Abstract
Objectives
This study aims to assess craniofacial dimensions in obstructive sleep apnea (OSA) patients treated with a mandibular advancement device (MAD) and to identify anatomic influences on OSA severity and MAD therapy outcomes.
Materials and methods
Twenty patients with OSA were prospectively treated with MAD. Clinical, cone-beam computed tomography and polysomnography exams were performed before treatment and 4–6 months after achieving the MAD therapeutic position. Polysomnographic exams and three-dimensional maxillary, mandibular and upper airway (UA) measurements were evaluated. Pearson’s correlation and t-tests were applied.
Results
Before MAD treatment, the transverse width measured at the frontomaxillary suture and the angle between the mandibular ramus and Frankfurt horizontal were statistically correlated with apnea and the hypopnea index (AHI), while the gonial angle was correlated with therapeutic protrusion. After MAD treatment, all patients showed a significant AHI reduction and an improvement in minimum oxyhemoglobin saturation. The total UA volume, superior and inferior oropharynx volume and area were statistically correlated with MAD therapeutic protrusion. The UA total area showed a statistical correlation with the improvement in AHI, and the superior oropharynx volume and area increased significantly.
Conclusions
The transversal frontomaxillary suture width and the mandibular ramus facial angle may influence OSA severity. The gonial angle, volume and area of all UA regions may indicate the amount of protrusion needed for successful MAD treatment.
Clinical relevance
The craniofacial characteristics reported as important factors for OSA severity and MAD treatment outcomes impact therapy planning for OSA patients, considering individual anatomic characteristics, prognosis and cost benefits.
Keywords: Cone-Beam Computed Tomography (CBCT), Anatomy, Sleep apnea, Obstructive, Airway Management, Occlusal Splints, Mandibular Advancement Device
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of apnea and/or hypopnea due to upper airway collapse during sleep. It is considered the most common sleep breathing disorder; it is more prevalent in males in their sixth decade and affects a total of 1 billion individuals worldwide[1–4]. OSA’s clinical manifestations include sleep and neurocognitive symptoms such as respiratory pauses during sleep, recurrent awakenings, intense and intermittent snoring, nonrestorative sleep, excessive daytime sleepiness, irritability, depression and anxiety[2]. Moreover, recurrent respiratory pauses may lead to intermittent hypoxemia that increases the risk of developing cardiovascular diseases such as arrhythmia, heart or coronary insufficiency, and stroke. These systemic consequences highlight the importance of OSA’s precocious diagnosis and influencing factors[5–8].
The diagnosis of OSA, obtained by polysomnographic examination, is characterized by more than 5 obstructive events per hour of sleep (apnea and hypopnea index - AHI ≥ 5 events/hour). In addition, among all the polysomnographic parameters, the AHI and oxyhemoglobin saturation (SpO2) determine the intensity of OSA, which can be classified as mild (AHI = 5–15 events/hour and SpO2 = 86 – 91%), moderate (AHI = 15–30 events/hour and SpO2 = 76 – 85%) or severe (AHI ≥30 events/hour and SpO2 ≤ 75%)[9].
Multiple aspects, such as genetic, neuromuscular, and anatomic dysfunctions, may be involved in the pathophysiology of OSA[10]. Among the anatomic factors, it is possible to identify craniofacial variations, which include alterations in the vertical, transversal, anteroposterior, linear, angular dimensions of the craniofacial skeleton, as possible predisposing factors for upper airway (UA) collapse[11–15]. Craniofacial pattern and bone phenotype characterization may be considerable parameters for diagnostic guidance and multidisciplinary planning of OSA treatment. Among the possible therapies, it is possible to identify continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) as options [16].
Although CPAP is considered the gold standard for OSA treatment, studies have shown MAD as an alternative treatment for patients who are not responsive or not suitable candidates for CPAP treatment. The mechanism of action of a MAD is based on the extension/distension between the oropharynx and the base of the tongue by mandibular advancement, preventing UA collapse. Thus, mandibular characteristics may affect the amount of advancement ability and, consequently, therapeutic outcomes. This fact indicates anatomic variation again as an essential factor, not only anticipating OSA occurrence but also identifying differences in movement patterns and outcomes when using MAD as a therapy option[16–20]. These anatomic components involved in OSA may be analyzed by cone-beam computed tomography (CBCT), which is a useful tool for identifying craniofacial and upper airway three-dimensional configurations with great resolutions and precision[21]. In addition, all anatomic mechanisms involved in OSA pathogenesis, which may play an important role in the patency of UA and in MAD treatment, and successful outcomes have not been totally elucidated[19]. Therefore, it is hypothesized that craniofacial anatomic variations may influence polysomnographic parameters and MAD therapeutic prognosis. This study aims to evaluate craniofacial linear, angular, area and volumetric dimensions on CBCT images of OSA patients treated with MAD and determine whether these dimensions influence OSA severity and outcomes of MAD treatment.
MATERIALS AND METHODS
Sample and ethical considerations
This observational longitudinal study was approved by the Research Ethics Committee of the Federal University of São Paulo – Brazil (number 0301/10). All volunteers signed the Informed Consent Form (ICF). Patients aged 18 to 65 with a clinical and polysomnographic diagnosis of OSA were consecutively referred for dentistry evaluation and MAD treatment. Sixty-three patients were initially recruited, but 21 patients did not match the eligibility criteria. Thus, 42 patients were selected for the research. Before starting the T1 follow-up, 2 volunteers dropped out of the study, and 20 others were removed for not having performed all the necessary exams, leading to a total sample of 20 patients of both genders. The inclusion criteria consisted of body mass index (BMI) ≤ 35 kg/m2; clinical and polysomnographic diagnosis of OSA (AHI≥ 5/h) according to the International Classification of Sleep Disorders; negative TMD - Temporomandibular Disorder diagnosis by the Research Diagnostic Criteria for Temporomandibular Disorders - RDC/TMD questionnaire (adapted to the Portuguese language)[22] and a mandibular protrusion of at least 7 mm clinically measured with the George Gauge device. This study excluded patients with unsatisfactory dental conditions (active periodontal disease, caries or insufficient teeth to retain the appliance); dental crown/dental root ratio ≤1; predominating central apnea in polysomnography (50% or more of central events of the absolute number of events); use of psychoactive medicines; decompensated clinical, neurological or psychiatric diseases; other sleep disorders; and those already undergoing previous OSA treatments.
Based on the study by Consellu et al.[23], who observed that there was a significant increase in the mean total airway volume (+1261.6 ± 1476.2 mm3) in patients with OSA after treatment with MAD, it is estimated that at least 16 patients need to be evaluated across two time points in the present study to obtain a sample to obtain 95% confidence intervals and 90% power for the alternative hypothesis of this work (examined via paired t-tests). Thus, the study sample was composed of 20 patients (mean age of 48.35 ± 10.42 years), with a mean weight of 72.90 ± 15.41 kg, height of 1.64 ± 0.10 and BMI of 27.10 ± 4.29. There were 9 males and 11 females. Before MAD treatment (T0), 15 patients showed mild OSA, 3 showed moderate OSA, and 2 showed severe OSA (Table 1).
