Abstract
The treatment of refractory Helicobacter pylori remains challenging in clinical practice. Factors that should be considered in the treatment of refractory H. pylori infection include treatment length, dosage of antibiotics and proton pump inhibitors (PPIs), number of drugs, and the selection of appropriate antibiotics. Extending the treatment length of triple therapy and non-bismuth quadruple therapy to 14 days may increase the eradication rate compared with a shorter period (7 or 10 days). The use of a higher dose of PPIs or vonoprazan may also increase the efficacy of triple therapy. Four-drug therapy, including bismuth or non-bismuth quadruple therapies, usually achieve higher eradication rates than triple therapy. The addition of bismuth or metronidazole to levofloxacin-amoxicillin-PPI therapy may also increase the eradication rate. Therefore, four-drug therapies containing a higher dose of PPIs for 14 days are recommended in the third-line treatment setting for refractory H. pylori infection. The selection of appropriate antibiotics may be guided by susceptibility testing or empirically by medication history. Tailored therapy guided by susceptibility testing or genotypic resistance is recommended whenever possible. However, properly designed empirical therapy based on prior medication history (i.e., avoid the reuse of clarithromycin or levofloxacin empirically) is an acceptable alternative to tailored therapy after considering accessibility, cost, and the preference of the patient.
Keywords: Helicobacter pylori, Refractory, Third-line, Eradication, Resistance
INTRODUCTION
Eradication of Helicobacter pylori reduces the recurrence rate of peptic ulcer disease, cures two-thirds of patients with mucosal-associated lymphoid tissue lymphoma, and reduces the risk of gastric cancer.1-4 Clarithromycin triple therapy is one of the most commonly used regimen in the first-line treatment.1,5 However, the eradication rate of clarithromycin triple therapy is now lower than 80% in the first-line treatment due to the global rising prevalence of clarithromycin resistance.6,7 Levofloxacin triple therapy and bismuth quadruple therapy are the most commonly used second-line rescue regimens.1,5,7,8 Yet, about 10% to 20% of patients cannot be cured with either one of them. Patients who experience treatment failure after two or more eradication therapies are usually termed as those with refractory H. pylori infection.9,10 Overall, it is estimated that 3% to 10% of H. pylori-infected subjects would require third-line rescue therapy for refractory H. pylori infection. Yet, treatment of refractory H. pylori infection remains a challenge in clinical practice and some patients are left untreated. Therefore, we reviewed current evidence and proposed strategies to optimize the treatment for refractory H. pylori infection.
SHOULD WE RECOMMEND RESCUE THERAPY FOR ALL PATIENTS WITH REFRACTORY H. PYLORI INFECTION?
There are contradictory viewpoints about whether patients with refractory H. pylori infection should be actively treated with rescue therapy or they may be left untreated. Some physicians considered that further rescue therapy is not mandatory because gastric cancer develops only in 1% to 3% of H. pylori-infected subjects and there are potential concerns about increased risk of antibiotic resistance at individual level as well as in the community.11,12 However, most experts considered that physicians should recommend rescue therapy for these patients since eradication of H. pylori reduces the risk of gastric cancer.2,3,4,7 Of course, the patients can make their own decisions according to their preference judging from the benefit and risk of rescue therapy. For example, patients who carry the higher risk of gastric cancer, such as the presence of premalignant lesions and positive family history are candidates for the rescue therapy.
FACTORS ATTRIBUTABLE TO TREATMENT FAILURE
Physicians should try to identify factors leading to treatment failure for their patients with refractory H. pylori infection. Common reasons for treatment failure include poor compliance to prior treatment, the presence of antibiotic resistance, insufficient delivery of drugs into the gastric mucous layer, rapid metabolism of treatment drugs, and insufficient treatment length.13 Poor compliance of therapy may result from adverse effects or the complexity of drug administration of prior regimens.13 If the patient’s compliance is good, the presence of antibiotic resistance is the most common reason for treatment failure.13 The high bacterial load makes it likely that antibiotic-resistant H. pylori strains will be present when antibiotic therapy is begun. The average H. pylori-infected stomach contains huge numbers of H. pylori such that if the spontaneous rate of development of resistance was only 1 in 10 million and 109 (100 million) organisms were present, one would expect that a resistant subpopulation of H. pylori would already be present and cause the therapy to fail.4 H. pylori can also rapidly acquire new genotypic resistance to many commonly used antimicrobials. Our study showed that the prevalence of clarithromycin resistance was 61% and 95% in patients who experienced treatment failures after one and two eradication therapies, respectively.14 Another study showed that the gyrase A and 23S rRNA mutant H. pylori strains were already present in patients who failed after levofloxacin-based and clarithromycin-based triple therapy.15 A proportion of H. pylori bacteria attach to gastric mucosal cells and form a biofilm, and some are intracellular, which means they are inaccessible to many antibiotics.16 This biofilm phenomenon which has been demonstrated with H. pylori in vitro and is likely also present in vivo. H. pylori can also survive intracellularly making them inaccessible to topical therapy and to drugs that penetrate cells poorly.16 Acetylcystein was shown to destroy the biofilm and may increase the efficacy of eradication therapy for refractory H. pylori infection in some studies, but the effect remains controversial.17 Most proton pump inhibitors (PPIs) are metabolized through the CYP2C19 pathway and the eradication rate is lower in patients with CYP2C19 extensive metabolizer.18 Increase the dosage of PPIs may be required to provide adequate acid suppression and higher eradication rate in such circumstances.18,19
OPTIMIZATION OF THIRD-LINE TREATMENT
Optimization of the regimens is important to achieve the best cure rates used in the treatment of refractory H. pylori infection. The proposed strategies to optimize the eradication rate are shown in Table 1. These include extending treatment length to 14 days, use of higher dosage or more potent acid suppression agents, optimization of dosage of antibiotics, use of bismuth or non-bismuth quadruple therapy, and selection of appropriate antibiotics according to susceptibility testing or empirically according to detailed medication history.19-26
Table 1.
