Abstract
The virucidal activities of 11 prepared disinfectant solutions (active ingredients of household sanitizers) and 10 household sanitizers against bacteriophage MS2 on plastic and stainless steel surfaces were studied. Among the prepared sanitizers, 70–90% ethanol and ethanol-based disinfectants resulted in 1–2.5 log PFU/mL reductions on both surfaces. The 70% isopropanol and isopropanol-based formula reduced MS2 by 0.7–1.5 log PFU/mL on both surfaces. Other disinfectants, containing 0.1% benzalkonium chloride (BAC), 0.5% hydrogen peroxide, or 4% acetic acid, showed significant (P < 0.05) lower log reductions (−0.17-0.55 log PFU/mL) compared with other treatments. At room temperature, the virucidal activities of 70% ethanol on plastic (1.46–1.64 log PFU/mL reductions) and stainless steel (0.84–0.93 log PFU/mL reductions) surfaces were not significantly (P > 0.05) affected by the treatment time (30–600 s). However, 85% ethanol-treated groups showed significant (P < 0.05) higher log reductions in 60 and 600 s treated groups (1.69–2.24 log PFU/mL) compared with those in 30 s treated groups (0.92–1.32 log PFU/mL). Their virucidal activities were further examined at low temperatures (4 and 8 °C). We observed that the surface inactivation efficacies were not affected by the low temperatures. In addition, the virucidal activities of household sanitizers revealed that sanitizers with 1.84% (pH = 12.5, ∼17,500 ppm free-chlorine concentrations) or 3% (pH = 13.1, ∼38,100 ppm free-chlorine concentrations) sodium hypochlorite (NaClO) reduced 4.15–6.23 log PFU/mL MS2 on hard surfaces after 60 s contact time. Furthermore, an approximately 1.5 log PFU/mL reduction was observed in groups treated by sanitizer H (active ingredients: 58% ethanol + 0.1% quaternary ammonium compound). Household products with BAC or organic acid resulted in −0.28-0.33 log reductions on two surfaces after 30 or 60 s treatment. Therefore, the use of ethanol and NaClO-based products should be considered as a potential surface decontamination strategy in the food industry.
Keywords: MS2, Plastic surface, Stainless steel surface, Low temperature, Sanitizers, Surrogate
Graphical abstract
Highlight
-
•
Inactivation efficacies of 21 sanitizers against MS2 on hard surfaces were tested.
-
•
Ethanol-based sanitizers resulted in 1–2.5 log PFU/mL reductions on both surfaces.
-
•
Low temperatures (4 and 8 °C) did not affect the disinfectant efficacy.
-
•
Sodium hypochlorite (1.84–3%) reduced 4.15–6.23 log PFU/mL MS2 on hard surfaces.
1. Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a serious threat to public health (Liu et al., 2020). As of December 28th, 2021, more than 279 million cases, including 5.40 million deaths, were reported to the World Health Organization (WHO, 2021). SARS-CoV-2 is generally transmitted via direct contact between individuals, and airborne droplets and aerosols produced when coughing or sneezing (Prather et al., 2020). In addition, recent studies have suggested that indirect contact via contaminated surfaces is a secondary transmission route, as SARS-CoV-2 is able to survive on hard surfaces (plastic, stainless steel, copper, etc.) for up to 72 h (Chin et al., 2020; Van Doremalen et al., 2020; Marquès and Domingo 2020). However, the Centers for Disease Control and Prevention (CDC) suggested that the risk of surface transmission route is low. Surface disinfection is recommended in indoor environment where there has been a suspected/confirmed COVID-19 case (CDC, 2021). The surface stability of SARS-CoV-2 also greatly impacts many value chains in the food supply system and threatens food security and safety on a global scale. The U.S. Food and Drug Administration (FDA) suggested that employers in food industry should clean and disinfect a sick worker's workspace after waiting 24 h (U.S. FDA, 2020).
In addition to SARS-CoV-2, many other viruses also exhibit surface stability. For instance, norovirus (NoV), the leading cause of human acute viral gastroenteritis, is able to survive and remain infectious on fomite surfaces for 2 weeks or more (Bolton et al., 2013). Concurrently, hepatitis A virus (HAV) causes the highest number of foodborne invasive viral infections in humans. Its environmental persistence enables contamination of kitchen surfaces (Rajiuddin et al., 2020). Indeed, the most frequent reported foodborne outbreaks resulted from NoV and HAV infections have originated from restaurants or canteens, where the kitchen surface environments and foods were contaminated by infectious food handlers (Boxman et al., 2011; Fankhauser et al., 2002; Franck et al., 2015). To reduce the risk of potential surface contamination and transmission of related viruses under current COVID-19 pandemic, a number of measures, including regular surface disinfection, have been adopted by the food industry (Aday and Aday, 2020; Rajiuddin et al., 2020).
Previous studies have examined the efficacies of various sanitizers on hard surfaces artificially contaminated by viruses (e.g., SARS-CoV-2 and HAV) or their surrogates (e.g., beta human coronaviruses 229 E and OC43, bacteriophages MS2 and ΦX174) (Hoelzer et al., 2013; Meyers et al., 2021). Ethanol (62–80%) and isopropanol (70–80%) solutions were shown to be effective against coronavirus 229 E on porcelain and ceramic tiles, resulting in >4 log reduction (Meyers et al., 2021). Furthermore, household sanitizer with 3% sodium hypochlorite (NaClO) resulted in >3 log reductions of murine norovirus on stainless steel (Girard et al., 2010). The inactive efficacies of sanitizers are affected by various factors, such as disinfectant type, pH, pKa, presence of metal cations, and physical properties of the viruses (Hoelzer et al., 2013). Generally, the active ingredients of household disinfectants contain alcohol, benzalkonium chloride (BAC), hydrogen peroxide (H2O2), acid, or NaClO (Hoelzer et al., 2013; Lin et al., 2020). However, little information about these sanitizers on food contact surfaces is available.
The aim of this research was to investigate the efficacies of 21 sanitizers, including 10 household sanitizers, in the inactivation of bacteriophage MS2 on plastic and stainless steel surfaces. MS2 is an Escherichia coli bacteriophage, and it is commonly used as a surrogate of human enteric viruses, such as NoV and HAV (Bozkurt et al., 2015). Moreover, MS2 can be used as a highly conservative surrogate for SARS-CoV-2. Similar with SARS-CoV-2, the MS2 is a positive-sense, single-stranded RNA virus. Unlike SARS-CoV-2, the MS2 phage lacks a lipid envelope, making it more resistant to disinfection (Zulauf et al., 2020). In this work, the inactivation potential of 11 sanitizers on MS2 at different exposure times was determined, followed by examining the effects of low temperatures (4 and 8 °C) on the disinfection efficacy of selected sanitizers. Although the risk of SARS-Cov-2 infection from cold chain food is very low, contamination of certain viruses (e.g., NoV and HAV) under low temperature is possible (Bozkurt et al., 2021; Lu et al., 2021; Mormann et al., 2015). Lastly, the disinfection performances of 10 household sanitizers were additionally examined.
