Abstract
Background/Aims
Although mucosal healing (MH) is acknowledged as the treatment target in the treat-to-target era, there are limitations on repeated endoscopic examinations, especially in pediatric patients. We aimed to investigate whether fecal calprotectin (FC) could serve as a surrogate marker for the assessment of MH in pediatric patients with Crohn’s disease (CD) who have achieved sustained clinical remission (CR) while treated with anti-tumor necrosis factor (TNF) agents.
Methods
This multicenter retrospective cross-sectional study included pediatric CD patients who had sustained a CR for at least 6 months with anti-TNF agents and who simultaneously underwent ileocolonoscopy and FC tests during follow-up. MH was defined as the absence of any ulcer on ileocolonoscopy.
Results
A total of 131 patients were included in this study. MH was observed in 87 patients (66.7%). The FC level was significantly lower in patients with MH than in those without MH (median 49.0 mg/kg vs 599.0 mg/kg; p<0.001). According to the multivariate logistic regression analysis, FC was the only factor associated with MH (odds ratio, 0.62; 95% confidence interval [CI], 0.52 to 0.73; p<0.001). According to the receiver operating characteristic curve analysis, the optimal cutoff value for FC for the association with MH was <140 mg/kg (area under the curve 0.890, 95% CI 0.829 to 0.951, sensitivity 78.2%, specificity 88.6%, p<0.001).
Conclusions
FC was associated with MH in pediatric patients with CD who had achieved a sustained CR for at least 6 months with anti-TNF agents. In these patients, FC can be used to stratify patients and guide decisions regarding ileocolonoscopy in the treat-to-target era.
Keywords: Crohn disease, Infliximab, Adalimumab, Child, Adolescent
INTRODUCTION
Crohn’s disease (CD) is a chronic, disabling inflammatory disease that affects the gastrointestinal tract.1 Untreated CD leads to critical complications including fibrostenosis and penetration of the bowel, which require surgery.2,3 Pediatric-onset CD presents with a more severe phenotype and is more aggressive than adult-onset disease, requiring earlier and more intensive treatment with biologics.4-6 Meanwhile, mucosal healing (MH), which is evaluated by ileocolonoscopy, is the acknowledged therapeutic goal of CD in the treat-to-target era, which is recommended 6 to 9 months after treatment initiation.7,8 However, its feasibility in real-life practice is limited, especially in children and adolescents.
Fecal calprotectin (FC) is a sensitive surrogate marker in detecting endoscopic activity in CD. Studies have shown that FC level cutoffs of 70–250 mg/kg in adults are capable of detecting endoscopic remission with a sensitivity of 70% to 94% and a specificity of 62% to 92%.9-11 There are limited studies in the pediatric population. However, a study has shown that an FC level of <200 mg/kg is associated with endoscopic remission and that the positive likelihood ratio increases when the cutoff is increased to 500 mg/kg.12 Another study reported that the most accurate cutoff of FC that is associated with MH was <300 mg/kg, and for a composite of MH and transmural healing, a cutoff of <100 mg/kg is most accurate.13
Anti-tumor necrosis factor (TNF) agents are potent drugs that are capable of inducing and maintaining not only clinical remission (CR) but also MH in CD.14 Therefore, in real-life clinical practice, some patients not only respond well to anti-TNF treatment but also sustain CR.5 When faced with this clinical scenario of pediatric patients with CD in well-sustained CR with anti-TNF agents, pediatric gastroenterologists question the necessity of frequent and repetitive endoscopic follow-up evaluation considering the limitation of ileocolonoscopic examination in children and the likeliness of a substantial portion of these patients will have MH. However, there is a lack of evidence whether noninvasive markers such as FC are capable of distinguishing those with and without MH in pediatric patients with CD in sustained CR with anti-TNF agents.
Therefore, we aimed to investigate whether FC could serve as a surrogate marker for assessing MH in pediatric patients with CD in sustained CR for at least 6 months while on treatment with anti-TNF agents, and to seek the FC cutoff levels associated with MH in this specific group of patients.
MATERIALS AND METHODS
1. Study design and participants
This study was approved by the institutional review board of all participating centers (Kyungpook National University Chilgok Hospital IRB number: 2017-10-002) and was conducted in accordance with the Declaration of Helsinki. Informed consent was waived owing to the retrospective nature of this study.