Optimization of Rescue Therapy for Refractory Helicobacter pylori Infection
| Strategy | Recommendation |
|---|---|
| Duration of therapy | 14 Days |
| Dosage of drugs | |
| PPIs | Higher dosage PPIs (omeprazole 40 mg or equivalent twice daily) or vonoprazan 20 mg twice daily |
| Amoxicillin | 2,000–3,000 mg per day in 2–4 divided doses |
| Levofloxacin | 500 mg per day or 250 mg twice daily |
| Sitafloxacin | 100 mg twice daily |
| Metronidazole | 1,500–1,600 mg per day in 3–4 divided doses |
| Tetracycline | 1,500–2,000 mg per day in 3–4 divided doses |
| Rifabutin | 300 mg per day in 2 divided doses |
| Clarithromycin | 800–1,000 mg per day in 2 divided doses |
| Number of drugs | We recommended 4-drug therapy (bismuth or non-bismuth quadruple therapy) for refractory H. pylori infection |
| How to choose antibiotics | Guided by susceptibility testing or genotypic resistance whenever possible |
| Empirical therapy to avoid reuse of clarithromycin and levofloxacin may be an acceptable alternative considering availability, cost, and preference of patient |
PPIs, proton pump inhibitors.
The intragastric location of H. pylori complicates therapy as it requires consideration of many variables as the infection is both outside the body, attached to cells, and even within gastric cells.27 Factors that should be considered to recommend an optimal regimen include optimum drugs, formulations, routes of administration, doses, dosing intervals, relation to meals, adjuvants, and duration of therapy.27 Optimum is defined as the best or most effective therapy possible in a particular situation. In subjects adherent to treatment, regimens are usually expected to achieve cure rates reliably equal to or greater than 95% for infectious diseases.4,27,28
1. Duration of therapy
Duration is based on overcoming the persister effect and takes into account that PPIs do not achieve full effectiveness until after 3 or 4 days of administration.4,27,28 Extending the treatment length of triple therapy for 14 days was superior to the same regimen given for 7 days or 10 days in the first-line treatment.22 Thus, various guidelines have recommended duration of 14 days in the first-line treatment unless a shorter duration is locally proven to be non-inferior and produce a reliably high success rate.1,5,8,29 In the second-line or third-line treatment, the cure rates of levofloxacin triple therapy were 58.3%, 68.2%, and 93.3% when the treatment length were 7, 10, and 14 days, respectively.30 However, it is noteworthy that the benefit of extending the treatment length to 14 days is minimal in susceptible strains.29 However, the eradication rate can be increased in strains with clarithromycin resistance, which is attributable to the effect for PPIs-amoxicillin dual therapy.18 Taken together, we recommend 14-day therapy for refractory H. pylori infection, but further well designed trials are needed.
2. Dosage of PPIs
PPIs vary greatly in relative potency such that it is impossible to compare regimens using different PPIs unless these differences are taken into account. For H. pylori eradication, 20 mg omeprazole equivalents, twice daily is regarded as low dose PPI and 40 mg omeprazole equivalents, twice daily regarded as high or double dose.19 Randomized trials showed that the use of higher dosage of PPIs may increase the efficacy of triple therapy.24 Therefore, it has been recommended to give double dose PPIs because of the benefits obtained by increasing the anti-secretory effect with dual PPIs amoxicillin therapy. More recently, vonoprazan, a potassium-competitive acid blocker, is shown to be more potent than PPIs, especially in those with CYP2C19 extensive metabolizer. Vonoprazan-based triple therapy for 7 days was shown to be superior to lansoprazole-based triple therapy for 7 days in Japanese, especially in those infected with clarithromycin-resistant strains.25 A recent randomized trial further showed that vonoprazan-based sitafloxacin triple therapy was superior to PPIs-based sitafloxacin triple therapy in the third-line treatment of H. pylori infection.31
3. Optimal dosage of antibiotics in rescue therapy
Earlier studies showed that the use of higher dosage of metronidazole (up to 1,600 to 2,000 mg per day) may partly overcome the metronidazole resistance.13 Recent studies also showed that the use of higher dosage of amoxicillin (up to 750 mg three times or four times a day) may increase the efficacy of dual therapy.32 The recommended dosage of tetracycline is 500 mg four times a day in bismuth quadruple therapy.1,5,8 In contrast, increase in clarithromycin or levofloxacin dosage cannot overcome the resistance to these two antibiotics, respectively.
4. Number of drugs
Several randomized trials showed that four-drug regimens, including bismuth quadruple therapy and non-bismuth quadruple therapies (concomitant therapy, sequential therapy, hybrid therapy) were more effective than triple therapy in the first-line treatment when given for the same duration.20,21,23,26 Concomitant or sequential therapy for 14 days, but not 10 days, was superior to 14-day triple therapy in the first-line treatment.23,33 Triple therapy containing esomeprazole, amoxicillin and metronidazole for 2 weeks was suboptimal in the third-line therapy after failure from clarithromycin-based therapy and fluoroquinolone-based therapy.34 The eradication rates were 64% and 37% in metronidazole-naive and metronidazole experienced patients, respectively.34 Systematic review and meta-analysis showed that the efficacy of levofloxacin triple therapy was lower than 80% in the second-line treatment.35 Hsu et al.36 showed that addition of bismuth to levofloxacin triple therapy cured 84% of patients (31/37) in the third-line treatment of H. pylori infection. The addition of bismuth to rifabutin triple therapy (96.6%, 28/29) was shown to increase the eradication rate of rifabutin-based triple therapy (66.7%, 18/27) in the third-line treatment.37 Taken together, it is suggested to provide four-drug regimens (bismuth or non-bismuth quadruple therapy) as third-line or fourth-line salvage therapy.
