Abstract
Reduction of the risk of asthma attacks is a major goal of current asthma management. We propose to derive a risk scale predicting asthma attacks based on the blood eosinophil count and exhaled nitric oxide (FeNO). Biomarker-stratified trial-level attack rates were extracted and pooled from the control arms of the Novel START, CAPTAIN, QUEST, Benralizumab Phase 2b, PATHWAY, STRATOS 1–2 and DREAM trials (n=3051). These were used to derive rate ratios and the predicted asthma attack rate for different patient groups. The resultant prototype risk scale shows potential to predict asthma attacks, which may be prevented by anti-inflammatory treatment.
Keywords: asthma, exhaled airway markers, eosinophil biology, asthma epidemiology, respiratory measurement, pulmonary eosinophilia, clinical epidemiology, allergic lung disease
Introduction
Assessment and reduction of the risk of attacks are a major goal of asthma management.1 However, our ability to do this is limited for several reasons. First, the extent to which the risk associated with clinical characteristics is independent of the inflammatory phenotype has not been defined. Second, some acknowledged risk factors are difficult to identify and/or modify, for example, non-adherence and obesity, respectively. Third, some parameters can be modified independent of an effect on asthma attacks; for example, symptom burden improves following bronchodilator monotherapy without an effect on asthma attacks.2 These limitations mean that a precise estimation of the risk of asthma attacks and the likely benefit of treatment is not possible.
Recently, five analyses of clinical trials across the spectrum of asthma severity have assessed the independent relationship between blood eosinophils, fractional exhaled nitric oxide (FeNO) and the risk of asthma attacks.3–7 Collectively, these studies show that the prognostic importance of these biomarkers is similar in strength and additive to the independent risk seen with more established risk factors such as a history of an attack in the last year and Global Initiative for Asthma (GINA) treatment step.8 In four out of the five studies, the prognostic value of blood eosinophils and FeNO was additive.3 5–7
These findings suggest that the blood eosinophil count and FeNO could form the basis of a useful risk scale analogous to those that have had a large impact in cardiovascular medicine.9 We have explored this hypothesis by developing a prototype risk scale.
Methods
We designed a scale presenting the modifiable risk of asthma attacks associated with blood eosinophils and FeNO on the background of the unmodifiable risk associated with GINA treatment step, a recent history of an asthma attack and the presence of less modifiable risk factors. Asthma attacks were defined as episodes of acute asthma requiring treatment with systemic steroids ≥3 days and/or hospitalisation.
We used control arm data3–7 from the trials described in the supplementary table (see online supplemental file 1) to derive frequency-weighted rate ratios of asthma attacks by biomarker combinations using established cut points for blood eosinophil counts and FeNO (table 1). Individual trial rate ratios were calculated as follows: [(absolute asthma attack rate for subgroup 1)×(frequency n1 )] ÷ [(frequency-weighted mean for the remaining subgroups 2–9)×(Σ(n2 to 9 ))]. Aggregate rate ratios (rightmost column of table) were calculated as frequency-weighted means of the individual trial’s rate ratios for each biomarker combination. In effect, an aggregate rate ratio is a mean fold change in the asthma attack rate for patients with that biomarker combination compared with others.
Table 1.
Biomarker-stratified data and rate ratios derived from included trials
| Blood Eos (×109/L) |
FeNO (ppb) |
Novel START4 | CAPTAIN5 | Pooled AZ trials: Benralizumab 2b, PATHWAY, STRATOS 1–27 |
QUEST6 | DREAM3 | Aggregate data for the prototype risk scale | |||||||||||