2. Material and methods
2.1. Bacteriophage, bacterial host, and disinfectants
Freeze-dried MS2 bacteriophage (phage; ATCC 15597-B1) was purchased from Cedarlane Labs (Burlington, ON, Canada), and Escherichia coli (E. coli) ATCC 15597 was used as the host organism for phage propagation. Table 1 shows the information on selected disinfectants. Their active ingredients were the major compounds of disinfectants recommended by Health Canada (2021b). Moreover, 10 recommended household sanitizers with different active ingredients (BAC, H2O2, alcohol, acid, NaClO, and quaternary ammonium compound) were selected for the surface inactivation test.
Table 1.
Selected disinfectants used in this study.
| Prepared disinfectants | Active ingredient(s) | Household sanitizers | Active ingredient(s) |
|---|---|---|---|
| i | 70% isopropanol | Sanitizer A | 0.1% alkyl dimethyl benzyl ammonium chloride |
| ii | 0.1% benzalkonium chloride | Sanitizer B | 70% isopropanol |
| iii | 0.1% sodium hypochlorite | Sanitizer C | 1.84% sodium hypochlorite |
| iv (modified WHO II formula) | 75% (w/w) isopropanol, 0.725 glycerol, 0.125% hydrogen peroxide | Sanitizer D | 0.26%, w/w, alkyl dimethyl benzyl ammonium chloride |
| v | 0.5% hydrogen peroxide | Sanitizer E | 3% sodium hypochlorite |
| vi | 4% acetic acid | Sanitizer F | 0.2% alkyl dimethyl benzyl ammonium chloride, 0.15% octyl decyl dimethyl ammonium chloride, 0.075% didecyl dimethyl ammonium chloride, 0.075% dioctyl dimethyl ammonium chloride |
| vii | 70% ethanol | Sanitizer G | 0.19%, w/w, L-lactic acid |
| viii (modified WHO I formula) | 80% (w/w) ethanol, 0.725% glycerol, 0.125% hydrogen peroxide | Sanitizer H | 58%, w/w, ethanol, 0.1%, w/w, n-alkyl dimethyl benzyl ammonium saccharinate |
| ix | 85% ethanol | Sanitizer I | 0.15% alkyl dimethyl benzyl ammonium chloride, 0.15% alkyl dimethyl ethylbenzyl ammonium chloride |
| x | 85% ethanol, 5% isopropanol | Sanitizer J | 1.75%, w/w, glycolic acid |
| xi | 90% ethanol |
2.2. Propagation and quantification of MS2 phage
Lyophilized MS2 phage was rehydrated in 1.5 ml Tryptic Soy Broth (TSB; Difco, Becton Dickinson, NJ, USA) before use. Phage propagation and quantification were performed according to the methods described by Fong et al. (2019) and Wong et al. (2020). Briefly, fresh E. coli culture was prepared by colony inoculation into 10 mL TSB, followed by incubation at 37 °C for 16 h at 170 rpm. Reconstituted MS2 was ten-fold serially diluted in salt-magnesium (SM) buffer (0.05 M Tris-HCl, 0.1 M NaCl, and 0.01 M MgSO4; pH 7.5). Then, 50 μL dilutions were spotted on bacterial overlays composed of 300 μL 1:10 (v/v) diluted E. coli culture and 4 mL 0.7% (w/v) soft tryptic soy agar (TSA, Becton Dickinson). The overlays were scraped into 5 mL SM buffer after incubating at 37 °C for 4 h. The mixtures were centrifuged at 4500×g for 20 min, and the supernatants were filtered through 0.45 μm filters. Obtained phage suspensions were stored at 4 °C before use. Phage enumeration was conducted according to the method described by Fong et al. (2017) with modifications. The filtrates were spotted onto the prepared bacterial overlays as previously described, and the plates were then incubated for 18 ± 2 h at 37 °C before plaques were counted. Phage concentration was expressed as plaque forming units (PFU)/mL. In this study, phage filtrate with high titer (around 4.0 × 1011 PFU/mL) was stored at 4 °C as stock solution.
2.3. Cytotoxicity control, interference test, and neutralizer validation
The cytotoxicity of neutralized sanitizers, neutralizer, and SM buffer (control) on host bacterial cells was examined according to the methods described in the American Society for Testing and Materials (ASTM) standard number E2197-17e1 (ASTM International, 2017). The ASTM method was used as the reference since it was recommended by Health Canada (2021a) for disinfectant testing. The 21 disinfectants were 1:20 (v/v) and 1:200 (v/v) quenched by neutralizer Difco™ D/E (Dey/Engley) neutralizing Broth (BD Biosciences, Mississauga, ON, Canada). The neutralizer was selected according to the method described in ASTM E1054-08 (ASTM International, 2008). Twenty drops (each drop comprised 5 μL solution) of each prepared solution were spotted onto the bacterial overlays and air-dried at room temperature followed by incubation at 37 °C for 16 h. The experiments were performed in triplicate.
The ability of the prepared solutions to interfere with virus infectivity was tested. The bacterial overlays with air-dried neutralized solution spots were prepared as previously mentioned. MS2 phage drops (5 μL each drop) containing 500–1000 log PFU/mL were added onto the air-dried spots, and the plates were further incubated for 16 h at 37 °C. Plaques were counted to determine if the neutralized disinfectants, neutralizer, and SM buffer interfered with plaque formation. SM buffer was used as a control. The experiments were performed in triplicate.
Neutralization validation was conducted by diluting MS2 phage in neutralized sanitizer solutions (1:20 and 1:200 dilutions), neutralizer, and SM buffer (Morin et al., 2015). Twenty drops (5 μL each drop) of each phage solution were spotted onto the bacterial overlays, and the plates were incubated at 37 °C (16 h). Plaques were counted to validate neutralization efficacy of the disinfectants.
2.4. Carrier test on plastic and stainless steel
Plate recovery control was conducted to determine the phage concentration applied on the surface disinfection study. Working stocks of MS2 (previously prepared in 2.2) were ten-fold serially diluted in SM buffer. The phage dilutions (10 μL) were individually spotted onto the test surfaces and air dried in a biosafety cabinet for 30 min. The plastic surface (untreated 24-well polypropylene microplate) and sterilized stainless steel discs (1 cm diameter discs of AISI type 304 stainless steel) were purchased from Pegen Industries Inc. (Ottawa, ON, Canada). SM buffer (50 μL) was subsequently added onto the air-dried phage spots. Then, 1 mL neutralizer was added, and the phage enumeration was further performed (Fong et al., 2017).
