Summary
The elevation of glycolysis in autoreactive T cells is a key target for the prevention and treatment of T cell-related autoimmune diseases, such as type 1 diabetes (T1D). Here, we describe a simple and efficient protocol for isolating human peripheral blood mononuclear cells (PBMCs) and T cells, and the subsequent assessment of T cell glycolysis using Seahorse analyzer. This protocol is useful to analyze different subsets of T cells and applicable to different autoimmune disease models (i.e., T1D, multiple sclerosis).
For complete details on the use and execution of this profile, please refer to Kong et al. (2021).
Subject areas: Classification Description: Cell Biology, Cell isolation, Cell-based Assays, Immunology, Metabolism
Graphical abstract
Highlights
-
•
Human T cell metabolism can be easily and quickly measured
-
•
Basal and activated T cell metabolism measurement using Seahorse analyzer
-
•
Various types of human T cell subset can be assessed in response to drugs of interest
-
•
T cells from different types of autoimmune disease model can be assessed
The elevation of glycolysis in autoreactive T cells is a key target for the prevention and treatment of T cell-related autoimmune diseases, such as type 1 diabetes (T1D). Here, we describe a simple and efficient protocol for isolating human peripheral blood mononuclear cells (PBMCs) and T cells, and the subsequent assessment of T cell glycolysis using Seahorse analyzer. This protocol is useful to analyze different subsets of T cells and applicable to different autoimmune disease models (i.e., T1D, multiple sclerosis).
Before you begin
The main steps of the protocol include human T cell isolation and real-time metabolic flux assessment of therapeutic drugs in real-time T cell activation. This protocol requires both 37°C/CO2 and 37°C/non-CO2 incubator throughout the protocol. Work fast, keep cells cold, and use pre-cooled solutions. The entire protocol should be performed within the same day. Users need to have an access of Seahorse XFe24 equipment to carry out this experiment. Also, note that this protocol requires institutional permission for recruitment of human patients and healthy controls. For this protocol, one healthy control was enrolled from the Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University Hospital (IRB no. 1808-151-967).
Peripheral blood mononuclear cell (PBMC) isolation
Timing: 3 h
Below we provide a detailed protocol on isolating PBMC using Ficoll-PaqueTM (ρ=1.077 g/mL) and density gradient centrifugation. This protocol is optimized for PBMCs from human blood only. For mouse blood, it is recommended to use either Ficoll-paqueTM or OptiPrepTM ((ρ=1.320 g/mL) (Liu et al., 1996; Mendez-David et al., 2013). It is important to note that the maximum blood volume obtained from different species (i.e., mouse (1–1.5 mL from 30 g/mice), rat (7–10 mL from 400 g/rat), rabbit (30–300 mL from 1 kg/rabbit)) vary highly (Wolfensohn & Lloyd, Handbook of Laboratory Animal Management and Welfare, Third Edition, 2003). Thus, the blood dilution with PBS-EDTA, volume of Ficoll-paqueTM, and centrifugation time may vary accordingly (Liu et al., 1996).
Note: This protocol was performed using freshly isolated cells from human donors. Cryopreservation could affect T cell viability and function depending on thawing time and temperature (Buhl et al., 2012; Farrant et al., 1973; Germann et al., 2013; Heo et al., 2009; Knight et al., 1972) that cryopreserved T cells should be compared for T cell function before assessing them with freshly isolated cells.
-
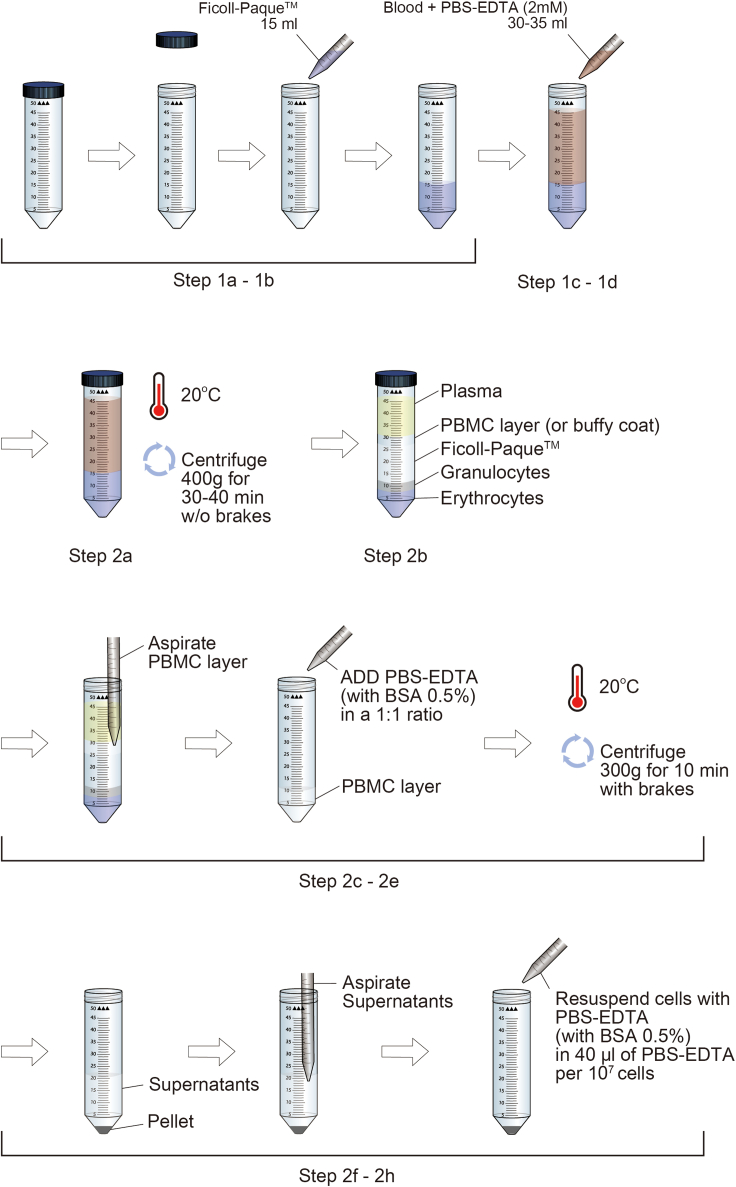
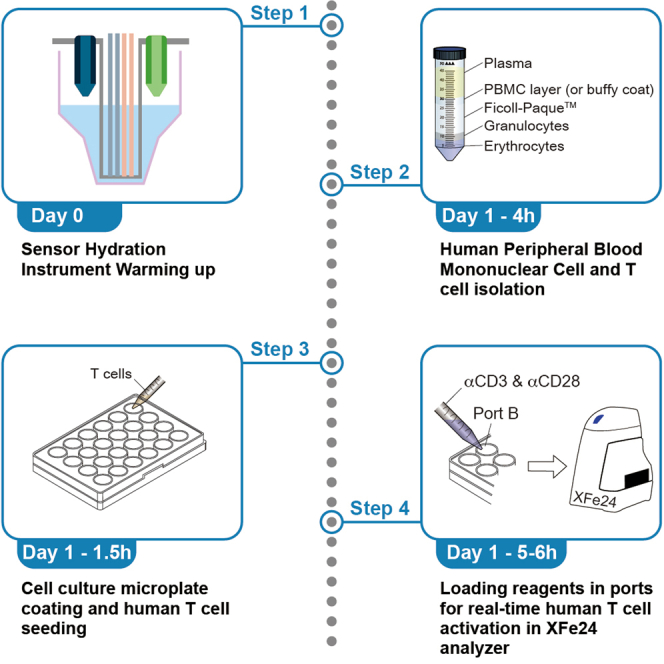
1.Preparation before density gradient separation (Figure 1).
