Abstract
During their lifespan, T cells are tasked with patrolling the body for potential pathogens. To do so, T cells migrate through numerous distinct anatomical sites and tissue environments with different biophysical characteristics. To migrate through these different environments, T cells use various motility strategies that rely on actin network remodeling to generate shape changes and mechanical forces. In this review we initially discuss the migratory journey of T cells, then cover the actin polymerization effectors at play in T cells, and finally we focus on the function of these effectors of actin cytoskeleton remodeling in mediating T cell migration through diverse tissue environments. Specifically, we will discuss the current state of the field pertaining to our understanding of the roles in T cell migration played by members of the three main families of actin polymerization machinery: the Arp2/3 complex; formin proteins; and Ena/VASP proteins.
Keywords: Actin, cytoskeleton, migration, motility, extravasation, T cells, lymphocyte, Arp2/3, Formin, VASP
Graphical Abstract
To perform their functions, T cell migrate through tissue environments with varying biophysical properties. Migration within these different tissues relies on actin network remodeling and the use of various motility strategies. In this review we discuss the role of actin polymerizing proteins, including Formins, Ena/VASP proteins, and the Arp2/3 complex, in enabling T cell migration modes through diverse tissue environments.
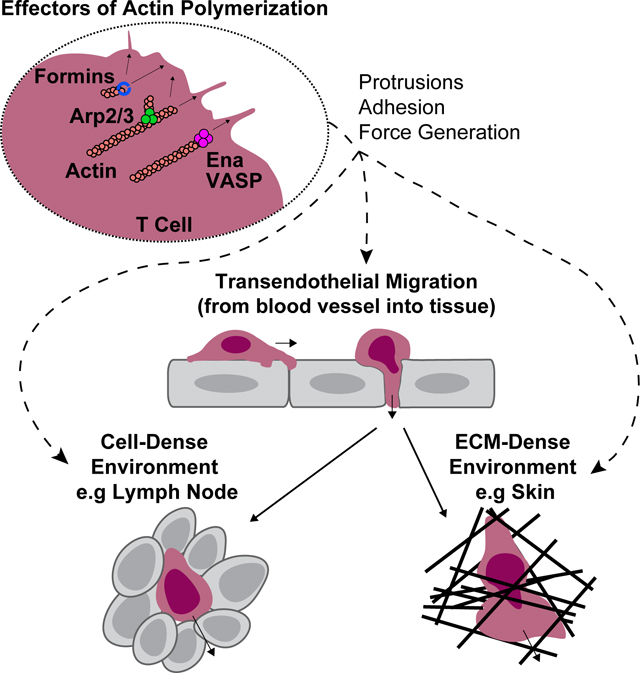
T cell Migration and Trafficking Overview:
A T cell’s journey begins as a progenitor cell in the bone marrow which travels to the thymus to undergo development. Pre-T cells enter the thymus by exiting from the blood vasculature, a process that will be discussed in detail later in this review. However, during early embryonic development, before the thymus is vascularized, progenitor cells can also enter the thymus by a vascular independent route [1]. Interestingly, so far, there has only been one report of mutations in actin cytoskeletal regulators that prevent the entry of progenitor cells into the thymus [2], suggesting that this is a relatively permissive process.
Actin cytoskeleton rearrangements can play important roles in thymic selection by regulating both thymocyte migration as well as immunological synapse formation and T cell receptor (TCR) signaling. Within the thymus, the pre-T cell must navigate through a dense cell-packed environment to undergo the processes of positive selection in the thymus cortex and negative selection in the medulla by interacting with thymic epithelial cells (TECs) and resident dendritic cells (DCs) [1]. In the absence of autoreactivity, positively selected thymocytes move quickly through the medulla along set migratory paths. However, as observed via multi-photon microscopy, some autoreactive thymocytes encountering a negative selecting ligand are induced to slow their speed and follow motility patterns that keep them confined to the medulla. Within these ‘confinement zones’ autoreactive T cells make frequent transient interactions with resident DCs, enhancing the efficiency of the negative selection process [3]. Once a T cell has matured it will exit the thymus to enter circulation in response to sphingosine-1-phosphate (S1P) gradients [4]. This can happen either by reverse extravasation into blood vessels or by entry into the lymphatics, which eventually drain into the blood stream through the thoracic duct [5,6].
Mature naïve T cells in the blood circulation will home to secondary lymphoid organs such as lymph nodes, spleen, and mucosal-associated lymphoid tissues, where they patrol for foreign antigens. To enter these and other peripheral tissues, T cells exit the blood stream through a multistep process known as extravasation or transendothelial migration (TEM) [7]. Canonically, TEM consists of four main sequential stages: rolling, adhering, crawling, and diapedesis [7].
Intravascular T cells are subjected to substantial shear stress due to the rapid flow of the bloodstream, ranging from approximately 1 dyne/cm2 in the high endothelial venules (HEVs) of lymph nodes to greater than 6 dyne/cm2 in the post-capillary venules of other tissues [8–10]. Transient interactions between selectins and oligosaccharides related to sialyl-Lewisx enable T cells to resist these forces and initiate TEM by rolling on the vascular endothelial cell wall [11–13]. Low-affinity interactions between integrins and their immunoglobulin super family ligands can also facilitate rolling in some tissue-specific contexts, such as integrin alpha-4 in the Central Nervous System [14]. This rolling process enables T cells to subsequently encounter chemokines attached to the glycocalyx of the endothelium [15–17]. Signaling by these chemokines as well as shear forces acting on T cells serve to activate integrins into a high-affinity conformation [13,16–20]. Subsequent binding of high-affinity integrins to their ligands on the endothelium promotes the full arrest and attachment of T cells to the vascular wall [16,17,21].
Post-arrest, T cells respond to chemotactic cues and crawl on the endothelial luminal surface, relying on integrin-mediated adhesions for traction [16,17]. During this process T cells adopt an amoeboid morphology, with a ruffled, protrusive structure known as the lamellipodium at the leading edge of the cell and a contractile structure known as the uropod at the trailing edge [22–24]. Additionally, during crawling, micro-protrusions known as podosomes and filopodia extend from the ventral side and leading edge of T cells into the endothelium, to probe for chemokine signals and permissive sites for transmigration across the endothelium [16,21,25].
During diapedesis, the final stage of TEM, T cells translocate across the vascular cell wall. This process predominantly occurs between endothelial cells at cell-cell junctions via the paracellular route. However, in certain tissues, such as the Central Nervous System, diapedesis also occurs through the endothelial cell body via the transcellular route [25–30]. Ligation of endothelial adhesion molecules such as Intracellular Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) by T cell integrins drives signaling events within the endothelial cells that promote the internalization of VE-cadherin [30–32]. Internalization of VE-cadherin loosens endothelial cell-cell junctions and thus facilitates subsequent diapedesis of T cells [30–32]. Extension of actin-dependent protrusions, such as filopodia and podosomes, may also induce changes within the endothelial cells to initiate diapedesis [16,25,29,30]. During diapedesis T cells extrude their body through and underneath the endothelium [16,29,30,33,34]. As T cells migrate beneath the endothelium, they retract their uropod to complete the process [23,29,30,33].
The nucleus is the most rigid cellular structure and, as such, presents a significant obstacle to the completion of diapedesis [23,33–37]. It has been proposed that the segmented and deformable shape of the neutrophil nucleus facilitates the rapid migration of these cells through restrictive environments [27,35,38]. In contrast, T cells exhibit a more rigid, ovoid nucleus and rely on significant contractile forces in the uropod to transmigrate the nucleus through the endothelium during diapedesis [22,33,36]. Thus, nucleus translocation can be a rate-limiting step for T cell migration through restrictive environments and, consequently, diapedesis [39,40].
Upon entering secondary lymphoid organs such as lymph nodes, T cells once again encounter a cell-packed environment, including dendritic cell networks and stromal cells such as fibroblastic reticular cells (FRCs), which guide their migration [41–43]. Soluble and cell surface-immobilized chemokines play a key role in guiding interstitial T cell migration in part by regulating actin cytoskeleton remodeling [44,45]. Within lymph nodes T cells undergo semi-random migration to maximize encounters with dendritic cells in search for their cognate antigen [46,47]. If T cells do not detect the presence of such antigens after 12–24 hours [48], naïve T cells will leave the lymph node through the lymphatic sinuses to enter lymphatic circulation and drain to another lymph node or return to the blood circulation through the thoracic duct and continue their patrolling. If, during their meandering migration within a lymph node, a T cell encounters an antigen-presenting cell (APC) bearing its cognate antigen, the T cell will temporarily stop crawling to interact with the APC and become activated [46,47,49,50]. The specifics of immunological synapse formation, T cell activation, and the role of the actin cytoskeleton in these processes has been reviewed in depth elsewhere, for example in references [51–53]. Within the lymph node, T cell interactions with dendritic cells can occur in three distinct phases. During the first phase, T cells engage in short, serial interactions with cognate DCs (ranging from about 30 minutes to 1 hour). Gradually, in the second phase, these transition into longer, more stable interactions, in which T cells remain in contact with DCs for 8–12 hours and begin producing IL-2 and other cytokines. Finally, in the third phase, T cells disassociate from DCs, and begin migrating rapidly and proliferating vigorously, still forming transient interactions with DCs that grow shorter over time [49]. These transient interactions, known as kinapses, observed in the first and third phases of primary T cell activation demonstrate the ability of a T cell to integrate activation signals from APCs over a series of interactions [54]. T cells that are being activated will remain in the lymph node for approximately 3 days, as regulated by the relative balance of S1PR and CD69 expression [4], before reentering circulation. Effector T cells will then be recruited from the circulation to the site of inflammation by extravasating through the vasculature of the inflamed tissue.
