Abstract
Antigens in a 4-week-old culture filtrate (CF) of Mycobacterium avium subsp. avium were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and identified by Western blotting. The culture had minimal lysis of bacilli, giving a CF preparation consisting mainly of secreted proteins. Comparison with a similar CF of Mycobacterium tuberculosis with almost no contamination with intracellular proteins showed the presence of cross-reactive antigens homologous to the four components of the antigen 85 complex, as well as MPT32. These were major constituents of the M. avium subsp. avium CF. In addition, there were several low-molecular-mass bands (<15 kDa) in both species that did not cross-react with polyclonal and polyvalent rabbit antibodies in Western blotting. Furthermore, these bands were not detected in corresponding sonicate preparations, indicating high localization indexes, which is typical of soluble secreted proteins. A 14-kDa protein was selected for purification and more detailed characterization. The N-terminal amino acid sequence was determined, and a matching gene was found within the genomic sequence of M. avium subsp. avium which was highly homologous to Rv0455c of M. tuberculosis. The gene encoded a signal peptide typical of secreted mycobacterial proteins. A rabbit antiserum was raised against the purified protein, and the antigen was demonstrated by Western blotting in CFs of M. avium subsp. avium, Mycobacterium avium subsp. paratuberculosis, Mycobacterium intracellulare, and Mycobacterium scrofulaceum but was not detected in M. tuberculosis. This is a new example of a highly homologous gene being differentially expressed by different mycobacterial species.
The Mycobacterium avium complex consists of the subspecies Mycobacterium avium subsp. avium, Mycobacterium avium subsp. paratuberculosis, and Mycobacterium avium subsp. silvaticum (29). M. avium subsp. avium causes disseminated disease in birds and also granulomatous lesions in various animals. The bacterium can also cause severe infections in immunocompromised humans, and the human immunodeficiency virus epidemic has led to an increasing interest in this bacterium that contributes considerably to mortality in human immunodeficiency virus-infected patients (4). M. avium subsp. paratuberculosis is a pathogenic bacterium which causes a chronic granulomatous infection of the intestines of ruminants. Only 10 to 15% of infected animals develop overt paratuberculosis, or Johne's disease, but subclinically infected animals result in decreased production, with financial consequences for farmers. M. avium subsp. paratuberculosis has also been suggested as the etiologic agent of Crohn's disease in humans, which has similarities to paratuberculosis in ruminants (5, 18). Yet another subspecies of the M. avium complex, M. avium subsp. silvaticum, can cause enteritis in ruminants, as well as disseminated infections in other hosts.
Protection against mycobacterial infections is dependent on cellular immune responses, and early diagnosis of tuberculosis in animals is currently based on the skin test measuring delayed-type hypersensitivity reactions or in vitro tests measuring gamma interferon production (37). Tests measuring humoral immune responses have little value, especially in subclinically infected animals. A number of studies have thus focused on identifying the antigens responsible for the cellular immune responses seen in the early stages of the infections. Proteins secreted by the bacteria during growth may be important in this respect, and several secreted antigens from the Mycobacterium tuberculosis complex have been identified and characterized (10, 20, 25–27, 31, 34). These antigens have also received a lot of attention in attempts to improve diagnostic tests and in the design of new vaccines against tuberculosis (2, 11, 13). In comparison, relatively little is known about secreted proteins in the M. avium complex. Such proteins have been demonstrated by using monoclonal antibodies (19) or 35S methionine labeling of proteins in short-term cultures (30). Only a few secreted proteins have been further characterized, including components of the antigen 85 complex, which are major antigens of Mycobacterium bovis BCG (7, 8, 20, 32). Ohara et al. have described the presence of 85B and 85D (MPT51) (22) in M. avium subsp. avium culture fluid, and a component of the 85 complex from M. avium subsp. paratuberculosis has also been purified and characterized (28). Plum et al. have described a macrophage-induced protein of M. avium subsp. avium grown in human macrophages (24). A ferric reductase (12), as well as a putative serine protease (3), from M. avium subsp. paratuberculosis has also been studied. Some of these proteins have been shown to induce humoral immune responses in animals with paratuberculosis, but the applicability of the proteins in diagnostic assays has not been evaluated.
