Abstract
Objective
Helicobacter pylori infection is mostly a family-based infectious disease. To facilitate its prevention and management, a national consensus meeting was held to review current evidence and propose strategies for population-wide and family-based H. pylori infection control and management to reduce the related disease burden.
Methods
Fifty-seven experts from 41 major universities and institutions in 20 provinces/regions of mainland China were invited to review evidence and modify statements using Delphi process and grading of recommendations assessment, development and evaluation system. The consensus level was defined as ≥80% for agreement on the proposed statements.
Results
Experts discussed and modified the original 23 statements on family-based H. pylori infection transmission, control and management, and reached consensus on 16 statements. The final report consists of three parts: (1) H. pylori infection and transmission among family members, (2) prevention and management of H. pylori infection in children and elderly people within households, and (3) strategies for prevention and management of H. pylori infection for family members. In addition to the ‘test-and-treat’ and ‘screen-and-treat’ strategies, this consensus also introduced a novel third ‘family-based H. pylori infection control and management’ strategy to prevent its intrafamilial transmission and development of related diseases.
Conclusion
H. pylori is transmissible from person to person, and among family members. A family-based H. pylori prevention and eradication strategy would be a suitable approach to prevent its intra-familial transmission and related diseases. The notion and practice would be beneficial not only for Chinese residents but also valuable as a reference for other highly infected areas.
Keywords: helicobacter pylori, gastric cancer, 13C-urea breath test, mucosal infection, helicobacter pylori - gastritis
Introduction
Helicobacter pylori has infected half of the world population and is a major health threat for Chinese families and society. It is also a heavy economic and healthcare burden for the country due mostly to the high infection rate and healthcare costs that are attributed to its related gastrointestinal (GI) and extra-GI diseases.1–4 A portion of infected people will develop various degrees of GI diseases, such as dyspepsia (5%–10%), chronic gastritis (90%), peptic ulcers (15%–20%) and gastric malignancies (1%); H. pylori infection is also closely associated with a number of extra-GI diseases,1 5 such as iron-deficiency anaemia, idiopathic thrombocytopenic purpura, autoimmune diseases, cardiovascular and cerebrovascular diseases, etc. Eradication of H. pylori is recommended to reduce the infection rate and occurrence of related diseases.1–4
Over the past three to four decades, the general H. pylori infection rate in China has slowly declined due to the continued intervention, education, improved sanitary condition and drinking water quality (online supplemental figures 1–4).6–8 One meta-analysis in 2020, which includes 670 572 participants from 26 provinces of mainland China, found that during 1983–2018, H. pylori prevalence declined by 0.9% annually. The overall prevalence was 63.8% in 1983–1994, 57.5% in 1995–2005 and 46.7% in 2006–2018. Infection rate varies greatly among different geographical areas and is much higher in rural areas.6 In addition, the age-standardised gastric cancer (GC) incidence rate has decreased from 37.56 per 100 000 in 1990 to 30.64 per 100 000 in 2019, with an estimated annual percentage change of −0.41, but GC incidence has increased from 317.34 thousand in 1990 to 612.82 thousand in 2019. The cause for increased GC incidence appears due to an increase in the middle to elderly population (≥40 years of age), along with the growth of the general population (online supplemental figures 2 and 3). It is estimated that in the next 25 years, the numbers of new GC cases and deaths will continue increase, while the rates of incidence and death should steadily decline.7
gutjnl-2021-325630supp001.pdf (1.3MB, pdf)
During this period, China’s social and family structure have also profoundly changed (online supplemental figure 5),9 with an increase in the total population and family number of the nation from 1.13 billion and 278.6 million in 1990 to 1.41 billion and 494.1 million, respectively, in 2021. The traditional multiple-generation family structure has shrank toward fewer generation and smaller size, with a decrease in average family size from 4.05 person/family in 1990 to 2.62 person/family in 2021,9 probably due to urbanisation, industrialisation and, previously, the ‘one-child policy’. All these are important factors related to H. pylori infection.
Since 2017, four major consensus reports for H. pylori infection control and related disease prevention have been published in China.1 2 10 11 These include the ‘Fifth Chinese National Consensus Report on the Management of Helicobacter pylori Infection’,1 the ‘Consensus on Chronic Gastritis in China (2017, Shanghai)’,10 ‘National Integrated Traditional Chinese and Western Medicine Management of Helicobacter pylori-related Diseases’11 and the ‘Consensus on Eradication of Helicobacter pylori and Prevention and Control of Gastric Cancer in China (2019, Shanghai)’.2 In addition, important international consensus reports have been published to guide the management of H. pylori infection and related disease prevention at the global level.12–18 However, no corresponding guidelines and strategies have been designated for the management of H. pylori infection in the general public and among family members to block its transmission and development of related diseases.
H. pylori infection is largely a family-based disease, with the advances in clinical practice and public awareness, the detrimental effects of family-based H. pylori infection have been increasingly recognised,19–21 which requires attention from physicians and public health administrative officials. Traditionally, the ‘test-and-treat’ and ‘screen-and-treat’ strategies are available for various infected populations,1 12 13 and they are critical in guiding global H. pylori eradication programmes over the past decades. However, clinical practice has found the need for refinement on these strategies,19–22 as the daily practice has met challenges and found that it is hard to differentiate a low-prevalence area from many highly infected communities in China, and the treatment processes are also affected by patient adherence, selection of treatment population and cost–benefit estimations. Furthermore, it is difficult to control infection from source without the infected family members being engaged; therefore, development of novel comprehensive strategies is desired to solve these problems and to facilitate bacterial eradication.20 21
Population-wide screening and eradication of H. pylori to prevent GC in highly infected areas have recently been proposed by several consensus reports and supported by large-scale investigations.12–14 17 22–28 The notion of ‘family-based H. pylori infection control and management’ was also recently introduced in China to block its intrafamilial transmission, and it appears to be an effective, practical and promising strategy to reduce H. pylori infection and to prevent related diseases.20 21 The National Clinical Research Centre for Digestive Diseases (Shanghai); Gastrointestinal Early Cancer Prevention & Treatment Alliance of China; Helicobacter pylori Study Group of Chinese Society of Gastroenterology, Chinese Medical Association (CMA); and Chinese Alliance for Helicobacter pylori Study therefore decided to hold a joint meeting to discuss and adopt the notion of family-based H. pylori infection control and management as a practical strategy to curb H. pylori intrafamilial transmission and development of related diseases in order to reduce its infection rates, related disease and GC burden for the nation in the coming decades.
The consensus suggestions will be instrumental to prevent H. pylori spread among family members and subsequently reduce related disease, GC incidence and medical expenditure. It will also be helpful to optimise household living style and to improve public awareness and health for Chinese residents and society. Experience from this practice would be beneficial not only for China, but also valuable as a reference for international communities that have high infection rates and related disease burdens.
Methods
Preparation and construction of consensus-related clinical questions (CQs) and statements followed a search and systematic review of relevant documents; 57 experts from 41 major universities and institutions in 20 provinces and regions of Mainland China were invited to review evidence and to modify statements. Key questions related to family-based H. pylori infection control and management were searched and drafted, and 23 original statement items were formed. The consensus drafting process refers to the population, intervention, comparator and outcome principle,29 and we also referred to the international consensus formulation processes.12–14
Evaluation of quality of evidence and strength of recommendations: grading of recommendations assessment, development and evaluation system is used to evaluate the quality of evidence and strength of recommendations.30 Quality of evidence was divided into four levels: high, moderate, low and very low. Strength of recommendations was divided into two levels: strong recommendation (the benefit is significantly greater than the risk or vice versa) and conditional recommendation (benefit is greater than the risk or vice versa). Quality of evidence is only one of the factors that determine the strength of recommendation, and low-quality evidence may also get strong recommendations. Experts provide recommendations based on both the available evidence and their personal opinion and experience.
Consensus reaching process: Delphi method was applied to reach a consensus on relevant CQs and statements. The constructed CQs and statements were first sent to all experts by email; feedbacks from experts were incorporated into the statements. After two rounds of consultation and modification of statements, the initial consensus statements were reached. On 30 January 2021, an online meeting was organised using the ‘Zoom Cloud Meetings’ platform (because the COVID-19 pandemic prevented a face-to-face meeting), which included 35 available experts from 27 institutions in 18 provinces to discuss and make necessary changes on the statements. Experts discussed the original CQs and 23 statements on family-based H. pylori infection transmission, control and management, and reached consensus on 16 statements with modifications.
Experts voted anonymously through the Chinese online ‘Questionnaire Star’ platform, and electronic ballots were automatically calculated; this process was monitored by the meeting secretary. Voting opinions were divided into six levels: (1) agree strongly; (2) agree, with minor reservations; (3) agree, with larger reservations; (4) disagree, with larger reservations; (5) disagree, with minor reservations; and (6) disagree completely. Voting results of 1+2 ≥80% were considered to reach consensus.
The voting results were later finalised, and the contents were prepared for publication. Three versions of the consensus report were drafted for different audiences: (1) a Chinese version of the report31 was published by Chinese Journal of Digestion, which is the official journal of the Chinese Society of Gastroenterology, CMA and circulated mostly in mainland China; (2) a popular science version of the report32 which was modified from the professional version and published in Chinese by Health World, the CMA-affiliated popular science magazine, and circulated in mainland China aimed at the general audience; and (3) this English language version of the report, which is aimed at international communities.
Results
The 16 statements that reached consensus are summarised in table 1 and detailed as follows; 7 statements that failed to reach consensus are presented in online supplemental table 1.
Table 1.
Summary of the 16 statements
| Statements | Consensus level (%) |
| Section 1: Helicobacter pylori infection and transmission among family members in the household | |
| Statement 1: H. pylori is a bacterial pathogen that is transmissible from person to person and especially among family members. | 94.7 |
| Statement 2: H. pylori is transmitted by oral route, and intrafamilial transmission is one of the major sources of infection. | 94.3 |
| Statement 3: Family members infected by H. pylori are potential sources of infection and have the possibility for continued transmission. | 92.1 |
| Statement 4: Most H. pylori infections occur during childhood and adolescents but can also be acquired in adulthood. | 84.2 |
| Statement 5: For all H. pylori-infected adult family members in a household, eradication should be considered. | 81.5 |
| Section 2: Prevention and management of H. pylori infection in children and elderly people within the household | |
| Statement 6: The relationship between H. pylori infection and gastric mucosal precancerous lesions in children and adolescents needs further investigation. | 86.6 |
| Statement 7: H. pylori infection in children needs to be managed based on risk–benefit assessment and related disease status. | 89.4 |
| Statement 8: For elderly members of the family, strategies for treating H. pylori infection should be formatted based on individual conditions. | 97.3 |
| Section 3: Strategies for prevention and management of H. pylori infection among family members | |
| Statement 9: ‘Family-based H. pylori infection control and management’ is an important strategy to prevent intrafamilial transmission and infection. | 86.8 |
| Statement 10: Concurrent treatment of H. pylori-infected family members is helpful to reduce the chance of reinfection after its eradication. | 81.5 |
| Statement 11: For patients with gastric cancer or gastric mucosal precancerous lesions, H. pylori infection should be screened and treated for their family members living in the same household. | 84.2 |
| Statement 12: The treatment regimens proposed by the ‘Fifth National Consensus Report on the Management of H. pylori Infection’ are suitable for H. pylori eradication among family members. | 94.7 |
| Statement 13: The concept of ‘eradicating H. pylori at the first-time treatment’ is applicable in the management of H. pylori infection among family members. | 94.7 |
| Statement 14: Urea breath tests, serum antibody tests and stool antigen tests are suitable methods to detect H. pylori infection among family members. | 92.1 |
| Statement 15: Family-based H. pylori infection control and management is an essential part of comprehensive H. pylori infection prevention and control strategies at the general public and community levels. | 92.1 |
| Statement 16: While an H. pylori vaccine is not available, preventing new infections and eradicating existing infections are both effective approaches for infection prevention and control | 89.7 |
gutjnl-2021-325630supp002.pdf (196.8KB, pdf)
Section 1: H. pylori infection and transmission among family members in the household
CQ1. Is H. pylori transmissible within family units or among family members?
Statement 1: H. pylori is a bacterial pathogen that is transmissible from person to person and especially among family members.