The carrier test was conducted to determine the surface inactivation efficacies of selected disinfectants. The methods described in ASTM E2197-17e1 and ASTM E1053-20 (ASTM International, 2017; ASTM International, 2020) were applied with modifications. The phage inoculum was prepared by mixing 340 μL of phage solution with 160 of μL soil load, comprised of 25 μL bovine serum albumin solution (50 mg/mL in PBS, pH 7.2), 35 μL 50 mg/mL yeast extract stock, and 100 μL 4 mg/mL mucin. The mixture was vortexed, and 10 μL suspension drops were spotted on a sterilized plastic plate (untreated 24-well polypropylene microplate) and sterilized stainless steel discs (1 cm diameter discs of AISI type 304 stainless steel, Pegen Industries Inc., Ottawa, ON, Canada). Polypropylene was chosen as the plastic material because it is one of the most common polymers used in the food packaging industry due to its good functionality and relatively low cost (Paiva et al., 2021). The drops were air-dried at room temperature in a biosafety cabinet for 30 min.
The virucidal trials were performed by adding 50 μL of disinfectant onto the phage spots, and allowing appropriate contact time. SM buffer (50 μL) was used as a negative control. At the end of contact, 1 mL of the neutralizer was immediately applied to the droplets. For sanitizer E, 2 mL of the neutralizer was applied due to insufficient neutralization of 1 mL D/E neutralizing broth. The treated viruses were eluted from stainless steel discs by vortexing for 30 s in Nalgene vials, while the samples in plastic wells were pipetted up and down to obtain the eluted solutions. Phage enumeration was performed (as previously described in section 2.2) to determine viral survival.
The effect of temperature on the virucidal activities of selected disinfectants was tested. The MS2 phage inoculum was prepared as mentioned above in section 2.4. The virus drops were spotted on stainless steel and plastic surfaces, and air-dried under room temperature. The carriers and selected disinfectants were incubated at 4, 8, and 21.8 °C before inactivation treatment. The eluted solutions were collected for phage quantification.
2.5. Statistical analysis
Data were analyzed statistically using analysis of variance (ANOVA), and means were compared using the least significant difference (LSD) in SPSS (Version 22.0, IBM, Armonk, NY, USA). Differences with a P value < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Surrogate phage MS2, cytotoxicity and interference control, and validation
The surface stability of certain viruses (e.g., SARS-CoV-2, NoV, and HAV) has led to concerns of potential surface transmission throughout the food supply chain (Bolton et al., 2013; González et al., 2021; Rajiuddin et al., 2020). In this study, the inactivation efficacies of selected disinfectants against surrogate MS2 on food contact surfaces were tested, to reinforce both personal and food hygiene principles during the COVID-19 pandemic.
None of the neutralized sanitizers nor neutralizers themselves exhibited significant (P < 0.05) cytotoxicity to the E. coli host cell, compared with the control group. In addition, the interference test revealed that 1:20 diluted disinfectants, such as 70% isopropanol, 0.1% BAC, 0.1% NaClO, 70% ethanol, 85% ethanol, 85% ethanol +5% isopropanol, and 90% ethanol, did not notably (P > 0.05) affect the plaque counts, compared with that in the control group. Moreover, neutralization validation showed that a 1:20 dilution of the sanitizers (except household sanitizer E, which was 1:40 diluted) with D/E neutralizing broth effectively quenched the virucidal activity. Therefore, the 21 selected disinfectants and neutralizer were used for subsequent carrier tests.
3.2. Surface inactivation efficacies of prepared disinfectants on a plastic surface
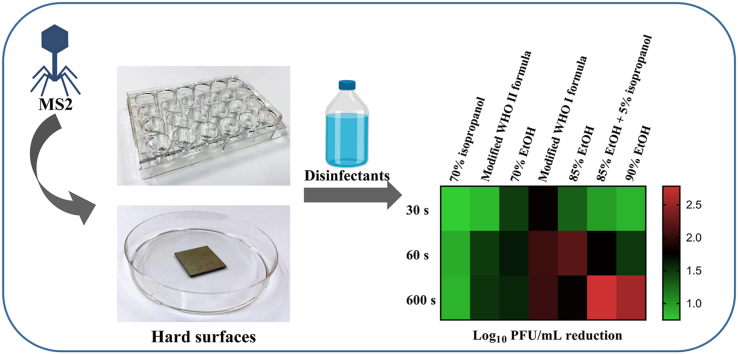
The inactivation efficacies of the selected disinfectants against MS2 on plastic surfaces are shown in Table 2. Application of seven disinfectants (70% isopropanol, modified WHO II formula, 70% ethanol, modified WHO I formula, 85% ethanol, 85% ethanol + 5% isopropanol, and 90% ethanol) significantly reduced (P < 0.05) the viral load after 30 s of treatment (Table 2). Virucidal effects were increased by 12.33–80.61% upon treatment for 60 s. Generally, a treatment time of 600 s treatments did not enhance the virucidal activity, although, 85% ethanol +5% isopropanol, and 90% ethanol, further lowered the viral load by 2.78 and 2.53 log PFU/mL, respectively. Moreover, 0.1% NaClO (1000 ppm free-chlorine concentration), which is recommended by the WHO (2020), displayed 0.40 and 0.54 log PFU/mL viral reductions after contact for 60 and 600 s, respectively. Interestingly, application of 70% isopropanol exhibited a significant (P < 0.05) lower virucidal activity than 70% ethanol (Table 2). Similar results were also observed by Moorer (2003): ethanol is a better antiviral agent compared with isopropanol.
Table 2.
Inactivation efficacy of prepared disinfectants against MS2 on a plastic surface.
| Disinfectant | Active ingredient(s) | Log reduction (30 s)a | Log reduction (60 s)a | Log reduction (600 s)a |
|---|---|---|---|---|
| i | 70% isopropanol | 0.88 ± 0.08aBC | 0.85 ± 0.30aD | 0.98 ± 0.02aD |
| ii | 0.1% BACb | −0.02 ± 0.05bD | 0.00 ± 0.13bEF | 0.24 ± 0.10aEF |
| iii | 0.1% sodium hypochlorite | 0.10 ± 0.09bD | 0.40 ± 0.12aE | 0.54 ± 0.11aE |
| iv | Modified WHO II formulac | 0.79 ± 0.27bC | 1.58 ± 0.02aC | 1.67 ± 0.03aBC |
| v | 0.5% hydrogen peroxide | 0.15 ± 0.10aD | 0.12 ± 0.13aEF | 0.04 ± 0.16aF |
| vi | 4% acetic acid | 0.20 ± 0.16aD | −0.17 ± 0.68aF | 0.08 ± 0.43aF |
| vii | 70% ethanol | 1.46 ± 0.42aA | 1.64 ± 0.04aC | 1.58 ± 0.05aC |
| viii | Modified WHO I formulad | 1.79 ± 0.06bA | 2.74 ± 0.00aA | 2.04 ± 0.55bB |
| ix | 85% ethanol | 1.32 ± 0.38bAB | 2.24 ± 0.33aB | 1.74 ± 0.32abBC |
| x | 85% ethanol+5% isopropanol | 0.98 ± 0.09cBC | 1.78 ± 0.18bC | 2.78 ± 0.05aA |
| xi | 90% ethanol | 0.93 ± 0.58bBC | 1.46 ± 0.16bC | 2.53 ± 0.22aA |
In each column, values with the same upper letter do not differ significantly at P < 0.05; in each row, values with the same lower letter do not differ significantly at P < 0.05.