-
a.Collect 30–40 mL of blood in heparinized tubes from both healthy and type 1 diabetes donors according to institutionally-approved protocols.Note: Samples from no more than 8 subjects/conditions, including healthy controls and patients, can be carried out at once for Seahorse XFe24 users.Note: Other types of anti-coagulant (i.e., EDTA, citrate) coated tubes can be considered instead of heparinized tubes. Previous reports suggest different type of anti-coagulant may show different immune cell proliferation, frequency, and cytokine production (Cedrone et al., 2017; Diks et al., 2019; Duvigneau et al., 2007). Previous work on EDTA has shown slightly purer mononuclear cell population compared to heparin (Boyum, 1976). Thus, we recommend using the same type of tube throughout the experiment rather than using several types of tubes.
-
b.Load 15 mL of Ficoll-PaqueTM (ρ = 1.077 g/mL) in a 50 mL conical tube.
-
c.Mix blood samples with PBS-EDTA (2 mM, pH 7.2) buffer in a 1:1 to 1:3 ratio.Note: The more diluted the blood sample, the better the purity of the mononuclear cells.Note: EDTA can be replaced by other supplements such as anti-coagulant citrate dextrose formula-A (ACD-A) or citrate phosphate dextrose (CPD). Anti-coagulant is needed in the PBS buffer to keep blood samples from clotting.
-
d.Pour 30–35 mL of the blood and PBS-EDTA (2 mM) buffer mixture from step 1c over the Ficoll-paqueTM from step 1b.
-
a.
Note: This step should be done in a very gentle and slow manner by slightly slanting the tube or by rotating the tube while pouring the mixture on the tube wall.
-
2.Density gradient separation by centrifugation (Figure 1).
-
a.Centrifuge the mixture from step 1d at 400 g for 30–40 min at 20°C without applying brakes.Note: Higher g Force centrifugation (800 g) will require shorter time (20–30 min) (https://www.miltenyibiotec.com/_Resources/Persistent/6d7e0fb8a3eab613958d142051e3c7050eb0c8a9/SP_MC_PB_density_gradient.pdf, https://www.sigmaaldrich.com/KR/ko/technical-documents/protocol/clinical-testing-and-diagnostics-manufacturing/hematology/recommended-standard-method). Lower g Force centrifugation (400 g) will require longer time (30–40 min) (https://www.stemcell.com/isolating-mononuclear-cells-from-whole-blood-by-density-gradient-centrifugation.html).
-
b.After centrifugation, the PBMC layer (also known as buffy coat) would appear as a thin white layer between the yellow plasma and white Ficoll-paque layer.
-
c.Aspirate the buffy coat into a new 50 mL conical tube.Note: Avoid the red portion of the layer when aspirating the buffy coat.
-
d.Add PBS-EDTA (2 mM, with BSA 0.5%) buffer in a 1:1 ratio.Note: PBMCs may be stored in the refrigerator overnight in PBS containing 0.5% BSA or autologous serum.
-
e.Centrifuge at 300 g for 10 min at 20°C with brakes applied.
-
f.Confirm the pellet after centrifuging and discard the supernatant.
-
g.Resuspend the PBMC pellet in PBS-EDTA buffer (with BSA 0.5%).
-
i.To count cells and check cell viability, stain cells with trypan blue and perform analysis. Use 1:1 ratio for trypan blue versus cell suspension to count cells.
-
i.
-
h.Resuspend the PBMC in 40 μL of PBS-EDTA (with BSA 0.5%) per 107 cells.
-
a.
Figure 1.
Isolation of peripheral blood mononuclear cells (PBMCs) using density gradient centrifugation
This protocol is optimized for the preparation of PBMCs from human blood using Ficoll-paqueTM. This figure is to show detailed steps for “before you begin”
T cell isolation
Timing: 1 h
-
3.T cell labeling with magnetic microbeadsNote: For human CD4+ T cell separation, please refer to the manufacturer’s instructions: https://www.miltenyibiotec.com/upload/assets/IM0001983.PDF.For human CD8+ T cell separation, please refer to the manufacturer’s instructions: https://www.miltenyibiotec.com/upload/assets/IM0001984.PDF). These two protocols were used for the following protocols for T cell labeling with magnetic microbeads.
-
a.Add 10 μL of either CD4+ or CD8+ T cell Biotin-antibody cocktail per 107 total cells to PBMC.
