Abstract
An early detection and intervention for dementia represent tremendous unmet clinical needs and priorities in society. A shared feature of neurodegenerative diseases causing dementia is the abnormal accumulation and spreading of pathological protein aggregates, which affect the selective vulnerable circuit in a disease-specific pattern. The advancement in positron emission tomography (PET) biomarkers has accelerated the understanding of the disease mechanism and development of therapeutics for Alzheimer’s disease and Parkinson’s disease. The clinical utility of amyloid-β PET and the clinical validity of tau PET as diagnostic biomarker for Alzheimer’s disease continuum have been demonstrated. The inclusion of biomarkers in the diagnostic criteria has introduced a paradigm shift that facilitated the early and differential disease diagnosis and impacted on the clinical management. Application of disease-modifying therapy likely requires screening of patients with molecular evidence of pathological accumulation and monitoring of treatment effect assisted with biomarkers. There is currently still a gap in specific 4-repeat tau imaging probes for 4-repeat tauopathies and α-synuclein imaging probes for Parkinson’s disease and dementia with Lewy body. In this review, we focused on recent development in molecular imaging biomarkers for assisting the early diagnosis of proteinopathies (i.e., amyloid-β, tau, and α-synuclein) in dementia and discussed future perspectives.
Keywords: amyloid-β, tau, α-synclein, positron emission tomography (PET), Alzheimer’s disease, Parkinson’s disease, Lewy bodies, frontotemporal dementia (FTD)
Introduction
Today, nearly 50 million worldwide live with dementia. This number is projected to reach 152 million in 2050 as the population ages (Bhatt et al., 2019; Cummings et al., 2021b). An early detection and intervention for dementia represent tremendous unmet clinical needs and priorities in the aging societies. Neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB), are the most common causes of dementia. In these diseases, the abnormal accumulation of aggregates of the pathological protein activates a cascade of biochemical changes and affects the selective vulnerable circuit in a disease-specific pattern (Pievani et al., 2014; Bang et al., 2015; Goedert, 2015; De Strooper and Karran, 2016; Jucker and Walker, 2018; Soto and Pritzkow, 2018; Park et al., 2020). AD is pathologically hallmarked by amyloid-β (Aβ) plaque, neurofibrillary tangle (NFT), and neuronal loss (Knopman et al., 2021; Scheltens et al., 2021). Clinically, AD is characterized by the progressive loss of memory and cognitive functions, gradually affecting the daily life of patients. In vivo imaging studies in AD have shown that molecular changes in the brain precede the occurrence of clinical symptoms of cognitive decline by a long period, up to 15 years (Palmqvist et al., 2021). FTD includes a spectrum of tauopathy diseases, including corticobasal disease (CBD), progressive supranuclear palsy (PSP), and Pick’s disease (Spillantini and Goedert, 2013), clinically characterized by progressive executive, behavioral, or language dysfunctions depending on the disease types (Spillantini and Goedert, 2013). DLB, PD, and multiple system atrophy (MSA) are pathologically characterized by the appearance of Lewy bodies and Lewy neurites, composed of aggregated α-synuclein fibrils (Fares et al., 2021). The loss of dopaminergic neurons in the substantia nigra is the major pathological hallmark of PD (Poewe et al., 2017). The clinical diagnosis of PD is based on the motor dysfunction symptoms, including bradykinesia, rigidity, and resting tremor due to the nigrostriatal degeneration. Overlapping clinical symptoms and comorbidities in different diseases impose challenges on the accurate disease diagnosis, especially at a prodromal or early disease stage (Irwin et al., 2013). For example, the clinical symptoms in PD overlap with that in MSA and PSP to a certain extent (Politis, 2014). AD overlaps in the symptom or in pathological features with vascular dementia, FTD, and DLB (Knopman et al., 2021). Thus, a highly specific biomarker or combinations of biomarkers for increasing the diagnostic accuracy and enabling optimal treatment strategy are highly desired. In this review, we focused on the recent developments in positron emission tomography (PET) tracers for the detection of proteinopathies (i.e., Aβ, tau, and α-synuclein) in neurodegenerative diseases.
Positron Emission Tomography for Proteinopathies in Neurodegenerative Diseases
The advances in molecular imaging using PET, structural and functional imaging using magnetic resonance imaging, cerebrospinal fluid assays for detecting disease pathological hallmarks have facilitated the early and differential diagnosis and clinical management in AD, as well as the understanding of the disease mechanism and development of therapeutics (Sevigny et al., 2016; Crunkhorn, 2017; Boxer et al., 2019; Rabinovici et al., 2019; Chételat et al., 2021; Hansson, 2021). [18F]fluorodeoxyglucose (FDG)-PET has been used for detecting the cerebral glucose hypometabolism in disease-specific brain regions in patients with AD, FTD (Foster et al., 2007; Chételat et al., 2020), and idiopathic PD and atypical parkinsonism associated with dementia improving the diagnostic accuracy (Walker et al., 2018). There is a rapid advancement in recent 20 years in the development of specific PET tracers for pathological proteinopathies, neuroinflammation, and synaptic density markers in neurodegenerative diseases. Several prerequisites need to be fulfilled for an ideal PET tracer, including low molecular weight, sensitivity, specificity (i.e., low off-target binding), high affinity, moderate lipophilicity, solubility, blood-brain barrier entrance (i.e., sufficient brain uptake), reversible binding property, and pharmacokinetics, as well as no radiolabeled metabolites in the brain (Pike, 2009).
Amyloid-β Imaging
Amyloid-β is produced by proteolytic processing of the amyloid precursor protein on the neurons and glial cells. An imbalance between the production and clearance of Aβ leads to its abnormal cerebral accumulation (i.e., accumulation of oligomers, protofibrils, fibrils, and amyloid plaques), which plays a central role in the pathogenesis of AD both in animal models and in patients (Lesné et al., 2006; Haass and Selkoe, 2007; Lambert et al., 2007; Shankar et al., 2008; Selkoe and Hardy, 2016). The spread of Aβ follows a specific pattern, starting from neocortical regions to regions that receive neuronal projections and later to subcortical regions such as the striatum and the cerebellum (Thal et al., 2002). Using amyloid PET imaging combined with a functional MRI, the earliest accumulation of Aβ is found within the default mode network and, concurrently, affects the brain connectivity (Altmann et al., 2015; Palmqvist et al., 2017; Sepulcre et al., 2017; Hanseeuw et al., 2019; Rabinovici et al., 2019; Vogel et al., 2020). Amyloid PET tracers detect the β-sheet structures and are mainly benzothiazole and benzoxazole derivatives (Table 1; Klunk et al., 2004; Rowe et al., 2008; Furukawa et al., 2009; Nelissen et al., 2009; Nyberg et al., 2009; Hostetler et al., 2011; Cselenyi et al., 2012; Rodriguez-Vieitez et al., 2015; Sehlin et al., 2016; Grimmer et al., 2020; Meier et al., 2021; Ni, 2021; Ni et al., 2021). Among these tracers, three have been approved by Food and Drug Administration (FDA) and European Medicines Agency for clinical usage, namely, [18F]flutemetamol (Vizamyl), [18F]florbetapir (Amyvid), and [18F]florbetaben (Neuraceq) (Clark et al., 2011; Curtis et al., 2015; Sabri et al., 2015). PET studies using amyloid probes [11C]PiB, [18F]flutemetamol, [18F]florbetapir, [18F]florbetaben, and [18F]flutafuranol (AZD4694) have demonstrated higher cortical fibrillar Aβ loads in patients with mild cognitive impairment and AD compared with those in healthy controls (Klunk et al., 2005; Clark et al., 2011; Curtis et al., 2015; Sabri et al., 2015; Jack et al., 2018; Wolk et al., 2018). A robust in vivo congruence between aforementioned tracers and an in vivo postmortem correlation have been demonstrated in the human brain (Villemagne et al., 2012; Rowe et al., 2013; Ni et al., 2017, 2021; Su et al., 2019; Ikonomovic et al., 2020). It is noted that Aβ deposits are detected in the non-demented control, with the incidence associating with increasing age (Pike et al., 2007). In the context of a structured 5-phase development framework, amyloid PET using aforementioned tracers has already reached the clinical utility phase (Cotta Ramusino et al., 2021). It has been established as a pathological biomarker for early and differential diagnosis of AD continuum based on both the international working group and the National Institute on Aging-Alzheimer’s Association research AT(N) framework (Frisoni et al., 2017; Jack et al., 2018; Dubois et al., 2021) and recently proposed ATX(N) conceptual framework (Hampel et al., 2021). To further ensure a standardized outcome measure, and to reduce the disagreement across amyloid-PET imaging, the readouts have been converted into Centiloid units (Klunk et al., 2015). This is based on the normalization of the data from [18F] amyloid tracers relative to [11C]PiB, with young controls as zero and averages from typical patients with mild-moderate AD as 100 (Klunk et al., 2015). Recent probes with an improved binding specificity and lower bone uptake, such as [18F]FIBT, [18F]FACT, and [18F]D15FSP (Ito et al., 2014; Grimmer et al., 2020; Xiao et al., 2021), or that detect diffuse amyloid, such as benzoselenazole derivative [18F]fluselenamyl, have been developed (Sundaram et al., 2016). Antibody-based PET/single-photon emission computed tomography (SPECT) tracers [124I]RmAb158-scFv8D3 and [124I]8D3-F(ab’)2-h158 have showed sufficient blood-brain barrier entrance, by conjugating to transferrin receptor antibodies, in several transgenic mouse models of amyloidosis (Sehlin et al., 2016; Meier et al., 2021).
TABLE 1.
Positron emission tomography and SPECT imaging tracers for detecting proteinopathies; in vivo evaluation in human and in animal models.
