Abstract
Testicular germ cell regression is a rare, generally metastatic phenomenon which describes the spontaneous, complete, or partial regression of a testicular germ cell tumour. As a result, studies have focused on defining specific histopathological criteria to establish if the resected testis is the primary source of the germ cell tumour. There are few publications which describe its presentation in the absence of distant metastases with elevated tumour markers and suspicious findings on testicular ultrasound. We present the clinical presentation and radiological features of a non-metastatic regressed testicular germ cell tumour following scrotal trauma in a post pubertal male.
Keywords: Testicle, Tumour regression, Burned-out tumour, Germ cell tumour, Teratoma
Abbreviations: GCNIS, germ cell neoplasia in-situ; GCT, Germ cell tumour(s)
1. Introduction
The World Health Organisation discriminates testicular teratomas into postpubertal-type and prepubertal-type teratoma, the former being derived from germ cell neoplasia in-situ (GCNIS).1,2 GCNIS is widely accepted as the precursor of adult malignant testicular germ cell tumours (GCT), thus patients with apparently pure testicular teratomas often have GCNIS in the testis and may develop metastases consisting of teratoma or other GCT.2 GCT also include a rare histotype known as regressed, or ‘burned out’ GCT which represents a primary testicular GCT that has spontaneously regressed and manifests with metastatic disease.1,2 We present a diagnosis of a non-metastatic regressed testicular GCT with residual teratoma and GCNIS following scrotal trauma in a post pubertal male.
2. Case presentation
A 33-year-old male presented to the ER with a history of progressive, painless left testicular swelling following an inadvertent knee to the scrotum one month prior.
On examination, his left hemi-scrotum was uniformly enlarged with an area of discrete hardness posteriorly. His right testis and abdomen examined normally, urinalysis was clear, white cell count and CRP were unremarkable, and he was apyrexial. Ultrasound (US) testes reported a left-sided hydrocele (131ml) and an enlarged, diffusely heterogenous left testis with increased vascularity (Fig. 1). There was also some discontinuity of the tunica at the posterosuperior aspect, and in the context of trauma, the presence of a testicular rupture could not be excluded. Serum tumour markers were sampled to rule out a testicular malignancy as the diagnosis was unclear (Table 1). The patient was prescribed a two-week course of oral antibiotics and an outpatient appointment 12 days later was arranged.