5. How to choose antibiotics in third-line or fourth-line rescue therapies?
1) Susceptibility guided therapy
Ideally, therapy should be tailored by susceptibility testing whenever possible. Tailored therapy is recommended by international consensus reports for patients with refractory H. pylori infection although the evidence level is low for such recommendation.1,4 The efficacy of susceptibility testing-guided therapy for refractory H. pylori infection has been reported in nine studies, as shown in Table 2.38-47 Eight of them are noncontrolled case series, one is non-randomized controlled study, and another one is a randomized control trial. E-test was the most commonly used method to detect antibiotic resistance (Table 2). The successful rate of culture ranged from 74% to 98%. The resistance rate to clarithromycin, metronidazole, and levofloxacin ranged from 51%–95%, 43%–100%, and 6%–52%, respectively. Bismuth quadruple therapy including a PPI, bismuth and another two susceptible antibiotics or non-bismuth quadruple therapy including a PPI plus another three antibiotics were the most commonly used regimens. The treatment length varied from 7 to 14 days. The overall eradication rate of susceptibility testing-guided therapy ranged from 60% to 90% (Table 2).
Table 2.
Susceptibility Testing-Guided Therapy in Third-Line Treatment for Helicobacter pylori Infection
| Author (year) | Study design |
No. of Tx | Test used | Culture success rate |
CLA/LEV/MET resistance rate, % |
Rules to choose regimen | Duration, day |
No. of cases |
ITT analysis | PP analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Gasbarrini et al. (2000)38 | NC | 2 | E-test | 80 (39/49) | 56/-/56 | Quadruple: PPIs, bismuth, plus 2 antibiotics | 7 | 49 | 61 (30/49) | 77 (30/39) |
| Vicente et al. (2002)39 | NC | 2 | E-test | 97.6 | 51/-/43 | Quadruple: PPIs, bismuth, plus 2 antibiotics | 14 | 40 | 60 (24/40) | 63 (24/38) |
| Cammarota et al. (2004)40 | NC | 2 | E-test | 96 (94/98) | 95/31/100 | Quadruple: PPIs, bismuth, doxycycline and amoxicillin or triple PPIs, amoxicillin and levofloxacin or clarithromycin | 7 | 94 | 90 (85/94) | 91 (85/93) |
| Yahav et al. (2006)41 | NC | 1 or 2 | E-test | 100 (49/49) | 59/-/47 | Triple therapy or quadruple therapy | 7 | 49 | 86 (42/49) | 86 (42/49) |
| Tay et al. (2012)42 | NC | 1 or 2 | E-test | 98.7 (306/310) | 94/6/68 | Quadruple: PPIs, amoxicillin, ciprofloxacin, and rifabutin; PPIs, bismuth, furazolidone, amoxicillin or rifabutin; PPIs, bismuth, tetracycline, furazolidone or amoxicillin | 10 | 310 | 94 (291/310) | 94 (291/310) |
| Fiorini et al. (2013)43 | NC | 1 or 2 | E-test | 93 (236/254) | 92/44/73 | Triple: PPI, amoxicillin, levofloxacin or rifabutin | 10–12 | 254 | 83 (211/254) | 90 (212/236) |
| Liou et al. (2013)44 | NC | 2 or more | PCR and agar dilution | 95 (128/135)/ 74 (100/135) |
87/47/58 | Non-bismuth quadruple: PPIs, amoxicillin, metronidazole, levofloxacin or clarithromycin or tetracycline | 14 | 135 | 81 (109/135) | 83 (109/132) |
| Costa et al. (2017)45 | NC | 2 | E-test | 100 | 86/52/67 | Triple therapy with PPIs, amoxicillin, susceptible drug or PPIs, doxycycline, and rifampicin | 8–14 | 42 | 60 (25/42) | 62 (24/39) |
| Liou et al. (2018)46 | RCT | 2 or more | PCR | 97.8 | 90/61/66 | Non-bismuth quadruple: PPI, amoxicillin, metronidazole, levofloxacin or clarithromycin or tetracycline | 14 | 205 | 78 (160/205) | 78 (156/199) |
| Yu et al. (2019)47 | NC | 1 or more | Agar dilution | 95.8 (206/215) | 94/93.5/81 | Triple therapy with PPI, amoxicillin plus clarithromycin or metronidazole, or levofloxacin | 14 | 200 | 95 (189/200) | 97 (186/192) |
| Bismuth quadruple for multidrug-resistant infections: PPI, bismuth, amoxicillin, and metronidazole |
Data are presented percent (number/number).
No. of Tx, number of prior eradication therapy; CLA, clarithromycin; LEV, levofloxacin; MET, metronidazole; ITT, intention-to-treat; PP, per protocol; NC, noncontrolled study; RCT, randomized controlled trial; PCR, polymerase chain reaction; PPIs, proton pump inhibitors.