Finally, within peripheral tissues, recruited effector T cells (and resident memory T cells) encounter a variety of environmental conditions depending on the tissue composition of stromal cells, extracellular matrix (ECM), and inflammatory state [55,56]. In these tissues T cells will migrate towards the source of inflammation following chemotactic cues and navigating through complex ECM structures with varying sizes of openings to squeeze through [57,58]. To efficiently survey tissues, particularly in inflamed sites, T cells can adopt a type of motility termed Levy walk, which is a modified random walk that entails alternate periods of confined motility and more straight migration to optimize target searching efficiency [59]. Interestingly, T-helper (Th) 1 cells and Th2 cells in the inflamed skin can use different searching strategies, with Th1 cells having more confined chemokine-dependent motility and Th2 cells relying on integrin alpha-V/beta-3 to search a larger tissue volume. Hence, intrinsic differences resulting from T cell differentiation tune effector T cell sensitivity to environmental factors, leading to T cell subset-specific migration patterns [60]. In a recent in vivo study, intravital imaging revealed that the homeostatic patrolling of tissue resident memory T cells in the salivary glands is both integrin and chemokine independent but relies instead on the topology of resident macrophages to serve as a frictional interface to drive motility [61]. Similarly, the ECM provides a three-dimensional (3D) scaffold that can direct T cell migration within tissues, and this process is often modulated by environmental inflammation. For example, during toxoplasma infection, Secreted Protein, Acidic, Rich in Cysteine (SPARC), is upregulated in the brain, inducing the formation of a network of ECM fibers. These fibers serve as tracks to guide T cell migration towards the source of the infection [62]. However, these ECM tracks can also be appropriated as a method of immune evasion by tumors. As observed in the lung, tumors are often surrounded by densely aligned ECM fibers, restricting T cell migration to circling the perimeter, and stopping T cell infiltration in the tumor [63].
T cell motility modes:
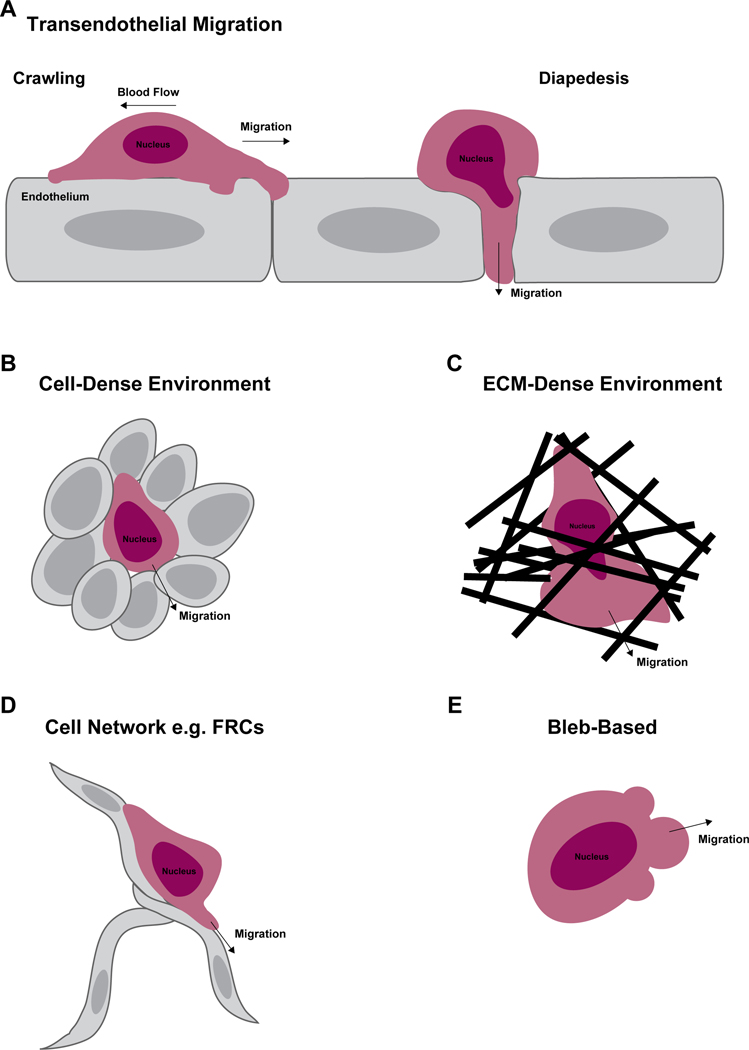
To adapt to different environmental conditions and optimize migration, T cells use various motility strategies that require actin network remodeling to generate mechanical forces and shape changes [22]. These motility modes include migration on flat surfaces (such as on endothelial cells), migration in cell-packed environments (as in lymph nodes), and migration through confined spaces (such as during diapedesis and motility through the ECM) (Figure 1 A–D). Furthermore, T cells can also adopt a bleb-based motility mode which is characterized by myosin-dependent hydrostatic pressure-driven round protrusions of the cell membrane which drive the cell forward (Figure 1E). Bleb-based motility in T cells has recently been suggested to be induced in response to S1P signaling [64].
Figure 1. T cell motility modes.
During their lifetime T cells encounter many different environments and physical barriers. Depending on the context, T cells can adopt various motility strategies that facilitate their migration. Depicted here are some of the main motility modes employed by T cells. To enter tissues from the blood stream T cells undergo the multistep process of transendothelial migration (TEM) (A). This process includes both the crawling phase where T cells migrate over the endothelial monolayer (a 2D type of migration) in response to chemotactic cues and the diapedesis phase where T cells squeeze across the endothelial barrier. Within tissue parenchyma T cells may encounter a cell-dense environment (B), such as in secondary lymphoid organs, or an extracellular matrix (ECM)-dense environment (C), such as in the skin. ECM-dense environments may contain restrictive openings requiring substantial morphological changes to enable migration. In some tissues, T cells migrate along connected cell networks such as the fibroblastic reticular cells (FRCs) found in lymph nodes (D). T cells have also been reported to be able to switch to a bleb-based migration (E), where the formation of membrane blebs rather than a lamellipod or pseudopod directs migration at the leading edge.
In terms of modulating motility, actin polymerization can only drive forward momentum in cells when it is coupled with a transfer of force to the surroundings. To date, in most cases the molecular mechanisms that regulate T cell motility modes in complex environments have been extrapolated by using in vitro model systems to mimic environmental conditions found in tissues. These studies show that migration on flat 2D surfaces, as with migration over endothelial blood vessel walls, requires some form of adhesion to transmit forces to the substrate to enable motility. In this context, integrins provide a clutch mechanism to resist vascular shear forces and transfer force to the substrate [29]. During this adhesion-dependent intravascular crawling on the endothelial cell wall, the T cell cytoskeleton modulates integrin function as well as motility [17,19]. In contrast, T cell migration is mostly adhesion-independent within lymph nodes [65,66], with integrins only accounting for ~15–35% of the motility speed in this setting [67]. A mechanism by which T cells can adapt their motility mode to their environment is the modulation of actin polymerization (mainly driven by chemotactic signals) in different conditions of force transfer to the substrate. In naïve T cells, the balance between CCR7 signaling and integrin adhesiveness regulates actin polymerization rate, with an inverse correlation between retrograde actin flow and adhesion to the substrate [68]. With increased adhesion to the surrounding environment, both the actin polymerization rate and retrograde flow decrease, allowing the T cell to efficiently translate actin polymerization into forward migration. However, in complex 3D environments T cells are not strictly dependent on substrate adhesion and can use shape changes and environmental topography to transmit cytoskeletal-generated forces to the surrounding environment for motility [69]. For example, using microchannels with smooth or serrated walls Reversat et al. showed that T cells are able to migrate normally in both conditions but T cells lacking talin, and thus unable to engage integrin adhesion, only migrate in the serrated wall channels [69]. This ability to transfer forces to the substrate even in the absence of integrin adhesion can enable T cell motility in tissues such as lymph nodes [65,66]. This could also be the case in some peripheral tissues where actin filaments can push directly against the ECM or other cells in their environment as T cells navigate through the tissue (as in Figure 4). However, in certain constrictive, ECM-dense environments found in non-lymphoid peripheral tissues such as the inflamed skin, it appears that integrin-dependent adhesion is important for efficient T cell motility [57].
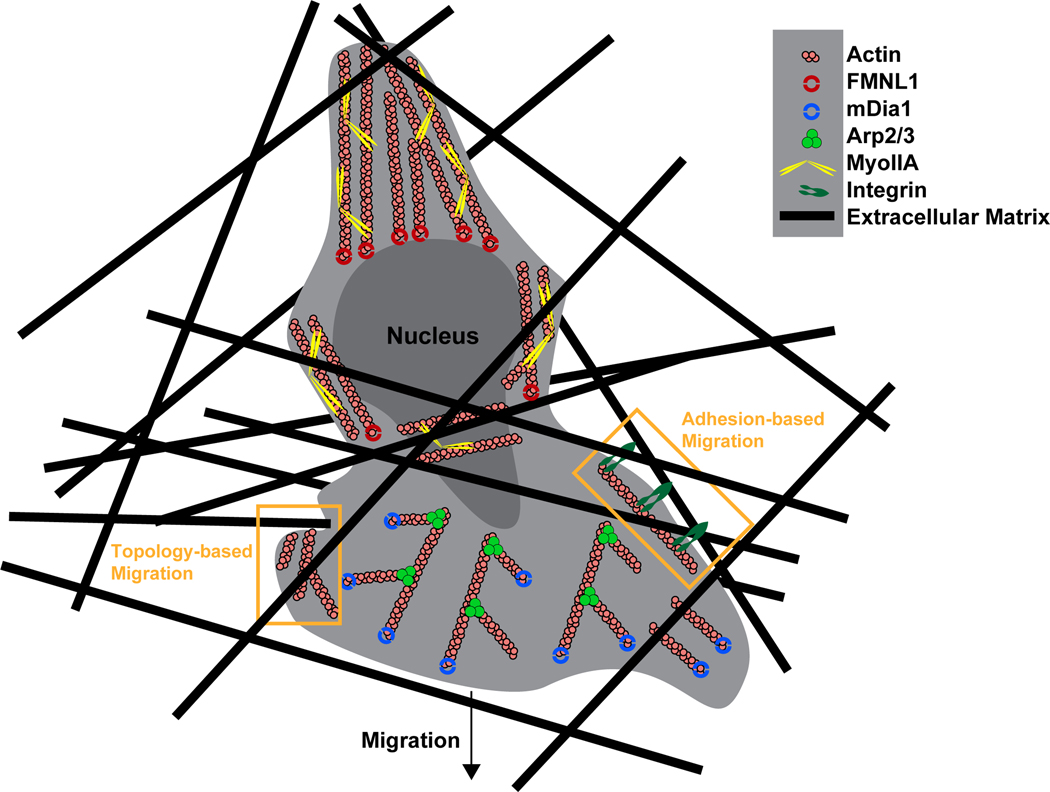
Figure 4. Model of the actin polymerization machinery involved in T cell interstitial migration.