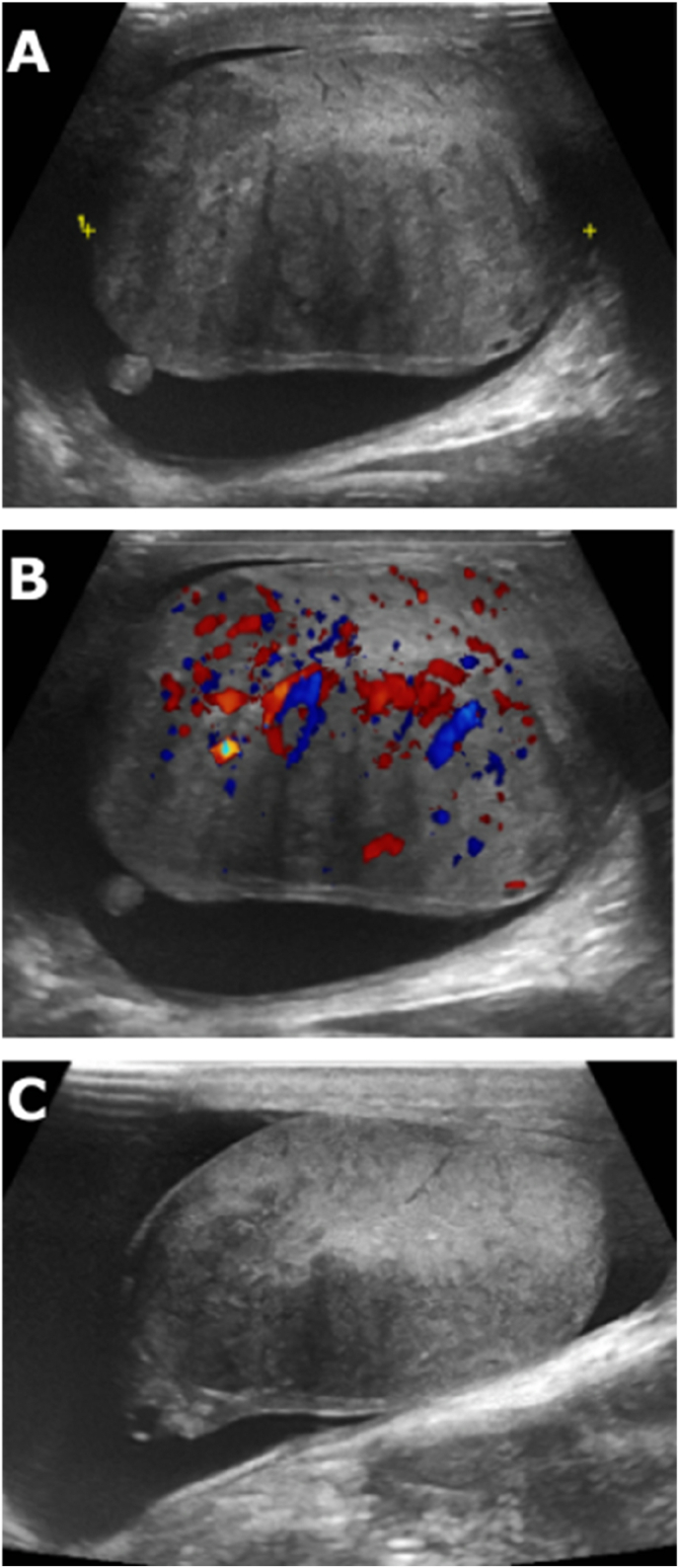
Fig. 1.
Diffusely heterogenous left testis (A) with increased vascularity (B) seen on US on initial presentation. Overall volume of the left testis had reduced significantly on follow-us US testes (C).
Table 1.
Serum tumour markers levels.
| Tumour Markers | On Presentation (July 02, 2021) | Pre-operative (August 06, 2021) | Day of Surgery (August 24, 2021) | Day 3 Postoperative | Day 10 Postoperative |
|---|---|---|---|---|---|
| βhCG IU/L (<2) | 4995 | 50.5 | 5.8 | 2.9 | <1.0 |
| αFP IU/ml (0–6.64) | 47.8 | 1.4 | 0.8 | 0.8 | 0.8 |
| LDH U/L (135–250) | 350 | 161 | 155 | 157 | 140 |
βhCG = human chorionic gonadotropin; FP = αalpha-fetoprotein; LDH = lactate dehydrogenase.
At follow-up, the left testis examined as it had on presentation. The previously sent tumour markers were strikingly elevated (Table 1.), however subsequent history revealed recreational marijuana use which clouded the interpretation somewhat. Overall, the case was now considered highly suspicious for malignancy and a left radical orchidectomy was recommended. The patient, however, refused surgery. His case was discussed urgently at a urology multidisciplinary meeting (MDM) which confirmed a heterogenous left testis highly suspicious for malignancy. Again, urgent radical orchidectomy was recommended which once more was declined by the patient.
On short interval telephone follow-up, the patient reported reduced swelling of the left testis and requested repeat imaging. This was organised urgently and redemonstrated an extensive heterogenous appearance of the left testis. Surprisingly, the overall volume of the left testis had reduced significantly (56.7 cc–39.2 cc), raising the possibility of a resolving inflammatory process. Subsequent clinical examination confirmed reduced swelling of the left testis and repeat tumour markers spontaneously and dramatically decreased in the absence of any intervention (Table 1). Overall, findings were less suspicious for malignancy, however it remained a concerning differential and the case was rediscussed urgently at MDM. Based on the persistent heterogenous appearance of the left testis on US, the initial tumour marker levels, and extensive multidisciplinary opinion, 10 days later the patient agreed to undergo a radical left-sided orchidectomy which was uneventful.
