Abstract
Neurocysticercosis (NCC) caused by infection with the larvae of Taenia solium is an important cause of neurological disease worldwide. In order to establish an enzyme-linked immunosorbent assay (ELISA) for this infection using recombinant proteins, we carried out molecular cloning and identified four candidates as diagnostic antigens (designated Ag1, Ag1V1, Ag2, and Ag2V1). Except for Ag2V1, these clones could encode a 7-kDa polypeptide, and Ag2V1 could encode a 10-kDa polypeptide. All of the clones were very similar. Except for Ag2V1, recombinant proteins were successfully expressed using an Escherichia coli expression system. Immunoblot analysis of NCC patient sera detected recombinant proteins, but because reactivity to recombinant Ag1 was too weak, Ag1 was not suitable as an immunodiagnostic antigen. So, Ag1V1 and Ag2 were chosen as ELISA antigens, and the Ag1V1/Ag2 chimeric protein was expressed. Of 49 serum samples from NCC patients confirmed to be seropositive by immunoblot analysis, 44 (89.7%) were positive by ELISA. No assays of serum samples from patients with other parasitic infections recognized the Ag1V1/Ag2 chimeric protein. The Ag1V1/Ag2 chimeric protein obtained in this study had a high value for differential immunodiagnosis.
The larval stage of the pork tapeworm Taenia solium is responsible for cysticercosis. Humans are accidentally infected with T. solium by ingestion of eggs excreted with the feces of individuals harboring the adult tapeworm in the intestinal tract. The larvae migrate throughout the body, invade skeletal muscle, subcutaneous tissue, or the central nervous system (CNS), the latter of which is known as neurocysticercosis (NCC), and encyst to form cysticerci. Cysticercosis is a crucial emerging disease in developing countries (4, 20, 22, 26, 27).
Diagnosis of NCC has been achieved by clinical criteria, computed tomography (CT), and nuclear magnetic resonance imaging (MRI). The imaging techniques are useful for diagnosis, but the infection can be overlooked by these methods when the number of parasites is few and/or the figures are not clear or typical. Moreover, these techniques are not suitable for diagnosis in areas of endemicity because of the cost. Therefore, the development of an immunodiagnostic test that detects specific antibodies either in sera or in cerebrospinal fluid is urgently required because of its simplicity and reliability, especially in sera. For these reasons, several immunodiagnostic methods have been developed using crude or partially purified antigens of T. solium cyst fluid or cyst tissue extract (1, 6, 14, 18, 23, 25). Parkhouse and Harrison (18) and Tsang et al. (25) purified the glycoproteins (GPs) by lentil-lectin affinity chromatography and reported that seven bands around 15 to 30 kDa were highly specific to neurocysticercosis. However, these GPs prepared by lentil-lectin affinity chromatography showed cross-reactivity when used as enzyme-linked immunosorbent assay (ELISA) antigens. Recently, we developed a simple method to purify diagnostic antigens (10- to 26-kDa antigens under reducing conditions) by preparative isoelectric-focusing electrophoresis (IEFE) from cyst fluid available for both ELISA and immunoblot analysis (10), and we demonstrated the sensitivity and specificity of this method for differential serodiagnosis of NCC. Nevertheless, for preparation of diagnostic antigens, we still need to find naturally infected pigs or to maintain infected pigs, which is not practicable. Therefore, as reported in this study, we have carried out molecular cloning, characterization of immunodiagnostic antigens, and expression of recombinant proteins for an ELISA-based diagnostic system.
MATERIALS AND METHODS
Parasite materials.
T. solium metacestodes used for construction of an expression cDNA library and extraction of genomic DNA were obtained from naturally infected pigs in China. After washing with phosphate-buffered saline (PBS), T. solium metacestodes were mechanically disrupted and kept in RNAlater reagent (Ambion, Austin, Tex.) at 4°C.
Serum samples.