Evidence quality: high.
Strength of recommendation: strong recommendation 84.2%, conditional recommendation 15.8%.
Consensus level: 94.7%.
Comments: Interpersonal and intrafamilial transmissions of H. pylori are important routes for bacterial spread among family members. Accumulating evidence from a large number of studies have clearly demonstrated that H. pylori infection has obvious family cluster infection.33–39 Regarding the role and infection status of parents in the transmission of H. pylori to children, both clinical surveys and molecular biology studies such as random amplified polymorphic DNA fingerprinting analysis have indicated that the infected parents, especially mothers, play a key role in H. pylori transmission within a household.35 40 When parents are infected by H. pylori, the infection rate of their children increases significantly; spread also occurs between spouses and among siblings.33–39 These results suggest that intrafamilial transmission is an important cause of H. pylori infection, but the risk of infection among family members varies from family to family. Factors such as living habits, race, hygiene conditions, socioeconomic status and family size all contribute to transmission. Bacterial spread among family members also varies greatly between different geographical regions.38 41 42
All H. pylori-infected patients will develop histological gastritis,1 12 and clinical manifestations after H. pylori infection are very different. In some infected people, H. pylori infection does not have any symptoms or signs, and it is only discovered during medical examinations or routine checkups. A portion of infected people will follow Correa’s cascade and develop chronic non-atrophic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia and GC after years or decades of slow development.1 12 43
CQ2. What are the transmission routes for H. pylori spread within family units or among family members?
Statement 2: H. pylori is transmitted by oral route, and intrafamilial transmission is one of the major sources of infection.
Evidence quality: high.
Recommendation strength: strong recommendation 78.9%, conditional recommendation 21.1%.
Consensus level: 94.3%.
Comments: Mounting evidence has demonstrated that H. pylori is transmitted through oral–oral, fecal–oral routes and water sources39 44–46 (table 2). H. pylori can be isolated and cultured from vomitus, as well as occasionally from saliva and cathartic stools of infected people,44 45 and the bacterium can be detected in dental plaque, cavities, saliva specimens and dental pulp using in vitro molecular detection methods such as PCR and western blots.47–50 In addition, H. pylori can also be detected in a variety of animals,46 51 52 and isolated and cultured in the gastric mucosa of sheep,46 goats and cows.52
Table 2.
Common Helicobacter pylori transmission routes and preventive measures
| Types of transmission | Transmission routes | Measures for prevention |
| 1. Oral–oral transmission. | Chewing food before feeding children; kissing; consuming contaminated water, meat, milk, vegetables and other foods; poor hygiene practice, etc | Avoid chewing food before feeding to infants and young children; eat healthy and safe foods; implement good personal hygiene practice. |
| 2. Shared utensils or equipment transmission. | Sharing food utensils, such as dishes, bowls, chopsticks, spoons and other food containers; using contaminated dental equipment, etc | Do not share food utensils such as plates and food wares; implement individual dining and serving; separate use of chopsticks, spoons, etc; use safe dental equipment. |
| 3. Fecal–oral transmission. | Drinking water or eating food contaminated by excrement, such as well water and untreated water | Consume only hygienic and safe food and water. |
| 4. Iatrogenic transmission. | Intimate contact with people infected with H. pylori or contaminated equipment, medical equipment that has not been thoroughly disinfected, etc | Avoid intimate contact with H. pylori-infected persons and suspicious equipment; thoroughly disinfect medical equipment. |
Studies have also found that H. pylori can survive for a certain period in food stuffs, such as milk, juice, vegetables, ready-to-eat foods and different kinds of meats.53 Investigations in Latin America using molecular techniques or bacterial culture methods have found that H. pylori is also present in drinking water, fresh water, well water, estuarine water, seawater and marine products.54–58 Although the specific routes of transmission remain to be confirmed, these results strongly indicate that they are one of the major causes of infection among family members. H. pylori infection is thus speculated as a foodborne or waterborne disease, with humans and animals the likely reservoirs.53 58
Due to its contagious nature, H. pylori infection is usually spread among family members, and the infected strains among members can be completely same or very similar after mutation.33–39 59 Infected individuals may also carry strains of different origins, suggesting that there is also a possibility of exogenous infection.35 40 60 Further investigations are warranted to clarify the proportion and importance of both infection routes.
CQ3. Are H. pylori-infected family members a source of transmission to other family members?
Statement 3: Family members infected by H. pylori are potential sources of infection and have the possibility for continued transmission.
Evidence quality: moderate.
Strength of recommendation: strong recommendation 73.6%, conditional recommendation 26.4%.
Consensus level: 92.1%.
Comments: Individuals infected by H. pylori usually will not clear by itself without proper treatment; family members infected by H. pylori are always a potential source of infection and have the possibility for continued transmission.33–39 When living in the same household with one or more H. pylori-infected family members, other members such as spouses and children are exposed with increased infection risk.38 39 Although H. pylori may not necessarily infect every member of a household, the likelihood of infection depends on close contact, living habits and hygiene conditions.38 39 61 Therefore, education of family members about good hygiene practices, eating habits, awareness of H. pylori and its detrimental effects, handwashing, and avoidance of raw or contaminated food and water are all important to help prevent cross-contamination and reduce the risk of infection (table 3).
Table 3.
Helicobacter pylori infection control and management strategies
| Strategies | Characteristics | Applications and limitations |
| 1. Test and treat. | Recommended for uninvestigated young patients with dyspeptic symptoms, but not for older patients or persons with alarm symptoms | Not suitable for areas with high H. pylori infection rates and high incidences of GC |
| 2. Screen and treat. | Recommended for patients with family history of GC and alarm symptoms; not suitable for areas with low H. pylori infection rates | Suitable for areas with high H. pylori infection rates and high incidences of GC |
| 3. Family-based control and management. | Targeting H. pylori-infected individuals within the family; screening, treating and following up H. pylori-infected family members within the household | Application areas are not affected by H. pylori infection rates or incidences of GC. |
GC, gastric cancer.
For Chinese families, a common problem that deserves close attention is the use of serving/public chopsticks and spoons. As Chinese families have this historical tradition, and some of them usually share foods in the same dish or bowl, sometimes using the same utensils, which are sources of H. pylori cross-contamination. Therefore, changing habit and promoting separate meals/serving for individual family members are desirable, especially during family gatherings.
CQ4. When and where do most people get H. pylori infection in their lifetime?
Statement 4: Most H. pylori infections occur during childhood and adolescents but can also be acquired in adulthood.
Evidence quality: moderate.
Strength of recommendation: strong recommendation 63.1%, conditional recommendation 36.9%.
Consensus level: 84.2%.
Comments: Studies have shown that intrafamilial transmission is a major source of H. pylori transmission to children and adolescents (10–19 years of age), and infection is mainly transmitted by parents, especially by mothers.33 34 38 39 Common routes of transmission among family members include sharing utensils and food, chewing food before feeding children, kissing, drinking contaminated water and poor hygiene habits.37 38
One 2006 survey of childhood H. pylori infection conducted in Shanghai, China, included 1119 healthy school children and adolescents62; the results showed that infection rate among asymptomatic children in the 7-year-old age group was 30.9%, with an average annual increase of 3.2%, reaching the adulthood infection level at the age of 12. In 2014, a retrospective survey in China that included 1634 paediatric inpatients undergoing endoscopy found that among children aged under 3, 4–6, 7–10 and 11–18 years groups, H. pylori infection rates were 24.6%, 27.2%, 32.9%, and 34.8%, respectively.63 In recent years, due to improved living conditions and healthcare, the overall H. pylori infection rate has declined.6 63–65 One H. pylori infection survey in 2011 conducted in three major Chinese cities (Beijing, Guangzhou and Chengdu) included 3491 children; the results showed that in children aged 1–3, 4–6, 7–9, 10–12, 13–15 and 16–18 years, the infection rates were 0.6%–4.9%, 5.6%–9.7%, 3.9%–7.1%, 8.6%–12.1%, 6.2%–17.2% and 13.0%–33.0%, respectively.64 However, for children and adolescents living in rural areas, due to limited access to healthcare and poor sanitation conditions, the infection rates are expected to be higher than those in cities.6 Further investigations are required to determine rural infection conditions.
These results demonstrate that H. pylori infection rate in children increases with age. Infection is mainly acquired during childhood and adolescent stages,62–64 and it can also be acquired during adulthood, although the chances are slightly less. In addition, family living style, living environment, education level, socioeconomic status and family size are factors that affect its spread.
CQ5. Should all H. pylori-infected adult family members be treated to eliminate the infection?
Statement 5: For all H. pylori-infected adult family members in a household, eradication should be considered.
Evidence quality: moderate.
Recommendation strength: strong recommendation 52.6%, conditional recommendation 47.4%.
Consensus level: 81.5%.
Comments: For infected adult family members, H. pylori eradication is recommended following guidelines from the ‘Fifth Chinese National Consensus Report on the Management of Helicobacter pylori Infection’, unless there are competing considerations.1 The 2015 ‘Kyoto Global Consensus Report on Helicobacter pylori Gastritis’ also indicated that H. pylori gastritis is an infectious disease. Eradicating H. pylori can eliminate the source of infection, reduce infection rate of the population and prevent serious complications.12 Other guidelines and consensus suggestions including the 2017 ‘Management of Helicobacter pylori Infection—The Maastricht V/Florence Consensus Report’, consensus of the USA, Japan and Asia-Pacific region,12–18 and results from several large-scale clinical observations23–26 have all recommended eradicating H. pylori before the occurrence of gastric mucosal atrophy and intestinal metaplasia to reduce the risk of GC. Therefore, unless there are competing considerations, eradicating H. pylori infection is recommended for all adults within the family.
The phenomenon of family cluster infections can also partially explain the fact that gastric mucosal precancerous lesions or GC are present in one or more infected family members at different times of their lives, indicating that H. pylori infection may play a critical role in disease progression.66–68
Section 2: Prevention and management of H. pylori infection in children and elderly people within the household
CQ6. Does H. pylori infection induce gastric mucosal precancerous lesions in children and adolescents?
Statement 6: The relationship between H. pylori infection and gastric mucosal precancerous lesions in children and adolescents needs further investigation.
Evidence quality: moderate.
Strength of recommendation: strong recommendation 50.0%, conditional recommendation 50.0%.
Consensus level: 86.8%.
Comments: Because H. pylori infection usually results in mild or no gastric mucosal lesions in paediatric patients, few studies have been performed on the relationship between H. pylori infection and gastric mucosal lesions in children.42 69 Recent reports have noted that gastric mucosal atrophy and intestinal metaplasia are present in H. pylori-infected children, even in those who are very young.42 63 69–72 Earlier small studies indicated that atrophy was present in 0%–72% of the samples studied.69 However, recent surveys of 524 children in China63 and 131 children in Japan70 showed that the incidence of atrophic gastritis in H. pylori-infected children was 4.4% and 10.7%, respectively. In stage II and III atrophic gastritis of H. pylori-positive Japanese children, the incidence of intestinal metaplasia is 4.6% in both the antrum and body of the stomach.70 A small Mexican study in 2014 on gastric biopsies from 82 children with chronic gastritis found that 8.5% of the samples had gastric mucosal atrophy, and 6.1% had intestinal metaplasia; and among the 36 H. pylori-infected children, six (16.7%) had atrophic gastritis.72 In Tunisia, an H. pylori-prevalent area, one study in 2009 found that patients with gastric mucosal atrophy accounted for 9.3% (32/345 cases) of the enrolled children and 14.5% (32/221 cases) of the enrolled patients with chronic gastritis; among these 32 children with atrophic gastritis, 30 were infected by H. pylori.73
However, research results are inconsistent between different regions and countries. For example, in a 2012 study of 96 H. pylori-infected children in Brazil, gastric mucosal atrophy was not found, and gastric mucosal atrophy was relatively rare in French and Austrian children infected by H. pylori.74–76 Although further research is needed, these data suggest that gastric mucosal atrophy and intestinal metaplasia in children and adolescents may present in a similar way to adult infection and are probably more common in H. pylori highly infected areas than they were previously thought. Therefore, active intervention based on infection and disease status is required. It is also necessary to explore further on its natural course, consequences, potential carcinogenic risks and factors that lead to atrophy and metaplasia during H. pylori infection.
