Abstract
Background: Yogurt is known to be nutrient-rich and probiotic content, which gather optimism due to their potential role in preventing and managing cancers. The effect of yogurt consumption on colorectal cancer (CRC) is inconsistent.
Objective: This study aims to investigate the association of yogurt consumption with the risk of CRC.
Methods: Three databases, namely, PubMed, Web of Science, and Embase, were searched for all relevant studies from July 2021 on the association of yogurt consumption with CRC risk. We pooled the odds ratios (ORs) and their 95% CIs using a random-effects meta-analysis to assess the association.
Results: Finally, 16 studies met the inclusion criteria and were chosen in the meta-analysis. Yogurt consumption was significant with lower risk of CRC risk in the overall comparison (OR = 0.87, 95% CI: 0.81–0.94), in the cohort studies (OR = 0.91, 95% CI: 0.86–0.97), and case-control studies (OR = 0.75, 95% CI: 0.65–0.85). With regard to subgroup analyses by study region, cancer type, publication year, and sex, yogurt consumption significantly decreased overall CRC, colon cancer, and distal colon cancer risks. In stratified analyses, we observed significantly decreased CRC risk in Europe and Africa and published after 2010 and overall population. Sensitivity analysis indicated the result is stable and there is no publication bias in the meta-analysis.
Conclusions: Overall, this study indicated that yogurt intake was related to a decreased risk of CRC.
Keywords: yogurt, colorectal risk, systematic review, meta-analysis, cohort studies
Introduction
Colorectal cancer (CRC) is the third most common cancer among men and women in the world (1–4). Some known risk factors for the development of CRC have been identified, such as genetic predisposition and epigenetic factors, tobacco use, overweight and obesity, and low physical activity (5–8). Moreover, CRC is also easily influenced by a wide range of dietary factors, such as regular alcohol consumption (9, 10), low fruit and vegetables diet (11–13), low-fiber and high-fat diet, or a diet high in processed meats (14, 15). Over the past decade, a growing number of epidemiological studies have suggested that the gut microbiome builds a unique ecosystem inside the gastrointestinal tract to maintain homeostasis and that gut microbiome compositional changes are highly related to the risk of CRC (16–20). Previous studies have suggested that the equilibrium of gut microbiota is affected by diet factors and any change may create an environment that might foster or prevent tumorigenesis of the intestinal system (21, 22). Thus, the gut microbiota is proposed to play a crucial mediator role in the association of dietary factors with CRC. The gut microbiota is a complex composed of trillions of viruses and microbial cells, which affect many aspects of physiology and human health (23–29).
Fermented food contains a large number of live microorganisms, so it can be used as probiotics to enrich the intestinal tract with beneficial bacteria. It helps the body to absorb nutrients and enhance immune function by preventing inflammation and stimulating phagocytosis (30). Yogurt is one of the representatives and popular fermented foods worldwide, and consumption of yogurt has been reported to associate with a wide range of health benefits in different populations (31–35). The potential mechanisms are complicated, but have been identified as producing immune-modulating metabolites, such as short-chain fatty acids (36); preventing pathogens from entering the intestinal epithelium (37); generating antimicrobial compounds (38); producing proteolytic enzymes (39); reducing the fecal enzyme activity of azoreductase, nitroreductase, and b-glucoronidase, which convert the procarcinogens to carcinogens in the colon (40). Over the past several decades, many epidemiological pieces of evidence have reported that yogurt consumption is associated with decreased risk of metabolic syndrome (41), hip fracture (42), type 2 diabetes (43), cardiovascular diseases (44), etc. However, nutritional information and health-related properties of yogurt in disease progression are limited. Disregarding a growing number of observational studies that have been performed to assess the association of yogurt consumption with CRC risk, the available evidence was inconsistent, several epidemiological studies have indicated an inverse association (45–49), while several other epidemiological studies reported non-significant associations (30, 50–60). More recently, Godos et al. (61) performed an umbrella review of observational studies on the associations of dairy foods with health and reported that yogurt intake may be associated with various health outcomes, yet with too limited evidence to draw definite conclusions. Thus, it is necessary to further clarify the association between yogurt intake and the risk of CRC.
To the best of our knowledge, previous reviews always included the small number of epidemiological studies and did not reach a consensus (62–64). In view of the inconsistent findings in the literature, and lack of a comprehensive systematic review and meta-analysis of the existing literature, an updated systematic review and meta-analysis is needed to further clarify the associations. We performed a meta-analysis of observational studies to clarify the association of yogurt intake with the risk of CRC. Our hypothesis was that higher yogurt intake is associated with a lower risk of CRC.
Methods
Protocol and Research Question
This study was presented according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statements (65). We provided the PRISMA checklist in Supplementary Table 1. The participant, exposure, comparison, outcome, and study design (PECOS) are grouped in Supplementary Table 2. The research question of this study is presented as follows: among the general population, is higher yogurt intake related to a lower risk of CRC?
Data Source and Search Strategy
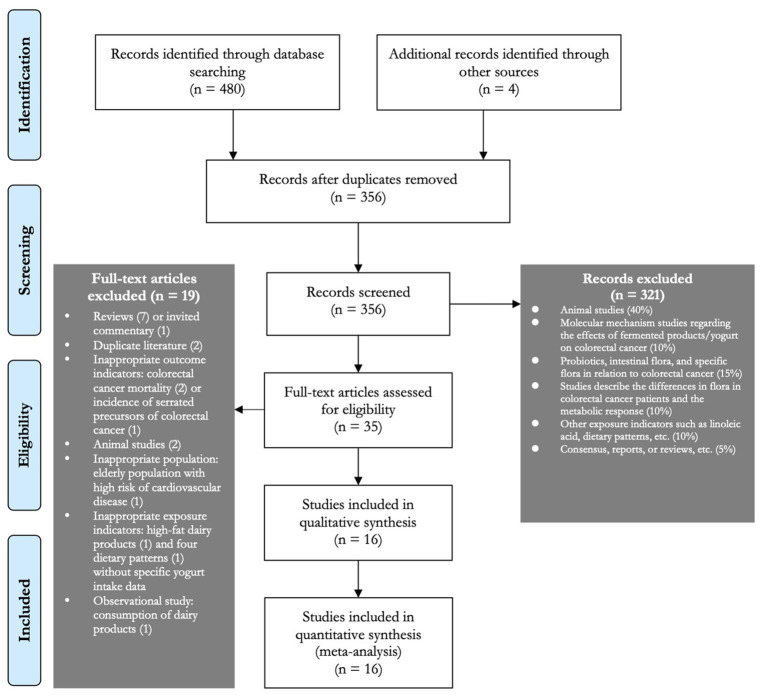
PubMed, Embase, and Web of Science literature databases were searched dated up to July 2021, using the combinations of keywords related to yogurt and CRC. Keywords for exposure (yogurt consumption) included “yogurt,” “yogurt,” and “cultured milk products,” while keywords for the outcome (risk of CRC) included “colorectal cancer” and “colorectal neoplasms.” The detailed search terms used in each literature database are summarized in Supplementary Table 3. In addition, the reference lists of the chosen studies and any relevant systematic reviews were also checked for any potentially eligible studies not previously identified in this review. Figure 1 depicts the search process.