The aim of this study was to identify secreted proteins in the M. avium complex using polyclonal antisera raised against culture filtrate (CF) proteins and antisera against individual secreted proteins from M. tuberculosis and M. bovis. Secreted proteins that are present in the M. avium complex and not in M. bovis and M. tuberculosis could help distinguish between paratuberculosis and tuberculosis in cattle and also between M. avium complex infections and tuberculosis in humans by using assays measuring cellular or humoral immune responses.
MATERIALS AND METHODS
Bacterial culture and antigen preparation.
M. tuberculosis and M. bovis CF proteins have been described previously (20, 33). M. tuberculosis CF was a gift from S. Nagai, Osaka, Japan. The other strains were obtained from the National Veterinary Institute, Oslo, Norway. All strains were kept on Middlebrook's 7H10 medium (with mycobactin [2 μg/ml] for the M. avium subsp. paratuberculosis strain). The reference strains M. avium subsp. paratuberculosis strain 2E and M. avium subsp. avium strain D4 were cultivated on the surface of synthetic Reid's medium at 37°C for 1 month. Mycobacterium intracellulare, Mycobacterium scrofulaceum, Mycobacterium fortuitum, Mycobacterium kansasii, and Mycobacterium phlei were cultured on the same medium for 1 to 3 weeks until sufficient growth was obtained. The bacteria were sequentially removed by paper filtration, and the CF was subsequently passed through a sandwich filtration system (pore sizes, 1.0, 0.5, and 0.22 μm). Concentration of CF was done by precipitation with 80% ammonium sulfate (550 g/liter). The bacteria were washed three times in phosphate-buffered saline (PBS), and 2 g of bacteria in 10 ml of PBS was kept on ice and sonicated 20 times for 1 min each time. The samples were centrifuged, followed by filtration (0.22-μm-pore-size filter) of the supernatant. The protein concentration was determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.).
Antisera.
The antisera against cellular antigens of M. avium subsp. paratuberculosis strain 2E and M. avium subsp. avium strain D4 were obtained from Dako (Glostrup, Denmark). Antisera against CF proteins from M. avium subsp. paratuberculosis and M. avium subsp. avium were produced in rabbits by three subcutaneous administrations of concentrated CF proteins (0.5 mg) in Freund's incomplete adjuvant, with 2 weeks between injections. The rabbits were bled 2 weeks after the last injection, and the sera were used in Western blotting at a dilution of 1:1,000. Antisera against purified M. tuberculosis and M. bovis CF proteins have been described previously (20, 33). Antiserum against a purified 14-kDa protein was made by immunizing two rabbits with 40 μg of purified protein in Freund's incomplete adjuvant three times, with 2 weeks between injections. The rabbits were bled 2 weeks after the last injection, and the sera were used in Western blotting at a dilution of 1:200.
SDS-PAGE with immunoblotting.
The antigens were separated under reducing and nonreducing conditions by horizontal SDS-PAGE in precast gradient Excel gel 8 to 18% (Pharmacia, Uppsala, Sweden), using a Multiphor II unit model 2117 (Pharmacia). After separation, the proteins were transferred to a nitrocellulose membrane (pore size, 0.2 μm) (Schleicher & Schuell, Dassel, Germany) by diffusion blotting (23), and the proteins remaining in the gel were stained with Coomassie brilliant blue (CBB). The membranes were blocked with PBS containing 2% bovine serum albumin and 1% gelatin and incubated with antisera over night. Bound antibodies were recognized by horseradish peroxidase-labeled anti-rabbit immunoglobulins. As a substrate, 3,3′ diaminobenzidine was added to visualize the bound antibodies.
Purification of a 14-kDa protein.
The purification procedures for the 14-kDa protein were developed on the basis of combinations of ion-exchange chromatography and gel filtration using a Gradi Frac system equipped with a P-50 pump, a UV monitor, and a conductivity monitor (Pharmacia). Fractions were analyzed by SDS-PAGE with diffusion blotting (23), and the nitrocellulose membrane was incubated with polyvalent anti-M. avium subsp. avium antiserum against CF proteins. Proteins remaining in the gel after blotting were stained with CBB. The fractions containing proteins with the estimated molecular masses were collected.