However, there is limited evidence to show the superiority of tailored therapy over empirical therapy in rescue therapies. In the first-line therapy, susceptibility testing-guided therapy was more effective than empirical triple therapy for 7 or 10 days in the first-line treatment in a meta-analysis of randomized trials.48 Yet, two randomized trials showed that empirical bismuth quadruple therapy and empirical non-bismuth quadruple therapy were not inferior to tailored therapy in China and Korea where the clarithromycin-resistant rate was higher than 15% to 20%.49,50 Moreover, tailored therapy was not superior to empirical therapy in three trials that recruited patients failed after one eradication therapy.48 Of the only one randomized trial that compared the efficacy of tailored therapy versus empirical therapy for patients who failed after at least two eradication therapies, Liou et al.46 showed that the eradication rate of genotypic resistance guided therapy and empirical therapy were 78% and 72%, respectively. Therefore, our suggestion is that susceptibility testing or genotypic resistance should be determined for patients with refractory H. pylori infection whenever possible. However, properly chosen empirical therapy according to the detailed medication history may be an as effective alternative considering accessibility to susceptibility testing, patient preference, and cost.
2) Empirical therapy
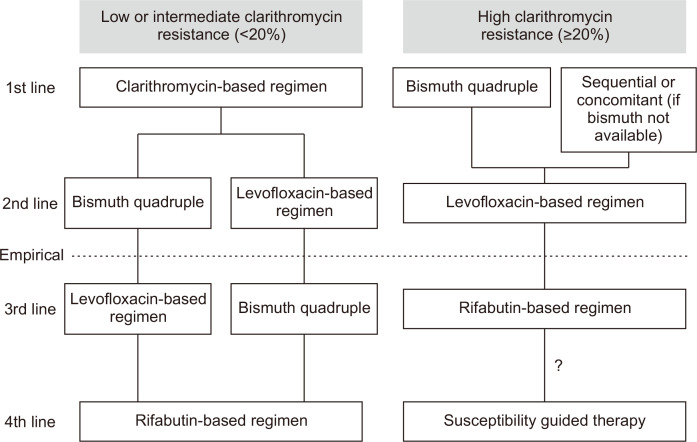
Since the resistance rates are high in patients who fail after regimens containing clarithromycin and levofloxacin, these two antibiotics should not be reused empirically.1,8,51 The strategy to choose antibiotics for third-line and fourth-line therapy is shown in Fig. 1.1,8,51 For patients who have received regimens containing clarithromycin and levofloxacin in their prior therapies, bismuth quadruple therapy is recommended. For those who have not been treated with levofloxacin-containing regimen in their prior treatment, levofloxacin triple therapy, bismuth enhanced levofloxacin triple therapy, or non-bismuth quadruple therapy containing levofloxacin may be used. For those who have received bismuth quadruple therapy and regimens clarithromycin and levofloxacin in their prior treatments, rifabutin-based triple or quadruple therapy may be used as rescue therapy. Whether re-treatment with bismuth quadruple therapy is an option remains controversial, although a retrospective study in Korea showed that re-treatment with bismuth quadruple therapy cured 75% of patients who failed after the same regimen in the second-line treatment.52 Sitafloxacin-based triple therapy was shown to be effective in patients who harbor gyrase A mutations.53 However, there is limited evidence to support the use of sitafloxacin-based therapy for treatment after failure from levofloxacin-based therapy.
Fig. 1.
How to choose antibiotics empirically in rescue therapies. Avoid the reuse of clarithromycin or levofloxacin empirically in third-line rescue treatment.
"?" indicates that although susceptibility testing guided therapy is recommended for patients who fail after a rifabutin-based regimen, there is limited evidence to support this recommendation.
There are limited data on the efficacy of empirical bismuth quadruple therapy and levofloxacin-based therapy in the third-line treatment of refractory H. pylori infection (Table 3).30,36,52-57 The reported efficacy of 7- to 14-day bismuth quadruple therapy containing PPIs, bismuth, tetracycline, and metronidazole varied from 65% to 80% in the third-line treatment.54,55 The efficacy of 7- to 14-day levofloxacin triple therapy or bismuth enhanced levofloxacin triple therapy ranged from 43.3% to 84% in the third-line treatment (Table 3).30,36,53,56,57 The reported efficacy of sitafloxacin-based triple therapy varied from 54% to 93% in the third-line treatment (Table 4).53,58-66 Meta-analysis of these studies revealed that the eradication rate of sitafloxacin-based triple therapy was 80% (74.6% to 84.9%) (Table 4). The presence of gyrase A mutation was associated with increased risk of treatment failure (risk ratio, 1.3; 95% confidence interval, 1.2 to 1.4; p<0.001). The eradication rate appeared to be higher when more potent acid secretion inhibitor was used. A randomized trial showed higher efficacy of sitafloxacin-based triple therapy than that of levofloxacin-based triple therapy.53 Therefore, sitafloxacin may be preferable to levofloxacin in the treatment of refractory H. pylori infection if it is available.
Table 3.