Tau Imaging
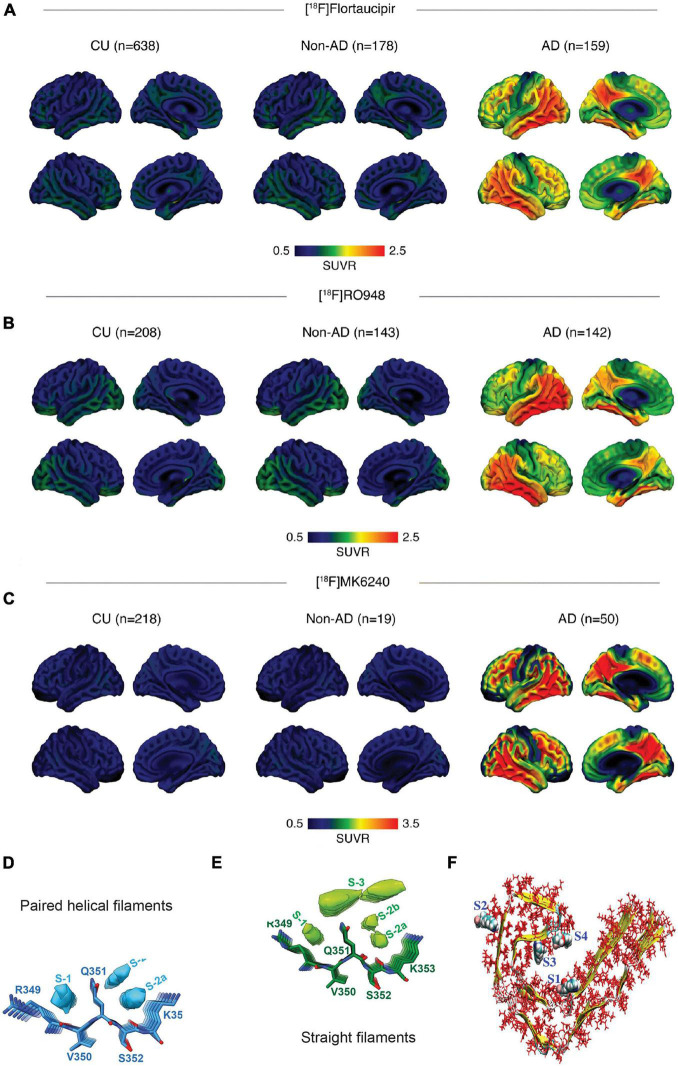
Microtubule-associated tau protein (MAPT) is located inside the neurons and is produced by alternative splicing from MAPT gene on chromosome 17. Tau has important physiological functions in regulating the axonal transport and neurite outgrowth and maintaining the microtubule stability (Chang et al., 2021). In AD brain, both 3-repeat (3R) and 4-repeat (4R) tau are presented, as 4R tau in CBD and PSP brain and 3R tau in Pick’s disease brain (Iqbal et al., 2010; Shi et al., 2021b). Tau is abnormally hyperphosphorylated forming oligomer, fibrils, and NFTs (Iqbal et al., 2010; Spillantini and Goedert, 2013). In the AD brain, tangles accumulate first in the entorhinal cortex (Braak and Braak, 1991) and, subsequently, spread from the entorhinal cortex to the hippocampus and neocortex via neuronal projection, leading to the disruption of the microtubule stability and cell death (Holmes et al., 2014; Franzmeier et al., 2019). MRI readouts of neurodegeneration and functional network alterations associate with tau and Aβ accumulation detected by PET in patients with mild cognitive impairment and AD (Jacobs et al., 2018; Franzmeier et al., 2019; La Joie et al., 2020; Vogel et al., 2020). Several tau tracers have been developed, including first-generation [18F]flortaucipir (Johnson et al., 2016), [11C]PBB3 (Maruyama et al., 2013), [11C]THK-523 (Fodero-Tavoletti et al., 2011), [18F]THK-5117 (Okamura et al., 2013), [18F]THK-5105 (Okamura et al., 2014), and [18F]THK-5351 (Harada et al., 2016) and second-generation [18F]MK6240 (Lohith et al., 2019), [18F]RO948 (Leuzy et al., 2020), [18F]PI2620 (Mueller et al., 2019), [18F]PM-PBB3 (APN-1607) (Tagai et al., 2020), [18F]JNJ-64326067 (Schmidt et al., 2020), and [18F]GTP1 (Sanabria Bohórquez et al., 2019). In the context of a structured 5-phase development framework of biomarkers for AD, the first- and second-generation tau PET tracers are currently considered at the clinical validity phase (Bischof et al., 2021; Chiotis et al., 2021; Wolters et al., 2021). Among these tracers, [18F]flortaucipir (Tauvid) has been approved by FDA for imaging tauopathy in patients with cognitive impairments undergoing evaluation for AD. [18F]flortaucipir has been used in clinical trials to monitor the development of regional tauopathy in patients with AD during immunotherapy, targeting Aβ (Cummings et al., 2021a; Knopman et al., 2021). The off-target binding to monoamine oxidase-B (MAO-B) and in the choroid plexus was reported with the first-generation tracers, namely, [18F]flortaucipir, [18F]THK-5117, and (S)-[18F]THK-5117 (Sander et al., 2016; Lemoine et al., 2018; Wren et al., 2018; Murugan et al., 2019). In addition, Hansen et al. showed a decrease in the [18F]flortaucipir binding to neuromelanin in the midbrain of patients with PD compared with controls, reflecting the loss of pigmented neurons in the substantial nigra (Hansen et al., 2016). With the improved design, no clear off-target binding was reported for the second-generation tau imaging probe in the choroid plexus in vivo (Mueller et al., 2019; Sanabria Bohórquez et al., 2019; Leuzy et al., 2020; Pascoal et al., 2020; Schmidt et al., 2020; Tagai et al., 2020) or to MAO-B in postmortem investigations (Yap et al., 2021). Leuzy et al. (2021) reported a multicenter comparison study and suggested that a common temporal lobe region of interest and cut-off can be used for the differential diagnosis of patients with dementia with [18F]flortaucipir, [18F]RO948, and [18F]MK6240 tau PET (Figures 1A–C). For the primary tauopathy diseases, Kroth et al. (2019) and Brendel et al. (2020) showed that [18F]PI2620 showed a higher uptake in the basal ganglia of patients with PSP compared with that in controls by PET, with a high specificity in the brain from patients with PSP at the postmortem. Tagai et al. (2020) showed a distinct tau distribution pattern using PET with [18F]PM-PBB3 in patients with PSP in the basal ganglia and patients with AD in the cortex and hippocampus compared with that in control. Yap et al. (2021) recently compared the second-generation probes PI2620, RO948, MK6240, and JNJ-64326067 in postmortem brain tissues from patients with AD, PSP, CBD, and Pick’s disease by using autoradiography and immunohistochemistry and demonstrated that these tracers could detect cortical paired-helical-filament tau and a lower binding to cortical inclusions of primary tauopathies.
FIGURE 1.
In vivo and postmortem comparison of tau imaging probes in the human brain (A–C) multicenter comparison of positron emission tomography (PET) imaging using [18F]flortaucipir, [18F]RO948, and [18F]MK6240, standardized uptake value ratios (SUVRs) across all participants within diagnostic groups; non-demented controls (CU), Alzheimer’s disease (AD); reproduced from Leuzy et al. (2021) with permission from Springer Nature; (D,E) binding of APN-1607 (PM-PBB3) to tau-paired helical filaments and straight filaments is based on cryo-EM, top views and side views of the extra densities in the PM-PBB3 binding sites of paired helical filaments (D) and straight filaments (E) maps. The models of PM-PBB3 are shown near these extra densities at the same scale. Reproduced from Shi et al. (2021a) with permission from Springer Nature; and (F) various high-affinity binding sites of tau protofibril. The sites 1, 3, and 4 are termed core sites as they are buried inside the fibril, whereas site 2 is termed a surface site as it is exposed to a greater amount of solvent molecules. Reproduced from Murugan et al. (2018) with permission from American Chemical Society.
There is currently a lack of tracers specific for 4R tau. Animal models, such as P301L and P301S, that recapitulate pathological features of 4R tauopathy have been developed with mutations in the MAPT gene, (Lewis et al., 2000; Gotz et al., 2001; SantaCruz et al., 2005; Spires et al., 2006), as well as hTau and knock in animal models (Saito et al., 2019; Hosokawa et al., 2021). The in vivo imaging of tau has been demonstrated in animal models using [18F]APN-1607, [11C]PBB3, [11C]mPBB5, [18F]THK5117, and [18F]JNJ64349311 (Maruyama et al., 2013; Brendel et al., 2016, 2018; Declercq et al., 2017; Ishikawa et al., 2018; Ni et al., 2018; Tagai et al., 2020; Vagenknecht et al., 2021), as well as SPECT using [123I]PIP-NHMe (Watanabe et al., 2021). It is noted that [18F]flortaucipir did not detect tau in the rTg4510 (P301L) 4R-tau mouse model (Marquié et al., 2015; Ni et al., 2018). More recently, tracer pyridinyl-indole derivative [18F]CBD-2115 has shown 4R-specific detection and promising brain uptake in mouse, rat, as well as non-human primate (Lindberg et al., 2021).