A total of 50 serum samples of NCC confirmed by image analysis (CT and/or MRI) and/or serology of immunoblot analysis using the antigens purified by preparative isoelectric focusing of cyst fluid of T. solium metacestodes (10) were examined for this study. Of these samples, 10, 10, 22, and 8 were from the Centers for Disease Control and Prevention (CDC), Atlanta, Ga., Ecuador, China, and Japan, respectively. Ten NCC serum samples from CDC, clinically and serologically confirmed at CDC, were thoroughly confirmed at Asahikawa Medical College under a blind test for differentiation of NCC from alveolar echinococcosis (AE) and cystic echinococcosis (CE) (11). The technical quality of samples for diagnosis of NCC by immunoblotting was very similar between those from CDC (25) and Asahikawa Medical College (10). Ten NCC case samples from CDC were from seven Hispanic immigrants to the United States and three cases in Peru. Ten NCC cases in Ecuador were confirmed by CT and by serology to detect circulating antigens (2) at the Zoonoses International Research Center, Central University of Ecuador. Twenty-two NCC cases in Xiling Province, China, were confirmed at Cysticercosis Hospital in Changchun by CT and serology. All serum samples from the United States (CDC), Ecuador, and China were serologically confirmed at Asahikawa Medical College. Eight patients with imported NCC in Japan were treated either by surgery or by administration of praziquantel. They all were suspected of exposure to the eggs of T. solium in countries of endemicity outside Japan (in Asia and Latin America). Five samples were from patients with single cysts in the brain; four of these were Japanese, and one was Nepali (12, 16; Ito et al., unpublished data). Three samples were from patients with multiple cysts in the brain, one from Brazil and two from Peru. Seven of the eight NCC cases were serologically confirmed to be NCC at Asahikawa Medical College too, whereas the single patient that was surgically treated and confirmed parasitologically was seronegative (17). All 50 NCC sera examined in this study, therefore, were from clinical patients who had presented with seizures or other neurological signs and symptoms and in whom CT or other neuroimaging had demonstrated cystic lesions and/or calcifications compatible with NCC, and 49 of these cases were serologically confirmed to be NCC by immunoblot analysis. All other serum samples from parasitic diseases other than NCC (AE, CE, or others) were from the stock sera examined previously (10, 11).
Construction and screening of T. solium metacestodes cDNA library.
A λSCREEN cDNA library was constructed from poly(A)+ RNA isolated from T. solium metacestodes with a FastTrack 2.0 kit (Invitrogen, Carlsbad, Calif.). The oligo(dT)-primed cDNA was synthesized from 5 μg of poly(A)+ RNA, using a cDNA synthesis kit (Takara, Tokyo, Japan). The resulting cDNA fragments were ligated to directional EcoRI/HindIII linker DNA (Novagen, Madison, Wis.), digested with restriction enzymes (EcoRI and HindIII), and finally ligated with λSCREEN arms (Novagen). The recombinant DNA was packaged using a Phage Maker in vitro packaging system (Novagen).
The cDNA library was first immunoscreened with sera from rabbits immunized with low-molecular-weight antigens of T. solium cyst fluid purified by IEFE (10). Positive clones reacting with rabbit sera were isolated, and cDNA inserts were amplified by PCR with a vector primer set according to manufacturer's instructions. The amplified PCR products were used for plaque hybridization to isolate the complete-length cDNA.
Southern blot hybridization.
Genomic DNA (5 μg) was digested with restriction enzymes (EcoRI, HindIII, and BamHI), electrophoresed on 0.8% agarose gel, and transferred onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) with transfer solution (0.4 M NaOH–1.5 M NaCl) overnight. cDNA fragments used as probes were labeled with fluorescein isothiocyanate (FITC)-dUTP (Amersham Pharmacia Biotech) by PCR. The membrane was washed three times with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% sodium dodecyl sulfate (SDS) at room temperature and once for 30 min at 55°C or 65°C with 0.1× SSC containing 0.1% SDS. Signals on blots hybridized with FITC-labeled probes were visualized using a Gene Images CDP-Star detection module (Amersham Pharmacia Biotech).
DNA sequencing.
The cDNA nucleotide sequences were determined using a Thermo Sequenase dye terminator cycle sequencing pre-mix kit (Amersham Pharmacia Biotech) and an automated DNA sequencer (Applied Biosystems model 377; Perkin-Elmer, Foster City, Calif.).
Expression and purification of recombinant antigens.
The coding region without a signal sequence was amplified by PCR with primer sets containing a restriction enzyme BamHI (underlined below) recognition sequence added to the 5′ end to facilitate cloning of the PCR products. The primers used were 5′-TTGGATCCGGAGAAAAATAAAACGGATGG-3′ (Ag1/F), 5′-TTGGATCCTTAAGCGGTTTTGTTCTTGA-3′ (Ag1/R), 5′-TTGGATCCGGAGAAAAACAAACCGAAGTG-3′ (Ag1V1/F), 5′-TTGGATCCGAAGGAAACTAAACCAGAGGA-3′ (Ag1V1/R), 5′-TTGGATCCGAAGGAAACTAAACCAGAGGA-3′ (Ag2/F), and 5′-TTGGATCCTTAAGCAGCTTCGTTCTTGA-3′ (Ag2/R). The PCRs were performed in a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% (wt/vol) gelatin, 0.1 μM concentrations of each primer, 0.2 mM concentrations of each dNTP, 1 ng of cDNA, and 0.5 U of Taq DNA polymerase (AmpliTaq Gold; Perkin-Elmer) and cycling conditions were 30 s at 94°C (first cycle, 10 min at 94°C), 30 s at 50°C, and 30 s at 72°C for 35 cycles. The PCR products were digested with BamHI and cloned into bacterial expression vector pET-32b(+) (Novagen) to produce a fusion protein with thioredoxin (TRX) and a His tag. The orientation of insert DNA was confirmed by sequencing. The cloned plasmid was transfected into an E. coli BL21(DE3)pLysS strain. Expression of the recombinant protein was induced by addition of 1 mM isopropyl-β-d-thiogalactoside to the culture. The expressed recombinant proteins were purified using a His Trap kit (Amersham Pharmacia Biotech) and were dialyzed against PBS. Protein concentrations were determined with a BCA protein assay kit (Pierce, Rockford, Ill.).