Bismuth Quadruple Therapy and Levofloxacin-Based Therapy in the Third-Line Treatment Setting
| Author (year) | Design | Dosing frequency | Duration, day | Eradication rate, % (No./No.) | |
|---|---|---|---|---|---|
| ITT analysis | PP analysis | ||||
| Bismuth quadruple therapy | |||||
| Gisbert et al. (2014)54 | Prospective (observational) | PPI (standard dose b.i.d.), bismuth subcitrate (120 mg q.i.d. or 240 mg b.i.d.), tetracycline (from 250 mg t.i.d. to 500 mg q.i.d.) and metronidazole (from 250 mg t.i.d. to 500 mg q.i.d.) | 7–14 | 65.5 (131/200) | 66.7 (128/192) |
| Rodríguez de Santiago et al. (2017)55 | Prospective (observational) | Pylera® (three-in-one capsules containing metronidazole 125 mg, bismuth subcitrate potassium 140 mg, and tetracycline 125 mg) 3 tablets q.i.d. and a PPI b.i.d. | 10 | 80.2 (81/102) | 84.4 (82/97) |
| Hsu et al. (2011)36 | Prospective | Rabeprazole (20 mg b.i.d.), bismuth subcitrate (300 mg q.i.d.), amoxicillin (500 mg q.i.d.) and levofloxacin (500 mg o.d.) | 10 | 83.8 (31/37) | 83.8 (31/37) |
| Levofloxacin-based therapy | |||||
| Noh et al. (2016)30 | NC | PPI standard dose b.i.d., levofloxacin 500 mg q.d., amoxicillin 1 g b.i.d. | 7 | 58.3 (7/12) | 58.3 (7/12) |
| 10 | 62.5 (15/24) | 68.2 (15/22) | |||
| 14 | 73.7 (14/19) | 93.3 (14/15) | |||
| Lim et al. (2017)56 | Retrospective | Levofloxacin-based therapy | 7 | - | 80.6 (25/31) |
| 10 | - | 64.0 (16/25) | |||
| 14 | - | 68.8 (22/32) | |||
| Okimoto et al. (2014)57 | RCT | Rabeprazole 10 mg b.i.d., amoxicillin 750 mg b.i.d., levofloxacin 500 mg q.d. | 10 | 45.8 (11/24) | 45.8 (11/24) |
| Murakami et al. (2013)53 | RCT | Lansoprazole 30 mg b.i.d., amoxicillin 750 mg b.i.d., levofloxacin 300 mg b.i.d. | 7 | 43.3 (28/65) | 43.7 (28/64) |
| Lansoprazole 30 mg b.i.d., amoxicillin 750 mg b.i.d., sitafloxacin 100 mg b.i.d. | 7 | 70.4 (49/70) | 72.1 (49/68) | ||
| Tursi et al. (2012)58 | NC | PPI plus amoxicillin 1 g for the first 5 days, followed by PPI, levofloxacin 500 mg and tetracycline 500 mg for the remaining 5 days (all b.i.d.). | 10 | 67.2 (80/119) | 68.4 (80/117) |
ITT, intention-to-treat; PP, per protocol; NC, noncontrolled study; RCT, randomized controlled trial; PPI, proton pump inhibitor; q.d., once a day; b.i.d., twice a day; q.i.d., four times a day.
Table 4.
Sitafloxacin Triple Therapy in the Third-Line Treatment Setting
| Author (year) | Dosing frequency | Duration, day |
Eradication rate, % (No./No.) | ||
|---|---|---|---|---|---|
| Overall | Gyrase A wild | Gyrase A mutant | |||
| Mori et al. (2019)59 | Esomeprazole (20 mg, b.i.d.), amoxicillin (500 mg, q.i.d.), and sitafloxacin (100 mg, b.i.d.) | 10 | 81.6 (31/38) | 94.7 (18/19) | 68.4 (13/19) |
| Saito et al. (2019)60 | Esomeprazole (20 mg, b.i.d.), amoxicillin (750 mg, b.i.d.), and sitafloxacin (100 mg, b.i.d.) | 7 | 54.2 (13/24) | 66.7 (12/18) | 20.0 (1/5) |
| Vonoprazan (20 mg, b.i.d.), amoxicillin (750 mg, b.i.d.), and sitafloxacin (100 mg, b.i.d.) | 7 | 93.0 (53/57) | 96.4 (27/28) | 91.7 (11/12) | |
| Sue et al. (2019)31 | Vonoprazan 20 mg b.i.d., amoxicillin 750 mg b.i.d., and sitafloxacin 100 mg b.i.d. | 7 | 75.8 (25/33) | - | - |
| Esomeprazole 20 mg b.i.d., rabeprazole 10 mg b.i.d., or lansoprazole 30 mg b.i.d.; amoxicillin 750 mg b.i.d.; and sitafloxacin 100 mg b.i.d. | 7 | 53.3 (16/30) | - | - | |
| Hirata et al. (2016)61 | Esomeprazole 20 mg b.i.d., amoxicillin 750 mg b.i.d., and sitafloxacin 100 mg b.i.d. | 7 | 83.3 (25/30) | - | - |
| Mori et al. (2016)62 | Esomeprazole (20 mg, b.i.d.), amoxicillin (500 mg, q.i.d.), and sitafloxacin (100 mg, b.i.d.) | 10 | 81.0 (51/63) | 100 (24/24) | 70.3 (26/37) |
| Esomeprazole (20 mg, b.i.d.), metronidazole (250 mg, b.i.d.), and sitafloxacin (100 mg, b.i.d.) | 10 | 72.4 (42/58) | 100 (16/16) | 66.7 (26/39) | |
| Sugimoto et al. (2015)63 | PPI, amoxicillin 750 mg b.i.d. and clarithromycin 200 or 400 mg b.i.d. | 7 | 88.3 (83/94) | - | - |
| Furuta et al. (2014)64 | Rabeprazole 10 mg b.i.d./q.i.d., amoxicillin 500 mg q.i.d., and sitafloxacin 100 mg b.i.d. | 7 | 84.1 (37/44) | - | - |
| Rabeprazole 10 mg b.i.d./q.i.d., amoxicillin 500 mg q.i.d., and sitafloxacin 100 mg b.i.d. | 14 | 88.9 (40/45) | - | - | |
| Rabeprazole 10 mg b.i.d./q.i.d., metronidazole 250 mg b.i.d., and sitafloxacin 100 mg b.i.d. | 7 | 90.9 (40/44) | - | - | |
| Rabeprazole 10 mg b.i.d./q.i.d., metronidazole 250 mg b.i.d., and sitafloxacin 100 mg b.i.d. | 14 | 87.2 (41/47) | - | - | |
| Murakami et al. (2013)53 | LPZ 30 mg b.i.d. + amoxicillin 750 mg b.i.d. + sitafloxacin 100 mg b.i.d. | 7 | 70.0 (49/70) | 72.0 (28/39) | 50.0 (1/2) |
| Matsuzaki et al. (2012)65 | Rabeprazole (10 mg, q.i.d.), amoxicillin (500 mg, q.i.d.), and sitafloxacin (100 mg, b.i.d.) | 7 | 78.2 (61/78) | 93.5 (29/31) | 68.1 (32/47) |
| Hirata et al. (2012)66 | Rabeprazole 10 mg b.i.d., amoxicillin 750 mg b.i.d., and sitafloxacin 100 mg b.i.d. | 7 | 75.0 (21/28) | 100 (1/1) | 66.7 (2/3) |
| Meta-analysis | 80.2 (74.6–84.9)* |
||||
b.i.d., twice a day; q.i.d., four times a day; PPI, proton pump inhibitor.