Figure 1.
Flow diagram of study selection process.
Study Eligibility Criteria
The inclusion criteria were (1) human (>18 years old) epidemiological studies (cross-sectional, cohort, or case-control design) that focused on the association of yogurt consumption with an incidence of CRC, such as total CRC, colon or rectal cancer, or proximal or distal colon cancer; (2) studies provided estimates of the odds ratio [OR], relative risk [RR], or hazard ratio [HR] with their 95% CIs for the data synthesis or reported sufficient data that could be used to calculate the estimates was presented; and (3) studies evaluated the intake of yogurt through the use of validated food questionnaires. We excluded studies that (1) were not written in the English language; (2) were not original studies, such as review, meta-analysis, commentary, letter, or editorial; and (4) studies assessed CRC mortality as an outcome of interest.
Data Extraction
The following data were extracted from each chosen study: name of the first author, year of publication, country, study design (duration of follow-up for cohort studies), sample size, mean age of study participants, dietary assessment, outcome assessment, number of cases, categories of yogurt intake, reported risk estimates (HRs, RRs, or ORs) with their 95% CIs, and the adjusted confounders in the final multivariable regression models. If two effect estimates based on the sex of study participants were reported in a study, we firstly pooled them using fixed-effect meta-analysis and then put the pooled estimate in the main meta-analysis. If studies report the crude and confounding adjusted risk estimates for CRC, we selected the effect estimates from the full-adjusted model.
Study Quality Evaluation
To assess the quality of each study, we applied the widely used quality assessment tool for an observational study, which is Newcastle-Ottawa Scale (NOS) (66). Two of the authors evaluated the study quality independently using the following criteria: (1) the study selection (maximum 4 points); (2) the adequacy of the outcome in cohort studies and the adequacy of the exposure in case-control studies (maximum 3 points); and (3) the comparability of the studies (maximum 2 points). A study was categorized as high quality if it was assigned with a score ≥7; otherwise, low quality was indicated. Any discrepancies were solved by a group discussion to reach a consensus.
Meta-Analysis
The reported effect estimates (ORs, RRs, or HRs) were used as the measures of the association of yogurt consumption with the risk of CRC. Following previous practices (67, 68), we considered that standardized risk estimates (e.g., ORs, RRs, and HRs) were equivalent and pooled HRs and RRs with ORs and we used ORs as the indicator of pooled effect size; this is acceptable in the present situation where the outcome is rare (69). To calculate the pooled effect estimates, we compared the highest vs. the lowest categories of yogurt intake, we conducted random-effects or fixed-effect meta-analysis depending on the between-study heterogeneity. When substantial heterogeneity was found, a random-effects meta-analysis was used; otherwise, fixed-effect meta-analysis would be used. The between-study heterogeneity was evaluated using the I2 statistic (70) and the P-value from the Chi-squared test of heterogeneity. We considered an I2 value ≥50% to indicate substantial heterogeneity and a P-value ≤ 0.1 to indicate the presence of statistically significant heterogeneity (71). To test the robustness of the result, sensitivity analysis was performed with the “leave-one-out” method. The potential risk of publication bias was assessed using funnel plot and Egger's test. The sources of heterogeneity were explored by subgroup analyses where available. In the present study, STATA 15.0 (Stata Corp LLC, College Station, TX, USA) was used to perform all analyses.
Results
A total of 484 studies (PubMed: 108, Web of Science: 248, EMBASE: 124, and other sources: (4) were chosen through the literature search (Figure 1). We excluded 321 papers based on the title/abstract screen, and a brief screening of the full-text article after the duplicated studies (n = 128) was excluded. Nineteen studies that did not meet the inclusion criteria were excluded and produced a total of 16 studies were included in the systematic review and meta-analysis (35 studies were detailed assessed). The reference lists of all the 19 studies were also screened, and we found that all the potentially included articles were already chosen. Finally, a total of 16 studies were chosen for the systematic review and meta-analysis.
Table 1 summarizes the main characteristics of the epidemiological studies included in the review. Among the 16 included studies, 9 were cohort studies and 7 were case-control studies. The number of study participants in each study who were ranged from 392 to 477,122 and different kinds of food frequency questionnaires were used to assess the consumption of yogurt, and the ascertainment of cases were always from national or regional cancer registers. Eight studies were performed in Europe, 3 in North American, 2 in Asia, 2 in Africa, and 1 in multiple European countries. Almost all of the included studies adjusted the confounders when investigated the association of yogurt consumption with CRC risk. With regard to the quality assessment, almost all of the included studies were appraised as moderate to high quality (Table 1).
Table 1.
Characteristics of studies investigated the association of yogurt consumption and colorectal cancer risk.