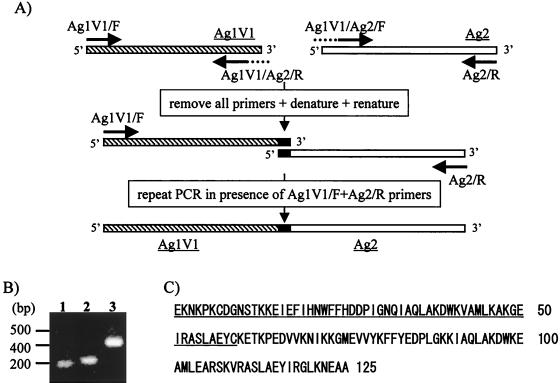
For production of Ag1V1/Ag2 chimeric protein, sequential PCR mutagenesis was carried out (see Fig. 3 below). Briefly, PCR products that had been amplified from Ag1V1 cDNA with primers Ag1V1/F and Ag1V1/Ag2/R and from Ag2 cDNA with primers Ag2/R and Ag1V1/Ag2/F were further amplified with primers Ag1V1/F and Ag2/R in order to obtain an Ag1V1/Ag2 chimeric gene. Ag1V1/Ag2/R and Ag1V1/Ag2/F primer sequences were 5′-TTGTTTAGTTTCCTTGCAGTACTCAGCCAGTGACGC-3′ and 5′-CTGGCTGAGTACTGCAAGGAAACTAAACCAGAGGAC-3′, respectively. The PCR products were digested with BamHI and cloned into bacterial expression vector pET-16b(+) (Novagen) to produce a fusion protein with a His tag.
FIG. 3.
(A) The scheme of PCR mutagenesis for production of an Ag1V1/Ag2 chimeric gene. Details of the PCR mutagenesis to obtain Ag1V1/Ag2 chimeric gene are described in Materials and Methods. (B) Analysis of the resultant PCR products in a 2.0% agarose gel. Molecular size markers are indicated on the left. Lane 1, first PCR product of the Ag1V1 gene; lane 2, first PCR product of the Ag2 gene; lane 3, second PCR product of the Ag1V1/Ag2 chimeric gene. (C) Amino acid sequence of Ag1V1/Ag2 chimeric protein. The underlined amino acids were derived from an Ag1V1 (20E to 78C) clone, and the remains were derived from an Ag2 (20K to 85A) clone.
SDS-PAGE and immunoblot analysis.
Protein analysis by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by the method of Laemmli (13). Proteins were solubilized with a SDS sample buffer (10 mM Tris-HCl [pH 6.8], containing 2% SDS, 5% 2-mercaptoethanol, and 10% glycerol) at 100°C for 5 min and separated electrophoretically in a 12.5% polyacrylamide gel. For immunoblot analysis, the separated proteins were electrophoretically transferred onto a polyvinylodene difluoride membrane sheet (Millipore, Tokyo, Japan) as described by Towbin et al. (24). The sheet was blocked with 3% skim milk (Morinaga, Tokyo, Japan) and probed with patients' or healthy human sera followed by peroxidase-conjugated anti-human immunoglobulin G antibodies (Cappel, West Chester, Pa.). 4-Chloro-1-naphtol was used for color development.
ELISA.
ELISA plates (Nunc-Immuno plate with a MaxiSorp surface; Nalge Nunc International, Tokyo, Japan) were coated with 0.2 μg of recombinant proteins. The wells were blocked with 200 μl of casein buffer at 37°C for 1 to 2 h. After the wells had been washed twice with PBS containing 0.05% Tween 20 (PBST), 100 μl of serum samples diluted 1:100 in PBST containing 1% bovine serum albumin was added and incubated at 37°C for 1 h. The wells then were washed five times with PBST, incubated with 100 μl of anti-human immunoglobulin G antibodies conjugated with peroxidase (Cappel) at 37°C for 1 h, and washed five times with PBST. After incubation with 100 μl of substrate (0.4 mM 2,2′-azino-di-[3-ethyl-benzthiazoline sulfonate] in 0.2 M citric acid buffer [pH 4.7]) for 30 min at room temperature, the optical density at 405 nm (OD405) of each well was determined using an ELISA plate reader (model 450; Bio-Rad Laboratories, Hercules, Calif.). Serum samples giving OD405 values greater than the mean OD405 plus four standard deviations for healthy human controls were considered seropositive.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the low-molecular-weight-protein genes obtained in this study are as follows: AB044080 for Ag1, AB044081 for Ag1V1, AB044082 for Ag2, and AB044083 for Ag2V1.