*95% confidence interval.
RIFABUTIN-BASED THERAPY IN THE FOURTH-LINE TREATMENT OF H. PYLORI INFECTION
There are limited data regarding the efficacy of rifabutin-based therapy in the fourth-line treatment. In a prospective noncontrolled trial, Gisbert et al.67 showed that rifabutin-based triple therapy containing rifabutin, amoxicillin and PPIs (standard dose twice daily) for 10 days was 50% (50/100). In another study, Mori et al.68 showed that the efficacy of rifabutin-based triple therapy containing esomeprazole (20 mg, four times a day), amoxicillin (500 mg four times a day), and rifabutin (300 mg, once daily) in the third-line or fourth-line treatment were 83.3% (10/12) for the 10-day group and 94.1% (16/17) for the 14-day group. Therefore, rifabutin-based therapy may be used as the fourth-line rescue treatment empirically for patients who have received clarithromycin-based therapy, levofloxacin-based therapy, and bismuth quadruple therapy in their prior treatments (Fig. 1).37,67,68
CONCLUSIONS
In conclusion, four-drug therapies containing higher dosage of PPIs for 14 days are recommended in the third-line treatment of refractory H. pylori infection. Susceptibility testing or genotypic resistance guided therapy is recommended whenever possible. However, properly designed empirical therapy, based on prior medication history (i.e., avoid reuse of clarithromycin or levofloxacin empirically), is an acceptable alternative to susceptibility testing-guided therapy after consideration of accessibility, cost, and preference of patient. Rifabutin-based therapy may be used as the fourth-line rescue therapy for those who have previously been treated with clarithromycin-based therapy, levofloxacin or sitafloxacin-based therapy, and bismuth quadruple therapy. Further large scale, randomized trials are warranted to identify the best strategy in the treatment of refractory H. pylori infection.
ACKNOWLEDGEMENTS
The authors received grants from the Ministry of Science and Technology (grant number: TCTC 109-2321-B-002-035 and MOST 108-2314-B-002-187, 109-2314-B-002-090-MY3, 109-2314-B-002-202), the Ministry of Health and Welfare (grant number: MOHW109-TDU-B-211-114002, MOHW109-CDC-C-114-122118), “Center of Precision Medicine” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) (grant number: NTU-109L901401), National Taiwan University Hospital (grant number: NTUH 109–P05), and the Liver Disease Prevention & Treatment Research Foundation, Taiwan. The funding source had no role in study design, data collection, analysis or interpretation, report writing or the decision to submit this paper for publication.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Liou JM, Lee YC, El-Omar EM, Wu MS. Efficacy and long-term safety of H. pylori eradication for gastric cancer prevention. Cancers (Basel) 2019;11:593. doi: 10.3390/cancers11050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. doi: 10.1136/gutjnl-2020-322368. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 7.Liou JM, Lin JT, Chang CY, et al. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59:572–578. doi: 10.1136/gut.2009.198309. [DOI] [PubMed] [Google Scholar]
- 8.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Liou JM, Bair MJ, Chen CC, et al. Levofloxacin sequential therapy vs levofloxacin triple therapy in the second-line treatment of Helicobacter pylori: a randomized trial. Am J Gastroenterol. 2016;111:381–387. doi: 10.1038/ajg.2015.439. [DOI] [PubMed] [Google Scholar]
- 10.Liou JM, Chen CC, Chen MJ, et al. Empirical modified sequential therapy containing levofloxacin and high-dose esomeprazole in second-line therapy for Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2011;66:1847–1852. doi: 10.1093/jac/dkr217. [DOI] [PubMed] [Google Scholar]
- 11.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–545. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liou JM, Chen CC, Chang CM, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis. 2019;19:1109–1120. doi: 10.1016/S1473-3099(19)30272-5. [DOI] [PubMed] [Google Scholar]
- 13.Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Liou JM, Chang CY, Chen MJ, et al. The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors: a nationwide study. PLoS One. 2015;10:e0124199. doi: 10.1371/journal.pone.0124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liou JM, Chang CY, Sheng WH, et al. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55:1123–1129. doi: 10.1128/AAC.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cammarota G, Sanguinetti M, Gallo A, Posteraro B. Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment Pharmacol Ther. 2012;36:222–230. doi: 10.1111/j.1365-2036.2012.05165.x. [DOI] [PubMed] [Google Scholar]
- 17.Fontes LES, Martimbianco ALC, Zanin C, Riera R. N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2019;2:CD012357. doi: 10.1002/14651858.CD012357.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter. 2016;21:85–90. doi: 10.1111/hel.12287. [DOI] [PubMed] [Google Scholar]
