Abstract
Background:
Neurofilament light chain (NfL) protein is a blood-based marker of neuroaxonal injury. We sought to 1) compare plasma NfL levels in children with chronic kidney disease (CKD) and healthy peers, 2) characterize the relationship between NfL level and kidney function, and 3) evaluate NfL as a predictor of abnormal brain structure in CKD.
Methods:
Sixteen children with CKD due to congenital kidney anomalies and 23 typically developing peers were included. Plasma NfL was quantified using single-molecule array immunoassay. Participants underwent structural magnetic resonance imaging. Multiple linear regression models were used to evaluate the association between plasma NfL levels, kidney function, and brain structure.
Results:
An age × group interaction was identified whereby NfL levels increased with age in the CKD group only (estimate = 0.65; CI = 0.08–1.22; p = 0.026). Decreased kidney function was associated with higher NfL levels (estimate = −0.10; CI = −0.16 to −0.04; p = 0.003). Lower cerebellar gray matter volume predicted increased plasma NfL levels (estimate = −0.00024; CI = −0.00039–0.00009; p = 0.004) within the CKD group.
Conclusion:
Children with CKD show accelerated age-related increase in NfL levels. NfL level is associated with lower kidney function and abnormal brain structure in CKD.
INTRODUCTION
Pediatric chronic kidney disease (CKD) is most commonly due to congenital anomalies of the kidney and urinary tract (CAKUT). Children with CKD due to CAKUT are at risk of lifelong disease burden. Neurocognitive difficulties specific to attention regulation, academic underachievement, and executive functions are common even among children with mild/moderate CKD (i.e., pre-transplant/pre-dialysis).1–3 Although profound cognitive impairment is rare, the presence of even subtle cognitive concerns, particularly in the domain of executive functioning, is known to pose barriers to learning and social functioning that affects the overall quality of life.1,4,5 We recently demonstrated that cognitive difficulties in pediatric CKD are accompanied by volumetric abnormalities within the regional cerebellar gray matter.6 These findings point to potential neurobiological underpinnings of neurocognitive deficits in CKD.
Neurofilament light chain (NfL) protein is a component of the neuronal cytoskeleton that provides structural support to axons.7 Axonal damage and/or neuronal degeneration results in the release of NfL into the cerebrospinal fluid (CSF) and blood. NfL has emerged as a sensitive marker for central nervous system (CNS) injury in adults with neurological diseases.8,9 NfL can be reliably detected in the blood,8 and this makes it a promising and easily available biomarker for evaluating brain pathology early in the course of the disease. Higher NfL levels have also been associated with decreased gray matter volume in adults with underlying neurological disease.10 In context of these previous findings, as well as our own observation of abnormal cerebellar and cerebral gray matter volumes in patients with CKD,6 we tried to 1) identify whether plasma NfL levels are elevated in children with mild/moderate, non-dialysis-dependent CKD; 2) characterize the relationship between plasma NfL levels and kidney function; and 3) examine the utility of NfL as a predictor of abnormal brain structure in children with mild/moderate CKD.
METHODS
Study Sample
Children aged 6–16 years who were diagnosed with CKD due to CAKUT were recruited for a single study visit between 2015 and 2018. Children with CKD may have normal or decreased kidney function in the presence of abnormal kidney structure. Thus, the inclusion criteria for children with CKD were 1) a clinical diagnosis of CKD due to CAKUT, and 2) the presence of mild/moderate CKD as quantified using the estimated glomerular filtration rate (eGFR) (between 30 and 90 ml/min/1.73 m2) within 6 months of scheduled study participation.
The exclusion criteria for children with CKD were 1) current dialysis or a history of dialysis in past 6 weeks or more, 2) a history of kidney transplant, 3) premature birth (<32 weeks), 4) a history of seizures requiring antiepileptic medication, 5) known anatomical CNS malformations, 6) chromosomal anomalies, 7) a history of congenital cardiac disease, 8) diagnosis of intellectual disability, and 9) a history of traumatic brain injury.
Healthy age-matched controls, without any developmental or neurological diagnoses and with presumed normal kidney function, were recruited from local areas through electronic advertisements during the same timeframe. The exclusion criteria for the control group were otherwise as per the exclusion criteria for the CKD group.
Patient Consent
This study was approved by the University of Iowa Institutional Review Board. All participants and parents/caregivers were informed that the study results would not be disclosed. All the participants and parents/caregivers provided their written consent/assent on the day of study participation.