In a typical run, the initial protein concentrate (100 mg of M. avium subsp. paratuberculosis CF proteins) was applied to a column of Q Sepharose Fast Flow (Pharmacia) with a bed volume of 15 ml and eluted at 7.5 ml/min using a gradient of 0 to 500 mM NaCl with 30 mM Tris-HCl, pH 8.7, and 3% Methyl Cellosolve (Sigma Chemical Co., St. Louis, Mo.). The fractions containing the protein were diluted 1:10 in 30 mM Tris-HCl, pH 8.7, and 3% Methyl Cellosolve and separated on a 6-ml Resource Q column (Pharmacia) followed by elution at 3 ml/min using a gradient of 0 to 400 mM NaCl. The fractions containing the protein were concentrated to a sample of 5 ml on the Resource Q column by first diluting the sample 1:5 with 30 mM Tris-HCl, pH 8.7, and 3% Methyl Cellosolve followed by elution in the same buffer supplemented with 200 mM NaCl. The concentrated sample containing the 14-kDa protein was gel filtered on a Superdex 75 column (26/60) in PBS at a flow rate of 2 ml/min and then applied to the Resource Q column and eluted at 3 ml/min using a gradient of 0 to 300 mM ammonium sulfate in Tris-HCl, pH 7.5. The 14-kDa protein was present in two fractions.
N-terminal amino acid sequencing.
Automatic Edman degradation was performed by Knut Sletten at the Biotechnology Centre, University of Oslo, Oslo, Norway, using a 477A protein sequencer with an on-line 120-A phenylthiohydantoin amino acid analyzer (Applied Biosystems, Foster City, Calif.).
Databases.
The Institute for Genomic Research and Sanger databases http://www.tigr.org/tdb/mdb/mdb.htlm and http://www.sanger.ac.uc/Datasearch/, respectively) were searched using the N-terminal amino acid sequence.
RESULTS
Characterization of M. avium subsp. avium and M. avium subsp. paratuberculosis CFs and antisera.
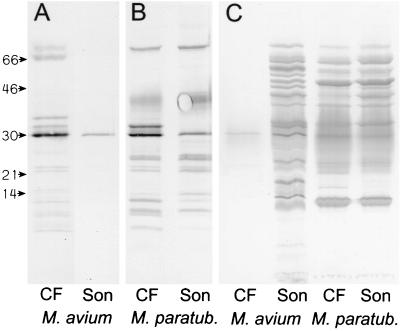
Concentrated CF protein samples were tested in SDS-PAGE and Western blotting using the antisera raised against the CF proteins. The antiserum against M. avium subsp. avium CF proteins reacted mainly with CF proteins and only to a limited extent with sonicate antigens, while antiserum against M. avium subsp. paratuberculosis CF reacted with several bands in both the CF and the sonicate preparations (Fig. 1). The Dako antiserum against M. avium subsp. avium was raised against a sonicate preparation of bacilli and hence consisted mainly of antibodies directed against cellular proteins. In Western blotting, this antiserum gave multiple bands, with the sonicate preparations from both subspecies showing the large extent of cross-reactions. The antiserum also reacted with many bands in M. avium subsp. paratuberculosis CF, while only one distinct band at 30 kDa was seen in M. avium subsp. avium CF (Fig. 1). This showed that the M. avium subsp. avium CF was not contaminated with any substantial amounts of intracellular antigens while the M. avium subsp. paratuberculosis CF contained a large number of cellular proteins, probably due to extensive lysis of the bacteria in the culture.
FIG. 1.
M. avium subsp. paratuberculosis (M. paratub.) and M. avium subsp. avium (M. avium) CF and sonicate proteins were separated in SDS-PAGE and blotted onto nitrocellulose membranes. The amounts of total protein applied were 1 μg for lanes A and B and 5 μg for lanes C. The molecular masses (in kilodaltons) are indicated. The two lanes A were incubated with antiserum against M. avium subsp. avium CF, and the two lanes B were incubated with antiserum against M. avium subsp. paratuberculosis CF. The four lanes C were incubated with antiserum against M. avium subsp. avium sonicate (Dako).
Comparison of M. avium subsp. avium CF and M. tuberculosis CF.