α-Synuclein Imaging
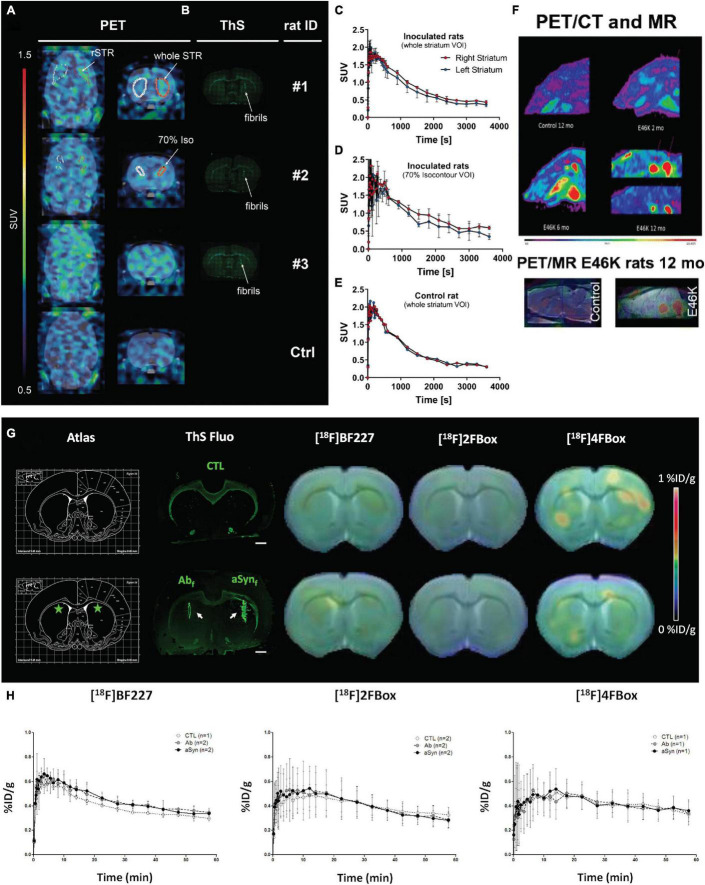
A highly desired, but so far unmet clinical need, is the in vivo visualization of the cerebral accumulation of α-synuclein in individuals with α-synucleinopathies, including patients with PD, DLB, and MSA (Poewe et al., 2017; Attems et al., 2021). The α-synuclein inclusions are mainly located in the presynaptic neurons in PD and DLB, while in oligodendroglial cells in MSA. Dopamine transporter imaging using [18F]DOPA PET or [123I]FP-CIT SPECT (DAT scan) is commonly utilized to visualize dopaminergic deficits in PD (Maltais et al., 2020). [18F]FDG PET visualizes the cerebral glucose metabolism in DLB- and MSA-related patterns (Niethammer and Eidelberg, 2012) and differentiates between patients with classical PD, atypical parkinsonian syndromes, and healthy control. The accumulation of misfolded α-synuclein occurs at Braak stage 1 in PD, preceding the loss of dopaminergic neurons that occurs at Braak stage 4 in the substantia nigra (Politis, 2014). Thus, the development of PET imaging for α-synuclein deposits would enable an early diagnosis of α-synucleinopathies and facilitate clinical trials targeting α-synuclein (Poewe et al., 2017; Attems et al., 2021). A few structures and imaging tracers for α-synuclein, such as BF-227 alike compounds, [11C]PBB3, [18F]C05-05, [11C]MODAG-001, [18F]FS3 (or DABTA-11, –7, –8), [18F]ACI-Cpd-AE, [18F]ACI-12589, [18F]4FBox, and [18F]2FBox, have been identified and evaluated in vitro (Yu et al., 2012; Bagchi et al., 2013; Koga et al., 2017; Verdurand et al., 2018; Hooshyar Yousefi et al., 2019; Capotosti et al., 2020; Ono et al., 2020; Uzuegbunam et al., 2021; Table 1). Many of the current α-synuclein PET tracers display insufficient selectivity, inadequate brain uptake, or pharmacokinetics. Among these, only four tracers have so far been evaluated in human subjects with α-synucleinopathy, namely, (1) in vivo PET using [11C]BF-227 PET in patients with MSA showed higher brain accumulation compared with healthy control (Kikuchi et al., 2010). However, BF-227 also detects Aβ pathology and is insensitive to α-synuclein in brain from α-synuclein transgenic mouse model (Levigoureux et al., 2014). (2) In vivo PET using [11C]PBB3 has been performed in patients with MSA. However, the signal source was inconclusive due to the comorbidity in the brain (Perez-Soriano et al., 2017). [11C]PBB3 showed a lower affinity and selectivity binding to α-synuclein fibrils compared with tau fibrils in vitro. Given the nanomolar concentration of [11C]PBB3 in in vivo PET, α-synuclein pathology is likely below the detection threshold (Koga et al., 2017); (3) [18F]FS3 showed nanomolar affinity to α-synuclein fibrils (around 100-folds selectivity over Aβ and tau fibrils), brain uptake in human, as well as in the medulla oblongata of E46K α-synuclein rat model (Yousefi et al., 2016; Aboagye and Kraeber-Bodéré, 2017; Hooshyar Yousefi et al., 2019; Figure 2F); and (4) [18F]-ACI-Cpd-AE demonstrated a fast brain uptake, low non-specific binding, rapid metabolism, and 10% higher relative standard uptake value in the substantia nigra of patients with PD compared with those in healthy controls (Capotosti et al., 2020).
FIGURE 2.
In vivo α-synuclein imaging in animal models. (A–E) In vivo binding of (d3)-[11C]MODAG-001 in α-synuclein-inoculated rats. Coronal and transversal PET images (2.5–60 min) (A). Images show increased tracer accumulation in the α-synuclein fibril-inoculated right striatum compared with the vehicle-injected contralateral striatum. Thioflavin-S staining (B) indicated α-synuclein fibrils in the right striatum of fibril-inoculated rats (B). (C–E) Time activity curves of (d3)-[11C]MODAG-001 higher signal in the right (α-synuclein injected) than left (vehicle injected) striatum; α-SYN, α-synuclein; rSTR, right striatum; ThS, thioflavin S; Ctrl, control; SUV, standardized uptake value, DVR-1, distribution volume ratio-1; VOI, voxel of interest; Reproduced from Kuebler et al. (2021) with permission from Springer Nature. (F) [18F]DABTA-11 PET images in E46K rats show accumulation of the tracer in the medulla oblongata. The accumulation is apparent even at 2 months of age and is more prominent at 6 and 12 months of age with detectable uptake in the substantia nigra. PET/MRI and rat brain atlas confirm the regional uptake of the tracer. Reproduced from Yousefi et al. (2016) and Aboagye and Kraeber-Bodéré (2017) with permission from Springer Nature. (G,H) small-animal PET imaging with [18F]BF227, [18F]2FBox, and [18F]4FBox in control and fibril-injected rats. (G) Summed PET images were coregistered with CT images, and the radioactivity index was reflected by a color scale representing %ID/g. ThS fluorescence staining of Aβ42 and α-syn fibrils injected in the striata is presented (white arrows), with the corresponding stereotaxic brain atlas region (green stars representing injection sites). Scale bar represents 1 mm on ThS fluorescence staining. (H) Time activity curves (expressed in %ID/g over time) for each radiotracer are presented. Values (mean ± SD) were extracted from the striata regions based on an in-house-made MRI atlas that was coregistered to PET-CT images. Reproduced from Verdurand et al. (2018) with permission from American Chemical Society.
Several new α-synuclein probes of different scaffolds have been reported recently with in vitro/in vivo evaluation in animal models. Verdurand et al. (2018) reported two new probes [18F]4FBo and [18F]2FBox that bind to α-synuclein and Aβ fibrils that show sufficient brain uptake in a rat model but not in a mouse model with α-synucleinopathy (Figures 2G,H). Kaide et al. (2020) developed a bisquinoline derivative [18F]BQ2 and showed a moderate brain uptake (i.e., 1.59% ID/g at 2 min and 1.35% ID/g at 60 min post injection) in the brain of a mouse model. Maurer et al. (2020) reported that diphenyl pyrazoles derivative [11C]anle253b, based on α-synuclein oligomer modulator anle138b (Wagner et al., 2013; Wegrzynowicz et al., 2019), exhibited a good penetration in the blood-brain barrier, brain uptake, and low background binding to the non-pathological brain. Kuebler et al. (2021) reported that diphenyl pyrazole derivative [11C]MODAG-001 showed a high-affinity binding to α-synuclein (i.e., 0.6 nM, 30-fold higher than to tau and Aβ fibrils) and a sufficient brain uptake in α-synuclein-inoculated rats (Figures 2A–E). Ono et al. recently reported that [18F]C-05-05, a compound developed based on the PBB3 structure, showed specific detection of ps129 antibody-positive phosphorylated α-synuclein in a mouse model, as well as in the non-human primate (Ono et al., 2020). In addition, several recent probes [18F]WC-58a (Chu et al., 2015), XW01-04, XW01-64 (Sun et al., 2021), [3H]BF2846, and [3H]Tg-190b (Ferrie et al., 2020) demonstrated > 30-fold selectivity to α-synuclein over Aβ (in silico and in vitro binding to fibrils) and in autoradiograph/staining in postmortem brain tissues.
Discussion
The advances in PET detection of disease-specific pathological proteinopathy have facilitated the personalized and timely diagnosis of dementia and offers a window for therapeutic intervention (Hansson, 2021). The integration of PET imaging, assays of cerebrospinal fluid, MRI biomarkers, and forthcoming blood tests further increases the diagnostic power in early and differential diagnosis (Altomare et al., 2021; Cullen et al., 2021; Palmqvist et al., 2021). The application of new disease-modifying treatment such as immunotherapy will likely require screening in prodromal patients for pathological evidence, e.g., cerebral Aβ, tau, or α-synuclein accumulation, and monitoring of treatment effects (Sevigny et al., 2016; Boxer et al., 2019; Rabinovici et al., 2019). In addition, proteinopathy imaging combined with PET for synaptic loss, mitochondria dysfunction, and neuroinflammation (e.g., astrocytosis and microgliosis) enables a more comprehensive understanding of the mechanism underlying neurodegeneration associated with proteinopathies (Calsolaro et al., 2021; Pascoal et al., 2021; Zhou R. et al., 2021).
Structural variations in Aβ fibrils may contribute to variations in the disease onset and the progression rate of AD. The cryo-EM study has shown polymorphism of Aβ fibrils from the AD brain tissue (Kollmer et al., 2019). The in vivo imaging and postmortem studies have demonstrated different detection patterns of Aβ conformational variants in different autosomal-dominant AD (Schöll et al., 2012; Ni et al., 2017; Chen et al., 2021). In silico studies have implied six binding sites on Aβ fibrils, and amyloid tracers of different structures detect different sites on Aβ fibrils or conformations (Murugan et al., 2016; Kuang et al., 2019). Tau molecular diversity and posttranslational modification are important contributors for the clinical heterogeneity in patients with AD (Dujardin et al., 2020). Four trajectories of diverse tau deposition pattern have been identified in the AD brain (Vogel et al., 2021). Shi et al. recently proposed a structure-based classification of tauopathy diseases underlined the tau strain heterogeneity and challenge in developing imaging probes specific for certain tau strain (Shi et al., 2021b). In vivo and postmortem comparative studies using different tau tracers indicated even more divergent patterns among tracers in primary tauopathies than in AD (Ono et al., 2017; Schonhaut et al., 2017; Chen et al., 2018; Endo et al., 2019; Ikeda et al., 2019; Leuzy et al., 2019; Arakhamia et al., 2020; Brendel et al., 2020; Tagai et al., 2020; Yap et al., 2021). Several recent in silico modeling studies suggest four binding sites on AD tau and highlighted the heterogeneity among probes binding to different tau strains: For the first-generation tracers, MK6240 and flortaucipir bind only to major binding site 1, while THK5351 binds to site 1 and 3, and PBB3 detects all four binding sites. For the second-generation tracers, PI2620, CBD-2115, and PM-PBB3 showed higher binding affinities to CBD tau compared with the 3R/4R tracer MK6240, and CBD-2115 and PM-PBB3 demonstrated higher binding affinities to AD tau compared with PI2620 (Figure 1F; Murugan et al., 2018, 2021; Kuang et al., 2020; Zhou Y. et al., 2021). Recent study reported that PM-PBB3 showed similar binding sites in cryo-EM study toward tau filaments from AD, posterior cortical atrophy, and primary age-related tauopathy (Figures 1D,E; Fitzpatrick et al., 2017; Shi et al., 2021a). Further investigations are anticipated for elucidating the tracers binding with cryo-EM structures of tau filaments from CBD and PSP and for rational designing of disease (strain) specific to develop tracers with an increased specificity and binding activity (Fitzpatrick et al., 2017; Zhang et al., 2020). The challenges of α-synuclein imaging stem from the intracellular location of α-synuclein inclusions, distinct α-synuclein strains, presence across different neurodegenerative diseases, and difficulty in finding a tracer with selectivity to α-synuclein over Aβ and tau fibrils (Yamasaki et al., 2019; Berg et al., 2021). Moreover, the cryo-EM structures of α-synuclein filaments from the brains of patients with MSA differ from in vitro recombinant α-synuclein fibrils (Schweighauser et al., 2020). The different α-synuclein strains contribute to the disease heterogeneity in animal models and in patients (Holec and Woerman, 2021). Klingstedt et al. (2019) and Shahnawaz et al. (2020) demonstrated a differential binding of a fluorescence luminescent-conjugated oligothiophenes probe to α-synuclein fibrils derived from patients with MSA with that from patients with PD.