Brain Structure
The participants underwent non-sedated, non-contrast-enhanced structural magnetic resonance imaging (sMRI) of the brain after scanner safety screening. Participants who were assessed before June 2016 completed sMRI on the Siemens TrioTim® 3T scanner (N = 6). sMRI scans obtained after June 2016 were acquired using the GE Discovery ® 750 W 3T scanner (N = 33). Both machines were equipped with a 32-channel head coil. Anatomical T1-weighted images were acquired using the following parameters for the GE machine (parameters for the Siemens machine are provided in parentheses): coronal brain volume imaging (magnetization-prepared rapid gradient-echo), repetition time (TR) = 8.392 (2300) ms; echo time (TE) = 3.184 (2.82) ms; inversion time (TI) = 450 (900) ms; flip angle = 12 (10)°; field of view (FOV) = 282 × 282 × 264 mm; and matrix = 256 × 256 × 240. The T2-weighted images were acquired using the following parameters: coronal, TR = 3000 (4800) ms; TE = 85.925 (430) ms; FOV = 256 × 256 × 224 mm; and matrix = 256 × 256 × 160. A prospective motion correction sequence was used to prospectively correct any motion artifacts.11 After the scan, all images were processed and visually inspected by a trained neuroimaging specialist. Scans that contained too many artifacts to produce reliable data (N = 4 & 2 scans in the CKD and control groups, respectively) were flagged and removed before the analysis.
The sMR images were processed using the BRAINSAutoWorkup pipeline, which optimizes tissue classification through an iterative framework, produces robust parcellation of the brain regions, and works in a multi-scanner setting.12 BRAINSAutoWorkup labels the brain regions using a multi-atlas, similarity-weighted, majority-vote procedure (joint label fusion),13 using a set of expert-segmented templates adapted from the Desikan-Killiany atlas.14 Brain regions included the cortical and subcortical regions, separated by the hemispheres and tissue type (gray or white matter), where appropriate. The BRAINSAutoWorkup pipeline automatically derives the volume of different brain structures. Inter-scanner variation was harmonized using the empirical Bayesian approach (ComBat harmonization) as implemented by the ez.combat toolbox in RStudio.15–17 We confirmed that the scanner did not predict the region of interest.6 All subsequent analyses were performed using the harmonized data.
Neurofilament Light Chain
Plasma and serum samples can be utilized for NfL analysis, and the measured plasma NfL levels show excellent correlation to those obtained from CSF samples.18 Furthermore, NfL levels remain stable between freeze and thaw cycles.19–21 For this study, whole blood samples were centrifuged, and plasma was stored at −80 °C. The plasma samples were analyzed using the single-molecule array (Simoa®) immunoassay at Quanterix Laboratories. Previously published research has evaluated and described the sensitivity of the Simoa® immunoassay for NfL quantification.22 Samples were analyzed in duplicate according to the kit protocols.23 The average concentration of duplicates for each sample was computed, and the sample was then diluted to achieve the dilution-corrected concentration. Calibration curves and controls for dilution-corrected concentration values were reviewed for acceptance. Any sample with CV greater than 20% underwent repeat measurement. Any sample that had a retained CV greater than 20% was discarded from analysis (N = 1). The dilution-corrected concentration (pg/mL) for retained samples was utilized in data analysis.
Assessment of Kidney Function
Blood samples were collected on the day of study participation to determine the eGFR (ml/min/1.73 m2) in participants from both the groups. Data were also extracted from the medical records of the patients with CKD to collect information on serum bicarbonate level (mEq/L), hemoglobin level (g/dL), and proteinuria within 6 months prior to assessment.
Statistical Analysis
CKD is more common in males. In the current study, only two female participants with CKD were recruited. Sex-based developmental differences in the brain are difficult to account for in a small- to moderate-sized sample that includes both male and female participants.24–26 Thus, only data from the male participants were analyzed in this study.
We used the Akaike Information Criterion (AIC) to select the statistical models, wherein we considered the key predictor variables of interest (i.e., group [aim 1], kidney function [aim 2], and brain region of interest [aim 3]) in addition to the variables such as participants’ age and age × predictor variable interactions.
We first compared the plasma NfL levels in patients with CKD and healthy controls. Linear regression analysis was conducted using the dilution-corrected NfL levels as the dependent variable. The predictor variable of interest was group (CKD and control).
Thereafter, we examined the association between serum NfL level and kidney function in the CKD group alone. Separate linear regression models were applied within the CKD group using NfL level as the dependent variable with eGFR (ml/min/1.73 m2), proteinuria (urine protein/creatinine ratio [mg/mg]), serum bicarbonate level (mEq/L), systolic and diastolic blood pressures (mmHg), and hemoglobin level (g/dL) as independent (predictor) variables.