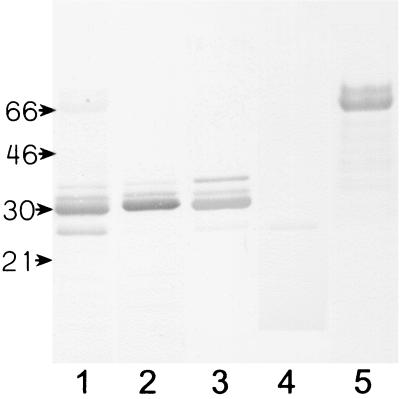
The various bands in M. avium subsp. avium CF were tested with rabbit antisera made against purified proteins from M. tuberculosis or M. bovis BCG (Fig. 2). The mobility in SDS-PAGE for the four bands reacting with sera against the 85 complex corresponded to molecular masses of 36, 33, 30, and 26 kDa. The most prominent band of the 85 complex seemed to be 85B, with a mobility corresponding to 30 kDa, while the band reacting weakly with anti-85D had a mobility corresponding to 26 kDa. The two bands that reacted strongly with the MPT32 antiserum had molecular masses of about 65 and 74 kDa, which are considerably higher than the molecular masses of 43 and 45 kDa of this antigen in M. tuberculosis. No bands were detected in M. avium subsp. avium CF with sera against MPB83, MPB70, MPT63, MPT46, and ESAT6, but a weak band with a molecular mass of 26 kDa was detected with rabbit MPT64 that was probably due to a cross-reaction with antigen 85D, since an MPT64 homologue was not found in the M. avium subsp. avium genomic sequence.
FIG. 2.
M. avium subsp. avium CF proteins (5 μg of total protein) were separated in SDS-PAGE, blotted onto nitrocellulose membranes, and incubated with various antisera. Lanes: 1, M. tuberculosis CF antiserum; 2, anti-85B antiserum; 3, anti-85C antiserum; 4, anti-85D antiserum; 5, anti-MPT32 antiserum. The molecular masses (in kilodaltons) are indicated.
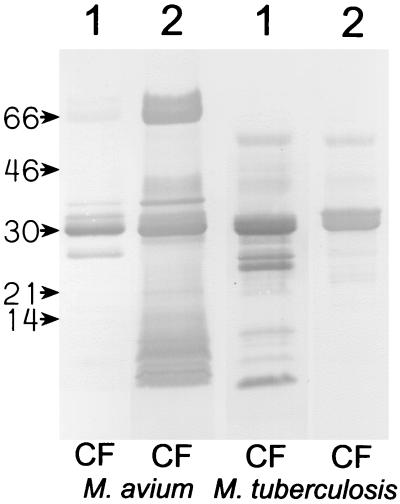
M. avium subsp. avium CF and M. tuberculosis CF were also tested in SDS-PAGE with Western blotting, using the polyclonal antisera against CF from the two species in order to identify possible M. avium complex-specific proteins (Fig. 3). The M. avium subsp. avium CF antiserum reacted with several bands with molecular masses of less than 15 kDa in M. avium subsp. avium that were not present in M. tuberculosis CF, in addition to the already identified antigen 85 complex and MPT32. The M. tuberculosis CF antiserum reacted with the antigen 85 complex and MPT32 in M. avium subsp. avium and gave faint staining of two or three bands among the low-molecular-mass proteins. In contrast, this antiserum reacted distinctly with several low-molecular-mass proteins in M. tuberculosis that did not have apparent counterparts in M. avium subsp. avium. These results indicate that there are major differences in the low-molecular-mass areas of the two species.
FIG. 3.
M. avium subsp. avium (M. avium) CF proteins and M. tuberculosis CF proteins (5 μg of total protein) were separated in SDS-PAGE and blotted onto nitrocellulose membranes. The molecular masses (in kilodaltons) are indicated. Lanes 1 were incubated with antiserum against M. tuberculosis CF proteins, and lanes 2 were incubated with antiserum against M. avium subsp. avium CF proteins.
Purification and N-terminal sequence of a CF protein.