Table 1:
Sample anthropometric, protrusive and rotational characteristics in OSA distribution.
| T0 - OSA Severity | ||||
|---|---|---|---|---|
|
| ||||
| Anthropometric | Total Sample (n=20) | Mild (n=15) | Moderate (n=3) | Severe (n=2) |
|
|
||||
| Age | 48.35±10.42 | 48.27±11.50 | 50±8.88 | 46.5±4.5 |
| Weight | 72.90±15.41 | 72.87±16.07 | 65.33±14.01 | 84.5±5.5 |
| Height | 1.64±0.10 | 1.62±0.09 | 1.65±0.18 | 1.72±0.02 |
| BMI | 27.10±4.29 | 9.93±4.95 | 23.8±1.73 | 28.7±2.4 |
| Sex (M/F) | 9/11 | 6/9 | 1/2 | 2/0 |
| Protrusion | ||||
| Maximum | 11.00±2.22 | 10.93±2.43 | 11.66±1.52 | 10.5±1.5 |
| Therapeutic | 10.88±2.20 | 10.80±2.43 | 11.5±1.32 | 10.5±1.5 |
| Mandibular rotation | ||||
| Mandibular linear anterior (anteroposterior) | 2.49±2.63 | 2.45±2.47 | 4.32±2.76 | 0.02±2.86 |
| Mandibular linear anterior (superoinferior) | −9.38±2.92 | −9.00±3.18 | −10.10±2.13 | −11.2±0.83 |
| Mandibular ramus angular | −3.93±1 | −3.89 ±1.11 | −4.02±0.65 | −4.075±0.61 |
| Mandibular anterior angular | −4.09±1.2 | −4.04±0.63 | −4.07±1.22 | −4.91±0.685 |
OSA = Obstructive sleep apnea. BMI = Body mass index. M = Male. F = Female.
Study protocol
Exams
All patients underwent clinical, CBCT and polysomnography (PSG) exams at two time points: before treatment (T0) and after achieving the MAD therapeutic position (T1). Therapeutic protrusion (TP) was achieved from 4 to 6 months after MAD placement, and 30 to 48 days after TP was established, volunteers performed the final exams (T1).
Variables
This study analyzed clinical/demographic, polysomnographic and 3D imaging variables. The clinical variables included anthropometric characteristics: sex, age, weight, height, and BMI. Polysomnographic variables included AHI and minimum and medium SpO2. Three-dimensional image analysis variables included maxillary and mandibular linear, angular, and volumetric measurements, as well as linear and volumetric measurements of the UA.
MAD treatment and measurement of protrusion
For OSA treatment, the MAD used was the Brazilian dental appliance (BRD)[9], which is a maxillomandibular individualized device that allows gradual mandibular advances. The initial advancement was 50% of the total mandibular maximum protrusion ability. Mandibular advancement was made gradually until TP was achieved. TP was on average 97.4 ± 4.8% of the maximum protrusion, ranging from 85 to 100% of the mandible’s maximum anterior displacement. The amount of TP was also determined by the improvement of the signs/symptoms recorded in the medical record, and the treatment time until achieving TP was 4–6 months.
Polysomnography
All-night PSGs were performed at the Sleep Disorders Institute with digital-based polysomnography (Embla® N7000, Embla Systems, Inc., Broomfield, CO, USA). Surface electrodes were used for recording electroencephalography, submental and tibial electromyography, bilateral electrooculogram, and electrocardiography. Breathing was monitored with a nasal cannula with nasal flow measurement by a pressure transducer and oronasal thermistor, and respiratory effort was assessed by chest and abdomen inductance plethysmography. Pulse oximetry was used to measure oxyhemoglobin saturation. The body position for decubitus recording was made using a sensor placed over the sternum bone region. A cervical microphone was used to register the snoring. In this study, an AHI reduction below 5 obstructive events per hour (AHI <5) was considered a criterion for success since OSA treatment success is usually expressed as a ≥50% AHI reduction from baseline or at least an AHI of <10 events/hour[24].
CBCT acquisition protocol
CBCTs were performed at a private dental radiological clinic (Sao Paulo, Brazil) using the i-CAT® device (Imaging Sciences International, Hatfield, PA), configured with 120 Kvp, 3–8 mA, a 0.4-mm voxel size and a field of view (FOV) of 23 cm x 17 cm, allowing total vertical head framing[25, 26]. During the CBCT initial exam, all patients were awake, with a natural head position (Camper’s horizontal plane parallel to the ground) and to keep the gaze fixed at a stationary point on the wall. In T0, they were instructed to keep the occluded jaw in the maximum intercuspal position, and in T1, they were instructed with the intraoral appliance placed[25, 27]. The volunteers were instructed to not move, swallow, or take deep breaths to avoid changes in the UA volume during the exam[28, 29]. All images were stored in Digital Imaging and Communications in Medicine (DICOM) files.
Image processing
All CBCT data from T0 and T1 were processed with open-source imaging platforms. The segmentation and mandibular cropping required for image processing were performed using ITK-SNAP 2.4 software (https://www.itksnap.org). The DICOM files were converted into NIfTI files using the same software. To orient and register patients’ scans/segmentations, as well as to determine all the linear, angular, and volumetric measurements, Slicer CMF 4.0 software (www.slicer.org) was used.
To apply the 3D head orientation for all T0 scans, the models were moved by orienting its Frankfurt horizontal, midsagittal and transporionic planes to match the axial, sagittal and coronal planes, respectively, at a standard coordinate system in the Slicer software. The cranial registration of T1 scans was made after manual approximation to T0 scan oriented[30]. To perform all measurements, a list of 3D landmarks was used for the maxilla, mandible, and UA (Tables S1, S2, and S3). All linear, angular, area and volumetric dimensions were obtained in millimeters (mm), degrees (°), squared millimeters (mm2) and cubic millimeters (mm3), respectively.
a). Maxillary measurements
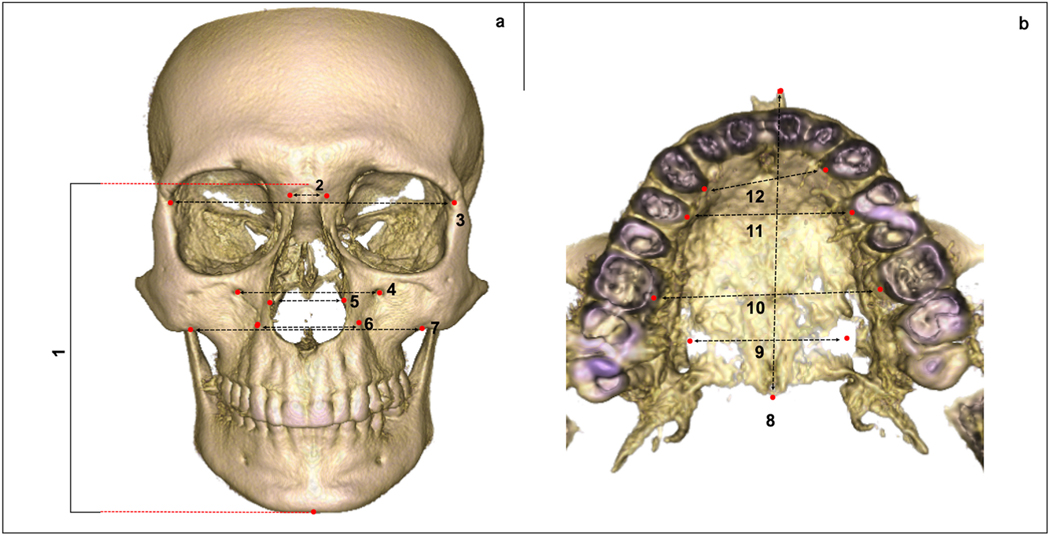
Fifteen measurements, linear and angular, were performed on maxillary bone: palatal alveolar bone crest M width, palatal alveolar bone crest PM width, palatal alveolar bone crest C width, intercanine eminence distance, greater palatine foramen distance, nasal width, inferior margin of the zygomaticomaxillary suture distance, infraorbital foramen distance, anterior border of the frontozygomatic suture distance, lateral border of the frontomaxillary suture distance, facial height, ANS-PNS, SNA, SNB and ANB (Table S4). To characterize the craniofacial aspects for these patients, all maxillary measurements were required only for T0 images (Figure 1).