Further high-throughput screening and structure-activity relationship studies are needed to map the ligand binding site topology on 4R-tau and α-synuclein fibrils, to guide the development of tracers with a higher affinity and selectivity. In addition, deep learning-based drug development such as using AlphaFold or RoseTTaFold and on-chip pharmacokinetics may speed up the development and optimization of imaging tracers (Schneider, 2018; Bhhatarai et al., 2019; Baek et al., 2021; Jumper et al., 2021). Multiscale simulation pipeline combining methods with different accuracy/efficiency such as molecular docking, molecular dynamics simulation, and free energy calculation, will likely provide a high degree of validation of the simulations (Araki et al., 2021).
In summary, amyloid and tau PET imaging have a profound impact on the early and differential diagnosis of dementia and facilitated the development of disease-modifying therapeutics. Further development of 4R tau and α-synuclein specific tracers is needed to fill the unmet need and move toward precision medicine in dementia.
Author Contributions
RN wrote the draft manuscript. Both authors contributed to the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
RN received funding from Helmut Horten Stiftung, Jubiläumsstiftung von SwissLife, and Vontobel Stiftung, UZH Entrepreneur Fellowship of the University of Zurich, reference no. [MEDEF-20-021].
References
- Aboagye E., Kraeber-Bodéré F. (2017). Highlights lecture EANM 2016: “Embracing molecular imaging and multi-modal imaging: a smart move for nuclear medicine towards personalized medicine”. Eur. J. Nucl. Med. Mol. Imaging 44 1559–1574. 10.1007/s00259-017-3704-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A., Ng B., Landau S., Jagust W., Greicius M. (2015). Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain 138 3734–3746. 10.1093/brain/awv278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare D., Caprioglio C., Assal F., Allali G., Mendes A., Ribaldi F., et al. (2021). Diagnostic value of amyloid-PET and tau-PET: a head-to-head comparison. Eur. J. Nucl. Med. Mol. Imaging 48 2200–2211. 10.1007/s00259-021-05246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakhamia T., Lee C. E., Carlomagno Y., Duong D. M., Kundinger S. R., Wang K., et al. (2020). Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 180 633–644.e12. 10.1016/j.cell.2020.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M., Matsumoto S., Bekker G.-J., Isaka Y., Sagae Y., Kamiya N., et al. (2021). Exploring ligand binding pathways on proteins using hypersound-accelerated molecular dynamics. Nat. Commun. 12:2793. 10.1038/s41467-021-23157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J., Toledo J. B., Walker L., Gelpi E., Gentleman S., Halliday G., et al. (2021). Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 141 159–172. 10.1007/s00401-020-02255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M., DiMaio F., Anishchenko I., Dauparas J., Ovchinnikov S., Lee G. R., et al. (2021). Accurate prediction of protein structures and interactions using a three-track neural network. Science 373 871–876. 10.1126/science.abj8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D. P., Yu L., Perlmutter J. S., Xu J., Mach R. H., Tu Z., et al. (2013). Binding of the radioligand SIL23 to α-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a Parkinson disease imaging agent. PLoS One 8:e55031. 10.1371/journal.pone.0055031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang J., Spina S., Miller B. L. (2015). Frontotemporal dementia. Lancet 386 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D., Borghammer P., Fereshtehnejad S. M., Heinzel S., Horsager J., Schaeffer E., et al. (2021). Prodromal Parkinson disease subtypes – key to understanding heterogeneity. Nat. Rev. Neurol. 17 349–361. 10.1038/s41582-021-00486-9 [DOI] [PubMed] [Google Scholar]
- Bhatt J., Comas Herrera A., Amico F., Farina N., Wong J., Orange J. B., et al. (2019). The World Alzheimer Report 2019: Attitudes to dementia. [Google Scholar]
- Bhhatarai B., Walters W. P., Hop C. E. C. A., Lanza G., Ekins S. (2019). Opportunities and challenges using artificial intelligence in ADME/Tox. Nat. Mater. 18 418–422. 10.1038/s41563-019-0332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof G. N., Dodich A., Boccardi M., van Eimeren T., Festari C., Barthel H., et al. (2021). Clinical validity of second-generation tau PET tracers as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur. J. Nucl. Med. Mol. Imaging 48 2110–2120. 10.1007/s00259-020-05156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer A. L., Qureshi I., Ahlijanian M., Grundman M., Golbe L. I., Litvan I., et al. (2019). Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 18 549–558. 10.1016/S1474-4422(19)30139-5 [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82 239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Brendel M., Barthel H., van Eimeren T., Marek K., Beyer L., Song M., et al. (2020). Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol. 77 1408–1419. 10.1001/jamaneurol.2020.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M., Jaworska A., Probst F., Overhoff F., Korzhova V., Lindner S., et al. (2016). Small-animal PET imaging of tau pathology with 18F-THK5117 in 2 transgenic mouse models. J. Nucl. Med. 57 792–798. 10.2967/jnumed.115.163493 [DOI] [PubMed] [Google Scholar]
- Brendel M., Yousefi B. H., Blume T., Herz M., Focke C., Deussing M., et al. (2018). Comparison of 18F-T807 and 18F-THK5117 PET in a mouse model of tau pathology. Front. Aging Neurosci. 10:174. 10.3389/fnagi.2018.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsolaro V., Matthews P. M., Donat C. K., Livingston N. R., Femminella G. D., Guedes S. S., et al. (2021). Astrocyte reactivity with late-onset cognitive impairment assessed in vivo using 11C-BU99008 PET and its relationship with amyloid load. Mol. Psychiatry 10.1038/s41380-021-01193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosti F., Vokali E., Molette J., Tsika E., Ravache M., Juergens T., et al. (2020). Developing a novel alpha-synuclein positron emission tomography (PET) tracer for the diagnosis of synucleinopathies. Alzheimers Dement. 16:e043249. [Google Scholar]
- Chang C.-W., Shao E., Mucke L. (2021). Tau: enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science 371:eabb8255. 10.1126/science.abb8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. D., Joseph-Mathurin N., Sinha N., Zhou A., Li Y., Friedrichsen K., et al. (2021). Comparing amyloid-β plaque burden with antemortem PiB PET in autosomal dominant and late-onset Alzheimer disease. Acta Neuropathol. 142 689–706. 10.1007/s00401-021-02342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li Y., Pirraglia E., Okamura N., Rusinek H., de Leon M. J. (2018). Quantitative evaluation of tau PET tracers (18)F-THK5351 and (18)F-AV-1451 in Alzheimer’s disease with standardized uptake value peak-alignment (SUVP) normalization. Eur. J. Nucl. Med. Mol. Imaging 45 1596–1604. 10.1007/s00259-018-4040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Ono M., Kimura H., Kagawa S., Nishii R., Saji H. (2010). A novel 18F-labeled pyridyl benzofuran derivative for imaging of β-amyloid plaques in Alzheimer’s brains. Bioorg. Med. Chem. Lett. 20 6141–6144. 10.1016/j.bmcl.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Chételat G., Arbizu J., Barthel H., Garibotto V., Lammertsma A. A., Law I., et al. (2021). Finding our way through the labyrinth of dementia biomarkers. Eur. J. Nucl. Med. Mol. Imaging. 48 2320–2324. 10.1007/s00259-021-05301-7 [DOI] [PubMed] [Google Scholar]
- Chételat G., Arbizu J., Barthel H., Garibotto V., Law I., Morbelli S., et al. (2020). Amyloid-PET and (18)F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 19 951–962. 10.1016/S1474-4422(20)30314-8 [DOI] [PubMed] [Google Scholar]
- Chiotis K., Dodich A., Boccardi M., Festari C., Drzezga A., Hansson O., et al. (2021). Clinical validity of increased cortical binding of tau ligands of the THK family and PBB3 on PET as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur. J. Nucl. Med. Mol. Imaging 48 2086–2096. 10.1007/s00259-021-05277-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W., Zhou D., Gaba V., Liu J., Li S., Peng X., et al. (2015). Design, synthesis, and characterization of 3-(Benzylidene)indolin-2-one derivatives as ligands for α-synuclein fibrils. J. Med. Chem. 58 6002–6017. 10.1021/acs.jmedchem.5b00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. M., Schneider J. A., Bedell B. J., Beach T. G., Bilker W. B., Mintun M. A., et al. (2011). Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 305 275–283. 10.1001/jama.2010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta Ramusino M., Perini G., Altomare D., Barbarino P., Weidner W., Salvini Porro G., et al. (2021). Outcomes of clinical utility in amyloid-PET studies: state of art and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 48 2157–2168. 10.1007/s00259-020-05187-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunkhorn S. (2017). Antisense oligonucleotide reverses tau pathology. Nat. Rev. Drug Discov. 16 166–166. 10.1038/nrd.2017.37 [DOI] [PubMed] [Google Scholar]
- Cselenyi Z., Jonhagen M. E., Forsberg A., Halldin C., Julin P., Schou M., et al. (2012). Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J. Nucl. Med. 53 415–424. 10.2967/jnumed.111.094029 [DOI] [PubMed] [Google Scholar]
- Cullen N. C., Leuzy A., Janelidze S., Palmqvist S., Svenningsson A. L., Stomrud E., et al. (2021). Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat. Commun. 12:3555. 10.1038/s41467-021-23746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J., Lee G., Zhong K., Fonseca J., Taghva K. (2021b). Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (N Y) 7:e12179. 10.1002/trc2.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J., Aisen P., Lemere C., Atri A., Sabbagh M., Salloway S. (2021a). Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res. Ther. 13:98. 10.1186/s13195-021-00838-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C., Gamez J. E., Singh U., Sadowsky C. H., Villena T., Sabbagh M. N., et al. (2015). Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 72 287–294. 10.1001/jamaneurol.2014.4144 [DOI] [PubMed] [Google Scholar]