RESULTS
Cloning and characterization of diagnostic antigen candidate genes.
To identify immunodiagnostic antigen genes, the T. solium metacestode expression cDNA library was immunoscreened with sera from rabbits immunized with diagnostic low-molecular-weight antigens as described by Ito et al. (10). Immunoscreening allowed the selection of two clones expressing protein epitopes recognized by immunized-rabbit sera. NCC patient sera also recognized protein epitopes expressed by these clones (data not shown). Because DNA sequencing indicated that these clones did not contain full-length cDNAs, DNA hybridization screening was carried out using a cDNA clone as a probe. Finally, four clones, named Ag1, Ag1V1, Ag2, and Ag2V1, having full-length cDNA determined by DNA sequencing were isolated (Fig. 1). These clones ranged from 325 to 415 bp in length and encoded polypeptides with 85 to 112 amino acids and with predicted molecular masses of 9.6 to 13 kDa. These clones showed 53 to 94% similarity at the amino acid level. Putative N-linked glycosylation sites were found at positions 22, 59, and 82 in Ag1 and at positions 29 and 83 in Ag1V1 (Fig. 1, boxes) but not in clones Ag2 and Ag2V1. All clones had N-terminal hydrophobic regions, which were thought to be signal sequences (Fig. 1, underlines), and each signal sequence cleavage site was predicted by the method described by Nielsen et al (15). After cleavage of the signal sequence, the predicted molecular mass of mature polypeptide ranged from 7.0 to 10.0 kDa. The amino acid sequence IAQLAKDW (Fig. 1, italic letters) was conserved among all clones. A sequence homology search revealed that all clones were related to the 14- and 18-kDa GPs of T. solium (GenBank accession numbers AF082828, AF082829, AF098073, AF098074, AF098075, AF158184, and AF257776), the cysticercosis-specific antigen of T. solium (3) (GenBank accession number AF076609), the immunodiagnostic antigen of Taenia crassiceps (29) (GenBank accession number U07150), and antigen B of Echinococcus granulosus (5, 21) (GenBank accession numbers M36774, U15001, and Z26482).
FIG. 1.
Alignment of amino acid sequences of four diagnostic-antigen candidate clones. The amino acid sequences predicted from cDNA clones were aligned by using the CLUSTAL V program (7). Asterisks indicate residues which are identical to the Ag1 sequence, while gaps introduced by the CLUSTAL V program are symbolized by dashes. Features within the sequences are denoted as follows: underlined letters at the N terminal, the putative signal sequences; boxed letters, N-linked glycosylation sites; circled letters, cysteine residues; the amino acid sequence conserved among all four clones is italicized.
Southern blot analysis.
To estimate the copy number of isolated genes per genome, Southern blot analysis was performed (data not shown). Genomic DNA derived from T. solium metacestodes was digested with restriction enzyme (EcoRI, HindIII, and BamHI) and electrophoresed, transferred onto a nylon membrane, and probed with the Ag1 cDNA insert. Several bands (at least four) in each digest were detected under mildly stringent washing conditions (55°C for 30 min), and this hybridization pattern did not change under highly stringent washing conditions (65°C for 1 h; data not shown). None of the cDNA clones possessed a recognition site for restriction enzymes used in Southern blot analysis within the transcribing regions. In order to confirm whether recognition sites for restriction enzymes used in Southern blot analysis existed within each gene in genomic DNA, PCR was performed on T. solium genomic DNA using specific primer sets that amplified the region from the initiation codon to the termination codon of each gene, and restriction enzyme digestion analysis was performed. Each primer set specific to the Ag1, Ag1V1, Ag2, and Ag2V1 genes generated DNA fragments of 390 bp in length, approximately 130 bp larger than predicted from the cDNA sequences, while the Ag2V1 fragment was approximately 50 bp larger than predicted from the cDNA sequence (data not shown). Those PCR products were not digested by the restriction enzymes used in Southern blot analysis (data not shown), which indicated that no restriction enzyme recognition sites were present within genes in the genomic DNA. Complex banding patterns generated in Southern blots of T. solium genomic DNA digested with restriction enzymes which could not cut within each gene were consistent with either the existence of multiple copies of these clones throughout the genome of parasites or of a gene family. The latter might be supported by the fact that these clones showed similarity to each other.
Expression of recombinant antigens and immunoblot analysis.