Lastly, we evaluated the association between serum NfL levels and regional brain volumes. We used a hypothesis-driven approach that limits the analysis to regions of interest previously identified as being different among children with CKD, specifically the cerebral and cerebellar gray matter. These brain regions of interest were included as the predictor variables along with age.
Statistical significance tests were two-sided, with p < 0.05 set as the threshold. Analyses were performed using R version 3.6.3.
Data Availability
Anonymized data are not available in a public repository, however, will be shared upon request from any qualified investigator.
RESULTS
Sample
The final sample included 23 healthy males (control) and 16 male patients with CKD. All children within the final sample had complete data for analysis. Case and control participants were matched on chronological age. The demographic data of the participants are summarized in Table 1. The medical variables commonly associated with disease burden in CKD are shown in Table 2. Among the 16 patients with CKD, 6 (33%) were prescribed antihypertensive agents, 2 (11%) were receiving growth hormones, and 1 (5.5%) required alkalinizing agent therapy because of a history of metabolic acidosis. None of the participants were on erythropoietin-stimulating agents or diuretics at the time of assessment. The healthy controls did not receive any of these medications. Given that all the patients with CKD had disease due to CAKUT, the duration of the disease was considered equivalent to the patients’ chronological age.
Table 1.
Demographic characteristics of the patients with pediatric chronic kidney disease (CKD) and the controls.
| Controls (N=23) | CKD (N=16) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 11.9 (3.03) | 12.0 (3.29) |
| Median [Min, Max] | 11.8 [6.33, 16.9] | 12.8 [6.25, 16.6] |
| Race | ||
| Multiracial | 1 (4.3%) | 1 (6.2%) |
| White | 22 (95.7%) | 13 (81.2%) |
| Asian American | 0 (0%) | 1 (6.2%) |
| Black or African American | 0 (0%) | 1 (6.2%) |
| Ethnicity | ||
| Hispanic/Latino | 1 (4.3%) | 3 (18.8%) |
| Not Hispanic/Latino | 22 (95.7%) | 13 (81.2%) |
| BMI Percentile | ||
| Mean (SD) | 53.4 (29.6) | 69.4 (27.8) |
| Median [Min, Max] | 63.7 [7.10, 96.9] | 85.4 [27.4, 99.3] |
| Weight (kg) | ||
| Mean (SD) | 45.0 (15.0) | 52.1 (20.7) |
| Median [Min, Max] | 42.1 [23.2, 81.6] | 53.9 [17.0, 84.2] |
| Height (cm) | ||
| Mean (SD) | 154 (18.4) | 153 (24.3) |
| Median [Min, Max] | 153 [126, 184] | 156 [108, 182] |
| Parental Socioeconomic Status | ||
| Professional Degree | 3 (13.0%) | 1 (6.2%) |
| College or Advanced Degree | 14 (60.9%) | 7 (43.8%) |
| White Collar/Semi-skilled Worker, High School Education | 5 (21.7%) | 8 (50.0%) |
| Semi-skilled Worker/laborer Below Secondary Education | 1 (4.3%) | 0 (0%) |
| Maternal Education (years) | ||
| Mean (SD) | 16.4 (2.31) | 15.0 (2.25) |
| Median [Min, Max] | 16.0 [12.0, 20.0] | 14.5 [12.0, 20.0] |
P values were derived from linear models with group as the predictor variable, except for socioeconomic status, for which a chi-square test was performed.
Table 2.
Clinical characteristics of the patients with chronic kidney disease (CKD).
| CKD (N = 16) | |
|---|---|
| eGFR (ml/min/1.73 m2) | |
| Mean (SD) | 75.0 (27.6) |
| Median [Min, Max] | 75.7 [22.7, 116] |
| Serum Bicarbonate (mEq/L) | |
| Mean (SD) | 24.7 (2.33) |
| Median [Min, Max] | 25.0 [20.0, 31.0] |
| Venous pH | |
| Mean (SD) | 7.34 (0.0301) |
| Median [Min, Max] | 7.34 [7.29, 7.40] |
| Missing | 4 (25.0%) |
| Urine Protein/Creatinine Ratio (mg/mg) | |
| Mean (SD) | 0.399 (0.775) |
| Median [Min, Max] | 0.135 [0.0400, 3.16] |
| Hemoglobin (g/dL) | |
| Mean (SD) | 13.6 (1.43) |
| Median [Min, Max] | 14.1 [11.1, 15.8] |
| Systolic Blood Pressure (mmHg) | |
| Mean (SD) | 114 (11.1) |
| Median [Min, Max] | 118 [96.0, 138] |
| Diastolic Blood Pressure (mmHg) | |
| Mean (SD) | 62.4 (7.71) |
| Median [Min, Max] | 62.0 [47.0, 80.0] |
eGFR: estimated glomerular filtration rate
Age-Related Changes in Intergroup Plasma NfL Levels
The model including the group × age interaction had the lowest AIC (eTable 1). The group × age interaction predicted the NfL levels (estimate = 0.65; CI = 0.08 to 1.22; p = 0.026), whereby the NfL levels remained relatively stable across age in the controls but increased with age in the CKD group (Figure 1).