-
b.Mix well and incubate for 5 min at 2°C–8°C.
-
c.Add 30 μL of PBS-EDTA (with BSA 0.5%) buffer per 107 total cells.
-
d.Add 20 μL of either CD4+ or CD8+ T cell Microbead cocktail per 107 total cells.Note: For purification, cells incubated with CD4+ T cell Biotin-antibody cocktail needs to be mixed with CD4+ microbead cocktail. The same procedure is applied for CD8+ T cells. When both types are required for analysis, PBMC need to be split before staining cells with Biotin-antibody cocktail at step 3a.
-
e.Mix well and incubate for 10 min at 2°C–8°C.
-
f.Proceed to subsequent magnetic cell separation.
-
a.
-
4.Magnetic cell separation
-
a.Place LS Columns in the magnetic field of MiniMACSTM, MidiMACSTM, or QuadroMACSTM.Note: Depending on a patient, 30–40 mL blood samples from a patient will give you 2–5 × 107 PBMCs. Thus, MiniMACSTM, MidiMACSTM, or QuadroMACSTM will be suitable for magnetic cell separation. Less than 10% will be CD4+ T cells (2–5 × 106 cells).
-
b.Put 0.5 mL of PBS-EDTA (2 mM) with BSA (0.5%) in the column. Wait until the buffer completely passes through the column.
-
c.Apply cell suspension onto the column.
-
d.Collect the unlabeled cells that pass through, representing CD4+ or CD8+ T cells.
-
e.Wash the column with 3 mL of PBS-EDTA (2 mM) with BSA (0.5%) buffer. Collect unlabeled cells that pass through, representing the enriched CD4+ or CD8+ T cells, and combine with the effluent from step d.
-
a.
Note: To have higher purity of T cells, we recommend performing the step. 4, two times.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Purified NA/LE mouse Anti-Human CD3, 4 μg/mL | BD Biosciences | Cat#555329 |
| Purified NA/LE mouse Anti-Human CD28, 20 μg/mL | BD Biosciences | Cat#555725 |
| Chemicals, peptides, and recombinant proteins | ||
| Cell-TakTM | Corning | Cat#354240 |
| l-Glutamine | Agilent | Cat#103579-100 |
| NaOH solution 0.1N | Merck | Cat#1310-73-2 |
| EDTA 0.5M, pH 8.0 | Invitrogen | Cat#15575020 |
| BSA | Merck Millipore | Cat#820451 |
| Poly-d-lysine | Sigma-Aldrich | P7405 |
| DPBS | Welgene | LB 201-02 |
| Biological samples | ||
| Human, Age: 32, Sex: Male | Seoul National University Hospital | IRB no. 1808-151-967 |
| Critical commercial assays | ||
| CD8+ T cell isolation kit, human | Miltenyi Biotec | Cat#130-096-495 |
| CD4+ T cell isolation kit, human | Miltenyi Biotec | Cat#130-096-533 |
| LS columns | Miltenyi Biotec | Cat#130-042-401 |
| MiniMACSTM starting kit | Miltenyi Biotec | Cat#130-090-312 |
| Seahorse XFe24 FluxPaks (microplate/cartridge) | Agilent | Cat#102340-100 |
| Seahorse XF RPMI Medium, pH 7.4 | Agilent | Cat#103576-100 |
| Ficoll-paqueTM | Cytiva | Cat#17-1440-02 |
| Heparinized tube | BD | Cat#367874 |
| Software and algorithms | ||
| Wave | Agilent | https://www.agilent.com/ko-kr/product/cell-analysis/real-time-cell-metabolic-analysis/xf-software/seahorse-wave-desktop-software-740897 |
| Prism v8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Seahorse XFe24 equipment | Agilent | 102238 |
Materials and equipment
XF running medium
| Reagent | Final concentration | Amount |
|---|---|---|
| l-glutamine | 1 mM (50 μL per 10 mL XF media) | 250 μL |
| XF medium (w/o phenol-red, pH 7.4) | 49.75 mL | |
| Total | n/a | 50 mL |
Pass through sterile filter and keep warm in a water bath, medium should be freshly prepared for every new experiment.
Coating solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Poly-d-lysine | 50 μg/mL | 50 μL/well |
| Cell-TakTM | 1 μg/cm2 | 50 μL/well |
Note: Poly-d-lysine (2°C–8°C, 2 years) and Cell TakTM (4°C, 3 months) can be used as an alternative. 50 μL of either poly-d-lysine (50 μg/mL in DPBS, 1 h, room temperature (RT; 15°C–30°C)) or Cell TakTM (5.74 μL dissolved in 0.5 mL of 0.1 M sodium carbonate, pH 8.0 for 15–20 min, RT) is needed for a well. Use either coating solution to coat wells before cell seeding.
Testing compounds
| Reagent | Final concentration | Amount |
|---|---|---|
| Drug of interest | 8× concentration | Depends on the drug |
| Purified NA/LE mouse Anti-Human CD3 | 4 μg/mL | 2.5 μL |
| Purified NA/LE mouse Anti-Human CD28 | 20 ug/mL | 12.5 μL |
Drug of interest is separately dissolved in XF running medium. CD3 and CD28 antibodies are mixed in a separate XF running medium.
CRITICAL: Note that glucose and sodium pyruvate are not added in this protocol.
Step-by-step method details
Real-time human T cell activation analysis using metabolic flux analyzer
Timing: 6–8 h
Below we provide a detailed step-by-step protocol about the real-time human T cell activation by using Seahorse XFe24 (Figures 2, 3, 4, and 5).
Note: Glycolysis and mitochondrial respiration of T cells (i.e., murine splenocytes, CD4+ T cells, CD8+ T cells, or Jurkat cells) can be measured by using commercially available kits (OXPHOS: https://www.agilent.com/ko-kr/product/cell-analysis/real-time-cell-metabolic-analysis/xf-assay-kits-reagents-cell-assay-media/seahorse-xf-cell-mito-stress-test-kit-740885; Glycolysis: https://www.agilent.com/ko-kr/product/cell-analysis/real-time-cell-metabolic-analysis/xf-assay-kits-reagents-cell-assay-media/seahorse-xf-glycolysis-stress-test-kit-740886) and by following previous protocols (Gotoh et al., 2021; Gu et al., 2021). However, this protocol does not require the purchase of these commercial kits.