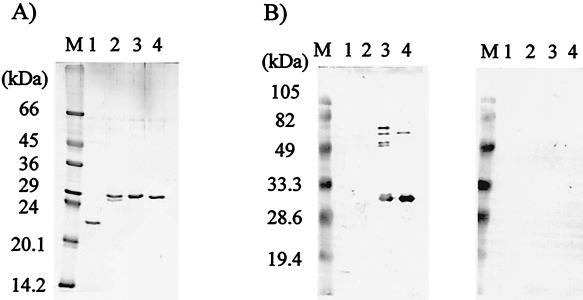
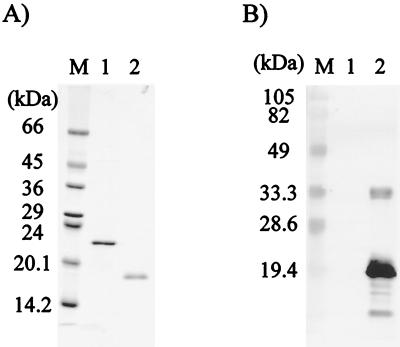
In order to obtain recombinant proteins, an E. coli-based expression system was established. Recombinant proteins without N-terminal hydrophobic regions were expressed as TRX/His tag fusion proteins. In a preliminary observation, the expression of recombinant Ag2V1 (rAg2V1) was successful, but its yield was too low due to its cytotoxicity against E. coli (data not shown). The other three recombinant proteins (rAg1, rAg1V1, and rAg2) were, therefore, selected for further experiment. Figure 2 shows the results of purification of these three recombinant proteins and of immunoblot analyses with NCC and AE patient sera. Recombinant proteins were recognized by NCC patient sera (Fig. 2B, left panel, lanes 3 and 4), but not by AE patient sera (Fig. 2B, right panel, lanes 3 and 4). Notably, rAg1 was recognized by NCC patient serum (Fig. 2B, left panel, lane 2), but its reaction was too weak. This indicated that antigenicity of the Ag1 polypeptide was low in natural infection and that it was not suitable for diagnostic applications. For this reason, rAg1V1 and rAg2 were chosen as diagnostic polypeptides, and we tried to express an Ag1V1/Ag2 chimeric polypeptide using a PCR technique, as illustrated in Fig. 3A. This Ag1V1/Ag2 chimeric protein contained a His tag for purification but not TRX. As shown in Fig. 4A, Ag1V1/Ag2 chimeric protein migrated to 17.0 kDa in SDS-PAGE analysis, which was in agreement with the size predicted from the cDNA sequence. In immunoblot analysis (Fig. 4B), the Ag1V1/Ag2 chimeric protein was strongly recognized by NCC patient sera.
FIG. 2.
(A) Analysis by SDS-PAGE of purified recombinant proteins stained with Coomassie blue. (B) Immunoblot analysis using a pooled serum from NCC patients (left panel) or AE patients (right panel). Molecular size markers are indicated on the left. Lane 1, TRX; lane 2, rAg1; lane 3, rAg1V1; lane 4, rAg2.
FIG. 4.
(A) Analysis by SDS-PAGE of purified Ag1V1/Ag2 chimeric protein stained with Coomassie blue. (B) Immunoblot analysis using a pooled serum from NCC patients. Molecular size markers are indicated on the left. Lane 1, TRX; lane 2, Ag1V1/Ag2 chimeric protein.
Evaluation of Ag1V1/Ag2 chimeric protein for diagnostic value using ELISA.
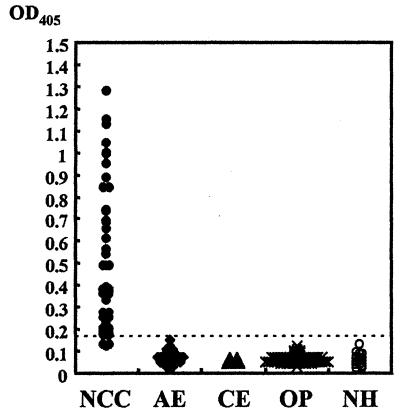
To assess the diagnostic value of Ag1V1/Ag2 chimeric protein, we further tested its immunoreactivity by ELISA using individual sera from patients with various parasitic infections (Fig. 5). A positive reaction to Ag1V1/Ag2 chimeric protein was observed in 89.7% (44 of 49 cases) of sera from NCC patients confirmed to be seropositive by immunoblot analysis based on a cutoff value of 0.17. Of the six NCC patient sera judged negative by this ELISA system, five showed very weak reaction to native GPs by immunoblot analysis (data not shown), and one serum sample was negative by both immunoblot analysis (17) and ELISA. No positive results were observed with sera from patients with other parasitic infections (AE, 35 cases; CE, 10 cases; clonochiasis, 10 cases; sparganosis, 10 cases; fascioliasis, 8 cases; paragonimiasis, 32 cases; and schistosomiasis, 10 cases) and sera from healthy controls.