Figure 1.
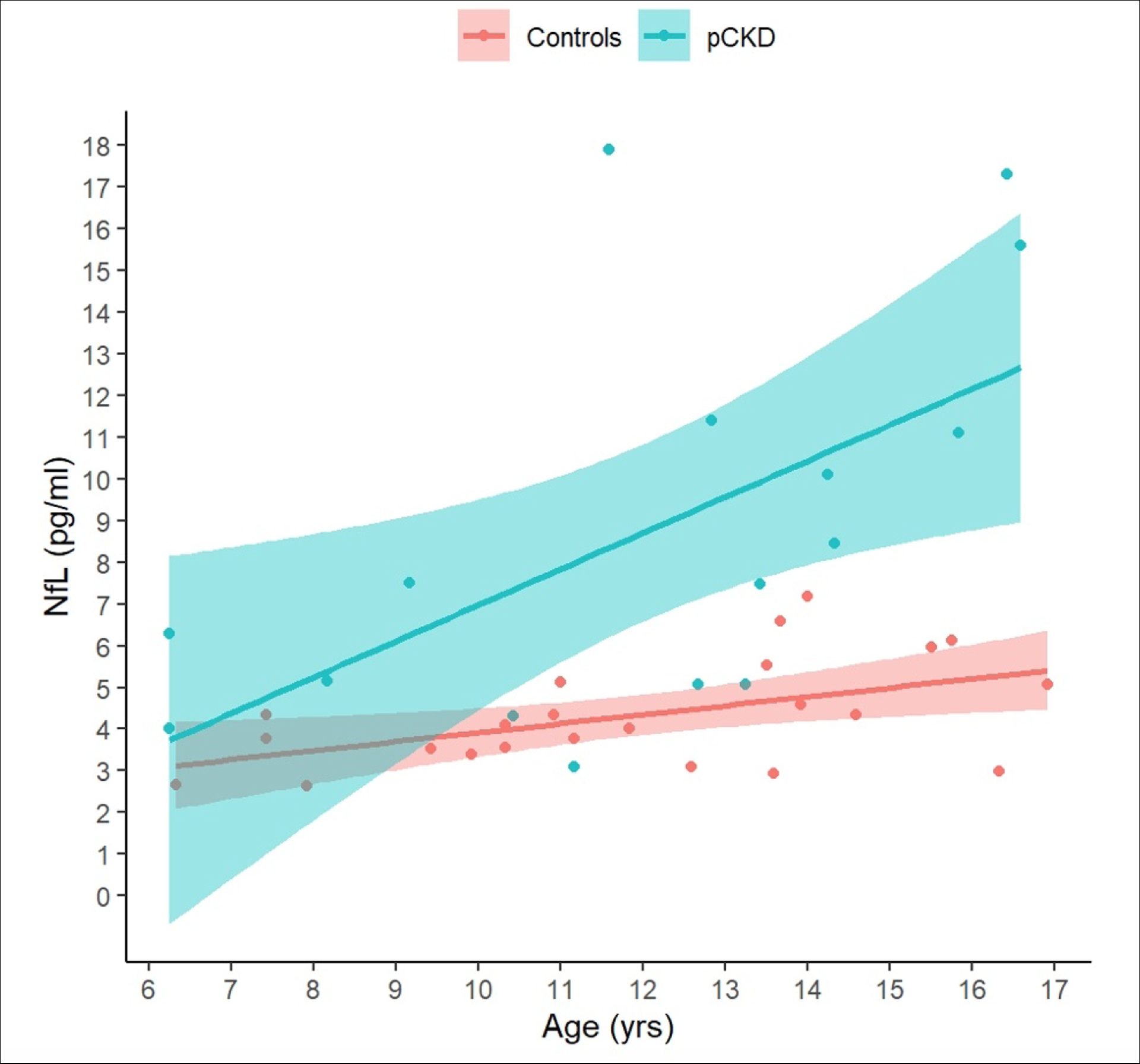
Age-related changes in neurofilament light chain (NfL) protein levels in chronic kidney disease (CKD) and control groups.