-
1.Start sensor hydration and instrument warm up.Note: Sensor hydration has to be prepared a day before the experiment. Instrument warm up has to start minimum 4 h before the experiment.
-
a.This preparation starts a day before the experiment.
-
b.In the clean bench, take out the ‘Sensor Cartridge (green) & Utility plate (pink)’ from Seahorse XFe24 Fluxpaks.
-
c.Add autoclaved ddH2O 1 mL/well in Utility plate.
-
d.Place the Sensor Cartridge over the Utility plate and incubate this in non-CO2 incubator (37°C), 24 h.
-
e.Pour 25 mL of ‘XF Calibrant (pH 7.4)’ in conical tube and incubate 24 h in non-CO2 incubator (37°C).
-
f.Turn on XFe24 analyzer minimum 4 h before the experiment.
-
g.1 h before the experiment, take out both ‘XF Calibrant’ and ‘Sensor Cartridge & Utility plate.’
-
h.Remove all ddH2O in Utility plate.
-
i.Add XF Calibrant 1 mL/well in Utility plate.
-
j.Incubate the ‘Sensor Cartridge & Utility plate’ with ‘XF Calibrant’ in non-CO2 incubator.
-
a.
Note: If the experiment starts in the morning, turn of XFe24 analyzer a day prior to the experiment.
-
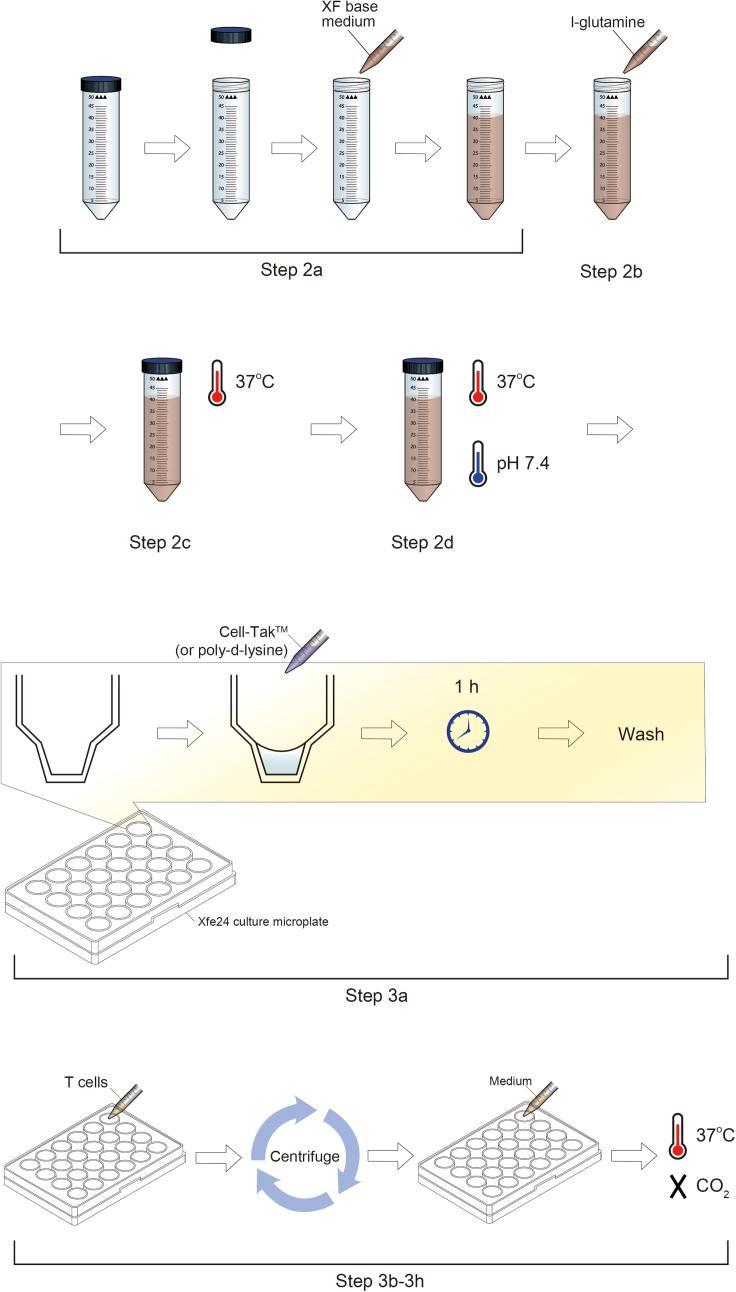
2.Prepare XF running medium (Figure 2).
-
a.Adjust pH level of pre-warmed XF RPMI media with L-glutamine to pH level of 7.4 using NaOH 0.1 N.Note: For adjustment of pH level to 7.4, approximately 80–100 μL of NaOH 0.1N needs to be added for 40 mL XF RPMI medium. From this step, all XF RPMI media used in the experiment should contain L-glutamine.
-
b.Keep the medium warm at 37°C in a water bath.
-
a.
CRITICAL: It is critical to keep the medium at 37°C, because pH level fluctuates depending on the temperature of the XF RPMI medium. For non-phenol XF RPMI medium with pH 7.4, this step is not required. Since ECAR equation involves the conversion of pH changes Fluctuation of pH level affects the ECAR level.