CQ7. Within a household, should all H. pylori-infected children be treated to eliminate the infection?
Statement 7: H. pylori infection in children needs to be managed based on risk–benefit assessment and related disease status.
Evidence quality: high.
Strength of recommendation: strong recommendation 60.5%, conditional recommendation 39.5%.
Consensus level: 89.4%.
Comments: Management of H. pylori infection in children is recommended primarily based on risk–benefit assessment, related disease status and depending on the infection rate in different geographical locations.42 77–79 In 2015, the Gastroenterology Group of the Chinese Paediatric Association, CMA, published a guideline on the management of H. pylori infection in children.77 The consensus recommends that H. pylori must be eradicated in children with peptic ulcer and patients with gastric mucosa-associated lymphoid tissue (MALT) lymphoma. H. pylori eradication can be offered for children with chronic gastritis, those with a family history of GC or unexplained refractory iron-deficiency anaemia, those planning to take long-term non-steroidal anti-inflammatory drugs (NSAIDs, including low-dose aspirin), and those whose guardians or older siblings strongly demand it. The indications to detect H. pylori infection include these conditions and children with a family history of GC in first-degree relatives, but H. pylori testing is not recommended as a routine assay.77
For North America and Europe, the joint ESPGHAN/NASPGHAN guidelines for the management of H. pylori in children and adolescents (Update 2016) recommended against a test-and-treat strategy for H. pylori infection in children but recommended testing and treating H. pylori in children with gastric or duodenal ulcer diseases.78 As these recommendations are primarily based on and for the settings of North America and Europe, where H. pylori infection rates are low and decreasing, along with the related disease burden, the guideline may therefore not apply to other areas of the world where the infection rate and related disease burden are high, such as Asia, Latin America and many developing countries.
In 2020, the Japanese Society of Paediatric Gastroenterology, Hepatology and Nutrition published a revised guideline managing H. pylori infection in children.79 The guideline recommends that H. pylori must be eradicated in children with gastric or duodenal ulcers, histological gastric mucosal atrophy, gastric MALT lymphoma, protein-losing gastroenteropathy, iron-deficiency anaemia and chronic idiopathic thrombocytopenic purpura. The report also recommends using at least two H. pylori tests, such as two non-invasive tests (eg, breath test and stool antigen test) or a biopsy-based and a non-invasive test (eg, breath test) for more accurate diagnosis of active infection.
This guideline79 also recommends that for children aged 5 years or older with active infections, H. pylori eradication therapy can be offered when reinfection possibility is considered. Consideration of H. pylori eradication therapy is recommended for children who have active gastritis, undergo gastroscopy due to abdominal symptoms, have a family history of GC in first-degree or second-degree relatives, and in whom active H. pylori infection has been found. However, H. pylori eradication therapy is not recommended for chronic primary urticaria. A test-and-treat approach is not recommended for asymptomatic children for preventing GC or preventing reinfection in adults in families where H. pylori infection has been eradicated. Compared with its previous versions, the updated recommendations have further clarified the indications for H. pylori detection and eradication in children.
Regarding the methods for diagnosis and confirmation of H. pylori eradication in children and adolescents, commonly applied 13C-urea breath test (UBT), stool antigen test and serum antibody test, as well as invasive methods including rapid urease test, histological examination and culture, have been recommended. These methods have different accuracy and application limitations, and can be offered based on the indications mentioned in the aforementioned guidelines.76–78 At least two tests are recommended for more accurate diagnosis.79 Eradication therapies for children and adolescents are slightly different from adult regimens, and body weight, dose calculation-based triple therapies are recommended, including proton pump inhibitor (PPI) plus two antibiotics, such as amoxicillin and clarithromycin or metronidazole; the course is 7–14 days in Japan79 to 10–14 days in China, North America and Europe regimens. Bismuth is not approved in Japan79 but is recommended in the consensus reports from China, North America and Europe.77 78 To achieve maximal therapeutic effects, consultation with parents or guardians before eradication therapy is mandatory.
CQ8. Should all H. pylori-infected elderly family members be treated for the infection?
Statement 8: For elderly members of the family, strategies for treating H. pylori infection should be formulated based on individual conditions.
Evidence quality: moderate.
Recommendation strength: strong recommendation 65.7%, conditional recommendation 34.3%.
Consensus level: 97.3%.
Comments: H. pylori infection rate is higher in elderly population (60–65 years or older). Eradication of H. pylori can improve GI symptoms in this group of patients, and, to a certain extent, prevent, delay or even partially reverse gastric mucosal atrophy and intestinal metaplasia, as well as reducing the incidence of GC.80–83 Studies have shown that the elderly population has no significantly increased resistance to antibiotics commonly used to eradicate H. pylori such as amoxicillin and furazolidone, but resistance to quinolone (levofloxacin) and clarithromycin has increased.83 84 Eradication treatment should be considered for this population unless competing considerations exist.
The elderly population often has one or more disease conditions at the time of treatment, such as heart, lung, cerebrovascular and kidney diseases, or uses long-term NSAIDs. Therefore, caution should be exercised and risk–benefit assessment should be carried out before H. pylori eradication. Individual or personalised treatment plans should be selected based on the patient’s drug intake history, physiopathological condition, disease status and adverse drug reactions.80–83 Discussing treatment regimens with family members or carers is recommended. Proper instruction and education about eradication processes before and during treatment can improve adherence and render the individualised treatment safe and effective.
Section 3: Strategies for prevention and management of H. pylori infection among family members
CQ9. Is family-based H. pylori infection control and management a practical strategy to prevent H. pylori intrafamilial transmission and infection?
Statement 9: Family-based H. pylori infection control and management is an important strategy to prevent intrafamilial transmission and infection.
Evidence quality: moderate.
Strength of recommendation: strong recommendation 76.3%, conditional recommendation 23.4%.
Consensus level: 86.8%.
Comments: Two strategies are currently available for the management of H. pylori infection (table 2): (1) test and treat, which is recommended for young patients with uninvestigated dyspepsia but is unsuitable for older patients and those with alarm symptoms; and (2) screen and treat, which is for patients who have a family history of GC, have alarm symptoms or live in GC-prevalent areas.1 12 13 In areas where the infection rate is low, the benefits of population-based screen-and-treat strategy are limited due to declining H. pylori infection rates and related disease burden such as peptic ulcers, precancerous lesions and GC. For areas where the infection rate and related disease burden are high, this strategy is beneficial, cost-effective and worth population-wide screening and intervention (table 2).1 13 17
H. pylori is transmitted orally, mostly among family members during childhood and adolescents.36 38 Prevention and control of intrafamilial transmission among family members is therefore a practical approach to block the transmission chain and to reduce the infection rate in a society; this approach has been suggested to reduce the infection rate and related diseases.20 21 85 The notion of family-based H. pylori infection control and management was also recently introduced as a third important strategy to block H. pylori transmission and infection in China19 20 31 32 (figure 1), and the strategy appears practical, easily manageable, with good family member adherence, and does not require differentiating high-infection and low-infection areas. Although large-scale household-based clinical trials to prove its efficacy are currently lacking, meta-analyses and scattered small reports have begun to demonstrate that whole family-based cotreatment approach is a superior strategy to regimens treating only infected individuals in reducing H. pylori reinfection rates.19 85 86 This is in line with the concept of mass screening and eradication of H. pylori in populations to reduce its related disease burden.17 25 26 It is also necessary to investigate further and to formulate detailed strategies to control household H. pylori infection, ideally by randomised controlled trials (RCTs).
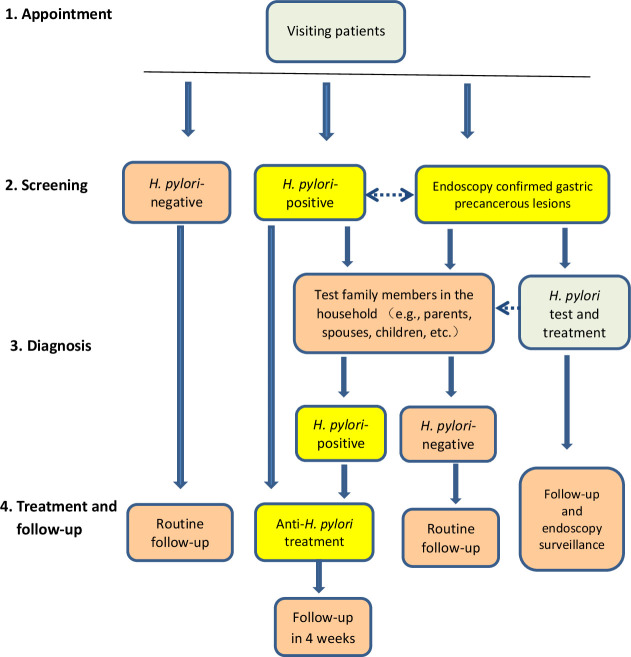
Figure 1.
Flowchart of family-based Helicobacter pylori infection control and management. In clinical settings, visiting patients are questioned for symptoms and signs, and Helicobacter pylori infection status is screened by urease breath tests, serological tests or stool antigen tests. If the patient is H. pylori-positive, their family members are recommended to test for H. pylori using one or more of these methods. Family members usually include parents, spouses, children and others living in the same household. The infected patients and family members are advised to treat the infection based on individual condition and follow-up in 4 weeks. If patients or their family members are H. pylori-negative, routine follow-up and no treatment are required. For patients with endoscopy-confirmed gastric precancerous lesions such as atrophy, intestinal metaplasia and intraepithelial neoplasia, H. pylori infection status should be tested, and if it is positive, eradication therapy should be offered and regular endoscopy surveillance should be performed regardless of H. pylori infection status. Dashed line with arrow indicates interaction and close relationship.
CQ10. Is concurrent treatment of H. pylori-infected family members helpful to reduce the chance of reinfection among family members?
Statement 10: Concurrent treatment of H. pylori-infected family members is helpful to reduce the chance of reinfection after its eradication.
Evidence quality: moderate.
Recommendation strength: strong recommendation 65.7%, conditional recommendation 34.3%.
Consensus level: 81.5%.
Comments: One critical issue after H. pylori eradication is its reinfection and recurrence; study results from different regions have yielded slightly different outcomes, ranging from 0% to 12%.14 87–92 One Chinese study in 2019 showed that recurrence rates of 1 and 3 years in adults after H. pylori eradication (including recrudescence within 1 year and reinfection over 1 year after eradication) were 1.75% and 4.61%, respectively. Low income and poor hygiene conditions are independent risk factors responsible for recurrence.87 A systematic review in 2017 showed that the global annual H. pylori recurrence, reinfection and recrudescence rates were 4.3%, 3.1% and 2.2%, respectively.88 However, these results vary by regions and countries. For example, a Korean study in 2013 showed that the long-term (37.1 months) average reinfection rate was 10.9%, and the annual reinfection rate was 3.5% after H. pylori eradication.89 The results may also differ between developing and developed countries92 and between urban and rural areas because residents may experience different healthcare accessibilities, living environments and sanitation conditions.87 However, very few studies have compared the reinfection rate in H. pylori-infected households versus the general infection conditions in a community.
H. pylori infection among family members can occur before, during or after treatment. Family members living with H. pylori-infected individuals are exposed to an increased infection risk due to factors such as similar living habits, sharing food or food wares, intimate contact and chewing food before feeding children. This could also be the reason that some patients are reinfected after eradication.61 85 87 Concurrent treatment of all H. pylori-infected family members could theoretically eliminate its cross-infection and spread within the household because the familial source of infection is removed.21 85 86 Recent studies have begun to show that family-based cotreatment of H. pylori infection could partially reduce recurrence rates and increase eradication rates compared with the approach of treating only infected individuals.19 85 86 93
A small, single-centre, non-RCT observation in China93 in 2014 showed that the 24-month cumulative recurrence rate in the single-infected patient treatment group was 19.7%, and in the family cotreatment group, it was only 7.4%. These results are in line with a previous report by Sari et al in Turkey19 in 2008. However, in countries or regions where H. pylori infection rates are low, no difference exists between these two treatment approaches. For example, one study in the UK94 in 2004 found no difference between the two treatment groups, even after 62.2 months of follow-up on 50 families. One German study95 in 2002 that was conducted with a 2-year follow-up of 108 cases of H. pylori-eradicated patients found no reinfection; even some family members were H. pylori-positive. In 2002, Gisbert et al in Spain96 followed up 120 cases of H. pylori-eradicated patients and found 6.8% of the patients whose spouses were H. pylori-positive were reinfected per year. However, molecular biology study showed that bacterial strains of the reinfected patients were different from those of their partners, suggesting that reinfected H. pylori strains are from different sources.