Fig. 1.
Maxillary linear measurements (T0). (a) Measurements in a frontal view; (1) Facial height; (2) Lateral border of the frontomaxillary suture distance; (3) Anterior border of the frontozygomatic suture distance; (4) Infraorbital foramen distance; (5) Nasal width; (6) Intercanine eminence distance; (7) Inferior margin of the zygomaticomaxillary suture distance. (b) Measurements in an occlusal view; (8) ANS-PNS; (9) greater palatine foramen distance; (10) palatal alveolar bone crest M width; (l1) palatal alveolar bone crest PM width; (12) palatal alveolar bone crest C width. Landmark placement and measurements described in Tables S1 and S4.
b). Mandibular measurements
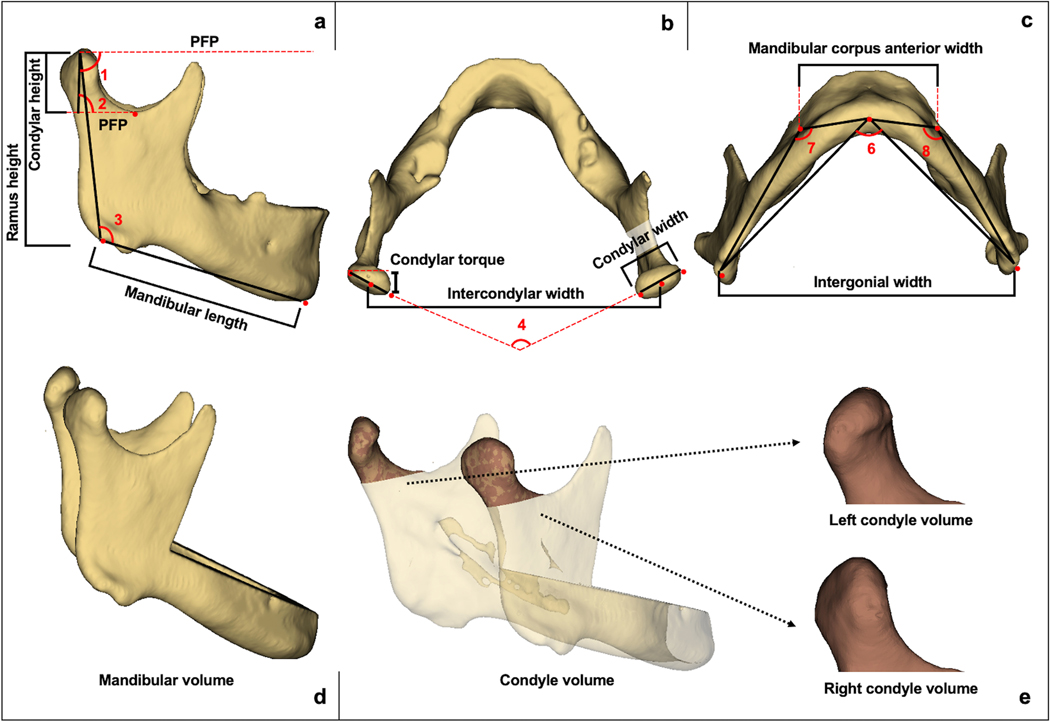
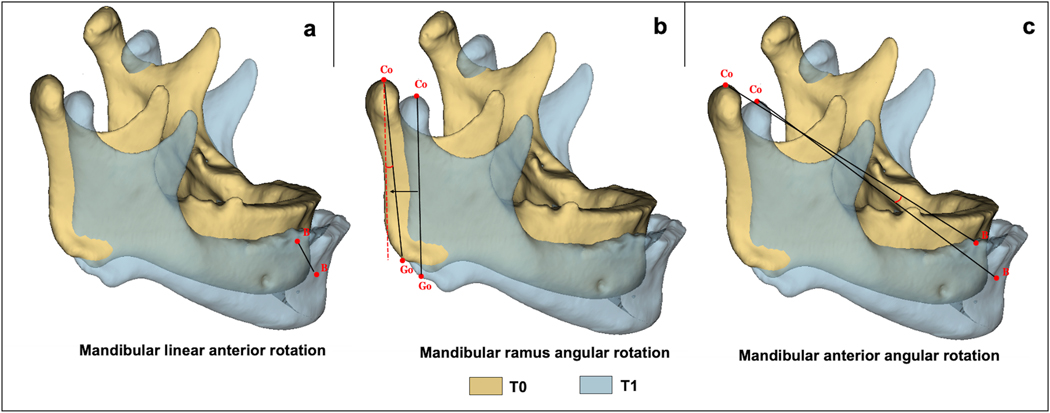
Mandibular dimensions were assessed with 19 measurements, including linear (condylar height, condylar width, condylar torque, ramus height, mandibular length, intergonial width, intercondylar width, mandibular corpus anterior width and mandibular linear anterior rotation), angular (mandibular ramus facial angle, gonial angle, condylar inclination, mandibular corpus posterior angle, mandibular corpus curve angle, intercondylar angle, mandibular ramus angular rotation and mandibular anterior angular rotation) and volumetric (condylar volume and total mandibular volume) 3D evaluations (Table S5). All these evaluations were made only in T0 scans (Figure 2), except for the last mandibular linear evaluation (mandibular linear anterior rotation) and the two last mandibular angular measurements (mandibular ramus angular rotation and mandibular anterior angular rotation), which were made comparing landmarks between T0 and T1 images (Figure 3).
Fig. 2.
Mandibular linear, angular and volumetric measurements (T0). (a) Linear and angular measurements in a lateral view; Ramus height; Condylar height; Mandibular length; (1) Mandibular ramus facial angle; (2) Condylar inclination; (3) Gonial angle; (PFP) Parallel line to Frankfurt plane. (b) Condylar torque; Condylar width; Intercondylar width; (4) Intercondylar angle. (c) Mandibular corpus anterior width; Intergonial width; (5) Mandibular corpus posterior angle; (6) Right mandibular corpus curve angle; (7) Left mandibular corpus curve angle. (d) Model without teeth used to calculate mandibular bone volume. (e) Condylar models used to calculate right and left condylar volumes. Landmark placement and measurements described in Tables S2 and S5.
Fig. 3.
Mandibular linear and angular measurements comparing T0 and T1. (a) Mandibular linear anterior rotation. (b) Mandibular ramus angular rotation. (c) Mandibular anterior angular rotation. Go = Gonion; Co = Condilyon; B = B point. Landmark placement and measurements described in Tables S2 and S5.
c). Upper airway measurements
Linear, volumetric, and surface area measurements were performed in UA (Table S6). The shape of UA in second (C2I) and fourth (C4S) vertebrae point slices was set only in T0 to identify the influence of UA shape on OSA severity and therapy outcomes. The UA shape was estimated based on the modification of the equation developed by Abramson et al.[31]. In addition, 3 volumetric measurements (total upper airway volume, superior oropharynx volume and inferior oropharynx volume) were made in T0 and T1 images to compare changes in this anatomic region before and after MAD treatment (Figure 4).
Fig. 4.