-
3.Seed cells in XF cell culture microplate (Figure 2)
-
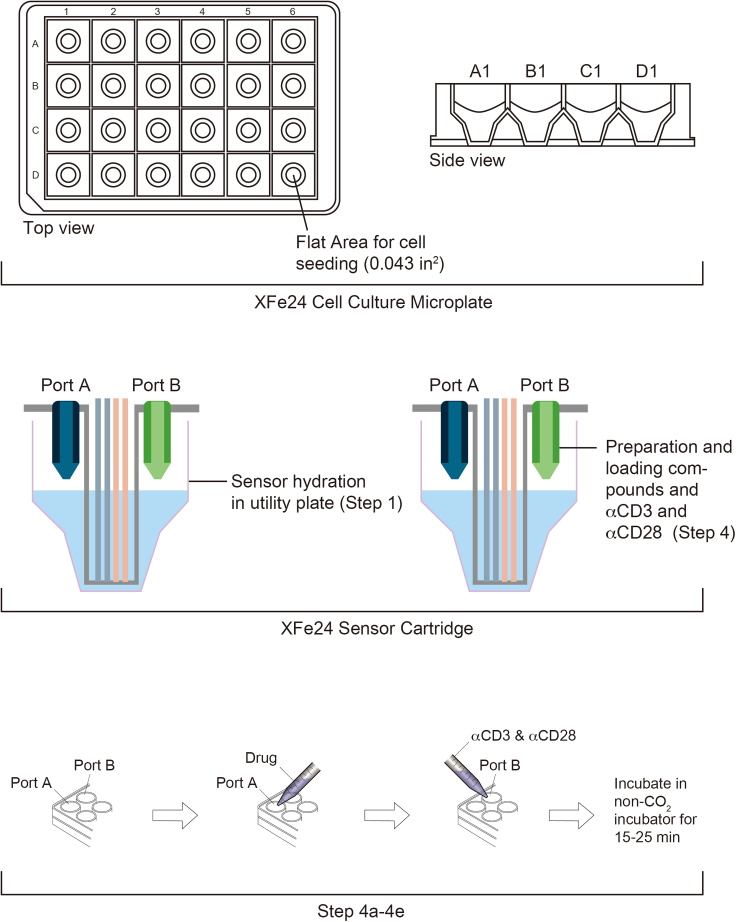
a.Coat the XF cell culture microplate, which is included in Seahorse XFe24 Fluxpaks: Use either coating solution to coat wells before cell seeding.Note: Suspension cells (i.e., T cells) require this step. Strongly adherent immune cells (i.e., macrophages) do not require this step if cells were cultured and incubated 1 hour before the experiment. Less adherent cell types may take 5–6 h for adhesion prior to the experiment.Note: Poly-d-lysine and Cell TakTM can be used as an alternative. 50 μL of either poly-d-lysine (50 μg/mL in DPBS, 1 h, RT) or Cell TakTM (5.74 μL dissolved in 0.5 mL of 0.1 M sodium carbonate, pH 8.0 for 15–20 min, RT) is needed for a well. Tilt and stir the plate slightly so that the coating solution can be dispersed evenly in the well.Note: The wells of XF cell culture microplate are smaller in size compared to the conventional 24-well plate, as illustrated (Figure 3). The seeding surface of microplate is similar to that of a typical 96-well (0.275 cm2). Coated XF cell culture microplate can be stored for 1 week in 4°C.
 CRITICAL: All wells are needed to be coated with either poly-d-lysine or Cell TakTM. Do not seed cells on A1, B4, C3, D6 wells (for blank/background correction wells in XFe24; blank/background correction wells are preset settings in Wave program, but they can be assigned manually in Wave program, which is used for all types of Seahorse analyzers (i.e., XFe24)). Blank/Background correction wells are used to filter out artifacts, such as temperature and buffering capacity, that can affect oxygen and pH that are not due to changes in metabolism (https://www.agilent.com/en/support/cell-analysis/purpose-of-background-wells). Thus, 4 wells needed to be used for blank/background correction, and only 20 wells can be used for samples.
CRITICAL: All wells are needed to be coated with either poly-d-lysine or Cell TakTM. Do not seed cells on A1, B4, C3, D6 wells (for blank/background correction wells in XFe24; blank/background correction wells are preset settings in Wave program, but they can be assigned manually in Wave program, which is used for all types of Seahorse analyzers (i.e., XFe24)). Blank/Background correction wells are used to filter out artifacts, such as temperature and buffering capacity, that can affect oxygen and pH that are not due to changes in metabolism (https://www.agilent.com/en/support/cell-analysis/purpose-of-background-wells). Thus, 4 wells needed to be used for blank/background correction, and only 20 wells can be used for samples. -
b.Aspirate off the coating media and wash the wells with autoclaved ddH2O twice and let wells to air dry.
-
c.Adjust the concentrations to 1.0 × 106 cells/mL of T cells with XF running media containing L-glutamine.Note: All samples needed to be seeded in duplicates or triplicates. Also, optimal cell seeding numbers vary widely, though are typically between 10,000–80,000 cells per well (http://www.agilent.com/cell-reference-database/).
-
d.Add 100 μL of cells into the bottom of the wells (=1 × 105 cells/well for XFe24).Note: This is due to the structure of the XF cell culture microplate as illustrated in (Figure 2). The plate’s well is 2-layered that adding cells in one step will seed cells in an upper level of the well, which the sensor cannot detect and measure.
-
e.Centrifuge 5 min at 400 g to allow the cells to settle down as a monolayer at the bottom of the plate.
-
f.Slowly add 450 μL of XF media to each well making sure not to disrupt the monolayer. The total volume of each well will be 550 μL for XF24.
-
g.Observe the cells under a microscope to determine that all groups have a monolayer of cells without any that have dislodged.
-
h.Incubate the plate at 37°C in a non-CO2 incubator for 30–60 min.
-
a.
Note: The incubation is necessary for de-gassing the plate, allowing for CO2 diffusion from the cells, medium, and plate (https://www.agilent.com/en/support/cell-analysis/why-non-co2-incubation). The contributions of CO2 to total extracellular acidification have been considered negligible in the measurement platform, XFe24 analyzer (Mookerjee and Brand, 2015; Wu et al., 2007).