BAC: benzalkonium chloride.
Modified WHO II formula: 75% (w/w) isopropanol, 0.725 glycerol, and 0.125% hydrogen peroxide (H2O2).
Modified WHO I formula: 80% (w/w) ethanol, 0.725% glycerol, and 0.125% H2O2.
On the contrary, disinfectants containing BAC, H2O2, or acetic acid exhibit limited inactivation efficacies (<0.24 log PFU/mL) against MS2 on plastic surfaces (Table 2). In a previous study, BAC at 0.1–0.5 mg/mL resulted in 1.74–2.00 log PFU/mL MS2 reduction after 2 h treatment in suspension (Su & D'Souza, 2012). These different performances may be a result of different reaction environments (liquid suspension vs. plastic surface) and contact time (2 h vs. 30–600 s). Generally, longer contact time or higher concentration of disinfectants are required for effective virucidal activity on hard surfaces, compared with those in liquid suspension (Mileto et al., 2021).
Acetic acid had a limited decontamination effect against MS2. Inactivation rates of other organic acids have been tested on MS2. Very limited reductions (0.00–0.06 log PFU/mL) of MS2 and murine norovirus were observed after treatment with tannic and gallic acid (room temperature, 2 h exposure) (Su & D'Souza, 2012). However, gallic acid demonstrated antiviral activities against herpes simplex virus type B. Its 50% inhibitory concentration (IC50) was 33.56 μM, which was much lower than the 50% cytotoxic concentration (CC50), 1000 μM (Kratz et al., 2008). The antiviral effects of organic acids are affected by various factors, including type and nature of virus and organic acid, treatment time and method. Based on our study, it should be noted that many of the disinfectants that incurred a > one log PFU/ml reduction are isopropanol or ethanol-based (Table 2). Alcohol-based products have historically been shown to be effective against various viruses. Maillard, Beggs, Day, Hudson, and Russell (1994) reported that 70% ethanol reduced MS2 by 3.68 log PFU/mL in suspension, while 100% ethanol demonstrated a reduction of 1.53 log PFU/mL: the inclusion of water in the alcohol biocidal system increases the disinfectant efficacy, as water facilitates a faster denaturation of proteins (Lin et al., 2020). Furthermore, 100% isopropanol inactivated MS2 by 1.50 log PFU/mL after contact for 20 min. Overall, alcohol-based products appear to demonstrate consistent inactivation rates in the decontamination of viruses in a variety of systems, including plastic surfaces.
3.3. Surface inactivation efficacies of prepared disinfectants on a stainless steel surface
The sanitizing efficacies of 11 prepared disinfectants against MS2 on a stainless steel surface were tested (Table 3). Most of the sanitizers resulted in a <1 log PFU/mL reduction after contact for 30 s. Four sanitizers (modified WHO I formula, 85% ethanol, 85% ethanol + 5% isopropanol, and 90% ethanol), inactivated >1 log PFU/mL MS2 on stainless steel surface following 60 s treatment. After contact for 600 s, the sanitizing effects of 85% ethanol +5% isopropanol was further significantly (P < 0.05) increased to 2.35 log PFU/mL. Alcohols, specially isopropanol and ethanol, are capable of inactivating a wide spectrum of microorganisms through disruption of the cell membrane and denaturation of intracellular proteins. Viruses are particularly susceptible to this mode of action (Boyce, 2018).
Table 3.
Inactivation efficacy of prepared disinfectants against MS2 on a stainless steel surface.
| Disinfectant | Active ingredient(s) | Log reduction (30 s)a | Log reduction (60 s)a | Log reduction (600 s)a |
|---|---|---|---|---|
| i | 70% isopropanol | 0.22 ± 0.05aEF | 0.16 ± 0.07aFG | 0.12 ± 0.03aG |
| ii | 0.1% BACb | −0.01 ± 0.02cF | 0.06 ± 0.03bFG | 0.16 ± 0.01aG |
| iii | 0.1% sodium hypochlorite | 0.37 ± 0.12bDE | 0.42 ± 0.10bEF | 0.71 ± 0.09aEF |
| iv | Modified WHO II formulac | 0.70 ± 0.11aBC | 0.63 ± 0.05aDE | 0.75 ± 0.06aEF |
| v | 0.5% hydrogen peroxide | 0.19 ± 0.07aEF | 0.15 ± 0.09aFG | 0.22 ± 0.07aG |
| vi | 4% acetic acid | 0.04 ± 0.13bEF | −0.06 ± 0.08bG | 0.55 ± 0.11aF |
| vii | 70% ethanol | 0.84 ± 0.12aABC | 0.93 ± 0.14aCD | 0.86 ± 0.04aD |
| viii | Modified WHO I formulad | 1.08 ± 0.16aA | 1.40 ± 0.57aAB | 1.47 ± 0.31aC |
| ix | 85% ethanol | 0.92 ± 0.14bAB | 1.69 ± 0.18aA | 1.89 ± 0.00aB |
| x | 85% ethanol+5% isopropanol | 0.59 ± 0.43cCD | 1.75 ± 0.01bA | 2.35 ± 0.20aA |
| xi | 90% ethanol | 0.10 ± 0.21bEF | 1.21 ± 0.29aBC | 1.58 ± 0.19aC |
In each column, values with the same upper letter do not differ significantly at P < 0.05; in each row, values with the same lower letter do not differ significantly at P < 0.05.
BAC: benzalkonium chloride.
Modified WHO II formula: 75% (w/w) isopropanol, 0.725 glycerol, and 0.125% hydrogen peroxide (H2O2).
Modified WHO I formula: 80% (w/w) ethanol, 0.725% glycerol, and 0.125% H2O2.
Similar to what we observed on plastic surfaces, BAC, H2O2, and acetic acid, exhibited a relatively low range of activity (<0.55 log PFU/mL reduction) on the stainless steel surface (Table 3). Interestingly, the tested disinfectants on stainless steel exhibited lower effectiveness compared with those on plastic surfaces. Similar results were observed in a previous study: electrochemical oxidants could reduce MS2 more effectively on plastic compared with that on stainless steel surface (Julian et al., 2014). Longer treatment (steam-ultrasound) time was also required to reduce murine norovirus on stainless steel to below the theoretical limit of detection, compared with that on plastic (Rajiuddin et al., 2020). It is apparent that surface materials (plastic and stainless steel) may interfere with the sanitizing effects of disinfectants (Møretrø et al., 2012), but, the mechanistic basis for this interference is unknown and warrants further investigation.