During interstitial migration actin polymerization by Arp2/3 and mDia1 at the leading edge of the cell promote expansion of the leading edge and formation of protrusions to facilitate environmental sensing. Depending on the tissue environment, actin polymerization can enable motility in the absence of adhesions, in that actin structures may create a sufficient frictional interface by pushing directly against the surroundings, such as the ECM or other cells (topology-based migration). However, in constrictive ECM-dense environments such as the skin, integrin adhesion can play an important role in interstitial migration, as greater force transfer may be required to enable migration. During this adhesion-based migration, adhesion receptors such as chemokine-activated integrins, which are coupled to actin structures, can bind to the ECM, serving as a clutch to engage the ‘motor’ of actin polymerization, and provide the traction force necessary to move cells forward. At the rear of the cell, actin filament contraction by MyoIIA allows cellular and nucleus deformation for navigating small openings. Similar to its role in diapedesis, FMNL1 is likely also involved in facilitating squeezing of the nucleus through constrictive barriers within tissues.
Various studies have imaged the motility behavior of T cells in lymph nodes as well as in some peripheral tissues in the presence and absence of antigen. However, mainly due to technical challenges, there is little direct observation of actin dynamics in vivo. Recently, Yan et al. have visualized actin dynamics in T cells during extravasation and migration within lymph nodes [70] using T cells from LifeAct-GFP mice [71], which enables real-time visualization of sites of actin polymerization. This in vivo LifeAct imaging showed that during the diapedesis step F-actin is enriched at the sides of the T cell as it squeezes through the HEV endothelial barrier in lymph nodes. In contrast, imaging during lymph node migration suggested highly dynamic actin rearrangements and protrusion formation [70]. Overall, these approaches and tools will be key in determining the molecular mechanisms of how actin polymerization effectors regulate T cell motility within tissues in vivo. However, in relation to the actin polymerization machinery, only mDia1 knock-out (KO) T cells have been imaged during lymph node migration thus far [72]. Thus, the relative contribution of specific actin polymerization machinery to different motility strategies within tissue environments is still mostly unknown. Another outstanding question is how T cells sense the physical characteristics of their environment (such as topology and stiffness) and signal to engage their cytoskeletal machinery to adapt their motility.
Actin Networks and Cytoskeletal Effectors Overview:
Actin is found in two forms in cells, monomeric globular actin (G-actin) and polymerized filamentous actin (F-actin). Nucleation of new actin filaments is thermodynamically unfavorable. However, once an initial oligomer has formed, actin polymerization can proceed in a polarized fashion, with polymerization occurring 10x faster at the dynamic barbed end than the less active pointed end [73]. To control this dynamic network, cells rely on effectors of actin polymerization to efficiently nucleate and elongate actin filaments, as well as actin filament capping proteins to stop polymerization or severing proteins to break filaments. Like other cells, actin networks in T cells are comprised of branched and linear filaments and different networks can play selective functions in T cell migration [24,74]. Importantly, the amount of actin monomer available for use in actin filaments at steady state is limited; thus, branched and linear polymerization remain in competition with each other. Consequently, inhibition of one cytoskeletal effector protein can lead to deregulation and overactivity of others, complicating our understanding of individual protein functions [75]. Immediately beneath the plasma membrane, a network of actin filaments known as the cortical cytoskeleton is important for cell shape maintenance and changes. At the leading edge, a branched actin network is responsible for the generation of pseudopod protrusions. In 2D migration settings this takes the form of a lamellipodium, a large and flat membrane structure important for motility. Furthermore, the polymerization of actin at the leading edge generates force to push the T cell membrane forward [76]. In the uropod, actin filaments are cross-linked by the motor protein non-muscle Myosin-IIA (MyoIIA) to generate contractile forces at the back of the cell [23]. As discussed above, the combination of these forces when transmitted to the extracellular environment facilitates the migration of T cells.
Branched actin networks are initiated through the activity of the actin related protein 2/3 (Arp2/3) complex. Downstream of Rac and Cdc42 signaling, Wiskott-Aldrich syndrome protein (WASP) and WAVE/SCAR family proteins bind Arp2/3 to form an activated complex that can recruit profilin-bound actin monomers to nucleate new actin filaments (Figure 2). This complex binds to pre-existing acting filaments to nucleate new filaments at an angle of 70 degrees, thus developing a branched actin network [73,76]. In migrating T cells, these branched actin networks expand at the leading edge of the cell, pushing the membrane forward to create the lamellipodium [77]. Arp2/3 and branched actin networks may also be involved in initiating filopodia, thin membrane projections containing linear actin at the leading edge of cells [78]. Additionally, WASP and Arp2/3 contribute to the formation and maintenance of the immune synapse [79,80].
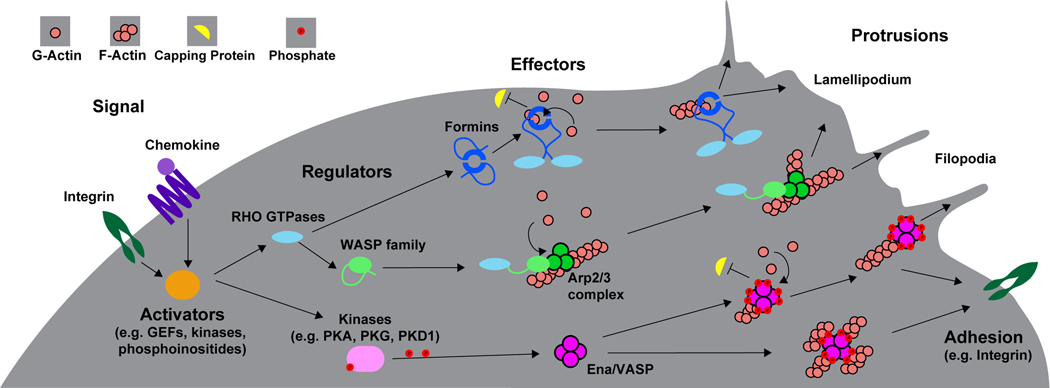
Figure 2. Regulation of actin polymerization effectors and their functions.
Upon binding their ligands, chemokine receptors and integrins induce local phosphoinositide production, kinase activity and guanine nucleotide exchange factor (GEFs) activity. The GEFs can in turn activate Rho GTPases which orchestrate cytoskeletal rearrangements through the activation of terminal effector proteins that directly interact with the actin cytoskeleton. In the case of formins, direct binding by Rho GTPases disrupts an autoinhibitory interaction enabling formin homodimers to nucleate new actin filaments (F-actin) and processively elongate them via recruitment of actin monomers (G-actin) (typically bound to profilin, not depicted) and by anti-capping activity. Rho GTPases can also disrupt the autoinhibitory regulation of WASP family members enabling them to associate with the Arp2/3 complex causing conformational changes that allow Arp2/3 to bind to F-actin. This complex recruits G-actin to nucleate a new F-actin branch from the existing actin filament. Kinases such as PKA, PKG and PKD1 downstream of integrin or chemokine signaling can phosphorylate Ena/VASP family members at multiple sites enabling them to bind actin. Ena/VASP proteins can then promote actin filament elongation via recruitment of G-actin as well as anti-capping activity. The tetrameric structure of Ena/VASP facilitates bundling of multiple actin filaments together. Actin polymerization by these various effectors pushes on the cell membrane, driving membrane protrusion formation including the broad fan-like structure of the lamellipodium and long thin membrane protrusions such as filipodia. Additionally, actin polymerization and crosslinking can affect integrin adhesion or transmit force to the surrounding environment.
Linear actin polymerization is mediated by two major families in T cells: the Ena/VASP family and the formin family [81–83]. These cytoskeletal effector families can also contribute to branched actin networks by elongating filaments nucleated by Arp2/3. The Ena/VASP family is comprised by three members: Vasodilator-stimulated phosphoprotein (VASP), Ena/VASP-like (EVL), and mammalian-enabled (Mena). However, Mena is not typically expressed in lymphocytes [81,84]. These proteins facilitate monomeric actin recruitment to the barbed end of the actin filament, prevent actin filament capping, and can play a role in actin filament bundling [85–89] (Figure 2). EVL and VASP share significant structural homology and are defined by an N-terminal EVH1 domain, which regulates cellular localization, and a C-terminal EVH2 domain, which facilitates tetramerization, binds F-actin, and is thought to be responsible for actin polymerization [90–93]. Several phosphorylation sites are present in Ena/VASP proteins which regulate localization as well as actin binding [90,94,95]. These phosphorylation sites are regulated by kinases such as PKA, PKG and PKD1 [96].
Formins remodel the actin cytoskeleton both by nucleating new actin filaments and processively elongating them via recruitment of profilin-bound G-actin and by anti-capping activity [97] (Figure 2). While mammals have 15 formin family members [98], two are highly expressed in primary T cells: Diaphanous-related formin 1 (mDia1, Diaph1) and Formin like-1 (FMNL1) [99–103]. T cell can also express the formins INF2 and FHOD1 at lower levels [104,105]. Formins form homodimers to carry out their function [97,98] and regulation of these formins is controlled by an autoinhibition mechanism [97,98,106–108]. Binding of Rho GTPases to a GTPase binding domain (GBD) disrupts an autoinhibitory interaction between the N-term and C-term domains to activate the formin [97,98,107,108]. GTPase binding has also been suggested to regulate the localization of mDia1 and FMNL1 to the cell membrane [106]. In this manner, formins serve as terminal cytoskeletal effectors for Rho GTPases to induce actin network remodeling.
Regulation of Migration and Trafficking by Actin Cytoskeletal Effectors:
We will now discuss what is currently known about how specific actin polymerization effectors, as well as some of their upstream regulators, modulate T cell migration during trafficking through various anatomical sites. Specifically, in this review, we focus on the roles of the direct actin polymerization machinery including Arp2/3, formins, and Ena/VASP proteins. We also include some examples of the roles played by upstream cytoskeletal regulators, particularly where direct evidence for the role of actin polymerization effectors is currently limited. A summary of this knowledge can be found in Table 1.