- De Strooper B., Karran E. (2016). The cellular phase of Alzheimer’s disease. Cell 164 603–615. [DOI] [PubMed] [Google Scholar]
- Declercq L., Rombouts F., Koole M., Fierens K., Mariën J., Langlois X., et al. (2017). Preclinical evaluation of 18F-JNJ64349311, a novel PET tracer for tau imaging. J. Nucl. Med. 58 975–981. 10.2967/jnumed.116.185199 [DOI] [PubMed] [Google Scholar]
- Dubois B., Villain N., Frisoni G. B., Rabinovici G. D., Sabbagh M., Cappa S., et al. (2021). Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 20 484–496. 10.1016/S1474-4422(21)00066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S., Commins C., Lathuiliere A., Beerepoot P., Fernandes A. R., Kamath T. V., et al. (2020). Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 26 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H., Shimada H., Sahara N., Ono M., Koga S., Kitamura S., et al. (2019). In vivo binding of a tau imaging probe, [11C]PBB3, in patients with progressive supranuclear palsy. Mov. Disord. 34 744–754. 10.1002/mds.27643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares M. B., Jagannath S., Lashuel H. A. (2021). Reverse engineering Lewy bodies: how far have we come and how far can we go? Nat. Rev. Neurosci. 22 111–131. 10.1038/s41583-020-00416-6 [DOI] [PubMed] [Google Scholar]
- Ferrie J. J., Lengyel-Zhand Z., Janssen B., Lougee M. G., Giannakoulias S., Hsieh C.-J., et al. (2020). Identification of a nanomolar affinity α-synuclein fibril imaging probe by ultra-high throughput in silico screening. Chem. Sci. 11 12746–12754. 10.1039/d0sc02159h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick A. W. P., Falcon B., He S., Murzin A. G., Murshudov G., Garringer H. J., et al. (2017). Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher A. S., Pontecorvo M. J., Devous M. D., Sr., Lu M., Arora A. K., Truocchio S. P., et al. (2020). Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 77 829–839. 10.1001/jamaneurol.2020.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodero-Tavoletti M. T., Okamura N., Furumoto S., Mulligan R. S., Connor A. R., McLean C. A., et al. (2011). 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 134 1089–1100. 10.1093/brain/awr038 [DOI] [PubMed] [Google Scholar]
- Foster N. L., Heidebrink J. L., Clark C. M., Jagust W. J., Arnold S. E., Barbas N. R., et al. (2007). FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 130 2616–2635. 10.1093/brain/awm177 [DOI] [PubMed] [Google Scholar]
- Franzmeier N., Rubinski A., Neitzel J., Kim Y., Damm A., Na D. L., et al. (2019). Functional connectivity associated with tau levels in ageing, Alzheimer’s, and small vessel disease. Brain 142 1093–1107. 10.1093/brain/awz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni G. B., Boccardi M., Barkhof F., Blennow K., Cappa S., Chiotis K., et al. (2017). Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 16 661–676. 10.1016/S1474-4422(17)30159-X [DOI] [PubMed] [Google Scholar]
- Furukawa K., Okamura N., Tashiro M., Waragai M., Furumoto S., Iwata R., et al. (2009). Amyloid PET in mild cognitive impairment and Alzheimer’s disease with BF-227: comparison to FDG–PET. J. Neurol. 257 721–727. 10.1007/s00415-009-5396-8 [DOI] [PubMed] [Google Scholar]
- Furumoto S., Okamura N., Furukawa K., Tashiro M., Ishikawa Y., Sugi K., et al. (2013). A 18F-labeled BF-227 derivative as a potential radioligand for imaging dense amyloid plaques by positron emission tomography. Mol. Imaging Biol. 15 497–506. 10.1007/s11307-012-0608-5 [DOI] [PubMed] [Google Scholar]
- Goedert M. (2015). Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349:1255555. 10.1126/science.1255555 [DOI] [PubMed] [Google Scholar]
- Gotz J., Chen F., van Dorpe J., Nitsch R. M. (2001). Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293 1491–1495. 10.1126/science.1062097 [DOI] [PubMed] [Google Scholar]
- Grimmer T., Shi K., Diehl-Schmid J., Natale B., Drzezga A., Förster S., et al. (2020). (18)F-FIBT may expand PET for β-amyloid imaging in neurodegenerative diseases. Mol. Psychiatry 25 2608–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. (2007). Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8 101–112. 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- Hampel H., Cummings J., Blennow K., Gao P., Jack C. R., Jr., Vergallo A. (2021). Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 17 580–589. 10.1038/s41582-021-00520-w [DOI] [PubMed] [Google Scholar]
- Hanseeuw B. J., Betensky R. A., Jacobs H. I. L., Schultz A. P., Sepulcre J., Becker J. A., et al. (2019). Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 76 915–924. 10.1001/jamaneurol.2019.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A. K., Knudsen K., Lillethorup T. P., Landau A. M., Parbo P., Fedorova T., et al. (2016). In vivo imaging of neuromelanin in Parkinson’s disease using 18 F-AV-1451 PET. Brain 139 2039–2049. 10.1093/brain/aww098 [DOI] [PubMed] [Google Scholar]
- Hansson O. (2021). Biomarkers for neurodegenerative diseases. Nat. Med. 27 954–963. 10.1093/jnen/nlaa041 [DOI] [PubMed] [Google Scholar]
- Harada R., Okamura N., Furumoto S., Furukawa K., Ishiki A., Tomita N., et al. (2015). [18F]THK-5117 PET for assessing neurofibrillary pathology in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 42 1052–1061. 10.1007/s00259-015-3035-4 [DOI] [PubMed] [Google Scholar]
- Harada R., Okamura N., Furumoto S., Furukawa K., Ishiki A., Tomita N., et al. (2016). 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J. Nucl. Med. 57 208–214. 10.2967/jnumed.115.164848 [DOI] [PubMed] [Google Scholar]
- Higashi T., Nishii R., Kagawa S., Kishibe Y., Takahashi M., Okina T., et al. (2018). 18F-FPYBF-2, a new F-18-labelled amyloid imaging PET tracer: first experience in 61 volunteers and 55 patients with dementia. Ann. Nucl. Med. 32 206–216. 10.1007/s12149-018-1236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holec S. A. M., Woerman A. L. (2021). Evidence of distinct α-synuclein strains underlying disease heterogeneity. Acta Neuropathol. 142 73–86. 10.1007/s00401-020-02163-5 [DOI] [PubMed] [Google Scholar]
- Holmes B. B., Furman J. L., Mahan T. E., Yamasaki T. R., Mirbaha H., Eades W. C., et al. (2014). Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. U. S. A. 111 E4376–E4385. 10.1073/pnas.1411649111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshyar Yousefi B., Shi K., Arzberger T., Wester H. J., Schwaiger M., Yakushev I., et al. (2019). Translational study of a novel alpha-synuclein PET tracer designed for first-in-human investigating. Nuklearmedizin 58 L25. [Google Scholar]
- Hosokawa M., Masuda-Suzukake M., Shitara H., Shimozawa A., Suzuki G., Kondo H., et al. (2021). Development of a novel tau propagation mouse model endogenously expressing 3 and 4 repeat tau isoforms. Brain 10.1093/brain/awab289 [DOI] [PubMed] [Google Scholar]
- Hostetler E. D., Sanabria-Bohórquez S., Fan H., Zeng Z., Gammage L., Miller P., et al. (2011). [18F]Fluoroazabenzoxazoles as potential amyloid plaque PET tracers: synthesis and in vivo evaluation in rhesus monkey. Nucl. Med. Biol. 38 1193–1203. [DOI] [PubMed] [Google Scholar]
- Hostetler E. D., Walji A. M., Zeng Z., Miller P., Bennacef I., Salinas C., et al. (2016). Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J. Nucl. Med. 57 1599–1606. 10.2967/jnumed.115.171678 [DOI] [PubMed] [Google Scholar]
- Ikeda A., Shimada H., Nishioka K., Takanashi M., Hayashida A., Li Y., et al. (2019). Clinical heterogeneity of frontotemporal dementia and Parkinsonism linked to chromosome 17 caused by MAPT N279K mutation in relation to tau positron emission tomography features. Mov. Disord. 34 568–574. 10.1002/mds.27623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic M. D., Buckley C. J., Abrahamson E. E., Kofler J. K., Mathis C. A., Klunk W. E., et al. (2020). Post-mortem analyses of PiB and flutemetamol in diffuse and cored amyloid-β plaques in Alzheimer’s disease. Acta Neuropathol. 140 463–476. 10.1007/s00401-020-02175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C. X., Grundke-Iqbal I. (2010). Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer. Res. 7 656–664. 10.2174/156720510793611592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D. J., Lee V. M. Y., Trojanowski J. Q. (2013). Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 14 626–636. 10.1038/nrn3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A., Tokunaga M., Maeda J., Minamihisamatsu T., Shimojo M., Takuwa H., et al. (2018). In vivo visualization of tau accumulation, microglial activation, and brain atrophy in a mouse model of tauopathy rTg4510. J. Alzheimers Dis. 61 1037–1052. 10.3233/JAD-170509 [DOI] [PubMed] [Google Scholar]
- Ito H., Shinotoh H., Shimada H., Miyoshi M., Yanai K., Okamura N., et al. (2014). Imaging of amyloid deposition in human brain using positron emission tomography and [18F]FACT: comparison with [11C]PIB. Eur. J. Nucl. Med. Mol. Imaging 41 745–754. 10.1007/s00259-013-2620-7 [DOI] [PubMed] [Google Scholar]
- Jack C. R., Jr., Bennett D. A., Blennow K., Carrillo M. C., Dunn B., Haeberlein S. B., et al. (2018). NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. I. L., Hedden T., Schultz A. P., Sepulcre J., Perea R. D., Amariglio R. E., et al. (2018). Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat. Neurosci. 21 424–431. 10.1038/s41593-018-0070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Schultz A., Betensky R. A., Becker J. A., Sepulcre J., Rentz D., et al. (2016). Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79 110–119. 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M., Walker L. C. (2018). Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 21 1341–1349. 10.1038/s41593-018-0238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596 583–589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaide S., Watanabe H., Shimizu Y., Iikuni S., Nakamoto Y., Hasegawa M., et al. (2020). Identification and evaluation of bisquinoline scaffold as a new candidate for α-synuclein-PET imaging. ACS Chem. Neurosci. 11 4254–4261. 10.1021/acschemneuro.0c00523 [DOI] [PubMed] [Google Scholar]