On histological examination the tumour itself was poorly defined but appeared to measure 30mm in maximum dimension and appeared to abut the tunica albuginea and rete testis. On microscopic examination, there was marked atrophy of the testicular tubules with Leydig cell hyperplasia. The tubules showed GCNIS and normal spermatogenesis was not seen. The macroscopically described tumour above showed widespread fibrosis, haemosiderin deposition, inflammation, and multiple foci of geographic necrosis (Fig. 2). Within the fibrotic stroma, there were residual glandular structures with bland nuclear features most in keeping with a mature teratoma. Overall, features were in keeping with regressed GCT with residual teratoma and GCNIS. The tumour was confined to the testis (pT1). Staging computed tomography of the thorax, abdomen and pelvis was negative for metastatic disease and tumour markers normalised in the post-operative period (Table 1). The patient was referred to medical oncology, however, did not require adjuvant treatment.
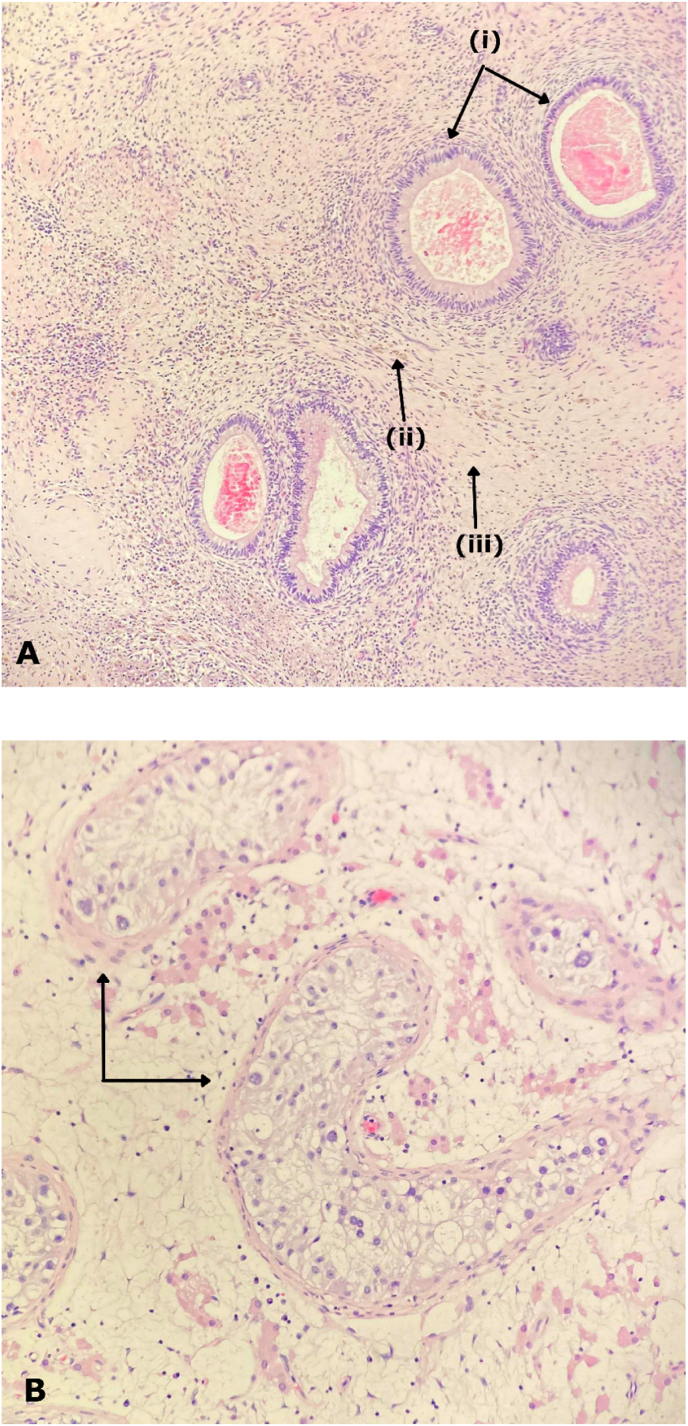
Fig. 2.
(A) Image at 20× magnification showing glandular structures with bland nuclear features representing residual teratoma (i), haemosiderin deposition (ii) and fibrosis, inflammation (iii). (B) Image at 20× magnification showing intratubular proliferation of large, atypical, germ cells with a mostly peripheral distribution.