Upper airway linear measurements in (T0) and volumetric measurements comparing T0 and T1. (a) Model used to calculate the total upper airway volume. (b) Model used to calculate superior oropharynx volume and superior oropharynx surface to identify shape in slice C2I. (c) Model used to calculate inferior oropharynx volume and inferior oropharynx surface to identify shape in slice C4S. L-R = maximum width; A-P = maximum anteroposterior linear distance. Landmark placement and measurements described in Tables S3 and S6.
Study error
To avoid potential sources of bias, intraexaminer reliability was made by repeating the 3D measurements with an interval of 15 days. The data were exported to Microsoft Excel spreadsheets (Microsoft Corporation, Redmond, WA) and analyzed using the Statistical Package for the Social Sciences (SPSS®) version 20.0 for Windows (IBM Corporation, Sommers, NY). The following analyses were performed: (1) intraclass correlation coefficient (ICC) analysis to evaluate systematic errors regarding numerical data; (2) Dahlberg’s formula for assessing casual errors of measurements performed.
Statistical approach
The data were stored in Microsoft Excel and exported to SPSS® software version 20.0 for Windows, in which the analysis was performed adopting 95% confidence intervals. Tomographic and polysomnographic measurements, analyzed by the Kolmogorov-Smirnov normality test and Pearson’s correlation, were expressed as the mean and standard deviation. The variables were compared between degrees of severity (mild versus moderate/severe) using Student’s t-test and between assessment moments (T0 and T1) or sides (right and left) using the paired t-test (parametric data).
RESULTS
Study error
The intraexaminer repeatability of angular and linear measurements showed excellent correlation coefficients (ICCs greater than 0.9). Volume measurements showed adequate ICCs greater than 0.75. Dahlberg’s coefficient of at least 0.01 was obtained.
Mandibular protrusion and advancement
The means of maximum and therapeutic protrusion were 11.00±2.22 mm and 10.88±2.20 mm, respectively. Mandibular advancement measurements at point B (mandibular linear anterior rotation) demonstrated an average anterior displacement of 2.49±2.63 mm and inferior displacement of −9.38±2.92 mm. The mandibular ramus and mandibular angular anterior rotation presented on average −3.93±1° (backward rotation) and −4.09±1.2° (downward rotation), respectively (Table 1 and Figure 3).
Polysomnographic findings
All patients showed a marked reduction (p <0.001) in AHI with a mean variation of −6.86±5.23 between T0 and T1. The mean SpO2 only showed a variance of −0.35±1.11 and did not significantly improve with treatment (p=0.181), while the minimum SpO2 demonstrated a range of 3.15±3.39 and was significantly improved with MAD treatment (0.001). The AHI at T0 was not correlated with the maximum (p=0.197; r=0.301) or therapeutic protrusion (p=0.229; r=0.282), and similar results were found with MAD treatment.
Maxillary measurements
A significant correlation was found between the ANS-PNS linear dimension and maximum protrusion [p=0.043 (r=−0.457)]. The transverse width of the frontomaxillary suture was statistically correlated with the AHI before MAD treatment [p=0.019 (r=−0.519)]. All other linear and angular measurements of the maxilla were not correlated with AHI at baseline or the AHI variation between T1-T0 (Table 2).
Table 2:
Correlation between maxillary measurements with AHI and protrusion.
| AHI | Protrusion | |||
|---|---|---|---|---|
|
|
||||
| Maxillary variables | T0 | Δ | Maximum | Therapeutic |
|
| ||||
| p-value (r-value) | p-value (r-value) | p-value (r-value) | p-value (r-value) | |
| Linear | ||||
| Palatal alveolar bone crest M | 0.862 −0.042) | 0.401 (−0.199) | 0.260 (0.264) | 0.292 (0.248) |
| Palatal alveolar bone crest PM | 0.711 (−0.088) | 0.295 (0.246) | 0.366 (0.214) | 0.358 (0.217) |
| Palatal alveolar bone crest | 0.894 (−0.032) | 0.320 (0.234) | 0.419 (0.191) | 0.435 (0.185) |
| Intercanine eminence | 0.837 (0.049) | 0.842 (−0.048) | 0.835 (0.050) | 0.904 (0.029) |
| Nasal width | 0.062 (0.425) | 0.372 (0.211) | 0.569 (0.136) | 0.466 (0.173) |
| Greater palatine foramen | 0.372 (−0.211) | 0.443 (0.182) | 0.424 (0.189) | 0.563 (0.137) |
| Anterior border of the frontozygomatic suture | 0.864 (0.041) | 0.707 (0.090) | 0.590 (0.128) | 0.702 (0.091) |
| Lateral border of the frontomaxillary suture | * 0.019 (−0.519) | 0.291 (0.249) | 0.370 (0.212) | 0.377 (0.209) |
| Inferior margin of the zygomaticmaxillary suture | 0.692 (0.094) | 0.631 (−0.114) | 0.628 (0.116) | 0.671 (0.101) |
| Infraorbital foramens | 0.408 (−0.196) | 0.998 (0.001) | 0.648 (0.109) | 0.647 (0.109) |
| Facial height | 0.614 (0.120) | 0.486 (−0.165) | 0.649 (0.108) | 0.677 (0.099) |
| ANS - PNS | 0.664 (−0.104) | 0.827 (0.052) | * 0.043 (−0.457) | 0.116 (−0.363) |
| Angular | ||||
| SNA | 0.378 (0.208) | 0.556 (0.140) | 0.982 (−0.005) | 0.626 (0.116) |
| SNB | 0.785 (−0.065) | 0.249 (0.270) | 0.676 (−0.100) | 0.802 (−0.060) |
| ANB | 0.058 (0.431) | 0.313 (−0.238) | 0.351 (0.220) | 0.133 (0.348) |
p<0.05, Pearson’s correlation. AHI = Apnea and hypopnea index.
Mandibular measurements
Since no significant differences were identified between the left and right sides, we utilized the average measurements for all bilateral measurements. The mandibular ramus facial angle was correlated with AHI values at T0 [p=0.031 (r=0.896)], and the gonial angle demonstrated a correlation with therapeutic protrusion [p=0.049 (r=0.837]). The mandibular linear dimensions, volume and area were not significantly correlated with AHI at T0, the AHI variation with therapy, or protrusion (Table 3).
Table 3:
Correlation between mandibular measurements with AHI and Protrusion.