This study was conducted between May 2016 and April 2017 at the department of pediatrics of five tertiary medical centers in the Republic of Korea; Kyungpook National University Children’s Hospital, Yonsei University Children’s Hospital, Keimyung University Dongsan Medical Center, Soonchunhyang University Hospital Bucheon, and Samsung Medical Center.
The study included pediatric patients with CD who had been diagnosed before the age of 19 years and had sustained CR for at least 6 months with an anti-TNF agent, and who had simultaneously undergone ileocolonoscopy and FC test, as well as other laboratory tests. CD was diagnosed in accordance with the ESPGHAN-Porto criteria.15 The disease classification and behavior were based on the Paris classification.16 CR was defined as a pediatric Crohn’s disease activity index (PCDAI) score of <10.17 Patients with a history of bowel resection distal to the terminal ileum (TI) at follow-up ileocolonoscopy, with failure of intubation up to the TI, and who did not undergo FC level test the day before ileocolonoscopy were excluded.
Baseline demographic and clinical data, including sex, age, disease phenotype, and growth indicators, were obtained from electronic medical records. Data corresponding to the point of ileocolonoscopy examination were also collected from electronic charts or electronic test results, including age at follow-up, disease duration, anti-TNF type, anti-TNF duration, previous bowel resection history, PCDAI score, white blood cell count, hematocrit, platelet count, serum albumin level, erythrocyte sedimentation rate, C-reactive protein (CRP), FC levels, and simple endoscopic score for CD18 at follow-up. Blood tests were conducted either the day before or on the same day as ileocolonoscopy, and FC level was tested on the day before ileocolonoscopy. FC levels were measured using fluorometric enzyme immunoassay at Soonchunhyang University Hospital Bucheon and Kyungpook National University Children’s Hospital. FC levels were measured using enzyme-linked immunosorbent assay in the other three centers. The associations between FC and MH were investigated. MH was defined as the absence of ulcer on ileocolonoscopy.
2. Statistical methods
For statistical comparison between groups, the Student t-test or Wilcoxon rank-sum test was used for continuous variables and a chi-square test or Fisher exact test was used for categorical variables. Comparative data for continuous variables are reported as mean (standard deviation) or median (interquartile range). The Pearson correlation was used to investigate the correlation between continuous variables. Univariate and multivariate logistic regression analyses were performed to examine the association between MH and the variables. A univariate logistic regression analysis was first conducted to investigate the crude odds ratio for each factor. Thereafter, a multivariate logistic regression analysis was conducted using a stepwise selection procedure with a p<0.1 significance level for a covariate to enter or remain in the model. The results were expressed as adjusted odds ratios with 95% confidence intervals. Receiver operating characteristic curve analysis was performed to derive the best cutoff point for continuous variables that were identified as statistically significant in the multivariate logistic regression analysis. De Long test was used to compare the area under the receiver operating characteristic curve (AUC) for FC and CRP in detecting MH and the presence of ulcers. Data were considered statistically significantly different if p-value was <0.05. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
1. Patient inclusion and baseline characteristics at diagnosis
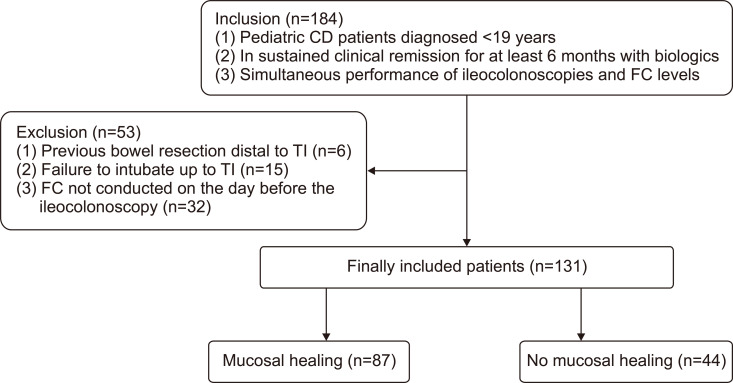
A total of 184 patients were included in this study. Among these patients, a history of bowel resection distal to the TI at follow-up ileocolonoscopy was noted in six patients, failure of intubation up to the TI was observed in 15 patients, and FC level was not tested the day before the ileocolonoscopy in 32 patients. These 53 patients were excluded, leaving 131 patients for final inclusion in the study. Among these 131 patients, MH was observed in 87 patients (66.7%) (Fig. 1). The comparison of baseline characteristics between the groups divided according to MH status is summarized in Table 1.