In 2021, a meta-analysis demonstrated that whole-family H. pylori eradication is a superior strategy to the single-infected patient treatment approach.85 The overall recurrence rate at 1-year follow-up in the pooled data was 2.7% for the whole-family treatment group, and 10.5% for single-infected treatment patient group. Studies in this area currently lack large-scale, multicentre RCTs, and further stratified investigations are warranted.
CQ11. Should H. pylori be screened and treated among family members living in the same household with patients who have GC or gastric mucosal precancerous lesions?
Statement 11: For patients with GC or gastric mucosal precancerous lesions, H. pylori infection should be screened and treated for their family members living in the same household.
Evidence quality: moderate.
Recommendation strength: strong recommendation 78.9%, conditional recommendation 21.1%.
Consensus level: 84.2%.
Comments: H. pylori has the characteristic of family clustering infections. Persistent H. pylori infection is the most important factor for the initiation and development of gastric mucosal precancerous lesions, such as atrophic gastritis and intestinal metaplasia,97 98 and H. pylori-infected family members of patients with GC have increased risk of developing gastric mucosal precancerous lesions and GC.66–68 99 Although H. pylori infection-related diseases are more frequently present in adulthood, infections are mostly acquired during childhood and adolescents; the chronic gastritis progresses into atrophic gastritis, intestinal metaplasia, dysplasia and GC after years of slow development.12 13 Despite other factors such as genetics, chemical factors, diet, living habits and age are also closely related to histological gastric mucosal atrophy and intestinal metaplasia; H. pylori is the most important known cause of these precancerous lesions and GC,98 100 especially for patients who carry the CagA-positive genotype strains.101 102 As the high-risk group of individuals for GC, family members of patients with GC should be tested for H. pylori, and those who are H. pylori-positive should receive eradication therapy. For family members with gastric mucosa precancerous lesions, H. pylori infection status should be tested and eradicated if it is positive. In addition, regular endoscopic examination is recommended for early GC surveillance in these patients.1 2
Patients with peptic ulcers usually have higher H. pylori infection rates (90.4%),13 103 and family members living with the patient are also exposed to a higher risk of infection. For H. pylori-infected adult patients in the family, eradication therapy is recommended unless competing considerations exist.1 13 Recent studies on the genotyping of infected persons also show that type I H. pylori strains (CagA and VacA positive) are the major type of H. pylori infection in Chinese residents,65 103 104 and it induces severer inflammation, gastric mucosal lesions and are more prevalent in patients with GC than type II (CagA and VacA negative) strains. This is also supported by population-based studies and confirmed by basic science research experiments.101 105–108 Future investigations in this area are of great importance for preventing gastric mucosal lesion and GC in first-degree relatives of patients with GC.66 109
The concept of eradicating H. pylori infection in whole family unit is also reflected in existing international consensus. For example, in the 2018 ‘Helicobacter pylori Management in ASEAN: the Bangkok Consensus Report’,14 statement 4 recommends that ‘eradication of H. pylori reduces the risk of GC and family members of patients with GC should be screened and treated’. The 2020 ‘Screening and Eradication of Helicobacter pylori for Gastric Cancer Prevention: the Taipei Global Consensus’ also recommended ‘screening and eradication of H. pylori for gastric cancer prevention in populations with a high incidence or high risk of gastric cancer’ (statement 6), and that this approach should be included in the national health insurance plan (statement 12).17 The 2016 ‘Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States’ also included several family member-related testing and treatment statements.18
Additionally, a number of clinical observations and international consensuses have clearly highlighted that eradication of H. pylori can relieve the inflammatory level, the risk of gastric mucosal atrophy, intestinal metaplasia, dysplasia and even the incidence of GC.23 26 28 Young generations would benefit most from this strategy because eliminating H. pylori infection can reduce the risk of precancerous lesions, along with GC in later life. It also reduces the chance of transmission to their children.17 Even at the stage of atrophic gastritis and intestinal metaplasia, eradicating H. pylori is also beneficial in preventing the deterioration of gastric mucosal lesions,23 26 and reducing the occurrence of metachronous cancer in patients with GC after endoscopic resection treatment.80 110
CQ12. What are the suitable treatment regimens for Chinese residents to eradicate H. pylori among family members?
Statement 12: The treatment regimens proposed by the ‘Fifth National Consensus Report on the Management of Helicobacter pylori Infection’ are suitable for H. pylori eradication among family members.
Evidence quality: high.
Recommendation strength: strong recommendation 78.9%, conditional recommendation 21.1%.
Consensus level: 94.7%.
Comments: In 2017, the Helicobacter pylori Study Group of Chinese Society of Gastroenterology, CMA, published its ‘Fifth National Consensus Report on the Treatment of Helicobacter pylori Infection’, which proposed seven different combinations of bismuth and PPI-containing quadruple treatment regimens (BQT) as the main empiric therapy to eradicate H. pylori. The course of treatment is 10 or 14 days (PPI+bismuth+two antibiotics). These regimens are currently commonly applied in clinical practice with high eradication rates1 (table 4). They are also suitable for family-based H. pylori eradication in the household. Infected family members should be offered a quadruple regimen with known high curative rate locally and low adverse drug reaction rate as recommended.1 Bismuth does not have drug resistance and is safe and convenient in short-term application. Therefore, unless there are competing considerations, the consensus recommends the empirical bismuth quadruple regimen to treat H. pylori infection. Its current eradication rate is above 85% and, in some instances, above 90% (table 4),1 111–114
Table 4.
Bismuth and PPI containing quadruple therapies currently recommended for Helicobacter pylori eradication by the ‘Fifth National Consensus Report on the Management of Helicobacter pylori Infection’1
| Regimens | Antibiotic 1 | Antibiotic 2 |
| 1 | Amoxicillin 1000 mg, two times a day | Clarithromycin 500 mg, two times a day |
| 2 | Amoxicillin 1000 mg, two times a day | Levofloxacin 500 mg, one time a day; or 200 mg, two times a day |
| 3 | Amoxicillin 1000 mg, two times a day | Furazolidone 100 mg, two times a day |
| 4 | Tetracycline 500 mg, three or four times a day | Metronidazole 400 mg, three or four times a day |
| 5 | Tetracycline 500 mg, three or four times a day | Furazolidone 100 mg, two times a day |
| 6 | Amoxicillin 1000 mg, two times a day | Metronidazole 400 mg, three or four times a day |
| 7 | Amoxicillin 1000 mg, two times a day | Tetracycline 500 mg, three or four times a day |
Standard dose isPPI+bismuth compound (two times/day, orally half an hour before meals)+two antibiotics (orally after meals). The standard doses of PPIs are esomeprazole 20 mg, rabeprazole 10 mg (or 20 mg), omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg and prazole 5 mg. The standard dose of bismuth is 220 mg of potassium bismuth citrate (the standard dose of bismuth pectin is to be determined).1
PPI, proton pump inhibitor.
However, the actual eradication rates also have variations, depending on different regimens used and geographical location.115 116 One large-scale clinical trial that involves a total of 94 101 subjects in 2016 found that the overall H. pylori eradication rate was only 72.9% in the infection group by using the omeprazole, tetracycline, metronidazole and bismuth citrate 10-day regimen. Gender, body mass index, history of stomach disease, baseline delta over baseline value of 13C-UBT, missed medication doses, smoking and drinking were independent predictors of eradication failure.115 While in other studies, where eradication therapy that uses different regimens such as esomeprazole, amoxicillin, clarithromycin and bismuth citrate for 14 days, the eradication rates are around to or above 85%.116 In addition to China, the BQT therapies are also recommended as treatment strategies by several international consensus reports, such as the ‘Management of Helicobacter pylori Infection-The Maastricht V/Florence Consensus Report’, the ‘Toronto Consensus for the Treatment of H. pylori Infection in Adults’, and ‘Helicobacter pylori Management in ASEAN: the Bangkok Consensus Report”.13–15
Two concerns for the widespread use of antibiotics are their adverse effects and H. pylori resistance.117 118 In 2019, one Chinese study investigated H. pylori resistance rates and found that in a treatment-naïve adult group, the resistance rates for metronidazole, clarithromycin, levofloxacin, amoxicillin, rifampicin and tetracycline were 78.4%, 19.0%, 23.3%, 1.2%, 1.7% and 2.3%, respectively. The previously treated adult group had significantly higher resistance rates for metronidazole (99.2%), clarithromycin (58.3%) and levofloxacin (52.3%).119 Recently, double-dose, high-frequency PPI+amoxicillin combination therapy without bismuth has shown promising results in H. pylori eradication. The regimen has been reported to achieve eradication rates similar to quadruple therapy in several individual trials, with eradication rates ranging from 84.7% to 95.3%.113 114 120 This regimen is relatively simple and easy to implement, with high patient adherence and fewer adverse drug reactions, providing clinicians an alternative option in practice. However, it has limitations: this approach is not suitable for patients who are allergic to amoxicillin, and a history of amoxicillin use may lead to the risk of drug resistance. Drug susceptibility tests are required in cases of repeated eradication failures.114 120 121
In addition to the aforementioned PPI-based regimen, potassium-competitive acid blocker (P-CAB)-based regimen was also introduced recently.122–125 P-CABs are a novel and heterogeneous class of drugs that competitively block the potassium-binding site of gastric H+/K+-ATPase. Vonoprazan (VPZ), a P-CAB, has shown a strong inhibitory effect on gastric acid secretion, which lasts longer and is not affected by CYP2C19 gene polymorphism. This provides physicians a new regimen option for eradicating H. pylori.122–124 Studies have compared the efficacy of VPZ versus PPIs in the treatment of acid-related disorders and for H. pylori eradication. Optimised VPZ–amoxicillin dual therapy can reliably achieve eradication rates equal to or higher than 95%.122–125 VPZ-based dual or triple therapy has shown similar or even higher eradication rates than the corresponding PPI-based therapy in intention-to-treat and per-protocol treatments for H. pylori eradication, potentially overcoming the limitations of PPIs.122 124 125 Further large-scale, prospective, multicentre randomised trials will be required to verify its efficacy.
CQ13. What is the management strategy in treating H. pylori infection among family members?
Statement 13: The concept of ‘eradicating H. pylori at the first-time treatment’ is applicable in the management of H. pylori infection among family members.
Evidence quality: high.
Recommendation strength: strong recommendation 86.8%, conditional recommendation 13.2%.
Consensus level: 94.7%.
Comments: The nature of H. pylori eradication is similar to the treatment of other commonly found pathogenic bacterial infections, but their characteristics are different. H. pylori eradication rates can be improved by using high-efficiency acid-suppressing PPIs to raise the stomach pH and therefore increase antibiotic bioavailability.126 In recent years, with widespread H. pylori eradication in many hospitals and clinics in China, the H. pylori resistance rate to antibiotics has been gradually increasing, which is accompanied with a decline in the empirical eradication rate.3 127 Failure of the first-time eradication may lead to the development of bacterial resistance and reduce the range of antibiotic options for future retreatment.118 127 Hence, successfully eradicating H. pylori infection in the first treatment is desirable.
H. pylori resistance to antibiotics is usually related to local bacteria resistance patterns and previous antibiotic usage.3 119 128 Before selecting an empirical treatment regimen, the cure rate, adverse drug reactions, convenience, accessibility, adherence and costs, along with the antibiotic resistance situation of the patient, should be evaluated to achieve the maximal therapeutic effect. It should also avoid misuse or unnecessary use of antibiotics.17 129 When treating H. pylori infection in children and adolescents, the safety and benefits of eradication should be assessed carefully based on individual infection history, antibiotic usage and disease status; doses of antibiotics should be adjusted according to body weight before eradication is implemented.16 77 78
Regarding the efficacy of susceptibility-guided individualised treatment and empirical therapy in eradicating H. pylori infection, a recent Chinese study confirmed that susceptibility-guided individualised therapy and previously proven locally highly effective empiric therapy are highly and equally effective.130 However, for patients who have repeatedly failed eradication, individualised therapy guided by drug-sensitivity testing is recommended.118 130 In addition, if the patient’s history of antibiotic use can be incorporated into the selection of empirical treatment regimen, it will help to increase the first-time H. pylori eradication rate and reduce the chance of developing drug resistance, and only therapies that are known to be highly effective locally should be used empirically.