NfL levels increase with age in the CKD group, whereas they remain stable in the control group. The x-axis shows age in years and the y-axis shows NfL levels. Regression lines with 95% confidence limits for the controls (pink) and children with CKD (blue) are superimposed on individual observations (colored circles).
Association between NfL Levels and CKD Clinical Variables
The AIC identified two models wherein the inclusion of kidney function variables (eGFR and bicarbonate level) resulted in a better model than the one including age alone (eTables 2 and 3). Decreased kidney function (eGFR) predicted NfL levels in the CKD group (estimate = −0.10; SD = 0.027; t(13) = −3.65; p = 0.003; Figure 2A). Serum bicarbonate level was also inversely associated with the NfL level, but the results were not statistically significant (estimate = −0.83; SD = 0.40; p = 0.056). None of the remaining CKD-associated variables predicted the NfL levels in the CKD group.
Figure 2.
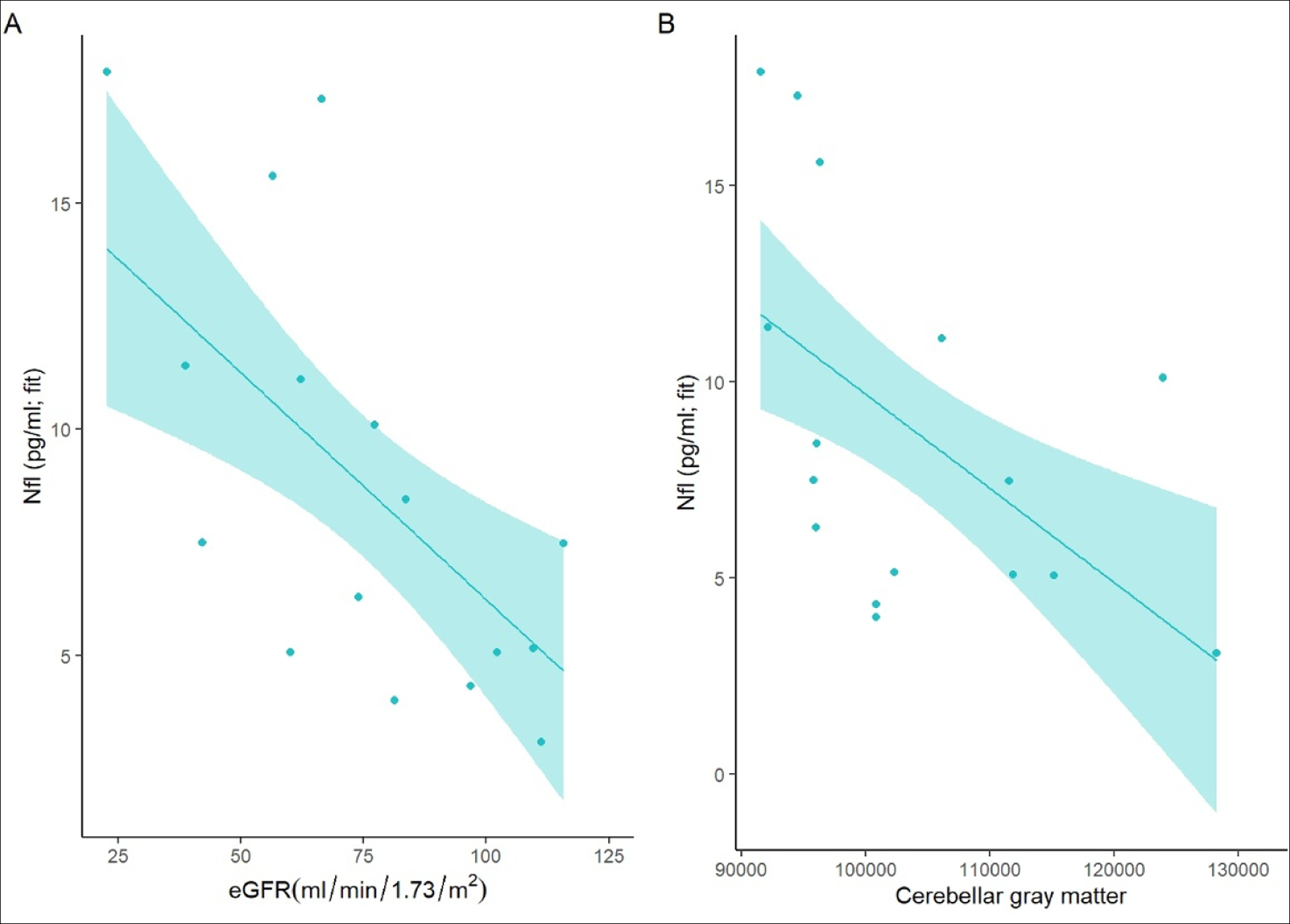
Association between neurofilament light chain (NfL) protein levels and disease burden (A) and cerebellar gray matter volume (B).