Fig. 1.
Patient inclusion and exclusion.
CD, Crohn’s disease; FC, fecal calprotectin; TI, terminal ileum.
Table 1.
Comparison of Baseline Variables between Patients with and without Mucosal Healing
| Baseline characteristics at diagnosis | MH (n=87) | No MH (n=44) | p-value |
|---|---|---|---|
| Male sex | 58 (66.7) | 30 (68.2) | 1.000 |
| Age, median (IQR), yr | 14.0 (12.4–16.1) | 13.7 (11.7–15.3) | 0.232 |
| Age according to Paris classification | 0.809 | ||
| A1a | 7 (8.0) | 5 (11.4) | |
| A1b | 68 (78.2) | 33 (75.0) | |
| A2 | 12 (13.8) | 6 (13.6) | |
| Lower GI tract involvement | 0.534 | ||
| L1 | 12 (13.8) | 3 (6.8) | |
| L2 | 4 (4.6) | 2 (4.5) | |
| L3 | 71 (81.6) | 39 (88.7) | |
| None (isolated L4) | 0 | 0 | |
| Upper GI tract involvement | 0.732 | ||
| None | 31 (35.6) | 16 (36.4) | |
| L4a | 19 (21.8) | 12 (27.3) | |
| L4a+b | 14 (16.1) | 8 (18.2) | |
| L4b | 23 (26.4) | 8 (18.2) | |
| Luminal disease behavior | 0.452 | ||
| B1 | 75 (86.2) | 35 (79.6) | |
| B2 | 8 (9.2) | 7 (15.9) | |
| B3 | 4 (4.6) | 2 (4.5) | |
| Perianal fistulizing disease | 38 (43.7) | 28 (63.6) | 0.049 |
| Linear growth failure | 21 (24.1) | 11 (25.0) | 0.667 |
| Baseline laboratory results at IFX start | |||
| WBC, median (IQR),/μL | 8,460 (6,355–10,615) | 9,145 (6,945–10,810) | 0.387 |
| Hematocrit, mean±SD, % | 35.8±5.4 | 35.7±6.1 | 0.905 |
| Platelet count, median (IQR), ×103/µL | 398 (341–515) | 429 (336–538) | 0.697 |
| Albumin, median (IQR), g/dL | 3.9 (3.5–4.0) | 3.8 (3.4–4.0) | 0.347 |
| ESR, median (IQR), mm/hr | 38 (20–62) | 38 (25–84) | 0.336 |
| CRP, median (IQR), mg/dL | 1.2 (0.3–4.9) | 1.8 (0.6–4.6) | 0.517 |
| FC, median (IQR), mg/kg (n=16) | 1,749.1 (917.1–3,234.2) | 2,408.2 (789.5–4,469.3) | 0.828 |
Data are presented as number (%) unless otherwise indicated.
MH, mucosal healing; IQR, interquartile range; A1a, 0–9 years; A1b, 10–16 years; A2, 17–18 years; GI, gastrointestinal; L1, distal 1/3 ileum±limited cecal disease; L2, colonic disease; L3, ileocolonic disease; L4a, upper disease proximal to ligament of Treitz; L4b, upper disease distal to the ligament of Treitz and proximal to the distal 1/3 ileum; L4a+b, upper disease involving both L4a and L4b; B1, nonstricturing, nonpenetrating; B2, stricturing; B3, penetrating; IFX, infliximab; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FC, fecal calprotectin.
2. Association between FC level and MH
At follow-up ileocolonoscopic examinations, no significant differences were observed between the two groups except PCDAI scores and FC levels (Table 2). FC level was significantly lower in patients with MH than in those without (median 49.0 mg/kg vs 599.0 mg/kg; p<0.001). According to the Pearson correlation, a significant correlation was observed between FC level and simple endoscopic score for CD (ρ=0.68, p<0.001).
Table 2.