| Step 1 asthma; low risk; 9% with attack in past 12 months | Step 4 asthma; high risk; 62% with attack in past 12 months | 1% step 3 asthma, 50% step 4 asthma, 49% step 5 asthma; high risk; with attack in past 12 months | 47% step 4 asthma, 53% step 5 asthma; high risk; with attack in past 12 months | Step 5 asthma; high risk; with attack in past 12 months | ||||||||||||||
| N† | Attack rate‡ | Rate ratio | N | Attack rate‡ | Rate ratio | N† | Attack rate | Rate ratio | N | Attack rate | Rate ratio | N | Attack rate | Rate ratio | N | Rate ratio | ||
| <0.15 | <25 | 18 | 0.05 | 0.98 | 228 | 0.85 | 0.54 | 199 | 0.58 | 0.81 | 106 | 0.56 | 0.52 | 23 | 1.98 | 0.76 | 574 | 0.65 |
| 25–<50 | 23 | 0.00 | 0.00 | 40 | 0.10 | 1.11 | 82 | 0.46 | 0.64 | 35 | 0.62 | 0.61 | (9) | (1.78) | (0.71) | 180 | 0.66 | |
| ≥50 | 8 | 0.00 | 0.00 | 17 | 0.15 | 1.74 | 23 | 0.57 | 0.81 | 21 | 0.53 | 0.53 | 69 | 0.86 | ||||
| 0.15–<0.30 | <25 | 19 | 0.07 | 1.50 | 240 | 0.07 | 0.82 | 191 | 0.56 | 0.76 | 96 | 0.82 | 0.80 | 12 | 1.54 | 0.59 | 558 | 0.81 |
| 25–<50 | 42 | 0.02 | 0.36 | 87 | 0.07 | 0.79 | 173 | 0.67 | 0.96 | 53 | 1.14 | 1.17 | (23) | (2.70) | (1.07) | 355 | 0.88 | |
| ≥50 | 32 | 0.01 | 0.24 | 24 | 0.12 | 1.43 | 52 | 1.29 | 1.93 | 25 | 0.48 | 0.47 | 133 | 1.16 | ||||
| ≥0.30 | <25 | 4 | 0.30 | 6.35 | 248 | 0.11 | 1.29 | 102 | 0.58 | 0.82 | 89 | 0.84 | 0.84 | 18 | 1.95 | 0.75 | 461 | 1.12 |
| 25–<50 | 22 | 0.00 | 0.00 | 147 | 0.09 | 1.00 | 133 | 0.87 | 1.30 | 97 | 1.24 | 1.31 | (66) | (3.08) | (1.22) | 399 | 1.12 | |
| ≥50 | 51 | 0.13 | 4.40 | 66 | 0.18 | 2.14 | 107 | 1.01 | 1.53 | 98 | 1.78 | 2.12 | 322 | 2.29 | ||||
| Analysed | 219 | 0.05 | 1.00 | 1097 | 0.09 | 1.00 | 1062 | 0.70 | 1.00 | 620 | 0.99 | 1.00 | 151 | 2.52 | 1.00 | 3051 | 1.00 | |
| Missing* | 4 | 121 | 120 | 14 | 4 | 262 | ||||||||||||
| Total | 223 | 1218 | 1182 | 634 | 155 | 3313 | ||||||||||||
Aggregate ratios in bold (rightmost column) are those included to derive the prototype risk scale: in effect, an aggregate rate ratio is a mean fold change in the asthma attack rate for patients with that biomarker combination. Numbers between brackets were extracted to calculate frequency-weighted rate ratios but were not used to derive the scale, as this analysis was stratified using only two cut points for fractional exhaled nitric oxide (FeNO <25 or ≥25 ppb).
Blood Eos, peripheral blood eosinophil count; n, number of patients.
*Missing data were excluded from analyses.
†For Novel START and the pooled AstraZeneca (AZ) trials, we regrouped the data of patients with a baseline FeNO of 20–<50 ppb into our 25–<50 ppb group, as the difference of 5 ppb in FeNO is not clinically relevant.
‡For both the Novel START and CAPTAIN, only the percentage of patients with one or more severe attack(s) in the 52 weeks of follow-up was reported, so we imputed the annualised rate as –log10(1 − %incidence).
thoraxjnl-2021-217325supp001.pdf (106.5KB, pdf)
We used asthma attack rates from a US population study involving 222 817 patients to derive a predicted asthma attack rate by GINA step.8 We further stratified by a history of an asthma attack in the last year (which we assumed increased risk by a factor of 2.8)8 and the presence of two or more additional potential risk factors (which we assumed increased risk by a factor of 1.3). Our estimate of the additional risk associated with two or more additional potential risk factors was based on the difference in asthma attack rates in the CAPTAIN population,5 who had persistent symptoms and airflow obstruction, compared with the Novel START population,4 who had neither.
To populate each cell of the prototype risk scale, the reference rate for GINA treatment steps 1, 2, 3, 4 and 5 was multiplied by the appropriate risk pertaining to that group for example, the figure’s rightmost column’s rates are calculated as [aggregate biomarker-stratified rate ratio] × [GINA treatment step-specific attack rate] × 2.8 × 1.3.
A frequency-weighted intraclass correlation coefficient (two-way mixed model for absolute agreement of single measures) and 95% CIs were computed between the predicted and observed asthma attack rates using the derivation trials in SPSS V.27.