- Kepe V., Huang S. C., Small G. W., Satyamurthy N., Barrio J. R. (2006). Visualizing pathology deposits in the living brain of patients with Alzheimer’s disease. Methods Enzymol. 412 144–160. 10.1016/S0076-6879(06)12010-8 [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Takeda A., Okamura N., Tashiro M., Hasegawa T., Furumoto S., et al. (2010). In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain 133 1772–1778. 10.1093/brain/awq091 [DOI] [PubMed] [Google Scholar]
- Klingstedt T., Ghetti B., Holton J. L., Ling H., Nilsson K. P. R., Goedert M. (2019). Luminescent conjugated oligothiophenes distinguish between α-synuclein assemblies of Parkinson’s disease and multiple system atrophy. Acta Neuropathol. Commun. 7:193. 10.1186/s40478-019-0840-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk W. E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D. P., et al. (2004). Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55 306–319. 10.1002/ana.20009 [DOI] [PubMed] [Google Scholar]
- Klunk W. E., Koeppe R. A., Price J. C., Benzinger T. L., Devous M. D., Sr., Jagust W. J., et al. (2015). The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 11 1–15.e1–4. 10.1016/j.jalz.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk W. E., Lopresti B. J., Ikonomovic M. D., Lefterov I. M., Koldamova R. P., Abrahamson E. E., et al. (2005). Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J. Neurosci. 25 10598–10606. 10.1523/JNEUROSCI.2990-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D. S., Amieva H., Petersen R. C., Chételat G., Holtzman D. M., Hyman B. T., et al. (2021). Alzheimer disease. Nat. Rev. Dis. Primers. 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S., Ono M., Sahara N., Higuchi M., Dickson D. W. (2017). Fluorescence and autoradiographic evaluation of tau PET ligand PBB3 to α-synuclein pathology. Mov. Disord. 32 884–892. 10.1002/mds.27013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmer M., Close W., Funk L., Rasmussen J., Bsoul A., Schierhorn A., et al. (2019). Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 10:4760. 10.1038/s41467-019-12683-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroth H., Oden F., Molette J., Schieferstein H., Capotosti F., Mueller A., et al. (2019). Discovery and preclinical characterization of [(18)F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur. J. Nucl. Med. Mol. Imaging 46 2178–2189. 10.1007/s00259-019-04397-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang G., Murugan N. A., Ågren H. (2019). Mechanistic Insight into the Binding Profile of DCVJ and α-synuclein fibril revealed by multiscale simulations. ACS Chem. Neurosci. 10 610–617. 10.1021/acschemneuro.8b00465 [DOI] [PubMed] [Google Scholar]
- Kuang G., Murugan N. A., Zhou Y., Nordberg A., Ågren H. (2020). Computational insight into the binding profile of the Second-Generation PET tracer PI2620 with tau fibrils. ACS Chem. Neurosci. 11 900–908. 10.1021/acschemneuro.9b00578 [DOI] [PubMed] [Google Scholar]
- Kudo Y., Okamura N., Furumoto S., Tashiro M., Furukawa K., Maruyama M., et al. (2007). 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6- (2-[fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J. Nucl. Med. 48 553–561. 10.2967/jnumed.106.037556 [DOI] [PubMed] [Google Scholar]
- Kuebler L., Buss S., Leonov A., Ryazanov S., Schmidt F., Maurer A., et al. (2021). [(11)C]MODAG-001-towards a PET tracer targeting α-synuclein aggregates. Eur. J. Nucl. Med. Mol. Imaging 48 1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara H., Comley R. A., Borroni E., Honer M., Kitmiller K., Roberts J., et al. (2018). Evaluation of 18F-RO-948 PET for quantitative assessment of tau accumulation in the human brain. J. Nucl. Med. 59 1877–1884. 10.2967/jnumed.118.214437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R., Visani A. V., Baker S. L., Brown J. A., Bourakova V., Cha J., et al. (2020). Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci. Transl. Med. 12:eaau5732. 10.1126/scitranslmed.aau5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. P., Velasco P. T., Chang L., Viola K. L., Fernandez S., Lacor P. N., et al. (2007). Monoclonal antibodies that target pathological assemblies of Aβ. J. Neurochem. 100 23–35. 10.1111/j.1471-4159.2006.04157.x [DOI] [PubMed] [Google Scholar]
- Lemoine L., Leuzy A., Chiotis K., Rodriguez-Vieitez E., Nordberg A. (2018). Tau positron emission tomography imaging in tauopathies: the added hurdle of off-target binding. Alzheimers Dement. (Amst.) 10 232–236. 10.1016/j.dadm.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., et al. (2006). A specific amyloid-β protein assembly in the brain impairs memory. Nature 440 352–357. 10.1038/nature04533 [DOI] [PubMed] [Google Scholar]
- Leuzy A., Chiotis K., Lemoine L., Gillberg P. G., Almkvist O., Rodriguez-Vieitez E., et al. (2019). Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 24 1112–1134. 10.1038/s41380-018-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A., Pascoal T. A., Strandberg O., Insel P., Smith R., Mattsson-Carlgren N., et al. (2021). A multicenter comparison of [(18)F]flortaucipir, [(18)F]RO948, and [(18)F]MK6240 tau PET tracers to detect a common target ROI for differential diagnosis. Eur. J. Nucl. Med. Mol. Imaging 48 2295–2305. 10.1007/s00259-021-05401-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzy A., Smith R., Ossenkoppele R., Santillo A., Borroni E., Klein G., et al. (2020). Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 77 955–965. 10.1001/jamaneurol.2020.0989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levigoureux E., Lancelot S., Bouillot C., Chauveau F., Verdurand M., Verchere J., et al. (2014). Binding of the PET radiotracer [18F]BF227 does not reflect the presence of alpha-synuclein aggregates in transgenic mice. Curr. Alzheimer Res. 11 955–960. [DOI] [PubMed] [Google Scholar]
- Lewis J., McGowan E., Rockwood J., Melrose H., Nacharaju P., Van Slegtenhorst M., et al. (2000). Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25 402–405. 10.1038/78078 [DOI] [PubMed] [Google Scholar]
- Lindberg A., Knight A. C., Sohn D., Rakos L., Tong J., Radelet A., et al. (2021). Radiosynthesis, in vitro and in vivo evaluation of [(18)F]CBD-2115 as a first-in-class radiotracer for imaging 4R-tauopathies. ACS Chem. Neurosci. 12 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohith T. G., Bennacef I., Vandenberghe R., Vandenbulcke M., Salinas C. A., Declercq R., et al. (2019). Brain imaging of Alzheimer dementia patients and elderly controls with (18)F-MK-6240, a PET tracer targeting neurofibrillary tangles. J. Nucl. Med. 60 107–114. 10.2967/jnumed.118.208215 [DOI] [PubMed] [Google Scholar]
- Maltais D. D., Jordan L. G., Min H. K., Miyagawa T., Przybelski S. A., Lesnick T. G., et al. (2020). Confirmation of (123)I-FP-CIT SPECT quantification methods in dementia with lewy bodies and other neurodegenerative disorders. J. Nucl. Med. 61 1628–1635. 10.2967/jnumed.119.239418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquié M., Normandin M. D., Vanderburg C. R., Costantino I. M., Bien E. A., Rycyna L. G., et al. (2015). Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol. 78 787–800. 10.1002/ana.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M., Shimada H., Suhara T., Shinotoh H., Ji B., Maeda J., et al. (2013). Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79 1094–1108. 10.1016/j.neuron.2013.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer A., Leonov A., Ryazanov S., Herfert K., Kuebler L., Buss S., et al. (2020). (11) C radiolabeling of anle253b: a putative PET tracer for Parkinson’s disease that binds to α-synuclein fibrils in vitro and crosses the blood-brain barrier. ChemMedChem 15 411–415. 10.1002/cmdc.201900689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S. R., Sehlin D., Roshanbin S., Lim Falk V., Saito T., Saido T. C., et al. (2021). (11)C-PIB and (124)I-antibody PET provide differing estimates of brain amyloid-beta after therapeutic intervention. J. Nucl. Med. [Epub ahead of print]. 10.2967/jnumed.121.262083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Azpiazu P., Svedberg M., Higuchi M., Ono M., Jia Z., Sunnemark D., et al. (2020). Identification and in vitro characterization of C05-01, a PBB3 derivative with improved affinity for alpha-synuclein. Brain Res. 1749:147131. 10.1016/j.brainres.2020.147131 [DOI] [PubMed] [Google Scholar]
- Mueller A., Bullich S., Barret O., Madonia J., Berndt M., Papin C., et al. (2019). Tau PET imaging with (18)F-PI-2620 in patients with Alzheimer’s disease and healthy controls: a first-in-human study. J. Nucl. Med. 61 911–919. 10.2967/jnumed.119.236224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan N. A., Chiotis K., Rodriguez-Vieitez E., Lemoine L., Agren H., Nordberg A. (2019). Cross-interaction of tau PET tracers with monoamine oxidase B: evidence from in silico modelling and in vivo imaging. Eur. J. Nucl. Med. Mol. Imaging 46 1369–1382. 10.1007/s00259-019-04305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan N. A., Halldin C., Nordberg A., Långström B., Ågren H. (2016). The culprit is in the cave: the core sites explain the binding profiles of amyloid-specific tracers. J. Phys. Chem. Lett. 7 3313–3321. 10.1021/acs.jpclett.6b01586 [DOI] [PubMed] [Google Scholar]
- Murugan N. A., Nordberg A., Ågren H. (2018). Different positron emission tomography tau tracers bind to multiple binding sites on the tau fibril: insight from computational modeling. ACS Chem. Neurosci. 9 1757–1767. 10.1021/acschemneuro.8b00093 [DOI] [PubMed] [Google Scholar]
- Murugan N. A., Nordberg A., Ågren H. (2021). Cryptic sites in tau fibrils explain the preferential binding of the AV-1451 PET tracer toward Alzheimer’s tauopathy. ACS Chem. Neurosci. 12 2437–2447. 10.1021/acschemneuro.0c00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen N., Van Laere K., Thurfjell L., Owenius R., Vandenbulcke M., Koole M., et al. (2009). Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J. Nucl. Med. 50 1251–1259. 10.2967/jnumed.109.063305 [DOI] [PubMed] [Google Scholar]