Comparison of Variables at Follow-up Ileocolonoscopy between Patients with and without Mucosal Healing
| Variable | MH (n=87) | No MH (n=44) | p-value |
|---|---|---|---|
| Age, mean±SD, yr | 17.4±3.4 | 17.7±3.6 | 0.659 |
| Disease duration, median (IQR), yr | 2.5 (1.2–5.0) | 3.9 (1.6–6.9) | 0.109 |
| Duration from diagnosis to first anti-TNF agent, median (IQR), yr | 0.27 (0.04–1.59) | 0.46 (0.06–1.29) | 0.704 |
| Current anti-TNF agent | 0.915 | ||
| Adalimumab | 24 (27.6) | 11 (25.0) | |
| Infliximab | 63 (72.4) | 33 (75.0) | |
| Treatment duration of current anti-TNF agent, median (IQR), yr | 1.3 (1.0–1.2) | 1.5 (0.5–2.8) | 0.821 |
| Previous anti-TNF agent usage | 6 (6.9) | 6 (13.6) | 0.217 |
| PCDAI, median (IQR) | 0 (0–2.5) | 2.5 (0–5) | 0.002 |
| WBC, mean±SD,/µL | 6,501±1,362 | 6,561±1,564 | 0.820 |
| Hematocrit, mean±SD, % | 41.7±4.4 | 41.8±4.2 | 0.917 |
| Platelet count, median (IQR), ×103/µL | 257 (226–306) | 274 (230–322) | 0.351 |
| Albumin, median (IQR), g/dL | 4.5 (4.4–4.7) | 4.4 (4.3–4.7) | 0.123 |
| ESR, median (IQR), mm/hr | 8 (4–17) | 12 (7–22) | 0.051 |
| ESR <20 mm/hr | 71 (81.6) | 32 (72.7) | 0.344 |
| CRP, median (IQR), mg/dL | 0.03 (0.03–0.08) | 0.04 (0.03–0.20) | 0.223 |
| CRP <0.3 mg/dL | 83 (95.4) | 37 (84.1) | 0.043 |
| FC, median (IQR), mg/kg | 49.0 (25.2–107.2) | 599.0 (273.9–1,000.0) | <0.001 |
| FC <50 mg/kg | 48 (55.2) | 2 (4.5) | <0.001 |
| FC <200 mg/kg | 71 (81.6) | 7 (15.9) | <0.001 |
| FC <600 mg/kg | 84 (96.6) | 22 (50.0) | <0.001 |
| SES-CD, median (IQR) | 0 (0–0) | 6 (3–8) | <0.001 |
Data are presented as number (%) unless otherwise indicated.
MH, mucosal healing; IQR, interquartile range; TNF, tumor necrosis factor; PCDAI, pediatric Crohn’s disease activity index; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FC, fecal calprotectin; SES-CD, simple endoscopic score for Crohn’s disease.
When the patients were divided according to lower gastrointestinal location, there was no significant difference in MH rates between groups (L1, 12/15 [80%]; L2, 4/6 [66.7%]; L3, 71/110 [64.5%]; p=0.534). FC levels were also comparable between groups divided according to lower gastrointestinal location (L1, 55.7 [38.0–458.5] mg/kg; L2, 65.2 [23.6–577.0] mg/kg; L3, 99.0 [37.1–416.3] mg/kg; p=0.761). Additionally, among patients with MH (n=87), there was no significant difference in FC levels between these groups (L1 [n=12], 49.0 [26.6–97.6] mg/kg; L2 [n=4], 27.5 [15.9–65.2] mg/kg; L3 [n=71], 49.2 [25.1–118.9] mg/kg; p=0.427).
3. Factors associated with MH
According to the univariate analysis, concomitant perianal fistulizing disease, PCDAI, erythrocyte sedimentation rate, CRP, and FC×102 levels were significantly associated with MH (Table 3). When factors with p-values of <0.1 were included in the multivariate analysis using a stepwise selection procedure, only FC×102 level was associated with MH (odds ratio, 0.62; 95% confidence interval, 0.52 to 0.73; p<0.001) (Table 3).
Table 3.