| AHI | Protrusion | |||
|---|---|---|---|---|
|
|
||||
| Mandibular variables | T0 | Δ | Maximum | Therapeutic |
| p-value (r-value) | p-value (r-value) | p-value (r-value) | p-value (r-value) | |
| Linear | ||||
| Condylar height | 0.666 (−0.103) | 0.558 (−0.139) | 0.496 (−0.161) | 0.534 (−0.148) |
| Condylar width | 0.712 (0.088) | 0.750 (−0.076) | 0.742 (0.078) | 0.974 (0.008) |
| Condylar width | 0.061 (−0.426) | 0.593 (0.127) | 0.171 (−0.319) | 0.191 (−0.305) |
| Ramus height | 0.897 (−0.031) | 0.664 (0.104) | 0.841 (−0.048) | 0.845 (0.047) |
| Mandibular length | 0.619 (−0.119) | 0.460 (0.175) | 0.955 (−0.013) | 0.613 (−0.121) |
| Intergonial width | 0.317 (−0.236) | 0.417 (0.192) | 0.966 (0.010) | 0.425 (−0.189) |
| Intercondylar width | 0.851 (−0.045) | 0.886 (0.034) | 0.416 (0.192) | 0.718 (0.086) |
| Mandibular corpus anterior width | 0.553 (0.141) | 0.839 (−0.049) | 0.586 (−0.130) | 0.636 (−0.113) |
| Mandibular linear anterior rotation (anteroposterior) | 0.480(−0.168) | 0.411 (−0.195) | 0.826 (0.052) | 0.957 (0.013) |
| Mandibular linear anterior rotation (superoinferior) | 0.199 (−0.300) | 0.336 (0.227) | 0.857 (0.043) | 0.792 (0.063) |
| Angular | ||||
| Mandibular ramus facial angle | * 0.031 (0.896) | 0.097 (0.685) | 0.075 (0.752) | 0.156 (0.511) |
| Gonial angle | 0.117 (0.624) | 0.110 (0.645) | 0.109 (0.647) | * 0.049 (0.837) |
| Condylar inclination | 0.105 (0.659) | 0.179 (0.450) | 0.295 (0.206) | 0.331 (0.153) |
| Mandibular corpus posterior angle | 0.174 (0.464) | 0.166 (0.486) | 0.223 (0.344) | 0.270 (0.249) |
| Mandibular corpus curve angle | 0.343 (0.139) | 0.291 (0.213) | 0.267 (0.255) | 0.255 (0.277) |
| Intercondylar angle | 0.397 (0.083) | 0.266 (0.258) | 0.188 (0.427) | 0.224 (0.343) |
| Mandibular ramus angular rotation | 0.178 (0.452) | 0.077 (0.748) | 0.160 (0.501) | 0.252 (0.285) |
| Mandibular anterior angular rotation | 0.189 (−0.306) | 0.835 (−0.050) | 0.827 (0.052) | 0.922 (0.024) |
| Volume/area | ||||
| Mandibular volume | 0.424 (0.189) | 0.935 (0.019) | 0.473 (−0.170) | 0.415 (−0.193) |
| Condyle volume | 0.154 (0.331) | 0.885 (−0.035) | 0.937 (−0.019) | 0.603 (−0.124) |
p <0.05, Paired t test (mean ± SD). AHI = Apnea and hypopnea index.
Upper airway measurements
The superior oropharynx volume (p=0.003) and surface area (p=0.001) presented highly significant increases with MAD treatment (variances of 1694.77 ± 2228.89 and 349.99 ± 416.77, respectively). The changes between T0-T1 in UA total volume (p=0.108) and surface area (p=0.470), as well as the inferior oropharynx volume (p=0.458) and surface area (p=237), were not statistically significant.
The total volume of the UA at baseline and its variation with MAD treatment were correlated with maximum protrusion, p=0.004 (r=0.615) and p=0.005 (r=0.604), therapeutic protrusion, respectively p=0.011 (r=0.556) and p=0.011 (r=0.558). The total area of the UA in T0 was correlated with AHI at baseline [p=0.016 (r=−0.533)] and with maximum [p=0.007 (r=0.579)] and therapeutic protrusion [p=0.008 (r=0.572)]. The superior oropharynx and inferior oropharynx volume and area in T0 were statistically correlated with both protrusion variables (Table 4). The total volume of the UA was not correlated with the AHI. UA linear variables were not correlated with AHI at baseline or AHI changes with treatment or protrusion.
Table 4:
Correlation between UA volumes and protrusion.
| IAH | Protrusion | |||
|---|---|---|---|---|
|
|
||||
| UA variables | T0 | Δ | Maximum | Therapeutic |
|
| ||||
| p-value (r-value) | p-value (r-value) | p-value (r-value) | p-value (r-value) | |
| Linear | ||||
| Lateral C2I slice | 0.371 (0.211) | 0.872 (−0.038) | 0.196 (0.302) | 0.111 (0.367) |
| Anteroposterior C2I slice | 0.955 (−0.013) | 0.523 (0.152) | 0.711 (0.089) | 0.926 (−0.022) |
| Lateral C2I slice | 0.303 (−0.243) | 0.154 (0.331) | 0.382 (−0.207) | 0.146 (−0.338) |
| Anteroposterior C4S slice | 0.245 (0.273) | 0.445 (−0.181) | 0.067 (0.418) | 0.033 (0.478) |
| Shape in C4S slice | 0.795 (0.062) | 0.980 (−0.006) | 0.891 (−0.033) | 0.999 (0.000) |
| Shape in C4S slice | 0.605 (−0.123) | 0.533 (0.148) | 0.570 (−0.135) | 0.575 (−0.133) |
| Volumetric | ||||
| UA total volume | ||||
| T0 | 0.278 (0.255) | 0.667 (−0.102) | * 0.004 (0.615) | * 0.011 (0.556) |
| T1 -T0 | 0.154 (0.331) | 0.302 (−0.243) | * 0.005 (0.604) | * 0.011 (0.558) |
| UA total surface area | ||||
| T0 | 0.235 (0.278) | * 0.016 (−0.533) | * 0.007 (0.579) | * 0.008 (0.572) |
| T1 - T0 | 0.315 (−0.236) | 0.353 (−0.219) | 0.797 (0.061) | 0.616 (0.119) |
| Superior oropharynx volume | ||||
| T0 | 0.257 (0.266) | 0.527 (−0.150) | * 0.018 (0.523) | * 0.042 (0.458) |
| T1 - T0 | 0.952 (−0.014) | 0.159 (−0.327) | 0.220 (0.287) | 0.251 (0.269) |
| Superior oropharynx surface area | ||||
| T0 | 0.229 (0.282) | 0.207 (−0.295) | * 0.012 (0.552) | * 0.020 (0.517) |
| T1 -T0 | 0.859 (−0.042) | 0.524 (−0.151) | 0.763 (0.072) | 0.769 (0.070) |
| Inferior oropharynx volume | ||||
| T0 | 0.378 (0.208) | 0.832 (−0.051) | * 0.005 (0.606) | * 0.009 (0.570) |
| T1 - T0 | 0.397 (−0.201) | 0.264 (−0.263) | 0.962 (−0.011) | 0.836 (0.050) |
| Inferior oropharynx surface area | ||||
| T0 | 0.135 (0.346) | 0.569 (−0.135) | * 0.007 (0.583) | * 0.014 (0.542) |
| T1 - T0 | 0.113 (−0.366) | 0.255 (−0.267) | 0.916 (−0.025) | 0.807 (0.058) |
p <0.05, Pearson’s correlation. UA = Upper airway, C2I = Most inferior and anterior point of the second cervical vertebra
C4S= Most superior and anterior point of the fourth cervical vertebra.
DISCUSSION
This study tested associations between 3D craniofacial and upper airway anatomy measurements and OSA severity, outcomes of treatment with a mandibular advancement device, and the amount of protrusion needed for successful therapy. The precise 3D measurements of the maxilla, mandible and upper airway in this study were performed in standardized head orientation during image acquisition and image analysis procedures. Previous authors have evaluated the relationship between anatomic skeletal classes and the development of obstructive sleep apnea and the efficacy of the mandibular advancement device in OSA treatment[32–37]. However, the literature lacks research involving tomographic assessment of the craniofacial anatomy and UA as impact factors in OSA severity and its treatment prognosis.
In the present study, the therapeutic protrusion obtained with MAD was 10.88±2.20 mm. This amount of protrusion measured in the appliance resulted in anterior (2.49±2.63 mm) and inferior (−9.38±2.92 mm) displacement of the mandible measured at point B (mandibular anterior rotation). The overall mandibular displacement with the appliance measured in 3D superimposition relative to the cranial base showed an amount of vertical movement of the mandible higher than anterior movement, a finding also shown in Kim et al.[38]. These outcomes indicate that MAD placement may increase the vertical dimension, leading to mandibular clockwise rotation. It has been reported that the range of mandibular protrusion reduces 0.3 mm for each 1 mm of vertical displacement[39]. Interestingly, there was no correlation between the improvement in AHI and the amount of therapeutic protrusion or the amount of displacement of the mandible.