- Ni R. (2021). Positron emission tomography in animal models of Alzheimer’s disease amyloidosis: translational implications. Pharmaceuticals 14:1179. 10.3390/ph14111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni R., Gillberg P. G., Bogdanovic N., Viitanen M., Myllykangas L., Nennesmo I., et al. (2017). Amyloid tracers binding sites in autosomal dominant and sporadic Alzheimer’s disease. Alzheimers Dement. 13 419–430. 10.1016/j.jalz.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Ni R., Ji B., Ono M., Sahara N., Zhang M. R., Aoki I., et al. (2018). Comparative in-vitro and in-vivo quantifications of pathological tau deposits and their association with neurodegeneration in tauopathy mouse models. J. Nucl. Med. 59 960–966. 10.2967/jnumed.117.201632 [DOI] [PubMed] [Google Scholar]
- Ni R., Röjdner J., Voytenko L., Dyrks T., Thiele A., Marutle A., et al. (2021). In vitro characterization of the regional binding distribution of amyloid PET tracer florbetaben and the glia tracers deprenyl and PK1195 in autopsy Alzheimer’s brain tissue. J. Alzheimers Dis. 80 1723–1737. 10.3233/JAD-201344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M., Eidelberg D. (2012). Metabolic brain networks in translational neurology: concepts and applications. Ann. Neurol. 72 635–647. 10.1002/ana.23631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg S., Jonhagen M. E., Cselenyi Z., Halldin C., Julin P., Olsson H., et al. (2009). Detection of amyloid in Alzheimer’s disease with positron emission tomography using [11C]AZD2184. Eur. J. Nucl. Med. Mol. Imaging 36 1859–1863. 10.1007/s00259-009-1182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Furumoto S., Fodero-Tavoletti M. T., Mulligan R. S., Harada R., Yates P., et al. (2014). Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain 137 1762–1771. 10.1093/brain/awu064 [DOI] [PubMed] [Google Scholar]
- Okamura N., Furumoto S., Harada R., Tago T., Yoshikawa T., Fodero-Tavoletti M., et al. (2013). Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J. Nucl. Med. 54 1420–1427. 10.2967/jnumed.112.117341 [DOI] [PubMed] [Google Scholar]
- Ono M., Doi Y., Watanabe H., Ihara M., Ozaki A., Saji H. (2016). Structure-activity relationships of radioiodinated diphenyl derivatives with different conjugated double bonds as ligands for α-synuclein aggregates. RSC Adv. 6 44305–44312. 10.1039/C6RA02710E [DOI] [Google Scholar]
- Ono M., Sahara N., Kumata K., Ji B., Ni R., Koga S., et al. (2017). Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain 140 764–780. 10.1093/brain/aww339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Takahashi M., Shimozawa A., Fujinaga M., Mori W., Nagai Y., et al. (2020). In vivo visualization of propagating α-synuclein pathologies in mouse and marmoset models by a bimodal imaging probe. SocArXiv [Preprint]. 10.1101/2020.10.23.349860. [DOI] [Google Scholar]
- Palmqvist S., Schöll M., Strandberg O., Mattsson N., Stomrud E., Zetterberg H., et al. (2017). Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 8:1214. 10.1038/s41467-017-01150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S., Tideman P., Cullen N., Zetterberg H., Blennow K., Dage J. L., et al. (2021). Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat. Med. 27 1034–1042. 10.1038/s41591-021-01348-z [DOI] [PubMed] [Google Scholar]
- Parent M. J., Zimmer E. R., Shin M., Kang M. S., Fonov V. S., Mathieu A., et al. (2017). Multimodal imaging in rat model recapitulates Alzheimer’s disease biomarkers abnormalities. J. Neurosci. 37 12263–12271. 10.1523/JNEUROSCI.1346-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L., Hochrainer K., Hattori Y., Ahn S. J., Anfray A., Wang G., et al. (2020). Tau induces PSD95-neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration. Nat. Neurosci. 23 1079–1089. 10.1038/s41593-020-0686-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoal T. A., Benedet A. L., Ashton N. J., Kang M. S., Therriault J., Chamoun M., et al. (2021). Microglial activation and tau propagate jointly across Braak stages. Nat. Med. 27 1592–1599. 10.1038/s41591-021-01456-w [DOI] [PubMed] [Google Scholar]
- Pascoal T. A., Therriault J., Benedet A. L., Savard M., Lussier F. Z., Chamoun M., et al. (2020). 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143 2818–2830. 10.1093/brain/awaa180 [DOI] [PubMed] [Google Scholar]
- Perez-Soriano A., Arena J. E., Dinelle K., Miao Q., McKenzie J., Neilson N., et al. (2017). PBB3 imaging in Parkinsonian disorders: evidence for binding to tau and other proteins. Mov. Disord. 32 1016–1024. 10.1002/mds.27029 [DOI] [PubMed] [Google Scholar]
- Pievani M., Filippini N., van den Heuvel M. P., Cappa S. F., Frisoni G. B. (2014). Brain connectivity in neurodegenerative diseases–from phenotype to proteinopathy. Nat. Rev. Neurol. 10 620–633. 10.1038/nrneurol.2014.178 [DOI] [PubMed] [Google Scholar]
- Pike K. E., Savage G., Villemagne V. L., Ng S., Moss S. A., Maruff P., et al. (2007). Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130 2837–2844. 10.1093/brain/awm238 [DOI] [PubMed] [Google Scholar]
- Pike V. W. (2009). PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol. Sci. 30 431–440. 10.1016/j.tips.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W., Seppi K., Tanner C. M., Halliday G. M., Brundin P., Volkmann J., et al. (2017). Parkinson disease. Nat. Rev. Dis. Primers 3:17013. [DOI] [PubMed] [Google Scholar]
- Poisnel G., Dhilly M., Moustié O., Delamare J., Abbas A., Guilloteau D., et al. (2012). PET imaging with [18F]AV-45 in an APP/PS1-21 murine model of amyloid plaque deposition. Neurobiol. Aging 33 2561–2571. 10.1016/j.neurobiolaging.2011.12.024 [DOI] [PubMed] [Google Scholar]
- Politis M. (2014). Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat. Rev. Neurol. 10 708–722. 10.1038/nrneurol.2014.205 [DOI] [PubMed] [Google Scholar]
- Rabinovici G. D., Gatsonis C., Apgar C., Chaudhary K., Gareen I., Hanna L., et al. (2019). Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321 1286–1294. 10.1001/jama.2019.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Vieitez E., Ni R., Gulyas B., Toth M., Haggkvist J., Halldin C., et al. (2015). Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur. J. Nucl. Med. Mol. Imaging 42 1119–1132. 10.1007/s00259-015-3047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts F. J. R., Declercq L., Andrés J. I., Bottelbergs A., Chen L., Iturrino L., et al. (2019). Discovery of N-(4-[18F]fluoro-5-methylpyridin-2-yl)isoquinolin-6-amine (JNJ-64326067), a new promising tau positron emission tomography imaging tracer. J. Med. Chem. 62 2974–2987. 10.1021/acs.jmedchem.8b01759 [DOI] [PubMed] [Google Scholar]
- Rominger A., Brendel M., Burgold S., Keppler K., Baumann K., Xiong G., et al. (2013). Longitudinal assessment of cerebral β-amyloid deposition in mice overexpressing Swedish mutant β-amyloid precursor protein using 18F-florbetaben PET. J. Nucl. Med. 54 1127–1134. 10.2967/jnumed.112.114660 [DOI] [PubMed] [Google Scholar]
- Rowe C. C., Ackerman U., Browne W., Mulligan R., Pike K. L., O’Keefe G., et al. (2008). Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 7 129–135. 10.1016/S1474-4422(08)70001-2 [DOI] [PubMed] [Google Scholar]
- Rowe C. C., Pejoska S., Mulligan R. S., Jones G., Chan J. G., Svensson S., et al. (2013). Head-to-head comparison of 11C-PiB and 18F-AZD4694 (NAV4694) for β-amyloid imaging in aging and dementia. J. Nucl. Med. 54 880–886. 10.2967/jnumed.112.114785 [DOI] [PubMed] [Google Scholar]
- Sabri O., Sabbagh M. N., Seibyl J., Barthel H., Akatsu H., Ouchi Y., et al. (2015). Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 11 964–974. 10.1016/j.jalz.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Saito T., Mihira N., Matsuba Y., Sasaguri H., Hashimoto S., Narasimhan S., et al. (2019). Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J. Biol. Chem. 294 12754–12765. 10.1074/jbc.RA119.009487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria Bohórquez S., Marik J., Ogasawara A., Tinianow J. N., Gill H. S., Barret O., et al. (2019). [(18)F]GTP1 (Genentech Tau Probe 1), a radioligand for detecting neurofibrillary tangle tau pathology in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 46 2077–2089. 10.1007/s00259-019-04399-0 [DOI] [PubMed] [Google Scholar]
- Sander K., Lashley T., Gami P., Gendron T., Lythgoe M. F., Rohrer J. D., et al. (2016). Characterization of tau positron emission tomography tracer [(18)F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 12 1116–1124. 10.1016/j.jalz.2016.01.003 [DOI] [PubMed] [Google Scholar]
- SantaCruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., et al. (2005). Tau suppression in a neurodegenerative mouse model improves memory function. Science 309 476–481. 10.1126/science.1113694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C. E., et al. (2021). Alzheimer’s disease. Lancet 397 1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. E., Janssens L., Moechars D., Rombouts F. J. R., Timmers M., Barret O., et al. (2020). Clinical evaluation of [(18)F] JNJ-64326067, a novel candidate PET tracer for the detection of tau pathology in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 47 3176–3185. 10.1007/s00259-020-04880-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. (2018). Automating drug discovery. Nat. Rev. Drug Discov. 17 97–113. 10.1038/nrd.2017.232 [DOI] [PubMed] [Google Scholar]
- Schöll M., Wall A., Thordardottir S., Ferreira D., Bogdanovic N., Långström B., et al. (2012). Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology 79 229–236. 10.1212/WNL.0b013e31825fdf18 [DOI] [PubMed] [Google Scholar]
- Schonhaut D. R., McMillan C. T., Spina S., Dickerson B. C., Siderowf A., Devous M. D., Sr., et al. (2017). (18) F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: a multicenter study. Ann. Neurol. 82 622–634. 10.1002/ana.25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighauser M., Shi Y., Tarutani A., Kametani F., Murzin A. G., Ghetti B., et al. (2020). Structures of α-synuclein filaments from multiple system atrophy. Nature 585 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlin D., Fang X. T., Cato L., Antoni G., Lannfelt L., Syvanen S. (2016). Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat. Commun. 7:10759. 10.1038/ncomms10759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Hardy J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J., Grothe M. J., Sabuncu M., Chhatwal J., Schultz A. P., Hanseeuw B., et al. (2017). Hierarchical organization of tau and amyloid deposits in the cerebral cortex. JAMA Neurol. 74 813–820. 10.1001/jamaneurol.2017.0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J., Chiao P., Bussière T., Weinreb P. H., Williams L., Maier M., et al. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537 50–56. [DOI] [PubMed] [Google Scholar]