| Author, year of publication (country) | Study design (follow-up, years) | Cohort name, sample size and study period | Age (mean±SD or range, years) | Dietary assessment | Outcome assessment | Reported risk estimates | Adjusted confounders |
|---|---|---|---|---|---|---|---|
| Kampman et al. (57) (U.S.) | Case-control (NA) | The HPFS and the NHS cohort studies, 18,398, 1986–1990 and 1980–1988 | NA | Semi-quantitative food-frequency questionnaire | Diagnosis of adenocarcinoma polyps of the colon or rectum | HPFS C5 vs. C1: RR = 1.06 (0.72, 1.57) NHS (1984–1988) C5 vs. C1: RR = 0.75 (0.51, 1.11) NHS (1980–1988) C5 vs. C1: RR = 0.89 (0.63, 1.25) |
Age, total energy, family history, and saturated fat intake |
| Kampman et al. (53) (Netherlands) | Cohort study (9) | The Netherlands Cohort Study, 120,852, 1986–1989 | 55–69 | Validated FFQ (150 items) | Record linkage to cancer registries and a nationwide pathology register | 64 g/day: Ref 181 mg/day: RR = 1.01 (0.69, 1.48) 287 mg/day: RR = 1.29 (0.89, 1.88) 397 mg/day: RR = 1.18 (0.80, 1.72) 634 mg/day: RR = 1.14 (0.77, 1.68) |
Age, gender, family history of colorectal cancer, intake of energy, energy-adjusted intake of fat and dietary fiber, BMI, history of gallbladder surgery |
| Boutron et al. (58) (France) | Case-control (NA) | NA, 1268, 1985–1990 | Cases: 64.2 ± 10.3 Controls: 62.1 ± 11.6 | Detailed 2-h questionnaire about the diet in the past year | Registry of Digestive Tumors of Burgundy | Tertile 1: Ref Tertile 2: RR = 1.0 (0.7, 1.7) Tertile 3: RR = 1.0 (0.6, 1.6) |
Age, sex and caloric intake |
| Kearney et al., (59) (U.S.) | Cohort study (6) | The HPFS cohort study, 47,935, 1986–1992 | 40–75 | Validated FFQ (131 items) | Self-reported, then confirmed by hospital records and pathology reports | <1/month: Ref 1–4/month: RR = 0.70 (0.45, 1.09) 2–4/week: RR = 0.81 (0.51, 1.26) 5–7/week: RR = 0.96 (0.51, 1.26) > 1/day: RR = 1.09 (0.70, 1.72) |
Age, total calories, family history for colon cancer, previous potyp, screening, past history of smoking, alcohol, aspirin, physical activity, BMI, red meat, saturated fat, and dietary fiber |
| Jarvinen et al. (54) (Finland) | Cohort study (15) | Population cohort from a large-scale health examination survey performed by the Social Insurance Institution's Mobile Clinic, 9959, 1966–1991 | > 15 | Performed questionnaire | Linkage to the Finish Cancer Registry | Colon cancer Q4 vs. Q1: RR = 0.79 (0.34, 1.79) Rectum cancer RR = 2.67 (0.91, 7.80) Both cancers RR = 1.28 (0.68, 2.40) |
Age, sex, BMI, occupation, geographical area, and intake of energy |
| Terry et al. (55) (Sweden) | Cohort study (11.3) | Swedish Mammography Screening Cohort, 61,463, 1987–2000 | The average age at diagnosis was 67 for colon cancer cases and 68 for rectal cancer cases | FFQ (67 items) | Linkage to regional cancer registry | Colorectal cancer Q4 vs. Q1: RR = 0.90 (0.72, 1.13) Colon cancer RR = 0.76 (0.57, 1.01) Proximal colon cancer RR = 0.67 (0.44, 1.03) Distal colon cancer RR = 0.80 (0.47, 1.35) Rectal cancer RR = 1.28 (0.87, 1.89) |
Age, BMI, educational level, total energy, and quartiles of red meat, alcohol, and energy-adjusted folic acid and vitamin C intake |
| Sanz et al. (49) (Spain) |
Case-control (NA) | NA, 392, 1998 | Cases: 61.7 ± 10.8 Controls: 61.6 ± 9.8 | Questionnaire | Linkage to cancer register | 0.97 (0.95, 0.98) | Age, sex and geographical area |
| Kojima et al. (60) (Japan) | Cohort study (9.9) | Japan Collaborative Cohort Study, 107,824, 1988–1999 | 40–79 | Validated FFQ in Japanese diet (33 items) | The resident registration records of municipalities | Colon cancer: Seldom: Ref 1–2 per month: HR = 1.32 (0.74, 2.35) 1–7 per week: HR = 0.80 (0.42, 1.51) Rectal cancer: Seldom: Ref 1–2 per month: HR = 0.80 (0.39, 1.62) 1–7 per week: HR = 0.46 (0.21, 1.02) |
Age, family history of CRC, BMI, frequency of alcohol intake, current smoking status, walking time per day, and educational level and stratified by regions of enrollment |
| Pala et al. (45) (Italy) | Cohort study (12) | EPIC-Italy cohort, 45,241, 1993–1998 | 30–86 | Three validated semi-quantitative food questionnaires | Linkage of the study cohort to the databases of the regional cancer registries | 0–1 g/day: Ref 1–25 g/day: HR = 0.86 (0.65, 1.15) 25–87.5 g/day: HR = 0.65 (0.48, 0.89) |
Energy, animal fat, red meat intake, dietary calcium, dietary fiber and simple sugars, BMI, alcohol consumption, smoking, education level, recreational activity, sporting and type of work |
| Kinany et al. (47) (Morocco) |
Case-control (NA) | NA, 2906, 2009–2017 | 41–71 | Validated FFQ (225 items) | Anatomo- pathology reports | CRC ≤44.0 g/day: Ref. > 44.0 g/day: OR = 0.74 (0.64, 0.86) Colon cancer ≤44.0 g/day: Ref. >44.0 g/day: OR = 0.72 (0.58, 0.89) Rectal cancer ≤44.0 g/day: Ref. > 44.0 g/day: OR = 0.76 (0.61, 0.93) |
Age in years, residence, education level, monthly income, physical activity intensity, smoking status, BMI categories, NSAIDS, total energy intake, intakes of red processed meat and dietary fiber, family history of CRC |
| Michels et al. (30) (U.S.) | Cohort study (32) | The NHS and HFPS cohort studies 126,323, 1980–2012 and 1986–2012 | 40–75 | Validated FFQ (61 items and 131 items) | Self-report and then confirmed by medical records and pathology reports | CRC Never or < 1 serving/month: Ref 1–3 servings/month: HR = 0.97 (0.87, 1.07) 1+ servings/week: HR = 0.89 (0.80, 1.00) Colon cancer Never or < 1 serving/month: Ref 1–3 servings/month: HR = 0.97 (0.86, 1.09) 1+ servings/week: HR = 0.87 (0.76, 0.99) Proximal colon cancer Never or < 1 serving/month: Ref 1–3 servings/month: HR = 0.92 (0.79, 1.08) 1+ servings/week: HR = 0.84 (0.70, 0.99) Distal cancer Never or < 1 serving/month: Ref 1–3 servings/month: HR = 1.04 (0.86, 1.25) 1+ servings/week: HR = 0.91 (0.74, 1.12) Rectal cancer Never or < 1 serving/month: Ref 1–3 servings/month: HR = 0.93 (0.75, 1.17) 1+ servings/week: HR = 0.95 (0.76, 1.21) |