Panel A shows that lower kidney function, as determined using the estimated glomerular filtration rate (eGFR; x-axis), is associated with higher NfL levels (y-axis) in children with chronic kidney disease (CKD). Panel 2 shows the relationship between the cerebellar gray matter volume (x-axis) and NfL levels (y-axis). The regression lines represent the model fits and the circles represent unadjusted observations.
Association with the Brain Structure
The model including age and cerebellar gray matter as the main effects was selected (eTable 4). Lower cerebellar gray matter volume predicted the NfL levels (estimate = −0.0002401; SD = 0.00007; p = 0.004; Figure 2B). Cerebral gray matter was not associated with NfL levels in patients with CKD (eTable 5).
DISCUSSION
Brain development in a typically developing child involves an intricate process of dendritic pruning within the gray matter. We have previously described decreased cerebellar gray matter volumes in a well-characterized cohort of children with non-dialysis-dependent CKD.6 Elevation of the neuroaxonal injury marker NfL has also been described in relation to both gray and white matter volume loss within the brain.9,10,19 In this study, we provide evidence of accelerated age-related increase in NfL levels in children with CKD than in healthy controls. We also demonstrated that increased NfL levels are associated with relevant variables of kidney function and brain structure. These results suggest that decreased kidney function may be linked to abnormal neuronal integrity in patients with pediatric CKD.
Although NfL is often considered a marker of neurodegenerative disease, Barro et al.27 noted that increased NfL levels are not specific to neurodegenerative diseases. Rather, more common factors such as age, cardiovascular disease, and increased body mass index can result in increased blood NfL levels. Thus, elevated NfL levels in young individuals may primarily reflect the underlying disease pathology rather than aging-related processes. However, in older individuals, age becomes a notable contributor to the elevated NfL levels in addition to the presence of any underlying neurodevelopmental aberration or neurological disease.27 NfL can be released additionally from the peripheral nervous system. Comorbidities like diabetes that are common among individuals with adult-onset CKD, may result in neuropathy. As described earlier, these comorbidities are less common in patients with pediatric CDK due to congenital causes. Further, the demonstrated association between NfL and cerebellar gray matter underscores the potential role of CNS pathology presumably associated NfL levels in this sample.
Adult-onset acquired kidney disease is typically caused by hypertension and diabetes mellitus, whereas pediatric CKD is largely due to congenital causes with disease onset and progression coinciding with crucial stages of brain development.28 The relationship between NfL levels and kidney function in adult-onset acquired kidney disease is under debate, with some evidence suggesting that NfL levels are related to aging and cardiovascular disease but not to decreased kidney function.27 Contrary to this, two studies29,30 demonstrated that among adults with kidney disease due to diabetes mellitus, decreased eGFR was associated with higher serum NfL levels. Notably, diabetes mellitus is associated with an increased risk of cerebrovascular disease; thus, it is difficult to determine whether the potential association between kidney function and NfL levels in adult samples is reflective of diminished kidney function or small-vessel cerebrovascular disease as a secondary effect of diabetes. In contrast, children with CKD in our sample did not have a history of hypertension or other cerebrovascular risk factors, making it less likely that our results were affected by cardiovascular comorbidities. More work is needed to better understand how blood NfL level is impacted by CKD due to congenital anomalies of the kidney and urinary tract.
Compared to controls, children with CKD showed a disproportionate increase in blood NfL levels with increasing age. Given the congenital nature of CKD in children included in the present study, age can arguably be considered a proxy for disease duration. Although kidney disease in this population is often considered to be caused by “isolated congenital defects” of the kidney and urinary tract, several genetic drivers of the kidney disease are also linked to neurodevelopmental abnormalities.31 For example, prenatal cerebellar development is linked to homeobox genes such as PAX-2 and fibroblast growth factor 8.32–34 These genes also play critical roles in early kidney development and are associated with congenital kidney anomalies, namely, disorders of nephron development, renal agenesis, and vesicoureteral reflux.35–40 Considering this, we postulate a “diathesis-stress” model, wherein the presence of CKD (i.e., diathesis) renders the cerebellum particularly vulnerable to systemic sequalae (i.e., stress) associated with advancing CKD.