Factors Associated with Mucosal Healing
| Factor | Univariate logistic regression | Multivariate logistic regression with stepwise selection | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value | ||
| Sex (male vs female) | 0.93 | 0.43–2.03 | 0.862 | ||||
| Age at diagnosis, yr | 1.08 | 0.95–1.22 | 0.222 | ||||
| Any TI involvement (yes vs no) | 0.99 | 0.17–5.62 | 0.989 | ||||
| Any colonic involvement (yes vs no) | 0.46 | 0.12–1.71 | 0.246 | ||||
| Upper GI tract involvement (yes vs no) | 1.03 | 0.49–2.20 | 0.934 | ||||
| B1 disease behavior (yes vs no) | 1.61 | 0.62–4.17 | 0.329 | ||||
| Concomitant perianal fistulizing disease (yes vs no) | 0.44 | 0.21–0.93 | 0.033 | 0.43 | 0.16–1.18 | 0.100 | |
| Disease duration, yr | 0.87 | 0.76–1.00 | 0.056 | ||||
| Duration from diagnosis to first anti-TNF agent, yr | 0.93 | 0.75–1.14 | 0.487 | ||||
| Current anti-TNF agent (IFX vs ADL) | 0.88 | 0.38–2.00 | 0.752 | ||||
| Treatment duration of current anti-TNF agent, yr | 0.84 | 0.66–1.07 | 0.158 | ||||
| Previous anti-TNF agent usage (yes vs no) | 0.47 | 0.14–1.55 | 0.215 | ||||
| PCDAI | 0.81 | 0.70–0.93 | 0.003 | 0.84 | 0.70–1.00 | 0.051 | |
| Albumin, g/dL | 3.16 | 0.80–12.44 | 0.099 | ||||
| ESR, mm/hr | 0.97 | 0.94–1.00 | 0.042 | ||||
| CRP, mg/dL | 0.13 | 0.02–0.96 | 0.046 | ||||
| FC, ×102 mg/kg | 0.61 | 0.51–0.73 | <0.001 | 0.62 | 0.52–0.73 | <0.001 | |
OR, odds ratio; CI, confidence interval; TI, terminal ileum; GI, gastrointestinal; B1, nonstricturing nonpenetrating; TNF, tumor necrosis factor; IFX, infliximab; ADL, adalimumab; PCDAI, pediatric Crohn’s disease activity index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FC, fecal calprotectin.
4. FC cutoff levels for MH association
According to the receiver operating characteristic curve analysis, the optimal FC cutoff level associated with MH was 140 mg/kg with an AUC of 0.890 (95% confidence interval, 0.829 to 0.951; sensitivity 78.2%, specificity 88.6%, PPV 93.2%, NPV 67.2%, p<0.001). The cutoff levels of FC associated with MH are shown in Table 4. According to De Long test, the AUC of FC for detecting MH was significantly higher than that of CRP for detecting MH (Z=5.82, p<0.001) (Fig. 2).
Table 4.
Cutoff Levels of FC for the Assessment of Mucosal Healing
| FC cutoff, mg/kg |
Sensitivity, % |
Specificity, % |
PPV, % |
NPV, % |
|---|---|---|---|---|
| <50 | 95.5 | 55.2 | 51.9 | 96.0 |
| <100 | 90.9 | 74.7 | 64.5 | 94.2 |
| <140 | 88.6 | 78.2 | 67.2 | 93.2 |
| <200 | 84.1 | 81.6 | 69.8 | 91.0 |
| <300 | 70.5 | 85.1 | 70.5 | 85.1 |
| <400 | 61.4 | 92.0 | 79.4 | 82.5 |
| <500 | 56.8 | 95.4 | 86.2 | 81.4 |
| <600 | 50.0 | 96.6 | 88.0 | 79.3 |
FC, fecal calprotectin; PPV, positive predictive value; NPV, negative predictive value.
Fig. 2.
ROC curves for FC and CRP for the differentiation of patients with and without mucosal healing.
ROC, receiver operating characteristic; FC, fecal calprotectin; CRP, C-reactive protein; AUC, area under the curve; CI, confidence interval.
DISCUSSION
In this study, we investigated whether FC could serve as a surrogate marker for assessing MH among pediatric patients with CD in sustained CR for at least 6 months with anti-TNF agents. FC level was significantly associated with MH, and an FC cutoff of <140 mg/kg was associated with MH with a sensitivity of 78.2% and specificity of 88.6%.