CQ14. What are the suitable methods to detect H. pylori infection among family members?
Statement 14: UBTs, serum antibody tests and stool antigen tests are suitable methods to detect H. pylori infection among family members.
Evidence quality: high.
Strength of recommendation: strong recommendation 76.3%, conditional recommendation 23.7%.
Consensus level: 92.1%.
Comments: Non-invasive H. pylori tests include UBTs, serum antibody tests and stool antigen tests. These have been recommended by consensus reports to detect H. pylori infection both in China and internationally.1 13 14 They are currently commonly used in clinical settings and are suitable to detect H. pylori infection among family members. However, each of these methods has its own advantages and restrictions, and they can be selected based on their characteristics and applied either alone or in combination to obtain accurate results and to avoid false-positive and false-negative results (table 5).131–133
Table 5.
Accuracy of current commonly used Helicobacter pylori diagnosis methods
| H. pylori diagnosis tests | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
| Non-invasive tests | ||||
| 13C-UBT135 138 161 | 90.0%–96.0% | 94%–98.0% | 97.4%–99.2% | 90.8%–91.2% |
| 14C-UBT135 136 148 162 | 96%–97.3% | 91%–97.0% | 93.31%–96.8% | 94.3%–97.6% |
| Serum anti-H. pylori IgG antibody test142–144
Serum anti-H. pylori protein array UreB/CagA/VacA145 |
72.7%–90.0% 93.4%/95.4%/96.04% |
68.4%–100.0% 94.8%/94.4%/97.5% |
66.7%–100.0% N/A |
74.3%–83.3% N/A |
| HpSA ELISA test133 146–148 | 73.9%–95.0% | 86.8%–100.0% | 85%–100.0% | 76.5%–92.0% |
| Invasive tests | ||||
| RUT148 149 161 | 85.0%–99.0% | 92.4%–94.1% | 90.5%–97.4% | 86.5%–99.2% |
| Histology138 150 151 161 | 83%–95.5% | 95.4%–100.0% | 94.6%–100.0% | 88.6%–97.0% |
| Culture148 150 152 | 67.9%–96.0% | 79.4%–100.0% | 88.7%–100.0% | 50.9%–95.0% |
HpSA, Helicobacter pylori stool antigen test; N/A, not available; RUT, rapid urease test; UBT, urea breath test.
13C-UBT and 14C-UBT have the advantages of accuracy, relatively high specificity, convenience and being unaffected by patchy distribution of H. pylori in the stomach; their sensitivity and specificity are all above 90%.131 134–137 However, when test results are close to cut-off values, they must be treated with caution or verified by another method or repeated assay.132 138 Furthermore, 13C-UBT and 14C-UBT results are easily affected by prior use of antibiotics, PPIs and certain Chinese medicines1, 13; residual foods in the stomach, partial gastrectomy, gastric bleeding, gastric tumours, severe gastric mucosal atrophy or low amount H. pylori bacteria numbers in the gastric mucosa also interfere with test results, and false negatives and false positives may occur.128 132 Serum antibodies and stool antigen tests are not affected by these factors and are good alternative methods for confirmation, or they can be assayed together for more accurate results.139 140 On the occasion of serum tests being positive in untreated patients, they are more likely infected by H. pylori, and treatment options can be considered if active infection is confirmed. In addition, although both 13C-UBT and 14C-UBT are considered safe and efficient, for children and pregnant women, 13C-UBT is the preferred test for these groups of population to avoid the possible radiation exposure.141
Serum H. pylori antibody testing has been widely used in hospitals in China with sensitivity and specificity around 72.7%–90.7% and 68.4%–100.0%,142–144 respectively. H. pylori genotyping antibody testing (for CagA, VacA and UreB) showed higher sensitivity and specificity all above 90%.145 However, for patients whose H. pylori has been eradicated, due to the long-term presence of serum antibodies, these tests cannot differentiate between past and current infections and are not suitable for follow-up; 13C-UBT and 14C-UBT, or stool antigen testing, can make up for these shortcomings. Detection of H. pylori antigen in stools with monoclonal antibodies for the diagnosis of infection is currently available in China146 147; the sensitivity and specificity are around 73.9%–95% and 86.8%–100%, respectively (table 5). Therefore, combined use of multiple methods is of great value to effectively differentiate H. pylori infection status among family members. Commercial products such as H. pylori detection kits for oral plaque or saliva are also simple and rapid, but their sensitivity and specificity need improvements, and they are expected to be the preliminary methods for household family member screening in the future.
Other invasive methods for H. pylori detection include rapid urease tests,148–150 histological examination of gastric mucosal biopsies,150 151 cultures148 152 and molecular techniques.139 140 These are available in various clinical settings, but they are inconvenient and require endoscopy to obtain biopsy samples. H. pylori cultures can be used for drug susceptibility testing and bacteriological research, and molecular techniques can be used with specimens such as faeces or gastric mucosal tissues, especially for DNA genotyping of bacterial strains and detection of drug-resistant gene mutations. These methods are applied in different scenarios to detect family member infection.
CQ15. Do we need an H. pylori infection control and management strategy at the general public or community levels to prevent its infection and related disease?
Statement 15: Family-based H. pylori infection control and management is an essential part of comprehensive H. pylori infection prevention and control strategies at the general public and community levels.
Evidence quality: moderate.
Strength of recommendation: strong recommendation 73.6%, conditional recommendation 26.4%.
Consensus level: 92.1%.
Comments: intrafamilial spread is one of the major routes of H. pylori transmission and infection. In addition to the test-and-treat and screen-and-treat strategies, the newly introduced concept of family-based H. pylori infection control and management is another important strategy.1 13 21 31 The strategy is to screen, identify, treat and follow-up all H. pylori-infected family members and is expected to reduce the overall infection burden for the nation in the coming decades. As family members become engaged and motivated, it would be easier to implement H. pylori eradication programmes beginning from family units and to extend them to the general public at the national level.20 21
The advantage of this approach (figure 1) is that it proposes not only to treat the visiting patients but also to engage non-visiting, untreated but infected family members. It also considers the gastric mucosal lesion progression of infected family members. Patients’ adherence will be improved with the participation and encouragement from family relatives; therefore, prevention and management of H. pylori infection at public and community levels should include the notion of family-based H. pylori infection control and management to reduce the sources of infection.20 21 31 These approaches will be helpful to block the H. pylori transmission chain and to reduce the likelihood of reinfection. It is currently the most economical, practical and manageable approach to reduce H. pylori infection burden for the nation. At the general public and community levels, this can be achieved through advocacy by government health administrative officials, education programmes from multiple forms of media to increase public awareness about the detrimental effects of H. pylori and to promote healthy lifestyles and habits.153
One concern about this strategy is that it might overscreen family members who are not infected. Despite the fact that cost–benefit assessments on large scale, population-wide treatment programmes have yet to be determined, previous results have shown that population-wide screening is cost-effective in GC and related disease prevention.4 154–156 In addition, as non-invasive urease breath tests, serological tests and stool antigen tests are more affordable, accessible and efficient, this strategy has provided a practical solution for whole family-based H. pylori infection prevention and control, which is especially suitable for areas of high H. pylori infection and related disease prevalence. Clinicians including family doctors should also guide visiting patients in clinical practice to avoid unnecessary waste of medical resources and panic about H. pylori while diagnosing and treating infected persons and their family members.
CQ16. Will an H. pylori vaccine prevent infection?
Statement 16: While an H. pylori vaccine is not available, preventing new infections and eradicating existing infections are both effective approaches for infection prevention and control.
Evidence quality: moderate.
Strength of recommendation: strong recommendation 76.3%, conditional recommendation 23.7%.
Consensus level: 89.7%.
Comments: Regarding the development of H. pylori vaccine, many attempts have been made over the past decades.157–159 Due to the complexity of H. pylori antigen preparation and the human immune response, it is still at laboratory developmental stage and not yet suitable for clinical application. Therefore, prevention and control of H. pylori infection will still need to use antibiotics to control infection and prevent recurrence. Until an effective vaccine is available, preventing new infections is as important as eradicating existing infections among family members, and both are effective measures for H. pylori infection prevention and control.
Discussion
H. pylori infection is a family-based, population-wide infectious disease. In addition to the traditional test-and-treat and screen-and-treat strategies,1 12 13 this consensus introduced the third novel family-based H. pylori infection control and management strategy for H. pylori infection prevention and control at the national level. The strategy is to screen, identify, treat and follow up on all H. pylori-infected family members (figure 1). It is an extension of the previous consensus to eradicate H. pylori and is able to increase family members’ engagement and awareness of infection, prevent or reduce the spread of bacteria within households, and save later medical expenses. It is expected that future practice will confirm the convenience and efficacy of this approach, and provide clinical practice a better option for global H. pylori eradication and prevention.
The advantage of this strategy is that it changed the current clinical practice from only treating H. pylori-infected individuals into actively caring for the infection and disease status of the entire family, including non-visiting, untreated but infected family members, and trying to eliminate the source of infection and reduce the possibility of reinfection within households.1 12 13 20 21 31 Family members who are at high stakes are easily motivated and engaged to receive test and treatment. The strategy also integrates the relevant gastric mucosal lesions and disease progression that was already present in the infected family members, thus subsequently reducing the chance for further deterioration of mucosal inflammation and precancerous lesions.20 21 30 In addition, the practice is also expected to positively influence or improve the family’s hygiene habit and prevent other infectious diseases. Family doctors and physicians should also provide family members and the general public relevant information about H. pylori and, at the same time, avoid unnecessary waste of medical recourses.
Despite the consensus statements and conclusions being well supported by the current available evidence, this strategy has limitations. First, the concept of family-based H. pylori infection control and management is introduced to the field for only several years before reaching current consensus19–21; therefore, experience from this practice is very limited and future refinement is necessary. Second, this consensus is based on and for the setting of China and areas with high infection rates and related disease burden; some of the statements may not be suitable for other countries or areas, as infection condition and treatment strategies vary greatly between different countries or communities. Third, available evidences supporting the strategy on health economics/cost-effectiveness, and several other statements are actually weak, and they are in much need to be improved and strengthened by future investigations. Fourth, massive population-wide eradication-related problems, such as antibiotic resistance, drugs’ adverse effects and refractory cases, may increase with the increasing number of patients treated, which deserves serious attention from clinicians, pharmacists and health administrative officials. This version of the consensus will need to be improved and revised based on future clinical practice and evidence-based medicine.
For future perspectives, there are several important areas that need to be improved: first, one critical issue discussed in statement 6 is that if H. pylori might induce gastric mucosal precancerous lesions in children and adolescents, and if the CagA-positive and VacA-positive strains might have different effects compared with CagA-negative and VacA-negative strains in inducing gastric atrophy and intestinal metaplasia in this population, future investigations in these areas are critical to provide evidence to guide future clinical practice and consensus recommendations. Second, another statement that caused much debate and barely passed the consensus is statement 10, which tackles the problem if concurrent treatment of H. pylori-infected family members might reduce the chance of reinfection among family members. As large-scale, randomised controlled clinical trials are actually lacking in these areas, further studies are required for more convincing evidence. Third, future investigations on the molecular mechanisms that H. pylori infection induced gastric precancerous lesion and cancer are critically important to increase our understanding on the carcinogenesis,105 160 and to help identify therapeutic targets for intervention and prevention. Fourth, techniques for reliable diagnosis methods for H. pylori infection, confirmation of eradication, and detection drug resistance to guide antibiotics selections are also needed for more convenient and easy access; future development in these areas is of great value to assist accurate detection and susceptibility-guided H. pylori eradication.