Table 1.
Summary of the roles in T cell migration and trafficking of actin polymerization effectors and Myosin-IIA
| Effector | Arp2/3 | FMNL1 | mDia1 | EVL | VASP | MyoIIA |
|---|---|---|---|---|---|---|
| Localization | enriched at leading edge | enriched at uropod, perinuclear | enriched at leading edge | unknown | unknown | enriched at uropod |
| Cellular Role | branched actin filaments, lamellipodia | linear actin filaments | linear actin filaments, lamellipodia | linear actin filaments | linear actin filaments | actin crosslinking, contraction |
| Role in Thymus | thymic egress (based on upstream regulator phenotype) | dispensable | thymic egress | dispensable | dispensable | Germline KO is embryonic lethal |
| Role in trafficking | naïve T cell trafficking to lymphoid tissues (based on upstream regulators) | effector T cell trafficking to restrictive non-lymphoid tissues | effector and naïve t cell trafficking to lymphoid and non-lymphoid tissues | effector T cell trafficking to lymphoid and non-lymphoid tissues (redundant with VASP) | effector T cell trafficking to lymphoid and non-lymphoid tissues (redundant with EVL) | effector T cell trafficking to lymphoid and non-lymphoid tissues |
| Role in Transendothelial Migration | chemotaxis | diapedesis, nucleus passage | adhesion, chemotaxis | diapedesis, α4 integrin function (redundant with VASP) | diapedesis, α4 integrin function (redundant with EVL) | diapedesis, nucleus passage |
| Role in Interstitial Migration | general motility, cellular integrity | unknown, likely involved in constrictive environments | motility in lymph nodes, unknown in other tissues | unknown | unknown | motility in lymph nodes, unknown in other tissues |
| Primary References | [78,111–116,121–126] | [110] | [100,102,109,120,145] | [117] | [117] | [23,33,129] |
1. Thymocyte development and egress
While thymocyte development is not impaired in T cells deficient in mDia1, this formin has a role in thymic egress. This is evidenced by the observation that mDia1 KO mice have increased mature thymocytes in the thymus, reduced peripheral T cell populations, and that mDia1-deficient thymocytes have impaired chemotaxis in vitro [100,109]. However, FMNL1, the other highly expressed formin in T cells, does not appear to regulate T cell thymic development or egress, suggesting that these formins maintain distinct roles [110].
Arp2/3 complex deficiency in humans, mainly in the form of mutations in the hematopoietic-specific regulatory component ARPC1B, has been reported to cause T cell development abnormalities with reduced naïve T cells in the periphery of some patients [111,112]. However, ARPC1B and ARPC2 deficient mice appear to have normal T cell development and egress but have impaired T cell numbers and T cell functions in the periphery [113,114], possibly suggesting issues with T cell homeostasis and survival. Nevertheless, a role in thymic egress for the Arp2/3 regulator Coronin 1A has been identified in both mice and humans [115,116].
Ena/VASP proteins do not appear to be necessary for thymocyte development since double-knock out mice for EVL and VASP (the two Ena/VASP proteins expressed in T cells) do not have defects in generating mature T cells in the thymus or in the ability of these T cells to egress into the periphery [117].
Regarding other actin filament regulators, a conditional KO of Cofilin-1, an actin depolymerizing factor, has been shown to regulate thymocyte motility in collagen matrices and promote differentiation to the single positive thymocyte stage [118]. Furthermore, mice lacking L-plastin, an actin filament bundling protein, show reduced thymic egress [119].
2. Extravasation
T cells rely on the extensive vascular network to patrol for pathogens and to reach disparate tissues throughout the body. Whether for a naïve T cell entering a lymph node or an activated T cell entering an inflamed tissue, the process of extravasation is key for the subsequent execution of their functions. As discussed above, the process of transendothelial migration (TEM) consists of 4 sequential stages: rolling, adhering, crawling, and diapedesis [7]. While the stages of TEM are similar in different tissues, differences in vascular architecture and barriers to extravasation can further define the roles and requirements for different cytoskeletal effectors. The use of multi-photon microscopy has enabled visualization of the extravasation process in vivo. However, in many non-lymphoid tissues, extravasation events can be rare and optical resolution limited. Thus, much of our mechanistic understanding of the function of cytoskeletal effectors in TEM comes from in vitro model systems of this process.
Some cytoskeletal effectors seem to have broad roles throughout the process of TEM while others have more selective functions. Formins can have important roles in T cell trafficking through various mechanisms. Transwell assays suggest mDia1-deficient T cells display impaired chemotaxis even though chemokine receptor expression levels are unaltered [72,100,109]. Likewise, integrin expression appears to be unaltered in mDia1-deficient T cells, although adhesion to integrin ligands is impaired [72,102,109]. In vivo, loss of mDia1 in T cells impairs trafficking to both lymphoid organs and inflamed skin [72,100]. Thus, mDia1 seems to have a role in general responsiveness to chemokines as well as adhesion, which leads to broad trafficking defects in its absence. While the role of mDia1 in the TEM process hasn’t been directly analyzed in T cells, data from B cell leukemia show that mDia1 deficiency causes a defect in TEM completion [120].
Compared to the other cytoskeletal effectors, FMNL1 appears unique in that it seems specifically involved in the trafficking of activated T cells and only to non-lymphoid tissues [110]. In vitro models suggest that the requirement for FMNL1 is dependent on the restrictiveness of the barrier to be crossed, with FMNL1 deficiency only impairing T cell migration through narrow pores or constrictions, such as those found in non-lymphoid tissue endothelial barriers. This is consistent with in vitro TEM experiments where FMNL1 was only important for the diapedesis step of TEM [110]. During this process FMNL1 localized directly behind the nucleus, whose rigid structure has been proposed to be a significant barrier to cellular deformation required for completing diapedesis [35]. Indeed, in the absence of FMNL1 T cells were impaired in squeezing their nuclei through the endothelial barrier [110].
Regarding the Arp2/3 complex, its role in TEM has not been directly addressed. However, TEM studies on lymphocytes from WAS patients (characterized by mutated or deficient WASP) suggest that the Arp2/3 regulator WASP is particularly important in mediating transcellular diapedesis [25]. Interestingly, in vivo studies with lymphocytes from WASP KO mice showed altered trafficking to Peyer’s patches but not to other secondary lymphoid organs [121]. Additionally, previous in vitro studies on WASP and WIP (WASP-interacting protein) support that the Arp2/3 complex plays a key role in T cell migration in 2D settings [121,122]. However, data from an actin binding mutant of WIP suggest that WIP may regulate migration through additional WASP-independent mechanisms [123]. More recent in vitro studies using 2D migration and transwell assays show that the Arp2/3 complex modulates mouse and human T cell migration and chemotaxis [112,124], partly through regulation of lamellipodia formation [124,125]. Mutations in Hem1, a key component of the WAVE regulatory complex, also cause defects in membrane protrusions and reduced 2D migration, and can lead to immunodeficiency [126]. Mutations in WASP and other regulators of Arp2/3 function, such as WIP and Coronin 1A, have also been shown to cause immunodeficiency, due in part to impaired lymphocyte migration and trafficking. As is the case for WIP, since Coronin 1A has additional non-Arp2/3 related functions it is possible that Coronin 1A deficiency affects motility through a different mechanism, such as actin filament disassembly [127]. Taken together these studies support a potentially broad role for Arp2/3 in regulating T cell chemotaxis and motility. However, its requirement at specific stages of TEM may be somewhat tissue dependent. Some of these tissue specific roles may depend on which WASP family members and other members of the regulatory complex are utilized downstream of tissue-specific cues and characteristics.
Ena/VASP protein deficiency selectively impairs activated T cell trafficking [117]. This is likely because loss of EVL and VASP impairs the expression and function of alpha-4 integrin, which is only expressed on activated T cells. These trafficking defects extended to both lymphoid and non-lymphoid tissues. Surprisingly, in a model of TEM, this alteration of integrin alpha-4 does not impact EVL/VASP-deficient T cell adhesion to, or crawling on, the endothelium but rather inhibits the diapedesis step [117]. This suggests that instead of affecting 2D motility, Ena/VASP proteins have either a role in providing anchoring support for the physically demanding diapedesis step, or a role in endothelial cell/T cell cross-talk via integrin ligands, which can lead to the internalization of endothelial junctional molecules [30].
Finally, from 2D migration models, MyoIIA-driven contractile forces have been shown to contribute to the retraction of the uropod and to limit adhesion as the T cell moves forward [23,33,128]. Additionally, contractile forces generated by MyoIIA also promote transmigration of the T cell nucleus across the endothelium during TEM [33]. In vivo, MyoIIA deficiency impairs activated T cell trafficking to lymphoid and non-lymphoid organs [33,129]. Together, these findings suggest a role for MyoIIA in both the crawling and diapedesis steps at disparate tissue sites.
While many effectors of actin polymerization have been implicated in T cell trafficking, how they impact specific stages of TEM is still being elucidated. In combining our current knowledge from both in vitro and in vivo systems it appears some effectors such as Arp2/3 and mDia1 have roles at multiple stages of TEM while others such as FMNL1 and EVL/VASP only seem to be critical for diapedesis (Figure 3). Consistent with the idea of diapedesis being a rate-limiting step in TEM, it seems to be particularly sensitive to perturbations in actin cytoskeletal machinery as evidenced by multiple effectors being important for this process (e.g. FMNL1, EVL/VASP, Arp2/3, and MyoIIA). While it is well established that integrin-mediated adhesion and coupling with the cytoskeleton are important for the initial attachment and crawling phases, there appears to be substantial redundancy among effectors of actin polymerization in achieving these initial TEM steps. Further use of actin polymerization reporters in conjunction with disruption of specific actin effectors or fluorescent reporters of their activity will be critical for understanding the contributions and mechanisms of individual effectors in mediating the stages of TEM.