Therapy with the MAD appliance significantly reduced the AHI in all patients evaluated in this investigation. After MAD treatment, an improvement in the AHI was demonstrated, with 15 patients (75% of the sample) showing an AHI lower than 5, indicating a successful outcome[35, 40]. These findings are in agreement with Metz et al.[35], who identified that the same appliances successfully treated OSA of all severities with efficacy. Although this study demonstrated an AHI decrease in all patients, the AHI in severe cases remained at values greater than 15 awakes per hour, leading to a moderate severity instead of a mild severity. Different findings were reported in an orthognathic surgery systematic review that identified a mean AHI decrease from 63.9/h to 9.5/h (p < 0.001), indicating that even the most severe cases could show an AHI lower than 15 associated with mild severity[41].
The increase in the minimum SpO2 observed in our results was also significantly improved, aiding treatment success. Oxygen saturation findings were also reported by Zhan et al.[42], who identified that the lowest oxygen saturation was significantly higher after MAD therapy.
We assessed measures that may influence OSA severity, as determined by AHI values before MAD treatment (T0). The T0 craniofacial characteristics revealed that the facial width at the level of the frontomaxillary suture and the inclination of the mandibular ramus relative to the Frankfurt horizontal plane (mandibular ramus facial angle) were significantly associated with OSA severity. The frontomaxillary suture was also analyzed by Bruwier et al.[43], who observed that the Basion-superior pituitary tubercle-frontomaxillary suture angle in OSA patients was higher than in non-OSA individuals (133.4±6.00 and 132.9±5.2, respectively), showing in agreement to this study the role that frontomaxallary may play in influencing OSA development. Nonetheless, volumetric dimensions of the mandibular skeletal morphology or the airway were not statistically correlated with AHI at baseline. These findings corroborate those studies that described maxillary transversal anatomic variations as an important factor for OSA development[44–46]. However, those authors reported that mandibular linear measurements, such as mandibular length, mandibular width and mandibular height, were correlated with AHI, while the present study only found a correlation of the mandibular ramus facial angle with AHI values. Our results also differ from the results reported by Johal et al.[47], who found that the vertical maxillary anatomy and angular dimensions (SNA, SNA, ANB) are critical structures for OSA prediction[44, 46–48].
Comparing the UA volume before and after MAD treatment, all patients showed a significant increase in superior oropharynx volume. Similar outcomes were also described by Pahkala et al.[49], who demonstrated that MAD therapy protrusion significantly increased the oropharyngeal volume. In the present study, no significant difference was identified in the inferior oropharynx volume. The inferior oropharynx region demonstrated a volume decrease with treatment (−321.24 ± 1897.24 mm3), which can likely be explained by the MAD clockwise rotation effect[39].
Therapeutic protrusion with MAD was significantly and proportionally correlated with the gonial angle, and the mandibular ramus facial angle (Co-Go to the Frankfurt plane) was statistically and proportionally correlated with a more severe OSA diagnosis. This fact implies that clinicians may anticipate a greater therapeutic advance for a successful treatment in dolichofacial patients. The mandibular anterior width and intergonial width were not statistically associated with the AHI or the amount of protrusion. Sutherland et al. [40], demonstrated different results, showing that excess intramandibular space area was associated with successful MAD therapy. UA total, superior and inferior oropharynx volume and surface area measured at baseline were also correlated with therapeutic protrusion. These variables may be considered important factors to identify the amount of MAD advancement required for successful OSA treatment. The amount of therapeutic protrusion, expressed in both anterior and vertical mandibular movement, increased the overall airway volume.
CBCT is an imaging modality of great value for craniofacial anatomic evaluations. While most commercially available software allows adjustment of head position after image acquisition, the standardization of head position and stage of respiration during image acquisition is critical for consistent measurements of airway volume and area across different patients and timepoints[50, 51]. In our study, all patients were carefully instructed to maintain an adequate natural head position and avoid swallowing during image acquisition. Furthermore, the 3D image analysis of hard and soft tissue structures was performed in a standardized head orientation at T0, as defined by Ruellas et al.[30], and all T0 and T1 scans were registered relative to the cranial base. The airway volume and area performed in this study were measured using ITK-SNAP semiautomatic segmentation, which has been reported to be comparable to well-known commercial software, such as Dolphin imaging[52, 53]. The present study data did not find correlations between the volume and shape of the airway and OSA severity at baseline. However, UA’s total area was significantly correlated with AHI improvement with treatment and is considered a factor that may influence OSA treatment and outcomes with MAD. While previous studies[54, 55] reported correlations between airway linear, area and/or volume measurements and AHI severity at baseline, Svaza et al.[55] utilized only 2D measurements, Abramson et al.[31] did not standardize head position during or after image acquisition, and Ozer et al.[54] utilized CT scans taken in the supine position. For these reasons, the findings of those studies are not directly comparable to the present study results.
The present study findings indicate that anatomic craniofacial factors may influence OSA severity, MAD outcomes and the amount of protrusion for resolutive mandibular advancement therapy. Such results grant better knowledge to use MAD as a possible treatment option while also considering the indication for consolidated and predictable surgical therapies[41, 56, 57]. Importantly, the findings of this study highlight clinical decisions considering individual characteristics, prognosis and interests.
As many of the 3D image variables assessed as influencing OSA severity and MAD outcome had not yet been tested in the literature, the comparisons with previous study findings were limited. Moreover, the present study is limited by the lack of a control group. However, it is unrealistic to follow OSA patients without treatment or place the MAD appliance without proper diagnostic indications for ethical reasons. Importantly, our prospective study sample was longitudinally evaluated with polysomnography exams and CBCT images before and after 4–6 months of MAD treatment. Future studies with an adequate sample size of mild, moderate, and severe AHI subgroups are essential, especially considering the possibility of applying more robust statistical evaluations, such as regression analysis and receiver operating characteristic curve (ROC curve), whether these anatomic variances are risk factors for OSA development and MAD treatment prognosis. Such future studies have the potential to provide further insight to validate craniofacial aspects that may anticipate OSA severity and therapeutic response to MAD.
CONCLUSIONS
In conclusion, 3D anatomic craniofacial measurements play an essential role in influencing OSA severity, MAD outcomes for OSA treatment and the amount of protrusion for resolutive mandibular advancement therapy. A greater transverse width of the frontomaxillary suture may indicate a diagnosis of a less severe OSA, while a greater mandibular ramus facial angle may suggest a more severe OSA. Moreover, greater measurements of gonial angle, UA total, superior and inferior oropharynx volume and surface area were considered anatomic factors that may anticipate the knowledge about greater amount of protrusion needed for a successful MAD treatment. The total UA area also influenced MAD outcomes in patients with OSA.
Supplementary Material
ACKNOWLEDGEMENTS
The authors express gratitude to the Coordination for the Improvement of Higher Education Personnel (CAPES) CAPES/PRINT - Call no. 41/2017 file number 88887.465681/2019–00 and to the Brazilian National Council for Scientific and Technological Development (CNPq), which provided Dr. Fabio Costa a PQ fellowship in category 2.