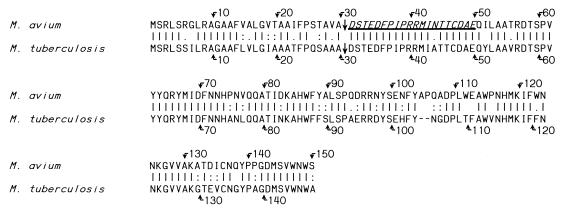
A 14-kDa protein was purified from M. avium subsp. paratuberculosis CF as described above. The purified protein was run in SDS-PAGE and stained with CBB and also blotted onto nitrocellulose and incubated with M. avium subsp. paratuberculosis antiserum. Only a 14-kDa band was detected using either method. The N-terminal amino acid sequence was obtained by Edman degradation, and homologous proteins with unknown functions were found in M. avium subsp. avium and M. tuberculosis when the Institute for Genomic Research and Sanger databases were searched (Fig. 4). The calculated molecular mass of the mature protein in M. avium subsp. avium was 14.1 kDa, fitting with its observed mobility in SDS-PAGE. The gene encoded a signal peptide typical of secreted proteins, with a cleavage site consisting of the amino acids AVA just upstream of the mature protein. The predicted cleavage site was confirmed by the N-terminal sequence of the mature protein, and the first 20 N-terminal amino acids were identical to those found in the M. avium subsp. avium genome. The overall homology between the proteins from M. avium subsp. avium and M. tuberculosis was 76% by the Lipman and Pearson alignment (16).
FIG. 4.
Complete amino acid sequence of the 14-kDa protein identified in M. avium subsp. avium and the homologous protein of M. tuberculosis. The N-terminal amino acid sequence obtained by Edman degradation is italicized and underlined, and straight arrows indicate the cleavage sites for the signal peptide. The positions of amino acids are indicated with bent arrows. |, identical amino acid; :, conservative exchange of amino acid; ., less conservative exchange of amino acid.
Specificity of the 14-kDa protein.
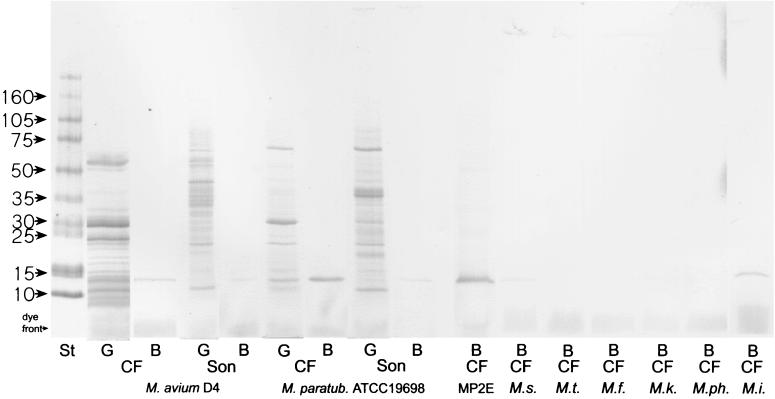
CFs and sonicated antigens of several mycobacterial strains were tested in SDS-PAGE with Western blotting using a monospecific antiserum raised in rabbits against the purified protein in order to identify cross-reacting proteins in the various species (Fig. 5). The monospecific antiserum reacted with a 14-kDa band in M. avium subsp. paratuberculosis, M. avium subsp. avium, M. intracellulare, and M. scrofulaceum but not with the other strains that were tested, including M. tuberculosis. The bands were distinct in the CFs from these four species and barely visible in the sonicate when the same amount of total protein was applied to the gel, indicating that the 14-kDa protein is actively secreted by the bacteria during growth. This method has been shown to be reliable in M. tuberculosis for identification of secreted proteins (35).
FIG. 5.
SDS-PAGE with CBB staining and Western blot of sonicate (Son) and CF antigens of various mycobacterial species. The molecular masses (in kilodaltons) of the standard (St) are indicated. G, proteins in gel stained with CBB; B, lanes blotted onto nitrocellulose and incubated with antiserum against the 14-kDa protein isolated from M. avium subsp. paratuberculosis; MP2E, M. avium subsp. paratuberculosis strain 2E; M.s., M. scrofulaceum; M.t., M. tuberculosis; M.f., M. fortuitum; M.k., M. kansasii; M.ph, M. phlei; M.i, M. intracellulare.