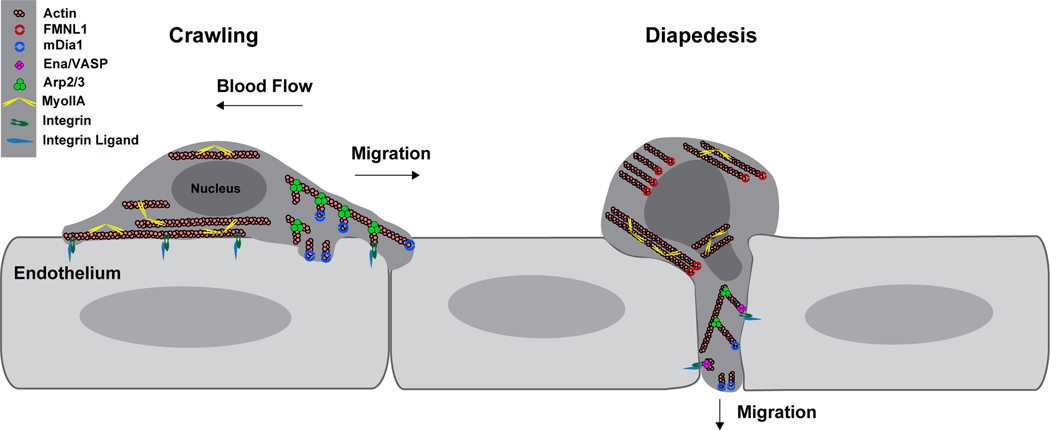
Figure 3. Model of the actin polymerization machinery involved in T cell transendothelial migration.
During the crawling phase of diapedesis (left half of figure), actin polymerization at the leading edge of the cell by Arp2/3 and likely mDia1 promote expansion of the lamellipod, driving the T cell forward. In this 2-dimensional migration setting, adhesion to the substrate, typically through integrins, is critical to couple actin polymerization into forward motion. Linear actin filaments extended by mDia1 may also be involved in generating membrane protrusions and providing adhesive connections to the endothelium and sensing of the environment. At the rear of the cell, contraction of actin filaments by MyoIIA provides force and detaches the uropod from the endothelium facilitating forward migration. During the diapedesis step (right half of figure), actin polymerization at the leading edge by Arp2/3 and mDia1 drives extension of the cell body through the endothelium. Through regulation of α4 integrins, EVL/VASP proteins may provide anchoring support or crosstalk with the endothelium. Transmigration of the rigid structure of the nucleus through the endothelial barrier is a rate-limiting step in diapedesis. Contraction of cortical actin by MyoIIA deforms the nucleus enabling passage through the narrow space in the endothelial barrier. FMNL1 also promotes nucleus passage during diapedesis, either by providing actin filaments for MyoIIA to contract or by providing independent force generation via actin polymerization at the rear of the cell.
3. Migration within tissues
After leaving the blood stream and entering tissues, T cells encounter environments with very different characteristics, including varying composition and densities of ECM as well as stromal cells with different morphology and distribution. A challenge that T cells encounter while migrating within non-lymphoid tissues is the need to navigate through small openings within the tissue parenchyma. Similarly to diapedesis, the T cell nucleus can be an obstacle and a rate limiting step for migration through small openings [35,40]. To migrate through these small openings within the tissue parenchyma, T cells need to deform their nucleus and/or mechanically enlarge the opening. Both events require force generation and the actin polymerization effectors involved in this process are only beginning to be elucidated. In dendritic cells the Arp2/3 complex has been shown to have a key role in mediating perinuclear actin polymerization to squeeze the nucleus through constrictions [130]. In T cells, using a microchannel system with constriction points, we have recently shown that actin polymerization by FMNL1 allows efficient pushing of the T cell nucleus through such constriction points [110]. An important open question is related to how T cells sense environmental cues such as the topology, stiffness, adhesiveness, and mechanical forces at play in their surroundings, a process generally known as mechanosensing. Leukocytes have been shown to be able to discriminate between constriction points of different sizes and select the more permissive ones, with front positioning of their nucleus promoting this pore selection process [131]. Interestingly, formins have the capacity to sense and respond to mechanical cues [132]. In particular, mDia1 can respond to pulling forces by increasing actin filament polymerization rate and exerting tension on actin filaments [133]. In the context of nucleus deformation during migration, we can thus speculate that formins, and specifically FMNL1, may act as a mechanosensing hub to enable T cells to squeeze their nucleus when confronted with constriction points. However, the molecular mechanism and actin structures at play for nucleus deformation and squeezing in T cells are still unclear.
Using in vivo imaging, efficient motility within lymph nodes has been shown to require the activity of mDia1 [72]. Although mDia1 regulates actin polymerization in response to chemokine stimulation in T cells [100], its function in T cell migration is also related to mDia1’s regulation of microtubule dynamics [72]. As discussed in the section on extravasation, previous in vitro studies on the Arp2/3 complex and its regulators indicate that the Arp2/3 complex plays a role in T cell migration. Recent work using Arp3 knock-down and a zebrafish transfer model shows that the Arp2/3 complex is also needed for efficient motility in vivo. This manifested as a switch to a bleb-based motility with reduced speed and displacement by Arp3 knock-down T cells [125]. The role of actin polymerization effectors such as FMNL1 or Ena/VASP proteins has not yet been directly assessed in interstitial motility in vivo. However, many other cytoskeletal effectors and upstream regulators can promote efficient T cell motility in tissues. Due to length limitations, in this review we will only discuss a few key examples. MyoIIA and its regulator Rho-associated protein kinase (ROCK) have a role in regulating motility and interaction with tissue components in lymph nodes and in the lung [23,129,134]. Rho GTPases, such as Rac1 and Rac2, and their regulators such as the guanine exchange factor DOCK2 also promote efficient T cell motility in lymph nodes [135,136]. Instead, rather than affecting chemotaxis, DOCK8 is key in maintaining cell shape and integrity, and in preventing cell death during migration in confined environments [137]. Furthermore, upstream cytoskeletal regulators such as Coronin 1A and the actin bundling protein L-plastin are important for interstitial migration of T cells [115,119].
Overall, based on currently available data, Arp2/3 and mDia1 are likely to exert their functions at the leading edge of the T cell by generating protrusions for environmental sensing and actin filament treadmilling for propulsion [124,125]. On the other hand, FMNL1 acts at the T cell rear, independently or in concert with MyoIIA [33], to generate force to push or deform the nucleus and promote passage through small openings [110] (Figure 4). Little is known about the role of Ena/VASP proteins in interstitial T cell migration; however, while important for diapedesis, this protein family is not required for 2D migration of T cells [117].
Closing Remarks and Open Questions
T cells can migrate at high speed within various environments by adapting their motility mode to the biophysical characteristics of the environments they encounter. The actin cytoskeleton through actin polymerization and acto-myosin contraction provides the shape changes and forces necessary for migration. Great strides have been made in understanding the molecular mechanisms that regulate T cell motility, especially through the use of advanced imaging techniques in vivo and in vitro (e.g. multi-photon, TIRF, super-resolution and lattice lightsheet microscopy), the ingenious use of microfabricated structures to model different environmental parameters, and the development of biosensors such as the LifeAct-GFP construct [71]. However, there is still a lack of understanding at the molecular level of the specific functions of individual actin polymerization effectors and of how these effectors may work together or through parallel mechanisms to drive motility and pathfinding within complex environments. For example, it is still unclear which specific actin polymerization effectors contribute to actin filament elongation and retrograde flow shown to be crucial for different T cell motility modes. Additionally, measurements of the forces generated by the actin cytoskeleton and transferred to the environment in T cells are still scarce. Furthermore, the actin structures and mechanisms required for nucleus squeezing through small openings in tissues or during diapedesis are only beginning to be uncovered. Mechanosensing is also emerging as a key property of T cells to efficiently navigate complex tissue environments, but very little is known about how T cells sense these mechanical cues, or how actin cytoskeletal effectors work to respond to these cues. Finally, while small molecule inhibitors such as blebbistatin, CK-666 and SMIFH2 [138–140] have provided valuable insights into potential roles for cytoskeletal effectors in some in vitro contexts, the broad specificity, potential for toxicity and poor bioavailability mean these tools should be used with caution and are of limited use for in vivo studies [140–142].
The continued use and development of advanced imaging techniques, such as the combination of adaptive optics with multi-photon microscopy [143] and lattice lightsheet microscopy [144], paired with the use of biosensors, optogenetics, and biophysical readouts will allow the development of a more comprehensive understanding of the molecular mechanisms by which each actin effector contributes to the efficient motility and spatiotemporal localization of T cells. The ability to interrogate the role of cytoskeletal effectors in actin dynamics and motility behaviors in vivo, paired with the ability to image the various elements and structures of the surrounding environment will greatly increase our mechanistic knowledge of T cell motility within physiological environments. As migration is an essential component of T cell function, this improved molecular understanding of T cell migration could then be translated into therapeutic approaches for clinical intervention.