In summary, ethanol and ethanol-based disinfectants showed the most effective virucidal activity against MS2 on both plastic and stainless steel surfaces. These results agree with previous observations where ethanol with concentrations ranging from 60% to 95% showed effective inactivation abilities (>3.2 log reductions) against various viruses, such as SARS-CoV, mouse hepatitis virus, etc. (Singh et al., 2020).
3.4. Effect of low temperature on the surface inactivation efficacy of ethanol
Factors, such as sanitizer concentration, contact time, interfering matter, and pH, affect the inactivation effectiveness to various degrees. Temperature is a key factor affecting effectiveness of virucides in practical use (Pinto et al., 2010; Vong et al., 2018). Unlike bacterial pathogens, low temperature is not a mitigation strategy for viral pathogens, as persistence of enteric viruses (e.g., norovirus) is higher at low temperatures (Bozkurt et al., 2015). It raises questions about disinfectant efficacy in low temperature environments. In the carrier tests on plastic and stainless steel surfaces, ethanol and ethanol-based disinfectants showed good (>0.84 log PFU/mL reduction) virucidal activity against MS2 (Table 2, Table 3), therefore, the effect of low temperature (i.e., 4 and 8 °C) on the surface inactivation efficacy of ethanol was assessed.
Overall, low temperatures did not affect the efficacies of 70% ethanol against MS2 on plastic nor stainless steel (Table 4), compared with those under room temperature (21.8 °C). Approximately one log PFU/mL reduction was observed on a plastic surface, while lower reductions (approximately 0.17–0.64 log PFU/mL) were observed on stainless steel. The data were consistent with the results in Table 2, Table 3 It has been shown that MS2 aggregates at low temperature (<20 °C), leading to similar log PFU/mL reductions at 10 and 20 °C upon treatment (Pinto et al., 2010).
Table 4.
Effect of temperature on the inactivation efficacy of 70% ethanol (60 s) against MS2 on plastic and stainless steel surfaces.
| Surface | Temperature | Log reductiona |
|---|---|---|
| Plastic | 4 °C | 0.89 ± 0.31A |
| 8 °C | 0.77 ± 0.32A | |
| 21.8 °C | 1.03 ± 0.16A | |
| Stainless steel | 4 °C | 0.64 ± 0.10B |
| 8 °C | 0.77 ± 0.44A | |
| 21.8 °C | 0.17 ± 0.13B |
In each column, values with the same upper letter do not differ significantly at P < 0.05.
Table 5 describes the effect of 85% ethanol against MS2 on hard surfaces for 1 min at 4, 8, and 21.8 °C. Higher reductions (1.36–2.10 log PFU/mL and 1.04–1.38 log PFU/mL on plastic and stainless steel surfaces, respectively) were observed with 85% ethanol treatments, compared with those in the 70% ethanol treated groups (Table 4, Table 5), in accordance with the data obtained in Table 2, Table 3 In line with our results, it was reported the increased concentration of ethanol from 80% to 95% led to an increase in the reduction factors against SARS coronavirus from 4.25 to 5.5 log PFU/mL (Rabenau et al., 2005). On a plastic surface, treatment with 85% ethanol resulted in an approximately 1.4 log PFU/mL reductions of MS2 at 4 and 8 °C. However, the reduction (2.10 log PFU/mL) at 21.8 °C was significantly (P < 0.05) higher than those at 4 and 8 °C. Moreover, approximately 1 log PFU/mL MS2 was inactivated when using this treatment on stainless steel at all test temperatures. It can be concluded that low temperatures 4 and 8 °C, did not negatively affect (except 85% ethanol treatment on a plastic surface) the virucidal activities of 70% and 85% ethanol against MS2 on plastic and stainless steel surfaces. In contrast, other sanitizers have been shown to be unstable at low temperature, for instance, the QACs at high concentration (0.05%, w/v) had no virucidal effect on equine herpesvirus type 1 after 10 min reaction time at 0 °C (Tsujimura et al., 2015).
Table 5.
Effect of temperature on the inactivation efficacy of 85% ethanol (60 s) against MS2 on plastic and stainless steel surfaces.
| Surface | Temperature | Log reductiona |
|---|---|---|
| Plastic | 4 °C | 1.36 ± 0.40B |
| 8 °C | 1.44 ± 0.42B | |
| 21.8 °C | 2.10 ± 0.07A | |
| Stainless steel | 4 °C | 1.38 ± 0.11B |
| 8 °C | 1.17 ± 0.12B | |
| 21.8 °C | 1.04 ± 0.10B |
In each column, values with the same upper letter do not differ significantly at P < 0.05.
3.5. Surface inactivation efficacies of household sanitizers
Ten household sanitizers were tested against MS2 on plastic and stainless steel in this study (Table 6). Sanitizers, containing BAC (0.1–0.26%), QAC (0.3–0.5%), or organic acid (0.19% lactic acid and 1.75% glycolic acid), showed limited virucidal activities (−0.28-0.33 log PFU/mL reductions) against MS2. Three sanitizers, in particular, named sanitizer C, E, and H, exhibited promising surface inactivation efficacies. Sanitizer C, with 1.84% NaClO, reduced >4 and 5 log PFU/mL of MS2 on plastic and stainless steel surface, respectively. Sanitizer E, with 3% NaClO, also resulted in >5 log PFU/mL MS2 reductions. Furthermore, around 1.5 log PFU/mL reductions were recorded in sanitizer H treated groups (Table 6). The 30 s and 60 s contact times produced comparable results.
Table 6.
Inactivation efficacy of household disinfectants against MS2 on plastic and stainless steel surfaces.