DISCUSSION
There are several possible approaches to an attempt to identify secreted proteins. Today, when the whole genome of M. tuberculosis has been sequenced, secreted proteins can be recognized by searching the genome for proteins with a typical signal sequence (9). The secretory pathway is probably the main mechanism for protein export in mycobacteria, and most of the actively secreted proteins will have a typical signal sequence (21). Construction of libraries with M. tuberculosis genomic DNA fused with leaderless genes for phosphatase A (15) or beta-lactamase (6) have been successfully used to identify secreted proteins and have allowed a more detailed characterization of the mycobacterial signal peptide (15, 36). A reliable approach is to search for proteins found in abundance in CFs and in minor quantities in cellular extracts. The classification of such proteins as cellular or secreted can be based on calculation of a localization index (LI) as described earlier (33). The low-molecular-mass proteins identified in M. avium subsp. avium CF in the present study clearly had high LIs. They were found in abundance in CF, while they were barely detectable in the corresponding sonicate preparation. With proper antibody reagents, secreted proteins can be identified in old lysed cultures by the LI method, but it is helpful to use cultures with a minimal extent of cellular lysis, especially when trying to identify novel proteins. This can sometimes be achieved by using short-term cultures (1), but it can also be obtained by cultivating the bacteria as a surface pellicle (33).
The present study has focused on identifying secreted antigens in the M. avium complex using polyclonal antisera raised against crude CF proteins from this species and antisera against known proteins from M. tuberculosis and M. bovis. The extensive cellular lysis seen in the culture of M. avium subsp. paratuberculosis, with release of cellular proteins to the CF, made the identification of novel secreted proteins in this subspecies difficult. This extensive lysis was in marked contrast to what was seen in M. avium subsp. avium CF, which appeared to consist mainly of secreted proteins. The quality of the M. avium subsp. avium CF in this study is comparable to that of the M. tuberculosis CFs produced by S. Nagai (20, 33), which also had negligible amounts of intracellular proteins. Both the CF made here and Nagai's CFs were produced by traditional surface pellicle cultures grown on synthetic media for several weeks. This shows that it is not necessary to use short-term cultures to obtain CFs specially adapted for studying secreted proteins. The advantage of using longer cultivation times is a higher yield.
It is not known whether a low degree of lysis can be obtained by refining the cultivation technique or whether it is a feature of particular strains. If there is variation in lysis between strains and species of bacteria, it will be of consequence for the amounts of cellular proteins present in CFs and therefore also in purified protein derivatives made from the various cultures. Therefore, caution should be taken in interpretation of immune responses elicited against purified protein derivative as responses towards secreted proteins.
The diagnosis of mycobacterial disease, especially in subclinically infected animals, is still a problem and is often based on measuring cellular immune responses. The specificity of such tests is dependent on the antigens used to stimulate the cells, and specific proteins from M. tuberculosis have been shown to be able to differentiate between tuberculosis and M. avium complex infections (14, 17). We have shown that several secreted low-molecular-mass proteins present in the M. avium complex are without overt counterparts in M. tuberculosis. It is likely that some of these are also good candidates for use in diagnostic assays based on cellular and humoral immune responses to distinguish between infections with these bacteria. We have identified and purified one 14-kDa protein that was detected by Western blotting only in the M. avium-M. intracellulare complex including M. scrofulaceum. It is a secreted protein found in much larger amounts in the CF than in the sonicate and with a signal sequence typical of secreted mycobacterial proteins, thus fulfilling the basic criteria to establish it as a secreted protein (35). A homologous gene (Rv0455c) with an unknown function is present in the M. tuberculosis H37Rv genome, but the monospecific and polyclonal antiserum raised against the purified protein did not react with any proteins in CF from M. tuberculosis. Given the strong homology between the proteins, a likely explanation for this finding is that the protein is expressed in significantly larger amounts in the M. avium complex. However, it might also be due to structural differences between the two proteins. A Jameson-Wolf antigenic index showed that one of the most likely areas for B-cell epitopes is between amino acids 90 and 100, and the differences in amino acid sequence between M. tuberculosis and M. avium subsp. avium in this area were quite extensive.