Acknowledgments:
This work was supported in part by NIH grant R01AI125553 (to JJ). MMW was supported in part by NIH Training Grant T32AI007405. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- 3D
three-dimensional
- APC
antigen-presenting cell
- Arp2/3
actin related protein 2/3
- DCs
dendritic cells
- mDia1
Diaphanous-related formin 1
- ECM
extracellular matrix
- EVL
Ena/VASP-like
- FMNL1
Formin like-1
- FRCs
fibroblastic reticular cells
- F-actin
filamentous actin
- G-actin
globular actin
- GBD
GTPase binding domain
- HEV
high endothelial venules
- ICAM-1
Intracellular Adhesion Molecule-1
- KO
knock-out
- Mena
mammalian-enabled
- MyoIIA
Myosin-IIA
- ROCK
Rho-associated protein kinase
- S1P
sphingosine-1-phosphate
- SPARC
Secreted Protein, Acidic, Rich in Cysteine
- TCR
T cell receptor
- TECs
thymic epithelial cells
- TEM
transendothelial migration
- Th
T-helper
- VASP
Vasodilator-stimulated phosphoprotein
- VCAM-1
Vascular Cell Adhesion Molecule-1
- WASP
Wiskott-Aldrich syndrome protein
- WIP
WASP-interacting protein
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Takahama Y (2006) Journey through the thymus: Stromal guides for T-cell development and selection. Nat Rev Immunol 6, 127–135. [DOI] [PubMed] [Google Scholar]
- 2.He L, Valignat M, Zhang L, Gelard L, Zhang F, Le Guen V, Audebert S, Camoin L, Fossum E, Bogen B, Wang H, Henri S, Roncagalli R, Theodoly O, Liang Y, Malissen M & Malissen B (2021) ARHGAP45 controls naïve T- and B-cell entry into lymph nodes and T-cell progenitor thymus seeding. EMBO Rep 22, e52196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK & Robey EA (2009) The impact of negative selection on thymocyte migration in the medulla. Nat Immunol 10, 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyster JG & Schwab SR (2012) Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annu Rev Immunol 30, 69–94. [DOI] [PubMed] [Google Scholar]
- 5.Schwab SR & Cyster JG (2007) Finding a way out: Lymphocyte egress from lymphoid organs. Nat Immunol 8, 1295–1301. [DOI] [PubMed] [Google Scholar]
- 6.Kato S (1997) Thymic microvascular system. Microsc Res Tech 38, 287–299. [DOI] [PubMed] [Google Scholar]
- 7.Nourshargh S & Alon R (2014) Leukocyte Migration into Inflamed Tissues. Immunity 41, 694–707. [DOI] [PubMed] [Google Scholar]
- 8.Abadier M, Pramod AB, McArdle S, Marki A, Fan Z, Gutierrez E, Groisman A & Ley K (2017) Effector and Regulatory T Cells Roll at High Shear Stress by Inducible Tether and Sling Formation. Cell Rep 21, 3885–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickard JE & Ley K (2009) Micro-PTV Measurement of the Fluid Shear Stress Acting on Adherent Leukocytes In Vivo. Biophys J 96, 4249–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley K & Gaehtgens P (1991) Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res 69, 1034–1041. [DOI] [PubMed] [Google Scholar]
- 11.Gallatin WM, Weissman IL & Butcher EC A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304, 30–4. [DOI] [PubMed] [Google Scholar]
- 12.Ley K (2003) The role of selectins in inflammation and disease. Trends Mol Med 9, 263–8. [DOI] [PubMed] [Google Scholar]
- 13.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA & Ley K (2008) PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRγ to induce slow leukocyte rolling. J Exp Med 205, 2339–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vajkoczy P, Laschinger M & Engelhardt B (2001) Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest 108, 557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao X, Moseman EA, Saito H, Petryniak B, Petryanik B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, von Andrian UH & Fukuda M (2010) Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33, 817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G & Alon R (2009) Lymphocyte Crawling and Transendothelial Migration Require Chemokine Triggering of High-Affinity LFA-1 Integrin. Immunity 30, 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alon R & Shulman Z (2011) Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res 317, 632–641. [DOI] [PubMed] [Google Scholar]
- 18.Alon R & Ley K (2008) Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr Opin Cell Biol 20, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicente-Manzanares M, Choi CK & Horwitz AR (2009) Integrins in cell migration - The actin connection. J Cell Sci 122, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD & Hogg N (2005) A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol 170, 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, Klein E, Shinder V, Stoler-Barak L, Feigelson SW, Meshel T, Nurmi SM, Goldstein I, Hartley O, Gahmberg CG, Etzioni A, Weninger W, Ben-Baruch A & Alon R (2012) Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol 13, 67–76. [DOI] [PubMed] [Google Scholar]
- 22.Krummel MF, Friedman RS & Jacobelli J (2014) Modes and mechanisms of T cell motility: roles for confinement and Myosin-IIA. Curr Opin Cell Biol 30C, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano SF, Hons M, Schumann K, Kumar V, Dennier TJ, Lyck R, Sixt M & Stein J V (2011) In vivo analysis of uropod function during physiological T cell trafficking. J Immunol 187, 2356–64. [DOI] [PubMed] [Google Scholar]
- 24.Dupré L, Houmadi R, Tang C & Rey-Barroso J (2015) T lymphocyte migration: an action movie starring the actin and associated actors. Front Immunol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carman C V, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM& Springer TA(2007) Transcellular Diapedesis Is Initiated by Invasive Podosomes. Immunity 26, 784–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoefl GI & Miles W an A by RE(1972) The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A reexamination. J Exp Med 136, 568–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng D, Nagy JA, Pyne K, Dvorak HF & Dvorak AM (1998) Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med 187, 903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodfin A, Voisin M-B, Beyrau M, Colom B, Caille D, Diapouli F-M, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA & Nourshargh S (2011) The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nourshargh S, Hordijk PL & Sixt M (2010) Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol 11, 366–378. [DOI] [PubMed] [Google Scholar]
- 30.Vestweber D (2015) How leukocytes cross the vascular endothelium. Nat Rev Immunol 15, 692–704. [DOI] [PubMed] [Google Scholar]
- 31.Vockel M & Vestweber D (2013) How T cells trigger the dissociation of the endothelial receptor phosphatase VE-PTP from VE-cadherin. Blood 122, 2512–22. [DOI] [PubMed] [Google Scholar]
- 32.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF & Vestweber D (2014) Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol 15, 223–30. [DOI] [PubMed] [Google Scholar]
- 33.Jacobelli J, Estin Matthews M, Chen S & Krummel MF (2013) Activated T Cell Trans-Endothelial Migration Relies on Myosin-IIA Contractility for Squeezing the Cell Nucleus through Endothelial Cell Barriers. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barzilai S, Yadav SK, Morrell S, Roncato F, Klein E, Stoler-Barak L, Golani O, Feigelson SW, Zemel A, Nourshargh S & Alon R (2017) Leukocytes Breach Endothelial Barriers by Insertion of Nuclear Lobes and Disassembly of Endothelial Actin Filaments. Cell Rep 18, 685–699. [DOI] [PubMed] [Google Scholar]
- 35.Friedl P, Wolf K & Lammerding J (2011) Nuclear mechanics during cell migration. Curr Opin Cell Biol 23, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S, Marcel N, Talwar S, Garg RIM. Perumalsamy LR, Sarin A & Shivashankar GV. (2012) Developmental Heterogeneity in DNA Packaging Patterns Influences T-Cell Activation and Transmigration. PLoS One 7, e43718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav SK, Feigelson SW, Roncato F, Antman-Passig M, Shefi O, Lammerding J & Alon R (2018) Frontline Science: Elevated nuclear lamin A is permissive for granulocyte transendothelial migration but not for motility through collagen I barriers. J Leukoc Biol 104, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jill Mackarel A, Cottell DC, Russell KJ, FitzGerald MX & O’Connor CM (1999) Migration of Neutrophils across Human Pulmonary Endothelial Cells Is Not Blocked by Matrix Metalloproteinase or Serine Protease Inhibitors. Am J Respir Cell Mol Biol 20, 1209–1219. [DOI] [PubMed] [Google Scholar]
- 39.Wolf K, Müller R, Borgmann S, Bröcker E-B, Friedl P, Urbano-Márquez A, Yagüe J & Cid MC (2003) Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–9. [DOI] [PubMed] [Google Scholar]
- 40.Wolf K, te Lindert M, Krause M, Alexander S, te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ & Friedl P (2013) Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201, 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajénoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N & Germain RN (2006) Stromal Cell Networks Regulate Lymphocyte Entry, Migration, and Territoriality in Lymph Nodes. Immunity 25, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajénoff M, Glaichenhaus N & Germain RN (2008) Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol 181, 3947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katakai T, Habiro K & Kinashi T (2013) Dendritic Cells Regulate High-Speed Interstitial T Cell Migration in the Lymph Node via LFA-1/ICAM-1. J Immunol 191, 1188–1199. [DOI] [PubMed] [Google Scholar]
- 44.Worbs T, Mempel TR, Bölter J, Von Andrian UH & Förster R (2007) CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med 204, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada T & Cyster JG (2007) CC Chemokine Receptor 7 Contributes to Gi-Dependent T Cell Motility in the Lymph Node. J Immunol 178, 2973–2978. [DOI] [PubMed] [Google Scholar]
- 46.Miller MJ, Wei SH, Parker I & Cahalan MD (2002) Two-Photon Imaging of Lymphocyte Motility and Antigen Response in Intact Lymph Node. Science (80- ) 296, 1869–1873. [DOI] [PubMed] [Google Scholar]
- 47.Stoll S, Delon J, Brotz TM & Germain RN (2002) Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science (80- ) 296, 1873–1876. [DOI] [PubMed] [Google Scholar]
- 48.Mandl JN, Liou R, Klauschen F, Vrisekoop N, Monteiro JP, Yates AJ, Huang AY & Germain RN (2012) Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naïve CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A 109, 18036–18041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mempel TR, Henrickson SE & Von Andrian UH (2004) T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. [DOI] [PubMed] [Google Scholar]