In conclusion, the current work summarises previous studies and presents novel consensus recommendations on family-based H. pylori prevention and management. Despite the fact that it just started from China, the practice actually paves the road to explore a global whole family-based H. pylori eradication project; therefore, it has the potential to provide clinical practice a novel avenue for the management of H. pylori infection at a broader level. It is expected to play an important role in reducing H. pylori spread among the Chinese population, increase family members’ awareness of infection, improve public health and reduce related diseases and GC burden in the coming decades. In addition to China, the notion and practice could also be a valuable reference and benefit other H. pylori highly infected areas or communities globally.
Footnotes
SZD, YQD, HL and WHW contributed equally.
Contributors: Conception and design: SZD, YQD, HL, WHW, LYZ, NHL, YSY, ZSL.
Faculty members SZD, YQD, HL, WHW, CHL, CH, JBW, JMX, GBY, GXZ, JZZ, ZYZ and YL prepared and drafted clinical questions (CQs), statements and comments, which were further revised by senior members LYZ, NHL, YSY and ZSL.
Faculty members HC, SYC, MHC, WCC, YC, JYF, HJG, MZG, YH, XHH, FLH, BJ, HXJ, CHL, JNL, YL, YQL, JL, YML, BL, YYL, YLM, YZN, JMQ, JQS, CWT, FW, HHW, JBW, JTW, JPW, XHW, KCW, XZX, WFX, YX, JMX, CQY, GBY, YY, ZRZ, BYZ, GYZ, GXZ, JZZ, ZYZ, PYZ, YZ and XLZ performed system review on the statements and critically revised manuscript.
Faculty members SZD, YQD, HL, WHW, HC, SYC, WCC, YC, HJG, FLH, CHL, JNL, YL, YQL, YLM, YZN, FW, HHW, JBW, JTW, XHW, WFX, YX, JMX, GBY, YY, ZRZ, GYZ, GXZ, JZZ, ZYZ, PYZ, NHL, YSY and ZSL, participated in voting and edited the CQ, statements and comments after voting.
ZSL, YSY, NHL, LYZ and SZD share the senior authorship and are co-corresponding authors of this work.
Funding: This work was supported by the National Clinical Research Center for Digestive Diseases (Shanghai), Gastrointestinal Early Cancer Prevention & Treatment Alliance of China, Helicobacter pylori Study Group of Chinese Society of Gastroenterology, CMA and Chinese Alliance for Helicobacter pylori Study, with no external funding support. Participating faculty members are supported by their own national, provincial or regional research grants. These funding sources had no role in the study design, data collection and interpretation, meeting organisation, manuscript writing or submission for publication.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein) or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Liu W-Z, Xie Y, Lu H, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter 2018;23:e12475. 10.1111/hel.12475 [DOI] [PubMed] [Google Scholar]
- 2. Du Y, Zhu H, Liu J, et al. Consensus on eradication of Helicobacter pylori and prevention and control of gastric cancer in China (2019, Shanghai). J Gastroenterol Hepatol 2020;35:624–9. 10.1111/jgh.14947 [DOI] [PubMed] [Google Scholar]
- 3. Hu Y, Zhu Y, Lu N-H. Recent progress in Helicobacter pylori treatment. Chin Med J 2020;133:335–43. 10.1097/CM9.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Q, Liang X, Long X, et al. Cost-effectiveness analysis of screen-and-treat strategy in asymptomatic Chinese for preventing Helicobacter pylori-associated diseases. Helicobacter 2019;24:e12563. 10.1111/hel.12563 [DOI] [PubMed] [Google Scholar]
- 5. Santos MLC, de Brito BB, da Silva FAF, et al. Helicobacter pylori infection: Beyond gastric manifestations. World J Gastroenterol 2020;26:4076–93. 10.3748/wjg.v26.i28.4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li M, Sun Y, Yang J, et al. Time trends and other sources of variation in Helicobacter pylori infection in mainland China: A systematic review and meta-analysis. Helicobacter 2020;25:e12729. 10.1111/hel.12729 [DOI] [PubMed] [Google Scholar]
- 7. Zhang T, Chen H, Yin X, et al. Changing trends of disease burden of gastric cancer in China from 1990 to 2019 and its predictions: findings from global burden of disease study. Chin J Cancer Res 2021;33:11–26. 10.21147/j.issn.1000-9604.2021.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang T, Sun D, Zhang Q, et al. China's drinking water sanitation from 2007 to 2018: a systematic review. Sci Total Environ 2021;757:143923. 10.1016/j.scitotenv.2020.143923 [DOI] [PubMed] [Google Scholar]
- 9. National Bureau of Statistics of China . China statistical Yearbook 2020. China Statistical Press, 2020. [Google Scholar]
- 10. Fang J-Y, YQ D, Liu W-Z, et al. Consensus on chronic gastritis in China (2017, Shanghai). Chin J Dig 2017;37:721–38. [Google Scholar]
- 11. Hu F-L, Zhang S-S. National integrated traditional Chinese and Western medicine management of Helicobacter pylori-related diseases. Chin J Gastroenterol Hepatol 2018;27:1008–16. [Google Scholar]
- 12. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]
- 14. Mahachai V, Vilaichone R-K, Pittayanon R, et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol 2018;33:37–56. 10.1111/jgh.13911 [DOI] [PubMed] [Google Scholar]
- 15. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51–69. 10.1053/j.gastro.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 16. Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter 2019;24:e12597. 10.1111/hel.12597 [DOI] [PubMed] [Google Scholar]
- 17. Liou J-M, Malfertheiner P, Lee Y-C, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020;69:2093–112. 10.1136/gutjnl-2020-322368 [DOI] [PubMed] [Google Scholar]
- 18. El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002. 10.1016/j.cgh.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sari Y-S, Can D, Tunali V, et al. H pylori: treatment for the patient only or the whole family? World J Gastroenterol 2008;14:1244–7. 10.3748/wjg.14.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding S-Z. Focusing on whole family based-Helicobacter pylori infection management and clinical research to prevent gastric mucosal diseases and gastric cancer. Zhonghua Yi Xue Za Zhi 2019;99:1446–8. 10.3760/cma.j.issn.0376-2491.2019.19.003 [DOI] [PubMed] [Google Scholar]
- 21. Ding S-Z. Global whole family based-Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J Gastroenterol 2020;26:995–1004. 10.3748/wjg.v26.i10.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malfertheiner P. Helicobacter pylori treatment for gastric cancer prevention. N Engl J Med 2018;378:1154–6. 10.1056/NEJMe1800147 [DOI] [PubMed] [Google Scholar]
- 23. Li W-Q, Ma J-L, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst 2014;106:dju116. 10.1093/jnci/dju116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong BC-Y, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–94. 10.1001/jama.291.2.187 [DOI] [PubMed] [Google Scholar]
- 25. Lee Y-C, Chen TH-H, Chiu H-M, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82. 10.1136/gutjnl-2012-302240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiang T-H, Chang W-J, Chen SL-S, et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 2021;70:243–50. 10.1136/gutjnl-2020-322200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori Infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–9. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 28. Ma J-L, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. 10.1093/jnci/djs003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson KA, Saldanha IJ, McKoy NA. Development of a framework to identify research gaps from systematic reviews. J Clin Epidemiol 2011;64:1325–30. 10.1016/j.jclinepi.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 30. Qaseem A, Snow V, Owens DK, et al. Clinical guidelines Committee of the American College of physicians. the development of clinical practice guidelines and guidance statements of the American College of physicians: summary of methods. Ann Intern Med 2010;153:194–9. [DOI] [PubMed] [Google Scholar]
- 31. National Clinical Research Center for Digestive Diseases (Shanghai), Gastrointestinal Early Cancer Prevention & Treatment Alliance of China, Helicobacter pylori Study Group of Chinese Society of Gastroenterology, Chinese Alliance for Helicobacter pylori Study . The Chinese consensus report on family based-Helicobacter pylori infection control and management (2021). Chin J Dig 2021;41:221–33. [Google Scholar]
- 32. National Clinical Research Center for Digestive Diseases (Shanghai), Gastrointestinal Early Cancer Prevention & Treatment Alliance of China, Helicobacter pylori Study Group of Chinese Society of Gastroenterology, Chinese Alliance for Helicobacter pylori Study . The Chinese consensus report on family based-Helicobacter pylori infection control and management (2021, popular science edition). Health World 2021;28:10–18. [Google Scholar]
- 33. Drumm B, Perez-Perez GI, Blaser MJ, et al. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med 1990;322:359–63. 10.1056/NEJM199002083220603 [DOI] [PubMed] [Google Scholar]
- 34. Georgopoulos SD, Mentis AF, Spiliadis CA, et al. Helicobacter pylori infection in spouses of patients with duodenal ulcers and comparison of ribosomal RNA gene patterns. Gut 1996;39:634–8. 10.1136/gut.39.5.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothenbacher D, Winkler M, Gonser T, et al. Role of infected parents in transmission of Helicobacter pylori to their children. Pediatr Infect Dis J 2002;21:674–9. 10.1097/00006454-200207000-00014 [DOI] [PubMed] [Google Scholar]
- 36. Perry S, de la Luz Sanchez M, Yang S, et al. Gastroenteritis and transmission of Helicobacter pylori infection in households. Emerg Infect Dis 2006;12:1701–8. 10.3201/eid1211.060086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garg PK, Perry S, Sanchez L, et al. Concordance of Helicobacter pylori infection among children in extended-family homes. Epidemiol Infect 2006;134:450–9. 10.1017/S0950268805005352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nahar S, Kibria KMK, Hossain ME, et al. Evidence of intra-familial transmission of Helicobacter pylori by PCR-based RAPD fingerprinting in Bangladesh. Eur J Clin Microbiol Infect Dis 2009;28:767–73. 10.1007/s10096-008-0699-8 [DOI] [PubMed] [Google Scholar]
- 39. Osaki T, Konno M, Yonezawa H, et al. Analysis of intra-familial transmission of Helicobacter pylori in Japanese families. J Med Microbiol 2015;64:67–73. 10.1099/jmm.0.080507-0 [DOI] [PubMed] [Google Scholar]
- 40. Kivi M, Johansson ALV, Reilly M, et al. Helicobacter pylori status in family members as risk factors for infection in children. Epidemiol Infect 2005;133:645–52. 10.1017/s0950268805003900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blecker U, Lanciers S, Mehta DI, et al. Familial clustering of Helicobacter pylori infection. Clin Pediatr 1994;33:307–8. 10.1177/000992289403300511 [DOI] [PubMed] [Google Scholar]
- 42. Okuda M, Lin Y, Kikuchi S. Helicobacter pylori infection in children and adolescents. Adv Exp Med Biol 2019;1149:107–20. 10.1007/5584_2019_361 [DOI] [PubMed] [Google Scholar]
- 43. Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 44. Thomas JE, Gibson GR, Darboe MK, et al. Isolation of Helicobacter pylori from human faeces. Lancet 1992;340:1194–5. 10.1016/0140-6736(92)92894-l [DOI] [PubMed] [Google Scholar]
- 45. Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA 1999;282:2240–5. 10.1001/jama.282.23.2240 [DOI] [PubMed] [Google Scholar]
- 46. Dore MP, Sepulveda AR, El-Zimaity H, et al. Isolation of Helicobacter pylori from sheep-implications for transmission to humans. Am J Gastroenterol 2001;96:1396–401. 10.1111/j.1572-0241.2001.03772.x [DOI] [PubMed] [Google Scholar]
- 47. Yee JKC. Helicobacter pylori colonization of the oral cavity: A milestone discovery. World J Gastroenterol 2016;22:641–8. 10.3748/wjg.v22.i2.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kadota T, Hamada M, Nomura R, et al. Distribution of Helicobacter pylori and Periodontopathic Bacterial Species in the Oral Cavity. Biomedicines 2020;8:161. 10.3390/biomedicines8060161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flores-Treviño CE, Urrutia-Baca VH, Gómez-Flores R, et al. Molecular detection of Helicobacter pylori based on the presence of cagA and vacA virulence genes in dental plaque from patients with periodontitis. J Dent Sci 2019;14:163–70. 10.1016/j.jds.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsami A, Petropoulou P, Kafritsa Y, et al. The presence of Helicobacter pylori in dental plaque of children and their parents: is it related to their periodontal status and oral hygiene? Eur J Paediatr Dent 2011;12:225–30. [PubMed] [Google Scholar]