The four components of the antigen 85 complex and an MPT32 homologue were the quantitatively dominant secreted proteins in M. avium subsp. avium. Ohara et al. have previously claimed that only 85B and 85D are expressed in large amounts in M. avium subsp. avium based on the fact that they managed to obtain N-terminal sequences of only these two proteins when eluting spots from a two-dimensional nitrocellulose blot (22). We found that antisera raised against 85A, 85B, 85C, and 85D reacted with at least four bands in M. avium subsp. avium CF, showing that all four of these proteins probably are expressed in this species. The genes encoding the four proteins are present in M. avium subsp. avium (22), which is consistent with our findings. We cannot exclude the possibility that our results are due to the presence of other proteins that cross-react with the antigen 85 complex, but no genes encoding other homologous proteins were found when the M. avium subsp. avium genome was searched.
The present work has shown that the low-molecular-mass areas of the M. avium complex and M. tuberculosis contain different repertoires of secreted proteins. These findings are probably due to differences in protein expression, as well as the existence of species-specific proteins. It is likely that some of these proteins can be useful in the attempt to improve current immunological assays to help differentiate among infections with these species. We have purified and characterized one of these proteins with a molecular mass of 14 kDa that will be further evaluated for its potential role as an antigenic target in mycobacterial infections.
ACKNOWLEDGMENTS
We thank Inger Austrheim Heffernan for excellent technical assistance.
This work was supported by grants from the Norwegian Research Council (project no. 116086/122).
REFERENCES
- 1.Abou-Zeid C, Smith I, Grange J M, Ratliff T L, Steele J, Rook G A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988;134:531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron R M, Stevenson K, Inglis N F, Klausen J, Sharp J M. Identification and characterization of a putative serine protease expressed in vivo by Mycobacterium avium subsp. paratuberculosis. Microbiology. 1994;140:1977–1982. doi: 10.1099/13500872-140-8-1977. [DOI] [PubMed] [Google Scholar]
- 4.Chin D P, Hopewell P C, Yajko D M, Vittinghoff E, Horsburgh C R, Jr, Hadley W K, Stone E N, Nassos P S, Ostroff S M, Jacobson M A. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289–295. doi: 10.1093/infdis/169.2.289. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini R J, Van Kruiningen H J, Merkal R S, Thayer W R, Jr, Coutu J A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984;20:966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chubb A J, Woodman Z L, Silva Tatley F M, Hoffmann H J, Scholle R R, Ehlers M R. Identification of Mycobacterium tuberculosis signal sequences that direct the export of a leaderless beta-lactamase gene product in Escherichia coli. Microbiology. 1998;144:1619–1629. doi: 10.1099/00221287-144-6-1619. [DOI] [PubMed] [Google Scholar]
- 7.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 2000;396:190. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Gomez M, Johnson S, Gennaro M L. Identification of secreted proteins of Mycobacterium tuberculosis by a bioinformatic approach. Infect Immun. 2000;68:2323–2327. doi: 10.1128/iai.68.4.2323-2327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe M, Nagai S, Patarroyo M E, Torres M L, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harboe M, Wiker H G, Duncan J R, Garcia M M, Dukes T W, Brooks B W, Turcotte C, Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990;28:913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homuth M, Valentin-Weigand P, Rohde M, Gerlach G F. Identification and characterization of a novel extracellular ferric reductase from Mycobacterium paratuberculosis. Infect Immun. 1998;66:710–716. doi: 10.1128/iai.66.2.710-716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lein A D, von Reyn C F, Ravn P, Horsburgh C R, Jr, Alexander L N, Andersen P. Cellular immune responses to ESAT-6 discriminate between patients with pulmonary disease due to Mycobacterium avium complex and those with pulmonary disease due to Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 1999;6:606–609. doi: 10.1128/cdli.6.4.606-609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim E M, Rauzier J, Timm J, Torrea G, Murray A, Gicquel B, Portnoi D. Identification of Mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J Bacteriol. 