FIG. 5.
Results of ELISA using Ag1V1/Ag2 chimeric protein with sera from 50 patients with neurocysticercosis (NCC), 35 with alveolar echinococcosis (AE), 10 with cystic echinococcosis (CE), and 70 with other parasitic diseases (OP; 10 clonochiasis, 10 sparganosis, 8 fascioliasis, 32 paragonimiasis, 10 schistosomiasis) and from 20 healthy humans (NH). The dotted line shows the cutoff value (0.17).
DISCUSSION
For diagnosis of NCC, in addition to imaging techniques (CT and MRI), detection of patient serum antibodies with low-molecular-weight antigens derived from cystic fluid of T. solium cysts is very important. Recently we developed a simple method to purify low-molecular-weight antigens using IEFE (10). But for the stable production of immunodiagnostic antigens, we have needed naturally infected pigs or the capacity to maintain infected pigs, which is not practicable. Therefore, the purpose of this study was to isolate immunodiagnostic-antigen genes from T. solium metacestodes and to express the recombinant proteins in E. coli to establish an immunodiagnostic method based on the ELISA system.
By immunoscreening with sera from rabbits immunized with low-molecular-weight antigens and DNA hybridization screening, we could identify four cDNA clones (Ag1, Ag1V1, Ag2, and Ag2V1). These clones, except Ag2V1, could encode a 7-kDa polypeptide, and Ag2V1 could encode a 10-kDa polypeptide (Fig. 1). These clones showed 53 to 94% similarity at the amino acid level. Analysis of their hydrophobicity profiles by the method of Hopp and Woods (8) revealed that these clones appeared to have an N-terminal hydrophobic region that was expected to be a signal sequence and no other transmembrane regions. This finding indicated that proteins encoded by these clones might be secretory proteins, in agreement with the detection of these proteins in cystic fluid of T. solium cysts (10). Some clones (Ag1, Ag1V1, and Ag2V1) had a cysteine residue that was involved in disulfide binding. Under nonreducing conditions, immunodiagnostic antigens recognized by NCC patient sera were detected at around 50 and 28 kDa, but under reducing conditions, those bands disappeared and several smaller bands (<28 kDa) were detected (data not shown). Based on these results, cysteine residues in these proteins might contribute to form functional complexes. Putative N-linked glycosylation sites were found at positions 22, 59, and 82 in Ag1 and positions 29 and 83 in Ag1V1, but not in Ag2 and Ag2V1 (Fig. 1). In this study, we could not confirm whether N-linked glycosylation occurred. But, the difference between the polypeptide size predicted from a cDNA sequence and the native antigen size detected by immunoblot analysis suggested the occurrence of N-linked glycosylation. Plancarte et al. (19) carried out the characterization of T. solium low-molecular-weight GPs and determined their N-terminal amino acid sequences and glycan portions. The N-terminal amino acid sequence determined (12) was almost identical to our clone sequences, which strongly suggested that these GPs belong to the same family. Analyses of the glycan portion of GP identified mannose, N-acetyl-d-glucosamine, and galactose detected in N-linked GP but not N-acetylgalactosamine, N-acetylneuraminic acid, fucose, or sialic acid, which were found in N- and O-linked glycoproteins. These results also supported the occurrence of N-linked glycosylation. Further characterizations of posttranslational modification, especially glycosylation of parasite proteins and more information on other variant genes, are necessary.