Funding This study was funded in part by the Coordination for the Improvement of Higher Education Personnel (CAPES) - Finance Code 001, which supported a sandwich Doctorate Program. The research image analysis tools were supported by the National Institute of Dental and Craniofacial Research and the National Institute of Biomedical Imaging and Bioengineering under R01DE024450.
DECLARATIONS
Ethical approval The study was approved by the Research Ethics Committee of the Federal University of São Paulo – Brazil (number 0301/10). All volunteers signed the Informed Consent Form (ICF).
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K and Malhotra A (2019) Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 7:687–698. doi: 10.1016/s2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prisant LM, Dillard TA and Blanchard AR (2006) Obstructive sleep apnea syndrome. J Clin Hyperten 8:746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sateia MJ (2014) International classification of sleep disorders-third edition: highlights and modifications. Chest 146:1387–1394. doi: 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 4.Wolkove N, Elkholy O, Baltzan M and Palayew M (2007) Sleep and aging: 1. Sleep disorders commonly found in older people. Cmaj 176:1299–304. doi: 10.1503/cmaj.060792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin KA and Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima AMJ, Franco RMC, Castro BMMC, Bezerra AA, Ataide L Jr, Halpern A (2008) Contribution of Obstructive Sleep Apnea to Oxidative Obesity Stress. Arch Endocrinol Metab 52:668–676. [DOI] [PubMed] [Google Scholar]
- 7.Polonis K, Sompalli S, Becari C, Xie J, Covassin N, Schulte PJ, Druliner BR, Johnson RA, Narkiewicz K, Boardman LA, Singh P and Somers VK (2019) Telomere Length and Risk of Major Adverse Cardiac Events and Cancer in Obstructive Sleep Apnea Patients. Cells 8. doi: 10.3390/cells8050381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R and Hla KM (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Fabbro CD, Chaves CM Jr, Bittencourt LRA and Tufik S. (2010) Clinical and polysonographic assessment of the BRD Appliance in the treatment of obstructive sleep apnea syndrome. Dental Press J Orthod 15:107–117. [Google Scholar]
- 10.Sonnesen L (2010) Associations between the Cervical Vertebral Column and Craniofacial Morphology. Int J Dent 2010:295728. doi: 10.1155/2010/295728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battagel JM, Johal A and Kotecha B (2000) A cephalometric comparison of subjects with snoring and obstructive sleep apnoea. Eur J Orthod 22:353–65. doi: 10.1093/ejo/22.4.353 [DOI] [PubMed] [Google Scholar]
- 12.Grauer D, Cevidanes LS, Styner MA, Ackerman JL and Proffit WR (2009) Pharyngeal airway volume and shape from cone-beam computed tomography: relationship to facial morphology. Am J Orthod Dentofacial Orthop 136:805–14. doi: 10.1016/j.ajodo.2008.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe AA, Fleetham JA, Adachi S and Ryan CF (1995) Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop 107:589–95. doi: 10.1016/s0889-5406(95)70101-x [DOI] [PubMed] [Google Scholar]
- 14.Miles PG, Vig PS, Weyant RJ, Forrest TD and Rockette HE Jr., (1996) Craniofacial structure and obstructive sleep apnea syndrome--a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop 109:163–72. doi: 10.1016/s0889-5406(96)70177-4 [DOI] [PubMed] [Google Scholar]
- 15.Zheng ZH, Yamaguchi T, Kurihara A, Li HF and Maki K (2014) Three-dimensional evaluation of upper airway in patients with different anteroposterior skeletal patterns. Orthod Craniofac Res 17:38–48. doi: 10.1111/ocr.12029 [DOI] [PubMed] [Google Scholar]
- 16.An HJ, Baek SH, Kim SW, Kim SJ and Park YG (2020) Clustering-based characterization of clinical phenotypes in obstructive sleep apnoea using severity, obesity, and craniofacial pattern. Eur J Orthod 42:93–100. doi: 10.1093/ejo/cjz041 [DOI] [PubMed] [Google Scholar]
- 17.Cunali PA, Almeida FR, Santos CD, Valdrichi NY, Nascimento LS, Dal-Fabbro C, Tufik S and Bittencourt LR (2011) Mandibular exercises improve mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath 15:717–27. doi: 10.1007/s11325-010-0428-2 [DOI] [PubMed] [Google Scholar]
- 18.García M, Cabrera JA, Bataller A, Vila J and Mayoral P (2020) Mandibular movement analisys by means of a kinematic model applied to the design of oral appliances for the treatment of obstructive sleep apnea. Sleep Med 73:29–37. doi: 10.1016/j.sleep.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Than KD, Chen KS, Wang AC, La Marca F and Park P (2014) Sleep apnea and cervical spine pathology. Eur Spine J 23:641–7. doi: 10.1007/s00586-013-3046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuiki S, Lowe AA, Almeida FR and Fleetham JA (2004) Effects of an anteriorly titrated mandibular position on awake airway and obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop 125:548–55. doi: 10.1016/j.ajodo.2003.05.006 [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Bumann A and Mah J (2005) Three-dimensional radiographic analysis in orthodontics. J Clin Orthod 39:421–8. [PubMed] [Google Scholar]
- 22.de Lucena LB, Kosminsky M, da Costa LJ and de Góes PS (2006) Validation of the Portuguese version of the RDC/TMD Axis II questionnaire. Braz Oral Res 20:312–7. doi: 10.1590/s1806-83242006000400006 [DOI] [PubMed] [Google Scholar]
- 23.Cossellu G, Biagi R, Sarcina M, Mortellaro C and Farronato G (2015) Three-dimensional evaluation of upper airway in patients with obstructive sleep apnea syndrome during oral appliance therapy. J Craniofac Surg 26:745–8. doi: 10.1097/scs.0000000000001538 [DOI] [PubMed] [Google Scholar]
- 24.Knappe SW and Sonnesen L (2018) Mandibular positioning techniques to improve sleep quality in patients with obstructive sleep apnea: current perspectives. Nat Sci Sleep 10:65–72. doi: 10.2147/nss.S135760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moshiri M, Scarfe WC, Hilgers ML, Scheetz JP, Silveira AM and Farman AG (2007) Accuracy of linear measurements from imaging plate and lateral cephalometric images derived from cone-beam computed tomography. Am J Orthod Dentofacial Orthop 132:550–60. doi: 10.1016/j.ajodo.2006.09.046 [DOI] [PubMed] [Google Scholar]
- 26.Scarfe WC, Farman AG and Sukovic P (2006) Clinical applications of cone-beam computed tomography in dental practice. J Can Dent Assoc 72:75–80. [PubMed] [Google Scholar]
- 27.Kim YJ, Hong JS, Hwang YI and Park YH (2010) Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. Am J Orthod Dentofacial Orthop 137:306.e1–11; discussion 306–7. doi: 10.1016/j.ajodo.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 28.Tso HH, Lee JS, Huang JC, Maki K, Hatcher D and Miller AJ (2009) Evaluation of the human airway using cone-beam computerized tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:768–76. doi: 10.1016/j.tripleo.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 29.El H and Palomo JM (2010) Measuring the airway in 3 dimensions: A reliability and accuracy study. Am J Orthod Dentofacial Orthop 137:S50–S52. doi: 10.1016/j.ajodo.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 30.Ruellas ACDO, Tonello C, Gomes LR, Yatabe MS, MacRon L, Lopinto J, Goncalves JR, Garib Carreira DG, Alonso N, Souki BQ, Coqueiro RDS and Cevidanes LHS (2016) Common 3-dimensional coordinate system for assessment of directional changes. Am J Orthod Dentofacial Orthop 149:645–656. doi: 10.1016/j.ajodo.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abramson Z, Susarla S, Troulis M and Kaban L (2009) Age-related changes of the upper airway assessed by 3-dimensional computed tomography. J Craniofac Surg 20:657–663. doi: 10.1097/SCS.0b013e318193d521 [DOI] [PubMed] [Google Scholar]