Age, 2-year follow-up cycle, family history of CRC, history of lower gastrointestinal endoscopy, BMI, height, physical activity, pack-years of smoking before age 30, current multivitamin use, regular aspirin or NSAIDs use, parity in women and age at first birth in women, menopausal status and age at menopause, menopausal status and hormone use in women, total caloric intake, alcohol consumption, and energy-adjusted intake of folate, calcium, vitamin D, total fiber, unprocessed red meat, and processed meat |
| Negrichi et al. (48) (Algeria) | Case-control (NA) | NA, 400, 2016–2019 | 55.6 ± 13.0 (control) 55.2 ± 17.0 (case) | Validated FFQ | Medical diagnosed | Rarely: Ref Frequently: OR = 0.63 (0.41, 0.96) |
No adjustment was made for multiple testing |
| Nilsson et al. (56) (Sweden) | Cohort study (30) | Northern Sweden Health and Disease Study, 101,235, 1986–2016 | 45.9 ± 9.4 (referents) 54.9 ±8.3 (any cancer) | Semi-quantitative FFQ | Linkage to Sweden Cancer Register | Q5 vs. Q1 HR = 0.98 (0.77, 1.25) (men) HR = 0.90 (0.70, 1.15) (women) |
Age, screening year, dairy product category, BMI, civil status, education level, physical activity in leisure time, smoking status, recruitment cohort, and quintiles of fruit-and vegetables, alcohol, and energy intake |
| Barrubés et al. (50) (Spain) | Cohort study (9) | PREvencion con DIeta MEDiterranea study, 7216, 2003–2012 | 55–80 | Validated FFQ (137 items) | Medical records | 8 (1–22) g/day: Ref 65 (54–85) g/day: HR = 1.15 (0.70, 1.90) 128 (122–186) g/day: HR = 0.94 (0.56, 1.59) |
Intervention group, sex, age, leisure time physical activity, BMI, current smoker, former smoker, never smoker, family history of cancer, education level, history of diabetes and use of aspirin at baseline, tertiles of cumulative average consumption during the follow-up of vegetables, fruits, legumes, cereals, fish, meat, olive oil and nuts (all in g/day) and alcohol (g/day and quadratic term) |
| Tayyem et al. (51) (Jordan) | Case-control (NA) | NA, 501, 2010–2012 | ≥ 18 | Validated Arabic FFQ (30 items) | Face-to-face interview | Rarely: Ref. Monthly: OR = 1.06 (0.31, 3.62) Weekly: OR = 0.82 (0.29, 2.32) Daily: OR = 0.76 (0.25, 2.32) |
Age, sex, total energy, physical activity, smoking, education level, marital status, work, income, other health problems and CRC history |
| Murphy et al. (46) (Europe) | Cohort study (11) | EPIC, 477,122 (8), 1992–2010 | ≥ 35 | Diet and lifestyle questionnaires | Population cancer registries, kin health insurance records, cancer and pathology registries | CRC ≥ 109 g/day vs. 0 g/day: HR = 0.90 (0.81, 0.99) All colon cancer ≥ 109 g/day vs. 0 g/day: HR = 0.88 (0.77, 1.00) Proximal ≥ 109 g/day vs. 0 g/day: HR = 0.94 (0.79, 1.13) Distal ≥ 109 g/day vs. 0 g/day: HR = 0.84 (069, 1.02) Rectal cancer ≥ 109 g/day vs. 0 g/day: HR = 0.93 (0.79, 1.10) |
Total energy intake, body mass index, physical activity index, smoking status and intensity, education status, ever use of contraceptive pill, ever use of menopausal hormone therapy, menopausal status, alcohol consumption and intakes of red and processed meat and fiber, and stratified by age, sex and center |
HPFS, Health Professionals Follow-up Study; NHS, Nurses' Health Study; EPIC, the EuropeanProspective Investigation into Cancer and Nutrition; SD, standardized deviation; OR, odds ratio; RR, relative risk; HR, hazard ratio; BMI, body mass index; FFQ, food frequency questionnaire; CRC, colorectal cancer; TCPS, Tennessee colorectal polyp study; U.S., United States; NSAIDS, non-steroidal anti-inflammatory drugs; HRT, hormone replacement therapy; NA, not available.
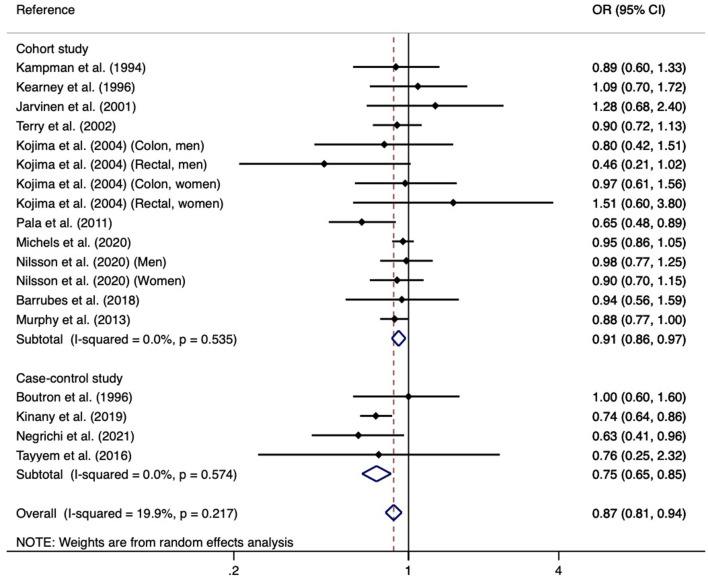
In the meta-analysis, we have found that higher yogurt intake was associated with a lower risk of CRC (pooled OR for the highest compared with the lowest consumption groups: 0.87; 95% CI: 0.81, 0.94; Figure 2). There was no substantial heterogeneity between studies (I2 = 19.9%; P-heterogeneity = 0.217). When performed stratified meta-analyses (Table 2), there is a stronger positive association for case-control studies than in cohort studies (OR = 0.75, 95% CI: 0.65, 0.85 vs. OR = 0.91, 95% CI: 0.86, 0.97). Subgroup analysis by sex indicated no significant associations of yogurt consumption with the risk of CRC in any specific subpopulations. When stratified by publication year, only studies published after 2010 indicated a significant association. When studies restricted to the exposure as fermented milk included yogurt, there is also no significant association with CRC risk. Subgroup analyses by CRC subtype and geographic location revealed significant associations in overall CRC, colon, distal colon, Europe, and Africa. In the sensitivity analysis, each individual study was omitted at a time that did not change the summary effect estimate substantially and the pooled ORs ranged from 0.79 to 0.96. We further excluded one study that has some overlap data, the result was also not changed substantially. The funnel plot in combination with Egger's test for asymmetry (p-value = 0.820) did not indicate the presence of publication bias (Figure 3).
Figure 2.
Forest plot of the association between yogurt consumption and risk of colorectal cancer.
Table 2.
Subgroup analysis of studies investigated the association of yogurt consumption with risk of colorectal cancer.
| Subgroup factors | n of studies | OR (95% CI) | I2, % |
|---|---|---|---|
| Study design | |||
| Cohort study | 10 | 0.91 (0.86, 0.97) | 0 |
| Case-control study | 4 | 0.75 (0.65, 0.85) | 0 |
| Study region | |||
| Asian | 2 | 0.85 (0.62, 1.17) | 4.4 |
| Europe | 8 | 0.89 (0.82, 0.97) | 0 |
| Africa | 2 | 0.73 (0.63, 0.84) | 0 |
| North American | 2 | 0.96 (0.87, 1.05) | 0 |
| Cancer subtype | |||
| Colorectal cancer | 12 | 0.87 (0.80, 0.94) | 25.8 |
| Colon | 6 | 0.86 (0.78, 0.96) | 6.0 |
| Rectal | 6 | 0.95 (0.78, 1.16) | 57.6 |
| Proximal colon | 3 | 0.91 (0.81, 1.03) | 8.7 |
| Distal colon | 3 | 0.87 (0.77, 0.99) | 0 |
| Yogurt solely | |||
| Yes | 9 | 0.83 (0.74, 0.93) | 38.1 |
| No | 5 | 0.94 (0.84, 1.07) | 0 |
| Publication year | |||
| Before 2010 | 6 | 0.93 (0.81, 1.08) | 0 |
| After 2010 | 8 | 0.85 (0.77, 0.94) | 44.5 |
| Sex | |||
| All | 10 | 0.84 (0.76, 0.94) | 40.6 |
| Men | 4 | 0.77 (0.55, 1.08) | 58.4 |
| Women | 4 | 0.89 (0.77, 1.02) | 0 |
Figure 3.