Recombinant proteins were specifically recognized with NCC patient sera but their immunoreactivity to rAg1 appeared to be too weak (Fig. 2). As Ag1 had three N-linked glycosylation sites, it might be more highly glycosylated than other clones in their native condition, and it might be difficult to produce antibodies to polypeptides. Nevertheless, the possibility that conformational epitopes but not linear epitopes exist in Ag1 polypeptides could not be ruled out. Based on the results of immunoblot analysis, we thought that Ag1 was not suitable to be used as an immunodiagnostic antigen, so Ag1V1 and Ag2 were chosen as good candidate antigens for ELISA and expressed as an Ag1V1/Ag2 chimeric protein. Using the PCR technique, Ag1V1 and Ag2 genes were ligated to produce the Ag1V1/Ag2 chimeric protein as shown in Fig. 3, which had the advantage of simplifying the preparation of both recombinant antigens by a one-step purification procedure. Ag1V1/Ag2 chimeric proteins were expressed successfully at a molecular mass of 17.0 kDa and confirmed their antigenicity (Fig. 4). Using an Ag1V1/Ag2 chimeric protein, we developed an immunodiagnostic method based on the ELISA system (Fig. 5). Of 49 serum samples from NCC patients confirmed to be seropositive by immunoblot analysis (10, 11, 12, 17), 44 (89.7%) were positive by ELISA. Two of five NCC patients with a single cyst (imported cases in Japan) were negative by ELISA. By immunoblot analysis, one of two negative cases by ELISA was also negative by immunoblot analysis (17), and another showed a very weak reaction to native diagnostic antigens (12). Immunoblot analysis detected 98% of parasitologically proven cases with multiple cysts, whereas it was less sensitive (between 60 and 80%) in cases with a single cyst (28). This suggested that a single cyst was not always efficient to stimulate host responses that produced measurable antibody to diagnostic antigens. In addition, since Ag1V1/Ag2 chimeric protein used in this study was expressed using an E. coli system, this protein was not glycosylated. Native antigens might be highly glycosylated, and the carbohydrates were thought to be key antigenic parts for immunodiagnostic sensitivity. We, however, speculated that the carbohydrate components showed similarity among various parasites and were not suitable targets for differential immunodiagnosis. Indeed, when native antigens purified by affinity chromatography using monoclonal antibodies to T. solium low-molecular-weight antigen were used as ELISA antigens, some serum samples from AE patients showed a strong reaction (our unpublished observation). So, with regards to differential diagnosis, parasite-specific polypeptides should be utilized. Some researchers have developed immunodiagnostic methods based on immunoblotting or on ELISA systems using recombinant antigens (3, 9). Their sensitivity was similar to that of our recombinant protein, but some cross-reactions were observed. In our experiments, serum samples from other parasitic-infection patients (AE, CE, clonochiasis, sparganosis, fascioliasis, paragonimiases, and schistosomiasis patients) did not recognize recombinant protein (100% specificity). Therefore, it is expected that Ag1V1/Ag2 is a valuable target antigen for differential diagnosis.
Hereafter, we will determine the B-cell epitopes on these molecules for the development of a synthetic peptide-based ELISA system that may be suitable for stable and high-quality diagnoses.
ACKNOWLEDGMENTS
We are grateful to Peter M. Schantz, Centers for Disease Control and Prevention, Atlanta, Ga., and Washington B. Ortiz, Zoonoses International Research Center, Central University of Ecuador, Ecuador, for providing NCC patient serum samples.
This work was supported by a grants-in-aid for Encouragement of Young Scientists (12770122) to Y. S. and for Scientific Research (A) (11694259) and (B) (12557024) to A. I. from the Ministry of Education, Science, Sports and Culture, Japan, and by a research grant from the Japan Health Sciences Foundation to T. I.
REFERENCES
- 1.Baily G G, Mason P R, Trijssenar F E, Lyons N F. Serological diagnosis of neurocysticercosis: evaluation of ELISA tests using cyst fluid and other components of Taenia solium cysticerci as antigens. Trans R Soc Trop Med Hyg. 1988;82:295–299. doi: 10.1016/0035-9203(88)90451-8. [DOI] [PubMed] [Google Scholar]
- 2.Brandt J R, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, Falla N. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–477. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 3.Chung J Y, Bahk Y Y, Huh S, Kang S Y, Kong Y, Cho S Y. A recombinant 10-kDa protein of Taenia solium metacestodes specific to active neurocysticercosis. J Infect Dis. 1999;180:1307–1315. doi: 10.1086/315020. [DOI] [PubMed] [Google Scholar]
- 4.Craig P S, Rogan M T, Allan J C. Detection, screening and community epidemiology of taeniid cestode zoonoses: cystic echinococcosis, alveolar echinococcosis and neurocysticercosis. Adv Parasitol. 1996;38:169–250. doi: 10.1016/s0065-308x(08)60035-4. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez V, Ferreira H B, Fernandez C, Zaha A, Nieto A. Molecular characterisation of a novel 8-kDa subunit of Echinococcus granulosus antigen B. Mol Biochem Parasitol. 1996;77:247–250. doi: 10.1016/0166-6851(96)02602-3. [DOI] [PubMed] [Google Scholar]