| Disinfectant | Active ingredient(s) | Surface | Log reduction (30 s)a | Log reduction (60 s)a |
|---|---|---|---|---|
| Sanitizer A | 0.1% BACb | Plastic | 0.09 ± 0.06aEF | 0.25 ± 0.23aD |
| Stainless steel | 0.25 ± 0.23aEF | −0.02 ± 0.20aD | ||
| Sanitizer B | 70% isopropanol | Plastic | 0.66 ± 0.06aDE | 0.55 ± 0.36aD |
| Stainless steel | 0.29 ± 0.11aEF | 0.41 ± 0.13aD | ||
| Sanitizer C | 1.84% sodium hypochlorite | Plastic | 4.64 ± 2.43aB | 4.15 ± 1.80aB |
| Stainless steel | 5.95 ± 0.22aA | 5.90 ± 0.41aA | ||
| Sanitizer D | 0.26% BACb | Plastic | −0.28 ± 0.26aF | −0.27 ± 0.27aD |
| Stainless steel | 0.01 ± 0.38aEF | 0.32 ± 0.26aD | ||
| Sanitizer E | 3% sodium hypochlorite | Plastic | 5.81 ± 0.19aA | 5.60 ± 0.48aA |
| Stainless steel | 5.72 ± 0.52aA | 6.23 ± 0.06aA | ||
| Sanitizer F | 0.5% QACc | Plastic | −0.08 ± 0.25aEF | −0.01 ± 0.37aD |
| Stainless steel | 0.09 ± 0.02aEF | 0.08 ± 0.08aD | ||
| Sanitizer G | 0.19% L-lactic acid | Plastic | −0.11 ± 0.29aEF | 0.16 ± 0.52aD |
| Stainless steel | 0.16 ± 0.18aEF | −0.02 ± 0.44aD | ||
| Sanitizer H | 58% ethanol, 0.1% QACc | Plastic | 1.62 ± 0.25aC | 1.59 ± 0.30aC |
| Stainless steel | 1.26 ± 0.13aCD | 1.48 ± 0.38aC | ||
| Sanitizer I | 0.3% QACc | Plastic | 0.15 ± 0.36aEF | −0.04 ± 0.48aD |
| Stainless steel | 0.06 ± 0.07aEF | 0.25 ± 0.38aD | ||
| Sanitizer J | 1.75% glycolic acid (1:64 dilution) | Plastic | 0.06 ± 0.34aEF | 0.33 ± 0.23aD |
| Stainless steel | −0.12 ± 0.60aEF | 0.06 ± 0.59aD |
In each column, values with the same upper letter do not differ significantly at P < 0.05; in each row, values with the same lower letter do not differ significantly at P < 0.05.
BAC: benzalkonium chloride.
QAC: quaternary ammonium compound.
NaClO solution has been widely used to inactivate pathogens on surfaces in the food industry, because of its effectiveness and low cost. NaClO is a strong oxidizing agent, and it can form hypochlorous acid when dissolved in water. The ratios of hypochlorous acid, Cl2, and OCl− in solution are pH dependent. The hypochlorous acid can further interact with peptide bonds and thiol groups, resulting in oxidization of proteins and other biomolecules (Fukuzaki, 2006). The WHO (2020) recommended a conservative concentration (0.1%) of hypochlorite-based products in the context of COVID-19. Moreover, its virucidal efficacy against various viruses also has been widely reported (Chen et al., 2019; Martin et al., 2013). Generally, NaClO solutions with higher concentrations present increased disinfectant efficacies (Meyers et al., 2021). The NaClO-based (1.84–3%) household sanitizers (sanitizer C and E) showed particularly good virucidal properties and outperformed that 0.1% NaClO (Table 2, Table 3). Therefore, NaClO solutions with higher concentrations (1–3%) should be tested in the future.
Sanitizer H was the only sanitizer containing ethanol (58%). Its surface disinfection performance was generally similar to the prepared ethanol solutions. In a previous study, a household sanitizer (active ingredients: 79% ethanol + 0.1% QAC) resulted in >3 log PFU/mL reductions of mouse hepatitis virus on a Petri dish (Dellanno et al., 2009). The present study showed lower (∼1.5 log PFU/mL) reductions of MS2 with Sanitizer H, which may be reflective of the different ethanol concentrations tested. In addition, viral structures might also contribute to the different virucidal activities. MS2 is a non-enveloped virus, which is more resistant to sanitizers compared with the enveloped virus, such as mouse hepatitis virus and SARS-CoV-2 (Ahmed et al., 2020; Kumar et al., 2020).
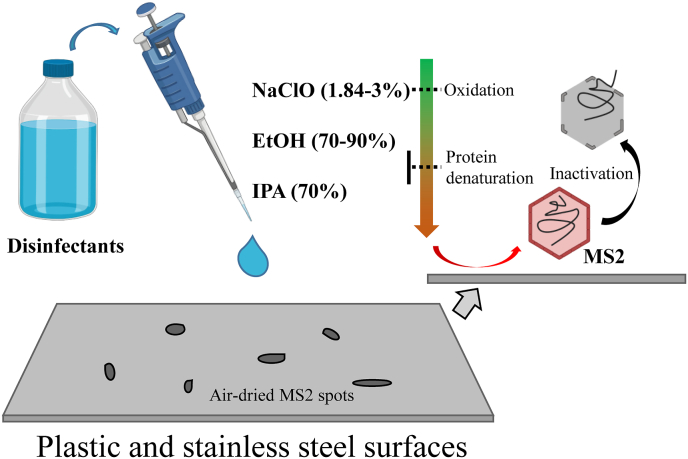
Based on our data, NaClO solutions with the appropriate concentrations (in this study, we tested a concentration of 1.84%) appear to be the most effective against MS2 on food packing and contact materials, particularly plastic and stainless steel (Fig. 1). The strong oxidizing property of NaClO solution contributes to the virucidal activity (Fukuzaki, 2006). Ethanol-based disinfectants also exhibited good inactivation performance on both plastic and stainless steel surfaces, where it has been shown to effectively denature the proteins and further disrupt the capsid (Lin et al., 2020). Lastly, isopropanol-based disinfectants ranked third, with higher efficacy on plastic surfaces. It has been shown previously that its lipophilic properties are more suitable for inactivation of enveloped viruses (McDonnell and Russell, 1999).
Fig. 1.
Illustration of MS2 disinfection using sodium hypochlorite (NaClO), ethanol (EtOH), and isopropanol (IPA) solutions.
In this study, the efficacies of different sanitizers against MS2 on plastic and stainless steel surfaces were tested. The surface inactivation test of MS2, a traditional surrogate of human enteric viruses (e.g., NoV and HAV), provide valuable information for hygiene regulation in food industry. Moreover, it could serve as a conservative surrogate for SARS-CoV-2. It provided a facile system for rapid quantitative evaluation of respirator disinfection. Because of its higher resistance to sanitizers compared to SARS-CoV-2, the substantial reduction in viable MS2 by certain sanitizers (e.g., sanitizers with 1.84–3% NaClO) gives confidence in an appropriate safety margin for SARS-CoV-2 surface decontamination.
4. Conclusion
To reinforce the hygienic operations in the food industry during the COVID-19 pandemic, the virucidal effects of frequently used sanitizers against MS2 on food contact surfaces (plastic and stainless steel) were examined. Overall, our results showed that isopropanol and isopropanol-based modified WHO II formula reduced 0.7–1.5 log PFU/mL MS2 on plastic and stainless steel surfaces. Moreover, ethanol and ethanol-based disinfectants exhibited promising inactivation effectiveness, resulting in around 1–2.5 log PFU/mL reductions. The effect of low temperature (4 and 8 °C) showed that low temperature generally did not affect the surface inactivation efficacies of 70% and 85% ethanol solutions, emphasizing its utility in the Agri-Food sector. Lastly, the disinfectant activities of 10 household sanitizers were determined to provide consumer reference information. We observed that sanitizers containing appropriate NaClO concentrations (1.84–3%) presented excellent surface decontamination performance of 4.15–6.31 log PFU/mL reductions of MS2. In conclusion, our data provide valuable information for hygiene regulation on a variety of surfaces in the Agri-Food sector.