Treat-to-target has recently emerged as a strategy to better treat patients with CD and to minimize future complications. The main principles of the treat-to-target strategy are based on risk stratification and timely appropriate treatment, regular monitoring of disease activity, and subsequent adjustment of treatment when inflammation persists.7 According to the Selecting Therapeutic Targets in Inflammatory Bowel Disease program, the acknowledged target for treat-to-target in CD is clinical/patient-reported outcome remission, and endoscopic remission, which is recommended 6 to 9 months from treatment initiation.8 For those who cannot be adequately assessed with ileocolonoscopy, cross-sectional imaging studies are recommended as an alternative exam.8 Meanwhile, biomarker remission, defined as a normal CRP and FC level was considered an adjunctive target.8 Despite these recommendations of Selecting Therapeutic Targets in Inflammatory Bowel Disease program, limitations regarding cost, availability, invasiveness, and complexity exists with ileocolonoscopy and cross-sectional imaging, questioning whether they are feasible as modalities for repetitive monitoring.19
Considering the limitations in repetitive performances of ileocolonoscopy in children, pediatric gastroenterologists should have a clear treatment plan before they conduct a follow-up ileocolonoscopy. In pediatric patients with CD who are in CR but not in MH during treatment with conventional drugs, stepping up treatment to an anti-TNF agent would be a reasonable choice. However, if patients are already receiving treatment with an intensified dose of an anti-TNF agent and are in CR but not MH, it is questionable whether these patients should change their drugs. Of course, there should be a role of therapeutic drug monitoring in these situations.20-22 However, it is still questionable whether their anti-TNF drug should be changed when they are in CR and have only mild disease on ileocolonoscopy, especially in children with CD who only have two licensed drugs to use, namely infliximab and adalimumab.
Therefore, instead of conducting mere repetitive endoscopic exams in these patients, knowing which patients will likely be in MH or not is important for planning future treatment. In patients who are likely in MH based on FC levels, postponing a regular ileocolonoscopy to a near future and thus lengthening the intervals between regular exams may be better to avoid frequent repetitive exams. Meanwhile, in patients who are not likely in MH based on FC levels, the initially scheduled endoscopic evaluation would be necessary. Additionally, because FC can vary according to environmental factors such as diet,23 consecutive elevated FC levels may be better than a single elevated FC level for deciding whether to postpone an ileocolonoscopy or not.24-26 However, this approach does not imply that FC can substitute ileocolonoscopy. Because of the limitations regarding the relatively low sensitivity of FC in detecting MH and uncertainty in optimal cutoffs, ileocolonoscopy still should be the mainstay to evaluate MH and disease activity in CD in the treat-to-target era.8
While high FC levels suggest mucosal inflammation and a normal FC is associated with endoscopic, and potentially radiographic and histologic remission, uncertainty remains regarding optimal test cut-points.19 Studies have suggested different cutoff values of FC for discriminating between active and inactive CD. D’Haens et al.9 demonstrated in a cohort of 87 adult patients with CD that an FC cutoff of <250 μg/g was capable of detecting endoscopic remission with 94% sensitivity and 62.2% specificity. Sipponen et al.10 reported in a study of 77 adult patients with CD that an FC cutoff of <200 mg/kg was capable of detecting endoscopically inactive CD with 70% sensitivity and 92% specificity. In a study in children, Zubin and Peter12 investigated the correlation between endoscopic disease activity and FC level by examining 32 newly diagnosed CD patients with a median age of 13.5 years, who were offered exclusive enteral nutrition or steroid as induction therapy and were initiated on early thiopurines. They suggested that the adult FC cutoff value of <200 mg/kg was not appropriate in the pediatric population for detecting endoscopic disease activity and that an FC cutoff of <500 mg/kg provided a greater sensitivity of 64% and specificity of 80% and was more appropriate for children.12 According to another prospective study in 58 pediatric patients aged 11.8 to 14.9 years, the optimal cutoff of FC to discriminate between remission and active disease was 143 mg/kg, which had a sensitivity of 94% and a specificity of 64%.27
In this study, an FC cutoff level of <140 mg/kg was capable of detecting MH with a sensitivity of 78.2% and specificity of 88.6%, which revealed a higher specificity at lower FC cutoff levels than the previous studies in children, but rather similar to the aforementioned study in adults by Sipponen et al. The difference with previous pediatric studies may be due to the difference in the population included in this study. First, the median age of patients at follow-up ileocolonoscopy was 17.5 years, which is older than that of previous studies conducted in children of which the mean age was 13.9 to 14.2 years.13,27 Second, patients in sustained CR with anti-TNF agents for at least 6 months were included in this study. Therefore, the proportion of patients in MH in this study (66.4%) was higher than that of a previous study in children of which MH rates were only 20%.13 Another possible explanation is that among patients with MH, the majority may have achieved further histologic remission. Moreover, considering that all patients in this study were receiving treatment with anti-TNF agents, the proportion of patients who had achieved a more stringent histologic remission in addition to endoscopic remission may have been higher in this study. Studies in patients with ulcerative colitis have revealed a stronger correlation between FC level and histologic remission than between FC level and endoscopic remission.28,29 This may have resulted in the derivation of a lower cutoff value for FC in discriminating between endoscopically active and inactive CD.