-
4.Prepare compounds to assess real-time T cell activation (Figure 3)
-
a.Prepare 8× compound, which will be tested as a drug of interest (i.e., MOTS-c), diluted with XF running medium to be loaded into Drug Port A of sensor cartridge as below:
-
i.The final concentration is calculated based on the combined final volume [(drug of interest (8×) mixed with XF medium). Thus, 8× compound is added into Drug Port A.Note: To assess the effects of a drug (i.e., MOTS-c), you will need to prepare 8× concentration of a drug (i.e., 80 μM of MOTS-c). 75 μL of 8× drug is loaded into the drug port of sensor cartridge. The drug loaded in the drug port of sensor cartridge will be automatically injected from the drug port of sensor cartridge into the 550 μL of cell culture microplate during the assessment. The final concentration will be 1× concentration of drug after the injection (i.e., 10 μM of MOTS-c).
-
i.
-
b.Prepare αCD3 and αCD28 in 4 μg/mL and 20 μg/mL.
-
i.The final concentration is calculated based on the combined final volume [(drug in the port (75 μL) mixed with XF medium). Thus, 4 μg/mL (αCD3) and 20 μg/mL (αCD28) is mixed and added into Drug Port B.Note: Until this stage, the experiment will take approximately 50–60 min.
-
i.
-
c.Take out Sensor Cartridge + Utility plate, which was incubated for 1 h, from non-Co2 incubator from Step 1.
-
d.Add prepared drug of interest to Port A and mixtures of αCD3 and αCD28 to Port B of Sensor Cartridge + Utility plate.
-
e.Incubate Sensor Cartridge + Utility plate with drugs loaded in the ports in non-Co2 incubator for 15–25 min.
-
a.
-
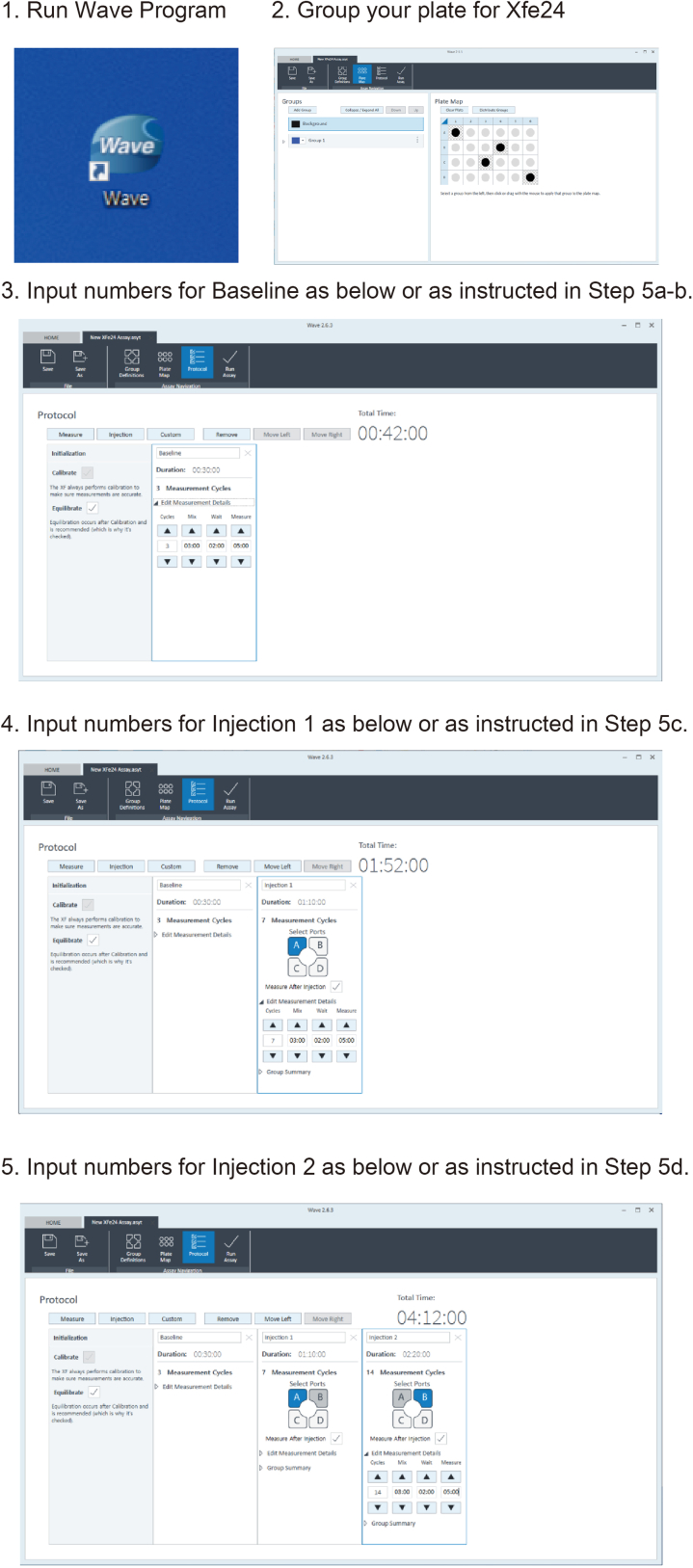
5.Set up Seahorse program (XFe24) for real-time human T cell activation (Figure 4)
-
a.Initialization:12 min equilibrate
-
b.Basal Measurement: 2–3 min mixture; 5 min measurement (3 times)
-
c.Injection 1 (Drug of interest): 2–3 min wait; 5 min measurement (7 times)
-
d.Injection 2 (αCD3&αCD28): 2–3 min wait; 5 min measurement (14 times)
-
a.
Note: The αCD3/αCD28 will be injected from the port to the cell after step c. and will be measured for 49–56 min. Then, after the injection of drug at step d, 98–112 min will be measured. With the interval time in between the injection, the total measurement period is around 3–4 h.
Figure 2.
Preparation of XF running medium and XF cell culture microplate for human T cells
This figure is to show detailed steps for “step-by-step method details”. pH and temperature of XF running medium is important factor in the experiment. Also, coating cell culture microplate for human T cells is essential to allow cells to be positioned at the right spot for the measurement by sensor cartridge.
Figure 3.