Funding sources
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC Alliance AWD-014333).
CRediT authorship contribution statement
Lin Chen: Data curation, Formal analysis, Visualization, Writing – original draft. Win-ju Lee: Investigation. Yvonne Ma: Investigation, Project administration. Sung Sik Jang: Funding acquisition, Methodology, Writing - review & editing. Karen Fong: Funding acquisition, Methodology, Writing - review & editing. Siyun Wang: Conceptualization, Supervision, Methodology, Funding acquisition, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aday S., Aday M.S. Impact of COVID-19 on the food supply chain. Food Qual. Saf. 2020;4(4):167–180. [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTM E1053-20 . ASTM International; West Conshohocken, PA: 2020. Standard Practice to Assess Virucidal Activity of Chemicals Intended for Disinfection of Inanimate, Nonporous Environmental Surfaces. [Google Scholar]
- ASTM E1054-08 . ASTM International; West Conshohocken, PA: 2008. Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents. [Google Scholar]
- ASTM E2197-17e1 . ASTM International; West Conshohocken, PA: 2017. Standard Quantitative Disk Coupon Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals. [Google Scholar]
- Bolton S.L., Kotwal G., Harrison M.A., Law S.E., Harrison J.A., Cannon J.L. Sanitizer efficacy against murine norovirus, a surrogate for human norovirus, on stainless steel surfaces when using three application methods. Appl. Environ. Microbiol. 2013;79(4):1368–1377. doi: 10.1128/AEM.02843-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J.M. Alcohols as surface disinfectants in healthcare settings. Infect. Control Hosp. Epidemiol. 2018;39(3):323–328. doi: 10.1017/ice.2017.301. [DOI] [PubMed] [Google Scholar]
- Boxman I.L., Verhoef L., Dijkman R., Hägele G., Te Loeke N.A., Koopmans M. Year-round prevalence of norovirus in the environment of catering companies without a recently reported outbreak of gastroenteritis. Appl. Environ. Microbiol. 2011;77(9):2968–2974. doi: 10.1128/AEM.02354-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt H., D'Souza D.H., Davidson P.M. Thermal inactivation of foodborne enteric viruses and their viral surrogates in foods. J. Food Protect. 2015;78(8):1597–1617. doi: 10.4315/0362-028X.JFP-14-487. [DOI] [PubMed] [Google Scholar]
- Bozkurt H., Phan-Thien K.Y., van Ogtrop F., Bell T., McConchie R. Outbreaks, occurrence, and control of norovirus and hepatitis a virus contamination in berries: a review. Crit. Rev. Food Sci. Nutr. 2021;61(1):116–138. doi: 10.1080/10408398.2020.1719383. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2021 April 5. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments.https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html [PubMed] [Google Scholar]
- Chen L., Zhang H., Liu Q., Pang X., Zhao X., Yang H. Sanitising efficacy of lactic acid combined with low-concentration sodium hypochlorite on Listeria innocua in organic broccoli sprouts. Int. J. Food Microbiol. 2019;295:41–48. doi: 10.1016/j.ijfoodmicro.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Chin A.W., Chu J.T., Perera M.R., Hui K.P., Yen H.L., Chan M.C., Peiris M., Poon L.L. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellanno C., Vega Q., Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am. J. Infect. Control. 2009;37(8):649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser R.L., Monroe S.S., Noel J.S., Humphrey C.D., Bresee J.S., Parashar U.D., Ando T., Glass R.I. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 2002;186(1):1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- Fong K., LaBossiere B., Switt A.I., Delaquis P., Goodridge L., Levesque R.C., Danyluk M.D., Wang S. Characterization of four novel bacteriophages isolated from British Columbia for control of non-typhoidal Salmonella in vitro and on sprouting alfalfa seeds. Front. Microbiol. 2017;8:2193. doi: 10.3389/fmicb.2017.02193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K., Tremblay D.M., Delaquis P., Goodridge L., Levesque R.C., Moineau S., Suttle C.A., Wang S. Diversity and host specificity revealed by biological characterization and whole genome sequencing of bacteriophages infecting Salmonella enterica. Viruses. 2019;11(9):854. doi: 10.3390/v11090854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck K.T., Lisby M., Fonager J., Schultz A.C., Böttiger B., Villif A., Absalonsen H., Ethelberg S. Sources of calicivirus contamination in foodborne outbreaks in Denmark, 2005–2011—the role of the asymptomatic food handler. J. Infect. Dis. 2015;211(4):563–570. doi: 10.1093/infdis/jiu479. [DOI] [PubMed] [Google Scholar]
- Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11(4):147–157. doi: 10.4265/bio.11.147. [DOI] [PubMed] [Google Scholar]
- Girard M., Ngazoa S., Mattison K., Jean J. Attachment of noroviruses to stainless steel and their inactivation, using household disinfectants. J. Food Protect. 2010;73(2):400–404. doi: 10.4315/0362-028x-73.2.400. [DOI] [PubMed] [Google Scholar]
- González N., Marquès M., Domingo J.L. Respiratory viruses in foods and their potential transmission through the diet: a review of the literature. Environ. Res. 2021 doi: 10.1016/j.envres.2021.110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada . 2021, August 19. Guidance Document: Disinfectant Drugs.https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents.html 2020. [Google Scholar]
- Health Canada . 2021, July 09. Hard-surface Disinfectants and Hand Sanitizers (COVID-19): List of Disinfectants with Evidence for Use against COVID-19.https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/list.html [Google Scholar]
- Hoelzer K., Fanaselle W., Pouillot R., Van Doren J.M., Dennis S. Virus inactivation on hard surfaces or in suspension by chemical disinfectants: systematic review and meta-analysis of norovirus surrogates. J. Food Protect. 2013;76(6):1006–1016. doi: 10.4315/0362-028X.JFP-12-438. [DOI] [PubMed] [Google Scholar]
- Julian T.R., Trumble J.M., Schwab K.J. Evaluating efficacy of field-generated electrochemical oxidants on disinfection of fomites using bacteriophage MS2 and mouse norovirus MNV-1 as pathogenic virus surrogates. Food Environ. Virol. 2014;6(2):145–155. doi: 10.1007/s12560-014-9136-6. [DOI] [PubMed] [Google Scholar]
- Kratz J.M., Andrighetti-Fröhner C.R., Leal P.C., Nunes R.J., Yunes R.A., Trybala E., Bergström T., Barardi C.R.M., Simões C.M.O. Evaluation of anti-HSV-2 activity of gallic acid and pentyl gallate. Biol. Pharm. Bull. 2008;31(5):903–907. doi: 10.1248/bpb.31.903. [DOI] [PubMed] [Google Scholar]