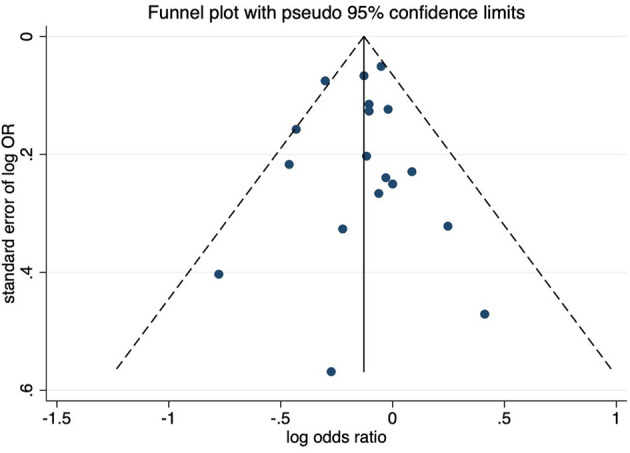
Funnel plot of the association between yogurt consumption and risk of colorectal cancer.
Discussion
The present meta-analysis identified 16 studies that included a total of 1,129,035 participants. When compared with the lowest category of yogurt intake, the highest category of yogurt consumption was associated with a lower risk of CRC. Importantly, yogurt intake was related to a decreased risk of CRC in both case-control and cohort studies. The effect was more pronounced in case-control studies than in cohort studies. The conclusion of this study is generally in line with evidence from previous meta-analyses that suggested an inverse association of yogurt consumption with the risk of other diseases (33, 43, 72–75).
Over the past few years, the beneficial effects of yogurt consumption on lowing risk of CRC have been supported by a growing number of human epidemiological studies (30, 45, 46, 51, 52, 62). Although the findings were inconsistent, several clinical and epidemiological studies have indicated the important role of yogurt intake in managing weight (76–78). Obesity is a well-known risk factor of CRC (79–81); thus, the above studies indirectly support the beneficial role of yogurt intake in decreasing the risk of CRC. Furthermore, regular yogurt consumption is a good habit and thus may also be associated with decreasing the risk of CRC. This study is in agreement with two previous systematic reviews and meta-analyses that reported yogurt consumption was associated with decreased risk of CRC (62, 63). However, this study has updated the available evidence and is more comprehensive (Table 3). The non-significant association reported from previous original studies can be attributed to the following factors: (1) the definition of exposure is not precise (yogurt has different associations with other food items), and number of living bacteria in the yogurt could also have reduced the power to elaborate the association between the two of previous studies; (2) there are different types of methods used by fermentation processes in different regions, depending on the starter organisms used. The obtained varied yogurt types might give different effects to the results of previous epidemiological studies; and (3) few studies have classified the subtypes of CRC, and yogurt consumption may exert different effects on each subtype of CRC. A possible explanation for the differences in associations between yogurt consumption and CRC risk by different subgroups is that the number of included studies might influence the results. For example, almost half of the included studies (n = 8) were conducted in European countries and the dairy products consumption varies greatly among different regions. Europe is the region with the highest dairy products consumption (82). The possible reason for the studies that were published after 2010 showed significant association is that the follow-up durations of the included studies were long enough for the outcome to occur. An only significant association was found for the overall population that has also been reported in the study of Pala et al. (45), the possible reason is that most of the included studies were insufficiently powered to detect a supposed small difference between women and men regarding the protective effect.
Table 3.
The comparison of protocols between previous systematic reviews and our systematic review.
| Barrubés et al. (63) | Zhang et al. (62) | Our systematic review | Observation | |
|---|---|---|---|---|
| Protocol | ||||
| Databases | MEDLINE(PubMed), Cochrane Library, CINAHL, and ScienceDirect | PubMed, Embase and CNKI | Web of Science, PubMed, and EMBASE | – |
| Keywords | Dairy products (i.e., “dairy” or “dairy products”) and subtypes of dairy products (i.e., “milk” or “yogurt” or “yogurt” or “cheese” or “cultured milk products”) in combination with keywords related to CRC events (i.e., “colorectal cancer” or “colorectal neoplasms”) | “Fermented food or cheese or fermented milk or cultured milk or yogurt or lactic acid bacteria” and “cancer” | “Yogurt,” “yogurt” and “cultured milk products” in combination with “colorectal cancer” and “colorectal neoplasms” | The keywords of Zhang et al. (62) also focused on other cancers, but Barrubés et al., (63) and our study only focused on CRC. Furthermore, the two previous studies also focused on other dairy products |
| Searching period Guideline |
4 June, 2018 Cochrane Handbook for Systematic Reviews of Interventions MOOSE PRISMA |
Before July 2018 Not reported |
Before July, 2021 PRISMA | Our study included 7 additional studies due to the updated search; PRISMA guideline is recommended for systematic reviews |
| Exposure of interest | Total dairy products High-fat dairy products Low-fat dairy products Total milk Whole milk Low-fat milk Fermented dairy products Total yogurt Cultured milk Cheese |
Yogurt Cheese |
Yogurt | Our exposure analysis is more specific |
| Outcome of interest for meta-analysis | CRC Colon cancer Colon cancer by site (proximal or distal colon) Rectal cancer |
Cancers | CRC Colon cancer Colon cancer by site (proximal or distal colon) Rectal cancer |
No difference, all the three studies have assessed CRC |
| Exclusion criteria | Not report | Not report | Articles does not our inclusion criteria were excluded | – |
| Types of studies | Case-control and prospective cohort studies | Cohort study or case–control study that published in English language | Epidemiological studies with cohort, cross-sectional, or case-control designs | – |
| Quality assessment | NOS | None | NOS | NOS is widely used to assess the quality of cohort and case-control studies |
| Number of included studies | 29 studies Yogurt: 7 studies |
61 studies Yogurt and CRC: 9 studies |
16 studies | Our study included more studies |
| Statistical analysis Subgroup |
Not reported; Study design CRC subsite |
Fixed-effects model or random-effects model Study design | Random-effects or fixed-effect meta-analysis | – |
| Test of heterogeneity | Q test I2 statistic |
Q test I2 statistic |
Q test I2 statistic | Q test and I2 statistic are valid test for heterogeneity |
| Sensitivity analysis | None | Leave-one-out method | Leave-one-out method | To observe the robustness of pooled analysis, sensitivity analysis is recommended |
| Publication bias | None | Funnel plot Begg's test |
Funnel plot Egger's test |
To assess the publication bias, funnel plot and Egger's test are recommended by the Cochrane handbook |
| Main findings | Yogurt consumption is associated with lower risk of CRC in cohort studies, but not in case-control studies | Yogurt consumption was significantly with decreased CRC risk | Yogurt consumption was significantly with decreased CRC risk | Our study provided more information due to the available of subgroup analyses |
Aune et al. (64) assessed the associations of dairy products with colorectal cancer using systematic review and meta-analysis, but only included two studies and reported an estimate of 1.00 (95% CI: 0.67, 1.48) was thus not compared with our study.
PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-analyses; NOS, Newcastle-Ottawa Scale; CRC, colorectal cancer.
For a long time, people have believed that yogurt and other fermented dairy products are beneficial to the health of the gastrointestinal tract. Therefore, several pathogenic mechanisms that may have a protective effect on CRC have been proposed. Yogurt can exert anti-tumor effects by reducing the level of carcinogens in the intestine, for example, by reducing the activity of intestinal enzymes, such as nitro reductase and fecal bacterial enzymes, and reducing the level of soluble fecal bile acids, all of which are related to colon carcinogenesis (83, 84). Lactobacillus bulgaricus (L. bulgaricus) has been shown to prevent tumor induction caused by 1,2-dimethylhydrazine in mouse models (85), and both streptococcus thermophilus and lactobacillus delbrueckii subsp. bulgaricus produce antigenotoxic metabolites that act as blocking agents to prevent initiation carcinogenesis (86).
Compared with previous systematic and meta-analyses focused on the association of fermented dairy foods intake and risk of cancer (62), this is the first meta-analysis that further performed the stratified analyses. All the included studies are appraised as moderate to high quality and evidence from the present meta-analysis is reliable.
Several strengthens should be acknowledged for this study. To our knowledge, this is the first meta-analysis to investigate the association of yogurt intake with risks of CRC and its different subtypes. Moreover, the robustness of the results was tested by performing some sensitivity analyses, and the potential risk of publication bias was also evaluated. Disregarding the strengthens of this study, some limitations should be acknowledged as (1) the number of included studies is relatively small and thus precluded us perform meta-regression analysis to explore source(s) of heterogeneity. Moreover, we only included studies published in the English language so that some other language papers may be omitted; (2) we are unable to explore the dose-response curve of yogurt consumption with CRC risk due to the limited data provided by the included studies; (3) most of the included studies did not distinguish colon and rectal cancers and analyzed them together. In spite of these cancers are always considered together, potential etiological factors for colon and rectal cancers may be different and site-specific mechanisms of carcinogenesis have been indicated (87); (4) although most of the included studies have controlled some important confounders, other potential unmeasured confounders cannot be ruled out and thus influence the results of the meta-analysis; (5) most of the chosen studies were performed in developed countries and thus prohibited us to generalize the results to other countries. Considering that the consumption and making methods of yogurt vary greatly from country to country (88, 89), region-difference should be considered in future studies; (6) the findings were sourced from observational studies and thus cannot establish the causal relationship.
Conclusion
To conclude, this systematic review and meta-analysis suggested that yogurt consumption is related to a lower risk of CRC. However, in consideration of the aforementioned limitation, these findings should be confirmed by further longitudinal studies with improved yogurt consumption assessment, better CRC, such as subtypes of CRC case ascertainment and comprehensive control of confounders in clarifying the association. If such a conclusion is supported, we would recommend regular yogurt intake as a healthy lifestyle behavior in decreasing the risk of CRC in adults.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JSu and JSo conceived the idea, performed the statistical analysis, and drafted this meta-analysis. JY, LC, and ZW selected and retrieved relevant papers. MD and SY assessed each study. JSu was the guarantor of the overall content. CH and QB supervised the whole study process and contributed to the critical revision of the manuscript. All authors revised and approved the final manuscript.
Funding
This study was supported by Anhui Province Natural Science Foundation (1908085MG233), Quality Engineering for Research Projects of the Anhui Province (2020wyxm108, 2020SJJXSFK1341), and Key Projects of Natural Science Research of Anhui Provincial Department of Education (KJ2020A0163).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.789006/full#supplementary-material
References
- 1.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. (2018) 4:1553–68. 10.1001/jamaoncol.2018.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 5.Xu W, He Y, Wang Y, Li X, Young J, Ioannidis JPA, et al. Risk factors and risk prediction models for colorectal cancer metastasis and recurrence: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. (2020) 18:172. 10.1186/s12916-020-01618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–732. 10.1038/s41575-019-0189-8 [DOI] [PubMed] [Google Scholar]
- 7.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. (2013) 24:1207–22. 10.1007/s10552-013-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gausman V, Dornblaser D, Anand S, Hayes RB, O'Connell K, Du M, et al. Risk factors associated with early-onset colorectal cancer. Clin Gastroenterol Hepatol. (2020) 18:2752–59. 10.1016/j.cgh.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Menezes RF, Bergmann A, Thuler LC. Alcohol consumption and risk of cancer: a systematic literature review. Asian Pac J Cancer Prev. (2013) 14:4965–72. 10.7314/APJCP.2013.14.9.4965 [DOI] [PubMed] [Google Scholar]
- 10.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. (2015) 112:580–93. 10.1038/bjc.2014.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Laure Preterre A, Iqbal K, et al. Food groups and risk of colorectal cancer. Int J Cancer. (2018) 142:1748–58. 10.1002/ijc.31198 [DOI] [PubMed] [Google Scholar]
- 12.Mafiana RN, Al Lawati AS, Waly MI, Al Farsi Y, Al Kindi M, Al Moundhri M. Association between dietary and lifestyle indices and colorectal cancer in oman: a case-control study. Asian Pac J Cancer Prev. (2018) 19:3117–22. 10.31557/APJCP.2018.19.11.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. (2017) 18:e457–71. 10.1016/S1470-2045(17)30411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. (2015) 148:1244–60.e16. 10.1053/j.gastro.2014.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baena R, Salinas P. Diet and colorectal cancer. Maturitas. (2015) 80:258–64. 10.1016/j.maturitas.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 16.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. (2019) 16:690–704. 10.1038/s41575-019-0209-8 [DOI] [PubMed] [Google Scholar]