- 19.Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter. 2019;24:e12554. doi: 10.1111/hel.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205–213. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 21.Liou JM, Chen CC, Chang CY, et al. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut. 2016;65:1784–1792. doi: 10.1136/gutjnl-2015-310142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;(12):CD008337. doi: 10.1002/14651858.CD008337.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Liou JM, Fang YJ, Chen CC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2016;388:2355–2365. doi: 10.1016/S0140-6736(16)31409-X. [DOI] [PubMed] [Google Scholar]
- 24.McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414–425. doi: 10.1111/j.1365-2036.2012.05211.x. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou JM, Chen CC, Fang YJ, et al. 14 day sequential therapy versus 10 day bismuth quadruple therapy containing high-dose esomeprazole in the first-line and second-line treatment of Helicobacter pylori: a multicentre, non-inferiority, randomized trial. J Antimicrob Chemother. 2018;73:2510–2518. doi: 10.1093/jac/dky183. [DOI] [PubMed] [Google Scholar]
- 27.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/S0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 28.Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800–808. doi: 10.1016/j.cgh.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liou JM, Chen PY, Kuo YT, Wu MS Taiwan Gastrointestinal Disease and Helicobacter Consortium, author. Toward population specific and personalized treatment of Helicobacter pylori infection. J Biomed Sci. 2018;25:70. doi: 10.1186/s12929-018-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noh HM, Hong SJ, Han JP, et al. Eradication rate by duration of third-line rescue therapy with levofloxacin after Helicobacter pylori treatment failure in clinical practice. Korean J Gastroenterol. 2016;68:260–264. doi: 10.4166/kjg.2016.68.5.260. [DOI] [PubMed] [Google Scholar]
- 31.Sue S, Shibata W, Sasaki T, et al. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol. 2019;34:686–692. doi: 10.1111/jgh.14456. [DOI] [PubMed] [Google Scholar]
- 32.Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015;13:895–905. doi: 10.1016/j.cgh.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MJ, Chen CC, Chen YN, et al. Systematic review with meta-analysis: concomitant therapy vs. triple therapy for the first-line treatment of Helicobacter pylori infection. Am J Gastroenterol. 2018;113:1444–1457. doi: 10.1038/s41395-018-0217-2. [DOI] [PubMed] [Google Scholar]
- 34.Puig I, González-Santiago JM, Molina-Infante J, et al. Fourteen-day high-dose esomeprazole, amoxicillin and metronidazole as third-line treatment for Helicobacter pylori infection. Int J Clin Pract. 2017;71:e13004. doi: 10.1111/ijcp.13004. [DOI] [PubMed] [Google Scholar]
- 35.Chen PY, Wu MS, Chen CY, et al. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;44:427–437. doi: 10.1111/apt.13712. [DOI] [PubMed] [Google Scholar]
- 36.Hsu PI, Wu DC, Chen A, et al. Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur J Clin Invest. 2008;38:404–409. doi: 10.1111/j.1365-2362.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 37.Ciccaglione AF, Tavani R, Grossi L, Cellini L, Manzoli L, Marzio L. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: two pilot studies. Helicobacter. 2016;21:375–381. doi: 10.1111/hel.12296. [DOI] [PubMed] [Google Scholar]
- 38.Gasbarrini A, Ojetti V, Armuzzi A, et al. Efficacy of a multistep strategy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:79–83. doi: 10.1046/j.1365-2036.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 39.Vicente R, Sicilia B, Gallego S, Revillo MJ, Ducóns J, Gomollón F. Helicobacter pylori eradication in patients with peptic ulcer after two treatment failures: a prospective culture-guided study. Gastroenterol Hepatol. 2002;25:438–442. doi: 10.1016/S0210-5705(02)70283-5. [DOI] [PubMed] [Google Scholar]
- 40.Cammarota G, Martino A, Pirozzi G, et al. High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;19:789–795. doi: 10.1111/j.1365-2036.2004.01910.x. [DOI] [PubMed] [Google Scholar]
- 41.Yahav J, Samra Z, Niv Y, et al. Susceptibility-guided vs. empiric retreatment of Helicobacter pylori infection after treatment failure. Dig Dis Sci. 2006;51:2316–2321. doi: 10.1007/s10620-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 42.Tay CY, Windsor HM, Thirriot F, et al. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther. 2012;36:1076–1083. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
- 43.Fiorini G, Vakil N, Zullo A, et al. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2013;11:507–510. doi: 10.1016/j.cgh.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Liou JM, Chen CC, Chang CY, et al. Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2013;68:450–456. doi: 10.1093/jac/dks407. [DOI] [PubMed] [Google Scholar]
- 45.Costa S, Soares JB, Gonçalves R. Efficacy and tolerability of culture-guided treatment for Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2017;29:1258–1263. doi: 10.1097/MEG.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 46.Liou JM, Chen PY, Luo JC, et al. Efficacies of genotypic resistance-guided vs empirical therapy for refractory Helicobacter pylori infection. Gastroenterology. 2018;155:1109–1119. doi: 10.1053/j.gastro.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 47.Yu L, Luo L, Long X, et al. Susceptibility-guided therapy for Helicobacter pylori infection treatment failures. Therap Adv Gastroenterol. 2019;12:1756284819874922. doi: 10.1177/1756284819874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447–2455. doi: 10.1093/jac/dkv155. [DOI] [PubMed] [Google Scholar]