- Kumar G.D., Mishra A., Dunn L., Townsend A., Oguadinma I.C., Bright K.R., Gerba C.P. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front. Microbiol. 2020;11:1351. doi: 10.3389/fmicb.2020.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Lim J.Y., Xue K., Yew P.Y.M., Owh C., Chee P.L., Loh X.J. Sanitizing agents for virus inactivation and disinfection. View. 2020;1(2) doi: 10.1002/viw2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lu L.C., Quintela I., Lin C.H., Lin T.C., Lin C.H., Wu V.C., Lin C.S. A review of epidemic investigation on cold‐chain food‐mediated SARS‐CoV‐2 transmission and food safety consideration during COVID‐19 pandemic. J. Food Saf. 2021 doi: 10.1111/jfs.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard J.-Y., Beggs T., Day M., Hudson R., Russell A. Effect of biocides on MS2 and K coliphages. Appl. Environ. Microbiol. 1994;60(6):2205–2206. doi: 10.1128/aem.60.6.2205-2206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès M., Domingo J.L. Contamination of inert surfaces by SARS-CoV-2: persistence, stability and infectivity. A review. Environ. Res. 2020;193 doi: 10.1016/j.envres.2020.110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H., Soumet C., Fresnel R., Morin T., Lamaudière S., Le Sauvage A., Deleurme K., Maris P. Comparison of the virucidal efficiency of peracetic acid, potassium monopersulfate and sodium hypochlorite on hepatitis A and enteric cytopathogenic bovine orphan virus. J. Appl. Microbiol. 2013;115(4):955–968. doi: 10.1111/jam.12297. [DOI] [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Kass R., Goldenberg D., Milici J., Alam S., Robison R. Ethanol and isopropanol inactivation of human coronavirus on hard surfaces. J. Hosp. Infect. 2021;107:45–49. doi: 10.1016/j.jhin.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileto D., Mancon A., Staurenghi F., Rizzo A., Econdi S., Gismondo M.R., Guidotti M. Inactivation of SARS-CoV-2 in the liquid phase: are aqueous hydrogen peroxide and sodium percarbonate efficient decontamination agents? ACS Chem. Health Saf. 2021 doi: 10.1021/acs.chas.0c00095. [DOI] [PubMed] [Google Scholar]
- Moorer W.R. Antiviral activity of alcohol for surface disinfection. Int. J. Dent. Hyg. 2003;1(3):138–142. doi: 10.1034/j.1601-5037.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Møretrø T., Heir E., Nesse L.L., Vestby L.K., Langsrud S. Control of Salmonella in food related environments by chemical disinfection. Food Res. Int. 2012;45(2):532–544. [Google Scholar]
- Morin T., Martin H., Soumet C., Fresnel R., Lamaudière S., Le Sauvage A., Deleurme K., Maris P. Comparison of the virucidal efficacy of peracetic acid, potassium monopersulphate and sodium hypochlorite on bacteriophages P001 and MS 2. J. Appl. Microbiol. 2015;119(3):655–665. doi: 10.1111/jam.12870. [DOI] [PubMed] [Google Scholar]
- Mormann S., HEIßENBERG C.A., Pfannebecker J., Becker B. Tenacity of human norovirus and the surrogates feline calicivirus and murine norovirus during long-term storage on common nonporous food contact surfaces. J. Food Protect. 2015;78(1):224–229. doi: 10.4315/0362-028X.JFP-14-165. [DOI] [PubMed] [Google Scholar]
- Paiva R., Wrona M., Nerín C., Veroneze I.B., Gavril G.L., Cruz S.A. Importance of profile of volatile and off-odors compounds from different recycled polypropylene used for food applications. Food Chem. 2021;350 doi: 10.1016/j.foodchem.2021.129250. [DOI] [PubMed] [Google Scholar]
- Pinto F., Maillard J.Y., Denyer S.P. Effect of surfactants, temperature, and sonication on the virucidal activity of polyhexamethylene biguanide against the bacteriophage MS2. Am. J. Infect. Control. 2010;38(5):393–398. doi: 10.1016/j.ajic.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Rabenau H., Kampf G., Cinatl J., Doerr H.W. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005;61(2):107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajiuddin S.M., Vigre H., Musavian H.S., Kohle S., Krebs N., Hansen T.B., Gantzer C., Schultz A.C. Inactivation of hepatitis A virus and murine norovirus on surfaces of plastic, steel and raspberries using steam-ultrasound treatment. Food Environ. Virol. 2020;12(4):295–309. doi: 10.1007/s12560-020-09441-1. [DOI] [PubMed] [Google Scholar]
- Singh D., Joshi K., Samuel A., Patra J., Mahindroo N. vol. 148. Epidemiology & Infection; 2020. (Alcohol-based Hand Sanitisers as First Line of Defence against SARS-CoV-2: a Review of Biology, Chemistry and Formulations). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., D'Souza D.H. Inactivation of human norovirus surrogates by benzalkonium chloride, potassium peroxymonosulfate, tannic acid, and gallic acid. Foodborne Pathog. Dis. 2012;9(9):829–834. doi: 10.1089/fpd.2012.1155. [DOI] [PubMed] [Google Scholar]
- Tsujimura K., Murase H., Bannai H., Nemoto M., Yamanaka T., Kondo T. Efficacy of five commercial disinfectants and one anionic surfactant against equine herpesvirus type 1. J. Vet. Med. Sci. 2015:15–30. doi: 10.1292/jvms.15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . 2020 July 17. What to Do if You Have a COVID-19 Confirmed Positive Worker or Workers Who Have Been Exposed to a Confirmed Case of COVID-19.https://www.fda.gov/food/food-safety-during-emergencies/what-do-if-you-have-covid-19-confirmed-positive-worker-or-workers-who-have-been-exposed-confirmed [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong W.C., Hua X.Y., Liu S.Q. Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT-Food Sci. Technol. 2018;90:316–322. [Google Scholar]
- Wong C.W., Delaquis P., Goodridge L., Lévesque R.C., Fong K., Wang S. Inactivation of Salmonella enterica on post-harvest cantaloupe and lettuce by a lytic bacteriophage cocktail. Curr. Res. Food Sci. 2020;2:25–32. doi: 10.1016/j.crfs.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020 May 16. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19.https://www.who.int/publications/i/item/cleaning-and-disinfection-of-environmental-surfaces-inthe-context-of-covid-19 [Google Scholar]
- World Health Organization . 2021, December 28. https://covid19.who.int/ (WHO Coronavirus (COVID-19) Dashboard). [Google Scholar]
- Zulauf K.E., Green A.B., Nguyen Ba A.N., Jagdish T., Reif D., Seeley R., Dale A., Kirby J.E. Microwave-generated steam decontamination of N95 respirators utilizing universally accessible materials. mBio. 2020;11(3) doi: 10.1128/mBio.00997-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]