- Shahnawaz M., Mukherjee A., Pritzkow S., Mendez N., Rabadia P., Liu X., et al. (2020). Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578 273–277. 10.1038/s41586-020-1984-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., et al. (2008). Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 14 837–842. 10.1038/nm1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang W., Yang Y., Murzin A. G., Falcon B., Kotecha A., et al. (2021b). Structure-based classification of tauopathies. Nature 598 359–363. 10.1038/s41586-021-03911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Murzin A. G., Falcon B., Epstein A., Machin J., Tempest P., et al. (2021a). Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol. 141 697–708. 10.1007/s00401-021-02294-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman A., Rokka J., Lopez-Picon F. R., Eskola O., Wilson I., Farrar G., et al. (2012). Pharmacokinetics of [18F]flutemetamol in wild-type rodents and its binding to beta amyloid deposits in a mouse model of Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 39 1784–1795. 10.1007/s00259-012-2178-9 [DOI] [PubMed] [Google Scholar]
- Soto C., Pritzkow S. (2018). Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21 1332–1340. 10.1038/s41593-018-0235-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M. G., Goedert M. (2013). Tau pathology and neurodegeneration. Lancet Neurol. 12 609–622. 10.1016/s1474-4422(13)70090-5 [DOI] [PubMed] [Google Scholar]
- Spires T. L., Orne J. D., SantaCruz K., Pitstick R., Carlson G. A., Ashe K. H., et al. (2006). Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am. J. Pathol. 168 1598–1607. 10.2353/ajpath.2006.050840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Flores S., Wang G., Hornbeck R. C., Speidel B., Joseph-Mathurin N., et al. (2019). Comparison of Pittsburgh compound B and florbetapir in cross-sectional and longitudinal studies. Alzheimers Dement. (Amst.) 11 180–190. 10.1016/j.dadm.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Admane P., Starosolski Z. A., Eriksen J. L., Annapragada A. V., Tanifum E. (2021). Structure-guided design, synthesis, and evaluation of 1-indanone and 1,3-indandione derivatives as ligands for misfolded α-synuclein aggregates. ChemRxiv [Preprint] 10.26434/chemrxiv.13817243.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram G. S. M., Dhavale D. D., Prior J. L., Yan P., Cirrito J., Rath N. P., et al. (2016). Fluselenamyl: a novel benzoselenazole derivative for PET detection of amyloid plaques (Aβ) in Alzheimer’s disease. Sci. Rep. 6:35636. 10.1038/srep35636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagai K., Ono M., Kubota M., Kitamura S., Takahata K., Seki C., et al. (2020). High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron 109 42–58.e8. 10.1016/j.neuron.2020.09.042 [DOI] [PubMed] [Google Scholar]
- Teng E., Kepe V., Frautschy S. A., Liu J., Satyamurthy N., Yang F., et al. (2011). [F-18]FDDNP microPET imaging correlates with brain Aβ burden in a transgenic rat model of Alzheimer disease: effects of aging, in vivo blockade, and anti-Aβ antibody treatment. Neurobiol. Dis. 43 565–575. 10.1016/j.nbd.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D. R., Beach T. G., Zanette M., Heurling K., Chakrabarty A., Ismail A., et al. (2015). [18F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: specific detection of advanced phases of amyloid-β pathology. Alzheimers Dement. 11 975–985. 10.1016/j.jalz.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Thal D. R., Rub U., Orantes M., Braak H. (2002). Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58 1791–1800. 10.1212/wnl.58.12.1791 [DOI] [PubMed] [Google Scholar]
- Uzuegbunam B. C., Librizzi D., Hooshyar Yousefi B. (2020). PET radiopharmaceuticals for Alzheimer’s disease and Parkinson’s disease diagnosis, the current and future landscape. Molecules 25:977. 10.3390/molecules25040977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzuegbunam B. C., Paslawski W., Zhou Y., Ågren H., Längström B., Weber W. A., et al. (2021). Optimized disarybisthiazole derivatives with high affinity to alpha-synuclein aggregates and improved pharmacokinetics. Nuklearmedizin 60:144. [Google Scholar]
- Vagenknecht P., Ono M., Luzgin A., Ji B., Higuchi M., Noain D., et al. (2021). Non-invasive imaging of tau-targeted probe uptake by whole brain multi-spectral optoacoustic tomography. bioRxiv [Preprint]. 10.1101/2021.07.10.451626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdurand M., Levigoureux E., Zeinyeh W., Berthier L., Mendjel-Herda M., Cadarossanesaib F., et al. (2018). In silico, in vitro, and in vivo evaluation of new candidates for α-synuclein PET imaging. Mol. Pharm. 15 3153–3166. [DOI] [PubMed] [Google Scholar]
- Villemagne V. L., Mulligan R. S., Pejoska S., Ong K., Jones G., O’Keefe G., et al. (2012). Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 39 983–989. 10.1007/s00259-012-2088-x [DOI] [PubMed] [Google Scholar]
- Vogel J. W., Iturria-Medina Y., Strandberg O. T., Smith R., Levitis E., Evans A. C., et al. (2020). Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat. Commun. 11:2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. W., Young A. L., Oxtoby N. P., Smith R., Ossenkoppele R., Strandberg O. T., et al. (2021). Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 27 871–881. 10.1038/s41591-021-01309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Ryazanov S., Leonov A., Levin J., Shi S., Schmidt F., et al. (2013). Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 125 795–813. 10.1007/s00401-013-1114-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z., Gandolfo F., Orini S., Garibotto V., Agosta F., Arbizu J., et al. (2018). Clinical utility of FDG PET in Parkinson’s disease and atypical parkinsonism associated with dementia. Eur. J. Nucl. Med. Mol. Imaging 45 1534–1545. 10.1007/s00259-018-4031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Kishimoto T., Kaide S., Tarumizu Y., Tatsumi H., Iikuni S., et al. (2021). Characterization and optimization of benzimidazopyrimidine and pyridoimidazopyridine derivatives as Tau-SPECT probes. ACS Med. Chem. Lett. 12 805–811. 10.1021/acsmedchemlett.1c00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzynowicz M., Bar-On D., Calo’ L., Anichtchik O., Iovino M., Xia J., et al. (2019). Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol. 138 575–595. 10.1007/s00401-019-02023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk D. A., Sadowsky C., Safirstein B., Rinne J. O., Duara R., Perry R., et al. (2018). Use of flutemetamol F 18-labeled positron emission tomography and other biomarkers to assess risk of clinical progression in patients with amnestic mild cognitive impairment. JAMA Neurol. 75 1114–1123. 10.1001/jamaneurol.2018.0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters E. E., Dodich A., Boccardi M., Corre J., Drzezga A., Hansson O., et al. (2021). Clinical validity of increased cortical uptake of [18F]flortaucipir on PET as a biomarker for Alzheimer’s disease in the context of a structured 5-phase biomarker development framework. Eur. J. Nucl. Med. Mol. Imaging 48 2097–2109. 10.1007/s00259-020-05118-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren M. C., Lashley T., Årstad E., Sander K. (2018). Large inter- and intra-case variability of first generation tau PET ligand binding in neurodegenerative dementias. Acta Neuropathol. Commun. 6:34. 10.1186/s40478-018-0535-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Choi S. R., Zhao R., Ploessl K., Alexoff D., Zhu L., et al. (2021). A new highly deuterated [18F]AV-45, [18F]D15FSP, for imaging β-Amyloid plaques in the brain. ACS Med. Chem. Lett. 12 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T. R., Holmes B. B., Furman J. L., Dhavale D. D., Su B. W., Song E. S., et al. (2019). Parkinson’s disease and multiple system atrophy have distinct α-synuclein seed characteristics. J. Biol. Chem. 294 1045–1058. 10.1074/jbc.RA118.004471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap S. Y., Frias B., Wren M. C., Schöll M., Fox N. C., Årstad E., et al. (2021). Discriminatory ability of next-generation tau PET tracers for Alzheimer’s disease. Brain 144:2284–2290. 10.1093/brain/awab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi B. H., Mohring M., Arzberger T., Hoglinger G., Wester H., Schwaiger M. (2016). Novel compounds for specific visualization of alpha-synucleinopathies by PET. Eur. J. Nucl. Med. Mol. Imaging 43 S22–S23. [Google Scholar]
- Yousefi B. H., von Reutern B., Scherübl D., Manook A., Schwaiger M., Grimmer T., et al. (2015). FIBT versus florbetaben and PiB: a preclinical comparison study with amyloid-PET in transgenic mice. EJNMMI Res. 5:20. 10.1186/s13550-015-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Cui J., Padakanti P. K., Engel L., Bagchi D. P., Kotzbauer P. T., et al. (2012). Synthesis and in vitro evaluation of α-synuclein ligands. Bioorg. Med. Chem. 20 4625–4634. 10.1016/j.bmc.2012.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Tarutani A., Newell K. L., Murzin A. G., Matsubara T., Falcon B., et al. (2020). Novel tau filament fold in corticobasal degeneration. Nature 580 283–287. 10.1038/s41586-020-2043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Ji B., Kong Y., Qin L., Ren W., Guan Y., et al. (2021). PET imaging of neuroinflammation in Alzheimer’s disease. Front. Immunol. 12:739130. 10.3389/fimmu.2021.739130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li J., Nordberg A., Ågren H. (2021). Dissecting the binding profile of PET tracers to corticobasal degeneration tau fibrils. ACS Chem. Neurosci. 12 3487–3496. 10.1021/acschemneuro.1c00536 [DOI] [PMC free article] [PubMed] [Google Scholar]