Meanwhile, the superiority of FC over CRP in detecting endoscopic activity has been reported in several studies.11,30,31 Although CRP and FC are one of the most used biomarkers for assessing disease activity in CD, the limitation of CRP as a surrogate marker for endoscopic activity is its poor sensitivity.31 Studies in pediatric patients with CD have shown that adding CRP to FC is better than using FC alone in detecting endoscopic remission.12,32 However, the AUC curve of CRP was consistently below that of FC in this study, indicating that there was no additional benefit of adding CRP to FC for discriminating those with and without MH on endoscopy.
This study has some limitations. First, this was a retrospective, cross-sectional study with certain limitations compared to studies with a prospective design. Moreover, because FC tests have been available in Korea since 2016, the majority of the patients lacked FC levels at diagnosis and at the initiation of infliximab treatment. Therefore, we were unable to confirm whether the change in FC level could reflect the change in endoscopic activity, and also the impact of baseline FC on achieving MH during treatment. Second, selection bias may have been introduced by excluding patients in whom intubation up to the TI was impossible. Additionally, despite the low proportion of patients whose disease involvement was only proximal to the ileocecal valve (11.5%), FC levels in these patients may have not well reflected the degree of inflammation in the small bowel. There are speculations that the diagnostic accuracy of FC in CD may be influenced by different disease location.33 Several studies have shown that FC levels were lower in patients with small bowel disease compared to those with large bowel involvement.10,34 However, we were unable to observe such findings probably due to the small number of patients with L1 and L2 disease. Third, differences in FC measurement tests among centers may have affected the results of this study. However, according to a study, the accuracy of enzyme-linked immunosorbent assay and fluorometric enzyme immunoassay in measuring FC level was comparable.35 Fourth, we were unable to investigate the correlation between FC level and histologic remission. Biopsies were not conducted in some patients with MH. Moreover, as CD involves the bowel discontinuously, there are also limitations in obtaining tissue biopsies from all previously involved sites. Nonetheless, Zittan et al.29 suggested that FC is a noninvasive biomarker for both endoscopic healing and histological remission in adult patients with colonic CD. According to this study, FC levels of <100 mg/kg had the strongest negative predictive value and the highest sensitivity for histological remission compared with endoscopic remission and CR. Further prospective studies are required to determine whether FC level correlates with histologic inflammation in CD.
In conclusion, FC may serve as a good surrogate marker for detecting MH in pediatric patients with CD in sustained CR for at least 6 months with anti-TNF agents. In these patients, FC may guide in stratifying those who require their scheduled ileocolonoscopy from those who may postpone their regular ileocolonoscopy to a near future.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2017R1C1B5076980), and the Soonchunhyang University Research Fund (No. 20200033).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: B.K. Data curation: Y.M.L., S.C., H.J.J., S.K., E.S.K., M.J.K. Formal analysis: B.H.C., H.K., Y.H.C., B.K. Funding acquisition: B.K. Methodology: Y.H.C., B.K. Project administration: B.K. Visualization: Y.M.L., S.C., E.S.K., B.K. Writing - original draft: Y.M.L., S.C., H.J.J., S.K., E.S.K., B.K. Writing - review & editing: M.J.K., B.H.C., H.K., Y.H.C, B.K. Approval of final manuscript: all authors.