Loading ports in the microplate cartridge for drug and soluble αCD3 and αCD28 injections
This figure is to show detailed steps for “step-by-step method details”. The schematic diagram of XFe24 cell culture microplate shows that the wells are double-layered in the top view. The side view of XFe24 cell culture microplate also shows that cells and medium should be added separately to avoid cells seeding on the top-level. The XFe24 Sensor Cartridge diagram has to be hydrated in utility plate before use. Also, the compounds and soluble αCD3 and αCD28 are loaded in this component as shown in steps 8 and 9.
Figure 4.
Diagram showing the Wave program set up for real-time T cell activation assessment
This figure is to show detailed steps for “step-by-step method details”. Designing the program for the experiment is shown here. The program set-up can be done before the experiment and the template can be saved for future analyses.
Figure 5.
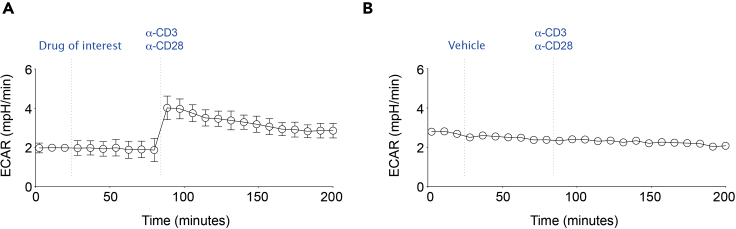
Successful (left) and failed (right) outcome after performing metabolic flux analysis in human T cells
(A) The successful outcome shows that the ECAR level of human T cells after the treatment of soluble αCD3 and αCD28 is increased from 2 to 4 mpH/min. Data are presented as mean ± standard error of mean (SEM)
(B) However, the failed outcome shows that the treatment of soluble αCD3 and αCD28 did not increase the ECAR level of human T cells. Data are presented as mean ± standard error of mean (SEM)
Expected outcomes
The overall goal of this experiment is to find out whether a drug of interest can lower the T cell activation, measured by ECAR, induced by αCD3/αCD28. For example, αCD3/αCD28 increased ECAR level from the basal level of 2 mpH/min to 4 mpH/min. However, ECAR level of MOTS-c treated T cells had subtle changes of ECAR after αCD3/αCD28 in our previous work (Kong et al., 2021). MOTS-c treatment can cause a variety of metabolic changes in different cell types Ahn et al., 2020, Lee et al., 2015.
The ECAR (mpH/min) during the initialization/basal measurement step will be constant. The activation of CD4+ or CD8+ T cells by αCD3/αCD28 will increase ECAR instantly (Figure 5A). Failure to increase ECAR is indicative of unsuccessful T cell activation (Figure 5B).
Background or blank measurements are automatically measured and calculated by the program that additional subtraction of values are not required. Furthermore, after the assay is done, you are able to transfer the data in excel or prism files with graphs that no further editing is needed.
Limitations
In this experiment, we have utilized soluble αCD3 and αCD28. One of the main restrictions of real-time stimulation of T cells is that the responsiveness to antibodies differs depending on the subset of T cells. It is reported that CD4+ T cells show higher responsiveness to beads, whereas soluble αCD3 are more effective in CD8+ T cells (Li and Kurlander, 2010).
Subsets of double-positive and double-negative T cells for CD4 and CD8 have been identified in autoimmune and chronic inflammatory diseases (Overgaard et al., 2017; Parel et al., 2007). Even though the function of these cells remains controversial, these subset populations may lower the purity of T cell isolation.
The drugs can be tested one at a time in this protocol design. Thus, to test multiple drugs at once, you have to use multiple plates for number of drugs you are testing. This will require greater number of T cells, meaning higher volume of blood from patients and healthy controls.
Other immune cells (sorted or unsorted) can be analyzed and measured for activation. However, it will require optimization for different cell types as they react differently to the same stimuli (i.e., αCD3/αCD28).
Acquiring stable cell number from patients or healthy controls is critical factor in this experiment. Thus, it is crucial to know how much cells can be yielded from a density gradient separation by centrifugation. 40 mL of blood will provide 2–5 × 107 cells of PBMC in healthy controls. Patients with immunoregulatory or immunosuppressive drugs will provide lesser amount of PBMC to start. Thus, higher volume of blood might be needed to acquire the same amount of PBMC as healthy controls.
Troubleshooting
Problem 1
Viability of PBMCs and purified T cells (steps 1–4 in “before you begin”).
Potential solution
Since gradient separation of blood and magnetic bead isolation can lead to cell loss and death, we recommend using 30–40 mL of blood at the start to obtain a high number of cells.
Problem 2
Insufficient activation of CD4+ or CD8+ T cells (step 4).
Potential solution
We recommend adding αCD3 and αCD28 antibodies immediately prior to Seahorse measurements. αCD3 and αCD28 antibodies should be aliquoted and stored at 4°C in appropriate volumes. Long-term storage can reduce the efficacy of αCD3 and αCD28 antibodies. Also, we recommend trying CD3 and CD28 microbeads when soluble CD3 and CD28 antibodies are insufficient for T cell activation. Other T cell activation protocols can be utilized, but needs optimization and confirmation.
Problem 3
If the measurements are low on seahorse (step 5).
Potential solution
Basal measurement, which is measured after the initialization, is measurements without any stimuli. Thus, these measurements have to stay steady. If these values are lower or higher than usual, two factors should be considered, which are temperature and pH levels. These two factors influence greatly to the ECAR level of T cells.
Problem 4
If the response after the drug injection is poor during seahorse measurements (step 5).
Potential solution
Drugs are prepared in different types of solvents. Since drugs loaded in the drug ports are injected through a channel that is less than 6 mm in diameter, crystallization of drugs can stop drugs from injection. We recommend testing serial dilution of drugs and the optimum solubility under XF RPMI medium.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, [Young Min Cho] ymchomd@snu.ac.kr
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea (2017R1A2B4007166) to Y.M.C., and NIH grants (R01AG052258 and R01GM136837) to C.L.
Author contributions
B.S.K., C.L., and Y.M.C. conceptualized the experiments, interpreted data, drafted the manuscript, and prepared figures. All authors reviewed and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Byung Soo Kong, Email: byungsoo_kong@hotmail.com.
