowan N., D'Alessandro S., Le Dang N., Delves M., DeRisi J.L., Du A.Y., Duffy S., Abd El-Salam El-Sayed S., Ferdig M.T., Fernandez Robledo J.A., Fidock D.A., Florent I., Fokou P.V., Galstian A., Gamo F.J., Gokool S., Gold B., Golub T., Goldgof G.M., Guha R., Guiguemde W.A., Gural N., Guy R.K., Hansen M.A., Hanson K.K., Hemphill A., Hooft van Huijsduijnen R., Horii T., Horrocks P., Hughes T.B., Huston C., Igarashi I., Ingram-Sieber K., Itoe M.A., Jadhav A., Naranuntarat Jensen A., Jensen L.T., Jiang R.H., Kaiser A., Keiser J., Ketas T., Kicka S., Kim S., Kirk K., Kumar V.P., Kyle D.E., Lafuente M.J., Landfear S., Lee N., Lee S., Lehane A.M., Li F., Little D., Liu L., Llinas M., Loza M.I., Lubar A., Lucantoni L., Lucet I., Maes L., Mancama D., Mansour N.R., March S., McGowan S., Medina Vera I., Meister S., Mercer L., Mestres J., Mfopa A.N., Misra R.N., Moon S., Moore J.P., Morais Rodrigues da Costa F., Muller J., Muriana A., Nakazawa Hewitt S., Nare B., Nathan C., Narraidoo N., Nawaratna S., Ojo K.K., Ortiz D., Panic G., Papadatos G., Parapini S., Patra K., Pham N., Prats S., Plouffe D.M., Poulsen S.A., Pradhan A., Quevedo C., Quinn R.J., Rice C.A., Abdo Rizk M., Ruecker A., St Onge R., Salgado Ferreira R., Samra J., Robinett N.G., Schlecht U., Schmitt M., Silva Villela F., Silvestrini F., Sinden R., Smith D.A., Soldati T., Spitzmuller A., Stamm S.M., Sullivan D.J., Sullivan W., Suresh S., Suzuki B.M., Suzuki Y., Swamidass S.J., Taramelli D., Tchokouaha L.R., Theron A., Thomas D., Tonissen K.F., Townson S., Tripathi A.K., Trofimov V., Udenze K.O., Ullah I., Vallieres C., Vigil E., Vinetz J.M., Voong Vinh P., Vu H., Watanabe N.A., Weatherby K., White P.M., Wilks A.F., Winzeler E.A., Wojcik E., Wree M., Wu W., Yokoyama N., Zollo P.H., Abla N., Blasco B., Burrows J., Laleu B., Leroy D., Spangenberg T., Wells T., Willis P.A. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viseras J., Garcia-Fernandez P., Adroher F.J. Isolation and establishment in in vitro culture of a Theileria annulata--infected cell line from Spain. Parasitol. Res. 1997;83:394–396. doi: 10.1007/s004360050270. [DOI] [PubMed] [Google Scholar]
- Wallis O.C. The effect of pentamidine on ribosomes of the parasitic flagellate Crithidia (Strigomonas) oncopelti. J. Protozool. 1966;13:234–239. doi: 10.1111/j.1550-7408.1966.tb01900.x. [DOI] [PubMed] [Google Scholar]
- Wlodkowic D., Telford W., Skommer J., Darzynkiewicz Z. Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol. 2011;103:55–98. doi: 10.1016/B978-0-12-385493-3.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.