3. Discussion
Historically, regressed testicular GCTs were labelled as ‘primary’ retroperitoneal tumours, a phenomenon which described manifestation exclusively by metastasis.2 Currently, it's believed that these tumours uniformly represent a ‘burned out’ testicular primary malignancy which have metastasised and spontaneously regressed.2 Rarely, as in the case described above, do they present in the absence of metastases necessitating a high index of suspicion on clinical and radiological work-up.2,3
In 2017 Rocher et al. reported that the US findings of 10 patients with burned-out testicular tumours appeared as poorly delineated hypoechoic lesions in all cases except one where the entire testis was hyperechoic.4 The authors added that nodular and ghost-like hypoechoic areas without any vascularization associated with microliths following colour doppler US are highly suggestive features of regression.4 These findings are inconsistent with those reported on US in our case where diffuse heterogeneity and increased vascularity was seen. It has been hypothesised that ischemia may be caused by the high metabolic rate of the neoplasm outgrowing its blood supply.5 It is possible therefore that the increased vascularity seen on initial US in our case captured the preceding elements of this phenomenon.
Mosillo et al. also reported a case of burned-out phenomenon in teratoma and remarked that the patient reported testicular inflammation 1 year prior to presentation.5 We hypothesize that presentation following trauma in our case resulted in an early and somewhat serendipitous diagnosis of a regressed testicular GCT that otherwise would have likely remained clinically silent until metastatic spread. Moreover, our patient's initial refusal of treatment and the resultant time lag offered a unique insight into the radiological and biochemical behaviour of an actively regressing GCT in real time.
4. Conclusion
To our knowledge, we present the only described case of an actively regressing GCT in the literature to date. This case highlights the importance of a multidisciplinary approach and provides a rare insight into the radiological and biochemical behaviours of an actively regressing GCT in real time. Additionally, it strengthens the 2016 ISUP recommendation that pathologists must consider the possibility of GCT regression for any testicular ‘scar’.2 A missed tissue diagnosis in this case would almost certainly have resulted in a later presentation with metastatic disease.
Consent
Consent was obtained from the patient.
Credit roles
Lianne Pickett: Conceptualisation, data curation, validation, writing – original draft; Richard Liddy: data curation, writing-review and editing; Niall Davis: Writing – review and editing; Paul Foran: data curation, writing – review and editing; Jaipreet Singh: writing – review and editing; Mark Quinlan: Supervision, writing – review and editing.
Declaration of competing interest
None.
References
- 1.Ulbright T. Recently described and clinically important entities in testis tumors: a selective review of changes incorporated into the 2016 classification of the World Health organization. Arch Pathol Lab Med. 2018;143(6):711–721. doi: 10.5858/arpa.2017-0478-RA. [DOI] [PubMed] [Google Scholar]
- 2.Williamson S., Delahunt B., Magi-Galluzzi C., et al. The World Health organization 2016 classification of testicular germ cell tumours: a review and update from the international society of urological pathology testis consultation panel. Histopathology. 2016;70(3):335–346. doi: 10.1111/his.13102. https://doiorg.proxy.library.rcsi.ie/10.1111/his.13102 [DOI] [PubMed] [Google Scholar]
- 3.Patel M., Patel B. Sonographic and magnetic resonance imaging appearance of a burned-out testicular germ cell neoplasm. J Ultrasound Med. 2007;26(1):143–146. doi: 10.7863/jum.2007.26.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Rocher L., Glas L., Bellin M., et al. Burned-out testis tumors in asymptomatic infertile men: multiparametric sonography and MRI findings. J Ultrasound Med. 2016;36(4):821–831. doi: 10.7863/ultra.15.08037. https://doi-org.proxy.library.rcsi.ie/10.7863/ultra.15.08037 [DOI] [PubMed] [Google Scholar]
- 5.Mosillo C., Scagnoli S., Pomati G., et al. Burned-out testicular cancer: really a different history. Case Rep Oncol. 2017;10(3):846–850. doi: 10.1159/000480493. [DOI] [PMC free article] [PubMed] [Google Scholar]