- 50.Miller MJ, Safrina O, Parker I & Cahalan MD (2004) Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med 200, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy NH & Burkhardt JK (2018) The actin cytoskeleton: A mechanical intermediate for signal integration at the immunological synapse. Front Cell Dev Biol 6, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gérard A, Beemiller P, Friedman RS, Jacobelli J & Krummel MF (2013) Evolving immune circuits are generated by flexible, motile, and sequential immunological synapses. Immunol Rev 251, 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammer JA, Wang JC, Saeed M & Pedrosa AT (2019) Origin, Organization, Dynamics, and Function of Actin and Actomyosin Networks at the T Cell Immunological Synapse. Annu Rev Immunol 37, 201–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dustin ML (2008) T-cell activation through immunological synapses and kinapses. Immunol Rev 221, 77–89. [DOI] [PubMed] [Google Scholar]
- 55.Sorokin L (2010) The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10, 712–723. [DOI] [PubMed] [Google Scholar]
- 56.Fowell DJ & Kim M (2021) The spatio-temporal control of effector T cell migration. Nat Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun Y-M, Lambert K, Acharya M, Billroth-MacLurg AC, Rosenberg AF, Topham DJ, Yagita H, Kim M, Lacy-Hulbert A, Meier-Schellersheim M & Fowell DJ (2013) Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat Immunol 14, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes NRJ, Reilly NS, Schrock DC, Hocking DC, Oakes PW & Fowell DJ (2020) CD4+ T Cell Interstitial Migration Controlled by Fibronectin in the Inflamed Skin. Front Immunol 11, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris TH, Banigan EJ, Christian DA, Konradt C, Wojno EDT, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ, Hunter CA, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ & Hunter CA (2012) Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486, 545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaylo-Moynihan A, Prizant H, Popović M, Fernandes NRJ, Anderson CS, Chiou KK, Bell H, Schrock DC, Schumacher J, Capece T, Walling BL, Topham DJ, Miller J, Smrcka A V., Kim M, Hughson A & Fowell DJ (2019) Programming of Distinct Chemokine-Dependent and -Independent Search Strategies for Th1 and Th2 Cells Optimizes Function at Inflamed Sites. Immunity 51, 298–309.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stolp B, Thelen F, Ficht X, Altenburger LM, Ruef N, Krishna Inavalli VVG, Germann P, Page N, Moalli F, Raimondi A, Keyser KA, Morteza Seyed Jafari S, Barone F, Dettmer MS, Merkler D, Iannacone M, Sharpe J, Schlapbach C, Fackler OT, Valentin Nägerl U & Stein J V. (2020) Salivary gland macrophages and tissue-resident CD8+ T cells cooperate for homeostatic organ surveillance. Sci Immunol 5. [DOI] [PubMed] [Google Scholar]
- 62.McGovern KE, Nance JP, David CN, Harrison RES, Noor S, Worth D, Landrith TA, Obenaus A, Carson MJ, Morikis D & Wilson EH (2021) SPARC coordinates extracellular matrix remodeling and efficient recruitment to and migration of antigen-specific T cells in the brain following infection. Sci Rep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F & Donnadieu E (2012) Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 122, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson TF, Chengappa P, Gomez Atria D, Wu CF, Avery L, Roy NH, Maillard I, Petrie RJ & Burkhardt JK (2021) Lymphocyte egress signal sphingosine-1-phosphate promotes ERM-guided, bleb-based migration. J Cell Biol 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG & Alon R (2007) Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol 8, 1076–1085. [DOI] [PubMed] [Google Scholar]
- 66.Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman C V., Von Andrian UH& Shimaoka M (2010) Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood 115, 1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katakai T & Kinashi T (2016) Microenvironmental control of high-speed interstitial T cell migration in the lymph node. Front Immunol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hons M, Kopf A, Hauschild R, Leithner A, Gaertner F, Abe J, Renkawitz J, Stein J V. & Sixt M (2018) Chemokines and integrins independently tune actin flow and substrate friction during intranodal migration of T cells. Nat Immunol 19, 606–616. [DOI] [PubMed] [Google Scholar]
- 69.Reversat A, Gaertner F, Merrin J, Stopp J, Tasciyan S, Aguilera J, de Vries I, Hauschild R, Hons M, Piel M, Callan-Jones A, Voituriez R & Sixt M (2020) Cellular locomotion using environmental topography. Nature 582, 582–585. [DOI] [PubMed] [Google Scholar]
- 70.Yan SLS, Hwang IY, Kamenyeva O & Kehrl JH (2019) In Vivo F-Actin Filament Organization during Lymphocyte Transendothelial and Interstitial Migration Revealed by Intravital Microscopy. iScience 16, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M & Wedlich-Soldner R (2008) Lifeact: a versatile marker to visualize F-actin. Nat Methods 5, 605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong B, Zhang SS, Gao W, Su H, Chen J, Jin F, Bhargava A, Chen X, Jorgensen L, Alberts AS, Zhang J & Siminovitch KA (2013) Mammalian diaphanous-related formin 1 regulates GSK3β-dependent microtubule dynamics required for T cell migratory polarization. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blanchoin L, Boujemaa-Paterski R, Sykes C & Plastino J (2014) Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiol Rev 94, 235–263. [DOI] [PubMed] [Google Scholar]
- 74.Vicente-Manzanares M & Sánchez-Madrid F (2004) Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol 4, 110–122. [DOI] [PubMed] [Google Scholar]
- 75.Burke TA, Christensen JR, Barone E, Suarez C, Sirotkin V & Kovar DR (2014) Homeostatic actin cytoskeleton networks are regulated by assembly factor competition for monomers. Curr Biol 24, 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pollard TD & Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465. [DOI] [PubMed] [Google Scholar]
- 77.Ridley AJ (2011) Life at the leading edge. Cell 145, 1012–1022. [DOI] [PubMed] [Google Scholar]
- 78.Johnston SA, Bramble JP, Yeung CL, Mendes PM & Machesky LM (2008) Arp2/3 complex activity in filopodia of spreading cells. BMC Cell Biol 9, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR & Dustin ML (2007) Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell 129, 773–85. [DOI] [PubMed] [Google Scholar]
- 80.Calvez R, Lafouresse F, De Meester J, Galy A, Valitutti S & Dupré L (2011) The Wiskott-Aldrich syndrome protein permits assembly of a focused immunological synapse enabling sustained T-cell receptor signaling. Haematologica 96, 1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krause M, Dent EW, Bear JE, Loureiro JJ & Gertler FB (2003) Ena/VASP Proteins: Regulators of the Actin Cytoskeleton and Cell Migration. Annu Rev Cell Dev Biol 19, 541–564. [DOI] [PubMed] [Google Scholar]
- 82.Chesarone M a DuPage AG & Goode BL(2010) Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 11, 62–74. [DOI] [PubMed] [Google Scholar]
- 83.Faix J & Grosse R (2006) Staying in shape with formins. Dev Cell 10, 693–706. [DOI] [PubMed] [Google Scholar]
- 84.Vanderzalm P & Garriga G (2007) Losing Their Minds: Mena/VASP/EVL Triple Knockout Mice. Dev Cell 13, 757–758. [DOI] [PubMed] [Google Scholar]
- 85.Hansen SD & Mullins RD (2010) VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J Cell Biol 191, 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly I V., Chaga OY, Cooper JA, Borisy GG& Gertler FB(2002) Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521. [DOI] [PubMed] [Google Scholar]
- 87.Bear JE, Gertler FB, Sechi AS, Domann E, Gerstel B, Machesky LM, Chakraborty T & Wehland J (2009) Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci 122, 1947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chesarone MA & Goode BL (2009) Actin nucleation and elongation factors: mechanisms and interplay NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Applewhite DA, Barzik M, Kojima SI, Svitkina TM, Gertler FB & Borisy GG (2007) Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell 18, 2579–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambrechts A, Kwiatkowski A V., Lanier LM, Bear JE, Vandekerckhove J, Ampe C & Gertler FB(2000) cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem 275, 36143–36151. [DOI] [PubMed] [Google Scholar]
- 91.Bailly M (2004) Ena/VASP family: New partners, bigger enigma. Dev Cell 7, 462–463. [DOI] [PubMed] [Google Scholar]
- 92.Ferron F, Rebowski G, Lee SH & Dominguez R (2007) Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J 26, 4597–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T & Renné T (2009) Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci 122, 3954–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Döppler HR, Bastea LI, Lewis-Tuffin LJ, Anastasiadis PZ & Storz P (2013) Protein kinase D1-mediated phosphorylations regulate Vasodilator-stimulated phosphoprotein (VASP) localization and cell migration. J Biol Chem 288, 24382–24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Janssens K, De Kimpe L, Balsamo M, Vandoninck S, Vandenheede JR, Gertler F & Van Lint J (2009) Characterization of EVL-I as a protein kinase D substrate. Cell Signal 21, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Döppler H & Storz P (2013) Regulation of VASP by phosphorylation Consequences for cell migration. Cell Adhes Migr 7, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kühn S & Geyer M (2014) Formins as effector proteins of Rho GTPases. Small GTPases 5, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schönichen A & Geyer M (2010) Fifteen formins for an actin filament: A molecular view on the regulation of human formins. Biochim Biophys Acta - Mol Cell Res 1803, 152–163. [DOI] [PubMed] [Google Scholar]
- 99.Yayoshi-Yamamoto S, Taniuchi I & Watanabe T (2000) FRL, a Novel Formin-Related Protein, Binds to Rac and Regulates Cell Motility and Survival of Macrophages. Mol Cell Biol 20, 6872–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N & Narumiya S (2007) Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med 204, 2031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Painter MW, Davis S, Hardy RR, Mathis D, Benoist C & Immunological Genome Project Consortium TIGP (2011) Transcriptomes of the B and T lineages compared by multiplatform microarray profiling. J Immunol 186, 3047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vicente-Manzanares M, Rey M, Pérez-Martínez M, Yáñez-Mó M, Sancho D, Cabrero JR, Barreiro O, de la Fuente H, Itoh K & Sánchez-Madrid F (2003) The RhoA effector mDia is induced during T cell activation and regulates actin polymerization and cell migration in T lymphocytes. J Immunol 171, 1023–34. [DOI] [PubMed] [Google Scholar]
- 103.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ & Billadeau DD (2007) Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity 26, 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrés-Delgado L, Antón OM, Bartolini F, Ruiz-Sáenz A, Correas I, Gundersen GG & Alonso MA (2012) INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol 198, 1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heng TSP, Painter MW, Elpek K, Lukacs-Kornek V, Mauermann N, Turley SJ, Koller D, Kim FS, Wagers AJ, Asinovski N, Davis S, Fassett M, Feuerer M, Gray DHD, Haxhinasto S, Hill JA, Hyatt G, Laplace C, Leatherbee K, Mathis D, Benoist C, Jianu R, Laidlaw DH, Best JA, Knell J, Goldrath AW, Jarjoura J, Sun JC, Zhu Y, Lanier LL, Ergun A, Li Z, Collins JJ, Shinton SA, Hardy RR, Friedline R, Sylvia K & Kang J (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9, 1091–1094. [DOI] [PubMed] [Google Scholar]