Despite these strengths, our study has some limitations. Pediatric CKD is a rare childhood disease, and this makes it difficult to recruit a large sample at a single center. Furthermore, CKD is often considered a silent disease with prolonged asymptomatic progression. Therefore, possibility exists that we might have included controls with undiagnosed mild CKD. Selection bias is a particular risk in case-control studies whereby cases (or controls) are included/excluded due to a particular characteristic related to the factor under evaluation. We have attempted to mitigate the risk of selection bias by utilizing the stringent inclusion/exclusion criteria at the study outset and recruiting controls from the broad community to provide a representative view of the typically developing child. Lastly, we used a hypothesis-driven approach to evaluate the association between serum NfL levels and brain structure. This approach may be subjected to confirmation bias. We feel that our approach is justified within the context of our previous findings and limitations of the sample size; however, future studies should aim to evaluate the entire brain in a larger sample of children with CKD to confirm our findings.
Our data, nonetheless, lend support to biomarker-based neurobiological differences that correspond with brain abnormalities and decreased kidney function in children with CKD. The findings of this study underscore that our understanding of CKD progression and multisystem sequelae, such as neurodevelopment, may be complicated by the developmental origins of CKD itself. These results should serve as catalysts for replication in a larger, longitudinal study including a wider age range of patients with CKD to identify changes in NfL levels even earlier in the disease process. Future studies should also seek to further evaluate the relationship between modifiable CKD-associated medical variables, such as the control of metabolic acidosis and NfL levels. Additionally, more research is warranted to understand whether elevated NfL levels are present among children with acquired causes of CKD, such as lupus nephritis, and whether the elevation in NfL levels abate after transplantation. The chronic and lifelong nature of CKD underscores the importance of expanding our understanding of the kidney-brain relationship in this disease.
Supplementary Material
Impact:
Neurofilament light chain (NfL) is a component of the neuronal cytoskeleton providing structural axonal support. Elevated NfL has been described in relation to gray and white matter brain volume loss. We have previously described abnormal cerebellar gray matter in chronic kidney disease (CKD).
We explored the relationship between NfL, chronic kidney disease, and brain volume
There is an accelerated, age-related increase in NfL level in CKD. Within the CKD sample, NfL level is associated with abnormal kidney function and brain structure. Decreased kidney function may be linked to abnormal neuronal integrity in pediatric CKD.
Funding Source:
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK110443 - Harshman).
Footnotes
Disclosure statement: There are no financial relationships to disclose or conflict of interest for the authorship team.
Informed Consent: Informed consent and/or assent, as applicable, was obtained from participants and legal guardians at the time of participation.
REFERENCES
- 1.Hooper SR et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN 6, 1824–1830, doi: 10.2215/CJN.09751110 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendley SR et al. Duration of chronic kidney disease reduces attention and executive function in pediatric patients. Kidney Int 87, 800–806, doi: 10.1038/ki.2014.323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harshman LA et al. Academic achievement in children with chronic kidney disease: a report from the CKiD cohort. Pediatr Nephrol, doi: 10.1007/s00467-018-4144-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harshman LA & Hooper SR The brain in pediatric chronic kidney disease-the intersection of cognition, neuroimaging, and clinical biomarkers. Pediatric nephrology 35, 2221–2229, doi: 10.1007/s00467-019-04417-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RJ et al. A longitudinal examination of parent-reported emotional-behavioral functioning of children with mild to moderate chronic kidney disease. Pediatric nephrology 35, 1287–1295, doi: 10.1007/s00467-020-04511-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon MA et al. Early pediatric chronic kidney disease is associated with brain volumetric gray matter abnormalities. Pediatr Res, doi: 10.1038/s41390-020-01203-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disanto G et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81, 857–870, doi: 10.1002/ana.24954 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne LM et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 16, 601–609, doi: 10.1016/S1474-4422(17)30124-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thebault S, Booth RA & Freedman MS Blood Neurofilament Light Chain: The Neurologist’s Troponin? Biomedicines 8, doi: 10.3390/biomedicines8110523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakimovski D et al. Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann Clin Transl Neurol 6, 1757–1770, doi: 10.1002/acn3.50872 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White N et al. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med 63, 91–105, doi: 10.1002/mrm.22176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierson R et al. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage 54, 328–336, doi: 10.1016/j.neuroimage.2010.06.047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H et al. Multi-Atlas Segmentation with Joint Label Fusion. IEEE Trans Pattern Anal Mach Intell 35, 611–623, doi: 10.1109/TPAMI.2012.143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desikan RS et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980, doi: 10.1016/j.neuroimage.2006.01.021 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Fortin JP et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 167, 104–120, doi: 10.1016/j.neuroimage.2017.11.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson WE, Li C & Rabinovic A Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127, doi: 10.1093/biostatistics/kxj037 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Koscik TR ez.combat, <www.github.com/TKosik/ez.combat> (2018).