- 17.Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology. (2020) 158:322–40. 10.1053/j.gastro.2019.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao R, Gao Z, Huang L, Qin H. Gut microbiota and colorectal cancer. Eur J Clin Microbiol Infect Dis. (2017) 36:757–69. 10.1007/s10096-016-2881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. (2016) 22:501–18. 10.3748/wjg.v22.i2.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. (2016) 70:395–411. 10.1146/annurev-micro-102215-095513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. (2019) 10:S17–s30. 10.1093/advances/nmy078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H, Rao MC, Chang EB. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat Rev Gastroenterol Hepatol. (2021) 17:2. 10.1038/s41575-021-00452-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:73. 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- 25.Järbrink-Sehgal E, Andreasson A. The gut microbiota and mental health in adults. Curr Opin Neurobiol. (2020) 62:102–14. 10.1016/j.conb.2020.01.016 [DOI] [PubMed] [Google Scholar]
- 26.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. (2020) 113:2019–40. 10.1007/s10482-020-01474-7 [DOI] [PubMed] [Google Scholar]
- 27.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. (2018) 362:776–80. 10.1126/science.aau5812 [DOI] [PubMed] [Google Scholar]
- 28.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 29.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. (2019) 76:473–93. 10.1007/s00018-018-2943-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michels KB, Willett WC, Vaidya R, Zhang X, Giovannucci E. Yogurt consumption and colorectal cancer incidence and mortality in the nurses' health study and the health professionals follow-up study. Am J Clin Nutr. (2020) 112:1566–75. 10.1093/ajcn/nqaa244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk, and health: a systematic review. Nutr Rev. (2021) 79:599–614. 10.1093/nutrit/nuaa013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He A, Chin J, Lomiguen CM. Benefits of probiotic yogurt consumption on maternal health and pregnancy outcomes: a systematic review. Cureus. (2020) 12:e9408. 10.7759/cureus.9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. (2017) 32:269–87. 10.1007/s10654-017-0243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez MA, Panahi S, Daniel N, Tremblay A, Marette A. Yogurt and cardiometabolic diseases: a critical review of potential mechanisms. Adv Nutr. (2017) 8:812–29. 10.3945/an.116.013946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drouin-Chartier JP, Brassard D, Tessier-Grenier M, Côté JA, Labonté M, Desroches S, et al. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. (2016) 7:1026–40. 10.3945/an.115.011403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiruvengadam M, Subramanian U, Venkidasamy B, Thirupathi P, Samynathan R, Shariati MA, et al. Emerging role of nutritional short-chain fatty acids (SCFAs) against cancer via modulation of hematopoiesis. Crit Rev Food Sci Nutr. (2021) 28:1–18. 10.1080/10408398.2021.1954874 [DOI] [PubMed] [Google Scholar]
- 37.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. (2003) 52:988–97. 10.1136/gut.52.7.988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de los Reyes-Gavilán CG, Fernández M, Hudson JA, Korpela R. Role of microorganisms present in dairy fermented products in health and disease. Biomed Res Int. (2015) 2015:204173. 10.1155/2015/204173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramchandran L, Shah NP. Proteolytic profiles and angiotensin-I converting enzyme and alpha-glucosidase inhibitory activities of selected lactic acid bacteria. J Food Sci. (2008) 73:M75–81. 10.1111/j.1750-3841.2007.00643.x [DOI] [PubMed] [Google Scholar]
- 40.Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr. (2004) 80:245–56. 10.1093/ajcn/80.2.245 [DOI] [PubMed] [Google Scholar]
- 41.Jin S, Je Y. Dairy consumption and risk of metabolic syndrome: results from korean population and meta-analysis. Nutrients. (2021) 13:1574. 10.3390/nu13051574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidayat K, Du X, Shi BM, Qin LQ. Systematic review and meta-analysis of the association between dairy consumption and the risk of hip fracture: critical interpretation of the currently available evidence. Osteoporos Int. (2020) 31:1411–25. 10.1007/s00198-020-05383-3 [DOI] [PubMed] [Google Scholar]
- 43.Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, et al. Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: a systematic review and meta-analysis. Adv Nutr. (2020) 11:834–63. 10.1093/advances/nmaa030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K, Chen X, Zhang L, Deng Z. Fermented dairy foods intake and risk of cardiovascular diseases: a meta-analysis of cohort studies. Crit Rev Food Sci Nutr. (2020) 60:1189–94. 10.1080/10408398.2018.1564019 [DOI] [PubMed] [Google Scholar]
- 45.Pala V, Sieri S, Berrino F, Vineis P, Sacerdote C, Palli D, et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int J Cancer. (2011) 129:2712–9. 10.1002/ijc.26193 [DOI] [PubMed] [Google Scholar]
- 46.Murphy N, Norat T, Ferrari P, Jenab M, Bueno-de-Mesquita B, Skeie G, et al. Consumption of dairy products and colorectal cancer in the European prospective investigation into cancer and nutrition (EPIC). PLoS ONE. (2013) 8:e72715. 10.1371/journal.pone.0072715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Kinany K, Mint Sidi Deoula M, Hatime Z, Boudouaya HA, Huybrechts I, El Asri A, et al. Consumption of modern and traditional Moroccan dairy products and colorectal cancer risk: a large case control study. Eur J Nutr. (2020) 59:953–963. 10.1007/s00394-019-01954-1 [DOI] [PubMed] [Google Scholar]
- 48.Negrichi S, Taleb S. Hereditary, environmental, and dietary risk factors of colorectal cancer: a case-control study in the Algerian East. Environ Sci Pollut Res Int. (2021) 28:12372–81. 10.1007/s11356-020-10378-y [DOI] [PubMed] [Google Scholar]
- 49.Sanz MJ, Llora TS, Purón MEC, Hernández DM, Navarro AG, Rojas VD. Influence of the diet on the development of colorectal cancer in a population of Madrid. Rev Clin Esp. (2004) 204:355–61. 10.1157/13063526 [DOI] [PubMed] [Google Scholar]
- 50.Barrubés L, Babio N, Mena-Sánchez G, Toledo E, Ramírez-Sabio JB, Estruch R, et al. Dairy product consumption and risk of colorectal cancer in an older mediterranean population at high cardiovascular risk. Int J Cancer. (2018) 143:1356–66. 10.1002/ijc.31540 [DOI] [PubMed] [Google Scholar]
- 51.Tayyem RF, Bawadi HA, Shehadah I, AbuMweis SS, Agraib LM, Al-Jaberi T, et al. Meats, milk and fat consumption in colorectal cancer. J Hum Nutr Diet. (2016) 29:746–56. 10.1111/jhn.12391 [DOI] [PubMed] [Google Scholar]
- 52.Zheng X, Wu K, Song M, Ogino S, Fuchs CS, Chan AT, et al. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. (2020) 69:970–2. 10.1136/gutjnl-2019-318374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kampman E, Goldbohm RA, van den Brandt PA, van 't Veer P. Fermented dairy products, calcium, and colorectal cancer in The Netherlands Cohort Study. Cancer Res. (1994) 54:3186–90. [PubMed] [Google Scholar]
- 54.Järvinen R, Knekt P, Hakulinen T, Aromaa A. Prospective study on milk products, calcium and cancers of the colon and rectum. Eur J Clin Nutr. (2001) 55:1000–7. 10.1038/sj.ejcn.1601260 [DOI] [PubMed] [Google Scholar]