- 51. Dimola S, Caruso ML. Helicobacter pylori in animals affecting the human habitat through the food chain. Anticancer Res 1999;19:3889–94. [PubMed] [Google Scholar]
- 52. Ranjbar R, Chehelgerdi M. Genotyping and antibiotic resistance properties of Helicobacter pylori strains isolated from human and animal gastric biopsies. Infect Drug Resist 2018;11:2545–54. 10.2147/IDR.S187885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quaglia NC, Dambrosio A. Helicobacter pylori: A foodborne pathogen? World J Gastroenterol 2018;24:3472–87. 10.3748/wjg.v24.i31.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moreno Y, Ferrús MA. Specific detection of cultivable Helicobacter pylori cells from wastewater treatment plants. Helicobacter 2012;17:327–32. 10.1111/j.1523-5378.2012.00961.x [DOI] [PubMed] [Google Scholar]
- 55. Holman CB, Bachoon DS, Otero E, et al. Detection of Helicobacter pylori in the coastal waters of Georgia, Puerto Rico and Trinidad. Mar Pollut Bull 2014;79:354–8. 10.1016/j.marpolbul.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 56. Abdel-Moein KA, Saeed H, Samir A. Novel detection of Helicobacter pylori in fish: A possible public health concern. Acta Trop 2015;152:141–4. 10.1016/j.actatropica.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 57. Reavis C. Rural health alert: Helicobacter pylori in well water. J Am Acad Nurse Pract 2005;17:283–9. 10.1111/j.1745-7599.2005.0047.x [DOI] [PubMed] [Google Scholar]
- 58. Santiago P, Moreno Y, Ferrús MA. Identification of viable Helicobacter pylori in drinking water supplies by cultural and molecular techniques. Helicobacter 2015;20:252–9. 10.1111/hel.12205 [DOI] [PubMed] [Google Scholar]
- 59. Raymond J, Thiberg J-M, Chevalier C, et al. Genetic and transmission analysis of Helicobacter pylori strains within a family. Emerg Infect Dis 2004;10:1816–21. 10.3201/eid1010.040042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kivi M, Tindberg Y, Sörberg M, et al. Concordance of Helicobacter pylori strains within families. J Clin Microbiol 2003;41:5604–8. 10.1128/JCM.41.12.5604-5608.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bruce MG, Bruden DL, Morris JM, et al. Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol Infect 2015;143:1236–46. 10.1017/S0950268814001770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu W, CD X, RP X, et al. Relation of environment to the prevalence of Helicobacter pylori infection in children. Zhong Guo Shi Yong Er Ke Za Zhi 2006:19–21. Article in Chinese. [Google Scholar]
- 63. Yu Y, Su L, Wang X, et al. Association between Helicobacter pylori infection and pathological changes in the gastric mucosa in Chinese children. Intern Med 2014;53:83–8. 10.2169/internalmedicine.53.0918 [DOI] [PubMed] [Google Scholar]
- 64. Ding Z, Zhao S, Gong S, et al. Prevalence and risk factors of Helicobacter pylori infection in asymptomatic Chinese children: a prospective, cross-sectional, population-based study. Aliment Pharmacol Ther 2015;42:1019–26. 10.1111/apt.13364 [DOI] [PubMed] [Google Scholar]
- 65. Zhang F, Pu K, Wu Z, et al. Prevalence and associated risk factors of Helicobacter pylori infection in the Wuwei cohort of north-western China. Trop Med Int Health 2021;26:290–300. 10.1111/tmi.13517 [DOI] [PubMed] [Google Scholar]
- 66. El-Omar EM, Oien K, Murray LS, et al. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology 2000;118:22–30. 10.1016/s0016-5085(00)70410-0 [DOI] [PubMed] [Google Scholar]
- 67. Rokkas T, Sechopoulos P, Pistiolas D, et al. Helicobacter pylori infection and gastric histology in first-degree relatives of gastric cancer patients: a meta-analysis. Eur J Gastroenterol Hepatol 2010;22:1128–33. 10.1097/MEG.0b013e3283398d37 [DOI] [PubMed] [Google Scholar]
- 68. Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol 2010;44:e34–9. 10.1097/MCG.0b013e3181a159c4 [DOI] [PubMed] [Google Scholar]
- 69. Dimitrov G, Gottrand F. Does gastric atrophy exist in children? World J Gastroenterol 2006;12:6274–9. 10.3748/wjg.v12.i39.6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kato S, Nakajima S, Nishino Y, et al. Association between gastric atrophy and Helicobacter pylori infection in Japanese children: a retrospective multicenter study. Dig Dis Sci 2006;51:99–104. 10.1007/s10620-006-3091-5 [DOI] [PubMed] [Google Scholar]
- 71. Kato S, Kikuchi S, Nakajima S. When does gastric atrophy develop in Japanese children? Helicobacter 2008;13:278–81. 10.1111/j.1523-5378.2008.00611.x [DOI] [PubMed] [Google Scholar]
- 72. Villarreal-Calderon R, Luévano-González A, Aragón-Flores M, et al. Antral atrophy, intestinal metaplasia, and preneoplastic markers in Mexican children with Helicobacter pylori-positive and Helicobacter pylori-negative gastritis. Ann Diagn Pathol 2014;18:129–35. 10.1016/j.anndiagpath.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boukthir S, Mrad SM, Kalach N, et al. Gastric atrophy and Helicobacter pylori infection in children. Trop Gastroenterol 2009;30:107–9. [PubMed] [Google Scholar]
- 74. Hoepler W, Hammer K, Hammer J. Gastric phenotype in children with Helicobacter pylori infection undergoing upper endoscopy. Scand J Gastroenterol 2011;46:293–8. 10.3109/00365521.2010.533383 [DOI] [PubMed] [Google Scholar]
- 75. Kalach N, Papadopoulos S, Asmar E, et al. In French children, primary gastritis is more frequent than Helicobacter pylori gastritis. Dig Dis Sci 2009;54:1958–65. 10.1007/s10620-008-0553-y [DOI] [PubMed] [Google Scholar]
- 76. Carvalho MA, Machado NC, Ortolan EVP, et al. Upper gastrointestinal histopathological findings in children and adolescents with nonulcer dyspepsia with Helicobacter pylori infection. J Pediatr Gastroenterol Nutr 2012;55:523–9. 10.1097/MPG.0b013e3182618136 [DOI] [PubMed] [Google Scholar]
- 77. Gastroenterology Group of Chinese Pediatric Association . Expert consensus on the diagnosis and treatment of H. pylori infection in children. Chin J Pediatr 2015;53:496–8. Article in Chinese. [Google Scholar]
- 78. Jones NL, Koletzko S, Goodman K. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nut 2017;64:991–1003. [DOI] [PubMed] [Google Scholar]
- 79. Kato S, Shimizu T, Toyoda S, et al. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr Int 2020;62:1315–31. 10.1111/ped.14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Choi IJ, Kook M-C, Kim Y-I, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018;378:1085–95. 10.1056/NEJMoa1708423 [DOI] [PubMed] [Google Scholar]
- 81. Nguyen CT, Davis KA, Nisly SA, et al. Treatment of Helicobacter pylori in special patient populations. Pharmacotherapy 2019;39:1012–22. 10.1002/phar.2318 [DOI] [PubMed] [Google Scholar]
- 82. Wang J-B. Benefit/risk assessment and issues related to antibiotic use of Helicobacter pylori eradication in elderly individuals. Zhonghua Yi Xue Za Zhi 2020;100:2343–5. 10.3760/cma.j.cn112137-20200524-01640 [DOI] [PubMed] [Google Scholar]
- 83. Boyanova L, Gergova G, Markovska R, et al. Primary Helicobacter pylori resistance in elderly patients over 20 years: A Bulgarian study. Diagn Microbiol Infect Dis 2017;88:264–7. 10.1016/j.diagmicrobio.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 84. Ji Z, Han F, Meng F, et al. The association of age and antibiotic resistance of Helicobacter pylori: a study in Jiaxing City, Zhejiang Province, China. Medicine 2016;95:e2831. 10.1097/MD.0000000000002831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhao J-B, Yuan L, Yu X-C, et al. Whole family-based Helicobacter pylori eradication is a superior strategy to single-infected patient treatment approach: a systematic review and meta-analysis. Helicobacter 2021;26:e12793. 10.1111/hel.12793 [DOI] [PubMed] [Google Scholar]
- 86. Zhou G. Helicobacter pylori recurrence after eradication therapy in Jiangjin District, Chongqing, China. Gastroenterol Res Pract 2020;2020:7510872. 10.1155/2020/7510872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xue Y, Zhou L-Y, Lu H-P, et al. Recurrence of Helicobacter pylori infection: incidence and influential factors. Chin Med J 2019;132:765–71. 10.1097/CM9.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hu Y, Wan J-H, Li X-Y, et al. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther 2017;46:773–9. 10.1111/apt.14319 [DOI] [PubMed] [Google Scholar]
- 89. Kim MS, Kim N, Kim SE, et al. Long-term follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter 2013;18:135–42. 10.1111/hel.12018 [DOI] [PubMed] [Google Scholar]
- 90. Ryu KH, Yi SY, Na YJ, et al. Reinfection rate and endoscopic changes after successful eradication of Helicobacter pylori. World J Gastroenterol 2010;16:251–5. 10.3748/wjg.v16.i2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xie Y, Song C, Cheng H, et al. Long-term follow-up of Helicobacter pylori reinfection and its risk factors after initial eradication: a large-scale multicentre, prospective open cohort, observational study. Emerg Microbes Infect 2020;9:548–57. 10.1080/22221751.2020.1737579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Niv Y, Hazazi R. Helicobacter pylori recurrence in developed and developing countries: meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter 2008;13:56–61. 10.1111/j.1523-5378.2008.00571.x [DOI] [PubMed] [Google Scholar]
- 93. Jiang C-L, Cai Q-Z, Mao W-H, et al. Role of comprehensive treatment in Helicobacter pylori eradication. Chin J Clin Gastroenterol 2014;26:203–5. Article in Chinese. [Google Scholar]
- 94. Farrell S, Milliken I, Doherty GM, et al. Total family unit Helicobacter pylori eradication and pediatric re-infection rates. Helicobacter 2004;9:285–8. 10.1111/j.1083-4389.2004.00240.x [DOI] [PubMed] [Google Scholar]
- 95. Knippig C, Arand F, Leodolter A, et al. Prevalence of H. pylori-infection in family members of H. pylori positive and its influence on the reinfection rate after successful eradication therapy: a two-year follow-up. Z Gastroenterol 2002;40:383–7. 10.1055/s-2002-32128 [DOI] [PubMed] [Google Scholar]
- 96. Gisbert JP, Arata IG, Boixeda D, et al. Role of partner's infection in reinfection after Helicobacter pylori eradication. Eur J Gastroenterol Hepatol 2002;14:865–71. 10.1097/00042737-200208000-00009 [DOI] [PubMed] [Google Scholar]
- 97. Rugge M, Genta RM, Di Mario F, et al. Gastric cancer as preventable disease. Clin Gastroenterol Hepatol 2017;15:1833–43. 10.1016/j.cgh.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 98. Amieva M, Peek RM. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 2016;150:64–78. 10.1053/j.gastro.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Leung WK, Ng EKW, Lam CCH, et al. Helicobacter pylori infection in 1st degree relatives of Chinese gastric cancer patients. Scand J Gastroenterol 2006;41:274–9. 10.1080/00365520510024269 [DOI] [PubMed] [Google Scholar]
- 100. Chooi EYH, Chen H-M, Miao Q, et al. Chronic atrophic gastritis is a progressive disease: analysis of medical reports from Shanghai (1985-2009). Singapore Med J 2012;53:318–24. [PubMed] [Google Scholar]
- 101. Hatakeyama M. Malignant Helicobacter pylori-associated diseases: gastric cancer and MALT lymphoma. Adv Exp Med Biol 2019;1149:135–49. 10.1007/5584_2019_363 [DOI] [PubMed] [Google Scholar]
- 102. Miura M, Ohnishi N, Tanaka S, et al. Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int J Cancer 2009;125:2497–504. 10.1002/ijc.24740 [DOI] [PubMed] [Google Scholar]
- 103. Yuan L, Zhao J-B, Zhou Y-L, et al. Type I and type II Helicobacter pylori infection status and their impact on gastrin and pepsinogen level in a gastric cancer prevalent area. World J Gastroenterol 2020;26:3673–85. 10.3748/wjg.v26.i25.3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pan K-F, Formichella L, Zhang L, et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int J Cancer 2014;134:2118–25. 10.1002/ijc.28560 [DOI] [PubMed] [Google Scholar]