- 18.Gray E et al. A multi-center study of neurofilament assay reliability and inter-laboratory variability. Amyotroph Lateral Scler Frontotemporal Degener 21, 452–458, doi: 10.1080/21678421.2020.1779300 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Gaiottino J et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 8, e75091, doi: 10.1371/journal.pone.0075091 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhle J et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler 19, 1597–1603, doi: 10.1177/1352458513482374 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Keshavan A, Heslegrave A, Zetterberg H & Schott JM Stability of blood-based biomarkers of Alzheimer’s disease over multiple freeze-thaw cycles. Alzheimers Dement (Amst) 10, 448–451, doi: 10.1016/j.dadm.2018.06.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhle J et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 54, 1655–1661, doi: 10.1515/cclm-2015-1195 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Simoa NF-Light Advantage Kit: Kit Instructions for HD-1 (Quanterix Corporation, Lexington, MA, 2018). [Google Scholar]
- 24.Lenroot RK et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073, doi: 10.1016/j.neuroimage.2007.03.053 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaan RA et al. Gender differences in white matter microstructure. PLoS One 7, e38272–e38272, doi: 10.1371/journal.pone.0038272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasung L, Fischi-Gomez E & Huppi PS Multimodality evaluation of the pediatric brain: DTI and its competitors. Pediatric Radiology 43, 60–68, doi: 10.1007/s00247-012-2515-y (2013). [DOI] [PubMed] [Google Scholar]
- 27.Barro C, Chitnis T & Weiner HL Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol 7, 2508–2523, doi: 10.1002/acn3.51234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown TT & Jernigan TL Brain development during the preschool years. Neuropsychol Rev 22, 313–333, doi: 10.1007/s11065-012-9214-1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akamine S et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep 10, 20350, doi: 10.1038/s41598-020-76990-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korley FK et al. Serum NfL (Neurofilament Light Chain) Levels and Incident Stroke in Adults With Diabetes Mellitus. Stroke 50, 1669–1675, doi: 10.1161/STROKEAHA.119.024941 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbitsky M et al. Genomic Disorders and Neurocognitive Impairment in Pediatric CKD. J Am Soc Nephrol 28, 2303–2309, doi: 10.1681/ASN.2016101108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namm A, Arend A & Aunapuu M Expression of Pax2 protein during the formation of the central nervous system in human embryos. Folia Morphol (Warsz) 73, 272–278, doi: 10.5603/FM.2014.0043 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, Harada H & Nakamura H Nuclear translocation of FGF8 and its implication to induce Sprouty2. Dev Growth Differ 54, 463–473, doi: 10.1111/j.1440-169X.2012.01332.x (2012). [DOI] [PubMed] [Google Scholar]
- 34.Wang VY & Zoghbi HY Genetic regulation of cerebellar development. Nat Rev Neurosci 2, 484–491, doi: 10.1038/35081558 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Dziarmaga A, Quinlan J & Goodyer P Renal hypoplasia: lessons from Pax2. Pediatr Nephrol 21, 26–31, doi: 10.1007/s00467-005-2039-x (2006). [DOI] [PubMed] [Google Scholar]
- 36.Martinovic-Bouriel J et al. PAX2 mutations in fetal renal hypodysplasia. Am J Med Genet A 152A, 830–835, doi: 10.1002/ajmg.a.33133 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y et al. The significance of Pax2 expression in the ureter epithelium of children with vesicoureteric reflux. Hum Pathol 46, 963–970, doi: 10.1016/j.humpath.2015.01.007 (2015). [DOI] [PubMed] [Google Scholar]
- 38.de Miranda DM et al. PAX2 polymorphisms and congenital abnormalities of the kidney and urinary tract in a Brazilian pediatric population: evidence for a role in vesicoureteral reflux. Mol Diagn Ther 18, 451–457, doi: 10.1007/s40291-014-0096-1 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Boualia SK et al. Vesicoureteral reflux and other urinary tract malformations in mice compound heterozygous for Pax2 and Emx2. PLoS One 6, e21529, doi: 10.1371/journal.pone.0021529 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates CM Role of fibroblast growth factor receptor signaling in kidney development. Am J Physiol Renal Physiol 301, F245–251, doi: 10.1152/ajprenal.00186.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are not available in a public repository, however, will be shared upon request from any qualified investigator.