- 55.Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutr Cancer. (2002) 43:39–46. 10.1207/s15327914nc431_4 [DOI] [PubMed] [Google Scholar]
- 56.Nilsson LM, Winkvist A, Esberg A, Jansson JH, Wennberg P, van Guelpen B, et al. Dairy products and cancer risk in a northern sweden population. Nutr Cancer. (2020) 72:409–20. 10.1080/01635581.2019.1637441 [DOI] [PubMed] [Google Scholar]
- 57.Kampman E, Giovannucci E, van't Veer P, Rimm E, Stampfer MJ, Colditz GA, et al. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am J Epidemiol. (1994) 139:16–29. 10.1093/oxfordjournals.aje.a116931 [DOI] [PubMed] [Google Scholar]
- 58.Boutron MC, Faivre J, Marteau P, Couillault C, Senesse P, Quipourt V. Calcium, phosphorus, vitamin D, dairy products and colorectal carcinogenesis: a French case–control study. Br J Cancer. (1996) 74:145–51. 10.1038/bjc.1996.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kearney J, Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Wing A, Kampman E, Willett WC. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. American journal of epidemiology. (1996) 143:907–17. 10.1093/oxfordjournals.aje.a008834 [DOI] [PubMed] [Google Scholar]
- 60.Kojima M, Wakai K, Tamakoshi K, Tokudome S, Toyoshima H, Watanabe Y, et al. Diet and colorectal cancer mortality: results from the japan collaborative cohort study. Nutr Cancer. (2004) 50:23–32. 10.1207/s15327914nc5001_4 [DOI] [PubMed] [Google Scholar]
- 61.Godos J, Tieri M, Ghelfi F, Titta L, Marventano S, Lafranconi A, et al. Dairy foods and health: an umbrella review of observational studies. Int J Food Sci Nutr. (2020) 71:138–51. 10.1080/09637486.2019.1625035 [DOI] [PubMed] [Google Scholar]
- 62.Zhang K, Dai H, Liang W, Zhang L, Deng Z. Fermented dairy foods intake and risk of cancer. Int J Cancer. (2019) 144:2099–108. 10.1002/ijc.31959 [DOI] [PubMed] [Google Scholar]
- 63.Barrubés L, Babio N, Becerra-Tomás N, Rosique-Esteban N, Salas-Salvadó J. Association between dairy product consumption and colorectal cancer risk in adults: a systematic review and meta-analysis of epidemiologic studies. Adv Nutr. (2019) 10(suppl_2):S190–s211. 10.1093/advances/nmy114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aune D, Lau R, Chan DSM, Vieira R, Greenwood DC, Kampman E, et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. (2012) 23:37–45. 10.1093/annonc/mdr269 [DOI] [PubMed] [Google Scholar]
- 65.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 67.Ba DM, Ssentongo P, Beelman RB, Muscat J, Gao X, Richie JP. Higher mushroom consumption is associated with lower risk of cancer: a systematic review and meta-analysis of observational studies. Adv Nutr. (2021) Mar 16. 10.1093/advances/nmab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simin J, Fornes R, Liu Q, Olsen RS, Callens S, Engstrand L, et al. Antibiotic use and risk of colorectal cancer: a systematic review and dose-response meta-analysis. Br J Cancer. (2020) 123:1825–32. 10.1038/s41416-020-01082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. 10.1093/oxfordjournals.epirev.a036298 [DOI] [PubMed] [Google Scholar]
- 70.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neuenschwander M, Barbaresko J, Pischke CR, Iser N, Beckhaus J, Schwingshackl L, et al. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective observational studies. PLoS Med. (2020) 17:e1003347. 10.1371/journal.pmed.1003347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L, Li M, Li H. Milk and yogurt intake and breast cancer risk: a meta-analysis. Medicine. (2019) 98:e14900. 10.1097/MD.0000000000014900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao X, Jia HY, Chen GC, Li CY, Hao M. Yogurt intake reduces all-cause and cardiovascular disease mortality: a meta-analysis of eight prospective cohort studies. Chin J Integr Med. (2020) 26:462–468. 10.1007/s11655-020-3085-8 [DOI] [PubMed] [Google Scholar]
- 74.Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y. Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J. (2016) 15:91. 10.1186/s12937-016-0210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu L, Sun D. Consumption of yogurt and the incident risk of cardiovascular disease: a meta-analysis of nine cohort studies. Nutrients. (2017) 9(3). 10.3390/nu9030315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eales J, Lenoir-Wijnkoop I, King S, Wood H, Kok FJ, Shamir R, et al. Is consuming yoghurt associated with weight management outcomes? results from a systematic review. Int J Obes (Lond). (2016) 40:731–46. 10.1038/ijo.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. (2014) 99(5 Suppl):1229s–34s. 10.3945/ajcn.113.073031 [DOI] [PubMed] [Google Scholar]
- 78.Panahi S, Tremblay A. The potential role of yogurt in weight management and prevention of type 2 diabetes. J Am Coll Nutr. (2016) 35:717–31. 10.1080/07315724.2015.1102103 [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE. (2013) 8:e53916. 10.1371/journal.pone.0053916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. (2016) 14:21. 10.1186/s12967-016-0772-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soltani G, Poursheikhani A, Yassi M, Hayatbakhsh A, Kerachian M, Kerachian MA. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr Disord. (2019) 19:113. 10.1186/s12902-019-0444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee MS, Wahlqvist ML, Peng CJ. Dairy foods and health in Asians: Taiwanese considerations. Asia Pac J Clin Nutr. (2015) 24:S14–20. 10.6133/apjcn.2015.24.s1.03 [DOI] [PubMed] [Google Scholar]
- 83.Perdigón G, de Moreno de LeBlanc A, Valdez J, Rachid M. Role of yoghurt in the prevention of colon cancer. Eur J Clin Nutr. (2002) 56:S65–8. 10.1038/sj.ejcn.1601490 [DOI] [PubMed] [Google Scholar]
- 84.Gobbato N, Rachid M, Perdigón G. Anti-inflammatory effect of yoghurt in an experimental inflammatory bowel disease in mouse. J Dairy Res. (2008) 75:497–504. 10.1017/S0022029908003579 [DOI] [PubMed] [Google Scholar]
- 85.Lo PR, Yu RC, Chou CC, Huang EC. Determinations of the antimutagenic activities of several probiotic bifidobacteria under acidic and bile conditions against benzo[a]pyrene by a modified Ames test. Int J Food Microbiol. (2004) 93:249–57. 10.1016/j.ijfoodmicro.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 86.Wollowski I, Ji ST, Bakalinsky AT, Neudecker C, Pool-Zobel BL. Bacteria used for the production of yogurt inactivate carcinogens and prevent DNA damage in the colon of rats. J Nutr. (1999) 129:77–82. 10.1093/jn/129.1.77 [DOI] [PubMed] [Google Scholar]
- 87.Liu L, Tabung FK, Zhang X, Nowak JA, Qian ZR, Hamada T, et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain fusobacterium nucleatum. Clin Gastroenterol Hepatol. (2018) 16:1622–1631.e3. 10.1016/j.cgh.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fisberg M, Machado R. History of yogurt and current patterns of consumption. Nutr Rev. (2015) 73:4–7. 10.1093/nutrit/nuv020 [DOI] [PubMed] [Google Scholar]
- 89.Gómez-Gallego C, Gueimonde M, Salminen S. The role of yogurt in food-based dietary guidelines. Nutr Rev. (2018) 76:29–39. 10.1093/nutrit/nuy059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.