- 106.Seth A, Otomo C & Rosen MK (2006) Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol 174, 701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM & Narumiya S (1997) p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J 16, 3044–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watanabe N, Kato T, Fujita A, Ishizaki T & Narumiya S (1999) Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol 1, 136–143. [DOI] [PubMed] [Google Scholar]
- 109.Eisenmann KM, West R a, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch K a. & Alberts AS(2007) T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem 282, 25152–25158. [DOI] [PubMed] [Google Scholar]
- 110.Thompson SB, Sandor AM, Lui V, Chung JW, Waldman MM, Long RA, Estin ML, Matsuda JL, Friedman RS & Jacobelli J (2020) Formin-like 1 mediates effector T cell trafficking to inflammatory sites to enable T cell-mediated autoimmunity. Elife 9, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Volpi S, Cicalese MP, Tuijnenburg P, Tool ATJ, Cuadrado E, Abu-Halaweh M, Ahanchian H, Alzyoud R, Akdemir ZC, Barzaghi F, Blank A, Boisson B, Bottino C, Brigida I, Caorsi R, Casanova JL, Chiesa S, Chinn IK, Dückers G, Enders A, Erichsen HC, Forbes LR, Gambin T, Gattorno M, Karimiani EG, Giliani S, Gold MS, Jacobsen EM, Jansen MH, King JR, Laxer RM, Lupski JR, Mace E, Marcenaro S, Maroofian R, Meijer AB, Niehues T, Notarangelo LD, Orange J, Pannicke U, Pearson C, Picco P, Quinn PJ, Schulz A, Seeborg F, Stray-Pedersen A, Tawamie H, van Leeuwen EMM, Aiuti A, Yeung R, Schwarz K & Kuijpers TW (2019) A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol 143, 2296–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brigida I, Zoccolillo M, Cicalese MP, Pfajfer L, Barzaghi F, Scala S, Oleaga-Quintas C, Álvarez-Álvarez JA, Sereni L, Giannelli S, Sartirana C, Dionisio F, Pavesi L, Benavides-Nieto M, Basso-Ricci L, Capasso P, Mazzi B, Rosain J, Marcus N, Lee YN, Somech R, Degano M, Raiola G, Caorsi R, Picco P, Velez MM, Khourieh J, Arias AA, Bousfiha A, Issekutz T, Issekutz A, Boisson B, Dobbs K, Villa A, Lombardo A, Neven B, Moshous D, Casanova JL, Franco JL, Notarangelo LD, Scielzo C, Volpi S, Dupré L, Bustamante J, Gattorno M & Aiuti A (2018) T-cell defects in patients with ARPC1B germline mutations account for combined immunodeficiency. Blood 132, 2362–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuijpers TW, Tool ATJ, van der Bijl I, de Boer M, van Houdt M, de Cuyper IM, Roos D, van Alphen F, van Leeuwen K, Cambridge EL, Arends MJ, Dougan G, Clare S, Ramirez-Solis R, Pals ST, Adams DJ, Meijer AB & van den Berg TK (2017) Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol 140, 273–277.e10. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Shen H, Liu H, Feng H, Liu Y, Zhu X & Liu X (2017) Arp2/3 complex controls T cell homeostasis by maintaining surface TCR levels via regulating TCR+ endosome trafficking. Sci Rep 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Föger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM & Cyster JG (2008) The actin regulator coronin 1A is mutant in a thymic egress–deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol 9, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU & Puck JM (2009) Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol 131, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Estin ML, Thompson SB, Traxinger B, Fisher MH, Friedman RS & Jacobelli J (2017) Ena/VASP proteins regulate activated T-cell trafficking by promoting diapedesis during transendothelial migration. Proc Natl Acad Sci U S A 114, E2901–E2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salz A, Gurniak C, Jönsson F & Witke W (2020) Cofilin1-driven actin dynamics controls migration of thymocytes and is essential for positive selection in the thymus. J Cell Sci 133. [DOI] [PubMed] [Google Scholar]
- 119.Morley SC, Wang C, Lo W-L, Lio C-WJ, Zinselmeyer BH, Miller MJ, Brown EJ & Allen PM (2010) The Actin-Bundling Protein l-Plastin Dissociates CCR7 Proximal Signaling from CCR7-Induced Motility. J Immunol 184, 3628–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thompson SB, Wigton EJ, Krovi SH, Chung JW, Long RA & Jacobelli J (2018) The Formin mDia1 Regulates Acute Lymphoblastic Leukemia Engraftment, Migration, and Progression in vivo. Front Oncol 8, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallego MD, de la Fuente MA, Anton IM, Snapper S, Fuhlbrigge R & Geha RS (2006) WIP and WASP play complementary roles in T cell homing and chemotaxis to SDF-1α. Int Immunol 18, 221–232. [DOI] [PubMed] [Google Scholar]
- 122.Haddad E, Zugaza JL, Louache F, Debili N, Crouin C, Schwarz K, Fischer A, Vainchenker W & Bertoglio J (2001) The interaction between Cdc42 and WASP is required for SDF-1-induced T-lymphocyte chemotaxis. Blood 97, 33–38. [DOI] [PubMed] [Google Scholar]
- 123.Massaad MJ, Oyoshi MK, Kane J, Koduru S, Alcaide P, Nakamura F, Ramesh N, Luscinskas FW, Hartwig J & Geha RS (2014) Binding of WIP to Actin Is Essential for T Cell Actin Cytoskeleton Integrity and Tissue Homing. Mol Cell Biol 34, 4343–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Randzavola LO, Strege K, Juzans M, Asano Y, Stinchcombe JC, Gawden-Bone CM, Seaman MNJ, Kuijpers TW & Griffiths GM (2019) Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity. J Clin Invest 129, 5600–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Obeidy P, Ju LA, Oehlers SH, Zulkhernain NS, Lee Q, Galeano Niño JL, Kwan RYQ, Tikoo S, Cavanagh LL, Mrass P, Cook AJL, Jackson SP, Biro M, Roediger B, Sixt M & Weninger W (2020) Partial loss of actin nucleator actin-related protein 2/3 activity triggers blebbing in primary T lymphocytes. Immunol Cell Biol 98, 93–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cook SA, Comrie WA, Poli MC, Similuk M, Oler AJ, Faruqi AJ, Kuhns DB, Yang S, Vargas-Hernández A, Carisey AF, Fournier B, Anderson DE, Price S, Smelkinson M, Chahla WA, Forbes LR, Mace EM, Cao TN, Coban-Akdemir ZH, Jhangiani SN, Muzny DM, Gibbs RA, Lupski JR, Orange JS, Cuvelier GDE, Al Hassani M, Al Kaabi N, Al Yafei Z, Jyonouchi S, Raje N, Caldwell JW, Huang Y, Burkhardt JK, Latour S, Chen B, El Ghazali G, Rao VK, Chinn IK & Lenardo MJ (2020) HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science (80- ) 369, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gandhi M, Achard V, Blanchoin L & Goode BL (2009) Coronin Switches Roles in Actin Disassembly Depending on the Nucleotide State of Actin. Mol Cell 34, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morin NA, Oakes PW, Hyun Y-M, Lee D, Chin YE, Chin EY, King MR, Springer TA, Shimaoka M, Tang JX, Reichner JS & Kim M (2008) Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med 205, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil A-MM, Piel M, Sorensen CM, Adelstein RS & Krummel MF (2010) Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol 11, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thiam H-R, Vargas P, Carpi N, Crespo CL, Raab M, Terriac E, King MC, Jacobelli J, Alberts AS, Stradal T, Lennon-Dumenil A-M & Piel M (2016) Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat Commun 7, 10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Renkawitz J, Kopf A, Stopp J, de Vries I, Driscoll MK, Merrin J, Hauschild R, Welf ES, Danuser G, Fiolka R & Sixt M (2019) Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature 568, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le S, Yu M, Bershadsky A & Yan J (2020) Mechanical regulation of formin-dependent actin polymerization. Semin Cell Dev Biol 102, 73–80. [DOI] [PubMed] [Google Scholar]
- 133.Jégou A, Carlier MF & Romet-Lemonne G (2013) Formin mDia1 senses and generates mechanical forces on actin filaments. Nat Commun 4, 1–7. [DOI] [PubMed] [Google Scholar]
- 134.Mrass P, Oruganti SR, Fricke GM, Tafoya J, Byrum JR, Yang L, Hamilton SL, Miller MJ, Moses ME & Cannon JL (2017) ROCK regulates the intermittent mode of interstitial T cell migration in inflamed lungs. Nat Commun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faroudi M, Hons M, Zachacz A, Dumont C, Lyck R, Stein J V. & Tybulewicz VLJ (2010) Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood 116, 5536–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martínez-A C. Fukui Y, Von Andrian UH& Stein JV. (2007) A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med 204, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Q, Dove CG, Hor JL, Murdock HM, Strauss-Albee DM, Garcia JA, Mandl JN, Grodick RA, Jing H, Chandler-Brown DB, Lenardo TE, Crawford G, Matthews HF, Freeman AF, Cornall RJ, Germain RN, Mueller SN & Su HC (2014) DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med 211, 2549–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR & Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science (80- ) 299, 1743–1747. [DOI] [PubMed] [Google Scholar]
- 139.Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, Hartman J, Sakowicz R & Pollard TD (2009) Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature 460, 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rizvi SA, Neidt EM, Cui J, Feiger Z, Skau CT, Gardel ML, Kozmin SA & Kovar DR (2009) Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem Biol 16, 1158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Isogai T, van der Kammen R & Innocenti M (2015) SMIFH2 has effects on Formins and p53 that perturb the cell cytoskeleton. Sci Rep 5, 9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Képiró M, Várkuti BH, Végner L, Vörös G, Hegyi G, Varga M & Málnási-Csizmadia A (2014) Para-nitroblebbistatin, the non-cytotoxic and photostable myosin II inhibitor. Angew Chemie - Int Ed 53, 8211–8215. [DOI] [PubMed] [Google Scholar]
- 143.Aviles-Espinosa R, Filippidis G, Hamilton C, Malcolm G, Weingarten KJ, Südmeyer T, Barbarin Y, Keller U, Santos SIC., Artigas D & Loza-Alvarez P (2011) Compact ultrafast semiconductor disk laser: targeting GFP based nonlinear applications in living organisms. Biomed Opt Express 2, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu TL, Upadhyayula S, Milkie DE, Singh V, Wang K, Swinburne IA, Mosaliganti KR, Collins ZM, Hiscock TW, Shea J, Kohrman AQ, Medwig TN, Dambournet D, Forster R, Cunniff B, Ruan Y, Yashiro H, Scholpp S, Meyerowitz EM, Hockemeyer D, Drubin DG, Martin BL, Matus DQ, Koyama M, Megason SG, Kirchhausen T & Betzig E (2018) Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science (80- ) 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS & Siminovitch KA (2009) The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol 182, 3837–45. [DOI] [PubMed] [Google Scholar]