REFERENCES
- 1.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S. Surgery in pediatric Crohn's disease: indications, timing and post-operative management. Pediatr Gastroenterol Hepatol Nutr. 2017;20:14–21. doi: 10.5223/pghn.2017.20.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang B, Choe YH. Early biologic treatment in pediatric Crohn's disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr. 2018;21:1–11. doi: 10.5223/pghn.2018.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal Crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis. 2016;10:1279–1286. doi: 10.1093/ecco-jcc/jjw086. [DOI] [PubMed] [Google Scholar]
- 6.Kang B, Kim JE, Jung JH, et al. Korean children and adolescents with Crohn's disease are more likely to present with perianal fistulizing disease at diagnosis compared to their European counterparts. Pediatr Gastroenterol Hepatol Nutr. 2020;23:49–62. doi: 10.5223/pghn.2020.23.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:1042–1050. doi: 10.1016/j.cgh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 9.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 10.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 11.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the simple endoscopic score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 12.Zubin G, Peter L. Predicting endoscopic Crohn's disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis. 2015;21:1386–1391. doi: 10.1097/MIB.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn's disease. Clin Gastroenterol Hepatol. 2018;16:1089–1097. doi: 10.1016/j.cgh.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. Intest Res. 2019;17:285–310. doi: 10.5217/ir.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 16.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 17.Turner D, Griffiths AM, Walters TD, et al. Appraisal of the pediatric Crohn's disease activity index on four prospectively collected datasets: recommended cutoff values and clinimetric properties. Am J Gastroenterol. 2010;105:2085–2092. doi: 10.1038/ajg.2010.143. [DOI] [PubMed] [Google Scholar]
- 18.Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/S0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 19.Ma C, Battat R, Parker CE, Khanna R, Jairath V, Feagan BG. Update on C-reactive protein and fecal calprotectin: are they accurate measures of disease activity in Crohn's disease? Expert Rev Gastroenterol Hepatol. 2019;13:319–330. doi: 10.1080/17474124.2019.1563481. [DOI] [PubMed] [Google Scholar]
- 20.Kang B, Choi SY, Choi YO, et al. Infliximab trough levels are associated with mucosal healing during maintenance treatment with infliximab in paediatric Crohn's disease. J Crohns Colitis. 2019;13:189–197. doi: 10.1093/ecco-jcc/jjy155. [DOI] [PubMed] [Google Scholar]
- 21.Kang B, Choi SY, Choi YO, et al. Subtherapeutic infliximab trough levels and complete mucosal healing are associated with sustained clinical remission after infliximab cessation in paediatric-onset Crohn's disease patients treated with combined immunosuppressive therapy. J Crohns Colitis. 2018;12:644–652. doi: 10.1093/ecco-jcc/jjy021. [DOI] [PubMed] [Google Scholar]
- 22.Ma C, Battat R, Jairath V, Vande Casteele N. Advances in therapeutic drug monitoring for small-molecule and biologic therapies in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2019;17:127–145. doi: 10.1007/s11938-019-00222-9. [DOI] [PubMed] [Google Scholar]
- 23.Mendall MA, Chan D, Patel R, Kumar D. Faecal calprotectin: factors affecting levels and its potential role as a surrogate marker for risk of development of Crohn's Disease. BMC Gastroenterol. 2016;16:126. doi: 10.1186/s12876-016-0535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2016;22:1042–1048. doi: 10.1097/MIB.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 25.Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23:894–902. doi: 10.1097/MIB.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, Dominguez-Muñoz JE. Accuracy of consecutive fecal calprotectin measurements to predict relapse in inflammatory bowel disease patients under maintenance with anti-TNF therapy: a prospective longitudinal cohort study. J Clin Gastroenterol. 2018;52:229–234. doi: 10.1097/MCG.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 27.Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547–553. doi: 10.1016/j.dld.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflamm Bowel Dis. 2017;23:1600–1604. doi: 10.1097/MIB.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 29.Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm Bowel Dis. 2016;22:623–630. doi: 10.1097/MIB.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 30.Kostas A, Siakavellas SI, Kosmidis C, et al. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J Gastroenterol. 2017;23:7387–7396. doi: 10.3748/wjg.v23.i41.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 32.Cozijnsen MA, Ben Shoham A, Kang B, et al. Development and validation of the mucosal inflammation noninvasive index for pediatric Crohn's disease. Clin Gastroenterol Hepatol. 2020;18:133–140. doi: 10.1016/j.cgh.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Simon EG, Wardle R, Thi AA, Eldridge J, Samuel S, Moran GW. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn's disease? A systematic review. Intest Res. 2019;17:160–170. doi: 10.5217/ir.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn's disease. Scand J Gastroenterol. 2015;50:841–847. doi: 10.3109/00365521.2015.1008035. [DOI] [PubMed] [Google Scholar]
- 35.Prell C, Nagel D, Freudenberg F, Schwarzer A, Koletzko S. Comparison of three tests for faecal calprotectin in children and young adults: a retrospective monocentric study. BMJ Open. 2014;4:e004558. doi: 10.1136/bmjopen-2013-004558. [DOI] [PMC free article] [PubMed] [Google Scholar]