- 32.Aarab G, Lobbezoo F, Hamburger HL and Naeije M (2011) Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnea: A randomized, placebo-controlled trial. Respiration 81:411–419. doi: 10.1159/000319595 [DOI] [PubMed] [Google Scholar]
- 33.Alqahtani ND, Algowaifly MI, Almehizia FA, Alraddadi ZA, Al-Sehaibany FS, Almosa NA, Albarakati SF and Bahammam AS (2018) The characteristics of dental occlusion in patients with moderate to severe obstructive sleep apnea in Saudi Arabia. Saudi Med J 39:928–934. doi: 10.15537/smj.2018.9.22750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF and de Bont LG (2008) Obstructive sleep apnea therapy. J Dent Res 87:882–7. doi: 10.1177/154405910808700917 [DOI] [PubMed] [Google Scholar]
- 35.Metz JE, Attarian HP, Harrison MC, Blank JE, Takacs CM, Smith DL and Gozal D (2019) High-resolution pulse oximetry and titration of a mandibular advancement device for obstructive sleep apnea. Front Neurol 10. doi: 10.3389/fneur.2019.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB and Cistulli PA (2013) Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am J Respir Crit Care Med 187:879–887. doi: 10.1164/rccm.201212-2223OC [DOI] [PubMed] [Google Scholar]
- 37.Schütz TCB, Cunha TCA, Moura-Guimaraes T, Luz GP, Ackel-D’Elia C, Alves ES, Pantiga Junior G, de Mello MT, Tufik S and Bittencourt L (2013) Comparison of the effects of continuous positive airway pressure, oral appliance and exercise training in obstructive sleep apnea syndrome. Clinics 68:1168–1174. doi: 10.6061/clinics/2013(08)17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DI, Lagravère Vich M, Mayoral P. and Miguez M (2020) Three-Dimensional Changes in Skeletal/ Dental Landmarks With Use of Mandibular Advancement Devices. J Dent Sleep Med 7. [Google Scholar]
- 39.Mayoral P, Lagravère MO, Míguez-Contreras M and Garcia M (2019) Antero-posterior mandibular position at different vertical levels for mandibular advancing device design. BMC Oral Health 19. doi: 10.1186/s12903-019-0783-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, Kushida CA and Cistulli PA (2014) Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 10:215–27. doi: 10.5664/jcsm.3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holty JE and Guilleminault C (2010) Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 14:287–97. doi: 10.1016/j.smrv.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 42.Zhan X, Fang F, Wu C, Pinto JM and Wei Y (2018) A retrospective study to compare the use of the mean apnea-hypopnea duration and the apnea-hypopnea index with blood oxygenation and sleep patterns in patients with obstructive sleep apnea diagnosed by polysomnography. Med Sci Monit 24:1887–1893. doi: 10.12659/MSM.909219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruwier A, Poirrier R, Albert A, Maes N, Limme M, Charavet C, Milicevic M, Raskin S and Poirrier AL (2016) Three-dimensional analysis of craniofacial bones and soft tissues in obstructive sleep apnea using cone beam computed tomography. Int Orthod 14:449–461 [DOI] [PubMed] [Google Scholar]
- 44.Neelapu BC, Kharbanda OP, Sardana HK, Balachandran R, Sardana V, Kapoor P, Gupta A and Vasamsetti S (2017) Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med Rev 31:79–90. doi: 10.1016/j.smrv.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 45.Perri RA, Kairaitis K, Cistulli P, Wheatley JR and Amis TC (2014) Surface cephalometric and anthropometric variables in OSA patients: Statistical models for the OSA phenotype. Sleep Breath 18:39–52. doi: 10.1007/s11325-013-0845-0 [DOI] [PubMed] [Google Scholar]
- 46.Seto BH, Gotsopoulos H, Sims MR and Cistulli PA (2001) Maxillary morphology in obstructive sleep apnoea syndrome. Eur J Orthod 23:703–714. doi: 10.1093/ejo/23.6.703 [DOI] [PubMed] [Google Scholar]
- 47.Johal A and Conaghan C (2004) Maxillary morphology in obstructive sleep apnea: A cephalometric and model study. Angle Orthodontist 74:648–656. [DOI] [PubMed] [Google Scholar]
- 48.Barrera JE, Pau CY, Forest VI, Holbrook AB and Popelka GR (2017) Anatomic measures of upper airway structures in obstructive sleep apnea. World J Otorhinolaryngol Head Neck Surg 3:85–91. doi: 10.1016/j.wjorl.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pahkala R, Seppä J, Myllykangas R, Tervaniemi J, Vartiainen VM, Suominen AL and Muraja-Murro A (2020) The impact of oral appliance therapy with moderate mandibular advancement on obstructive sleep apnea and upper airway volume. Sleep Breath 24:865–873. doi: 10.1007/s11325-019-01914-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cevidanes LHS, Chaves CM Jr, Nguyen T, Moro A, Borges SW, Porto P, Yatabe MS, Ioshida MM, Ruellas ACO (2018) Critical Concepts In The Diagnosis Of The Airway Using 3D Images. [Google Scholar]
- 51.Obelenis Ryan DP, Bianchi J, Ignácio J, Wolford LM and Gonçalves JR (2019) Cone-beam computed tomography airway measurements: Can we trust them? Am J Orthod Dentofacial Orthop 156:53–60. doi: 10.1016/j.ajodo.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 52.Lo Giudice A, Ronsivalle V, Grippaudo C, Lucchese A, Muraglie S, Lagravère MO and Isola G (2020) One Step before 3D Printing-Evaluation of Imaging Software Accuracy for 3-Dimensional Analysis of the Mandible: A Comparative Study Using a Surface-to-Surface Matching Technique. Materials (Basel) 13. doi: 10.3390/ma13122798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinheiro ML, Yatabe M, Ioshida M, Orlandi L, Dumast P and Trindade-Suedam IK (2018) Volumetric reconstruction and determination of minimum crosssectional area of the pharynx in patients with cleft lip and palate: comparison between two different softwares. J Appl Oral Sci 26:e20170282. doi: 10.1590/1678-7757-2017-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Özer T, Selçuk A, Yılmaz Z, Voyvoda N, Çam İ, Özel HE, Özdoğan F, Esen E, Genç G and Genç S (2018) The role of upper airway morphology in apnea versus hypopnea predominant obstructive sleep apnea patients: an exploratory study. Br J Radiol 91:20170322. doi: 10.1259/bjr.20170322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svaza J, Skagers A, Cakarne D and Jankovska I (2011) Upper airway sagittal dimensions in obstructive sleep apnea (OSA) patients and severity of the disease. Stomatologija 13:123–7. [PubMed] [Google Scholar]
- 56.Alcalde LFA, Faria PEP, Nogueira RLM, Chihara L and Sant’Ana E (2019) Computed tomography visualizing alterations in the upper airway after orthognathic surgery. J Craniomaxillofac Surg 47:1041–1045. doi: 10.1016/j.jcms.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 57.Sistla SK, Paramasivan VK and Agrawal V (2019) Anatomic and Pathophysiologic Considerations in Surgical Treatment of Obstructive Sleep Apnea. Sleep Med Clin 14:21–31. doi: 10.1016/j.jsmc.2018.11.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.