1995;177:59–65. doi: 10.1128/jb.177.1.59-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipman D J, Pearson W R. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 17.Manca C, Lyashchenko K, Colangeli R, Gennaro M L. MTC28, a novel 28-kilodalton proline-rich secreted antigen specific for the Mycobacterium tuberculosis complex. Infect Immun. 1997;65:4951–4957. doi: 10.1128/iai.65.12.4951-4957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFadden J J, Butcher P D, Chiodini R, Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987;25:796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutharia L M, Moreno W, Raymond M. Analysis of culture filtrate and cell wall-associated antigens of Mycobacterium paratuberculosis with monoclonal antibodies. Infect Immun. 1997;65:387–394. doi: 10.1128/iai.65.2.387-394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Ohara N, Ohara-Wada N, Kitaura H, Nishiyama T, Matsumoto S, Yamada T. Analysis of the genes encoding the antigen 85 complex and MPT51 from Mycobacterium avium. Infect Immun. 1997;65:3680–3685. doi: 10.1128/iai.65.9.3680-3685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen I, Wiker H G. Diffusion blotting for rapid production of multiple identical imprints from sodium dodecyl sulfate polyacrylamide gel electrophoresis on a solid support. J Immunol Methods. 1998;220:77–84. doi: 10.1016/s0022-1759(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 24.Plum G, Brenden M, Clark-Curtiss J E, Pulverer G. Cloning, sequencing, and expression of the mig gene of Mycobacterium avium, which codes for a secreted macrophage-induced protein. Infect Immun. 1997;65:4548–4557. doi: 10.1128/iai.65.11.4548-4557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romain F, Laqueyrerie A, Militzer P, Pescher P, Chavarot P, Lagranderie M, Auregan G, Gheorghiu M, Marchal G. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect Immun. 1993;61:742–750. doi: 10.1128/iai.61.2.742-750.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkrands I, Weldingh K, Jacobsen S, Hansen C V, Florio W, Gianetri I, Andersen P. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimensional gel electrophoresis, microsequencing and immunodetection. Electrophoresis. 2000;21:935–948. doi: 10.1002/(SICI)1522-2683(20000301)21:5<935::AID-ELPS935>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugden E A, Brooks B W, Young N M, Watson D C, Nielsen K H, Corner A H, Turcotte C, Michaelides A, Stewart R B. Chromatographic purification and characterization of antigens A and D from Mycobacterium paratuberculosis and their use in enzyme-linked immunosorbent assays for diagnosis of paratuberculosis in sheep. J Clin Microbiol. 1991;29:1659–1664. doi: 10.1128/jcm.29.8.1659-1664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorel M F, Krichevsky M, Levy-Frebault V V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 30.Valentin-Weigand P, Moriarty K M. Protein antigens secreted by Mycobacterium paratuberculosis. Zentbl Veterinarmed. 1992;39:762–766. doi: 10.1111/j.1439-0450.1992.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 31.Weldingh K, Rosenkrands I, Jacobsen S, Rasmussen P B, Elhay M J, Andersen P. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect Immun. 1998;66:3492–3500. doi: 10.1128/iai.66.8.3492-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiker H G, Harboe M, Lea T E. Purification and characterization of two protein antigens from the heterogeneous BCG85 complex in BCG. Int Arch Allergy Appl Immunol. 1986;81:298–306. doi: 10.1159/000234153. [DOI] [PubMed] [Google Scholar]
- 33.Wiker H G, Harboe M, Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991;137:875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]
- 34.Wiker H G, Harboe M, Nagai S, Patarroyo M E, Ramirez C, Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81:307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- 35.Wiker H G, Michell S L, Hewinson R G, Spierings E, Nagai S, Harboe M. Cloning, expression and significance of MPT53 for identification of secreted proteins of Mycobacterium tuberculosis. Microb Pathog. 1999;26:207–219. doi: 10.1006/mpat.1998.0267. [DOI] [PubMed] [Google Scholar]
- 36.Wiker H G, Wilson M A, Schoolnik G K. Extracytoplasmic proteins of Mycobacterium tuberculosis—mature secreted proteins often start with aspartic acid and proline. Microbiology. 2000;146:1525–1533. doi: 10.1099/00221287-146-7-1525. [DOI] [PubMed] [Google Scholar]
- 37.Wood P R, Rothel J S. In vitro immunodiagnostic assays for bovine tuberculosis. Vet Microbiol. 1994;40:125–135. doi: 10.1016/0378-1135(94)90051-5. [DOI] [PubMed] [Google Scholar]