Results
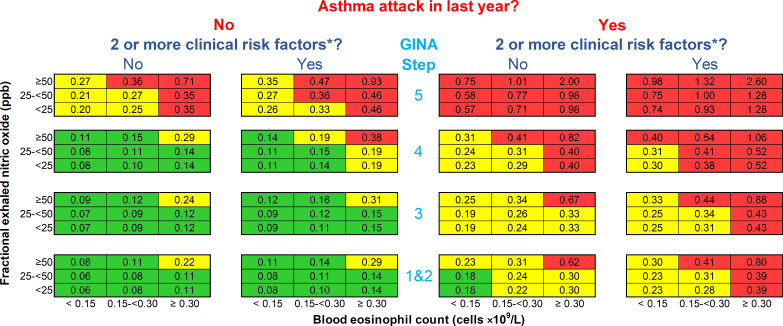
The resulting prototype risk scale is shown in the figure 1: each cell represents the predicted annual asthma attack rate for a given scenario if treatment is not changed. The predicted asthma attack rates range from 0.06 to 2.60 per year; they are comparable to observed attack rates in the derivation trial control patients (intraclass correlation coefficient: 0.83 (95% CI 0.78 to 0.86)).
Figure 1.
Prototype asthma attack risk scale. Numbers in each cell are predicted annual asthma attack rates for patients over the age of 12 if treatment is not changed. An asthma attack is an episode of acute asthma requiring treatment with systemic steroids ≥3 days and/or hospitalisation. The blood eosinophil count is contemporaneous or the highest result in the last 12 months; fractional exhaled nitric oxide level is contemporaneous. *Risk factors are defined by the Global Initiative for Asthma (GINA) guidelines1: poor symptom control (Asthma Control Questionnaire score ≥1.5), low lung function (forced expiratory volume in 1 second <80% predicted), adherence issues, reliever overuse (>200 dose of salbutamol cannister/month), intubation or intensive care unit admission for asthma previously, comorbidities (one of chronic rhinosinusitis, obesity and psychiatric disease) and environmental exposures (one of smoking, allergen and pollution).
Discussion
We designed a prototype risk scale based on trial-level data that shows potential to predict asthma attacks which may be modified by anti-inflammatory treatment. As is the case with cardiovascular risk, the relative risk associated with biomarkers was consistent across populations, but the absolute risk conferred by type 2 airway inflammation was greater in a population at higher background risk.
The fact that blood eosinophils and FeNO provide additive prognostic information is predictable, as both biomarkers provide different and complementary mechanistic information: FeNO reflects airway type 2 activity and the chemotactic pull to the airways, while blood eosinophils reflect the systemic pool of available effector cells and circulating interleukin 5.10 In contrast, symptom scores do not correlate with airway inflammation nor with airflow limitation10 and do not reliably predict exacerbations when the inflammatory phenotype is considered.11
An important feature of the prototype risk scale is that it centres attention on biomarkers that are not only closely associated with the mechanism of asthma attacks but are also easily modified with therapy directed against this mechanism. For example, the excess risk of asthma attacks associated with the highest biomarker combination compared with the lowest was effectively removed by low-dose inhaled corticosteroids (ICS) in mild asthma,4 an increased dosage of ICS in moderate asthma5 and biologics in severe asthma.3 In many cases, this reduction in risk is associated with a proportionate reduction in biomarkers.
We emphasise that the proposed risk scale is a prototype and several assumptions have been made in its derivation. First, there were some inconsistencies in the relationship between FeNO and the risk of asthma attacks in the mild asthma population,4 which likely reflect the small sample sizes. However, a difference in the mechanism of asthma attacks or a relatively greater prognostic value of FeNO in ICS-treated patients cannot be excluded. Larger studies are required to investigate these possibilities. Second, we categorised risk factors, and since the independent risk conferred by these risk factors over and above that associated with type 2 biomarkers is unknown, we derived the multiplier for having ≥2 risk factors by comparing the Novel START4 and CAPTAIN5 populations. The resultant multiplier of 1.3 suggests that the independent impact of these factors is modest, but further work is needed to confirm this. Third, although the biomarker-stratified rate ratios were adjusted for each other, we concede that the other covariates were not perfectly adjusted for one another. Fourth, the prototype features categories rather than the absolute values of blood eosinophils, FeNO and clinical risk factors. We did this as this was the only data available to us. It also allowed us to tabulate risk across the spectrum of patients and biomarkers in an accessible way. This approach has been very successful in cardiovascular risk reduction, but we acknowledge that there may be better ways of representing the continuous risk associated with these factors.
We speculate that a risk scale based on this prototype could facilitate better treatment decisions by doctors and patients by providing a framework for a preventive, treatable, trait-based management. This hypothesis needs to be tested, and it is also important that the scale is refined using individual patient data from large and well-characterised populations.