- 49.Chen Q, Long X, Ji Y, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther. 2019;49:1385–1394. doi: 10.1111/apt.15273. [DOI] [PubMed] [Google Scholar]
- 50.Ong S, Kim SE, Kim JH, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: a multicenter randomized controlled trial. Helicobacter. 2019;24:e12654. doi: 10.1111/hel.12654. [DOI] [PubMed] [Google Scholar]
- 51.Liou JM, Lee YC, Wu MS. Treatment of Helicobacter pylori infection and its long-term impacts on gut microbiota. J Gastroenterol Hepatol. 2020;35:1107–1116. doi: 10.1111/jgh.14992. [DOI] [PubMed] [Google Scholar]
- 52.Lee SK, Lee SW, Park JY, et al. Effectiveness and safety of repeated quadruple therapy in Helicobacter pylori infection after failure of second-line quadruple therapy: repeated quadruple therapy as a third-line therapy. Helicobacter. 2011;16:410–414. doi: 10.1111/j.1523-5378.2011.00870.x. [DOI] [PubMed] [Google Scholar]
- 53.Murakami K, Furuta T, Ando T, et al. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48:1128–1135. doi: 10.1007/s00535-012-0731-8. [DOI] [PubMed] [Google Scholar]
- 54.Gisbert JP, Perez-Aisa A, Rodrigo L, et al. Third-line rescue therapy with bismuth-containing quadruple regimen after failure of two treatments (with clarithromycin and levofloxacin) for H. pylori infection. Dig Dis Sci. 2014;59:383–389. doi: 10.1007/s10620-013-2900-x. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez de Santiago E, Martín de Argila de Prados C, Marcos Prieto HM, et al. Limited effectiveness with a 10-day bismuth-containing quadruple therapy (Pylera®) in third-line recue treatment for Helicobacter pylori infection. A real-life multicenter study. Helicobacter. 2017;22:e12423. doi: 10.1111/hel.12423. [DOI] [PubMed] [Google Scholar]
- 56.Lim JH, Kim SG, Song JH, et al. Efficacy of levofloxacin-based third-line therapy for the eradication of Helicobacter pylori in peptic ulcer disease. Gut Liver. 2017;11:226–231. doi: 10.5009/gnl16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okimoto K, Arai M, Saito K, et al. Efficacy of levofloxacin based triple and high-dose PPI-amoxicillin dual eradication therapy for Helicobacter pylori after failures of first- and second-line therapies. Int Sch Res Notices. 2014;2014:631501. doi: 10.1155/2014/631501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tursi A, Picchio M, Elisei W. Efficacy and tolerability of a third-line, levofloxacin-based, 10-day sequential therapy in curing resistant Helicobacter pylori infection. J Gastrointestin Liver Dis. 2012;21:133–138. [PubMed] [Google Scholar]
- 59.Mori H, Suzuki H, Matsuzaki J, Masaoka T, Kanai T. 10-Year trends in Helicobacter pylori eradication rates by sitafloxacin-based third-line rescue therapy. Digestion. 2020;101:644–650. doi: 10.1159/000501610. [DOI] [PubMed] [Google Scholar]
- 60.Saito Y, Konno K, Sato M, et al. Vonoprazan-based third-line therapy has a higher eradication rate against sitafloxacin-resistant Helicobacter pylori. Cancers (Basel) 2019;11:116. doi: 10.3390/cancers11010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirata Y, Serizawa T, Shichijo S, et al. Efficacy of triple therapy with esomeprazole, amoxicillin, and sitafloxacin as a third-line Helicobacter pylori eradication regimen. Int J Infect Dis. 2016;51:66–69. doi: 10.1016/j.ijid.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Mori H, Suzuki H, Matsuzaki J, et al. Efficacy of 10-day sitafloxacin-containing third-line rescue therapies for Helicobacter pylori strains containing the gyrA mutation. Helicobacter. 2016;21:286–294. doi: 10.1111/hel.12286. [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto M, Sahara S, Ichikawa H, Kagami T, Uotani T, Furuta T. High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment Pharmacol Ther. 2015;42:477–483. doi: 10.1111/apt.13280. [DOI] [PubMed] [Google Scholar]
- 64.Furuta T, Sugimoto M, Kodaira C, et al. Sitafloxacin-based third-line rescue regimens for Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2014;29:487–493. doi: 10.1111/jgh.12442. [DOI] [PubMed] [Google Scholar]
- 65.Matsuzaki J, Suzuki H, Nishizawa T, et al. Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob Agents Chemother. 2012;56:1643–1645. doi: 10.1128/AAC.05941-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirata Y, Ohmae T, Yanai A, et al. Sitafloxacin resistance in Helicobacter pylori isolates and sitafloxacin-based triple therapy as a third-line regimen in Japan. Int J Antimicrob Agents. 2012;39:352–355. doi: 10.1016/j.ijantimicag.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Gisbert JP, Castro-Fernandez M, Perez-Aisa A, et al. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941–947. doi: 10.1111/j.1365-2036.2012.05053.x. [DOI] [PubMed] [Google Scholar]
- 68.Mori H, Suzuki H, Matsuzaki J, et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: a pilot study. United European Gastroenterol J. 2016;4:380–387. doi: 10.1177/2050640615618043. [DOI] [PMC free article] [PubMed] [Google Scholar]