- 6.Gottstein B, Tsang V C, Schantz P M. Demonstration of species-specific and cross-reactive components of Taenia solium metacestode antigens. Am J Trop Med Hyg. 1986;35:308–313. doi: 10.4269/ajtmh.1986.35.308. [DOI] [PubMed] [Google Scholar]
- 7.Higgins D G, Bleasby A J, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 8.Hopp T P, Woods K R. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983;20:483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- 9.Hubert K, Andriantsimahavandy A, Michault A, Frosch M, Muhlschlegel F A. Serological diagnosis of human cysticercosis by use of recombinant antigens from Taenia solium cysticerci. Clin Diagn Lab Immunol. 1999;6:479–482. doi: 10.1128/cdli.6.4.479-482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito A, Plancarte A, Ma L, Kong Y, Flisser A, Cho S Y, Liu Y H, Kamhawi S, Lightowlers M W, Schantz P M. Novel antigens for neurocysticercosis: simple method for preparation and evaluation for serodiagnosis. Am J Trop Med Hyg. 1998;59:291–294. doi: 10.4269/ajtmh.1998.59.291. [DOI] [PubMed] [Google Scholar]
- 11.Ito A, Ma L, Schantz P M, Gottstein B, Liu Y H, Chai J J, Abdel Hafez S K, Altintas N, Joshi D D, Lightowlers M W, Pawlowski Z S. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (EM18) Am J Trop Med Hyg. 1999;60:188–192. doi: 10.4269/ajtmh.1999.60.188. [DOI] [PubMed] [Google Scholar]
- 12.Ito A, Nakao M, Ito Y, Yuzawa I, Morishima H, Kawano N, Fujii K. Neurocysticercosis case with a single cyst in the brain showing dramatic drop in specific antibody titers within 1 year after curative surgical resection. Parasitol Int. 1999;48:95–99. doi: 10.1016/s1383-5769(99)00005-7. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Larralde C, Laclette J P, Owen C S, Madrazo I, Sandoval M, Bojalil R, Sciutto E, Contreras L, Arzate J, Diaz M L, Govezensky T, Montoya R M, Goodsaid F. Reliable serology of Taenia solium cysticercosis with antigens from cyst vesicular fluid: ELISA and hemagglutination tests. Am J Trop Med Hyg. 1986;35:965–973. doi: 10.4269/ajtmh.1986.35.965. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi K, Murata M, Nakane M, Takemura N, Tsuchida T, Nakamura T. Cerebral cysticercosis. Intern Med. 1993;32:569–573. doi: 10.2169/internalmedicine.32.569. [DOI] [PubMed] [Google Scholar]
- 17.Ohsaki Y, Matsumoto A, Miyamoto K, Kondoh N, Araki K, Ito A, Kikuchi K. Neurocysticercosis without detectable specific antibody. Intern Med. 1999;38:67–70. doi: 10.2169/internalmedicine.38.67. [DOI] [PubMed] [Google Scholar]
- 18.Parkhouse R M, Harrison L J. Cyst fluid and surface associated glycoprotein antigens of Taenia sp. metacestodes. Parasite Immunol. 1987;9:263–268. doi: 10.1111/j.1365-3024.1987.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 19.Plancarte A, Hirota C, Martinez J O, Hernandez G M, Zenteno E, Flisser A. Characterization of GP39–42 and GP24 antigens from Taenia solium cysticerci and of their antigenic GP10 subunit. Parasitol Res. 1999;85:680–684. doi: 10.1007/s004360050615. [DOI] [PubMed] [Google Scholar]
- 20.Schantz P M, Moore A C, Munoz J L, Hartman B J, Schaefer J A, Aron A M, Persaud D, Sarti E, Wilson M, Flisser A. Neurocysticercosis in an Orthodox Jewish community in New York City. N Engl J Med. 1992;327:692–695. doi: 10.1056/NEJM199209033271004. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd J C, Aitken A, McManus D P. A protein secreted in vivo by Echinococcus granulosus inhibits elastase activity and neutrophil chemotaxis. Mol Biochem Parasitol. 1991;44:81–90. doi: 10.1016/0166-6851(91)90223-s. [DOI] [PubMed] [Google Scholar]
- 22.Simanjuntak G M, Margono S S, Okamoto M, Ito A. Taeniasis/Cysticercosis in Indonesia as an emerging disease. Parasitol Today. 1997;13:321–323. [Google Scholar]
- 23.Sloan L, Schneider S, Rosenblatt J. Evaluation of enzyme-linked immunoassay for serological diagnosis of cysticercosis. J Clin Microbiol. 1995;33:3124–3128. doi: 10.1128/jcm.33.12.3124-3128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang V C, Brand J A, Boyer A E. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 26.Wandra T, Subahar R, Simanjuntak G M, Margono S S, Suroso T, Okamoto M, Nakao M, Sako Y, Nakaya K, Schantz P M, Ito A. Resurgence of cases of epileptic seizures and burns associated with cysticercosis in Assologaima, Jayawijaya, Irian Jaya, Indonesia, 1991–95. Trans R Soc Trop Med Hyg. 2000;94:46–50. doi: 10.1016/s0035-9203(00)90433-4. [DOI] [PubMed] [Google Scholar]
- 27.White A C., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–113. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M, Bryan R T, Fried J A, Ware D A, Schantz P M, Pilcher J B, Tsang V C. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164:1007–1009. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]
- 29.Zarlenga D S, Rhoads M L, al Yaman F M. A Taenia crassiceps cDNA sequence encoding a putative immunodiagnostic antigen for bovine cysticercosis. Mol Biochem Parasitol. 1994;67:215–223. doi: 10.1016/0166-6851(94)00132-4. [DOI] [PubMed] [Google Scholar]