Acknowledgments
Audun Nilsen, who participated in early elaboration of the concept, and colleagues and patients in the Oxford University Hospitals Foundation Trust’s severe asthma clinic for inspiration, advice and proofreading.
Footnotes
Twitter: @simcouillard, @SanjayResp, @HinksLab
Correction notice: This article has been corrected since it was published Online First. The abstract has been modified.
Contributors: SC drafted the manuscript and developed the risk scale. AL proposed the initial concept and approved the manuscript. MJ, SR, JM and TH contributed to data analysis and interpretation and reviewed/approved the manuscript. IP is the guarantor of this publication, contributed to the writing of the manuscript and reviewed and approved the final version. All authors have accessed and verified the underlying data.
Funding: This work was supported by the Oxford Respiratory NIHR BRC. This research was funded in part by the Wellcome Trust (211050/Z/18/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any author-accepted manuscript version arising from this submission.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: SC: has received a non-restricted research grant from Sanofi Genzyme for investigator-initiated type 2 innovation research and speaker honoraria from Sanofi/Regeneron, AstraZeneca and GlaxoSmithKline (GSK), all outside the submitted work. AL is an employee of Sanofi Norway. MJ has received grants from the University of Oxford and National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). SR reports non-financial support from AstraZeneca and other sources of revenue from Australian Government Research Training Program and the NIHR Oxford BRC. JM has received grants from the NIHR Oxford BRC. TH has received grants from Pfizer, the University of Oxford, the Wellcome Trust, the Guardians of the Beit Fellowship and the NIHR Oxford BRC outside the submitted work. He has received personal fees from AstraZeneca, Teva and PeerVoice outside the submitted work. In the last 5 years, IP has received speaker’s honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine AB, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini and GSK and payments for organising educational events from AstraZeneca, GSK, Sanofi/Regeneron and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi and Knopp and payments to support FDA approval meetings from GSK. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva and Chiesi. He has received a grant from Chiesi to support a phase 2 clinical trial in Oxford. He is a copatent holder of the rights to the Leicester Cough Questionnaire and has received payments for its use in clinical trials from Merck, Bayer and Insmed. In 2014–2015, he was an expert witness for a patent dispute involving AstraZeneca and Teva.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The studies described herein were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines. Study protocols received independent ethics committee approval at each study site.
References
- 1. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention (2021 update), 2021. Available: https://ginasthma.org/
- 2. Lazarus SC, Boushey HA, Fahy JV, et al. Long-Acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583–93. 10.1001/jama.285.20.2583 [DOI] [PubMed] [Google Scholar]
- 3. Shrimanker R, Keene O, Hynes G, et al. Prognostic and Predictive Value of Blood Eosinophil Count, Fractional Exhaled Nitric Oxide, and Their Combination in Severe Asthma: A Post Hoc Analysis. Am J Respir Crit Care Med 2019;200:1308–12. 10.1164/rccm.201903-0599LE [DOI] [PubMed] [Google Scholar]
- 4. Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med 2020;8:671–80. 10.1016/S2213-2600(20)30053-9 [DOI] [PubMed] [Google Scholar]
- 5. Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med 2021;9:69–84. 10.1016/S2213-2600(20)30389-1 [DOI] [PubMed] [Google Scholar]
- 6. Busse WW, Wenzel SE, Casale TB, et al. Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the liberty asthma quest study: a post-hoc analysis. Lancet Respir Med 2021. 10.1016/S2213-2600(21)00124-7. [Epub ahead of print: 25 Jun 2021]. [DOI] [PubMed] [Google Scholar]
- 7. Kraft M, Brusselle G, Mark FitzGerald J, et al. Patient characteristics, biomarkers, and exacerbation risk in severe, uncontrolled asthma. Eur Respir J 2021. 10.1183/13993003.00413-2021. [Epub ahead of print: 10 Jun 2021]. [DOI] [PubMed] [Google Scholar]
- 8. Suruki RY, Daugherty JB, Boudiaf N, et al. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med 2017;17:74. 10.1186/s12890-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the score project. Eur Heart J 2003;24:987–1003. 10.1016/S0195-668X(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 10. Couillard S, Shrimanker R, Chaudhuri R, et al. FeNO non-suppression identifies corticosteroid-resistant type-2 signaling in severe asthma. Am J Respir Crit Care Med 2021. 10.1164/rccm.202104-1040LE. [Epub ahead of print: 15 Jun 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaw D, Green R, Berry M, et al. A cross-sectional study of patterns of airway dysfunction, symptoms and morbidity in primary care asthma. Prim Care Respir J 2012;21:283–7. 10.4104/pcrj.2012.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-217325supp001.pdf (106.5KB, pdf)