- 105. Ding S-Z, Goldberg JB, Hatakeyama M. Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol 2010;6:851–62. 10.2217/fon.10.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ansari S, Yamaoka Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins 2019;11:667. 10.3390/toxins11110677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res 2008;68:379–87. 10.1158/0008-5472.CAN-07-0824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A 2008;105:1003–8. 10.1073/pnas.0711183105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Matos JI, de Sousa HAC, Marcos-Pinto R, et al. Helicobacter pylori CagA and VacA genotypes and gastric phenotype: a meta-analysis. Eur J Gastroenterol Hepatol 2013;25:1431–41. 10.1097/MEG.0b013e328364b53e [DOI] [PubMed] [Google Scholar]
- 110. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–7. 10.1016/S0140-6736(08)61159-9 [DOI] [PubMed] [Google Scholar]
- 111. Liu W-Z. The safety of using bismuth compounds in the treatment of Helicobacter pylori. Chin J Dig 2017;37:877–8. Article in Chinese. [Google Scholar]
- 112. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–8. 10.1136/gutjnl-2015-311019 [DOI] [PubMed] [Google Scholar]
- 113. Yang J-C, Lin C-J, Wang H-L, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2015;13:895–905. 10.1016/j.cgh.2014.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang J, Zhang Y, Fan L, et al. Correction: Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol 2019;114:835. 10.14309/ajg.0000000000000230 [DOI] [PubMed] [Google Scholar]
- 115. Pan K-F, Zhang L, Gerhard M, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 2016;65:9–18. 10.1136/gutjnl-2015-309197 [DOI] [PubMed] [Google Scholar]
- 116. Yi D-M, Yang T-T, Chao S-H, et al. Comparison the cost-efficacy of furazolidone-based versus clarithromycin-based quadruple therapy in initial treatment of Helicobacter pylori infection in a variable clarithromycin drug-resistant region, a single-center, prospective, randomized, open-label study. Medicine 2019;98:e14408. 10.1097/MD.0000000000014408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cui R, Song Z, Suo B, et al. Correlation analysis among genotype resistance, phenotype eesistance and eradication effect of Helicobacter pylori. Infect Drug Resist 2021;14:1747–56. 10.2147/IDR.S305996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Luo L, Huang Y, Liang X, et al. Susceptibility-guided therapy for Helicobacter pylori-infected penicillin-allergic patients: a prospective clinical trial of first-line and rescue therapies. Helicobacter 2020;25:e12699. 10.1111/hel.12699 [DOI] [PubMed] [Google Scholar]
- 119. Liu D-S, Wang Y-H, Zhu Z-H, et al. Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob Resist Infect Control 2019;8:192. 10.1186/s13756-019-0632-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sapmaz F, Kalkan IH, Atasoy P, et al. A non-inferiority study: modified dual therapy consisting higher doses of rabeprazole is as successful as standard quadruple therapy in eradication of Helicobacter pylori. Am J Ther 2017;24:e393–8. 10.1097/MJT.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 121. Yang X, Wang J-X, Han S-X, et al. High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: a systematic review and meta-analysis. Medicine 2019;98:e14396. 10.1097/MD.0000000000014396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther 2017;46:106–14. 10.1111/apt.14130 [DOI] [PubMed] [Google Scholar]
- 123. Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of Vonoprazan for Helicobacter pylori eradication. Intern Med 2020;59:153–61. 10.2169/internalmedicine.2521-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut 2020;69:1019–26. 10.1136/gutjnl-2019-319954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rokkas T, Gisbert JP, Malfertheiner P, et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: a network meta-analysis. Gastroenterology 2021;161:495–507. 10.1053/j.gastro.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 126. Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019;24:e12554. 10.1111/hel.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zou Y, Qian X, Liu X, et al. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: a systematic review and meta-analysis. Helicobacter 2020;25:e12714. 10.1111/hel.12714 [DOI] [PubMed] [Google Scholar]
- 128. Camargo MC, García A, Riquelme A, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol 2014;109:485–95. 10.1038/ajg.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics 2020;9:671. 10.3390/antibiotics9100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chen Q, Long X, Ji Y, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther 2019;49:1385–94. 10.1111/apt.15273 [DOI] [PubMed] [Google Scholar]
- 131. Best LM, Takwoingi Y, Siddique S, et al. Non-Invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev 2018;3:CD012080. 10.1002/14651858.CD012080.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Godbole G, Mégraud F, Bessède E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter 2020;25 Suppl 1:e12735. 10.1111/hel.12735 [DOI] [PubMed] [Google Scholar]
- 133. Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol 2006;101:1921–30. 10.1111/j.1572-0241.2006.00668.x [DOI] [PubMed] [Google Scholar]
- 134. Logan RP. Urea breath tests in the management of Helicobacter pylori infection. Gut 1998;43 Suppl 1:S47–50. 10.1136/gut.43.2008.S47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol 2015;21:1305–14. 10.3748/wjg.v21.i4.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhou Q, Li L, Ai Y, et al. Diagnostic accuracy of the 14C-urea breath test in Helicobacter pylori infections: a meta-analysis. Wien Klin Wochenschr 2017;129:38–45. 10.1007/s00508-016-1117-3 [DOI] [PubMed] [Google Scholar]
- 137. Alzoubi H, Al-Mnayyis Asma'a, Al Rfoa I, et al. The use of 13C-urea breath test for non-invasive diagnosis of Helicobacter pylori infection in comparison to endoscopy and stool antigen test. Diagnostics 2020;10:448. 10.3390/diagnostics10070448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wong BC, Wong WM, Wang WH, et al. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther 2001;15:505–11. 10.1046/j.1365-2036.2001.00947.x [DOI] [PubMed] [Google Scholar]
- 139. Wang Y-K, Kuo F-C, Liu C-J, et al. Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol 2015;21:11221–35. 10.3748/wjg.v21.i40.11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Atkinson NSS, Braden B. Helicobacter pylori Infection: Diagnostic strategies in primary diagnosis and after therapy. Dig Dis Sci 2016;61:19–24. 10.1007/s10620-015-3877-4 [DOI] [PubMed] [Google Scholar]
- 141. Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007;20:280–322. 10.1128/CMR.00033-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xia HH-X, Wong BCY, Wong WM, et al. Optimal serological tests for the detection of Helicobacter pylori infection in the Chinese population. Aliment Pharmacol Ther 2002;16:521–6. 10.1046/j.1365-2036.2002.01176.x [DOI] [PubMed] [Google Scholar]
- 143. H-J Su, Ge Z-Z, Xiang Z-Q, et al. Evalation of detecting methods of Helicoabter pylori infection histology, serology, 13C-urea breath test, and rapid urease test. Chin J Dig 1998;18:260–2. Article in Chinese. [Google Scholar]
- 144. Leung WK, Ng EK, Chan FK, et al. Evaluation of three commercial enzyme-linked immunosorbent assay kits for diagnosis of Helicobacter pylori in Chinese patients. Diagn Microbiol Infect Dis 1999;34:13–17. 10.1016/S0732-8893(99)00002-4 [DOI] [PubMed] [Google Scholar]
- 145. Han F-C, Li X-J, Jiang H, et al. Detection of H. pylori antibody profile in serum by protein array. World J Gastroenterol 2006;12:4044–8. 10.3748/wjg.v12.i25.4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Li Y-H, Guo H, Zhang P-B, et al. Clinical value of Helicobacter pylori stool antigen test, ImmunoCard STAT HpSA, for detecting H pylori infection. World J Gastroenterol 2004;10:913–4. 10.3748/wjg.v10.i6.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wong BCY, Xia HHX, Cheung HKL, et al. Evaluation of two stool antigen tests for the detection of Helicobacter pylori infection in the Chinese population. J Gastroenterol Hepatol 2003;18:26–31. 10.1046/j.1440-1746.2003.02926.x [DOI] [PubMed] [Google Scholar]
- 148. Hussein RA, Al-Ouqaili MTS, Majeed YH. Detection of Helicobacter pylori infection by invasive and non-invasive techniques in patients with gastrointestinal diseases from Iraq: a validation study. PLoS One 2021;16:e0256393. 10.1371/journal.pone.0256393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McNicholl AG, Ducons J, Barrio J, et al. Accuracy of the ultra-rapid urease test for diagnosis of Helicobacter pylori infection. Gastroenterol Hepatol 2017;40:651–7. 10.1016/j.gastrohep.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 150. Aftab H, Yamaoka Y, Ahmed F, et al. Validation of diagnostic tests and epidemiology of Helicobacter pylori infection in Bangladesh. J Infect Dev Ctries 2018;12:305–12. 10.3855/jidc.9841 [DOI] [PubMed] [Google Scholar]
- 151. Hartman DJ, Owens SR. Are routine ancillary stains required to diagnose Helicobacter infection in gastric biopsy specimens? An institutional quality assurance review. Am J Clin Pathol 2012;137:255–60. 10.1309/AJCPD8FFBJ5LSLTE [DOI] [PubMed] [Google Scholar]
- 152. Allahverdiyev AM, Bagirova M, Caliskan R, et al. Isolation and diagnosis of Helicobacter pylori by a new method: microcapillary culture. World J Gastroenterol 2015;21:2622–8. 10.3748/wjg.v21.i9.2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Luo M, Hao Y, Tang M, et al. Application of a social media platform as a patient reminder in the treatment of Helicobacter pylori. Helicobacter 2020;25:e12682. 10.1111/hel.12682 [DOI] [PubMed] [Google Scholar]
- 154. Zheng H, Xie Q, Zhan M, et al. Cost‑effectiveness analysis of Helicobacter pylori eradication therapy in first-degree relatives of patients with gastric cancer. Patient Prefer Adherence 2021;15:77–85. 10.2147/PPA.S286860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Han Y, Yan T, Ma H, et al. Cost-effectiveness analysis of Helicobacter pylori eradication therapy for prevention of gastric cancer: A Markov Model. Dig Dis Sci 2020;65:1679–88. 10.1007/s10620-019-05910-1 [DOI] [PubMed] [Google Scholar]
- 156. Yeh JM, Kuntz KM, Ezzati M, et al. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer 2009;124:157–66. 10.1002/ijc.23864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zeng M, Mao X-H, Li J-X, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1457–64. 10.1016/S0140-6736(15)60310-5 [DOI] [PubMed] [Google Scholar]
- 158. Stubljar D, Jukic T, Ihan A. How far are we from vaccination against Helicobacter pylori infection? Expert Rev Vaccines 2018;17:935–45. 10.1080/14760584.2018.1526680 [DOI] [PubMed] [Google Scholar]
- 159. Malfertheiner P, Selgrad M, Wex T, et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: a randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol Hepatol 2018;3:698–707. 10.1016/S2468-1253(18)30125-0 [DOI] [PubMed] [Google Scholar]
- 160. Ding S-Z, Zheng P-Y. Helicobacter pylori infection induced gastric cancer; advance in gastric stem cell research and the remaining challenges. Gut Pathog 2012;4:18. 10.1186/1757-4749-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ramírez-Lázaro MJ, Lite J, Lario S, et al. Good diagnostic accuracy of a chemiluminescent immunoassay in stool samples for diagnosis of Helicobacter pylori infection in patients with dyspepsia. J Investig Med 2016;64:388–91. 10.1136/jim-2015-000004 [DOI] [PubMed] [Google Scholar]
- 162. Miftahussurur M, Windia A, Syam AF, et al. Diagnostic value of 14C-urea breath test for Helicobacter pylori detection compared by histopathology in indonesian dyspeptic patients. Clin Exp Gastroenterol 2021;14:291–6. 10.2147/CEG.S306626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-325630supp001.pdf (1.3MB, pdf)
gutjnl-2021-325630supp002.pdf (196.8KB, pdf)