Changhan Lee, Email: changhan.lee@usc.edu.
Young Min Cho, Email: ymchomd@snu.ac.kr.
Data and code availability
Original/source data for figures in the paper is available in [https://doi.org/10.1016/j.celrep.2021.109447].
References
- Ahn C.H., Choi E.H., Kong B.S., Cho Y.M. Effects of MOTS-c on the mitochondrial function of cells harboring 3243 A to G mutant mitochondrial DNA. Molecular Biology Reports. 2020;47:4029–4035. doi: 10.1007/s11033-020-05429-z. [DOI] [PubMed] [Google Scholar]
- Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. 1976;Suppl 5:9–15. [PubMed] [Google Scholar]
- Buhl T., Legler T.J., Rosenberger A., Schardt A., Schon M.P., Haenssle H.A. Controlled-rate freezer cryopreservation of highly concentrated peripheral blood mononuclear cells results in higher cell yields and superior autologous T-cell stimulation for dendritic cell-based immunotherapy. Cancer Immunol. Immunother. 2012;61:2021–2031. doi: 10.1007/s00262-012-1262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedrone E., Neun B.W., Rodriguez J., Vermilya A., Clogston J.D., McNeil S.E., Barenholz Y., Szebeni J., Dobrovolskaia M.A. Anticoagulants influence the performance of in vitro assays intended for characterization of nanotechnology-based formulations. Molecules. 2017;23:12. doi: 10.3390/molecules23010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diks A.M., Bonroy C., Teodosio C., Groenland R.J., de Mooij B., de Maertelaere E., Neirynck J., Philippe J., Orfao A., van Dongen J.J.M., et al. Impact of blood storage and sample handling on quality of high dimensional flow cytometric data in multicenter clinical research. J. Immunol. Methods. 2019;475:112616. doi: 10.1016/j.jim.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Duvigneau J.C., Sipos W., Hartl R.T., Bayer M., Moldzio R., Stevenson L., Adair B., Gemeiner M. Heparin and EDTA as anticoagulant differentially affect cytokine mRNA level of cultured porcine blood cells. J. Immunol. Methods. 2007;324:38–47. doi: 10.1016/j.jim.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Farrant J., Knight S.C., O'Brien J.A., Morris G.J. Selection of leukaemic cell populations by freezing and thawing. Nature. 1973;245:322–323. doi: 10.1038/245322a0. [DOI] [PubMed] [Google Scholar]
- Germann A., Oh Y.J., Schmidt T., Schon U., Zimmermann H., von Briesen H. Temperature fluctuations during deep temperature cryopreservation reduce PBMC recovery, viability and T-cell function. Cryobiology. 2013;67:193–200. doi: 10.1016/j.cryobiol.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Gotoh K., Takata Y., Nakashima Y., Mizuguchi S., Komori K., Kang D. Metabolic analysis of mouse bone-marrow-derived dendritic cells using an extracellular flux analyzer. STAR Protoc. 2021;2:100401. doi: 10.1016/j.xpro.2021.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Ma Y., Liu Y., Wan Q. Measurement of mitochondrial respiration in adherent cells by seahorse XF96 cell mito stress test. STAR Protoc. 2021;2:100245. doi: 10.1016/j.xpro.2020.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo Y.J., Son C.H., Chung J.S., Park Y.S., Son J.H. The cryopreservation of high concentrated PBMC for dendritic cell (DC)-based cancer immunotherapy. Cryobiology. 2009;58:203–209. doi: 10.1016/j.cryobiol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Knight S.C., Farrant J., Morris G.J. Separation of populations of human lymphocytes by freezing and thawing. Nat. New Biol. 1972;239:88–89. doi: 10.1038/newbio239088a0. [DOI] [PubMed] [Google Scholar]
- Kong B.S., Min S.H., Lee C., Cho Y.M. Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep. 2021;36:109447. doi: 10.1016/j.celrep.2021.109447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Zeng J., Drew B.G., Sallam T., Martin-Montalvo A., Wan J., Kim S.-J., Mehta H., Hevener A.L., de Cabo R., Cohen P. The Mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metabolism. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kurlander R.J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl Med. 2010;8:104. doi: 10.1186/1479-5876-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.C., Hoyt D.B., Coimbra R., Junger W.G. Proliferation assays with human, rabbit, rat, and mouse lymphocytes. In Vitro Cell Dev. Biol. Anim. 1996;32:520–523. doi: 10.1007/BF02722976. [DOI] [PubMed] [Google Scholar]
- Mendez-David I., El-Ali Z., Hen R., Falissard B., Corruble E., Gardier A.M., Kerdine-Romer S., David D.J. A method for biomarker measurements in peripheral blood mononuclear cells isolated from anxious and depressed mice: beta-arrestin 1 protein levels in depression and treatment. Front Pharmacol. 2013;4:124. doi: 10.3389/fphar.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee S.A., Brand M.D. Measurement and analysis of extracellular acid production to determine glycolytic rate. J. Vis. Exp. 2015:e53464. doi: 10.3791/53464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard N.H., Cruz J.L., Bridge J.A., Nel H.J., Frazer I.H., La Gruta N.L., Blumenthal A., Steptoe R.J., Wells J.W. CD4(+)CD8beta(+) double-positive T cells in skin-draining lymph nodes respond to inflammatory signals from the skin. J. Leukoc. Biol. 2017;102:837–844. doi: 10.1189/jlb.1AB0217-065R. [DOI] [PubMed] [Google Scholar]
- Parel Y., Aurrand-Lions M., Scheja A., Dayer J.M., Roosnek E., Chizzolini C. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum. 2007;56:3459–3467. doi: 10.1002/art.22927. [DOI] [PubMed] [Google Scholar]
- Wu M., Neilson A., Swift A.L., Moran R., Tamagnine J., Parslow D., Armistead S., Lemire K., Orrell J., Teich J., et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original/source data for figures in the paper is available in [https://doi.org/10.1016/j.celrep.2021.109447].